Abstract
Objective:
This pilot study aimed to evaluate the effectiveness of motor imagery training (MIT) in enhancing maximal voluntary contraction (MVC) force among healthy older adults and inducing neural adaptations measured by electromyography (EMG) signals of both agonist and antagonist muscles, as well as the co-contraction index (CCI).
Methods:
Conducted with a single MIT group using a within-subject design, the study involved 12 right-handed elderly participants, with nine completing the 8-week training (5 sessions/week and 30 min/session). Elbow flexion MVC force and MVC EMG for biceps brachii (agonist) and triceps brachii (antagonist) were recorded pre- and post-training.
Results:
Significant improvements in muscle strength were observed following the 8-week MIT, with a notable 22% increase (p < 0.05). Additionally, there was a significant 27% increase in EMG amplitude for the agonist muscle (p < 0.05), with no notable change in EMG amplitude for the antagonist muscle. Notably, this study is the first to demonstrate a significant decrease in CCI (−32%, p < 0.05) following MIT.
Conclusion:
These findings provide further evidence of MIT’s efficacy in enhancing voluntary muscle strength through neural adaptations, particularly beneficial and safer for older individuals encountering challenges with conventional strength training methods.
Introduction
Aging is accompanied by a decline in voluntary muscle strength. While the age-related loss of muscle mass is a significant contributor to muscle weakness, another crucial factor is the central neural drive (Yue et al., 1999; Ranganathan et al., 2001; Sale and Semmler, 2005). This encompasses reduced activity in agonist muscles and heightened activity in antagonist muscles, both of which play a pivotal role in muscle weakness (Narici et al., 2008).
Growing evidence indicates that traditional muscle strength training paradigms enhance descending cortical drive and strengthen muscle output in the elderly. The increased maximal voluntary contraction (MVC) force could result from increased net excitation of motoneurones, increased activation of prime movers, more appropriate co-contraction of synergists, and increased inhibition of antagonists (Sale, 1986). In addition to an intensified neural drive to the motor neuron pools of the prime mover muscles with strength training, changes can involve both the relative activation of different motor neuron pools and the connectivity within and between pools. These adaptations can modulate the amount of antagonist co-activation and the levels of activation of the different synergist muscles.
Co-contraction, traditionally defined as the simultaneous activation of the antagonist muscle with the agonist muscles, can affect muscle force (Sale, 1986). Research indicates that training can modify the level of co-activation. For instance, resistance training has been found to result in a significant reduction in co-contraction and an increase in maximal voluntary contraction (MVC) force in both young (Carolan and Cafarelli, 1992; LaRoche et al., 2008) and elderly individuals (Simoneau et al., 2006; LaRoche et al., 2008).
Motor imagery refers to the internal simulation of actual movements without overt movements (Jeannerod and Decety, 1995). Research indicates that motor imagery is task-specific, with the same neural correlates selectively activated during the imagery of a movement as during the actual movement (Park and Li, 2011). Training with motor imagery has proven effective in enhancing muscle force in both young (Yao et al., 2013) and elderly populations (Jiang et al., 2016; Yue et al., 2024). Additionally, research indicates that motor imagery training (MIT) can lead to a bilateral transfer effect in both motor skill acquisition and muscle strength improvement (Yao et al., 2023). A most recent meta-analysis study (Liu et al., 2023) suggests that older adults may derive greater benefits from MIT in terms of muscle force gains compared to young adults. While research consistently attributes increase in muscle strength to neural adaptations in the central nervous system (CNS) (Yue et al., 1996; Yao et al., 2013; Ranganathan et al., 2004), such as heightened CNS commands following MIT, it remains uncertain whether MIT exerts a similar impact on both agonist and antagonist muscles, potentially affecting the level of co-contraction. MIT holds significant promise in rehabilitation medicine, particularly for weak patients or frail older adults who may find conventional high-intensity strength training programs challenging or unsafe (Jackson et al., 2001). Understanding the effects of MIT on co-contraction index (CCI) of antagonist and agonist muscles will provide valuable insights for practitioners, such as physical therapists, in optimizing rehabilitation interventions. Therefore, the aim of this pilot study was to evaluate the effectiveness of MIT in reducing CCI among older adults by analyzing alterations in both agonist and antagonist muscles, specifically in terms of their force production and electromyography (EMG) amplitudes subsequent to MIT.
Based on previous studies demonstrating the effectiveness of MIT in improving muscle strength through neural adaptations (Yue and Cole, 1992; Yao et al., 2013; Ranganathan et al., 2004; Dos Anjos et al., 2022), it was hypothesized that MIT would decrease CCI, a link between the CNS and muscle activities. Consequently, this alteration of CCI was expected to lead to an increase in MVC force among older adults.
