- 1Department of Pharmacy, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Hubei Province Clinical Research Center for Precision Medicine for Critical Illness, Wuhan, China
- 3Department of Pharmacy, Hubei Hospital of Traditional Chinese Medicine, Wuhan, China
Although perinatal depression (PND) has garnered increasing attention, few specific pharmacological treatments exist, particularly for breastfeeding mothers concerned about antidepressant safety. The misconception that “natural is harmless” merits caution; herbal remedies and dietary supplements should be regarded as supplementary interventions pending robust safety evidence. This review summarizes recent advances in PND pathogenesis (neurotransmitter dysregulation, inflammation, hormonal imbalance, and microbiota alterations) and emerging drug development strategies, alongside clinical evidence for herbal and dietary supplements. Randomized controlled trial (RCT) findings reveal that while interventions like saffron and vitamin D show promise, significant limitations persist, including inconsistent efficacy, limited long-term safety data, and potential interactions with perinatal physiology. Caution is warranted until comprehensive studies validate the safety and reliability of natural interventions. This review underscores the need for rigorous trials to identify safe, effective PND treatments, particularly for vulnerable populations.
1 Introduction
Perinatal psychiatric disorders encompass a spectrum of mental health challenges occurring during pregnancy and postpartum, varying in clinical presentation and severity. Among these, baby blues (postpartum blues) represent the most prevalent yet transient condition, affecting nearly 40% of women within the first week postpartum (Rezaie-Keikhaie et al., 2020). Characterized by mood lability, anxiety, and irritability, baby blues typically resolve spontaneously without intervention. In contrast, postnatal psychosis, a severe neuropsychiatric disorder, involves hallucinations, delusions, and manic episodes, necessitating immediate psychiatric care (Perry et al., 2021). Perinatal depression (also known as postpartum depression) is one of the most common obstetric complications for mothers during pregnancy and after delivery. According to a study by Al-Abri et al. (2023), which analyzed 128 systematic reviews, the overall average prevalence of perinatal depression is 26.3% (SD = 11.6, median = 23.8%). This indicates that one in four perinatal women experiences depression. The burden of PND is particularly pronounced among high-risk populations, including those exposed to intimate partner violence, HIV infection, or the aftermath of natural disasters (Roddy Mitchell et al., 2023). Beyond its immediate psychological burden, untreated PND disrupts maternal–infant bonding, impairs child cognitive development, and increases long-term risks of chronic mood disorders. Despite its profound public health implications, PND universally remains underdiagnosed and undertreated, with fewer than 20% of affected women receiving adequate care, especially in low-resource settings where care accessibility is severely limited (Roddy Mitchell et al., 2023; Payne and Maguire, 2019). Studies have indicated that the causes of PND can have various sources, including physiological ones, such as hormone fluctuations (Worthen and Beurel, 2022), psychological ones, such as family pressures and a lack of social support (Upadhyay et al., 2017; Pillai et al., 2023), such as societal expectations (Klainin and Arthur, 2009), and genetic factors, such as those involved in monoamine neurotransmitters, key molecules of the HPA axis and kynurenine pathways, as well as genes associated with immune regulation (Mehta et al., 2021). Unfortunately, existing treatments lack specificity for PND (Frieder et al., 2019). Despite the extensive understanding of these factors, current treatment options remain limited and often inadequate, with antidepressants being the primary pharmaceutical approach. However, concerns over potential risks to both maternal health and infant safety—especially during breastfeeding—have led many women to seek alternative therapies (Dubreucq et al., 2022; Johansen et al., 2019).
First-line pharmacological interventions for PND—primarily selective serotonin reuptake inhibitors (SSRIs) such as sertraline and fluoxetine—are largely extrapolated from major depressive disorder (MDD) protocols (Brown et al., 2021). While SSRIs demonstrate moderate efficacy, their use in perinatal populations is complicated by two critical factors: Firstly, up to 5–10% of maternal SSRI doses transfer to breast milk, raising concerns about neonatal neurobehavioral outcomes, albeit with conflicting evidence (Heinonen et al., 2021). Secondly, most women with PND face the dilemma of choosing between the risk of depression and the risks of antidepressants. While risks of antidepressants are often overstated, the consequences of untreated depression during pregnancy and postpartum are gravely underestimated. Untreated moderate-to-severe perinatal depression poses critical threats to maternal and infant wellbeing, including heightened risks of maternal substance use and suicidal ideation (Latendresse et al., 2017; Stohl et al., 2016). The impact on the long-term development of newborns could make sick mothers more careful about the choice of drug treatment. Thus, being able to treat PND while breastfeeding is an important challenge at present. This therapeutic impasse has fueled interest in non-pharmacological alternatives, particularly plant-based remedies (e.g., St. John’s wort, saffron) and dietary supplements (e.g., omega-3 fatty acids, vitamin D) because they tend to be perceived as less intrusive and “safer.” However, this perception is fraught with scientific ambiguity and caution should always be exercised until sufficient reliable studies.
In particular, although herbal medicines (including Chinese herbs) are often considered “natural remedies, “their pharmacological components can still be transmitted through breast milk or cause adverse reactions. Therefore, this article will carefully distinguish the potential risks of plant-derived therapy as a “drug” when discussing it, and emphasize the need for caution in its clinical application. The use of herbal medicine in treating mental disorders has a long tradition. St. John’s wort (SJW), a traditional European herbal medicine remedy, has been widely used to treat anxiety, depression, and even sleep disorders (Canenguez Benitez et al., 2022). Its low level of excretion into breast milk makes it a popular choice among postpartum mothers (Avila et al., 2018). However, the fact that part of the use of SJW exceeds prescribed dosages and lacks guidance from medical professionals creates safety risks. Saffron is commonly used by women in the field of food and medicine; it is taken mainly from the flower’s stigma. Clinical studies predominantly focus on its anti-inflammatory, antioxidant, antidepressant, and anti-anxiety activities (El Midaoui et al., 2022). In recent years, studies have evaluated its safety and compared its efficacy with SSRIs in the treatment of PND (Tabeshpour et al., 2017; Kashani et al., 2017). Additionally, herbs like lavender and basil, known for their volatile essential oils, are often used in aromatherapy to alleviate PND (Cho and Kim, 2023). Despite their popularity, these “natural” remedies are not without risks. SJW, for instance, can interact with other medications, reducing their efficacy, and many herbal preparations show significant variability in their bioactive compounds.
Although mothers in many cultures follow special care routines during the perinatal period, including less physical activity and changes in diet, recent evidence suggests that changes in diet are beneficial. Two recent cross-sectional studies from China with 939 and 1,659 participants, respectively, collected data on 24-h meal recall and food frequency to assess the association between dietary patterns and PND risk (Yang et al., 2021; Cao et al., 2020). The results showed that diet imbalance was associated with an increased risk of PND, while the healthy pattern of vegetables, fruits, and nuts was significantly associated with a decreased risk of PND; this was similar to the conclusion of a recent cross-sectional study in Iran (Dehghan-Banadaki et al., 2023). Therefore, diet during the perinatal period and the dietary supplements chosen are critically important.
This review addresses three critical gaps: (1) synthesizing recent advances in PND pathogenesis (e.g., neurosteroid fluctuations, gut-brain axis dysregulation) to establish mechanistic rationales for alternative therapies; (2) critically evaluating the safety profiles of herbal and dietary interventions, with emphasis on lactation risks and herb-drug interactions; and (3) proposing a clinically actionable framework to prioritize evidence-backed interventions (e.g., saffron) while flagging those requiring safety validation (e.g., TCM). By bridging preclinical insights and clinical realities, this work aims to guide clinicians and patients in navigating the complex trade-offs between conventional and alternative PND treatments.
2 Literature review
We searched PubMed, Web of Science, and Cochrane Library from 2000 to 2024 using the following key search terms: “postpartum depression,” “perinatal depression,” “treatment efficacy,” “pharmacological interventions,” “psychological therapies,” “alternative medicine,” “herbal medicine,” “psychotherapy approaches,” “maternal mental health,” “breastfeeding safety,” “randomized controlled trials,” “meta-analysis,” “longitudinal studies,” and “observational research.” We prioritized randomized controlled trials (RCTs) and meta-analyses, followed by high-quality longitudinal and cohort studies. We included all English language, peer-reviewed publications with sufficient methodological rigor, applying stricter thresholds for study size and quality in pharmacological intervention studies compared to psychological therapies. When multiple large RCTs were available, we did not include smaller case series or uncontrolled studies. We incorporated pilot studies and observational research only when robust clinical trials were lacking. We also performed manual reference checking of all included articles to identify additional relevant studies.
2.1 Prenatal depression pathogenesis
In the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) and the International Classification of Diseases (ICD-10), PND is considered a specific period of depression; that is, symptoms occur within 4 weeks after giving birth or during pregnancy. Therefore, although some evidence supports PND as a unique disorder (Di Florio and Meltzer-Brody, 2015), it still shares the same diagnostic criteria as other non-perinatal depression, such as anxiety, stress, and emotional impairment, among others. Multiple cohort studies have shown that the increased risk of PND is independently related to psychosocial, genetic, and perinatal complications (Koutra et al., 2014; Koutra et al., 2018; Walker et al., 2021). The studies on this have looked at the mental fragility and emotional damage caused by PND, and explored the physiological material changes and functional impairment of pregnant mothers (Stewart and Vigod, 2019).
2.1.1 The neurotransmitter
2.1.1.1 Serotonin (5-HT)
In As an affective disorder, PND is characterized by low mood. It is widely believed that emotional changes are closely related to monoamine neurotransmitters. A number of clinical and pre-clinical studies have focused on the correlation between low 5-HT levels and depressive emotions (Jenkins et al., 2016; Young and Leyton, 2002; Toker et al., 2010). At the same time, the transmission of monoamine neurotransmitters, represented by 5-HT, is considered to be highly correlated with the onset and development of PND. Abnormal serotonin neurotransmission is viewed as an important factor in the onset of depression, so SSRIs, targeting 5-HT transporters, are widely used as first-line drugs for antidepression treatment (Latendresse et al., 2017; Haase and Brown, 2015; Reilly et al., 1997). Studies have shown that pregnant and postpartum women have high levels of serotonin and its metabolites in cerebrospinal fluid, and in late pregnancy and early postnatal, this is accompanied by low levels of tryptophan (Pawluski et al., 2019). In a longitudinal cohort study, Borgsted et al. (2022) evaluated the effect of the central serotonin system on maternal mental distress during the prenatal to postnatal transition. Results of high-performance liquid-chromatography (HPLC) analysis of maternal cerebrospinal fluid showed that higher levels of postpartum mental distress were positively correlated, at trend level, with levels of serotonin metabolite 5-hydroxy indole acetic acid (5-HIAA) in high-yield pre-cerebrospinal fluid (Borgsted et al., 2022).
2.1.1.2 Dopamine
During pregnancy and motherhood, there are dynamic, continuous changes in brain structure, function, and behavior. This includes the adaptation of the brain’s reward system, the mesolimbic dopamine (DA) system. These alterations are thought to be adaptive and promote the initiation and maintenance of maternal behavior, which is essential for offspring survival (Barba-Muller et al., 2019). A number of perinatal studies on rodents have focused on time-dependent changes in DA function, showing that increasing perinatal dopaminergic activity is crucial for maternal and infant care (Stolzenberg and Numan, 2011; Rincon-Cortes and Grace, 2020). In other words, PND can occur if this adaptive change is disrupted or defective. In their study, Post and Leuner (2019) summarized nine neuroimaging studies on the mesolimbic dopamine system in patients with PND. Their results showed that the striatum with high depressive symptoms had weakened function and was more sluggish in responding to positive stimuli, which was consistent with the anhedonia symptom of PND (Post and Leuner, 2019).
