Abstract
University students face multiple stressors, which can impair their physical and mental health without timely intervention. Despite the close link between nature and wellbeing, as well as the growing interest in using natural substances for health management, the effectiveness of Cunninghamia lanceolata essential oil (C. lanceolata EO) in alleviating emotional disorders among university students remains underexplored. This study investigated the physiological and psychological effects of inhaling C. lanceolata EO among 40 healthy university students (mean age: 21.75 ± 1.82 years). Subjects inhaled room air (control) and C. lanceolata EO for 5 min each. Electroencephalograms (EEG), heart rate variability (HRV), blood pressure (BP), and the Profile of Mood States (POMS) were used as assessment indicators. The findings showed that, at the physiological level, compared with inhaling room air (control), the mean theta (θ) wave in the frontal and parietal lobes (p < 0.05) and the mean alpha (α) wave in the whole brain (p < 0.001) were significantly higher during inhalation of C. lanceolata EO. Simultaneously, the mean beta (β) wave in the frontal, temporal, and parietal lobes was significantly lower (p < 0.05); stress-related indices, including heart rate (HR), BP, low-frequency power (LF), and the LF/HF ratio, were significantly lower (p < 0.01), while the Standard Deviation of Normal-to-Normal intervals (SDNN) and high-frequency power (HF) were significantly higher (p < 0.01), indicating a more relaxed physiological state. Psychologically, during inhalation of C. lanceolata EO, scores for negative emotions (tension, depression, and fatigue) and total emotional disturbance (TMD) on the POMS were significantly lower (p < 0.05), while scores for positive emotions (energy and self-esteem) were significantly higher (p < 0.01). These results suggest that inhaling C. lanceolata EO may have a relaxing effect on the physiological and psychological states of university students, indicating that inhaling C. lanceolata EO has a positive impact on promoting their mental and physical relaxation. Future long-term empirical studies could be conducted to further explore the actual stress-relieving effects of C. lanceolata EO.
1 Introduction
University students are in a unique transitional period of their lives. They face stressors from various aspects, such as academics, the economy, social life, employment, and family (Elena et al., n.d.). Excessive stress can reduce quality of life, happiness, and life satisfaction (김남정 and Lim, 2012), and may lead to common emotional symptoms such as anxiety and depression (Amaro et al., 2024). Furthermore, severe emotional disorders can affect physical and mental health, potentially leading to suicidal thoughts (Duffy et al., 2019). Therefore, there is an urgent need to develop simple, effective physical and mental health programs that address the specific needs of university students.
Among the various methods of physical and mental regulation, aromatherapy has been proven to significantly reduce anxiety, improve sleep quality, and relieve pain when used as an adjuvant therapy (Hedigan et al., 2023; Yin et al., 2024; Akbas Uysal et al., 2024). The primary methods of aromatherapy are inhalation, oral administration, massage, and bathing, all of which help prevent the onset of, alleviate, or treat psychological and emotional disorders (Farrar and Farrar, 2020). One common form of aromatherapy is inhalation, in which aromatic particles enter the body through the nasal cavity and stimulate olfactory receptors. These signals are then transmitted to the limbic system, which triggers emotional responses such as pleasure, relaxation, and instinctive behaviors. The limbic system consists of the amygdala, hippocampus, cingulate gyrus, hypothalamus, and other neural structures in the brain. These structures are primarily involved in important physical and psychological activities such as emotion regulation, memory formation, and sleep cycle regulation (Kremen et al., 2025). The limbic system also acts on the autonomic nervous system, regulating physiological processes such as heart rate, blood pressure, respiratory rate, memory, and stress responses (Florian et al., 2025).
Essential oils (EOs) are complex, volatile mixtures extracted from aromatic plants that usually contain ketones, aldehydes, and aromatic compounds (Vora et al., 2024). Numerous studies have demonstrated the efficacy of EOs in alleviating anxiety, regulating negative emotions, and enhancing sleep quality in various populations, including women (Moon et al., 2020), drivers (Hu et al., 2024), teachers (Liu et al., 2013). In particular, a growing number of studies have consistently shown positive effects of aromatherapy on university students. For instance, lavender EO reduced anxiety levels in freshmen with social anxiety during test evaluations (Solomon et al., 2024); bergamot EO improved sleep quality by promoting psychophysiological relaxation (Wakui et al., 2023); Black pepper EO may alleviate stress in university students by inhibiting excessive sympathetic nervous system (SNS) excitation and promoting local blood flow (Dilrukshi et al., 2024).
However, many studies have also shown that exposure to scents does not always reduce stress or anxiety. For example, one study found that lower doses of lavender EO had an anti-anxiety effect, while higher doses were comparable to the control group and did not show significant differences, suggesting that we need to precisely control the appropriate dosage (Angulo et al., 2025). Another study validated the auxiliary effects of EOs in progressive muscle relaxation (PMR) training, and results showed that regardless of whether scented nose clips were used, all subjects experienced a significant decrease in heart rate after PMR training (p < 0.001), but there was no significant difference between the scented group and the non-scent group (Fs < 1; F-statistic from ANOVA), and scent also did not alleviate stress during the second phase of the stress task. Possible reasons for the lack of significant results include: some subjects in the no-scent group may have been psychologically influenced by being told that the “scent concentration was extremely low”; subjects selected their own scents (e.g., lavender was the most common), and individual differences may have diluted the overall effect; the experimental task (word association) did not elicit sufficient anxiety or fear. Additionally, since subjective anxiety scores were not recorded, relying solely on heart rate metrics may have obscured differences in emotional experiences (Mahmut et al., 2023). Another study found that subjects who received the expectation cue showed significantly shorter reaction times on cognitive tasks under stress conditions, along with reduced N200/P300 latency (indicating more efficient cognitive function). However, the lavender group and the control group showed no significant differences across all measured indicators, suggesting that lavender aroma did not influence cognitive task performance under stress conditions. Possible reasons for the lack of significant aromatic effects include: small effect sizes, which require larger sample sizes to detect; cognitive tasks being too simple to reach the effect detection threshold; task duration inducing fatigue effects; issues with the method of EO exposure (e.g., the dose of 3 drops of EO may be insufficient, and the simple cotton pad application method may not ensure consistent exposure); failure to activate specific neural pathways involved in aroma effects; and differences in the nature of experimental stress (cognitive tasks) versus clinical stress (e.g., medical settings) (Chamine and Oken, 2015).
Furthermore, existing research on the health benefits of EOs mostly focuses on flowers (Pourshaikhian et al., 2024; Genc and Saritas, 2020), herbs (Lucena et al., 2024; Mank-halati et al., 2024), and shrubs (Bozova et al., 2024; Doner et al., 2024; Goncalves et al., 2024). There are relatively few studies on EOs from ligneous angiosperms, especially C. lanceolata. Additionally, the evaluation indicators in existing research on EOs of ligneous angiosperms are not comprehensive. For example, a study investigating the effects of Japanese cedar EO on improving employee mental health included only 9 male subjects in its sample, which is limited by its small sample size and lack of gender diversity. In terms of physiological indicator testing, only saliva stress markers were analyzed, and important stress-related indicators such as autonomic nervous system function were not covered (Matsubara et al., 2017).
To more scientifically and accurately explore the regulatory mechanism of aromatherapy on emotions, electroencephalography (EEG) technology has become an important research tool. Because odor is closely related to emotions, the impact of aromatic odors on brain activity can be measured quantitatively through EEG (Kato et al., 2022). EEG records electrical signals emitted by the brain, which in turn reflect its real-time activity (Fox et al., 2024). The power spectrum is divided into distinct frequency bands, including delta (δ) wave (0.5–4 Hz), theta (θ) wave (4–8 Hz), alpha (α) wave (8–13 Hz), beta (β) wave (13–30 Hz), and gamma (γ) wave (30–50 Hz). Each frequency band corresponds to a different brain state (Sowndhararajan and Kim, 2016). For instance, theta wave is associated with cognition and memory, as well as with deep relaxation (Jacobs et al., 1996), while α wave indicates a relaxed, quiet, and awake state (Grassini et al., 2022). β wave, on the other hand, reflects a tense state of brain activity and is commonly found in an alert state (Lee et al., 2014). These brain waves play a key role in emotional regulation and cognitive processing. Changes in their EEG signals can reveal the intervention mechanism of EOs and evaluate their anxiolytic and relaxing effects. Beyond frequency bands, EEG also enables analysis of brain activity across different anatomical regions. EEG regional analysis focuses on four major lobes: the frontal, temporal, parietal, and occipital lobes, which govern emotional regulation, olfactory perception, somatosensory processing, and visual integration, respectively (Juran et al., 2016; Maksimenko et al., 2018; Nickel and Seitz, 2005; Nejati et al., 2021). Based on data from the central and autonomic nervous systems, EEG can reliably and objectively analyze subtle emotional changes. Previous studies have used EEG to explore the potential therapeutic value of various plant EOs. For instance, a study has used EEG to demonstrate that exposure to peppermint, rose, and lavender EOs can improve subjective perception and enhance positive emotions (Zhang et al., 2025). Additionally, EEG studies on the relaxing effects of lavender EO have indicated that θ and α waves associated with relaxation significantly increased in subjects’ frontal lobes (Afghan et al., 2024). Furthermore, other studies have used EEG to investigate the effects of Cinnamomum camphora EO on human emotional states, demonstrating that C. camphora EO can promote cognitive processes such as memory consolidation and retention while improving emotional states (Gong et al., 2024). EEG can accurately reflect functional differences in frequency bands and brain regions, and it has demonstrated significant value in researching the effects of plant EOs.
Cunninghamia lanceolata (Lamb.) Hook., a common coniferous tree species in southern China, belongs to the evergreen genus of the Cupressaceae (Cui and Matsumura, 2020). Its EO contains various volatile organic compounds with biological activity, including terpenoids, alcohols, naphthalenes, and terpenoid esters (Xin et al., 2012; Su et al., 2012; Li et al., 2020; Kuspradini et al., 2016). Research has confirmed that C. lanceolata EO has anti-inflammatory and analgesic effects (Su et al., 2012; Xin et al., 2012). The main component, α-cedrene, has antibacterial and antioxidant properties (Usman et al., 2024; Bellioua et al., 2024). Cedrol exhibits antibacterial, anti-inflammatory, and neuroprotective properties (Forouzanfar et al., 2025; Asgharzade et al., 2025; Rastegar-Moghaddam et al., 2024), and β-cedrene inhibits the growth of human intestinal bacteria (Kim and Lee, 2015). These findings demonstrate the high medicinal value of C. lanceolata EO. Previous studies have revealed the regulatory effects of Cupressaceae EOs on emotional and physiological states. Specifically, an earlier study experimentally found that when subjects inhaled hinoki EO (a species of Cupressaceae), changes occurred in blood pressure (BP) and heart rate (HR), with decreased sympathetic nerve activity and increased parasympathetic nerve activity. These results suggested that Cupressaceae EOs may improve emotional states (Chen et al., 2015). Subsequently, a comparative study determined that inhaling Cupressaceae EOs was more effective in reducing tension and depression and providing a sense of natural comfort than Pinaceae EOs (Gho et al., 2023). Meanwhile, studies on workplace settings further confirmed that using Cupressaceae EOs in office environments can help relieve employees’ stress and promote their physical and mental health (Matsubara et al., 2023). These studies on Cupressaceae EOs imply that C. lanceolata EO, as a member of this family, may share similar mood-regulating effects. However, research on the effect of C. lanceolata EO on human emotions is limited, and its therapeutic potential for emotional health remains underexplored.
In conclusion, given the limited research on the emotional regulatory effects of C. lanceolata EO and the lack of comprehensive evaluation metrics in previous studies on woody plant EOs, this study aims to investigate the potential effects of C. lanceolata EO on physiological and psychological relaxation in university students from a multidimensional perspective, using EEG, heart rate variability (HRV), BP, and the Profile of Mood States (POMS) psychological questionnaire.
To achieve this objective, we compared the physiological and psychological indicators of university students during inhalation of C. lanceolata EO and room air under daily stress conditions, and discussed the findings in conjunction with indicator definitions, trend implications, and previous conclusions. Additionally, we improved the research methodology to address issues identified in previous studies: subjects were not informed of any information regarding the scent beforehand to avoid psychological expectations influencing the experimental results; a standardized diffusion device was used to optimize the aroma exposure method, ensuring consistency in exposure; we employed a within-subject experimental design to reduce individual trait differences with self as control; we also eliminated fatigue differences caused by temporal sequence; and we did not artificially impose additional stress, instead we conducted it under the normal stress conditions of university students. The study also expanded the measurement indicators for a multidimensional quantitative analysis of the potential benefits of C. lanceolata EO.
The relevant research hypotheses are shown below (all compared with inhalation of room air (control)):
Hypothesis 1: Physiological recovery.
H1a: During inhalation of C. lanceolata EO, the subjects’ mean θ and mean α waves will be significantly higher, while the mean β wave will be significantly lower.
