- 1Department of Engineering, University of the Ryukyus, Nishihara, Okinawa, Japan
- 2Department of Physical Therapy, Okinawa Rehabilitation Welfare College, Yonabaru, Okinawa, Japan
- 3H2L Inc., Tokyo, Japan
- 4Future Robotics Organization, Waseda University, Tokyo, Japan
- 5Department of Life Sciences, Graduate School of Arts and Sciences, The University of Tokyo, Tokyo, Japan
- 6Department of Systems Innovation, School of Engineering, The University of Tokyo, Tokyo, Japan
Introduction: Conditioning involves the regulation of psychological health, physical fitness, and overall well-being, all of which are essential for optimal athletic performance. While psychological aspects have been extensively studied, the relationship between muscle activity and psychological factors such as anxiety and depression remains underexplored.
Methods: This study investigated the relationship between anxiety, depression, and forearm muscle activity in amateur athletes (mean age: 28 ± 9 years; sports experience: 13 ± 5 years) who participate in sports requiring upper extremity use. Muscle activity was evaluated using autocorrelation coefficients derived from muscle deformation data collected via a sensor array. Anxiety and depression levels were assessed using validated questionnaires, and their associations with muscle deformation were analyzed.
Results: Muscle deformation suggested a task-dependent relationship with psychological factors. A significant correlation was observed between anxiety and Hand tasks (r = −0.57, p = 0.004, pfdr = 0.05) and a trend was found between depression and random tasks such as HPI randomness (r = 0.46, p = 0.04, pfdr = 0.15).
Discussion: These findings suggest that chronic psychological stress may impair sustained muscle contraction during cyclic movements and promote fixed motor patterns during random tasks. This study elucidates how anxiety and depression affect muscle activity under different task conditions, providing a basis for optimizing training protocols and psychological stress management strategies in athletic conditioning.
1 Introduction
Sports science focuses extensively on enhancing athletes’ performance, particularly the critical role of conditioning in improving physical and mental health (1). Conditioning involves optimizing psychological well-being, physical fitness, and overall health, which are essential for peak performance (2, 3). Despite comprehensive efforts, achieving this ideal state presents challenges, including slumps (4), and performance plateaus. Athletes may also face specific issues like “yips” (5, 6)—a sudden loss of fine motor skills and dystonia (7, 8), which stem from psychological stress, physical health concerns, environmental factors, and learning dynamics.
Psychological stress can be divided into acute and chronic stress. Acute stress is a temporary response to external stimuli or stressors. Chronic stress persists for weeks to several months or longer and can affect the body, mind, and behavior (9). Prolonged psychological load disrupts leading to sustained activation of the hypothalamic—pituitary—adrenal (HPA) axis and chronically elevated glucocorticoid secretion (10), Such HPA-axis dysregulation has been implicated in insomnia, depressive symptoms, and the development of stress-related lifestyle disorders (11). Chronic exposure to high levels of glucocorticoids in adulthood has been associated with depressive disorders (12) Additionally, high doses of glucocorticoids are believed to cause muscle dysfunction, including decreased skeletal muscle size, mass, and weakness (10, 13). A chronic stress history reduces basal activity below control levels and abolishes the activation response to acute stress (14).
The reduction of acute activation has been reported to be similar to depression (15). The importance of anxiety and other emotional and personality factors in athletic competition has long been recognized (15–17); performance and competition anxiety in sports are closely related to each other and have been shown to reinforce each other negativelys (18). In addition, Chronic stress changes have also been reported in relation to physical functioning, with low grip strength being cross-sectionally and longitudinally associated with depressive symptoms. (19, 20). However, it is unclear whether anxiety and depression affect physical function, particularly the skeletal muscle status, in athletes. Therefore, this study aimed to determine the relationship between the effects of anxiety and depression on forearm muscle activity in athletes performing various exercise tasks. The findings of this study are expected to provide basic data for stress assessment and for designing targeted mental-intervention strategies in athletic settings.
2 Methods
2.1 Participations
Twenty-one healthy males (age: 28 9 years, height: 173 7 cm, weight: 70 12 kg, sports experience: 13 5 years) participated in the study (Table 1). The participants were amateur athletes between the ages of 18 and 59 years with at least 5 years of continuous experience in sports involving the upper extremities. Open recruitment was conducted among those who played upper-limb sports such as baseball and golf, in which slumps and yips are known to be common, had at least 5 years of continuous experience, and subjectively experienced a decline in performance within 3 months. Exclusion criteria included cardiac disease (use of a pacemaker), psychiatric disease, no serious illness, and any injury or disease for which treatment or rehabilitation had not been completed. Pregnant or possibly pregnant, have skin atopy, or have a job or hobby (playing a musical instrument) that involves wrist use (Table 2).
2.2 Equipment
2.2.1 Experimental procedures
2.2.1.1 Muscle deformation data acquisition
Muscle deformation data were compared by performing exercises with different loading amounts on the muscles, hand-push tasks, and movements with competition characteristics. The training with different amounts of the load was based on the report of Miyake et al. (21).
Tasks with different loading amounts as shown in Figure 1, participants performed the following five training tasks.
