- 1Engineering Research Center of Edible and Medicinal Fungi, Ministry of Education, Jilin Agricultural University, Changchun, China
- 2Department of Biological Sciences, University of Arkansas, Fayetteville, AR, United States
Myxomycetes (plasmodial slime molds) are eukaryotic protist predators that are associated with wood, leaf litter, and soil in forests, where they feed on bacteria, protozoans, and (to a more limited extent) fungi. The health of crop plants is essential because they represent a primary food source for humans. However, when myxomycetes produce numerous fruiting bodies on the stems and leaves of crop plants, which is herein referred to as a myxomycete colonization, this has the potential of interfering with plant photosynthesis, transpiration and respiration by blocking out light and covering stomata. Myxomycetes are not pathogens, but their occurrence on plants can be mistakenly interpreted as some type of infection. However, this phenomenon has been largely ignored. This paper provides a comprehensive overview of the taxonomic and economic diversity of the organisms involved in myxomycete colonization. In addition, the various types of myxomycete colonization reported in the literature are described and discussed, a number of images provided, and cultural and chemical prevention and control measures are summarized. The latter should be of significant relevance for local production of crops and plant protective stations. While myxomycetes are not pathogens of crop plants, some species can seriously impact commercially grown mushrooms. Reports of myxomycetes affecting mushrooms are also described in this paper.
1 Introduction
The health of crop plants has major public implications when farmers are able to access basic crop healthcare and services from relevant authorities to evaluate infested or suspected infested crop plants. By extension, this is also advantageous for human health (Vega et al., 2020; Jia et al., 2023). Over the past few decades, people-crop plant studies have increasingly focused on empirically demonstrating relationships between crop plants and health (Grandin, 2022). There is a vast array of diseases in the natural environment of crop plants, such as fungal diseases (Ceasar and Ignacimuthu, 2012; Wang, 2023), bacterial diseases (Hikichi, 2016; Li et al., 2023), viral diseases (Bhat et al., 2022; Zhang et al., 2022), and nematode-caused diseases (Kaloshian and Teixeira, 2019; Bhat et al., 2023). These crop plant diseases constitute a huge economic and environmental threat to agricultural and forestry production. However, there is increasing difficulty in identifying new plant diseases and what mistakingly appear as plant diseases as a result of ongoing environmental change (Jones, 2016; Jain et al., 2019). There is a need to identify the factors influencing the emergence and the increasing incidences of these diseases.
