- National Key Laboratory of Crop Biology, College of Life Sciences, Shandong Agricultural University, Taian, China
The male–female interactions in pollination mediate pollen hydration and germination, pollen tube growth and fertilization. Reactive oxygen species (ROS) derived from both male and female tissues play regulatory roles for the communication between the pollen/pollen tube and female tissues at various stages, such as pollen hydration and germination on the stigma, pollen tube growth in the pistil and pollen tube reception in the female gametophyte. In this minireview, we primarily summarize the recent progress on the roles of ROS signaling in male–female interactions during pollination and discuss several ROS-regulated downstream signaling pathways for these interactions. Furthermore, several ROS-involved downstream pathways are outlined, such as Ca2+ signaling, cell wall cytomechanics, the redox modification of CRP, and cell PCD. At the end, we address the roles of ROS in pollen tube guidance and fertilization as future questions that merit study.
Highlights
ROS as signal function in male–female interactions during pollination, including pollen hydration and germination on the stigma, pollen tube growth in the pistil and pollen tube reception in the female gametophyte.
Introduction
Pollination is a critical step for sexual plant reproduction. After landing on the stigma, the pollen undergoes adhesion and hydration before it germinates to create a pollen tube. The polar tip growth of the pollen tube guides it through the maternal tissues toward the female gametophyte. On arrival at the female gametophyte, the rupture of the pollen tube releases two sperms in a degenerated synergid cell for fertilization. The interactions between the pollen (pollen tube) and maternal tissues (stigma, style, ovule and female gametophyte) are critical for pollen hydration and germination, pollen tube growth in the pistil tissues, guidance to the female gametophyte, reception of the female gametophyte and sperm-egg cell fusion (Johnson et al., 2019; Lopes et al., 2019). Reactive oxygen species (ROS; e.g., O2•−, H2O2, OH•, 1O2) in cells that serve as signaling molecules are involved in various biological processes (Waszczak et al., 2018). ROS play roles in plant development, stress responses, and sexual plant reproduction, such as pollen development, pollen tube tip growth, embryo sac development and fertilization (Cárdenas et al., 2006; Liu et al., 2009; Martin et al., 2013; Lassig et al., 2014; Xie et al., 2014; Huang et al., 2019; Jiménez-Quesada et al., 2019). In contrast, under heat stress conditions, the increased ROS in pollen tubes inhibits tube growth, and flavonols control the pollen tube growth and integrity by regulating ROS homeostasis (Muhlemann et al., 2018). During pollination, ROS derived from both the pollen and female tissues are involved in their communications at various stages. In this review, we summarize the recent progress of the ROS signaling roles concentrating on the male–female interactions in pollination.
ROS Involved In Pollen Hydration and Germination on the Stigma
Pollen grains undergo adhesion and hydration after landing on the surface of the stigma and germinate to create pollen tubes. The pollen–stigma interaction is critical for pollen adhesion, hydration and germination, and many factors, such as proteins and lipids located on the surface of pollen, have been shown to be involved in this process (Hiscock and Allen, 2008; Dresselhaus and Franklin-Tong, 2013; Doucet et al., 2016). Arabidopsis KINβγ is a plant-specific subunit of the SNF1-related protein kinase 1 complex, which functions in the biogenesis of mitochondria and peroxisomes in pollen (Gao X.-Q. et al., 2016). In the null mutant of Arabidopsis KINβγ, the ROS levels of the pollen grains are reduced, and the pollen adhesion and hydration on the stigma surface is compromised. Additionally, the ROS signal might regulate the expression of the inward shaker K+ channel SPIK in pollen, which is important for pollen hydration and germination on the stigma and pollen tube growth in the pistil (Mouline et al., 2002; Li et al., 2017). Thus, the ROS signaling that originates from the interior of the pollen grains mediates the pollen–stigma interactions (Figure 1A).
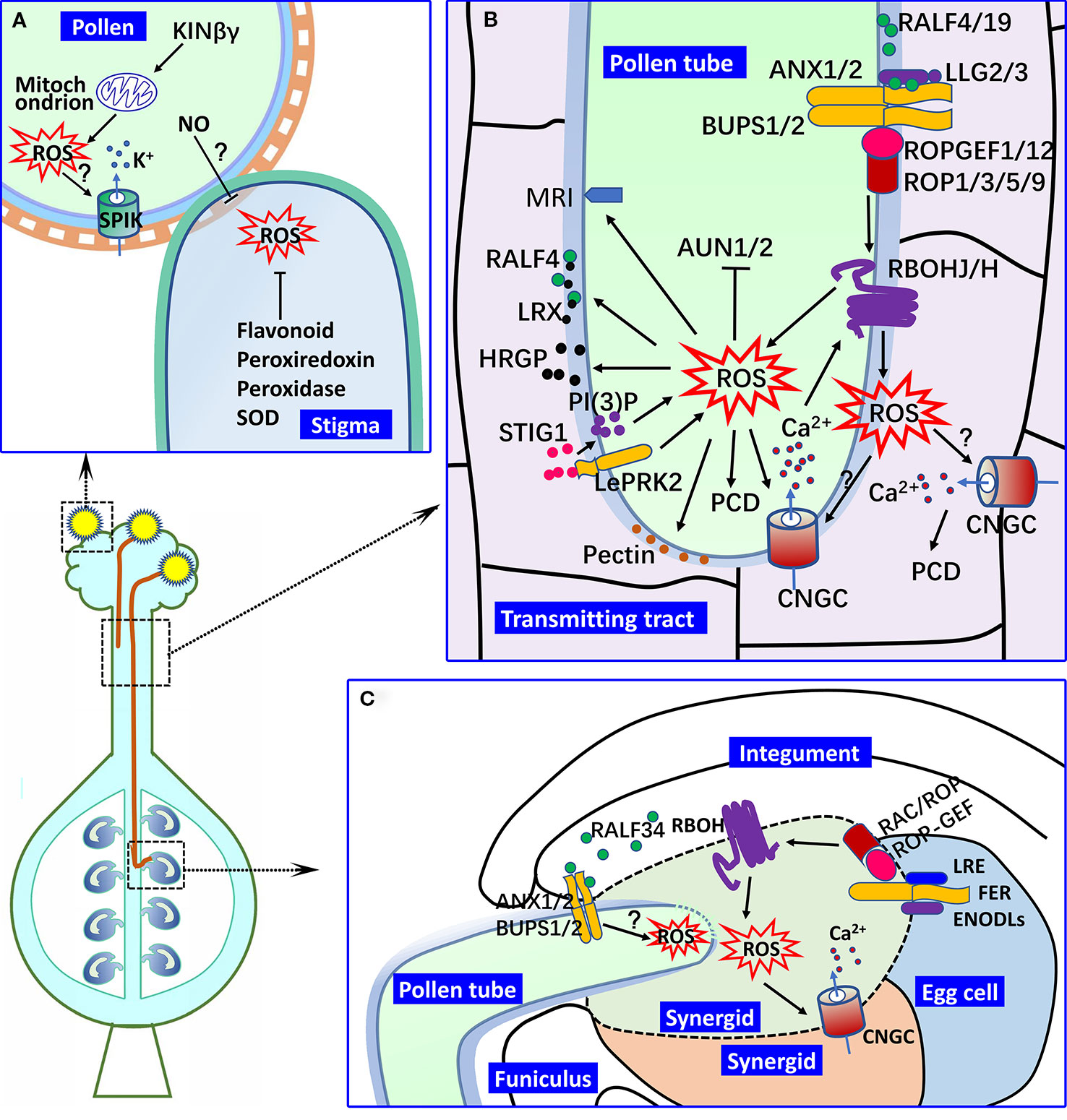
Figure 1 ROS in male–female interactions. (A) Pollen hydration and germination on the stigma. In Arabidopsis pollen, KINβγ mediates the biogenesis of mitochondrion and ROS levels, which regulate pollen hydration and germination on the stigma. In this process, the expression of SPIK might be regulated by ROS signaling, by which ROS signaling mediates K+ transport and pollen hydration on the stigma. ROS accumulation is found in receptive stigma, which is important for pollen attachment, but its decrease is required for the germination of compatible pollen. The ROS levels of the stigma are controlled by various oxidoreductase and flavonoids and could also be regulated by NO from the pollen. (B) Pollen tube growth in the pistil. NAD(P)H oxidase RBOHH/J-mediated apoplastic ROS production in the growing tip of the pollen tube is important for pollen tube integrity and growth in the pistil by regulating the activities of calcium channels (such as the CNGCs), the secretion of HRGPs (such as LRXs), and the metabolism of wall materials (such as