- 1Nanhai College of Arts and Technology, Haikou University of Economics, Haikou, China
- 2College of arts, Chonbuk National University, Jeonju, Republic of Korea
- 3Zhangjiajie Hospital of Traditional Chinese Medicine, Zhangjiajie, China
- 4Department of International Cultural Education, Chodang University, Muan, Republic of Korea
Objective: This meta-analysis aimed to evaluate the effectiveness of art therapy interventions in improving motor function performance in patients with Parkinson’s Disease (PD), with a focus on identifying the most effective modalities.
Method: Randomized controlled trials were identified through searches in PubMed, Embase, Web of Science, and Cochrane Library. Twenty-six studies were included, assessed for quality, and analyzed following PRISMA guidelines (PROSPERO: CRD42024611770). Subgroup analyses were performed for primary outcomes (UPDRS, TUG, Mini−BESTest), while secondary outcomes (Stride Length, FOG, 6MWT, and Gait Speed) were evaluated using forest and funnel plots to estimate pooled effects.
Results: Art therapy significantly improved motor function, as evidenced by reductions in UPDRS III scores (SMD = −0.44, 95% CI [−0.61, −0.26], p < 0.05), TUG scores (SMD = −0.25, 95% CI [−0.41, −0.10], p < 0.05), and increases in Mini-BESTest scores (SMD = 0.41, 95% CI [0.10, 0.72], p < 0.05). Among the interventions, dance therapy demonstrated the most significant effects on motor function (UPDRS III: SMD = −0.52, 95% CI [−0.78, −0.26], p < 0.05; TUG: SMD = −0.37, 95% CI [−0.58, −0.17], p < 0.05; Mini-BESTest: SMD = 0.56, 95% CI [0.25, 0.87], p < 0.05). Secondary outcomes revealed small to moderate improvements in gait speed (SMD = 0.34, p < 0.05), 6MWT (SMD = 0.41, p < 0.05), FOG (SMD = −0.33, p < 0.05), and stride length (SMD = 0.59, p < 0.05). Although the findings were robust, high heterogeneity in certain outcomes highlights the need for standardized intervention protocols to ensure consistency and reproducibility.
Conclusion: This study underscores the clinical significance of art therapy in improving motor functions in PD patients. Among the interventions, dance therapy exhibited the most pronounced effects, highlighting its potential as a pivotal component in multidisciplinary neurorehabilitation programs.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42024611770, identifier (CRD42024611770).
1 Introduction
The disability and mortality rates of PD (PD), an incurable progressive neurodegenerative disorder, are rising faster than those of any other neurological condition (Playfer, 1997; World Health Organization, n.d.). Early loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) leads to dopamine deficiency in the basal ganglia and dysfunction in the basal ganglia-supplementary motor area (BG-SMA) interaction (Cunnington et al., 1996), resulting in movement disorders characterized by classic Parkinsonian motor symptoms (Kalia and Lang, 2015). These motor symptoms include resting tremors, bradykinesia, rigidity, postural instability, freezing of gait, and balance difficulties (Kuhlman et al., 2019; Morris et al., 1994). Motor decline also directly affects non-motor functions, such as depression, anxiety, obsessive-compulsive behaviors, cognitive decline, reduced quality of life, and autonomic dysfunction (e.g., bladder and sexual issues) (Chaudhuri and Schapira, 2009; Postuma and Montplaisir, 2009). The diagnosis of PD relies on clinical motor signs as key indicators (Postuma et al., 2015), Reduction in the amplitude of step size (hypokinesia) is a regular feature of PD (Morris et al., 1994) and gait disturbances are closely associated with disease severity (Iansek et al., 2006). Gait disturbances and impaired motor function are major contributors to the decline in quality of life and loss of independence among PD patients, profoundly impacting their physical and mental health.
Dopaminergic medications are the standard treatment for PD and are effective in managing motor symptoms during early stages (Cotzias et al., 1969), however, they often fail to address the full spectrum of motor symptoms (Fahn, 2008; Bohannon, 1993). Over time, complications masked by these medications may emerge (Lang and Lozano, 1998). The progressive worsening of PD symptoms, combined with the high costs of traditional treatments (Balestrino and Schapira, 2020), underscores the urgent need for adjunctive interventions that are cost-effective, safe, and effective in clinical practice.
Art therapy is an umbrella term for disciplines that use creative expression within therapeutic relationships to facilitate emotional exploration, self-awareness, and healing. This heterogeneous field encompasses distinct modalities: visual art therapy employs non-verbal expression through painting or sculpting to process emotions; dance/movement therapy integrates rhythmic auditory cues and body awareness to enhance motor-cognitive connections; music therapy uses structured sound patterns for neurological retraining; and drama therapy focuses on role-play for interpersonal skill development (Kaplan, 2003). As a safe and reliable adjunctive intervention, art therapy has demonstrated effectiveness across clinical contexts including stroke rehabilitation (Kongkasuwan et al., 2016), cancer care (Boehm et al., 2014), mental health (Moo and Ho, 2024; Zhang et al., 2024), and diabetes management (Krishnan et al., 2015; Yang et al., 2021). The current review specifically examines movement-based creative interventions (dance therapies) within Parkinson’s disease (PD) care, where their multisensory integration mechanisms—combining rhythmic auditory stimulation, visual prompts, and choreographed movements—synergize with social engagement to target motor dysfunction. Culturally specific approaches, such as Biodanza, tango, and Qi dance, enhance gait rhythm through rhythmic auditory stimulation and improve adherence through social engagement. Studies suggest these methods positively impact gait speed, stride length, freezing of gait, motor function, attention, and cognition (Morris et al., 1994).
Despite several clinical trials, no comprehensive meta-analysis has evaluated the effects of art therapy on physical function and gait in PD. This study addresses this gap by providing evidence-based insights to support cost-effective, personalized, non-pharmacological interventions for clinical practice and research. In this study, “body function” is defined as a multidimensional concept encompassing both gait and dynamic balance, with gait being a core component.
2 Methods
The study strictly follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting checklist and has been registered in PROSPERO (CRD42024611770). Subgroup analyses were performed exclusively for primary outcomes (UPDRS III, TUG and Mini-BESTest), which are central to evaluating motor function in PD. Secondary outcomes (Stride Length, FOG, 6MWT, and Gait Speed) were quantitatively analyzed using forest plots and funnel Plots to estimate pooled effects.
2.1 Search strategy
A thorough literature search was performed across multiple electronic databases, including the Cochrane Library, Web of Science, PubMed, and EMBASE, covering publications from their inception up to July 31, 2024. Using a comprehensive search strategy that combined Medical Subject Headings (MeSH) and free-text terms, we screened titles, abstracts, keywords, and subsequently conducted a full-text review. The temporal range of this review spanned from January 1900 to July 2024, with non-English language literature excluded. These time and language restrictions were applied to focus the research team on the latest, relevant studies within their language proficiency, thereby enhancing the quality and validity of the findings. The search strategy, initially based on PubMed, was extended to additional databases. A manual search was also conducted by reviewing the bibliographies of pertinent articles and references within the selected studies.
The detailed search formula was as follows:
((“1900/01/01”[Date - Publication]: “2024/07/31”[Date - Publication])) AND (((((trial[Title/Abstract]) OR (random*[Title/Abstract])) OR (“Randomized Controlled Trial” [Publication Type])) AND ((((((“singing”[Title/Abstract] OR “dancing”[Title/Abstract] OR “painting”[Title/Abstract] OR “drawing”[Title/Abstract] OR “calligraphy”[Title/Abstract] OR “music”[Title/Abstract] OR “sculpture”[Title/Abstract] OR “collage”[Title/Abstract] OR “poetry”[Title/Abstract] OR “drama”[Title/Abstract] OR “clay”[Title/Abstract] OR “theater”[Title/Abstract]) OR (“visual art therapy”[Title/Abstract])) OR (“yoga”[Title/Abstract])) OR (“mindfulness”[Title/Abstract])) OR (“meditation therapy”[Title/Abstract])) OR (“Art Therapy”[Mesh]))) AND ((“Parkinson Disease”[Mesh]) OR ((((((((((((Idiopathic Parkinson’s Disease[Title/Abstract]) OR (Lewy Body Parkinson’s Disease[Title/Abstract])) OR (Parkinson’s Disease, Idiopathic[Title/Abstract])) OR (Parkinson’s Disease, Lewy Body[Title/Abstract])) OR (Parkinson Disease, Idiopathic[Title/Abstract])) OR (Parkinson’s Disease[Title/Abstract])) OR (Idiopathic Parkinson Disease[Title/Abstract])) OR (Lewy Body Parkinson Disease[Title/Abstract])) OR (Primary Parkinsonism[Title/Abstract])) OR (Parkinsonism, Primary[Title/Abstract])) OR (Paralysis Agitans[Title/Abstract])) OR (Parkinson[Title/Abstract])))). Additionally, a manual search was conducted through the bibliographies of relevant review articles and studies cited in the selected papers. The full search strategies for each database are documented in Table 1.
2.2 Inclusion and exclusion criteria
2.2.1 Inclusion criteria
The selection process follows the PICOS framework (Population, Intervention, Comparison, Outcome, and Study Design) to ensure consistency and rigor:
Population: (1) Individuals clinically diagnosed with PD by qualified clinicians or established diagnostic guidelines. (2) Aged ≥ 40 years, capable of standing for at least 30 min, and able to walk independently for a minimum of 3 meters (with or without assistive devices). (3) Excludes participants with psychiatric conditions other than PD or those unable to continue observation due to severe physical illness.
Intervention: (1) Experimental group: Any form of art therapy (e.g., singing, dancing, painting, yoga, or other artistic expressions), including combined therapies. (2) Control group: Standard care, no intervention, or non-art-based therapies.
Outcome: (1) Primary outcome measures include UPDRS III, TUG, Ministerial. (2) Secondary outcomes include 6MWT, Stride Length, FOG, and Gait Speed.
Study design: (1) Only randomized controlled trials (RCTs) published in English and reporting original clinical data. (2) Excludes case reports, editorials, reviews, conference abstracts, and other non-clinical formats.
2.2.2 Exclusion criteria
(1) Literature with titles and abstracts in English (or American English) but with the full text in a different language will not be considered.
(2) Non-randomized studies, such as reviews, animal studies, study protocols, conference abstracts, case reports, and online reports, are ineligible.
(3) Studies lacking complete experimental data or having missing information will not be included.
(4) Observational studies will be excluded.
Overall, the inclusion criteria for the meta-analysis are designed to include only high-quality and rigorously conducted studies.
2.3 Study selection and data extraction
Records that align with the specified search criteria will be imported into Endnote 20 for deduplication purposes. Two independently evaluated the relevance of search results and extracted data using a standardized form, an initial review was conducted, and any differences between the reviewers were discussed until a consensus was reached. If an agreement could not be achieved, a third reviewer was consulted to make a final decision after discussions with the original two reviewers. To measure the agreement between the two primary reviewers during abstract and full-text screening, kappa values were calculated following established guidelines. Prioritizing intention−to−treat data over completer analysis data when available. They recorded details from each article, such as publication year, study location, diagnostic criteria, sample size, mean/median age, percentage of male participants, registration number, intervention method, and outcomes of interest. For studies with missing or unextractable data, attempts were made to contact the authors for additional information.