The myxomycetes (true slime molds or plasmodial slime molds) are a monophyletic taxon within the phylum Amoebozoa as the class Myxomycetes or Myxogastrea (Adl et al., 2012; Kang et al., 2017b). These organisms have a peculiar life cycle that encompasses a microscopic amoeboflagellates (the first tropic stage), a multinuclearte plasmodium (the second trophic stage), and a macroscopic fruiting body (the reproductive stage) within which spores are produced (Everhart and Keller, 2008; Stephenson and Feest, 2012; Stephenson and Rojas, 2017). Like many other protist predators, myxomycetes feed on bacteria and other microorganisms. As such, they represent an ecologically important component of terrestrial nutrient cycles (Walker et al., 2019). Well known microhabitats for myxomycetes include decaying wood (Fukasawa et al., 2018), aerial plant litter, ground plant litter (Pecundo et al., 2017), the bark of living and dead trees, and dung (Abdel-Raheem, 2002). They even occur on the inflorescences of Neotropical herbs (Schnittler and Stephenson, 2002) and in aquatic habitats (Lindley et al., 2007).
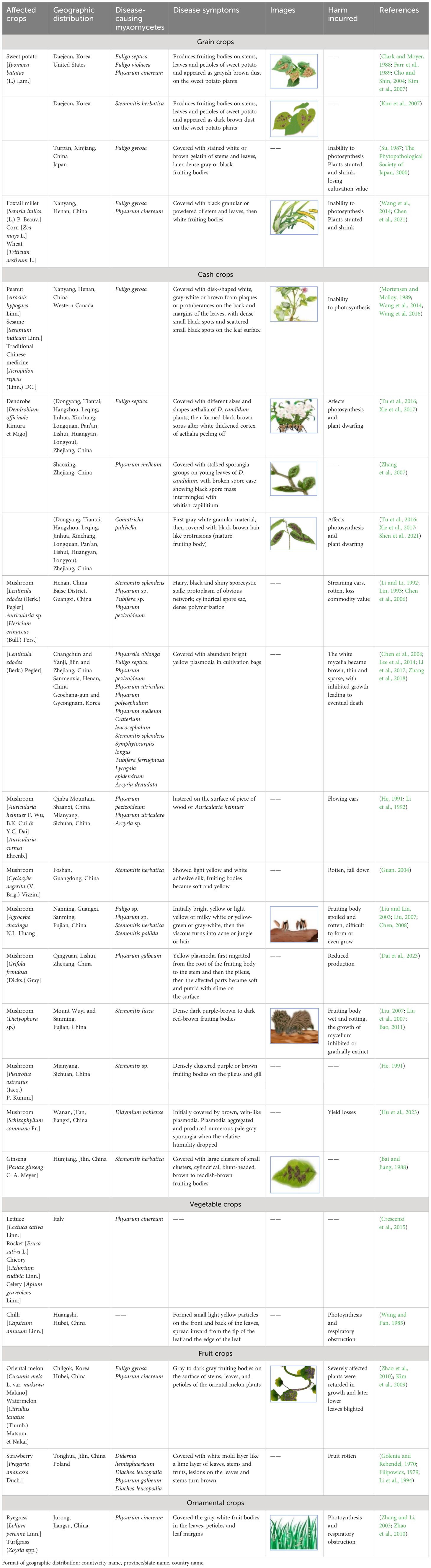
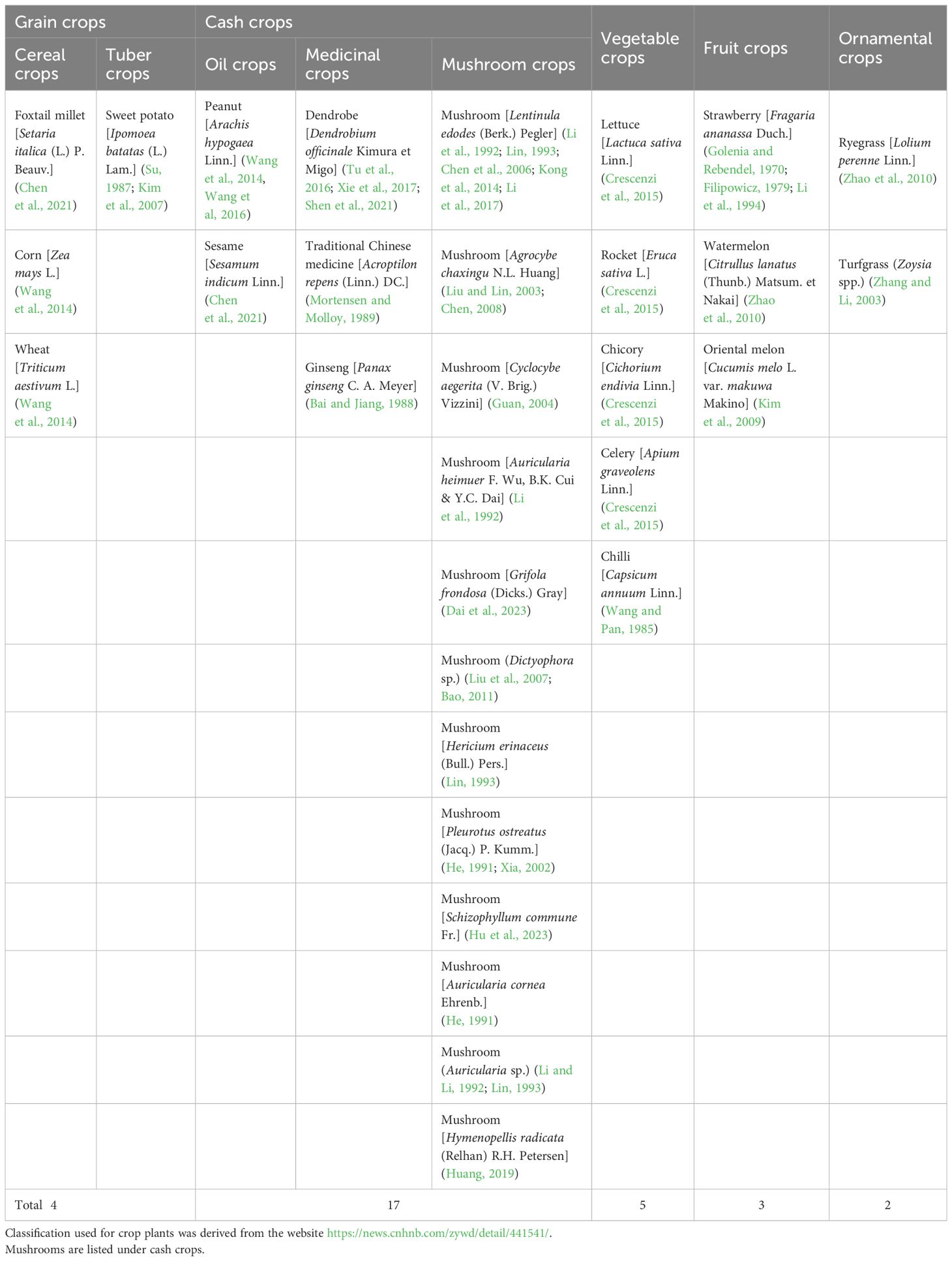
It is worth noting that myxomycetes have been reported as a common crop plant “diseases” despite the fact that they are not pathogenic. Su (Su, 1987) observed the occurrence of myxomycetes on sweet potato seedlings over a period of six years, and considered this as a “disease”. Li (Li et al., 1994) reported a new slime mold “disease” on strawberries. Also, Couch (Couch, 1995) used the term “disease” for myxomycetes covering plant leaves. Herein, we refer to a myxomycete colonization as simply referring to the occurrence of myxomycetes, typically the fruiting bodies, on crop plants but not implying that myxomycetes are in any sense true pathogens. Reports of this phenomenon come from seven countries throughout the world, with China having the most widespread instances of myxomycete colonization (Figure 1). These reports include affected grain crops (Su, 1987; Kim et al., 2007; Wang et al., 2014; Chen et al., 2021), cash crops (Mortensen and Molloy, 1989; Li et al., 1992; Guan, 2004; Kong et al., 2014; Wang et al., 2014; Tu et al., 2016; Wang et al., 2016; Xie et al., 2017; Huang, 2019; Chen et al., 2021), vegetable crops (Wang and Pan, 1985; Kim et al., 2009; Crescenzi et al., 2015), fruit crops (Golenia and Rebendel, 1970; Filipowicz, 1979; Li et al., 1994) and ornamental crops (Zhang and Li, 2003; Zhao et al., 2010) (Table 2). According to the statistics compiled from an intensive search of the published literature, a total of 31 crop plants have been reported to have “slime mold disease”. Among these, cash crops account for the highest proportion, with 17 crop species associated with myxomycetes, accounting for 54.8% of all crop plant types.
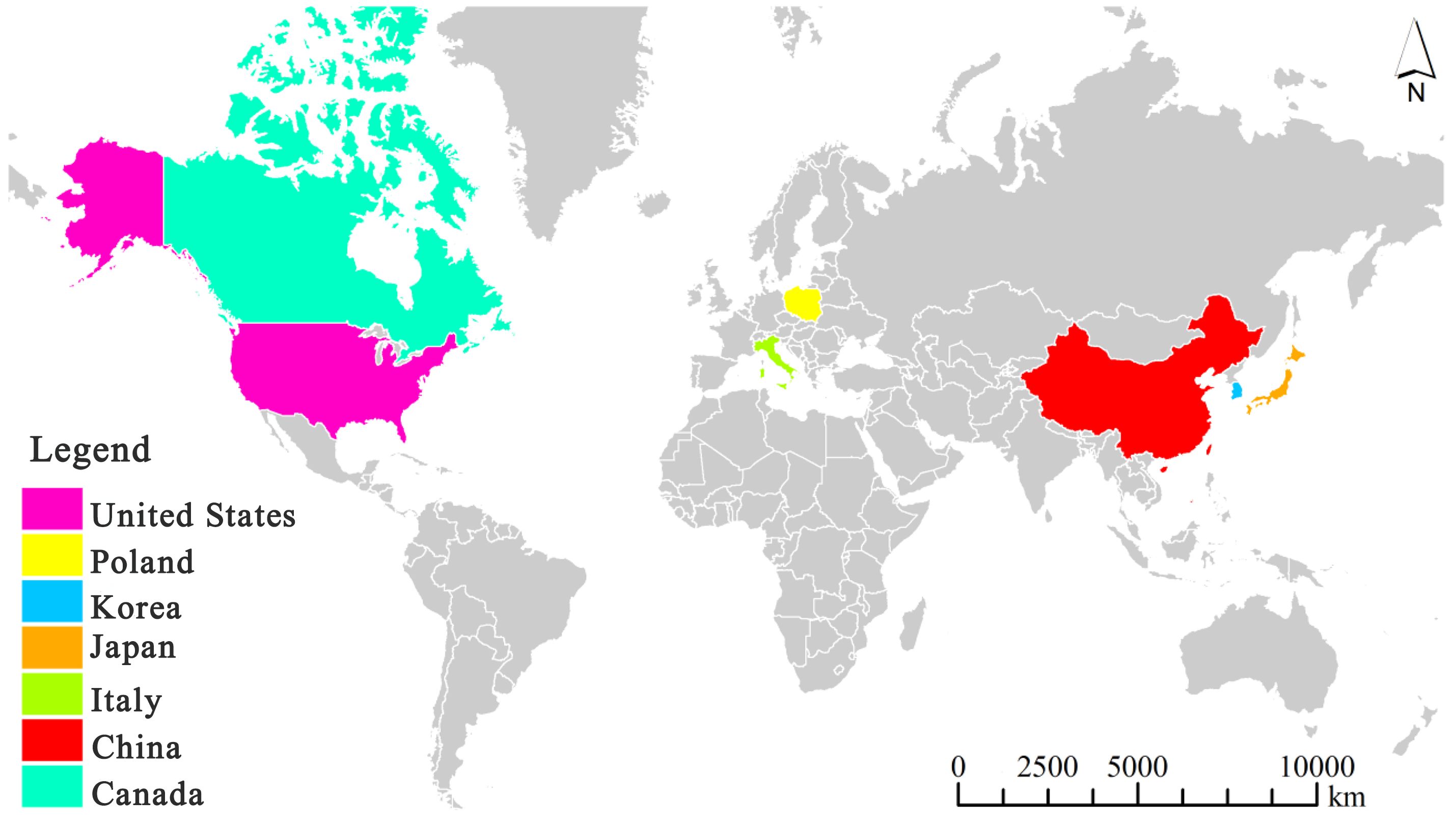
Figure 1 A map showing countries reporting myxomycete colonization by December 2023. Note: the map was drawn using ArcGIS software by summarizing the countries where the distribution of myxomycete colonization from Table 1, as shown in Table 1 for references.