2.1.1.3 GABA
Some scientists have been inspired to understand depression and develop therapeutic drugs from non-monoamine ideas. In this process, excitatory/inhibitory (E/I) regulation based on non-monoamine targets γ-aminobutyric (GABA) acid and glutamatergic (Glu) has become an important non-monoamine drug development strategy (Li, 2020). Based on studies on rodents, researchers have shown that the structure and function of GABAA receptors in the brain undergo significant changes in response to the fluctuation of neuroactive steroid levels during the perinatal period (Sanna et al., 2009). Some speculate that neurological and psychiatric disorders during this period may relate to the failure of these receptors to adapt to the changes (Maguire et al., 2009). Notably, Maguire and Mody (2008) identified a GABAA receptor subunit-deficient mouse model in their study. The mice exhibited symptoms similar to depression and anxiety only after delivery, suggesting that this could become a specific model for PND (rather than major depression) (Maguire and Mody, 2008). Neuroactive steroids (NAS) are modulators of the GABA system. The neuroactive steroid, allopregnanolone, has been shown to be involved in emotional regulation and play an important role in the pathophysiology of mood disorders, such as depression or anxiety (Meltzer-Brody and Kanes, 2020). Deligiannidis et al. (2016) evaluated plasma NAS and GABA levels in perinatal women at risk for PND in a prospective study. Their results show that perinatal GABA concentrations were lower in AR-PND women compared with the healthy cohorts and negatively correlated with the Hamilton Depression Rating Scale (HAM-D17) and the Hamilton Anxiety Rating Scale (HAM-A) scores. In their study, however, no correlation was found between individual NAS levels and GABA concentration. The GABA level during the whole perinatal period was also not monitored, so it was difficult to further reveal the potential dynamic change process of perinatal GABA level (Deligiannidis et al., 2016).
2.1.2 Nerve inflammation
Pregnancy is closely related to a body’s inflammatory response, and implanting and delivery are typical inflammatory processes (Romero et al., 2007). During labor, the uterus shifts from a relatively stable state to a relatively active state from the accumulation of a large number of proinflammatory signals (Leimert et al., 2021). Normal pregnancy relies on a rigorous balance of proinflammatory and anti-inflammatory signals, and abnormal inflammatory signals can lead to severe obstetric complications, such as preterm birth and eclampsia (Kalagiri et al., 2016; Challis et al., 2009). Given the high levels of proinflammatory cytokines in pregnant women during the perinatal trimester, they are also at a high risk for sleep disorders, postpartum pain, and even PND (Kendall-Tackett, 2007). In recent studies, emerging evidence has suggested that PND is also an inflammatory disease (Osborne and Monk, 2013).
Sluiter et al. (2020) analyzed levels of inflammatory epigenetic markers in blood samples from 148 Latina women in the United States. They found that high levels of DNA methylation in the FOXP3 and TNF-α promoter regions correlated prenatal perceptual discrimination with PND and anxiety symptoms (Sluiter et al., 2020). For the first time, Boufidou et al. (2009) evaluated elevated levels of the pro-inflammatory cytokines IL-6 and TNF-α in maternal cerebrospinal fluid (CSF). The results revealed a partial potential association between brain inflammation and depressive symptoms, given the high sensitivity of CSF tests to the central nervous system. Notably, Sathyanarayanan et al. (2019) studied inflammation levels in women with their first episode of PND. Their results showed that IL-6 levels rose immediately in healthy women (HP group) and in depressed women (PP group), likely representing a normal perinatal physiological response. However, the increase of IL-8 in the PP and HP groups was not expected compared with healthy non-maternal (HNP) groups. Thus, this may partially reveal changes in the specific inflammatory levels of perinatal depression (Sathyanarayanan et al., 2019). Notably, mothers with a history of MDD are accompanied by sensitization of the inflammatory response system (IRS), and the phenomenon of immune activation is more severe after delivery (Maes et al., 2001).
2.1.3 Hormone levels
As the body supports fetal development and prepares for maternal delivery, women undergo significant neuroendocrine changes during the perinatal period. Specifically, we look at the changes in gonadal hormones and glucocorticoids. Progesterone, isoprogesterone, and estradiol remain high for a long time during pregnancy, but decline rapidly during delivery (Galea and Frokjaer, 2019). Meanwhile, the plasma glucocorticoid levels of women during pregnancy are more than two times higher than those of non-pregnant women (Lindsay and Nieman, 2005). Although these changes are preparing women for motherhood, studies have shown that pregnant women are more vulnerable and susceptible to hormonal fluctuations, and that the endocrine changes associated with childbirth play a direct role in PND in certain women (Galea and Frokjaer, 2019).
2.1.4 Sex hormone
Perinatal estrogen decline leads to a state of estrogen withdrawal, which may be responsible for the emotional and behavioral changes in perinatal mothers. The estrogen withdrawal hypothesis is one of the most common explanations for the pathogenesis of PND (Schmidt et al., 2015). Many studies of rodents have shown that perinatal estrogen withdrawal (estradiol) leads to symptoms of depression and anxiety after delivery, and that sustained high levels of estradiol treatment can reverse these depression-like behaviors (Stoffel and Craft, 2004; Galea et al., 2001). To identify early biomarkers of PND in a longitudinal discovery cohort, Mehta et al. (2014) conducted a genome-wide study of 62 psychopathological women. Researchers identified 116 transcripts with differential expression, predicting PPD with 88% accuracy in both discovery and replication cohorts. These transcriptomes were significantly enriched in estrogen signaling pathways (represented by SP1 and TAF6). In addition, the estrogen receptor ESR1 site is the only transcription factor binding site that is significantly enriched. At the same time, PND women showed increased sensitivity to estrogen signals. It is worth mentioning that plasma estradiol and estriol levels of women in different groups did not show differences (Mehta et al., 2014).
2.1.5 Glucocorticoid
One of the most credible findings in depression-related psychiatry over the years has been the dysregulation of the hypothalamic–pituitary–adrenal axis (HPA) in major depression (Alam et al., 2024). Although PND is generally considered a subtype of major depression, the HPA disorder in PND is significantly different from that in MDD (Glynn et al., 2013). The data show that the changes in the female endocrine stress system during pregnancy mainly come from the production and growth of a new organ, the placenta. Different from the negative feedback effect of glucocorticoids on the hypothalamus, the placenta secretes the additional placental corticotropin-releasing hormone (CRH) during pregnancy, and glucocorticoids activate the placenta and stimulate CRH synthesis (Frim et al., 1988). This positive feedback loop leads to significant increases in the adrenocorticotropic hormone (ACTH), cortisol, and the corticotropin-releasing hormone in the mother throughout pregnancy (Lindsay and Nieman, 2005). Animal models of postpartum depression studying the HPA axis have focused on pregnancy stress and/or exposure to high levels of corticosterone. Chronic mild stress has been found to induce elevated corticosterone and estrogen levels at the end of pregnancy, and lead to postpartum depressive behavior in these mice (Brummelte and Galea, 2010a; Brummelte and Galea, 2010b).
2.1.6 Gut microbiota
The gut-brain axis is a bidirectional connection between the brain and the gastrointestinal tract. There is growing evidence that gut microbiota is involved in a number of mental disorders, including depression (Alam et al., 2024) Gut microbiota acts on the central nervous system (CNS) in three main ways: 1. The microbiome directly stimulates neurons to transmit signals to the brain via the vagus nerve; 2. The gut microbiota influence the regulation of neurotransmitters and glucocorticoids (HPA axis) through neuroendocrine pathways (Marano et al., 2023); 3. The gut microbiota affect the blood–brain barrier (BBB) and CNS function through immune cells. A prospective cohort study of 90 pregnant women found that microbial diversity was significantly associated with greater intestinal symptoms and feelings of helplessness in the late perinatal period (Long et al., 2023). Multiple studies evaluating the gut microbiota of patients with PND versus healthy women have shown significant differences between the two groups. Chen et al. (2021) analyzed stool samples from depressed women and healthy subjects. Bacteroidetes, Proteobacteria, and Clostriobacteria were highly enriched in patients with MDD, while firmicutes and actinobacteria were consistently higher in the control group (Chen et al., 2021). The number of microbiome specific genera in the stool of patients with PND correlated with clinical indicators and sex hormone levels (Zhou et al., 2020). However, because of limitations of sample size, homogeneity, age, diet, and other factors, the conclusions of these studies on microbial diversity changes in PPD patients are still controversial. More general consensus needs to be obtained in more reliable large-scale clinical trials.
2.2 Drug therapy for PND
In terms of current PND interventions, most are derived from the treatment of MDD (represented by SSRIs), with selective serotonin reuptake inhibitors (SSRIs) like sertraline and escitalopram serving as first-line therapies due to their established efficacy in reducing depressive symptoms via serotonin reuptake inhibition (O’Hara et al., 2019; Milgrom et al., 2015; Wisner et al., 2004; Misri et al., 2012). Few drugs are specifically approved for perinatal depression. Emerging therapies, as summarized in Table 1, include neuroactive steroids (e.g., brexanolone) showing symptom reduction in Phase 3 trials (Meltzer-Brody et al., 2018; Kanes et al., 2017; Deligiannidis et al., 2021). Hormonal interventions like transdermal estradiol with mixed evidence for efficacy (Li et al., 2020; Wisner et al., 2015; Gregoire et al., 1996). For women who are breastfeeding, antidepressants used for PND must be careful of adverse reactions during lactation. Future directions must prioritize personalized pharmacogenomic strategies and rigorous safety profiling in perinatal populations. The clinical studies (RCT dominated) on the drug treatment or prevention of PND to date are presented in Table 1.
2.3 Botanicals/natural products
Given the unique pathophysiological mechanisms of postpartum depression and the special physiological conditions of perinatal women, traditional antidepressants including SSRIs demonstrate certain limitations in treatment efficacy (Eke et al., 2016). Therefore, natural products extracted from plants have become attractive sources of alternatives to drugs. Plants, such as saffron and hypericum perforatum, have shown potential. The evidence from Tables 2, 3 indicates that plants/herbs have shown both clinical and preclinical efficacy in improving PND.