pectin and callose). A type one protein phosphatase (AUN1/2) and plasma membrane-localized receptor-like cytoplasmic kinase MRI also function downstream of ROS signaling in pollen tube growth. The RALF-LLG-BUPS-ANX receptor–ligand interaction is involved in the active regulation of RBOHs and ROS generation, which is mediated by ROPGEFs and ROPs. Pistil-derived STIG1 induces the ROS production of the growing pollen tube in the transmitting tract mediated by LePRK2 and PI(3)P. Apoplastic ROS of the pollen tube might induce PCD of the transmitting tract by mediating CNGC activity and Ca2+ signaling. (C) Pollen tube reception in the female gametophyte. Pollen tube rupture in the synergid is controlled by ROS from RBOHs in the female gametophyte. LRE and ENODLs serve as the co-receptors of FER to regulate the activity of RBOHs and ROS generation in the synergid, and RAC/ROP might mediate this process. RALF34 primarily derived from the inner integument controls the pollen tube rupture in degenerated synergid by binding to the BUPS/ANX receptor complex in the pollen tube, during which ROS might act downstream of the BUPS/ANX receptor complex. (? indicates the putative regulation.).
The compromised adhesion and germination of the pollen grains on the non-stigma surfaces indicate that the stigma factors are important for the pollen–stigma interaction (Ma et al., 2012). ROS accumulation is found in the stigmas of various angiosperm species, including Magnolia (a primitive angiosperm) and Arabidopsis (McInnis et al., 2006; Zafra et al., 2016). Stigma receptivity is correlated with the activity of ROS-related enzymes, such as superoxide dismutase and peroxidase (McInnis et al., 2005; Sharma and Bhatla, 2013), indicating that ROS accumulation in the stigma is a self-regulated process. Recently, the ROS accumulation controlled by flavonoids and the ROS metabolic enzymes were identified in the stigma of ornamental kale (Brassica oleracea var. acephala), a self-incompatible (SI) species (Lan et al., 2017). The decreased ROS levels in the ornamental kale stigma after treatment with exogenous flavonoid (kaempferol) do not compromise the SI response of the stigma, but the attachment and germination of the compatible pollen is drastically reduced. In contrast, the adherence of pollen grains that trigger the decrease of ROS in the stigma and nitric oxide (NO) from the adhesive pollen as the inducing factor for ROS decrease have been suggested (Hiscock et al., 2007; Serrano et al., 2011; Sharma and Bhatla, 2013), which further supports the suggestion that regulation of the ROS in the stigma is involved in the signaling for pollen–stigma interactions (Hiscock and Allen, 2008; Zafra et al., 2016). Therefore, a possible scenario is that the levels of higher ROS in the mature stigma are favorable for the early stage of the pollen–stigma interaction, e.g., pollen adhesion and hydration, and the decrease in the ROS in the stigma after pollen landing might provide a surrounding for compatible pollen tube growth in the stigma tissue (Figure 1A).
ROS Regulate Pollen Tube Growth in the Pistil
The facilitation of the pollen tube growth in the pistil tissues by the apoplastic ROS has been well studied. Arabidopsis respiratory burst oxidase homologs (RBOHs) are plasma membrane-localized NAD(P)H oxidases, which are essential for pollen tube penetration into the transmitting tract of pistil by mediating apoplastic ROS production in the growing tip of the pollen tube (Kaya et al., 2014; Kaya et al., 2015). The pollen tube of the rbohh,j double mutant exhibits bursting in vitro and retarded growth in the pistil. In contrast, in the self-incompatible pollen tube, the increase in ROS levels triggers programmed cell death (PCD) and the self-incompatibility response (Serrano et al., 2015) (Figure 1B).
An Arabidopsis receptor complex was reported to control the maintenance of pollen tube integrity during its growth in the style, which is composed of pollen-specific CrRLK1L subfamily receptor-like kinases ANXUR1/2 (ANX1/2), Buddha's Paper Seal 1/2 (BUPS1/2) and LORELEI-like-GPI-anchored protein 2/3 (LLG2/3). This complex is localized in the apical membrane of the pollen tube and functions by perceiving the autocrine peptide ligands, rapid alkalinization factor 4/19 (RALF4/19) (Boisson-Dernier et al., 2009; Miyazaki et al., 2009; Ge et al., 2017; Mecchia et al., 2017; Feng et al., 2019; Ge et al., 2019b). The pollen tube of the ralf4,19 double mutant also displays precocious rupture in vitro and inhibited growth in the transmitting tract, which is similar to that of the llg2,3, bups1,2 and anx1,2 double mutants. However, RALF4 induced the production of ROS in the pollen tube that stimulates the pollen tube growth and inhibits the pollen burst in vitro (Feng et al., 2019). An Arabidopsis llg2,3 double mutant pollen tube exhibited reduced ROS levels and burst after germination in vitro, and the application of exogenous H2O2 rescued the rupture of the pollen tube (Feng et al., 2019). As suggested (Boisson-Dernier et al., 2013), the LLG-BUPS-ANX receptor complex functions upstream of RBOHH/J and regulates their activities to coordinate ROS production and Ca2+ homeostasis in regulating the pollen tube growth in the pistil. In this process, the activities of RBOHs are mediated by RopGEF-ROP downstream of ANX1/2 (Zhu et al., 2018; Feng et al., 2019) (Figure 1B).