Lister (Lister, 1888) reported that the plasmodium of Badhamia utricularis could feed upon a number of basidiomycetes and would consume a fruiting body of Stereum hirsutum within a few hours. Later, Madelin (Madelin et al., 1975) demonstrated that the plasmodium of B. utricularis showed a positive chemotaxis towards a substance produced by S. hirsutum. In mushroom cultivation sites, artificial manipulation of the environment provides excessive moisture and large amounts of dead plant material, which favors the growth of myxomycetes. Chung (Chung et al., 1998) summarized the myxomycetes (29 species and 4 varieties) that had been recorded from various edible mushroom cultivation sites in Taiwan, China. The production of edible mushrooms has developed rapidly in China (Fang et al., 2014), and a myxomycete colonization that is not non-pathogenic has affected sustainable and development of a number of different types of mushrooms, including Lentinula edodes (Berk.) Pegler (Li et al., 1992), Pleurotus ostreatus (Jacq.) P. Kumm (He, 1991), Auricularia heimuer F. Wu, B.K. Cui & Y.C. Dai (Li et al., 1992), Hericium erinaceus (Bull.) Pers (Lin, 1993), and various other species. This fact implies that slime molds might be a serious problem in some situations. In addition, reports on mushroom diseases caused by slime molds may be overlooked because of a failure in identification. Some mushroom farmers have misunderstand myxomycete colonization and misuse pesticides for prevention and control, which delays the application of truly effective prevention methods and causes unnecessary economic losses (Bao, 2011).
While previous literature on what we refer herein as myxomycete “disease” has predominantly focused on reporting species and cultural or chemical control measures targeting specific species. While these reviews have made significant contributions to the field, they still have limitations in a number of different aspects. First, what is the total number of species of myxomycetes that cause a “disease”, and what is their worldwide distribution? Second, what are the symptoms exhibited by these myxomycete on different crop plants? Third, what measures have been taken to address these “disease” in crop plants? Therefore, the main contribution of this review lies in systematically summarizing myxomycete colonization and the cultural and chemical strategies for the prevention and control of this phenomenon, derived from the published reports that have appeared thus far, which are expected to provide new guidance for research and practice in this field.
2 Detection and diagnosis of myxomycetes
Detecting and diagnosing myxomycetes is crucial for understanding their ecology, distribution, and impact. The initial step in detection involves regular field surveys to identify potential habitats for myxomycetes. Habitats such as decaying logs, soil, leaf litter, and mossy areas should be carefully examined for the presence of their fruiting bodies, slime trails, or other characteristic signs of myxomycete activity. The use of a hand lens or magnifying glass can aid in the detection of small or inconspicuous fruiting bodies. Once potential myxomycete habitats have been identified, targeted sampling techniques should be employed to collect specimens for further analysis (Tu et al., 2016). This can involve the use of sterile tools to collect fruiting bodies, slime trails, or soil samples containing plasmodia. The samples should be collected in sterile containers and labeled with relevant information such as location, date, and habitat type.
The initial step in diagnosis involves morphological analysis of the collected samples. This includes examining the shape, color, and texture of fruiting bodies, as well as the structure and behavior of myxamoebae and plasmodia under a microscope (Zhao et al., 2010; Song and Chen, 2024). The use of light microscopy and staining techniques can aid in the determination of characteristic features. For more accurate identification, molecular analysis techniques can be employed. This involves extracting DNA from the samples and amplifying specific genetic markers (Fiore-Donno et al., 2008; Prikhodko et al., 2023) using PCR (polymerase chain reaction). The amplified DNA fragments can then be sequenced and compared to reference databases (https://www.ncbi.nlm.nih.gov/) to identify the species. Based on the results of morphological and molecular analyses, the particular species of myxomycetes can be identified.
3 Symptoms of myxomycete colonization
The symptoms of the myxomycete colonization on crops are presented in Table 1. We found that myxomycete colonization is primarily manifested on the stems, leaves, and leaf margins of crop plants in the form of fruiting bodies or less commonly plasmodia (Kim et al., 2007, Kim et al, 2009). In some cases, the “disease” expands to cover much of the entire plant. This could potentially interfere with plant photosynthesis, transpiration and respiration by blocking out light and covering stomata (Wang and Pan, 1985; Couch, 1995; Tu et al., 2016; Chen et al., 2021; Shen et al., 2021), causing the loss of plant biomass and thus cultivation value, which will seriously lead to the drying of leaves, the death of the whole plant, and serious shortage of seedlings. The color of the “disease” affecting different crops varies according to the species of myxomycete involved, which can range from white to yellow or orange to black. These could alter the appearance of the crop and make it less aesthetically pleasing.