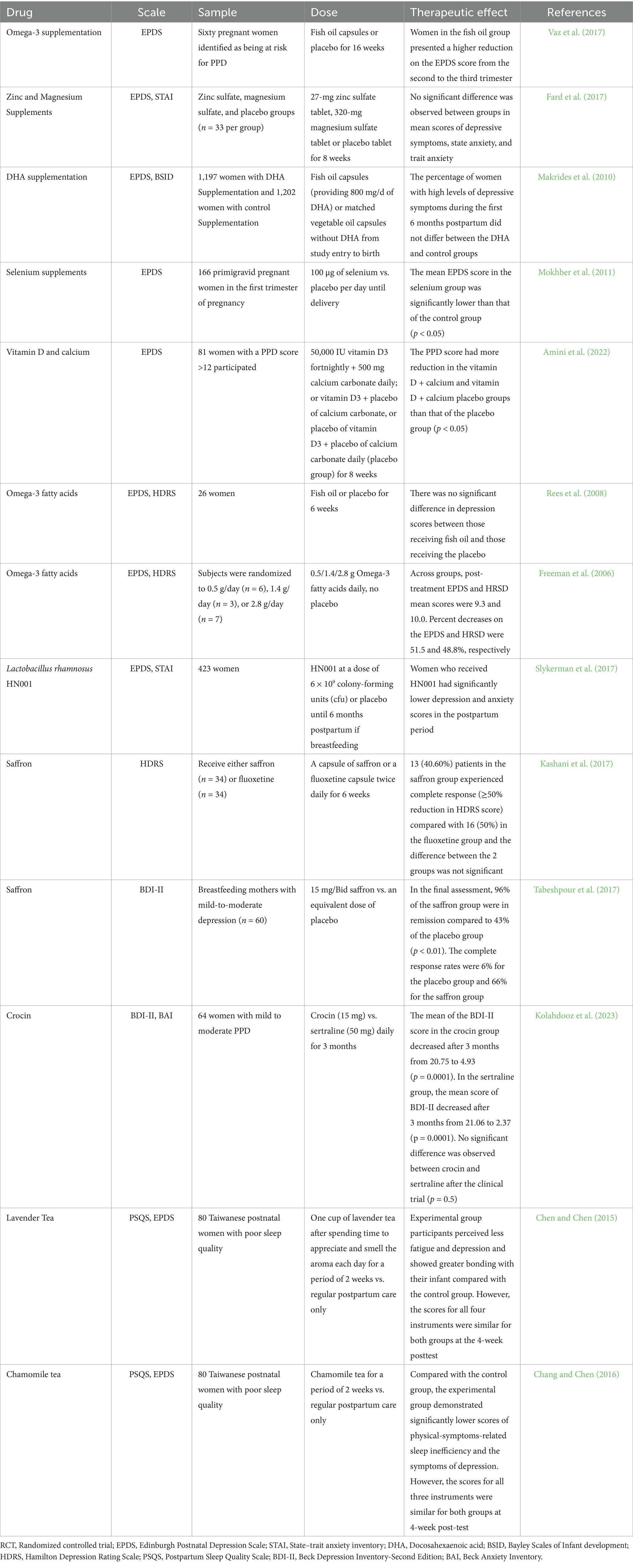
Table 2. Clinical trials of dietary supplements and herbal medicines related to perinatal depression (mainly RCT).
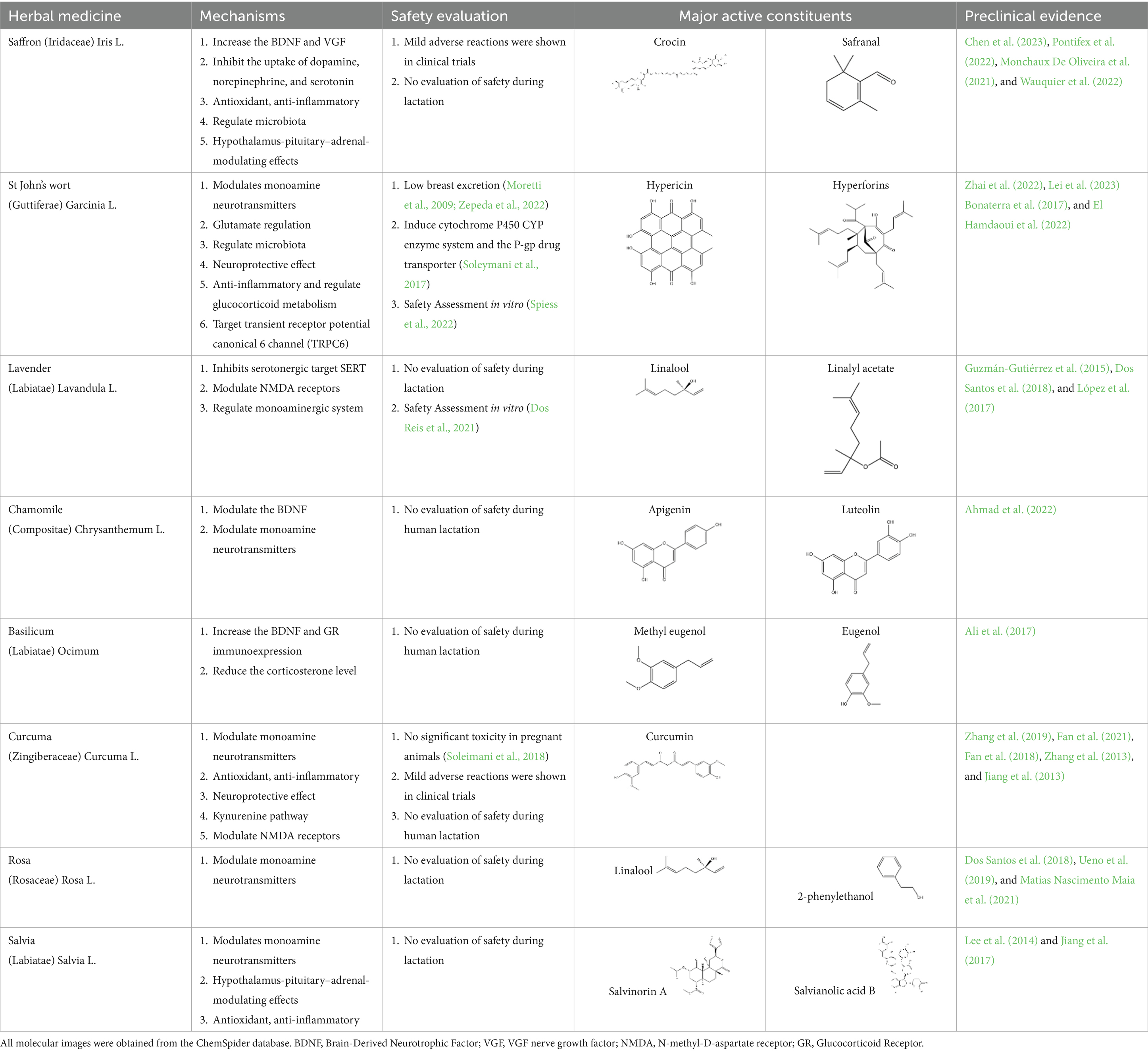
Table 3. Herbal-based interventions of postpartum depression and their mechanisms of action (focusing on preclinical evidence from the last decade).
2.3.1 Saffron
In vivo and in vitro studies suggest that saffron’s antidepressant effects arise from polypharmacological mechanisms targeting the modulation of the microbiota, neuroinflammation (TNF-α/IL-6 via NF-κB inhibition), monoamine modulation (dopamine, norepinephrine, and serotonin downregulation), HPA axis regulation (cortisol downregulation), and neuroprotection (BDNF upregulation) (Chen et al., 2023; Pontifex et al., 2022; Monchaux De Oliveira et al., 2021; Wauquier et al., 2022). Kashani et al. (2017) evaluated the efficacy of saffron capsules and fluoxetine in the treatment of mild to moderate PND in a randomized controlled trial (RCT). Of the saffron patients, 40.60% (13 cases) had complete responses (50% ≥ HDRS score reduction), compared with 50% (16 cases) in the fluoxetine group. There was no significant difference between the groups in efficacy and adverse reactions (Kashani et al., 2017). Tabeshpour et al. (2017) conducted a randomized placebo-controlled trial on saffron. In the final evaluation, the complete response rate was 6% in the placebo group and 66% in the saffron group. These clinical results support the basic anti-PND studies of saffron, suggesting that saffron may be a safe alternative for improving PND symptoms (Tabeshpour et al., 2017). Despite these promising results, larger trials and further safety evaluations are needed before saffron can be recommended as a first-line treatment for PND.
2.3.2 St. John’s wort
St. John’s wort has been used for a long time to treat everything from anxiety to sleep disorders. Hypericins and hyperforins are the main pharmacodynamic components of St. John’s wort (Table 3). St. John’s wort extract can increase the concentration of dopamine, norepinephrine through multiple pathways and have a broad spectrum antidepressant effect (Linde et al., 2008). Notably, much of its use goes beyond medical guidelines, which also creates potential risks for users. Linde et al. (2005) summarized the double-blind controlled trials on St. John’s wort extract, most using the HAMD score as the efficacy criterion. St. John’s wort was shown to be at least as effective as conventional antidepressants and significantly better than the placebo group. Regarding the significant breastfeeding concern, there is evidence that hypericin from St. John’s wort is excreted into breast milk at low levels (Klier et al., 2006). It is noteworthy that St. John’s Wort serves as a potent inducer of CYP 3A4. Additionally, evidence indicates that it may act as a weak monoamine oxidase inhibitor (MAOI). Consequently, the potential for drug interactions represents a significant safety concern, particularly when co-administered with other SSRIs (e.g., sertraline and Paroxetine), which could elevate the risk of 5-HT Syndrome (Zhou et al., 2004; Sacher et al., 2011). However, although there is partial preclinical evidence and perinatal safety evaluations of St. John’s wort, there is still a lack of reliable large RCT evidence on the safety and efficacy of its use for PND.
2.3.3 Aromatic essential oils (EOs)
Aromatherapy is mainly based on the volatile components of natural products. Plants rich in volatile oils, such as labiaceae and ruticaceae, are often chosen in the treatment of mental disorders (Tsai et al., 2020). Aromatherapy is used globally to alleviate insomnia, depression, anxiety, and certain cognitive disorders. A Taiwanese study assessed the benefits of indoor gardening and inhaling essential oils from Pseudotsuga menziesii and Lavandula angustifolia for 15 women. Physiological measurements showed decreased sympathetic and increased parasympathetic nerve activity, indicating relaxation. Psychological measurements showed reduced STAI-S anxiety scores after gardening and inhaling the essential oils (Chung et al., 2024). Hachul et al. conducted two placebo-controlled studies on the effects of lavender essential oil on sleep in postmenopausal women, comparing sunflower oil and lavender essential oil. Participants in the lavender group showed improvements in overall sleep patterns, quality, and efficiency, as well as benefits in almost all areas of quality of life (Lucena et al., 2024; Dos Reis et al., 2021). In addition, aromatherapy with lavender essential oil is believed to help manage Premenstrual Syndrome (PMS) by reducing symptoms such as anxiety, depressed mood, nervousness, pain, bloating, and negative thoughts. However, the study lacked a placebo (Uzunçakmak and Ayaz, 2018). In a clinical trial, 140 postpartum women were randomly assigned to either an aromatherapy group or a non-aromatherapy group (Kianpour et al., 2016). The aromatherapy intervention involved inhaling 3 drops of lavender essential oil every 8 h for 4 weeks, while the control group received usual care after hospital discharge. Results showed that stress, anxiety, and depression scores were significantly lower in the aromatherapy group compared to the control group.
Although essential oils have shown some advantages in improving depression and relieving anxiety, the current view that aromatherapy improves or treats depression is still controversial (Jafari-Koulaee et al., 2020; Chen et al., 2022). On the one hand, in the absence of sufficiently reliable medical evidence, placebo-controlled trials on aromatherapy are difficult to design because aromatherapy focuses on smell, and the subject can judge the group by the smell. Additionally, the use of essential oils carries potential risks, including allergic reactions, skin irritation, and toxicity if not properly managed. Further research is required to clarify the therapeutic potential and safety of aromatherapy for PND.
