- 1School of Psychological and Cognitive Sciences, Peking University, Beijing, China
- 2Beijing Key Laboratory of Behavior and Mental Health, Peking University, Beijing, China
- 3CAS Key Laboratory of Mental Health, Institute of Psychology, Beijing, China
- 4Department of Psychology, University of Chinese Academy of Sciences, Beijing, China
- 5Department of Pain Management, The State Key Clinical Specialty in Pain Medicine, The Second Affiliated Hospital of Guangzhou Medical University, Guangzhou, China
- 6PKU-IDG/McGovern Institute for Brain Research, Peking University, Beijing, China
Chronic back pain (CBP) is a leading cause of disability and results in considerable socio-economic burdens worldwide. Although CBP patients are commonly diagnosed and treated with a focus on the “end organ dysfunction” (i.e., peripheral nerve injuries or diseases), the evaluation of CBP remains flawed and problematic with great challenges. Given that the peripheral nerve injuries or diseases are insufficient to define the etiology of CBP in some cases, the evaluation of alterations in the central nervous system becomes particularly necessary and important. With the development of advanced neuroimaging techniques, extensive studies have been carried out to identify the cortical abnormalities in CBP patients. Here, we provide a comprehensive overview on a series of novel findings from these neuroimaging studies to improve our understanding of the cortical abnormalities originated in the disease. First, CBP patients normally exhibit central sensitization to external painful stimuli, which is indexed by increased pain sensitivity and brain activations in pain-related brain regions. Second, long-term suffering from chronic pain leads to emotional disorders, cognitive impairments, and the abnormalities of the relevant brain networks among CBP patients. Third, CBP is associated with massive cortical reorganization, including structural, functional, and metabolic brain changes. Overall, a deep insight into the neural mechanisms underlying the development and outcome of CBP through more sophisticated neuroimaging investigations could not only improve our current understanding of the etiology of CBP but also facilitate the diagnosis and treatment of CBP based on precision medicine.
Introduction
As a substantial worldwide health problem, chronic back pain (CBP) is one of the most frequent complaints and the second most common symptom reported by patients during their primary physical care visits (Mantyselka et al., 2001; Vogt et al., 2005). Being more prevalent in females (Hoy et al., 2012; Maher et al., 2017), CBP has been introduced as the first leading cause of years of lived with disability (YLDs) in 2016, with the incidence of 57.6 million YLDs all over the world (Vos et al., 2017). In western countries, the prevalence of lifetime CBP ranges from 49% to 70% (Koes et al., 2006), seriously impairing the quality of life in these patients (Ricci et al., 2006). In addition, CBP imposes considerable socio-economic burdens and leads to rigorous challenges for healthcare program developments (Vogt et al., 2005; Dagenais et al., 2008).
It is widely accepted that CBP can be caused by anatomical abnormalities or systemic diseases in/around the spinal cord (i.e., peripheral level), including lesions or degenerations in certain structures of the spine (Deyo et al., 1992; Dixit and Dickson, 2018). Therefore, in the diagnosis and treatment of CBP, it is reasonable that clinicians commonly focus on the “end organ dysfunction,” where structural and functional abnormalities could be found within the musculoskeletal system (Robinson and Apkarian, 2009; Wand et al., 2011). However, a specific pathoanatomical diagnosis of the pain generators cannot be precisely identified in 90% of CBP patients with apparent symptoms (Koes et al., 2006; Maher et al., 2017), i.e., most CBP patients are non-specific, and characterized by a range of biophysical, psychological, and social factors with an extreme variability in genesis (Hartvigsen et al., 2018). Therefore, it remains difficult to accurately diagnose and evaluate non-specific CBP to date. Consequently, there is no doubt that such imprecise diagnosis and evaluation of CBP hamper the individualized intervention based on the etiology of the disease itself (Wand and O’Connell, 2008; Peng et al., 2017), leading to the prolongation of treatment duration and the deterioration of the health condition in patients (Heithoff and Burton, 1985; Chou et al., 2007).
Theoretically, the imprecise diagnosis of CBP could be caused by the possible dissociation between nociception (nociceptive inputs caused by injuries or diseases at the peripheral level) and pain (a conscious experience in the brain) (Craufurd et al., 1990; Loeser, 1991; Mee et al., 2006; Hu and Iannetti, 2019). Pain can occur in the absence of nociception, and the link between nociception and pain is heavily dependent on various factors, including cognitive condition (e.g., attention, expectation, and context) and emotional state/trait (e.g., depression, anxiety, and catastrophizing) (Loeser, 1991; Rhudy et al., 2006). Based on these theoretical perspectives, accumulating evidence has revealed potential obstacles in diagnosis and treatment of CBP, spanning from the aspects of structural (Apkarian et al., 2004; Schmidt-Wilcke et al., 2006; Baliki et al., 2011b; Seminowicz et al., 2011, 2013; Ivo et al., 2013; Fritz et al., 2016), functional (Giesecke et al., 2004; Baliki et al., 2011a, 2014; Seminowicz et al., 2011; Berger et al., 2014; Mao et al., 2014; Yu et al., 2014; Pijnenburg et al., 2015; Hotz-Boendermaker et al., 2016; Letzen and Robinson, 2017), to metabolic abnormalities in the brain (Grachev et al., 2000, 2001, 2002, 2003; Gussew et al., 2011). Since the properties of the peripheral injury or disease are insufficient to characterize CBP, it would be important to evaluate the cortical abnormalities for a better understanding of the causes and consequences of CBP (Wand et al., 2011; Ng et al., 2018).
In clinical practice, an integrated diagnosis strategy that not only assesses injuries or diseases in/around the spinal cord but also evaluates cortical abnormalities is highly needed to optimize the treatment strategies for CBP patients, especially for non-specific CBP patients. In the present study, we overviewed findings in recent research that evaluates cortical abnormalities in CBP patients using advanced neuroimaging techniques, and discussed some perspectives on how to improve the diagnosis of the disease (please note that there are not enough studies exploring cortical abnormalities in specific CBP patients, and the possible differences of brain alterations in specific and non-specific CBP patients are not considered in the present study).
Cortical Evaluations Using Neuroimaging Techniques
Several non-invasive neuroimaging techniques with different underlying physical principles are widely adopted to evaluate the cortical abnormalities in CBP patients (Aine, 1995; Chen, 2001), including structural and functional magnetic resonance imaging (MRI; Table 1), electroencephalography (EEG; Table 2), magnetoencephalography (MEG; Table 2), and magnetic resonance spectroscopy (MRS; Table 3). In the following sections, we provided a comprehensive review of recent studies that investigated brain alterations in CBP patients by these techniques.
