Abstract
Background:
Anxiety and depression are leading contributors to disability worldwide. In Mexico, an upper-middle-income country (UMIC) with health-system constraints resembling those of low- and middle-income countries (LMICs), access to psychotherapy remains limited. Digital therapeutics provide a scalable approach to bridging this gap. Aurora is a Spanish-language, self-guided mobile application delivering cognitive behavioral therapy (CBT) through eight interactive modules. This study evaluated its feasibility, usability, and preliminary clinical impact among pharmacologically treated adults with anxiety and depression.
Methods:
We conducted a mixed-methods, pre–post feasibility study in Mexico between March and October 2024. Participants were 46 adults (18–45 years) with psychiatrist-confirmed mild-to-moderate anxiety and/or depression on stable pharmacotherapy; 38 (82.6%) completed baseline and follow-up. Usability was assessed with the System Usability Scale (SUS) and the user Mobile App Rating Scale (uMARS). Symptoms were measured with the Spanish Goldberg Anxiety and Depression Scale (GADS). The number of modules completed defined engagement, categorized as follows: low (0–2), moderate (3–5), and high (6–7). Analyses included paired t-tests, non-parametric trend tests, correlations, and adjusted regressions.
Results:
Anxiety scores decreased from 13.2 (SD 5.6) to 10.6 (SD 5.1) (t(37) = 3.81, p = 0.001; d = 0.49), and depression scores from 11.1 (SD 5.2) to 8.4 (SD 4.9) (t(37) = 4.75, p < 0.001; d = 0.54). Engagement showed a graded dose–response: high-engagement users (n = 17) achieved larger reductions (Δ anxiety = −3.9; Δ depression = −4.0) than low-engagement users (Δ anxiety = −0.9; Δ depression = −1.0). Regression confirmed each additional module predicted incremental reductions in both anxiety (β = −0.38, p = 0.009) and depression (β = −0.41, p = 0.006). Usability ratings were high (SUS = 78.5/100; uMARS = 4.2/5).
Conclusion:
Aurora was feasible, usable, and associated with moderate symptom reduction in pharmacologically treated patients. The observed dose–response underscores the clinical importance of sustained engagement. These findings highlight the potential of co-designed, culturally tailored digital therapeutics to expand access to mental health care in Spanish-speaking UMIC and LMIC settings, warranting confirmation in larger controlled trials.
1 Introduction
Mental health disorders are a leading contributor to the global burden of disease. Anxiety and depression together account for more than 10% of all years lived with disability worldwide, affecting over 600 million people annually (GBD 2019 Diseases and Injuries Collaborators, 2020). Despite the availability of evidence-based treatments such as cognitive behavioral therapy (CBT), more than 75% of individuals in low- and middle-income countries (LMICs) receive no adequate mental health care (Patel et al., 2018).
In Mexico, anxiety and depression are the most prevalent psychiatric disorders, with lifetime prevalence estimates of 14.3 and 9.2%, respectively (Medina-Mora et al., 2005). The national health system faces significant challenges: a shortage of fewer than three psychiatrists per 100,000 inhabitants, fragmented integration between psychological and pharmacological care, stigma, and high out-of-pocket costs (Berenzon Gorn et al., 2013). The COVID-19 pandemic further intensified unmet needs by increasing psychological distress and disrupting access to in-person treatment (Hernandez-Diaz et al., 2022). Although the World Bank classifies Mexico as an upper-middle-income country (UMIC), its health system continues to share critical structural limitations with LMICs, including resource scarcity, inequitable access, and digital divides. This contextual nuance highlights that findings from Mexico can inform broader Latin American strategies, as many middle-income health systems in the region face similar constraints.
Digital therapeutics represent a promising approach to bridge this gap. Mobile health (mHealth) applications can deliver validated psychological interventions at scale, overcoming barriers of geography, stigma, and limited specialist availability (Naslund et al., 2017). Robust evidence demonstrates that digital CBT reduces anxiety and depressive symptoms across diverse populations (Firth et al., 2017; Linardon et al., 2019). In Latin America, emerging studies have confirmed the feasibility and effectiveness of smartphone-based mental health interventions (Araya et al., 2021; Karyotaki et al., 2023; Kim et al., 2023). Nevertheless, most commercially available mental health apps lack clinical validation, cultural adaptation, and regulatory oversight (Larsen et al., 2016).
Aurora was developed to address these shortcomings. It is a Spanish-language, self-guided mobile application delivering structured CBT through eight interactive modules. Its content—behavioral activation, cognitive restructuring, and mindfulness-based practices—was selected based on evidence linking these elements to improved outcomes in digital CBT (Cuijpers et al., 2022; Furukawa et al., 2025). Development followed a co-creation framework, incorporating clinicians, patients, and usability experts to ensure both therapeutic fidelity and user-centered design. Importantly, engagement has emerged as a consistent mediator of digital CBT outcomes, with module completion predicting greater symptom reduction (Gan et al., 2021). Assessing usability and engagement was therefore a core objective of this feasibility study.
This pilot study aimed to evaluate the feasibility, usability, and preliminary clinical impact of Aurora in a sample of pharmacologically treated patients with anxiety and depression in Mexico. We hypothesized that Aurora would be associated with reductions in self-reported symptoms, demonstrate high usability, and show a graded relationship between engagement and clinical outcomes.
2 Methods
2.1 Study design and setting
We conducted a mixed-methods, pre–post feasibility study of Aurora, a Spanish-language, self-guided digital therapeutic mobile application based on CBT principles. The trial was carried out in Mexico between March and October 2024 and was not powered for definitive efficacy testing. We adhered to the CONSORT extension for pilot and feasibility trials (Eldridge et al., 2016) and simulation-based recommendations for pilot sample sizes (Teare et al., 2014; Whitehead et al., 2016). A sample of 38 completers was sufficient to estimate feasibility and usability parameters, as well as symptom change variance, to inform a future randomized controlled trial (RCT). The study followed an iterative human-centered design process incorporating co-design with expert stakeholders, clinical usability testing, and pre–post evaluation of anxiety and depression symptoms.