The NADPH oxidases RBOHs that serve as the primary sources of apoplastic/cytoplasmic ROS in the pollen tube are widely studied in male–female interactions. However, little is known about the roles of ROS from other sources, such as the mitochondrion, peroxisome, and various oxidases. In addition hypoxia induces ROS production and RBOHH expression in plants (Pucciariello and Perata, 2017; Yamauchi et al., 2017). The transmitting tract provides a hypoxic surrounding for the pollen tube growth within it, which results from the restricted oxygen diffusion and active carbohydrate metabolism in the growing pollen tube (Goetz et al., 2017). We found evidence for this in the fact that the expression of ethanol degradation-related genes has changed in the pollinated stigma and style (Xu et al., 2013; Yue et al., 2014). Considering the availability of oxygen in the pollen tube, the ROS metabolism of the pollen tube growing in the transmitting tract might be different from that of the growing tubes in vitro.
ROS Are Required for Pollen Tube Reception in the Female Gametophyte
The pollen tube grows into the micropyle and ruptures in a degenerated synergid to release two sperm cells that are ready for fertilization, which is under the control of the interaction between the pollen tube and synergid (Leydon et al., 2015). The pollination induces a ROS burst inside the embryo sac (Martin et al., 2013). It has been proven that the pollen tube rupture in the synergid is controlled by ROS from NADPH oxidases in the female gametophyte (Duan et al., 2014). FERONIA (FER), a universally expressed CrRLK1L family member, mediates the pollen tube rupture by inducing ROS generation at the entrance of the female gametophyte. Glycosylphosphatidylinositol-anchored protein LORELEI (LRE) and early nodulin-like protein functions (ENODLs) might be the co-receptors for FER signaling, which is also involved in ROS generation in the synergid (Duan et al., 2014; Li C. et al., 2015; Hou et al., 2016; Zhong and Qu, 2019). In the lre mutant, the ovule showed reduced levels of ROS, and the pollen tube revealed an overgrowth phenotype in the mutant ovule (Duan et al., 2014). However, the ectopic expression of LRE in the pollen tube could rescue the pollen tube rupture in the ovule of lre mutant (Liu et al., 2016). Thus, the LRE-FER signaling that was recovered could induce an instantaneous burst of ROS in the synergid cells that is adequate for pollen tube reception. The interactions between RAC/ROPs and FER and LRE indicate that RAC/ROPs mediate the activation of NADPH oxidase for ROS generation (Duan et al., 2010; Duan et al., 2014). Therefore, a FER-RAC/ROP-NADPH oxidase-ROS signaling pathway exists in the interactions between the pollen tube and female gametophyte (Li C. et al., 2015; Nissen et al., 2016). In addition, an ovule-expressed RALF peptide, RALF34, induces pollen tube rupture in vitro. RALF34 binds both BUPS1/2 and ANX1/2 in vitro, indicating that RALF34 may play its roles via the BUPS/ANX receptor complex (Ge et al., 2017). RBOHH- and RBOHJ-mediated ROS function downstream of the BUPS/ANX receptor complex that regulates pollen tube growth in the pistil (Boisson-Dernier et al., 2013). Thus, ROS may be involved in the RALF34-BUPS/ANX receptor complex signaling in the pollen tube rupture in the synergid cells (Figure 1C).
ROS Trigger Downstream Responses
RBOH-derived ROS that mediate pollen tube integrity are required for either pollen tube growth in the pistil or pollen tube reception in the female gametophyte. Ca2+ and Ca2+-mediated protein phosphorylation functions in the activation of RBOHH and RBOHJ in pollen tube growth (Kaya et al., 2014). It has been suggested that Ca2+ binding triggers the production of ROS, which can also act on Ca2+ channels (Wudick and Feijó, 2014). However, the ROS-activated Ca2+ channels in the pollen tube are elusive. The cyclic nucleotide gated channel (CNGC) family functions as Ca2+ channels in pollen tube growth and guidance (Tunc-Ozdemir et al., 2013; Gao Q.-F. et al., 2016). The pollen tube of a cngc7,8 double mutant shows a similar phenotype with that of the rbohj,h double mutant: bursting in vitro and sterility (Tunc-Ozdemir et al., 2013; Lassig et al., 2014). Thus, the activity of the CNGCs might be regulated by ROS in pollen tube growth, although experiments are required to test this hypothesis. The ROS-induced opening of the Ca2+ channels is required for pollen tube reception (Duan et al., 2014; Wudick and Feijó, 2014). The auto-inhibited Ca2+ ATPase 9 and CNGCs might be the downstream targets of ROS in pollen tube rupture (Schiøtt et al., 2004; Tunc-Ozdemir et al., 2013; Gao Q.-F. et al., 2016). ATUNIS1/2 (AUN1/2), a type one protein phosphatase, was identified to act downstream of ANX and RBOHH/J, and its activity is inhibited by ROS, which enables AUN1/2 to play its role as a negative regulator in pollen tube integrity (Franck et al., 2018). MARIS (MRI) is a plasma membrane-localized receptor-like cytoplasmic kinase that acts downstream of ROS and is mediated by both the ANXs and RBOHH/J in controlling pollen tube integrity and growth in the pistil (Boisson-Dernier et al., 2015; Liao et al., 2016). However, the manner in which AUN1/2 and MRI mediate the ROS signaling in pollen tube growth remains unknown (Figures 1B, C).
The regulation of pollen wall cytomechanics by ROS is suggested, e.g., •OH is involved in the loosening of the pollen intine in the germination pore region that might facilitate the enlargement of the pollen volume during hydration (Smirnova et al., 2014). However, little is known about how ROS regulate the wall cytomechanics of the growing pollen tube. ROS upregulation of pectin synthesis, PME activity and pectin demethylesterification in the root and other tissues was reported (Messenger et al., 2009; Xiong et al., 2015). The pollen tube wall is enriched in pectins, and pectin methylesterase activity is critical for pollen tube integrity and its growth in the transmitting tract (Jiang et al., 2005). RALF4 not only induces ROS production in the pollen tube but alters the composition of the pollen tube wall, such as callose and pectin, which are correlated with the pollen tube integrity and growth (Mecchia et al., 2017; Feng et al., 2019). A llg2,3 double mutant pollen tube showed reduced ROS levels and altered pectin and callose deposition at the tip wall of the pollen tube (Feng et al., 2019). Thus, ROS might be implicated in pollen tube integrity by regulating the metabolism of wall materials, such as pectin and callose (Figure 1B). Before the arrival of pollen tube at the synergid, the micropylar end of the synegid accumulates ROS that is controlled by FER (Duan et al., 2014; Li C. et al., 2015). The ROS in synergids might be involved in the development of a filiform apparatus, as suggested in phloem in which a ROS signal induces the formation of wall ingrowths in the transfer cells (Andriunas et al., 2013). After the arrival of the pollen tube, the high level of ROS at the micropylar end of the synegid might function in the regulation of pollen tube integrity by its implication in the metabolism of wall materials.
