- 1Institute of Mental Health, First Hospital of Hebei Medical University, Shijiazhuang, China
- 2Hebei Medical University and Hebei Technical Innovation Center for Mental Health Assessment and Intervention, Hebei Clinical Research Center for Mental Disorders and Institute of Mental Health, Department of Psychiatry, The First Hospital of Hebei Medical University, Shijiazhuang, China
- 3Department of Clinical Pharmacy, The First Hospital of Hebei Medical University, Shijiazhuang, China
- 4Department of Nursing, The First Hospital of Hebei Medical University, Shijiazhuang, China
- 5Department of Nephrology, The First Hospital of Hebei Medical University, Shijiazhuang, China
- 6Department of Hematology, The First Hospital of Hebei Medical University, Shijiazhuang, China
- 7Department of Hospital Infection Management, The First Hospital of Hebei Medical University, Shijiazhuang, China
Background and aim: Carbon monoxide (CO), a prevalent environmental pollutant, has been implicated in adverse mental health outcomes, but the mechanistic relationship between atmospheric CO levels and hospital admissions for mental and behavioral disorders remains unclear. This study investigates the epidemiological link between atmospheric CO and hospitalizations for mental health conditions in Shijiazhuang, China.
Methodology: Clinical data from patients hospitalized with mental and behavioral disorders at The First Hospital of Hebei Medical University between January 2014 and December 2020 were analyzed. Daily atmospheric CO levels, temperature, and relative humidity were concurrently monitored. A generalized additive model (GAM) was used to explore the correlation between CO levels and hospital admissions. Blood samples from patients with depressive disorders were analyzed for MAPK3 expression, and a mouse model of CO-induced depression was employed to further explore the molecular mechanisms.
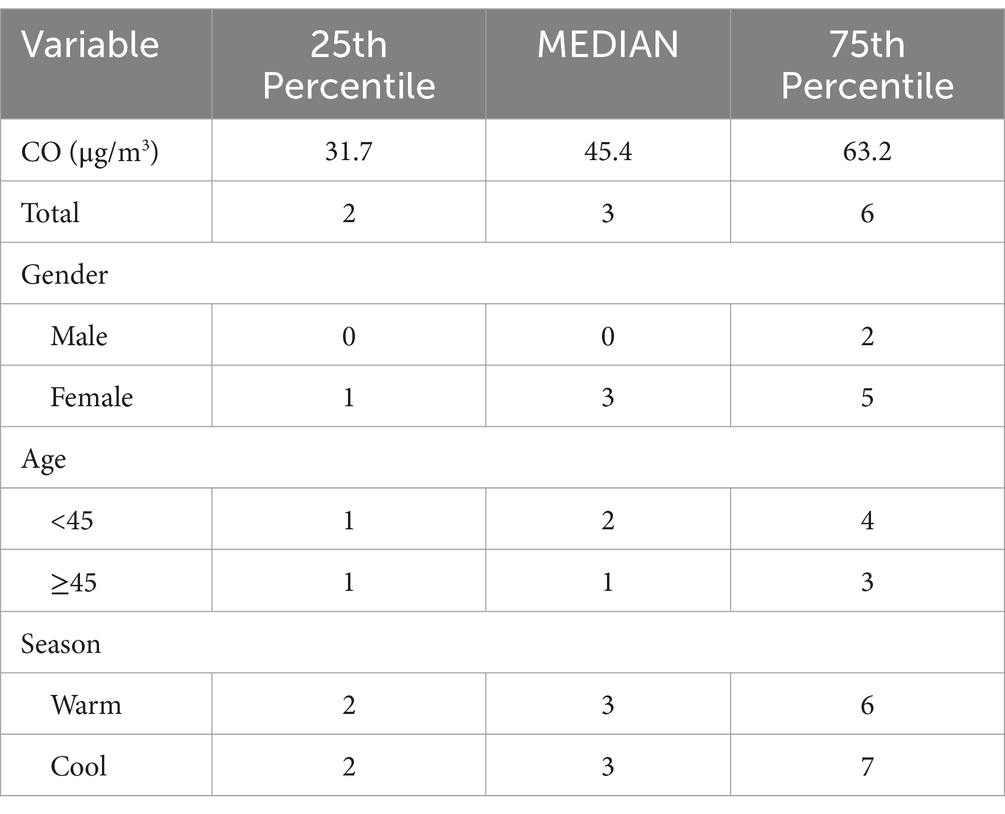
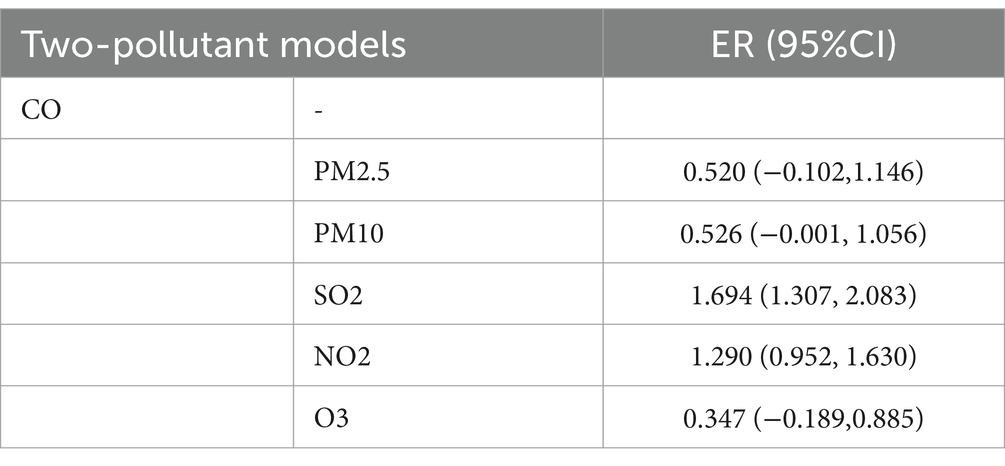
Results: A total of 15,890 hospitalization records were included. A significant positive correlation was identified between CO levels and the number of daily hospitalizations, with the strongest effects observed when CO concentrations exceeded 40 μg/m3. This association was most pronounced in males and individuals aged over 45, as well as during both warm and cold seasons. A two-pollutant model confirmed CO as a major factor affecting hospitalizations, independent of other pollutants like nitric oxide and sulfur dioxide. Additionally, elevated MAPK3 expression was found in the blood samples of depressed patients, and treatment with the MAPK inhibitor PD98059 alleviated CO-induced depression in a mouse model.
Conclusion: This study provides compelling evidence for a significant association between atmospheric CO and hospitalizations for mental and behavioral disorders. The findings suggest that CO exposure may exacerbate mental health conditions, particularly in vulnerable populations. These insights underline the importance of air quality management and highlight potential pathways for therapeutic interventions targeting CO-induced mental health disorders.
Introduction
As the frequency and intensity of severe air pollution events continue to increase in various regions around the world, the impact of air pollution on mental disorders has emerged as a matter of growing concern. Indeed, there are accumulated studies suggesting that air pollution may increase the risk of suicide, depression, autism, and anxiety during pregnancy (Bakian et al., 2015; Wang et al., 2018; Lin et al., 2017; Volk et al., 2013). The mechanistic link between air pollution and mental disorders remains to be fully elucidated, despite oxidized pollutants being suggested to be involved in the pathogenesis of depression (Guan et al., 2015). Carbon monoxide (CO), a commonly detected air pollutant resulting from incomplete organic combustion, is toxic by inhalation at high concentrations (Xu et al., 2020). CO binds to hemoglobin with high affinity, impairing oxygen delivery to tissues, leading to hypoxia. This hypoxic state triggers oxidative stress and lipid peroxidation in the central nervous system, resulting in neuronal injury. Additionally, CO exposure induces the release of excitatory amino acids like glutamate, causing excitotoxicity and apoptosis (Ho et al., 2012). These mechanisms collectively contribute to neuropsychiatric sequelae, including cognitive deficits, mood disorders, and delayed neuropsychiatric effects (Watt et al., 2018).
Szyszkowicz et al. indicated that atmospheric pollutants such as sulfur dioxide and CO would be associated with increases in the number of depression diagnoses in psychiatric emergency rooms, implicating the important role of CO in mental disorders (Szyszkowicz et al., 2016). More recently, the pro-oxidant constituents of vehicle exhaust including CO were demonstrated to cause memory deficits and anxiety and depression-like behaviors, which were accompanied with elevated levels of serum corticosterone and markers of oxidative stress and inflammatory markers in male rats (Kochi et al., 2021). Pollutants are also believed to impair normal cellular functions by affecting mitochondria (Kochi et al., 2023). Despite these reported adverse effects of CO on nervous system and so cognitive behaviors, on the other hand, there are also studies suggesting that CO might be a neuroprotective substance that can reduce inflammation, mediating neurotransmission and regulating hemodynamics in the brain (Hanafy et al., 2013). CO is suggested to act as a signaling molecule that promotes cellular survival pathways (Mahan, 2012). Besides, CO was shown to reduce oxidative stress and apoptosis in neuronal cells, thereby protecting against ischemic brain injuries (Guo et al., 2024). Additionally, CO’s ability to enhance neurogenesis and improve cognitive functions has been observed in animal models (Watt et al., 2018). An exogenous administration of CO was also found able to block LPS-induced depressive- and anxiety-like behaviors in mice (Luo et al., 2021). The impact of CO on mental disorders is therefore not entirely consistent.
