- 1Department of Medicinal Chemistry, School of Pharmacy, Weifang Medical University, Weifang, China
- 2School of Stomatology, Weifang Medical University, Weifang, China
- 3Shandong Analysis and Test Center, Qilu University of Technology (Shandong Academy of Sciences), Jinan, China
Inhibition of histone deacetylases (HDACs) has been extensively studied in the development of anticancer drugs. In the discovery of potent HDAC inhibitors with novel structures, the 2-substituted phenylquinoline-4-carboxylic acid group was introduced to the cap moiety of HDAC inhibitors. In total, 30 compounds were synthesized with hydroxamic acid or hydrazide zinc-binding groups. In the enzyme inhibitory test, active compound D28 and its analog D29 exhibited significant HDAC3 selectivity against HDAC1, 2, 3, and 6. However, compared with D28, the hydrazide-bearing compounds (D29 and D30) with remarkably improved enzyme inhibitory activities did not exhibit significant antiproliferative potency in the in vitro anticancer study. Further K562 cell-based mechanistic results revealed that induction of G2/M cell cycle arrest and promotion of apoptosis make important contributions to the anticancer effects of molecule D28. Collectively, an HDAC3 selective inhibitor (D28) with potent in vitro anticancer activity was developed as a lead compound for the treatment of cancer.
Introduction
Histone deacetylases (HDACs) are a group of enzymes that are responsible for the removal of acetyl group from ɛ-N-acetyl-lysine amino groups of histone proteins (Bernstein et al., 2000; de Ruijter et al., 2003). Regulated by HDACs and histone acetyltransferases (HATs), the balance of acetylation levels significantly contributes to the modulation of cellular functions and activities. In humans, a total of 18 HDAC isoforms have been identified and classified into four classes (Foglietti et al., 2006; Zhang et al., 2015). Among them, class I HDACs (HDACs 1, 2, 3, and 8), class II HDACs (HDACs 4, 5, 6, 7, 9, and 10), and class IV HDAC (HDAC 11) are zinc-dependent enzymes. In contrast, class III (sirtuins, Sirt1-7) HDACs are a family of NAD+-dependent enzymes which have remarkable structural differences from other classes of HDACs.
Overexpression and aberrant recruitment of HDACs are closely related to tumorigenesis and cancer aggravation (Marks et al., 2001; Zhang et al., 2019). Therefore, inhibition of HDACs has been extensively studied as a potential therapeutic target in the development of anticancer drugs. Vorinostat (SAHA) (Marks, 2007), romidepsin (FK-228) (Ueda et al., 1994), belinostat (PXD101) (Yang et al., 2010), and panobinostat (LBH589) (Neri et al., 2012) have been approved by United States FDA for the treatment of cutaneous T-cell lymphoma (CTCL), peripheral T-cell lymphoma (PTCL), and multiple myeloma, respectively. An increasing number of anticancer agents targeting HDACs are currently being developed in various stages.
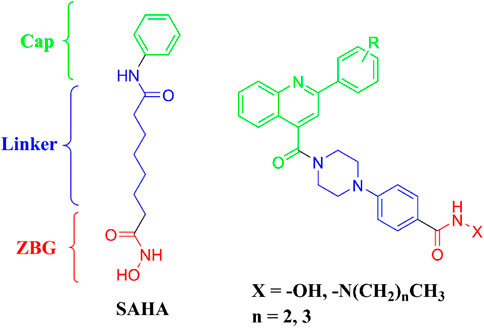
The pharmacophores of a typical HDAC inhibitor consists of a zinc-binding group (ZBG), a linker, and a cap region. According to ZBGs and chemical structures, HDAC inhibitors can be categorized into four types, including hydroxamic acids, benzamides, cyclic peptides, and aliphatic carboxylic acids (Zhang et al., 2010). Among the available ZBGs, the hydroxamic acid group had been widely utilized for the design of HDAC inhibitors. Cap moiety responsible for binding to the hydrophobic region at the opening of the HDAC active site, can also block other substrates from entering the HDAC catalytic pocket. The linker located in the channel of the HDAC active site was used to connect the ZBG and cap structures.
Cap group is an important feature in the design of novel HDAC inhibitors. Different cap structures play critical roles in the structural diversity of HDAC inhibitors. In the present study, the 2-substituted phenylquinoline-4-carboxylic acid group was introduced to the cap region for potent new HDAC inhibitors (Figure 1). The structure with multiple aromatic rings was designed to form strong hydrophobic interactions with residues in the opening of HDAC active site. The hydroxamic acid group was incorporated as the most commonly used ZBG and has exhibited advantages of both high HDAC (or zinc ion) affinity and potent antiproliferative activities (Zhang et al., 2018). Hydrazides as ZBGs have been revealed to have inhibitory selectivity of class I HDACs, especially in the inhibition of HDAC3 (Jiang et al., 2022). Therefore, hydrazides and the frequently used hydroxamic acid were utilized as ZBGs in the design of target compounds. The phenylpiperazine group was used as the linker to connect the substituted quinoline-4-carboxylic acid and ZBGs. The designed molecules were synthesized and evaluated in the in vitro enzyme inhibitory assay, antiproliferative study, cell cycle, and apoptosis tests.
Chemistry
Target compounds were synthesized as described in Scheme 1. The commercially available isatin (A) was used as the starting material for the compound synthesis. Intermediates B1-B29 were obtained by the coupling of isatin with substituted acetophenone through the Pfitzinger reaction (Swain et al., 2022). After that, key intermediates C1-C28 were derived by the condensation of the intermediates B1-B29 with 4-(piperazine-1-yl) methyl benzoate (Yamada et al., 2017). Target molecules D1-D28 were synthesized by treatment of intermediates C1-C28 with NH2OK in methanol. As to compounds D29-D30, hydrazides were introduced by hydrazinolysis of C28, and the subsequent condensation and reduction reactions.
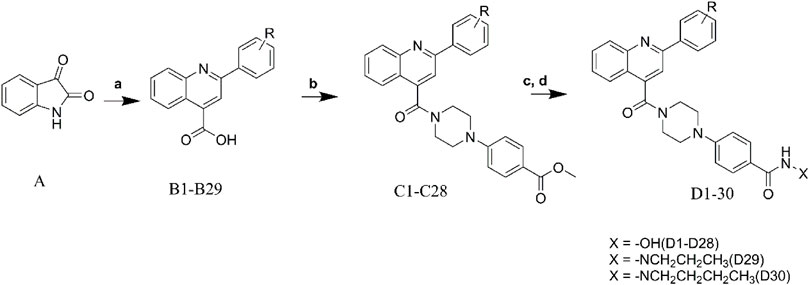
SCHEME 1. Synthesis of the designed compounds. Reagents and conditions: (A) substituted acetophenone, KOH, EtOH, 80°C; (B) TBTU, Et3N, DCM, 0°C; (C) NH2OK, CH3OH, rt; (D) NH2NH2.H2O, MeOH, reflux, 12–48 h; aliphatic aldehydes, NaBH4, rt, 4 h.
Results and Discussion
Enzymatic Inhibition Assay
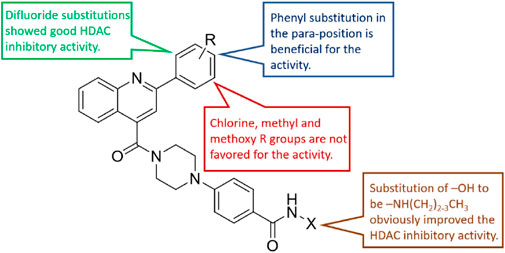
The HDAC enzyme inhibitory activities of synthesized compounds were evaluated against Hela nucleus extract containing a mixture of HDAC isoforms. Percentage inhibitory rate (PIR) was calculated to determine the activity of tested compounds (Table 1). The compounds (D1-28) were firstly synthesized for the activity screening. The results showed that compounds D11, D12, D23, D24, and D28 exhibited good HDAC inhibitory activity with PIR of 63.49%, 74.91%, 66.16%, and 68.00%, respectively, at a concentration of 2 μM. Compared with the unsubstituted D1, the difluoride-substitution and phenyl substitution can be conducive to the inhibitory activity. However, the chlorine-substituted compounds, such as D3 (PIR of 49.43%), D8 (PIR of 51.81%), and D14 (PIR of 49.74%), showed decreased activity compared with D1. Similarly, the methyl substitution and methoxy substitution in the phenyl ring reduced the HDAC inhibitory potency. As illustrated in Figure 2, the SAR analysis results provided evidence for further structural modification of current compounds. To avoid excessive molecular weight and increase the HDAC inhibitory activity, the R group with small size and molecular weight could be reserved for further structural modification.
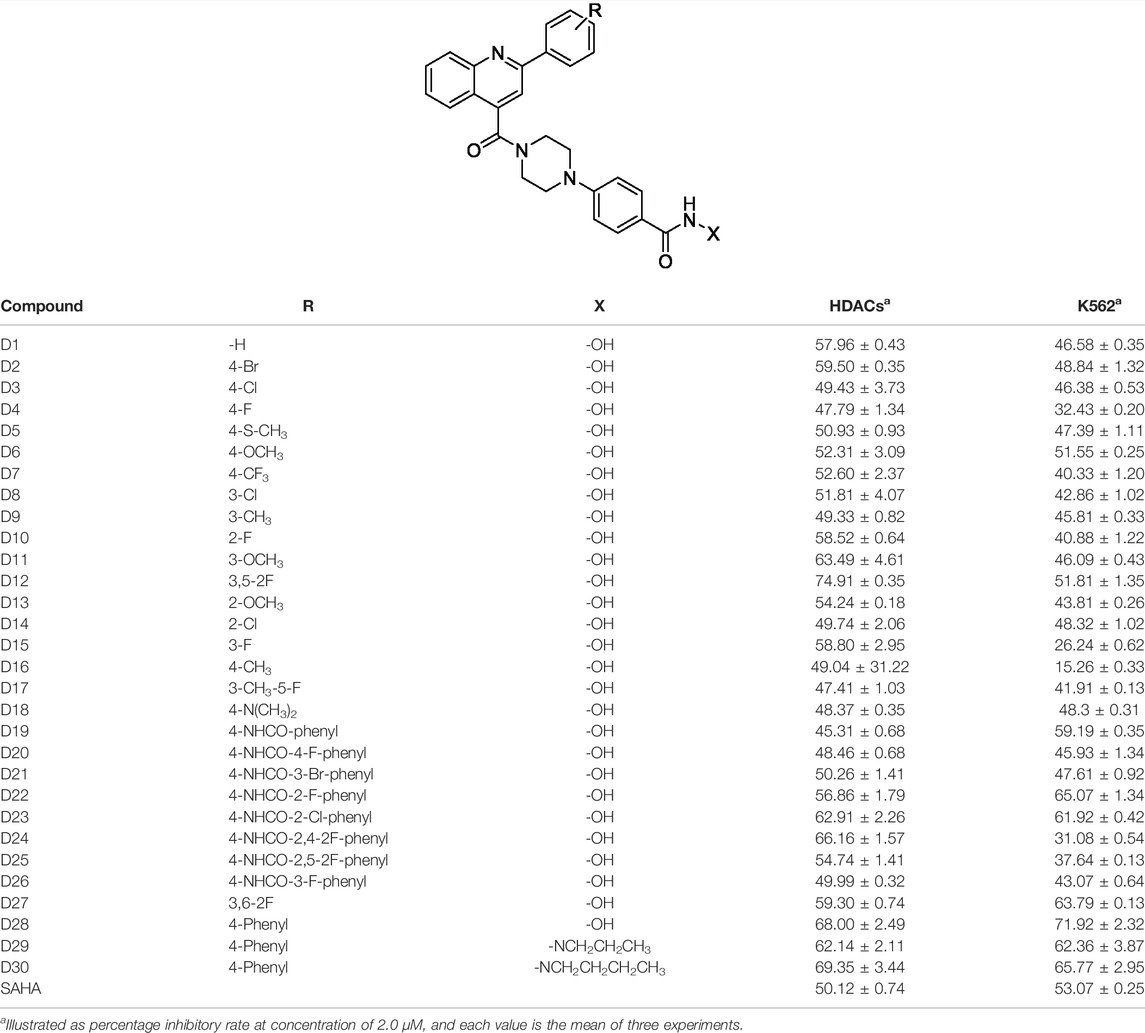
TABLE 1. Structure and inhibitory activity of target compounds at 2 µM against HDACs and K562 cells.
Molecule D28 exhibited significant activities in both enzymatic and K562 cell-based antiproliferative screening. Therefore, to evaluate the effects of different kinds of ZBGs, hydrazides were introduced to the ZBG part of D28. The synthesized D28 derivative D29 and D30 did not show obvious activity improvement in the Hela nucleus extract screening (Table 1). To study the inhibitory pattern of the derived molecules, the selectivities of molecules D28, D29, and D30 were investigated against various isoforms of HDACs using SAHA as a positive control (Table 2). As shown in Table 2, the tested compounds exhibited potential selectivity in inhibition of class I HDACs (HDAC1, 2, and 3) over HDAC6. Remarkably, molecules D29 and D30 showed improved inhibitory activities against HDAC 1, 2, and 3 compared with D28. The results also revealed that all the selected compounds, especially D28 and D29, have an inhibitory pattern of remarkable HDAC3 selectivity. It is indicated that hydrazide ZBGs are good at increasing the activity and selectivity of HDAC inhibitors.
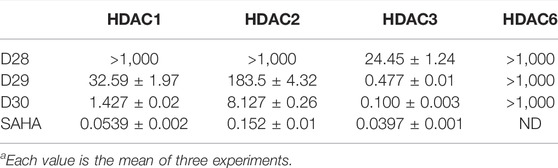
TABLE 2. Enzyme inhibitory selectivity of representative compounds compared with SAHA (IC50, µMa).
In Vitro Antiproliferative Test
To evaluate the in vitro anticancer activity of the active compounds, a CCK-8 assay was performed against a series of cancer cell lines. In the K652 cell-based screening, molecule D28 exhibited antiproliferative activity at a concentration of 2 µM. The hydrazide derivatives of D28 (D29 and D30) also showed in vitro anticancer potential in the test. Therefore, D28, D29, and D30 were selected for further evaluation. In screening of cell types that are sensitive to the derived compounds, both solid cancer cell lines were selected in the antiproliferative test (Table 3). Similar to the positive control SAHA, the selected compounds (D28, D29, and D30) showed higher inhibitory potency against hematologic cancer cells than the activity against solid cancer cell lines. Compared with SAHA, molecule D28 exhibited potency in inhibiting the growth of the test cell lines, especially in inhibition of the hematologic K562, U266 and U937 cell lines. Although compound D29 and D30 exhibited significantly increased enzyme inhibitory activities compared with the hydroxamic acid containing D28, the antiproliferative activities of D29 and D30 decreased markedly in the cancer cell-based test. Compound D29 and D30 did not show effective potency in the growth inhibition of the tested solid tumor cell lines, such as Fadu, MDA-MB-231, MDA-MB-468, A549, A2780, and HepG2 cells. The results revealed that hydrazide substitutions of D28 lead to a decrease in in vitro anticancer effects. Among the derived compounds, molecule D28 could be used as a lead compound for further evaluation.