Research methods
Subjects
Twelve right-handed elderly individuals (73.9 ± 8.0 years, ranging from 68 to 87, 10 females) participated in the study. Hand dominance was determined by the Edinburgh Handedness Inventor (Oldfield, 1971).
Training protocol
In this within-subject design, all participants underwent motor imagery training (MIT) for 30 min per day, 5 days a week for 8 weeks. Non-dominant (left) side elbow flexion (EF) strength was emphasized during first-person perspective MI of maximal EF contractions (internal or kinesthetic imagery). In other words, in doing that he/she attempted to activate the brain in a same manner as in a physical MVC (Yao et al., 2013; Ranganathan et al., 2004). During each trial, the participants were instructed to imagine their left arm (focus on elbow flexors) pushing maximally against the force transducer that was used for the strength measurements during the pre-training tests or against a heavy object. There were 30 trials (3 sets, 10 trials in each set) in each training session; there was a 20-s rest between trials and 3-min rest between sets; and a total of 40 training sessions were performed. Each trial started and terminated by an auditory signal that was timed and recorded on a tape recorder. To ensure that MIT was performed without overt muscle movement, we monitored EMG signal from the two elbow flexor muscles, the biceps brachii and brachioradialis, which are accessible from the skin surface. On average, the EMG amplitude during the MIT was less than 2% of the MVC EMG recorded during the pre-training strength test, which further confirms that no overt muscle activity occurred during the MIT.
Strength measurement
In the assessment of primary elbow flexor muscles for the task of elbow flexion, which include the biceps brachii (BB), the brachialis, and the brachioradialis muscles, the BB was selected as the primary agonist muscle for testing. The antagonist was the triceps brachii muscle. The maximal elbow-flexion isometric force was evaluated to determine the effect of training on this force. The elbow-flexion force was measured with the subject seated and the left arm abducted slightly (~10° shoulder abduction) so that the arm was in a sagittal plane and the elbow (flexed ~90°) resting on a padded support at about hip height. The forearm was in an intermediate position between supination and pronation so that the palm of the hand is facing inward. The wrist was placed in the wrist cuff that is attached to an O-shaped metal frame connected to the force transducer (JR3, 45E15A-U760 100 L450, Woodland, CA). In each test session, 5 trials of elbow-flexion MVC force were performed. The timing of the MVC force task was based on a verbal count during which the subject graded the contraction force from zero to maximum in about 2 s and then maintain this force for about 3 s. The subjects were verbally encouraged to maximize the force. The transducer output for the elbow-flexion force was displayed on an oscilloscope to provide feedback for the subject and recorded on the hard disk of a personal computer. The force signals were sampled at 200 Hz. All the strength measurement conditions were kept the same across all measurement sessions. For more detailed information on the procedures used to measure and calculate the MVC force data, please refer to Ranganathan et al. (2004) and Yao et al. (2013).
MVC EMG
Surface EMG was recorded from the skin overlying the belly of the biceps brachii (BB) and triceps brachii (TB) muscles during the strength measurement trials. All recordings were bipolar with the electrodes (8-mm diameter) fixed over the belly of the test muscles. All surface EMG signals were amplified (500–5,000 times), bandpass filtered (10 Hz to 1 kHz), displayed on an oscilloscope, digitized (1,000 Hz) and recorded on the computer hard disk. EMG signals were analyzed offline using the Spike2 data analysis system (Spike2, Cambridge Electronic Design, Ltd., Cambridge, UK), and custom-designed software. The signals of each trial were first full-wave rectified and then averaged across data points of 1-s duration that covered the timing of the highest force in that trial. Similar to the quantification of the MVC force, the greatest EMG amplitude among all the 5 MVC trials were taken as the MVC EMG. For more detailed information on the procedures used to measure and calculate the MVC EMG, please refer to Ranganathan et al. (2004) and Yao et al. (2013) (Figure 1).