2.2 Ethical considerations
The study complied with the Declaration of Helsinki and the ethical standards of the Comisión Nacional de Bioética (CONBIOÉTICA) in Mexico. The protocol (INNOVA 2024-01) received approval from the Comité de Ética en Investigación (Folio CEI-000002) and the Comité de Investigación (Folio CI-000002) of Médica Sur, S.A.B. de C.V. Review covered all procedures, including consent forms, participant interaction materials, and data protection safeguards. Written informed consent was obtained from all participants. As the intervention was a non-invasive digital therapeutic without pharmacological components, no additional physical risk was posed.
2.3 Expert advisory and co-creation panels
A multidisciplinary advisory board, comprising psychiatrists, psychologists, digital health specialists, and UX designers, guided the development of the app. Three activities structured this process: (i) workshops with psychiatrists and psychologists (n = 20) to assess clinical validity and therapeutic coherence; (ii) heuristic and accessibility evaluation by UX experts based on Nielsen’s principles, which identified issues such as excessive cognitive load, unclear navigation, and insufficient accessibility; and (iii) structured focus groups with psychiatric patients (n = 12) to assess emotional needs, use contexts, and perceived value. These activities informed successive redesigns of the interface and content.
2.4 Participants and recruitment
Participants were recruited through referrals from psychiatrists and online outreach across metropolitan areas in Mexico. Inclusion criteria were: age 18–45 years; a psychiatrist-confirmed diagnosis of mild-to-moderate anxiety and/or depression; stable pharmacotherapy for at least 1 month; and access to a smartphone with internet. Exclusion criteria were severe psychiatric comorbidity (e.g., psychosis, active suicidality) or concurrent psychotherapy. Excluding psychotherapy reduced co-intervention bias and reflected prior digital CBT trials (Johansson et al., 2019; Kambeitz-Ilankovic et al., 2022). By contrast, stable pharmacotherapy was permitted to reflect real-world practice. Of the 46 participants who initiated app use, 38 completed both pre- and post-assessments and were included in the analyses. Demographics, treatment history, and engagement were collected via digital questionnaires.
2.5 Intervention: Aurora app
Aurora is a Spanish-language, self-guided digital therapeutic classified as a Software as a Medical Device (SaMD) designed to complement pharmacotherapy for anxiety and depression. Its development followed human-centered design principles, informed by clinical guidelines, heuristic evaluation, and patient co-creation. The app integrates CBT elements with established digital efficacy, including cognitive restructuring, behavioral activation, and mindfulness training, which are prioritized both by the advisory board and by evidence from meta-analyses.
Aurora comprises eight sequentially unlockable modules targeting emotional self-regulation and cognitive restructuring (Figure 1). Modules incorporate audio-guided breathing exercises, mindfulness training, psychoeducation, behavioral activation, and structured activities to promote reframing and emotional awareness (Figure 2). At module completion, users receive personalized reports summarizing activities and symptom trajectories. Activity logs and gamified progression reinforce adherence. The interface was iteratively refined using Nielsen’s heuristics, with accessibility features such as high-contrast design, simplified icons, captions, and text-to-audio conversion. The app is compatible with both Android and iOS devices and requires continuous internet access for synchronization and to receive updated content. All data were encrypted and managed in accordance with the Mexican Federal Law on the Protection of Personal Data Held by Private Parties.
Figure 1
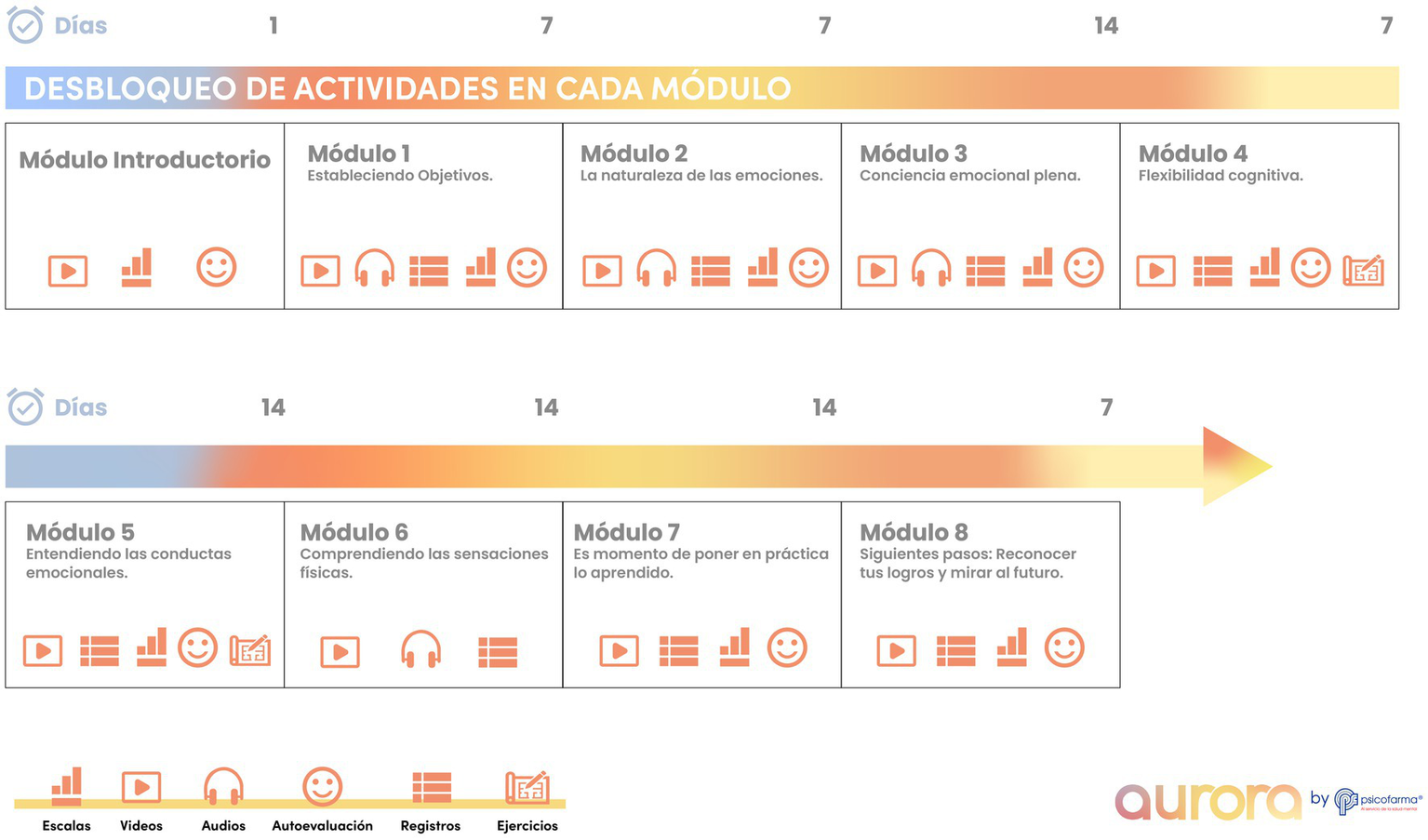