2.4 Quality assessment
The methodological quality of the included studies was assessed using the Cochrane Risk of Bias Tool 2.0. This tool evaluates key domains, including randomization process, deviations from intended interventions, missing outcome data, measurement of outcomes, and selection of reported results, ensuring a comprehensive assessment of potential bias. Two independent reviewers conducted the evaluations, and any discrepancies were discussed to reach consensus. If consensus was not achievable, a third reviewer provided the final decision. This rigorous quality assessment establishes a robust foundation for the meta-analysis, enhancing the validity and reliability of the findings. Which examines the generation of random sequences, allocation concealment, blind method for implementers and participants, blinding of outcome assessors, incomplete data, selective reporting, and other sources of bias. Studies were classified as “low risk,” “unclear,” or “high risk” of bias (Figure 1).
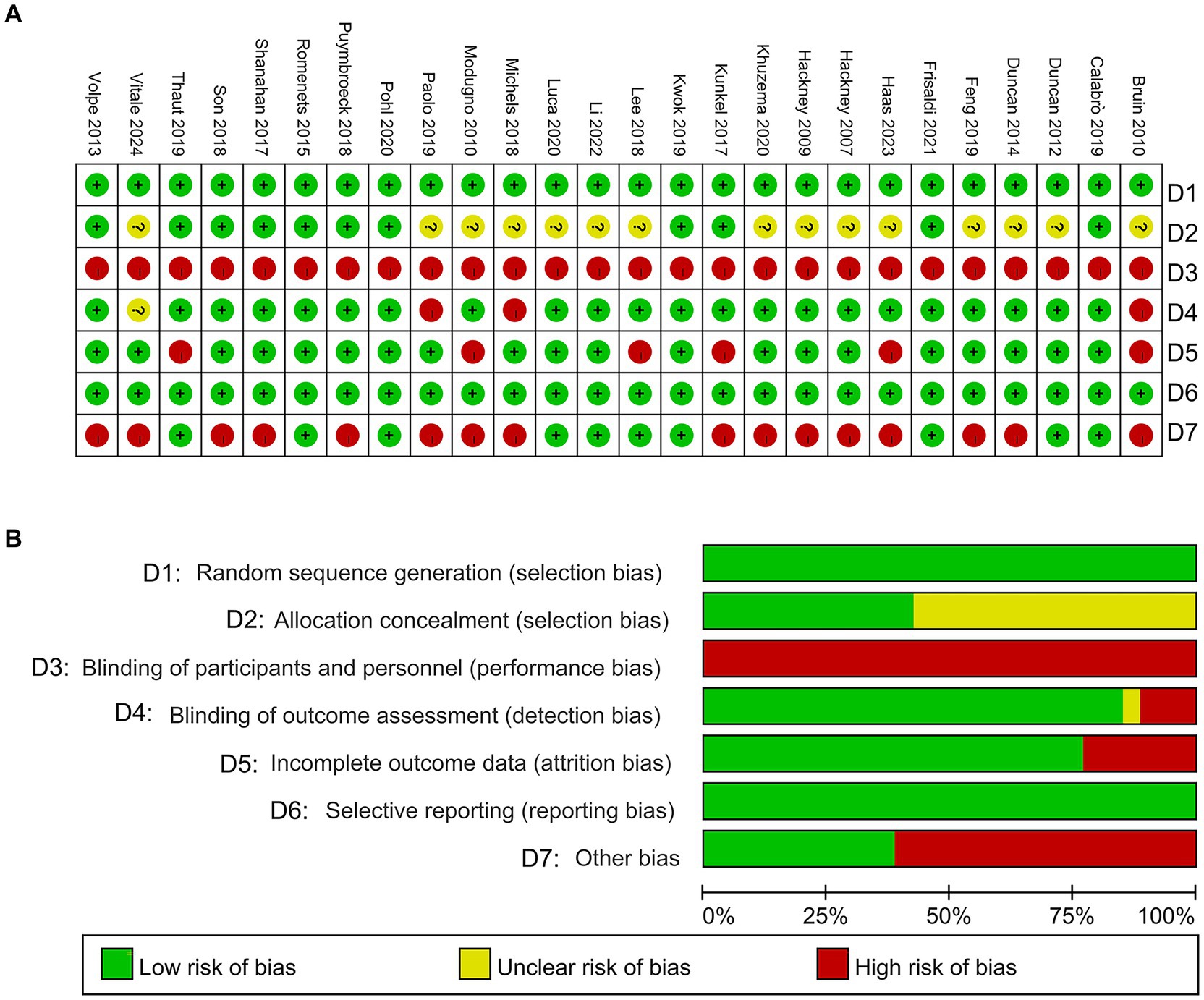
Figure 1. (A) Methodological quality assessment of included studies. (B) Distribution of methodological quality across included studies.
D1: For random sequence generation, 26 studies (Volpe et al., 2013; Vitale et al., 2024; Van Puymbroeck et al., 2018; Thaut et al., 2019; Son and Choi, 2018; Solla et al., 2019; Shanahan et al., 2017; Romenets et al., 2015; Pohl et al., 2020; Modugno et al., 2010; Michels et al., 2018; Li et al., 2022; Lee et al., 2018; Kwok et al., 2019; Kunkel et al., 2017; Khuzema et al., 2020; Hackney et al., 2007; Hackney and Earhart, 2009; Haas et al., 2024; Frisaldi et al., 2021; Feng et al., 2019; Duncan and Earhart, 2014; Duncan and Earhart, 2012; De Luca et al., 2020; de Bruin et al., 2010; Calabrò et al., 2019) demonstrated a low risk of bias, accounting for 100%.
D2: Concerning allocation concealment, 11 studies (Volpe et al., 2013; Van Puymbroeck et al., 2018; Thaut et al., 2019; Son and Choi, 2018; Shanahan et al., 2017; Romenets et al., 2015; Pohl et al., 2020; Kwok et al., 2019; Kunkel et al., 2017; Frisaldi et al., 2021; Calabrò et al., 2019) were found to have a low risk of bias (42.31%), while the remaining 57.69% had an unclear risk.
D3: 28 studies (Volpe et al., 2013; Vitale et al., 2024; Van Puymbroeck et al., 2018; Thaut et al., 2019; Son and Choi, 2018; Solla et al., 2019; Shanahan et al., 2017; Romenets et al., 2015; Pohl et al., 2020; Modugno et al., 2010; Michels et al., 2018; Li et al., 2022; Lee et al., 2018; Kwok et al., 2019; Kunkel et al., 2017; Khuzema et al., 2020; Hackney et al., 2007; Hackney and Earhart, 2009; Haas et al., 2024; Frisaldi et al., 2021; Feng et al., 2019; Duncan and Earhart, 2014; Duncan and Earhart, 2012; De Luca et al., 2020; de Bruin et al., 2010; Calabrò et al., 2019) exhibited a high risk of bias in the blinding of participants and personnel, with no studies at low risk.
D4: For blinding of outcome assessment, 24 studies (Volpe et al., 2013; Van Puymbroeck et al., 2018; Thaut et al., 2019; Son and Choi, 2018; Shanahan et al., 2017; Romenets et al., 2015; Pohl et al., 2020; Modugno et al., 2010; Lee et al., 2018; Kwok et al., 2019; Kunkel et al., 2017; Khuzema et al., 2020; Hackney et al., 2007; Hackney and Earhart, 2009; Haas et al., 2024; Frisaldi et al., 2021; Feng et al., 2019; Duncan and Earhart, 2014; Duncan and Earhart, 2012; De Luca et al., 2020; Calabrò et al., 2019) were assessed as having a low risk of bias (84.62%), with 11.54% showing high risk and 3.84% remaining unclear.
D5: In terms of incomplete outcome data, 20 studies (Volpe et al., 2013; Vitale et al., 2024; Van Puymbroeck et al., 2018; Son and Choi, 2018; Solla et al., 2019; Shanahan et al., 2017; Romenets et al., 2015; Pohl et al., 2020; Michels et al., 2018; Li et al., 2022; Kwok et al., 2019; Khuzema et al., 2020; Hackney et al., 2007; Hackney and Earhart, 2009; Haas et al., 2024; Frisaldi et al., 2021; Feng et al., 2019; Duncan and Earhart, 2014; Duncan and Earhart, 2012; De Luca et al., 2020; Calabrò et al., 2019) were found to have a low risk of bias (76.92%), while 23.08% were rated as high risk.
D6: Regarding selective reporting and outcome reporting, 28 studies (Volpe et al., 2013; Vitale et al., 2024; Van Puymbroeck et al., 2018; Thaut et al., 2019; Son and Choi, 2018; Solla et al., 2019; Shanahan et al., 2017; Romenets et al., 2015; Pohl et al., 2020; Modugno et al., 2010; Michels et al., 2018; Li et al., 2022; Lee et al., 2018; Kwok et al., 2019; Kunkel et al., 2017; Khuzema et al., 2020; Hackney et al., 2007; Hackney and Earhart, 2009; Haas et al., 2024; Frisaldi et al., 2021; Feng et al., 2019; Duncan and Earhart, 2014; Duncan and Earhart, 2012; De Luca et al., 2020; de Bruin et al., 2010; Calabrò et al., 2019) showed a low risk of bias, making up 100%.
D7: For other types of bias, 11 studies (Thaut et al., 2019; Romenets et al., 2015; Pohl et al., 2020; Li et al., 2022; Lee et al., 2018; Kwok et al., 2019; Frisaldi et al., 2021; Duncan and Earhart, 2012; De Luca et al., 2020; Calabrò et al., 2019) exhibited a low risk (38.46%), while 61.54% were found to have a high risk.
2.5 Data analysis
We conducted analyses using R software (version 4.1.2) with the “meta” package. For each intervention group, standardized mean differences (SMD) and their corresponding standard deviations were calculated. To compare various art therapies with control groups, pairwise meta-analyses were performed. Heterogeneity was assessed using the Cochrane Q statistic and I2 index, with thresholds indicating low (0–25%), moderate (25–50%), and substantial (>50%) heterogeneity. When heterogeneity exceeded 30%, a random-effects model was applied; otherwise, a common-effects model was used. Statistical significance was determined by a two-sided p-value of less than 0.05. Publication bias was assessed through Egger’s test and funnel plot visualization.
Setting a 30% threshold for heterogeneity assessment facilitates the early detection of variability across studies included in this meta-analysis. Compared to the conventional 50% threshold, the choice of a 30% cutoff enables the application of a random-effects model under moderate heterogeneity, thereby more comprehensively accounting for inter-study differences (Higgins et al., 2003). This approach improves the accuracy and robustness of the meta-analysis findings.