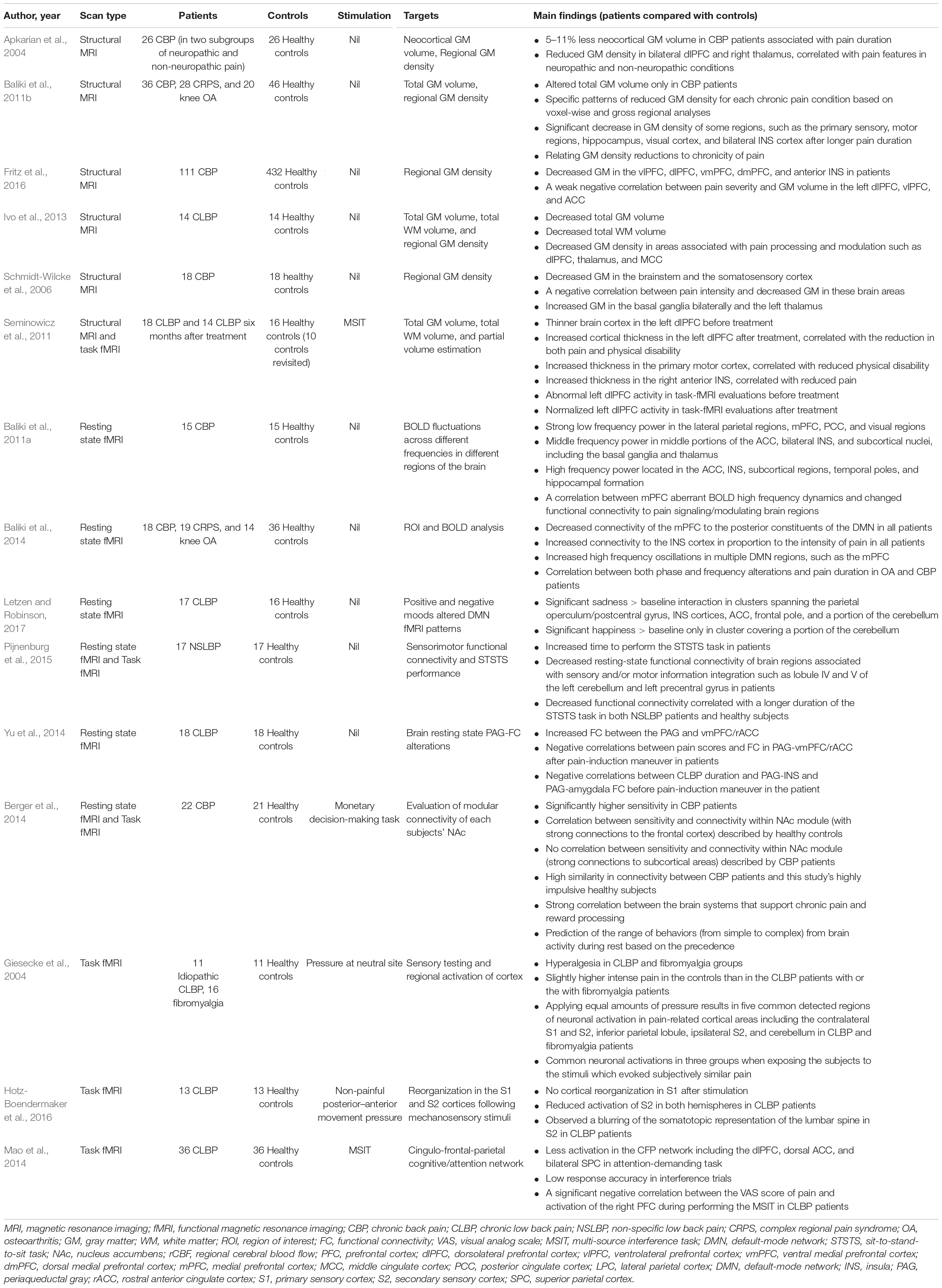
Table 1. Evaluation of cortical alterations in CBP patients using structural and functional MRI techniques.
Structural and Functional MRI Studies
The structural MRI could provide anatomical information of the brain with high spatial resolution, and the functional MRI (fMRI) is used to determine the location of the “activate” brain regions during cognitive tasks (Aine, 1995; Lindquist, 2008; Huettel et al., 2009; Sadek, 2012). Both techniques are helpful to provide information about brain organization and offer potential new criteria for assessing the neurological status and neurosurgical risk; thus they are widely employed to characterize structural or functional brain alterations among CBP patients under clinical settings (Wand et al., 2011; Ng et al., 2018). Since these neuroimaging techniques have distinct advantages, we reviewed studies that explore the cortical abnormalities in CBP patients using structural MRI, resting state fMRI, and task fMRI, respectively.
By extracting morphological features from structural MRI through some advanced analysis techniques (e.g., voxel-based morphometry), several crucial anatomical changes have been observed in CBP patients. In a pilot study, CBP patients exhibited decreased neocortical gray matter (GM) volume (5–11% less than healthy controls), with the magnitude equivalent to the loss quantity caused by 10–20 years of normal aging (Apkarian et al., 2004). Meanwhile, several studies have reported that the GM density of CBP patients was significantly reduced in a series of pain-related brain regions, including the dorsolateral prefrontal cortex (dlPFC) (Apkarian et al., 2004; Schmidt-Wilcke et al., 2006; Fritz et al., 2016), dorsomedial prefrontal cortex (dmPFC) (Fritz et al., 2016), ventrolateral prefrontal cortex (vlPFC) (Fritz et al., 2016), ventromedial prefrontal cortex (vmPFC) (Fritz et al., 2016), primary somatosensory cortex (S1) (Schmidt-Wilcke et al., 2006; Baliki et al., 2011b; Ivo et al., 2013), secondary somatosensory cortex (S2) (Schmidt-Wilcke et al., 2006; Baliki et al., 2011b; Ivo et al., 2013), insula (INS) (Baliki et al., 2011b; Fritz et al., 2016), middle cingulate cortex (Ivo et al., 2013), thalamus (Apkarian et al., 2004; Ivo et al., 2013), and brainstem (Schmidt-Wilcke et al., 2006). Please note that these brain regions are also functionally associated with some clinical symptoms (e.g., subjective ratings of pain intensity, emotional disorders, and cognitive impairments) of CBP, and the relevant findings are detailed in the following paragraphs. In addition, the GM densities of some brain regions (dorsal rostral pons and somatosensory cortices) demonstrated significant negative correlations with both the subjective ratings of pain intensity and unpleasantness (Schmidt-Wilcke et al., 2006). Importantly, the structural abnormalities in the brain can be reversed by effective CBP treatments. For example, increased cortical thickness in the left dlPFC after treatment could be observed in CBP patients compared to that before treatment, and such improvement in brain structure was positively correlated with the reduction of pain and physical disability (Seminowicz et al., 2011, 2013). All these morphological findings indicated that CBP is accompanied by brain atrophy in regions commonly associated with pain processing and modulation, which has a great influence on pain chronicity (Li and Hu, 2016).