The mechanistic links between exposure to environmental pollutants with severe mental disorders have been investigated in different studies. The hypothalamic–pituitary–adrenal axis, which regulates stress responses, has been identified as a key pathway that is often disrupted by pollutants including CO (Thomson et al., 2019). Pollutant exposure can affect neurotransmitter systems like the serotoninergic system, leading to imbalances that contribute to mood disorders (Thomson et al., 2019). Additionally, the mitogen-activated protein kinase (MAPK) was demonstrated to play a key role in modulating neuronal survival, synaptic plasticity, and neuroinflammation upon pollutant exposure (Ijomone et al., 2021). The studies have indicated the complex interplay between environmental pollutants and mental health, emphasizing the need for further research to develop effective preventive measurement and therapeutic intervention (Catapano et al., 2024).
In view of the debated role of CO in mental disorders, the present longitudinal study was aimed to explore the impact of atmospheric CO on the number of hospitalizations for mental disorders in Shijiazhuang City of Hebei Province of the People’s Republic of China. Shijiazhuang City was selected as the research location because the atmospheric CO has been increasing due to the rapid development of transportation and industrial and mining enterprises over the past few years. Being one of the important central cities in the Beijing-Tianjin-Hebei region having a permanent population of 11.2 million and an urbanization rate of 71.4%, findings from Shijiazhuang City would provide valuable insights into the relationship between CO and mental disorders in populated cities around the world. At the same time, with an initial hope to identify new therapeutic target for treatment of CO-induced depression and given the potential role of MAPK in such disorder, the present study also determined the blood MAPK3 transcript among patients with depression and characterized the role of MAPK3 in a mouse model of CO-induced depression.
Methodology
Clinical data collection
Clinical records of patients who were hospitalized for mental and behavioral disorders into The First Hospital of Hebei Medical University from January 2014 to December 2020 were retrieved. There is strong patient adhesion in Shijiazhuang and the surrounding areas, with most hospitalized patients coming from Shijiazhuang and its vicinity. The disorders were defined according to the International Classification of Diseases (10th edition, ICD-10) with the code F01-F99. Data were stratified into subgroups of patients’ age (i.e., < 45 and ≥45 years), gender (i.e., male and female) and season of hospitalization (i.e., cold [October to April] and warm [May to September]). The study was approved by the Ethics Committee of First Hospital of Hebei Medical University (IRB approval number: 20190592) and the requirement for informed consent was waived because the review of the patient data was retrospective and anonymous.
Air pollution and meteorological data collection
Atmospheric levels of pollutants including CO were retrieved from the website of the Ministry of Ecology and Environment of the State Council of the People’s Republic of China. This data was collected real-time from seven fixed stations in the urban areas of Shijiazhuang City. The stations are far away from main roads, industrial sources, residential areas, and emissions from burning coal, oil, or waste; therefore, the monitored results would truly reflect urban air pollution levels rather than local pollution sources. Daily meteorological data like mean temperature (MT) and relative humidity (RH) were also obtained from the National Meteorological Information Centre of China Meteorological Data Service Centre.
Determination of blood MAPK3 mRNA
RNA was extracted from whole blood samples collected from patients with depression as well as healthy individuals for first-strand cDNA synthesis. MAPK3 and GAPDH were then amplified using real-time PCR. The relative level of MAPK3 was calculated using 2-∆∆CT approach. This technique begins by determining the CT (threshold cycle) values, which indicate the cycle number at which the PCR product becomes detectable; lower CT values correspond to higher gene expression levels. The first step involves calculating the ∆CT for each sample by subtracting the CT value of a reference (housekeeping) gene from that of the target gene. Next, to assess the relative expression between experimental and control groups, the ∆∆CT is calculated by finding the difference in ∆CT values between these groups. Finally, the relative expression level of the target gene is derived using the formula 2^(−∆∆CT).
Mouse model of CO-induced depression
To further decipher the molecular pathogenesis of CO-induced mental disorders, the functional role of MAPK3 and its associated pathway activation was studied in mice with depression induced by CO exposure. C57BL/6J mice, aged 6–8 weeks with initial weight upon arrival was 18–20 grams, were purchased (Henan Skobes Biotechnology, Henan, China). The active and exploratory behavior of this mouse strain makes them a good model for behavioral studies. The mice were housed at a constant temperature of 23 ± 2°C and subjected to a 12-h light/dark cycle (lights on from 8 AM to 8 PM), with free access to food and water. After a two-week acclimatization period, the mice were randomly divided into four groups: Control group (n = 6), CO-induced depression model (i.e., CO, n = 6), Control group + MAPK3 inhibition group (i.e., Control + PD98059, n = 6), and CO-mediated depression model + MAPK3 inhibition group (i.e., CO + PD98059, n = 6). PD98059 is a selective potent inhibitor to MAPK3.
Depression was induced in mice by exposing mice to a 3,000 ppm CO environment for 40 min using a static inhalation exposure method. This exposure condition would ensure robust and uniform exposure of mice to CO. After CO exposure, the mice received injections of saline, as the negative control, to assess the effects of CO alone on their behavior. The mice would exhibit corresponding depressive behaviors, such as anhedonia, despair, significant reduction in appetite, social withdrawal, and cognitive impairment. PD98059 was prepared as solution (10 mg/ml, dimethyl sulfoxide [DMSO]) and was injected intraperitoneally at a dosage of 10 mg/kg.
The effect of MAKP3 treatment on CO-inflicted mice was studied by examining their behaviors by different tests including sucrose preference test (SPT), open field test (OFT), and Y-maze test. After the tests, mice were humanly sacrificed. Protein levels of brain-derived neurotrophic factor (BDNF) and MAPK3 in brain tissues were then examined using western blotting.
Statistical analysis
To investigate the effect of individuals’ age and gender and season on the occurrence of mental and behavioral disorders, data were stratified into subgroups for in-depth analysis. The time series regression method was used to study the acute impact of particulate matter (i.e., CO) on the hospitalization of patients with mental and behavioral disorders. Given that data about patients’ hospitalization days usually follow a quasi-Poisson distribution, the over dispersed generalized additive model (GAM) was used to analyze the relationship between CO and hospitalization days in patients with mental and behavioral disorders. To control for potential confounding effects, several covariates were introduced, including (i) natural cubic regression as a smooth function of calendar time with 7 degrees of freedom (df) per year, excluding unmeasured long-term and seasonal trends beyond 2 months; (ii) natural smooth functions of average temperature (6, df) and relative humidity (3, df) to control weather conditions affected by nonlinear confounding effect; and (iii) any indicator variables representing “day of the week” and public holidays.
The main model was described as follows: logE (Yt) = βzt + ns (time, df) + ns (temperature, 6) + ns (humidity, 3) + dow + intercept, where e (yt) represents the number of hospitalizations for mental behavioral disorders on day t expected number of people; β represents the logarithmic correlation rate of admissions for mental and behavioral disorders associated with a unit increase in particulate matter pollutants; zt represents the pollutant concentration on day t; dow represents a dummy variable for the day of the week; ns represents the natural cubic regression smoothing function.
After the basic model was established, we further introduced single-day lag 0–7 and multi-day moving average exposure, including lag0-1, 0–2, 0–3, 0–4, 0–5, 0–6. By adding three df natural spline functions to the model, the exposure-response relationship curve between CO and hospitalization for mental behavioral disorders was drawn.
To test the stability of the model, a sensitivity analysis was performed. Second, we built a two-pollutant model to examine the robustness of effect estimates after adjusting for co-pollutants.
In addition, we conducted three levels of analysis to explore potential effects by gender, age (<45 years and ≥45 years) and season (warm: April to September, cold: October and January to April). We further tested the statistical significance of the differences between estimates of formation effects by calculating 95% confidence intervals (CI) using the following equation.
Where and are the two class estimates, and are their respective standard errors.
The statistical test was a two-sided test, and p < 0.05 was defined as a statistically significant difference. All statistical models were run in R software (version 3.3.3) using the “MGCV” package. The change rate and 95% CI of mental and behavioral disorders caused by every 10 μg/m3 increase in CO were used as indicators.
For the determination of MAPK3 transcript in patients’ blood samples and mouse model of CO-induced depression, the comparison was done using non-parametric tests.
Results
Cohort characteristics
During the study period, there were a total of 15,890 cases of hospitalization for mental and behavioral disorders in Shijiazhuang City. The majority was male (n = 10,083), and there were 9,143 cases being aged <45 years. Slightly more cases were seen in cold than in warm seasons (cold season, 8,378 cases; warm season, 7,512 cases). The daily average concentration of CO was 45.4 μg/m3, with the daily average temperature and relative humidity as 15 degree and 56%, respectively (Table 1). Results showed that there was a significant lag effect of CO, with lagging for 2 days (i.e., lag02) being the maximum estimated value (Figure 1). A E-R with positive correlation between lag02 and daily number of hospitalizations was seen, in which a peak was achieved when the concentration of CO was greater than 40 μg/m3 (Figure 2).
