Abstract
Biological motion (BM), the movement generated by living entities, transmits signals of life and conveys vital cues for animacy perception. In this review, we synthesize empirical findings from human and non-human animal studies to reveal how BM enjoys a unique position in visual perception as an animate motion and how it elicits animacy perception. Compared to non-biological and inanimate motions, BM engages specialized perceptual processing mechanisms and a dedicated cortical–subcortical network. Local motion cues, especially the foot movements of terrestrial animals, are pivotal in driving such specificity, and emerging evidence supports the existence of an innate, evolutionarily conserved “Life Detector” or “Step Detector” tuned to such information in the human and other vertebrate brains. The direct perception of animacy from BM relies on the processing of low-level kinematic features and mid-level motion features embedded in both intrinsic joint movements and extrinsic body motion. While ecological constraints and implied internal energy sources may serve as generic factors affecting animacy perception from visual motion, how precise BM features (both in intrinsic and extrinsic movements) combine to influence animacy percepts and the neural implementation remain largely unexplored. Addressing these gaps will help establish a framework for understanding BM through the lens of animate motion processing. This approach will offer deeper insights into how the life detection system hardwired in the vertebrate brain distinguishes animate from inanimate motion, further uncovering its broader cognitive and evolutionary implications.
1 Introduction
Biological motion (BM)—the movement produced by humans or other living creatures—conveys prominent visual cues that elicit a perception of life (Giese and Poggio, 2003; Pavlova, 2012). Compared with static properties of living beings (e.g., forms or surface textures of faces and bodies), BM is more powerful and robust in signaling animacy. It enables rapid detection and efficient perception of animate beings, even under conditions of blurred vision, small object sizes, or great distances (Borst and Egelhaaf, 1989). This ability is essential for survival and reproduction in complex natural environments (Wheatley et al., 2007) and may serve as a gateway to higher-level cognitive processes, such as action understanding and social interaction (Thurman and Lu, 2014; Zanon et al., 2024).
Over the past half-century, numerous studies have revealed the remarkable ability of BM perception in humans (Blake and Shiffrar, 2007), as well as in many other species (Cheng et al., 2025; De Agrò et al., 2021; Jastorff et al., 2012; Larsch and Baier, 2018; Lu et al., 2024; Ma et al., 2022; Nakayasu and Watanabe, 2014; Vallortigara et al., 2005). Human observers could immediately recognize BM from sparse point-light displays that portray only the movement of major body joints (Johansson, 1973). This capacity is inheritable (Wang Y. et al., 2014; Wang et al., 2018), and a visual preference for BM emerges early in life in human infants and visually inexperienced chicks (Bardi et al., 2014, 2011; Simion et al., 2008; Vallortigara et al., 2005). These findings suggest the existence of innate mechanisms, potentially conserved across vertebrates, underlying their superior sensitivity to life motion signals (Lu et al., 2024; Troje and Chang, 2023; Troje and Westhoff, 2006).
While existing theoretical accounts imply that BM serves as a signal of life that is inherently associated with animacy perception (Chang and Troje, 2008; Johnson, 2006; Troje and Westhoff, 2006), surprisingly few studies have directly explored the mechanisms for the perception of animacy from BM. Animacy perception refers to perceiving an entity as alive or possessing a lifelike quality (Chang and Troje, 2008; Scholl and Tremoulet, 2000; Tremoulet and Feldman, 2000). In the literature, it has been discussed across various contexts, engaging multiple mechanisms at perceptual and cognitive levels (Huang et al., 2023; Rosa Salva et al., 2015). Some research concerns the fast, automatic, and irresistible detection and perception of animate properties from static or motion stimuli (e.g., faces, BM, self-propelled motion) based on visual analysis (Chang and Troje, 2008; Koldewyn et al., 2014; Stewart, 1982). Other studies go further and explore how to infer an entity’s goals and even “mind” based on its interaction with others or the environment (Gao et al., 2010; Heider and Simmel, 1944; Scholl and Tremoulet, 2000), eliciting an impression of an intentional agent (i.e., autonomous and volitional control of action) beyond mere animacy (i.e., being alive, lifelikeness). Here, we focus on animacy perception elicited by non-interactive BM (Figure 1), approaching it primarily from the perspective of visual processing rather than the higher-level mentalizing or social causality comprehension processes. Investigating this issue will expand our understanding of how the human brain processes BM as a signal of life and achieves animacy perception.
Figure 1
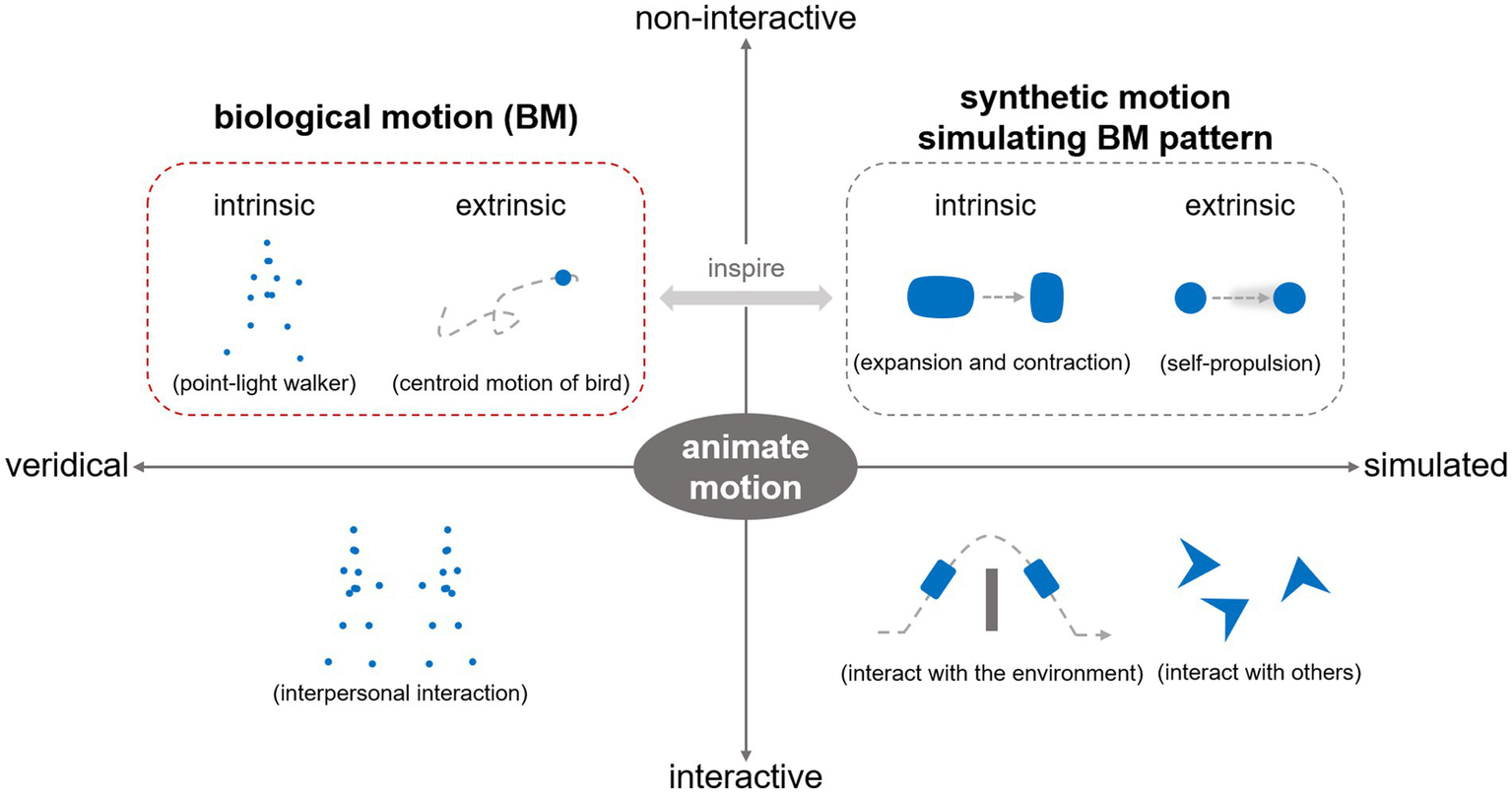
A conceptual framework for decomposing and investigating animate motion. In the literature, the term “animate motion” generally refers to motion that elicits animacy perception. It can be categorized along two dimensions. First, based on engagement with others or the environment, it can be classified as interactive or non-interactive motion. These motion stimuli elicit animate perception via different mechanisms, either through social cognitive inferences or visual feature analysis. Second, based on the motion-generating entity, animate motion can be distinguished as veridical motion or simulated motion. Along this dimension, veridical BM represents the most essential form of animate motion in reality. It consists of intrinsic joint motion and extrinsic body motion components. The area outlined by the red dashed box (i.e., non-interactive BM) represents the primary focus of this review. Additionally, some studies outlined by the gray dashed box also provide insights for understanding animacy perception from BM patterns and are addressed in the third section of the review.
