- 1Graduate School of Media and Governance, Keio University, Fujisawa, Japan
- 2Faculty of Environment and Information Studies, Keio University, Fujisawa, Japan
- 3Japan Society for the Promotion of Science, Tokyo, Japan
Controlling physiological and psychological states before a performance is essential for professional musicians to realize their full potential. However, the characteristics of the optimal pre-performance state remain unclear. While an increase in sympathetic nervous system activity is typically observed before performance, when associated with anxiety, it can degrade the performance quality. This study examined whether recalling positive autobiographical performance memories enhances subjective performance achievement, accompanied by increased emotional arousal, valence, and autonomic nervous system activity. Thirty-six professional wind instrument players participated in the study. Prior to performing musical pieces, participants engaged in one of three conditions: (1) recalling positive autobiographical memories, (2) recalling negative autobiographical memories, or (3) imagining routine pre-performance activities (no-memory condition). During the memory recall phase, heart rate was measured. After each performance, participants rated their subjective arousal, valence, and performance achievement. We calculated the heart rate variability indices, specifically SD1 (reflecting parasympathetic nervous system activity) and SD2/SD1 (used as a relative indicator of sympathetic predominance). The results showed that performance achievement, arousal, and valence were significantly higher in the positive than in the negative condition. Our path analysis further revealed that an increase in SD2/SD1 did not directly predict performance achievement; instead, it was associated with an increase in emotional valence, which in turn led to improved performance. These findings suggest that recalling positive performance memories activates sympathetic nervous system activity and fosters positive emotions, thereby enhancing the performance achievement of professional musicians.
1 Introduction
For professional musicians, controlling their psychophysiological state before a performance is crucial not only for delivering high-quality performance, but also for sustaining long-term careers. However, managing this state is often challenging because of various uncontrollable factors, such as the audience, program, venue, and fellow performers, and the performer’s uncertainty or limited control over these elements (Baumeister, 1984; Burin and Osório, 2017; Furuya et al., 2021; Kenny, 2011). These factors activate the sympathetic nervous system (SNS), a division of the autonomic nervous system (ANS), leading to physiological responses, such as increased heart rate, elevated respiratory rate, and sweating. These physiological responses are correlated with psychological reactions, including heightened anxiety (Abel and Larkin, 1990; Brouwer and Hogervorst, 2014; Mulcahy et al., 1990; Yoshie et al., 2009a). Such psychophysiological changes can degrade performance skills, resulting in unsatisfactory outcomes for musicians (Yoshie et al., 2009b).
Conversely, some studies indicate that SNS activation can coincide with richer emotional expressions during performance, thereby enhancing performance quality. For example, increased sympathetic activity has been observed during emotionally expressive performance (Nakahara et al., 2011). Moreover, a music competition winner demonstrated lower anxiety and superior performance despite exhibiting similar levels of sympathetic activation compared to other contestants (Yoshie et al., 2009c). These findings suggest that, even when SNS is activated, high-quality performance can be achieved by managing this activation. However, the mechanisms through which musicians regulate sympathetic activation to enhance performance quality remain unclear.
One promising approach to enhancing performance is to cultivate positive emotions during SNS activation prior to performance. Research on musical performance has shown that heart rate significantly increases, and SNS activity, closely linked to the fight-or-flight response preparing the body for action, intensifies immediately before taking the stage (Craske and Craig, 1984; Nakahara et al., 2011; Yoshie, 2008). Previous studies have demonstrated that positive emotional experiences can balance the activity of the sympathetic and parasympathetic nervous systems (PNS) (Kop et al., 2011; Kreibig, 2010; McCraty et al., 1995). The PNS is associated with rest-and-digest functions (Guyton and Hall, 2006; Saper, 2002) and can suppress SNS overactivation (Berntson et al., 1991). In contrast, negative emotional experiences are suggested to activate SNS activity, while having little effect on PNS activity (Kop et al., 2011; Kreibig, 2010; McCraty et al., 1995). Additionally, according to theories of emotion and ANS functioning, the interpretation of physiological responses leads to subjective emotional experiences (Craig, 2009; Damasio, 1996; Schachter and Singer, 1962). Therefore, even if SNS is activated, the body’s response can vary depending on whether the subjective experience is positive or negative.
One way that performers manage their emotional state before taking the stage is through mental strategies such as imagery. Mental imagery has been widely studied in sports and performance psychology as a method for enhancing emotional control and performance quality under pressure (Orbach and Blumenstein, 2022). This technique often involves imagining successful past performances in vivid detail, including associated physical actions, emotional experiences, and contextual elements. Such imagery has been shown to reduce anxiety, boost confidence and self-efficacy, and improve performance outcomes (Li et al., 2024; Volgemute et al., 2024). Psychological research further indicates that recalling emotionally significant autobiographical memories can influence mood and stress responses. For instance, recalling positive experiences has been associated with the activation of brain regions involved in reward processing and emotion regulation, including areas of the prefrontal cortex (Speer and Delgado, 2017; van Schie et al., 2019). These activations are thought to contribute to reduced stress and improved emotional states. In contrast, recalling negative experiences has been linked to increased physiological arousal and heightened negative affect (MacNamara, 2018; Olsson, 2024), underscoring the role of emotional valence in shaping subsequent affective responses.
The present study aimed to examine whether recalling positive emotions enhances emotional valence even when SNS is activated and whether this leads to improved performance achievement in professional musicians. Since previous research suggests that autobiographical memories influence ANS responses (Kop et al., 2011; Strohm et al., 2021), we hypothesized that recalling a positive performance memory would activate the SNS, mitigate reductions in PNS activity, increase emotional arousal and valence, and enhance performance achievement compared with recalling negative memories. Additionally, we explored whether pre-performance ANS activity directly predicts post-performance achievement or indirectly influences emotional arousal and valence during performance, which, in turn, affects achievement. We hypothesized that ANS activity would influence emotional arousal and valence, thereby enhancing performance.
2 Materials and methods
2.1 Participants
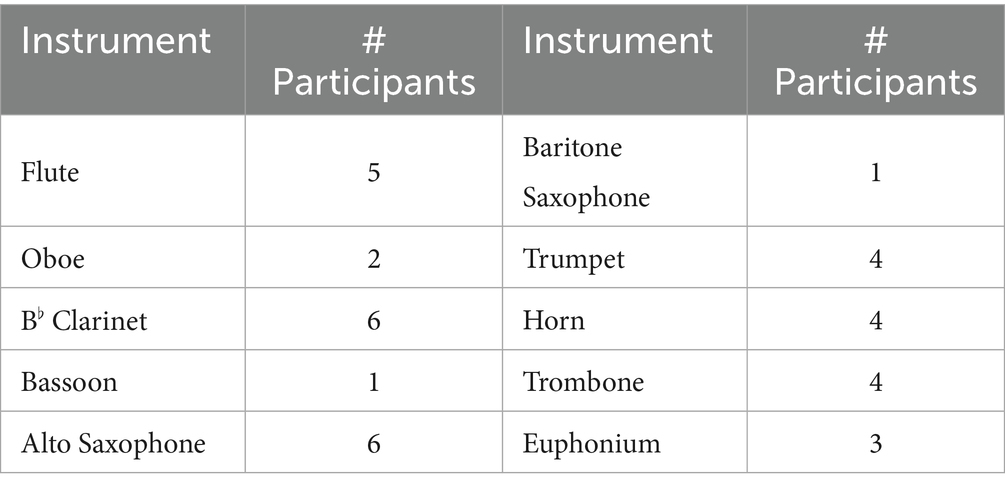
Thirty-six active Japanese professional classical wind instrumentalists (10 males, 26 females; mean age ± SD: 37.69 ± 8.79 years, range: 24–60 years) participated in this study. All participants graduated from music universities and specialized in the instruments they played during the experiment (see Table 1). Their professional experience ranged from 3 to 38 years (mean ± SD: 15.72 ± 8.75 years). To ensure extensive performance experience and the ability to provide expert evaluations, the following inclusion criteria were established: participants had graduated from a music university or received equivalent professional training and had at least 3 years of professional experience as wind instrumentalists. Participants were recruited through the internet and social media platforms.
All participants reported no history of cardiovascular or mental health issues and confirmed their physical and mental well-being. Ethical approval for this study was obtained from the Research Ethics Committee of the Keio University Shonan Fujisawa Campus (Approval Number 507). The study was conducted in accordance with the Declaration of Helsinki, and written informed consent was obtained from all the participants. The experiment was conducted in soundproofed music studios located in Tokyo and Aichi Prefecture, Japan, where temperature and humidity were controlled.