As an effective technique to map white matter (WM) tractography in the brain (Pierpaoli et al., 1996; Basser and Jones, 2002), diffusion tensor imaging (DTI) has been used to study the WM architecture and integrity in CBP patients (Buckalew et al., 2010; Čeko et al., 2015). Lower WM integrity of the splenium of the corpus callosum was found in disabled CBP patients (Buckalew et al., 2010), and importantly, negative correlation between total months of CBP and WM integrity of the splenium of the corpus callosum was observed. In addition, Vania Apkarian and his colleagues tracked brain properties in subacute back pain patients longitudinally for 1 year (Mansour et al., 2013) and 3 years (Vachon-Presseau et al., 2016), as their pain either recovered or transitioned to chronic pain. Testing the role of the corticolimbic system in the development of CBP, Vania Apkarian and his colleagues observed that the dorsal medial prefrontal cortex (mPFC)-amygdala-nucleus accumbens network contributing to risk of chronic pain, which suggested that corticolimbic neuroanatomical factors were important features to predispose subacute back pain patients to recover from or transition to chronic pain (Vachon-Presseau et al., 2016).
The resting state fMRI and task fMRI are normally applied to investigate functional alterations in CBP patients by measuring the spontaneous blood-oxygen-level dependent (BOLD) activities of brain networks at resting state (Baliki et al., 2006; Barkhof et al., 2014; Vachon-Presseau et al., 2019; Zhang et al., 2019) and the evoked BOLD responses during pre-defined tasks/stimuli (Aine, 1995; Lindquist, 2008), respectively. Previous studies using resting state fMRI revealed that CBP patients exhibited reduced deactivation in the mPFC, amygdala, and posterior cingulate cortex, which were considered as key brain regions in the default mode network (DMN) (Baliki et al., 2008). The disruptions of the DMN were related to the cognitive and behavioral impairments associated with chronic pain (Baliki et al., 2008). Additionally, the resting state functional connectivity (FC) of the DMN network was reported to be influenced by negative mood in CBP patients, which implied that the abnormalities of the DMN were related to the information processing of affective-motivational aspect of pain (Letzen and Robinson, 2017). Apart from the DMN network, decreased resting state FC of the sensorimotor network was also observed in CBP patients, which was associated with the performance of a dynamic sensorimotor task (i.e., the duration of performing the sit-to-stand-to-sit task) (Pijnenburg et al., 2015). Notably, increased resting state FC between periaqueductal gray (PAG, a key region in the descending pain modulation pathway) and vmPFC/rostral anterior cingulate cortex (ACC) was represented in CBP patients compared to the one in healthy controls, suggesting an abnormal function of the PAG-centered descending pain modulation system in CBP patients (Yu et al., 2014). Additionally, abnormal FC between mPFC/ACC and brain regions within the DMN was observed in CBP patients, and the abnormal FC was also found to be correlated with pain duration, pain severity, and pain interference (Tu et al., 2019a). Importantly, Vania Apkarian and his colleagues focused on investigating the neural mechanism associated with pain chronification, and obtained several novel findings. For example, they found that corticostriatal FC (between nucleus accumbens and prefrontal cortex) is an accurate predictor of the transition from acute to chronic pain (Baliki et al., 2012; Vachon-Presseau et al., 2016). In addition, they observed that brain activity associated with acute/subacute back pain is limited to regions involved in acute pain, while brain activity related to chronic pain is confined to emotion-related circuitry (Hashmi et al., 2013). This observation suggested that brain representation for back pain can undergo large-scare shifts in brain activity with pain chronification.
Consistently, evidence from the task fMRI revealed that CBP patients exhibited abnormal brain functions related to pain processing (Giesecke et al., 2004). For example, relative to the healthy controls, the CBP patients reported significant higher pain intensity when received painful pressure with fixed physical intensity, and showed stronger activations in several pain-related brain regions, including the contralateral S1, bilateral S2, inferior parietal lobule, and cerebellum (Giesecke et al., 2004), which indicated that CBP patients have increased pain sensitivity. In contrast, when receiving non-painful movement pressure, CBP patients showed a decreased somatosensory acuity and reduced activations of bilateral S2, suggesting a reorganization of higher order processing of sensory information in these patients (Hotz-Boendermaker et al., 2016). In addition, brain dysfunction in emotional and cognitive disorders caused by the maladaptation to chronic pain was frequently reported (Seminowicz et al., 2011; Berger et al., 2014; Mao et al., 2014). For example, in an attention-demanding cognitive task, the impaired cognitive ability and abnormal activation of cingulo-frontal-parietal (CFP) cognitive/attention network (Mao et al., 2014), especially in the dlPFC (Seminowicz et al., 2011), were observed in CBP patients. Further, risky monetary behavior and altered connectivity of the nucleus accumbens (a key brain region in reward processing) were observed in CBP patients (Berger et al., 2014), and such observation has been interpreted as a consequence of cognitive disorders or comorbidity of chronic pain.
EEG and MEG Studies
Different from MRI techniques that could provide massive spatial information related to cortical regions/networks involved in pain processing, EEG/MEG techniques can measure the cortical changes with a high temporal resolution, thus giving a deep insight into the dynamic process of pain information processing (Chen, 2001; Kucyi and Davis, 2015). Nowadays, crucial progress has been made in the evaluation of cortical dysfunction in CBP patients with EEG/MEG techniques. It is generally accepted that the central sensitization (represented by reduced pain threshold, pain tolerance, and increased perceived pain intensity) and the cortical processing of the sensory-discriminative aspect of pain were significantly enhanced in CBP patients (Flor et al., 1997b, 2004; Diers et al., 2007). For example, a larger amplitude of the early N80 component in somatosensory event-related potentials (ERPs) elicited by painful electrical intramuscular and intracutaneous stimuli was observed in CBP patients (Diers et al., 2007), indicating a central sensitization among these patients. Accordingly, when receiving intracutaneous electrical painful stimuli, CBP patients showed significant larger power of early evoked MEG response than healthy controls did, and the power of this early response was positively correlated with the chronicity in CBP patients (Flor et al., 1997a), which provided a strong evidence that pain chronicity is accompanied with central sensitization, resulting in the abnormal information processing of the sensory-discriminative aspect of pain (Flor et al., 1997a; Diers et al., 2007).