Aurora app architecture and therapeutic features. Schematic overview of Aurora, a Spanish-language, self-guided mobile application delivering cognitive behavioral therapy (CBT) for depression and anxiety. The app includes eight sequentially unlockable modules integrating breathing exercises, mindfulness training, psychoeducation, cognitive restructuring, and behavioral activation. Core functions include: (1) self-assessment tools for daily mood and symptom monitoring, (2) multimedia psychoeducational videos, (3) guided audio relaxation and CBT exercises, (4) structured activity logs and reflections, and (5) progressive unlocking of modules to encourage sustained engagement. Accessibility features include high-contrast icons, text-to-audio options, and captioned multimedia content.
Figure 2

Aurora user interface and digital experience. Representative screenshots of the Aurora app, illustrating core features and user journey. Panels show: (1) the home interface with module progression, (2) breathing and mindfulness exercises, (3) psychoeducational content on cognitive restructuring, (4) self-assessment scales for anxiety, depression, and positive emotions, (5) graphical feedback of scores, (6) access to technical support, and (7) automatically generated personal and medical reports. Multimedia integration, gamified feedback, and encrypted data management support user engagement and adherence.
2.6 Outcome measures
Primary outcomes. Usability was assessed with the Spanish-validated System Usability Scale (SUS) (Sevilla-Gonzalez et al., 2020) and the user Mobile App Rating Scale (uMARS) (Martin-Payo et al., 2021). Qualitative feedback was thematically analyzed to identify barriers, benefits, and design improvements. Engagement was measured via in-app analytics of time, module completion, and frequency of use, and categorized into low (0–2), moderate (3–5), and high (6–7) engagement groups to test dose–response associations (Gan et al., 2021).
Secondary outcomes. Self-reported symptoms of anxiety and depression were measured using the Spanish Goldberg Anxiety and Depression Scale (GADS), comprising two nine-item subscales. Validated versions in Latin America were used (Espinosa et al., 2015; Martín Carbonell et al., 2016; Reivan-Ortiz et al., 2019). Internal consistency was assessed in our sample, with Cronbach’s α reported in the Results. Details of scale items, scoring, and validation are provided in Supplementary Table S1.
2.7 Statistical analysis
Analyses were conducted in Python and SPSS v27. Within-group changes in anxiety and depression were tested using paired two-tailed t-tests, with Cohen’s d and 95% CIs reported. Engagement–outcome associations were examined using Kruskal–Wallis and Jonckheere–Terpstra tests, Spearman’s ρ, and adjusted OLS regressions (controlling for baseline severity, age, sex). Regression models reported β coefficients, SEs, 95% CIs, and p-values. Exploratory subgroup analyses were conducted stratifying participants by age, sex, and baseline severity. Feasibility metrics included recruitment, retention, and usability scores.
3 Results
3.1 Participant characteristics
A total of 46 individuals initiated the intervention, of whom 38 (82.6%) completed both the pre- and post-assessments and were included in the final analysis. Participants were predominantly female (73.7%), aged 18–45 years (mean = 31.2 years; SD = 6.4), with the majority holding a university-level education (78.9%). All participants had been previously diagnosed with anxiety and/or depression by a psychiatrist and were undergoing pharmacological treatment during the study period (mean = 1.4 psychotropic medications; range: 1–3). No participant received concurrent psychotherapy, as this was not in line with the eligibility criteria. Baseline demographic and clinical characteristics are shown in Table 1.
Table 1
| Characteristic | Value |
|---|---|
| Age, mean (SD), y | 31.2 (6.4) |
| Age range, y | 18–45 |
| Female sex, n (%) | 28 (73.7) |
| Education, university or higher, n (%) | 30 (78.9) |
| Diagnosis, n (%) | Anxiety only: 14 (36.8) Depression only: 10 (26.3) Both anxiety and depression: 14 (36.8) |
| Duration of pharmacological treatment, mean (SD), mo | 11.4 (7.8) |
| Psychotropic medication use, n (%) | 38 (100) |
| Number of medications, mean (range) | 1.4 (1–3) |
Baseline demographic and clinical characteristics of participants.
The Goldberg Anxiety and Depression Scale (GADS) demonstrated acceptable internal consistency in our sample (Cronbach’s α = 0.82 for anxiety; 0.80 for depression). The item structure and scoring are summarized in Supplementary Table S1.