2.2 Procedure and data acquisition
The experimental procedure is illustrated in Figure 1. The participants began by sitting to measure the electrocardiogram (ECG) to calculate their baseline heart rate and then transitioned to a standing position. In each condition, the participants were asked to recall autobiographical memories, perform an assigned musical piece, and evaluate their own performance. The order of the conditions was counterbalanced across participants to minimize order effects. On a separate day, the participants listened to the recordings of other participants’ performances and provided objective evaluations.
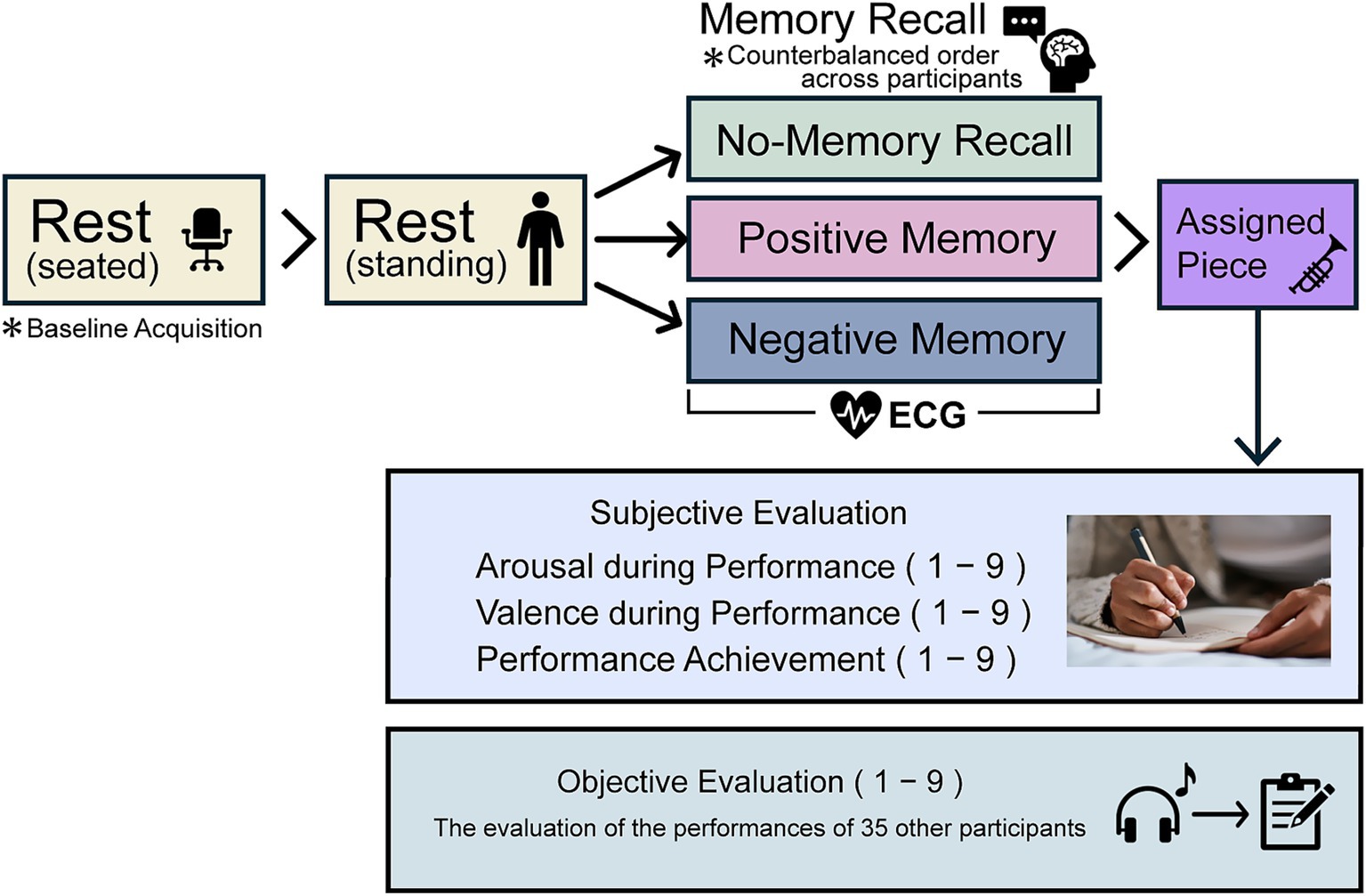
Figure 1. Experimental procedure. The three experimental conditions were counterbalanced across participants, and the entire procedure was repeated three times. Electrocardiogram (ECG) measurements were performed during the memory recall period. Subjective evaluations included arousal and valence, assessed retrospectively as indicators of the emotional state during the performance of the assigned piece, and performance achievement, evaluated based on the participants’ sense of accomplishment after completing the piece. On a separate day, each participant objectively evaluated the performance of other participants.
ECG data were recorded wirelessly via Bluetooth on a computer (HP ZBook Firefly, HP Inc., USA) using dedicated software (OpenSignals (r)evolution, PLUX Wireless Biosignals, Portugal) at a sampling rate of 1,000 Hz. Three pre-gelled Ag/AgCl electrodes (biosignalsplux, PLUX Wireless Biosignals, Portugal) were placed on the participants’ chests to ensure accurate ECG measurements.
All performances during the session were digitally recorded on a separate computer (MacBook Retina, Apple Inc., USA) using music production software (GarageBand, Apple Inc., USA) at a sampling rate of 44.1 kHz. A USB microphone (AT2020USB+, Audio-Technica, Japan) was used to capture the performed musical pieces. The microphone was positioned 1 m from the instrument at the height of the participant’s mouth to ensure consistent and high-quality audio recording.
2.2.1 Preparation and baseline measurement of ECG
Participants were instructed to abstain from alcohol the day before the experiment and avoid caffeine intake on the day of the experiment. Upon arrival at the music studio, disposable electrodes were attached to the chest to measure ECG, and ECG monitoring was initiated. A brief practice session familiarized the participants with the studio acoustics and performed with the electrodes attached, during which the sound recording levels were adjusted.
The participants sat on a chair positioned in front of a music stand and were instructed to rest quietly with their eyes open for 5 min. ECG data collected during the initial resting period served as baseline data. After an additional 5 min of standing rest, the participants began the first condition. Following each condition, the participants rested in a seated position for 5 min to allow their heart rate to return to baseline before starting the next condition.
2.2.2 Memory recall and ECG measurement
Participants completed three conditions in this study: (1) positive condition: participants performed the pieces after vividly recalling their most positive performance memory; (2) negative condition: participants performed the pieces after vividly recalling their most negative performance memory; and (3) no-memory condition: participants performed the assigned piece after imagining a scenario of waiting backstage, as if preparing to perform on stage, without recalling any specific memory.
To facilitate effective recall during the experiment, participants were instructed in advance to recall a specific performance during which they experienced their most positive (or negative) emotional state. They were asked to vividly remember what they experienced during the performance, including what they saw, heard, felt emotionally, and perceived in their bodies (e.g., breathing, muscle tension, or other bodily sensations). The instructions emphasized re-experiencing the subjective state of performing itself, rather than simply reflecting on the event as a whole, in order to simulate the emotional and physiological conditions associated with that moment.
The no-memory condition was designed to serve as a control that approximates a typical pre-performance state for professional musicians, without the cognitive load of recalling specific autobiographical performance memories. Since establishing a strictly emotionally neutral state is inherently difficult, participants were asked to imagine waiting backstage before the performance, a familiar and routine scenario for musicians, to minimize emotional and cognitive activation as much as possible while providing a realistic baseline.
ECG data recorded during the 5-min memory recall period for each task were used for heart rate variability (HRV) analysis. Following recommendations that ECG data collection for HRV analysis should last for at least 5 min (Electrophysiology TF of the ES, 1996), the memory recall duration for each condition was set accordingly.
2.2.3 Performed piece
In this study, an assigned musical piece was employed. Participants performed the piece using sheet music placed on a stand rather than playing from memory. The performance duration for the assigned piece was set to 5 min, and the piece was performed twice consecutively.
The assigned piece was “Ave Maria,” composed by Charles Gounod in 1859. This composition combines the Latin text “Ave Maria” with a melody accompanied by J. S. Bach’s Prelude No. 1 from The Well-Tempered Clavier, Book 1 (BWV 846). We selected this piece because of its accessibility to various wind instruments, manageable range, and low technical difficulty. Although it included exceptionally long phrases that required sustained and delicate breath control, as well as expressive nuance, these features likely helped wind instrument players maintain attentional focus and a moderate level of physiological arousal throughout the task.
Musical tempo and dynamics are known to influence cardiovascular and respiratory systems (Bernardi et al., 2006, 2009). To minimize physiological variability among participants owing to instrument-related differences, the piece’s moderate tempo and gentle dynamics were deemed appropriate. To further ensure ease of performance, participants were allowed to select the key and were instructed to prepare the piece at a performance tempo of 72 beats per minute (bpm).
