- 1Fujian Key Laboratory of Oral Diseases and Fujian Provincial Engineering Research Center of Oral Biomaterial and Stomatological Key Lab of Fujian College and University, School and Hospital of Stomatology, Fujian Medical University, Fuzhou, China
- 2Institute of Stomatology and Research Center of Dental Esthetics and Biomechanics, School and Hospital of Stomatology, Fujian Medical University, Fuzhou, China
- 3Inspection and Quarantine Technology Center, Fujian Entry-Exit Inspection and Quarantine Bureau, Fuzhou, China
- 4Department of Clinical Laboratory, School of Medical Technology and Engineering, Fujian Medical University, Fuzhou, China
CAD/CAM (computer-aided design and computer-aided manufacturing) technology has been widely applied in clinical dentistry, but the material safety remains a concern. To investigate the impacts of CAD/CAM metallic materials on trace metals and biocompatibilities, selective laser melted (SLM) cobalt-chromium (Co-Cr) alloys and computer numeric controlled milled (CNC milled) commercially pure titanium (CP-Ti) were placed on the maxilla of beagle dogs for 6 months. The trace metals in the oral mucosa, blood, liver, kidney, and hair were then determined by inductively coupled plasma mass spectrometry (ICP-MS). The histopathologic changes and biocompatibilities of tissues were examined by hematoxylin and eosin (H&E) staining, terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-digoxigenin nick end labeling (TUNEL) method, immunohistochemistry (IHC) assay, Western blot analysis, and liver and kidney function tests. Our results showed that trace metals released from these two CAD/CAM metallic materials accumulated transiently in the oral mucosa and whole blood. The trace metals released from SLM Co-Cr alloys could also transiently accumulate in the plasm and hair. In addition, these two CAD/CAM metallic materials both induced apoptosis and histopathologic changes in the oral mucosa, with SLM Co-Cr alloys inducing a higher level of apoptosis. In contrast, both materials did not exert autophagic effects on the oral mucosa or affect the trace metals, functions, or biocompatibilities of the liver and kidney. Therefore, this study suggests that CAD/CAM metallic materials should be selected carefully, especially in patients with metal- and apoptosis-related diseases, and CNC-milled CP-Ti can be recommended to patients on account of its better biocompatibility and safety.
Introduction
With the promotion and application of computer-aided design and computer-aided manufacturing (CAD/CAM) technology in dentistry, increased digital molding methods, such as selective laser melting (SLM) and computer numeric controlled (CNC) milling, have been used to fabricate metallic dental prostheses and implants. CAD/CAM technology covers a range of applications in modern industries, including the manufacturing of computer components and digital control devices. Additionally, this technology has great potential in producing complex-shaped and porous materials and components (Attarilar et al., 2021).
The SLM technique is an additive manufacturing technique that produces samples layer by layer based on 3D model data (Kandavalli et al., 2021). In contrast, the CNC milling technique is a subtractive manufacturing technique, which is a manufacturing process that controls the removal of unnecessary materials to obtain the desired shape by cutting, drilling or milling. There are many benefits associated with dental prostheses fabricated by CAD/CAM compared to traditional lost-wax casting techniques, including the access to better and almost defect-free pieces, an increase in quality, reproducibility, and efficiency, and an improvement in accuracy and precision (Patel, 2014; Bilgin et al., 2016; AhmED, 2018). These digitized molding methods mainly use cobalt-chromium (Co-Cr) alloy and commercial pure titanium (CP-Ti) as raw materials for dental prostheses because of their excellent mechanical properties and acceptable price. Among them, SLM Co-Cr alloys and CNC-milled CP-Ti are most commonly used in the clinic. The main reason is that Co-Cr has a lower grindability than CP-Ti and thus is more difficult to mill (Ohkubo et al., 2006), and the CNC-milled titanium has higher precision than additive manufacturing titanium (Tan et al., 2019; Sulaiman, 2020). Therefore, in this research, the SLM Co-Cr alloy and CNC-milled CP-Ti were used as the representatives of CAD/CAM metallic materials.
Previous studies have found that trace metal ions can be released from dental alloys and titanium into saliva and nearby oral tissues due to the complex oral microenvironment, and then likely disseminated around the whole body through the blood system, thus causing potential cytotoxic effects on not only local oral tissues but also distant organs for a long time (Siddharth et al., 2015; Noumbissi et al., 2019; Vaicelyte et al., 2020). Our previous studies found that trace metals released from casted Co-Cr alloy and CP-Ti could be accumulated transiently in the oral mucosa, blood, liver and kidney, which might cause apoptosis-based cell death via the mitochondrial pathway and trigger autophagic regulators. The results also showed that casted CP-Ti has better chemical stability and biocompatibility than casted Co-Cr alloy (Liu et al., 2020; Pan et al., 2020).
In addition, metal ions released from metallic dental materials can induce local and systemic biological effects. Studies showed that metal elements can penetrate into different live tissues, thus cause toxicity and inflammations depending on their chemical characteristics (Attarilar et al., 2020). Baricevic et al.(2012) reported that metal ions released from Co-Cr alloys could cause DNA damage to human oral mucosal cells (Baričević et al., 2012). Faccioni et al.(2003) showed that metal ions released from orthodontic appliances could lead to increased apoptosis of peripheral venous blood lymphocytes and oral mucosal epithelial cells (Faccioni et al., 2003). Similarly, our previous studies confirmed that trace metals released from Co-Cr alloy and CP-Ti could induce time-dependent early apoptosis through the intrinsic pathway (Pan et al., 2017; Pan et al., 2020). Moreover, Fatima et al. found that chromium compounds could lead to nephrotoxicity to the kidney in rats through the damage to the renal brush border membrane (Fatima et al., 2005). It is also controversial whether the biocompatibility of digitally molded dental alloys is better than that of traditional casted alloys. Xin et al.(2014) found that SLM Co-Cr alloy had better cell proliferation and biocompatibility than cast Co-Cr alloy, but studies by Boldbayar and colleagues suggested that there was no significant difference in biocompatibility between them (Ganbold et al., 2019).
However, studies on CAD/CAM metallic materials have been conducted primarily in vitro. In particular, while the mechanical and chemical properties of SLM Co-Cr alloys and CNC-milled CP-Ti have been widely studied, research focusing on trace metals released from these two CAD/CAM metallic materials in surrounding tissues, peripheral blood, distant organs, and even hair, remains rare. Moreover, their biocompatibilities in vivo have not yet been studied. The aim of the present study was to investigate the trace metals and biocompatibilities of SLM Co-Cr alloy and CNC-milled CP-Ti in a beagle canine model. We hypothesized that these two CAD/CAM metallic materials could affect the accumulation of metal ions and biological effects in local and systemic tissues, and CNC-milled CP-Ti might have better biocompatibility. This study could provide insight into the selection of clinical dental restoration materials.
Materials and Methods
Establishment of Beagle Canine Oral Mucosa Irritation Tests
The studies involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of the Affiliated Hospital of Stomatology, Fujian Medical University [Reference No.: 2018 (02)]. Nine beagle dogs (18 months old and weighing 10–16 kg) were used for the experiments and were divided equally into three groups (SLM Co-Cr alloy group, CNC-milled CP-Ti groups, and the negative control group). Anesthesia was induced by injecting Sumianxin II (Shengda Pharmaceutical Co., Ltd., China, 1.6 mg/ kg intramuscularly), followed by a subsequent intravenous injection of 3% pentobarbital [MERK, Germany, 30 mg/( kg h)] for around 10–15 min. After cleaning and preparing the teeth, the impressions of the maxilla were produced with silicone rubber and sent to the laboratory for fabrication of maxillary metal plates with bands (Figure 1A).