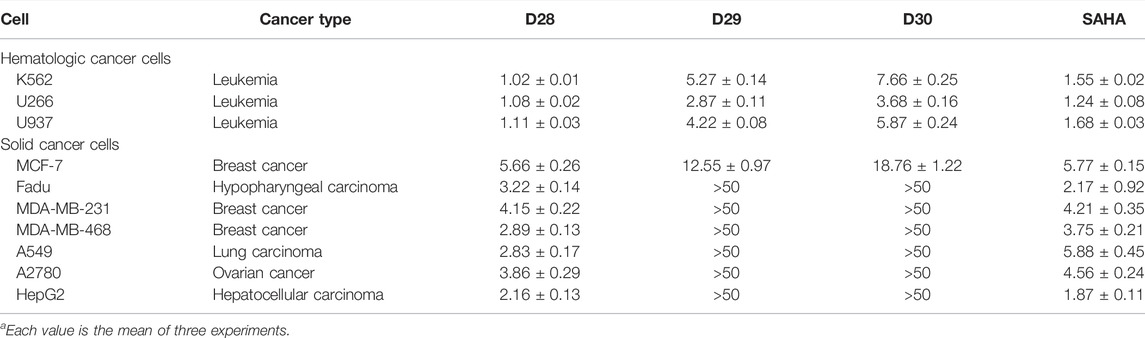
TABLE 3. Antiproliferative activities of representative molecules against various cancer cell lines (IC50, μMa).
Cell Cycle Analysis
Deregulation of the cell cycle divided into G0/G1 phase, S phase, and G2/M phase is one of the most frequent alterations during cancer development. Thus, the cell cycle analysis is usually performed in the anticancer evaluation. Herein, the most sensitive K562 cell line was utilized to investigate the effects of active molecule D28 on the cell cycle. After treating K562 cells with 1 and 2 µM of D28 and SAHA for 24 h, the cell cycle distribution was analyzed by flow cytometry. The results revealed different effects of D28 on the K562 cell cycle compared with SAHA (Figure 3). Molecule D28 increased cell proportion at the G2/M phase in a dose-dependent manner, while SAHA induced cell cycle arrest at G0/G1 phase. As shown in Figure 3, molecule D28 increased the G2/M phase ratio of K562 cells from 3.44% of the control to 5.95% and 32.57% at a dose of 1 and 2 μM, respectively. It is suggested that the promotion of G2/M phase cell cycle arrest contributes to the anticancer effects of compound D28.
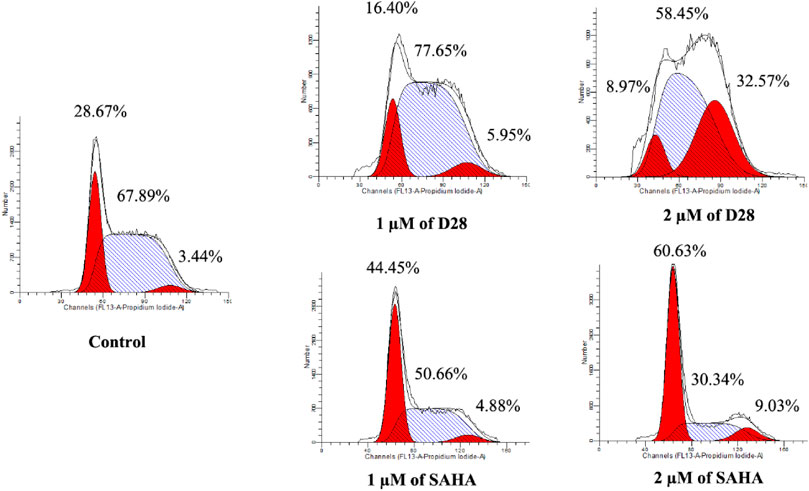
FIGURE 3. Molecule D28 induces G2/M cell cycle arrest in K562 cells. Cells were treated with 1.0 and 2.0 µM of D28 and SAHA for 24 h, and then flow cytometry was used to detect the percentage of cells in various phases of the cell cycle.
Cellular Apoptosis Study
Cancer is characterized by too little occurrence of apoptosis. Therefore, induction of apoptosis plays an important role in the treatment of cancer. To evaluate the effects of D28 on cell apoptosis, K562 cells were treated with various concentrations (1 μM, 2, and 4 µM) of D28 and SAHA, respectively. The results showed that molecule D28 promoted apoptosis K562 cells in a dose-dependent manner compared with SAHA (Figure 4). Significantly, molecule D28 increased apoptotic cell proportion from 1.14% of the control to 10.10%, 15.53%, and 27.92% at a dose of 1, 2, and 4 μM, respectively, compared with SAHA (apoptotic rate of 1.39%, 3.36%, 19.75% at doses of 1, 2, and 4 μM, respectively). The results revealed the important role of apoptosis in the anticancer effects of molecule D28.
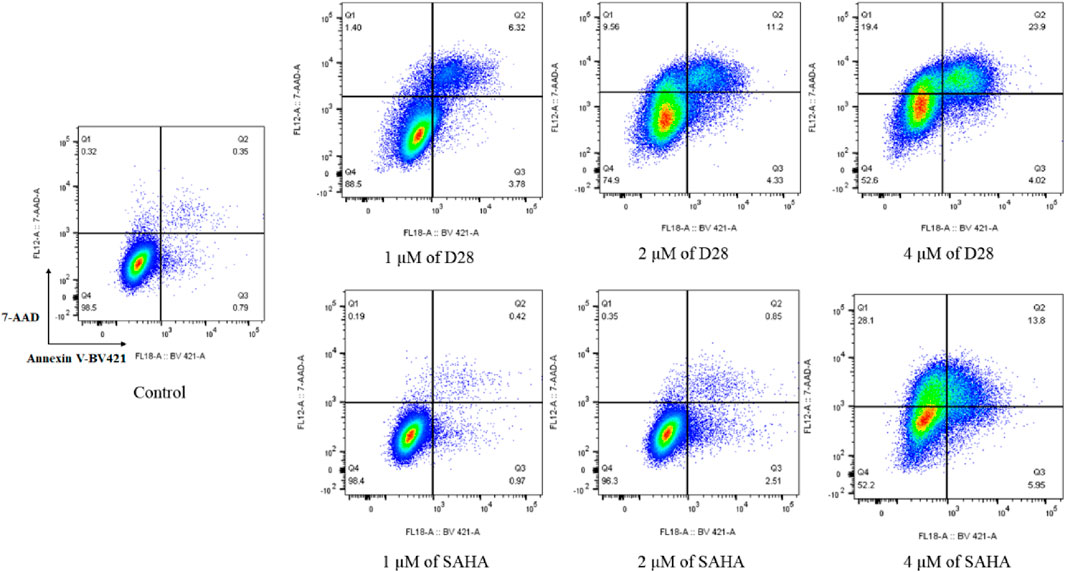
FIGURE 4. Molecule D28 induces cellular apoptosis of K562 cells. Cells were treated with 1.0, 2.0 and 4.0 µM of D28 and SAHA for 24 h, and cell apoptosis was determined by flow cytometric analysis.
Conclusion
Inhibition of HDACs has been extensively studied in the development of anticancer drugs. The discovery of selective HDAC inhibitors has emerged as a hotspot in the epigenetic therapy (He et al., 2020). Selective inhibitors are considered to be promising for cancer treatment by targeting a particular tumor type or a specific mechanism in the tumorigenesis. Compared with non-selective inhibitors, the isoform-selective HDAC inhibitors are considered to be characterized by therapeutic specificity and good safety profiles. HDAC3, the unique HDAC isoform that binds to the nuclear receptor corepressor NCOR1/SMRT, has been revealed to play a specific role in the emergence and development of cancer (Zhang et al., 2019). Selective inhibition of HDAC3 has shown a vast prospect in the treatment of cancer.
In the discovery of novel HDAC inhibitors for the treatment of cancer, the 2-substituted phenylquinoline-4-carboxylic acid group was introduced to the cap moiety of HDAC inhibitors. A total of 30 final compounds were synthesized for the SAR analysis, and various types of ZBGs (hydroxamic acid and hydrazide) were evaluated in the activity test. In the HDAC inhibitory assay, molecule D28 exhibited HDAC3 selectivity (IC50 value of 24.45 µM) without inhibition of HDAC1, 2, and 6. The hydrazide derivative of D28 showed remarkably improved inhibitory activities compared with D28, especially D29 with IC50 values of 32.59, 183.5, 0.477, and >1,000 µM against HDAC1, 2, 3, and 6, respectively. It is indicated that the hydrazide ZBG has the advantage of high potency and HDAC3 selectivity. In the in vitro anticancer test, molecule D28 exhibited good potency in the growth inhibition of tested cell lines with IC50 values of 1.02, 1.08, 1.11, 5.66, 3.22, 4.15, 2.89, 2.83, 3.86, and 2.16 µM against K562, U266, U937, MCF-7, Fadu, MDA-MB-231, MDA-MB-468, A549, A2780 and HepG2 cells compared with SAHA. However, molecules D29 and D30 with hydrazide ZBG exhibited reduced antiproliferative activities compared with D28 in the in vitro anticancer test. In the flow cytometry, promotion of G2/M cell cycle arrest and induction of apoptosis were revealed to be involved in the anticancer effects of molecule D28. Collectively, the current results exhibited the potential of HDAC3 selective inhibition in the treatment of cancer. The discovered HDAC3 selective inhibitors D28 and D29 could be utilized as lead compounds for further structural modification in the anticancer drug development.
Materials and Methods
All chemicals were obtained from commercial suppliers and can be used without further refinement. All reactions were detected by TLC using a 0.25 mm silica gel plate (60GF-254). UV light and ferric chloride were used to show TLC spots. All the synthesized target compounds were classified to be of new structures that have not been previously synthesized. 1H NMR and 13C NMR spectra were recorded on a Bruker DRX spectrometer at 500 MHz, using TMS as an internal standard. High-resolution mass spectra were recorded using a Thermo Scientific Q Exactive hybrid quadrupole-orbitrap mass spectrometer from Weifang Medical University.
Preparation of B1 and its analogs: derivatives B2–B28 were prepared as described for B1 (see below).
2-Phenylquinoline-4-carboxylic acid (B1). Isatin (0.5 g, 3.4 mmol) was dissolved in 10 ml 33% KOH solution. Then, 20 ml of acetophenone (0.45 g, 3.74 mmol) ethanol solution was slowly added, and the mixture refluxed at 85°C for 8 h. The solvent was removed by rotary evaporator, and 100 ml of water was added, then 10 ml of 3 M HCl was used to adjust the PH to 5–6. Compound B1 was obtained by filtration as yellow powder (0.3 g, 35% yield). HRMS C16H11NO2 [M + H+] calc. 250.08233 found 250.08180. 1H NMR (400 MHz, DMSO) δ 8.67 (d, J = 8.5 Hz, 1H), 8.48 (s, 1H), 8.31 (d, J = 7.4 Hz, 2H), 8.18 (d, J = 8.4 Hz, 1H), 7.87 (t, J = 7.6 Hz, 1H), 7.72 (t, J = 7.6 Hz, 1H), 7.57 (dq, J = 14.1, 7.0 Hz, 3H).
2-(4-Bromophenyl)Quinoline-4-Carboxylic Acid (B2)
Crystallization in EtOAc as a white solid (0.27g, 21.2% yield). HRMS C16H10BrNO2 [M + H+] calc. 327.99285 found 327.99585. 1H NMR (400 MHz, DMSO) δ 14.07 (s, 1H), 8.65 (d, J = 8.5 Hz, 1H), 8.48 (s, 1H), 8.28 (d, J = 8.0 Hz, 2H), 8.18 (d, J = 8.4 Hz, 1H), 7.87 (s, 1H), 7.82–7.67 (m, 3H).
2-(4-Chlorophenyl)Quinoline-4-Carboxylic Acid (B-3).
Crystallization in EtOAc as a white solid (0.28 g, 29.0% yield). HRMS C16H10ClNO2 [M + H+] calc. 284.04336 found 284.04630. 1H NMR (400 MHz, DMSO) δ 8.66 (d, J = 8.5 Hz, 1H), 8.48 (s, 1H), 8.35 (d, J = 8.2 Hz, 2H), 8.18 (d, J = 8.4 Hz, 1H), 7.88 (t, J = 7.6 Hz, 1H), 7.73 (t, J = 7.6 Hz, 1H), 7.64 (d, J = 8.2 Hz, 2H).
2-(4-Fluorophenyl)Quinoline-4-Carboxylic Acid (B4).
Crystallization in EtOAc as a white solid (0.26 g, 28.5% yield). HRMS C16H10FNO2 [M + H+] calc. 268.07291 found 268.07605. 1H NMR (400 MHz, DMSO) δ 14.00 (s, 1H), 8.69–8.56 (m, 1H), 8.39 (dd, J = 23.2, 12.3 Hz, 2H), 8.26 (d, J = 8.1 Hz, 1H), 8.13 (dd, J = 17.7, 8.5 Hz, 1H), 7.94–7.77 (m, 1H), 7.72–7.62 (m, 1H), 7.40 (t, J = 8.6 Hz, 1H), 7.10 (d, J = 8.6 Hz, 1H).
2-(4-(Methylthio)Phenyl)Quinoline-4-Carboxylic Acid (B5)
Crystallization in EtOAc as a yellow solid (0.52 g, 51.7% yield). HRMS C17H13NO2S [M + H+] calc. 296.07005 found 296.07318. 1H NMR (400 MHz, DMSO) δ 8.63 (d, J = 8.5 Hz, 1H), 8.39 (s, 1H), 8.26 (d, J = 8.2 Hz, 2H), 8.12 (d, J = 8.2 Hz, 1H), 7.82 (s, 1H), 7.66 (s, 1H), 7.44 (d, J = 8.0 Hz, 2H), 2.56 (s, 3H).
2-(4-Methoxyphenyl)Quinoline-4-Carboxylic Acid (B6)
Crystallization in EtOAc as a white solid (0.60 g, 63.0% yield). HRMS C17H13NO3 [M + H+] calc. 280.09290 found 280.09592. 1H NMR (400 MHz, DMSO) δ 13.97 (s, 1H), 8.62 (d, J = 8.5 Hz, 1H), 8.41 (s, 1H), 8.28 (d, J = 8.5 Hz, 2H), 8.12 (d, J = 8.4 Hz, 1H), 7.83 (t, J = 7.6 Hz, 1H), 7.66 (t, J = 7.6 Hz, 1H), 7.13 (d, J = 8.5 Hz, 2H), 3.85 (d, J = 7.7 Hz, 3H).
2-(4-(Trifluoromethyl)Phenyl)Quinoline-4-Carboxylic Acid (B7)
Crystallization in EtOAc as a white solid (0.36 g, 33.4% yield). HRMS C17H10F3NO2 [M + H+] calc. 318.06972 found 318.07202. 1H NMR (400 MHz, DMSO) δ 14.13 (s, 1H), 8.68 (d, J = 8.5 Hz, 1H), 8.53 (d, J = 8.7 Hz, 3H), 8.21 (d, J = 8.4 Hz, 1H), 7.91 (dd, J = 20.8, 8.0 Hz, 3H), 7.76 (t, J = 7.6 Hz, 1H).
2-(3-Chlorophenyl)Quinoline-4-Carboxylic Acid (B8)
Crystallization in EtOAc as a white solid (0.56 g, 58.0% yield). HRMS C16H10ClNO2 [M + H+] calc. 284.04336 found 284.04587. 1H NMR (400 MHz, DMSO) δ 14.09 (s, 1H), 8.64 (d, J = 8.4 Hz, 1H), 8.49 (s, 1H), 8.37 (s, 1H), 8.28 (d, J = 3.5 Hz, 1H), 8.20 (d, J = 8.4 Hz, 1H), 7.88 (t, J = 7.6 Hz, 1H), 7.73 (t, J = 7.6 Hz, 1H), 7.61 (d, J = 4.5 Hz, 2H).