Figure 1
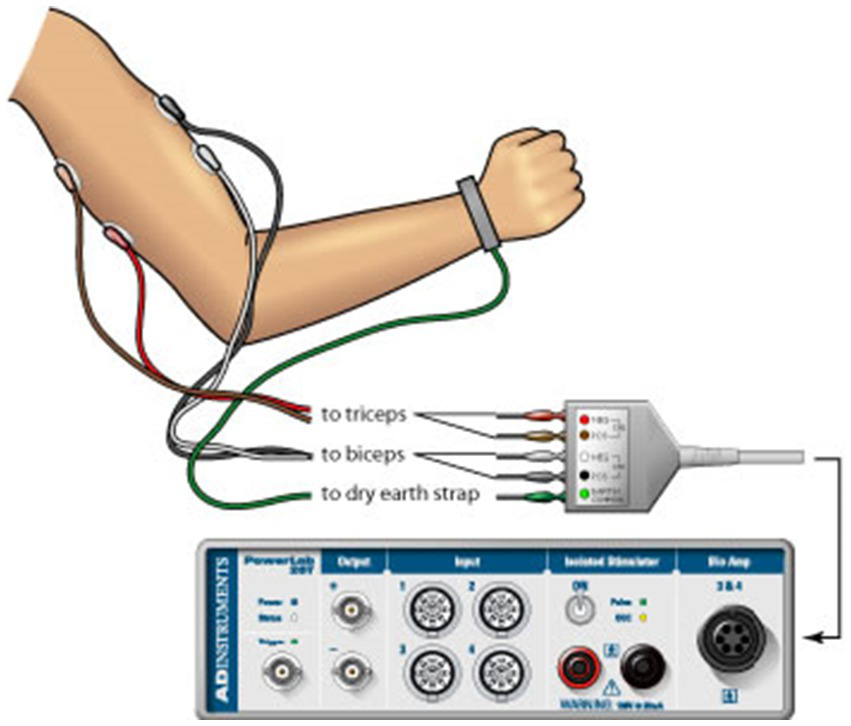
Surface EMG used to record muscle activity from the skin overlying the belly of the biceps brachii and triceps brachii muscles during the strength measurement trials.
Co-contraction index
The CCI was determined by calculating the ratio of the EMG amplitude of the antagonist muscle to the sum of the EMG amplitudes of both the agonist and antagonist muscles during maximal voluntary contraction (MVC):
Statistical analysis
Three of the 12 participants did not complete the eight-week’s training. Thus, the analysis of MVC force and EMG amplitude were conducted using the data from the nine participants (7 females) who successfully completed the training (75.6 ± 8.5 years).
Previous studies consistently demonstrate the beneficial effects of MIT on enhancing muscle strength (Paravlic et al., 2018; Liu et al., 2023) and improving EMG amplitude on agonist muscles (Yue and Cole, 1992; Yao et al., 2013; Ranganathan et al., 2004). Therefore, the data of the MVC force, EMG amplitude, and CCI before and after the MIT were analyzed with paired-samples one-tailed t-tests in SPSS and the significant level was set at p ≤ 0.05. Given the small sample size, individual t-tests were chosen to minimize the risk of Type II errors and maximize the ability to detect potential meaningful changes in the outcomes.
To account for multiple comparisons, the Holm-Bonferroni correction was applied to adjust the p-values. This ensured that any reported findings remained statistically significant even after the adjustment for multiple tests.
Results
MVC force
Following the training, there was a substantial increase (22%) in the elbow flexion (EF) MVC force. Specifically, the MVC force was 78.5 ± 38.5 N before the training, and it rose to 95.3 ± 31.3 N after the training. This difference was statistically significant, as indicated by t(8) = 2.09, p < 0.05. See Figure 2 for MVC forces.
Figure 2
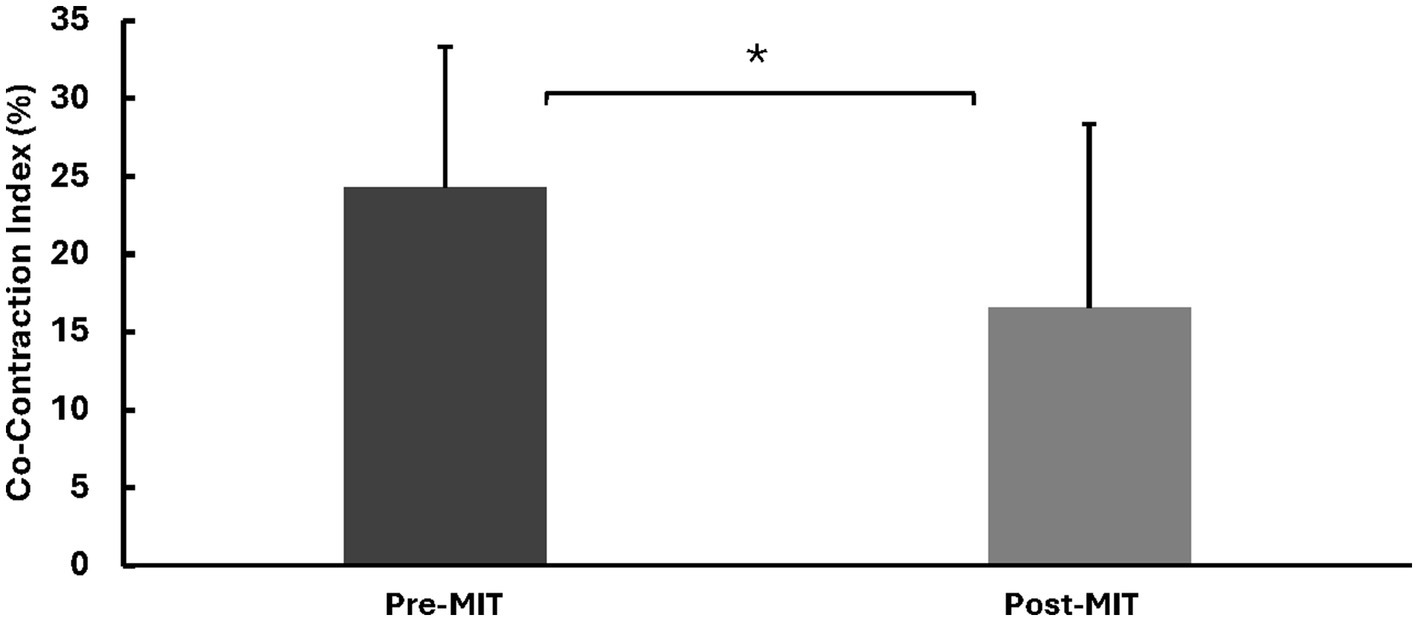
Means and standard deviations of the left EF MVC force in Newtons (N). The results reveal a significant increase of 22% (p < 0.05) in MVC force following the MIT. *Statistically significant at p < 0.05.