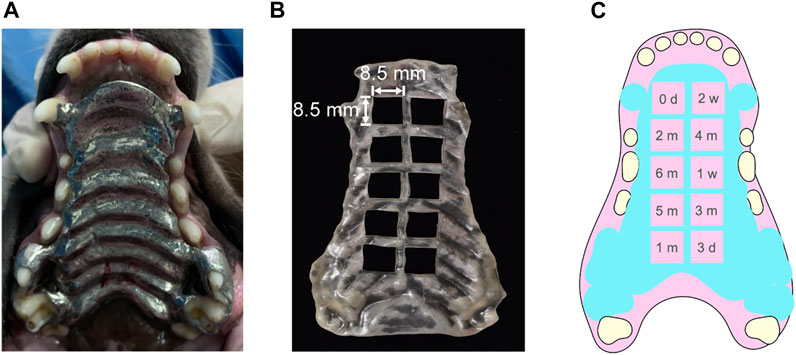
FIGURE 1. (A) CAD/CAM metallic dental prosthesis, which was personalized by taking impression of the Beagle’s maxilla with silicone rubber, (B) 3-dimensional printed resin guide board for taking oral mucosa samples, and (C) schematic diagram of the order for taking oral mucosa samples.
According to the instructions of the manufacturers, SLM Co-Cr alloy plates and CNC-milled CP-Ti plates were fabricated by the SLM system (Space Traveler Tr150, Profeta Intelligent Technology, Nanjing, China) and the CAD/CAM system (DT400, Jiansan Automation Technology Co., Ltd., Guangzhou, China), respectively. The compositions of the Co-Cr alloy and CP-Ti are shown in Supplementary Table S1 and the manufacturing technical parameters are shown in Supplementary Tables S2–S3. Orthodontic vacuum-formed retainer films (SIMONA, Germany) were selected as a negative control. After the preparation of the plates, the beagle dogs were anesthetized in the same manner as above, and the resin-reinforced glass ionomer adhesive (RelyX™ Luting 2, Minnesota Mining and Manufacturing Company, United States) was then used to bond the maxillary plates to the beagle maxillary canines, fourth premolars, and first molars. The palatal plates and the mucosa were ensured fit closely.
Blood Sample Collection
Blood samples were collected after the beagle dogs adapted to their feeding conditions. Beagle dogs were fasted overnight before collection of blood and tissue samples. Blood samples were harvested from the cephalic veins by vacutainers with spray-coated K2EDTA (Becton, Dickinson and Company, United States) at 0 days, 3 days, 1 week, 2 weeks, 1 month, 2 months, 3 months, 4 months, 5 months, and 6 months after plate bonding. A 500-μL blood sample was frozen for ICP-MS analysis of whole blood. The rest of the blood samples were kept at 37°C in a water bath for 30 min and centrifuged at 3,000 rpm for 10 min for inductively coupled plasma mass spectrometry (ICP-MS) analysis of the plasma. For serum biochemical evaluation, 1 ml of blood was collected in empty vacutainers (Changgeng Medical Devices Co., Ltd., China) at the same time points and centrifuged at 3,000 rpm for 10 min after holding at 37°C in a water bath for 30 min.
Oral Mucosa, Hair, and Organ Samples Collection
Animals were anaesthetized in the same manner as 3.1.1. After anaesthetization, oral mucosa samples in contact with the plates were collected in order of the guide board at 0 days, 3 days, 1 week, 2 weeks, 1 month, 2 months, 3 months, 4 months, 5 months, and 6 months after bonding the plates (Figures 1B,C) and divided into two parts, with one part being weighed for ICP-MS analysis and the other part being fixed in 4% paraformaldehyde prepared for H&E staining, IHC (immunochemistry) assay, and TUNEL analysis. Hairs on the inside of the extremities were collected at 0 and 3 days, 1 and 2 weeks, and 1, 2, 3, 4, 5, and 6 months after plate bonding. The hair samples were washed with neutral detergent and dried. The deeply anesthetized beagle dogs were euthanized humanely at 6 months. Afterward, the liver and kidney were collected and divided into three parts, with one of them being weighed for ICP-MS analysis, another being frozen at −80°C for Western blot analysis, and the rest being fixed in 4% paraformaldehyde prepared for H&E staining and TUNEL assay.
ICP-MS Analysis
The concentrations of trace metal ions in the oral mucosa, blood, liver, kidney, and hair were then determined by ICP-MS analysis according to our previous publication (Pan et al., 2020).
Liver and Kidney Function Tests
The liver and kidney function tests were performed according to our previous publication (Pan et al., 2020).
H and E Staining
Following fixation, oral mucosa and organ tissues were dehydrated, embedded in paraffin, sectioned and stained with H and E. Histologic evaluation was performed according to the standard procedure for microscopy described in ISO 10993–10 (ISO 10993-10, 2010); (Supplementary Tables S4–S5).
TUNEL Assays
Sections of paraffin-embedded tissues were subjected to the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-digoxigenin nick end labeling (TUNEL) assay to determine apoptosis-related DNA breaks in the oral mucosa, liver, and renal cells following the manufacturer’s protocol (ab66110, Abcam, United States). Images were acquired with an Olympus BX53 microscope and five high-power fields ( × 400) in each slice were selected randomly. The total number of nuclei (blue stained with DAPI) and the DNA fragmentation with red staining were counted. The apoptotic index was determined by calculating the number of apoptotic cells per sample.
IHC Assays
Serial sections of paraffin-embedded tissues were dewaxed, and endogenous peroxidase was blocked with 1% hydrogen peroxide for 12 min, followed by incubation with the primary antibodies anti-cleaved Caspase-3 (1:500, #9661, Cell Signaling Technology, United States), anti-SQSTM1/p62 (1:5000, NBP1–48320, Novus Biologicals, United States), and anti-Beclin1 (1:400, NB110–87318, Novus Biologicals, United States) in a moist chamber at room temperature for 1 h. After washing with phosphate-buffered saline (PBS) solution, sections were incubated with secondary antibodies (Kit-9902, MXB Biotechnologies, China). DAB Kid (DAB-1031, MXB Biotechnologies, China) was used as the horseradish peroxidase, substrate and the sections were further counterstained with hematoxylin. Finally, Image-Pro Plus software was used to measure the integrated optical density (IOD) values of the positive cells in each field of view.
Western Blot Analysis
The liver and kidney samples were weighed and ground to powder under the protection of liquid nitrogen. The tissue powder was then collected and lysed with radioimmunoprecipitation assay (RIPA) buffer containing 1 mM phenylmethane sulfonyl fluoride (PMSF, ST506, Beyotime Biotechnology, China) on ice for 40 min. The supernatant was obtained by centrifugation at 12,000 × g for 5 min at 4°C, and the protein concentration of the supernatant was determined with a bicinchoninic acid (BCA) assay kit (Beyotime Biotechnology, China) according to the manufacturer’s instructions. A total of 50 μg of protein was loaded per lane, separated by 15% SDS–PAGE, and then transferred to polyvinyl difluoride membranes (Millipore, United States) by electrophoresis. The membranes were blocked with 7% nonfat milk at room temperature for 2 h, followed by incubation with the primary antibody anti-SQSTM1/p62 (1:4,000, NBP1–48320, Novus Biologicals, United States) or anti-Beclin1 (1:1,000, NB110–87318, Novus Biologicals, United States) at 4°C overnight. The membrane was then incubated with a horseradish peroxidase-conjugated secondary antibody (1:3,000, Affinity Bioscience, United States), visualized with an electrochemiluminescence kit (ECL, P0018FS, Beyotime Biotechnology, China) in the dark room, and quantified by ImageJ software. β-Actin (1:1,000, T0022, Affinity Bioscience, United States) was included as the endogenous control. The gray values of the target protein and β-actin were analyzed by ImageJ software (National Institutes of Health, United States).
Statistical Analysis
All data were normally distributed and expressed as the means ± SDs (standard deviations). Statistical comparisons of metal concentrations as well as biological indices among various time points were conducted by one-way analyses of variance (ANOVA) with repeated measures and continued with the least significant difference (LSD) t-test. In addition, the comparisons of metal concentrations as well as biological indices in the liver and kidney were analyzed by one-way ANOVA, followed by the LSD t-test. Semiquantitative data were converted by the formula and then analyzed in the same way as the quantitative data. Correlations between metal concentrations and biological indices were analyzed by Pearson correlation. All data were analyzed by Statistical Package for Social Sciences software (SPSS, Chicago, IL, United States), and p values < 0.05 were considered statistically significant.