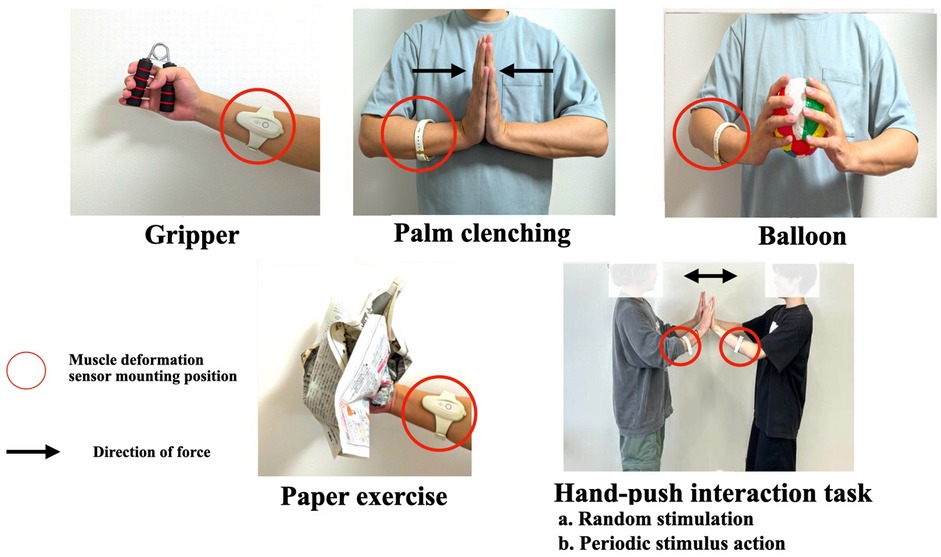
Figure 1. Performing exercises. The gripper and paper tasks were isotonic exercises, and the other tasks were isometric exercises. Hand-push interaction (HPI) was a task with periodic and random stimuli. In addition, crumpling pieces of paper exercise (paper) involves randomness because the resistance force of the paper changes depending on the shape and layers of the paper.
For the first 5 s, the forearm orientation was the same as that used in the task to reduce the effects of skin deformation. During calibration data acquisition, the wrist angle was set to the forearm at to relax the skeletal muscles. After the first 5 s had elapsed, a training movement, such as grasping, was initiated for 10 s (Figure 2). Calibration was performed for each assignment.
• Gripper: Grip and release a 25 kg hand-gripper approximately eight times for 10 s.
• Palm clenching (Hand): Keep pushing the hand in front of the chest for 10 s. Pressing with the entire palm.
• Balloon: Hold a paper balloon in front of the chest and apply pressure with arms for 10 s, making sure not to crush the balloon.
• Paper exercise (Paper): Asked to keep crumpling a layer of newspaper for 10 s.The gripper and paper tasks were isotonic exercises, and the other tasks were isometric exercises. The gripper and tasks were selected as the most typical hand-gripping training, where the human moves the skeletal muscles periodically. In addition, crumpling pieces of paper involves randomness because the resistance force of the paper changes depending on the shape and layers of the paper.
• Hand-push interaction task: The subject and the experimenter performed a hand-push interaction for 10 s with their hands clasped before their chests (Figure 1). The following two tasks were performed during the hand-push interaction:
a. Random stimulation (HPI Randomness)The experimenter applied random perturbations to the subjects three times, and the subjects maintained their balance.
b. Periodic stimulus action (HPI Periodicity)A metronome was played at 60 BPM, and the experimenter and the subject alternately pushed each other at 1 s intervals. This interaction was repeated ten times per trial.

Figure 2. Summary of measurements and equipment used in the experiment. The entire training task was performed for 15 s. The first 5 s were used as the serenity phase, and the remaining 10 s were used as the moving phase. The moving phase consisted of grasping and other movements for 10 s. The muscle deformation sensor array used for the experimental task contained 14 optical sensors. When skeletal muscles are activated, the skeletal muscle deforms by muscle contraction. The reading value in each channel of the muscle deformation sensor changes when skeletal muscle or skin deforms. The reading values were sent to a computer from a muscle deformation sensor through Bluetooth Low Energy. The device is also equipped with a gyro sensor and a 3-axis acceleration sensor, making it possible to acquire quaternion data.
2.2.1.2 Psychological factor
Psychological conditions were first checked by questionnaire before the experiment. The questionnaires used the Kessler 6 scale(K6) (Table 3).
The K6 is a brief 6-item self-report measure designed to assess non-specific psychological distress (such as anxiety and depression) experienced over the past 30 days (22). Higher scores indicate a greater risk of psychological distress, including anxiety and depression. The K6 was originally written in English, and a Japanese version has been prepared. The Japanese version was used in this study, (23). The K-6 has demonstrated very good internal consistency and excellent predictive validity (24). The K6 has also been utilized in studies involving athletes, including both general sports practitioners and elite athlete populations. Among these groups, the K6 has demonstrated high internal consistency (25–27). Furthermore, it has been employed as a criterion measure to validate athlete-specific psychological assessments, such as the Athlete Psychological Strain Questionnaire (APSQ), showing strong convergent validity (r = 0.80) (28).