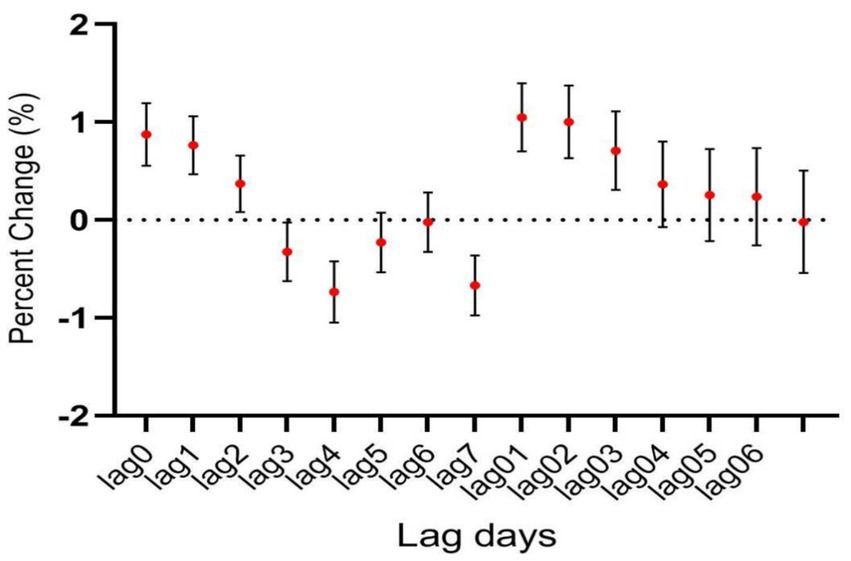
Figure 1. The relationship between atmospheric CO concentration and hospitalization for mental and behavioral disorders.
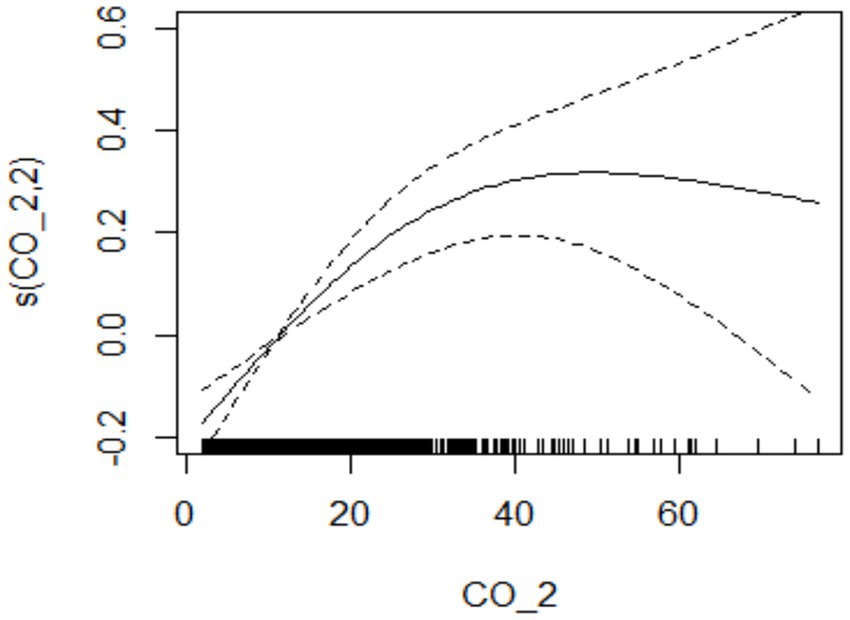
Figure 2. The relationship between atmospheric CO concentration and hospitalization for mental and behavioral disorders at lag02.
Effect of individuals’ age and gender and season
In male patients, CO was significantly associated with hospitalization for psychiatric and behavioral disorders (OR, 1.336; 95% CI, 0.041–1.972) (Figure 3A), however, such positive correlation was not seen in female patients despite there was slight increase in female patient hospitalizations along with the increasing CO level (Figure 3B).
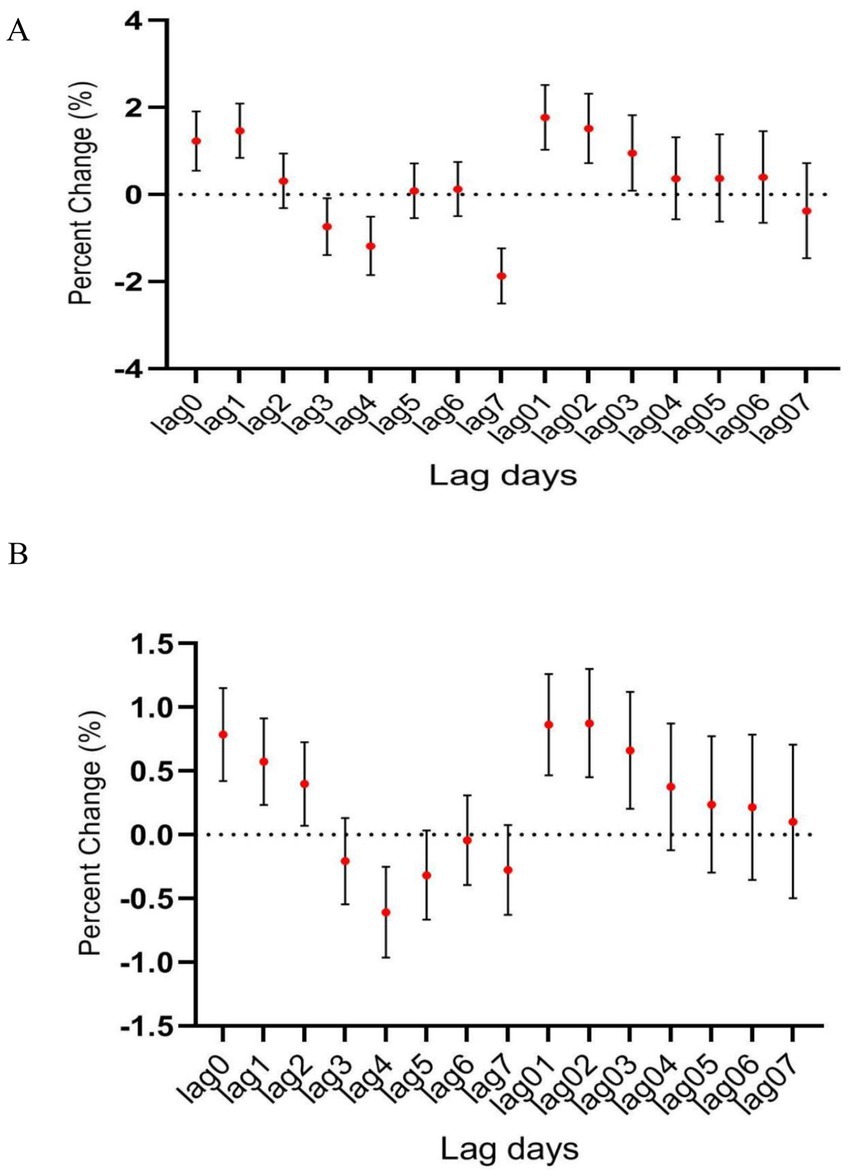
Figure 3. The relationship between atmospheric CO concentration and hospitalization for mental and behavioral disorders of (A) male and (B) female patients.
In age-specific analyses, the association was more significant among patients aged ≥45 years than among those younger than 45 years (Figure 4). Interestingly, the association between CO level and hospitalization number was more significant in warm than in cold seasons, despite the number of hospitalization cases being more in cold than in warm seasons (Figure 5). In the two pollutant models, after adjustment for nitric oxide and sulfur dioxide, the correlation of CO level with hospitalization number was still statistically significant (Table 2).
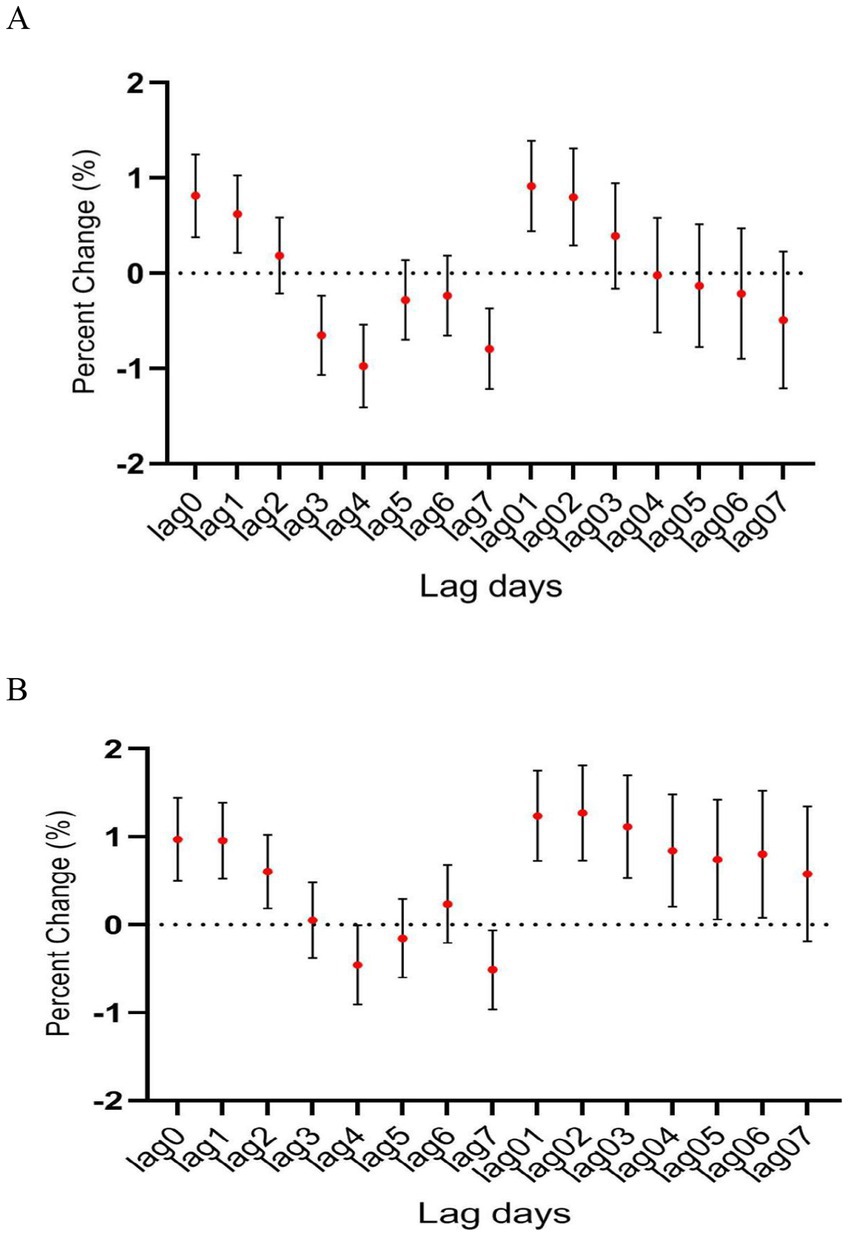
Figure 4. The relationship between atmospheric CO concentration and hospitalization for mental and behavioral disorders of patients aged (A) <45 years and (B) ≥ 45 years.