To facilitate the penetration of the pollen tube into the pistil tissues, cell wall modification and softening and cell separation in pistil tissues is required (Marsollier and Ingram, 2018). Hydroxyproline-rich glycoproteins (HRGPs), such as leucine-rich repeat extensins (LRXs), are localized at the pollen tube surface and in the intercellular matrix. It has been suggested that these proteins function to separate the cell walls of pistil tissues, by serving as lubricating functions for pollen tube growth in the pistil (Marsollier and Ingram, 2018; Sede et al., 2018). Stigma-specific protein 1 (STIG1), a cysteine-rich protein expressed in pistil tissues in tobacco and petunia, promotes pollen tube growth (Verhoeven et al., 2005; Huang et al., 2014). STIG1 controls the secretion of the HRGP-rich extracellular matrix and the ROS production of the pollen tube in both PI(3)P-dependent and LePRK2-dependent manners. There might be a linkage between the pistil factor-induced ROS elevation in the pollen tube and the HRGP secretion-facilitated pollen tube growth in the pistil tissues. Arabidopsis GRIM REAPER (GRI) is a secreted protein that is similar to STIG1. GRI promotes the superoxide production that triggers cell death (Wrzaczek et al., 2015). Thus, STIG1-promoted pollen tube growth in the pistil tissues might function via mediating ROS-induced PCD of the transmitting tract, as in the rice style (Xu et al., 2017). Rice OsCNGC13, a pistil-preferentially expressed CNGC member, plays roles in Ca2+ signal-inducing pistil PCD, which facilitates the penetration of the pollen tube in the pistil (Xu et al., 2017). Considering that pollination is an inducer for the PCD of transmitting tissue (Wang et al., 1996; Xu et al., 2017), it is tempting to study whether the apoplastic ROS of the pollen tube growing in transmitting tissue could diffuse into the pistil tissue to trigger the OsCNGC13 activity and pistil PCD in rice (Figure 1B).
Redox regulation for thiol/disulfide-containing proteins is involved in sexual plant reproduction (Traverso et al., 2013). ROS that act as signaling molecules function in the oxidation of a critical cysteine thiol group within redox-sensitive proteins (Reczek and Chandel, 2015; Sevilla et al., 2015). Cysteine-rich peptides (CRPs) expressed in either male or female reproductive tissues and cysteine-rich proteins as receptor complex subunits, such as LRE family members (LRE and LLG1-3) (Liu et al., 2016; Feng et al., 2019), are involved in male–female interactions, as was recently reviewed (Zhong and Qu, 2019). The modified eight-cysteine motif in the LRE is required for pollen tube reception (Liu et al., 2016). Recently, the N-terminus of the RALF23 peptides was identified to be involved in the binding to LLGs to assemble the LLG-FRE receptor complex to regulate immune signaling (Xiao et al., 2019), but the functions of the C-terminal region of RALF23 peptides with the conserved cysteine residues are unknown (Ge et al., 2019a; Ge et al., 2019b). Whether ROS functions in the redox modification and activity regulation of the cysteine-rich peptides and proteins in male–female interactions merits further investigation.
Are ROS Involved in Pollen Tube Guidance Growth and Fertlization?
There are less data about the roles of ROS in pollen tube guidance growth and fertilization. CRPs secreted from the female gametophyte as a signal are required for pollen tube guidance (Takeuchi and Higashiyama, 2012; Li H.-J. et al., 2015; Meng et al., 2019; Zhong et al., 2019). Arabidopsis pollen-expressed GPI-AP COBRA-LIKE 10 (COBL10) and its modification play roles in the guidance of pollen tube growth (Li et al., 2013; Cheung et al., 2014; Dai et al., 2014). The Arabidopsis COBRA-LIKE protein family harbors at least 12 conserved cysteine residues, and several intramolecular disulfide bonds in COBL10 were predicted (Supplemental Data 1). Whether ROS is involved in the thiol-based redox modification of these CRP proteins and these modifications function in pollen tube guidance growth await further study. In addition, small cysteine-rich EGG CELL 1 proteins secreted from the egg cell in Arabidopsis are necessary for sperm cell activation in male–female gamete interactions for fertilization (Sprunck et al., 2012; Cyprys et al., 2019). As mentioned previously, the cysteine residues in CRPs are the potential targets of ROS signaling; thus, the implication of ROS in pollen tube guidance and fertilization is expected by mediating the redox modification of the CRPs. Higher ROS levels in the central cell of the female gametophyte before fertilization has been reported in Arabidopsis (Martin et al., 2013). Cytosolic ascorbate peroxidase (cAPX) is a central component in the metabolism of ROS. Abundant cAPXs in rice egg cells were identified, indicating that ROS scavenging is required for fertilization (Uchiumi et al., 2007). Thus, an open question is how the ROS signaling in the gametes (egg and central cell) is implicated in male–female gamete recognition in fertilization.