H1b: During inhalation of C. lanceolata EO, the subjects’ HR, low-frequency power (LF), and LF/HF ratio will be significantly lower, while Normal-to-Normal intervals (SDNN) and HF will be significantly higher.
H1c: During inhalation of C. lanceolata EO, the subjects’ systolic blood pressure (SBP) and diastolic blood pressure (DBP) will be significantly lower.
Hypothesis 2: Psychological recovery.
H2a: During inhalation of C. lanceolata EO, the subjects’ negative emotion indicators (tension, depression, anger, fatigue, and panic) will be significantly lower, while the positive emotion indicators (energy and self-esteem) will be significantly higher.
H2b: During inhalation of C. lanceolata EO, the subjects will have significantly lower total mood disturbance (TMD) scores.
2 Materials and methods
2.1 Subjects
This study recruited 41 volunteers from Fujian Agriculture and Forestry University. Their ages ranged from 18 to 26 years. However, due to technical issues with the equipment, data from one subject could not be successfully collected, resulting in a final valid sample of 40 subjects, with 22 females and 18 males. All subjects were university students who were about to take exams within a week, had no cardiovascular diseases, neuropsychiatric symptoms, or nasal diseases, and did not experience any physical discomfort. They also did not have any allergic reactions to EOs. Subjects were required to avoid wearing perfume and consuming food, beverages, or medications that might affect the study results for 24 h before the experiment. They were also required to keep their scalps clean and dry. Before the experiment began, subjects were informed of the purpose of the study and its duration. After fully understanding the experimental procedures, all subjects voluntarily signed a written informed consent form. This study was approved by the Human Research Ethics Committee of Fujian Provincial Hospital (K2019-03-006).
2.2 Olfactory stimulation
The C. lanceolata EO used in this study was provided by the Department of Wood-Based Materials and Design, National Chiayi University. The oil was extracted from C. lanceolata leaves by steam distillation and diluted to a concentration of 3% using dipropylene glycol as a solvent. The diluted C. lanceolata EO was then atomized and released through an aromatherapy diffuser at a flow rate of 3 L/min for 5 min. This ensured the stability and controllability of the EO release concentration (Figure 1A). The experimental environment was kept at a constant temperature of 25 °C and relative humidity of 50%. The experimental room measured 4 × 3 × 2.5 m. Subjects smelled the odor through a tube connected to a funnel-shaped dispenser installed on the table. The funnel was 10 cm from the subjects’ noses. The device was designed to diffuse the odor evenly and maintain a relatively stable stimulation intensity. This effectively reduced the interference of external factors, such as airflow and distance, on the experimental results. Before the experiment began, the subjects were not informed of the specific odor. They were also blindfolded to avoid influencing the results by subjective expectations.
Figure 1
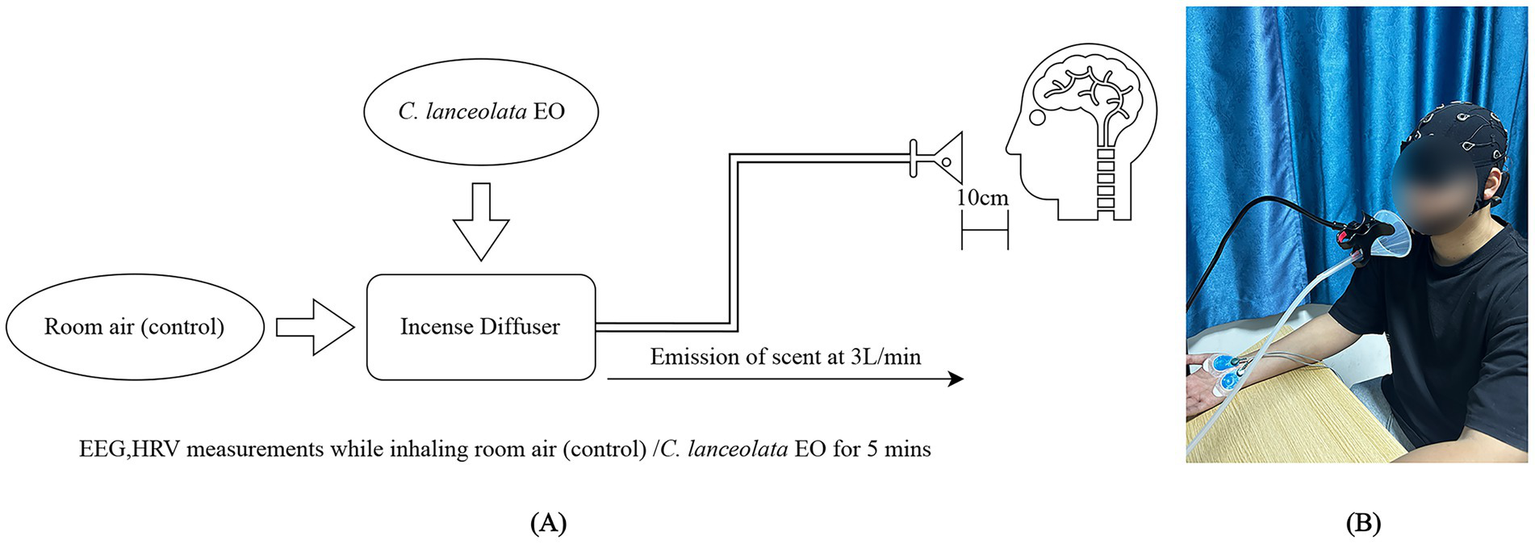
Specification of the experimental setup (A) Aromatherapy diffuser; (B) Experimental scene.
2.3 Experimental design
This study adopted a within-subject experimental design. Each subject used room air as a control and compared differences in physiological and psychological indicators between inhaling room air and inhaling C. lanceolata EO under normal stress conditions to infer the effects of C. lanceolata EO on the physiology and psychology of university students. To minimize the effects of sequence and fatigue, approximately half of the subjects first inhaled room air (control) for 5 min, and then inhaled C. lanceolata EO for 5 min. The remaining subjects first inhaled C. lanceolata EO for 5 min, then inhaled room air (control) for 5 min. A 10-min rest interval was set between the two odor exposures to ensure that the subjects were in as homogeneous a state as possible before inhaling each odor. This design effectively controls for individual variability and is suitable for observing differences in the same group of subjects under different intervention conditions. Figure 2 shows the experimental scheme (the experimental sequence is outlined therein), and the specific implementation is as follows:
Figure 2
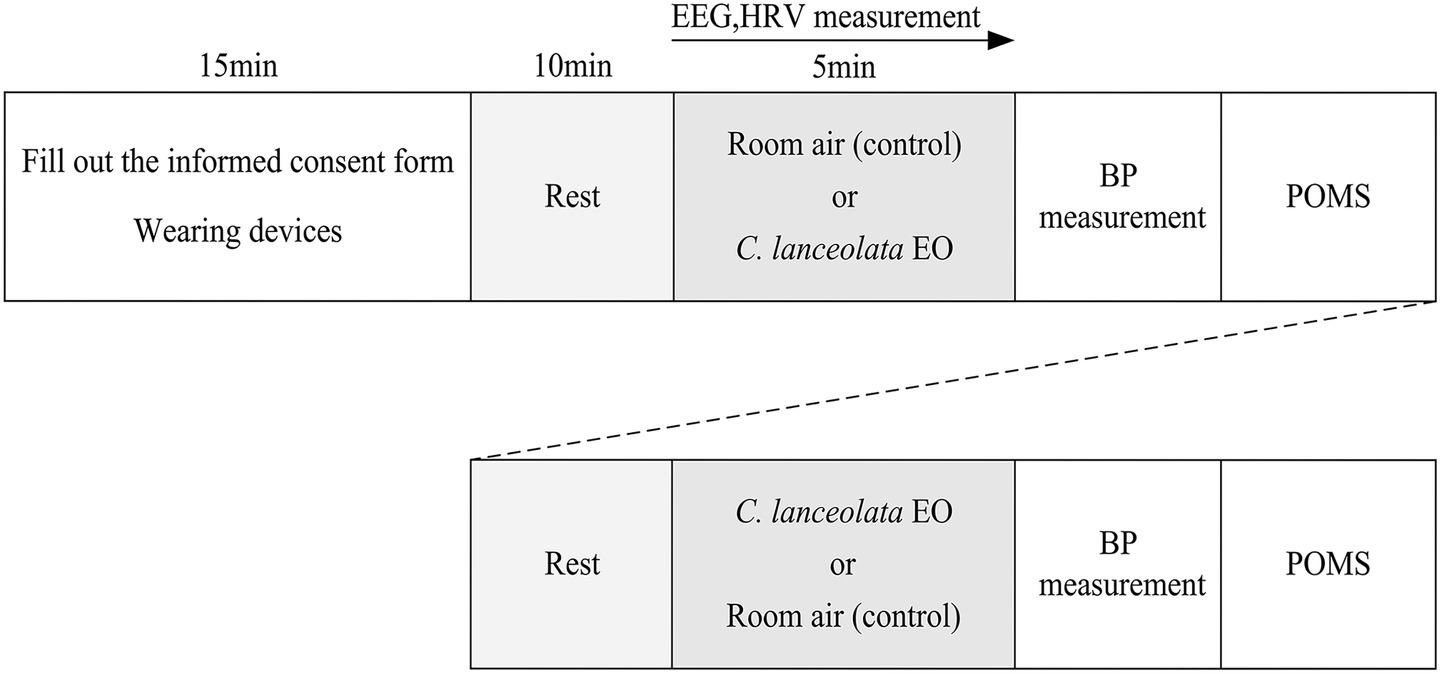
Experimental procedure.
After entering the experimental environment, the subjects were informed of the experimental procedures and purpose. They filled out and signed the informed consent form, and the experimenters assisted them in wearing the physiological monitoring equipment, which took about 15 min. Then, the subjects were given 10 min to rest and adapt to reach a stable physiological and psychological state. After resting, the subjects inhaled either room air or C. lanceolata EO for 5 min. Then, after another 10 min of rest, they inhaled the other odor. While inhaling the gas, their physiological data, such as Electroencephalograms (EEG) and HRV were continuously collected. After inhaling each gas, BP data were immediately collected and subjects completed the POMS psychological questionnaire shortly thereafter. After the experiment, the physiological monitoring equipment was removed, and the entire experimental session lasted about 60 min.
2.4 GC–MS analysis of C. lanceolata EO
The volatile aromatic components of C. lanceolata EO were identified using gas chromatography–mass spectrometry (GC–MS). The analysis was performed using a YLSZJ-SB-258 GC–MS system equipped with an HP-5MS quartz capillary column (30 m × 0.25 mm × 0.25 μm). The injector temperature was set at 250 °C, and the initial column temperature was maintained at 60 °C for 2 min, followed by an increase to 200 °C at a rate of 3 °C/min, where it was held for 15 min. The injection volume was 1 μL, and helium was used as the carrier gas at a flow rate of 1 mL/min and a pressure of 0.15 MPa. The injector and ion source temperatures were set at 220 °C and 230 °C, respectively. The components were analyzed qualitatively and quantitatively by comparing their mass spectra with the National Institute of Standards and Technology (NIST) library. The relative percentages of the components were quantified via area normalization. A total of 16 major components were identified with similarity matches greater than 90%, accounting for approximately 69% of the total composition (Table 1).
Table 1
| S. number | Component | Retention time (min) | Formula | Peak area (%) |
|---|---|---|---|---|
| 1 | α-Cedrene | 33.60 | C₁₅H₂₄ | 19.04 |
| 2 | Cedrol | 38.49 | C₁₅H₂₆O | 15.65 |
| 3 | β-Cedrene | 33.81 | C₁₅H₂₄ | 6.86 |
| 4 | n-Hexadecanoic acid | 44.29 | C₁₆H₃₂O₂ | 4.04 |
| 5 | cis-Thujopsene | 34.01 | C₁₅H₂₄ | 3.00 |
| 6 | β-Elemene | 32.55 | C₁₅H₂₄ | 2.84 |
| 7 | α-Terpinol | 26.60 | C₁₀H₁₈O | 2.63 |
| 8 | α-Alaskene | 35.93 | C₁₅H₂₄ | 2.48 |
| 9 | β-Selinene | 35.46 | C₁₅H₂₄ | 2.41 |
| 10 | δ-Cadinene | 36.043 | C₁₅H₂₄ | 2.34 |
| 11 | β-Copaene | 35.193 | C₁₅H₂₄ | 1.78 |
| 12 | Di-epi-α-cedrene-(I) | 32.624 | C₁₅H₂₄ | 1.74 |
| 13 | Cedryl acetate | 41.089 | C₁7H2₈O2 | 1.59 |
| 14 | Octadecanoic acid | 48.172 | C₁8H36O2 | 1.41 |
| 15 | α-Selinene | 35.612 | C₁5H24 | 1.19 |
| 16 | α-Pinene | 15.491 | C10H16 | 0.76 |
The main volatile organic compounds of C. lanceolata EO.