3.2 Usability and user experience
Usability testing with a subset of 15 users identified barriers, including excessive text density, limited interaction feedback, and inconsistencies in navigation. Iterative refinements addressed these issues.
Final usability metrics indicated high acceptability, with a mean System Usability Scale score of 78.5/100, exceeding the commonly accepted threshold for “good” usability. On the uMARS, users rated engagement, functionality, esthetics, and information quality above 4.0/5, consistent with validated benchmarks.
Participants reported that the app was easy to use, relevant to their daily lives, and aligned with their therapeutic needs.
3.3 Perceived impact
Thirty-one of 38 participants (81.6%) reported perceivable psychological improvements attributed to Aurora, particularly in emotional self-regulation, anxiety control, and integration of breathing and mindfulness exercises into daily routines. The highest perceived impact was observed among users with high engagement.
Qualitative responses reinforced quantitative findings. Representative themes included: “learning how to manage thoughts more effectively,” “feeling calmer during the day,” and “feeling supported between therapy sessions.” Common barriers included limited offline access and the desire for more personalized options.
3.4 Changes in anxiety and depression symptoms
Use of the Aurora app was associated with statistically significant and clinically meaningful reductions in self-reported symptoms of anxiety and depression (Table 2).
Table 2
| Outcome | Baseline, mean (SD) | Post, mean (SD) | Mean Δ (SD) | t(df) | p-value | Cohen’s d (95% CI) |
|---|---|---|---|---|---|---|
| Anxiety score | 13.2 (5.6) | 10.6 (5.1) | −2.6 (3.5) | 3.81 (37) | 0.001 | 0.49 (0.22–0.76) |
| Depression score | 11.1 (5.2) | 8.4 (4.9) | −2.7 (3.4) | 4.75 (37) | <0.001 | 0.54 (0.28–0.80) |
Pre and post-changes in anxiety and depression symptoms.
Scores derived from the Spanish Goldberg Anxiety and Depression Scale. Higher scores reflect greater symptom severity.
Anxiety symptoms: Mean scores decreased from 13.2 (SD = 5.6) at baseline to 10.6 (SD = 5.1) post-intervention (Δ = −2.6, SD = 3.5), t(37) = 3.81, p = 0.001, Cohen’s d = 0.49, 95% CI [0.22, 0.76]. Figure 3 illustrates pre–post distributions.
Figure 3
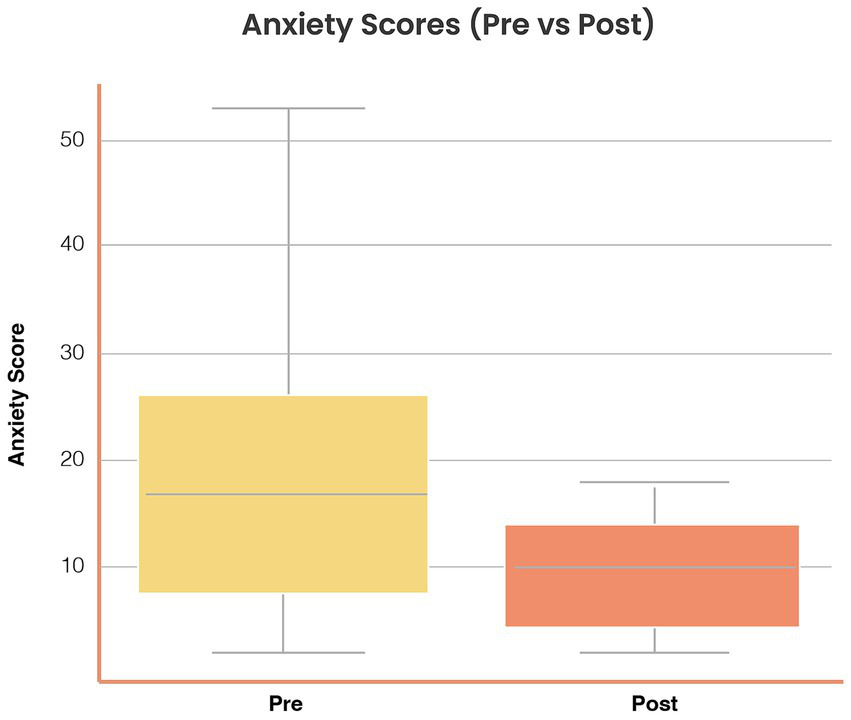
Reductions in anxiety symptoms following Aurora use. Distribution of anxiety scores (Goldberg Anxiety subscale, 0–21) at baseline (Pre) and post-intervention (Post). Mean anxiety scores decreased from 13.2 (SD = 5.6) to 10.6 (SD = 5.1), Δ = −2.6 (SD = 3.5), t(37) = 3.81, p = 0.001, Cohen’s d = 0.49 (95% CI [0.22, 0.76]). Boxplots display medians, interquartile ranges, and outliers. Symptom reductions were consistent across age and gender strata.
Depression symptoms: Mean scores decreased from 11.1 (SD = 5.2) to 8.4 (SD = 4.9) (Δ = −2.7, SD = 3.4), t(37) = 4.75, p < 0.001, Cohen’s d = 0.54, 95% CI [0.28, 0.80]. Figure 4 illustrates pre–post distributions.