2.2.4 Subjective evaluation
At the end of each condition, participants provided subjective evaluations of their performance using a 9-point Likert scale to assess performance achievement (1 = did not perform to potential at all, 9 = performed to potential extremely well).
The Affect Grid (Russell et al., 1989; Takada and Yukawa, 2014) was employed to retrospectively evaluate emotional valence and arousal during performance. This tool allowed participants to rate their current emotional states on a 9 × 9 grid consisting of 81 cells. The horizontal axis represents the pleasure–displeasure dimension (emotional valence), where a score of 9 indicates high pleasure and 1 indicates low pleasure. The vertical axis represents the arousal dimension, where a score of 9 indicates high arousal, and 1 indicates low arousal.
2.2.5 Objective evaluation
To evaluate whether the performances conveyed the intended emotions and qualities to the audience, the participants provided objective assessments of other musicians’ renditions of the assigned pieces. For this purpose, the first 20 s of each participant’s recording were extracted and edited into 60-s clips. These clips were counterbalanced across the three experimental conditions to minimize the order effects. Evaluations were conducted using Google Forms, with assessment sessions scheduled at least 1 month after the experiment. Participants were instructed to use their own computers and headphones in a quiet environment.
Initially, the participants were asked to set their computer volume to 25% of the maximum level and were provided with a demo sound to facilitate accurate volume adjustment. While listening to the demo, the participants adjusted the volume to a comfortable and clear level. A headphone check test was administered to confirm proper headphone use (Woods et al., 2017). After these preparations, the participants rated each of the three performances from the other 35 participants using a 9-point scale.
2.3 Analysis
2.3.1 Preprocessing of ECG
ECG data were exported from the OpenSignals (r)evolution software and processed using the Kubios HRV Premium software, version 4.1.0 (Kubios, Finland). During preprocessing, the automatic noise detection setting was configured to “Medium,” and the beat correction setting was set to “Automatic correction” to address artifacts. The R-R interval data were subsequently resampled at 4 Hz and any abnormal heart intervals were interpolated using a third-order spline, as recommended in a previous study (Tarvainen et al., 2014).
2.3.2 HRV calculation
We analyzed HRV using the Poincaré plot, a nonlinear method that displays the nth R-R interval on the x-axis and the (n + 1) th interval on the y-axis, providing a visual representation of HRV (Figure 2).
![Poincaré plot showing RR intervals of a participant during memory recall. Blue dots represent data points with axes labeled RR(n) [ms] and RR(n+1) [ms]. SD1 and SD2 are depicted with pink and orange arrows, respectively, indicating standard deviations within the data. An ellipse outlines the data distribution.](https://www.frontiersin.org/files/Articles/1544069/fpsyg-16-1544069-HTML/image_m/fpsyg-16-1544069-g002.jpg)
Figure 2. Example of Poincaré plot for a single participant. Each point represents a pair of consecutive R-R intervals: the x-axis shows the nth R-R interval and the y-axis shows the subsequent R-R interval. SD1 (pink line), the standard deviation along the short axis of the Poincaré plot, represents short-term fluctuations in R-R intervals and reflects parasympathetic activity. SD2 (orange line), the standard deviation along the long axis of the Poincaré plot, representing long-term variability influenced by both sympathetic and parasympathetic activities. The ellipse illustrates the overall distribution of these intervals defined by the SD1 and SD2 values.
From the Poincaré plot, we calculated SD1 (Equation 1.1) and SD2 (Equation 1.2), as follows:
where represents the variance of the nth R-R intervals, represents the variance of the differences between the consecutive nth and (n + 1) th R-R intervals, and represents the covariance between the consecutive nth and (n + 1) th R-R intervals. In this study, we analyzed HRV from a 5-min duration of memory recall for each condition set prior to performance. We examined the SD2/SD1 ratio and SD1 as changes from the baseline to account for individual differences at rest.
We selected this method because the Poincaré plot is considered relatively less sensitive to minor respiratory variability than some linear spectral measures (Toichi et al., 1997; Penttilä et al., 2001; Nakagawa, 2016; Mishima, 2021). We also intentionally did not impose respiratory control to avoid interfering with participants’ natural memory recall or subsequent performance.
SD1, the standard deviation along the short axis of the Poincaré plot, represents short-term fluctuations in R-R intervals and reflects parasympathetic activity. Short-term variability such as respiratory sinus arrhythmia is typically observed in the relaxed state. According to previous studies, SD1 correlates strongly with high-frequency (HF) components of HRV as well as the time-domain measure of the root mean square of successive differences (RMSSD), both reflecting parasympathetic activity. In fact, SD1 and RMSSD are mathematically related, with SD1 being equivalent to RMSSD divided by (Brennan et al., 2001; Shaffer and Ginsberg, 2017).
In contrast, SD2, the standard deviation along the long axis of the Poincaré plot, represents long-term fluctuations in R-R intervals and is influenced by both sympathetic and parasympathetic activities. Long-term variability tends to increase when sympathetic activity predominates. Meanwhile, the SD2/SD1 ratio has been reported to correlate with the low-frequency to high-frequency (LF/HF) ratio, which is often used as a relative indicator of sympathetic predominance (Goit et al., 2016; Guzik et al., 2007; Hsu et al., 2012).
2.3.3 Statistics
To evaluate the effects of memory recall on performance, we analyzed whether subjective performance achievement differed among conditions using the Friedman test, followed by Dunn’s test for multiple comparisons between the conditions. The same statistical tests were applied to emotional arousal and valence to determine whether these indicators varied across the conditions.
To examine whether SD2/SD1 and SD1 varied across conditions, we constructed two linear mixed-effects models: one with SD2/SD1 as the response variable, and the other with SD1. In both models, the condition was included as a fixed effect, and participant ID as a random effect. The linear mixed-effects model was run using the ‘lme4’ and ‘lmerTest’ packages in R (Bates et al., 2015; Kuznetsova et al., 2017). The model formulas (Equations 2.1, 2.2) are as follows:
Using the positive condition as a reference, we quantitatively evaluated the deviations in the negative and no-memory conditions. Additionally, the negative condition was used as a reference to assess differences between the positive and no-memory conditions. To estimate semi-standardized effect sizes for the fixed effects, we standardized the outcome variables (SD2/SD1 and SD1 differences from baseline) using z-scores and re-fitted the linear mixed-effects models. This approach enables interpretation of the condition effects in standard deviation units while preserving the categorical nature of the predictor variable (Lorah, 2018). Additionally, to assess the contribution of between-subject variance, we calculated the intraclass correlation coefficient (ICC) for each model using the performance package in R (Lüdecke et al., 2021). Model fit was assessed by calculating the marginal R2 (Rm2) and conditional R2 (Rc2) using the ‘partR2’ package in R (Stoffel et al., 2021). Rm2 represents the explanatory power of the fixed effects alone, whereas Rc2 reflects the explanatory power of both the fixed and random effects.
To explore the relationships between SD2/SD1, SD1, arousal, valence, and performance achievement, we conducted a path analysis using structural equation modeling (SEM). In the model, performance achievement was predicted by valence and arousal; valence and arousal were in turn predicted by SD2/SD1 and SD1. Additionally, performance achievement was directly predicted by SD2/SD1 and SD1. Data from all conditions were combined for this analysis without accounting for repeated measurements. SEM was performed using the ‘lavaan’ package in R (Rosseel, 2012).
Additionally, the Friedman test was used to analyze whether objective evaluations by other participants differed among the three conditions. Finally, Spearman’s rank correlation coefficient was calculated to investigate the relationship between the subjective evaluations of performance in the three conditions and objective evaluations by other participants.
For all statistical tests, the significance level (α) was set at 0.05. p-values for multiple comparisons were adjusted using the Bonferroni method and are denoted as pb in the Results section.
3 Results
3.1 Performance achievement, arousal, and valence
The mean performance achievement scores were 6.06 ± 1.59 (mean ± SD) for the positive condition, 5.03 ± 1.48 for the negative condition, and 5.81 ± 1.51 for the no-memory condition (Figure 3A). The Friedman test revealed a significant difference in the performance achievement scores among the conditions (χ2(2) = 9.09, p = 0.011). Post-hoc comparisons using Dunn’s test indicated that the positive condition had a significantly higher achievement score than the negative condition (z = 3.10, pb = 0.003), and the no-memory condition also scored higher than the negative condition (z = 2.34, pb = 0.029). However, there was no significant difference between positive and no-memory conditions (z = 0.76, pb = 0.670).