EMG (mV) of agonist and antagonist muscles
Along with the augmented MVC force, there was a significant increase in the MVC EMG amplitude of the agonist muscle (biceps brachii, BB) following MIT. The MVC EMG a for BB were 0.51 ± 0.28 mV before the training and 0.65 ± 0.26 mV after the training, with t(8) = 2.589, p < 0.05.
The EMG amplitude of the antagonist muscle (triceps brachii, TB) showed no significant difference before and after MIT. The mean and standard deviation of the MVC EMG amplitudes for TB were 0.11 ± 0.055 and 0.10 ± 0.065, respectively, with t(8) = 0.275, p = 0.395. The EMG results are summarized in Figure 3.
Figure 3
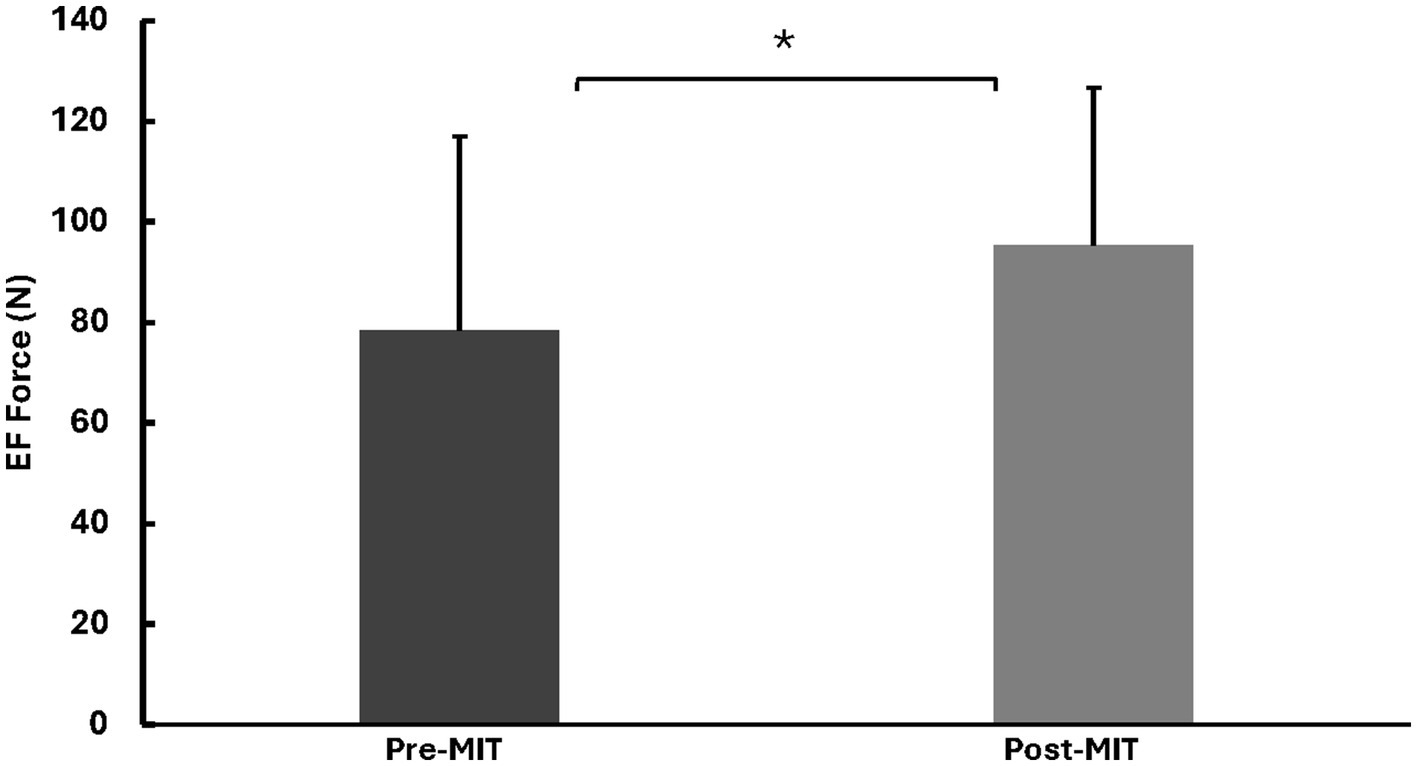
Means and standard deviations of the left BB and TB EMG amplitudes in millivolts (mV). The findings show a notable 27% (p < 0.05) increase for BB in EMG amplitude after the MIT and no notable difference in EMG amplitude for TB after the MIT. *Statistically significant at p < 0.05.