Figure 4
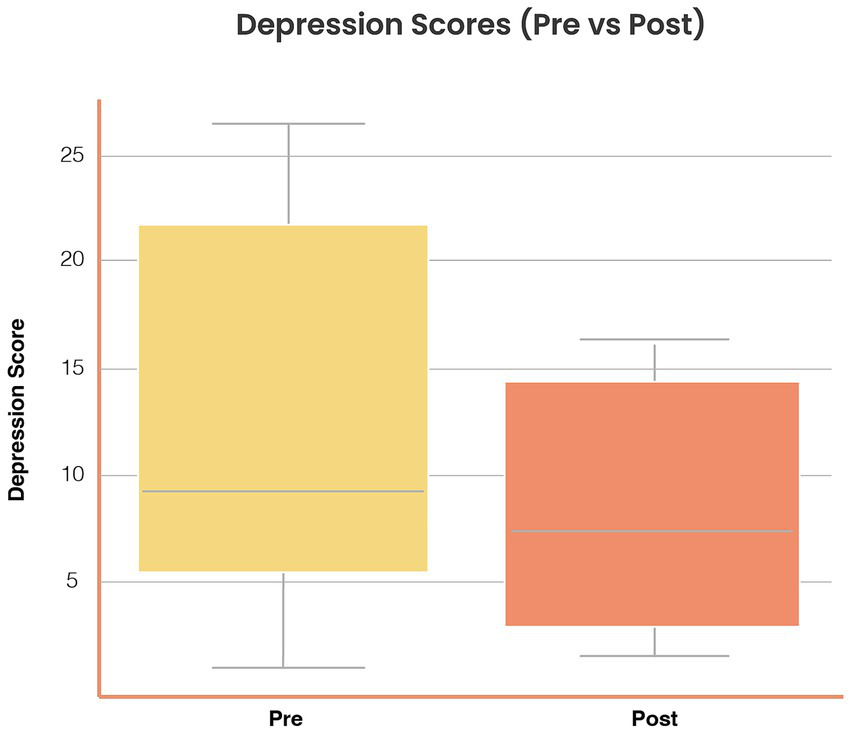
Reductions in depression symptoms following Aurora use. Distribution of depression scores (Goldberg Depression subscale, 0–21) at baseline and post-intervention. Mean depression scores decreased from 11.1 (SD = 5.2) to 8.4 (SD = 4.9), Δ = −2.7 (SD = 3.4), t(37) = 4.75, p < 0.001, Cohen’s d = 0.54 (95% CI [0.28, 0.80]). Boxplots show distributions with consistent reductions across subgroups.
Symptom improvements were consistent across subgroups stratified by age and gender, suggesting the broad applicability of Aurora across various user profiles.
3.5 App engagement profiles
Based on the number of modules completed, participants were categorized into three engagement groups: low (0–2 modules, n = 9), moderate (3–5 modules, n = 12), and high engagement (6–7 modules, n = 17). The median total time dedicated to in-app activities was 98 min (range, 22–193 min).
Dose–response analysis demonstrated a graded association between engagement and clinical benefit. Compared with the low-engagement group, participants with high engagement reported significantly larger reductions in both anxiety (Kruskal–Wallis H = 7.82, p = 0.02) and depression (H = 9.14, p = 0.01). A Jonckheere–Terpstra test confirmed an ordered trend (p = 0.01 for anxiety; p = 0.008 for depression).
Spearman’s correlations indicated significant associations between modules completed and change scores (ρ = −0.41, p = 0.01 for anxiety; ρ = −0.44, p = 0.007 for depression). In adjusted OLS regression, each additional completed module predicted a mean reduction of 0.38 points in anxiety (β = −0.38, SE = 0.14, 95% CI [−0.66, −0.10], p = 0.009) and 0.41 points in depression (β = −0.41, SE = 0.15, 95% CI [−0.71, −0.12], p = 0.006). These results are detailed in Supplementary Tables S2–S3 and illustrated in Figure 5.
Figure 5
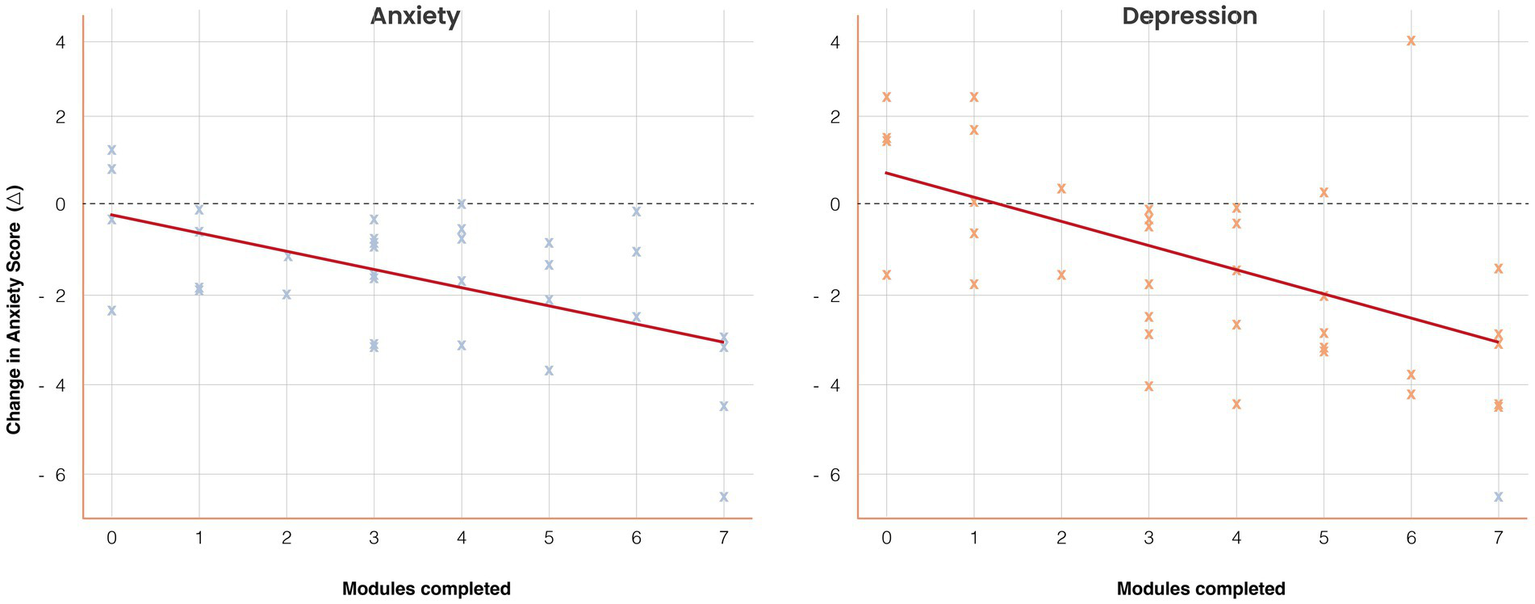
Dose–response association between engagement and symptom change. Scatterplots with regression lines illustrate the relationship between the number of modules completed (x-axis) and changes in anxiety and depression scores (y-axis). Higher engagement predicted greater symptom reduction: anxiety β = −0.38 (SE = 0.14), p = 0.009; depression β = −0.41 (SE = 0.15), p = 0.006. Shaded regions represent 95% confidence intervals. Group means (low = 0–2, moderate = 3–5, high = 6–7 modules) are overlaid for comparison. Results demonstrate a graded dose–response effect.