Results
Metals Released From the CAD/CAM Metallic Materials Were Accumulated Transiently in Oral Mucosa, Blood, and Hair, but did Not Affect the Concentrations of Trace Metals in Liver and Kidney
The concentrations of trace metal ions in the oral mucosa, whole blood, plasma, hair, liver, and kidney affected by SLM Co-Cr alloys and CNC-milled CP-Ti were measured and are illustrated in Figure 2. Metal ions released from the SLM Co-Cr alloys and CNC-milled CP-Ti were found to accumulate transiently in the oral mucosa. The main effect of time showed a statistically significant difference in the total metal ion concentration in the oral mucosa of both groups at the different time points (p SLM Co-Cr = 0.009 < 0.05, p CP-Ti = 0.000 < 0.05), with the total metal ion concentration peaking at 1 month and declining afterward.
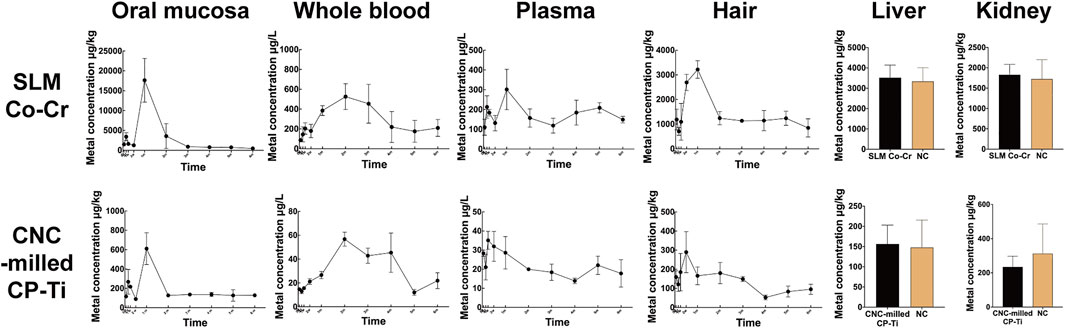
FIGURE 2. Total metal ion concentrations of trace metals in the oral mucosa, whole blood, plasma, hair, liver, and kidney affected by SLM Co-Cr alloys and CNC-milled CP-Ti. The line graphs and bar charts show that metals released from the SLM Co-Cr alloys and CNC-milled CP-Ti accumulated transiently in the oral mucosa, blood, and hair, but did not significantly affect the concentrations of trace metals in the liver and kidney.
There was a significant main effect of time on the total metal ion concentration in the whole blood of the SLM Co-Cr alloy and CNC-milled CP-Ti groups (p SLM Co-Cr = 0.002 < 0.05, p CP-Ti = 0.004 < 0.05), and the time periods of the highest metal ion concentration in the SLM Co-Cr alloy group and CNC-milled CP-Ti group were 2–4 months and 1–2 months respectively (p < 0.05).
There was a significant main effect of time on the total metal ion concentration in the plasma of the CNC-milled CP-Ti group (p SLM Co-Cr = 0.052 > 0.05, p CP-Ti = 0.002 < 0.05). The post hoc tests showed that the peak time of the SLM Co-Cr alloy group was at 1 month (p < 0.05), while no significant increase was found in the CNC-milled CP-Ti group (p > 0.05).
The main effect of time showed a statistically significant difference in the total metal ion concentration in the hair of both the SLM Co-Cr alloy and CNC-milled CP-Ti groups at the different time points (p SLM Co-Cr = 0.000 < 0.05, p CP-Ti = 0.004 < 0.05). In the SLM Co-Cr alloy group, post hoc tests showed that the total metal ion concentration from 2 weeks to 1 month was significantly higher than that at other time points (p < 0.05). However, there was no significant increase in the CNC-milled CP-Ti group (p > 0.05).
Independent sample t-tests showed that there was no statistically significant difference between the experimental groups and the negative control group (p SLM Co-Cr, liver = 0.965 > 0.05, p CP-Ti, liver = 0.867 > 0.05, p SLM Co-Cr, kidney = 0.271 > 0.05, p CP-Ti, kidney = 0.504 > 0.05).
CAD/CAM Metallic Materials did Not Affect Liver and Kidney Function Tests
As shown in Supplementary Figure S1, there was no significant difference in TP, AST, ALT, ALB, GLB, BUN, and CREA levels between the experimental groups and the concurrent control group, indicating that SLM Co-Cr alloy and CNC-milled CP-Ti did not affect them.
CAD/CAM Metallic Materials Led to Temporary Histopathologic Changes in the Oral Mucosa but did Not Affect Liver or Kidney Histopathology
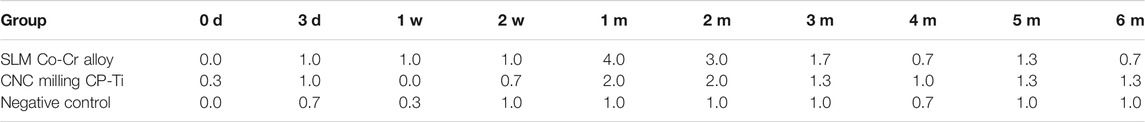
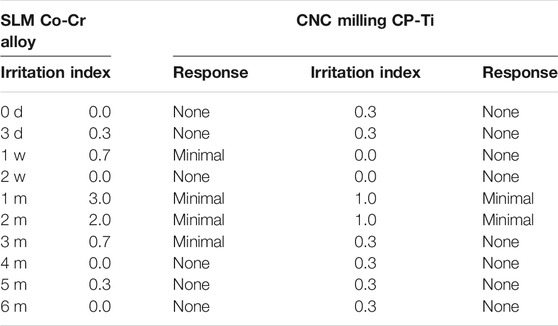
Tables 1–2; Figure 3A show representative H and E staining of the oral mucosa that was used for measurement of lesion morphology. In general, most of the oral mucosa in all groups exhibited intact epithelia, and the cell layers were clearly in order with minimal leucocyte infiltration (less than 25), and the responses of oral mucosa tissues to the irritation by SLM Co-Cr alloys and CNC-milled CP-Ti appeared minimal, with average grades between 0 and 4. Specifically, metaplasia could only be found in the SLM Co-Cr alloy groups at the one-month time point, and cell degeneration was more obvious in the experimental groups at the one- and two-month time points than in the other groups, implying that SLM Co-Cr alloys and CNC-milled CP-Ti would cause damage to the oral mucosa, which could be mitigated spontaneously.
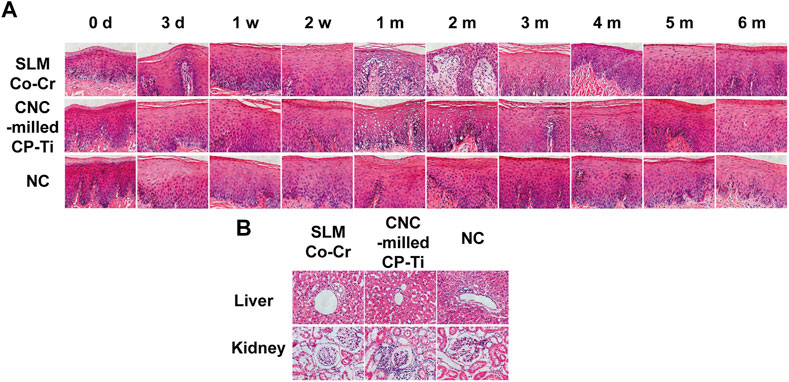
FIGURE 3. (A) H and E staining of the oral mucosa affected by SLM Co-Cr alloys and CNC-milled CP-Ti. Most of the oral mucosa in all groups exhibited intact epithelia, the cell layers were clearly in order with minimal leucocyte infiltration (less than 25), and the responses of oral mucosa tissues to irritation by SLM Co-Cr alloys and CNC-milled CP-Ti appeared minimal. Specifically, metaplasia could only be found in the SLM Co-Cr alloy groups at the one-month time point, and cell degeneration was more obvious in the experimental groups at the one- and two-month time points than in the other groups (Original magnification 400 × ). (B) H&E staining of the liver and kidney affected by SLM Co-Cr alloys and CNC-milled CP-Ti (original magnification 400 × ). No significant difference could be observed in liver and kidney pathology between the experimental groups and the control group.