In this article, we propose a framework for understanding the neurocognitive architecture of BM perception from the perspective of animate motion processing. We consider BM as the most essential form of animate motion in reality, examining the perceptual and neural mechanisms that underlie (1) its prominent position in visual processing relative to inanimate or less animate motions and (2) its capability to elicit a sense of animacy. We will discuss these topics in the next two sections, while also considering their ontogenetic and phylogenetic origins based on behavioral genetic, early developmental, and cross-species evidence.
Notably, the term BM is sometimes interpreted as the experimental stimulus, namely, point-light displays depicting articulated joint movements. Whereas, this review adopts a broader, ecological perspective, investigating BM as a natural phenomenon—that is, motion produced by living organisms (Giese and Poggio, 2003; Pavlova, 2012). Based on its manifestation, BM can further be decomposed into two components (Figure 1)—intrinsic joint/limb motion (i.e., the relative movements of body parts in the object-based reference system, typically depicted by point-light displays) and extrinsic motion (i.e., movements of the whole body across space) (Thurman and Lu, 2014). Studies on veridical BM sometimes draw inspiration from and inspire those on simulated BM stimuli. Considering the existing research, the second section of this review primarily addresses visual BM processing based on intrinsic motion, and the third section explores animacy perception from BM based on both intrinsic and extrinsic motions.
2 Processing BM as animate motion
The significance of BM as a crucial animate motion may have driven the evolution of specialized mechanisms for its perception. In this section, we review empirical findings on the domain-specific perceptual processing mechanisms and neural foundations of BM, as compared to those for inanimate or less animate motions (e.g., random motion, rigid motion, inverted BM). Two fundamental visual cues, namely the local motion capturing the movements of critical joints and the global configuration representing the skeletal structure of bodies, may contribute to these distinctive behavioral and neural responses. We introduce evidence highlighting the crucial contribution of local motion, which leads to theoretical hypotheses about the existence of a cross-species life detection system tuned to local BM cues hardwired in the vertebrate brain.
2.1 The specificity of BM perception revealed by human and non-human animal studies
Taking advantage of point-light BM displays (Figure 2a), extensive research has revealed the distinctive mechanisms underlying BM perception. Compared with inanimate motions, people recognize BM with higher accuracy and faster speed (Dittrich, 1993). The temporal summation of BM signals occurs over a longer time than that for translational motion and varies with motion speed (Neri et al., 1998). The differences between processing BM and inanimate motion may be traced back to their genetic and evolutionary basis. Twin studies suggest that perceiving the facing direction or motion direction of BM is governed by genetic factors, while the perception of inanimate motions like sphere rotation is largely determined by the environment (Wang Y. et al., 2014; Wang et al., 2018). Furthermore, human newborns spontaneously prefer to look at BM compared to random or rigid motion (Bardi et al., 2011; Simion et al., 2008). This innate preference also extends to other species, including terrestrial vertebrates (e.g., visually inexperienced chick) (Miura and Matsushima, 2012; Vallortigara et al., 2005; Vallortigara and Regolin, 2006) and aquatic vertebrates (e.g., zebrafish) (Larsch and Baier, 2018; Nakayasu and Watanabe, 2014). These findings suggest the existence of a heritable, evolutionarily conserved mechanism selectively dedicated to BM processing.
Figure 2
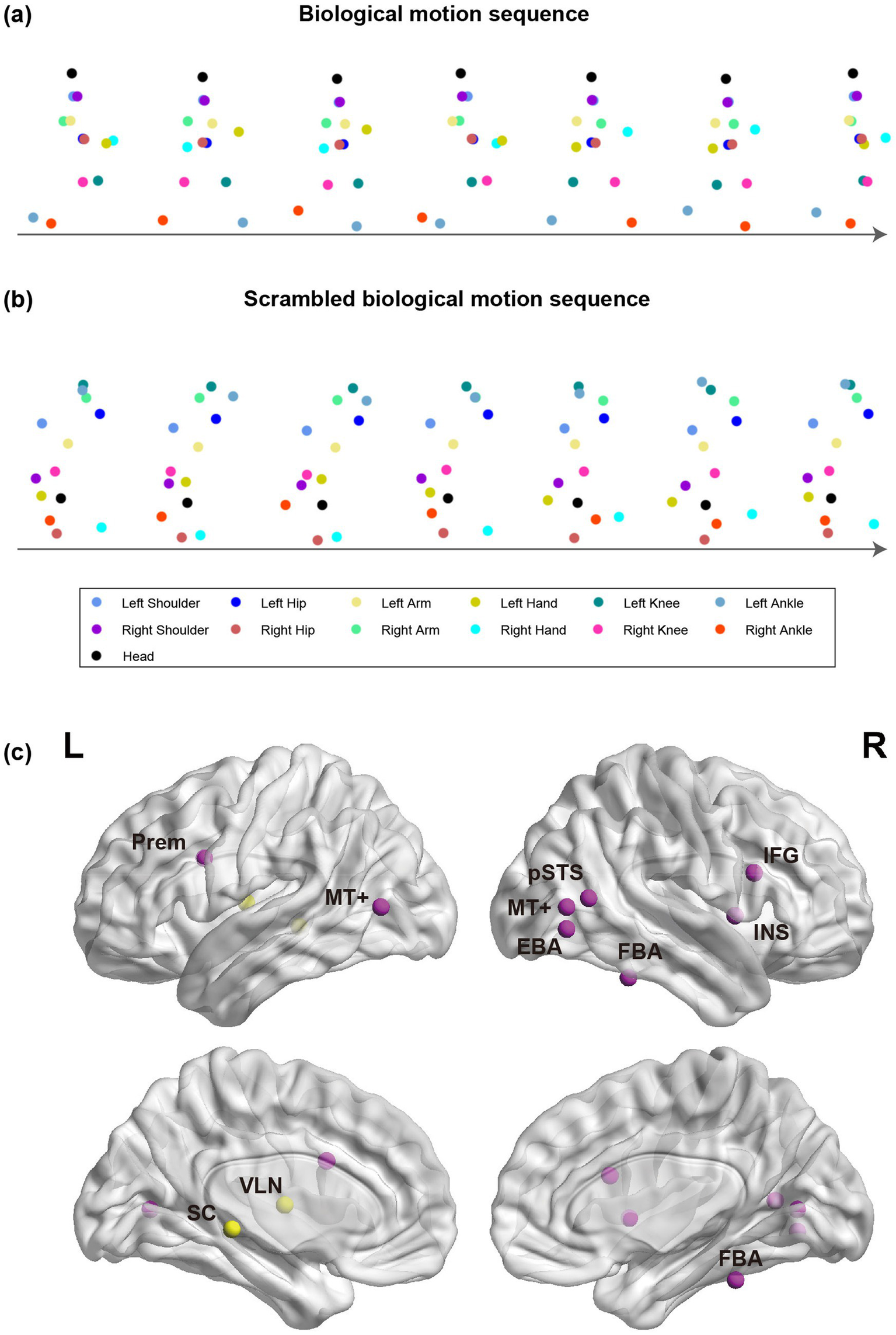
Illustrations of point-light BM sequences and the key brain regions involved in BM processing. (a,b) Intact and spatially scrambled BM sequences. (c) Cortical (purple) and subcortical (yellow) regions for BM processing in the human brain. SC, superior colliculus; VLN, ventral lateral nucleus; Prem, premotor; pSTS, posterior superior temporal sulcus; MT+, middle temporal complex; FBA, fusiform body area; EBA, extrastriate body area; IFG, inferior frontal gyrus; INS, insula.