4 Discussion
This feasibility study provides preliminary evidence that Aurora, a culturally adapted, Spanish-language mobile application delivering CBT, is usable, acceptable, and clinically relevant for patients with anxiety and depression in Mexico. Among participants on stable pharmacotherapy, the app was associated with moderate reductions in self-reported symptoms, high usability ratings, and a precise dose–response between engagement and outcomes. These findings extend the literature on digital therapeutics, which has been predominantly shaped by evidence from high-income countries, to an underrepresented Latin American context.
A central strength of this work is the co-creation methodology, which integrated clinicians, patients, and usability experts from the outset. This process ensured therapeutic fidelity while addressing common shortcomings of commercial mental health apps, including excessive cognitive load and inadequate cultural adaptation. The observed usability scores (mean SUS = 78.5/100; uMARS = 4.2/5) compare favorably with benchmarks reported in digital CBT interventions from high-income countries (Firth et al., 2017; Linardon et al., 2019), underscoring the potential for deployment in Spanish-speaking health systems with resource limitations.
The dose–response association between module completion and symptom reduction strengthens the case for Aurora’s clinical relevance. Participants in the high-engagement group achieved reductions of nearly four points on both anxiety and depression subscales, compared with minimal change in the low-engagement group. Regression analyses confirmed that each additional module completed predicted incremental symptom improvement, independent of baseline severity, age, and sex. This finding is consistent with meta-analytic results indicating that the intensity of use is a key mediator of digital CBT outcomes (Furukawa et al., 2025; Gan et al., 2021). Engagement thus emerges as a modifiable determinant of clinical benefit, with implications for future app design and implementation strategies.
This study also contributes to the debate on the external validity of digital therapeutics in UMICs. Although Mexico is classified as a UMIC, its mental health system continues to share critical constraints with LMICs, including workforce shortages, stigma, fragmented care, and high out-of-pocket costs. By situating our findings within this broader context, we show how scalable digital solutions may help bridge structural gaps across Latin America. Evidence from pragmatic trials in Chile, Brazil, and other LMICs has similarly demonstrated the feasibility and acceptability of mobile CBT interventions in under-resourced environments (Araya et al., 2021; Karyotaki et al., 2023; Kim et al., 2023).
Several limitations should temper interpretation. First, the absence of a control group precludes causal inference; symptom reductions may partly reflect regression to the mean, expectancy effects, or concurrent pharmacotherapy. Second, although all participants were receiving medication (mean, 1.4 drugs; range, 1–3), outcomes were not stratified by pharmacological class. Exploratory sensitivity analyses suggest that engagement–outcome associations were robust to medication count, but larger studies are needed to evaluate interactions between pharmacotherapy and digital CBT. Third, data on illness chronicity were not collected. Chronicity may moderate treatment response, and future trials should include this variable (Cuijpers et al., 2022; Kambeitz-Ilankovic et al., 2022; Haller et al., 2023; Sextl-Plotz et al., 2024). Fourth, although the Spanish GADS has been validated in Mexico and other Latin American populations (Espinosa et al., 2015; Martín Carbonell et al., 2016; Reivan-Ortiz et al., 2019), the minor linguistic adaptations applied here were not formally re-validated. We confirmed good internal consistency in this sample; however, psychometric validation in Mexican populations remains necessary. Finally, participants were predominantly urban and highly educated, which may limit the generalizability of the findings to rural or underserved populations.
Despite these limitations, the findings carry important implications. First, the results demonstrate that a self-guided CBT app can deliver clinically meaningful benefits as an adjunct to pharmacotherapy, supporting its potential role in integrated care. Second, the graded dose–response highlights the importance of engagement, suggesting that strategies such as gamification, reminders, or integration with telepsychiatry platforms could enhance effectiveness. Third, the co-creation framework—balancing clinical fidelity with user-centered design—offers a scalable model for adapting digital therapeutics to diverse cultural contexts.
Future research should prioritize adequately powered, randomized controlled trials with active comparators, longer follow-up periods, and clinician-rated outcomes. A collection of chronicity, medication regimens, and sociodemographic moderators will help identify who benefits most. Broader linguistic and cultural adaptation will also be essential before Aurora can be scaled across Latin America, but its modular architecture facilitates such expansion.