2.5 Physiological measurements
2.5.1 Electroencephalogram recording
This study used the Smarting Mobi portable EEG system (developed by mBrainTrain) to monitor subjects’ brain activity in real time. The study focused on the α, β, and θ frequency bands to evaluate the potential effects of aromatherapy on brain function (Figure 1B). The EEG system’s sampling frequency was set to 500 Hz, and the electrodes were placed precisely at specific positions according to the international 10–20 system. These positions included Fp1, Fp2, F3, F4, F7, F8, C3, C4, T7, T8, P3, P4, P7, P8, O1, O2, AFz, Fz, Cz, CPz, Pz, and POz. The reference electrodes were positioned at M1 and M2. During the experiment, GT5 medical EEG gel (produced by Wuhan GreenTech Co., Ltd., China) was injected into the electrode holes of the EEG cap. This ensured that the contact impedance between the scalp and each electrode remained below 5 kΩ, thereby allowing for the acquisition of high-quality signals.
To improve data quality, the original EEG signals collected from the scalp electrodes were first filtered using MATLAB R2013b to eliminate electrical artifacts caused by blinking or body movement. Second, the fast Fourier transform (FFT) was applied to convert the time-domain signals into frequency-domain signals. EEGLAB (running on MATLAB R2013b) software was then used to generate brain topographic maps, which intuitively compared the spatial distribution differences in the mean activity energy of whole-brain α, β, and θ waves during inhalation of room air (control) and C. lanceolata EO. This visualization method helps to analyze the effects of C. lanceolata EO on the activities of different brain regions from a spatial perspective, thereby providing a visual basis for exploring its anxiolytic and relaxing mechanisms.
2.5.2 Heart rate variability and blood pressure
In this study, a Mindware Mobile Instrument (produced by MindWare Technologies LTD.) was used to collect electrocardiogram (ECG) data focusing on heart rate variability (HRV). HRV software (RHMS-10-A22B; YoujiaLi Information Technology Co., Ltd., Jinan, China) was used to generate reports. HRV is a sensitive indicator of changes in heart rhythm and is widely recognized as a reliable marker of autonomic nervous system (ANS) activity (Carandina et al., 2021). It has been applied in multiple disciplines, such as psychology, medicine, and sports science (Perna et al., 2020). HRV was analyzed using the following key indicators: heart rate (HR), standard deviation of NN intervals (SDNN), low frequency (LF, 0.04–0.15 Hz), high frequency (HF, 0.15–0.4 Hz), and LF/HF ratio. HR reflects the frequency of heartbeats, while SDNN measures the overall variability of cardiac cycles. In frequency-domain analysis, LF and HF, respectively indicate the activities of the sympathetic and parasympathetic nervous systems (Pham et al., 2021), and the LF/HF ratio reflects ANS regulation balance. An increase in HR is typically indicative of anxiety and stress, whereas a decrease may suggest relaxation (Hongratanaworakit and Buchbauer, 2005). Higher SDNN reflects increased parasympathetic activity and decreased sympathetic activity (Bogdan et al., 2024). Increased HF activity indicates enhanced parasympathetic nerve activity, often associated with relaxation, whereas elevated LF activity correlates with heightened sympathetic activity, linked to arousal, tension, or anxiety (Yamashita et al., 2021). Fluctuations in the LF/HF ratio reveal the balance between sympathetic and parasympathetic activities. Decreased HR variability is associated with anxiety and depressive states (Dell’Acqua et al., 2020).
In addition to HRV, BP was also measured as an indicator of ANS regulation, as the autonomic nerves play a critical role in BP modulation (Tomitani et al., 2021). BP measurements complement HRV analysis in assessing ANS function. BP was recorded using a monitor (HEM-92200 L, Omron Dalian Co., Ltd.). BP is divided into systolic blood pressure (SBP), which reflects the maximum arterial pressure during ventricular contraction, and diastolic blood pressure (DBP), representing the minimum pressure during ventricular diastole. The BP is significantly higher in negative emotional states than in positive ones, indicating that emotions consistently and universally impact BP (Shahimi et al., 2022). Changes in these indicators effectively reflect the ANS’s response to emotions and stress. This thereby provides an objective basis for evaluating the relaxing effect of C. lanceolata EO in this study.
2.6 Psychological measurement
This study used the Profile of Mood States (POMS), one of the most widely used emotional psychological measurement tools in environmental psychology, to measure the psychological effects of olfactory stimulation (Albani et al., 2005). Initially developed by Grove and Prapavessis (1992) and later revised by Zhu (1995), the scale was adapted to establish a norm suitable for the Chinese population (Zhu, 1995). The 40-item questionnaire covers seven emotional dimensions: five negative states (“tension,” “depression,” “anger,” “fatigue,” and “panic”) and two positive states (“energy” and “self-esteem”). Each item is scored using a seven-point Likert scale, a commonly used method for quantifying subjects’ responses (Dolan, 1994). The Total Mood Disturbance (TMD) score is calculated as: (tension + depression + anger + fatigue + panic) − (energy + self-esteem) + 100 (Zhu, 1995). A higher TMD score indicates a more negative emotional state (Watanabe et al., 2024), and a lower TMD score reflects a more positive emotional state characterized by decreased stress and anxiety levels and increased calmness and relaxation (Grove and Prapavessis, 1992). According to psychological research standards, a Cronbach’s α value greater than 0.7 indicates acceptable reliability (0.8–0.9 is considered good) (Cronbach, 1951). In this study, Cronbach’s α was used to evaluate the internal consistency of each dimension in the two gas environments. After inhaling room air, the Cronbach’s α values were 0.797, 0.766, 0.874, 0.756, 0.915, 0.877, and 0.827. After inhaling C. lanceolata EO, the Cronbach’s α values were 0.918, 0.788, 0.718, 0.837, 0.906, 0.841, and 0.799. The α values of each psychological index in each dimension during inhalation of room air or C. lanceolata EO ranged from 0.718 to 0.918, exceeding the acceptable threshold and demonstrating good stability. These results confirm that the scale has ideal reliability and internal consistency, making it suitable for subsequent research.
2.7 Data analysis
In this study, EEG signals from the entire brain were systematically preprocessed. The time series of these signals were then segmented according to the experimental protocol to ensure consistent and accurate data processing. Subsequently, frequency-domain features of alpha (8–13 Hz), beta (13–30 Hz), and theta (4–8 Hz) waves were extracted from each subject’s electrode channels, focusing on EEG power in the frontal, temporal, parietal, and occipital lobes. Thereafter, the average power spectral density of these frequency bands in the selected brain regions was calculated. Brain topographic maps were then constructed using EEGLAB R2013b software to visually present the energy distribution of the different frequency bands.
The statistical data of the subjects, including EEG (α, β, θ waves), HRV (HR, SDNN, LF, HF, and LF/HF ratio), BP (SBP, DBP), and POMS scores, were analyzed using SPSS Statistics 27. Descriptive statistics, such as mean (M) and standard deviation (SD), were reported. To evaluate the differences between room air and C. lanceolata EO inhalation conditions, we conducted paired t-tests at a significance level of α = 0.05 (two-tailed). Cohen’s d was calculated for each dimension to quantify the effect size (d = 0.2 [small]; d = 0.5 [medium]; d = 0.8 [large]). Following the statistical analysis, GraphPad Prism 9 was used to visualize the results.
3 Results
3.1 Physiological responses
3.1.1 Comparison of differences in EEG topography maps during inhalation of room air (control) and C. lanceolata EO
Figure 3 shows the visual presentation of the spatial distribution of average α, β, and θ wave values in the whole brain when inhaling room air and C. lanceolata EO. The top, middle, bottom, and sides of the brain topography map correspond to the frontal lobe, parietal lobe, occipital lobe, and temporal lobe, respectively. Red represents higher values and blue represents lower values. The results showed that, compared to room air (control), inhalation of C. lanceolata EO was associated with significantly higher mean θ wave (4–8 Hz) in the frontal and parietal lobes and higher whole-brain mean α wave (8–13 Hz). In contrast, mean β wave (13–30 Hz) was significantly lower in the frontal, temporal, and parietal lobes. Additionally, the topography clearly showed that the α wave was significantly higher in the right-biased frontal lobe during inhalation of C. lanceolata EO, consistent with previous findings that “left-biased frontal α wave is positively correlated with depression severity, while right-biased frontal α wave is negatively correlated with depression severity” (Kopanska et al., 2025). These results suggest that inhaling C. lanceolata EO may influence activity in different brain regions, with θ and α wave power (linked to relaxation) and β wave power (linked to tension) contributing to differences in the EEG spectrum that reflect a potential relaxing effect.
Figure 3
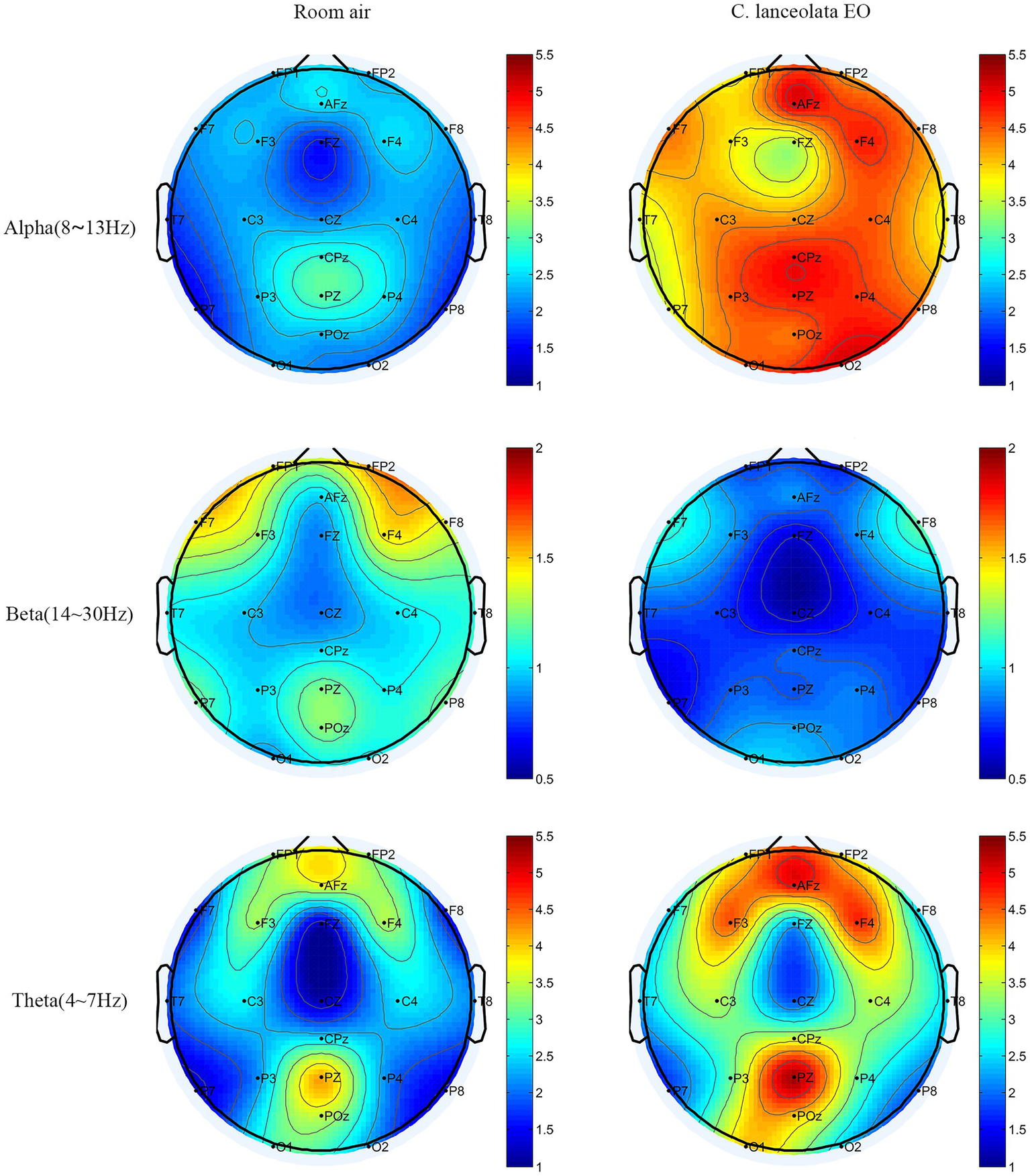
The t-mapping of EEG power spectrum changes during inhalation of room air and C. lanceolata EO.
3.1.2 Multiband EEG power spectrum
Paired-samples t-tests were performed to compare EEG differences during inhalation of room air (control) and C. lanceolata EO (Figure 4), and these results are summarized in Table 2. During inhalation of C. lanceolata EO, the alpha (α) wave power in all brain regions was significantly higher than that during inhalation of room air (control): frontal lobe 2.00 μV2/Hz (p < 0.001), temporal lobe 1.90 μV2/Hz (p < 0.001), parietal lobe 2.19 μV2/Hz (p < 0.001), occipital lobe 2.54 μV2/Hz (p < 0.001). In contrast, the beta (β) wave power in the frontal, temporal, and parietal lobes was significantly lower than that during inhalation of room air (control): frontal lobe 0.42 μV2/Hz (p < 0.01), temporal lobe 0.28 μV2/Hz (p < 0.05), parietal lobe 0.35 μV2/Hz (p < 0.001). The theta (θ) wave power in the frontal and parietal lobes was significantly higher than that during inhalation of room air (control): frontal lobe 1.01 μV2/Hz (p < 0.001), parietal lobe 0.85 μV2/Hz (p < 0.05). These results suggest that inhalation of C. lanceolata EO affects brain activity, mainly by enhancing α and θ wave activities throughout the brain and suppressing β wave activities in the frontal and temporal lobes.
