Introduction
Early studies reported enticing correlations between Big Five personality traits and brain structure (DeYoung et al., 2010; Kanai and Rees, 2011). Such findings supported the view that stable traits have identifiable neural substrates. However, over a decade of research revealed a more complicated picture: many reported brain-trait associations failed to replicate (e.g., Boekel et al., 2015), and the field struggled with so-called “voodoo correlations,” in which extremely high correlations were likely spurious (Vul et al., 2009). Subsequent critiques underscored that typical sample sizes were underpowered to detect the small effect sizes realistic for personality–brain links (Button et al., 2013; Dubois and Adolphs, 2016).
Small effects and the need for big data
Comprehensive investigations have found little to no evidence of robust brain–personality associations in large samples. For instance, Avinun et al. (2020) examined 1,107 individuals and reported no significant relationships between Big Five traits and multiple measures of brain morphometry. A systematic review and meta-analysis reached a similar conclusion, summarizing that there are no replicable structural brain differences as a function of Big Five traits (Chen and Canli, 2022). These results suggest that true associations, if present, are extremely small in magnitude. Power analyses and empirical work converge on the same message: detecting such tiny effects requires very large samples. Marek et al. (2022) showed that typical brain-wide association studies require thousands of subjects for reproducibility. Figure 1 illustrates this relationship: as effect size decreases, the sample size needed for 80% statistical power rises steeply. In practice, most historical studies in personality neuroscience were far too small, yielding underpowered analyses and spurious positives (Yarkoni, 2009; Button et al., 2013).
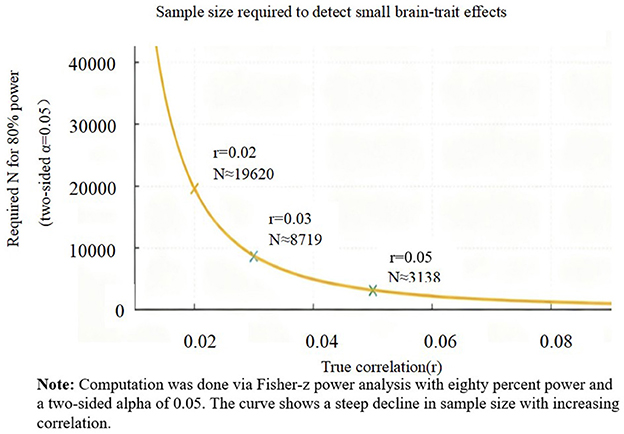
Figure 1. Sample size required to detect small brain–trait effects (80% power, two-sided α = 0.05). Markers indicate r = 0.02/0.03/0.05. Computed via Fisher-z power analysis.
Heterogeneity and the case for normative models
Heterogeneity is a key reason why averaging can obscure meaningful effects: individuals with the same trait score can show different neural patterns, and similar brain measures can accompany different trait expressions. Sex-dependent or subgroup-specific associations have been observed (Nostro et al., 2017), and idiosyncratic variation is the rule rather than the exception. Normative modeling provides a principled solution by estimating the expected distribution of brain measures given covariates (e.g., age, sex) and then characterizing each person by their deviation from that norm (Marquand et al., 2016, 2019). Instead of asking whether trait X correlates with region Y on average, we ask whether individuals with extreme trait values show atypical deviations relative to peers. This person-centered approach has already improved sensitivity in clinical domains, revealing heterogeneous deviation patterns in disorders such as schizophrenia and bipolar disorder (Wolfers et al., 2018). Resources like the Brain Charts for the human lifespan demonstrate how large multi-cohort datasets can be used to build robust normative references (Bethlehem et al., 2022).
From big associations to big practices
Adopting normative modeling as a default approach represents a shift from chasing elusive average effects to embracing big practices—robust analytical habits that match problem complexity. Practically, this entails: assembling adequately powered samples via collaboration and data sharing; deriving individual deviation scores for relevant brain measures; testing preregistered, out-of-sample hypotheses about how deviations relate to personality; and reporting full model performance and failures. This agenda pairs naturally with prediction-oriented analysis (Yarkoni and Westfall, 2017), multivariate methods, and open science. It aligns with the idea that personality is about what makes individuals unique—and our methods should quantify that uniqueness instead of averaging it away.
Conclusion
The era of small-N brain–personality correlation hunting is ending. By defaulting to normative modeling, personality neuroscience can better accommodate small effects and heterogeneity, leverage large datasets to establish meaningful baselines, and identify how and why certain individuals or subgroups deviate. Coupled with adequately powered designs and transparent, predictive workflows, this shift from big associations to big practices promises findings that are more robust, generalizable, and practically meaningful.