In addition, CBP patients showed evident abnormalities in emotional and cognitive functions. For example, when being assessed the emotional decision-making abilities using the Iowa gambling task, CBP patients scored much lower than healthy controls did, and their performance was significantly influenced by the duration and intensity of their chronic pain (Tamburin et al., 2014). Consistent with this behavioral result, the ERP data showed abnormal feedback processing in CBP patients during the Iowa gambling task (Tamburin et al., 2014). Specifically, the amplitude of feedback-related negativity (FRN) was higher in wins than in losses in healthy controls, while the opposite results were obtained in CBP patients; the amplitude of P300 was higher in wins than in losses in healthy controls, whereas no significant difference was observed in CBP patients. The abnormal feedback cognitive processing resulting in the impairments in the work and family settings were often reported by CBP patients (Tamburin et al., 2014). Moreover, CBP patients showed a lower amplitude of the later P260 component in somatosensory ERPs evoked by painful electrical stimuli, which also suggested the deficiency of higher cognitive functions in CBP patients (e.g., the function related to affective distress) (Diers et al., 2007).
Accompanied by the long-term changes of cortical function, cortical reorganization in CBP patients due to the processes of neuronal plasticity was well documented (Flor et al., 1997a; Wiech et al., 2000). Demonstrated by an MEG study, alterations in the somatotopic organization of the S1 were observed in CBP patients (Wiech et al., 2000). Specifically, being elicited by intracutaneous electrical stimuli with different intensities (from non-painful to painful), the maximal response in the primary somatosensory cortex was shifted more medially in CBP patients than in healthy controls (Flor et al., 1997a). Importantly, such brain reorganization was correlated with subjective pain ratings (Wiech et al., 2000). In summary, chronic pain is accompanied by cortical reorganization, an important neural marker indicating the persistence of the pain experience and the dysfunction of cortical processing. However, the potential relationships between findings obtained using EEG/MEG and MRI techniques in evaluating cortical alterations in CBP patients remain to be elucidated.
MRS Studies
Chemical changes in the brain of CBP patients can be detected using in vivo single-voxel proton MRS (1H-MRS), which is able to provide additional evidence on abnormal brain alterations associated with chronic pain (Gussew et al., 2011; Zhao et al., 2017). Several MRS studies showed that a reduced level of N-acetyl aspartate (NAA) was observed in several brain regions of CBP patients, including the dlPFC, orbitofrontal cortex (OFC), anterior INS, ACC, and thalamus (Grachev et al., 2000, 2003; Gussew et al., 2011; Sharma et al., 2011, 2012). In addition, some studies reported that CBP patients had reduced levels of glutamate (Glu) in the ACC (Gussew et al., 2011), glucose in the dlPFC (Grachev et al., 2000), and myo-inositol (mI) in the ACC and thalamus (Gussew et al., 2011). These brain chemical imbalances were negatively correlated with pain intensity in CBP patients (Grachev et al., 2000). Importantly, certain changes in brain chemistry were shown to be highly correlated with psychological factors (Siddall et al., 2006). For example, the levels of NAA in the right dlPFC and OFC were, respectively, correlated with depression (Grachev et al., 2003) and anxiety (Grachev et al., 2001, 2002) levels in CBP patients. Therefore, it would be reasonable to hypothesize that brain chemistry changes play an important role in the development and maintenance of CBP and its comorbidity (e.g., depression and anxiety) (Grachev et al., 2000, 2001, 2002, 2003). Given that the reduced Glu level may indicate disordered glutamatergic neurotransmission, and the reduced levels of NAA and mI could be related to the loss of neurons and glial cells (Gussew et al., 2011), these alterations in the brain biochemical profile in CBP patients could represent the cortical reorganization caused by long-term pain suffering (Grachev et al., 2000, 2001, 2002, 2003).
Discussion
Chronic back pain is a substantial worldwide health problem. The need for treatment of CBP based on a deeper understanding of its causes and outcomes is urgent and pressing in clinic. Considering that chronic pain is associated with several psychological disorders (e.g., depression, anxiety, and sleep disturbances) involving cortical dysfunction, more and more researchers shift their interests from the peripheral level to the cortical level. With the development of non-invasive neuroimaging techniques, a series of novel findings were obtained in this field.
First, despite CBP patients have sensorimotor impairments (e.g., decreased sensitivity to innocuous stimuli), they have enhanced central sensitization to external painful stimuli, manifested by increased subjective pain sensitivity and increased brain activations in pain-related brain regions. It has been reported that increased pain sensitization and decreased sensitivity to innocuous stimuli in CBP patients are associated with increased catastrophizing, which is also linked with increased clinical back pain (Meints et al., 2019). This central sensitization could serve as an overgeneralized protective function to prevent the injured spinal cord from being irritated by the harmful sensory inputs (Apkarian and Reckziegel, 2019). However, since the nociceptive signals induced by external stimuli could be amplified in the ascending pain modulation pathway at any level (e.g., the spinal cord, brainstem, and cerebral cortex), we could not determine the site of central sensitization based on the observation of overactivation in the brain (Apkarian and Reckziegel, 2019). In other words, the perceptual sensitization observed in the human brain could be caused by the changes in sensitivity of the spinal cord, of which the nociceptive information was amplified before propagated to the cortex. Integration of spinal cord and brain MRI/fMRI techniques is warranted to solve this issue (Wand et al., 2011; Ng et al., 2018), which could also allow us to identify relevant neural mechanisms to determine the target site for optimizing the CBP intervention (Wand et al., 2011; Ng et al., 2018).