Co-contraction index
Following the MIT, there was a significant decrease (−32%) in the CCI. The CCI values were 24.3 ± 9.01% before the training and 16.5 ± 11.7% after the training, with t(8) = 2.028, p < 0.05. See Figure 4 for CCI results.
Figure 4
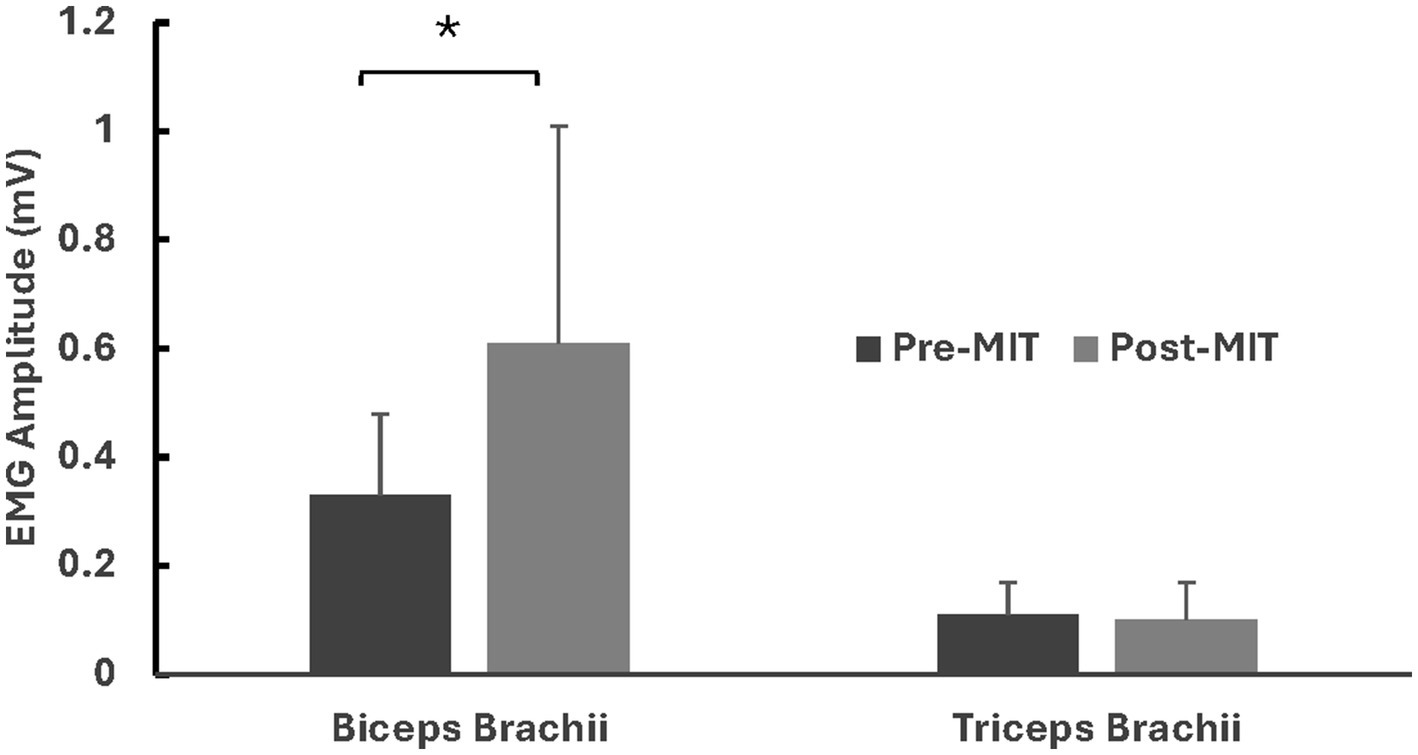
Means and standard deviations of co-contraction index (CCI). The findings reveal a significant −32% (p < 0.05) decrease in CCI after the MIT. *Statistically significant at p < 0.05.