2.2.1.3 Equipment used in this study
This study used a muscle deformation sensor array, FirstVR (H2L Inc, Tokyo, Japan) (29), as shown in Figure 2. The FirstVR is a band-type sensor based on near-infrared optical sensing, which consists of infra-light emitting and infra-light receiving. The muscle deformation sensor array contains 14 optical muscle deformation sensors. When skeletal muscles are activated, the skeletal muscle deforms by muscle contraction. The reading value in each channel of the FirstVR changes when the skeletal muscle or skin deforms. The attachment cover was modified by replacing the Urethane sponge from the original FirstVR to observe muscle deformation. The reading values were sent to a computer from FirstVR through Bluetooth Low Energy. The sampling frequency of the data recording was approximately 9 Hz. The sensor is mounted approximately one-half of the forearm length from the elbow. The device is also equipped with a gyro sensor and a 3-axis acceleration sensor, making it possible to acquire quaternion data (posture data of the part of the body wearing the device). The device can be worn by wrapping it around the forearm like a wristwatch to measure muscle deformation in the forearm and fingers (30).
2.2.1.4 Data processing and analysis
This study subjected the muscle deformation data obtained from the muscle deformation sensor array to moving average processing. The forearm muscle deformation data were categorized as follows. The forearm was defined as (1) Extensor muscles and (2) Flexor muscles. The observed flexor muscles are flexor carpi ulnaris muscle and flexor carpi radialis muscle. The observed extensor muscles are extensor carpi ulnaris muscle and extensor carpi radialis muscle. Autocorrelation coefficients were obtained based on muscle deformation time-series data obtained from each task. In this study, autocorrelation was used to evaluate the temporal regularity of muscle deformation patterns in response to task structure. This approach is justified by the fact that muscle activation during motor tasks often reflects rhythmic coordination when the external input is temporally structured. Conversely, random task conditions tend to disrupt this regularity. Autocorrelation thus serves as a physiologically relevant metric to assess how well muscle responses temporally align with the external task dynamics. The autocorrelation coefficient is the correlation between the original and time-shifted data. The autocorrelation coefficient is high if the time-series data are periodic (31). If the autocorrelation coefficient is low, it indicates that the data is random. This study used extreme values of the autocorrelation coefficient (except for the zero phase difference). Psychological distress levels were represented as a dummy variable based on the K6 score results. Statistical analysis examined the relationship between the autocorrelation coefficients of muscle deformation in each task and psychological distress measured by the K6. Statistical analysis was conducted with the effect size set at 0.6, the power of the test at 0.8, and a significance level of 0.05. Based on these parameters and the correlation test setup, the required sample size was calculated as 18 participants using the R pwr package. The first analysis was performed using the Shapiro-Wilk test to confirm the normality of the compared data. Subsequently, Pearson’s product-moment correlation coefficient or Spearman’s rank correlation coefficient was calculated depending on the distribution of the data. Furthermore, FDR correction using the Benjamini–Hochberg method was applied to account for multiple comparisons. Finally, additional group comparisons were made by dividing participants into two groups: the top five K6 scorers (four with six points and one with nine points) and the bottom five scorers (all with zero points). Group differences were assessed using the Wilcoxon rank-sum test.
2.2.1.5 Ethical considerations
The experiment was conducted in accordance with the Declaration of Helsinki. All participants were given a written and verbal explanation of the experiment. All experimental tasks were performed with the consent of the participants. They were also explained so that participants could discontinue the experiment or withdraw their consent at any time. This experiment was approved by the Japanese Society for Assistive Technology (No. 23-05).
3 Results
This study analyzed the relationship between the autocorrelation of muscle deformation and psychological factor in each task.
To examine how anxiety and depression are related to the autocorrelation coefficient of muscle deformations, the K6 scale was used. The results for K6 were: total score 3.2 2.6, Anxiety score (Q1–Q3) 1.8 1.4, Depression score (Q4–Q6) 1.5 1.5, Q1: 0.6 0.8, Q2: 0.5 0.5, Q3: 0.7 0.9, Q4: 0.5 0.7, Q5: 0.6 0.6, Q6: 0.4 0.7. The correlations between each task and the total K6 scores, anxiety item scores, depression item scores, and each question are shown in Tables 4, 5. K6 total scores showed a significant negative correlation with forearm extensor deformity in the Hand task (r = 0.57). Muscle deformation of the forearm extensor muscles correlated with the Hand task (r = 0.58) and the HPI randomness task (r = 0.46) in anxiety (Q1–Q3) and depression (Q4–Q6). Additionally, Table 5 and Figure 3 suggest associations between each K6 problem and specific tasks. Extensors in Figure 3A were significantly correlated with Q3 (r = 0.59) and the Hand, as well as with Q6 and the Handr = 0.60), HPI randomness task (r = 0.51). For the flexor muscles in Figure 3B, associations were found between Q3 and the gripper task (r = 0.47) and the HPI randomness task (r = 0.47). Finally, the five highest and lowest K6 scores were compared between the high- and low-stress groups. No statistically significant differences were found between the groups. However, moderate to large effect sizes were observed in extensor Gripper (r = 0.30), Hand (r = 0.60), balloon (r = 0.49) and HPI randomness (r = 0.54) and flexor balloon (r = 0.49) and HPI periodicity (r = 0.30) (Table 6).