Second, suffering from chronic pain, CBP patients commonly exhibited emotional and cognitive disorders, including depression, anxiety, catastrophizing, and sleep disturbances (Baliki et al., 2008). In line with these dysregulations, cortical abnormalities have been frequently observed in the regions, pathways, and networks associated with neural processing of emotional and cognitive information in CBP patients (Grachev et al., 2001, 2002, 2003; Baliki et al., 2008; Letzen and Robinson, 2017). In addition to the physiological and psychological factors that are important for the development and maintenance of chronic pain, a biopsychosocial model of pain, which also highlighted the social factors (e.g., interpersonal relationship), has been proposed to better identify the mechanisms of chronic pain (Peng et al., 2017). Importantly, the biopsychosocial model describes pain as a multidimensional and dynamic integration of physiological, psychological, and social factors, which are needed to be considered in the development, maintenance, and treatment of chronic pain (Riedel and Neeck, 2001; Peng et al., 2017). Indeed, more neuroimaging studies under the framework of the biopsychosocial model should be conducted in the future to achieve a comprehensive and sophisticated understanding of the neural mechanisms related to the causes and outcomes of CBP.
Additionally, accumulating evidence has demonstrated that CBP is associated with clear cortical reorganization and neuronal plasticity, which is normally quantified by structural (e.g., GM volume and density) (Apkarian et al., 2004), functional (e.g., cortical representation of the body, brain abnormalities of cortical regions and networks) (Baliki et al., 2008; Hotz-Boendermaker et al., 2016), and metabolic (e.g., levels of NAA, Glu, and mI) (Grachev et al., 2000, 2001, 2002, 2003; Gussew et al., 2011) changes in the brain. Interestingly, the cortical reorganization is reversible by effective treatment (Seminowicz et al., 2011, 2013), suggesting that the quantified brain changes could be used as important neural indicators to monitor the progress of CBP development and to evaluate the effectiveness of CBP treatments, such as acupuncture (Hashmi et al., 2014; Tu et al., 2019b), placebo (Vachon-Presseau et al., 2018), and other pain management approaches (Mülller-Schwefe et al., 2017; Foster et al., 2018).
To sum up, with the development of neuroimaging techniques, great progress has been made to improve our understanding of cortical alterations in CBP patients over the past few years. However, the neural mechanisms associated with the development of CBP remain largely mysterious, which hampers the improvement of the efficacy of CBP treatment. To address this issue, integration of neuroimaging techniques and other biotechnologies (e.g., genetic testing and psychological testing) would be important to achieve a comprehensive assessment of the risk factors (e.g., genetics, injuries, and mental health problems) of the development and maintenance of CBP. In addition, longitudinal studies are highly needed to assess the temporal relationship between chronic pain and neural plasticity. It is worthwhile to note that longitudinal studies would not only improve our understanding of the neural mechanisms associated with the causes and outcomes of CBP but also provide theoretical bases for accurate diagnoses of CBP patients. Integrating the results obtained from comprehensive and longitudinal studies is a promising way to identify the causes of pain and pain-associated comorbidities and deepen our understanding of the mechanisms involved in chronic pain, and ultimately promotes the development of more appropriate and effective treatments in CBP management.
Author Contributions
LZa, XZ, LW, and LH conceived of this topic. LZa conducted the literature search and wrote the manuscript. LZa, LZo, QR, TM, LW, XZ, and LH revised the work. LZa, XZ, LW, and LH edited the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 31671141 and 31822025), the 13th Five-year Informatization Plan of Chinese Academy of Sciences (No. XXH13506-306), and the Scientific Foundation Project of Institute of Psychology, Chinese Academy of Sciences (No. Y6CX021008).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Zhewen He for her suggestions.
References
Aine, C. J. (1995). A conceptual overview and critique of functional neuroimaging techniques in humans: I. MRI/fMRI and PET. Crit. Rev. Neurobiol. 9, 229–309.
Apkarian, A. V., and Reckziegel, D. (2019). Peripheral and central viewpoints of chronic pain, and translational implications. Neurosci. Lett. 702, 3–5. doi: 10.1016/j.neulet.2018.11.040
Apkarian, A. V., Sosa, Y., Sonty, S., Levy, R. M., Harden, R. N., Parrish, T. B., et al. (2004). Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J. Neurosci. 24, 10410–10415. doi: 10.1523/jneurosci.2541-04.2004
Baliki, M. N., Baria, A. T., and Apkarian, A. V. (2011a). The cortical rhythms of chronic back pain. J. Neurosci. 31, 13981–13990. doi: 10.1523/jneurosci.1984-11.2011
Baliki, M. N., Schnitzer, T. J., Bauer, W. R., and Apkarian, A. V. (2011b). Brain morphological signatures for chronic pain. PLoS One 6:e26010. doi: 10.1371/journal.pone.0026010
Baliki, M. N., Chialvo, D. R., Geha, P. Y., Levy, R. M., Harden, R. N., Parrish, T. B., et al. (2006). Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J. Neurosci. 26, 12165–12173. doi: 10.1523/jneurosci.3576-06.2006
Baliki, M. N., Geha, P. Y., Apkarian, A. V., and Chialvo, D. R. (2008). Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J. Neurosci. 28, 1398–1403. doi: 10.1523/jneurosci.4123-07.2008
Baliki, M. N., Mansour, A. R., Baria, A. T., and Apkarian, A. V. (2014). Functional reorganization of the default mode network across chronic pain conditions. PLoS One 9:e106133. doi: 10.1371/journal.pone.0106133
Baliki, M. N., Petre, B., Torbey, S., Herrmann, K. M., Huang, L. J., Schnitzer, T. J., et al. (2012). Corticostriatal functional connectivity predicts transition to chronic back pain. Nat. Neurosci. 15, 1117–1119. doi: 10.1038/nn.3153