2-(m-Tolyl)Quinoline-4-Carboxylic Acid (B9)
Crystallization in EtOAc as a white solid (0.44 g, 49.0% yield). HRMS C17H13NO2 [M + H+] calc. 264.09798 found 264.10056. 1H NMR (400 MHz, DMSO) δ 14.00 (s, 1H), 8.65 (d, J = 8.6 Hz, 1H), 8.45 (s, 1H), 8.21–8.05 (m, 3H), 7.86 (t, J = 7.5 Hz, 1H), 7.71 (t, J = 7.6 Hz, 1H), 7.47 (t, J = 7.5 Hz, 1H), 7.36 (d, J = 7.6 Hz, 1H), 2.46 (s, 3H).
2-(2-Fluorophenyl)Quinoline-4-Carboxylic Acid (B10)
Crystallization in EtOAc as a white solid (0.76 g, 59.7% yield). HRMS C16H10FNO2 [M + H+] calc. 268.07291 found 268.07291. 1H NMR (400 MHz, DMSO) δ 14.02 (s, 1H), 8.74 (d, J = 8.4 Hz, 1H), 8.31 (s, 1H), 8.19 (d, J = 8.4 Hz, 1H), 8.11 (t, J = 7.8 Hz, 1H), 7.89 (t, J = 7.7 Hz, 1H), 7.76 (t, J = 7.7 Hz, 1H), 7.65–7.54 (m, 1H), 7.42 (dd, J = 13.4, 5.8 Hz, 2H).
2-(3-Methoxyphenyl)Quinoline-4-Carboxylic Acid (B11)
Crystallization in EtOAc as a white solid (0.74 g, 65.5% yield). HRMS C17H13NO3 [M + H+] calc. 280.09290 found 280.09586. 1H NMR (400 MHz, DMSO) δ 14.03 (s, 1H), 8.64 (d, J = 8.5 Hz, 1H), 8.44 (s, 1H), 8.17 (d, J = 8.5 Hz, 1H), 7.93–7.80 (m, 3H), 7.71 (t, J = 7.6 Hz, 1H), 7.50 (t, J = 7.9 Hz, 1H), 7.11 (d, J = 8.3 Hz, 1H), 3.89 (s, 3H).
2-(3,5-Difluorophenyl)Quinoline-4-Carboxylic Acid (B12)
Crystallization in EtOAc as a white solid (0.84 g, 67.9% yield). HRMS C16H9F2NO2 [M + H+] calc. 286.06349 found 286.16583. 1H NMR (400 MHz, DMSO) δ 14.09 (s, 1H), 8.63 (d, J = 8.5 Hz, 1H), 8.52 (s, 1H), 8.20 (d, J = 8.4 Hz, 1H), 8.06 (d, J = 7.9 Hz, 2H), 7.89 (t, J = 7.7 Hz, 1H), 7.75 (t, J = 7.7 Hz, 1H), 7.44 (t, J = 8.8 Hz, 1H).
2-(2-Methoxyphenyl)Quinoline-4-Carboxylic Acid (B13)
Crystallization in EtOAc as a white solid (0.68 g, 51.1% yield). HRMS C17H13NO3 [M + H+] calc. 280.09290 found 280.09583. 1H NMR (400 MHz, DMSO) δ 13.88 (s, 1H), 8.70 (d, J = 8.5 Hz, 1H), 8.35 (s, 1H), 8.14 (d, J = 8.4 Hz, 1H), 7.84 (t, J = 6.4 Hz, 2H), 7.72 (t, J = 7.7 Hz, 1H), 7.51 (t, J = 7.8 Hz, 1H), 7.24 (d, J = 8.3 Hz, 1H), 7.14 (t, J = 7.4 Hz, 1H), 3.88 (s, 3H).
2-(2-Chlorophenyl)Quinoline-4-Carboxylic Acid (B14)
Crystallization in EtOAc as a white solid (0.78 g, 49.0% yield). HRMS C16H10ClNO2 [M + H+] calc. 284.04336 found 284.04639. 1H NMR (400 MHz, DMSO) δ 14.04 (s, 1H), 8.75 (d, J = 8.5 Hz, 1H), 8.16 (d, J = 9.4 Hz, 2H), 7.89 (t, J = 7.5 Hz, 1H), 7.81–7.71 (m, 2H), 7.66 (d, J = 7.8 Hz, 1H), 7.55 (dd, J = 9.3, 5.7 Hz, 2H).
2-(3-Fluorophenyl)Quinoline-4-Carboxylic Acid (B15)
Crystallization in EtOAc as a white solid (0.64 g, 49.0% yield). HRMS C16H10FNO2 [M + H+] calc. 268.07291 found 268.07581. 1H NMR (400 MHz, DMSO) δ 14.06 (s, 1H), 8.65 (d, J = 8.5 Hz, 1H), 8.50 (s, 1H), 8.17 (dd, J = 21.4, 11.4 Hz, 3H), 7.88 (t, J = 7.6 Hz, 1H), 7.74 (t, J = 7.7 Hz, 1H), 7.63 (dd, J = 14.6, 7.3 Hz, 1H), 7.39 (t, J = 8.2 Hz, 1H).
2-(o-Tolyl)Quinoline-4-Carboxylic Acid (B16)
Crystallization in EtOAc as a white solid (0.66 g, 52.6% yield). HRMS C17H13NO2 [M + H+] calc. 264.09798 found 264.10110. 1H NMR (400 MHz, DMSO) δ 13.96 (s, 1H), 8.72 (d, J = 8.2 Hz, 1H), 8.14 (d, J = 8.2 Hz, 1H), 8.04 (s, 1H), 7.87 (t, J = 7.3 Hz, 1H), 7.75 (t, J = 7.3 Hz, 1H), 7.56 (d, J = 6.7 Hz, 1H), 7.35 (d, J = 34.3 Hz, 3H), 2.41 (s, 3H).
2-(4-Fluoro-3-Methylphenyl)Quinoline-4-Carboxylic Acid (B17)
Crystallization in EtOAc as a white solid (0.54 g, 56.3% yield). HRMS C17H12FNO2 [M + H+] calc. 282.08856 found 282.08722. 1H NMR (400 MHz, DMSO) δ 8.62 (t, J = 9.7 Hz, 1H), 8.44 (s, 1H), 8.26 (d, J = 7.4 Hz, 1H), 8.21–8.10 (m, 2H), 7.85 (t, J = 7.6 Hz, 1H), 7.69 (dd, J = 16.9, 9.3 Hz, 1H), 7.33 (t, J = 9.1 Hz, 1H), 2.38 (s, 3H).
2-(4-(Dimethylamino)Phenyl)Quinoline-4-Carboxylic Acid (B18)
Crystallization in EtOAc as a red solid (0.55 g, 55.2% yield). HRMS C18H16N2O2 [M + H+] calc. 293.12453 found 293.12701. 1H NMR (400 MHz, DMSO) δ 8.58 (d, J = 8.4 Hz, 1H), 8.33 (s, 1H), 8.17 (d, J = 8.4 Hz, 2H), 8.05 (d, J = 8.4 Hz, 1H), 7.77 (t, J = 7.5 Hz, 1H), 7.59 (t, J = 7.6 Hz, 1H), 6.85 (d, J = 8.4 Hz, 2H).
2-(4-Benzamidophenyl)Quinoline-4-CarboxylicAcid (B19)
Crystallization in EtOAc as a white solid (0.27 g, 64.4% yield). HRMS C23H16N2O3 [M + H+] calc. 369.11945 found 369.12335. 1H NMR (400 MHz, DMSO) δ 10.49 (s, 1H), 8.64 (d, J = 8.5 Hz, 1H), 8.34–8.20 (m, 3H), 8.07 (d, J = 8.3 Hz, 1H), 8.01 (d, J = 7.9 Hz, 4H), 7.95 (d, J = 8.7 Hz, 1H), 7.76 (t, J = 7.5 Hz, 1H), 7.63–7.51 (m, 4H).
2-(4-(4-Fluorobenzamido)Phenyl)Quinoline-4-Carboxylic Acid (B20)
Crystallization in EtOAc as a white solid (0.41 g, 93.2% yield). HRMS C23H15FN2O3 [M + H+] calc. 387.11003 found 387.10703. 1H NMR (400 MHz, DMSO) δ 10.51 (s, 1H), 8.65 (d, J = 8.5 Hz, 1H), 8.48 (s, 1H), 8.34 (d, J = 8.3 Hz, 2H), 8.22–7.95 (m, 5H), 7.85 (t, J = 7.6 Hz, 1H), 7.69 (t, J = 7.6 Hz, 1H), 7.40 (t, J = 8.6 Hz, 2H).
2-(4-(3-Bromobenzamido)Phenyl)Quinoline-4-Carboxylic Acid (B21)
Crystallization in EtOAc as a white solid (0.36 g, 70.1% yield). HRMS C23H15BrN2O3 [M + H+] calc. 447.02996 found 447.03238. 1H NMR (400 MHz, DMSO) δ 10.60 (s, 1H), 8.64 (d, J = 7.8 Hz, 1H), 8.50–8.29 (m, 3H), 8.21 (s, 1H), 8.13 (d, J = 8.0 Hz, 1H), 7.99 (t, J = 14.1 Hz, 4H), 7.82 (s, 2H), 7.66 (d, J = 6.6 Hz, 1H), 7.54 (d, J = 7.5 Hz, 1H).
2-(4-(2-Fluorobenzamido)Phenyl)Quinoline-4-Carboxylic Acid (B22)
Crystallization in EtOAc as a white solid (0.44 g, 85.5% yield). HRMS C23H15FN2O3 [M + H+] calc. 387.11003 found 387.10721. 1H NMR (400 MHz, DMSO) δ 10.69 (s, 1H), 8.63 (d, J = 7.8 Hz, 1H), 8.58–8.22 (m, 3H), 8.14 (d, J = 7.8 Hz, 1H), 7.95 (d, J = 7.0 Hz, 2H), 7.82 (d, J = 6.6 Hz, 1H), 7.77–7.49 (m, 3H), 7.50–7.14 (m, 2H).
2-(4-(2-Chlorobenzamido)Phenyl)Quinoline-4-Carboxylic Acid (B23)
Crystallization in EtOAc as a white solid (0.73 g, 68.6% yield). HRMS C23H15FN2O3 [M + H+] calc. 403.08047 found 403.07718. 1H NMR (400 MHz, DMSO) δ 10.80 (s, 1H), 8.68 (d, J = 7.5 Hz, 1H), 8.27 (d, J = 7.2 Hz, 2H), 8.14 (s, 1H), 8.03 (d, J = 7.7 Hz, 1H), 7.93 (d, J = 7.1 Hz, 2H), 7.71 (s, 1H), 7.63 (s, 1H), 7.58 (s, 1H), 7.42 (dd, J = 65.7, 22.8 Hz, 4H).
2-(4-(2,4-Difluorobenzamido)Phenyl)Quinoline-4-Carboxylic Acid (B24)
Crystallization in EtOAc as a white solid (0.47 g, 94.7% yield). HRMS C23H14F2N2O3 [M + H+] calc. 405.10060 found 405.09412. 1H NMR (400 MHz, DMSO) δ 10.68 (s, 1H), 8.64 (d, J = 8.4 Hz, 1H), 8.43 (s, 1H), 8.33 (d, J = 8.3 Hz, 2H), 8.14 (d, J = 8.4 Hz, 1H), 7.93 (d, J = 8.4 Hz, 2H), 7.88–7.76 (m, 2H), 7.67 (t, J = 7.6 Hz, 1H), 7.46 (t, J = 9.9 Hz, 1H), 7.26 (t, J = 8.4 Hz, 1H), 5.29–5.25 (m, 1H).
2-(4-(3,5-Difluorobenzamido)Phenyl)Quinoline-4-Carboxylic Acid (B25)
Crystallization in EtOAc as a white solid (0.96 g, 89.4% yield). HRMS C23H14F2N2O3 [M + H+] calc. 405.10060 found 405.10413. 1H NMR (400 MHz, DMSO) δ 10.61 (s, 1H), 8.64 (d, J = 8.4 Hz, 1H), 8.43 (s, 1H), 8.35 (d, J = 8.2 Hz, 2H), 8.14 (d, J = 8.3 Hz, 1H), 8.01 (d, J = 8.2 Hz, 2H), 7.83 (t, J = 7.5 Hz, 1H), 7.80–7.60 (m, 3H), 7.55 (t, J = 8.5 Hz, 1H).
2-(4-(3-Fluorobenzamido)Phenyl)Quinoline-4-Carboxylic Acid (B26)
Crystallization in EtOAc as a white solid (0.67 g, 91.6% yield). HRMS C23H15FN2O3 [M + H+] calc. 387.11003 found 387.10410. 1H NMR (400 MHz, DMSO) δ 10.57 (s, 1H), 8.63 (d, J = 8.4 Hz, 1H), 8.41–8.28 (m, 3H), 8.11 (d, J = 8.4 Hz, 1H), 8.01 (d, J = 8.1 Hz, 2H), 7.90–7.75 (m, 3H), 7.62 (dd, J = 14.6, 7.2 Hz, 2H), 7.48 (t, J = 8.5 Hz, 1H).
2-(2,5-Difluorophenyl)Quinoline-4-Carboxylic Acid (B27)
Crystallization in EtOAc as a white solid (0.83 g, 85.3% yield). HRMS C16H9F2NO2 [M + H+] calc. 286.06349 found 286.06192. 1H NMR (400 MHz, DMSO) δ 8.75 (d, J = 8.5 Hz, 1H), 8.35 (s, 1H), 8.21 (d, J = 8.4 Hz, 1H), 7.91 (t, J = 7.5 Hz, 2H), 7.79 (t, J = 7.7 Hz, 1H), 7.59–7.33 (m, 2H).
2-([1,1′-Biphenyl]-4-yl)Quinoline-4-Carboxylic Acid (B28)
Crystallization in EtOAc as a yellow solid (0.98 g, 88.4% yield). HRMS C22H15NO2 [M + H+] calc. 326.11363 found 326.11176. 1H NMR (400 MHz, DMSO) δ 8.66 (d, J = 8.5 Hz, 1H), 8.52 (s, 1H), 8.42 (d, J = 7.9 Hz, 2H), 8.19 (d, J = 8.5 Hz, 1H), 7.88 (dd, J = 14.6, 7.9 Hz, 3H), 7.79 (d, J = 7.7 Hz, 2H), 7.71 (t, J = 7.6 Hz, 1H), 7.53 (t, J = 7.5 Hz, 2H), 7.43 (t, J = 7.2 Hz, 1H).
2-(4-Amino-Phenyl)-Quinoline-4-Carboxylic Acid (B29)
Crystallization in EtOAc as a white solid (0.54 g, 59.9% yield). HRMS C16H12N2O2 [M + H]+ calc. 265.0320, found 265.0960. 1H NMR (400 MHz, DMSO) δ 8.58 (d, J = 8.3 Hz, 1H), 8.32 (s, 1H), 8.05 (d, J = 8.1 Hz, 3H), 7.77 (t, J = 7.3 Hz, 1H), 7.59 (t, J = 7.4 Hz, 1H), 6.72 (d, J = 8.1 Hz, 2H).