In addition, the remarkable capability of BM perception is significantly impaired when the visual stimuli are inverted. Compared with inverted counterparts, people demonstrate greater sensitivity in detecting upright BM (Thomas and Shiffrar, 2010) and exhibit enhanced accuracy in discriminating its identity (Loula et al., 2005), walking direction (Troje and Westhoff, 2006), and audiovisual temporal relations (Saygin et al., 2008). Moreover, for both human infants and visually inexperienced chicks, there is an innate preference for upright BM over the inverted counterparts (Bardi et al., 2014; Vallortigara and Regolin, 2006). Since inversion disrupts gravity-compatible motion cues but not the low-level properties of upright BM, this cross-species disposition is considered a “gravity bias” that reflects the visual system’s selective tuning to life motion signals within the Earth’s gravitational field. This bias may, to some extent, contribute to the advantage of gravity-compatible BM in visual perception, presumably reflecting the adaptation of terrestrial life to Earth’s environment. Consistent with this assumption, a recent study has demonstrated that exposure to microgravity during spaceflight significantly reduced the inversion effect in BM perception, suggesting the significant role of the gravitational environment in shaping the visual sensitivity to BM (Wang et al., 2022).
2.2 The significance of local motion cues in life motion perception
BM perception relies on the processing of global configuration and local motion cues (Chang and Troje, 2009b). While early studies emphasized the significance of global configuration processing (Beintema and Lappe, 2002; Bertenthal and Pinto, 1994; Dittrich, 1993; Lange and Lappe, 2006; Reed et al., 2003; Shiffrar et al., 1997), later work by Troje and Westhoff proposed that local motion processing is another independent component of BM perception (Troje and Westhoff, 2006). Local motion cues can be extracted by spatially scrambling the point-light BM stimuli (Figure 2b), which disrupts the global configuration but preserves the movements of each joint. Troje and Westhoff found that humans can retrieve walking direction information from scrambled BM, whereas inverting the scrambled stimuli severely impaired visual perception, inducing an inversion effect comparable to that obtained with intact BM. Such an inversion effect is mainly driven by the motions of the feet, as inverting only the feet has a much stronger impact on walking direction discrimination compared to inverting all points except the feet (Troje and Westhoff, 2006). The following research suggests that local BM alone can modulate a variety of cognitive processes (e.g., visual attention, visual awareness, time perception, audiovisual integration) at levels comparable to those elicited by intact BM (Shen et al., 2023a; Shen et al., 2025a; Shi et al., 2010; Sun et al., 2022; Wang and Jiang, 2012; Wang L. et al., 2014). Moreover, both human newborns and newly hatched chicks exhibit comparable visual preferences toward intact BMs and scrambled ones (Bardi et al., 2011; Vallortigara et al., 2005), suggesting that the cross-species visual preference for BM may be primarily driven by local motion cues, even though the global configuration can modulate such effects (Bardi et al., 2014; Hirai et al., 2011). This is likely because local motion cues convey gravitational acceleration and exhibit semi-rigid characteristics, both of which have been implicated in eliciting spontaneous attention preferences (Bardi et al., 2011; Vallortigara and Regolin, 2006).
2.3 Theoretical accounts of life motion perception
The above findings have provided converging support for the “life detector” hypothesis originally proposed by Troje and Westhoff, which posits the existence of a specialized system in the human brain, and potentially in other vertebrates, that enables efficient detection of life motion signals based on distinctive local motion cues (Lemaire and Vallortigara, 2022; Troje and Chang, 2023; Troje and Westhoff, 2006). Recently, Hirai and Senju (2020) proposed a two-process theoretical model for the development of BM perception, parallel with the two-process model of face and gaze processing (Johnson et al., 2015; Senju and Johnson, 2009). According to this model, BM processing engages two systems: the Step Detector and the Bodily Action Evaluator. The Step Detector system quickly and coarsely processes the local feet motion and the feet-below-body information. It detects life motion signals generated by both conspecifics and other vertebrates and is largely innate. The Bodily Action Evaluator system slowly and precisely processes the global structure-from-motion information. It selectively responds to the motion from conspecifics and is mainly shaped by learning. The two-process model systematically develops and extends the earlier “Life Detector” hypothesis. The Step Detector system is generally compatible with the original Life Detector hypothesis but highlights the significance of feet-related motion and configurational information. The Bodily Action Evaluator engages in global configuration processing that was underrepresented in the Life Detector framework. The two-process model also characterizes the different developmental trajectories for the two systems, which are consistent with findings from a twin study that local and global BM perception are determined, respectively, by genetic and environmental factors (Wang et al., 2018). Based on the characteristics of the two systems, Hirai and Senju further hypothesized that the two systems involve distinct neural substrates, with the first system primarily relying on subcortical processing and the second system relying on cortical processing. In the next section, we will review the relevant evidence.
2.4 A cortical–subcortical network for BM processing
Previous reviews have provided comprehensive summaries of the human cortical network engaged in BM perception (Blake and Shiffrar, 2007; Giese and Poggio, 2003; Hirai and Senju, 2020). Figure 2c provides a brief overview of these cortical regions, including the superior temporal sulcus (STS), extrastriate body area (EBA), fusiform body area (FBA), middle temporal area (MT+), premotor cortex, inferior frontal gyrus (IFG), and insula. The STS is a core region that integrates form and motion cues to construct coherent representations of BM (Jastorff and Orban, 2009; Sokolov et al., 2018). Beyond human studies, a few non-human animal studies and cross-species studies offer insights into the evolution of this cortical network. By contrasting the neural response to BM versus that to static figures or non-BM, previous research has demonstrated STS activations in both monkeys and humans, regardless of whether the motion is generated by conspecifics or nonconspecifics, although with stronger responses to conspecific motions (Jastorff et al., 2012). By comparing the neural response to BM with that to inverted stimuli, a recent study found a selective response to conspecific motion in the human STS but not in the monkey STS, while the MT region in human as well as monkey brains responds to both conspecific and nonconspecific motions without conspecific preference (Cheng et al., 2025). Taken together, these findings indicate that upstream cortical regions (i.e., MT) may retain homologous functions across species (i.e., lack conspecific selectivity), and downstream cortical regions (i.e., STS) may have undergone differentiation and specialization throughout evolution.