Author contributions
PW: Funding acquisition, Investigation, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. YC: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The Outstanding Talent Cultivation Project under the High-Level and Subsidized Discipline Construction Program of Anhui Normal University (2025GFXK040) supported this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Gen AI was used in the creation of this manuscript. During manuscript preparation we used ChatGPT (OpenAI; model: GPT 5; accessed in September 2025 via chat.openai.com) only for language polishing.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Avinun, R., Israel, S., Knodt, A. R., and Hariri, A. R. (2020). Little evidence for associations between the Big Five personality traits and variability in brain gray or white matter. NeuroImage 220:117092. doi: 10.1016/j.neuroimage.2020.117092
Bethlehem, R. A. I., Seidlitz, J., White, S. R., Vogel, J. W., Anderson, K. M., Adamson, C., et al. (2022). Brain charts for the human lifespan. Nature 604, 525–533. doi: 10.1038/s41586-022-04554-y
Boekel, W., Wagenmakers, E. J., Belay, L., Verhagen, J., Brown, S., and Forstmann, B. U. (2015). A purely confirmatory replication study of structural brain–behavior correlations. Cortex 66, 115–133. doi: 10.1016/j.cortex.2014.11.019
Button, K. S., Ioannidis, J. P. A., Mokrysz, C., Nosek, B. A., Flint, J., Robinson, E. S. J., et al. (2013). Power failure: Why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 14, 365–376. doi: 10.1038/nrn3475
Chen, Y.-W., and Canli, T. (2022). “Nothing to see here”: No structural brain differences as a function of the Big Five personality traits from a systematic review and meta-analysis. Personal. Neurosci. 5:e8. doi: 10.1017/pen.2021.5
DeYoung, C. G., Hirsh, J. B., Shane, M. S., Papademetris, X., Rajeevan, N., and Gray, J. R. (2010). Testing predictions from personality neuroscience: brain structure and the Big Five. Psychol. Sci. 21, 820–828. doi: 10.1177/0956797610370159
Dubois, J., and Adolphs, R. (2016). Building a science of individual differences from fMRI. Trends Cognit. Sci. 20, 425–443. doi: 10.1016/j.tics.2016.03.014
Kanai, R., and Rees, G. (2011). The structural basis of inter-individual differences in human behaviour and cognition. Nat. Rev. Neurosci. 12, 231–242. doi: 10.1038/nrn3000
Marek, S., Tervo-Clemmens, B., Calabro, F. J., Montez, D. F., Kay, B. P., Hatoum, A. S., et al. (2022). Reproducible brain-wide association studies require thousands of individuals. Nature 603, 654–660. doi: 10.1038/s41586-022-04492-9
Marquand, A. F., Kia, S. M., Zabihi, M., Wolfers, T., Buitelaar, J. K., and Beckmann, C. F. (2019). Conceptualizing mental disorders as deviations from normative functioning. Mol. Psychiatry 24, 1415–1424. doi: 10.1038/s41380-019-0441-1
Marquand, A. F., Rezek, I., Buitelaar, J., and Beckmann, C. F. (2016). Understanding heterogeneity in clinical cohorts using normative models: Beyond case–control studies. Biol. Psychiatry 80, 552–561. doi: 10.1016/j.biopsych.2015.12.023
Nostro, A. D., Müller, V. I., Reid, A. T., and Eickhoff, S. B. (2017). Correlations between personality and brain structure: a crucial role of gender. Cereb. Cortex 28, 3698–3712. doi: 10.1093/cercor/bhw191
Vul, E., Harris, C., Winkielman, P., and Pashler, H. (2009). Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspect. Psychol. Sci. 4, 274–290. doi: 10.1111/j.1745-6924.2009.01125.x
Wolfers, T., Doan, N. T., Kaufmann, T., Alnæs, D., Moberget, T., Agartz, I., et al. (2018). Mapping the heterogeneous phenotype of schizophrenia and bipolar disorder using normative models. JAMA Psychiatry 75, 1146–1155. doi: 10.1001/jamapsychiatry.2018.2467
Yarkoni, T. (2009). Big correlations in little studies: inflated fMRI correlations reflect low statistical power—Commentary on Vul et al. (2009). Perspect. Psychol. Sci. 4, 294–298. doi: 10.1111/j.1745-6924.2009.01127.x
Keywords: personality neuroscience, small effect sizes, heterogeneity, normative modeling, brain-wide association, reproducibility, big data
Citation: Wu P and Chang Y (2025) From big associations to big practices—Why normative modeling should be the default in personality neuroscience. Front. Psychol. 16:1701166. doi: 10.3389/fpsyg.2025.1701166
Received: 08 September 2025; Accepted: 21 October 2025;
Published: 04 November 2025.
Edited by:
Kristina Stoyanova, Plovdiv Medical University, BulgariaReviewed by:
Stanislava Yordanova Stoyanova, South-West University “Neofit Rilski”, BulgariaCopyright © 2025 Wu and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peiqian Wu, eXpqd3BxQDE2My5jb20=
 Peiqian Wu
Peiqian Wu Yudie Chang
Yudie Chang