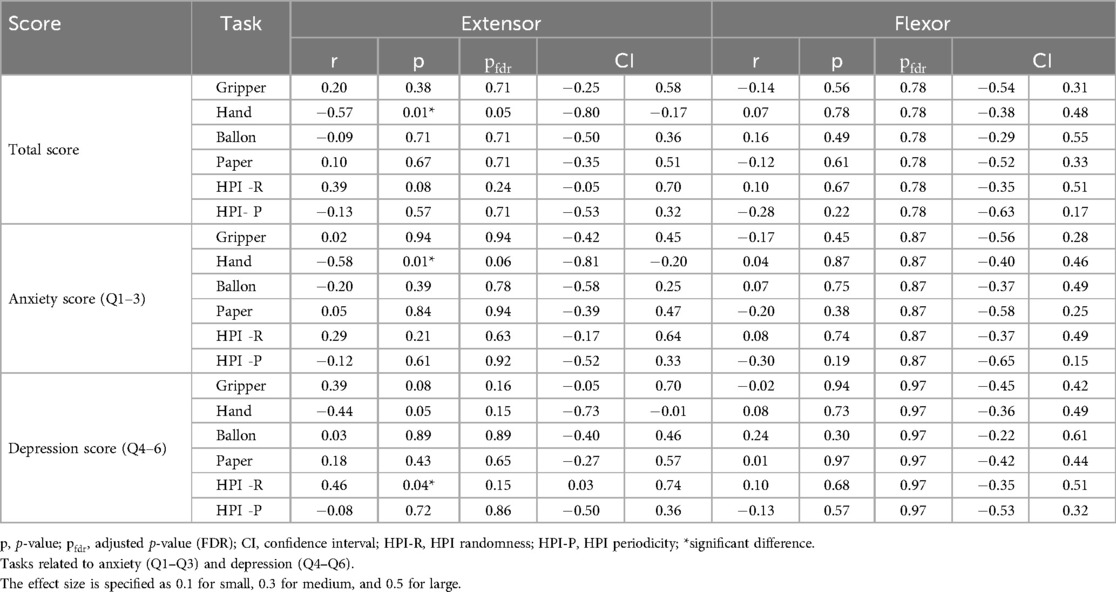
Table 4. Autocorrelation coefficient of muscle deformity in relation to K6 and total scores on anxiety and depression items for Each Training.
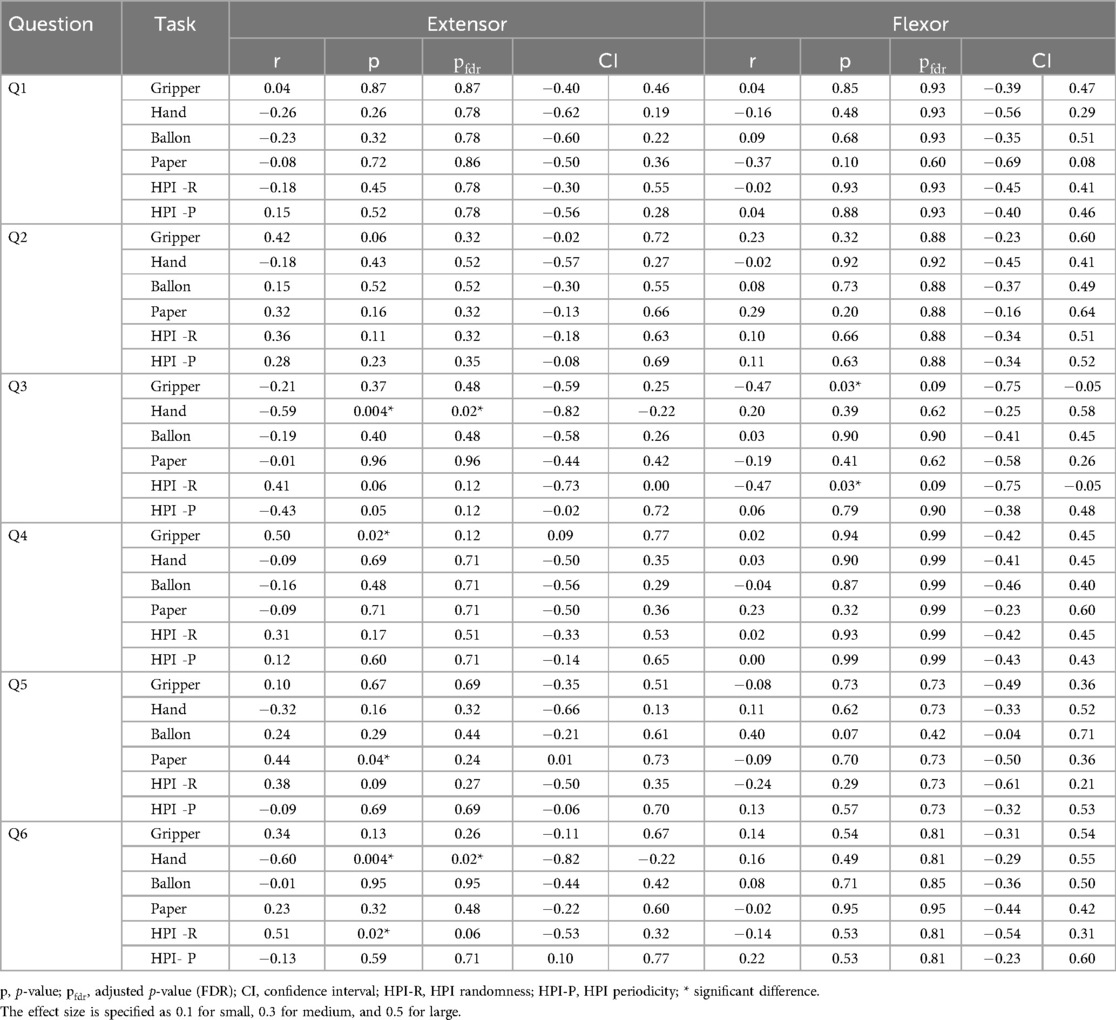
Table 5. Correlation between autocorrelation coefficient of muscle deformation and K6 for each training.