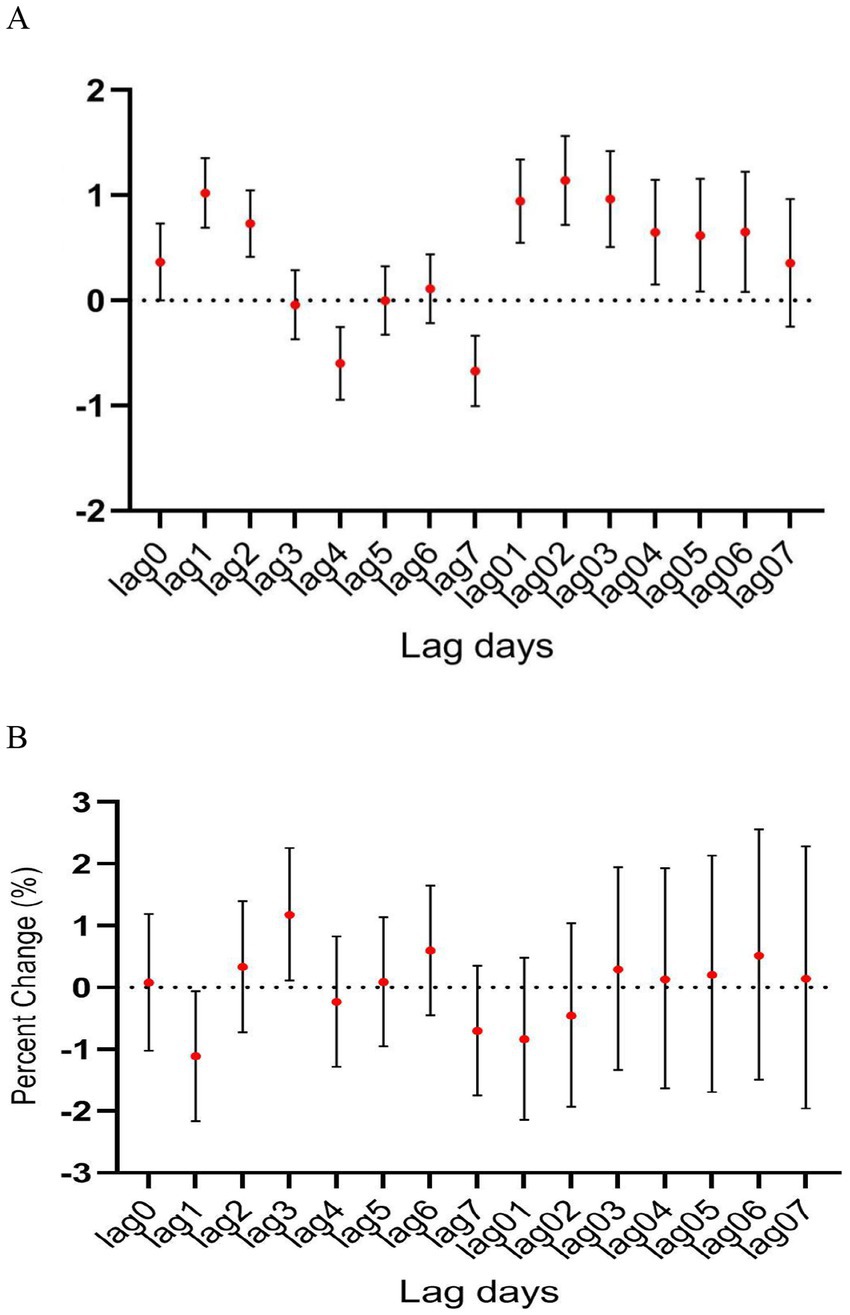
Figure 5. The relationship between atmospheric CO concentration and hospitalization for mental and behavioral disorders in (A) warm and (B) cold seasons.
Blood MAPK3 mRNA in patients with depression
Blood levels of MAPK3 mRNA in patients with depression (i.e., Case) were found significantly elevated by nearly 2 folds compared to those in healthy individuals (i.e., Control) (p < 0.05) (Figure 6). These results indicated the functional role of MAPK3 in the pathogenesis of depression, which would be validated in mouse model of CO-induced depression.
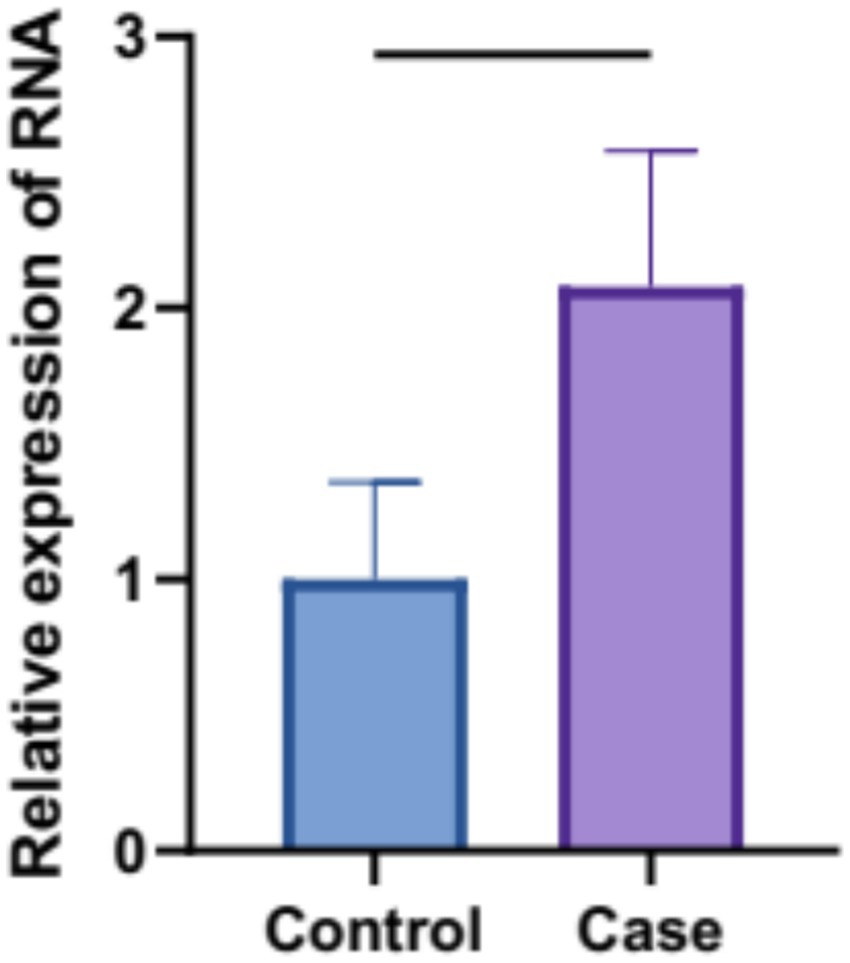
Figure 6. Blood MAPK3 transcript levels in patients with depression were determined using real-time PCR (p < 0.05).