2.4 Dietary habit and dietary supplements
For the treatment of PND through natural intervention strategies, some research has focused on the use of dietary supplements to prevent prodromal symptoms. There is evidence that deficiencies in omega-3 polyunsaturated fatty acids (PUFAs), vitamin D, and other key elements, such as iron, zinc, magnesium, and selenium, are highly associatead with PND symptoms (Rupanagunta et al., 2023). Herring fish oil supplements reduced hippocampal corticosterone concentration and plasma corticosterone levels, as well as proinflammatory cytokines (IL-1β, INF-γ and TNF-α) and improved depressive symptoms in model animals with PND (Arbabi et al., 2014). However, in several randomized placebo-controlled trials involving omega-3 in the prevention of PND, researchers found no significant benefit of PUFAs rich fish oil supplements compared with a placebo in the prevention of PND (Freeman et al., 2006; Rees et al., 2008; Vaz et al., 2017; Hulkkonen et al., 2021; Makrides et al., 2010). Amini et al. (2022) conducted the first clinical trial of the effect of vitamin D and calcium supplements on women with PND symptoms. The results showed that vitamin D supplements reduced PND scores; in addition, women taking vitamin D alone lowered their PND scores more than those taking vitamin D + Ca or the placebo (Amini et al., 2022). In another study of zinc and magnesium supplements, changes in depressive symptoms were not satisfactory (Fard et al., 2017).
Emerging evidence also highlights the potential of probiotics as adjunctive interventions for PND. A randomized, double-blind, placebo-controlled trial (n = 423) demonstrated that maternal supplementation with Lactobacillus rhamnosus HN001 during pregnancy and postpartum (until 6 months breastfeeding) significantly reduced postpartum depression (mean EPDS 7.7 vs. 9.0, p = 0.037) and anxiety scores (12.0 vs. 13.0, p = 0.014) compared to placebo, with a 56% lower risk of clinical anxiety (OR = 0.44, p = 0.002) (Slykerman et al., 2017). However, these effects were observed as secondary outcomes in a trial primarily designed to assess infant eczema, underscoring the need for dedicated trials. Another study in overweight women (n = 439) found no synergistic benefits of combining probiotics with fish oil for mood symptoms, though baseline dietary quality inversely correlated with depressive/anxiety trajectories (Hulkkonen et al., 2021). Despite promising safety profiles (breastfeeding-compatible), mechanistic insights into gut-brain axis modulation remain limited.
Despite their low cost and minimal side effects, dietary supplements cannot currently be recommended as a standalone treatment for PND due to insufficient evidence of their efficacy. More rigorous clinical trials are needed to confirm their effectiveness and establish appropriate dosages for perinatal populations.
3 Conclusion
Perinatal depression (PND), a multifactorial disorder involving neuroendocrine dysregulation and gut-brain axis disruption, demands innovative therapeutic strategies. This review highlights the potential of alternative interventions—such as saffron, St. John’s wort, and lavender aromatherapy—to alleviate symptoms, with saffron showing efficacy comparable to SSRIs in short-term trials. However, critical limitations persist: insufficient evidence on lactation safety (e.g., transfer of bioactive compounds into breast milk), variability in herbal formulations, and a lack of long-term outcome data. Dietary supplements like omega-3s and vitamin D yield inconsistent clinical results, underscoring the need for standardized dosing and robust trial designs. While these therapies offer safer perceptions for patients, their clinical application requires rigorous risk–benefit evaluation, particularly for breastfeeding mothers. Future research must prioritize large-scale RCTs, mechanistic studies, and safety monitoring to bridge the gap between preclinical promise and clinical utility. By integrating emerging evidence with practical guidelines, this work aims to support informed decision-making for PND management, balancing maternal mental health with infant safety.
4 Discussion
PND receives varying levels of attention in different countries/regions. Fisher et al. (2012) concluded that women in middle-and low-income countries had a higher incidence of perinatal mental disorders. A wealth of high-quality epidemiological, clinical, and healthcare-related evidence surrounding PND has been published from high-income/developed countries. In a study of 112 middle-and low-income countries, 80–90% of these least developed countries lacked local evidence on perinatal mental disorders (Fisher et al., 2012). Moreover, women from the most economically and socially vulnerable groups have the highest incidence rates. Because of constraints, such as the lack of access to healthcare (related to economic status), certain cultural and social factors (including discrimination against women, sex biases toward future generations, traditional cultural ostracism of psychiatric medicine, etc.), the legitimate needs of these women facing the risks of PND are not only ignored and neglected but their fundamental rights to perinatal preventive care are also violated (Fisher et al., 2010). Further research and action are needed to address the differences between countries and social groups in diagnosing and treating PND. This includes discussing potential interventions and policies to improve the identification and management of PND among low-income countries and marginalized populations, and focusing on cultural and social factors that can lead to shame and discrimination against women with perinatal depression. Cross-cultural and interdisciplinary cooperation on a global scale is needed to understand the etiology, prevention, and treatment of perinatal depression in different cultural backgrounds, and to provide better PND diagnosis and treatment for women all over the world.
Growing preclinical and clinical evidence suggests that non-traditional treatment modalities, such as the ones described in this review, are gaining increasing attention in the field of perinatal depression. These treatments include a wide range of interventions, such as herbal medicine, dietary supplements, and aromatherapy, all of which have shown some promise in alleviating symptoms associated with perinatal depression. However, current data are insufficient to recommend these as monotherapies for perinatal depression, and several issues cannot be ignored. First, the quality of these clinical trial data must be considered (including experimental design, dosing plan and intervention measures). In Table 2, two clinical trials of omega-3 involved fewer than 30 participants (Freeman et al., 2006; Rees et al., 2008). Regarding control group interventions, three clinical trials in Table 2 lacked placebo controls, and two were positive controls using fluoxetine or sertraline. The lack of a placebo control group similarly raises concerns about the quality of these clinical trials (Baldwin et al., 2003). Furthermore, the quality of trial design often lacks rigor. For instance, blinding is difficult to achieve in studies involving aromatherapy, leading to potential bias from both participants and researchers. The heterogeneity of interventions, including variations in the type, dosage, frequency, and duration of herbal and dietary supplements, complicates the comparison and evaluation of these studies. Heterogeneity is another concern, as omega-3 supplements in Table 2 come in many forms, with different omega-3 dosages and different proportions of EPA and DHA in different supplements, making it challenging to evaluate these studies.
The challenge with using herbal medicine in (PND) lies in the lack of human data. In Table 3, nine types of Chinese herbal medicines are listed with preclinical evidence for PND treatment, but only 2–3 of these herbs have clinical trial data involving women in the perinatal period in Table 2. For example, among the Chinese herbs mentioned in this article, saffron is more commonly used in women and has rarely been studied as a monotherapy for postpartum depression (Tabeshpour et al., 2017; Kashani et al., 2017). On the one hand, the metabolic pathways of drugs in animals and humans can differ significantly, and animal models often only partially mimic the pathological features of human diseases, failing to fully reproduce their complexity and diversity. This is especially true for psychiatric disorders, such as depression, where animal models can only simulate certain symptoms or pathological mechanisms. Common animal models for postpartum depression, such as hormone resistance models, chronic unpredictable stress models, and cortisol induction models, lack complexity and focus on single factors, which is a significant limitation. Therefore, clinical efficacy in humans cannot always be inferred from in vivo and in vitro pharmacodynamic evidence. Despite these limitations, such information does provide potential guidance for future research and helps in understanding the underlying mechanisms and potential therapeutic targets. Breastfeeding significantly influences researchers’ approach to using herbal medicine in perinatal mothers. There is a prevailing public belief that natural products are generally safe for pregnant women and fetuses; however, this assumption is often incorrect. A survey showed that nearly 40% of pregnant women use of the medicinal plants may not be safe (Bernstein et al., 2021). The use of St. John’s wort does not impact breastfeeding, but it may act as a CYP 3A4 inducer. Therefore, there is a potential risk for interaction with other herbal or traditional drugs during pregnancy (Dugoua et al., 2006; Markowitz and DeVane, 2001). The dilemma is the lack of studies or only partial studies on the efficacy and safety of most conventional drugs during pregnancy. Therefore, the view that “natural is harmless” needs to be cautioned against, and caution should always be exercised until sufficient reliable studies of perinatal use of herbal medicines are available. In addition to this, although omega-3 fatty acids have shown benefits in treating major depression, a significant number of studies have not supported their effectiveness in preventing postpartum depression. Similarly, other dietary supplements have also failed to demonstrate a clear preventive role in this context (Miller et al., 2013).
Although randomized controlled trials are regarded as the gold standard for evaluating monotherapies, this does not diminish the potential value of alternative therapies when used in combination. Herbal medicine often exerts a synergistic anti-depressant effect through the combination of various herbs and their ingredients. For example, the combination of bupleurum and peony can enhance the anti-depressant effect (Li et al., 2021). Herbal prescriptions like Xiaoyao powder and are commonly used for this purpose (Gao et al., 2022; Wang et al., 2023). Additionally, combining these herbal treatments with psychotherapy can further enhance their effectiveness. Medication combined with psychotherapy can enhance the treatment of specific symptoms of depression and anxiety (Bozzatello et al., 2020).
Compared with traditional antidepressants (including monoamine oxidase inhibitors (MAOs) and SSRIs), there is evidence that perinatal mothers are more likely to accept herbs or dietary supplements that appear to be safer, healthier, easier and cheaper. However, from our research, their effectiveness and safety data are not as strong as the public thinks. Another problem is that when consumers choose what they think is a safe treatment, many applications are actually outside the professional guidance of the clinician, which can create additional risks. Therefore, taking into account the fact that perinatal mothers prefer perinatal medications and alternative therapies, their knowledge of the effectiveness and potential risks of these interventions should be increased.