There was no significant difference in liver and kidney pathology between the experimental groups and the control group, indicating that SLM Co-Cr alloys and CNC-milled CP-Ti did not affect liver or kidney histopathology (Figure 3B).
CAD/CAM Metallic Materials Led to Temporary Apoptosis in the Oral Mucosa but did Not Affect Liver or Kidney Cell Apoptosis
As demonstrated in Figure 4, the TUNEL-positive levels in the oral mucosa were significantly different among the three groups (p = 0.004 < 0.05). In general, the TUNEL-positive levels in the oral mucosa of the SLM Co-Cr alloy group were higher than those of the CNC-milled CP-Ti group, and the negative control group retained the lowest levels. The main effect of time showed a statistically significant difference in the TUNEL-positive levels in the oral mucosa at the different time points (p = 0.000 < 0.05), with the TUNEL-positive levels peaking at 1 month in both the SLM Co-Cr alloy group and CNC-milled CP-Ti group and then decreasing afterward. The interaction between time and different experimental groups had a combined effect on the TUNEL-positive levels in the oral mucosa (p time * groups = 0.018 < 0.05), indicating that as the metal plates were worn for longer time, the change ranges of different groups were different, and the change range of the SLM Co-Cr alloy groups was the largest.
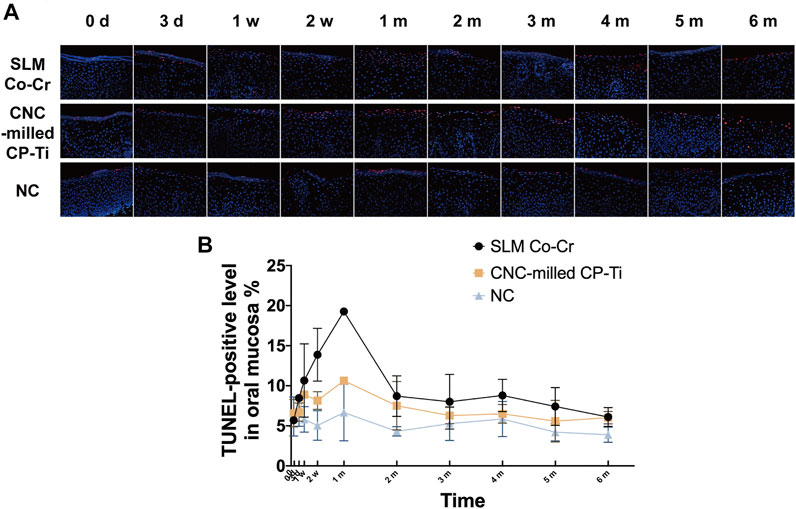
FIGURE 4. Representative images and quantification of TUNEL positivity in the oral mucosa affected by SLM Co-Cr alloys and CNC-milled CP-Ti. Panels show (A) representative TUNEL + labeling of double-stranded DNA in the oral mucosa with red staining, (B) quantification of TUNEL + labeling as performed (original magnification 400 × ). There was a statistically significant difference in the TUNEL-positive levels in the oral mucosa at the different time points, with the TUNEL-positive levels peaking at 1 month in both the SLM Co-Cr alloy group and CNC-milled CP-Ti group and then decreasing afterward. In addition, the TUNEL-positive levels in the oral mucosa of the SLM Co-Cr alloy group were higher than those of the CNC-milled CP-Ti group, and the negative control group retained the lowest levels.
Immunohistochemistry was used to identify cleaved Caspase-3, a marker of apoptosis. No significant differences (p = 0.796 > 0.05) in the expression of cleaved Caspase-3 in the oral mucosa among the three groups were found (Figure 5). The main effect of time showed a statistically significant difference in the expression of cleaved Caspase-3 protein in the oral mucosa (p = 0.001 < 0.05). Specifically, the expression of cleaved Caspase-3 protein in the SLM Co-Cr alloy group peaked only at the 1-month time point but remained relatively steady at other time points. Once again, these observations indicate that the interaction between time and different experimental groups has a combined effect on the expression of cleaved Caspase-3 protein in the oral mucosa (p time * groups = 0.003 < 0.05).
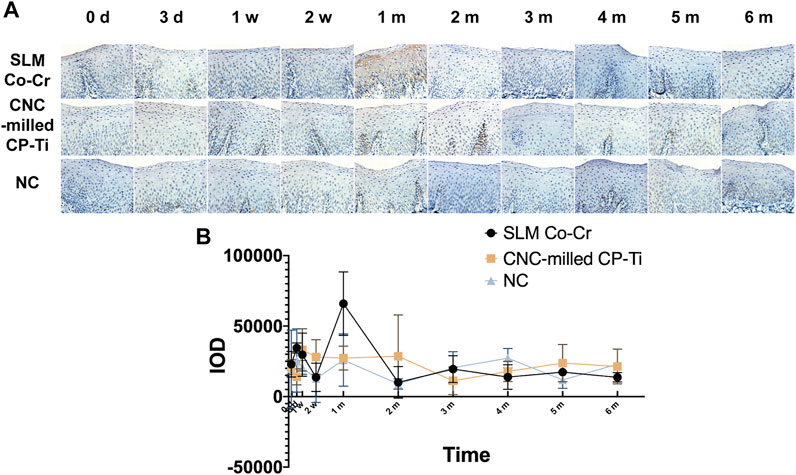
FIGURE 5. Representative images and quantification of cleaved Caspase-3 protein expression in the oral mucosa affected by SLM Co-Cr alloys and CNC-milled CP-Ti. Panels show (A) the expression of cleaved Caspase three protein with brown staining, (B) levels of cleaved Caspase-3 protein expression were evaluated by IOD using Image-Pro Plus software (original magnification 400 × ). The expression of cleaved Caspase-3 protein in the SLM Co-Cr alloy group peaked only at the 1-month time point but remained relatively steady at other time points, while that in the CNC-milled CP-Ti group was stable.
Pearson analysis illustrated the significantly positive correlation between TUNEL-positive levels and the expression of cleaved Caspase-3 protein in the oral mucosa, with a correlation coefficient of 0.535 (p < 0.01). Additionally, Pearson correlation analysis showed that TUNEL-positive levels and the expression of cleaved Caspase-3 protein were both positively correlated with the total metal ion concentration in the oral mucosa (p < 0.01). In particular, as shown in Tables 3–4, Pearson correlation analysis suggested that the TUNEL-positive levels were positively correlated with Cr, Ti, and Ni in the oral mucosa, and the expression levels of cleaved Caspase-3 protein were positively correlated with Co and Cr.
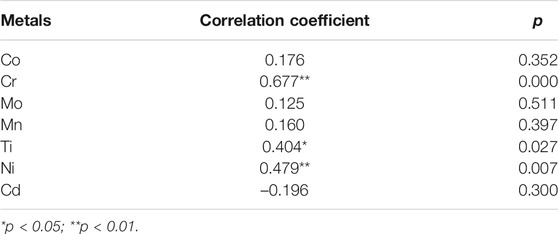
TABLE 3. Pearson correlation analysis between metal concentration and TUNEL-positive levels in oral mucosa.
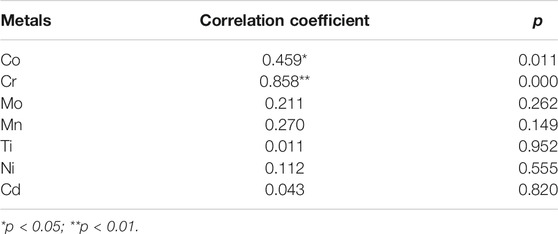
TABLE 4. Pearson correlation analysis between metal concentration and the expression of cleaved Caspase three protein in the oral mucosa.