Most myxomycetes most commonly occur under moist conditions and when wet virtually all types of organic matter provide suitable habitats for these organisms. Myxomycete colonization is sometimes present on a healthy plant. This was the case in one instance in which the myxomycete Comatricha pulchella (C. Bab.) Rostaf. were present on the plant, after rinsing or removing the sub-entity with water, the plant tissue at the site where the sub-entity was produced remained healthy (Wang et al., 2014; Tu et al., 2016). Therefore, strictly speaking, because it does not meet the Koch postulate (Ross and Woodward, 2016), a myxomycete colonization cannot be considered as a pathogen. One reason why myxomycete colonization occurs on crop plants in the field may be a result of the spread of humus to leaves as the humus is applied to supplement the soil. Myxomycetes would be expected to be abundant (as amoeboflagellates) in the humus. However, since the spores of myxomycetes are wind-dispersed, they have the potential to land anywhere, including plant surfaces and the soil out of which the plant is growing. As already noted, if the amoeboflagellates ultimately give rise first to plasmodia and then to fruiting bodies, the potential exists for this causing the leaves of crop leaves to have reduced photosynthesis and respiration, thus decreasing their cultivation value (Chen et al., 2021).
4 Characteristics of myxomycete colonization
4.1 Causes of the “disease”
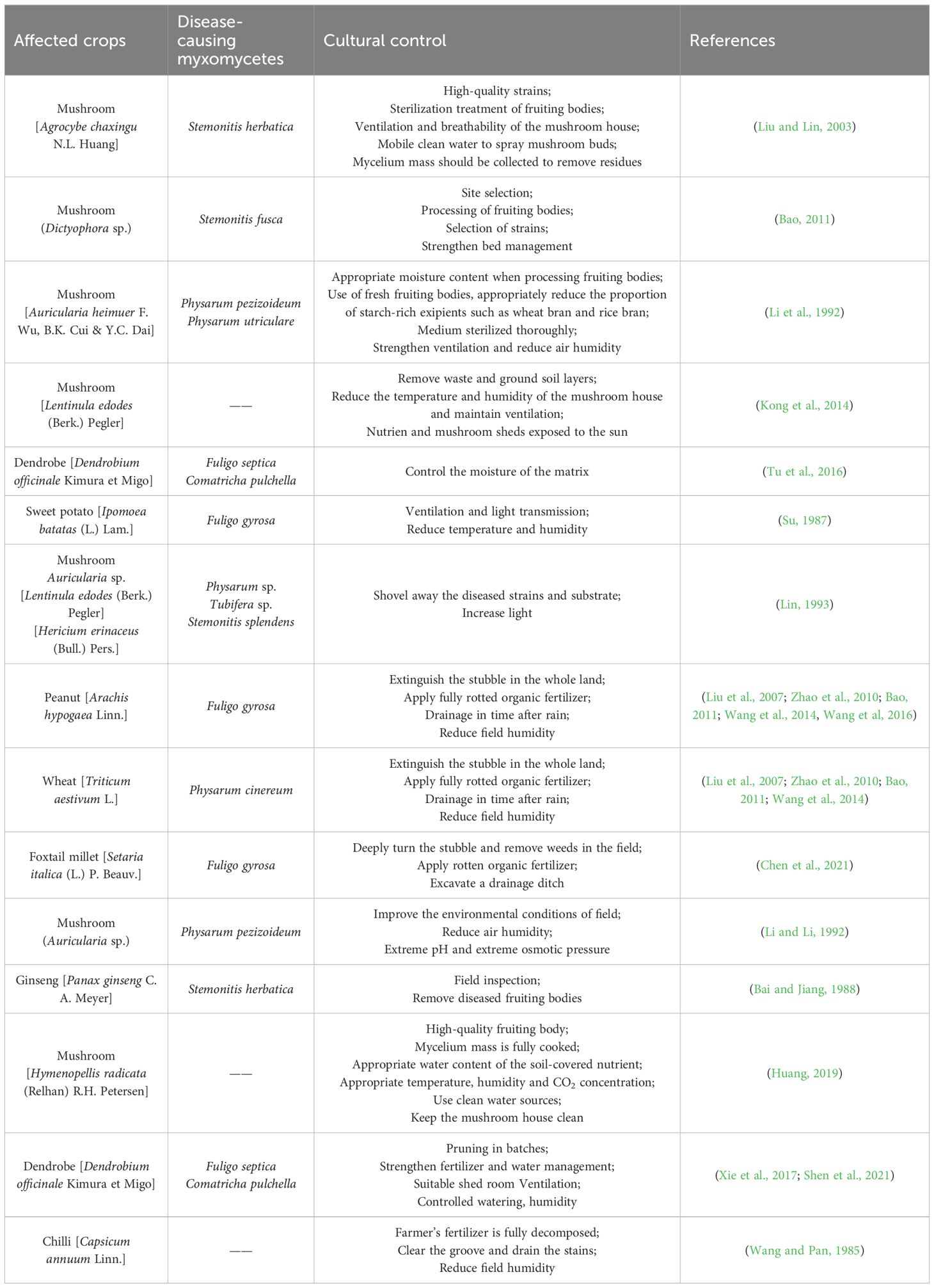
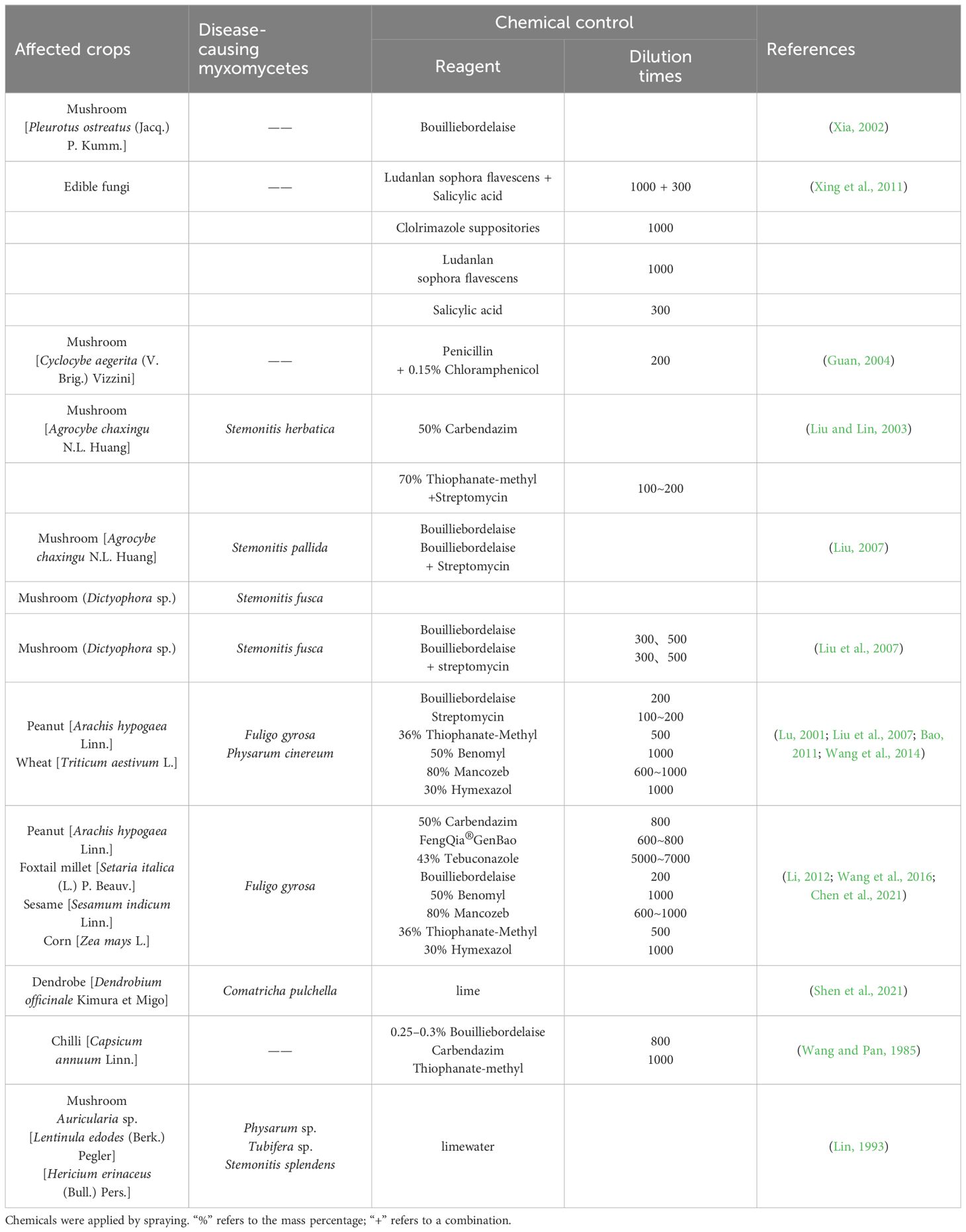
Myxomycetes are a group of polyphagous eukaryotes characterized by a distinctive life cycle, including a plasmodial stage and fruiting body stage (Hatano and Tazawa, 1968). Plasmodia feed on bacteria, organic substances, fragments of mushroom mycelia, and spores. However, bacteria represent the primary sources of their nutrition (Li et al., 2022). If any kind of organic material is infected with bacteria, or the environment is humid, this provides suitable conditions for myxomycetes. For example, the implementation of no tillage measures after wheat harvest provides a very suitable environment for the growth of myxomycetes. As such, large amount of undeveloped wheat stubble is likely to be affected by these organisms (Filipowicz, 1979; Chen et al., 2021; Dai et al., 2023).
4.2 Occurrence regularity
Some myxomycetes can be found, sometimes abundantly, in high-temperature (20–30°C) and high-humidity (about 90%) environments on moist dead grass, in crevices in dead logs, under bark, and on dead leaves and in fertile soils (Everhart et al., 2008; Trevino-Zevallos and Lado, 2020). They are well suited for occurring in places rich in organic matter. Therefore, since the period of July to August each year in much of Asia is a period of high temperature and humidity, this creates conditions for the frequent occurrence of the myxomycete colonization. This “disease” often occurs during hot and rainy seasons other places in the world (Kong et al., 2014). For example, in the process of mushroom cultivation during the growth of the mycelium, when the humidity of the air is too high and not breathable and the temperature also is too high, this makes it easy for a myxomycete colonization to invade the cultivation bag (Bao, 2011). When entering the stage of mushroom emergence, the growth of fruiting bodies requires weak light, high humidity, and a medium temperature environment. In addition, when substances such as bran are added, some bran is not completely decomposed by the tea tree mushroom hyphae. After being dripped with water, water accumulates in the bag, which can easily cause fermentation and acidification of the bran. This environment is suitable for the growth of myxomycetes, so they begin to invade from the bag mouth and gradually affect the mushroom fruiting bodies, causing these to wilt and rot (He, 1991; Li et al., 1992; Lin, 1993).
For this paper, we reviewed the published literature on myxomycetes reported to occur on crop plants reported since 1970, based on the latest 18S rDNA phylogeny (Leontyev et al., 2019) classification of the entire group and http://www.indexfungorum.org/names/Names.asp for current names. In this classification, myxomycetes mentioned in these reports are placed into four orders, five families, and thirteen genera, and twenty-nine species.
4.3 Transmission route
The occurrence of myxomycete colonization is closely related to a specific environment. The myxomycetes that occur on plants are probably brought in on soil and culture materials, and their occurrence does not affect the normal growth of crop plants if removed in time. Myxomycete spores are likely transmitted to affected crop plants via wind (Kamono et al., 2009), water (Lindley et al., 2007), insects (Nunes Lemos et al., 2010; Kataoka and Nakamori, 2020), other animals (Trimble, 2021), and human activities. In addition, the spores of myxomycetes can survive inhospitable environments. Wind dispersal of spores is considered to be most important way for myxomycetes to colonize new areas and/or substrates.