Methyl 4-(4-(2-Phenylquinoline-4-Carbonyl)Piperazin-1-yl)Benzoate (C1)
2-Phenylquinoline-4-carboxylic acid (B1) (0.51 g, 2.06 mmol) was dissolved in 20 ml of DCM. Under ice bath condition Et3N (0.48 g, 4.71 mmol) and TBTU (0.73 g, 2.27 mmol) were added. After 20 min, the ice bath was removed and 4-(Piperazine-1-yl) Methyl Benzoate (0.5 g, 2.27 mmol) was added. After 8 h, the solvent is removed by rotary evaporation, and the concentrated solution was dissolved in 100 ml of EtOAc, washed with NaHCO3 (3 × 20 ml) and NaCl (3 × 20 ml), and dried with MgSO4. The desired compound (C1) was obtained by filtration and recrystallization in EtOAc as a white solid (0.21 g, 23.1% yield). HRMS C28H25N3O3 [M + H+] calc. 452.19295 found 452.19568. 1H NMR (400 MHz, DMSO) δ 8.33 (d, J = 7.5 Hz, 2H), 8.20 (s, 1H), 8.16 (d, J = 8.3 Hz, 1H), 7.91–7.75 (m, 4H), 7.66 (t, J = 7.6 Hz, 1H), 7.57 (dd, J = 16.0, 8.8 Hz, 3H), 6.99 (d, J = 8.7 Hz, 2H), 3.95 (d, J = 19.7 Hz, 2H), 3.77 (s, 3H), 3.59 (s, 2H), 3.35 (s, 2H), 3.29–3.12 (m, 3H).
Methyl 4-(4-(2-(4-Bromophenyl)Quinoline-4-Carbonyl)Piperazin-1-yl)Benzoate (C2)
Crystallization in EtOAc as a yellow solid (0.17 g, 25.1% yield). HRMS C28H24BrN3O3 [M + H+] calc. 530.10346 found 530.10614. 1H NMR (400 MHz, DMSO) δ 8.30 (d, J = 8.2 Hz, 2H), 8.23 (s, 1H), 8.16 (d, J = 8.8 Hz, 1H), 7.82 (dt, J = 18.3, 8.4 Hz, 6H), 7.67 (t, J = 7.6 Hz, 1H), 6.99 (d, J = 8.6 Hz, 2H), 3.95 (d, J = 16.8 Hz, 2H), 3.77 (s, 3H), 3.59 (s, 2H), 3.33 (s, 2H), 3.25 (d, J = 18.2 Hz, 2H).
Methyl 4-(4-(2-(4-Chlorophenyl)Quinoline-4-Carbonyl)Piperazin-1-yl)Benzoate (C3)
Crystallization in EtOAc as a white solid (0.33 g, 41.1% yield). HRMS C28H24ClN3O3 [M + H+] calc. 486.15397 found 486.15885. 1H NMR (400 MHz, DMSO) δ 8.37 (d, J = 8.2 Hz, 1H), 8.14 (dd, J = 12.0, 8.6 Hz, 1H), 7.86 (t, J = 9.6 Hz, 3H), 7.80 (d, J = 8.8 Hz, 2H), 7.68 (d, J = 8.0 Hz, 1H), 7.64 (d, J = 8.6 Hz, 1H), 7.52 (t, J = 7.6 Hz, 1H), 7.06 (d, J = 8.7 Hz, 1H), 6.99 (d, J = 8.5 Hz, 2H), 3.78 (d, J = 7.6 Hz, 4H), 3.60 (dd, J = 21.8, 17.3 Hz, 4H), 3.35 (s, 4H).
Methyl 4-(4-(2-(4-Fluorophenyl)Quinoline-4-Carbonyl)Piperazin-1-yl)Benzoate (C4)
Crystallization in EtOAc as a white solid (0.13 g, 57.6% yield). HRMS C28H24FN3O3 [M + H+] calc. 470.18352 found 470.18607. 1H NMR (400 MHz, DMSO) δ 8.48–8.35 (m, 1H), 8.29 (d, J = 8.5 Hz, 1H), 8.25–8.06 (m, 2H), 7.90–7.73 (m, 4H), 7.64 (dd, J = 19.5, 7.7 Hz, 1H), 7.40 (t, J = 8.5 Hz, 1H), 7.09 (d, J = 8.6 Hz, 1H), 6.99 (d, J = 8.5 Hz, 2H), 3.95 (d, J = 17.2 Hz, 2H), 3.77 (s, 3H), 3.59 (s, 2H), 3.35 (s, 2H), 3.25 (d, J = 19.8 Hz, 2H).
Methyl 4-(4-(2-(4-(Methylthio)Phenyl)Quinoline-4-Carbonyl)Piperazin-1-yl)Benzoate (C5)
Crystallization in EtOAc as a yellow solid (0.21 g, 41.7% yield). HRMS C29H27N3O3S [M + H+] calc. 498.18067 found 498.18253. 1H NMR (400 MHz, DMSO) δ 8.29 (d, J = 8.1 Hz, 2H), 8.21–8.07 (m, 2H), 7.82 (dd, J = 19.3, 8.4 Hz, 4H), 7.64 (t, J = 7.6 Hz, 1H), 7.43 (d, J = 8.3 Hz, 2H), 6.99 (d, J = 8.6 Hz, 2H), 3.95 (d, J = 22.5 Hz, 2H), 3.78 (d, J = 8.2 Hz, 3H), 3.59 (s, 2H), 3.29–3.12 (m, 4H).
Methyl 4-(4-(2-(4-Methoxyphenyl)Quinoline-4-Carbonyl)Piperazin-1-yl)Benzoate (C6)
Crystallization in EtOAc as a white solid (0.18 g, 30.3% yield). HRMS C29H27N3O4 [M + H+] calc. 482.20351 found 482.19928. 1H NMR (400 MHz, DMSO) δ 8.32 (d, J = 8.2 Hz, 2H), 8.17–8.07 (m, 2H), 7.82 (dd, J = 11.9, 8.5 Hz, 4H), 7.63 (d, J = 7.7 Hz, 1H), 7.12 (d, J = 8.3 Hz, 2H), 6.99 (d, J = 8.5 Hz, 2H), 4.01–3.74 (m, 8H), 3.60 (d, J = 4.6 Hz, 2H), 3.35 (s, 1H), 3.33–3.14 (m, 3H).
Methyl 4-(4-(2-(4-(Trifluoromethyl)Phenyl)Quinoline-4-Carbonyl)Piperazin-1-yl)Benzoate (C7)
Crystallization in EtOAc as a white solid (0.16 g, 32.4% yield). HRMS C29H24F3N3O3 [M + H+] calc. 520.18033 found 520.18274. 1H NMR (400 MHz, DMSO) δ 8.56 (d, J = 8.1 Hz, 2H), 8.31 (s, 1H), 8.20 (d, J = 8.7 Hz, 1H), 8.00–7.83 (m, 4H), 7.80 (d, J = 8.4 Hz, 2H), 7.71 (t, J = 7.5 Hz, 1H), 6.99 (d, J = 8.7 Hz, 2H), 3.96 (d, J = 18.8 Hz, 2H), 3.77 (s, 3H), 3.60 (s, 2H), 3.33 (s, 2H), 3.21 (s, 2H).
Methyl 4-(4-(2-(3-Chlorophenyl)Quinoline-4-Carbonyl)Piperazin-1-yl)Benzoate (C8)
Crystallization in EtOAc as a white solid (0.25 g, 48.5% yield). HRMS C28H24ClN3O3 [M + H+] calc. 486.15397 found 486.15674. 1H NMR (400 MHz, DMSO) δ 8.40 (s, 1H), 8.30 (d, J = 17.4 Hz, 2H), 8.19 (d, J = 8.7 Hz, 1H), 7.87 (t, J = 7.2 Hz, 2H), 7.80 (d, J = 8.6 Hz, 2H), 7.68 (t, J = 7.7 Hz, 1H), 7.61 (d, J = 4.6 Hz, 2H), 6.99 (d, J = 8.6 Hz, 2H), 3.95 (d, J = 18.4 Hz, 2H), 3.77 (s, 3H), 3.60 (s, 2H), 3.34 (s, 2H), 3.22 (s, 2H).
Methyl 4-(4-(2-(m-Tolyl)Quinoline-4-Carbonyl)Piperazin-1-yl)Benzoate (C9)
Crystallization in EtOAc as a white solid (0.24 g, 45.2% yield). HRMS C29H27N3O3 [M + H+] calc. 466.20880 found 466.21140. 1H NMR (400 MHz, DMSO) δ 8.22–8.08 (m, 4H), 7.90–7.76 (m, 4H), 7.65 (t, J = 7.5 Hz, 1H), 7.45 (t, J = 7.6 Hz, 1H), 7.35 (d, J = 7.5 Hz, 1H), 6.99 (d, J = 8.6 Hz, 2H), 3.95 (d, J = 29.7 Hz, 2H), 3.77 (s, 3H), 3.59 (s, 2H), 3.33 (s, 2H), 3.25 (d, J = 21.0 Hz, 2H), 2.45 (s, 3H).
Methyl 4-(4-(2-(2-Fluorophenyl)Quinoline-4-Carbonyl)Piperazin-1-yl)Benzoate (C10)
Crystallization in EtOAc as a white solid (0.13 g, 24.7% yield). HRMS C28H24FN3O3 [M + H+] calc. 470.18352 found 470.18607. 1H NMR (400 MHz, DMSO) δ 8.18 (d, J = 8.3 Hz, 1H), 8.08 (t, J = 7.8 Hz, 1H), 7.90 (dd, J = 14.5, 7.3 Hz, 3H), 7.79 (d, J = 8.5 Hz, 2H), 7.71 (t, J = 7.5 Hz, 1H), 7.63–7.54 (m, 1H), 7.42 (dd, J = 14.0, 7.0 Hz, 2H), 6.98 (d, J = 8.6 Hz, 2H), 4.02–3.83 (m, 2H), 3.77 (s, 3H), 3.58 (s, 2H), 3.34 (s, 1H), 3.22 (s, 3H).
Methyl 4-(4-(2-(3-Methoxyphenyl)Quinoline-4-Carbonyl)Piperazin-1-yl)Benzoate (C11)
Crystallization in EtOAc as a white solid (0.20 g, 38.8% yield). HRMS C29H27N3O4 [M + H+] calc. 482.20351 found 482.20605. 1H NMR (400 MHz, DMSO) δ 8.21 (s, 1H), 8.16 (d, J = 8.4 Hz, 1H), 7.91 (d, J = 7.7 Hz, 1H), 7.90–7.82 (m, 3H), 7.80 (d, J = 8.6 Hz, 2H), 7.66 (t, J = 7.5 Hz, 1H), 7.48 (t, J = 8.0 Hz, 1H), 7.11 (d, J = 7.4 Hz, 1H), 6.99 (d, J = 8.7 Hz, 2H), 3.89 (s, 5H), 3.77 (s, 3H), 3.60 (s, 2H), 3.34 (s, 1H), 3.24 (d, J = 21.3 Hz, 2H), 1.99 (s, 1H).
Methyl 4-(4-(2-(3,5-Difluorophenyl)Quinoline-4-Carbonyl)Piperazin-1-yl)Benzoate (C12)
Crystallization in EtOAc as a white solid (0.20 g, 39.0% yield). HRMS C28H23F2N3O3 [M + H+] calc. 488.17410 found 488.17651. 1H NMR (400 MHz, DMSO) δ 8.31 (s, 1H), 8.19 (d, J = 8.7 Hz, 1H), 8.09 (d, J = 7.3 Hz, 2H), 7.94–7.85 (m, 2H), 7.80 (d, J = 8.6 Hz, 2H), 7.70 (t, J = 7.8 Hz, 1H), 7.42 (d, J = 9.5 Hz, 1H), 7.00 (d, J = 8.8 Hz, 2H), 3.95 (s, 2H), 3.77 (s, 3H), 3.60 (s, 2H), 3.34 (s, 2H), 3.25 (d, J = 16.6 Hz, 2H).
Methyl 4-(4-(2-(2-Methoxyphenyl)Quinoline-4-Carbonyl)Piperazin-1-yl)Benzoate (C13)
Crystallization in EtOAc as a white solid (0.21 g, 40.7% yield). HRMS C29H27N3O4 [M + H+] calc. 482.20351 found 482.20609. 1H NMR (400 MHz, DMSO) δ 8.12 (t, J = 10.6 Hz, 1H), 7.86 (ddd, J = 26.1, 16.7, 10.5 Hz, 6H), 7.66 (t, J = 7.6 Hz, 1H), 7.49 (t, J = 7.8 Hz, 1H), 7.21 (d, J = 8.3 Hz, 1H), 7.14 (t, J = 7.4 Hz, 1H), 6.98 (d, J = 8.4 Hz, 2H), 3.95 (d, J = 6.9 Hz, 2H), 3.87 (s, 3H), 3.78 (s, 3H), 3.56 (t, J = 4.6 Hz, 2H), 3.47 (s, 1H), 3.18 (d, J = 4.8 Hz, 3H).
Methyl 4-(4-(2-(2-Chlorophenyl)Quinoline-4-Carbonyl)Piperazin-1-yl)Benzoate (C14)
Crystallization in EtOAc as a white solid (0.20 g, 38.9% yield). HRMS C28H24ClN3O3 [M + H+] calc. 486.15397 found 486.15686. 1H NMR (400 MHz, DMSO) δ 8.15 (d, J = 8.5 Hz, 1H), 7.98–7.85 (m, 2H), 7.80 (d, J = 10.2 Hz, 3H), 7.73 (t, J = 7.6 Hz, 2H), 7.65 (d, J = 4.8 Hz, 1H), 7.57–7.51 (m, 2H), 6.99 (d, J = 8.7 Hz, 2H), 3.94 (s, 2H), 3.77 (s, 3H), 3.57 (s, 2H), 3.34 (s, 2H), 3.31–3.17 (m, 2H).
Methyl 4-(4-(2-(3-Fluorophenyl)Quinoline-4-Carbonyl)Piperazin-1-yl)Benzoate (C15)
Crystallization in EtOAc as a white solid (0.30 g, 57.0% yield). HRMS C28H24FN3O3 [M + H+] calc. 470.18352 found 470.18597. 1H NMR (400 MHz, DMSO) δ 8.26 (s, 1H), 8.24–8.12 (m, 3H), 7.87 (t, J = 7.2 Hz, 2H), 7.80 (d, J = 8.7 Hz, 2H), 7.65 (dt, J = 14.6, 7.8 Hz, 2H), 7.38 (t, J = 8.4 Hz, 1H), 6.99 (d, J = 8.8 Hz, 2H), 3.94 (s, 2H), 3.77 (s, 3H), 3.60 (s, 2H), 3.34 (s, 2H), 3.25 (d, J = 19.1 Hz, 2H).
Methyl 4-(4-(2-(o-Tolyl)Quinoline-4-Carbonyl)Piperazin-1-yl)Benzoate (C16)
Crystallization in EtOAc as a white solid (0.28 g, 52.7% yield). HRMS C29H27N3O3 [M + H+] calc. 466.20860 found 466.21133. 1H NMR (400 MHz, DMSO) δ 8.12 (d, J = 8.4 Hz, 1H), 7.93–7.82 (m, 2H), 7.80 (d, J = 8.6 Hz, 2H), 7.70 (dd, J = 15.2, 7.8 Hz, 2H), 7.56 (d, J = 7.2 Hz, 1H), 7.44–7.31 (m, 3H), 6.99 (d, J = 8.7 Hz, 2H), 3.93 (d, J = 37.1 Hz, 2H), 3.77 (s, 3H), 3.54 (d, J = 28.2 Hz, 2H), 3.34 (s, 2H), 3.31–3.10 (m, 2H), 2.42 (d, J = 10.6 Hz, 3H).