In conclusion, Aurora represents a feasible, usable, and culturally tailored digital therapeutic for anxiety and depression in Mexico. By combining evidence-based CBT with rigorous co-design and usability testing, it offers a scalable strategy to reduce treatment gaps in Spanish-speaking middle-income settings. If validated in larger controlled trials, Aurora and similar interventions could contribute to addressing one of the most urgent challenges in global mental health: the persistent inequity in access to effective treatment for common psychiatric disorders.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the study protocol (INNOVA 2024–01) was reviewed and approved by both the Comité de Ética en Investigación (Folio CEI-000002) and the Comité de Investigación (Folio CI-000002) of Médica Sur, S.A.B. de C.V. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AL-T: Formal analysis, Investigation, Writing – original draft. AP-G: Investigation, Writing – review & editing. CT-L: Formal analysis, Writing – review & editing. MC-G: Formal analysis, Writing – review & editing. RD-F: Formal analysis, Investigation, Writing – review & editing. AM-C: Formal analysis, Writing – review & editing. AR-T: Formal analysis, Software, Writing – review & editing. SN: Data curation, Formal analysis, Software, Validation, Writing – review & editing. DO-G: Funding acquisition, Supervision, Validation, Writing – review & editing. EZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. OA-C: Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The authors grateful to Psicofarma S.A. de C.V., whose funding made it possible to advance both the technological and economic development of Mexico. Psicofarma S.A. de C.V. was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Acknowledgments
The authors would like to express their gratitude to the Aurora Advisory Board, which included psychiatrists, psychologists, digital health experts, and user experience designers. Their collaborative feedback and expertise were instrumental in shaping the clinical and technical components of the app. We thank the patients and participants who generously contributed their time and insights to support the development and testing of Aurora. We are especially grateful for the dedication, professionalism, and heartfelt commitment of the team at Cinvestav Guadalajara, whose contributions were essential to the success of this project. We also thank Divertising.mx, whose creative collaboration made it possible to bring this product to life. The Department of Technology Strategy and Innovation has been the driving force behind this project. Through an open innovation framework, it enabled a truly transdisciplinary collaboration that brought together diverse areas of expertise.
Conflict of interest
SN, DO-G, EZ were employed by Psicofarma S.A. de C.V.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyg.2025.1659374/full#supplementary-material
References
1
Araya R. Menezes P. R. Claro H. G. Brandt L. R. Daley K. L. Quayle J. et al . (2021). Effect of a digital intervention on depressive symptoms in patients with comorbid hypertension or diabetes in Brazil and Peru: two randomized clinical trials. JAMA325, 1852–1862. doi: 10.1001/jama.2021.4348
2
Berenzon Gorn S. Saavedra Solano N. Medina-Mora Icaza M. E. Aparicio Basauri V. Galvan Reyes J. (2013). Evaluation of the mental health system in Mexico: where is it headed?Rev. Panam. Salud Publica33, 252–258. doi: 10.1590/S1020-49892013000400003
3
Cuijpers P. Ciharova M. Quero S. Miguel C. Driessen E. Harrer M. et al . (2022). The contribution of "individual participant data" meta-analyses of psychotherapies for depression to the development of personalized treatments: a systematic review. J. Pers. Med.12:93. doi: 10.3390/jpm12010093
4
Eldridge S. M. Chan C. L. Campbell M. J. Bond C. M. Hopewell S. Thabane L. et al . (2016). CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ355:i5239. doi: 10.1136/bmj.i5239
5
Espinosa M. C. Orozco L. A. Ybarra J. L. (2015). Síntomas de ansiedad, depresión y factores psicosociales en hombres que solicitan atención de salud en el primer nivel. Salud Mental38, 201–208. doi: 10.17711/SM.0185-3325.2015.028
6
Firth J. Torous J. Nicholas J. Carney R. Pratap A. Rosenbaum S. et al . (2017). The efficacy of smartphone-based mental health interventions for depressive symptoms: a meta-analysis of randomized controlled trials. World Psychiatry16, 287–298. doi: 10.1002/wps.20472
7
Furukawa T. A. Noma H. Tajika A. Toyomoto R. Sakata M. Luo Y. et al . (2025). Personalised & optimised therapy (POT) algorithm using five cognitive and behavioural skills for subthreshold depression. NPJ Digit. Med.8:531. doi: 10.1038/s41746-025-01906-6
8
Gan D. Z. Q. McGillivray L. Han J. Christensen H. Torok M. (2021). Effect of engagement with digital interventions on mental health outcomes: a systematic review and meta-analysis. Front. Digit. Health3:764079. doi: 10.3389/fdgth.2021.764079
9
GBD 2019 Diseases and Injuries Collaborators (2020). Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet396, 1204–1222. doi: 10.1016/S0140-6736(20)30925-9
10
Haller K. Becker P. Niemeyer H. Boettcher J. (2023). Who benefits from guided internet-based interventions? A systematic review of predictors and moderators of treatment outcome. Internet Interv.33:100635. doi: 10.1016/j.invent.2023.100635
11
Hernandez-Diaz Y. Genis-Mendoza A. D. Ramos-Mendez M. A. Juarez-Rojop I. E. Tovilla-Zarate C. A. Gonzalez-Castro T. B. et al . (2022). Mental health impact of the COVID-19 pandemic on Mexican population: a systematic review. Int. J. Environ. Res. Public Health19, –6953. doi: 10.3390/ijerph19116953