5 Prevention and control methods of myxomycete colonization
Across the globe, the problem of crop plant security has become more and more prominent. It is known to be significant impact ensuring economic growth, adjusting agricultural structure, impacting the livelihood of farmers, and affecting income (Kang et al., 2017a; Yu et al., 2022). Any increase in a myxomycete colonization can causes problems by reducing crop yield and thus losses in agricultural cultivation. The current research has considered only cultural and chemical control, so this manuscript provides a review of prevention and control strategies for myxomycetes, mainly from two aspects—cultural control and chemical control.
5.1 Cultural control
We have summarized cultural control for 11 types of myxomycete colonization for a total of 17 affected crop plants (Table 3). Among these, the myxomycete colonization of edible mushrooms accounts for more than 50% of the control method reported. This reflects the prevalence of myxomycete colonization and their potential threat to agricultural production. Due to the dominance of the control method of myxomycete colonization of edible mushrooms, it can be inferred that edible mushrooms may have high economic value in the agriculture or food industry for many human conditions and activities (Ghorai et al., 2009; Shen et al., 2022).
From the perspective of the statistical measures given in the table as a whole, for edible fungi one should adhere to the principle of “prevention first, comprehensive prevention and control”. The key to preventing and controlling myxomycete colonization is to improve the cultivation environment, control the moisture content of culture materials and deal with the incidence area in a timely manner (He, 1991; Lin, 1993; Kong et al., 2014; Li et al., 2017).
Cultural practices could include intercropping, crop rotation, and balanced doses of fertilizer, the specific recommendations often vary depending on the agro-ecological conditions, soil type, climate, and pest and disease pressure in a particular region. In general, selecting crops with complementary nutrient requirements and growth habits can enhance biodiversity and soil fertility, which may indirectly affect myxomycete populations (Liu et al., 2007; Zhao et al., 2010; Bao, 2011; Wang et al., 2014, Wang et al, 2016). For instance, legumes (such as beans and peas) can be intercropped with cereals (like wheat and maize) as they fix nitrogen from the air, which benefits the cereals (Wahbi et al., 2016). In crop rotation, rotating grains with legumes or vegetables can break disease cycles and improve soil structure (Ball et al., 2005; Ghosh et al., 2020). As for the role of balanced fertilizer doses in disease reduction, the key lies in maintaining optimal nutrient availability for plant growth while avoiding nutrient imbalances that can stress plants and make them more susceptible to diseases (Marouelli et al., 2015). Excessive nitrogen, for example, can promote vegetative growth but also make plants more vulnerable to fungal diseases (Jeon, 2019). By precisely applying the right mix of nutrients, including macronutrients (nitrogen, phosphorus, potassium) and micronutrients (like zinc and iron), we can promote healthy plant growth, strengthen the plant’s immune system, and thereby reduce disease pressure.
For cultivated crops such as peanuts, wheat, ginseng, chili peppers, and Dendrobium officinale, farmers should conduct regular field inspections. The fields cultivated should be incorporated into the local farming system by completing the stubble soon after harvesting, applying fully decomposed organic fertilizer, and adopting formula fertilization technology. Also, excess water should be drained soon after the rain to prevent the field from being too wet (Wang and Pan, 1985; Bai and Jiang, 1988; Wang et al., 2014; Shen et al., 2021).
5.2 Chemical control
Chemical control is known to result in environmental contamination of the biosphere and has become a debatable concern globally. Moreover, most methods are expensive and environmentally unfriendly (Naidu et al., 2021; Tudi et al., 2021). However, current chemical control measures that involve the spraying of traditional chemicals or antibiotics can have an effect on myxomycete colonization. Xing (Xing et al., 2011) selected low toxic and pollution free chemical reagents to conduct indoor toxicity tests to determine inhibition to myxomycetes; Then through indoor bioassay results to select the reagents with small effect on the growth of Pleurotus ostreatus. Chemical control that have been used for nine types of myxomycete “disease” in a total of 16 affected crop plants is summarized in Table 4.
The corresponding prevention and control strategies of the effective reagents reported thus far are outlined in Table 4. These data indicate that the different combinations of reagents and dilution ratios reflect the precise management tailored for different types of crops and edible mushrooms. The reason for this may be that regional differences have caused myxomycete colonization to display different patterns of occurrence on crops. Judging from the overall situation, the three most frequently used reagents are Bouilliebordelaise, Thiophanate-methyl, and Streptomycin, indicating that they show a certain degree of universality and effectiveness in controlling the “disease” on crops and edible mushrooms. However, in the future, chemical control methods should not be limited to specific types of reagents but also should include broader chemical control methods, especially those widely used synthetic fungicides.
6 Conclusions and future perspectives
Myxomycetes prefer humid conditions and are commonly occur in association with all kinds of organic material, including living plants. When myxomycete fruiting bodies are found on a living plant, the initial impression is that they are pathogenic. However, this is not the case. Nevertheless, myxomycetes can indeed affect the normal growth of plants by reducing photosynthesis and respiration. Most of the time it is inconsequential, but in some instances it is not. In time the spores produced by the fruiting bodies can adhere to other healthy plants as a result of being spread by wind and rain. Occurrence of myxomycete colonization is closely related to specific set of environmental conditions that include large amounts of decaying organic matter and the presence of a plentiful supply microorganisms, especially bacteria). Because of this, the “disease” has an obvious regional occurrence.
This review summarizes the current examples of myxomycetes affecting plant (including mushroom) growth and effective prevention and control measures, including cultural control and chemical control. Myxomycete colonization has caused serious economic losses to China’s local edible fungus industry. Therefore, we sincerely hope that more scholars will pay attention to myxomycete colonization in the future. For example, in production the relative air humidity in the growing shed can be controlled by constructing a suitable shed, maintaining proper ventilation in the shed, controlling watering, and sprinkling a reagent around the seedbed, thus controlling the occurrence of myxomycete colonization. However, we still face many challenges in the mechanism of myxomycete colonization and their understanding of the environment and ecosystem. Scientific questions that need to be answered in the future include: (1) Is there genetic exchange between the cultivation matrix of myxomycetes and mushrooms? What is the specific mechanism? (2) Will the bacteria carried by myxomycete cause plant diseases?
Future research on myxomycete “diseases” should focus on elucidating the genetic and molecular mechanisms underlying the “pathogenicity” of these organisms. Genomics and proteomics studies can provide insights into their virulence factors and how they interact with host crop plants. In addition, ecological studies examining the role of myxomycetes in ecosystems and their interactions with other organisms are needed to develop more targeted and sustainable management strategies. Current management strategies for myxomycete “diseases” face several challenges. One major concern is the development of resistance to chemical reagents, which threatens the effectiveness of traditional chemical control methods. Furthermore, the use of chemical reagents can have negative environmental impacts, including water pollution and disruption of ecological balance. Cost-effective and environmentally friendly alternative control methods need to be explored. Additionally, the variability in “disease” incidence and severity across different regions and conditions poses a challenge in developing universally applicable management strategies.
In summary, future research should have the objective of providing a deeper understanding of the biology and ecology of myxomycetes, while management strategies should focus on developing sustainable and cost-effective alternatives to chemical reagents.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the National Natural Science Foundation of China (Nos. 32370020, 32070009), the Natural Science Foundation of Jilin Province (No. 20220101187JC), and the Program of Creation and Utilization of Germplasm of Mushroom Crop of “111” Project (No. D17014).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdel-Raheem, A. M. (2002). Myxomycetes from upper Egypt. Microbiological Res. 157, 47–67. doi: 10.1078/0944-5013-00131
Adl, S. M., Simpson, A. G. B., Lane, C. E., Lukes, J., Bass, D., Bowser, S. S., et al. (2012). The revised classification of eukaryotes. J. Eukaryotic Microbiol. 59, 429–493. doi: 10.1111/j.1550-7408.2012.00644.x
Ball, B. C., Bingham, I., Rees, R. M., Watson, C. A., Litterick, A. (2005). The role of crop rotations in determining soil structure and crop growth conditions. Can. J. Soil Science. 85, 557–577. doi: 10.4141/S04-078
Bao, X. L. (2011). On the disease control of Dictyophora Stemonitis Fusca Roth. Friend Sci. Amateurs. 20, 159–160.