Recent studies have also explored the subcortical processing of BM (Figure 2c). One study found that the ventral lateral nucleus (VLN) is involved in the processing of both global configuration and local motion cues of BM (Chang et al., 2018). Another recent study based on 3 T and 7 T functional magnetic resonance imaging (fMRI) found that the superior colliculus (SC), a subcortical structure involved in early visual processing, is activated more strongly by upright scrambled BM compared to the inverted counterpart (Lu et al., 2024). Moreover, it reveals a subcortical–cortical functional pathway from SC through the MT to the pSTS in the human brain. More potential subcortical–cortical connectivities remain to be examined in the future. The activation of SC is observed not only in humans but also in macaque monkeys, suggesting a cross-species mechanism in the primate SC that facilitates the detection of local BM at the early stage of the visual processing stream. The subcortical encoding of BM has also been observed in non-primates. The preoptic area and septum of newly hatched chicks are involved in discriminating BM from rigid motion (Lorenzi et al., 2024; Mayer et al., 2017). The visual input to the preoptic area in chicks is mainly provided by the optic tectum, an area homologous to the mammalian SC and responsible for the detection of animate cues, especially dynamic motion cues (Rosa Salva et al., 2015). These findings suggest that the processing of local motion cues may recruit early and primitive brain areas in different species (e.g., SC, preoptic area), providing supporting evidence for the existence of a “Step Detector” (Hirai and Senju, 2020) or a “life detector” (Troje and Westhoff, 2006) system in the evolutionarily ancient subcortical structures.
In addition to the cerebrum, several neuroimaging studies have reported cerebellum activation during BM processing (Sokolov et al., 2012, 2018), although one lesion study found that cerebellum dysfunction did not impair the detection of BM (Jokisch et al., 2005). Furthermore, BM perception may also rely on embodied motor simulation (Arrighi et al., 2011; Bosbach et al., 2006; Cavallo et al., 2012; Wilson and Knoblich, 2005). By modulating the availability of online stimulation, a recent study suggests that sensorimotor simulation at peripheral effectors plays an irreplaceable role in processing BM, especially for local motion cues (Shen et al., 2025a). The brain–body entanglement mechanism in BM perception may provide a certain physiological basis for the timely execution of fight-or-flight and other action responses.
3 Animacy perception from BM
Although BM is a vital signal for the existence of life, how it leads to animacy perception remains largely unknown. In the current section, we review empirical evidence on this issue. Compared to other attributes of BM, animacy is a more fundamental and vital dimension associated with life, given its invariance across different circumstances and its role as a prerequisite for conveying other attributes (e.g., emotion) unique to living entities. Note that the perception of animacy and other attributes from BM is not perceptually independent. Chang and Troje (2008) found a significant correlation between walking direction discrimination and animacy ratings for point-light walking motion, suggesting potentially shared visual mechanisms underlying the two processes, such as visual motion analysis. However, perceiving animacy from BM should not be conflated with the processing of walking directions, as these processes serve distinct perceptual and cognitive functions. Walking direction conveys others’ goals and interests in the surroundings. Thus, perceiving and discerning walking direction enables rapid access to the direction of others’ intentions and automatic orienting toward it through a specialized social attentional mechanism (Ji et al., 2020; Shi et al., 2010; Wang L. et al., 2014; Wang et al., 2020; Zhao et al., 2014). In comparison, animacy perception refers to perceiving an entity as alive or possessing agency (Scholl and Tremoulet, 2000; Vallortigara, 2012). It enables organisms to differentiate between animate and inanimate objects and develop appropriate adaptive responses.
As previously mentioned, this article focuses on animacy perception elicited by non-interactive BM, exploring the mechanism for perceiving animacy based on visual motion processing rather than social cognitive inferences. We will introduce key factors and motion features that influence animacy perception based on intrinsic and extrinsic motion patterns, respectively, and explore the potential neural substrates.
3.1 Perceiving animacy from the intrinsic joint movements of BM
Only a few studies explored how intrinsic joint movements of BM lead to animacy perception. Most of them utilized walking stimuli, one of the most prevalent types of locomotion for terrestrial vertebrates, based on rating tasks or comparing visual stimuli on the animacy dimension. By measuring the animacy perception induced by point-light sequences of a walking human, cat, and pigeon, Chang and Troje (2008) found that upright stimuli were rated more animate than inverted stimuli. Such inversion effect of animacy rating also existed in scrambled point-light BM sequences, in which the local trajectories are preserved while the global configurations are disrupted. It suggests that at least part of animacy information is carried by the local motion cues without relying on global configuration. In addition, since the inverted point-light displays alter only the motion in the vertical direction while preserving the horizontal component, the inversion effect may indicate the influence of gravity direction on the animacy judgment of local motion. Thurman and Lu (2013) further found that gravity direction and the congruency between intrinsic and extrinsic motion have an interactive influence on animacy percept. Animacy ratings only increased for spatially scrambled point-light walkers with upright orientation and congruent intrinsic-extrinsic motion direction, compared with inverted or incongruent conditions. Taken together, these findings underscore the importance of local BM with ecological constraints (both physical and biological) in perceiving animacy. Combined with prior evidence for the influence of the gravitational environment on BM processing (Wang et al., 2022), these findings suggest that the ecological environment, along with the concomitant constraints and expectations, may shape the perception of animate motion information.
3.2 Perceiving animacy from extrinsic motion patterns
In addition to the intrinsic joint movement of animate agents, extrinsic motion patterns also constitute an integral component of BM and embody vital life signatures. While most studies on BM perception adopted point-light displays that eliminate extrinsic body locomotion, some evidence suggests that the extrinsic motion of BM also modulates its perception and animacy rating (Masselink and Lappe, 2015; Thurman and Lu, 2013). Even without intrinsic relative motion, observers can easily discriminate animate motion from inanimate one in the real world based on their low-level kinematics and trajectories of extrinsic motion (Han et al., 2023). Given the complexity of real animate motion, some researchers used a single inanimate object without animate form or texture (e.g., a dot or a geometrical shape) to simulate extrinsic motions, allowing the isolation of key motion factors that contribute to animacy impression under rigorous experimental control. Although these simulated motions are not identical to veridical BMs, identifying the critical motion factors in such stimuli that trigger animacy perception could offer insights into how we perceive animacy from the extrinsic motion patterns of living creatures.
A large number of studies have demonstrated that self-propelled motion elicited a stable perception of animacy. Observers attributed animacy to geometric shapes exhibiting autonomous motion initiation (Stewart, 1982), speed modulation, direction change, or alignment between the principal axis orientation and its motion direction (Tremoulet and Feldman, 2000). Another cue signaling animacy is motion against gravity. Szego and Rutherford (2008) demonstrated that extrinsic motions inconsistent with gravity elicited stronger animacy perception than those consistent with gravity. In particular, more participants judged the upward motions (against gravity) of a single dot as more animate compared with the downward ones presented on a vertically oriented computer screen. However, when the screen was horizontal (i.e., parallel to the ground), altering the directional relationship of the motion and the gravitational context, participants no longer showed preference in their animacy judgments. This gravitational modulation of animacy judgments suggests that gravity is an ecological constraint shaping animacy perception from extrinsic motion, and identifying motion against the gravitational force may be essential for animacy perception.
Although these findings were derived from the extrinsic motions along synthetic paths, terrestrial, aquatic, and aerial animals in the natural world are all able to initiate motion autonomously and move against gravity, driven by an internal energy source. Cross-species evidence suggests that the visual sensitivity to such motion patterns emerges early in life. Di Giorgio et al. (2017) first demonstrated human newborns’ ability to differentiate between self- and non-self-propelled objects and their visual preference toward self-produced motion. Studies on visually-naïve chicks demonstrated their spontaneous preference for a geometric shape’s motion exhibiting speed changes (Rosa-Salva et al., 2016), axis-path parallelism, or spontaneous rotation (Rosa-Salva et al., 2018), relative to constant-speed, non-parallel, or translational motions. Moreover, newborn chicks have a predisposition to approach stimuli moving against gravity (Bliss et al., 2023). These findings, together with the abovementioned evidence, open the possibility that identifying internal energy sources indicated by particular motion features (i.e., self-propelled, against-gravity) serves as a general mechanism for animacy perception from visual motion. They also suggest an early ontogenetic origin of the capacity to detect animate motion and perceive animacy based on some general animate kinematic features, possibly driven by a phylogenetically old mechanism shared among various vertebrates.