Figure 4
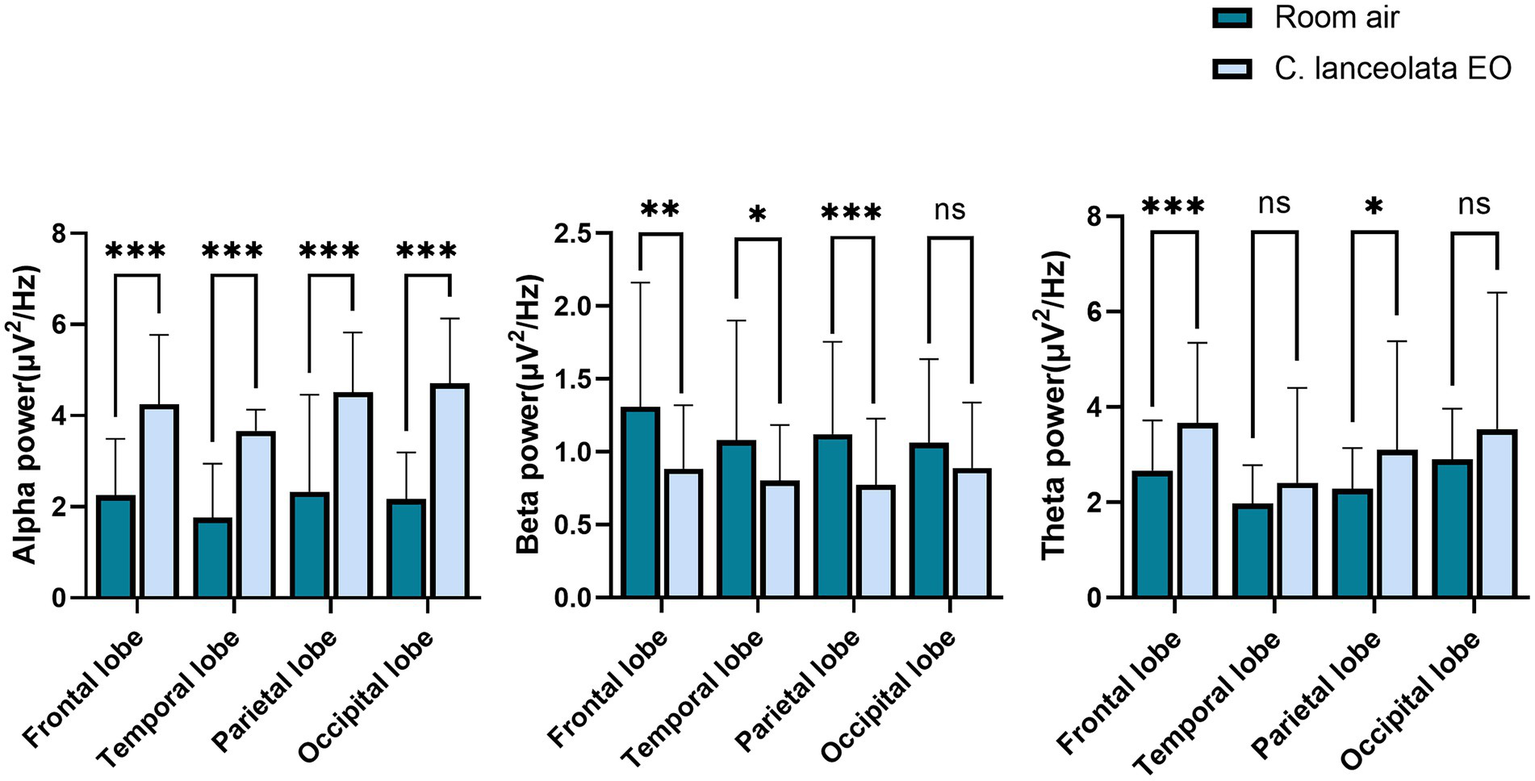
Paired t-test results for EEG power spectra changes during inhalation of room air and C. lanceolata EO.
Table 2
| Variables (Unit) | Site | Air | EO | t-test | p-value | Cohen’s d | ||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||||
| Alpha (μv2/Hz) | FL | 2.25 | 1.24 | 4.25 | 1.52 | −5.91 | 0.000*** | −0.93 |
| TL | 1.76 | 1.18 | 3.66 | 0.47 | −9.02 | 0.000*** | −1.43 | |
| PL | 2.33 | 2.13 | 4.52 | 1.31 | −5.06 | 0.000*** | −0.80 | |
| OL | 2.17 | 1.02 | 4.71 | 1.42 | −8.78 | 0.000*** | −1.39 | |
| Beta (μv2/Hz) | FL | 1.31 | 0.85 | 0.88 | 0.43 | 3.63 | 0.001** | 0.57 |
| TL | 1.08 | 0.82 | 0.80 | 0.38 | 2.30 | 0.027* | 0.36 | |
| PL | 1.12 | 0.63 | 0.77 | 0.45 | 4.88 | 0.000*** | 0.77 | |
| OL | 1.06 | 0.57 | 0.89 | 0.45 | 1.78 | 0.084 | 0.28 | |
| Theta (μv2/Hz) | FL | 2.66 | 1.06 | 3.67 | 1.67 | −4.26 | 0.000*** | −0.67 |
| TL | 1.98 | 0.80 | 2.21 | 1.99 | −0.70 | 0.490 | −0.11 | |
| PL | 2.27 | 0.86 | 3.13 | 2.26 | −2.57 | 0.014* | −0.41 | |
| OL | 2.89 | 1.07 | 3.03 | 2.86 | −0.34 | 0.732 | −0.05 | |
Paired t-test results of the EEG power spectrum during inhalation of room air (control) and C. lanceolata EO.
Air, room air; EO, C. lanceolata EO; FL, frontal lobe; TL, temporal lobe; PL, parietal lobe; OL, occipital lobe. N = 40; values are represented as mean ± SD. Significant differences: ns (not significant); *p < 0.05; **p < 0.01; ***p < 0.001.
3.1.3 Heart rate variability and hemodynamic responses
Paired-samples t-tests were conducted to evaluate significant differences in HRV and BP during inhalation of room air (control) versus C. lanceolata EO (Figure 5), with results summarized in Table 3. This analysis evaluated statistically significant differences between mean HR, SDNN, LF, HF, and LF/HF ratio during room air (control) inhalation versus C. lanceolata EO inhalation. The results showed that stress indicators during inhalation of C. lanceolata EO were significantly lower than those during inhalation of room air (control), while indicators related to relaxation were significantly higher. Specifically, compared with inhalation of room air (control), under the C. lanceolata EO inhalation condition, the HR of the subjects was significantly lower by 14.47 beats/min (p < 0.001). The mean SDNN was significantly higher by 14.48 ms (p < 0.001), the mean LF was significantly lower by 47.86 ms2 (p < 0.01), the mean HF was significantly higher by 78.05 ms2 (p < 0.01), and the mean LF/HF was significantly lower by 0.36 (p < 0.01). Additionally, the mean SBP was significantly lower by 4.0 mmHg (p < 0.01), and the mean DBP was significantly lower by 2.7 mmHg (p < 0.01). These results suggest that inhaling C. lanceolata EO can significantly improve heart rate variability and increase parasympathetic nerve activity while reducing sympathetic nerve activity.
Figure 5
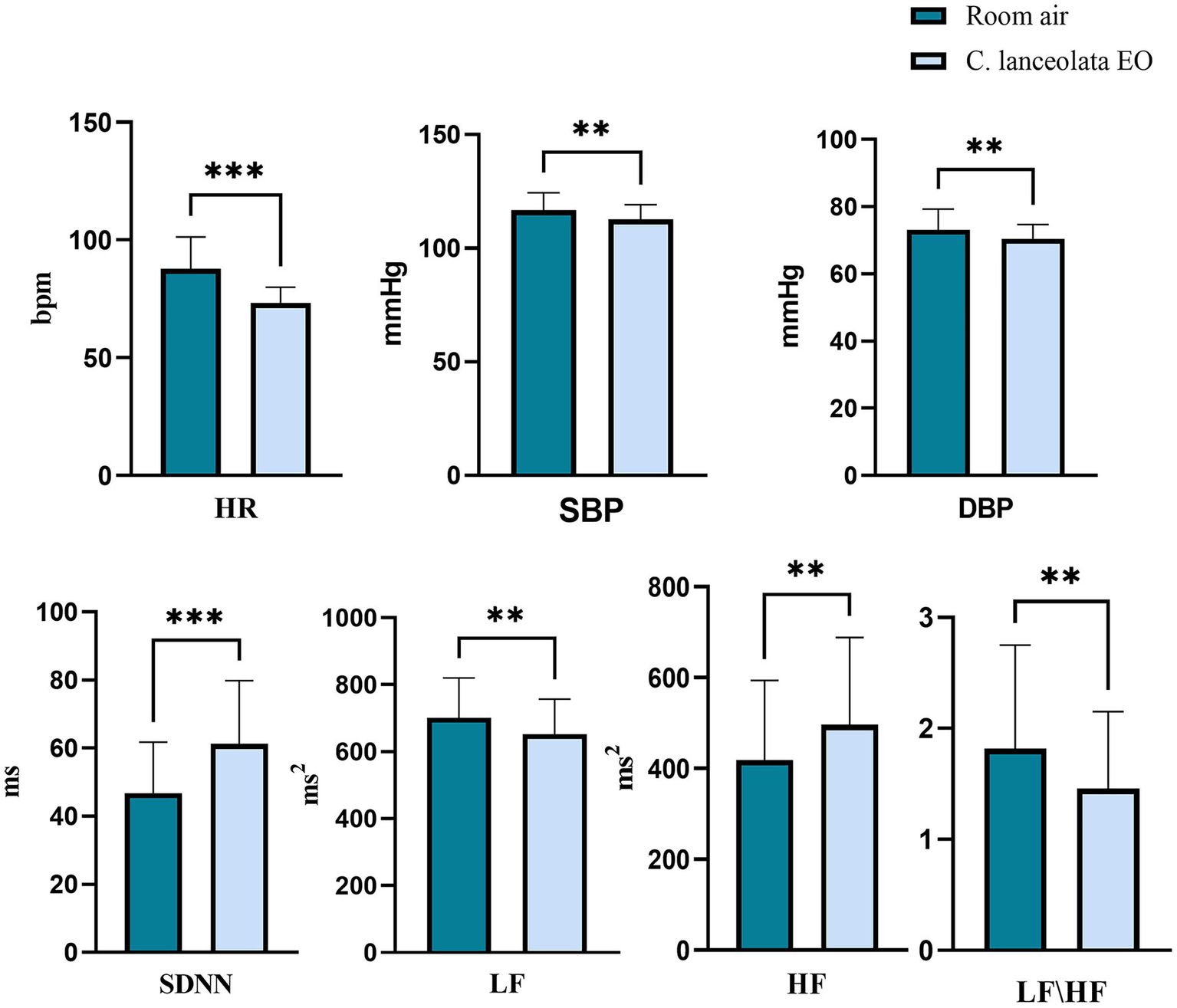
Paired t-test results for heart rate variability (HRV) and blood pressure (BP) during inhalation of room air (control) and C. lanceolata EO.
Table 3
| Variables (Unit) | Air | EO | t-test | p-value | Cohen’s d | ||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| HR (bmp) | 87.64 | 13.64 | 73.18 | 6.74 | 8.63 | 0.000*** | 1.37 |
| SBP (mmHg) | 116.63 | 7.84 | 112.63 | 6.50 | 3.32 | 0.002** | 0.53 |
| DBP (mmHg) | 73.13 | 6.14 | 70.45 | 4.29 | 3.51 | 0.001** | 0.56 |
| SDNN (ms) | 46.79 | 14.96 | 61.27 | 18.59 | −6.60 | 0.000*** | −1.04 |
| LF (ms2) | 701.14 | 119.67 | 653.29 | 103.62 | 3.21 | 0.003** | 0.51 |
| HF (ms2) | 418.08 | 175.78 | 496.14 | 191.96 | −2.88 | 0.006** | −0.46 |
| LF/HF | 1.82 | 0.94 | 1.46 | 0.69 | 2.98 | 0.005** | 0.47 |
Paired t-test of HRV and blood pressure (BP) during inhalation of indoor air (control) and C. lanceolata EO.