The results of the TUNEL assay on the liver and kidney are illustrated in Supplementary Figure S2. The results showed that there was no significant difference between the experimental groups and the control group (p > 0.05), indicating that SLM Co-Cr alloys and CNC-milled CP-Ti had no apoptotic effect on hepatocytes and renal cells.
CAD/CAM Metallic Materials did Not Affect the Autophagic Effects of the Oral Mucosa, Liver, or Kidney
Immunohistochemistry was also used to identify markers of autophagy (p62 and Beclin1) in the oral mucosa. As demonstrated in Supplementary Figures S3, S4, there was no significant difference in the expression of p62 and Beclin1 in the oral mucosa among the three groups (p p62 = 0.554 > 0.05, p Beclin1 = 0.474 > 0.05). Moreover, there was no significant main effect of time on the expression of p62 in the oral mucosa (p p62 = 0.055 > 0.05, p Beclin1 = 0.098 > 0.05), and the interaction between time and different experimental groups was not significant (p time * groups, p62 = 0.577 > 0.05, p time * groups, Beclin1 = 0.796 > 0.05).
The Western blot results for the protein expression of p62 and Beclin1 in the liver and kidney, as demonstrated in Supplementary Figure S5, showed no significant difference between the experimental groups and the control groups (p > 0.05), indicating that SLM Co-Cr alloys and CNC-milled CP-Ti had no autophagic effect on hepatocytes and renal cells.
Discussion
In recent years, with the rapid development of digital technology, CAD/CAM technology has been widely used in the production and processing of metallic dental restorations. Studies have suggested that after metallic dental restorations are placed into the mouth, they undergo chemical or electrochemical corrosion and release of metal ions, which can enter the body and might cause local and systemic adverse reactions for a long time (Elshahawy et al., 2009; Mikulewicz et al., 2014). However, almost all reports on the biocompatibilities of CAD/CAM metallic materials involve in vitro studies. It is important to conduct experimental research on animal models before clinical application to humans. In this study, beagle dogs were selected to establish animal models because they have an oral environment similar to that of humans and are widely used in dental research. In addition, studies have shown that beagle dogs can represent human exposure and metabolism, and they are not necessarily worse than primates in predicting human hepatotoxicity (Foster, 2005).
In the present study, the total metal ion concentrations in the oral mucosa of beagle dogs of both the SLM Co-Cr alloy group and CNC-milled CP-Ti group peaked at 1 month after plate bonding, indicating that metal ions would be released from different CAD/CAM metallic materials and accumulate in surrounding tissues, and the metal ion level in the oral mucosa could reach a maximum at approximately 1 month, which was similar to the results published by Agaoglu and colleagues (Agaoglu et al., 2001). The reason for the decrease in metal ion concentration during the later period may be that the Co-Cr alloys could switch into a passivated state after electrochemical corrosion in the mouth. Passivation refers to the phenomenon where, when the alloy materials are electrolyzed in the medium, after the current density increases to a certain value, the dissolution rate of metal ions will decrease sharply after maintaining a high current density for a while; that is, the overpotential of the metal materials increases, making the materials appear to exist in a stable status but no longer dissolve. It is also believed that the chromium and molybdenum in the alloy could improve the corrosion resistance of the alloy and form a continuous dense protective oxide film on the surface of the alloy, which is not prone to corrosion in corrosive media (Matković et al., 2004). At the same time, the Co-Cr alloy could form Cr-O and Cr-OH structures on the surface of the film, preventing the precipitation of metal ions (Huang, 2003). Similar mechanism can also be found in Ti, specifically, titanium could passivate both in synthetic saliva and in saliva with organics (Bilhan et al., 2007). Although titanium is chemically more active, it easily forms a well-structured oxide film, and its surface oxide film is firmly bonded to the base metal, preventing further corrosion (Ionescu et al., 2019). The formation of the thin and stable passivation oxide film stems from the high affinity of titanium for oxygen (Rolando A. Gittens et al., 2014). The oxide film formed on the titanium is usually composed of TiO2, but it may also coexist with other titanium oxides such as TiO and Ti2O3, and the thickness of the formed oxide film is less than 10 nm (Revathi et al., 2017). Studies have also shown that the organic constituents of saliva, such as mucine, IgA, urea, and lysozyme, could enhance the formation of a passive film layer on the titanium surface, thus inhibiting corrosion (Bilhan et al., 2007). Another possible reason for the decline in metal ion concentration in the later stage is that oral mucosal cells could return to a normal state after being stimulated (Natarajan et al., 2011). Moreover, the total concentration of metal ions in the two experimental groups increased slightly at the beginning of this study, which could be attributed to the dynamic load of the device under in vivo conditions (De Souza and De Menezes, 2008; Nayak et al., 2015).
Studies have also suggested that excessive accumulation of metal ions leads to inflammation, apoptosis, and autophagy (Faccioni et al., 2003; Pan et al., 2017; Pan et al., 2020). Therefore, this study explored the effects of CAD/CAM metallic materials on the oral mucosa from histopathologic as well as apoptotic and autophagic perspectives in mucosal cells. The results showed that the responses of oral mucosa tissues irritated by SLM Co-Cr alloys and CNC-milled CP-Ti appeared generally minimal, while metaplasia could only be found in the SLM Co-Cr alloy group at the 1 month time point, and cell degeneration was more serious in the experimental groups at one and 2 months after plate bonding, implying that SLM Co-Cr alloys and CNC-milled CP-Ti cause temporary damage to the oral mucosa. The results of the TUNEL assay indicated that both SLM Co-Cr alloy and CNC-milled CP-Ti could cause apoptosis and that SLM Co-Cr alloy showed a more harmful impact, mirroring the similar results obtained in our previous studies and those obtained by other researchers (Wang et al., 2010; Zhang et al., 2015; Pan et al., 2017). There are two theories available regarding the possible reasons for the high apoptotic rate triggered by SLM Co-Cr alloy. The first is that Co and Cr could induce DNA damage (Baričević et al., 2012) and the second refers to the reduction reactions in the alloy leach liquors (Haeri et al., 2012). Moreover, this study showed that the TUNEL-positive levels reached a peak at 1 month in both the SLM Co-Cr alloy and CNC-milled CP-Ti groups, and then decreased subsequently, while only the expression of cleaved Caspase-3 protein in the SLM Co-Cr alloy group peaked at 1 month. Our previous research also found that dental alloys could induce a transient increase in cell apoptosis (Pan et al., 2020). Furthermore, Pearson correlation analysis showed that TUNEL-positive levels and the expression of cleaved Caspase-3 protein were both positively correlated with the total metal ion concentration in the oral mucosa, indicating that the metal ions released from the CAD/CAM metallic materials that accumulated in the oral mucosa might induce apoptosis, which was similar to the results of studies by (Faccioni et al., 2003). Specifically, Pearson correlation analysis suggested that TUNEL-positive levels were positively correlated with the levels of Cr, Ti, and Ni in the oral mucosa, and the expression of cleaved Caspase-3 protein was positively correlated with the levels of Co and Cr. Many studies have confirmed that Co, Cr, Ni and Ti induce apoptosis; in particular, Co and Cr ions induce apoptosis via mitochondrial or endoplasmic pathways associated with Caspase-3, p52, Bcl-2, and bax, while Ni ions induce apoptosis through mitochondrial, Fas, and endoplasmic reticulum pathways, and Ti ions induce apoptosis through mitochondrial pathways related to Caspases 3 and 9, Bcl-2, Bax, cytochrome c, and ROS (Li et al., 2010; Guo et al., 2015, 2016).
In this study, the peak times of the total metal ion concentration in the whole blood of the experimental groups appeared later than those in the oral mucosa. There are two possible explanations. The first is that the oral mucosa is in direct contact with the metal plate, and the trace metal ions released from the materials could accumulate for the first time, although only a part of these was then transferred to the blood through the microcirculation system. The other explanation is that the trace metal ions in saliva can enter the blood after being absorbed in the epithelium of the gastrointestinal mucosa. Several studies have shown that metal ions would be released from dental alloys with saliva in the mouth over time (Agaoglu et al., 2001; De Souza and De Menezes, 2008). In addition, the main difference between whole blood and plasma is whether it contains blood cells. It has been discovered that some metal ions, such as chromium, can be selectively combined with red blood cells (Devoy et al., 2016), which presents a potential explanation for the differences in the trends of trace metal ion concentrations between whole blood and plasma.