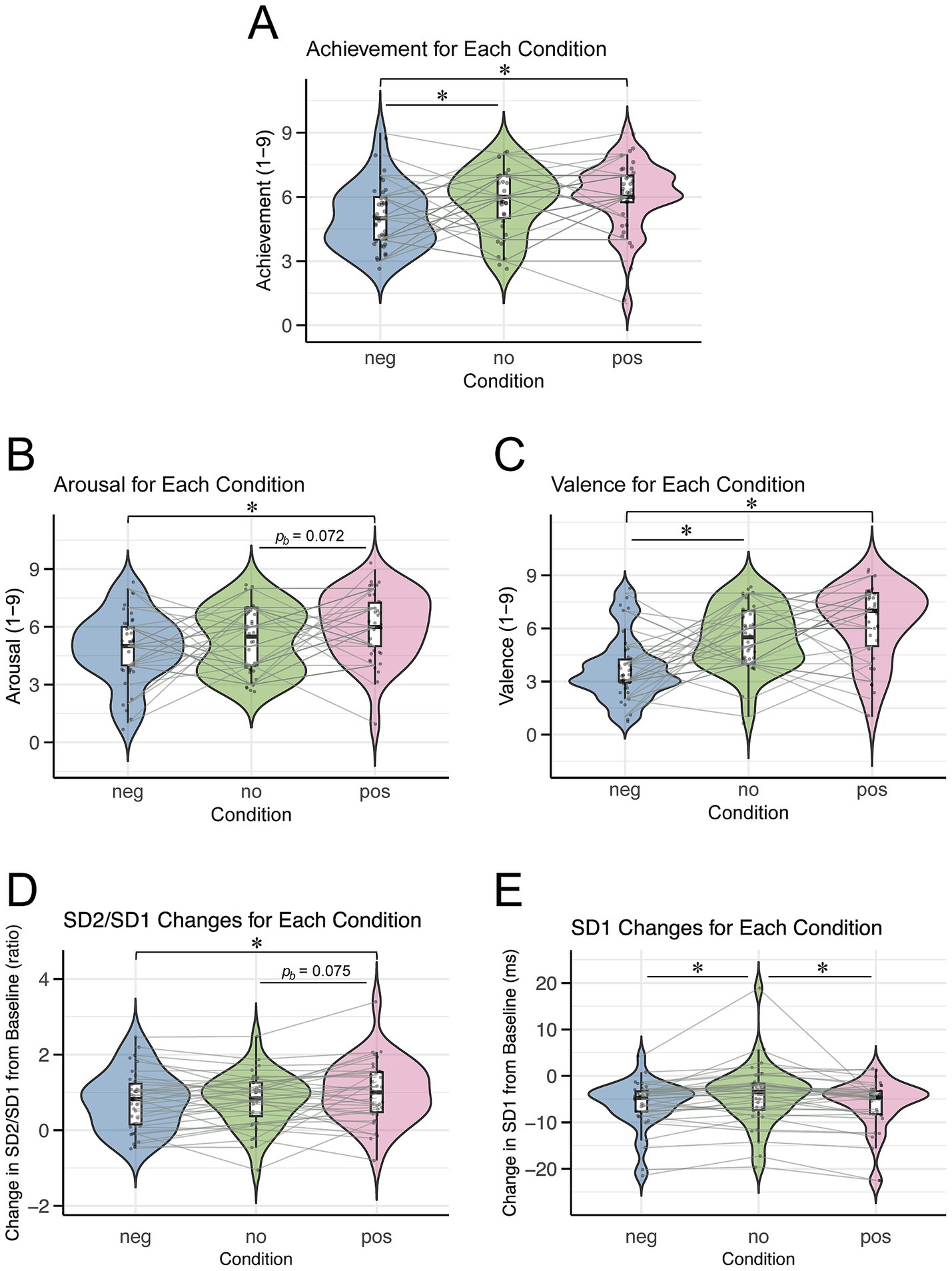
Figure 3. Violin plots illustrating subjective ratings and ANS indices for each condition. Each dot represents an individual participant, and asterisks (“*”) indicate significant differences between conditions (*pb < 0.05). Condition labels: “neg” = negative condition, “no” = no-memory condition, “pos” = positive condition. (A) Performance achievement. (B) Arousal. (C) Valence. (D) Changes in SD2/SD1 from the baseline. (E) Changes in SD1 from the baseline.
The mean arousal scores were 6.06 ± 1.76 for the positive condition, 4.89 ± 1.83 for the negative condition, and 5.28 ± 1.68 for the no-memory condition (Figure 3B). The Friedman test showed a significant difference in arousal scores across conditions (χ2(2) = 7.89, p = 0.019). Dunn’s post hoc analysis revealed a significant difference between the negative and positive conditions (pb = 0.012). However, no significant differences were found between the negative and no-memory conditions (pb = 0.739) or between the no-memory and positive conditions (pb = 0.072).
The mean valence scores were 6.31 ± 2.10 in the positive condition, 3.78 ± 1.87 in the negative condition, and 5.47 ± 1.76 in the no-memory condition (Figure 3C). The Friedman test indicated highly significant differences in valence scores across the conditions (χ2(2) = 18.38, p < 0.001). Post-hoc analysis using Dunn’s test identified significant differences between the negative and positive conditions (pb < 0.001) and between the negative and no-memory conditions (pb = 0.001). By contrast, no significant difference was observed between the no-memory and positive conditions (pb = 0.174).
3.2 SD2/SD1 and SD1
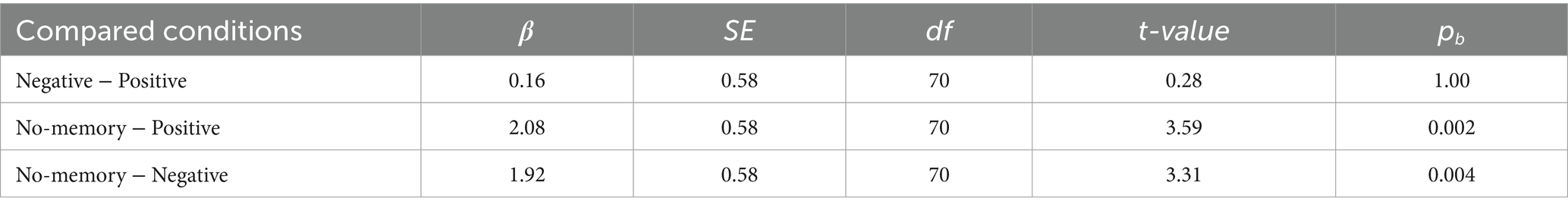
The mean changes in the SD2/SD1 ratio (reflecting SNS activity) from baseline were 1.00 ± 0.82 (mean ± SD) in the positive condition, 0.77 ± 0.77 in the negative condition, and 0.80 ± 0.71 in the no-memory condition (Figure 3D). Linear mixed-effects modeling revealed that SD2/SD1 was significantly higher in the positive condition than in the negative condition, whereas no significant difference was observed between the positive and no-memory conditions (Table 2). For SD2/SD1, the estimated effect sizes (β coefficients) ranged from 0.03 (no-memory vs. negative) to 0.31 (positive vs. negative). These values reflect the magnitude of the effect in terms of standard deviations, with the outcome variable standardized. Detailed results are provided in Supplementary Table 1. The model fit was assessed with R2m = 0.02 and R2c = 0.75.
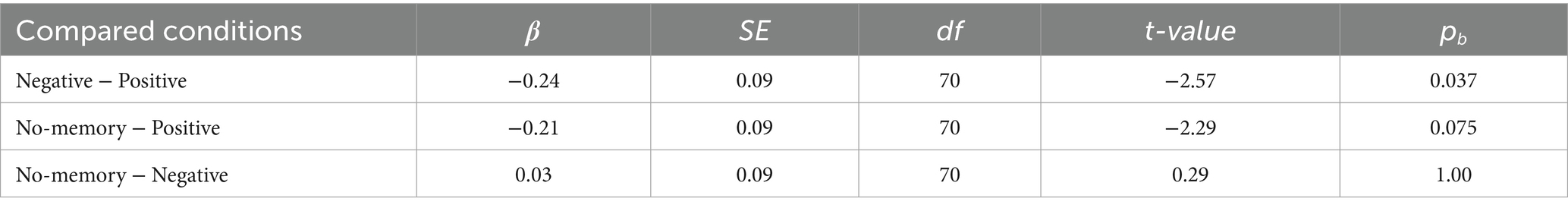
Table 2. Pairwise comparisons for the conditions in SD2/SD1 using the linear-mixed regression models.
The mean changes in SD1 (reflecting PNS activity) from baseline were −6.25 ± 5.52 ms in the positive condition, −6.09 ± 5.20 ms in the negative condition, and −4.16 ± 6.64 ms in the no-memory condition (Figure 3E). Linear mixed-effects modeling revealed that SD1 was significantly higher in the no-memory condition than in both the positive and negative conditions, whereas no significant difference was observed between the positive and negative conditions (Table 3). For SD1, the estimated β coefficients ranged from 0.03 (negative vs. positive) to 0.36 (no-memory vs. positive), likewise reflecting the effect magnitude in terms of standard deviations. Supplementary Table 2 provides the full results. The model fit was assessed with R2m = 0.03 and R2c = 0.83.