Discussion
This pilot study investigated the effects of Motor Imagery Training (MIT) on muscle strength among older adults and explored the underlying mechanisms through analyzing the electromyography (EMG) signals. Co-contraction index (CCI) of agonist and antagonist muscles has been shown to be an important factor to the alteration of muscle strength decades ago (Carolan and Cafarelli, 1992). Therefore, in addition to assessing isometric maximal-voluntary-contraction (MVC) elbow-flexion force and EMG amplitudes of the biceps brachii (the primary agonist muscle) and triceps brachii (the antagonist muscle) during MVC, this study also examined the CCI following MIT. To the best of our knowledge, this is the first investigation to examine the effect of MIT on CCI. Our findings revealed a significant increase in muscle strength following MIT, accompanied by a noteworthy reduction in the CCI. However, the observed changes in EMG amplitude between agonist and antagonist muscles post-MIT raise intriguing questions and warrant further discussion.
This study is notable for being the first to demonstrate a significant decrease in CCI by 32% (together perhaps with strengthened descending drive to agonist muscle) following MIT, leading to a marked enhancement in muscle strength (22%). This finding aligns with previous research suggesting that motor imagery can facilitate motor unit recruitment and/or discharge rate (Yue et al., 1996; Parsowith et al., 2023; Dos Anjos et al., 2022) and induce alteration of CCI (Dos Anjos et al., 2022), thereby promoting strength gains. The effectiveness of MIT in improving muscle strength in older adults underscores its potential as a valuable intervention for enhancing physical function and mitigating age-related declines in muscle mass and strength.
Interestingly, despite the significant increase in muscle strength, our analysis revealed no concurrent change in EMG amplitude of antagonist muscles following MIT. This outcome deviates from expectations based on studies employing traditional resistance training modalities, where a decrease in EMG amplitude of antagonist muscles often accompanies increases in agonist muscle activity (Carolan and Cafarelli, 1992). This discrepancy prompts consideration of potential underlying mechanisms and implications for motor control and neuromuscular adaptations.
One possible explanation for the differential effects on agonist and antagonist muscles post-MIT could relate to the nature of motor imagery itself. Unlike physical resistance training, which directly stimulates muscle contraction through external resistance, motor imagery primarily engages neural pathways involved in motor planning and execution. It is plausible that the neural adaptations induced by MIT prioritize enhancements in agonist muscle activation, while the influence on antagonist muscles may be less pronounced or indirect. Nevertheless, compared to an increased agonist EMG, the unchanged antagonist muscle activity resulted in a relative decline in antagonist muscle EMG, leading to a reduction in the CCI.
Moreover, the specific characteristics of the motor tasks employed during MIT may influence the observed EMG amplitudes. Variations in task complexity, movement dynamics, and attentional focus during motor imagery sessions could modulate the recruitment patterns of agonist and antagonist muscles differently. Further investigations into the nuances of motor imagery protocols, including task specificity and individual variability, may provide insights into optimizing its effectiveness for improving muscle strength and coordination in older adults.
Additionally, the absence of significant changes in EMG amplitude of antagonist muscles following MIT raises intriguing questions regarding the role of reciprocal inhibition and co-contraction in neuromuscular control. While traditional resistance training often aims to minimize co-contraction and optimize agonist–antagonist muscle balance (Carolan and Cafarelli, 1992), the observed maintenance of antagonist muscle activity post-MIT suggests a more nuanced interplay between neural mechanisms governing muscle coordination in the context of motor imagery.
It should be noted that a significant limitation of the current study is the absence of a control group, which restricts the interpretability of the results. Without a control comparison, it is difficult to definitively attribute the observed changes in muscle strength and CCI specifically to MIT rather than to other factors such as learning effects, increased familiarity with testing procedures, or the Hawthorne effect. To address this limitation, we compared our findings with controlled studies of similar interventions, such as those by Yao et al. (2013) and Ranganathan et al. (2004), which demonstrated that MIT led to significant strength gains compared to control groups. The pattern of improvements observed in our study, particularly the enhancement of muscle strength and the increase in agonist muscle’s EMG amplitude, aligns with the neural adaptations reported in these controlled studies. While these comparisons cannot fully compensate for the lack of a control group, they provide contextual support for the hypothesis that MIT was the primary driver of the observed changes.