The metal ions released from dental alloys may enter the body through local oral tissue, blood circulation, and the digestive tract. Rubio et al. implanted Co-Cr alloys and pure titanium into the hind legs of rats for 12 months and found that the concentration of metal ions in the liver and kidneys increased significantly, suggesting that metal ions from the prostheses could accumulate in the liver and kidneys (Rubio et al., 2008). Pan et al. fixed cast Co-Cr alloys and CP-Ti in the cheek pouch of golden hamsters and found that the trace metal ions released from dental alloys temporarily accumulated in the blood, liver, and kidney (Pan et al., 2020), which is different from the results of this study. The possible reason could be that the metal materials in this experiment were digitally molded, while Pan and colleagues used traditional casted alloys. Studies have found that CAD/CAM metallic materials have better corrosion resistance and less ion precipitation than traditional casted materials (Xin et al., 2014; Wu et al., 2016). Other reasons, such as animal species, breeding environment, stimulation time, and other conditions, may also lead to differences in results. This study found that the liver and kidney functional indices of the SLM Co-Cr alloy group and the CNC-milled CP-Ti group at various time points, as well as the histopathology, apoptosis and autophagy levels of liver and kidney cells in these two groups at 6 months, were not significantly different from those of the control group, suggesting that these two materials have favorable biocompatibility, which is similar to the conclusion of studies by Pan and coworkers (Pan et al., 2020).
Hair, as a terminal tissue, can retain metal ions. The results of this study showed that the concentration of total metal ions in the hair of the SLM Co-Cr alloy group increased transiently, which is consistent with the results of Jamshidi et al. (Jamshidi et al., 2018). However, there was no significant increase in the metal ion concentration in the hair of the beagle dogs during the period of CNC-milled CP-Ti wearing, which might be attributed to hairs being located on the body surface and being greatly affected by the external environment. Furthermore, previous studies showed that nickel ions released from dental alloys could be excreted through the urine, which would also affect the final accumulation of metal ions in the hair (Jensen et al., 2003; Menezes et al., 2007).
Based on the current findings, it is suggested that CAD/CAM metallic materials can induce transient increases in trace metals in the oral mucosa, blood, and hair. In addition, trace metals released from these two materials also lead to temporary apoptosis and histopathologic changes in the oral mucosa. Compared with CNC-milled CP-Ti, SLM Co-Cr alloys not only led to metaplasia, but also induced more severe cell degeneration and higher level of apoptosis in the oral mucosa (Table 5). Therefore, this study suggests that CNC-milled CP-Ti can be recommended to patients on account of its better biocompatibility. Furthermore, CAD/CAM metallic materials should be selected more carefully, especially in patients with metal- and apoptosis-related diseases. It is recommended to choose other biocompatible-friendly materials for patients suffering from removable partial denture (RPD) treatments.
There are several limitations of this study that should be acknowledged. First, groups with non-CAD/CAM metallic materials was not established in this study. There exists a possibility that these findings are also seen in the usual form of metals and could not be exactly related to the manufacturing method of CAD/CAM. However, previous researches, as well as our studies all demonstrated that the CAD/CAM metallic materials possessed better chemical stability and biocompatibility than the corresponding casted materials (Tuna et al., 2015; Liu et al., 2020). Second, the dimensions of released materials were not identified. It is possible that the different sizes of released trace metals may have different effects on biocompatibility. Nonetheless, it has been widely suggested in the literature that metallic dental prostheses, different than other materials such as artificial joint prostheses, directly release metal ions and act in the form of ions instead of wear particles, thanks to their high wear resistance and low clinical wear properties (Heintze et al., 2019; Noumbissi et al., 2019; Vaicelyte et al., 2020). Undoubtedly, investigations with rigorous design and decent sample sizes are warranted in the future.
Conclusion
CAD/CAM metallic materials can induce transient increases in total metal ion concentrations in the oral mucosa, blood, and hair. In addition, they might lead to temporary apoptosis and histopathologic changes in the oral mucosa but do not affect the autophagic effects, with the apoptotic level in the oral mucosa being positively correlated with the concentration of Co, Cr, Ti, and Ni ions. However, CAD/CAM metallic materials have no effect on trace metals, functions, histopathology, or apoptotic and autophagic effects in the liver and kidney. Compared with CNC-milled CP-Ti, SLM Co-Cr alloys not only led to metaplasia, but also induced more severe cell degeneration and higher level of apoptosis in the oral mucosa. Therefore, CNC-milled CP-Ti shows better biocompatibility and safety than SLM Co-Cr alloy and can be recommended to patients, especially those with metal- and apoptosis-related diseases.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of the Affiliated Hospital of Stomatology, Fujian Medical University [Reference No.: 2018 (02)].
Author Contributions
YL: Conceptualization, Methodology, Investigation, Writing–Original Draft, Funding acquisition. JC: Methodology, Investigation, Writing–Review and Editing. FJ: Formal analysis, Investigation, Writing–Review and Editing. YP: Methodology, Writing–Review and Editing, Funding acquisition. CX: Methodology, Resources. DL: Methodology, Resources. HL: Conceptualization, Methodology. LJ: Conceptualization, Methodology. DZ: Conceptualization. JQ: Methodology. HC: Conceptualization, Supervision, Project administration, Funding acquisition.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers 81870794 and 81641158); the Open Project of Stomatological Key Lab of Fujian College and University and Fujian Provincial Engineering Research Center of Oral Biomaterial (grant number 2019kq04); the Science and Technology Innovation Joint Project of Fujian Province (grant number 2018Y9103); the Medical Innovation Project, Health and Family Planning Commission of Fujian Province (grant number 2018-CXB-12); the Startup Research Fund of Fujian Medical University (grant number 2018QH 2040); and the Spring Buds Program, School and Hospital of Stomatology, Fujian Medical University (grant number 2017-KQCL-07).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank Prof. Zhijian Hu, Public Health School of Fujian Medical University, for assistance with statistical analysis. Thanks are also due to Mr. Huajian Liu, Technology Center of Fuzhou Customs District, for his guidance on ICP-MS analysis.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmats.2021.758873/full#supplementary-material
Supplementary Figure 1 | Liver (a) and kidney (b) function tests. There was no significant difference in TP, AST, ALT, ALB, GLB, BUN, and CREA levels between the experimental groups and the concurrent control group (p > 0.05).
Supplementary Figure 2 | Representative images and quantification of TUNEL positivity in the liver and kidney affected by SLM Co-Cr alloys and CNC-milled CP-Ti. Panels show (a) representative TUNEL+ labeling of double stranded DNA in the oral mucosa with red staining (pointed by arrows), (b) quantification of TUNEL+ labeling as performed. (Original magnification 400×) There was no significant difference in TUNEL-positive levels in the liver and kidney between the experimental groups and the control group (p > 0.05).
Supplementary Figure 3 | Representative images and quantification of p62 protein expression in the oral mucosa affected by SLM Co-Cr alloys and CNC-milled CP-Ti. Panels show (a) the expression of p62 protein with brown staining, (b) levels of p62 protein expression were evaluated by IOD using Image Pro Plus software. (Original magnification 400×) There was no significant difference in the protein expression of p62 in the oral mucosa between the experimental groups and the control group (p > 0.05).
Supplementary Figure 4 | Representative images and quantification of Beclin1 protein expression in the oral mucosa affected by SLM Co-Cr alloys and CNC-milled CP-Ti. Panels show (a) the expression of Beclin1 protein with brown staining, (b) levels of Beclin1 protein expression were evaluated by IOD using Image Pro Plus software. (Original magnification 400×) There was no significant difference in the protein expression of Beclin1 in the oral mucosa between the experimental groups and the control group (p > 0.05).