Future Directions
In recent years, ROS that function as critical signal molecules have resulted in significant advances in various stages of pollination, including the interactions between the pollen and stigma, pollen tube and transmitting tract, pollen tube and female gametophyte. However, there are still many gaps in understanding the ROS action as a signaling molecule in male–female interactions. For example, it remains to be studied whether ROS are involved in pollen tube guidance growth and fertilization. In most of the previous studies, ROS burst in pollen tube and embryo sac is generated by plasma membrane-localized NADPH oxidases. In fact, other subcellular compartments generate ROS in plant cells, such as cytosol, chloroplasts, mitochondria, and peroxisomes (Mignolet-Spruyt et al., 2016). However, the roles of these ROS sources in the male–female interactions during pollination are less known now. ROS homeostasis is under the control of diverse antioxidant system, such as thioredoxin and glutathione (Zhang et al., 2018). The mutations of Arabidopsis NADPH-dependent thioredoxin reductase A and glutathione reductase 1 disturb the transmission of male gametophyte, although the pollen development is normal (Marty et al., 2009). These indicate that the ROS homeostasis in pollen tube governed by thioredoxin and glutathione is critical for pollen tube growth in pistil or fertilization. Thus, the regulation of ROS homeostasis in male–female interactions can be expected.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This work is supported by National Natural Science Foundation of China (31770349), Youth Program of National Natural Science Foundation of China (31800267), and Major Research Plan from the Ministry of Science and Technology of China (2013CB945100).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2020.00177/full#supplementary-material
References
Andriunas, F., Zhang, H. M., Xia, X., Patrick, J. W., Offler, C. E. (2013). Intersection of transfer cells with phloem biology-broad evolutionary trends, function, and induction. Front. Plant Sci. 4, 221. doi: 10.3389/fpls.2013.00221
Boisson-Dernier, A., Roy, S., Kritsas, K., Grobei, M. A., Jaciubek, M., Schroeder, J. I., et al. (2009). Disruption of the pollen-expressed FERONIA homologs ANXUR1 and ANXUR2 triggers pollen tube discharge. Development 136, 3279–3288. doi: 10.1242/dev.040071
Boisson-Dernier, A., Lituiev, D. S., Nestorova, A., Franck, C. M., Thirugnanarajah, S., Grossniklaus, U. (2013). ANXUR receptor-like kinases coordinate cell wall integrity with growth at the pollen tube tip via NADPH oxidases. PloS Biol. 11, e1001719. doi: 10.1371/journal.pbio.1001719
Boisson-Dernier, A., Franck, C. M., Lituiev, D. S., Grossniklaus, U. (2015). Receptor-like cytoplasmic kinase MARIS functions downstream of CrRLK1L-dependent signaling during tip growth. Proc. Natl. Acad. Sci. U.S.A. 112, 12211–12216. doi: 10.1073/pnas.1512375112
Cárdenas, L., McKenna, S. T., Kunkel, J. G., Hepler, P. K. (2006). NAD (P) H oscillates in pollen tubes and is correlated with tip growth. Plant Physiol. 142, 1460–1468. doi: 10.1104/pp.106.087882
Cheung, A. Y., Li, C., Zou, Y. J., Wu, H. M. (2014). Glycosylphosphatidylinositol anchoring: control through modification. Plant Physiol. 166, 748–750. doi: 10.1104/pp.114.246926
Cyprys, P., Lindemeier, M., Sprunck, S. (2019). Gamete fusion is facilitated by two sperm cell-expressed DUF679 membrane proteins. Nat. Plants 5, 253–257. doi: 10.1038/s41477-019-0382-3
Dai, X. R., Gao, X. Q., Chen, G. H., Tang, L. L., Wang, H., Zhang, X. S. (2014). ABNORMAL POLLEN TUBE GUIDANCE1, an endoplasmic reticulum-localized mannosyltransferase homolog of GLYCOSYLPHOSPHATIDYLINOSITOL10 in yeast and PHOSPHATIDYLINOSITOL GLYCAN ANCHOR BIOSYNTHESIS B in human, is required for Arabidopsis pollen tube micropylar guidance and embryo development. Plant Physiol. 165, 1544–1556. doi: 10.1104/pp.114.236133
Doucet, J., Lee, H. K., Goring, D. R. (2016). Pollen acceptance or rejection, a tale of two pathways. Trends Plant Sci. 21, 1058–1067. doi: 10.1016/j.tplants.2016.09.004
Dresselhaus, T., Franklin-Tong, N. (2013). Male–female crosstalk during pollen germination, tube growth and guidance, and double fertilization. Mol. Plant 6, 1018–1036. doi: 10.1093/mp/sst061
Duan, Q., Kita, D., Li, C., Cheung, A. Y., Wu, H.-M. (2010). FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development. Proc. Natl. Acad. Sci. U.S.A. 107, 17821–17826. doi: 10.1073/pnas.1005366107
Duan, Q., Kita, D., Johnson, E. A., Aggarwal, M., Gates, L., Wu, H.-M., et al. (2014). Reactive oxygen species mediate pollen tube rupture to release sperm for fertilization in. Arabidopsis Nat. Commun. 5, 3129. doi: 10.1038/ncomms4129
Feng, H., Liu, C., Fu, R., Zhang, M., Li, H., Shen, L., et al. (2019). LORELEI-LIKE GPI-ANCHORED PROTEINS 2/3 regulate pollen tube growth as chaperones and coreceptors for ANXUR/BUPS receptor kinases in Arabidopsis. Mol. Plant 12, 1612–1623. doi: 10.1016/j.molp.2019.09.004
Franck, C. M., Westermann, J., Bürssner, S., Lentz, R., Lituiev, D. S., Boisson-Dernier, A. (2018). The protein phosphatases ATUNIS1 and ATUNIS2 regulate cell wall integrity in tip-growing cells. Plant Cell 30, 1906–1923. doi: 10.1105/tpc.18.00284
Gao, Q.-F., Gu, L.-L., Wang, H.-Q., Fei, C.-F., Fang, X., Hussain, J., et al. (2016). Cyclic nucleotide-gated channel 18 is an essential Ca2+ channel in pollen tube tips for pollen tube guidance to ovules in. Arabidopsis Proc. Natl. Acad. Sci. U.S.A. 113, 3096–3101. doi: 10.1073/pnas.1524629113
Gao, X.-Q., Liu, C. Z., Li, D. D., Zhao, T. T., Li, F., Jia, X. N., et al. (2016). The Arabidopsis KINβγ subunit of the SnRK1 complex regulates pollen hydration on the stigma by mediating the level of reactive oxygen species in pollen. PloS Genet. 12, e1006228. doi: 10.1371/journal.pgen