Bhat, A. I., Aman, R., Mahfouz, M. (2022). Onsite detection of plant viruses using isothermal amplification assays. Plant Biotechnol. J. 20, 1859–1873. doi: 10.1111/pbi.13871
Bhat, A. A., Shakeel, A., Waqar, S., Handoo, Z. A., Khan, A. A. (2023). Microbes vs. Nematodes: insights into biocontrol through antagonistic organisms to control root-knot nematodes. Plants-Basel. 12, 451. doi: 10.3390/plants12030451
Ceasar, S. A., Ignacimuthu, S. (2012). Genetic engineering of crop plants for fungal resistance: role of antifungal genes. Biotechnol. Letters. 34, 995–1002. doi: 10.1007/s10529-012-0871-1
Chen, S. Z. (2008). Reserach on comprehensive prevention and control for edible fungus II: occurrence and control of Polyma disease in Agrocybe chaxingu. Guangxi Agric. Sci. 39, 317–319.
Chen, P. Y., Qiang, X. J., Niu, Y. T., Ju, L., Zhou, X. J., Shen, J. D. (2021). First report of the damage of Physarum gyrosum Rostaf to Foxtail Millet (Setaria italica). Barley Cereal Sci. 38, 32–34. doi: 10.14069/j.cnki.32-1769/s.2021.06.007
Chen, Q. B., Wu, Q. C., Wu, Y. M. (2006). Investigation on diseases and pests of substitute shiitake mushrooms in Zhejiang Province. Acta Edulis Fungi. 13, 69–73. doi: 10.16488/j.cnki.1005-9873.2006.02.017
Cho, W. D., Shin, H. D. (2004). List of plant diseases in korea. 4th ed. (Suwon, Korea: The Korean Society of Plant Pathology.).
Chung, C. H., Liu, C. H., Tzean, S. S. (1998). Slime molds in edible mushroom cultivation sites. Plant Pathol. Bulletin. 7, 141–146.
Clark, C. A., Moyer, J. W. (1988). Compendium of sweet potato diseases (The American Phytopathological Society, St. Paul, Minnesota: APS Press).
Couch, H. B. (1995). Diseases of turfgrasses. 3rd ed (Florida, USA: Krieger Publishing Company), 421.
Crescenzi, A., Rana, G. L., Fanigliulo, A., Lahoz, E., Carrieri, R. (2015). First report of Physarum cinereum on lettuce, rocket, endive, and celery in Italy. Plant Disease. 99, 1272–1272. doi: 10.1094/PDIS-11-14-1121-PDN
Dai, D., Sun, P., Wu, X., Hu, J., Zhang, B., Wei, Y., et al. (2023). First report of yellow rot caused by Physarum galbeum in Grifola frondosa in China. Plant Disease. 107, 2250–2250. doi: 10.1094/PDIS-10-22-2419-PDN
Everhart, S. E., Keller, H. W. (2008). Life history strategies of corticolous myxomycetes: the life cycle, fruiting bodies, plasmodial types, and taxonomic orders. Fungal Diversity. 29, 1–16.
Everhart, S. E., Keller, H. W., Ely, J. S. (2008). Influence of bark pH on the occurrence and distribution of tree canopy myxomycete species. Mycologia. 100, 191–204. doi: 10.1080/15572536.2008.11832476
Fang, Y., Sun, X., Yang, W., Ma, N., Xin, Z., Fu, J., et al. (2014). Concentrations and health risks of lead, cadmium, arsenic, and mercury in rice and edible mushrooms in China. Food Chem. 147, 147–151. doi: 10.1016/j.foodchem.2013.09.116
Farr, D., Bills, G., Chamuris, G., Rossman, A. (1989). Fungi on plants and plant products in the United States (St. Paul, Minnesota: APS Press), 1252. doi: 10.13629/j.cnki.53–1054.2019.06.009
Filipowicz, A. (1979). Sluzowce (myxomycetes) patogenami truskawek [the slime molds (myxomycetes) as pathogens of strawberries]. Ochr. Rosl. 23, 15–16. doi: 10.1039/c5fo00497g
Fiore-Donno, A. M., Meyer, M., Baldauf, S. L., Pawlowski, J. (2008). Evolution of dark-spored Myxomycetes (slime-molds): Molecules versus morphology. Mol. Phylogenet. Evolution. 46, 878–889. doi: 10.1016/j.ympev.2007.12.011
Fukasawa, Y., Hyodo, F., Kawakami, S. I. (2018). Foraging association between myxomycetes and fungal communities on coarse woody debris. Soil Biol. Biochem. 121, 95–102. doi: 10.1016/j.soilbio.2018.03.006
Ghorai, S., Banik, S. P., Verma, D., Chowdhury, S., Mukherjee, S., Khowala, S. (2009). Fungal biotechnology in food and feed processing. Food Res. Int. 42, 577–587. doi: 10.1016/j.foodres.2009.02.019
Ghosh, P. K., Hazra, K. K., Venkatesh, M. S., Praharaj, C. S., Kumar, N., Nath, C. P., et al. (2020). Grain legume inclusion in cereal-cereal rotation increased base crop productivity in the long run. Exp. Agriculture. 56, 142–158. doi: 10.1017/S0014479719000243
Golenia, A., Rebendel, Z. (1970). Masowe wystepowanie sluzowca Diachea leucopodia (Bull.) Rost. na truskawkach [Mass occurrence of slime mold Diachea leucopodia (Bull.) Rost. on the strawberries]. Prace Nauk. IOR. 12, 267–271.
Grandin, T. (2022). Grazing cattle, sheep, and goats are important parts of a sustainable agricultural future. Animals. 12, 2092. doi: 10.3390/ani12162092
Guan, S. G. (2004). Prevention and control test of slime mold dieases of Cyclocybe aegerita. Edible Fungi. 4, 41–42.
Hatano, S., Tazawa, M. (1968). Isolation, purification and characterization of byosin B from myxomycete plasmodium. Biochim. Et Biophys. Acta 154, 507–519. doi: 10.1016/0005-2795(68)90011-1
He, X. S. (1991). Myxomycetes on the fruiting bodies of Auricular cornea and Pleurotus ostreatus. Edible Fungi. 1, 39.
Hikichi, Y. (2016). Interactions between plant pathogenic bacteria and host plants involved in establishment of susceptibility. Japanese J. Phytopathology. 82, 156–159. doi: 10.3186/jjphytopath.82.156
Hu, H., Xia, W., Li, M., Yang, Q., Zhai, Z., Song, H., et al. (2023). First report of Didymium bahiense on Cultivated Schizophyllum commune in China. Plant Disease. 107, 3295. doi: 10.1094/PDIS-04-23-0687-PDN
Huang, S. W. (2019). Biological characteristics and key points of cultivation technology of Hymenopellis radicata 17. Edible Medicinal Mushrooms. 27, 424–426.