Supplementary Figure 5 | Autophagic effects on the liver, and kidney of SLM Co-Cr alloys and CNC-milled CP-Ti. Representative images (a) and quantitative analysis (b) of p62 and Beclin1 expression in the liver and kidney determined by Western blot analysis. There was no significant difference in the protein expression of p62 and Beclin1 protein expression in the liver and kidney between the experimental groups and the control group (p > 0.05).
References
AhmED, K. E. (2018). We're Going Digital: The Current State of CAD/CAM Dentistry in Prosthodontics. Prim. Dent. J. 7, 30–35. doi:10.1177/205016841800700205
Attarilar, S., Ebrahimi, M., Djavanroodi, F., Fu, Y., Wang, L., and Yang, J. (2021). 3D Printing Technologies in Metallic Implants: A Thematic Review on the Techniques and Procedures. Int. J. Bioprint 7, 21–46. doi:10.18063/ijb.v7i1.306
Attarilar, S., Yang, J., Ebrahimi, M., Wang, Q., Liu, J., Tang, Y., et al. (2020). The Toxicity Phenomenon and the Related Occurrence in Metal and Metal Oxide Nanoparticles: A Brief Review from the Biomedical Perspective. Front. Bioeng. Biotechnol. 8 (822), 1–23. doi:10.3389/fbioe.2020.00822
Ağaoğlu, G., Arun, T., Izgi, B., Yarat, A., and Izgü, B. (2001). Nickel and Chromium Levels in the Saliva and Serum of Patients with Fixed Orthodontic Appliances. Angle Orthod. 71, 375–379. doi:10.1043/0003-3219(2001)071<0375:NACLIT>2.0
Baričević, M., Ratkaj, I., Mladinić, M., Želježić, D., Kraljević, S. P., Lončar, B., et al. (2012). In Vivo assessment of DNA Damage Induced in Oral Mucosa Cells by Fixed and Removable Metal Prosthodontic Appliances. Clin. Oral Invest. 16, 325–331. doi:10.1007/s00784-010-0489-4
Bilgin, M. S., Baytaroğlu, E. N., Erdem, A., and Dilber, E. (2016). A Review of Computer-Aided Design/computer-Aided Manufacture Techniques for Removable Denture Fabrication. Eur. J. Dent. 10, 286–291. doi:10.4103/1305-7456.178304
Bilhan, H., Bilgin, T., Cakir, A. F., Yuksel, B., and Von Fraunhofer, J. A. (2007). The Effect of Mucine, IgA, Urea, and Lysozyme on the Corrosion Behavior of Various Non-precious Dental Alloys and Pure Titanium in Artificial Saliva. J. Biomater. Appl. 22, 197–221. doi:10.1177/0885328207072557
Devoy, J., Géhin, A., Müller, S., Melczer, M., Remy, A., Antoine, G., et al. (2016). Evaluation of Chromium in Red Blood Cells as an Indicator of Exposure to Hexavalent Chromium: An In Vitro Study. Toxicol. Lett. 255, 63–70. doi:10.1016/j.toxlet.2016.05.008
Elshahawy, W., Watanabe, I., and Koike, M. (2009). Elemental Ion Release from Four Different Fixed Prosthodontic Materials. Dental Mater. 25, 976–981. doi:10.1016/j.dental.2009.02.004
Faccioni, F., Franceschetti, P., Cerpelloni, M., and Fracasso, M. E. (2003). In Vivo study on Metal Release from Fixed Orthodontic Appliances and DNA Damage in Oral Mucosa Cells. Am. J. Orthod. Dentofacial Orthopedics 124, 687–693. doi:10.1016/j.ajodo.2003.09.010
Fatima, S., Arivarasu, N. A., Banday, A. A., Yusufi, A. N. K., and Mahmood, R. (2005). Effect of Potassium Dichromate on Renal brush Border Membrane Enzymes and Phosphate Transport in Rats. Hum. Exp. Toxicol. 24, 631–638. doi:10.1191/0960327105ht585oa
Foster, J. R. (2005). Spontaneous and Drug-Induced Hepatic Pathology of the Laboratory Beagle Dog, the Cynomolgus Macaque and the Marmoset. Toxicol. Pathol. 33, 63–74. doi:10.1080/01926230590890196
Ganbold, B., Heo, S.-J., Koak, J.-Y., Kim, S.-K., and Cho, J. (2019). Human Stem Cell Responses and Surface Characteristics of 3D Printing Co-cr Dental Material. Materials 12, 3419–3512. doi:10.3390/ma12203419
Gittens, R. A., Scheideler, L., Rupp, F., Hyzy, S. L., Geis-Gerstorfer, J., Schwartz, Z., et al. (2014). A Review on the Wettability of Dental Implant Surfaces II: Biological and Clinical Aspects. Acta Biomater. 10, 2907–2918. doi:10.1016/j.actbio.2014.03.032.A
Guo, H., Chen, L., Cui, H., Peng, X., Fang, J., Zuo, Z., et al. (2015). Research Advances on Pathways of Nickel-Induced Apoptosis. Int. J. Mol. Sci. 17, 10. doi:10.3390/ijms17010010
Guo, H., Cui, H., Fang, J., Zuo, Z., Deng, J., Wang, X., et al. (2016). Nickel Chloride (NiCl2) in Hepatic Toxicity: Apoptosis, G2/M Cell Cycle Arrest and Inflammatory Response. Aging 8, 3009–3027. doi:10.18632/aging.101108
Haeri, M., Wӧllert, T., Langford, G. M., and Gilbert, J. L. (2012). Electrochemical Control of Cell Death by Reduction-Induced Intrinsic Apoptosis and Oxidation-Induced Necrosis on CoCrMo alloy In Vitro. Biomaterials 33, 6295–6304. doi:10.1016/j.biomaterials.2012.05.054
Heintze, S. D., Reichl, F.-X., and Hickel, R. (2019). Wear of Dental Materials: Clinical Significance and Laboratory Wear Simulation Methods -A Review. Dent. Mater. J. 38, 343–353. doi:10.4012/dmj.2018-140
Huang, H.-H. (2003). Effect of Fluoride and Albumin Concentration on the Corrosion Behavior of Ti-6Al-4V alloy. Biomaterials 24, 275–282. doi:10.1016/S0142-9612(02)00315-0
Ionescu, F., Reclaru, L., Ardelean, L. C., and Blatter, A. (2019). Comparative Analysis of the Corrosion Resistance of Titanium Alloys Intended to Come into Direct or Prolonged Contact with Live Tissues. Materials 12, 2841. doi:10.3390/ma12172841
ISO 10993-10 (2010). Biological Evaluation of Medical Devices-Part 10: Tests for Irritation and Delayed-type Hypersensitivity. Geneva: International Organization for Standardization.