Additionally, the study had a gender imbalance, with only 2 males among the 9 participants, which may limit the generalizability of the findings, particularly given potential differences in neuromuscular responses between males and females. Another concern is the use of multiple t-tests instead of a multivariate approach, such as MANOVA, which would have been more appropriate for analyzing multiple dependent variables. However, given the small sample size of this pilot study, a MANOVA may have been underpowered, increasing the risk of Type II errors. Therefore, individual t-tests were chosen to assess the effects of MIT while minimizing the likelihood of overlooking potentially meaningful changes.
In support of transparent and reproducible cognitive neuroscience research, we also recognize the growing need for standardized, open-access tools in data collection and analysis. Toolboxes such as the Brain Electrophysiological Recording & Stimulation (BEST) toolbox (Hassan et al., 2022) exemplify best practices for collecting, analyzing, and sharing neurophysiological datasets. Future studies, particularly those involving EEG monitoring during MIT, would benefit from integrating such toolkits to promote consistency, reproducibility, and data sharing within the field.
Despite these limitations, the study provides promising preliminary evidence that MIT can enhance muscle strength in older adults by facilitating neural adaptations. To strengthen the evidence base, future research should include control groups, ensure balanced gender representation, adopt multivariate statistical approaches with adequately powered sample sizes, assess MI ability, and monitor engagement during MIT using EEG. Furthermore, investigating the long-term effects of MIT on motor performance, CCI, and other neural factors in older adults would offer a deeper understanding of its potential physiological and functional benefits.
Conclusion
In conclusion, this pilot study provides preliminary evidence that MIT may enhance muscle strength and alter neuromuscular activation patterns, as indicated by the reduction in the CCI, in older adults. The observed improvements suggest that MIT could serve as a promising, non-invasive intervention to counteract age-related declines in muscle function. However, the study’s limitations—including the absence of a control group, gender imbalance, reliance on multiple t-tests, and lack of assessment of motor imagery (MI) ability and engagement—restrict the generalizability and interpretability of the results. These limitations highlight the need for caution in interpreting the findings, which should be viewed as preliminary evidence supporting the potential efficacy of MIT rather than definitive proof. To strengthen the evidence base, future research should incorporate controlled designs to account for potential confounding factors.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Center for Mobility and Rehabilitation Engineering Research, Kessler Foundation, United States. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
WY: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. BM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft. SG: Conceptualization, Data curation, Formal analysis, Writing – original draft. MB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. JW: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft. BJ: Conceptualization, Formal analysis, Writing – original draft. JZ: Conceptualization, Data curation, Formal analysis, Writing – original draft. GY: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was partially supported by NIH grants R01NS-035130 and R01HD-036725 awarded to GY.
Conflict of interest
BM was employed by Neurelis Inc. MB and GY were employed by Kessler Foundation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Carolan B. Cafarelli E. (1992). Adaptations in coactivation after isometric resistance training. J. Appl. Physiol.73, 911–917. doi: 10.1152/jappl.1992.73.3.911
2
Dos Anjos T. Guillot A. Kerautret Y. Daligault S. Di Rienzo F. (2022). Corticomotor plasticity underlying priming effects of motor imagery on force performance. Brain Sci.12:1537. doi: 10.3390/brainsci12111537
3
Hassan U. Pillen S. Zrenner C. Bergmann T. O. (2022). The brain electrophysiological recording & stimulation (best) toolbox. Brain Stimul.15, 109–115. doi: 10.1016/j.brs.2021.11.017
4
Jackson P. L. Lafleur M. F. Malouin F. Richards C. Doyon J. (2001). Potential role of mental practice using motor imagery in neurologic rehabilitation. Arch. Phys. Med. Rehabil.82, 1133–1141. doi: 10.1053/apmr.2001.24286
5
Jeannerod M. Decety J. (1995). Mental motor imagery: a window into the representational stages of action. Curr. Opin. Neurobiol.5, 727–732. doi: 10.1016/0959-4388(95)80099-9
6