3.3 Comparison of animacy perception between intrinsic and extrinsic motions
Beyond those general factors, cognitive processing mechanisms for intrinsic and extrinsic motion still differ in some aspects. For instance, the perception of extrinsic motion does not require the complex analysis and integration of intrinsic kinematic characteristics. This may account for the seemly contradictory findings that the gravity-incompatible intrinsic movements in inverted point-light BM reduce animacy perception while the extrinsic motion of a single point against gravity enhances animacy perception. Since the local kinematics of intrinsic joint ballistic movements reflect the combined influences of gravity and biomechanics, the inverted gravity-incongruent movements impair this inherent ecological validity and thus reduce perceived animacy. In comparison, the downward global extrinsic motion indicates only the gravitational constraint, therefore the ability to move against gravity can be interpreted as evidence of an internal energy source generated by an animate agent, prompting observers to perceive animacy.
While these findings can be understood within a unified framework considering fundamental physical and biological constraints, the similarities and differences in animacy impression mediated by intrinsic versus extrinsic motions and their integration remain underexplored. Thus, further investigation is required to advance our understanding of animacy perception from BM by considering both intrinsic and extrinsic motions.
3.4 Neural mechanism for animacy perception from motion cues
Most studies on the neural mechanism of point-light BM focused on its visual processing rather than the animacy percepts. Among the studies concerning animacy perception, most elicited higher-level social cognitive processes along with basic animacy impressions by imposing inter-object or object-environment interactions to simple objects. Only a limited number of studies have preliminarily investigated the neural substrates for animacy perception based on the visual processing of motion cues, and these studies typically examined the extrinsic motions of simple, non-interactive objects.
Schultz and Bülthoff (2019) have demonstrated that the intraparietal sulcus (IPS) serves as a key cortical region for perceiving animacy from extrinsic motion patterns. Using simulated animation sequences showing a single dot’s self-propelled motion transitioning through six morph levels from inanimate to animate, they found that the right IPS showed a higher response when observers perceived animacy compared with when they did not. However, they did not observe the involvement of some typical cortical regions associated with BM perception, such as the pSTS. This discrepancy may occur because perceiving animacy from such a simplified moving stimulus does not rely on form-motion integration or action recognition. It is likely that animacy perception from different motion cues, particularly the intrinsic joint movements in point-light displays and the global extrinsic motion of a single dot, involves distinct neural substrates. Therefore, it is necessary to reassess the functional contributions of brain regions engaged in complex BM processing and elucidate their role in perceiving animacy from motion cues.
Moreover, evidence from studies in humans and other animals indicates the role of subcortical structures in perceiving general animate motion with different forms. Previous studies have demonstrated that chicks exhibit innate predispositions for animate motion eliciting animacy perception in humans (Bliss et al., 2023; Rosa-Salva et al., 2016, 2018; Vallortigara et al., 2005; Vallortigara and Regolin, 2006). Given the substantial brain organization differences and phylogenetic distance between species, this cross-species mechanism potentially involves evolutionarily ancient neural substrates (Hirai and Senju, 2020; Johnson, 2006; Troje and Westhoff, 2006). More specifically, certain homologous subcortical structures may contribute to the cross-species capacity for innate and rapid perception of animacy, distinct from cortical elaboration. Lorenzi et al. (2017) found that the right septum and the preoptic area of newly hatched chicks exhibited higher neuronal activity when they were exposed to the motion of a disk with speed changes compared to that at a constant speed. These findings, combined with the recent empirical results of the preoptic area involvement in BM perception in newborn chicks (Lorenzi et al., 2024), establish evidence for the crucial role of the preoptic area, a conserved subcortical structure, in general animate motion processing at birth. In addition, the SC is a pivotal subcortical region for processing local motion cues of BM in humans and macaques (Lu et al., 2024), as previously discussed. Given the contributions of local motion in perceiving animacy (Chang and Troje, 2008; Thurman and Lu, 2013), not just in processing BM, the SC may also be closely related to animacy detection and perception in primates. However, since these findings are based on the visual processing of animate versus inanimate motion rather than being directly linked to animacy percept, the exact role of subcortical structures in perceiving animacy from motion cues remains to be delineated.
4 Discussion and future directions
In this review, we focus on the perceptual and neural processing of a vital type of animate motion, namely, BM, spanning from its visual perception to animacy perception based on visual motion analysis. By reviewing key findings in human and non-human animal studies, we have outlined the specialized processing of BM across different species and emphasized the role of local motion cues in driving the domain-specific mechanisms. Compared to the emerging understanding of the cortical–subcortical networks involved in BM processing, much less is known about the neural networks underlying animacy perception from BM. The latter topic requires more systematic and in-depth investigations.
In addition, what key motion features in BM elicit animacy perception remains to be investigated. The current “Life Detector” and “Step Detector” hypotheses emphasize the significance of low-level kinematic features, namely the acceleration profile of local BM and especially the foot motions, in perceiving walking direction as well as the animate properties of a creature (Chang et al., 2018; Chang and Troje, 2008, 2009a; Hirai and Senju, 2020; Johnson, 2006; Troje and Westhoff, 2006). Between processing low-level local motion and perceiving global BM, mid-level motion features provide structured motion units that capture spatiotemporal relations between joints. Previous research employing simulated motion stimuli suggests that some mid-level motion features, such as the combination of deformation and translation (Kawabe, 2017), as well as alternating contraction and expansion accompanied by translational movement (Parovel et al., 2018), could trigger the impression of animacy. Other mid-level features, such as opponent motion, are crucial for BM perception (Casile and Giese, 2005; Thurman and Grossman, 2008), while their role in animacy perception remains unclear. In addition, how low-level kinematic features and mid-level motion patterns are integrated to support animacy perception and agency attribution also awaits further investigation. Exploring these questions may not only help broaden our understanding of how the brain identifies BM as a signal of life but may also provide valuable insights for designing bio-robots capable of producing life-like movements.
Notably, rhythm is an inherent and intrinsic feature of basic locomotion patterns across many species, such as the walking of humans, the swimming of jellyfish, and the flying of butterflies. Moreover, like BM processing, rhythm processing also has an evolutionary basis (Kotz et al., 2018). These suggest a potential link between rhythm processing and animacy perception elicited by BM. Previous research has shown that neural oscillations will align to and continuously track the temporal structures in external and internal rhythms (Lakatos et al., 2019; Thut et al., 2011), a mechanism that enables humans to maintain perceptual and cognitive representations of rhythmic stimuli even after they have disappeared (Miller et al., 2013). Recent electroencephalograph (EEG) studies have found that the neural tracking of rhythmic structures in human walking movements contributes to domain-specific encoding of BM signals (Shen et al., 2023b; Shen et al., 2025b). These findings raise an important question: does the neural encoding of locomotion rhythms also contribute to the animacy perception elicited by BM?
Finally, future studies could integrate the investigation of neural mechanisms underlying animacy perception from visual motion cues with that from static cues. Previous work has revealed a lateral-to-medial functional gradient organization in the ventral visual pathway for distinguishing animate from inanimate categories through static cues (Grill-Spector and Weiner, 2014; Kriegeskorte et al., 2008). Apart from the ventral temporal cortex (VTC), the superior temporal sulcus (STS) is also sensitive to static animate cues such as face and body (Allison et al., 2000; Pinsk et al., 2009). Static animate images and dynamic animate cues seem to share a part of overlapping neural substrates, including the STS, despite differences in visual representation. However, it remains unclear whether static and motion cues elicit distinctive activation patterns in these regions, how integration occurs between the processing of static and motion cues, and whether they rely on a common core network or dissociable pathways in evoking animacy perception. Addressing these questions would lead to a more comprehensive understanding of the neurocognitive mechanism of animacy perception.