3 Results
3.1 Study identification
This search yielded 1,377 citations. After removing duplicates, 998 articles were left for screening. Of these, 914 were excluded due to issues with titles and abstracts, Non RCTs, Literature review, meta analyse, comments, Unrelated literature, Incomplete literature etc. The remaining 84 full−text articles were evaluated for eligibility; 20 were non-relevant outcomes, 7 were non-compliant study design, 7 were Incomplete or unsuitable full text, 6 were non-target populations, 13 Insufficient or inadequate data, 5 non-eligible intervention, Ultimately, 26 studies included in review (Figure 2).
3.2 Study characteristics
This meta-analysis incorporated 2,756 individuals from 26 randomized controlled trials. The studies were conducted between 2007 and 2024. All control groups were non-art therapy. The average age of participants in the experimental group was 65.98 ± 3.81 years, with 60.86% being male, and the mean disease duration was 4.01 ± 2.95 years. In the control group, the average age was 68.97 ± 6.08 years, with 51.98% being male, and the mean disease duration was 5.38 ± 3.38 years. 53.85% of the trials had a registration number. The studies included seven from Italy, seven from the United States one from Sweden, three from China (including Hong Kong), one from India, one from Brazil, one from Ireland, two from Korea, two from Canada, and one from the United Kingdom.
3.3 Characteristics of the intervention
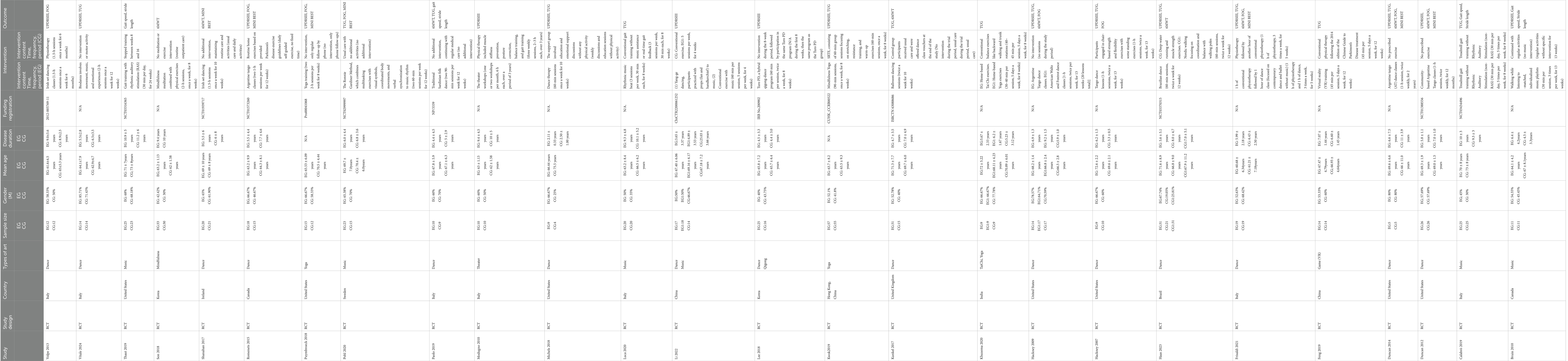
Among the 26 studies, the types of art-based interventions were diverse. Specifically, 5 studies utilized music, 14 focused on dance, 2 employed yoga, 1 incorporated mindfulness, 1 used theater, 1 employed game-based therapy through virtual reality (VR), 1 combined Tai Chi and yoga, and 1 combined Qigong and dance. This variety demonstrates the wide range of creative and therapeutic approaches used in the included studies. Four were combined art therapies. Training sessions lasted between 30 and 120 min, with program durations ranging from 4 weeks to 3 years. Control interventions varied widely: 18 of them were passive control conditions, including two wait−list controls and 13 usual care or no intervention. Ten of them were active control conditions, including physical interventions such as physical therapy, walking, Nordic Walking, Deep Water swimming, podcasts, stretching, flexibility exercises, Stretching and Resistance Training Exercises, and multimodal exercise programs. The UPDRS III was used in 19 of the 26 studies and the TUG in 20 studies, reflecting their frequent utilization (Table 2).