Ge, Z., Bergonci, T., Zhao, Y., Zou, Y., Du, S., Liu, M.-C., et al. (2017). Arabidopsis pollen tube integrity and sperm release are regulated by RALF-mediated signaling. Science 358, 1596–1600. doi: 10.1126/science.aao3642
Ge, Z., Dresselhaus, T., Qu, L.-J. (2019a). How CrRLK1L receptor complexes perceive RALF signals. Trends Plant Sci. 24, 978–981. doi: 10.1016/j.tplants.2019.09.002
Ge, Z., Zhao, Y., Liu, M.-C., Zhou, L.-Z., Wang, L., Zhong, S., et al. (2019b). LLG2/3 Are Co-receptors in BUPS/ANX-RALF Signaling to Regulate Arabidopsis Pollen Tube Integrity. Curr. Biol. 29, 3256–3265.e3255. doi: 10.1016/j.cub.2019.08.032
Goetz, M., Guivarćh, A., Hirsche, J., Bauerfeind, M. A., González, M.-C., Hyun, T. K., et al. (2017). Metabolic control of tobacco pollination by sugars and invertases. Plant Physiol. 173, 984–997. doi: 10.1104/pp.16.01601
Hiscock, S. J., Allen, A. M. (2008). Diverse cell signalling pathways regulate pollen-stigma interactions, the search for consensus. New Phytol. 179, 286–317. doi: 10.1111/j.1469-8137.2008.02457.x
Hiscock, S., Bright, J., McInnis, S. M., Desikan, R., Hancock, J. T. (2007). Signaling on the stigma, potential new roles for ROS and NO in plant cell signaling. Plant Signal. Behav. 2, 23–24. doi: 10.4161/psb.2.1.3644
Hou, Y., Guo, X., Cyprys, P., Zhang, Y., Bleckmann, A., Cai, L., et al. (2016). Maternal ENODLs are required for pollen tube reception in. Arabidopsis Curr. Biol. 26, 2343–2350. doi: 10.1016/j.cub.2016.06.053
Huang, W.-J., Liu, H.-K., McCormick, S., Tang, W.-H. (2014). Tomato pistil factor STIG1 promotes in vivo pollen tube growth by binding to phosphatidylinositol 3-phosphate and the extracellular domain of the pollen receptor kinase LePRK2. Plant Cell 26, 2505–2523. doi: 10.1105/tpc.114.123281
Huang, H., Ullah, F., Zhou, D. X., Yi, M., Zhao, Y. (2019). Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 10, 800. doi: 10.3389/fpls.2019.00800
Jiang, L., Yang, S. L., Xie, L. F., Puah, C. S., Zhang, X. Q., Yang, W. C., et al. (2005). VANGUARD1 encodes a pectin methylesterase that enhances pollen tube growth in the Arabidopsis style and transmitting tract. Plant Cell 17, 584–596. doi: 10.1105/tpc.104.027631
Jiménez-Quesada, M. J., Traverso, J. A., Potocký, M., Zarsky, V., Alché, J. D. D. (2019). Generation of superoxide by OeRbohH, a NADPH oxidase activity during olive (Olea europaea L.) pollen development and germination. Front. Plant Sci. 10, 1149. doi: 10.3389/fpls.2019.01149
Johnson, M. A., Harper, J. F., Palanivelu, R. (2019). A fruitful journey, Pollen tube navigation from germination to fertilization. Annu. Rev. Plant Biol. 70, 809–837. doi: 10.1146/annurev-arplant-050718-100133
Kaya, H., Nakajima, R., Iwano, M., Kanaoka, M. M., Kimura, S., Takeda, S., et al. (2014). Ca2+-activated reactive oxygen species production by Arabidopsis RbohH and RbohJ is essential for proper pollen tube tip growth. Plant Cell 26, 1069–1080. doi: 10.1105/tpc.113.120642
Kaya, H., Iwano, M., Takeda, S., Kanaoka, M. M., Kimura, S., Abe, M., et al. (2015). Apoplastic ROS production upon pollination by RbohH and RbohJ in. Arabidopsis. Plant Signal. Behav. 10, e989050. doi: 10.4161/15592324.2014.989050
Lan, X., Yang, J., Abhinandan, K., Nie, Y., Li, X., Li, Y., et al. (2017). Flavonoids and ROS play opposing roles in mediating pollination in ornamental kale (Brassica oleracea var. acephala). Mol. Plant 10, 1361–1364. doi: 10.1016/j.molp.2017.08.002
Lassig, R., Gutermuth, T., Bey, T. D., Konrad, K. R., Romeis, T. (2014). Pollen tube NAD(P)H oxidases act as a speed control to dampen growth rate oscillations during polarized cell growth. Plant J. 78, 94–106. doi: 10.1111/tpj.12452
Leydon, A. R., Tsukamoto, T., Dunatunga, D., Qin, Y., Johnson, M. A., Palanivelu, R. (2015). Pollen tube discharge completes the process of synergid degeneration that is initiated by pollen tube-synergid interaction in. Arabidopsis Plant Physiol. 169, 485–496. doi: 10.1104/pp.15.00528
Li, S., Ge, F. R., Xu, M., Zhao, X. Y., Huang, G. Q., Zhou, L. Z., et al. (2013). Arabidopsis COBRA-LIKE 10, a GPI-anchored protein, mediates directional growth of pollen tubes. Plant J. 74, 486–497. doi: 10.1111/tpj.12139
Li, C., Yeh, F.-L., Cheung, A. Y., Duan, Q., Kita, D., Liu, M.-C., et al. (2015). Glycosylphosphatidylinositol-anchored proteins as chaperones and co-receptors for FERONIA receptor kinase signaling in Arabidopsis eLife 4, e06587. doi: 10.7554/eLife.06587
Li, H. J., Zhu, S. S., Zhang, M. X., Wang, T., Liang, L., Xue, Y., et al. (2015). Arabidopsis CBP1 is a novel regulator of transcription initiation in central cell-mediated pollen tube guidance. Plant Cell 27, 2880–2893. doi: 10.1105/tpc.15.00370
Li, D. D., Guan, H., Li, F., Liu, C. Z., Dong, Y. X., Zhang, X. S., et al. (2017). Arabidopsis shaker pollen inward K+ channel SPIK functions in SnRK1 complex-regulated pollen hydration on the stigma. J. Integr. Plant Biol. 59, 604–611. doi: 10.1111/jipb.12563
Liao, H.-Z., Zhu, M.-M., Cui, H.-H., Du, X.-Y., Tang, Y., Chen, L.-Q., et al. (2016). MARIS plays important roles in Arabidopsis pollen tube and root hair growth. J. Integr. Plant Biol. 58, 927–940. doi: 10.1111/jipb.12484
Liu, P., Li, R. L., Zhang, L., Wang, Q. L., Niehaus, K., Baluška, F., et al. (2009). Lipid microdomain polarization is required for NADPH oxidase-dependent ROS signaling in Picea meyeri pollen tube tip growth. Plant J. 60, 303–313. doi: 10.1111/j.1365-313X.2009.03955.x
Liu, X., Castro, C., Wang, Y., Noble, J., Ponvert, N., Bundy, M., et al. (2016). The role of LORELEI in pollen tube reception at the interface of the synergid cell and pollen tube requires the modified eight-cysteine motif and the receptor-like kinase FERONIA. Plant Cell 28, 1035–1052. doi: 10.1105/tpc.15.00703