Statements
Author contributions
LS: Writing – original draft, Visualization, Conceptualization, Writing – review & editing, Funding acquisition. XY: Conceptualization, Visualization, Writing – review & editing, Writing – original draft. YJ: Funding acquisition, Writing – review & editing. YW: Writing – original draft, Writing – review & editing, Conceptualization, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by grants from the Space Medical Experiment Project of CMSP (HYZHXMR01002), the Ministry of Science and Technology of China (2021ZD0203800 and 2021ZD0204200), the National Natural Science Foundation of China (32171059 and 32430043), the Interdisciplinary Innovation Team (JCTD-2021-06), the Youth Innovation Promotion Association of the Chinese Academy of Sciences, the China Postdoctoral Science Foundation (2024M170993 and 2024M753476), and the Fundamental Research Funds for the Central Universities.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Allison T. Puce A. McCarthy G. (2000). Social perception from visual cues: role of the STS region. Trends Cogn. Sci.4, 267–278. doi: 10.1016/S1364-6613(00)01501-1
2
Arrighi R. Cartocci G. Burr D. (2011). Reduced perceptual sensitivity for biological motion in paraplegia patients. Curr. Biol.21, R910–R911. doi: 10.1016/j.cub.2011.09.048
3
Bardi L. Regolin L. Simion F. (2011). Biological motion preference in humans at birth: role of dynamic and configural properties. Dev. Sci.14, 353–359. doi: 10.1111/j.1467-7687.2010.00985.x
4
Bardi L. Regolin L. Simion F. (2014). The first time ever I saw your feet: inversion effect in newborns’ sensitivity to biological motion. Dev. Psychol.50, 986–993. doi: 10.1037/a0034678
5
Beintema J. A. Lappe M. (2002). Perception of biological motion without local image motion. Proc. Natl. Acad. Sci.99, 5661–5663. doi: 10.1073/pnas.082483699
6
Bertenthal B. I. Pinto J. (1994). Global processing of biological motions. Psychol. Sci.5, 221–225. doi: 10.1111/j.1467-9280.1994.tb00504.x
7
Blake R. Shiffrar M. (2007). Perception of human motion. Annu. Rev. Psychol.58, 47–73. doi: 10.1146/annurev.psych.57.102904.190152
8
Bliss L. Vasas V. Freeland L. Roach R. Ferrè E. R. Versace E. (2023). A spontaneous gravity prior: newborn chicks prefer stimuli that move against gravity. Biol. Lett.19:20220502. doi: 10.1098/rsbl.2022.0502
9
Borst A. Egelhaaf M. (1989). Principles of visual motion detection. Trends Neurosci.12, 297–306. doi: 10.1016/0166-2236(89)90010-6
10
Bosbach S. Knoblich G. Reed C. L. Cole J. Prinz W. (2006). Body inversion effect without body sense: insights from deafferentation. Neuropsychologia44, 2950–2958. doi: 10.1016/j.neuropsychologia.2006.06.018
11
Casile A. Giese M. A. (2005). Critical features for the recognition of biological motion. J. Vis.5:6. doi: 10.1167/5.4.6
12
Cavallo A. Becchio C. Sartori L. Bucchioni G. Castiello U. (2012). Grasping with tools: corticospinal excitability reflects observed hand movements. Cereb. Cortex22, 710–716. doi: 10.1093/cercor/bhr157
13
Chang D. H. F. Ban H. Ikegaya Y. Fujita I. Troje N. F. (2018). Cortical and subcortical responses to biological motion. NeuroImage174, 87–96. doi: 10.1016/j.neuroimage.2018.03.013
14
Chang D. H. F. Troje N. F. (2008). Perception of animacy and direction from local biological motion signals. J. Vis.8, 3–310. doi: 10.1167/8.5.3
15
Chang D. H. F. Troje N. F. (2009a). Acceleration carries the local inversion effect in biological motion perception. J. Vis.9:19. doi: 10.1167/9.1.19
16
Chang D. H. F. Troje N. F. (2009b). Characterizing global and local mechanisms in biological motion perception. J. Vis.9, 8.1–8.810. doi: 10.1167/9.5.8
17
Cheng Y. Xin Y. Lu X. Yang T. Ma X. Yuan X. et al . (2025). Shared and unique neural codes for biological motion perception in humans and macaque monkeys. Adv. Sci.12:2411562. doi: 10.1002/advs.202411562
18
De Agrò M. Rößler D. C. Kim K. Shamble P. S. (2021). Perception of biological motion by jumping spiders. PLoS Biol.19:e3001172. doi: 10.1371/journal.pbio.3001172
19
Di Giorgio E. Lunghi M. Simion F. Vallortigara G. (2017). Visual cues of motion that trigger animacy perception at birth: the case of self-propulsion. Dev. Sci.20:e12394. doi: 10.1111/desc.12394
20
Dittrich W. H. (1993). Action categories and the perception of biological motion. Perception22, 15–22. doi: 10.1068/p220015
21
Gao T. McCarthy G. Scholl B. J. (2010). The wolfpack effect: perception of animacy irresistibly influences interactive behavior. Psychol. Sci.21, 1845–1853. doi: 10.1177/0956797610388814
22
Giese M. A. Poggio T. (2003). Neural mechanisms for the recognition of biological movements. Nat. Rev. Neurosci.4, 179–192. doi: 10.1038/nrn1057
23
Grill-Spector K. Weiner K. S. (2014). The functional architecture of the ventral temporal cortex and its role in categorization. Nat. Rev. Neurosci.15, 536–548. doi: 10.1038/nrn3747
24
Han Y. Han W. Li L. Zhang T. Wang Y. (2023). Identifying critical kinematic features of animate motion and contribution to animacy perception. iScience26:107658. doi: 10.1016/j.isci.2023.107658
25
Heider F. Simmel M. (1944). An experimental study of apparent behavior. Am. J. Psychol.57:243. doi: 10.2307/1416950
26
Hirai M. Chang D. H. F. Saunders D. R. Troje N. F. (2011). Body configuration modulates the usage of local cues to direction in biological-motion perception. Psychol. Sci.22, 1543–1549. doi: 10.1177/0956797611417257
27
Hirai M. Senju A. (2020). The two-process theory of biological motion processing. Neurosci. Biobehav. Rev.111, 114–124. doi: 10.1016/j.neubiorev.2020.01.010
28
Huang M. Yang G.-Q. Wang Y. Jiang Y. (2023). Animacy perception from motion cues: cognitive and neural mechanisms. Adv. Psychol. Sci.31:1460. doi: 10.3724/SP.J.1042.2023.01460
29
Jastorff J. Orban G. A. (2009). Human functional magnetic resonance imaging reveals separation and integration of shape and motion cues in biological motion processing. J. Neurosci.29, 7315–7329. doi: 10.1523/JNEUROSCI.4870-08.2009
30
Jastorff J. Popivanov I. D. Vogels R. Vanduffel W. Orban G. A. (2012). Integration of shape and motion cues in biological motion processing in the monkey STS. NeuroImage60, 911–921. doi: 10.1016/j.neuroimage.2011.12.087
31
Ji H. Wang L. Jiang Y. (2020). Cross-category adaptation of reflexive social attention. J. Exp. Psychol. Gen.149, 2145–2153. doi: 10.1037/xge0000766
32
Johansson G. (1973). Visual perception of biological motion and a model for its analysis. Percept. Psychophys.14, 201–211. doi: 10.3758/BF03212378
33
Johnson M. H. (2006). Biological motion: a perceptual life detector?Curr. Biol.16, R376–R377. doi: 10.1016/j.cub.2006.04.008
34
Johnson M. H. Senju A. Tomalski P. (2015). The two-process theory of face processing: modifications based on two decades of data from infants and adults. Neurosci. Biobehav. Rev.50, 169–179. doi: 10.1016/j.neubiorev.2014.10.009
35
Jokisch D. Troje N. F. Koch B. Schwarz M. Daum I. (2005). Differential involvement of the cerebellum in biological and coherent motion perception. Eur. J. Neurosci.21, 3439–3446. doi: 10.1111/j.1460-9568.2005.04145.x
36
Kawabe T. (2017). Perceiving animacy from deformation and translation. I-Perception8:2041669517707767. doi: 10.1177/2041669517707767