3.4 Effects of art therapy on Parkinson’s disease
Meta-analysis of the difference: Given the significant differences between pre − and post−intervention, this study conducted a meta−analysis using SMD. This method directly captures the intervention’s effect while controlling for baseline differences, improving the accuracy and statistical power (Tables 3, 4).
Effect sizes are interpreted based on Cohen’s guidelines, defining small (SMD = 0.2), medium (SMD = 0.5), and large (SMD = 0.8) effects, and are further refined by relevant meta−analytic literature: Small Effect 0.2 ≤ SMD < 0.35, Slightly Medium Effect 0.35 ≤ SMD < 0.5, Medium Effect 0.5 ≤ SMD < 0.65, Slightly Large Effect 0.65 ≤ SMD < 0.8, Large Effect SMD ≥ 0.8.
3.4.1 Primary outcomes
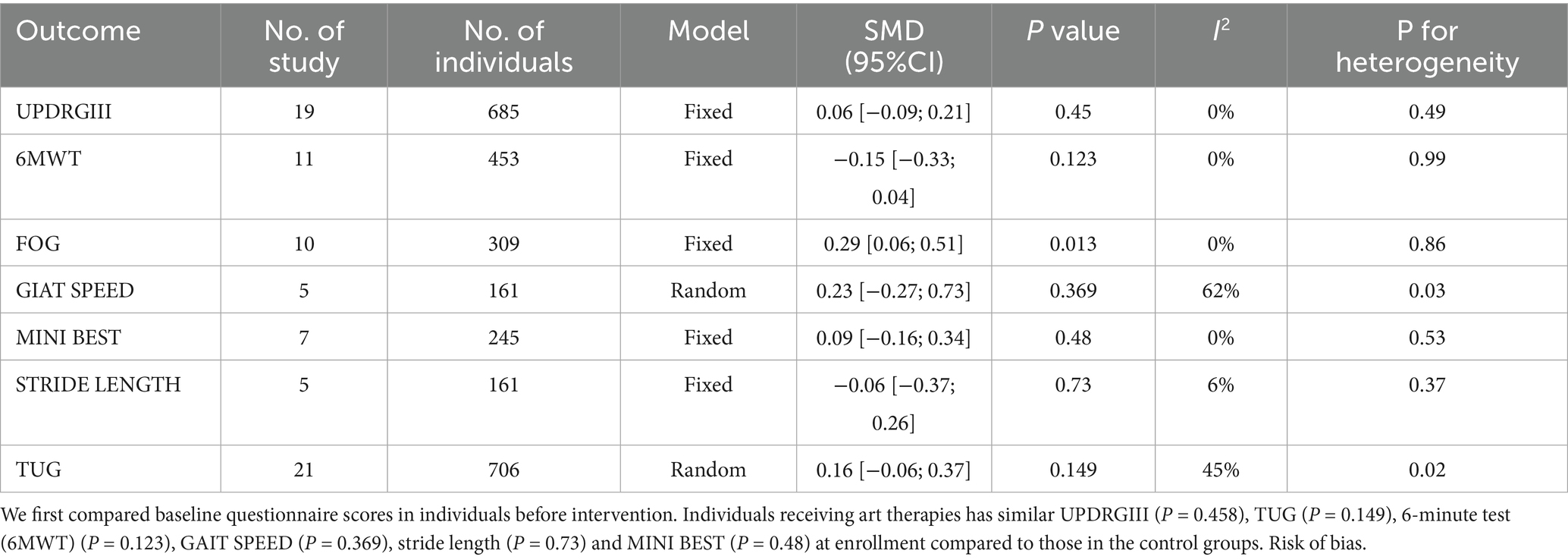
3.4.1.1 UPDRS III
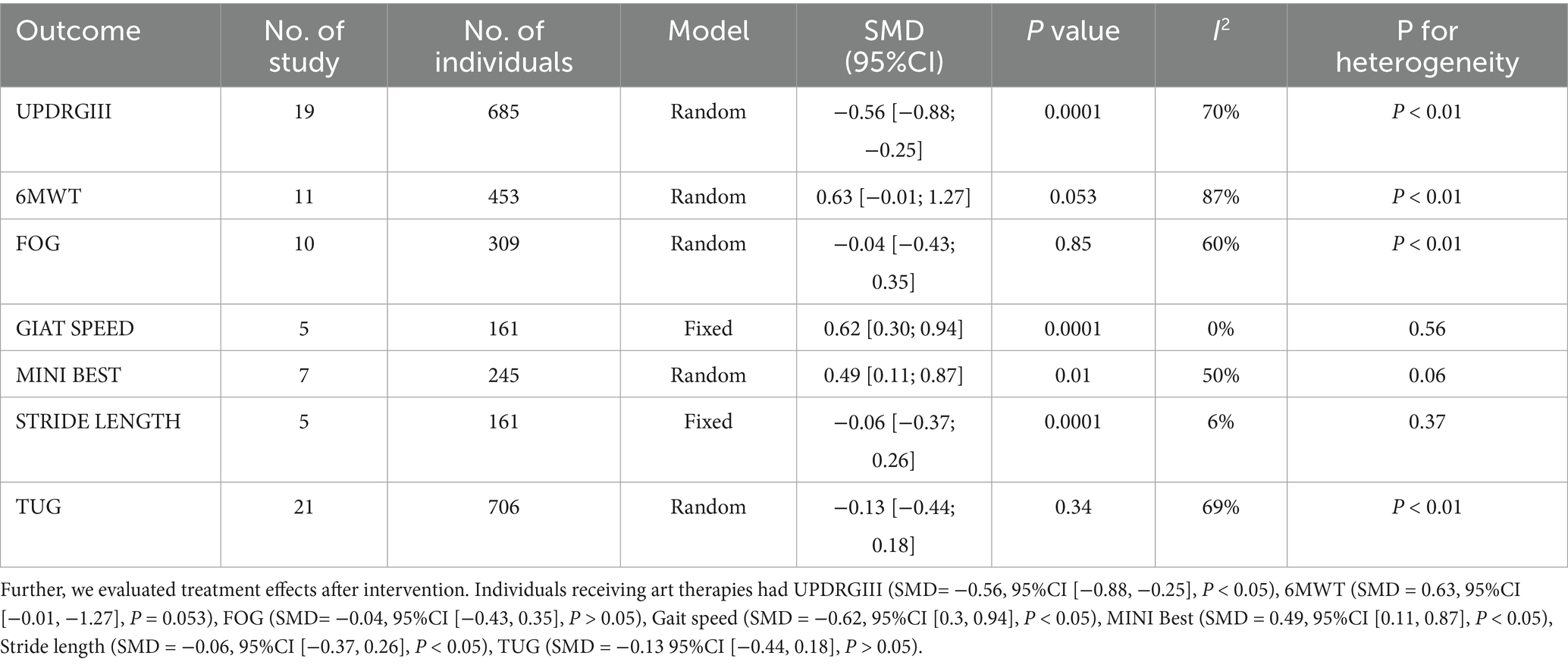
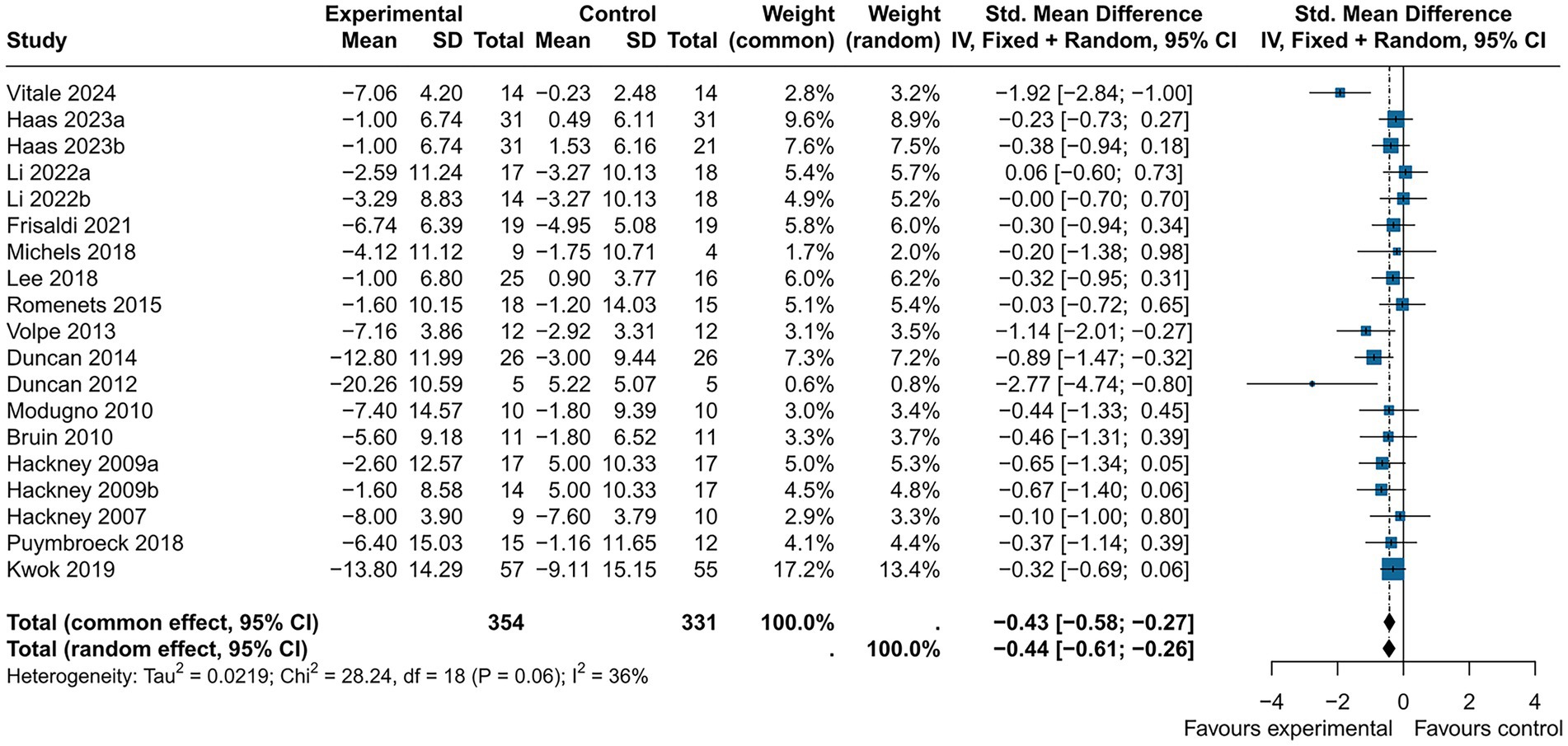
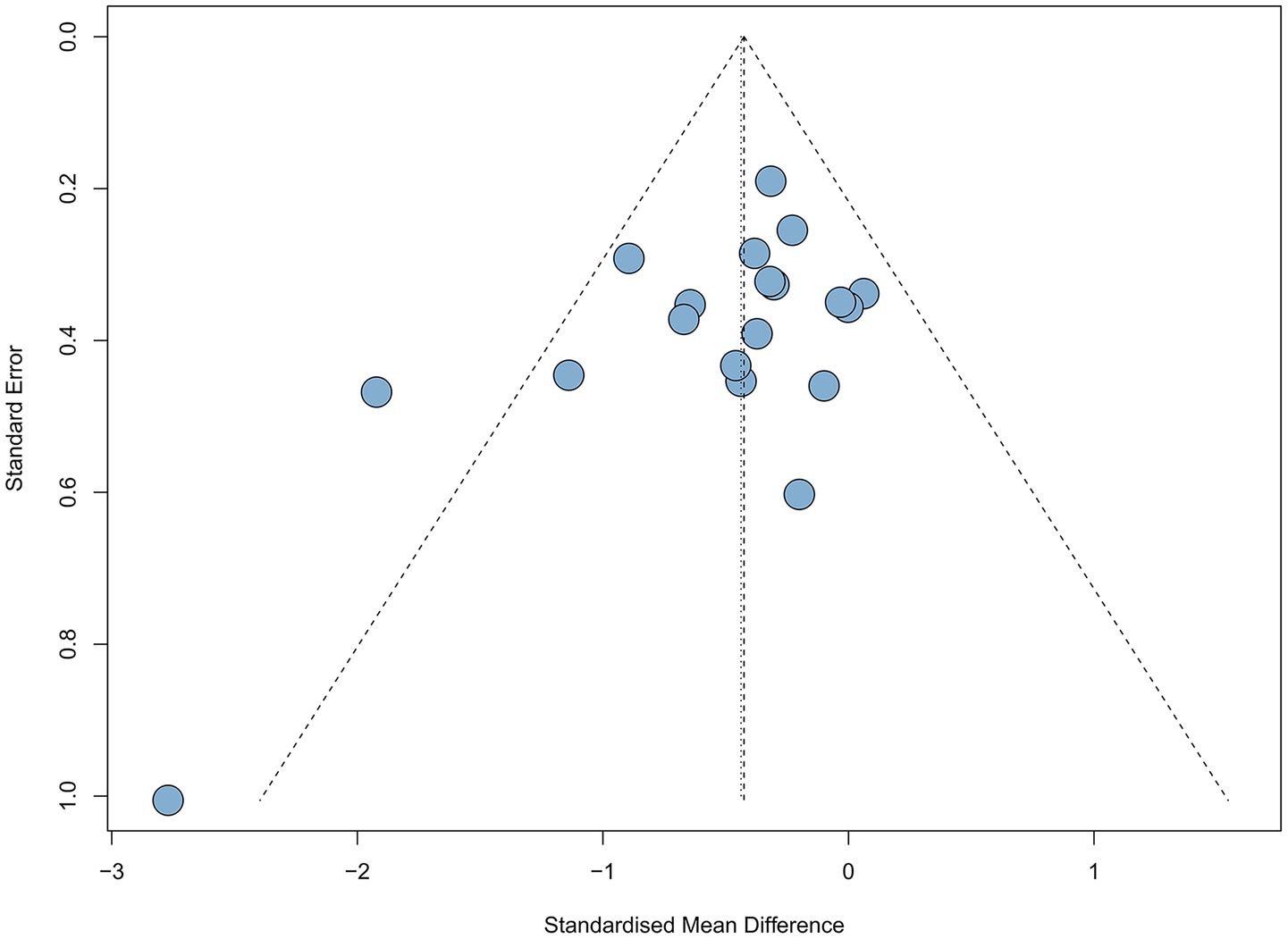
UPDRS III is used to measure motor function in PD patients, with lower scores after intervention indicating better motor function. Nineteen studies (n = 685). utilized the UPDRS III to assess treatment effects. Revealing a significant reduction in UPDRS III scores in the experimental group compared to the control group (SMD = −0.44, 95% CI [−0.61; −0.26], p < 0.05), with medium effect and statistical significance, with high heterogeneity (I2 = 36%) (Figure 3). Excluding the study by Vitale. reduced heterogeneity (I2 = 4%). Egger’s test indicated no publication bias (p = 0.07). Although the results were statistical significance, the high heterogeneity, possibly due to the small sample size, and the bias risk identified by Egger’s test suggest that these findings should be interpreted with caution. Which was further supported by the funnel plot (Figure 4).
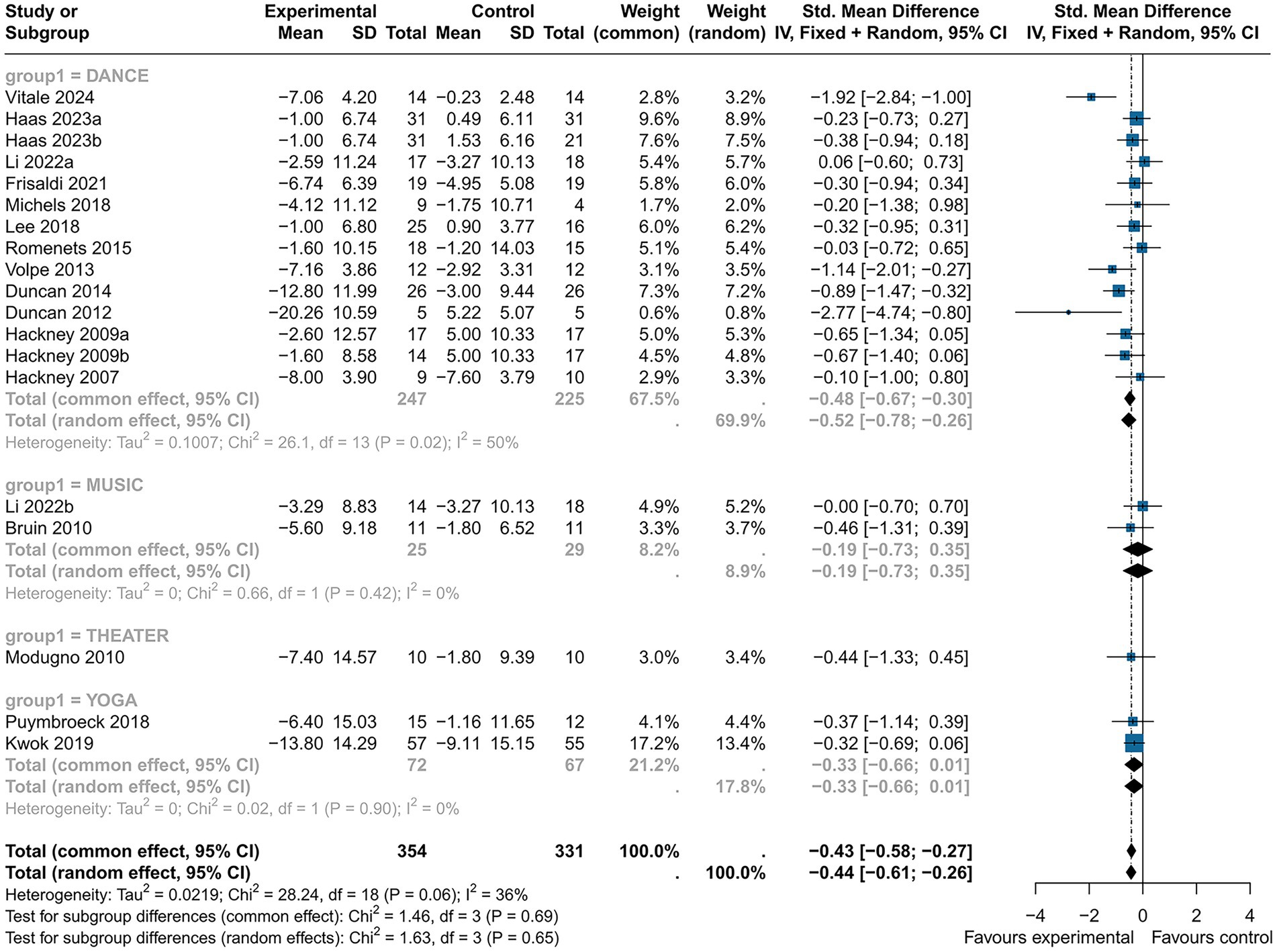
Subgroup analyses indicated that music (SMD = −0.19, 95% CI [−0.73; 0.35], p > 0.05), theater (SMD = −0.44, 95% CI [−1.33; 0.45], p > 0.05) and yoga intervention (SMD = −0.33, 95% CI [−0.66; −0.01], p > 0.05) were non-significant. Dance intervention (SMD = −0.52, 95% CI [−0.78; −0.26], p < 0.05) showed a medium effect and statistical significance, but with high heterogeneity (I2 = 50%) (Figure 5).