Air, Room air; EO, C. lanceolata EO; HRV, Heart Rate Variability; BP, Blood Pressure; HR, Heart Rate; SDNN, Standard Deviation of Normal - to - Normal Intervals; LF, Low-Frequency power; HF, High-Frequency power; LF/HF, Ratio of Low-Frequency to High-Frequency power; SBP, Systolic Blood Pressure; DBP, Diastolic Blood Pressure. N = 40, values are represented as mean ± SD. Significant differences: ns (not significant); *p < 0.05; **p < 0.01; ***p < 0.001.
3.2 Psychological responses
Paired-samples t-tests were conducted to determine whether the difference in the mean POMS scores and mean TMD scale scores of the subjects during inhalation of room air (control) and C. lanceolata EO was statistically significant (Table 4 and Figure 6). Compared to inhalation of room air (control), subjects who inhaled C. lanceolata EOs showed significantly lower scores on negative emotion dimensions, such as “tension,” “depression,” and “fatigue,” and significantly higher scores on positive emotional dimensions such as “energy” and “self-esteem.” Specifically, scores on the “tension” dimension were significantly lower by 6.93 (p < 0.001), scores on the “depression” dimension were significantly lower by 1.63 (p < 0.001), and scores on the “fatigue” dimension were significantly lower by 0.68 (p < 0.05). The scores on the “anger” and “panic” dimensions showed a lower trend, but were not statistically significant: the “anger” score was 1.18 (p > 0.05) lower and the “panic” score was 0.70 (p > 0.05) lower; the “energy” score, which is a measure of positive emotion, was significantly higher by 1.30 (p < 0.01), as did the “self-esteem” score, which was significantly higher by 1.53 (p < 0.001). Overall, the TMD score was 13.93 (p < 0.001) lower. These results suggest that inhaling C. lanceolata EO may have a positive effect on the subjects’ psychological indicators, and it is inferred that it has the potential to improve mood states.
Table 4
| Variables | Air | EO | t-test | p-value | Cohen’s d | ||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| Tension | 22.78 | 4.64 | 15.85 | 3.86 | 9.25 | 0.000*** | 1.46 |
| Depression | 22.85 | 4.51 | 21.23 | 2.98 | 3.54 | 0.001*** | 0.56 |
| Anger | 20.83 | 4.55 | 19.65 | 3.05 | 1.87 | 0.069 | 0.30 |
| Fatigue | 19.90 | 2.54 | 19.23 | 2.73 | 2.04 | 0.048* | 0.32 |
| Panic | 17.85 | 4.46 | 16.78 | 4.16 | 1.34 | 0.189 | 0.21 |
| Energy | 20.23 | 3.48 | 21.53 | 3.39 | −3.37 | 0.002** | −0.53 |
| Self-Esteem | 14.60 | 3.21 | 16.13 | 2.32 | −3.96 | 0.000*** | −0.63 |
| TMD | 169.38 | 10.56 | 155.45 | 9.74 | 8.16 | 0.000*** | 1.29 |
Paired t-test results of POMS between inhaling Room air (control) and inhaling C. lanceolata EO.
Air, Room air; EO, C. lanceolata EO; TMD, Total Mood Disturbance, N = 40, values are represented as mean ± SD. Significant differences: ns (not significant); *p < 0.05; **p < 0.01; ***p < 0.001.
Figure 6
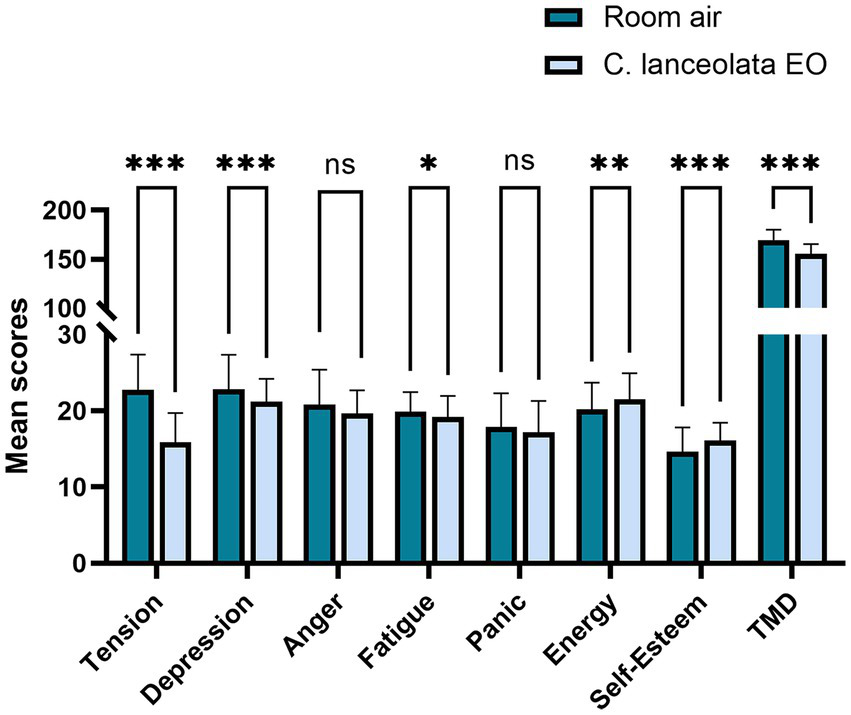
Paired t-test results for POMS scores during inhalation of room air (control) and C. lanceolata EO.
4 Discussion
This study explored the effects of inhaling C. lanceolata EO on the physiological and psychological relaxation of university students by using a comprehensive analysis of aromatic compounds in EO, subjects’ electroencephalogram (EEG), HRV, BP, and the POMS. Previous research has shown that the aromatic components in C. lanceolata EO can positively impact the physiology and psychology of subjects. The results of this study showed that physiologically, it affects the activity of α, β, and θ waves in EEG and improves HRV and BP indices. Psychologically, subjects’ scores are lower on negative emotions and TMD, while those on positive emotions are higher. Collectively, C. lanceolata EO improved the physiological indicators and emotional states of subjects. These findings suggest that inhaling C. lanceolata EO has the potential to promote physiological and psychological relaxation in humans.
Regarding the chemical composition, the C. lanceolata EO used in this study primarily contains α-cedrene (19.04%), cedrol (15.65%), β-cedrene (6.86%), and α-pinene (0.67%). These components serve as the basis for exploring their physiological and psychological effects. Previous studies have confirmed that cedrol induces obvious relaxation behaviors, demonstrating its relaxation effect at the behavioral level (Kagawa et al., 2003). Inhaling cedrol further affects the central nervous system, producing anti-anxiety and relaxation effects, which verifies its regulatory role in the nervous system through the inhalation pathway (Farimani et al., 2024). Olfactory stimulation by α-pinene significantly enhances parasympathetic nervous activity, improves subjective comfort scores, alleviates oxidative stress, and improves memory and mood (Ikei et al., 2016). Building on these findings, the present study hypothesizes that these components may exert similar effects in humans via the inhalation pathway. In conclusion, cedrol and α-pinene have anti-anxiety, relaxing, and physiological function-regulating effects. Although direct evidence for the anti-anxiety and relaxation effects of α-cedrene and β-cedrene is currently limited, the correlation between these compounds and the anti-anxiety and relaxation effects observed in other Cedrus, Cupressus, and Juniperus species suggests a potential role in similar mechanisms of action (Kumar et al., 2023; Lee, 2018; oezek et al., 2021). As common components of plant EOs, α-cedrene, cedrol, β-cedrene, and α-pinene may affect brain excitability via gamma-aminobutyric acid (GABA)-related pathways, producing anti-anxiety and relaxation effects. Olfactory stimulation using relevant EOs can enhance the degree of physiological relaxation, which is consistent with the findings of this study. These results suggest that the volatile components of C. lanceolata EO are beneficial to human physical health.
Physiologically, to explore the central nervous response, this study investigated the impact of aroma on EEG activity by examining the effect of C. lanceolata EO on brain activity through EEG spectral topographic maps. The results of the experiment showed that there were statistical differences in the α, β, and θ wave power in specific brain regions during inhalation of the two gases. These differences indicate that this EO may play a role in neural regulation by modulating the rhythmic power of multiple brain regions. Specifically, the energy of α waves in all brain regions was significantly higher during inhalation of C. lanceolata EO (p < 0.001). This difference is related to enhanced neuronal synchrony and cortical inhibition and is associated with changes in the EEG activity of the central nervous system during the relaxation response (Kim et al., 2013). Similar studies have reported that after subjects inhaled jasmine fragrance, the power of the θ and α bands in the frontal lobe increased, accompanied by an improvement in subjective relaxation (Kafaei et al., 2024); inhaling lavender EO increases α wave activity, indicating a relaxation effect (Sayorwan et al., 2012); inhaling sweet orange and lavender EOs significantly increases α wave activity, indicating a relaxation effect (Je et al., 2021). It was observed that, compared with inhaling room air (control), the α wave power in all brain regions was significantly higher during inhalation of C. lanceolata EO (p < 0.001), which is consistent with previous findings of a significant increase in α wave power in aromatherapy studies using various plant EOs. These similar differences in EEG activity suggest that C. lanceolata EO may have a neural regulatory mechanism similar to that of other plant EOs, providing a potential relaxation effect.
Compared with room air (control), β wave power in the frontal lobe, temporal lobe, and parietal lobe was significantly lower during inhalation of C. lanceolata EO (p < 0.05). These EEG differences align with prior aromatherapy research. As previously mentioned, a study reported that under relaxation response conditions, β wave power in the frontal lobe decreased significantly compared to the control condition. This indicates that the changing trend of β wave power can serve as one of the criteria for testing the relaxation effect. A study revealed that when subjects received the Copaiba EO intervention, a significant reduction in β wave activity occurred in the left frontal region, with this effect linked to a decrease in anxiety (Zhang et al., 2022). Additionally, a study further demonstrated that inhaling cannabis EO increased α and θ wave power while decreasing the relative power of β waves, corroborating the association between β wave reduction and anti-anxiety states (Gulluni et al., 2018). Temporal lobe β activity is usually associated with hypervigilance in cognitive tasks, and the reduction of β wave power may reflect decreased temporal cortex excitability, reduce cognitive load, and have a relaxing effect (정한나, 2012). Some scholars have investigated the effects of Citrus tangerina EO on brain waves and mood, and the results showed that at the threshold concentration, both β wave power was reduced and θ wave power was enhanced—presenting a calming characteristic, suggesting that this concentration promotes relaxation by inhibiting cortical excitability (Chandharakool et al., 2020). In conclusion, the significantly lower β wave power in the frontal, temporal, and parietal lobes during inhalation of C. lanceolata EO is consistent with previous aromatherapy study results showing a relaxation effect. Our research results suggest that C. lanceolata EO can shift the subject’s brain from an active, tense state to a relaxed, calm state.
Compared with inhalation of room air (control), θ waves were higher overall during inhalation of C. lanceolata EO, particularly significant in the frontal and parietal lobes (p < 0.05). As previously mentioned, θ waves are generally associated with cognition and memory, and also occur in a state of deep relaxation. This more significant change in the frontal lobe and parietal lobe may be related to the functional characteristics and neural connections of these two brain regions (Morgan et al., 2013). As noted earlier, the frontal lobe is responsible for executive functions, emotional regulation, decision-making, etc. The abundant distribution of neurotransmitter receptors makes it more sensitive to the stimulation of aromatic molecules. As a key hub for integrating sensory information, the parietal lobe may have extensive neural fiber projections with the frontal lobe (Andersen and Cui, 2009). The synergistic effect of the two might make the θ wave power show more obvious changes under the influence of the C. lanceolata EO. Some previous studies have shown that inhalation of the volatile components of Melia azedarach flowers can increase theta brainwave activity, playing a positive regulatory role in human physiological and psychological states (Lau et al., 2021); within 0–30 s after inhaling lemon EO, the θ and α waves in regions such as the frontal lobe were activated, suggesting that short-term inhalation of EOs can affect brain activities; after inhaling Chrysanthemum indicum EO, the overall θ wave increased, indicating that it may be related to psychophysiological relaxation after inhaling the EO (Kim et al., 2018). In this study, the overall θ wave was higher during inhalation of C. lanceolata EO, which is consistent with previous research findings and may also indicate that individuals have a tendency toward deep relaxation.
Overall, EEG power shows that the C. lanceolata EO positively impacts brain activity by altering the energy of α, β, and θ waves. This finding is similar to previous research on the anti-anxiety effects of EOs based on EEG. The results of this study indicate that, compared to the occipital lobe responsible for visual function, C. lanceolata EO has a more significant effect on the frontal lobe related to emotional regulation, the temporal lobe related to olfaction, and the parietal lobe related to cognition. Therefore, based on the functional connectivity of brain regions, it can be inferred that inhaling the scent of this EO may regulate emotional states, induce relaxation responses, and simultaneously promote cognitive processes such as memory and attention.