Jain, A., Sarsaiya, S., Wu, Q., Lu, Y., Shi, J. (2019). A review of plant leaf fungal diseases and its environment speciation. Bioengineered. 10, 409–424. doi: 10.1080/21655979.2019.1649520
Jeon, J. (2019). Phytobiome as a potential factor in nitrogen-induced susceptibility to the rice blast disease. Res. Plant Disease. 25, 103–107. doi: 10.5423/RPD.2019.25.3.103
Jia, Y., Kang, L., Wu, Y., Zhou, C., Li, D., Li, J., et al. (2023). Review on pesticide abiotic stress over crop health and intervention by various biostimulants. J. Agric. Food Chem. 71, 13595–13611. doi: 10.1021/acs.jafc.3c04013
Jones, R. A. C. (2016). “Future scenarios for plant virus pathogens as climate change progresses,” in Advances in virus research, vol. 95 . Eds. Kielian, M., Maramorosch, K., Mettenleiter, T. C., 87–147. doi: 10.1016/bs.aivir.2016.02.004
Kaloshian, I., Teixeira, M. (2019). Advances in plant-nematode interactions with emphasis on the notorious nematode genus Meloidogyne. Phytopathology. 109, 1988–1996. doi: 10.1094/PHYTO-05-19-0163-IA
Kamono, A., Kojima, H., Matsumoto, J., Kawamura, K., Fukui, M. (2009). Airborne myxomycete spores: detection using molecular techniques. Naturwissenschaften. 96, 147–151. doi: 10.1007/s00114-008-0454-0
Kang, S., Hao, X., Du, T., Tong, L., Su, X., Lu, H., et al. (2017a). Improving agricultural water productivity to ensure food security in China under changing environment: From research to practice. Agric. Water Management. 179, 5–17. doi: 10.1016/j.agwat.2016.05.007
Kang, S., Tice, A. K., Spiegel, F. W., Silberman, J. D., Panek, T., Cepicka, I., et al. (2017b). Between a pod and a hard test: the deep evolution of amoebae. Mol. Biol. Evolution. 34, 2258–2270. doi: 10.1093/molbev/msx162
Kataoka, M., Nakamori, T. (2020). Food preferences of Collembola for myxomycete plasmodia and plasmodium responses in the presence of Collembola. Fungal Ecology. 47, 100965. doi: 10.1016/j.funeco.2020.100965
Kim, W. G., Choi, H. W., Hong, S. K., Lee, Y. K., Lee, S. H. (2009). Occurrence of Fuligo gyrosa causing slime mold of oriental melon. Mycobiology. 37, 238–239. doi: 10.4489/MYCO.2009.37.3.238
Kim, W. G., Lee, S. Y., Cho, W. D. (2007). Two species of myxomycetes causing slime mold of sweet potato. Mycobiology. 35, 97–99. doi: 10.4489/MYCO.2007.35.2.097
Kong, W. L., Kang, Y. C., Zhang, Y. T., Yuan, R. Q., Kong, W. W., Han, Y. E., et al. (2014). Main diseases investigation of edible mushroom in Henan Province. Edible Fungi China. 33, 57–60. doi: 10.13629/j.cnki.53-1054.2014.04.020
Lee, J. H., Kim, D.-R., Kwak, Y.-S. (2014). First report of Stemonitis splendens Rostaf causing bark decay of oak logs used for shiitake cultivation in Korea. Mycobiology. 42, 279–281. doi: 10.5941/MYCO.2014.42.3.279
Leontyev, D. V., Schnittler, M., Stephenson, S. L., Novozhilov, Y. K., Shchepin, O. N. (2019). Towards a phylogenetic classification of the myxomycetes. Phytotaxa. 399, 209–238. doi: 10.11646/phytotaxa.399.3.5
Li, J. B. (2012). Occurrence of peanut diseases and pests in Xingye County and prevention and control measures. Modern Agric. Sci. Technology. 23, 142–144.
Li, R. X., Bai, J. K., Wang, Q., Li, Y. (1994). A new disease of strawberries-”mycosis”. J. Jilin Agric. University. 16, 92. doi: 10.13327/j.jjlau.1994.01.023
Li, Y. C., Li, Z. X. (1992). Separation and identification and biological characteristics of myxomycetes lyzing the fruitbodies of Auricularia Auricula and Auricularia Polytricha. J. Guangxi Agric. College. 11, 8–15.
Li, S., Qi, B., Wang, W., Peng, X., Gontcharov, A. A., Liu, B., et al. (2022). Diversity of bacterial communities in the plasmodia of myxomycetes. BMC Microbiol. 22, 314. doi: 10.1186/s12866-022-02725-5
Li, S. S., Qian, X. C., Xu, J. Z. (1992). Common hybrid bacteria in the production of black fungus shiitake mushrooms in Qinba Mountain and its prevention and control. Edible Fungi China. 11, 25–26. doi: 10.13629j.cnki.53-1054.1992.03.015
Li, P., Zhang, Y., Chen, Y. (2023). Bacterial wilt: pathogenic mechanism, disease control, bacteria-plant and bacteria-environmental microorganism interactions. Front. Microbiol. 14, 1–2. doi: 10.3389/fmicb.2023.1322189
Li, Y. S., Zhang, B., Jiang, S. C., Hsiang, T., Li, Y., Wang, X. L. (2017). First report of Physarella oblonga on Lentinula edodes in China. Plant Disease. 101, 2146–2147. doi: 10.1094/PDIS-02-17-0214-PDN
Lin, X. M. (1993). The harm of slime molds in the production of edible mushrooms in Henan. Edible Fungi China 1, 38.
Lindley, L. A., Stephenson, S. L., Spiegel, F. W. (2007). Protostelids and myxomycetes isolated from aquatic habitats. Mycologia. 99, 504–509. doi: 10.1080/15572536.2007.11832544
Lister, A. (1888). Notes on the plasmodium of Badhamia utricularis and Brefeldia maxima. Ann. Botany. 2, 1–24. doi: 10.1093/aob/os-2.1.1
Liu, Y. G. (2007). “Comprehensive prevention and treatment of Cyclocybe aegerita, Dictyophora sp. slime mold dieases and Trichoderma dieases of Ganoderma lucidum,” in Sanming edible mushroom technology promotion station.
Liu, Y. G., Lin, R. K. (2003). Symptoms and prevention and treatment technology of Agrocybe chaxingu mycosis. Edible Fungi. 3, 40.
Liu, Y. G., Xiao, S. G., Zhong, L. S., Weng, C. F., Lin, R. K., Liao, G. S. (2007). Stemonitis fusca of Dictyophora and its prevention and control. Edible Fungi. 4, 53–54.
Madelin, M. F., Audus, F., Knowlest, D. (1975). Attraction of plasmodia of the myxomycete, Badhamia utricularis, by extracts of the basidiomycete, Stereum hirsutum. J. Gen. Microbiol. 89, 229–234. doi: 10.1099/00221287-89-2-229
Marouelli, W. A., Guimaraes, T. G., Braga, M. B., de Carvalho e Silva, W. L. (2015). Optimal fractions of phosphorus fertilizer applied in preplanting and in drip fertigation of tomato crop. Pesquisa Agropecuaria Brasileira. 50, 949–957. doi: 10.1590/S0100-204X2015001000011
Mortensen, K., Molloy, M. M. (1989). Fungi detected on Acroptilon repens (Russian knapweed) during surveys from 1981 to 1988. Can. Plant Dis. Survev. 69, 143–145. doi: 10.13344/j.microbiol.China.200474
Naidu, R., Biswas, B., Willett, I. R., Cribb, J., Singh, B. K., Nathanail, C. P., et al. (2021). Chemical pollution: a growing peril and potential catastrophic risk to humanity. Environ. Int. 156, 106616. doi: 10.1016/j.envint.2021.106616
Nunes Lemos, D. B., Neves Nepomuceno Agra, ,.L., Iannuzzi, L., de Andrade Bezerra, M., Cavalcanti, L. (2010). Co-existence of myxomycetes and beetles in an Atlantic Rainforest remnant of Pernambuco, Brazil, with emphasis on staphylinids (Coleoptera: Staphylinidae). J. Natural History. 44, 1365–1376. doi: 10.1080/00222931003632724
Pecundo, M. H., Dagamac, N. H. A., Stephenson, S. L., Dela Crue, T. E. E. (2017). First myxomycete survey in the limestone forest of Puerto Princesa Subterranean River National Park, Palawan, Philippines. Nova Hedwigia. 104, 129–141. doi: 10.1127/nova_hedwigia/2016/0358
Prikhodko, I. S., Shchepin, O. N., Bortnikova, N. A., Novozhilov, Y. K., Gmoshinskiy, V. I., Moreno, G., et al. (2023). A three-gene phylogeny supports taxonomic rearrangements in the family Didymiaceae (Myxomycetes). Mycological Progress. 22, 11–34. doi: 10.1007/s11557-022-01858-1
Ross, L. N., Woodward, J. F. (2016). Koch’s postulates: an interventionist perspective. Stud. History Philosophy Biol. Biomed. Sci. 59, 35–46. doi: 10.1016/j.shpsc.2016.06.001
Schnittler, M., Stephenson, S. L. (2002). Inflorescences of Neotropical herbs as a newly discovered microhabitat for myxomycetes. Mycologia. 94, 6–20. doi: 10.1080/15572536.2003.11833244
Shen, T., Li, J. R., He, M. X. (2021). Occurrence regularity and pathogen identification of myxomycosis of Dendrobium Officinale. J. Jinhua Polytechnic. 21, 56–60. doi: 10.3969/j.issn.1671-3699.2021.06.010
Shen, X. J., Wang, Q., Liu, K. Y., Cai, J., Wang, H., Zhang, Q., et al. (2022). Main functional ingredients, nutritional, and medicinal values of common wild edible fungi: a review. Int. Food Res. J. 29, 1–9. doi: 10.47836/ifrj.29.1.01
Song, W. L., Chen, S. L. (2024). Arcyria similaris: A new myxomycete species from China. Mycologia. 116, 409–417. doi: 10.1080/00275514.2024.2312077
Stephenson, S. L., Feest, A. (2012). Ecology of soil eumycetozoans. Acta Protozoologica. 51, 201–208. doi: 10.4467/16890027ap.12.016.0762
Stephenson, S. L., Rojas, A. (2017). Myxomycetes: biology, systematics, biogeography, and ecology (United States: Academic Press).