3.4.1.2 TUG
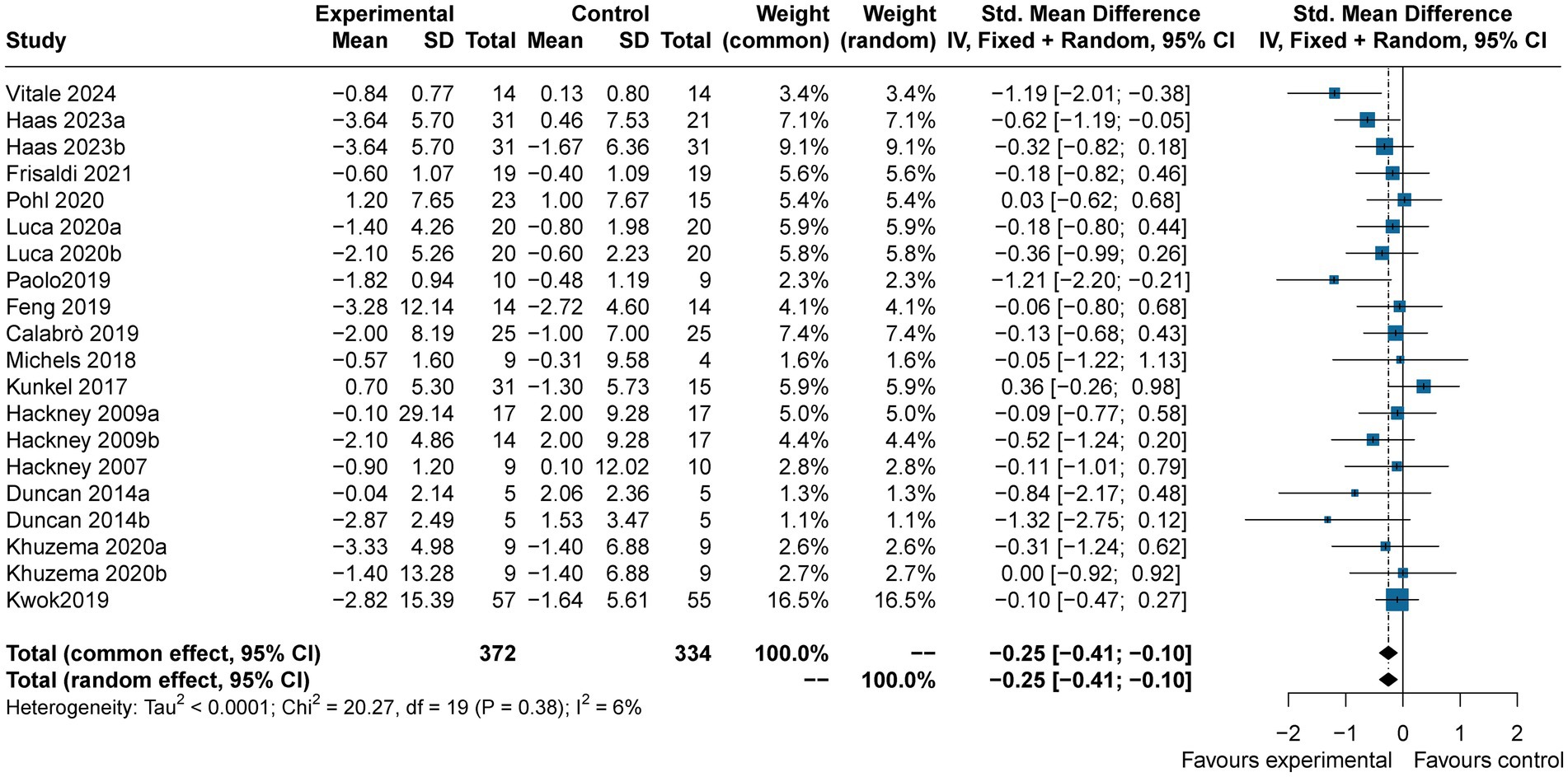
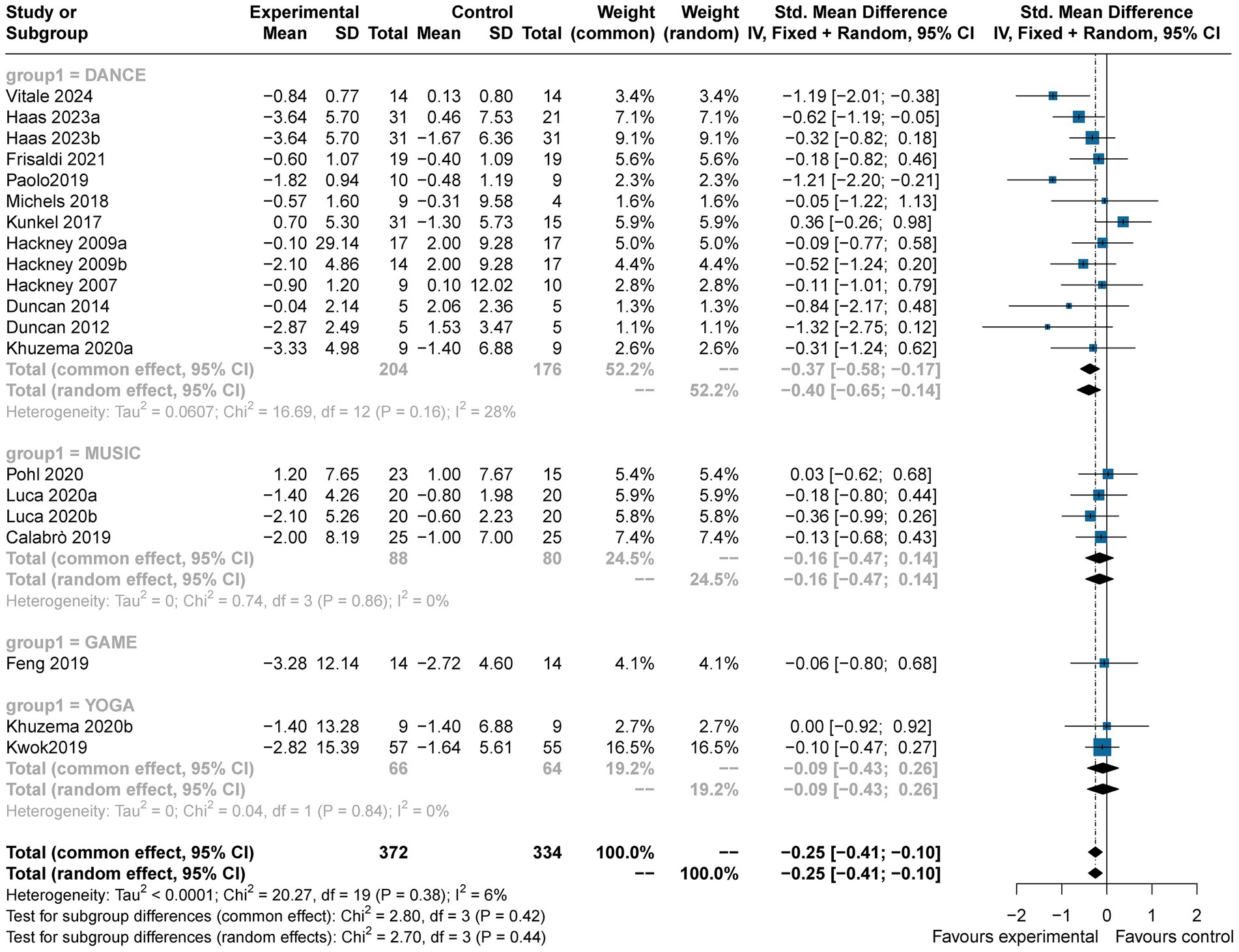
Twenty studies (n = 742) reported the effects of art therapy interventions on TUG scores. The TUG test is widely used to evaluate dynamic balance, gait function in PD patients. Results demonstrated a reduction in TUG scores in the experimental group compared to the control group (SMD = −0.25, 95% CI [−0.41, −0.10], p < 0.05), indicating a small effect size with low heterogeneity (I2 = 6%) (Figure 6). Egger’s test showed no evidence of publication bias (p = 0.08). These findings suggest art therapy has a modest but statistically significant impact on dynamic balance and gait function.
Subgroup analysis showed that music (SMD = −0.16, 95% CI [−0.47, 0.14], p > 0.05), game-based interventions (SMD = −0.06, 95% CI [−0.80, 0.68], p > 0.05), and yoga (SMD = −0.09, 95% CI [−0.43, 0.26], p > 0.05) yielded small, non-significant effects. However, dance interventions resulted in a greater reduction in TUG scores (SMD = −0.37, 95% CI [−0.58, −0.17], p < 0.05), with a slightly medium effect size and moderate heterogeneity (I2 = 28%). This underscores the potential of dance therapy to improve motor function in PD patients (Figure 7).