3.3 Relationship between psychological and physiological indicators
We examined the relationships between psychological and physiological indicators using structural equation modeling (SEM). Data from all the conditions were combined without repeated measurements (Figure 4A). The model tested the hypothesis that performance achievement is predicted by emotional valence and arousal, valence and arousal are predicted by SD2/SD1 and SD1, and post-performance achievement is predicted by SD2/SD1 and SD1. Model fit indices indicated a good fit (Comparative Fit Index = 1.00, Tucker-Lewis Index = 1.14, Root Mean Square Error of Approximation = 0.00, and Standardized Root Mean Square Residual = 0.01).

Figure 4. Relationship between psychological and physiological indicators. (A) Results of the path analysis showing how emotional valence, arousal, SD2/SD1, and SD1 influence performance achievement. Single-headed arrows represent direct predictions, whereas double-headed arrows represent correlations. Pink arrows indicate positive effects and purple (dotted) arrows indicate negative effects. The numbers next to each arrow are standardized estimates indicating the strength and direction of these effects (*p < 0.05). (B–F) Scatterplots illustrating pairwise relationships among the variables. Each dot represents an individual participant, with pink, blue, and green dots corresponding to the positive, negative, and no-memory conditions, respectively. The β coefficients and p-values shown here are consistent with those reported in the path analysis.
The scatter plots for each pairwise relationship are shown in Figures 4B–F. The analysis revealed that SD2/SD1 exerted a significant positive effect on valence (β = 0.19, p = 0.038; Figure 4F), and valence, in turn, had a significant positive effect on performance achievement (β = 0.68, p < 0.001; Figure 4E). Additionally, SD1 had a significant negative effect on achievement (β = −0.18, p = 0.012; Figure 4C).
In contrast, neither SD2/SD1 nor arousal significantly predicted achievement (β = −0.03, p = 0.685, β = −0.08, p = 0.333, respectively; Figures 4B,D). Similarly, neither SD2/SD1 nor SD1 significantly predicted arousal (β = −0.16, p = 0.098 and β = −0.09, p = 0.328, respectively), and SD1 did not significantly predict valence (β = 0.05, p = 0.496). All β values were standardized.
3.4 Correlation between subjective and objective evaluations
We additionally examined whether the performance effectively conveyed the intended emotions and quality to the audience. The Friedman test revealed no significant differences between conditions (χ2(2) = 0.58, p = 0.75, Figure 5A). In contrast, after combining the data for all conditions, we found a significant correlation between subjective performance achievement and objective evaluations after z-scoring for both variables (Spearman’s rank correlation coefficient = 0.23, p = 0.016; Figure 5B). The 95% confidence interval, calculated using the bootstrap method, ranged from 0.06 to 0.40, confirming a weak positive correlation between subjective performance achievement and objective evaluations.
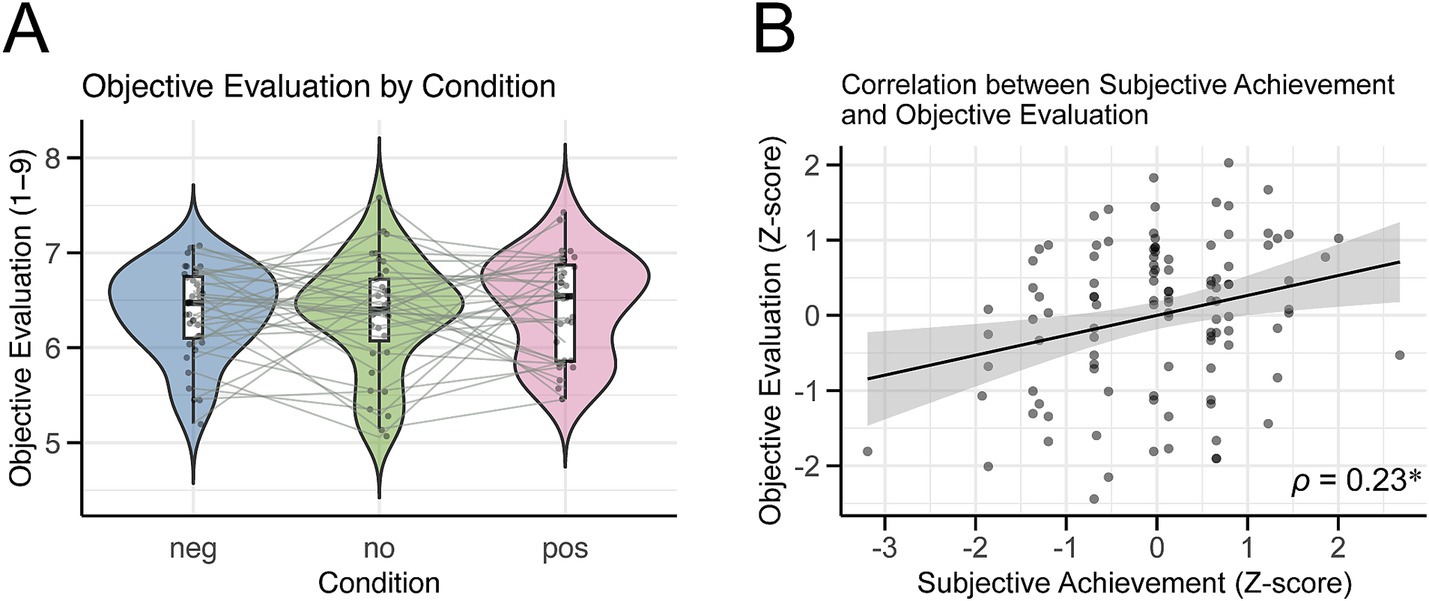
Figure 5. Results of the objective evaluation. (A) Violin plot showing the objective evaluation scores for each condition. Each dot represents the average score for each participant’s performance under these conditions. (B) Scatter plot illustrating the relationship between subjective achievement and objective evaluation. Each dot represents a z-score evaluation value for each participant. The regression line indicates the relationship between the two variables, and the grey band represents the standard error of the regression line. ρ is Spearman’s rank correlation coefficient, and a significant positive correlation was found (*p < 0.05).
4 Discussion
This study investigated whether recalling a positive performance memory could activate SNS, mitigate reductions in PNS activity, increase emotional arousal and valence, and ultimately enhance performance. We also explored whether pre-performance ANS activity directly predicted post-performance achievement or indirectly influenced emotional arousal and valence during performance, thereby affecting achievement.
4.1 Effects of recalling positive compared to negative performance memories
Our results revealed that subjective performance achievement was significantly higher when participants recalled positive performance memories than when they recalled negative ones (Figure 3A). This finding suggests that recalling positive memories can facilitate higher-level performances. In addition, emotional arousal and valence were both significantly greater in the positive condition than in the negative condition (Figures 3B,C), indicating that recalling positive memories not only heightens arousal, but also enhances emotional valence during performance.
We also found that the change in the SD2/SD1 ratio from baseline was significantly higher in the positive condition than in the negative condition (Figure 3D), suggesting a stronger SNS activation when recalling positive versus negative memories. This increase in sympathetic activity aligns with previous studies showing that recalling positive emotional experiences enhances sympathetic responses (McCraty et al., 1995), and that recalling autobiographical memories can elevate ANS responses (Strohm et al., 2021). Moreover, earlier research has established that SNS activity intensifies immediately before performance (Craske and Craig, 1984; Nakahara et al., 2011; Yoshie, 2008), presumably preparing the body for optimal action.
Considering that the SD2/SD1 findings align with our observation of higher subjective performance achievement, arousal, and valence in the positive condition than in the negative condition, these results support the hypothesis that recalling positive performance memories, accompanied by SNS activation, enhances valence and increases arousal during performance, ultimately contributing to improved performance. Furthermore, our results extend the previous findings (Speer and Delgado, 2017; van Schie et al., 2019; MacNamara, 2018; Olsson, 2024) that recalling positive or imagined experiences can influence mood and emotion regulation, by demonstrating that such memory-based strategies may also contribute to enhanced performance outcomes in professional musicians.
While these benefits were evident when comparing the positive and negative conditions, it is noteworthy that there was no significant difference in performance achievement between the positive and no-memory conditions. This pattern may reflect not only a detrimental effect of negative recall, but also the fact that the no-memory condition, which was designed to simulate each musician’s typical pre-performance state, may have facilitated a mental and physiological state that was more conducive to performance. Because the no-memory condition was not strictly emotionally neutral, future research should include a more carefully controlled neutral baseline to better examine the differential effects of positive and negative memory recall.