Effect of MAPK3 inhibition on mouse model of CO-induced depression
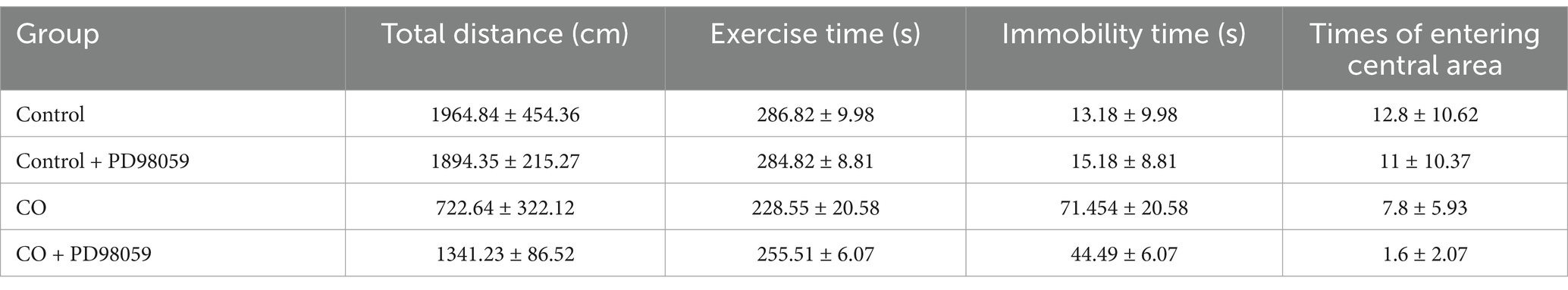
In the sucrose preference test, the CO group mice showed a significant reduction in the percentage of sucrose preference compared to the Control group (p < 0.0001). However, these effects were largely reversed after PD98059 injection, with a significant increase in the sucrose preference percentage observed relative to the CO group. The Control + PD98059 group did not show a substantial difference from the levels observed in the Control group, with no significant statistical differences (Table 3).
In open field test, 24 h after PD98059 and saline injection, the total distance moved by the CO-exposed group of mice was significantly reduced compared to the Control group (p < 0.0001), indicating that CO exposure negatively impacted the mice’s motor ability or exploratory behavior. Notably, the total distance moved by the CO + PD98059 group significantly increased compared to the CO group (p < 0.05). In terms of exercise time, the CO group showed a significant reduction compared to the Control group, while the time spent immobile correspondingly increased, reflecting increased anxiety behaviors in the CO-exposed mice. The exercise time of the CO + PD98059 group showed recovery, with reduced immobility time, indicating that PD98059 injection effectively alleviated anxiety behaviors induced by CO exposure (p < 0.05). The number of times the CO group mice entered the central area (7.8 ± 5.93 times) was significantly less than that of the Control group (12.8 ± 10.62 times, p < 0.0001), suggesting that CO exposure reduced exploration behavior, possibly accompanied by increased anxiety-like symptoms. There was no significant difference in the number of entries into the central area between the Control + PD98059 group and the Control group (11 ± 10.37 times), indicating that PD98059 alone did not affect the exploratory behavior of the mice. The CO + PD98059 group entered the central area (1.6 ± 2.07 times), which was significantly increased compared to the CO group (p < 0.05) (Table 4; Figure 7).
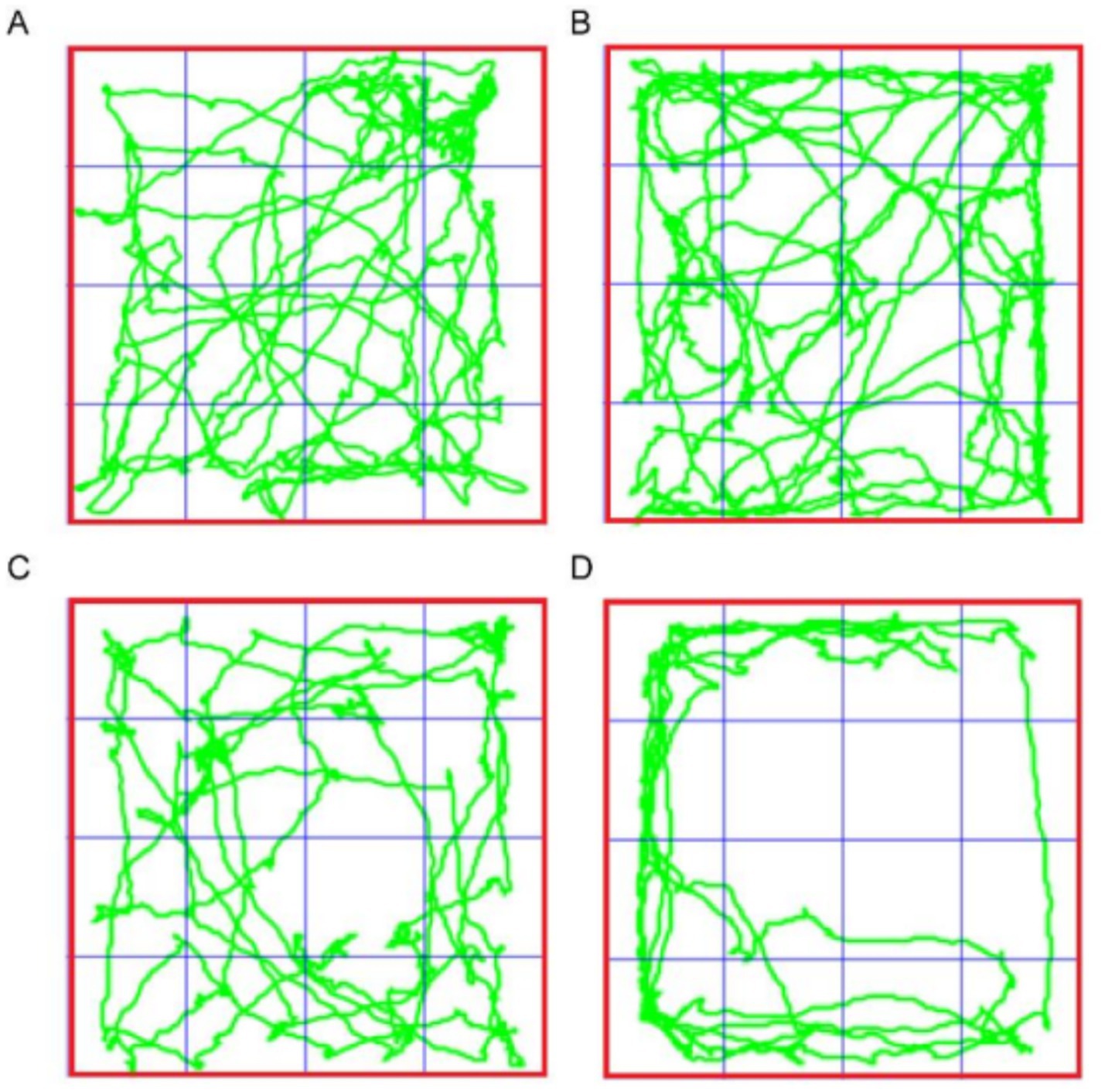
Figure 7. Open field test for mice of Control (A), Control + PD98059 (B), CO + PD98059 (C) and CO (D).
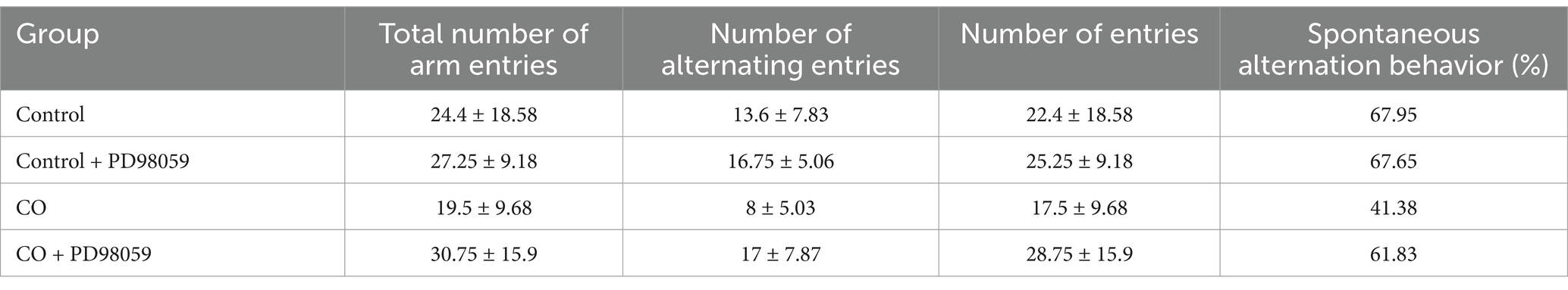
In the Y-maze test, the CO-exposed group of mice showed significant differences compared to the Control group, and the treatment with PD98059 affected these differences (Figure 8). The Control group and the Control + PD98059 group had higher scores for spontaneous alternation behavior, indicating good memory capability. The scores of the CO-exposed group significantly decreased (p < 0.01), while the scores of the CO + PD98059 group improved, suggesting that PD98059 has a certain ameliorative effect on CO-induced brain damage.