3.4.1.3 Mini–BESTest
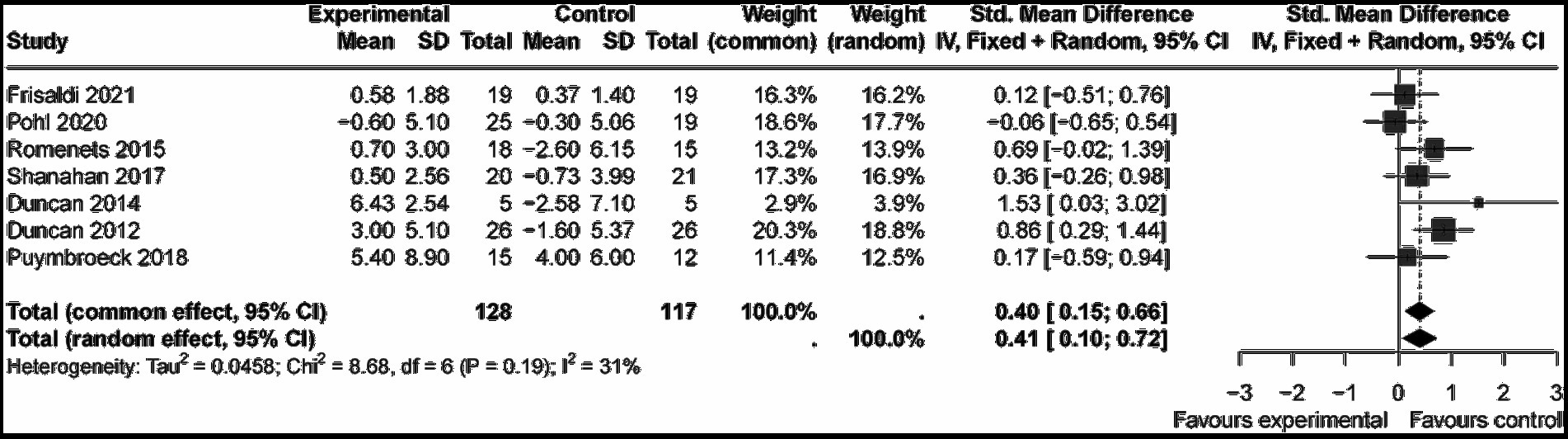
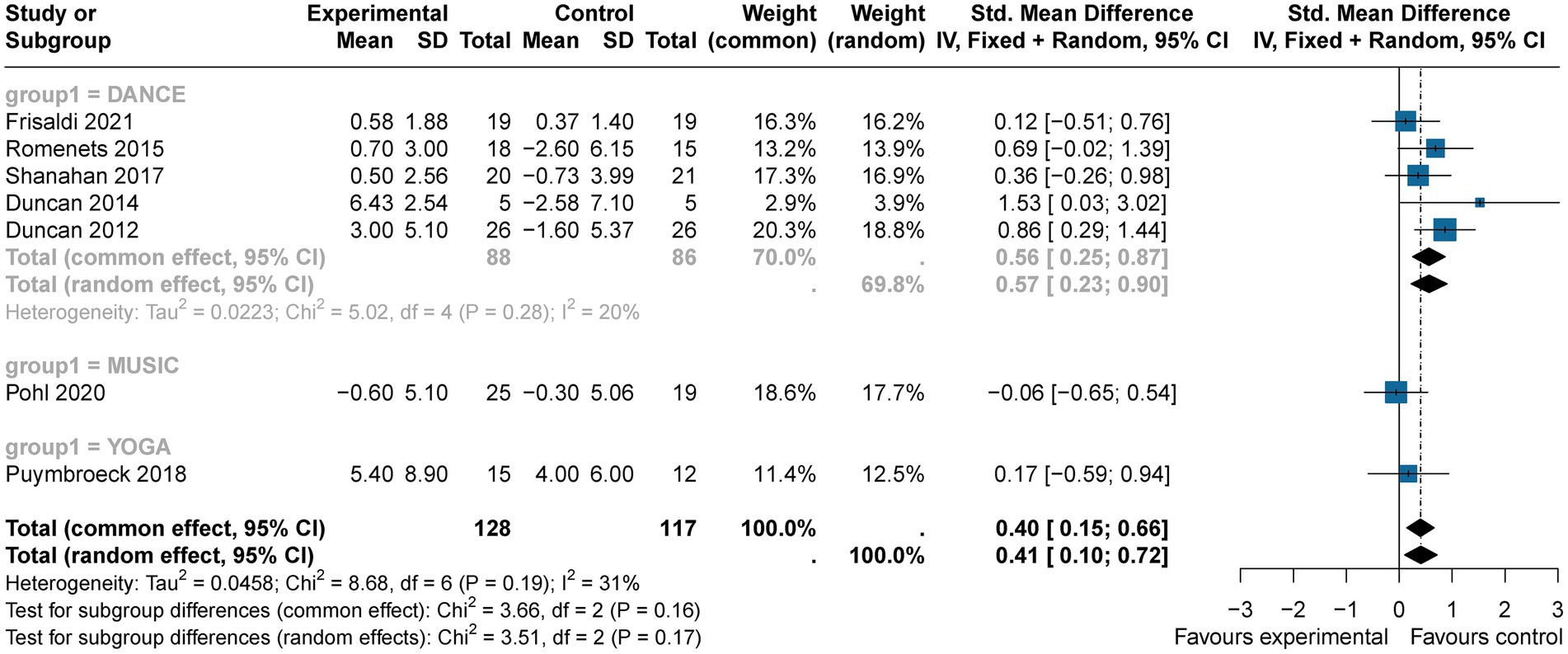
The Mini–BESTest, used to assess balance functions (e.g., postural control and gait stability), showed that art therapy participants had higher scores (SMD = 0.41, 95% CI [0.10; 0.72], p < 0.05) across seven studies (n = 245), with moderate heterogeneity (I2 = 31%) reduced to 9% upon excluding (Duncan, 2012) (Figure 8). Publication bias was not found (p = 0.356). Art therapy showed a slightly medium effect and statistical significance.
Subgroup analysis indicated that music (SMD = −0.06, 95% CI [−0.65; 0.54], p > 0.05) and yoga (SMD = 0.17, 95% CI [−0.59; 0.94], p > 0.05) were non-significant, while dance showed the strongest effect (SMD = 0.56, 95% CI [0.25, 0.87], p < 0.05) with low heterogeneity (I2 = 20%). This medium effect underscores dance as the most effective intervention with statistical significance (Figure 9).
3.4.2 Secondary outcomes
3.4.2.1 Gait speed
Gait speed, an indicator of walking performance, was assessed in five studies (n = 161). Art therapy participants demonstrated improved gait speed scores compared to controls (SMD = 0.34, 95% CI [0.03, 0.65], p < 0.05), with low heterogeneity (I2 = 0%) and no evidence of publication bias (p = 0.9), suggesting a small but statistically significant effect.
3.4.2.2 6MWT
The 6MWT, a measure of cardiovascular endurance and functional capacity, was evaluated in 11 studies (n = 453). Art therapy participants demonstrated higher 6MWT scores compared to controls (SMD = 0.41, 95% CI [0.11, 0.72], p < 0.05), indicating a slightly medium effect size with statistical significance. However, high heterogeneity was observed (I2 = 60%), which decreased to moderate levels (I2 = 43%) after excluding the study by Paolo et al. Publication bias was detected (p = 0.04).
3.4.2.3 FOG
The FOG scale, which assesses the severity and frequency of freezing episodes during walking, was evaluated in 10 studies (n = 309). Art therapy interventions demonstrated a small but statistically significant reduction in FOG scores compared to controls (SMD = −0.33, 95% CI [−0.58, −0.09], p < 0.05), with moderate heterogeneity (I2 = 32%) and no evidence of publication bias (p = 0.36). Excluding the study by Volpe reduced heterogeneity to 0%, further supporting the robustness of the results.
3.4.2.4 Stride length
Stride length, a measure of gait and walking ability, reflects improvements in walking efficiency, balance, and lower limb function when increased. Five studies (n = 161) reported a medium effect size for art therapy interventions (SMD = 0.59, 95% CI [0.09, 1.09], p < 0.05), indicating statistical significance. Moderate heterogeneity was observed (I2 = 57%) with no evidence of publication bias (p = 0.61). Excluding the Thaut’s study reduced heterogeneity to 0%, but subsequent analysis revealed no statistically significant difference in stride length between art therapy and conventional treatments. Sequential exclusion of the Paolo and Calabrò’s studies did not significantly alter heterogeneity, but reversed the findings, showing no statistical significance.
4 Discussion
PD significantly affects motor function and gait, both of which are essential for daily activities and independent living. As the disease progresses, impairments such as freezing of gait and reduced stride length lead to mobility loss, worsening disability, and diminishing quality of life (Noyes et al., 2006). Globally, PD prevalence has doubled over the past 25 years, with over 8.5 million cases reported in 2019. PD also accounts for the fastest-growing rates of disability and mortality among neurological disorders, with 5.8 million disability-adjusted life years (DALYs) and 329,000 deaths in 2019, representing increases of 81% and over 100%, respectively, since 2000 (World Health Organization, 2022).
Improving motor function and gait is critical to mitigating PD’s functional decline, enhancing independence, and reducing daily risks. However, current treatments are expensive, do not cure the disease, and often carry significant side effects. Medications like levodopa (L-Dopa), while initially effective, lose their therapeutic efficacy over time. They can also cause nausea, hypotension, L-Dopa-induced dyskinesia, and motor fluctuations, while COMT inhibitors may lead to gastrointestinal issues and, in severe cases, fatal liver toxicity. Surgical treatments such as deep brain stimulation (DBS) pose risks of infection, bleeding, cognitive decline, and speech or balance disorders. Despite these interventions, all available treatments fail to cure the disease, and PD remains a progressive disorder that ultimately leads to severe disability—particularly due to increasingly treatment-resistant motor and gait impairments, which are critical to maintaining functional independence (Poewe et al., 2017). Given the significant limitations and adverse effects of existing treatments for PD, exploring alternative therapeutic approaches has become imperative. Studies in neuroaesthetics show that art therapy engages prefrontal–limbic integration, enhances default mode network (DMN) connectivity for flow states, modulates cortisol and dopamine levels, promotes empathy via mirror neuron activation, and facilitates theta–gamma coupling—together mediating neuroplastic therapeutic effects. Art therapy holds significant promise in addressing both motor and non-motor symptoms in individuals with Parkinson’s disease (PD). This therapeutic potential is supported by evidence suggesting that art therapy can enhance neuroplasticity and activate dopaminergic pathways, thereby improving emotional regulation. These neural adaptations may help mitigate psychosocial stress and psychosomatic symptoms, highlighting the therapeutic promise of art-based interventions through well-defined neurobiological mechanisms (Lauring et al., 2019). This meta-analysis focuses on evaluating the impact of art therapy on motor function, particularly gait and dynamic balance, which are crucial for maintaining independence and reducing the risk of complications. By analysing validated studies, this research sheds light on the therapeutic potential of art therapy, especially dance, in enhancing physical function in PD patients. Dance therapy integrates rhythmic movement, music, and social engagement, enhancing motor coordination, neuroplasticity, and dopamine release while stimulating sensorimotor integration, emotional regulation, and cognitive-motor synchronization to mitigate Parkinson’s symptoms.
The motor function of this meta-analysis was defined as a multidimensional concept encompassing both gait and dynamic balance, with gait serving as a core component. Relevant studies that used validated scales to measure these aspects were included to ensure a comprehensive evaluation. This study demonstrates that art therapy significantly improves both motor and gait functions in patients with PD. Among the specific types of art therapy interventions evaluated, including drama, music, yoga, and dance, dance consistently demonstrated the most remarkable effects. The use of multifunctional assessment scales provided a comprehensive evaluation of body function, enabling simultaneous analysis of gait and dynamic balance-two closely interconnected components of motor performance. This meta-analysis presents strong evidence that art therapy enhances physical function in PD patients, with dance interventions yielding the most substantial benefits. Specifically, dance showed the strongest effects on gait and dynamic balance, with a moderate effect size and low heterogeneity. Furthermore, dance interventions also demonstrated significant improvements in overall body function. These findings underscore the exceptional value of dance as a targeted intervention for improving gait and dynamic balance, offering promising clinical implications for PD rehabilitation.