To examine whether these effects were influenced by individual differences in years of professional experience and trait-level performance anxiety, we conducted additional linear mixed-effects analyses including condition, years of experience, and trait anxiety as fixed effects, and subject as a random effect. The outcome variables were performance achievement, SD2/SD1, and SD1. The results revealed no significant effects of either experience or trait anxiety across any of the models (all ps > 0.05), and the direction and significance of condition effects remained consistent. These findings suggest that individual differences in professional experience and trait anxiety did not systematically confound the main results. Full statistical results are provided in Supplementary Tables 3–8.
Previous studies have shown that positive emotions enhance activity in the prefrontal cortex and anterior cingulate cortex (ACC) (Ashby et al., 1999), and imagining musical performance has been observed to activate functional connectivity between the angular gyrus (AG) and ACC (Tanaka and Kirino, 2019). Integrating these findings, it is possible that recalling positive performance memories in this study facilitated processes such as emotion regulation, decision-making, and attentional control, which may have contributed to the improvement in performance achievement. Although we did not measure neural activity in this study, the observed benefits of recalling positive performance memories on performance achievement may be mediated by the underlying neural mechanisms. Future research should examine whether activation of the prefrontal cortex, ACC, and AG occurs when musicians recall positive autobiographical memories.
4.2 Comparisons of PNS activity between recalling positive and negative memories
We observed no significant difference in the change in SD1 between positive and negative conditions (Figure 3E). Given the antagonistic relationship between the sympathetic and parasympathetic nervous systems (Guyton and Hall, 2006; Saper, 2002), and considering our initial hypothesis that the positive condition would yield a higher SD2/SD1 ratio, we expected a more pronounced decrease in parasympathetic activity in the positive condition. However, the SD1 results did not support this prediction.
Previous studies have shown that positive emotions can simultaneously enhance sympathetic and parasympathetic activity (Kop et al., 2011; Kreibig, 2010; McCraty et al., 1995). This dual enhancement may explain why SD1 did not decline as much as anticipated in the positive condition despite the elevated SD2/SD1 values. In contrast, negative emotional experiences are reported to increase sympathetic activity, while exerting relatively little influence on parasympathetic activity (Kop et al., 2011; Kreibig, 2010; McCraty et al., 1995). Consequently, in the negative condition, SD1 may have decreased more proportionally in response to changes in SD2/SD1.
Additionally, to further validate measurement stability of SD1, we ran a supplementary analysis. We calculated RMSSD, a widely used time-domain measure of parasympathetic activity, and compared it with SD1. Across all conditions, RMSSD and SD1 demonstrated extremely high correlations (neg: r = 0.997, no: r = 0.999, pos: r = 0.999; all p < 0.001), reinforcing the validity of SD1 as a proxy for short-term parasympathetic modulation. These findings are consistent with previous reports indicating that SD1 and RMSSD reflect comparable aspects of short-term HRV (Brennan et al., 2001; Guzik et al., 2007; Ciccone et al., 2017; Shaffer and Ginsberg, 2017).
4.3 Effects of no-memory recall compared to memory-recall conditions
Interestingly, we found that performance achievement, emotional valence, and SD1 were all significantly lower in the negative memory condition than in the no-memory condition (Figures 3A,C,E). These results suggest that recalling negative performance memories decreases achievement, valence, and PNS activity, as indicated by SD1, compared with simply imagining one’s routine pre-performance scenario. As SD1 reflects the rest-and-digest functions of the PNS (Guyton and Hall, 2006; Saper, 2002), its lower values in the negative condition may indicate that participants were less relaxed before performing. This reduced relaxation may have led to decreased valence during performance, ultimately contributing to a lower perceived achievement.
Another possible explanation is that the no-memory condition imposed a lower cognitive load than either memory recall condition. We also observed that SD1 was higher in the no-memory condition than in the positive condition (Figure 3E), suggesting that imagining a familiar pre-performance state allowed the participants to remain more relaxed. Additionally, we did not control individual differences in what the participants envisioned during the no-memory condition. These factors may have enabled participants to achieve a relatively more comfortable state, leading to enhanced valence and, ultimately, improved performance compared to the negative condition.
4.4 Relationship between psychological and physiological indicators
After combining data from all conditions, the path analysis revealed that valence had a significant positive effect on performance achievement, and the SD2/SD1 ratio exerted a significant positive influence on valence (Figure 4A). However, SD2/SD1 did not directly predict performance achievement. These results suggest that while activation of the sympathetic nervous system does not directly enhance performance achievement, it does increase emotional valence, which, in turn, improves musical performance. Although the standardized estimate of SD2/SD1’s effect on valence was small, these findings support our hypothesis that sympathetic activity, when accompanied by positive emotions, contributes to enhanced performance achievement.
Conversely, SD1 did not significantly affect arousal or valence and negatively predicted performance achievement (Figure 4A). Rather than implying that increased parasympathetic activity is harmful, we propose that an optimal level of physiological activation, rather than excessive relaxation, may be more beneficial for performance. This perspective aligns with the idea that a certain degree of sympathetic readiness may support effective musical expression, especially under performance conditions (Robazza et al., 2004; Yoshie et al., 2008).
4.5 Correlation of subjective and objective evaluation
We found no significant differences in objective evaluations across the conditions (Figure 5A). However, there was a positive correlation between subjective performance achievement and objective evaluation (Figure 5B). Notably, the objective evaluations were based only on the first 20 s of the piece, with approximately six or seven notes. Despite this limited amount of information, the positive correlation suggests that the enhanced quality of performances, potentially arising from recalling autobiographical performance memories, was perceptible to the listeners.
Future research should examine the criteria used in objective evaluations to determine which aspects of recorded performances are most effectively communicated. Understanding these criteria may offer insight into how positive autobiographical recall translates into perceptible improvements in musical output.
4.6 Limitations and future directions
This study had several limitations. First, because the experiment was conducted in a music studio, it remains unclear how these findings can be generalized to actual concert settings where professional musicians must avoid making mistakes. Although the performances were conducted without a live audience, all sessions were recorded, and participants were informed in advance that their performances would be evaluated by other professional musicians. This likely induced moderate performance pressure and social evaluative stress. Moreover, although the musical piece involved relatively few notes and a simple structure, it contained exceptionally long phrases that required sustained and delicate breath control, as well as expressive nuance, presenting substantial musical and physiological demands for wind instrumentalists. Importantly, our supplementary analyses indicate that the quality of performance was effectively conveyed to listeners (Figure 5B), suggesting that the findings may hold true in real concert halls as well. This raises the possibility that the present findings could generalize to more technically demanding repertoire. Future studies should further investigate how memory-induced changes influence motor performance under higher-arousal conditions and with more complex musical material.
Second, the study design involves methodological limitations in the assessment of ANS activity, specifically the exclusive reliance on HRV and the absence of respiratory monitoring. Because HRV analysis requires several minutes of data, it is challenging to capture transient dynamic changes in ANS activity (Malik, 1996; Shaffer and Ginsberg, 2017). Among the HRV-derived measures, the SD2/SD1 ratio is commonly used as a relative indicator of sympathetic dominance and has been shown to correlate with the LF/HF ratio, which itself does not directly reflect sympathetic outflow. To enhance the specificity of future studies, additional physiological indicators of sympathetic activity, such as pupil diameter (Bishop et al., 2021) and skin conductance (Brouwer and Hogervorst, 2014), should be incorporated.
In addition, we acknowledge that respiration was neither monitored nor controlled during ECG recording. SD1 and SD2/SD1 are considered relatively robust to minor respiratory fluctuations (Toichi et al., 1997; Penttilä et al., 2001; Nakagawa, 2016; Mishima, 2021), and our supplementary analyses suggested a certain degree of measurement stability in SD1. However, these indices are not entirely immune to respiratory influences. The lack of concurrent respiration monitoring is therefore a methodological limitation of this study. Future studies should incorporate respiration recordings to improve the physiological specificity of HRV analysis.
Third, the nature of the no-memory condition also presents limitations, particularly regarding differences in how participants mentally simulated the scenario. Although this condition was designed to minimize emotional activation by simulating a familiar pre-performance scenario, individual differences in participants’ mental imagery may have introduced variability in emotional or cognitive responses. Establishing a strictly emotionally neutral state is inherently difficult in experimental settings, particularly in studies involving professional musicians. Future studies may consider employing more rigorously controlled baseline conditions to further isolate the effects of autobiographical memory recall on autonomic and performance-related outcomes.
Fourth, we did not collect information regarding the menstrual cycle phase of female participants. Previous research suggests that autonomic nervous system reactivity may fluctuate across the menstrual cycle, with both sympathetic and parasympathetic activities peaking during the ovulatory phase (Little and Zahn, 1974). Although the within-subject design of the present study likely mitigated some inter-individual variability, the potential confounding effect of menstrual cycle phase cannot be entirely excluded. Future studies may benefit from incorporating the menstrual cycle tracking to further refine the assessment of autonomic function in female musicians.