Barkhof, F., Haller, S., and Rombouts, S. A. (2014). Resting-state functional MR imaging: a new window to the brain. Radiology 272, 29–49. doi: 10.1148/radiol.14132388
Basser, P. J., and Jones, D. K. (2002). Diffusion-tensor MRI: theory, experimental design and data analysis - a technical review. NMR Biomed. 15, 456–467. doi: 10.1002/nbm.783
Berger, S. E., Baria, A. T., Baliki, M. N., Mansour, A., Herrmann, K. M., Torbey, S., et al. (2014). Risky monetary behavior in chronic back pain is associated with altered modular connectivity of the nucleus accumbens. BMC Res. Notes 7:739. doi: 10.1186/1756-0500-7-739
Buckalew, N., Haut, M. W., Aizenstein, H., Morrow, L., Perera, S., Kuwabara, H., et al. (2010). Differences in brain structure and function in older adults with self-reported disabling and nondisabling chronic low back pain. Pain Med. 11, 1183–1197. doi: 10.1111/j.1526-4637.2010.00899.x
Čeko, M., Shir, Y., Ouellet, J. A., Ware, M. A., Stone, L. S., and Seminowicz, D. A. (2015). Partial recovery of abnormal insula and dorsolateral prefrontal connectivity to cognitive networks in chronic low back pain after treatment. Hum. Brain Mapp. 36, 2075–2092. doi: 10.1002/hbm.22757
Chen, A. C. N. (2001). New perspectives in EEG/MEG brain mapping and PET/fMRI neuroimaging of human pain. Int. J. Psychophysiol. 42, 147–159. doi: 10.1016/s0167-8760(01)00163-5
Chou, R., Qaseem, A., Snow, V., Casey, D., Cross, J. T., Shekelle, P., et al. (2007). Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American college of physicians and the American pain society. Ann. Intern. Med. 147, 478–491. doi: 10.7326/0003-4819-147-7-200710020-00006
Craufurd, D. I. O., Creed, F., and Jayson, M. I. V. (1990). Life events and psychological disturbance in patients with low-back pain. Spine 15, 490–494. doi: 10.1097/00007632-199006000-00011
Dagenais, S., Caro, J., and Haldeman, S. (2008). A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 8, 8–20. doi: 10.1016/j.spinee.2007.10.005
Deyo, R. A., Rainville, J., and Kent, D. L. (1992). What can the history and physical examination tell us about low back pain? JAMA 268, 760–765. doi: 10.1001/jama.1992.03490060092030
Diers, M., Koeppe, C., Diesch, E., Stolle, A. M., Holzl, R., Schiltenwolf, M., et al. (2007). Central processing of acute muscle pain in chronic low back pain patients: an EEG mapping study. J. Clin. Neurophysiol. 24, 76–83. doi: 10.1097/01.wnp.0000241093.00844.0e
Dixit, R. K., and Dickson, J. (2018). “Low back pain,” in ABC of Rheumatology, 5th Edn, eds A. Adebajo, and L. Dunkley (London: BMJ Books), 23–29.
Flor, H., Braun, C., Elbert, T., and Birbaumer, N. (1997a). Extensive reorganization of primary somatosensory cortex in chronic back pain patients. Neurosci. Lett. 224, 5–8. doi: 10.1016/s0304-3940(97)13441-3
Flor, H., Knost, B., and Birbaumer, N. (1997b). Processing of pain- and body-related verbal material in chronic pain patients: central and peripheral correlates. Pain 73, 413–421. doi: 10.1016/s0304-3959(97)00137-1
Flor, H., Diers, M., and Birbaumer, N. (2004). Peripheral and electrocortical responses to painful and non-painful stimulation in chronic pain patients, tension headache patients and healthy controls. Neurosci. Lett. 361, 147–150. doi: 10.1016/j.neulet.2003.12.064
Foster, N. E., Anema, J. R., Cherkin, D., Chou, R., Cohen, S. P., Gross, D. P., et al. (2018). Prevention and treatment of low back pain: evidence, challenges, and promising directions. Lancet 391, 2368–2383. doi: 10.1016/S0140-6736(18)30489-6
Fritz, H. C., McAuley, J. H., Wittfeld, K., Hegenscheid, K., Schmidt, C. O., Langner, S., et al. (2016). Chronic back pain is associated with decreased prefrontal and anterior insular gray matter: results from a population-based cohort study. J. Pain 17, 111–118. doi: 10.1016/j.jpain.2015.10.003
Giesecke, T., Gracely, R. H., Grant, M. A. B., Nachemson, A., Petzke, F., Williams, D. A., et al. (2004). Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 50, 613–623. doi: 10.1002/art.20063
Grachev, I. D., Fredrickson, B. E., and Apkarian, A. V. (2000). Abnormal brain chemistry in chronic back pain: an in vivo proton magnetic resonance spectroscopy study. Pain 89, 7–18. doi: 10.1016/s0304-3959(00)00340-7
Grachev, I. D., Fredrickson, B. E., and Apkarian, A. V. (2001). Dissociating anxiety from pain: mapping the neuronal marker N-acetyl aspartate to perception distinguishes closely interrelated characteristics of chronic pain. Mol. Psychiatry 6, 256–258. doi: 10.1038/sj.mp.4000834
Grachev, I. D., Fredrickson, B. E., and Apkarian, A. V. (2002). Brain chemistry reflects dual states of pain and anxiety in chronic low back pain. J. Neural Transm. 109, 1309–1334. doi: 10.1007/s00702-002-0722-7
Grachev, I. D., Ramachandran, T. S., Thomas, P. S., Szeverenyi, N. M., and Fredrickson, B. E. (2003). Association between dorsolateral prefrontal N-acetyl aspartate and depression in chronic back pain: an in vivo proton magnetic resonance spectroscopy study. J. Neural Transm. 110, 287–312. doi: 10.1007/s00702-002-0781-9
Gussew, A., Rzanny, R., Gullmar, D., Scholle, H. C., and Reichenbach, J. R. (2011). 1H-MR spectroscopic detection of metabolic changes in pain processing brain regions in the presence of non-specific chronic low back pain. Neuroimage 54, 1315–1323. doi: 10.1016/j.neuroimage.2010.09.039
Hartvigsen, J., Hancock, M. J., Kongsted, A., Louw, Q., Ferreira, M. L., Genevay, S., et al. (2018). What low back pain is and why we need to pay attention. Lancet 391, 2356–2367. doi: 10.1016/S0140-6736(18)30480-X
Hashmi, J. A., Baliki, M. N., Huang, L. J., Baria, A. T., Torbey, S., Hermann, K. M., et al. (2013). Shape shifting pain: chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain 136, 2751–2768. doi: 10.1093/brain/awt211
Hashmi, J. A., Kong, J., Spaeth, R., Khan, S., Kaptchuk, T. J., and Gollub, R. L. (2014). Functional network architecture predicts psychologically mediated analgesia related to treatment in chronic knee pain patients. J. Neurosci. 34, 3924–3936. doi: 10.1523/Jneurosci.3155-13.2014
Heithoff, K. B., and Burton, C. V. (1985). CT evaluation of the failed back surgery syndrome. Orthop. Clin. North Am. 16, 417–444.