37
Koldewyn K. Hanus P. Balas B. (2014). Visual adaptation of the perception of “life”: animacy is a basic perceptual dimension of faces. Psychon. Bull. Rev.21, 969–975. doi: 10.3758/s13423-013-0562-5
38
Kotz S. A. Ravignani A. Fitch W. T. (2018). The evolution of rhythm processing. Trends Cogn. Sci.22, 896–910. doi: 10.1016/j.tics.2018.08.002
39
Kriegeskorte N. Mur M. Bandettini P. (2008). Representational similarity analysis – connecting the branches of systems neuroscience. Front. Syst. Neurosci.2:4. doi: 10.3389/neuro.06.004.2008
40
Lakatos P. Gross J. Thut G. (2019). A new unifying account of the roles of neuronal entrainment. Curr. Biol.29, R890–R905. doi: 10.1016/j.cub.2019.07.075
41
Lange J. Lappe M. (2006). A model of biological motion perception from configural form cues. J. Neurosci.26, 2894–2906. doi: 10.1523/JNEUROSCI.4915-05.2006
42
Larsch J. Baier H. (2018). Biological motion as an innate perceptual mechanism driving social affiliation. Curr. Biol.28, 3523–3532.e4. doi: 10.1016/j.cub.2018.09.014
43
Lemaire B. S. Vallortigara G. (2022). Life is in motion (through a chick’s eye). Anim. Cogn.26, 129–140. doi: 10.1007/s10071-022-01703-8
44
Lorenzi E. Mayer U. Rosa-Salva O. Vallortigara G. (2017). Dynamic features of animate motion activate septal and preoptic areas in visually naïve chicks (Gallus gallus). Neuroscience354, 54–68. doi: 10.1016/j.neuroscience.2017.04.022
45
Lorenzi E. Nadalin G. Morandi-Raikova A. Mayer U. Vallortigara G. (2024). Noncortical coding of biological motion in newborn chicks’ brain. Cereb. Cortex34:bhae262. doi: 10.1093/cercor/bhae262
46
Loula F. Prasad S. Harber K. Shiffrar M. (2005). Recognizing people from their movement. J. Exp. Psychol. Hum. Percept. Perform.31, 210–220. doi: 10.1037/0096-1523.31.1.210
47
Lu X. Hu Z. Xin Y. Yang T. Wang Y. Zhang P. et al . (2024). Detecting biological motion signals in human and monkey superior colliculus: a subcortical-cortical pathway for biological motion perception. Nat. Commun.15:9606. doi: 10.1038/s41467-024-53968-x
48
Ma X. Yuan X. Liu J. Shen L. Yu Y. Zhou W. et al . (2022). Gravity-dependent animacy perception in zebrafish. Research2022. doi: 10.34133/2022/9829016
49
Masselink J. Lappe M. (2015). Translation and articulation in biological motion perception. J. Vis.15:10. doi: 10.1167/15.11.10
50
Mayer U. Rosa-Salva O. Morbioli F. Vallortigara G. (2017). The motion of a living conspecific activates septal and preoptic areas in naive domestic chicks (Gallus gallus). Eur. J. Neurosci.45, 423–432. doi: 10.1111/ejn.13484
51
Miller J. E. Carlson L. A. McAuley J. D. (2013). When what you hear influences when you see: listening to an auditory rhythm influences the temporal allocation of visual attention. Psychol. Sci.24, 11–18. doi: 10.1177/0956797612446707
52
Miura M. Matsushima T. (2012). Preference for biological motion in domestic chicks: sex-dependent effect of early visual experience. Anim. Cogn.15, 871–879. doi: 10.1007/s10071-012-0514-x
53
Nakayasu T. Watanabe E. (2014). Biological motion stimuli are attractive to medaka fish. Anim. Cogn.17, 559–575. doi: 10.1007/s10071-013-0687-y
54
Neri P. Morrone M. C. Burr D. C. (1998). Seeing biological motion. Nature395:6705. doi: 10.1038/27661
55
Parovel G. Guidi S. Kreß K. (2018). Different contexts change the impression of animacy. Atten. Percept. Psychophys.80, 553–563. doi: 10.3758/s13414-017-1439-x
56
Pavlova M. A. (2012). Biological motion processing as a Hallmark of social cognition. Cereb. Cortex22, 981–995. doi: 10.1093/cercor/bhr156
57
Pinsk M. A. Arcaro M. Weiner K. S. Kalkus J. F. Inati S. J. Gross C. G. et al . (2009). Neural representations of faces and body parts in macaque and human cortex: a comparative fMRI study. J. Neurophysiol.101, 2581–2600. doi: 10.1152/jn.91198.2008
58
Reed C. L. Stone V. E. Bozova S. Tanaka J. (2003). The body-inversion effect. Psychol. Sci.14, 302–308. doi: 10.1111/1467-9280.14431
59
Rosa Salva O. Mayer U. Vallortigara G. (2015). Roots of a social brain: developmental models of emerging animacy-detection mechanisms. Neurosci. Biobehav. Rev.50, 150–168. doi: 10.1016/j.neubiorev.2014.12.015
60
Rosa-Salva O. Grassi M. Lorenzi E. Regolin L. Vallortigara G. (2016). Spontaneous preference for visual cues of animacy in naïve domestic chicks: the case of speed changes. Cognition157, 49–60. doi: 10.1016/j.cognition.2016.08.014
61
Rosa-Salva O. Hernik M. Broseghini A. Vallortigara G. (2018). Visually-naïve chicks prefer agents that move as if constrained by a bilateral body-plan. Cognition173, 106–114. doi: 10.1016/j.cognition.2018.01.004
62
Saygin A. P. Driver J. de Sa V. R. (2008). In the footsteps of biological motion and multisensory perception: judgments of audiovisual temporal relations are enhanced for upright walkers. Psychol. Sci.19, 469–475. doi: 10.1111/j.1467-9280.2008.02111.x
63
Scholl B. J. Tremoulet P. D. (2000). Perceptual causality and animacy. Trends Cogn. Sci.4, 299–309. doi: 10.1016/S1364-6613(00)01506-0
64
Schultz J. Bülthoff H. H. (2019). Perceiving animacy purely from visual motion cues involves intraparietal sulcus. NeuroImage197, 120–132. doi: 10.1016/j.neuroimage.2019.04.058
65
Senju A. Johnson M. H. (2009). The eye contact effect: mechanisms and development. Trends Cogn. Sci.13, 127–134. doi: 10.1016/j.tics.2008.11.009
66
Shen L. Cheng X. Ma Z. Zhong H. Jiao X. Wang Y. et al . (2025a). Sensorimotor simulation and distributed processing of biological motion: insights from healthy and paraplegic adults. Psychon. Bull. Rev. doi: 10.3758/s13423-025-02689-3
67
Shen L. Li S. Tian Y. Wang Y. Jiang Y. (2025b). Cortical tracking of hierarchical rhythms orchestrates the multisensory processing of biological motion. eLife13:RP98701. doi: 10.7554/eLife.98701
68
Shen L. Lu X. Wang Y. Jiang Y. (2023a). Audiovisual correspondence facilitates the visual search for biological motion. Psychon. Bull. Rev.30, 2272–2281. doi: 10.3758/s13423-023-02308-z
69
Shen L. Lu X. Yuan X. Hu R. Wang Y. Jiang Y. (2023b). Cortical encoding of rhythmic kinematic structures in biological motion. NeuroImage268:119893. doi: 10.1016/j.neuroimage.2023.119893
70
Shi J. Weng X. He S. Jiang Y. (2010). Biological motion cues trigger reflexive attentional orienting. Cognition117, 348–354. doi: 10.1016/j.cognition.2010.09.001
71
Shiffrar M. Lichtey L. Chatterjee S. H. (1997). The perception of biological motion across apertures. Percept. Psychophys.59, 51–59. doi: 10.3758/BF03206847
72
Simion F. Regolin L. Bulf H. (2008). A predisposition for biological motion in the newborn baby. Proc. Natl. Acad. Sci.105, 809–813. doi: 10.1073/pnas.0707021105
73
Sokolov A. A. Erb M. Gharabaghi A. Grodd W. Tatagiba M. S. Pavlova M. A. (2012). Biological motion processing: the left cerebellum communicates with the right superior temporal sulcus. NeuroImage59, 2824–2830. doi: 10.1016/j.neuroimage.2011.08.039
74
Sokolov A. A. Zeidman P. Erb M. Ryvlin P. Friston K. J. Pavlova M. A. (2018). Structural and effective brain connectivity underlying biological motion detection. Proc. Natl. Acad. Sci.115, E12034–E12042. doi: 10.1073/pnas.1812859115
75
Stewart J. A. (1982). Perception of animacy[Unpublished PhD Thesis]: University of Pennsylvania.