Nevertheless, the present findings suggest that recalling positive autobiographical performance memories can help regulate emotional and physiological states before performance, contributing to enhanced subjective achievement. This offers a conceptual basis for future studies in more ecologically valid contexts and practical guidance for musicians and other performers seeking accessible, low-risk strategies to optimize their internal state before high-stakes performance.
5 Conclusion
We found that professional musicians who recalled memories of positive performance experienced greater performance achievement. Path analysis integrating data from all conditions revealed that, although SD2/SD1 did not directly predict performance achievement, increases in SD2/SD1 were associated with higher valence, which in turn led to improved achievement. These results suggest that consciously recalling positive memories before a performance in response to rising ANS activity may help professional musicians interpret their physiological state more positively, ultimately enhancing their on-stage performance.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Research Ethics Committee of the Keio University Shonan Fujisawa Campus (Approval Number 507). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AW: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. SK: Data curation, Formal analysis, Methodology, Supervision, Validation, Writing – review & editing. TS: Conceptualization, Methodology, Validation, Writing – review & editing. SF: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Taikichiro Mori Memorial Research Grants and the JST SPRING Grant (No. JPMJSP2123) (to AW), by the JST COI-NEXT Grant (No. JPMJPF2203), the JST Moonshot R&D Grant (No. JPMJMS2215-G3-7), and the JSPS KAKENHI Grant (No. 24H02199) (to SF).
Acknowledgments
We are deeply grateful to Dr. Junichi Ushiyama for insightful comments on data analysis, and Dr. Michiko Yoshie for helpful comments on the experimental design. We also extend our heartfelt thanks to Mr. Motohiko Murabayashi, representative of the General Incorporated Foundation Runde, and Ms. Junko Shimamura for their generous support in providing access to the music studio.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be interpreted as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyg.2025.1544069/full#supplementary-material
References
Abel, J. L., and Larkin, K. T. (1990). Anticipation of performance among musicians: physiological arousal, confidence, and state-anxiety. Psychol. Music 18, 171–182. doi: 10.1177/0305735690182006
Ashby, F. G., Isen, A. M., Turken, U., Turken, A., Barbara, S., Graduate, J., et al. (1999). A neuropsychological theory of positive affect and its influence on cognition. Psychol. Rev. 106, 529–550. doi: 10.1037/0033-295X.106.3.529
Bates, D., Mächler, M., Bolker, B., Walker, S., et al. (2015). Fitting linear mixed-effects models Usinglme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Baumeister, R. F. (1984). Choking under pressure: self-consciousness and paradoxical effects of incentives on skillful performance. J. Pers. Soc. Psychol. 46, 610–620. doi: 10.1037/0022-3514.46.3.610
Bernardi, L., Porta, C., Casucci, G., Balsamo, R., Bernardi, N. F., Fogari, R., et al. (2009). Dynamic interactions between musical, cardiovascular, and cerebral rhythms in humans. Circulation 119, 3171–3180. doi: 10.1161/circulationaha.108.806174
Bernardi, L., Porta, C., and Sleight, P. (2006). Cardiovascular, cerebrovascular, and respiratory changes induced by different types of music in musicians and non-musicians: the importance of silence. Heart 92, 445–452. doi: 10.1136/hrt.2005.064600
Berntson, G. G., Cacioppo, J. T., and Quigley, K. S. (1991). Autonomic determinism: the modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint. Psychol. Rev. 98, 459–487. doi: 10.1037/0033-295x.98.4.459
Bishop, L., Jensenius, A. R., and Laeng, B. (2021). Musical and bodily predictors of mental effort in string quartet music: an ecological pupillometry study of performers and listeners. Front. Psychol. 12:653021. doi: 10.3389/fpsyg.2021.653021
Brennan, M., Palaniswami, M., and Kamen, P. (2001). Do existing measures of Poincare plot geometry reflect nonlinear features of heart rate variability? IEEE Trans. Biomed. Eng. 48, 1342–1347. doi: 10.1109/10.959330
Brouwer, A.-M., and Hogervorst, M. A. (2014). A new paradigm to induce mental stress: the sing-a-song stress test (SSST). Front. Neurosci. 8:224. doi: 10.3389/fnins.2014.00224
Burin, A. B., and Osório, F. L. (2017). Music performance anxiety: a critical review of etiological aspects, perceived causes, coping strategies and treatment. Archiv Clinic. Psychiatry 44, 127–133. doi: 10.1590/0101-60830000000136
Ciccone, A. B., Siedlik, J. A., Wecht, J. M., Deckert, J. A., Nguyen, N. D., and Weir, J. P. (2017). Reminder: RMSSD and SD1 are identical heart rate variability metrics: issues & opinions: RMSSD and SD1. Muscle Nerve 56, 674–678. doi: 10.1002/mus.25573
Craig, A. D. B. (2009). How do you feel--now? The anterior insula and human awareness. Nat. Rev. Neurosci. 10, 59–70. doi: 10.1038/nrn2555
Craske, M. G., and Craig, K. D. (1984). Musical performance anxiety: the three-systems model and self-efficacy theory. Behav. Res. Ther. 22, 267–280. doi: 10.1016/0005-7967(84)90007-x
Damasio, A. R. (1996). The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 351, 1413–1420. doi: 10.1098/rstb.1996.0125
Electrophysiology TF of the ES (1996). Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation 93, 1043–1065. doi: 10.1161/01.CIR.93.5.1043
Furuya, S., Ishimaru, R., and Nagata, N. (2021). Factors of choking under pressure in musicians. PLoS One 16:e0244082. doi: 10.1371/journal.pone.0244082
Goit, R. K., Jha, S. K., and Pant, B. N. (2016). Alteration of cardiac autonomic function in patients with newly diagnosed epilepsy. Physiol. Rep. 4:e12826. doi: 10.14814/phy2.12826
Guyton, A. C., and Hall, J. E. (2006). Textbook of medical physiology. 11th Edn. Philadelphia: Elsevier.
Guzik, P., Piskorski, J., Krauze, T., Schneider, R., Wesseling, K. H., Wykretowicz, A., et al. (2007). Correlations between the Poincaré plot and conventional heart rate variability parameters assessed during paced breathing. J. Physiol. Sci. 57, 63–71. doi: 10.2170/physiolsci.RP005506
Hsu, C.-H., Tsai, M.-Y., Huang, G.-S., Lin, T.-C., Chen, K.-P., Ho, S.-T., et al. (2012). Poincaré plot indexes of heart rate variability detect dynamic autonomic modulation during general anesthesia induction. Acta Anaesthesiol Taiwan 50, 12–18. doi: 10.1016/j.aat.2012.03.002
Kenny, D. T. (2011). “The role of negative emotions in performance anxiety” in Handbook of music and emotion: Theory, research, applications. eds. P. N. Juslin and J. Sloboda (Oxford: Oxford University Press), 425–451.