Author contributions
QY: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. YL: Data curation, Formal analysis, Writing – original draft. SG: Data curation, Formal analysis, Writing – original draft. YZ: Writing – review & editing. XZ: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Natural Science Foundation of Hubei Province, China (2024AFB645), Key Research and Development Plan of Hubei Province, China (2023BCB030) and Chinese Pharmaceutical Association (CPA-Z05-ZC-2022-002).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmad, S., Azhar, A., Tikmani, P., Rafique, H., Khan, A., Mesiya, H., et al. (2022). A randomized clinical trial to test efficacy of chamomile and saffron for neuroprotective and anti-inflammatory responses in depressive patients. Heliyon 8:e10774. doi: 10.1016/j.heliyon.2022.e10774
Al-Abri, K., Edge, D., and Armitage, C. J. (2023). Prevalence and correlates of perinatal depression. Soc. Psychiatry Psychiatr. Epidemiol. 58, 1581–1590. doi: 10.1007/s00127-022-02386-9
Alam, M., Abbas, K., Dar, G. A., and Nosaiba, A. K. (2024). Nootropic property of Punica grantum extract as BDNF4 stimulant for treatment of major depressive disorder. Int. Neuropsychiatr. Dis. J. 21, 24–35. doi: 10.9734/indj/2024/v21i2424
Alam, M., Abbas, K., Mustafa, M., Usmani, N., and Habib, S. (2024). Microbiome-based therapies for Parkinson’s disease. Front. Nutr. 11:1496616. doi: 10.3389/fnut.2024.1496616
Ali, S. S., Abd El Wahab, M. G., Ayuob, N. N., and Suliaman, M. (2017). The antidepressant-like effect of Ocimum basilicum in an animal model of depression. Biotech. Histochem. 92, 390–401. doi: 10.1080/10520295.2017.1323276
Amini, S., Amani, R., Jafarirad, S., Cheraghian, B., Sayyah, M., and Hemmati, A. A. (2022). The effect of vitamin D and calcium supplementation on inflammatory biomarkers, estradiol levels and severity of symptoms in women with postpartum depression: a randomized double-blind clinical trial. Nutr. Neurosci. 25, 22–32. doi: 10.1080/1028415X.2019.1707396
Arbabi, L., Baharuldin, M. T., Moklas, M. A., Fakurazi, S., and Muhammad, S. I. (2014). Antidepressant-like effects of omega-3 fatty acids in postpartum model of depression in rats. Behav. Brain Res. 271, 65–71. doi: 10.1016/j.bbr.2014.05.036
Avila, C., Whitten, D., and Evans, S. (2018). The safety of St John’s wort (Hypericum perforatum) in pregnancy and lactation: a systematic review of rodent studies. Phytother. Res. 32, 1488–1500. doi: 10.1002/ptr.6099
Baldwin, D., Broich, K., Fritze, J., Kasper, S., Westenberg, H., and Möller, H. J. (2003). Placebo-controlled studies in depression: necessary, ethical and feasible. Eur. Arch. Psychiatry Clin. Neurosci. 253, 22–28. doi: 10.1007/s00406-003-0400-2
Barba-Muller, E., Craddock, S., Carmona, S., and Hoekzema, E. (2019). Brain plasticity in pregnancy and the postpartum period: links to maternal caregiving and mental health. Arch. Womens Ment. Health 22, 289–299. doi: 10.1007/s00737-018-0889-z
Bernstein, N., Akram, M., Yaniv-Bachrach, Z., and Daniyal, M. (2021). Is it safe to consume traditional medicinal plants during pregnancy? Phytother. Res. 35, 1908–1924. doi: 10.1002/ptr.6935
Bloch, M., Meiboom, H., Lorberblatt, M., Bluvstein, I., Aharonov, I., and Schreiber, S. (2012). The effect of sertraline add-on to brief dynamic psychotherapy for the treatment of postpartum depression: a randomized, double-blind, placebo-controlled study. J. Clin. Psychiatry 73, 235–241. doi: 10.4088/JCP.11m07117
Bonaterra, G. A., Schwendler, A., Hüther, J., Schwarzbach, H., Schwarz, A., Kolb, C., et al. (2017). Neurotrophic, cytoprotective, and anti-inflammatory effects of St. John’s wort extract on differentiated mouse hippocampal HT-22 neurons. Front. Pharmacol. 8:955. doi: 10.3389/fphar.2017.00955
Borgsted, C., Hogh, S., Hogsted, E. S., Fonnesbech-Sandberg, L., Ekelund, K., Albrechtsen, C. K., et al. (2022). The role of central serotonergic markers and estradiol changes in perinatal mental health. Acta Psychiatr. Scand. 146, 357–369. doi: 10.1111/acps.13461
Boufidou, F., Lambrinoudaki, I., Argeitis, J., Zervas, I. M., Pliatsika, P., Leonardou, A. A., et al. (2009). CSF and plasma cytokines at delivery and postpartum mood disturbances. J. Affect. Disord. 115, 287–292. doi: 10.1016/j.jad.2008.07.008
Bozzatello, P., Rocca, P., De Rosa, M. L., and Bellino, S. (2020). Current and emerging medications for borderline personality disorder: is pharmacotherapy alone enough? Expert. Opin. Pharmacother. 21, 47–61. doi: 10.1080/14656566.2019.1686482
Brown, J. V. E., Wilson, C. A., Ayre, K., Robertson, L., South, E., Molyneaux, E., et al. (2021). Antidepressant treatment for postnatal depression. Cochrane Database Syst. Rev. 2021:CD013560. doi: 10.1002/14651858.CD013560.pub2
Brummelte, S., and Galea, L. A. (2010a). Depression during pregnancy and postpartum: contribution of stress and ovarian hormones. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 34, 766–776. doi: 10.1016/j.pnpbp.2009.09.006
Brummelte, S., and Galea, L. A. (2010b). Chronic corticosterone during pregnancy and postpartum affects maternal care, cell proliferation and depressive-like behavior in the dam. Horm. Behav. 58, 769–779. doi: 10.1016/j.yhbeh.2010.07.012
Canenguez Benitez, J. S., Hernandez, T. E., Sundararajan, R., Sarwar, S., Arriaga, A. J., Khan, A. T., et al. (2022). Advantages and disadvantages of using St. John’s wort as a treatment for depression. Cureus 14:e29468. doi: 10.7759/cureus.29468
Cao, L., Liu, Y., Liang, X., Zheng, Y., Li, W., Yan, J., et al. (2020). Association between dietary patterns during the third trimester and the risk of postpartum depression in China. J. Affect. Disord. 264, 370–375. doi: 10.1016/j.jad.2019.11.054
Challis, J. R., Lockwood, C. J., Myatt, L., Norman, J. E., Strauss, J. F. 3rd, and Petraglia, F. (2009). Inflammation and pregnancy. Reprod. Sci. 16, 206–215. doi: 10.1177/1933719108329095
Chang, S. M., and Chen, C. H. (2016). Effects of an intervention with drinking chamomile tea on sleep quality and depression in sleep disturbed postnatal women: a randomized controlled trial. J. Adv. Nurs. 72, 306–315. doi: 10.1111/jan.12836
Chen, S. L., and Chen, C. H. (2015). Effects of lavender tea on fatigue, depression, and maternal-infant attachment in sleep-disturbed postnatal women. Worldviews Evid.-Based Nurs. 12, 370–379. doi: 10.1111/wvn.12122
Chen, M. L., Chen, Y. E., and Lee, H. F. (2022). The effect of bergamot essential oil aromatherapy on improving depressive mood and sleep quality in postpartum women: a randomized controlled trial. J. Nurs. Res. 30:e201. doi: 10.1097/jnr.0000000000000459
Chen, Z., Gu, J., Lin, S., Xu, Z., Xu, H., Zhao, J., et al. (2023). Saffron essential oil ameliorates CUMS-induced depression-like behavior in mice via the MAPK-CREB1-BDNF signaling pathway. J. Ethnopharmacol. 300:115719. doi: 10.1016/j.jep.2022.115719
Chen, Y. H., Xue, F., Yu, S. F., Li, X. S., Liu, L., Jia, Y. Y., et al. (2021). Gut microbiota dysbiosis in depressed women: the association of symptom severity and microbiota function. J. Affect. Disord. 282, 391–400. doi: 10.1016/j.jad.2020.12.143
Cho, K., and Kim, M. (2023). Effects of aromatherapy on depression: a meta-analysis of randomized controlled trials. Gen. Hosp. Psychiatry 84, 215–225. doi: 10.1016/j.genhosppsych.2023.08.003
Chung, Y. H., Chen, S. J., Lee, C. L., and Chang, Y. S. (2024). Kokedama and essential oils had a relaxing psychophysiological effect on Taiwanese women during the COVID-19 pandemic. Explore (N.Y.) 20, 371–379. doi: 10.1016/j.explore.2023.09.009
Dehghan-Banadaki, S., Hosseinzadeh, M., Madadizadeh, F., and Mozaffari-Khosravi, H. (2023). Empirically derived dietary patterns and postpartum depression symptoms in a large sample of Iranian women. BMC Psychiatry 23:422. doi: 10.1186/s12888-023-04910-w
Deligiannidis, K. M., Kroll-Desrosiers, A. R., Mo, S., Nguyen, H. P., Svenson, A., Jaitly, N., et al. (2016). Peripartum neuroactive steroid and gamma-aminobutyric acid profiles in women at-risk for postpartum depression. Psychoneuroendocrinology 70, 98–107. doi: 10.1016/j.psyneuen.2016.05.010
Deligiannidis, K. M., Meltzer-Brody, S., Gunduz-Bruce, H., Doherty, J., Jonas, J., Li, S., et al. (2021). Effect of Zuranolone vs placebo in postpartum depression: a randomized clinical trial. JAMA Psychiatry 78, 951–959. doi: 10.1001/jamapsychiatry.2021.1559
Di Florio, A., and Meltzer-Brody, S. (2015). Is postpartum depression a distinct disorder? Curr. Psychiatry Rep. 17:76. doi: 10.1007/s11920-015-0617-6
Donadon, M. F., Martin-Santos, R., and Lo, F. (2021). Oxytocin effects on the cognition of women with postpartum depression: a randomized, placebo-controlled clinical trial. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 111:110098. doi: 10.1016/j.pnpbp.2020.110098
Dos Reis, L. L., Dos Santos-Junior, J. G., Tufik, S., and Hachul, H. (2021). Lavender essential oil on postmenopausal women with insomnia: double-blind randomized trial. Complement. Ther. Med. 59:102726. doi: 10.1016/j.ctim.2021.102726
Dos Santos, É. R. Q., Maia, C. S. F., Fontes Junior, E. A., Melo, A. S., Pinheiro, B. G., and Maia, J. G. S. (2018). Linalool-rich essential oils from the Amazon display antidepressant-type effect in rodents. J. Ethnopharmacol. 212, 43–49. doi: 10.1016/j.jep.2017.10.013
Dubreucq, J., Kamperman, A. M., Al-Maach, N., Bramer, W. M., Pacheco, F., Ganho-Avila, A., et al. (2022). Examining the evidence on complementary and alternative therapies to treat peripartum depression in pregnant or postpartum women: study protocol for an umbrella review of systematic reviews and meta-analyses. BMJ Open 12:e057327. doi: 10.1136/bmjopen-2021-057327
Dugoua, J. J., Mills, E., Perri, D., and Koren, G. (2006). Safety and efficacy of St. John’s wort (hypericum) during pregnancy and lactation. Can. J. Clin. Pharmacol. 13, e268–e276.