Lopes, A. L., Moreira, D., Ferreira, M. J., Pereira, A. M., Coimbra, S. (2019). Insights into secrets along the pollen tube pathway in need to be discovered. J. Exp. Bot. 70, 2979–2992. doi: 10.1093/jxb/erz087
Ma, J.-F., Liu, Z.-H., Chu, C.-P., Hu, Z.-Y., Wang, X.-L., Zhang, X. S. (2012). Different regulatory processes control pollen hydration and germination in. Arabidopsis. Sex Plant Reprod. 25, 77–82. doi: 10.1007/s00497-011-0173-0
Marsollier, A.-C., Ingram, G. (2018). Getting physical, invasive growth events during plant development. Curr. Opin. Plant Biol. 46, 8–17. doi: 10.1016/j.pbi.2018.06.002
Martin, M. V., Fiol, D. F., Sundaresan, V., Zabaleta, E. J., Pagnussat, G. C. (2013). oiwa, a female gametophytic mutant impaired in a mitochondrial manganese-superoxide dismutase, reveals crucial roles for reactive oxygen species during embryo sac development and fertilization in. Arabidopsis. Plant Cell 25, 1573–1591. doi: 10.1105/tpc.113.109306
Marty, L., Siala, W., Schwarzländer, M., Fricker, M. D., Wirtz, M., Sweetlove, L. J., et al. (2009). The NADPH-dependent thioredoxin system constitutes a functional backup for cytosolic glutathione reductase in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 106, 9109–9114. doi: 10.1073/pnas.0900206106
McInnis, S. M., Costa, L. M., Gutiérrez-Marcos, J. F., Henderson, C. A., Hiscock, S. J. (2005). Isolation and characterization of a polymorphic stigma-specific class III peroxidase gene from Senecio squalidus L. (Asteraceae). Plant Mol. Biol. 57, 659–677. doi: 10.1007/s11103-005-1426-9
McInnis, S. M., Desikan, R., Hancock, J. T., Hiscock, S. J. (2006). Production of reactive oxygen species and reactive nitrogen species by angiosperm stigmas and pollen, potential signalling crosstalk? New Phytol. 172, 221–228. doi: 10.1111/j.1469-8137.2006.01875.x
Mecchia, M. A., Santos-Fernandez, G., Duss, N. N., Somoza, S. C., Boisson-Dernier, A., Gagliardini, V., et al. (2017). RALF4/19 peptides interact with LRX proteins to control pollen tube growth in Arabidopsis. Sci. 358, 1600–1603. doi: 10.1126/science.aao5467
Meng, J. G., Zhang, M. X., Yang, W. C., Li, H. J. (2019). TICKET attracts pollen tubes and mediates reproductive isolation between relative species in Brassicaceae. Sci. China Life Sci. 62, 1413–1419. doi: 10.1007/s11427-019-9833-3
Messenger, D. J., McLeod, A. R., Fry, S. C. (2009). The role of ultraviolet radiation, photosensitizers, reactive oxygen species and ester groups in mechanisms of methane formation from pectin. Plant Cell Environ. 32, 1–9. doi: 10.1111/j.1365-3040.2008.01892.x
Mignolet-Spruyt, L., Xu, E., Idänheimo, N., Hoeberichts, F. A., Mühlenbock, P., Brosché, M., et al. (2016). Spreading the news: Subcellular and organellar reactive oxygen species production and signalling. J. Exp. Bot. 67, 3831–3844. doi: 10.1093/jxb/erw080
Miyazaki, S., Murata, T., Sakurai-Ozato, N., Kubo, M., Demura, T., Fukuda, H., et al. (2009). ANXUR1 and 2, sister genes to FERONIA/SIRENE, are male factors for coordinated fertilization. Curr. Biol. 19, 1327–1331. doi: 10.1016/j.cub.2009.06.064
Mouline, K., Véry, A.-A., Gaymard, F., Boucherez, J., Pilot, G., Devic, M., et al. (2002). Pollen tube development and competitive ability are impaired by disruption of a shaker K+ channel in Arabidopsis. Genes Dev. 16, 339–350. doi: 10.1101/gad.213902
Muhlemann, J. K., Younts, T. L. B., Muday, G. K. (2018). Flavonols control pollen tube growth and integrity by regulating ROS homeostasis during high-temperature stress. Proc. Natl. Acad. Sci. U.S.A. 115, E11188–E11197. doi: 10.1073/pnas.1811492115
Nissen, K. S., Willats, W. G. T., Malinovsky, F. G. (2016). Understanding CrRLK1L function, cell walls and growth control. Trends Plant Sci. 21, 516–527. doi: 10.1016/j.tplants.2015.12.004
Pucciariello, C., Perata, P. (2017). New insights into reactive oxygen species and nitric oxide signalling under low oxygen in plants. Plant Cell Environ. 40, 473–482. doi: 10.1111/pce.12715
Reczek, C. R., Chandel, N. S. (2015). ROS-dependent signal transduction. Curr. Opin. Cell Biol. 33, 8–13. doi: 10.1016/j.ceb.2014.09.010
Schiøtt, M., Romanowsky, S. M., Bækgaard, L., Jakobsen, M. K., Palmgren, M. G., Harper, J. F. (2004). A plant plasma membrane Ca2+ pump is required for normal pollen tube growth and fertilization. Proc. Natl. Acad. Sci. U.S.A. 101, 9502–9507. doi: 10.1073/pnas.0401542101
Sede, A. R., Borassi, C., Wengier, D. L., Mecchia, M. A., Estevez, J. M., Muschietti, J. P. (2018). Arabidopsis pollen extensins LRX are required for cell wall integrity during pollen tube growth. FEBS Lett. 592, 233–243. doi: 10.1002/1873-3468
Serrano, I., Romero-Puertas, M. C., Rodríguez-Serrano, M., Sandalio, L. M., Olmedilla, A. (2011). Peroxynitrite mediates programmed cell death both in papillar cells and in self-incompatible pollen in the olive (Olea europaea L.). J. Exp. Bot. 63, 1479–1493. doi: 10.1093/jxb/err392
Serrano, I., Romero-Puertas, M. C., Sandalio, L. M., Olmedilla, A. (2015). The role of reactive oxygen species and nitric oxide in programmed cell death associated with self-incompatibility. J. Exp. Bot. 66, 2869–2876. doi: 10.1093/jxb/erv083
Sevilla, F., Camejo, D., Ortiz-Espín, A., Calderón, A., Lázaro, J., Jiménez, A. (2015). The thioredoxin/peroxiredoxin/sulfiredoxin system, current overview on its redox function in plants and regulation by reactive oxygen and nitrogen species. J. Exp. Bot. 66, 2945–2955. doi: 10.1093/jxb/erv146
Sharma, B., Bhatla, S. (2013). Accumulation and scavenging of reactive oxygen species and nitric oxide correlate with stigma maturation and pollen–stigma interaction in sunflower. Acta Physiol. Plant 35, 2777–2787. doi: 10.1007/s11738-013-1310-1