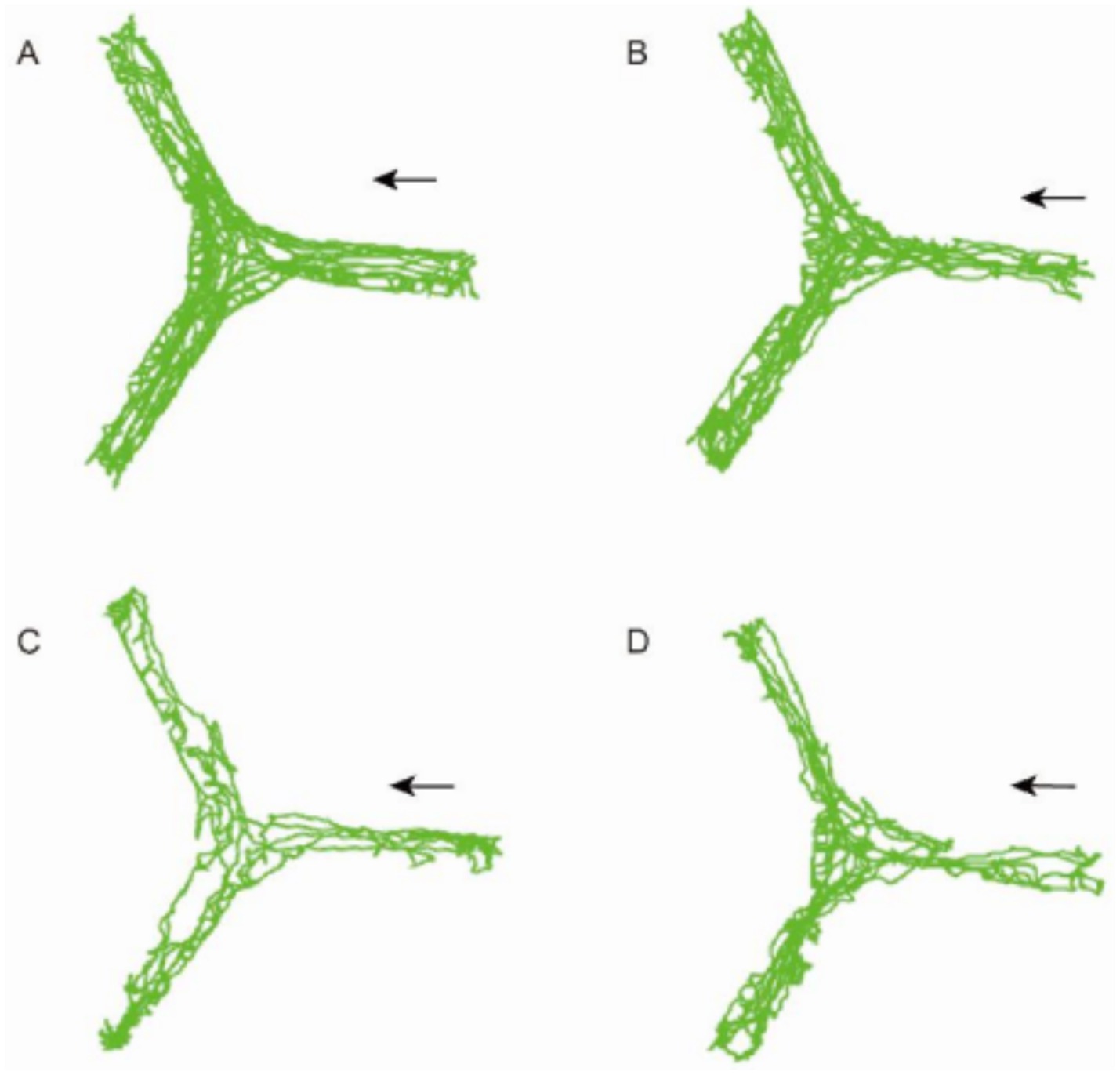
Figure 8. Y-maze field test for mice of Control (A), Control + PD98059 (B), CO + PD98059 (C) and CO (D). The arrow indicates the direction in which the mouse is placed.
The results of the Y-maze experiment indicate that CO exposure negatively impacts the spatial working memory of mice, while the treatment with PD98059 can partially reverse these effects. PD98059 may improve CO-induced cognitive dysfunction by inhibiting the MAPK3 signaling pathway (Table 5).
Effect of MAPK3 inhibition on mouse model of CO-induced depression
Whether the inhibition of MAPK3 would modulate signaling was studied by determining the protein levels of BDNF and MAPK by western blotting (Figure 9). The expression levels of BDNF protein in the Control and Control + PD98059 groups were similar, both maintained at high levels. However, in the CO group, BDNF expression significantly decreased (p < 0.01), suggesting that CO exposure may inhibit BDNF expression. The expression level of BDNF in the CO + PD98059 group was significantly higher than that in the CO-exposed group (p < 0.01), although it remained lower than that in the Control group. The expression levels of MAPK3 protein in the Control and Control + PD98059 groups were relatively stable. In contrast, CO exposure significantly increased the expression level of MAPK3 (p < 0.001). In the CO + PD98059 treatment group, the expression level of MAPK3 was significantly lower than that in the CO-exposed group (p < 0.05), approaching the levels of the Control group.
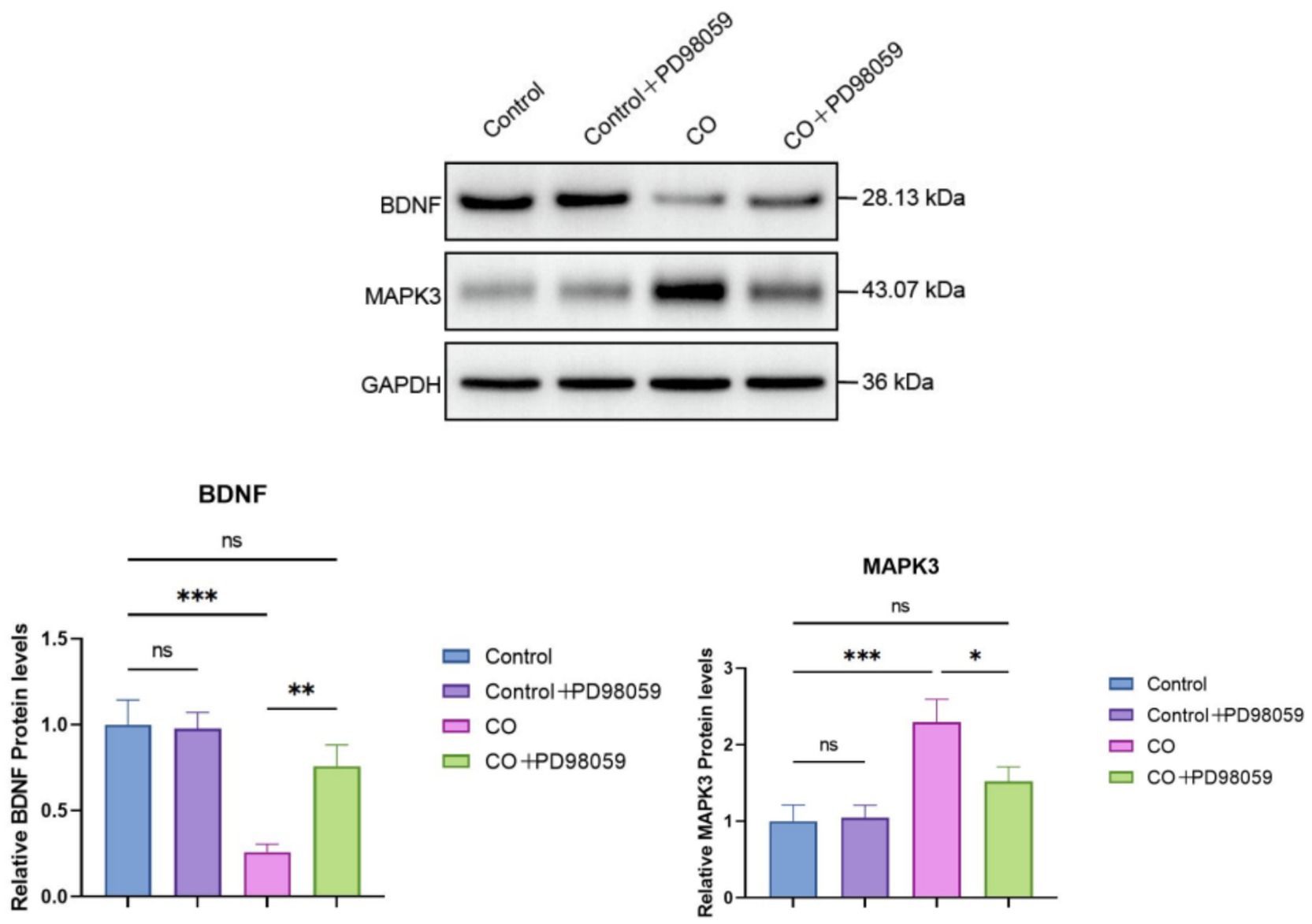
Figure 9. Protein levels of BDNF and MAPK3 in brain tissues of model mice were determined using western blotting. The band intensity was quantified and compared between treatment groups. *p < 0.05; **p < 0.01; ***p < 0.001; and ns, no significance.
Discussion
The present study is one of the few to investigate the relationship between atmospheric pollutant CO and psychiatric hospitalization rates in developing countries. Based on analysis on the daily data from a major hospital in Shijiazhuang from 2014 to 2020, the results of this longitudinal study showed that short-time exposure to CO pollution would be significantly associated with an increased risk of hospitalization for mental and behavioral disorders. The effect of CO was found to be the most substantial on day lag02, and of importance, this association remained robust even after adjusting for other gaseous pollutants in the two-pollutant model. The transcript levels of MAPK3 in the blood samples of patients with depressive disorder were also found significantly elevated compared to healthy individuals. Inhibition of MAPK3 could alleviate the adverse effect of CO-induced depressive disorder in mice, strengthening the notion that MAPK3 and its associated signaling would be essential to mental disorders associated with CO pollution.