In addition to EEG power, HRV and BP, which are closely related to the nervous and respiratory systems, are also commonly used to study the mechanisms by which EOs treat emotional disorders (Fung et al., 2021). Inhaling EOs stimulates the olfactory system and exerts influences on the neuroendocrine system, neurotransmitters, and neuromodulators (Angelucci et al., 2014). Through specific physiological pathways, this influence may ultimately induce varying degrees of change in these metrics (Kawai et al., 2020). For instance, studies have shown that inhaling EOs, such as lavender, hinoki, and petitgrain, can cause changes in HRV indicators, reflecting the oils’ ability to reduce stress and improve mood (Wu et al., 2020; Sahhoon et al., 2019; Huang and Capdevila, 2017). Previous studies have shown that the HRV indices of older adults were evaluated during gardening activities while inhaling Pseudotsuga menziesii and Lavandula angustifolia EOs. Results revealed that normalized low frequency (nLF) and LF/HF power ratios decreased, indicating a relaxing effect of the two EOs on older adults (Chung et al., 2022). Similarly, aromatherapy with lavender and Damask rose extracts reduced students’ pre-exam SBP, suggesting diminished stress-related physiological responses (Borzoo et al., 2025). In other studies, when dental patients inhaled Cymbopogon citratus EO, significant reductions in SBP, DBP, and HR were observed, implying that Cymbopogon citratus EO alleviates anxiety in this population (Maybodi et al., 2025). However, not all aromatic odors reduce stress indicators in humans; for example, one study confirmed that inhalation of grapefruit EO significantly increased DBP in healthy subjects through activation of the muscular sympathetic nerve activity (MSNA), suggesting that it may have some physiological pressure-boosting effect (Kawai et al., 2020). There have also been studies investigating the effects of inhaling rosemary oil on the sensations of test subjects, as well as its effects on various physiological parameters of the nervous system, which showed significant increases in blood pressure, heart rate, and respiratory rate following inhalation, confirming the stimulatory effects of rosemary oil (Sayorwan et al., 2013). The results of the study showed that compared with that during inhalation of room air (control), the stress indicators including HR, LF, and LF/HF ratio were significantly lower (p < 0.01) during inhalation of C. lanceolata EO, while SDNN and HF were significantly higher (p < 0.01) in the same condition. Additionally, both SBP and DBP were significantly lower (p < 0.01). These findings align with prior research on EOs-induced autonomic nervous system regulation, collectively suggesting that inhaling C. lanceolata EO promotes nervous system self-regulation and induces a relaxation response.
Psychologically, the results of this study show that C. lanceolata EO may help improve individual psychological characteristics, which also supports previous research on the benefits of EOs in mood improvement. For instance, a study used POMS to evaluate the psychological state of middle-aged women who inhaled fir EO, the study found significant improvements in subjects’ feelings of comfort, relaxation, natural feeling, and overall mood (Kim et al., 2023). Consistently, other studies have also used POMS to evaluate the mood-improving effects of inhaling EOs. For example, one study found that inhaling the aroma of Melia azedarach Linn flowers significantly reduced depression and tension, as well as anger and fatigue, using POMS. These results suggest that Melia azedarach Linn flowers or their main compounds have some mood-enhancing effects and can be used in aromatherapy (Lau et al., 2021). Another study used the POMS self-report scale to investigate the effects of exposure to the aroma of pink jasmine flowers on the mood of college students, and the results showed that the indicators of “tension,” “fatigue,” “depression,” “panic,” and TMD were significantly reduced after college students were exposed to the aroma, which supports the idea that “the aroma of pink jasmine flowers is able to improve mood state” (Xiong et al., 2023). Another previous study using the POMS test demonstrated that inhalation of Citrus junos Sieb. ex Tanaka oil significantly reduced scores on negative mood subscales such as “tension-anxiety,” “fatigue,” and reduced TMD, confirming its role in alleviating negative emotional stress (Matsumoto et al., 2016). It is also worth noting that another study assessed the effects of two lavender EOs and their chemical components on mood using POMS. The results showed that Lavandula angustifolia (high in linalyl acetate and linalool and low in camphor) significantly reduced the negative dimension scores of “tension-anxiety” and “fatigue” as well as the TMD, indicating a significant improvement in the mood state, whereas the reduction in the TMD for Lavandula spica (with camphor) was smaller (only 2.1 points) and non-significant. Some samples even showed a reverse effect. This may be due to the fact that the stimulating ingredients (e.g., camphor) in Lavandula spica weakened the effect of mood improvement, and even led to a slight discomfort in some subjects. It has been hypothesized that the camphor content (>9%) may antagonize the sedative effect, and therefore this type of EO is not suitable for mood regulation (Tomi et al., 2018).
In other words, not all EOs have an aromatherapeutic effect. Therefore, studies on the specific aromatherapy benefits of various types of EO need to exclude as many confounding factors as possible, while utilizing multiple lines of evidence for validation. In the present study, in addition to the above physiological indicators, the potential function of C. lanceolata EO was further demonstrated by POMS. Specifically, compared to that during inhalation of room air, subjects inhaling C. lanceolata EO showed significantly lower scores on the negative dimensions of the POMS scale: tension (6.93, p < 0.001), depression (1.63, p < 0.001), fatigue (0.68, p < 0.05), and TMD (13.93, p < 0.001). Meanwhile, scores on positive dimensions—such as energy (1.30, p < 0.01) and self-esteem (1.53, p < 0.001)—were significantly higher. These findings suggest that inhalation of C. lanceolata EO may alleviate negative emotions (such as “anxiety,” “depression,” and “fatigue”) and improve mood states significantly; this is hypothesized to be associated with its anxiolytic and relaxing effects.
5 Future recommendations
This study had several limitations. Firstly, the EEG cap used in the experiment was made of textile fabric, which may make subjects feel restricted when worn. Secondly, the conductive paste applied may cause discomfort for the subjects, which could induce a stress response and affect the experimental results. For follow-up trials, it is recommended that EEG cap developers replace traditional textile fabrics with medical-grade elastic silicone or breathable nylon-blend materials to enhance the cap’s ductility and reduce pressure on the face and scalp. Developers are also advised to explore dry electrode scalp patches to minimize discomfort caused by conductive paste. In addition, no artificial pressurization was performed during the experiment, but rather room air was used as a control under daily stress conditions, and only the differences with C. lanceolata EO intervention and the relaxation effects on university students’ physiology and psychology were analyzed, without exploring its specific stress indicator recovery ability. Future similar studies should consider incorporating artificial stressors and analyzing the intervention effects of EOs under high-stress states, which would enhance the reliability and persuasiveness of the findings. Moreover, the inhalation duration in this experiment was only 5 min. The short exposure time may affect the validity of the conclusions. Future research should design specific habituation tests to record the time it takes for subjects to habituate to the odor of C. lanceolata EO, from initial detection until they can no longer perceive it subjectively. On this basis, it should also extend the inhalation cycle to examine whether this habituation process diminishes the positive effects experienced from initially noticing the odor. Finally, the sample size was relatively small (n = 40) and consisted solely of university students, resulting in limitations related to a small sample size and a homogeneous population. To deeply explore the benefits of C. lanceolata EO, future research should expand the sample size and include subjects from different backgrounds for comparative analysis, thereby enhancing the universality and reliability of the results.
6 Conclusion
In summary, significant differences were observed in physiological and psychological indicators during inhalation of C. lanceolata EO and room air (control). The main manifestations were as follows: significantly higher α and θ wave power during inhalation of C. lanceolata EO, along with inhibited β wave power, characterized by lower alertness and higher relaxation, primarily affecting the frontal, temporal, and parietal regions; sympathetic nerve activity was significantly lower and parasympathetic nerve activity was higher; negative emotions such as tension, depression, and fatigue were lower, and positive emotions such as energy and self-esteem were higher. Based on the definitions and trend implications of the indicators and the results of previous studies, it is hypothesized that it may have a positive relaxation effect on the physiological and psychological state of university students and may have the potential to alleviate stress. This study has made advances in exploring the application value of C. lanceolata EO in aromatherapy and provides novel empirical support for integrating natural substances into research on physical and mental regulation and wellbeing.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Fujian Provincial Hospital (K2019-03-006), China. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JL: Writing – original draft, Software, Writing – review & editing, Conceptualization, Resources, Investigation, Project administration, Formal analysis, Validation, Visualization, Methodology, Data curation, Supervision. ML: Conceptualization, Writing – review & editing, Validation, Project administration, Investigation, Software, Data curation, Visualization. LP: Project administration, Writing – original draft, Investigation, Writing – review & editing, Software, Conceptualization. HH: Writing – review & editing, Validation, Methodology, Software, Project administration, Resources, Conceptualization. RS: Investigation, Writing – review & editing, Conceptualization, Methodology. WC: Funding acquisition, Resources, Writing – review & editing, Project administration, Validation, Supervision, Methodology.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Laboratory of Virtual Teaching and Research on Forest Therapy Specialty of Taiwan Strait, Fujian Agriculture and Forestry University (111TD2104).
Acknowledgments
We sincerely thank all volunteers for their active participation and Professor Jianzong Lin from the Department of Wood Science and Design at the National Pingtung University of Science and Technology for providing C. lanceolata essential oil; the Environmental Education and Forest Therapy Laboratory of Fujian Agriculture and Forestry University for its resource support and financial assistance; Huizhong Lin from the Department of Cardiovascular Medicine at Union Hospital Affiliated to Fujian Medical University for her guidance in collecting and analyzing physiological data; and Professor Lianghong Wang from the College of Physics and Information Engineering at Fuzhou University for his help with equipment use and the ethical review process.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyg.2025.1638492/full#supplementary-material
References
1
Afghan R. Heysieattalab S. Zangbar H. S. Ebrahimi-Kalan A. Jafari-Koshki T. Samadzadehaghdam N. (2024). Lavender essential oil inhalation improves attentional shifting and accuracy: evidence from dynamic changes of cognitive flexibility and power spectral density of electroencephalogram signals. J. Med. Signals Sens.14:12. doi: 10.4103/jmss.jmss_57_23
2
Akbas Uysal D. Senuzun Aykar F. Uyar M. (2024). The effects of aromatherapy and music on pain, anxiety, and stress levels in palliative care patients. Support Care Cancer32:632. doi: 10.1007/s00520-024-08837-0