12
Johansson O. Bjarehed J. Andersson G. Carlbring P. Lundh L. G. (2019). Effectiveness of guided internet-delivered cognitive behavior therapy for depression in routine psychiatry: a randomized controlled trial. Internet Interv.17:100247. doi: 10.1016/j.invent.2019.100247
13
Kambeitz-Ilankovic L. Rzayeva U. Volkel L. Wenzel J. Weiske J. Jessen F. et al . (2022). A systematic review of digital and face-to-face cognitive behavioral therapy for depression. NPJ Digit. Med.5:144. doi: 10.1038/s41746-022-00677-8
14
Karyotaki E. Miguel C. Panagiotopoulou O. M. Harrer M. Seward N. Sijbrandij M. et al . (2023). Digital interventions for common mental disorders in low- and middle-income countries: a systematic review and meta-analysis. Glob. Mental Health10:e68. doi: 10.1017/gmh.2023.50
15
Kim J. Aryee L. M. D. Bang H. Prajogo S. Choi Y. K. Hoch J. S. et al . (2023). Effectiveness of digital mental health tools to reduce depressive and anxiety symptoms in low- and middle-income countries: systematic review and meta-analysis. JMIR Mental Health10:e43066. doi: 10.2196/43066
16
Larsen M. E. Nicholas J. Christensen H. (2016). Quantifying app store dynamics: longitudinal tracking of mental health apps. JMIR Mhealth Uhealth4:e96. doi: 10.2196/mhealth.6020
17
Linardon J. Cuijpers P. Carlbring P. Messer M. Fuller-Tyszkiewicz M. (2019). The efficacy of app-supported smartphone interventions for mental health problems: a meta-analysis of randomized controlled trials. World Psychiatry18, 325–336. doi: 10.1002/wps.20673
18
Martín Carbonell M. Pérez Díaz R. Riquelme Marín A. (2016). Valor diagnóstico de la Escala de Ansiedad y Depresión de Goldberg (EAD-G) en adultos cubanos. Univ. Psychol.15, 177–192. doi: 10.11144/Javeriana.upsy15-1.vdea
19
Martin-Payo R. Carrasco-Santos S. Cuesta M. Stoyan S. Gonzalez-Mendez X. Fernandez-Alvarez M. D. M. (2021). Spanish adaptation and validation of the user version of the mobile application rating scale (uMARS). J. Am. Med. Inform. Assoc.28, 2681–2686. doi: 10.1093/jamia/ocab216
20
Medina-Mora M. E. Borges G. Lara C. Benjet C. Blanco J. Fleiz C. et al . (2005). Prevalence, service use, and demographic correlates of 12-month DSM-IV psychiatric disorders in Mexico: results from the Mexican National Comorbidity Survey. Psychol. Med.35, 1773–1783. doi: 10.1017/S0033291705005672
21
Naslund J. A. Aschbrenner K. A. Araya R. Marsch L. A. Unutzer J. Patel V. et al . (2017). Digital technology for treating and preventing mental disorders in low-income and middle-income countries: a narrative review of the literature. Lancet Psychiatry4, 486–500. doi: 10.1016/S2215-0366(17)30096-2
22
Patel V. Saxena S. Lund C. Thornicroft G. Baingana F. Bolton P. et al . (2018). The lancet commission on global mental health and sustainable development. Lancet392, 1553–1598. doi: 10.1016/S0140-6736(18)31612-X
23
Reivan-Ortiz G. Pineda-Garcia G. Leon-Parias B. D. (2019). Psychometric properties of the Goldberg anxiety and depression scale (GADS) in Ecuadorian population. Int. J. Psychol. Res.12, 41–48. doi: 10.21500/20112084.3745
24
Sevilla-Gonzalez M. D. R. Moreno Loaeza L. Lazaro-Carrera L. S. Bourguet Ramirez B. Vazquez Rodriguez A. Peralta-Pedrero M. L. et al . (2020). Spanish version of the system usability scale for the assessment of electronic tools: development and validation. JMIR Hum. Factors7:e21161. doi: 10.2196/21161
25
Sextl-Plotz T. Steinhoff M. Baumeister H. Cuijpers P. Ebert D. D. Zarski A. C. (2024). A systematic review of predictors and moderators of treatment outcomes in internet- and mobile-based interventions for depression. Internet Interv.37:100760. doi: 10.1016/j.invent.2024.100760
26
Teare M. D. Dimairo M. Shephard N. Hayman A. Whitehead A. Walters S. J. (2014). Sample size requirements to estimate key design parameters from external pilot randomised controlled trials: a simulation study. Trials15:264. doi: 10.1186/1745-6215-15-264
27
Whitehead A. L. Julious S. A. Cooper C. L. Campbell M. J. (2016). Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat. Methods Med. Res.25, 1057–1073. doi: 10.1177/0962280215588241
Summary
Keywords
digital therapeutics, cognitive behavioral therapy, mobile health, mHealth, anxiety and depression, usability testing, low- and middle-income countries
Citation
López-Tello A, Pérez-Gómez A, Toledo-Lozano CG, Callejas-Gómez MdP, Durón-Figueroa R, Moreno-Coutiño A, Ramirez-Treviño A, Nava S, Ocampo-Gutiérrez de Velasco DA, Zárate E and Arias-Carrión O (2025) Aurora: a mobile-based cognitive behavioral therapy intervention for anxiety and depression in Mexico. Front. Psychol. 16:1659374. doi: 10.3389/fpsyg.2025.1659374
Received
07 July 2025
Accepted
23 September 2025
Published
10 October 2025
Volume
16 - 2025
Edited by
Mini Han Wang, Zhuhai People's Hospital, China
Reviewed by
Susana Campos, University of Talca, Chile
Jay Jazul, University of Santo Tomas Research Center for Social Sciences and Education, Philippines
Updates
Copyright
© 2025 López-Tello, Pérez-Gómez, Toledo-Lozano, Callejas-Gómez, Durón-Figueroa, Moreno-Coutiño, Ramirez-Treviño, Nava, Ocampo-Gutiérrez de Velasco, Zárate and Arias-Carrión.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Edith Zárate, edith.zarate@psicofarma.com.mx; Oscar Arias-Carrión, ariasemc2@gmail.com
†ORCID: Christian Gabriel Toledo-Lozano, orcid.org/0000-0001-7418-1228
Ana Moreno-Coutiño, orcid.org/0000-0002-1601-9318
Antonio Ramirez-Treviño, orcid.org/0000-0003-3028-3446
Diego Antonio Ocampo-Gutiérrez de Velasco, orcid.org/0009-0008-8759-7116
Edith Zarate, orcid.org/0009-0000-2642-9831
Oscar Arias-Carrión, orcid.org/0000-0002-9982-7571
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.