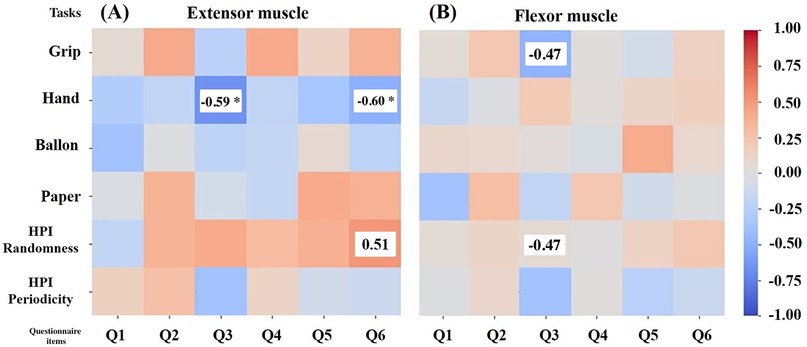
Figure 3. Correlation between Autocorrelation Coefficient of Muscle Deformation and K6 for Each Training. Heat-map of Spearman correlations between psychological items and muscle indices. (A) Extensor group; (B) Flexor group. The vertical axis of the figure shows the gripper, Hand, balloon, paper, HPI randomness and HPI periodicity tasks. The horizontal axis of the figure represents Q1–Q3 = anxiety items, Q4–Q6 = depression items. The items that showed predominant correlations were: (A) Q3 and Hand (r = 0.59), Q6 and Hand (r = 0.60), and HPI Randomness task (r = 0.51). (B) For flexors, Q3 and gripper (r = 0.47) and HPI Randomness task (r = 0.47). The heat map shows a positive correlation with a maximum of 1 in red and a negative correlation with a maximum of 1 in blue. Values shown in the heat map represent results after p-value correction. Values with an asterisk (*) represent , values without an asterisk (*) represent .
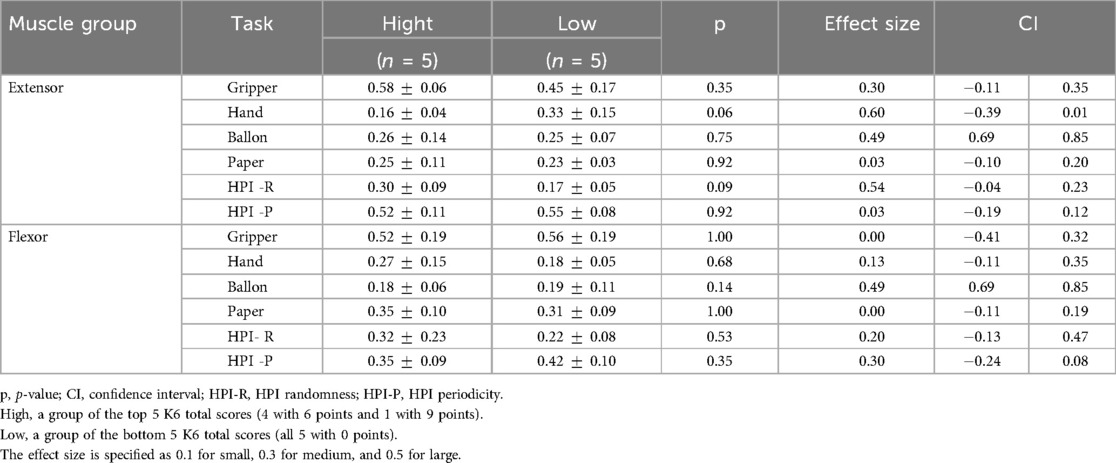
Table 6. Comparison of muscle activity metrics between high and low psychological distress groups (based on K6 scores).
4 Discussion
This study examined the relationship between forearm muscle deformation and psychological distress (anxiety and depression) during various training tasks. The following sections discuss how long-term psychological symptoms may influence muscle activity patterns.
The relationship between psychological stress and athletic performance has been widely studied, particularly in the context of performance failures such as the yips. Previous studies have reported elevated levels of stress and social anxiety in affected athletes, with the yips conceptualized along a continuum between anxiety-related choking and task-specific dystonia (32, 33). These findings are primarily based on psychological assessments rather than physiological measurements or muscle-specific data. In contrast, the present study focuses on the physiological correlates of psychological distress during motor task execution. Prior research has shown that pressure-induced motor disturbances, such as choking or the yips, are often associated with abnormal neuromuscular activity, including increased forearm tension, co-contraction of antagonist muscles, and reduced movement variability or speed (34, 35). These maladaptive motor responses have been attributed to disruptions in sensorimotor integration or excessive conscious monitoring of automated motor patterns. By analyzing forearm muscle deformation patterns across structured tasks, this study offers a biomechanical perspective that complements existing psychological models of stress-related performance breakdowns. This approach may contribute to a more comprehensive understanding of how chronic psychological stress alters neuromuscular control in high-pressure environments.
In this study, anxiety and depression were assessed using the K6 questionnaire. Associations were observed between K6 scores and muscle deformation patterns during training tasks. Although these associations did not consistently reach statistical significance after multiple comparison corrections, they suggested a potential relationship between psychological distress and task-specific muscle activity. Negative trends were observed between K6 scores and extensor muscle deformation in the Hand and HPI Periodicity tasks, while positive trends appeared in the Gripper, Paper, and HPI Randomness tasks.