Methyl 4-(4-(2-(4-Fluoro-3-Methylphenyl)Quinoline-4-Carbonyl)Piperazin-1-yl)Benzoate (C17)
Crystallization in EtOAc as a white solid (0.34 g, 43.9% yield). HRMS C29H26FN3O3 [M + H+] calc. 484.19917 found 484.20154. 1H NMR (400 MHz, DMSO) δ 8.29 (d, J = 7.2 Hz, 1H), 8.25–8.09 (m, 3H), 7.90–7.77 (m, 4H), 7.65 (t, J = 7.5 Hz, 1H), 7.32 (t, J = 9.0 Hz, 1H), 6.98 (d, J = 8.6 Hz, 2H), 4.07–3.84 (m, 2H), 3.77 (s, 4H), 3.59 (t, J = 4.7 Hz, 2H), 3.31–3.13 (m, 3H), 2.37 (s, 3H).
Methyl 4-(4-(2-(4-(Dimethylamino)Phenyl)Quinoline-4-Carbonyl)Piperazin-1-yl)Benzoate (C18)
Crystallization in EtOAc as a red solid (0.30 g, 35.4% yield). HRMS C30H30N4O3 [M + H+] calc. 495.23515 found 495.23041. 1H NMR (400 MHz, DMSO) δ 8.21 (d, J = 8.4 Hz, 2H), 8.05 (d, J = 10.8 Hz, 2H), 7.78 (dd, J = 15.2, 7.5 Hz, 4H), 7.55 (t, J = 7.5 Hz, 1H), 6.99 (d, J = 8.5 Hz, 2H), 6.85 (d, J = 8.5 Hz, 2H), 3.95 (d, J = 26.6 Hz, 2H), 3.78 (s, 3H), 3.59 (s, 2H), 3.25 (dd, J = 37.0, 14.9 Hz, 3H), 3.02 (s, 6H).
Methyl 4-(4-(2-(4-Benzamidophenyl)Quinoline-4-Carbonyl)Piperazin-1-yl)Benzoate (C19)
Crystallization in EtOAc as a white solid (0.28 g, 75.8% yield). HRMS C35H30N4O4 [M + H+] calc. 571.23006 found 571.23431. 1H NMR (400 MHz, DMSO) δ 10.49 (s, 1H), 8.37 (d, J = 8.5 Hz, 2H), 8.24–8.07 (m, 2H), 8.01 (dd, J = 7.8, 3.9 Hz, 4H), 7.83 (dd, J = 19.6, 8.3 Hz, 4H), 7.68–7.49 (m, 4H), 7.00 (d, J = 8.7 Hz, 2H), 3.96 (d, J = 16.8 Hz, 2H), 3.77 (s, 3H), 3.60 (s, 2H), 3.34 (d, J = 3.4 Hz, 3H), 3.24 (s, 1H).
Methyl 4-(4-(2-(4-(4-Fluorobenzamido)Phenyl)Quinoline-4-Carbonyl)Piperazin-1-yl)Benzoate (C20)
Crystallization in EtOAc as a white solid (0.33 g, 71.8% yield). HRMS C35H29FN4O4 [M + H+] calc. 589.22064 found 589.22241. 1H NMR (400 MHz, DMSO) δ 10.49 (s, 1H), 8.36 (d, J = 8.5 Hz, 2H), 8.19 (s, 1H), 8.16–8.05 (m, 3H), 7.99 (d, J = 8.3 Hz, 2H), 7.83 (dd, J = 19.7, 8.1 Hz, 4H), 7.64 (t, J = 7.6 Hz, 1H), 7.40 (t, J = 8.4 Hz, 2H), 7.00 (d, J = 8.6 Hz, 2H), 3.94 (s, 2H), 3.77 (s, 3H), 3.60 (s, 2H), 3.36 (s, 2H), 3.17 (s, 2H).
Methyl 4-(4-(2-(4-(3-Bromobenzamido)Phenyl)Quinoline-4-Carbonyl)Piperazin-1-yl)Benzoate (C21)
Crystallization in EtOAc as a white solid (0.23 g, 52.9% yield). HRMS C35H29BrN4O4 [M + H+] calc. 649.14057 found 649.14526. 1H NMR (400 MHz, DMSO) δ 10.58 (s, 1H), 8.37 (d, J = 8.4 Hz, 2H), 8.20 (s, 2H), 8.14 (d, J = 8.4 Hz, 1H), 8.00 (d, J = 8.1 Hz, 3H), 7.83 (dd, J = 20.0, 8.7 Hz, 5H), 7.64 (t, J = 7.4 Hz, 1H), 7.53 (t, J = 7.9 Hz, 1H), 7.00 (d, J = 8.7 Hz, 2H), 3.96 (d, J = 16.3 Hz, 2H), 3.77 (s, 3H), 3.60 (s, 2H), 3.35 (s, 2H), 3.24 (s, 2H).
Methyl 4-(4-(2-(4-(2-Fluorobenzamido)Phenyl)Quinoline-4-Carbonyl)Piperazin-1-yl)Benzoate (C22)
Crystallization in EtOAc as a white solid (0.21 g, 46.3% yield). HRMS C35H29FN4O4 [M + H+] calc. 589.22064 found 589.22437. 1H NMR (400 MHz, DMSO) δ 10.67 (s, 1H), 8.36 (d, J = 8.3 Hz, 2H), 8.19 (s, 1H), 8.14 (d, J = 8.6 Hz, 1H), 7.93 (d, J = 8.4 Hz, 2H), 7.83 (dd, J = 20.0, 8.3 Hz, 4H), 7.71 (t, J = 7.3 Hz, 1H), 7.68–7.57 (m, 2H), 7.44–7.31 (m, 2H), 7.00 (d, J = 8.6 Hz, 2H), 4.01–3.86 (m, 2H), 3.77 (s, 3H), 3.60 (s, 2H), 3.35 (s, 2H), 3.24 (s, 2H).
Methyl 4-(4-(2-(4-(2-Chlorobenzamido)Phenyl)Quinoline-4-Carbonyl)Piperazin-1-yl)Benzoate (C23)
Crystallization in EtOAc as a white solid (0.27 g, 45.1% yield). HRMS C35H29ClN4O4 [M + H+] calc. 605.19109 found 605.19525. 1H NMR (400 MHz, DMSO) δ 10.75 (s, 1H), 8.36 (d, J = 8.3 Hz, 2H), 8.18 (s, 1H), 8.14 (d, J = 8.6 Hz, 1H), 7.92 (d, J = 8.3 Hz, 2H), 7.83 (dd, J = 20.1, 8.3 Hz, 5H), 7.60 (d, J = 7.8 Hz, 2H), 7.51 (dt, J = 21.1, 7.3 Hz, 2H), 7.00 (d, J = 8.5 Hz, 2H), 3.93 (s, 2H), 3.77 (s, 3H), 3.60 (s, 2H), 3.35 (s, 2H), 3.17 (d, J = 5.2 Hz, 2H).
Methyl 4-(4-(2-(4-(2,4-Difluorobenzamido)Phenyl)Quinoline-4-Carbonyl)Piperazin-1-yl)Benzoate (C24)
Crystallization in EtOAc as a white solid (0.14 g, 31.2% yield). HRMS C35H28F2N4O4 [M + H+] calc. 607.21122 found 607.21466. 1H NMR (400 MHz, DMSO) δ 10.66 (s, 1H), 8.36 (d, J = 8.0 Hz, 2H), 8.26–8.05 (m, 2H), 7.98–7.71 (m, 7H), 7.64 (t, J = 7.7 Hz, 1H), 7.46 (t, J = 10.5 Hz, 1H), 7.26 (t, J = 8.6 Hz, 1H), 7.00 (d, J = 8.5 Hz, 2H), 4.06–3.87 (m, 2H), 3.77 (s, 3H), 3.60 (s, 2H), 3.40 (s, 1H), 3.17 (d, J = 5.1 Hz, 3H).
Methyl 4-(4-(2-(4-(3,5-Difluorobenzamido)Phenyl)Quinoline-4-Carbonyl)Piperazin-1-yl)Benzoate (C25)
Crystallization in EtOAc as a white solid (0.18 g, 40.1% yield). HRMS C35H28F2N4O4 [M + H+] calc. 607.21122 found 607.21472. 1H NMR (400 MHz, DMSO) δ 10.58 (s, 1H), 8.39 (d, J = 8.2 Hz, 2H), 8.20 (s, 1H), 8.14 (d, J = 8.4 Hz, 1H), 7.99 (d, J = 8.2 Hz, 2H), 7.89–7.77 (m, 4H), 7.74 (d, J = 7.2 Hz, 2H), 7.64 (t, J = 7.6 Hz, 1H), 7.56 (t, J = 9.1 Hz, 1H), 6.99 (d, J = 8.4 Hz, 2H), 3.96 (d, J = 15.3 Hz, 2H), 3.78 (s, 3H), 3.60 (s, 2H), 3.35 (s, 2H), 3.27 (d, J = 22.5 Hz, 2H).
Methyl 4-(4-(2-(4-(3-Fluorobenzamido)Phenyl)Quinoline-4-Carbonyl)piperazin-1-yl)Benzoate (C26)
Crystallization in EtOAc as a white solid (0.38 g, 53.3% yield). HRMS C35H29FN4O4 [M + H+] calc. 589.22064 found 589.21490. 1H NMR (400 MHz, DMSO) δ 10.55 (s, 1H), 8.37 (d, J = 8.3 Hz, 2H), 8.23–8.11 (m, 2H), 8.00 (d, J = 8.3 Hz, 2H), 7.86 (dd, J = 7.7, 4.3 Hz, 3H), 7.81 (s, 1H), 7.79 (s, 1H), 7.69–7.57 (m, 2H), 7.48 (t, J = 8.3 Hz, 1H), 7.00 (d, J = 8.5 Hz, 2H), 3.94 (s, 1H), 3.77 (s, 4H), 3.60 (s, 3H), 3.24 (s, 3H).
Methyl 4-(4-(2-(2,5-Difluorophenyl)Quinoline-4-Carbonyl)Piperazin-1-yl)Benzoate (C27)
Crystallization in EtOAc as a white solid (0.28 g, 36.3% yield). HRMS C28H23F2N3O3 [M + H+] calc. 488.17410 found 488.17487. 1H NMR (400 MHz, DMSO) δ 8.19 (d, J = 8.3 Hz, 1H), 7.92 (dd, J = 16.9, 7.9 Hz, 4H), 7.85–7.63 (m, 4H), 7.58–7.39 (m, 2H), 6.98 (d, J = 8.5 Hz, 2H), 3.94 (d, J = 23.1 Hz, 3H), 3.77 (s, 3H), 3.58 (s, 2H), 3.17 (d, J = 5.5 Hz, 3H).
Methyl 4-(4-(2-([1,1′-Biphenyl]-4-yl)Quinoline-4-Carbonyl)Piperazin-1-yl)Benzoate (C28)
Crystallization in EtOAc as a yellow solid (0.33 g, 40.6% yield). HRMS C34H29N3O3 [M + H+] calc. 528.22425 found 528.22449. 1H NMR (400 MHz, DMSO) δ 8.45 (d, J = 8.0 Hz, 2H), 8.27 (s, 1H), 8.18 (d, J = 8.3 Hz, 1H), 7.88 (d, J = 8.5 Hz, 4H), 7.83–7.76 (m, 4H), 7.67 (t, J = 7.5 Hz, 1H), 7.52 (t, J = 7.4 Hz, 2H), 7.42 (t, J = 7.2 Hz, 1H), 6.99 (d, J = 8.5 Hz, 2H), 3.97 (d, J = 16.6 Hz, 2H), 3.77 (s, 4H), 3.61 (d, J = 4.4 Hz, 2H), 3.23 (s, 3H).
N-Hydroxy-4-(4-(2-Phenylquinoline-4-Carbonyl)Piperazin-1-yl)Benzamide (D1)
Methyl 4-(4-(2-phenylquinoline-4-carbonyl)piperazin-1-yl)benzoate (C1) (0.26 g, 0.58 mmol) and 10 ml NH2OK methanol solution (3.76 M) was added as solvent. After 8 h, the solvent was removed by a rotary evaporator, and 3 mol/L HCl (50 ml) was added. The solution was extracted with EtOAc (3 × 30 ml) and washed with saturated NaCl solution, dried with MgSO4. The desired compound (D1) was obtained by filtration and recrystallization in EtOAc as a white solid (0.25 g, 95.0% yield).
Mp: 213.8–214.8°C; HRMS C27H24N4O3 [M + H+] calc. 453.18820, found 453.19052. 1H NMR (400 MHz, DMSO) δ 10.96 (s, 1H), 8.80 (s, 1H), 8.34 (d, J = 7.6 Hz, 2H), 8.24–8.10 (m, 2H), 7.85 (t, J = 7.4 Hz, 2H), 7.59 (tt, J = 14.3, 7.8 Hz, 7H), 6.96 (d, J = 8.4 Hz, 2H), 3.92 (s, 1H), 3.51 (s, 2H), 3.28 (s, 2H), 3.11 (s, 1H), 2.55 (d, J = 15.4 Hz, 1H). 13C NMR (101 MHz, DMSO) δ 171.78, 166.28, 156.37, 152.76, 148.14, 143.80, 138.54, 131.05, 130.45, 130.20, 129.38, 128.61, 127.91 (d, J = 19.9 Hz), 125.23, 123.35, 122.88, 116.08, 114.67, 48.03, 47.61, 46.60, 45.02, 41.35.
4-(4-(2-(4-Bromophenyl)Quinoline-4-Carbonyl)Piperazin-1-yl)-N-Hydroxybenzamide (D2)
Crystallization in EtOAc as a white solid (0.13 g, 56.5% yield). Mp: 231.7–232.8°C; HRMS C27H23BrN4O3 [M + H+] calc. 531.09871, found 531.10126. 1H NMR (400 MHz, DMSO) δ 10.96 (s, 1H), 8.80 (s, 1H), 8.30 (d, J = 8.3 Hz, 2H), 8.22 (s, 1H), 8.16 (d, J = 8.7 Hz, 1H), 7.86 (d, J = 8.3 Hz, 2H), 7.77 (d, J = 8.2 Hz, 2H), 7.66 (dd, J = 15.4, 8.1 Hz, 3H), 6.96 (d, J = 8.5 Hz, 2H), 4.01–3.85 (m, 2H), 3.50 (s, 2H), 3.27 (s, 2H), 3.11 (s, 1H), 2.69 (dd, J = 41.1, 15.7 Hz, 1H). 13C NMR (101 MHz, DMSO) δ 166.17, 155.23, 152.76, 148.08, 144.00, 137.70, 132.34, 131.20, 130.19, 129.83, 128.60, 128.24, 125.26, 124.24, 123.45, 122.89, 115.91, 114.68, 48.03, 47.61, 46.58, 41.36.
4-(4-(2-(4-Chlorophenyl)Quinoline-4-Carbonyl)Piperazin-1-yl)-N-Hydroxybenzamide (D3)
Crystallization in EtOAc as a white solid (0.17 g, 68.1% yield). Mp: 228.1–229.2°C; HRMS C27H23ClN4O3 [M + H+] calc. 487.14922, found 487.15167. 1H NMR (400 MHz, DMSO) δ 10.95 (s, 1H), 8.80 (s, 1H), 8.37 (d, J = 8.2 Hz, 2H), 8.23 (s, 1H), 8.16 (d, J = 8.6 Hz, 1H), 7.87 (d, J = 8.3 Hz, 2H), 7.72–7.58 (m, 6H), 6.96 (d, J = 8.6 Hz, 2H), 4.01–3.86 (m, 2H), 3.50 (s, 2H), 3.27 (s, 2H), 3.12 (d, J = 7.4 Hz, 1H). 13C NMR (101 MHz, DMSO) δ 166.18, 155.14, 152.76, 148.07, 143.99, 137.34, 135.38, 131.19, 130.19, 129.49 (d, J = 16.2 Hz), 128.60, 128.22, 125.25, 123.42, 122.89, 115.96, 114.68, 60.23, 48.03, 47.62, 46.59, 41.36.