Eke, A. C., Saccone, G., and Berghella, V. (2016). Selective serotonin reuptake inhibitor (SSRI) use during pregnancy and risk of preterm birth: a systematic review and meta-analysis. BJOG 123, 1900–1907. doi: 10.1111/1471-0528.14144
El Hamdaoui, Y., Zheng, F., Fritz, N., Ye, L., Tran, M. A., Schwickert, K., et al. (2022). Analysis of hyperforin (St. John’s wort) action at TRPC6 channel leads to the development of a new class of antidepressant drugs. Mol. Psychiatry 27, 5070–5085. doi: 10.1038/s41380-022-01804-3
El Midaoui, A., Ghzaiel, I., Vervandier-Fasseur, D., Ksila, M., Zarrouk, A., Nury, T., et al. (2022). Saffron (Crocus sativus L.): a source of nutrients for health and for the treatment of neuropsychiatric and age-related diseases. Nutrients 14:597. doi: 10.3390/nu14030597
Fan, C., Li, Y., Lan, T., Wang, W., Mao, X., and Yu, S. Y. (2021). Prophylactic treatment of curcumin in a rat model of depression by attenuating hippocampal synaptic loss. Food Funct. 12, 11202–11213. doi: 10.1039/D1FO02676C
Fan, C., Song, Q., Wang, P., Li, Y., Yang, M., and Yu, S. Y. (2018). Neuroprotective effects of curcumin on IL-1β-induced neuronal apoptosis and depression-like behaviors caused by chronic stress in rats. Front. Cell. Neurosci. 12:516. doi: 10.3389/fncel.2018.00516
Fard, F. E., Mirghafourvand, M., Mohammad-Alizadeh Charandabi, S., Farshbaf-Khalili, A., Javadzadeh, Y., and Asgharian, H. (2017). Effects of zinc and magnesium supplements on postpartum depression and anxiety: a randomized controlled clinical trial. Women Health 57, 1115–1128. doi: 10.1080/03630242.2016.1235074
Fisher, J., Cabral de Mello, M., Patel, V., Rahman, A., Tran, T., Holton, S., et al. (2012). Prevalence and determinants of common perinatal mental disorders in women in low- and lower-middle-income countries: a systematic review. Bull. World Health Organ. 90, 139–149H. doi: 10.2471/BLT.11.091850
Fisher, J., Tran, T., La, B. T., Kriitmaa, K., Rosenthal, D., and Tran, T. (2010). Common perinatal mental disorders in northern Viet Nam: community prevalence and health care use. Bull. World Health Organ. 88, 737–745. doi: 10.2471/BLT.09.067066
Freeman, M. P., Hibbeln, J. R., Wisner, K. L., Brumbach, B. H., Watchman, M., and Gelenberg, A. J. (2006). Randomized dose-ranging pilot trial of omega-3 fatty acids for postpartum depression. Acta Psychiatr. Scand. 113, 31–35. doi: 10.1111/j.1600-0447.2005.00660.x
Frieder, A., Fersh, M., Hainline, R., and Deligiannidis, K. M. (2019). Pharmacotherapy of postpartum depression: current approaches and novel drug development. CNS Drugs 33, 265–282. doi: 10.1007/s40263-019-00605-7
Frim, D. M., Emanuel, R. L., Robinson, B. G., Smas, C. M., Adler, G. K., and Majzoub, J. A. (1988). Characterization and gestational regulation of corticotropin-releasing hormone messenger RNA in human placenta. J. Clin. Invest. 82, 287–292. doi: 10.1172/JCI113585
Galea, L. A. M., and Frokjaer, V. G. (2019). Perinatal depression: embracing variability toward better treatment and outcomes. Neuron 102, 13–16. doi: 10.1016/j.neuron.2019.02.023
Galea, L. A., Wide, J. K., and Barr, A. M. (2001). Estradiol alleviates depressive-like symptoms in a novel animal model of post-partum depression. Behav. Brain Res. 122, 1–9. doi: 10.1016/S0166-4328(01)00170-X
Gao, Z., Wang, Y., and Yu, H. (2022). A Chinese classical prescription Chaihu Shugan powder in treatment of post-stroke depression: an overview. Medicina (Kaunas) 59:55. doi: 10.3390/medicina59010055
Glynn, L. M., Davis, E. P., and Sandman, C. A. (2013). New insights into the role of perinatal HPA-axis dysregulation in postpartum depression. Neuropeptides 47, 363–370. doi: 10.1016/j.npep.2013.10.007
Gregoire, A. J., Kumar, R., Everitt, B., Henderson, A. F., and Studd, J. W. (1996). Transdermal oestrogen for treatment of severe postnatal depression. Lancet 347, 930–933. doi: 10.1016/S0140-6736(96)91414-2
Guzmán-Gutiérrez, S. L., Bonilla-Jaime, H., Gómez-Cansino, R., and Reyes-Chilpa, R. (2015). Linalool and β-pinene exert their antidepressant-like activity through the monoaminergic pathway. Life Sci. 128, 24–29. doi: 10.1016/j.lfs.2015.02.021
Haase, J., and Brown, E. (2015). Integrating the monoamine, neurotrophin and cytokine hypotheses of depression — a central role for the serotonin transporter? Pharmacol. Ther. 147, 1–11. doi: 10.1016/j.pharmthera.2014.10.002
Hantsoo, L., Ward-O’Brien, D., Czarkowski, K. A., Gueorguieva, R., Price, L. H., and Epperson, C. N. (2014). A randomized, placebo-controlled, double-blind trial of sertraline for postpartum depression. Psychopharmacology 231, 939–948. doi: 10.1007/s00213-013-3316-1
Heinonen, E., Blennow, M., Blomdahl-Wetterholm, M., Hovstadius, M., Nasiell, J., Pohanka, A., et al. (2021). Sertraline concentrations in pregnant women are steady and the drug transfer to their infants is low. Eur. J. Clin. Pharmacol. 77, 1323–1331. doi: 10.1007/s00228-021-03122-z
Hulkkonen, P., Kataja, E. L., Vahlberg, T., Koivuniemi, E., Houttu, N., Pellonpera, O., et al. (2021). The efficacy of probiotics and/or n-3 long-chain polyunsaturated fatty acids intervention on maternal prenatal and postnatal depressive and anxiety symptoms among overweight and obese women. J. Affect. Disord. 289, 21–30. doi: 10.1016/j.jad.2021.04.006
Jafari-Koulaee, A., Elyasi, F., Taraghi, Z., Sadat Ilali, E., and Moosazadeh, M. (2020). A systematic review of the effects of aromatherapy with lavender essential oil on depression. Cent Asian J. Glob. Health 9:e442. doi: 10.5195/cajgh.2020.442
Jenkins, T. A., Nguyen, J. C., Polglaze, K. E., and Bertrand, P. P. (2016). Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients 8:56. doi: 10.3390/nu8010056
Jiang, P., Guo, Y., Dang, R., Yang, M., Liao, D., Li, H., et al. (2017). Salvianolic acid B protects against lipopolysaccharide-induced behavioral deficits and neuroinflammatory response: involvement of autophagy and NLRP3 inflammasome. J. Neuroinflammation 14:239. doi: 10.1186/s12974-017-1013-4
Jiang, H., Wang, Z., Wang, Y., Xie, K., Zhang, Q., Luan, Q., et al. (2013). Antidepressant-like effects of curcumin in chronic mild stress of rats: involvement of its anti-inflammatory action. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 47, 33–39. doi: 10.1016/j.pnpbp.2013.07.009
Johansen, S. L., Robakis, T. K., Williams, K. E., and Rasgon, N. L. (2019). Management of perinatal depression with non-drug interventions. BMJ 364:l322. doi: 10.1136/bmj.l322
Kalagiri, R. R., Carder, T., Choudhury, S., Vora, N., Ballard, A. R., Govande, V., et al. (2016). Inflammation in complicated pregnancy and its outcome. Am. J. Perinatol. 33, 1337–1356. doi: 10.1055/s-0036-1582397
Kanes, S., Colquhoun, H., Gunduz-Bruce, H., Raines, S., Arnold, R., Schacterle, A., et al. (2017). Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet 390, 480–489. doi: 10.1016/S0140-6736(17)31264-3
Kashani, L., Eslatmanesh, S., Saedi, N., Niroomand, N., Ebrahimi, M., Hosseinian, M., et al. (2017). Comparison of saffron versus fluoxetine in treatment of mild to moderate postpartum depression: a double-blind, randomized clinical trial. Pharmacopsychiatry 50, 64–68. doi: 10.1055/s-0042-115306
Kendall-Tackett, K. (2007). A new paradigm for depression in new mothers: the central role of inflammation and how breastfeeding and anti-inflammatory treatments protect maternal mental health. Int. Breastfeed. J. 2:6. doi: 10.1186/1746-4358-2-6
Kianpour, M., Mansouri, A., Mehrabi, T., and Asghari, G. (2016). Effect of lavender scent inhalation on prevention of stress, anxiety and depression in the postpartum period. Iran. J. Nurs. Midwifery Res. 21:197. doi: 10.4103/1735-9066.178248
Klainin, P., and Arthur, D. G. (2009). Postpartum depression in Asian cultures: a literature review. Int. J. Nurs. Stud. 46, 1355–1373. doi: 10.1016/j.ijnurstu.2009.02.012
Klier, C. M., Schmid-Siegel, B., Schäfer, M. R., Lenz, G., Saria, A., Lee, A., et al. (2006). St. John’s wort (Hypericum perforatum) and breastfeeding: plasma and breast milk concentrations of hyperforin for 5 mothers and 2 infants. J. Clin. Psychiatry 67, 305–309. doi: 10.4088/JCP.v67n0219
Kolahdooz, G., Vosough, I., Sepahi, S., and Mohajeri, S. A. (2023). The effect of crocin versus sertraline in treatment of mild to moderate postpartum depression: a double-blind, randomized clinical trial. Int. Clin. Psychopharmacol. 38, 9–15. doi: 10.1097/YIC.0000000000000426
Koutra, K., Vassilaki, M., Georgiou, V., Koutis, A., Bitsios, P., Chatzi, L., et al. (2014). Antenatal maternal mental health as determinant of postpartum depression in a population based mother-child cohort (Rhea study) in Crete, Greece. Soc. Psychiatry Psychiatr. Epidemiol. 49, 711–721. doi: 10.1007/s00127-013-0758-z
Koutra, K., Vassilaki, M., Georgiou, V., Koutis, A., Bitsios, P., Kogevinas, M., et al. (2018). Pregnancy, perinatal and postpartum complications as determinants of postpartum depression: the Rhea mother-child cohort in Crete, Greece. Epidemiol. Psychiatr. Sci. 27, 244–255. doi: 10.1017/S2045796016001062
Latendresse, G., Elmore, C., and Deneris, A. (2017). Selective serotonin reuptake inhibitors as first-line antidepressant therapy for perinatal depression. J. Midwifery Womens Health 62, 317–328. doi: 10.1111/jmwh.12607
Lee, K. B., Cho, E., and Kang, Y. S. (2014). Changes in 5-hydroxytryptamine and cortisol plasma levels in menopausal women after inhalation of clary sage oil. Phytother. Res. 28, 1599–1605. doi: 10.1002/ptr.5163
Lei, C., Li, N., Chen, J., and Wang, Q. (2023). Hypericin ameliorates depression-like behaviors via neurotrophin signaling pathway mediating m6A epitranscriptome modification. Molecules 28:3859. doi: 10.3390/molecules28093859
Leimert, K. B., Xu, W., Princ, M. M., Chemtob, S., and Olson, D. M. (2021). Inflammatory amplification: a central tenet of uterine transition for labor. Front. Cell. Infect. Microbiol. 11:660983. doi: 10.3389/fcimb.2021.660983
Li, Y. F. (2020). A hypothesis of monoamine (5-HT) - glutamate/GABA long neural circuit: aiming for fast-onset antidepressant discovery. Pharmacol. Ther. 208:107494. doi: 10.1016/j.pharmthera.2020.107494