3
Albani C. Blaser G. Geyer M. Schmutzer G. Brähler E. Bailer H. et al . (2005). Überprüfung der gütekriterien der deutschen kurzform des fragebogens "profile of mood states" (POMS) in einer repräsentativen bevölkerungsstichprobe. Psychother. Psychosom. Med. Psychol.55, 324–330. doi: 10.1055/s-2004-834727
4
Amaro P. Fonseca C. Afonso A. Jacinto G. Gomes L. Pereira H. et al . (2024). Depression and anxiety of Portuguese university students: a cross-sectional study about prevalence and associated factors. Depress. Anxiety2024, 1–14. doi: 10.1155/2024/5528350
5
Andersen R. A. Cui H. (2009). Intention, action planning, and decision making in parietal-frontal circuits. Neuron63, 568–583. doi: 10.1016/j.neuron.2009.08.028
6
Angelucci F. L. Silva V. V. Dal Pizzol C. Spir L. G. Praes C. E. O. Maibach H. (2014). Physiological effect of olfactory stimuli inhalation in humans: an overview. Int. J. Cosmetic Sci.36, 117–123. doi: 10.1111/ics.12096
7
Angulo S. M. Occhieppo V. B. Moya C. Crespo R. Bregonzio C. (2025). Anxiolytic-like effect characterization of essential oil from local lavender cultivation. Pharmaceuticals18:624. doi: 10.3390/ph18050624
8
Asgharzade S. Ahmadzadeh A. M. Pourbagher-Shahri A. M. Forouzanfar F. (2025). Protective effects of cedrol against transient global cerebral ischemia/reperfusion injury in rat. BMC Complement. Med. Ther.25:83. doi: 10.1186/s12906-025-04827-9
9
Bellioua S. Polito F. Dilagui I. Benrazzouk K. De Feo V. Bekkouche K. et al . (2024). Chemical composition, antioxidant, antimicrobial and antibiofilm activities of essential oil from the moroccan endemic plant, calendula maroccana (ball) B. D. Jacks. J Essent Oil Bear Plants27, 678–692. doi: 10.1080/0972060X.2024.2338174
10
Bogdan C. Apostol A. Ivan V. M. Sandu O. E. Petre I. Suciu O. et al . (2024). Heart rate variability and global longitudinal strain for prognostic evaluation and recovery assessment in conservatively managed post-myocardial infarction patients. J. Clin. Med.13:5435. doi: 10.3390/jcm13185435
11
Borzoo T. Tafazoli M. Ebrahimzadeh M. Kazemi-Arpanahi H. Tabahfar R. Kamyari N. et al . (2025). The effect of aromatherapy and music therapy on blood pressure and heart rate of nursing students before taking the exam. Sci. Rep.15:12783. doi: 10.1038/s41598-025-97199-6
12
Bozova B. Golukcu M. Giuffre A. M. (2024). The effect of different hydrodistillation times on the composition and yield of bergamot (citrus bergamia risso) peel essential oil and a comparison of the cold-pressing method. Flavour Fragr. J.39, 263–270. doi: 10.1002/ffj.3789
13
Carandina A. Rodrigues G. D. Di Francesco P. Filtz A. Bellocchi C. Furlan L. et al . (2021). Effects of transcutaneous auricular vagus nerve stimulation on cardiovascular autonomic control in health and disease. Auton Neurosci. Basic Clin.236:102893. doi: 10.1016/j.autneu.2021.102893
14
Chamine I. Oken B. S. (2015). Expectancy of stress-reducing aromatherapy effect and performance on a stress-sensitive cognitive task. Evid. Based Complement. Alternat. Med.2015:419812. doi: 10.1155/2015/419812
15
Chandharakool S. Koomhin P. Sinlapasorn J. Suanjan S. Phungsai J. Suttipromma N. et al . (2020). Effects of tangerine essential oil on brain waves, moods, and sleep onset latency. Molecules25:4865. doi: 10.3390/molecules25204865
16
Chen C. J. Kumar K. J. S. Chen Y. T. Tsao N. W. Chien S. C. Chang S. T. et al . (2015). Effect of hinoki and meniki essential oils on human autonomic nervous system activity and mood states. Nat. Prod. Commun.10, 1305–1308. doi: 10.1177/1934578X1501000742
17
Chung Y. H. Chen S. J. Lee C. L. Wu C. W. Chang Y. S. (2022). Relaxing effects of breathing pseudotsuga menziesii and lavandula angustifolia essential oils on psychophysiological status in older adults. Int. J. Environ. Res. Public Health19:15251. doi: 10.3390/ijerph192215251
18
Cronbach L. J. (1951). Coefficient alpha and the internal structure of tests. Psychometrika16, 297–334. doi: 10.1007/BF02310555
19
Cui X. Matsumura J. (2020). Weathering behaviour of cunninghamia lanceolata (lamb.) hook. under natural conditions. Forests11:1326. doi: 10.3390/f11121326
20
Dell’Acqua C. Dal Bo E. Messerotti Benvenuti S. Ambrosini E. Vallesi A. Palomba D. (2020). Reduced heart rate variability is associated with vulnerability to depression and impaired attentional control to unpleasant affective stimuli. Psychophysiology57:S40. doi: 10.1016/j.jadr.2020.100006
21
Dilrukshi E. A. C. Nishiyama Y. Ito K. Nomura S. (2024). Alleviation of acute stress response by black pepper aroma administration. J. Physiol. Anthropol.43:3. doi: 10.1186/s40101-023-00352-1
22
Dolan C. V. (1994). Factor analysis of variables with 2, 3, 5 and 7 response categories: A comparison of categorical variable estimators using simulated data. British Journal of Mathematical and Statistical Psychology, 47, 309–326. doi: 10.1111/j.2044-8317.1994.tb01039
23
Doner S. I. Tuzmen H. D. Duran B. Sunar F. (2024). The effect of aromatherapy massage with lemon and peppermint essential oil on menopausal symptoms: a double-blinded, randomized placebo controlled clinical trial. Explore J. Sci. Heal.20, 313–318. doi: 10.1016/j.explore.2023.09.001
24
Duffy M. E. Twenge J. M. Joiner T. E. (2019). Trends in mood and anxiety symptoms and suicide-related outcomes among US undergraduates, 2007-2018: evidence from two national surveys. J. Adolesc. Health65, 590–598. doi: 10.1016/j.jadohealth.2019.04.033
25
Elena S. Melanie S. -B. Claire B. Thomas M. Natalie C. Megan W. et al . Prevalence and risk factors for mental health problems in university undergraduate students: a systematic review with meta-analysis; Available online at: https://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=WWJD&dbname=GARJ2021_1&filename=SJES0EBBA38530243E40DE0E533E3487CEDC&v=
26
Farimani F. D. Hosseini M. Amirahmadi S. Akbarian M. Shirazinia M. Barabady M. et al . (2024). Cedrol supplementation ameliorates memory deficits by regulating neuro-inflammation and cholinergic function in lipopolysaccharide - induced cognitive impairment in rats. Heliyon10:e30356. doi: 10.1016/j.heliyon.2024.e30356
27
Farrar A. J. Farrar F. C. (2020). Clinical aromatherapy. Nurs. Clin. North Am.55, 489–504. doi: 10.1016/j.cnur.2020.06.015
28
Florian C. Hugo S. Vincent P. Francois M. Isabelle F. Marc G. et al . (2025). Cardiac autonomic responses to cortical electrical stimulation: A SEEG study. Neuroimage, 318, 121423. doi: 10.1016/j.neuroimage.2025.121423
29
Forouzanfar F. Hosseini M. Ahmadzadeh A. M. Pourbagher-Shahri A. M. (2025). Neuroprotective effect of cedrol in a male rat model of Parkinson’s disease. Physiol. Rep.13:e70309. doi: 10.14814/phy2.70309
30
Fox N. A. Perez-Edgar K. Morales S. Brito N. H. Campbell A. M. Cavanagh J. F. et al (2024). The development and structure of the HEALthy brain and child development (HBCD) study EEG protocol. Dev. Cogn. Neurosci.69:101447. doi: 10.1016/j.dcn.2024.101447
31
Fung T. K. H. Lau B. W. M. Ngai S. P. C. Tsang H. W. H. (2021). Therapeutic effect and mechanisms of essential oils in mood disorders: interaction between the nervous and respiratory systems. Int. J. Mol. Sci.22:4844. doi: 10.3390/ijms22094844
32
Genc H. Saritas S. (2020). The effects of lavender oil on the anxiety and vital signs of benign prostatic hyperplasia patients in preoperative period. Explore16, 116–122. doi: 10.1016/j.explore.2019.07.008
33
Gho J. Song A. Park (2023). Awakening and relaxing effects of essential oils from trees. 한국산림휴양학회지27, 55–67. doi: 10.34272/forest.2023.27.4.005
34
Goncalves S. Monteiro M. Gaivao I. Matos R. S. (2024). Preliminary insights into the antigenotoxic potential of lemon essential oil and olive oil in human peripheral blood mononuclear cells. Plants Basel13:1623. doi: 10.3390/plants13121623
35
Gong X. Yang Y. Xu T. Yao D. Lin S. Chang W. (2024). Assessing the anxiolytic and relaxation effects of cinnamomum camphora essential oil in university students: a comparative study of EEG, physiological measures, and psychological responses. Front. Psychol.15:1423870. doi: 10.3389/fpsyg.2024.1423870
36
Grassini S. Segurini G. V. Koivisto M. (2022). Watching nature videos promotes physiological restoration: evidence from the modulation of alpha waves in electroencephalography. Front. Psychol.13:871143. doi: 10.3389/fpsyg.2022.871143
37
Grove J. R. Prapavessis H. (1992). Preliminary evidence for the reliability and validity of an abbreviated profile of mood states. Int. J. Sport Psychol.23, 93–109.
38
Gulluni N. Re T. Loiacono I. Lanzo G. Gori L. Macchi C. et al . (2018). Cannabis essential oil: a preliminary study for the evaluation of the brain effects. Evid. Based Complement. Altern. Med.2018:1709182. doi: 10.1155/2018/1709182
39
Hedigan F. Sheridan H. Sasse A. (2023). Benefit of inhalation aromatherapy as a complementary treatment for stress and anxiety in a clinical setting-a systematic review. Complement. Ther. Clin. Pract.52:101750. doi: 10.1016/j.ctcp.2023.101750
40
Hongratanaworakit T. Buchbauer G. (2005). “Human behavioral and physiological reactions to inhalation of sweet orange oil” in WOCMAP III: quality, efficacy, safety, processing and trade in MAPs [internet]. eds. BrovelliE.ChansakaowS.FariasD.HongratanaworakitT.OmaryM. B.VejabhikulS. (Leuven 1: International Society Horticultural Science), 75–81. ACTA HORTICULTURAE
41
Hu F. Yao P. He K. Yang X. Gouda M. A. Zhang L. (2024). Effects of emotional olfactory stimuli on modulating angry driving based on an EEG connectivity study. Int. J. Neural Syst.34:2450058. doi: 10.1142/S0129065724500588
42
Huang L. Capdevila L. (2017). Aromatherapy improves work performance through balancing the autonomic nervous system. J. Altern. Complement. Med.23, 214–221. doi: 10.1089/acm.2016.0061
43
Ikei H. Song C. Miyazaki Y. (2016). Effects of olfactory stimulation by α-pinene on autonomic nervous activity. Journal of Wood Science, 62, 568–572. doi: 10.1007/s10086-016-1576-1
44
Jacobs G. D. Benson H. Friedman R. (1996). Topographic EEG mapping of the relaxation response. Biofeedback Self Regul.21, 121–129. doi: 10.1007/BF02284691
45
Je Y. An S. Ro H. Cho J. Seunghee B. (2021). Effects of the essential oils of sweet orange, lavender and amyris on EEG activity. 아시안뷰티화장품학술지19, 651–664. doi: 10.20402/ajbc.2021.0235
46
Juran S. A. Lundström J. N. Geigant M. Kumlien E. Fredrikson M. Åhs F. et al . (2016). Unilateral resection of the anterior medial temporal lobe impairs odor identification and valence perception. Frontiers in Psychology, 6. doi: 10.3389/fpsyg.2015.02015
47
Kafaei M. Burry J. Latifi M. Ciorciari J. (2024). “Designing a systematic experiment to investigate the effect of ambient smell on human emotions in the indoor space; introducing a mixed-method approach” in Phygital intelligence, CDRF 2023. eds. YanC.ChaiH.SunT.YuanP. F. (Singapore: Springer-Verlag Singapore Pte Ltd), 235–247. (Computational Design and Robotic Fabrication)
48
Kagawa D. Jokura H. Ochiai R. Tokimitsu I. Tsubone H. (2003). The sedative effects and mechanism of action of cedrol inhalation with behavioral pharmacological evaluation. Planta Med.69, 637–641. doi: 10.1055/s-2003-41114
49
Kato M. Okumura T. Tsubo Y. Honda J. Sugiyama M. Touhara K. et al . (2022). Spatiotemporal dynamics of odor representations in the human brain revealed by EEG decoding. Proc. Natl. Acad. Sci. USA119:e2114966119. doi: 10.1073/pnas.2114966119
50
Kawai E. Takeda R. Ota A. Morita E. Imai D. Suzuki Y. et al . (2020). Increase in diastolic blood pressure induced by fragrance inhalation of grapefruit essential oil is positively correlated with muscle sympathetic nerve activity. J. Physiol. Sci.70:2. doi: 10.1186/s12576-020-00733-6
51
Kim D. S. Goo Y. M. Cho J. Lee J. Lee D. Y. Sin S. M. et al . (2018). Effect of volatile organic chemicals in chrysanthemum indicum Linne on blood pressure and electroencephalogram. Molecules23:2063. doi: 10.3390/molecules23082063
52
Kim M. G. Lee H. S. (2015). Growth-inhibiting effects and chemical composition of essential oils extracted from Platycladus orientalis leaves and stems toward human intestinal bacteria. Food Sci. Biotechnol.24, 427–431. doi: 10.1007/s10068-015-0056-5
53
Kim S. C. Lee M. H. Jang C. Kwon J. W. Park J. W. (2013). The effect of alpha rhythm sleep on EEG activity and individuals’ attention. J. Phys. Ther. Sci.25, 1515–1518. doi: 10.1589/jpts.25.1515
54
Kim C. Lee G. Song C. (2023). The effect of short-term inhalation of fir essential oil on autonomic nervous activity in middle-aged women. Explore19, 820–826. doi: 10.1016/j.explore.2023.04.006