76
Sun Y. Wang X. Huang Y. Ji H. Ding X. (2022). Biological motion gains preferential access to awareness during continuous flash suppression: local biological motion matters. J. Exp. Psychol. Gen.151, 309–320. doi: 10.1037/xge0001078
77
Szego P. A. Rutherford M. D. (2008). Dissociating the perception of speed and the perception of animacy: a functional approach. Evol. Hum. Behav.29, 335–342. doi: 10.1016/j.evolhumbehav.2008.04.002
78
Thomas J. P. Shiffrar M. (2010). I can see you better if I can hear you coming: action-consistent sounds facilitate the visual detection of human gait. J. Vis.10:14. doi: 10.1167/10.12.14
79
Thurman S. M. Grossman E. D. (2008). Temporal “bubbles” reveal key features for point-light biological motion perception. J. Vis.8, 28–2811. doi: 10.1167/8.3.28
80
Thurman S. M. Lu H. (2013). Physical and biological constraints govern perceived animacy of scrambled human forms. Psychol. Sci.24, 1133–1141. doi: 10.1177/0956797612467212
81
Thurman S. M. Lu H. (2014). Perception of social interactions for spatially scrambled biological motion. PLoS One9:e112539. doi: 10.1371/journal.pone.0112539
82
Thut G. Schyns P. G. Gross J. (2011). Entrainment of perceptually relevant brain oscillations by non-invasive rhythmic stimulation of the human brain. Front. Psychol.2:170. doi: 10.3389/fpsyg.2011.00170
83
Tremoulet P. D. Feldman J. (2000). Perception of animacy from the motion of a single object. Perception29, 943–951. doi: 10.1068/p3101
84
Troje N. F. Chang D. H. F. (2023). Life detection from biological motion. Curr. Dir. Psychol. Sci.32, 26–32. doi: 10.1177/09637214221128252
85
Troje N. F. Westhoff C. (2006). The inversion effect in biological motion perception: evidence for a “life detector”?Curr. Biol.16, 821–824. doi: 10.1016/j.cub.2006.03.022
86
Vallortigara G. (2012). “Aristotle and the chicken: animacy and the origins of beliefs” in The theory of evolution and its impact. ed. FasoloA. (Milano: Springer), 189–199.
87
Vallortigara G. Regolin L. (2006). Gravity bias in the interpretation of biological motion by inexperienced chicks. Curr. Biol.16, R279–R280. doi: 10.1016/j.cub.2006.03.052
88
Vallortigara G. Regolin L. Marconato F. (2005). Visually inexperienced chicks exhibit spontaneous preference for biological motion patterns. PLoS Biol.3:e208. doi: 10.1371/journal.pbio.0030208
89
Wang L. Jiang Y. (2012). Life motion signals lengthen perceived temporal duration. Proc. Natl. Acad. Sci.109, E673–E677. doi: 10.1073/pnas.1115515109
90
Wang Y. Wang L. Xu Q. Liu D. Chen L. Troje N. F. et al . (2018). Heritable aspects of biological motion perception and its covariation with autistic traits. Proc. Natl. Acad. Sci.115, 1937–1942. doi: 10.1073/pnas.1714655115
91
Wang L. Wang Y. Xu Q. Liu D. Ji H. Yu Y. et al . (2020). Heritability of reflexive social attention triggered by eye gaze and walking direction: common and unique genetic underpinnings. Psychol. Med.50, 475–483. doi: 10.1017/S003329171900031X
92
Wang Y. Wang L. Xu Q. Liu D. Jiang Y. (2014). Domain-specific genetic influence on visual-ambiguity resolution. Psychol. Sci.25, 1600–1607. doi: 10.1177/0956797614535811
93
Wang L. Yang X. Shi J. Jiang Y. (2014). The feet have it: local biological motion cues trigger reflexive attentional orienting in the brain. NeuroImage84, 217–224. doi: 10.1016/j.neuroimage.2013.08.041
94
Wang Y. Zhang X. Wang C. Huang W. Xu Q. Liu D. et al . (2022). Modulation of biological motion perception in humans by gravity. Nat. Commun.13:2765. doi: 10.1038/s41467-022-30347-y
95
Wheatley T. Milleville S. C. Martin A. (2007). Understanding animate agents: distinct roles for the social network and mirror system. Psychol. Sci.18, 469–474. doi: 10.1111/j.1467-9280.2007.01923.x
96
Wilson M. Knoblich G. (2005). The case for motor involvement in perceiving conspecifics. Psychol. Bull.131, 460–473. doi: 10.1037/0033-2909.131.3.460
97
Zanon M. Lemaire B. S. Papeo L. Vallortigara G. (2024). Innate sensitivity to face-to-face biological motion. iScience27:108793. doi: 10.1016/j.isci.2024.108793
98
Zhao J. Wang L. Wang Y. Weng X. Li S. Jiang Y. (2014). Developmental tuning of reflexive attentional effect to biological motion cues. Sci. Rep.4:5558. doi: 10.1038/srep05558
Summary
Keywords
biological motion, animate motion, animacy perception, life detection, cortical–subcortical network, cross-species
Citation
Shen L, Yang X, Jiang Y and Wang Y (2025) Understanding biological motion through the lens of animate motion processing. Front. Psychol. 16:1630742. doi: 10.3389/fpsyg.2025.1630742
Received
18 May 2025
Accepted
30 July 2025
Published
12 August 2025
Volume
16 - 2025
Edited by
Giorgio Vallortigara, University of Trento, Italy
Reviewed by
Orsola Rosa Salva, University of Trento, Italy
Elena Lorenzi, Centre for Mind and Brain Sciences, University of Trento, Italy
Anastasia Morandi-Raikova, University of Trento, Italy
Updates
Copyright
© 2025 Shen, Yang, Jiang and Wang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Wang, wangying@psych.ac.cn
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.