Li, H. J., Martinez, P. E., Li, X., Schenkel, L. A., Nieman, L. K., Rubinow, D. R., et al. (2020). Transdermal estradiol for postpartum depression: results from a pilot randomized, double-blind, placebo-controlled study. Arch. Womens Ment. Health 23, 401–412. doi: 10.1007/s00737-019-00991-3
Li, X., Qin, X. M., Tian, J. S., Gao, X. X., Du, G. H., and Zhou, Y. Z. (2021). Integrated network pharmacology and metabolomics to dissect the combination mechanisms of Bupleurum chinense DC-Paeonia lactiflora pall herb pair for treating depression. J. Ethnopharmacol. 264:113281. doi: 10.1016/j.jep.2020.113281
Linde, K., Berner, M. M., and Kriston, L. (2008). St John’s wort for major depression. Cochrane Database Syst. Rev. 2008:Cd000448. doi: 10.1002/14651858.CD000448.pub3
Linde, K., Mulrow, C. D., Berner, M., and Egger, M. (2005). St John’s wort for depression. Cochrane Database Syst. Rev. 2:Cd000448. doi: 10.1002/14651858.CD000448.pub2
Lindsay, J. R., and Nieman, L. K. (2005). The hypothalamic-pituitary-adrenal axis in pregnancy: challenges in disease detection and treatment. Endocr. Rev. 26, 775–799. doi: 10.1210/er.2004-0025
Long, E. S., Penalver Bernabe, B., Xia, K., Azcarate-Peril, M. A., Carroll, I. M., Rackers, H. S., et al. (2023). The microbiota-gut-brain axis and perceived stress in the perinatal period. Arch. Womens Ment. Health 26, 227–234. doi: 10.1007/s00737-023-01300-9
López, V., Nielsen, B., Solas, M., Ramírez, M. J., and Jäger, A. K. (2017). Exploring pharmacological mechanisms of lavender (Lavandula angustifolia) essential oil on central nervous system targets. Front. Pharmacol. 8:280. doi: 10.3389/fphar.2017.00280
Lucena, L., Santos-Junior, J. G., Tufik, S., and Hachul, H. (2024). Effect of a lavender essential oil and sleep hygiene protocol on insomnia in postmenopausal women: a pilot randomized clinical trial. Explore (N.Y.) 20, 116–125. doi: 10.1016/j.explore.2023.07.004
Ma, J. H., Wang, S. Y., Yu, H. Y., Li, D. Y., Luo, S. C., Zheng, S. S., et al. (2019). Prophylactic use of ketamine reduces postpartum depression in Chinese women undergoing cesarean section (☆). Psychiatry Res. 279, 252–258. doi: 10.1016/j.psychres.2019.03.026
Maes, M., Ombelet, W., De Jongh, R., Kenis, G., and Bosmans, E. (2001). The inflammatory response following delivery is amplified in women who previously suffered from major depression, suggesting that major depression is accompanied by a sensitization of the inflammatory response system. J. Affect. Disord. 63, 85–92. doi: 10.1016/S0165-0327(00)00156-7
Maguire, J., Ferando, I., Simonsen, C., and Mody, I. (2009). Excitability changes related to GABAA receptor plasticity during pregnancy. J. Neurosci. 29, 9592–9601. doi: 10.1523/JNEUROSCI.2162-09.2009
Maguire, J., and Mody, I. (2008). GABA(a)R plasticity during pregnancy: relevance to postpartum depression. Neuron 59, 207–213. doi: 10.1016/j.neuron.2008.06.019
Makrides, M., Gibson, R. A., McPhee, A. J., Yelland, L., Quinlivan, J., and Ryan, P. (2010). Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: a randomized controlled trial. JAMA 304, 1675–1683. doi: 10.1001/jama.2010.1507
Marano, G., Mazza, M., Lisci, F. M., Ciliberto, M., Traversi, G., Kotzalidis, G. D., et al. (2023). The microbiota-gut-brain axis: psychoneuroimmunological insights. Nutrients 15:6. doi: 10.3390/nu15061496
Markowitz, J. S., and DeVane, C. L. (2001). The emerging recognition of herb-drug interactions with a focus on St. John’s wort (Hypericum perforatum). Psychopharmacol. Bull. 35, 53–64. doi: 10.1016/j.lfs.2005.04.055
Matias Nascimento Maia, W., Pereira, D. C., de Andrade, F., Alves Filgueiras, L., Nogueira Mendes, A., Fonseca Costa Assunção, A., et al. (2021). Antidepressant activity of rose oxide essential oil: possible involvement of serotonergic transmission. Heliyon 7:e06620. doi: 10.1016/j.heliyon.2021.e06620
Mehta, D., Grewen, K., Pearson, B., Wani, S., Wallace, L., Henders, A. K., et al. (2021). Genome-wide gene expression changes in postpartum depression point towards an altered immune landscape. Transl. Psychiatry 11:155. doi: 10.1038/s41398-021-01270-5
Mehta, D., Newport, D. J., Frishman, G., Kraus, L., Rex-Haffner, M., Ritchie, J. C., et al. (2014). Early predictive biomarkers for postpartum depression point to a role for estrogen receptor signaling. Psychol. Med. 44, 2309–2322. doi: 10.1017/S0033291713003231
Meltzer-Brody, S., Colquhoun, H., Riesenberg, R., Epperson, C. N., Deligiannidis, K. M., Rubinow, D. R., et al. (2018). Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet 392, 1058–1070. doi: 10.1016/S0140-6736(18)31551-4
Meltzer-Brody, S., and Kanes, S. J. (2020). Allopregnanolone in postpartum depression: role in pathophysiology and treatment. Neurobiol. Stress 12:100212. doi: 10.1016/j.ynstr.2020.100212
Milgrom, J., Gemmill, A. W., Ericksen, J., Burrows, G., Buist, A., and Reece, J. (2015). Treatment of postnatal depression with cognitive behavioural therapy, sertraline and combination therapy: a randomised controlled trial. Aust. N. Z. J. Psychiatry 49, 236–245. doi: 10.1177/0004867414565474
Miller, B. J., Murray, L., Beckmann, M. M., Kent, T., and Macfarlane, B. (2013). Dietary supplements for preventing postnatal depression. Cochrane Database Syst. Rev. 2013:CD009104. doi: 10.1002/14651858.CD009104.pub2
Misri, S., Abizadeh, J., Albert, G., Carter, D., and Ryan, D. (2012). Restoration of functionality in postpartum depressed mothers: an open-label study with escitalopram. J. Clin. Psychopharmacol. 32, 729–732. doi: 10.1097/JCP.0b013e31826867c9
Mokhber, N., Namjoo, M., Tara, F., Boskabadi, H., Rayman, M. P., Ghayour-Mobarhan, M., et al. (2011). Effect of supplementation with selenium on postpartum depression: a randomized double-blind placebo-controlled trial. J. Matern. Fetal Neonatal Med. 24, 104–108. doi: 10.3109/14767058.2010.482598
Monchaux De Oliveira, C., Pourtau, L., Vancassel, S., Pouchieu, C., Capuron, L., Gaudout, D., et al. (2021). Saffron extract-induced improvement of depressive-like behavior in mice is associated with modulation of monoaminergic neurotransmission. Nutrients 13:904. doi: 10.3390/nu13030904
Moretti, M. E., Maxson, A., Hanna, F., and Koren, G. (2009). Evaluating the safety of St. John’s wort in human pregnancy. Reprod. Toxicol. 28, 96–99. doi: 10.1016/j.reprotox.2009.02.003
Nonacs, R. M., Soares, C. N., Viguera, A. C., Pearson, K., Poitras, J. R., and Cohen, L. S. (2005). Bupropion SR for the treatment of postpartum depression: a pilot study. Int. J. Neuropsychopharmacol. 8, 445–449. doi: 10.1017/S1461145705005079
O’Hara, M. W., Pearlstein, T., Stuart, S., Long, J. D., Mills, J. A., and Zlotnick, C. (2019). A placebo controlled treatment trial of sertraline and interpersonal psychotherapy for postpartum depression. J. Affect. Disord. 245, 524–532. doi: 10.1016/j.jad.2018.10.361
Osborne, L. M., and Monk, C. (2013). Perinatal depression—the fourth inflammatory morbidity of pregnancy? Psychoneuroendocrinology 38, 1929–1952. doi: 10.1016/j.psyneuen.2013.03.019
Pawluski, J. L., Li, M., and Lonstein, J. S. (2019). Serotonin and motherhood: from molecules to mood. Front. Neuroendocrinol. 53:100742. doi: 10.1016/j.yfrne.2019.03.001
Payne, J. L., and Maguire, J. (2019). Pathophysiological mechanisms implicated in postpartum depression. Front. Neuroendocrinol. 52, 165–180. doi: 10.1016/j.yfrne.2018.12.001
Perry, A., Gordon-Smith, K., Jones, L., and Jones, I. (2021). Phenomenology, epidemiology and aetiology of postpartum psychosis: a review. Brain Sci. 11:47. doi: 10.3390/brainsci11010047
Pillai, L., Srivastava, S., Ajin, A., Rana, S. S., Mathkor, D. M., Haque, S., et al. (2023). Etiology and incidence of postpartum depression among birthing women in the scenario of pandemics, geopolitical conflicts and natural disasters: a systematic review. J. Psychosom. Obstet. Gynaecol. 44:2278016. doi: 10.1080/0167482X.2023.2278016
Pontifex, M. G., Connell, E., Le Gall, G., Pourtau, L., Gaudout, D., Angeloni, C., et al. (2022). Saffron extract (Safr’Inside™) improves anxiety related behaviour in a mouse model of low-grade inflammation through the modulation of the microbiota and gut derived metabolites. Food Funct. 13, 12219–12233. doi: 10.1039/D2FO02739A
Post, C., and Leuner, B. (2019). The maternal reward system in postpartum depression. Arch. Womens Ment. Health 22, 417–429. doi: 10.1007/s00737-018-0926-y
Rees, A. M., Austin, M. P., and Parker, G. B. (2008). Omega-3 fatty acids as a treatment for perinatal depression: randomized double-blind placebo-controlled trial. Aust. N. Z. J. Psychiatry 42, 199–205. doi: 10.1080/00048670701827267
Reilly, J. G., McTavish, S. F., and Young, A. H. (1997). Rapid depletion of plasma tryptophan: a review of studies and experimental methodology. J. Psychopharmacol. 11, 381–392. doi: 10.1177/026988119701100416
Rezaie-Keikhaie, K., Arbabshastan, M. E., Rafiemanesh, H., Amirshahi, M., Ostadkelayeh, S. M., and Arbabisarjou, A. (2020). Systematic review and meta-analysis of the prevalence of the maternity blues in the postpartum period. J. Obstet. Gynecol. Neonatal. Nurs. 49, 127–136. doi: 10.1016/j.jogn.2020.01.001
Rincon-Cortes, M., and Grace, A. A. (2020). Postpartum changes in affect-related behavior and VTA dopamine neuron activity in rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 97:109768. doi: 10.1016/j.pnpbp.2019.109768
Roddy Mitchell, A., Gordon, H., Lindquist, A., Walker, S. P., Homer, C. S. E., Middleton, A., et al. (2023). Prevalence of perinatal depression in low- and middle-income countries: a systematic review and meta-analysis. JAMA Psychiatry 80, 425–431. doi: 10.1001/jamapsychiatry.2023.0069
Romero, R., Gotsch, F., Pineles, B., and Kusanovic, J. P. (2007). Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr. Rev. 65, 194–202. doi: 10.1301/nr.2007.dec.S194-S202