Jiang C. Ranganathan V. K. Zhang J. Siemionow V. Yue G. H. (2016). Motor effort training with low exercise intensity improves muscle strength and descending command in aging. Medicine (Baltimore)95:e3291. doi: 10.1097/MD.0000000000003291
7
Laroche D. P. Roy S. J. Knight C. A. Dickie J. L. (2008). Elderly women have blunted response to resistance training despite reduced antagonist coactivation. Med. Sci. Sports Exerc.40, 1660–1668. doi: 10.1249/MSS.0b013e3181761561
8
Liu X. Ge S. Cordova A. Yaghi Z. Jiang B. Yue G. et al . (2023). Elderly may benefit more from motor imagery training in gaining muscle strength than young adults: a systematic review and meta-analysis. Front. Psychol.13:1052826. doi: 10.3389/fpsyg.2022.1052826
9
Narici M. V. Maffulli N. Maganaris C. N. (2008). Ageing of human muscles and tendons. Disabil. Rehabil.30, 1548–1554. doi: 10.1080/09638280701831058
10
Oldfield R. C. (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia9, 97–113. doi: 10.1016/0028-3932(71)90067-4
11
Paravlic A. H. Slimani M. Tod D. Marusic U. Milanovic Z. Pisot R. (2018). Effects and dose-response relationships of motor imagery practice on strength development in healthy adult populations: a systematic review and Meta-analysis. Sports Med.48, 1165–1187. doi: 10.1007/s40279-018-0874-8
12
Park W.-H. Li S. (2011). No graded responses of finger muscles to Tms during motor imagery of isometric finger forces. Neurosci. Lett.494, 255–259. doi: 10.1016/j.neulet.2011.03.027
13
Parsowith E. J. Stock M. S. Girts R. M. Beausejour J. P. Alberto A. Carr J. C. et al . (2023). The influence of resistance training experience on the efficacy of motor imagery for acutely increasing corticospinal excitability. Brain Sci.13:1635. doi: 10.3390/brainsci13121635
14
Ranganathan V. K. Siemionow V. Liu J. Z. Sahgal V. Yue G. H. (2004). From mental power to muscle power--gaining strength by using the mind. Neuropsychologia42, 944–956. doi: 10.1016/j.neuropsychologia.2003.11.018
15
Ranganathan V. K. Siemionow V. Sahgal V. Yue G. H. (2001). Effects of aging on hand function. J. Am. Geriatr. Soc.49, 1478–1484. doi: 10.1046/j.1532-5415.2001.4911240.x
16
Sale D. (1986). “Neural adaptation in strength and power training” in Human muscle power. eds. JonesN. L.MccartneyN.MccomasA. J. (Champaign, Iii: Human Kinetics).
17
Sale M. V. Semmler J. G. (2005). Age-related differences in corticospinal control during functional isometric contractions in left and right hands. J. Appl. Physiol. (1985)99, 1483–1493. doi: 10.1152/japplphysiol.00371.2005
18
Simoneau E. Martin A. Porter M. Van Hoecke J. (2006). Strength training in old age: adaptation of antagonist muscles at the ankle joint. Muscle Nerve33, 546–555. doi: 10.1002/mus.20492
19
Yao W. X. Ge S. Zhang J. Q. Hemmat P. Jiang B. Y. Liu X. J. et al . (2023). Bilateral transfer of motor performance as a function of motor imagery training: a systematic review and meta-analysis. Front. Psychol.14:1187175. doi: 10.3389/fpsyg.2023.1187175
20
Yao W. X. Ranganathan V. K. Allexandre D. Siemionow V. Yue G. H. (2013). Kinesthetic imagery training of forceful muscle contractions increases brain signal and muscle strength. Front. Hum. Neurosci.7:561. doi: 10.3389/fnhum.2013.00561
21
Yue G. Cole K. J. (1992). Strength increases from the motor program: comparison of training with maximal voluntary and imagined muscle contractions. J. Neurophysiol.67, 1114–1123. doi: 10.1152/jn.1992.67.5.1114
22
Yue G. H. Ranganathan V. K. Siemionow V. Liu J. Z. Sahgal V. (1999). Older adults exhibit a reduced ability to fully activate their biceps brachii muscle. J. Gerontol. A Biol. Sci. Med. Sci.54, M249–M253. doi: 10.1093/gerona/54.5.m249
23
Yue G. H. Wilson S. L. Cole K. J. Darling W. G. Yuh W. (1996). Imagined muscle contraction training increases voluntary neural drive to muscle. J. Psychophysiol.10, 198–208.
24
Yue G. Yao W. Mamone B. Bayram M. Wu J. Ge S. et al . (2024). Motor imagery training improves muscle strength and cortico-muscular connectivity in old adults. J. Psychiatry Behav. Sci.7:1091.
Summary
Keywords
motor imagery training, mental practice, muscle strength, muscle co-contraction, aging
Citation
Yao WX, Mamone B, Ge S, Bayram MB, Wu J, Jiang BY, Zhang JQ and Yue GH (2025) Effects of motor imagery training on muscle strength and co-contraction for older adults. Front. Psychol. 16:1441377. doi: 10.3389/fpsyg.2025.1441377
Received
31 May 2024
Accepted
30 June 2025
Published
14 July 2025
Volume
16 - 2025
Edited by
Sheng Li, University of Texas Health Science Center at Houston, United States
Reviewed by
Toshio Higashi, Nagasaki University, Japan
Umair Hassan, Stanford University, United States
Updates
Copyright
© 2025 Yao, Mamone, Ge, Bayram, Wu, Jiang, Zhang and Yue.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wan X. Yao, wanxiang.yao@utsa.eduGuang H. Yue, gyue@kesslerfoundation.org
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.