The K6 is a validated screening tool that assesses non-specific psychological distress, including anxiety and depression, over the past 30 days. These results suggest prolonged exposure to depressive symptoms may contribute to inconsistent muscle performance, particularly during tasks involving isometric contraction (e.g., Hand) and periodic movement (e.g., HPI Periodicity). Such patterns imply that chronic psychological stress may impair immediate motor output and interfere with the long-term adaptation and refinement of motor patterns during training. Although the results indicate possible associations, this study’s cross-sectional design limits causal interpretation. Therefore, these findings should be interpreted as correlational. The training tasks employed in this study included both open kinetic chain movements focusing on hand activity and closed kinetic chain modalities such as the HPI tasks (36). Motor execution can be functionally divided into focal and postural components. The focal component refers to movements that directly accomplish a task, whereas the postural component provides the necessary stabilization to support those movements (37). From a neurophysiological standpoint, these components are associated with distinct control pathways: the cortico-spinal tract, which governs fine motor control and dexterous finger movements (38), and the cortico-reticular-spinal tract (39), which primarily supports postural control (40). The former involves finger flexion and grasping, while the latter contributes to proximal stability, including shoulder and upper arm positioning. Effective use of forearm muscles depends on stable postural control, which requires predictive regulation by the central nervous system (41, 42). Based on the present findings, anxiety and depression appear to be associated with activity in both extensor and flexor forearm muscles during training. The cortico-reticular-spinal tract, which modulates extensor muscles, plays a key role in postural regulation. The observed association between extensor muscle deformation and psychological distress suggests that prolonged psychological stress may influence postural control. These changes may affect fine motor skills via cortico-spinal tracts and may also affect attentional control functions and have an impact on performance (43). Moreover, cerebellar mechanisms may also be involved in how anxiety and depression affect postural stability (44). Such involvement of central and peripheral pathways is consistent with previous reports showing that psychological stress can impair neuromuscular coordination by affecting timing, co-contraction, and movement stability and perception (45). These findings suggest that for individuals experiencing prolonged performance issues with comorbid anxiety and depression, incorporating elements of unpredictability and postural variability into training, rather than relying solely on repetitive, upper-extremity isometric exercises, may be beneficial for breaking maladaptive motor habits. Finally, the top and bottom five participants based on total K6 scores were classified into high- and low-stress groups for exploratory comparison. Although no statistically significant differences were found between groups, moderate to large effect sizes were observed in extensor tasks (Gripper, Hand, Balloon, HPI Randomness) and flexor tasks (Balloon, HPI Periodicity) (Table 6). The small sample size may have limited the statistical power of the analysis. Future studies should include a larger and more diverse sample, incorporating participants at different competitive levels to improve generalizability. Furthermore, as this study was cross-sectional, longitudinal research is needed to evaluate how anxiety and depression affect muscle performance and postural control over time.
5 Conclusions
This study was conducted with amateur athletes who complained of subjective discomfort. The physical state (changes in muscle status) during a training task was measured using a muscle deformation sensor array to determine the relationship between the autocorrelation coefficient of muscle deformation and anxiety and depression. The results showed that anxiety and depression showed a positive correlation in the randomness task and a negative correlation in the periodicity task. The correlation was mainly observed in the forearm extensor muscles. This study suggests that anxiety and depressive symptoms may hinder not only flexible muscle contraction responses but also continuous contraction and contraction-relaxation processes during random tasks. Therefore, understanding the psychological states of athletes, particularly those who subjectively perceive poor performance, is essential for interpreting muscle activity and the resulting physical performance. These findings provide valuable insights into performance impairments attributable to psychological factors. Importantly, our results support integrating anxiety-reducing strategies into athletic training programs. Interventions such as mental skills training, mindfulness-based techniques, relaxation strategies, or stress-targeted motor tasks may help improve neuromuscular coordination and motor adaptability under psychological stress. These approaches could enhance both mental resilience and physical performance, especially in athletes experiencing psychological burdens.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by The Japanese Society for Assistive Technology. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SS: Conceptualization, Data curation, Formal analysis, Investigation, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. TM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing – review & editing. TA: Data curation, Investigation, Writing – review & editing. CO: Conceptualization, Funding acquisition, Project administration, Resources, Writing – review & editing. DY: Conceptualization, Project administration, Writing – review & editing. ET: Conceptualization, Funding acquisition, Methodology, Resources, Software, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Japan Sports Agency.
Conflict of interest
Authors SS, TM, TA, CO, and ET were employed by H2L Inc, the company that developed First VR. Measurements in this study were performed using equipment provided by H2L.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mileva KN, Zaidell L. Sport and exercise science and health. In: Health Studies: An Introduction. Singapore: Springer Singapore (2022). p. 85–124.