4-(4-(2-(4-Fluorophenyl)Quinoline-4-Carbonyl)Piperazin-1-yl)-N-Hydroxybenzamide (D4)
Crystallization in EtOAc as a white solid (0.12 g, 54.5% yield). Mp: 163.5–164.8°C; HRMS C27H23FN4O3 [M + H+] calc. 470.17542, found 471.18057. 1H NMR (400 MHz, DMSO) δ 10.96 (s, 1H), 8.80 (s, 1H), 8.45–8.34 (m, 1H), 8.29 (d, J = 8.5 Hz, 1H), 8.22–8.04 (m, 2H), 7.84 (dd, J = 14.6, 9.9 Hz, 2H), 7.72–7.55 (m, 3H), 7.40 (t, J = 8.7 Hz, 1H), 7.09 (d, J = 8.5 Hz, 1H), 6.96 (d, J = 8.7 Hz, 2H), 3.92 (s, 2H), 3.50 (s, 2H), 3.19 (d, J = 64.9 Hz, 4H). 13C NMR (101 MHz, DMSO) δ 166.23, 155.33, 152.75, 148.07, 143.90, 131.11, 130.16, 129.29, 128.58, 128.03, 125.23, 123.26, 116.38, 116.17, 115.92, 115.15, 114.68, 63.75, 48.04, 47.62, 46.59, 41.35.
N-Hydroxy-4-(4-(2-(4-(Methylthio)Phenyl)Quinoline-4-Carbonyl)Piperazin-1-yl)Benzamide (D5)
Crystallization in EtOAc as a white solid (0.11 g, 73.3% yield). Mp: 177.2–179.6°C; HRMS C28H26N4O3S [M + H+] calc. 499.17592, found 499.17838. 1H NMR (400 MHz, DMSO) δ 10.95 (s, 1H), 8.80 (s, 1H), 8.30 (d, J = 8.0 Hz, 2H), 8.20–8.09 (m, 2H), 7.84 (d, J = 8.8 Hz, 2H), 7.65 (d, J = 8.2 Hz, 3H), 7.43 (d, J = 8.0 Hz, 2H), 6.96 (d, J = 8.4 Hz, 2H), 3.91 (s, 1H), 3.51 (s, 2H), 3.28–3.18 (m, 3H), 3.11 (s, 2H), 2.56 (s, 3H). 13C NMR (101 MHz, DMSO) δ 166.30, 155.80, 152.77, 148.14, 143.75, 141.47, 134.84, 131.03, 130.08, 128.60, 128.16, 127.82, 126.20, 125.20, 123.26, 122.89, 115.73, 114.67, 60.23, 48.03, 47.61, 46.59, 41.35, 21.24, 14.78, 14.56.
N-Hydroxy-4-(4-(2-(4-Methoxyphenyl)Quinoline-4-Carbonyl)Piperazin-1-yl)Benzamide (D6)
Crystallization in EtOAc as a white solid (0.11 g, 43.9% yield). Mp: 184.3–186.8°C; HRMS C28H26N4O4 [M + H+] calc. 483.19876, found 483.20083. 1H NMR (400 MHz, DMSO) δ 10.95 (s, 1H), 8.80 (s, 1H), 8.31 (d, J = 8.5 Hz, 2H), 8.18–8.05 (m, 2H), 7.82 (t, J = 6.5 Hz, 2H), 7.63 (dd, J = 15.7, 8.0 Hz, 3H), 7.11 (d, J = 8.4 Hz, 2H), 6.96 (d, J = 8.6 Hz, 2H), 3.99 (d, J = 8.2 Hz, 1H), 3.91 (s, 1H), 3.86 (s, 3H), 3.50 (s, 2H), 3.27 (s, 3H), 3.11 (s, 1H). 13C NMR (101 MHz, DMSO) δ 166.37, 161.39, 156.03, 152.77, 148.14, 143.60, 130.95 (d, J = 6.6 Hz), 129.97, 129.29, 128.60, 127.52, 125.17, 122.94 (d, J = 12.4 Hz), 115.59, 114.71 (d, J = 7.4 Hz), 60.23, 55.82, 48.03, 47.62, 46.59, 41.33.
N-Hydroxy-4-(4-(2-(4-(Trifluoromethyl)Phenyl)Quinoline-4-Carbonyl)Piperazin-1-yl)Benzamide (D7)
Crystallization in EtOAc as a white solid (0.09 g, 90.0% yield). Mp: 212.7–213.9°C; HRMS C28H23F3N4O3 [M + H+] calc. 521.17558, found 521.17804. 1H NMR (400 MHz, DMSO) δ 10.96 (s, 1H), 8.80 (s, 1H), 8.56 (d, J = 8.1 Hz, 2H), 8.31 (s, 1H), 8.20 (d, J = 8.7 Hz, 1H), 7.96–7.86 (m, 4H), 7.74–7.62 (m, 3H), 6.96 (d, J = 8.5 Hz, 2H), 3.96 (d, J = 30.8 Hz, 2H), 3.51 (s, 2H), 3.31–3.05 (m, 4H). 13C NMR (101 MHz, DMSO) δ 166.12, 154.87, 152.76, 148.09, 144.14, 142.33, 131.34, 130.35, 128.59, 126.28, 125.28, 123.66, 122.87, 116.37, 114.69, 60.25, 48.03, 47.61, 46.59, 41.38.
4-(4-(2-(3-Chlorophenyl)Quinoline-4-Carbonyl)Piperazin-1-yl)-N-Hydroxybenzamide (D8)
Crystallization in EtOAc as a white solid (0.06 g, 40.0% yield). Mp: 207.1–208.9°C; HRMS C27H23ClN4O3 [M + H+] calc. 487.14922, found 487.15164. 1H NMR (400 MHz, DMSO) δ 10.95 (s, 1H), 8.80 (s, 1H), 8.40 (s, 1H), 8.30 (d, J = 18.8 Hz, 2H), 8.19 (d, J = 8.7 Hz, 1H), 7.91–7.82 (m, 2H), 7.73–7.56 (m, 6H), 6.96 (d, J = 8.4 Hz, 2H), 3.92 (s, 1H), 3.51 (s, 2H), 3.28 (s, 3H), 3.11 (s, 1H). 13C NMR (101 MHz, DMSO) δ 166.13, 154.78, 152.75, 148.05, 144.07, 140.62, 134.39, 131.29, 130.25 (d, J = 7.0 Hz), 128.60, 128.39, 127.41, 126.43, 125.25, 123.60, 122.88, 116.14, 114.67, 48.02, 47.60, 46.58, 41.35.
N-Hydroxy-4-(4-(2-(m-Tolyl)Quinoline-4-Carbonyl)Piperazin-1-yl)Benzamide (D9)
Crystallization in EtOAc as a white solid (0.08 g, 53.3% yield). Mp: 199.2–199.7°C; HRMS C28H26N4O3 [M + H+] calc. 467.20385, found 467.20642. 1H NMR (400 MHz, DMSO) δ 10.96 (s, 1H), 8.80 (s, 1H), 8.14 (dd, J = 15.5, 9.3 Hz, 4H), 7.85 (t, J = 7.3 Hz, 2H), 7.65 (t, J = 8.2 Hz, 3H), 7.46 (t, J = 7.7 Hz, 1H), 7.35 (d, J = 7.2 Hz, 1H), 6.96 (d, J = 8.8 Hz, 2H), 3.90 (s, 1H), 3.51 (s, 2H), 3.27 (s, 3H), 3.11 (s, 2H), 2.45 (s, 3H). 13C NMR (101 MHz, DMSO) δ 166.30, 156.48, 152.75, 148.13, 143.74, 138.55 (d, J = 10.5 Hz), 131.05 (d, J = 7.2 Hz), 130.18, 129.28, 128.60, 128.32, 127.94, 125.10 (d, J = 19.6 Hz), 123.32, 122.87, 116.13, 114.67, 48.04, 47.60, 46.59, 41.33, 21.60.
4-(4-(2-(2-Fluorophenyl)Quinoline-4-Carbonyl)Piperazin-1-yl)-N-Hydroxybenzamide (D10)
Crystallization in EtOAc as a white solid (0.08 g, 80.0% yield). Mp: 172.5–173.3°C; HRMS C27H23FN4O3 [M + H+] calc. 471.17877, found 471.18130. 1H NMR (400 MHz, DMSO) δ 10.95 (s, 1H), 8.80 (s, 1H), 8.18 (d, J = 8.4 Hz, 1H), 8.08 (t, J = 7.9 Hz, 1H), 7.89 (d, J = 14.4 Hz, 3H), 7.77–7.52 (m, 4H), 7.42 (dd, J = 14.0, 7.1 Hz, 2H), 6.95 (d, J = 8.4 Hz, 2H), 3.89 (s, 2H), 3.49 (s, 2H), 3.19 (d, J = 64.2 Hz, 4H). 13C NMR (101 MHz, DMSO) δ 166.08, 161.82, 159.35, 152.76, 148.23, 143.13, 132.31, 131.81, 131.20, 130.20, 128.61, 127.30, 125.49, 125.24, 123.21, 122.82, 119.33, 117.01, 116.79, 114.69, 60.28, 48.03, 47.57, 46.58, 41.38, 14.54.
N-Hydroxy-4-(4-(2-(3-Methoxyphenyl)Quinoline-4-Carbonyl)Piperazin-1-yl)Benzamide (D11)
Crystallization in EtOAc as a white solid (0.10 g, 66.7% yield). Mp: 179.8–180.9°C; HRMS C28H26N4O4 [M + H+] calc. 483.19876, found 483.20111. 1H NMR (400 MHz, DMSO) δ 10.96 (s, 1H), 8.80 (s, 1H), 8.24–8.11 (m, 2H), 7.99–7.77 (m, 4H), 7.66 (t, J = 9.2 Hz, 3H), 7.48 (t, J = 8.0 Hz, 1H), 7.11 (d, J = 8.0 Hz, 1H), 6.96 (d, J = 8.4 Hz, 2H), 3.89 (s, 4H), 3.51 (s, 3H), 3.19 (d, J = 67.9 Hz, 4H). 13C NMR (101 MHz, DMSO) δ 166.27, 164.73, 160.29, 156.14, 152.75, 148.06, 143.77, 140.02, 131.04, 130.47, 130.23, 128.60, 128.05, 125.20, 123.43, 122.88, 120.22, 116.24, 114.67, 112.94, 55.80, 48.02, 47.60, 46.59, 41.35.
4-(4-(2-(3,5-Difluorophenyl)Quinoline-4-Carbonyl)Piperazin-1-yl)-N-Hydroxybenzamide (D12)
Crystallization in EtOAc as a white solid (0.09 g, 60.0% yield). Mp: 221.8–222.0°C; HRMS C28H26N4O4 [M + H+] calc. 489.16935, found 489.17206. 1H NMR (400 MHz, DMSO) δ 10.96 (s, 1H), 8.80 (s, 1H), 8.31 (s, 1H), 8.19 (d, J = 8.6 Hz, 1H), 8.10 (d, J = 7.7 Hz, 2H), 7.94–7.60 (m, 6H), 7.44 (t, J = 8.9 Hz, 1H), 6.96 (d, J = 8.6 Hz, 2H), 3.95 (d, J = 18.2 Hz, 2H), 3.51 (s, 2H), 3.20 (d, J = 62.7 Hz, 3H). 13C NMR (101 MHz, DMSO) δ 166.03, 162.15, 152.75, 147.90, 144.21, 131.36, 130.36, 128.65 (d, J = 8.0 Hz), 125.28, 123.82, 122.91, 116.13, 114.68, 111.12–110.93 (m), 110.79 (d, J = 26.8 Hz), 105.74, 48.01, 47.62, 46.58, 41.38. 13C NMR (101 MHz, DMSO) δ 166.03, 162.15, 152.75, 147.90, 144.21, 131.36, 130.36, 128.65 (d, J = 8.0 Hz), 125.28, 123.82, 122.91, 116.13, 114.68, 111.12–110.93 (m), 110.79 (d, J = 26.8 Hz), 105.74, 48.01, 47.62, 46.58, 41.38.
N-Hydroxy-4-(4-(2-(2-Methoxyphenyl)Quinoline-4-Carbonyl)Piperazin-1-yl)Benzamide (D13)
Crystallization in EtOAc as a white solid (0.11 g, 73.3% yield). Mp: 224.0–224.9°C; HRMS C28H26N4O4 [M + H+] calc. 483.19876, found 483.20151. 1H NMR (400 MHz, DMSO) δ 10.96 (s, 1H), 8.81 (s, 1H), 8.13 (d, J = 8.4 Hz, 1H), 7.90–7.79 (m, 4H), 7.66 (t, J = 8.9 Hz, 3H), 7.50 (t, J = 7.8 Hz, 1H), 7.21 (d, J = 8.3 Hz, 1H), 7.14 (t, J = 7.4 Hz, 1H), 6.96 (d, J = 8.4 Hz, 2H), 3.94 (d, J = 21.6 Hz, 2H), 3.87 (s, 3H), 3.48 (s, 3H), 3.23 (d, J = 52.7 Hz, 3H). 13C NMR (101 MHz, DMSO) δ 166.34, 157.51, 156.51, 152.75, 148.33, 141.55, 131.49 (d, J = 5.0 Hz), 130.64, 130.09, 128.62, 127.91, 125.22, 122.99 (d, J = 17.6 Hz), 121.28, 120.48, 114.66, 112.49, 56.27, 48.10, 47.64, 46.61, 41.36.
4-(4-(2-(2-Chlorophenyl)Quinoline-4-Carbonyl)Piperazin-1-yl)-N-Hydroxybenzamide (D14)
Crystallization in EtOAc as a white solid (0.09 g, 60.0% yield). Mp: 235.0–236.4°C; HRMS C27H23ClN4O3 [M + H+] calc. 487.14922, found 487.15176. 1H NMR (400 MHz, DMSO) δ 10.95 (s, 1H), 8.80 (s, 1H), 8.15 (d, J = 8.4 Hz, 1H), 7.90 (dd, J = 18.0, 8.2 Hz, 2H), 7.80 (s, 1H), 7.73 (t, J = 7.5 Hz, 2H), 7.64 (d, J = 7.9 Hz, 3H), 7.58–7.51 (m, 2H), 6.95 (d, J = 8.6 Hz, 2H), 3.90 (s, 1H), 3.48 (s, 3H), 3.32–3.21 (m, 3H), 3.15 (s, 1H). 13C NMR (101 MHz, DMSO) δ 165.93, 157.01, 152.75, 148.09, 142.42, 139.13, 132.30, 131.82, 131.13 (d, J = 9.9 Hz), 130.40, 130.15, 128.58, 128.01, 125.39, 123.25, 122.89, 119.91, 114.69, 48.07, 47.59, 46.62, 41.39.
4-(4-(2-(3-Fluorophenyl)Quinoline-4-Carbonyl)Piperazin-1-yl)-N-Hydroxybenzamide (D15)
Crystallization in EtOAc as a white solid (0.06 g, 40.0% yield). Mp: 225.0–226.3°C; HRMS C27H23FN4O3 [M + H+] calc. 471.17877, found 471.18146. 1H NMR (400 MHz, DMSO) δ 10.96 (s, 1H), 8.80 (s, 1H), 8.29–8.13 (m, 4H), 7.92–7.84 (m, 2H), 7.74–7.57 (m, 4H), 7.38 (t, J = 8.0 Hz, 1H), 6.96 (d, J = 8.6 Hz, 2H), 3.95 (d, J = 21.9 Hz, 2H), 3.51 (s, 2H), 3.20 (d, J = 65.1 Hz, 4H). 13C NMR (101 MHz, DMSO) δ 166.15, 154.94, 152.76, 148.03, 144.02, 141.07, 131.29 (d, J = 16.5 Hz), 130.28, 128.60, 128.35, 125.25, 123.85, 123.59, 122.89, 117.34, 116.14, 114.68, 114.44, 48.01, 47.61, 46.59, 41.37.