Kop, W. J., Synowski, S. J., Newell, M. E., Schmidt, L. A., Waldstein, S. R., and Fox, N. A. (2011). Autonomic nervous system reactivity to positive and negative mood induction: the role of acute psychological responses and frontal electrocortical activity. Biol. Psychol. 86, 230–238. doi: 10.1016/j.biopsycho.2010.12.003
Kreibig, S. D. (2010). Autonomic nervous system activity in emotion: a review. Biol. Psychol. 84, 394–421. doi: 10.1016/j.biopsycho.2010.03.010
Kuznetsova, A., Brockhoff, P., and Christensen, R. (2017). LmerTest package: tests in linear mixed effects models. J. Stat. Softw. 82, 1–26. doi: 10.18637/JSS.V082.I13
Li, Z., Moritz, S. E., and Liu, H. (2024). Motor imagery as a potential tool to alleviate choking under pressure in sports performance. J. Imagery Res. Sport Physic. Activity 19:20240012. doi: 10.1515/jirspa-2024-0012
Little, B. C., and Zahn, T. P. (1974). Changes in mood and autonomic functioning during the menstrual cycle. Psychophysiology 11, 579–590. doi: 10.1111/j.1469-8986.1974.tb01118.x
Lorah, J. (2018). Effect size measures for multilevel models: definition, interpretation, and TIMSS example. Large-Scale Assess. Educ. 6, 1–11. doi: 10.1186/s40536-018-0061-2
Lüdecke, D., Ben-Shachar, M., Patil, I., Waggoner, P., and Makowski, D. (2021). Performance: an R package for assessment, comparison and testing of statistical models. J. Open Source Softw. 6:3139. doi: 10.21105/joss.03139
MacNamara, A. (2018). In the mind’s eye: the late positive potential to negative and neutral mental imagery and intolerance of uncertainty. Psychophysiology 55:e13024. doi: 10.1111/psyp.13024
Malik, M. (1996). Heart rate variability: standards of measurement, physiological interpretation, and clinical use: task force of the European society of cardiology and the north American society for pacing and electrophysiology. Ann. Noninvasive Electrocardiol. 1, 151–181. doi: 10.1111/j.1542-474x.1996.tb00275.x
McCraty, R., Atkinson, M., Tiller, W. A., Rein, G., and Watkins, A. D. (1995). The effects of emotions on short-term power spectrum analysis of heart rate variability. Am. J. Cardiol. 76, 1089–1093. doi: 10.1016/s0002-9149(99)80309-9
Mishima, R. (2021). Challenges in interpreting autonomic nervous system evaluation through heart rate variability analysis: focusing on multiple parasympathetic activity indicators. Konan University Bulletin: Literature Edition. 171, 269–276. (in Japanese). Available at: https://konan-u.repo.nii.ac.jp/?action=repository_action_common_download&item_id=3809&item_no=1&attribute_id=22&file_no=1
Mulcahy, D., Keegan, J., Fingret, A., Wright, C., Park, A., Sparrow, J., et al. (1990). Circadian variation of heart rate is affected by environment: a study of continuous electrocardiographic monitoring in members of a symphony orchestra. Br. Heart J. 64, 388–392. doi: 10.1136/hrt.64.6.388
Nakagawa, C. (2016). Special issue 3: measurement methods for ergonomics (in Japanese). J. Human Ergon. 52, 6–12. doi: 10.5100/jje.52.6
Nakahara, H., Furuya, S., Masuko, T., Francis, P. R., and Kinoshita, H. (2011). Performing music can induce greater modulation of emotion-related psychophysiological responses than listening to music. Int. J. Psychophysiol. 81, 152–158. doi: 10.1016/j.ijpsycho.2011.06.003
Olsson, I. (2024). Exploring the persistence of negative emotional memories over positive ones: a qualitative study. Int. J. Psychophysiol. Stud. 16:26. doi: 10.5539/ijps.v16n4p26
Orbach, I., and Blumenstein, B. (2022). Preparatory routines for emotional regulation in performance enhancement. Front. Psychol. 13:948512. doi: 10.3389/fpsyg.2022.948512
Penttilä, J., Helminen, A., Jartti, T., Kuusela, T., Huikuri, H. V., Tulppo, M. P., et al. (2001). Time domain, geometrical and frequency domain analysis of cardiac vagal outflow: effects of various respiratory patterns. Clinic. Physiol. 21, 365–376. doi: 10.1046/j.1365-2281.2001.00337.x
Robazza, C., Pellizzari, M., and Hanin, Y. (2004). Emotion self-regulation and athletic performance: an application of the IZOF model. Psychol. Sport Exerc. 5, 379–404. doi: 10.1016/S1469-0292(03)00034-7
Rosseel, Y. (2012). Lavaan: an R package for structural equation modeling. J. Stat. Softw. 48, 1–36. doi: 10.18637/jss.v048.i02
Russell, J. A., Weiss, A., and Mendelsohn, G. A. (1989). Affect grid: a single-item scale of pleasure and arousal. J. Pers. Soc. Psychol. 57, 493–502. doi: 10.1037/0022-3514.57.3.493
Saper, C. B. (2002). The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu. Rev. Neurosci. 25, 433–469. doi: 10.1146/annurev.neuro.25.032502.111311
Schachter, S., and Singer, J. E. (1962). Cognitive, social, and physiological determinants of emotional state. Psychol. Rev. 69, 379–399. doi: 10.1037/h0046234
Shaffer, F., and Ginsberg, J. P. (2017). An overview of heart rate variability metrics and norms. Front. Public Health 5:258. doi: 10.3389/fpubh.2017.00258
Speer, M. E., and Delgado, M. R. (2017). Reminiscing about positive memories buffers acute stress responses. Nat. Hum. Behav. 1:0093. doi: 10.1038/s41562-017-0093
Stoffel, M. A., Nakagawa, S., and Schielzeth, H. (2021). partR2: partitioning R2 in generalized linear mixed models. PeerJ 9:e11414. doi: 10.7717/peerj.11414
Strohm, M., Siegesleitner, M., Kunze, A. E., Werner, G. G., Ehring, T., and Wittekind, C. E. (2021). Psychological and physiological effects of imagery rescripting for aversive autobiographical memories. Cogn. Ther. Res. 45, 1093–1104. doi: 10.1007/s10608-021-10233-5
Takada, T., and Yukawa, S. (2014). Effects of unrelatedly induced pleasantness and luck on the recklessness and conservativeness of gambling behavior (in Japanese). Jpn. J. Res. Emot. 22, 1–10. doi: 10.4092/jsre.22.1_1
Tanaka, S., and Kirino, E. (2019). Increased functional connectivity of the angular gyrus during imagined music performance. Front. Hum. Neurosci. 13:92. doi: 10.3389/fnhum.2019.00092
Tarvainen, M. P., Niskanen, J.-P., Lipponen, J. A., Ranta-aho, P. O., and Karjalainen, P. A. (2014). Kubios HRV – heart rate variability analysis software. Comput. Methods Prog. Biomed. 113, 210–220. doi: 10.1016/j.cmpb.2013.07.024
Toichi, M., Sugiura, T., Murai, T., and Sengoku, A. (1997). A new method of assessing cardiac autonomic function and its comparison with spectral analysis and coefficient of variation of R–R interval. J. Auton. Nerv. Syst. 62, 79–84. doi: 10.1016/S0165-1838(96)00112-9
van Schie, C. C., Chiu, C.-D., Rombouts, S. A. R. B., Heiser, W. J., and Elzinga, B. M. (2019). When I relive a positive me: vivid autobiographical memories facilitate autonoetic brain activation and enhance mood. Hum. Brain Mapp. 40, 4859–4871. doi: 10.1002/hbm.24742
Volgemute, K., Vazne, Z., and Krauksta, D. (2024). An intervention into imagery and self-efficacy: enhancing athletic achievements of alpine skiers. Educ. Sci. 14:513. doi: 10.3390/educsci14050513
Woods, K. J. P., Siegel, M. H., Traer, J., and McDermott, J. H. (2017). Headphone screening to facilitate web-based auditory experiments. Atten. Percept. Psychophys. 79, 2064–2072. doi: 10.3758/s13414-017-1361-2
Yoshie, M. (2008). Heart rate changes in pianists during competitions. Proc. Annu. Conv. Jpn. Psychol. Assoc. 72:1PM168. doi: 10.4992/pacjpa.72.0_1PM168
Yoshie, M., Kudo, K., Murakoshi, T., and Ohtsuki, T. (2009b). Music performance anxiety in skilled pianists: effects of social-evaluative performance situation on subjective, autonomic, and electromyographic reactions. Exp. Brain Res. 199, 117–126. doi: 10.1007/s00221-009-1979-y
Yoshie, M., Kudo, K., and Ohtsuki, T. (2009c). Motor/autonomic stress responses in a competitive piano performance. Ann. N. Y. Acad. Sci. 1169, 368–371. doi: 10.1111/j.1749-6632.2009.04786.x
Yoshie, M., Shigemasu, K., Kudo, K., and Ohtsuki, T. (2008). Multidimensional anxiety and music performance: an exploratory application of the zones of optimal functioning model. Stress and Anxiety: Application to Life Span Development and Health Promotion, 163–171. Available online at:https://books.google.co.jp/books?hl=ja&lr=lang_ja|lang_en&id=ccPkiJ06eoEC&oi=fnd&pg=PA163&dq=Multidimensional++anxiety++Yoshie&ots=0ThG0DtEjk&sig=UwXNxujGjLzDCVvefbPcKUcbtgo
Keywords: musical expression, professional musicians, performance achievement, autonomic nervous system, autobiographical memories, heart rate variability
Citation: Watanabe A, Kondoh S, Samma T and Fujii S (2025) Enhanced subjective performance achievement in wind instrument playing through positive memory recall: effects of sympathetic activation and emotional valence. Front. Psychol. 16:1544069. doi: 10.3389/fpsyg.2025.1544069
Edited by:
Maura Pilotti, Prince Mohammad bin Fahd University, Saudi ArabiaReviewed by:
Laura Bishop, University of Oslo, NorwayHidehiro Nakahara, Morinomiya University of Medical Sciences, Japan
Copyright © 2025 Watanabe, Kondoh, Samma and Fujii. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shinya Fujii, ZnVqaWkuc2hpbnlhQGtlaW8uanA=
 Aiko Watanabe
Aiko Watanabe Sotaro Kondoh
Sotaro Kondoh Tomohiro Samma
Tomohiro Samma Shinya Fujii
Shinya Fujii