Hotz-Boendermaker, S., Marcar, V. L., Meier, M. L., Boendermaker, B., and Humphreys, B. K. (2016). Reorganization in secondary somatosensory cortex in chronic low back pain patients. Spine 41, E667–E673. doi: 10.1097/brs.0000000000001348
Hoy, D., Bain, C., Williams, G., March, L., Brooks, P., Blyth, F., et al. (2012). A systematic review of the global prevalence of low back pain. Arthritis Rheum. 64, 2028–2037. doi: 10.1002/art.34347
Hu, L., and Iannetti, G. D. (2019). Neural indicators of perceptual variability of pain across species. Proc. Natl. Acad. Sci. U.S.A. 116, 1782–1791. doi: 10.1073/pnas.1812499116
Huettel, S. A., Song, A. W., and McCarthy, G. (2009). Functional Magnetic Resonance Imaging, 2nd Edn. Sunderland, MA: Sinauer.
Ivo, R., Nicklas, A., Dargel, J., Sobottke, R., Delank, K. S., Eysel, P., et al. (2013). Brain structural and psychometric alterations in chronic low back pain. Eur. Spine J. 22, 1958–1964. doi: 10.1007/s00586-013-2692-x
Koes, B. W., van Tulder, M. W., and Thomas, S. (2006). Diagnosis and treatment of low back pain. Br. Med. J. 332, 1430–1434. doi: 10.1136/bmj.332.7555.1430
Kucyi, A., and Davis, K. D. (2015). The dynamic pain connectome. Trends Neurosci. 38, 86–95. doi: 10.1016/j.tins.2014.11.006
Letzen, J. E., and Robinson, M. E. (2017). Negative mood influences default mode network functional connectivity in patients with chronic low back pain: implications for functional neuroimaging biomarkers. Pain 158, 48–57. doi: 10.1097/j.pain.0000000000000708
Li, X. Y., and Hu, L. (2016). The role of stress regulation on neural plasticity in pain chronification. Neural Plast. 2016:6402942. doi: 10.1155/2016/6402942
Maher, C., Underwood, M., and Buchbinder, R. (2017). Non-specific low back pain. Lancet 389, 736–747. doi: 10.1016/s0140-6736(16)30970-9
Mansour, A. R., Baliki, M. N., Huang, L. J., Torbey, S., Herrmann, K. M., Schnitzer, T. J., et al. (2013). Brain white matter structural properties predict transition to chronic pain. Pain 154, 2160–2168. doi: 10.1016/j.pain.2013.06.044
Mantyselka, P., Kumpusalo, E., Ahonen, R., Kumpusalo, A., Kauhanen, J., Viinamaki, H., et al. (2001). Pain as a reason to visit the doctor: a study in Finnish primary health care. Pain 89, 175–180. doi: 10.1016/s0304-3959(00)00361-4
Mao, C. P., Zhang, Q. L., Bao, F. X., Liao, X., Yang, X. L., and Zhang, M. (2014). Decreased activation of cingulo-frontal-parietal cognitive/attention network during an attention-demanding task in patients with chronic low back pain. Neuroradiology 56, 903–912. doi: 10.1007/s00234-014-1391-6
Mee, S., Bunney, B. G., Reist, C., Potkin, S. G., and Bunney, W. E. (2006). Psychological pain: a review of evidence. J. Psychiatr. Res. 40, 680–690. doi: 10.1016/j.jpsychires.2006.03.003
Meints, S. M., Mawla, I., Napadow, V., Kong, J., Gerber, J., Chan, S. T., et al. (2019). The relationship between catastrophizing and altered pain sensitivity in patients with chronic low-back pain. Pain 160, 833–843. doi: 10.1097/j.pain.0000000000001461
Mülller-Schwefe, G., Morlion, B., Ahlbeck, K., Alon, E., Coaccioli, S., Coluzzi, F., et al. (2017). Treatment for chronic low back pain: the focus should change to multimodal management that reflects the underlying pain mechanisms. Curr. Med. Res. Opin. 33, 1199–1210. doi: 10.1080/03007995.2017.1298521
Ng, S. K., Urquhart, D. M., Fitzgerald, P. B., Cicuttini, F. M., Hussain, S. M., and Fitzgibbon, B. M. (2018). The relationship between structural and functional brain changes and altered emotion and cognition in chronic low back pain brain changes. Clin. J. Pain 34, 237–261. doi: 10.1097/ajp.0000000000000534
Peng, W. W., Guo, X. L., Jin, Q. Q., Wei, H., Xia, X. L., Zhang, Y., et al. (2017). Biological mechanism of post-herpetic neuralgia: evidence from multiple patho-psychophysiological measures. Eur. J. Pain 21, 827–842. doi: 10.1002/ejp.985
Pierpaoli, C., Jezzard, P., Basser, P. J., Barnett, A., and DiChiro, G. (1996). Diffusion tensor MR imaging of the human brain. Radiology 201, 637–648. doi: 10.1148/radiology.201.3.8939209
Pijnenburg, M., Brumagne, S., Caeyenberghs, K., Janssens, L., Goossens, N., Marinazzo, D., et al. (2015). Resting-state functional connectivity of the sensorimotor network in individuals with nonspecific low back pain and the association with the sit-to-stand-to-sit task. Brain Connect. 5, 303–311. doi: 10.1089/brain.2014.0309
Rhudy, J. L., Williams, A. E., McCabe, K. M., Rambo, P. L., and Russell, J. L. (2006). Emotional modulation of spinal nociception and pain: the impact of predictable noxious stimulation. Pain 126, 221–233. doi: 10.1016/j.pain.2006.06.027
Ricci, J. A., Stewart, W. F., Chee, E., Leotta, C., Foley, K., and Hochberg, M. C. (2006). Back pain exacerbations and lost productive time costs in United States workers. Spine 31, 3052–3060. doi: 10.1097/01.brs.0000249521.61813.aa
Riedel, W., and Neeck, G. (2001). Nociception, pain, and antinociception: current concepts. Z. Rheumatol. 60, 404–415. doi: 10.1007/s003930170003
Robinson, J. P., and Apkarian, A. V. (2009). “Low back pain,” in Functional Pain Syndromes: Presentation and Pathophysiology, eds A. Mayer, and C. Bushnell (Seattle: IASP Press), 23–53.
Sadek, R. A. (2012). An improved MRI segmentation for atrophy assessment. Int. J. Comput. Sci. Issues 9, 569–574.