N-Hydroxy-4-(4-(2-(o-Tolyl)Quinoline-4-Carbonyl)Piperazin-1-yl)Benzamide (D16)
Crystallization in EtOAc as a white solid (0.07 g, 46.7% yield). Mp: 223.4–223.9°C; HRMS C28H26N4O3 [M + H+] calc. 467.20385, found 467.20605. 1H NMR (400 MHz, DMSO) δ 10.95 (s, 1H), 8.80 (s, 1H), 8.12 (d, J = 8.3 Hz, 1H), 7.87 (dd, J = 16.8, 8.3 Hz, 2H), 7.75–7.60 (m, 4H), 7.56 (d, J = 7.0 Hz, 1H), 7.39 (d, J = 7.9 Hz, 3H), 6.95 (d, J = 8.3 Hz, 2H), 3.86 (s, 1H), 3.49 (s, 4H), 3.13 (s, 2H), 2.43 (s, 4H). 13C NMR (101 MHz, DMSO) δ 166.18, 159.67, 152.77, 147.88, 142.82, 140.03, 136.23, 131.31, 130.95, 130.35, 130.03, 129.26, 128.61, 128.11, 126.49, 125.22, 122.79, 119.48, 114.69, 60.28, 48.06, 47.56, 46.60, 41.36, 20.66, 14.54.
4-(4-(2-(4-Fluoro-3-Methylphenyl)Quinoline-4-Carbonyl)Piperazin-1-yl)-N-Hydroxybenzamide (D17)
Crystallization in EtOAc as a white solid (0.16 g, 53.3% yield). Mp: 176.6–186.2°C; HRMS C28H25FN4O3 [M + H+] calc. 485.19442, found 485.19553. 1H NMR (400 MHz, DMSO) δ 10.96 (s, 1H), 8.81 (s, 1H), 8.30 (d, J = 7.4 Hz, 1H), 8.25–8.09 (m, 3H), 7.93–7.79 (m, 2H), 7.65 (d, J = 8.3 Hz, 3H), 7.32 (t, J = 9.1 Hz, 1H), 6.96 (d, J = 8.4 Hz, 2H), 3.91 (s, 1H), 3.51 (s, 3H), 3.32–3.02 (m, 4H), 2.42–2.24 (m, 3H). 13C NMR (101 MHz, DMSO) δ 166.26, 163.70, 161.25, 155.47, 152.75, 148.07, 143.83, 134.67, 131.16 (d, J = 17.0 Hz), 130.11, 128.61, 127.96, 127.45, 125.28 (d, J = 15.1 Hz), 123.21, 122.89, 115.91, 114.67, 48.04, 47.60, 46.59, 41.33.
4-(4-(2-(4-(Dimethylamino)Phenyl)Quinoline-4-Carbonyl)Piperazin-1-yl)-N-Hydroxybenzamide (D18)
Crystallization in EtOAc as a red solid (0.05 g, 16.7% yield). Mp: 200.6–201.2°C; HRMS C29H29N5O3 [M + H+] calc. 496.23039, found 496.23093. 1H NMR (400 MHz, DMSO) δ 10.95 (s, 1H), 8.80 (s, 1H), 8.20 (d, J = 8.5 Hz, 2H), 8.04 (d, J = 7.7 Hz, 2H), 7.80–7.67 (m, 2H), 7.65 (d, J = 8.4 Hz, 2H), 7.55 (t, J = 7.4 Hz, 1H), 6.95 (d, J = 8.4 Hz, 2H), 6.84 (d, J = 8.6 Hz, 2H), 3.94 (d, J = 32.6 Hz, 2H), 3.50 (s, 2H), 3.27 (s, 3H), 3.02 (s, 6H), -1.66 – -1.87 (m, 1H). 13C NMR (101 MHz, DMSO) δ 166.55, 156.52, 152.78, 151.97, 148.27, 143.20, 130.71, 128.68 (d, J = 16.6 Hz), 126.81, 125.70, 125.09, 122.78 (d, J = 19.0 Hz), 115.15, 114.66, 112.33, 47.61, 46.60, 41.31.
4-(4-(2-(4-Benzamidophenyl)Quinoline-4-Carbonyl)Piperazin-1-yl)-N-Hydroxybenzamide (D19)
Crystallization in EtOAc as a yellow solid (0.07 g, 46.7% yield). Mp: 173.9–174.8°C; HRMS C34H29N5O4 [M + H+] calc. 572.22531, found 572.22815. 1H NMR (400 MHz, DMSO) δ 10.96 (s, 1H), 10.48 (s, 1H), 8.80 (s, 1H), 8.37 (d, J = 8.4 Hz, 2H), 8.19 (s, 1H), 8.14 (d, J = 8.6 Hz, 1H), 8.05–7.97 (m, 4H), 7.85 (d, J = 7.9 Hz, 2H), 7.69–7.52 (m, 6H), 6.96 (d, J = 8.7 Hz, 2H), 3.93 (s, 1H), 3.51 (s, 4H), 3.13 (s, 1H), 2.67 (s, 2H). 13C NMR (101 MHz, DMSO) δ 207.54–131.11 (m), 207.54–128.98 (m), 207.54–129.02 (m), 207.54–128.67 (m), 207.54–127.84 (m), 207.54–125.39 (m), 207.54–123.27 (m), 218.32–122.78 (m), 120.69, 115.75, 114.68, 47.63.
4-Fluoro-N-(4-(4-(4-(4-(Hydroxycarbamoyl)Phenyl)Piperazine-1-Carbonyl)Quinolin-2-yl)Phenyl)Benzamide (D20)
Crystallization in EtOAc as a yellow solid (0.07 g, 46.7% yield). Mp: 178.3–180.3°C; HRMS C34H28FN5O4 [M + H+] calc. 590.21589, found 590.21875. 1H NMR (400 MHz, DMSO) δ 10.96 (s, 1H), 10.49 (s, 1H), 8.80 (s, 1H), 8.35 (t, J = 9.7 Hz, 2H), 8.19 (s, 1H), 8.14 (d, J = 8.8 Hz, 1H), 8.10 (s, 1H), 8.08 (d, J = 5.7 Hz, 1H), 7.99 (d, J = 8.4 Hz, 2H), 7.90 (d, J = 8.3 Hz, 1H), 7.84 (d, J = 8.2 Hz, 2H), 7.65 (d, J = 8.3 Hz, 3H), 7.40 (t, J = 8.6 Hz, 1H), 6.96 (d, J = 8.5 Hz, 2H), 3.93 (s, 1H), 3.51 (s, 2H), 3.29–3.20 (m, 2H), 3.12 (s, 1H), 2.69 (dd, J = 41.1, 15.4 Hz, 2H). 13C NMR (101 MHz, DMSO) δ 171.76, 165.09, 152.78, 131.04, 128.61, 128.26, 122.88, 120.73, 115.99, 115.77, 114.68, 72.87, 43.21.
3-Bromo-N-(4-(4-(4-(4-(Hydroxycarbamoyl)Phenyl)Piperazine-1-Carbonyl)Quinolin-2-yl)Phenyl)Benzamide (D21)
Crystallization in EtOAc as a yellow solid (0.12 g, 80.0% yield). Mp: 170.5–170.8°C; HRMS C34H28BrN5O4 [M + H+] calc. 650.13582, found 650.13867. 1H NMR (400 MHz, DMSO) δ 10.96 (s, 1H), 10.58 (s, 1H), 8.80 (s, 1H), 8.37 (d, J = 8.5 Hz, 2H), 8.19 (s, 2H), 8.14 (d, J = 8.6 Hz, 1H), 8.00 (d, J = 8.3 Hz, 3H), 7.84 (t, J = 7.8 Hz, 3H), 7.65 (d, J = 8.1 Hz, 3H), 7.53 (t, J = 7.9 Hz, 1H), 6.96 (d, J = 8.3 Hz, 2H), 3.96 (d, J = 24.8 Hz, 2H), 3.51 (s, 2H), 3.30–3.20 (m, 2H), 3.12 (s, 1H), 2.67 (s, 1H). 13C NMR (101 MHz, DMSO) δ 166.34, 164.63, 155.86, 152.77, 148.16, 141.19, 137.35, 134.94, 133.81, 131.17, 130.84, 128.63, 128.25, 127.50, 123.18, 122.88, 122.19, 120.84, 115.77, 114.67.
2-Fluoro-N-(4-(4-(4-(4-(Hydroxycarbamoyl)Phenyl)Piperazine-1-Carbonyl)Quinolin-2-yl)Phenyl)Benzamide (D22)
Crystallization in EtOAc as a yellow solid (0.05 g, 33.3% yield). Mp: 163.5–164.2°C; HRMS C34H28FN5O4 [M + H+] calc. 589.21253, found 588.20569. 1H NMR (400 MHz, DMSO) δ 10.96 (s, 1H), 10.66 (s, 1H), 8.80 (s, 1H), 8.36 (d, J = 8.3 Hz, 2H), 8.18 (s, 1H), 8.14 (d, J = 8.8 Hz, 1H), 7.93 (d, J = 8.5 Hz, 2H), 7.88–7.81 (m, 2H), 7.72 (t, J = 7.5 Hz, 1H), 7.66 (s, 1H), 7.64 (s, 2H), 7.61 (d, J = 7.8 Hz, 1H), 7.43–7.33 (m, 2H), 6.96 (d, J = 8.6 Hz, 2H), 3.92 (s, 1H), 3.51 (s, 2H), 3.30–3.21 (m, 2H), 3.12 (s, 1H), 2.79–2.57 (m, 2H). 13C NMR (101 MHz, DMSO) δ 171.76, 166.34, 163.46, 155.82, 152.77, 148.16, 143.72, 141.06, 133.81, 130.42, 128.61, 128.41, 125.11, 123.18, 120.19, 116.58, 115.75, 114.68.
2-Chloro-N-(4-(4-(4-(4-(Hydroxycarbamoyl)Phenyl)Piperazine-1-Carbonyl)Quinolin-2-yl)Phenyl)Benzamide (D23)
Crystallization in EtOAc as a yellow solid (0.07 g, 46.7% yield). Mp: 167.2–167.8°C; HRMS C34H28ClN5O4 [M + H+] calc. 606.18634, found 606.18951. 1H NMR (400 MHz, DMSO) δ 10.96 (s, 1H), 10.75 (s, 1H), 8.80 (s, 1H), 8.36 (d, J = 8.4 Hz, 2H), 8.20–8.09 (m, 2H), 7.92 (d, J = 8.3 Hz, 2H), 7.84 (t, J = 6.8 Hz, 3H), 7.69–7.45 (m, 9H), 6.96 (d, J = 8.6 Hz, 2H), 3.93–3.80 (m, 1H), 3.51 (s, 2H), 3.12 (s, 2H). 13C NMR (101 MHz, DMSO) δ 152.76, 130.30 (d, J = 23.8 Hz), 130.16–130.08 (m), 129.47, 128.42, 127.79, 123.17, 120.06, 114.68.
2,4-Difluoro-N-(4-(4-(4-(4-(Hydroxycarbamoyl)Phenyl)Piperazine-1-Carbonyl)Quinolin-2-yl)Phenyl)Benzamide (D24)
Crystallization in EtOAc as a red solid (0.03 g, 42.9% yield). Mp: 175.3–176.3°C; HRMS C34H27F2N5O4 [M + H+] calc. 608.20647, found 608.20923. 1H NMR (400 MHz, DMSO) δ 10.95 (s, 1H), 10.52 (d, J = 8.4 Hz, 1H), 8.80 (s, 1H), 8.38 (d, J = 8.3 Hz, 2H), 8.21–8.10 (m, 2H), 8.10–7.96 (m, 2H), 7.92 (d, J = 8.5 Hz, 2H), 7.85 (d, J = 8.8 Hz, 2H), 7.65 (d, J = 8.1 Hz, 3H), 6.96 (d, J = 8.4 Hz, 2H), 6.85 (dd, J = 16.0, 8.9 Hz, 2H), 6.68 (d, J = 8.4 Hz, 1H), 3.94 (s, 1H), 3.51 (s, 4H), 3.13 (s, 1H). 13C NMR (101 MHz, DMSO) δ 175.03, 171.76, 166.33, 155.80, 152.77, 148.16, 143.74, 140.85, 134.05, 131.03, 128.61, 128.32, 127.79, 123.20, 122.88, 120.87, 114.68, 111.85, 111.59, 72.90, 48.05, 43.16.
3,5-Difluoro-N-(4-(4-(4-(4-(Hydroxycarbamoyl)Phenyl)Piperazine-1-Carbonyl)Quinolin-2-yl)Phenyl)Benzamide (D25)
Crystallization in EtOAc as a yellow solid (0.11 g, 43.9% yield). Mp: 171.8–172.2°C; HRMS C34H27F2N5O4 [M + H+] calc. 608.20647, found 608.20923. 1H NMR (400 MHz, DMSO) δ 10.96 (s, 1H), 10.58 (s, 1H), 8.80 (s, 1H), 8.38 (d, J = 8.4 Hz, 2H), 8.20 (s, 1H), 8.14 (d, J = 8.7 Hz, 1H), 7.99 (d, J = 8.4 Hz, 2H), 7.84 (t, J = 7.0 Hz, 2H), 7.74 (d, J = 6.7 Hz, 2H), 7.64 (t, J = 7.5 Hz, 3H), 7.56 (t, J = 9.0 Hz, 1H), 6.96 (d, J = 8.5 Hz, 2H), 3.51 (s, 2H), 3.30–3.20 (m, 2H), 3.13 (s, 1H), 2.71 (dd, J = 41.4, 15.4 Hz, 3H). 13C NMR (101 MHz, DMSO) δ 175.03, 171.76, 166.33, 155.80, 152.77, 148.16, 143.74, 140.85, 134.05, 131.03, 128.61, 128.32, 127.79, 123.20, 122.88, 120.87, 114.68, 111.85, 111.59, 72.90, 48.05, 43.16.
3-Fluoro-N-(4-(4-(4-(4-(Hydroxycarbamoyl)Phenyl)Piperazine-1-Carbonyl)Quinolin-2-yl)Phenyl)Benzamide (D26)
Crystallization in EtOAc as a yellow solid (0.08 g, 32.0% yield). Mp: 232.6–232.9°C; HRMS C34H28FN5O4 [M + H+] calc. 590.21589, found 590.21637. 1H NMR (400 MHz, DMSO) δ 10.96 (s, 1H), 10.54 (s, 1H), 8.80 (s, 1H), 8.38 (d, J = 8.2 Hz, 2H), 8.23–8.07 (m, 2H), 8.00 (d, J = 8.3 Hz, 2H), 7.93–7.75 (m, 4H), 7.72–7.40 (m, 5H), 6.96 (d, J = 8.3 Hz, 2H), 3.96 (d, J = 25.2 Hz, 2H), 3.51 (s, 3H), 3.21 (d, J = 65.5 Hz, 3H). 13C NMR (101 MHz, DMSO) δ 175.08, 166.36, 155.92, 148.15, 143.66, 141.73, 132.85, 130.94, 130.01, 128.31, 125.34, 123.13, 120.18, 119.48, 116.39, 115.61, 47.18, 45.42, 29.60, 25.71.