55
Kopanska M. Ochojska D. Sarzynska I. Bartkowska O. Szczygielski J. (2025). Quantitative and qualitative electroencephalography in the diagnosis and monitoring of depression. A modern approach to clinical neurophysiology. Frontiers in Human Neuroscience, 19, 1624434. doi: 10.3389/fnhum.2025.1624434
56
Kremen V. Sladky V. Mivalt F. Gregg N. M. Brinkmann B. H. Balzekas I. et al . (2025). Modulating limbic circuits in temporal lobe epilepsy: impacts on seizures, memory, mood and sleep. Brain Commun.7:fcaf 106. doi: 10.1093/braincomms/fcaf106
57
Kuspradini H. Putri A. S. Sukaton E. Mitsunaga T. (2016). Bioactivity of essential oils from leaves of dryobalanops lanceolata, cinnamomum burmannii, cananga odorata, and scorodocarpus borneensis. Agric. Agric. Sci. Procedia9, 411–418. doi: 10.1016/j.aaspro.2016.02.157
58
Kumar E. N. Marathe P. A. Kamat S. K. Havaldar H. Eldhose M. Mall P. (2023). Experimental evaluation of hypnotic and antidepressant effect of pine needles of cedrus deodara. Journal of Ayurveda and Integrative Medicine, 14(2), 100707. doi: 10.1016/j.jaim.2023.100707
59
Lau K. M. Su W. S. Chien S. C. Wang S. Y. Kumar K. J. S. (2021). Melia azedarach flowers and their volatile components improved human physiological and psychological functions. J. Essent. Oil Bear. Plants24, 1200–1211. doi: 10.1080/0972060X.2021.1978869
60
Lee S.-M. (2018). A study on the influence of cypress (cupressus sempervirens L.) essential oil inhalation on relaxation of employment stress of college students. 패션과 니트16, 20–27. doi: 10.35226/kskd.2018.16.1.20
61
Lee B. G. Lee B. L. Chung W. Y. (2014). Mobile healthcare for automatic driving sleep-onset detection using wavelet-based EEG and respiration signals. Sensors14, 17915–17936. doi: 10.3390/s141017915
62
Li Y. Chen J. Yang Y. Li C. Peng W. (2020). Chemical compositions and functions of Chinese fir volatiles. Therm. Sci.24, 1853–1859. doi: 10.2298/TSCI190601073L
63
Liu S. H. Lin T. H. Chang K. M. (2013). The physical effects of aromatherapy in alleviating work-related stress on elementary school teachers in Taiwan. Evid. Based Complement. Altern. Med.2013:853809. doi: 10.1155/2013/853809
64
Lucena L. Santos-Junior J. G. Tufik S. Hachul H. (2024). Effect of a lavender essential oil and sleep hygiene protocol on insomnia in postmenopausal women: a pilot randomized clinical trial. Explore20, 116–125. doi: 10.1016/j.explore.2023.07.004
65
Mahmut M. K. Classen C. P. Croy I. Hummel T. (2023). Smelling an odor present during PMR does not impact heart rate during a stressful cognitive task. J. Sens. Stud.38:e12889. doi: 10.1111/joss.12889
66
Maksimenko V. A. Runnova A. E. Frolov N. S. Makarov V. V. Nedaivozov V. Koronovskii A. A. et al . (2018). Multiscale neural connectivity during human sensory processing in the brain. Phys. Rev. E97:052405. doi: 10.1103/PhysRevE.97.052405
67
Mank-halati M. S. Rezaei M. Farzaei M. H. Khatony A. (2024). Comparing the effects of rosemary aromatherapy and music therapy on anxiety levels in patients undergoing general surgery: a randomized controlled clinical trial. Explore20:102976. doi: 10.1016/j.explore.2024.01.002
68
Matsubara E. Matsui N. Kambara K. (2023). Utilization of essential oils mainly from cupressaceae trees in the work environment creates a psychophysiological stress-relieving effect. Wood Sci. Technol.57, 1197–1214. doi: 10.1007/s00226-023-01490-6
69
Matsubara E. Tsunetsugu Y. Ohira T. Sugiyama M. (2017). Essential oil of japanese cedar (cryptomeria japonica) wood increases salivary dehydroepiandrosterone sulfate levels after monotonous work. Int. J. Environ. Res. Public Health14:97. doi: 10.3390/ijerph14010097
70
Matsumoto T. Kimura T. Hayashi T. (2016). Aromatic effects of a Japanese citrus fruit-yuzu (citrus junos sieb. ex tanaka)-on psychoemotional states and autonomic nervous system activity during the menstrual cycle: a single-blind randomized controlled crossover study. Bio Psycho. Soc. Med.10:11. doi: 10.1186/s13030-016-0063-7
71
Maybodi F. R. Herandi V. Vaezpour M. S. (2025). Effect of aromatherapy with lemongrass (cymbopogon citratus) on the anxiety of patients undergoing scaling and root planning: a randomized clinical trial. BMC Complement. Med. Ther.25:100. doi: 10.1186/s12906-025-04834-w
72
Moon S. A. Bae J. Kim K. Cho S. Y. Kwon G. Lee R. et al . (2020). EEG revealed that fragrances positively affect menopausal symptoms in mid-life women. Exp. Neurobiol.29, 389–401. doi: 10.5607/en20036
73
Morgan H. M. Jackson M. C. van Koningsbruggen M. G. Shapiro K. L. Linden D. E. J. (2013). Frontal and parietal theta burst TMS impairs working memory for visual-spatial conjunctions. Brain Stimul.6, 122–129. doi: 10.1016/j.brs.2012.03.001
74
Nejati V. Majdi R. Salehinejad M. A. Nitsche M. A. (2021). The role of dorsolateral and ventromedial prefrontal cortex in the processing of emotional dimensions. Scientific Reports, 11, 1971. doi: 10.1038/s41598-021-81454-7
75
Nickel J. Seitz R. J. (2005). Functional clusters in the human parietal cortex as revealed by an observer-independent meta-analysis of functional activation studies. Anat. Embryol.210, 463–472. doi: 10.1007/s00429-005-0037-1
76
Oezek G. Schepetkin I. A. Yermagambetova M. oezek T. Kirpotina L. N. Almerekova S. S. et al . (2021). Innate immunomodulatory activity of cedrol, a component of essential oils isolated from juniperus species. Molecules 26, 7644. doi: 10.3390/molecules26247644
77
Perna G. Riva A. Defillo A. Sangiorgio E. Nobile M. Caldirola D. (2020). Heart rate variability: can it serve as a marker of mental health resilience? Special section on "translational and neuroscience studies in affective disorders" section editor, Maria Nobile MD, PhD. J. Affect. Disord.263, 754–761. doi: 10.1016/j.jad.2019.10.017
78
Pham T. Lau Z. J. Chen S. H. A. Makowski D. (2021). Heart rate variability in psychology: a review of HRV indices and an analysis tutorial. Sensors21:3998. doi: 10.3390/s21123998
79
Pourshaikhian M. Moghadamnia M. T. Leyli E. K. Kisomi Z. S. (2024). Effects of aromatherapy with matricaria chamomile essential oil on anxiety and hemodynamic indices in patients with acute coronary syndrome, 2021: a randomized controlled trial. BMC Complement. Med. Ther.24:17. doi: 10.1186/s12906-023-04326-9
80
Rastegar-Moghaddam S. H. Amirahmadi S. Akbarian M. Sharizina M. Beheshti F. Rajabian A. et al . (2024). Cardioprotective effect of cedrol in an inflammation systemic model induced by lipopolysaccharide: biochemical and histological verification. J. Cardiovasc. Thorac. Res.16, 120–128. doi: 10.34172/jcvtr.33112
81
Sahhoon P. Jeong H. S. Jang S. Kim S. J. Park J. S. (2019). Effects of chamaecyparis obtusa essential oil on the autonomic nervous system. 조선자연과학논문집12, 74–77. doi: 10.13160/ricns.2019.12.3.74
82
Sayorwan W. Ruangrungsi N. Piriyapunyporn T. Hongratanaworakit T. Kotchabhakdi N. Siripornpanich V. (2013). Effects of inhaled rosemary oil on subjective feelings and activities of the nervous system. Sci. Pharm.81, 531–542. doi: 10.3797/scipharm.1209-05
83
Sayorwan W. Siripornpanich V. Piriyapunyaporn T. Hongratanaworakit T. Kotchabhakdi N. Ruangrungsi N. (2012). The effects of lavender oil inhalation on emotional states, autonomic nervous system, and brain electrical activity. J. Med. Assoc. Thail.95, 598–606.
84
Shahimi N. H. Goh C. H. Mat S. Lim R. Koh V. C. A. Nyman S. R. et al . (2022). Psychological status and physical performance are independently associated with autonomic function. Biomed. Eng. Online21:29. doi: 10.1186/s12938-022-00996-7
85
Solomon D. A. Prasad N. Beautily V. Thenmozhi P. Madaswamy R. Deepika D. (2024). Effect of lavender oil on social anxiety among first-year college students. J. Pharm. Bioallied Sci.16, S2907–S2909. doi: 10.4103/jpbs.jpbs_601_24
86
Sowndhararajan K. Kim S. (2016). Influence of fragrances on human psychophysiological activity: with special reference to human electroencephalographic response. Sci. Pharm.84, 724–751. doi: 10.3390/scipharm84040724
87
Su Y. C. Hsu K. P. Wang E. I. C. Ho C. L. (2012). Composition, anticancer, and antimicrobial activities in vitro of the heartwood essential oil of cunninghamia lanceolata var. konishii from Taiwan. Nat. Prod. Commun.7, 1245–1247. doi: 10.1177/1934578X1200700938
88
Tomi K. Kitao M. Murakami H. Matsumura Y. Hayashi T. (2018). Classification of lavender essential oils: sedative effects of lavandula oils. J. Essent. Oil Res.30, 56–68. doi: 10.1080/10412905.2017.1377122
89
Tomitani N. Kanegae H. Suzuki Y. Kuwabara M. Kario K. (2021). Stress-induced blood pressure elevation self-measured by a wearable watch-type device. Am. J. Hypertens.34, 377–382. doi: 10.1093/ajh/hpaa139
90
Usman L. A. Ismaeel R. O. Kamaldeen M. A. (2024). Effect of time of harvest on the chemical composition and antioxidant potential of leaf essential oil of syzygium guineense growing in north Central Nigeria (Willd.) DC. Var.J. Mex. Chem. Soc.68, 201–209. doi: 10.29356/jmcs.v68i2.2035
91
Vora L. K. Gholap A. D. Hatvate N. T. Naren P. Khan S. Chavda V. P. et al . (2024). Essential oils for clinical aromatherapy: a comprehensive review. J. Ethnopharmacol.330:118180. doi: 10.1016/j.jep.2024.118180
92
Wakui N. Togawa C. Ichikawa K. Matsuoka R. Watanabe M. Okami A. et al . (2023). Relieving psychological stress and improving sleep quality by bergamot essential oil use before bedtime and upon awakening: a randomized crossover trial. Complement. Ther. Med.77:102976. doi: 10.1016/j.ctim.2023.102976
93
Wu C. Y. Lee H. F. Chang C. W. Chiang H. C. Tsai Y. H. Liu H. E. (2020). The immediate effects of lavender aromatherapy massage versus massage in work stress, burnout, and HRV parameters: a randomized controlled trial. Evid. Based Complement. Altern. Med.2020:8830083. doi: 10.1155/2020/8830083
94
Xin H. L. Zhai X. F. Zheng X. Zhang L. Wang Y. L. Wang Z. (2012). Anti-inflammatory and analgesic activity of total flavone of cunninghamia lanceolata. Molecules17, 8842–8850. doi: 10.3390/molecules17088842
95
Xiong X. Jin H. Hu W. Zeng C. Huang Q. Cui X. et al . (2023). Benefits of jasminum polyanthum’s natural aromas on human emotions and moods. Urban For. Urban Green.86:128010. doi: 10.1016/j.ufug.2023.128010
96
Yamashita K. Uto A. Uchino M. Shidou R. Kibe T. Sugimura M. (2021). Sympathetic nerve activity during tooth extraction in women is related to dental anxiety immediately after surgery. J. Oral Maxillofac. Surg.79, 2268.e1–2268.e5. doi: 10.1016/j.joms.2021.06.019
97
Yin X. J. Lin G. P. Wu X. Y. Huang R. Xu C. J. Yao M. Y. (2024). Effects of lavender essential oil inhalation aromatherapy on depression and sleep quality in stroke patients: a single-blind randomized controlled trial. Complement. Ther. Clin. Pract.55:101828. doi: 10.1016/j.ctcp.2024.101828
98
Zhang N. Chen J. Dong W. Yao L. (2022). The effect of copaiba oil odor on anxiety relief in adults under mental workload: a randomized controlled trial. Evid. Based Complement. Altern. Med.2022:3874745. doi: 10.1155/2022/3874745
99
Zhang N. Liu C. Ren Y. Li W. Chen R. Gao W. (2025). Effects of plant aromas on stress recovery and brain activity in university students. Build. Environ.273:112716. doi: 10.1016/j.buildenv.2025.112716
100
Zhu B. L. (1995). Brief Introduction of POMS Scale and Its Model for China. J. Tianjin Inst. Phys. Educ.1, 35–37.
101
김남정 Lim Y. S. (2012). The verification of intervening effect of youth activity on the relationship between stress and life satisfaction of adolescents. 청소년학연구19, 219–240.
102
정한나 J. C. (2012). Effects of origanum majorana essential oil aroma on the electroencephalograms of female young adults with sleep disorders. 생명과학회지22, 1077–1084. doi: 10.5352/JLS.2012.22.8.1077
Summary
Keywords
Cunninghamia lanceolata , essential oil, aromatherapy, electroencephalogram, natural therapy, health benefits
Citation
Liu J, Liu M, Peng L, He H, Sun R and Chang W (2025) Effects of inhaling Cunninghamia lanceolata essential oil on the physiological and psychological relaxation of university students. Front. Psychol. 16:1638492. doi: 10.3389/fpsyg.2025.1638492
Received
13 June 2025
Accepted
27 August 2025
Published
24 September 2025
Volume
16 - 2025
Edited by
Abdullah Akpinar, Adnan Menderes University, Türkiye
Reviewed by
Mehmet Mahmut, Macquarie University, Australia
Kenneth Y. T. Lim, Nanyang Technological University, Singapore
Updates
Copyright
© 2025 Liu, Liu, Peng, He, Sun and Chang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiyin Chang, 000q821019@fafu.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.