Schmidt-Wilcke, T., Leinisch, E., Ganssbauer, S., Draganski, B., Bogdahn, U., Altmeppen, J., et al. (2006). Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. Pain 125, 89–97. doi: 10.1016/j.pain.2006.05.004
Seminowicz, D. A., Shpaner, M., Keaser, M. L., Krauthamer, G. M., Mantegna, J., Dumas, J. A., et al. (2013). Cognitive-behavioral therapy increases prefrontal cortex gray matter in patients with chronic pain. J. Pain 14, 1573–1584. doi: 10.1016/j.jpain.2013.07.020
Seminowicz, D. A., Wideman, T. H., Naso, L., Hatami-Khoroushahi, Z., Fallatah, S., Ware, M. A., et al. (2011). Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J. Neurosci. 31, 7540–7550. doi: 10.1523/Jneurosci.5280-10.2011
Sharma, N. K., Brooks, W. M., Popescu, A. E., Vandillen, L., George, S. Z., McCarson, K. E., et al. (2012). Neurochemical analysis of primary motor cortex in chronic low back pain. Brain Sci. 2, 319–331. doi: 10.3390/brainsci2030319
Sharma, N. K., McCarson, K. E., Van Dillen, L., Lentz, A., Khan, T., and Cirstea, C. M. (2011). Primary somatosensory cortex in chronic low back pain–a 1H-MRS study. J. Pain Res. 4, 143–150. doi: 10.2147/jpr.s19297
Siddall, P. J., Stanwell, P., Woodhouse, A., Somorjai, R. L., Dolenko, B., Nikulin, A., et al. (2006). Magnetic resonance spectroscopy detects biochemical changes in the brain associated with chronic low back pain: a preliminary report. Anesth. Analg. 102, 1164–1168. doi: 10.1213/01.ane.0000198333.22687.a6
Tamburin, S., Maier, A., Schiff, S., Lauriola, M. F., Di Rosa, E., Zanette, G., et al. (2014). Cognition and emotional decision-making in chronic low back pain: an ERPs study during Iowa gambling task. Front. Psychol. 5:1350. doi: 10.3389/Fpsyg.2014.01350
Tu, Y. H., Jung, M. Y., Gollub, R. L., Napadow, V., Gerber, J., Ortiz, A., et al. (2019a). Abnormal medial prefrontal cortex functional connectivity and its association with clinical symptoms in chronic low back pain. Pain 160, 1308–1318. doi: 10.1097/j.pain.0000000000001507
Tu, Y. H., Ortiz, A., Gollub, R. L., Cao, J., Gerber, J., Lang, C., et al. (2019b). Multivariate resting-state functional connectivity predicts responses to real and sham acupuncture treatment in chronic low back pain. Neuroimage Clin. 23:101885. doi: 10.1016/j.nicl.2019.101885
Vachon-Presseau, E., Berger, S. E., Abdullah, T. B., Griffith, J. W., Schnitzer, T. J., and Apkarian, A. V. (2019). Identification of traits and functional connectivity-based neurotraits of chronic pain. PLoS Biol. 17:e3000349. doi: 10.1371/journal.pbio.3000349
Vachon-Presseau, E., Berger, S. E., Abdullah, T. B., Huang, L. J., Cecchi, G. A., Griffith, J. W., et al. (2018). Brain and psychological determinants of placebo pill response in chronic pain patients. Nat. Commun. 9:3397. doi: 10.1038/s41467-018-05859-1
Vachon-Presseau, E., Tetreault, P., Petre, B., Huang, L. J., Berger, S. E., Torbey, S., et al. (2016). Corticolimbic anatomical characteristics predetermine risk for chronic pain. Brain 139, 1958–1970. doi: 10.1093/brain/aww100
Vogt, M. T., Kwoh, C. K., Cope, D. K., Osial, T. A., Culyba, M., and Starz, T. W. (2005). Analgesic usage for low back pain: impact on health care costs and service use. Spine 30, 1075–1081. doi: 10.1097/01.brs.0000160843.77091.07
Vos, T., Abajobir, A. A., Abbafati, C., Abbas, K. M., Abate, K. H., Abd-Allah, F., et al. (2017). Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390, 1211–1259. doi: 10.1016/s0140-6736(17)32154-2
Wand, B. M., and O’Connell, N. E. (2008). Chronic non-specific low back pain - sub-groups or a single mechanism? BMC Musculoskelet. Disord. 9:11. doi: 10.1186/1471-2474-9-11
Wand, B. M., Parkitny, L., O’Connell, N. E., Luomajoki, H., McAuley, J. H., Thacker, M., et al. (2011). Cortical changes in chronic low back pain: current state of the art and implications for clinical practice. Man. Ther. 16, 15–20. doi: 10.1016/j.math.2010.06.008
Wiech, K., Preissl, H., and Birbaumer, N. (2000). Neuroimaging of chronic pain: phantom limb and musculoskeletal pain. Scand. J. Rheumatol. 29, 13–18. doi: 10.1080/030097400750001752-1
Yu, R. J., Gollub, R. L., Spaeth, R., Napadow, V., Wasan, A., and Kong, J. (2014). Disrupted functional connectivity of the periaqueductal gray in chronic low back pain. Neuroimage Clin. 6, 100–108. doi: 10.1016/j.nicl.2014.08.019
Zhang, B. L., Jung, M., Tu, Y. H., Gollub, R., Lang, C., Ortiz, A., et al. (2019). Identifying brain regions associated with the neuropathology of chronic low back pain: a resting-state amplitude of low-frequency fluctuation study. Br. J. Anaesth. 123, E303–E311. doi: 10.1016/j.bja.2019.02.021
Keywords: chronic back pain, cortical reorganization, neuroimaging techniques, central sensitization, emotional and cognitive disorders
Citation: Zhang L, Zhou L, Ren Q, Mokhtari T, Wan L, Zhou X and Hu L (2019) Evaluating Cortical Alterations in Patients With Chronic Back Pain Using Neuroimaging Techniques: Recent Advances and Perspectives. Front. Psychol. 10:2527. doi: 10.3389/fpsyg.2019.02527
Received: 06 September 2019; Accepted: 25 October 2019;
Published: 14 November 2019.
Edited by:
Qinghua He, Southwest University, ChinaCopyright © 2019 Zhang, Zhou, Ren, Mokhtari, Wan, Zhou and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Wan, d2FubGk1MDAwY25AMTYzLmNvbQ==; Xiaolin Zhou, eHoxMDRAcGt1LmVkdS5jbg==
 Li Zhang
Li Zhang Lili Zhou3,4
Lili Zhou3,4 Li Wan
Li Wan Xiaolin Zhou
Xiaolin Zhou Li Hu
Li Hu