Abstract
As a typical form of empathy, empathy for pain refers to the perception and appraisal of others’ pain, as well as the corresponding affective responses. Numerous studies investigated the factors affecting the empathy for pain, in which the exposure to violent video games (VVGs) could change players’ empathic responses to painful situations. However, it remains unclear whether exposure to VVG influences the empathy for pain. In the present study, in terms of the exposure experience to VVG, two groups of participants (18 in VVG group, VG; 17 in non-VVG group, NG) were screened from nearly 200 video game experience questionnaires. And then, the functional magnetic resonance imaging data were recorded when they were viewing painful and non-painful stimuli. The results showed that the perception of others’ pain were not significantly different in brain regions between groups, from which we could infer that the desensitization effect of VVGs was overrated.
Introduction
Recently, the society has witnessed the rapidly development in video game industry. A non-negligible issue is that most of the video games contain violent content (Yoon and Somers, 2003), which could be harmful to the players, even jeopardize the public safety. The relationship between exposure to media violence and its potential negative effects has been the subject of social, political, and scientific attention for decades. Playing violent games may heighten aggressive behavior, cognition, and affection; increase physiological arousal and hostility; and decrease the probability of helping others (e.g., Anderson and Bushman, 2001, 2002; Bushman and Anderson, 2001, 2009; Anderson et al., 2004, 2008; Gentile et al., 2004; Bartholow et al., 2005; Bushman and Huesmann, 2006). Playing violent video games (VVGs) also has a desensitizing physiological effect (Carnagey et al., 2007), and may also be associated with non-violent delinquent behaviors (Anderson and Dill, 2000; Ferguson and Kilburn, 2009; Desai et al., 2010; Gunter and Daly, 2012), such as cheating, skipping school, stealing, and substance abuse. Previous researches have investigated the relationship between empathy and exposure to video game violence. It has been suggested that exposure to VVG was associated with lower empathy (Funk et al., 2004; Anderson et al., 2010). However, the existing researches concerning desensitization effects of VVG are not inconsistent. For example, Ferguson and Kilburn (2010) conducted a meta-analysis and the results suggested that VVGs were not significantly associated with aggression, neither with prosocial behavior (Jerabeck and Ferguson, 2013; Elson and Ferguson, 2014; Tear and Nielsen, 2014). Instead, some of the VVGs could even increase the cognition abilities such as object tracing, spatial discrimination, and central attention (Green and Bavelier, 2006, 2007). Even the inconsistent researches are not quite much, given the publication bias and the validity of behavioral investigations, the null results of previous researches should also pay attention to. These indicated that the long-time effect of VVGs should be carefully examined further.
Empathy is a crucial component of human emotional experience and social interaction (Bernhardt and Singer, 2012), which is vital to our everyday communication and survival in a social environment (Fan et al., 2011). Usually, empathy refers to the capacity to understand and share the emotional and affective states of another person in relation to oneself (Decety and Jackson, 2004; Singer et al., 2006; Hein and Singer, 2008; Guo et al., 2012, 2013). The capacity for empathy allows us to understand others’ emotions, motivations, and behaviors, which help us to decide what we can do. Empathy for pain is a typical form of empathy. When witnessing other people suffering in pain, the observers often show compassion, sympathy and care-giving to them (Goubert et al., 2005). The empathy for pain is attracting increasing attention because of its survival value embodied in the capacity that positively correlates to prosocial behavior and behaviors conforming to our social norms (Hoffman, 2008).