Rupanagunta, G. P., Nandave, M., Rawat, D., Upadhyay, J., Rashid, S., and Ansari, M. N. (2023). Postpartum depression: aetiology, pathogenesis and the role of nutrients and dietary supplements in prevention and management. Saudi Pharm. J. 31, 1274–1293. doi: 10.1016/j.jsps.2023.05.008
Sacher, J., Houle, S., Parkes, J., Rusjan, P., Sagrati, S., Wilson, A. A., et al. (2011). Monoamine oxidase a inhibitor occupancy during treatment of major depressive episodes with moclobemide or St. John’s wort: an [11C]-harmine PET study. J. Psychiatry Neurosci. 36, 375–382. doi: 10.1503/jpn.100117
Sanna, E., Mostallino, M. C., Murru, L., Carta, M., Talani, G., Zucca, S., et al. (2009). Changes in expression and function of extrasynaptic GABAA receptors in the rat hippocampus during pregnancy and after delivery. J. Neurosci. 29, 1755–1765. doi: 10.1523/JNEUROSCI.3684-08.2009
Sathyanarayanan, G., Thippeswamy, H., Mani, R., Venkataswamy, M., Kumar, M., Philip, M., et al. (2019). Cytokine alterations in first-onset postpartum psychosis-clues for underlying immune dysregulation. Asian J. Psychiatr. 42, 74–78. doi: 10.1016/j.ajp.2019.03.012
Schmidt, P. J., Ben Dor, R., Martinez, P. E., Guerrieri, G. M., Harsh, V. L., Thompson, K., et al. (2015). Effects of estradiol withdrawal on mood in women with past perimenopausal depression: a randomized clinical trial. JAMA Psychiatry 72, 714–726. doi: 10.1001/jamapsychiatry.2015.0111
Sluiter, F., Incollingo Rodriguez, A. C., Nephew, B. C., Cali, R., Murgatroyd, C., and Santos, H. P. Jr. (2020). Pregnancy associated epigenetic markers of inflammation predict depression and anxiety symptoms in response to discrimination. Neurobiol. Stress 13:100273. doi: 10.1016/j.ynstr.2020.100273
Slykerman, R. F., Hood, F., Wickens, K., Thompson, J. M. D., Barthow, C., Murphy, R., et al. (2017). Effect of Lactobacillus rhamnosus HN001 in pregnancy on postpartum symptoms of depression and anxiety: a randomised double-blind placebo-controlled trial. EBioMedicine 24, 159–165. doi: 10.1016/j.ebiom.2017.09.013
Soleimani, V., Sahebkar, A., and Hosseinzadeh, H. (2018). Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: review. Phytother. Res. 32, 985–995. doi: 10.1002/ptr.6054
Soleymani, S., Bahramsoltani, R., Rahimi, R., and Abdollahi, M. (2017). Clinical risks of St John’s wort (Hypericum perforatum) co-administration. Expert Opin. Drug Metab. Toxicol. 13, 1047–1062. doi: 10.1080/17425255.2017.1378342
Spiess, D., Winker, M., Dolder Behna, A., Gründemann, C., and Simões-Wüst, A. P. (2022). Advanced in vitro safety assessment of herbal medicines for the treatment of non-psychotic mental disorders in pregnancy. Front. Pharmacol. 13:882997. doi: 10.3389/fphar.2022.882997
Stewart, D. E., and Vigod, S. N. (2019). Postpartum depression: pathophysiology, treatment, and emerging therapeutics. Annu. Rev. Med. 70, 183–196. doi: 10.1146/annurev-med-041217-011106
Stoffel, E. C., and Craft, R. M. (2004). Ovarian hormone withdrawal-induced “depression” in female rats. Physiol. Behav. 83, 505–513. doi: 10.1016/j.physbeh.2004.08.033
Stohl, H., Kohm, A. D., and Dossett, E. (2016). A rock and a hard place: the selective serotonin reuptake inhibitor dilemmas in addressing perinatal mood and anxiety disorders. J. Neonatal Perinatal Med. 9, 1–5. doi: 10.3233/NPM-16915057
Stolzenberg, D. S., and Numan, M. (2011). Hypothalamic interaction with the mesolimbic DA system in the control of the maternal and sexual behaviors in rats. Neurosci. Biobehav. Rev. 35, 826–847. doi: 10.1016/j.neubiorev.2010.10.003
Tabeshpour, J., Sobhani, F., Sadjadi, S. A., Hosseinzadeh, H., Mohajeri, S. A., Rajabi, O., et al. (2017). A double-blind, randomized, placebo-controlled trial of saffron stigma (Crocus sativus L.) in mothers suffering from mild-to-moderate postpartum depression. Phytomedicine 36, 145–152. doi: 10.1016/j.phymed.2017.10.005
Toker, L., Amar, S., Bersudsky, Y., Benjamin, J., and Klein, E. (2010). The biology of tryptophan depletion and mood disorders. Isr. J. Psychiatry Relat. Sci. 47, 46–55. doi: 10.1016/s0165-0327(02)00006-x
Tsai, S. S., Wang, H. H., and Chou, F. H. (2020). The effects of aromatherapy on postpartum women: a systematic review. J. Nurs. Res. 28:e96. doi: 10.1097/jnr.0000000000000331
Ueno, H., Shimada, A., Suemitsu, S., Murakami, S., Kitamura, N., Wani, K., et al. (2019). Anti-depressive-like effect of 2-phenylethanol inhalation in mice. Biomed. Pharmacother. 111, 1499–1506. doi: 10.1016/j.biopha.2018.10.073
Upadhyay, R. P., Chowdhury, R., Aslyeh, S., Sarkar, K., Singh, S. K., Sinha, B., et al. (2017). Postpartum depression in India: a systematic review and meta-analysis. Bull. World Health Organ. 95, 706–717C. doi: 10.2471/BLT.17.192237
Uzunçakmak, T., and Ayaz, A. S. (2018). Effect of aromatherapy on coping with premenstrual syndrome: a randomized controlled trial. Complement. Ther. Med. 36, 63–67. doi: 10.1016/j.ctim.2017.11.022
Vaz, J. D. S., Farias, D. R., Adegboye, A. R. A., Nardi, A. E., and Kac, G. (2017). Omega-3 supplementation from pregnancy to postpartum to prevent depressive symptoms: a randomized placebo-controlled trial. BMC Pregnancy Childbirth 17:180. doi: 10.1186/s12884-017-1365-x
Walker, A. L., de Rooij, S. R., Dimitrova, M. V., Witteveen, A. B., Verhoeven, C. J., de Jonge, A., et al. (2021). Psychosocial and peripartum determinants of postpartum depression: findings from a prospective population-based cohort. The ABCD study. Compr. Psychiatry 108:152239. doi: 10.1016/j.comppsych.2021.152239
Wang, J., Wu, Q., Ou, C., Lu, G., and Yu, H. (2023). Research on Xiaoyao powder in the treatment of depression based on epigenetics and quality markers. Front. Neurosci. 17:1223451. doi: 10.3389/fnins.2023.1223451
Wauquier, F., Boutin-Wittrant, L., Pourtau, L., Gaudout, D., Moras, B., Vignault, A., et al. (2022). Circulating human serum metabolites derived from the intake of a saffron extract (Safr’Inside(TM)) protect neurons from oxidative stress: consideration for depressive disorders. Nutrients 14:1511. doi: 10.3390/nu14071511
Wisner, K. L., Hanusa, B. H., Perel, J. M., Peindl, K. S., Piontek, C. M., Sit, D. K., et al. (2006). Postpartum depression: a randomized trial of sertraline versus nortriptyline. J. Clin. Psychopharmacol. 26, 353–360. doi: 10.1097/01.jcp.0000227706.56870.dd
Wisner, K. L., Perel, J. M., Peindl, K. S., Hanusa, B. H., Piontek, C. M., and Findling, R. L. (2004). Prevention of postpartum depression: a pilot randomized clinical trial. Am. J. Psychiatry 161, 1290–1292. doi: 10.1176/appi.ajp.161.7.1290
Wisner, K. L., Sit, D. K., Moses-Kolko, E. L., Driscoll, K. E., Prairie, B. A., Stika, C. S., et al. (2015). Transdermal estradiol treatment for postpartum depression: a pilot, randomized trial. J. Clin. Psychopharmacol. 35, 389–395. doi: 10.1097/JCP.0000000000000351
Worthen, R. J., and Beurel, E. (2022). Inflammatory and neurodegenerative pathophysiology implicated in postpartum depression. Neurobiol. Dis. 165:105646. doi: 10.1016/j.nbd.2022.105646
Yang, C., Zhao, A., Lan, H., Ren, Z., Zhang, J., Szeto, I. M., et al. (2021). Association between dietary quality and postpartum depression in lactating women: a cross-sectional survey in urban China. Front. Nutr. 8:705353. doi: 10.3389/fnut.2021.705353
Yonkers, K. A., Lin, H., Howell, H. B., Heath, A. C., and Cohen, L. S. (2008). Pharmacologic treatment of postpartum women with new-onset major depressive disorder: a randomized controlled trial with paroxetine. J. Clin. Psychiatry 69, 659–665. doi: 10.4088/JCP.v69n0420
Young, S. N., and Leyton, M. (2002). The role of serotonin in human mood and social interaction. Insight from altered tryptophan levels. Pharmacol. Biochem. Behav. 71, 857–865. doi: 10.1016/S0091-3057(01)00670-0
Yu, H. Y., Wang, S. Y., Quan, C. X., Fang, C., Luo, S. C., Li, D. Y., et al. (2019). Dexmedetomidine alleviates postpartum depressive symptoms following cesarean section in Chinese women: a randomized placebo-controlled study. Pharmacotherapy 39, 994–1004. doi: 10.1002/phar.2320
Zepeda, R. C., Juárez-Portilla, C., and T, M.-J. (2022). St. John’s wort usage in treating of perinatal depression. Front. Behav. Neurosci. 16:1066459. doi: 10.3389/fnbeh.2022.1066459
Zhai, X., Chen, Y., Han, X., Zhu, Y., Li, X., Zhang, Y., et al. (2022). The protective effect of hypericin on postpartum depression rat model by inhibiting the NLRP3 inflammasome activation and regulating glucocorticoid metabolism. Int. Immunopharmacol. 105:108560. doi: 10.1016/j.intimp.2022.108560
Zhang, W. Y., Guo, Y. J., Han, W. X., Yang, M. Q., Wen, L. P., Wang, K. Y., et al. (2019). Curcumin relieves depressive-like behaviors via inhibition of the NLRP3 inflammasome and kynurenine pathway in rats suffering from chronic unpredictable mild stress. Int. Immunopharmacol. 67, 138–144. doi: 10.1016/j.intimp.2018.12.012
Zhang, L., Xu, T., Wang, S., Yu, L., Liu, D., Zhan, R., et al. (2013). NMDA GluN2B receptors involved in the antidepressant effects of curcumin in the forced swim test. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 40, 12–17. doi: 10.1016/j.pnpbp.2012.08.017
Zhou, S., Chan, E., Pan, S. Q., Huang, M., and Lee, E. J. (2004). Pharmacokinetic interactions of drugs with St John’s wort. J. Psychopharmacol. 18, 262–276. doi: 10.1177/0269881104042632
Keywords: perinatal depression, pathophysiological mechanisms, non-drug intervention, herbal remedies, dietary supplements
Citation: Yang Q, Lv Y, Gao S, Zhang Y and Zhai X (2025) Supplementary approaches to perinatal depression: a review of pathogenesis, herbal interventions, and dietary supplements. Front. Psychol. 16:1529339. doi: 10.3389/fpsyg.2025.1529339
Edited by:
Mile Vuković, University of Belgrade, SerbiaReviewed by:
Mudassir Alam, Aligarh Muslim University, IndiaRafał Marecki, Medical University of Bialystok, Poland
Copyright © 2025 Yang, Lv, Gao, Zhang and Zhai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Zhang, d2h1aF96aGFuZ3l1QDEyNi5jb20=; Xuejia Zhai, emhhaXh1ZWppYUAxNjMuY29t
†These authors have contributed equally to this work
 Quancheng Yang
Quancheng Yang Yi Lv1,2†
Yi Lv1,2† Xuejia Zhai
Xuejia Zhai