2. Devi G. Sports performance and mental health of athletes. Sports Sci Health Adv. (2023) 1:46–9. doi: 10.60081/SSHA.1.1.2023.46-49
3. Beauchamp P, Kamis D, Stull T. Achieving mental health and peak performance in elite athletes. Psychiatr Clin North Am. (2021) 44:347–58. doi: 10.1016/j.psc.2021.04.002
4. Stead J, Poolton J, Alder D. Performance slumps in sport: a systematic review. Psychol Sport Exerc. (2022) 61:102136. doi: 10.1016/j.psychsport.2022.102136
5. Klämpfl MK, Lobinger BH, Raab M. How to detect the yips in golf. Hum Mov Sci. (2013) 32:1270–87. doi: 10.1016/j.humov.2013.04.004
6. Inoue K, Yamada T, Ohtsuki T. Relationships between throwing yips in baseball, experiential avoidance, cognitive fusion, values, and social factors. J Clin Sport Psychol. (2023) 18:1–22. doi: 10.1123/jcsp.2022-0057
7. Sadnicka A, Kassavetis P, Pareés I, Meppelink AM, Butler K, Edwards M. Task-specific dystonia: pathophysiology and management. J Neurol Neurosurg Psychiatry. (2016) 87:968–74. doi: 10.1136/jnnp-2015-311298
8. Altenmüller E, Ioannou CI. Maladaptive plasticity induces degradation of fine motor skills in musicians. Z Psychol. (2016) 224:80–90. doi: 10.1027/2151-2604/a000242
9. Bingham Thomas E, Edwards NM, Haug JD, Horsburgh KA. Advances in biocultural approaches to understanding stress in humans. Humans. (2024) 4:321–39. doi: 10.3390/humans4040021
10. Batsukh S, Oh S, Rheu K, Lee B-J, Choi CH, Son KH, et al. Rice germ attenuates chronic unpredictable mild stress-induced muscle atrophy. Nutrients. (2023) 15:2719. doi: 10.3390/nu15122719
11. Reul JM, Collins A, Saliba RS, Mifsud KR, Carter SD, Gutierrez-Mecinas M, et al. Glucocorticoids, epigenetic control and stress resilience. Neurobiol Stress. (2015) 15:44–59. doi: 10.1016/j.ynstr.2014.10.001
12. Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. (2009) 10:434–45. doi: 10.1038/nrn2639
13. Engelbrecht AM, Smith C, Neethling I, Thomas M, Ellis B, Mattheyse M, et al. Daily brief restraint stress alters signaling pathways and induces atrophy and apoptosis in rat skeletal muscle. Stress. (2010) 13:132–41. doi: 10.3109/10253890903089834
14. Metz GA, Schwab ME, Welzl H. The effects of acute and chronic stress on motor and sensory performance in male lewis rats. Physiol Behav. (2001) 72:29–35. doi: 10.1016/s0031-9384(00)00371-1
15. Kemarat S, Theanthong A, Yeemin W, Suwankan S. Personality characteristics and competitive anxiety in individual and team athletes. PLoS One. (2022) 17:e0262486. doi: 10.1371/journal.pone.0262486
16. Steca P, Baretta D, Greco A, D’Addario M, Monzani D. Associations between personality, sports participation and athletic success. A comparison of big five in sporting and non-sporting adults. Pers Individ Dif. (2018) 121:176–83. doi: 10.1016/j.paid.2017.09.040
17. Datcu RF, Brîndescu SP. Anxiety and athlete performance: a systematic narrative review of the mutual influence of these concepts. Timisoara Phys Educ Rehab J. (2021) 14:62–75. doi: 10.2478/tperj-2021-0007
18. Khan MK, Khan A, Khan SU, Khan S. Effects of anxiety on athletic performance. Res Inves Sports Med. (2017) 1:1–5. doi: 10.31031/RISM.2017.01.000508
19. Gan D, Wu X, Zhang Y, Gao Y, Huang X, Han S. Dietary influence on the link between depression and muscle mass and muscle strength: exploring interaction and mediation effects. BMC Public Health. (2025) 25:764. doi: 10.1186/s12889-025-21975-2
20. Volaklis K, Mamadjanov T, Meisinger C, Linseisen J. Association between muscular strength and depressive symptoms: a narrative review. Wien Klin Wochenschr. (2019) 131:255–64. doi: 10.1007/s00508-019-1491-8
21. Miyake T, Minakuchi T, Sato S, Okubo C, Yanagihara D, Tamaki E. Optical myography-based sensing methodology of application of random loads to muscles during hand-gripping training. Sensors. (2024) 24:1108. doi: 10.3390/s24041108
22. Kessler RC, Andrews G, Colpe LJ, Hiripi E, Mroczek DK, Normand S-LT, et al. Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med. (2002) 32:959–76. doi: 10.1017/S0033291702006074
23. Sakurai K, Nishi A, Kondo K, Yanagida K, Kawakami N. Screening performance of k6/k10 and other screening instruments for mood and anxiety disorders in Japan. Psychiatry Clin Neurosci. (2011) 65:434–41. doi: 10.1111/j.1440-1819.2011.02236.x
24. Furukawa TA, Kawakami N, Saitoh M, Ono Y, Nakane Y, Nakamura YK. The performance of the Japanese version of the k6 and k10 in the world mental health survey Japan. Int J Methods Psychiatr Res. (2008) 17:152–8. doi: 10.1002/mpr.257
25. Sullivan P, Blacker M, Murphy J, Cairney J. Levels of psychological distress of Canadian University student-athletes. Can J High Educ. (2019) 49:47–59. doi: 10.47678/cjhe.v49i1.188192
26. McLoughlin E, Arnold R, Fletcher D, Spahr CM, Slavich GM, Moore LJ. Assessing lifetime stressor exposure in sport performers: associations with trait stress appraisals, health, well-being, and performance. Psychol Sport Exerc. (2022) 58:102078. doi: 10.1016/j.psychsport.2021.102078
27. Malone TL, Kern A, Klueh E, Eisenberg D. Psychological distress and its association with subjective athletic performance. J Sport Behav. (2022) 45:173–84.