It is generally believed that particulate pollutants may cause various health problems, especially affecting respiratory and circulatory systems (Kan et al., 2008). In recent years, atmospheric particulate matter has also been considered as an important but unquantified risk factor affecting the mental system. There are indeed a few studies indicating the adverse effects of atmospheric pollutants (i.e., PM2.5, PM10, NO, etc.) on mental health (Calderón-Garcidueñas et al., 2015; Fan et al., 2020; Gu et al., 2020). There are few studies on its effect on mental health, despite environmental CO having been shown to have adverse effects on the circulatory and respiratory system (Liu et al., 2018; Orazzo et al., 2009). This study identified a significant association between CO and hospitalization for mental and behavioral disorders, of which the findings were in accordance with previous studies. A longitudinal study conducted in Shanghai indicated that the rate of hospitalization for mental disorders would be significantly associated with air pollutants (i.e., PM10, sulfur dioxide and CO) (Chen et al., 2018). It was also shown that increased ambient CO, nitrogen dioxide, and PM10 levels were associated with higher odds of depression among more than 120,000 Koreans (Shin et al., 2018).
Previous studies have shown that the elderly would be more susceptible to the effects of air pollution than young people (Song et al., 2018; Tong et al., 2016), which is basically consistent with the current findings, in which those aged more than 45 years were found more sensitive to the effects of CO. In terms of gender, this study showed that males would be more susceptible to the effects of air pollutants than females. The underlying mechanism has yet to be studied. An animal study indicated that the gender difference in neurotoxicity would be attributed to the differential expression of paraoxonase 2 between male and female (Costa et al., 2014). This study also found that the association between CO and hospitalization was stronger in warm than in cold seasons, which is consistent with a Canadian study (Szyszkowicz et al., 2009). It was believed that the strong association during warmer seasons might be due to greater outdoor activity so individuals would expose more to ambient air pollutants through ventilation (Zeger et al., 2000). There are also animal studies showing that the toxicity of air pollutants may increase under conditions of high temperature (Gordon et al., 2011). Additionally, heat stress has been found to promote neuroinflammation in mice, implicating high temperature may synergize with pollutants to promote neurodegeneration and subsequent sequelae of affective disorders (Lee et al., 2015). Interestingly, for other types of air pollutants, different seasonal patterns were shown. For example, Tong et al. found that the association between sulfur dioxide and nitrogen dioxide and psychiatric morbidity was stronger in cold season than in warm season in Tianjin (Tong et al., 2016). Lin et al. shown that there would be a close association between suicide rate and air pollution in cool season in Guangzhou (Lin et al., 2016). The seasonal pattern of the effect of air pollutants on mental health warrants further investigation, however, it is widely accepted that the differential patterns between pollutants would be due to complex factors such climatic conditions and exposure patterns in different geographic regions.
A complete picture about how air pollution would cause mental disorders has remained to be fully deciphered, however, studies have shown that air pollutants may alter normal brain functions and result in inflammatory responses and even pathological changes in brain tissues (Xu et al., 2016). The contribution of genetic and environmental factors to the development of mental disorders through activation of oxidative stress and inflammatory responses could be substantial. For example, an animal study revealed that exposure of the animals to particulate matters for 4 weeks could trigger depression-like response through upregulation of key neuroinflammatory cytokines like tumor necrosis factor-alpha and interleukin-1 beta (Hogan et al., 2015). Oxidative stress has been considered as one of the major pathways by which air pollutants cause damage to the cardiovascular and respiratory systems (Kelly, 2003). It is also evident that inhaled air pollutants can cause oxidative stress and system inflammation (Risom et al., 2005; Araujo, 2011), which would be able to affect psychological status (Ng et al., 2008; Hurley and Tizabi, 2013). The pathogenesis can be attributed to the damage of dopaminergic neurons by oxidative stress, which in turn depletes dopamine in the central nervous system, eventually resulting in depression and other mental disorders (Hasler, 2010; Block et al., 2004). Short-term exposure to CO pollution correlated with emergency department visits for mental disorders and related violent behavior (Tillakaratne et al., 2020), of which the underlying mechanism would also be due to the oxidative stress induced by CO.
The present study provided certain insights into how atmospheric CO level would affect hospitalization for mental disorders, however, since all CO measurements were done at fixed locations rather than individual measurements, the measurement of atmospheric CO level may have inevitable errors due to the fact that individual-level exposure can vary significantly based on factors such as time spent outdoors and personal activities. Besides, since data on more specific subtypes of mental disorders were not available for analysis, a comprehensive analysis on the CO-hospitalization relationship of the disorder subtypes was not possible. Analysis of short-term associations between specific air pollution and emergency room visits remains to be done as well. Due to her high level of urbanization, Shijiazhuang represents a model city for investigating how air pollution would affect mental health in China. Nevertheless, the findings of the present study require validations in other Chinese cities’ data, in which the repeated or planned hospitalization cases which are not inflicted with air pollution will be excluded. Other potential confounding variables like baseline health conditions, socioeconomic factors, and accessibility to healthcare service providers will also be studied.
This study also presented data to further support the important functional role of MAPK3 and its signaling pathway in CO-induced depression and mental disorders. MAPK3 transcript was found elevated in blood samples of patients with depression, while suppressing MAPK3 activity with an inhibitor could alleviate adverse effects in mouse model of CO-induced depression. These results might provide insights into the further development of MAPK3 inhibitors as potential therapeutics for treating CO-induced mental disorders in patients, although it is generally accepted that the CO exposure levels and conditions in the mouse model were highly controlled and may not reflect real-world human exposure.
This longitudinal study has demonstrated that short-term exposure to atmospheric CO would be associated with an increase in the number of hospitalizations for mental illness in Shijiazhuang city. The impact of CO was more obviously seen in the elderly, highlighting the urgent need to control CO emission to the atmosphere air pollution especially in China where the elderly population is growing rapidly. This study has also added to growing evidence for the adverse effects of air pollution on mental health and may help local policymakers develop and refine air pollution control measures, simultaneously, providing epidemiological perspectives for the future delineation of the causal relationship between particulate matter pollution and mental and behavioral disorders.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by First Hospital of Hebei Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the review of the patient data was retrospective and anonymous. The animal study was approved by First Hospital of Hebei Medical University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
PH: Conceptualization, Writing – original draft, Writing – review & editing. WL: Data curation, Writing – original draft, Writing – review & editing. XG: Data curation, Writing – original draft, Writing – review & editing. YL: Formal analysis, Writing – original draft, Writing – review & editing. YY: Formal analysis, Writing – original draft, Writing – review & editing. WY: Data curation, Writing – original draft, Writing – review & editing. LD: Methodology, Writing – original draft, Writing – review & editing. RR: Data curation, Investigation, Writing – original draft, Writing – review & editing. XW: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by Project of Clinical Medical Research Center for Psychiatric and Psychological Disorders of Hebei Province (No. 199776245D) and Provincial Science and Technology Program of Hebei Province (No. 21377711D).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CO, Carbon monoxide; MT, mean temperature; RH, relative humidity; GAM, generalized additive model; df, degrees of freedom; CI, confidence intervals.