Furthermore, the meta-analysis revealed significant overall improvements in secondary outcomes, including stride length, freezing of gait (FOG), the 6-min walk test (6MWT), and gait speed, following art therapy interventions. However, subgroup analyses were not conducted for these measures due to limited data availability, high heterogeneity in study designs and outcome measurements, and insufficient statistical power. These findings underscore the importance of future research with larger sample sizes, standardized protocols, and comprehensive data reporting to better explore subgroup-specific effects on secondary outcomes and provide more robust evidence for the role of art therapy in PD rehabilitation.
Studies demonstrate that art-based interventions significantly benefit motor function in Parkinson’s disease (PD). For instance, the study by Frisaldi et al. (2021) and Li et al. (2022) reported enhanced upper limb mobility using the DArT method and improved lower limb strength with Brazilian Dance Group (BDG) exercises. Similarly, Volpe et al. (2013) found that Irish dance, characterized by rhythmic music and large-amplitude movements, effectively addressed gait freezing, balance issues, and motor impairments. Hackney’s study (Hackney et al., 2007; Hackney and Earhart, 2009) showed that tango specifically targeted motor deficits related to gait and balance. de Bruin et al. (2010) and Puymbroeck’s studies (Van Puymbroeck et al., 2018) demonstrated the efficacy of music-assisted gait training and yoga in improving stride length, speed, rhythm, postural stability, and alleviating gait freezing. These findings suggest that rhythm-based therapies hold significant potential for managing motor symptoms, particularly in areas like gait and balance.
Art therapy also shows promise in addressing non-motor symptoms in PD. Pohl’s study suggests that group music interventions positively impact mental health and social engagement, though with minimal cognitive effects. Modugno et al. (2010) reported that combining active theater with conventional treatments enhanced cognitive, emotional, and motor functions, ultimately improving quality of life. Similarly, Kwok et al. (2019) highlighted that mindfulness yoga reduced anxiety and depression while enhancing motor function, mental health, and overall quality of life. Vitale et al. (2024) further demonstrated that Biodanza, an intervention combining movement, music, and group interaction, not only improved motor function but also strengthened cognitive and social outcomes. Despite these findings, some studies report that cognitive benefits and reductions in depressive symptoms often lack statistical significance, likely due to variations in sample size, intervention duration, or assessment sensitivity. This underscores the need for further research to refine art therapy protocols aimed specifically at cognitive outcomes.
Although the primary focus of this paper is on motor function, the long-term efficacy of art therapy in managing PD remains underexplored. Duncan’s longitudinal study (Duncan and Earhart, 2012) revealed that community dance programs could slow the progression of both motor and non-motor symptoms over 2 years, improving daily living activities and balance. However, challenges persist in evaluating the long-term effects of different art forms, particularly in mood and cognition. Volpe et al. (2013) noted the need for more studies quantifying the effects of various dance forms, such as modern dance, tango, and jazz ballet. Thaut et al. (2019) similarly emphasized the importance of exploring the sustained benefits of Rhythmic Auditory Stimulation (RAS) on non-motor symptoms.
Visual arts and painting, however, represent a significant research gap. While some studies examined Visual arts and painting as a tool for assessing disease progression (Kuosmanen et al., 2019; Celik et al., 2024), its direct therapeutic effects remain unexplored. This underscores the need for future research on the potential of visual arts, such as painting, in addressing non-motor symptoms like cognition and emotional health.
5 Future research directions
Emerging therapies such as clay therapy, game therapy, and virtual reality (VR) therapy also show promise. Although these fields are in their infancy and lack robust RCT data, integrating VR into therapeutic frameworks may offer innovative approaches to PD treatment. Future studies should explore these possibilities to enhance the scope of art-based interventions for PD.
6 Limitations
Dependence on secondary data from previously published RCTs introduces potential biases related to study design and reporting. Heterogeneity across studies—particularly in intervention types, sample sizes, and outcome measures—limits the consistency of conclusions. Notably, variability in art therapy implementation (session frequency, duration, and artistic mediums) creates challenges for cross-study synthesis. Additionally, the lack of participant and personnel blinding in some studies increases the risk of bias. The exclusion of non-English publications may introduce language bias in evidence selection; reliance on self-reported outcomes could lead to subjectivity, and the long-term sustainability of interventions remains uncertain without extended follow-up data. Future research should focus on larger, high−quality RCTs with standardized protocols and direct comparisons between different art therapy modalities.
7 Conclusion
This meta-analysis demonstrates that art therapy significantly improves motor and gait functions in patients with PD, offering robust evidence for its clinical utility. Motor function benefits were substantiated by reductions in UPDRS III and TUG scores and increases in Mini-BESTest scores. Additionally, secondary outcomes revealed small to moderate improvements in gait speed, 6MWT performance, FOG scores, and stride length, indicating a comprehensive impact on mobility and functional independence.
Notably, dance therapy exhibited the most pronounced effects across motor function outcomes, emphasizing its potential as a cornerstone intervention in multidisciplinary neurorehabilitation. Despite these promising results, the high heterogeneity observed in certain outcomes underscores the critical need for standardized protocols to ensure consistency, reproducibility, and broader applicability. Future research should focus on optimizing intervention designs, identifying patient-specific predictors of success, and exploring long-term benefits to maximize the therapeutic impact of art-based interventions for PD. These findings provide a compelling case for the integration of evidence-based art therapies, particularly dance therapy, into tailored treatment regimens aimed at enhancing quality of life and functional outcomes in PD populations.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
ZL: Supervision, Validation, Writing – original draft, Writing – review & editing. CH: Data curation, Software, Validation, Writing – original draft, Writing – review & editing. SL: Conceptualization, Data curation, Formal analysis, Project administration, Supervision, Writing – original draft, Writing – review & editing. YC: Investigation, Validation, Writing – original draft, Writing – review & editing. YZ: Investigation, Resources, Software, Writing – original draft, Writing – review & editing. WY: Conceptualization, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors sincerely thank their colleagues and mentors for their valuable support and guidance throughout the course of this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
PD, Parkinson’s Disease; UPDRSIII, The unified Parkinson’s disease rating scale III; TUG, Timed up and go test; 6MWT, 6–minute walk test; FOG, Freezing of gait questionnaire; SMD, Standardized mean difference.
References
Balestrino, R., and Schapira, A. H. (2020). Parkinson disease. Eur. J. Neurol. 27, 27–42. doi: 10.1111/ene.14108
Boehm, K., Cramer, H., Staroszynski, T., and Ostermann, T. (2014). Arts therapies for anxiety, depression, and quality of life in breast Cancer patients: a systematic review and Meta-analysis. Evid. Based Complement. Alternat. Med. 2014:103297. doi: 10.1155/2014/103297
Bohannon, R. W. (1993). Physical rehabilitation in neurologic diseases. Curr. Opin. Neurol. 6, 765–772. doi: 10.1097/00019052-199310000-00015
Calabrò, R. S., Naro, A., Filoni, S., Pullia, M., Billeri, L., Tomasello, P., et al. (2019). Walking to your right music: a randomized controlled trial on the novel use of treadmill plus music in Parkinson's disease. J. Neuroeng. Rehabil. 16:68. doi: 10.1186/s12984-019-0533-9
Celik, N. D., Durmaz Celik, N., Topal, A., Kuzu Kumcu, M., Ozkan, S., and Tezcan Aydemir, S. (2024). Sensitivity of clock drawing test alone to screen for cognitive impairment in patients with Parkinson's disease. SiSli Etfal Hastan. Tip Bul. 58, 381–388. doi: 10.14744/SEMB.2024.94758
Chaudhuri, K. R., and Schapira, A. H. V. (2009). Non-motor symptoms of Parkinson's disease: dopaminergic pathophysiology and treatment. Lancet Neurol. 8, 464–474. doi: 10.1016/S1474-4422(09)70068-7
Cotzias, G. C., Papavasiliou, P. S., and Gellene, R. (1969). L-dopa in parkinson’s syndrome. N. Engl. J. Med. 281:272. doi: 10.1056/NEJM196907312810517
Cunnington, R., Bradshaw, J. L., and Iansek, R. (1996). 3 the role of the supplementary motor area in the control of voluntary movement. Hum. Mov. Sci. 15, 627–647. doi: 10.1016/0167-9457(96)00018-8
de Bruin, N., Doan, J. B., Turnbull, G., Suchowersky, O., Bonfield, S., Hu, B., et al. (2010). Walking with music is a safe and viable tool for gait training in Parkinson's disease: the effect of a 13-week feasibility study on single and dual task walking. Parkinsons Dis. 2010:483530. doi: 10.4061/2010/483530
De Luca, R., Latella, D., Maggio, M. G., Leonardi, S., Sorbera, C., Di Lorenzo, G., et al. (2020). Do patients with PD benefit from music assisted therapy plus treadmill-based gait training? An exploratory study focused on behavioral outcomes. Int. J. Neurosci. 130, 933–940. doi: 10.1080/00207454.2019.1710147
Duncan, R. P., and Earhart, G. M. (2012). Randomized controlled trial of community-based dancing to modify disease progression in Parkinson disease. Neurorehabil. Neural Repair 26, 132–143. doi: 10.1177/1545968311421614
Duncan, R. P., and Earhart, G. M. (2014). Are the effects of community-based dance on Parkinson disease severity, balance, and functional mobility reduced with time? A 2-year prospective pilot study. J. Altern. Complement. Med. 20, 757–763. doi: 10.1089/acm.2012.0774
Fahn, S. (2008). The history of dopamine and levodopa in the treatment of Parkinson's disease. Mov. Disord. 23, S497–S508. doi: 10.1002/mds.22028
Feng, H., Li, C., Liu, J., Wang, L., Ma, J., Li, G., et al. (2019). Virtual reality rehabilitation versus conventional physical therapy for improving balance and gait in Parkinson's disease patients: a randomized controlled trial. Med. Sci. Monit. 25, 4186–4192. doi: 10.12659/MSM.916455
Frisaldi, E., Bottino, P., Fabbri, M., Trucco, M., de Ceglia, A., Esposito, N., et al. (2021). Effectiveness of a dance-physiotherapy combined intervention in Parkinson's disease: a randomized controlled pilot trial. Neurol. Sci. 42, 5045–5053. doi: 10.1007/s10072-021-05171-9
Haas, A. N., Delabary, M. S., Passos-Monteiro, E., Wolffenbuttel, M., Donida, R. G., Casal, M. Z., et al. (2024). The effects of Brazilian dance, deep-water exercise and nordic walking, pre-and post-12 weeks, on functional-motor and non-motor symptoms in trained PwPD. Arch. Gerontol. Geriatr. 118:105285. doi: 10.1016/j.archger.2023.105285
Hackney, M. E., and Earhart, G. M. (2009). Effects of dance on movement control in Parkinson's disease: a comparison of argentine tango and American ballroom. J. Rehabil. Med. 41, 475–481. doi: 10.2340/16501977-0362
Hackney, M. E., Kantorovich, S., Levin, R., and Earhart, G. M. (2007). Effects of tango on functional mobility in Parkinson's disease: a preliminary study. J. Neurol. Phys. Ther. 31, 173–179. doi: 10.1097/NPT.0b013e31815ce78b
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ 327, 557–560. doi: 10.1136/bmj.327.7414.557
Iansek, R., Huxham, F., and McGinley, J. (2006). The sequence effect and gait festination in Parkinson disease: contributors to freezing of gait? Mov. Disord. 21, 1419–1424. doi: 10.1002/mds.20998
Kalia, L. V., and Lang, A. E. (2015). 4 Parkinson's disease. Lancet 386, 896–912. doi: 10.1016/S0140-6736(14)61393-3
Kaplan, F. F. (2003). “Approaches to art therapy: theory and technique” in Contemporary Psychology-Apa Review of Books, vol. 48. 2nd ed, 776–778.