Su, J. M. (1987). Observation on the occurrence of myxomycosis in sweet potato seedlings. Plant Prot. 28.
The Phytopathological Society of Japan (2000). Common names of plant diseases in Japan. 1st ed. (Tokyo, Japan: Japan Plant Protection Association), 857.
Trevino-Zevallos, I. F., Lado, C. (2020). Myxomycete diversity in a humid montane forest on the eastern slopes of the Peruvian Andes. Plant Ecol. Evolution. 153, 390–398. doi: 10.5091/plecevo.153.3
Trimble, C. (2021). Spore dispersal of slime molds and higher fungi via animal vectors University of Arkansas, Dissertation/Thesis.
Tu, Y. L., Xiao, F., Zhang, J. Z., Lu, Q. Q., Wang, L. F. (2016). Two “myxomycete diseases” occurred on cultivated fields of Dendrobium candidum. J. Zhejiang Univ. (Agric Life Sci.). 42, 137–142. doi: 10.3785/j.issn.1008-9209.2015.06.091
Tudi, M., Ruan, H. D., Wang, L., Lyu, J., Sadler, R., Connell, D., et al. (2021). Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 18, 1112. doi: 10.3390/ijerph18031112
Vega, D., Gazzano Santos, M. I., Salm-Zapata, W., Poggio, S. L. (2020). Revising the concept of crop health from an agroecological perspective. Agroecology Sustain. Food Systems. 44, 215–237. doi: 10.1080/21683565.2019.1643436
Wahbi, S., Maghraoui, T., Hafidi, M., Sanguin, H., Oufdou, K., Prin, Y., et al. (2016). Enhanced transfer of biologically fixed N from faba bean to intercropped wheat through mycorrhizal symbiosis. Appl. Soil Ecology. 107, 91–98. doi: 10.1016/j.apsoil.2016.05.008
Walker, L. M., Cedeno-Sanchez, M., Carbonero, F., Herre, E. A., Turner, B. L., Wright, S. J., et al. (2019). The response of litter-associated myxomycetes to long-term nutrient addition in a lowland tropical forest. J. Eukaryotic Microbiol. 66, 757–770. doi: 10.1111/jeu.12724
Wang, H. (2023). Epidemiology and control of fungal diseases in crop plants. Agronomy. 13, 2327. doi: 10.3390/agronomy13092327
Wang, M., Pan, F. M. (1985). A newly discovered disease-chili slime mold disease. Hubei Agric. Science. 11, 16. doi: 10.14088/j.cnki.issn0439-8114.1985.11.006
Wang, J. Y., Song, J. F., Song, J. C., Zhang, X. G., Li, S. Z. (2016). The occurrence rules and prevention and control measures of peanut Fuligo gyrosa in Nanyang Basin. Modern Agric. Sci. Technology. 7, 121.
Wang, Z. Y., Song, J. C., Zhang, H. X., Feng, X. S., Cui, X. W. (2014). Occurrence and control of Physarum gyrosum on peanut. J. Peanut Science. 43, 54–56. doi: 10.14001/j.issn.1002-4093.2014.04.009
Xia, Z. X. (2002). Bordeaux mixture skillfully treats myxomycetes of Pleurotus ostreatus. Agric. Technol. Services. 1, 45.
Xie, Y. H., Fang, L., Wang, L. P., Wang, H. R. (2017). Occurrence and investigation of Dendrobium disease in Zhejiang Province. J. Zhejiang Agric. Sci. 58, 1757–1759. doi: 10.16178/j.issn.0528-9017.20171027
Xing, L. J., Liu, H. G., Yuan, F. R., Han, X. D., Zhang, X. Y., Duan, X. J. (2011). Chemical prevention of myxomycetes disease in edible fungi. J. Anhui Agric. Sci. 39, 9024–9025. doi: 10.13989/j.cnki.0517-6611.2011.15.179
Yu, T., Mahe, L., Li, Y., Wei, X., Deng, X., Zhang, D. (2022). Benefits of crop rotation on climate resilience and its prospects in China. Agronomy. 12, 436. doi: 10.3390/agronomy12020436
Zhang, S., Griffiths, J. S., Marchand, G., Bernards, M. A., Wang, A. (2022). Tomato brown rugose fruit virus: an emerging and rapidly spreading plant RNA virus that threatens tomato production worldwide. Mol. Plant Pathology. 23, 1262–1277. doi: 10.1111/mpp.13229
Zhang, Z. G., Li, D. W. (2003). Modern lawn management China Forestry (Beijing: China Forestry Press).
Zhang, B., Li, Y. S., Li, T. H., Li, Y. (2018). First report of a new myxogastria (Stemonaria longa) causing rot disease on shiitake logs (Lentinula edodes) in China. Plant Disease. 102, 1032–1033. doi: 10.1094/PDIS-05-17-0719-PDN
Zhang, J. Z., Liu, L. N., Fiore-Donno, A.-M., Xu, T. (2007). Ultrastructural characters of a Physarum melleum on living leaves of Dendrobium candidum in China. J. Zhejiang Univ. Sci. B. 8, 896–899. doi: 10.1631/jzus.2007.B0896
Keywords: plasmodial slime molds, crop health, colonization, mushroom crop, cultural control, chemical control
Citation: Zhang Z, Zhai C, Li Y, Stephenson SL and Liu P (2024) Slime molds (Myxomycetes) causing a “disease” in crop plants and cultivated mushrooms. Front. Plant Sci. 15:1411231. doi: 10.3389/fpls.2024.1411231
Received: 02 April 2024; Accepted: 23 May 2024;
Published: 10 June 2024.
Edited by:
Abhay K. Pandey, North Bengal Regional R & D Center, IndiaReviewed by:
Paul Bayman, University of Puerto Rico, Río Piedras Campus, Puerto RicoJuiYu Chou, National Changhua University of Education, Taiwan
Copyright © 2024 Zhang, Zhai, Li, Stephenson and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pu Liu, pul@jlau.edu.cn
 Zhaojuan Zhang
Zhaojuan Zhang Chao Zhai1
Chao Zhai1 Yu Li
Yu Li Steven L. Stephenson
Steven L. Stephenson Pu Liu
Pu Liu