Jamshidi, S., Rahmati Kamel, M., Mirzaie, M., Sarrafan, A., Khafri, S., and Parsian, H. (2018). Evaluation of Scalp Hair Nickel and Chromium Level Changes in Patients with Fixed Orthodontic Appliance: a One-Year Follow-Up Study. Acta Odontologica Scand. 76, 1–5. doi:10.1080/00016357.2017.1372624
Jensen, C. S., Menné, T., Lisby, S., Kristiansen, J., and Veien, N. K. (2003). Experimental Systemic Contact Dermatitis from Nickel: A Dose-Response Study. Contact Dermatitis 49, 124–132. doi:10.1111/j.0105-1873.2003.00157.x
Kandavalli, S. R., Wang, Q., Ebrahimi, M., Gode, C., Djavanroodi, F., Attarilar, S., et al. (2021). A Brief Review on the Evolution of Metallic Dental Implants: History, Design, and Application. Front. Mater. 8 (646383), 1–16. doi:10.3389/fmats.2021.646383
Li, N., Duan, Y., Hong, M., Zheng, L., Fei, M., Zhao, X., et al. (2010). Spleen Injury and Apoptotic Pathway in Mice Caused by Titanium Dioxide Nanoparticules. Toxicol. Lett. 195, 161–168. doi:10.1016/j.toxlet.2010.03.1116
Liu, Y., Hu, Q., Pan, Y., Wang, Y., Jiang, L., Lin, H., et al. (2021). The Apoptotic and Autophagic Effects of Cast Au-Pt, and Differently Manufactured Co-cr and Cp-Ti on Three-Dimensional Oral Mucosal Model. Mater. Sci. Eng. C 120, 111672. doi:10.1016/j.msec.2020.111672
Matković, T., Matković, P., and Malina, J. (2004). Effects of Ni and Mo on the Microstructure and Some Other Properties of Co-cr Dental Alloys. J. Alloys Comp. 366, 293–297. doi:10.1016/j.jallcom.2003.07.004
Matos de Souza, R., and Macedo de Menezes, L. (2008). Nickel, Chromium and Iron Levels in the Saliva of Patients with Simulated Fixed Orthodontic Appliances. Angle Orthod. 78, 345–350. doi:10.2319/111806-466.1
Menezes, L. M., Quintão, C. A., and Bolognese, A. M. (2007). Urinary Excretion Levels of Nickel in Orthodontic Patients. Am. J. Orthod. Dentofacial Orthopedics 131, 635–638. doi:10.1016/j.ajodo.2005.07.022
Mikulewicz, M., Wołowiec, P., Janeczek, M., Gedrange, T., and Chojnacka, K. (2014). The Release of Metal Ions from Orthodontic appliances Animal Tests. The Angle Orthodontist 84, 673–679. doi:10.2319/090213-641.1
Natarajan, M., Padmanabhan, S., Chitharanjan, A., and Narasimhan, M. (2011). Evaluation of the Genotoxic Effects of Fixed Appliances on Oral Mucosal Cells and the Relationship to Nickel and Chromium Concentrations: An In-Vivo Study. Am. J. Orthod. Dentofacial Orthopedics 140, 383–388. doi:10.1016/j.ajodo.2010.07.027
Nayak, R. S., Khanna, B., Pasha, A., Vinay, K., Narayan, A., and Chaitra, K. (2015). Evaluation of Nickel and Chromium Ion Release during Fixed Orthodontic Treatment Using Inductively Coupled Plasma-Mass Spectrometer: An In Vivo Study. J. Int. Oral Health 7, 14–20. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26464533%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC4588781.
Noumbissi, S., Scarano, A., and Gupta, S. (2019). A Literature Review Study on Atomic Ions Dissolution of Titanium and its Alloys in Implant Dentistry. Materials 12, 368. doi:10.3390/ma12030368
Ohkubo, C., Hosoi, T., Ford, J. P., and Watanabe, I. (2006). Effect of Surface Reaction Layer on Grindability of Cast Titanium Alloys. Dental Mater. 22, 268–274. doi:10.1016/j.dental.2005.04.020
Pan, Y., Jiang, L., Lin, H., and Cheng, H. (2017). Cell Death Affected by Dental Alloys: Modes and Mechanisms. Dent. Mater. J. 36, 82–87. doi:10.4012/dmj.2016-154
Pan, Y., Lin, Y., Jiang, L., Lin, H., Xu, C., Lin, D., et al. (2020). Removal of Dental Alloys and Titanium Attenuates Trace Metals and Biological Effects on Liver and Kidney. Chemosphere 243, 125205. doi:10.1016/j.chemosphere.2019.125205
Patel, N. (2014). Contemporary Dental CAD/CAM: Modern Chairside/lab Applications and the Future of Computerized Dentistry. Compend. Contin. Educ. Dent 35, 739–756.
Revathi, A., Borrás, A. D., Muñoz, A. I., Richard, C., and Manivasagam, G. (2017). Degradation Mechanisms and Future Challenges of Titanium and its Alloys for Dental Implant Applications in Oral Environment. Mater. Sci. Eng. C 76, 1354–1368. doi:10.1016/j.msec.2017.02.159
Rubio, J. C., Garcia-Alonso, M. C., Alonso, C., Alobera, M. A., Clemente, C., Munuera, L., et al. (2008). Determination of Metallic Traces in Kidneys, Livers, Lungs and Spleens of Rats with Metallic Implants after a Long Implantation Time. J. Mater. Sci. Mater. Med. 19, 369–375. doi:10.1007/s10856-007-3002-0
Siddharth, R., Gautam, R., Chand, P., Agrawal, K., Singh, R., and Singh, B. (2015). Quantitative Analysis of Leaching of Different Metals in Human Saliva from Dental Casting Alloys: An In Vivo Study. J. Indian Prosthodont. Soc. 15, 206–210. doi:10.4103/0972-4052.164906
Sulaiman, T. A. (2020). Materials in Digital Dentistry-A Review. J. Esthet. Restor. Dent. 32, 171–181. doi:10.1111/jerd.12566
Tan, F.-B., Song, J.-L., Wang, C., Fan, Y.-B., and Dai, H.-W. (2019). Titanium Clasp Fabricated by Selective Laser Melting, CNC Milling, and Conventional Casting: a Comparative In Vitro Study. J. Prosthodontic Res. 63, 58–65. doi:10.1016/j.jpor.2018.08.002
Tuna, S. H., Özçiçek Pekmez, N., and Kürkçüoğlu, I. (2015). Corrosion Resistance Assessment of Co-cr alloy Frameworks Fabricated by CAD/CAM Milling, Laser Sintering, and Casting Methods. The J. Prosthetic Dentistry 114, 725–734. doi:10.1016/j.prosdent.2015.02.031
Vaicelyte, A., Janssen, C., Le Borgne, M., and Grosgogeat, B. (2020). Cobalt-Chromium Dental Alloys: Metal Exposures, Toxicological Risks, CMR Classification, and EU Regulatory Framework. Crystals 10, 1151. doi:10.3390/cryst10121151
Wang, X., Xia, Y., Liu, L., Liu, M., Gu, N., Guang, H., et al. (2010). Comparison of Mtt Assay, Flow Cytometry, and Rt-Pcr in the Evaluation of Cytotoxicity of Five Prosthodontic Materials. J. Biomed. Mater. Res. 95B, 357–364. doi:10.1002/jbm.b.31723
Wu, M.-K., Song, N., Liu, F., Kou, L., Lu, X.-W., Wang, M., et al. (2016). Corrosion Behaviours of the Dental Magnetic Keeper Complexes Made by Different Alloys and Methods. Int. J. Oral Sci. 8, 155–163. doi:10.1038/ijos.2016.21
Xin, X.-Z., Chen, J., Xiang, N., Gong, Y., and Wei, B. (2014). Surface Characteristics and Corrosion Properties of Selective Laser Melted Co-cr Dental alloy after Porcelain Firing. Dental Mater. 30, 263–270. doi:10.1016/j.dental.2013.11.013
Keywords: computer-aided design, computer-aided manufacturing, dental alloys, titanium, metal, ions, apoptosis, autophagy
Citation: Lin Y, Chen J, Jiang F, Pan Y, Xu C, Lin D, Lin H, Jiang L, Zheng D, Qiu J and Cheng H (2021) Impacts of CAD/CAM Metallic Materials on Trace Metals and Biocompatibilities: An in vivo Study in Beagle Dogs. Front. Mater. 8:758873. doi: 10.3389/fmats.2021.758873
Received: 15 August 2021; Accepted: 30 September 2021;
Published: 18 October 2021.
Edited by:
Michael Danquah, University of Tennessee at Chattanooga, United StatesReviewed by:
Shokouh Attarilar, Shanghai Jiao Tong University, ChinaHeshu Sulaiman Rahman, University of Sulaimani, Iraq
Copyright © 2021 Lin, Chen, Jiang, Pan, Xu, Lin, Lin, Jiang, Zheng, Qiu and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Cheng, Y2hfZnVqaWFuQDE2My5jb20=
†These authors have contributed equally to this work
 Yunzhi Lin
Yunzhi Lin Jinbing Chen1†
Jinbing Chen1† Dali Zheng
Dali Zheng Jing Qiu
Jing Qiu Hui Cheng
Hui Cheng