Khuzema, A., Brammatha, A., and Selvan, V. A. (2020). Effect of home-based tai chi, yoga or conventional balance exercise on functional balance and mobility among persons with idiopathic Parkinson's disease: an experimental study. Hong Kong Physiother. J. 40, 39–49. doi: 10.1142/S1013702520500055
Kongkasuwan, R., Voraakhom, K., Pisolayabutra, P., Maneechai, P., Boonin, J., and Kuptniratsaikul, V. (2016). Creative art therapy to enhance rehabilitation for stroke patients: a randomized controlled trial. Clin. Rehabil. 30, 1016–1023. doi: 10.1177/0269215515607072
Krishnan, S., Tokar, T. N., Boylan, M. M., Griffin, K., Feng, D., Mcmurry, L., et al. (2015). Zumba® dance improves health in overweight/obese or type 2 diabetic women. Am. J. Health Behav. 39, 109–120. doi: 10.5993/AJHB.39.1.12
Kuhlman, G. D., Flanigan, J. L., Sperling, S. A., and Barrett, M. J. (2019). Predictors of health-related quality of life in Parkinson's disease. Parkinsonism Relat. Disord. 65, 86–90. doi: 10.1016/j.parkreldis.2019.05.009
Kunkel, D., Fitton, C., Roberts, L., Pickering, R. M., Roberts, H. C., Wiles, R., et al. (2017). A randomized controlled feasibility trial exploring partnered ballroom dancing for people with Parkinson's disease. Clin. Rehabil. 31, 1340–1350. doi: 10.1177/0269215517694930
Kuosmanen, E., Kan, V., Visuri, A., Boudjelthia, A., Krizou, L., and Ferreira, D.. Measuring Parkinson's disease motor symptoms with smartphone-based drawing tasks. ACM International Joint Conference on Pervasive and Ubiquitous Computing (UbiComp) / ACM International Symposium on Wearable Computers (ISWC). (2019). London.
Kwok, J. Y. Y., Kwan, J. C. Y., Auyeung, M., Mok, V. C. T., Lau, C. K. Y., Choi, K. C., et al. (2019). Effects of mindfulness yoga vs stretching and resistance training exercises on anxiety and depression for people with Parkinson disease a randomized clinical trial. JAMA Neurol. 76, 755–763. doi: 10.1001/jamaneurol.2019.0534
Lang, A. E., and Lozano, A. M. (1998). Parkinson's disease - first of two parts. N. Engl. J. Med. 339, 1044–1053. doi: 10.1056/NEJM199810083391506
Lauring, J. O., Pelowski, M., Specker, E., Ishizu, T., Haugbøl, S., Hollunder, B., et al. (2019). Parkinson's disease and changes in the appreciation of art: a comparison of aesthetic and formal evaluations of paintings between PD patients and healthy controls. Brain Cogn. 136:103597. doi: 10.1016/j.bandc.2019.103597
Lee, H. J., Kim, S. Y., Chae, Y., Kim, M. Y., Yin, C., Jung, W. S., et al. (2018). Turo (qi dance) program for Parkinson's disease patients: randomized, Assessor blind, waiting-list control. Partial crossover study. Explore (NY) 14, 216–223. doi: 10.1016/j.explore.2017.11.002
Li, F., Wang, D., Ba, X., Liu, Z., and Zhang, M. (2022). The comparative effects of exercise type on motor function of patients with Parkinson's disease: a three-arm randomized trial. Front. Hum. Neurosci. 16:1033289. doi: 10.3389/fnhum.2022.1033289
Michels, K., Dubaz, O., Hornthal, E., and Bega, D. (2018). “Dance therapy” as a psychotherapeutic movement intervention in Parkinson’s disease. Complement. Ther. Med. 40, 248–252. doi: 10.1016/j.ctim.2018.07.005
Modugno, N., Iaconelli, S., Fiorlli, M., Lena, F., Kusch, I., and Mirabella, G. (2010). Active theater as a complementary therapy for Parkinson's disease rehabilitation: a pilot study. ScientificWorldJournal 10, 2301–2313. doi: 10.1100/tsw.2010.221
Moo, J. T. N., and Ho, R. T. H. (2024). Family-centered creative arts therapies for children with autism: a configurative systematic review. Fam. Relat. 74, 412–429. doi: 10.1111/fare.13092
Morris, M. E., Iansek, R., Matyas, T. A., and Summers, J. J. (1994). The pathogenesis of gait Hypokinesia in Parkinsons-disease. Brain 117, 1169–1181. doi: 10.1093/brain/117.5.1169
Noyes, K., Liu, H., Li, Y., Holloway, R., and Dick, A. W. (2006). Economic burden associated with Parkinson's disease on elderly medicare beneficiaries. Mov. Disord. 21, 362–372. doi: 10.1002/mds.20727
Playfer, J. R. (1997). Parkinson's disease. Postgrad. Med. J. 73, 257–264. doi: 10.1136/pgmj.73.859.257
Poewe, W., Seppi, K., Tanner, C. M., Halliday, G. M., Brundin, P., Volkmann, J., et al. (2017). Parkinson disease. Nat. Rev. Dis. Primers 3:17013. doi: 10.1038/nrdp.2017.13
Pohl, P., Wressle, E., Lundin, F., Enthoven, P., and Dizdar, N. (2020). Group-based music intervention in Parkinson's disease - findings from a mixed-methods study. Clin. Rehabil. 34, 533–544. doi: 10.1177/0269215520907669
Postuma, R. B., Berg, D., Stern, M., Poewe, W., Olanow, C. W., Oertel, W., et al. (2015). MDS clinical diagnostic criteria for Parkinson's disease. Mov. Disord. 30, 1591–1601. doi: 10.1002/mds.26424
Postuma, R. B., and Montplaisir, J. (2009). Predicting Parkinson's disease - why, when, and how? Parkinsonism Relat. Disord. 15, S105–S109. doi: 10.1016/S1353-8020(09)70793-X
Romenets, S. R., Anang, J., Fereshtehnejad, S. M., Pelletier, A., and Postuma, R. (2015). Tango for treatment of motor and non-motor manifestations in Parkinson's disease: a randomized control study. Complement. Ther. Med. 23, 175–184. doi: 10.1016/j.ctim.2015.01.015
Shanahan, J., Morris, M. E., Bhriain, O. N., Volpe, D., Lynch, T., and Clifford, A. M. (2017). Dancing for Parkinson disease: a randomized trial of Irish set dancing compared with usual care. Arch. Phys. Med. Rehabil. 98, 1744–1751. doi: 10.1016/j.apmr.2017.02.017
Solla, P., Cugusi, L., Bertoli, M., Cereatti, A., Della Croce, U., Pani, D., et al. (2019). Sardinian folk dance for individuals with Parkinson's disease: a randomized controlled pilot trial. J. Altern. Complement. Med. 25, 305–316. doi: 10.1089/acm.2018.0413
Son, H. G., and Choi, E. O. (2018). The effects of mindfulness meditation-based complex exercise program on motor and nonmotor symptoms and quality of life in patients with Parkinson's disease. Asian Nurs. Res. 12, 145–153. doi: 10.1016/j.anr.2018.06.001
Thaut, M. H., Rice, R. R., Braun Janzen, T., Hurt-Thaut, C. P., and McIntosh, G. C. (2019). Rhythmic auditory stimulation for reduction of falls in Parkinson's disease: a randomized controlled study. Clin. Rehabil. 33, 34–43. doi: 10.1177/0269215518788615
Van Puymbroeck, M., Walter, A. A., Hawkins, B. L., Sharp, J. L., Woschkolup, K., Urrea-Mendoza, E., et al. (2018). Functional improvements in Parkinson's disease following a randomized trial of yoga. Evid. Based Complement. Alternat. Med. 2018:8516351. doi: 10.1155/2018/4523743
Vitale, C., Rosa, R., Agosti, V., Siciliano, M., Barra, G., Maggi, G., et al. (2024). Effects of Biodanza (®) SRT on motor, cognitive, and behavioral symptoms in patients with Parkinson's disease: a randomized controlled study. J. Pers. Med. 14:588. doi: 10.3390/jpm14060588
Volpe, D., Signorini, M., Marchetto, A., Lynch, T., and Morris, M. E. (2013). A comparison of Irish set dancing and exercises for people with Parkinson's disease: a phase II feasibility study. BMC Geriatr. 13:54. doi: 10.1186/1471-2318-13-54
World Health Organization. (2022). Launch of WHO's Parkinson disease technical brief. Available online at: https://www.who.int/news/item/14-06-2022-launch-of-who-s-parkinson-disease-technical-brief (Accessed November 26, 2024).
World Health Organization. Launch of WHO's Parkinson disease technical brief. Available online at: https://www.who.int/news/item/14-06-2022-launch-of-who-s-parkinson-disease-technical-brief (Accessed June 14, 2022).
Yang, Q. Q., Shao, Q., Xu, Q., Shi, H., and Li, L. (2021). Art therapy alleviates the levels of depression and blood glucose in diabetic patients: a systematic review and Meta-analysis. Front. Psychol. 12:639626. doi: 10.3389/fpsyg.2021.639626
Keywords: Parkinson’s disease, art therapy, dance therapy, gait, motor function, meta-analysis
Citation: Liu Z, Hui C, Liu S, Chen Y, Zhang Y and Ye W (2025) The effectiveness of art therapy on motor function in Parkinson’s disease: a systematic review and meta-analysis. Front. Psychol. 16:1542405. doi: 10.3389/fpsyg.2025.1542405
Edited by:
Ana-Maria Cebolla, Université libre de Bruxelles, BelgiumReviewed by:
Juliet L. King, George Washington University, United StatesYunYun Wu, Hainan General Hospital, China
Copyright © 2025 Liu, Hui, Liu, Chen, Zhang and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenliang Ye, eW91c2Vlc3k2N0BnbWFpbC5jb20=
†Present address: Zexi Liu,School of Journalism and Communication, Huaiyin Normal University, Huai’an, Jiangsu, China
 Zexi Liu
Zexi Liu Chen Hui3
Chen Hui3