4-(4-(2-(2,5-Difluorophenyl)Quinoline-4-Carbonyl)Piperazin-1-yl)-N-Hydroxybenzamide (D27)
Crystallization in EtOAc as a white solid (0.15 g, 53.6% yield). Mp: 196.9–198.0°C; HRMS C27H22F2N4O3 [M + H+] calc. 488.16600, found 489.17139. 1H NMR (400 MHz, DMSO) δ 10.96 (s, 1H), 8.81 (s, 1H), 8.20 (d, J = 8.3 Hz, 1H), 7.91 (dd, J = 15.9, 7.5 Hz, 4H), 7.73 (t, J = 7.6 Hz, 1H), 7.65 (d, J = 8.5 Hz, 2H), 7.56–7.38 (m, 2H), 6.95 (d, J = 8.5 Hz, 2H), 3.94 (d, J = 30.4 Hz, 2H), 3.49 (s, 2H), 3.20 (d, J = 56.1 Hz, 4H). 13C NMR (101 MHz, DMSO) δ 165.97, 152.76, 148.12, 143.40, 131.36, 130.28, 129.06–128.94 (m), 128.73 (d, J = 25.7 Hz), 125.26, 123.40, 122.82, 119.08, 117.67, 114.70, 48.02, 47.56, 46.57, 41.39.
4-(4-(2-([1,1′-Biphenyl]-4-yl)Quinoline-4-Carbonyl)Piperazin-1-yl)-N-Hydroxybenzamide (D28)
Crystallization in EtOAc as a white solid (0.14 g, 56.9% yield). Mp: 203.5–205.6°C; HRMS C33H28N4O3 [M + H+] calc. 529.21950, found 529.22034. 1H NMR (400 MHz, DMSO) δ 10.96 (s, 1H), 8.81 (s, 1H), 8.45 (d, J = 8.0 Hz, 2H), 8.27 (s, 1H), 8.18 (d, J = 8.5 Hz, 1H), 7.92–7.82 (m, 4H), 7.79 (d, J = 7.6 Hz, 2H), 7.67 (t, J = 7.9 Hz, 3H), 7.52 (t, J = 7.4 Hz, 2H), 7.42 (t, J = 7.3 Hz, 1H), 6.96 (d, J = 8.4 Hz, 2H), 3.97 (d, J = 25.3 Hz, 2H), 3.55 (d, J = 25.0 Hz, 2H), 3.32–3.04 (m, 3H). 13C NMR (101 MHz, DMSO) δ 166.30, 155.91, 152.78, 148.20, 143.80, 141.98, 139.81, 137.51, 131.09, 130.20, 129.54, 128.61, 128.40, 128.02, 127.59, 127.23, 125.23, 123.39, 122.88, 116.04, 114.68, 48.04, 47.61, 46.61, 41.38.
4-(4-(2-([1,1′-Biphenyl]-4-yl)Quinoline-4-Carbonyl)Piperazin-1-yl)-N′-Propylbenzohydrazide (D29)
Crystallization in EtOAc as a white solid (0.14 g, 35.5% yield). Mp: 223.3–224.2°C; HRMS C36H35N5O2 [M + H+] calc. 570.28243, found 570.28455. 1H NMR (400 MHz, DMSO) δ 9.83 (s, 1H), 8.50 (d, J = 7.9 Hz, 2H), 8.32 (s, 1H), 8.23 (d, J = 8.5 Hz, 1H), 7.98–7.88 (m, 4H), 7.84 (d, J = 7.5 Hz, 3H), 7.78 (d, J = 8.3 Hz, 2H), 7.73 (d, J = 7.5 Hz, 1H), 7.57 (t, J = 7.4 Hz, 2H), 7.48 (d, J = 7.2 Hz, 1H), 7.01 (d, J = 8.4 Hz, 2H), 3.99 (s, 1H), 3.57 (s, 2H), 3.34 (s, 1H), 3.19 (d, J = 8.2 Hz, 1H), 2.76 (dd, J = 12.9, 5.8 Hz, 2H), 1.50 (dd, J = 14.4, 7.2 Hz, 2H), 1.24 (dd, J = 17.0, 10.0 Hz, 2H), 0.99–0.86 (m, 4H). 13C NMR (101 MHz, DMSO) δ 166.30, 165.56, 155.91, 152.82, 148.21, 143.80, 141.98, 139.81, 137.51, 131.08, 130.20, 129.53, 128.80, 128.39, 128.00, 127.59, 127.23, 125.24, 123.39, 116.04, 114.58, 60.24, 53.74, 47.61, 46.60, 41.38, 21.34, 12.16.
4-(4-(2-([1,1′-Biphenyl]-4-yl)Quinoline-4-Carbonyl)Piperazin-1-yl)-N′-Butylbenzohydrazide (D30)
Crystallization in EtOAc as a white solid (0.14 g, 43.9% yield). Mp: 225.3–227.3°C; HRMS C37H37N5O2 [M + H+] calc. 584.29808, found 584.29950. 1H NMR (400 MHz, DMSO) δ 8.45 (d, J = 8.0 Hz, 2H), 8.27 (s, 1H), 8.18 (d, J = 8.4 Hz, 1H), 7.87 (dd, J = 11.7, 7.9 Hz, 4H), 7.78 (t, J = 8.3 Hz, 3H), 7.73 (d, J = 8.3 Hz, 1H), 7.70–7.62 (m, 1H), 7.52 (t, J = 7.4 Hz, 2H), 7.42 (t, J = 7.2 Hz, 1H), 7.11–6.86 (m, 2H), 6.54 (s, 1H), 3.94 (s, 1H), 3.69–3.40 (m, 3H), 3.32–3.23 (m, 2H), 3.15 (s, 1H), 2.75 (t, J = 6.7 Hz, 1H), 2.22 (d, J = 5.8 Hz, 1H), 1.70–1.21 (m, 4H), 0.90 (dt, J = 14.2, 7.1 Hz, 4H). 13C NMR (101 MHz, DMSO) δ 166.35, 163.86, 155.85, 148.18, 143.69, 141.33, 139.30, 137.73, 133.49, 132.84, 131.63, 130.90 (d, J = 15.8 Hz), 130.05, 128.53, 127.71, 127.07, 125.20, 124.33, 123.19, 122.81, 120.19, 119.74, 115.68, 50.90, 41.75.
In Vitro HDAC Inhibitory Assay
All HDAC enzymes were purchased from BPS Bioscience. In short, HeLa cell nuclear extract solution (60 μl) was mixed with a 2.0 μM compound sample (40 μl), 60 μl of recombinant HDAC enzyme solution was mixed with various concentrations of test compound (40 μl, with concentrations from 1 nM to 100 μM), and then incubated at 37°C for 30 min (Fan et al., 2021). The reaction was terminated by adding 100 μl of imaging agent containing trypsin and trichostatin A (TSA). After standing for 20 min, the fluorescence intensity was measured at the excitation and emission wavelengths of 360 and 460 nm with a microplate reader. The inhibition rate was calculated from the fluorescence intensity readings of the test wells relative to the control wells, and the IC50 curve and value were determined by GraphPad Prism 6.0 software.
In Vitro Antiproliferative Assay
The proliferation of cancer cells was tested by CCK-8 assay. Briefly, cells were seeded in a 96-well plate with about 5 × 103 cells in each well. In the K652 cell screening, cells were treated with 2.0 μM of tested compounds. In the IC50 calculation, cells were treated with tested compounds (with concentrations from 0.5 to 20 μM) after 24 h of incubation. CCK-8 reagent (10 ml) was added to each well after 72 h of incubation, and cells were incubated at 37°C for 4 h. The light absorbance at 450 nm was measured by using an Opsys microplate reader (Dynex Technologies, Chantilly, VA, United States). Results are illustrated as a percent of cell viability normalized to DMSO-treated control cells.
Cell Cycle Analysis
K562 cells were incubated with different doses of molecule D28 and SAHA for 24 h. After treatment, cells were collected and fixed with 70% pre-cold ethanol in PBS and stored at −20°C overnight. Then, the cells were washed with PBS twice and incubated with 100 μg/ml RNase I (Solarbio, China) at 37°C for 1 h and stained with propidium iodide (PI, 10 μg/ml, Solarbio, China) for 30 min avoiding light at room temperature. Finally, DNA content was measured by flow cytometry (FACSAriaIII, Becton Dickinson, United States). The data was analyzed and fitted by ModFit software.
Cell Apoptosis Analysis
K562 cells were treated with various concentrations of molecule D28 and SAHA for 24 h, cells were harvested and PBS washed twice, then resuspended with binding buffer (Becton Dickinson, United States). Cells were incubated with Annexin V-BV421(Becton Dickinson, United States) and 7-AAD (Becton Dickinson, United States) double labeling for 30 min in the dark at room temperature and measured by flow cytometry (FACSAriaIII, Becton Dickinson, United States). The data was analyzed by using Flowjo-V10 software.
Statistical Analysis
All experiments were repeated at least three times unless otherwise stated. The data were represented as mean ± SD. Statistical analyses were performed with Student’s t-test for two-group comparisons and using one-way ANOVA with Tukey’s post-hoc test for multigroup comparisons.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author Contributions
LeZ designed the project; QH synthesized the molecules; LiZ performed the enzymatic screening; JF performed the in vitro antitumor experiments; LeZ analyzed the data and wrote the manuscript.
Funding
This work was supported by the Natural Foundation of Shandong Province (Youth Found., Grant No. ZR2019QH005), the Science and Technology Support Plan for Youth Innovation in Universities of Shandong Province (Grant No. 2019KJM001), and the Ideological Project of Weifang University (Grant No. 2022-SZ-29).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2022.937225/full#supplementary-material
References
Bernstein, B. E., Tong, J. K., and Schreiber, S. L. (2000). Genomewide Studies of Histone Deacetylase Function in Yeast. Proc. Natl. Acad. Sci. U.S.A. 97 (25), 13708–13713. doi:10.1073/pnas.250477697
Fan, W., Zhang, L., Wang, X., Jia, H., and Zhang, L. (2021). Discovery of Potent Histone Deacetylase Inhibitors with Modified Phenanthridine Caps. J. Enzyme Inhibition Med. Chem. 36 (1), 707–718. doi:10.1080/14756366.2021.1892089
Foglietti, C., Filocamo, G., Cundari, E., De Rinaldis, E., Lahm, A., Cortese, R., et al. (2006). Dissecting the Biological Functions of Drosophila Histone Deacetylases by RNA Interference and Transcriptional Profiling. J. Biol. Chem. 281 (26), 17968–17976. doi:10.1074/jbc.M511945200
He, X., Li, Z., Zhuo, X.-T., Hui, Z., Xie, T., and Ye, X.-Y. (2020). Novel Selective Histone Deacetylase 6 (HDAC6) Inhibitors: A Patent Review (2016-2019). Pra 15 (1), 32–48. doi:10.2174/1574892815666200217125419
Jiang, Y., Xu, J., Yue, K., Huang, C., Qin, M., Chi, D., et al. (2022). Potent Hydrazide-Based HDAC Inhibitors with a Superior Pharmacokinetic Profile for Efficient Treatment of Acute Myeloid Leukemia In Vivo. J. Med. Chem. 65 (1), 285–302. doi:10.1021/acs.jmedchem.1c01472
Marks, P. A. (2007). Discovery and Development of SAHA as an Anticancer Agent. Oncogene 26 (9), 1351–1356. doi:10.1038/sj.onc.1210204
Marks, P. A., Rifkind, R. A., Richon, V. M., Breslow, R., Miller, T., and Kelly, W. K. (2001). Histone Deacetylases and Cancer: Causes and Therapies. Nat. Rev. Cancer 1 (3), 194–202. doi:10.1038/35106079
Neri, P., Bahlis, N. J., and Lonial, S. (2012). Panobinostat for the Treatment of Multiple Myeloma. Expert Opin. Investigational Drugs 21 (5), 733–747. doi:10.1517/13543784.2012.668883
Ruijter, A. J. M. d., Gennip, A. H. v., Caron, H. N., Kemp, S., and Kuilenburg, A. B. P. v. (2003). Histone Deacetylases (HDACs): Characterization of the Classical HDAC Family. Biochem. J. 370 (Pt 3), 737–749. doi:10.1042/BJ20021321
Swain, B., Sahoo, S. K., Singh, P., Angeli, A., Yaddanapudi, V. M., Supuran, C. T., et al. (2022). Exploration of 2-Phenylquinoline-4-Carboxamide Linked Benzene Sulfonamide Derivatives as Isoform Selective Inhibitors of Transmembrane Human Carbonic Anhydrases. Eur. J. Med. Chem. 234, 114247. doi:10.1016/j.ejmech.2022.114247
Ueda, H., Nakajima, H., Hori, Y., Fujita, T., Nishimura, M., Goto, T., et al. (1994). FR901228, a Novel Antitumor Bicyclic Depsipeptide Produced by Chromobacterium Violaceum No. 968. I. Taxonomy, Fermentation, Isolation, Physico-Chemical and Biological Properties, and Antitumor Activity. J. Antibiot. 47 (3), 301–310. doi:10.7164/antibiotics.47.301
Yamada, K., Levell, J., Yoon, T., Kohls, D., Yowe, D., Rigel, D. F., et al. (2017). Optimization of Allosteric With-No-Lysine (WNK) Kinase Inhibitors and Efficacy in Rodent Hypertension Models. J. Med. Chem. 60 (16), 7099–7107. doi:10.1021/acs.jmedchem.7b00708
Yang, L., Xue, X., and Zhang, Y. (2010). Simple and Efficient Synthesis of Belinostat. Synth. Commun. 40 (17), 2520–2524. Pii 925252787. doi:10.1080/00397910903277870
Zhang, L., Chen, Y., Jiang, Q., Song, W., and Zhang, L. (2019). Therapeutic Potential of Selective Histone Deacetylase 3 Inhibition. Eur. J. Med. Chem. 162, 534–542. doi:10.1016/j.ejmech.2018.10.072
Zhang, L., Fang, H., and Xu, W. (2010). Strategies in Developing Promising Histone Deacetylase Inhibitors. Med. Res. Rev. 30 (4), 585–602. doi:10.1002/med.20169
Zhang, L., Han, Y., Jiang, Q., Wang, C., Chen, X., Li, X., et al. (2015). Trend of Histone Deacetylase Inhibitors in Cancer Therapy: Isoform Selectivity or Multitargeted Strategy. Med. Res. Rev. 35 (1), 63–84. doi:10.1002/med.21320
Keywords: histone deacetylase, inhibitor, anticancer, selectivity, cell cycle, apoptosis
Citation: Hui Q, Zhang L, Feng J and Zhang L (2022) Discovery of 2-Phenylquinoline-4-Carboxylic Acid Derivatives as Novel Histone Deacetylase Inhibitors. Front. Chem. 10:937225. doi: 10.3389/fchem.2022.937225
Received: 06 May 2022; Accepted: 13 June 2022;
Published: 14 July 2022.
Edited by:
Mohamed Radwan, Kumamoto University, JapanReviewed by:
A. Ganesan, University of East Anglia, United KingdomAmaç Fatih Tuyun, Istanbul University, Turkey
Deena S. Lasheen, Ain Shams University, Egypt
Copyright © 2022 Hui, Zhang, Feng and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Zhang, bGVpemhhbmdjaGVtaWNhbEBnbWFpbC5jb20=
†These authors have contributed equally to this work
 Qian Hui1†
Qian Hui1† Lei Zhang
Lei Zhang