Smirnova, A., Matveyeva, N., Yermakov, I. (2014). Reactive oxygen species are involved in regulation of pollen wall cytomechanics. Plant Biol. 16, 252–257. doi: 10.1111/plb.12004
Sprunck, S., Rademacher, S., Vogler, F., Gheyselinck, J., Grossniklaus, U., Dresselhaus, T. (2012). Egg cell-secreted EC1 triggers sperm cell activation during double fertilization. Science 338, 1093–1097. doi: 10.1126/science.1223944
Takeuchi, H., Higashiyama, T. (2012). A species-specific cluster of defensin-like genes encodes diffusible pollen tube attractants in Arabidopsis. PloS Biol. 10, e1001449. doi: 10.1371/journal.pbio.1001449
Traverso, J. A., Pulido, A., Rodriguez-Garcia, M. I., Alche, J. D. (2013). Thiol-based redox regulation in sexual plant reproduction, new insights and perspectives. Front. Plant Sci. 4, 465. doi: 10.3389/fpls.2013.00465
Tunc-Ozdemir, M., Rato, C., Brown, E., Rogers, S., Mooneyham, A., Frietsch, S., et al. (2013). Cyclic nucleotide gated channels 7 and 8 are essential for male reproductive fertility. PloS One 8, e55277. doi: 10.1371/journal.pone.0055277
Uchiumi, T., Shinkawa, T., Isobe, T., Okamoto, T. (2007). Identification of the major protein components of rice egg cells. J. Plant Res. 120, 575–579. doi: 10.1007/s10265-007-0095-y
Verhoeven, T., Feron, R., Wolters-Arts, M., Edqvist, J., Gerats, T., Derksen, J., et al. (2005). STIG1 controls exudate secretion in the pistil of petunia and tobacco. Plant Physiol. 138, 153–160. doi: 10.1104/pp.104.054809
Wang, H., Wu, H. M., Cheung, A. Y. (1996). Pollination induces mRNA poly(A) tail-shortening and cell deterioration in flower transmitting tissue. Plant J. 9, 715–727. doi: 10.1046/j.1365-313x.1996.9050715.x
Waszczak, C., Carmody, M., Kangasjärvi, J. (2018). Reactive oxygen species in plant signaling. Annu. Rev. Plant Biol. 69, 209–236. doi: 10.1146/annurev-arplant-042817-040322
Wrzaczek, M., Vainonen, J. P., Stael, S., Tsiatsiani, L., Gauthier, A., Kaufholdt, D., et al. (2015). GRIM REAPER peptide binds to receptor kinase PRK5 to trigger cell death in Arabidopsis. EMBO J. 34, 55–66. doi: 10.15252/embj.201488582
Wudick, M. M., Feijó, J. A. (2014). At the intersection, merging Ca2+ and ROS signaling pathways in pollen. Mol. Plant 7, 1595–1597. doi: 10.1093/mp/ssu096
Xiao, Y., Stegmann, M., Han, Z., DeFalco, T. A., Parys, K., Xu, L., et al. (2019). Mechanisms of RALF peptide perception by a heterotypic receptor complex. Nature 572, 270–274. doi: 10.1038/s41586-019-1409-7
Xie, H.-T., Wan, Z.-Y., Li, S., Zhang, Y. (2014). Spatiotemporal production of reactive oxygen species by NADPH oxidase is critical for tapetal programmed cell death and pollen development in Arabidopsis. Plant Cell 26, 2007–2023. doi: 10.1105/tpc.114.125427
Xiong, J., Yang, Y., Fu, G., Tao, L. (2015). Novel roles of hydrogen peroxide (H2O2) in regulating pectin synthesis and demethylesterification in the cell wall of rice (Oryza sativa) root tips. New Phytol. 206, 118–126. doi: 10.1111/nph.13285
Xu, X. H., Wang, F., Chen, H., Sun, W., Zhang, X. S. (2013). Transcript profile analyses of maize silks reveal effective activation of genes involved in microtubule-based movement, ubiquitin-dependent protein degradation, and transport in the pollination process. PloS One 8, e53545. doi: 10.1371/journal.pone.0053545
Xu, Y., Yang, J., Wang, Y., Wang, J., Yu, Y., Long, Y., et al. (2017). OsCNGC13 promotes seed-setting rate by facilitating pollen tube growth in stylar tissues. PloS Genet. 13, e1006906. doi: 10.1371/journal.pgen
Yamauchi, T., Yoshioka, M., Fukazawa, A., Mori, H., Nishizawa, N. K., Tsutsumi, N., et al. (2017). An NADPH oxidase RBOH functions in rice roots during lysigenous aerenchyma formation under oxygen-deficient conditions. Plant Cell 29, 775–790. doi: 10.1105/tpc.16.00976
Yue, X., Gao, X. Q., Wang, F., Dong, Y., Li, X., Zhang, X. S. (2014). Transcriptional evidence for inferred pattern of pollen tube-stigma metabolic coupling during pollination. PloS One 9, e107046. doi: 10.1371/journal.pone.0107046
Zafra, A., Rejón, J. D., Hiscock, S. J., Alché, J. (2016). Patterns of ROS accumulation in the stigmas of angiosperms and visions into their multi-functionality in plant reproduction. Front. Plant Sci. 7, 1112. doi: 10.3389/fpls.2016.01112
Zhang, H., Zhang, T. T., Liu, H., Wang, M., Bie, X. M., Li, X. G., et al. (2018). Thioredoxin-mediated ROS homeostasis explains natural variation in plant regeneration. Plant Physiol. 176, 2231–2250. doi: 10.1104/pp.17.00633
Zhong, S., Qu, L.-J. (2019). Peptide/receptor-like kinase-mediated signaling involved in male–female interactions. Curr. Opin. Plant Biol. 51, 7–14. doi: 10.1016/j.pbi.2019.03.004
Zhong, S., Liu, M., Wang, Z., Huang, Q., Hou, S., Xu, Y. C., et al. (2019). Cysteine-rich peptides promote interspecific genetic isolation in. Arabidopsis Sci. 364, eaau9564. doi: 10.1126/science.aau9564
Keywords: reactive oxygen species, stigma, style, female gametophyte, pollen
Citation: Zhang MJ, Zhang XS and Gao X-Q (2020) ROS in the Male–Female Interactions During Pollination: Function and Regulation. Front. Plant Sci. 11:177. doi: 10.3389/fpls.2020.00177
Received: 26 November 2019; Accepted: 05 February 2020;
Published: 28 February 2020.
Edited by:
Zhong-Nan Yang, Shanghai Normal University, ChinaReviewed by:
Ravishankar Palanivelu, University of Arizona, United StatesJose A. Traverso, University of Granada, Spain
Copyright © 2020 Zhang, Zhang and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin-Qi Gao, gaoxq@sdau.edu.cn
†ORCID: Xin-Qi Gao, orcid.org/0000-0002-2530-7776
 Ming Jun Zhang
Ming Jun Zhang Xian Sheng Zhang
Xian Sheng Zhang Xin-Qi Gao
Xin-Qi Gao