28. Ojio Y, Matsunaga A, Kawamura S, Horiguchi M, Yoshitani G, Hatakeyama K, et al. Validating a Japanese version of the athlete psychological strain questionnaire. Sports Med Open. (2021) 7:1–8. doi: 10.1186/s40798-021-00385-9
29. Tamaki E, Hosono S, Iwasaki K. FirstVR: a muscle deformation sensors array device to detect finger gestures and noise reduction case. In: Proceedings of the 2019 2nd International Conference on Electronics, Communications and Control Engineering, Phuket, Thailand. New York, NY: Association for Computing Machinery (2019). p. 21–4. doi: 10.1145/3324033.3324041
30. Shimabukuro S, Miyake T, Tamaki E. Analyzing the differences in the degree of force application between novice and expert physiotherapists using a muscle deformation sensor. Cureus. (2024) 16(5):e59801. doi: 10.7759/cureus.59801
31. Nelson-Wong E, Howarth S, Winter DA, Callaghan JP. Application of autocorrelation and cross correlation analyses in human movement and rehabilitation research. J Orthop Sports Phys Therapy. (2009) 39:287–95. doi: 10.2519/jospt.2009.2969
32. Clarke P, Sheffield D, Akehurst S. Personality predictors of yips and choking susceptibility. Front Psychol. (2020) 10:479670. doi: 10.3389/fpsyg.2019.02784
33. Smith AM, Malo SA, Laskowski ER, Sabick M, Cooney WPF. A multidisciplinary study of the “yips” phenomenon in golf: an exploratory analysis. Sports Med. (2000) 30:423–37. doi: 10.2165/00007256-200030060-00004
34. Aoyama T, Ae K, Taguchi T, Kawamori Y, Sasaki D, Kawamura T, et al. Spatiotemporal patterns of throwing muscle synergies in yips-affected baseball players. Sci Rep. (2024) 14:2649. doi: 10.2165/00007256-200030060-00004
35. Sekiya H, Tanaka Y. Movement modifications related to psychological pressure in a table tennis forehand task. Percept Mot Skills. (2019) 126:143–56. doi: 10.2165/00007256-200030060-00004
36. Rivera JE. Open versus closed kinetic chain rehabilitation of the lower extremity: a functional and biomechanical analysis. J Sport Rehabil. (1994) 3:154–67. doi: 10.1123/jsr.3.2.154
37. Duarte M, Latash ML. Effects of postural task requirements on the speed–accuracy trade-off. Exp Brain Res. (2007) 180:457–67. doi: 10.1007/s00221-007-0871-x
38. Lemon R. Recent advances in our understanding of the primate corticospinal system. F1000Res. (2019) 8:F1000. doi: 10.12688/f1000research.17445.1
39. Jang SH, Lee SJ. Corticoreticular tract in the human brain: a mini review. Front Neurol. (2019) 10:490457. doi: 10.3389/fneur.2019.01188
40. Yanagihara D. Role of the cerebellum in postural control. J Phys Fit Sports Med. (2014) 3:169–72. doi: 10.7600/jpfsm.3.169
41. Marchese SM, Farinelli V, Bolzoni F, Esposti R, Cavallari P. Overview of the cerebellar function in anticipatory postural adjustments and of the compensatory mechanisms developing in neural dysfunctions. Appl Sci. (2020) 10:5088. doi: 10.3390/app10155088
42. Konosu A, Funato TM, Fujita A, Sakai R, Yanagihara D. A model of predictive postural control against floor tilting in rats. Front Syst Neurosci. (2021) 15:785366. doi: 10.3389/fnsys.2021.785366
43. Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: attentional control theory. Emotion. (2007) 7:336–53. doi: 10.1037/1528-3542.7.2.336
44. Konosu A, Matsuki Y, Fukuhara K, Funato T, Yanagihara D. Roles of the cerebellar vermis in predictive postural controls against external disturbances. Sci Rep. (2024) 14:3162. doi: 10.1038/s41598-024-53186-x
Keywords: muscle deformation, sports condition, anxiety and depression, autocorrelation coefficient, amateur athlete
Citation: Shimabukuro S, Miyake T, Akamine T, Ookubo C, Yanagihara D and Tamaki E (2025) Investigating the relationship between anxiety, depression, and forearm muscle deformation in amateur athletes using time-series analysis. Front. Sports Act. Living 7:1561808. doi: 10.3389/fspor.2025.1561808
Received: 16 January 2025; Accepted: 17 July 2025;
Published: 2 September 2025.
Edited by:
José Eduardo Teixeira, Instituto Politécnico da Guarda, PortugalReviewed by:
Pedro Forte, Higher Institute of Educational Sciences of the Douro, PortugalDaniel Leite Portella, Universidade Municipal de São Caetano do Sul, Brazil
Copyright: © 2025 Shimabukuro, Miyake, Akamine, Ookubo, Yanagihara and Tamaki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Satoshi Shimabukuro, azIyODY3MUBpZS51LXJ5dWt5dS5hYy5qcA==
 Satoshi Shimabukuro
Satoshi Shimabukuro Tamon Miyake
Tamon Miyake Tomo Akamine
Tomo Akamine Chihiro Ookubo3
Chihiro Ookubo3 Dai Yanagihara
Dai Yanagihara