References
Araujo, J. A. (2011). Particulate air pollution, systemic oxidative stress, inflammation, and atherosclerosis. Air Qual. Atmos. Health 4, 79–93. doi: 10.1007/s11869-010-0101-8
Bakian, A. V., Huber, R. S., and Coon, H. (2015). Acute air pollution exposure and risk of suicide completion. Am. J. Epidemiol. 181, 295–303. doi: 10.1093/aje/kwu341
Block, M. L., Wu, X., and Pei, Z. (2004). Nanometer size diesel exhaust particles are selectively toxic to dopaminergic neurons: the role of microglia, phagocytosis, and NADPH oxidase. FASEB J. 18, 1618–1620. doi: 10.1096/fj.04-1945fje
Calderón-Garcidueñas, L., Calderón-Garcidueñas, A., and Torres-Jardón, R. (2015). Air pollution and your brain: what do you need to know right now. Prim. Health Care Res. Dev. 16, 329–345. doi: 10.1017/S146342361400036X
Catapano, P., Luciano, M., Cipolla, S., D'Amico, D., Cirino, A., Della Corte, M. C., et al. (2024). What is the relationship between exposure to environmental pollutants and severe mental disorders? A systemic review on shared biological pathways. Brain Behav. Immun. Health 43:100922. doi: 10.1016/j.bbih.2024.100922
Chen, C., Liu, C., and Chen, R. (2018). Ambient air pollution and daily hospital admissions for mental disorders in Shanghai, China. Sci. Total Environ. 613-614, 324–330. doi: 10.1016/j.scitotenv.2017.09.098
Costa, L. G., Cole, T. B., and Coburn, J. (2014). Neurotoxicants are in the air: convergence of human, animal, and in vitro studies on the effects of air pollution on the brain. Biomed. Res. Int. 2014, 1–8. doi: 10.1155/2014/736385
Fan, S. J., Heinrich, J., and Bloom, M. S. (2020). Ambient air pollution and depression: a systematic review with meta-analysis up to 2019. Sci. Total Environ. 701:134721. doi: 10.1016/j.scitotenv.2019.134721
Gordon, C. J., Johnstone, A. F. M., and Aydin, C. (2011). Thermal stress and toxicity. Compr. Physiol. 4, 995–1016. doi: 10.1002/cphy.c130046
Gu, H., Yan, W., and Elahi, E. (2020). Air pollution risks human mental health: an implication of two-stages least squares estimation of interaction effects. Environ. Sci. Pollut. Res. 27, 2036–2043. doi: 10.1007/s11356-019-06612-x
Guan, D. F., Ren, P. Y., and Hu, W. (2015). The mGluR5 positive allosteric modulator CDPPB inhibits SO2-induced protein radical formation and mitochondrial dysfunction through activation of Akt in mouse hippocampal HT22 cells. Cell. Mol. Neurobiol. 35, 573–583. doi: 10.1007/s10571-014-0153-7
Guo, X. J., Huang, L. Y., Gong, S. T., Li, M., Wang, W., Chen, J., et al. (2024). Peroxynitrite-triggered carbon monoxide donor improves ishemic stroke outcome by inhibiting neuronal apoptosis and ferroptosis. Mol. Neurobiol. 61, 10629–10644. doi: 10.1007/s12035-024-04238-w
Hanafy, K. A., Oh, J., and Otterbein, L. E. (2013). Carbon monoxide and the brain: time to rethink the dogma. Curr. Pharm. Des. 19, 2771–2775. doi: 10.2174/1381612811319150013
Hasler, G. (2010). Pathophysiology of depression: do we have any solid evidence of interest to clinicians? World Psychiatry 9, 155–161. doi: 10.1002/j.2051-5545.2010.tb00298.x
Ho, R. C. M., Cheng, W., Chua, A. N. C., and Mak, A. (2012). Neuropsychiatric aspects of carbon monoxide poisoning: diagnosis and management. Adv. Psychiatr. Treat. 18, 94–101. doi: 10.1192/apt.bp.110.008441
Hogan, M. K., Kovalycsik, T., and Sun, Q. (2015). Combined effects of exposure to dim light at night and fine particulate matter on C3H/HeNHsd mice. Behav. Brain Res. 294, 81–88. doi: 10.1016/j.bbr.2015.07.033
Hurley, L. L., and Tizabi, Y. (2013). Neuroinflammation, neurodegeneration, and depression. Neurotox. Res. 23, 131–144. doi: 10.1007/s12640-012-9348-1
Ijomone, O. M., Iroegbu, J. D., Aschner, M., and Bornhorst, J. (2021). Impact of environmental toxicants on p38- and ERK-MAPK signaling pathways in the central nervous system. Neurotoxicology 86, 166–171. doi: 10.1016/j.neuro.2021.08.005
Kan, H., London, S. J., and Chen, G. (2008). Season, sex, age, and education as modifiers of the effects of outdoor air pollution on daily mortality in Shanghai, China: the public health and air pollution in Asia (PAPA) study. Environ. Health Perspect. 116, 1183–1188. doi: 10.1289/ehp.10851
Kelly, F. J. (2003). Oxidative stress: its role in air pollution and adverse health effects. Occup. Environ. Med. 60, 612–616. doi: 10.1136/oem.60.8.612
Kochi, C., Ahmad, S., and Salim, S. (2023). The effects of air pollution toxicants on the mitochondria. Mitochond. Intox., 147–166. doi: 10.1016/B978-0-323-88462-4.00004-3
Kochi, C., Salvi, A., Atrooz, F., and Salim, S. (2021). Simulated vehicle exhaust exposure induces sex-dependent behavioral deficits in rats. Environ. Toxicol. Pharmacol. 86:103660. doi: 10.1016/j.etap.2021.103660
Lee, W., Moon, M., and Kim, H. G. (2015). Heat stress-induced memory impairment is associated with neuroinflammation in mice. J. Neuroinflammation 12, 1–13. doi: 10.1186/s12974-015-0324-6
Lin, G. Z., Li, L., and Song, Y. F. (2016). The impact of ambient air pollution on suicide mortality: a case-crossover study in Guangzhou, China. Environ. Health 15, 1–8. doi: 10.1186/s12940-016-0177-1
Lin, Y., Zhou, L., and Xu, J. (2017). The impacts of air pollution on maternal stress during pregnancy. Sci. Rep. 7:40956. doi: 10.1038/srep40956
Liu, C., Yin, P., and Chen, R. (2018). Ambient carbon monoxide and cardiovascular mortality: a nationwide time-series analysis in 272 cities in China. Lancet Planet. Health 2, e12–e18. doi: 10.1016/S2542-5196(17)30181-X
Luo, Y., Ullah, R., Wang, J., Du, Y., Huang, S., Meng, L., et al. (2021). Exogenous carbon monoxide produces rapid antidepressant- and anxiolytic like effects. Fronit. Pharmacol. 12:757417. doi: 10.3389/fphar.2021.757417
Mahan, V. L. (2012). Neuroprotective, neurotherapeutic, and neurometabolic effects of carbon monoxide. Med. Gas Res. 2, 32–39. doi: 10.1186/2045-9912-2-32
Ng, F., Berk, M., and Dean, O. (2008). Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int. J. Neuropsychopharmacol. 11, 851–876. doi: 10.1017/S1461145707008401
Orazzo, F., Nespoli, L., and Ito, K. (2009). Air pollution, aeroallergens, and emergency room visits for acute respiratory diseases and gastroenteric disorders among young children in six Italian cities. Environ. Health Perspect. 117, 1780–1785. doi: 10.1289/ehp.0900599
Risom, L., Møller, P., and Loft, S. (2005). Oxidative stress-induced DNA damage by particulate air pollution. Mutat. Res. 592, 119–137. doi: 10.1016/j.mrfmmm.2005.06.012
Shin, J., Park, J. Y., and Choi, J. (2018). Long-term exposure to ambient air pollutants and mental health status: a nationwide population-based cross-sectional study. PLoS One 13:e0195607. doi: 10.1371/journal.pone.0195607
Song, J., Zheng, L., and Lu, M. (2018). Acute effects of ambient particulate matter pollution on hospital admissions for mental and behavioral disorders: a time-series study in Shijiazhuang, China. Sci. Total Environ. 636, 205–211. doi: 10.1016/j.scitotenv.2018.04.187
Szyszkowicz, M., Kousha, T., and Kingsbury, M. (2016). Air pollution and emergency department visits for depression: a multicity case-crossover study. Environm. Health Insights 10:EHI.S40493. doi: 10.4137/EHI.S40493
Szyszkowicz, M., Rowe, B. H., and Colman, I. (2009). Air pollution and daily emergency department visits for depression. Int. J. Occup. Med. Environ. Health 22, 355–362. doi: 10.2478/v10001-009-0031-6
Thomson, E. M., Filiatreault, A., and Guenette, J. (2019). Stress hormones as potential mediators of air pollutant effects on the brain: rapid induction of glucocorticoid-responsive genes. Environ. Res. 178:108717. doi: 10.1016/j.envres.2019.108717
Tillakaratne, R. A., Malig, B. J., and Basu, R. (2020). Examining the relationship between ambient carbon monoxide, nitrogen dioxide, and mental health-related emergency department visits in California, USA. Sci. Total Environ. 746:140915. doi: 10.1016/j.scitotenv.2020.140915
Tong, L., Li, K., and Zhou, Q. (2016). Season, sex and age as modifiers in the association of psychosis morbidity with air pollutants: a rising problem in a Chinese metropolis. Sci. Total Environ. 541, 928–933. doi: 10.1016/j.scitotenv.2015.09.066
Volk, H. E., Lurmann, F., and Penfold, B. (2013). Traffic-related air pollution, particulate matter, and autism. JAMA Psychiatry 70, 71–77. doi: 10.1001/jamapsychiatry.2013.266
Wang, F., Liu, H., and Li, H. (2018). Ambient concentrations of particulate matter and hospitalization for depression in 26 Chinese cities: a case-crossover study. Environ. Int. 114, 115–122. doi: 10.1016/j.envint.2018.02.012
Watt, S., Prado, C. E., and Crowe, S. F. (2018). Immediate and delayed neuropsychological effects of carbon monoxide poisoning: a meta-analysis. J. Int. Neuropsychol. Soc. 24, 405–415. doi: 10.1017/S1355617717001035
Xu, C., Fan, Y. N., and Liang, Z. (2020). Unexpected association between increased levels of ambient carbon monoxide and reduced daily outpatient visits for vaginitis: a hospital-based study. Sci. Total Environ. 723:137923. doi: 10.1016/j.scitotenv.2020.137923
Xu, X., Ha, S. U., and Basnet, R. (2016). A review of epidemiological research on adverse neurological effects of exposure to ambient air pollution. Front. Public Health 4:157. doi: 10.3389/fpubh.2016.00157
Keywords: carbon monoxide, air pollution, mental health, hospitalization, MAPK3, behavioral disorders, epidemiology
Citation: Hu P, Lu W, Gao X, Li Y, Yang Y, Yin W, Dong L, Ren R and Wang X (2025) Atmospheric carbon monoxide and hospitalization for mental and behavioral disorders: insights from a longitudinal study in Shijiazhuang. Front. Psychol. 16:1573556. doi: 10.3389/fpsyg.2025.1573556
Edited by:
Giulia Menculini, University of Perugia, ItalyReviewed by:
Matteo Di Vincenzo, University of Campania Luigi Vanvitelli, ItalyPierluigi Catapano, University of Campania Luigi Vanvitelli, Italy
Giulio Longo, Marche Polytechnic University, Italy
Copyright © 2025 Hu, Lu, Gao, Li, Yang, Yin, Dong, Ren and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xueyi Wang, MDcwQGhlYm11LmVkdS5jbg==
 Peihua Hu1
Peihua Hu1 Xueyi Wang
Xueyi Wang