There are a growing number of functional magnetic resonance imaging (fMRI) studies that focus on empathy for pain. Research demonstrates that the first-hand experience of pain and the observation of others in pain activate similar neural circuits. These neural circuits consist of areas encoding different dimensions of pain perception. The primary and secondary somatosensory cortex mainly subserve the sensory-discriminative dimension of pain (e.g., Bushnell et al., 1999; Avenanti et al., 2005; Valeriani et al., 2008; Akitsuki and Decety, 2009), whereas the supplementary motor area (SMA), cerebellum, insula, anterior cingulate cortex (ACC), and the anterior mid-cingulate cortex (aMCC) mainly subserve the affective-motivational dimension of pain (e.g., Singer et al., 2004; Danziger et al., 2006; Gu and Han, 2007; Lamm et al., 2007; Akitsuki and Decety, 2009). These two dimensions are highly correlated (Decety, 2011). There are also brain regions encoding the cognitive-evaluative dimension of pain, such as the temporoparietal junction (TPJ) and the orbitofrontal cortex (OFC), which are involved in social interaction, intention, and belief (e.g., Walter et al., 2004; Amodio and Frith, 2006; Moll and de Oliveira-Souza, 2007). Other regions, like the amygdala, thalamus, and periaqueductal gray (PAG) may also be activated when watching others in pain (e.g., Phelps et al., 2001; Adolphs, 2002; Winston et al., 2003). Furthermore, empathy or empathy-related neural networks may interact with (and be modulated by) the activity of other neural networks relevant for social cognition, such as mentalizing, cognitive control, and emotion regulation (Bufalari and Ionta, 2013). Based on these findings, it is apparent that pain empathy is associated with cognitive and emotional regions such as TPJ, OFC, and ACC, which were vital in cognitive control and moral judgment (Molenberghs et al., 2015). We could speculate that lack of empathy for pain in others may lead to terrible consequences, not only to the individuals themselves, but also the whole society.
Long-time exposure to VVGs may blunt this capacity and result in undesirable consequences. In this research, we mainly focused on the relationship between long-term exposure to video game violence and empathy to investigate the negative effects of media violence on empathy, especially empathy for pain for others. According to former researches, desensitization to video game violence may be a core factor to low capacity of empathy for pain in others. It has been repeatedly proved that longtime exposure to video game violence leads to desensitization, which refers to impaired emotional response to negative feelings coupling with aggressive consequences. This may cause numb senses of knowing the pain and suffering of others which may result in low empathy for others’ pain. A negative correlation was confirmed between long-term video game violence exposure and empathy (Funk et al., 2004; Bartholow et al., 2005; Krahé and Möller, 2010). There are also neuropsychological evidences to support this argument. Playing VVGs can affect some regions or neural circuits of the frontal lobe (e.g., Davidson et al., 2000; Mathiak and Weber, 2006; Wang et al., 2009), and this may affect the response of gamers to emotional stimuli (Kühn et al., 2011). Similar results were found by Gentile et al. (2016) that long time exposure to VVGs may lead to suppression in regions relating to emotional response regions and cognitive regions and abnormal activities in cognitive control regions (Gentile et al., 2016). Moreover, Montag et al. (2012) assumed that due to a frequent confrontation with violent scenes, the first-person-shooter-video-gamers might have habituated to the effects of unpleasant stimuli, resulting in lower brain activation. Coincidentally, Guo et al. (2013) explored participants’ empathic responses after short-term exposure to violent videos, using fMRI. They found that short-term exposure to media violence reduced the activation of the aMCC and insula, and proposed that exposure to media violence had a desensitizing effect. These indicated that longtime exposure to media violence is associated with empathy for pain in others. In addition, it also should be noted that extant literature still exist some inconsistency between VVGs and desensitization effect, which needs further exploration.
What’s more, there currently is few fMRI research exploring the relationship between exposure to video game violence and pain empathy. This kind of research can help us to understand the neural mechanism of empathy and to identify the influence of violent games on the brain. Based on past research on empathy among healthy participants (e.g., Akitsuki and Decety, 2009; Fan et al., 2011, 2014; Lamm et al., 2011) and certain types of groups (e.g., Mathews and MacLeod, 2005; Decety et al., 2009), as well as research on the affective processing and empathy among violent video gamers (e.g., Anderson et al., 2010; Barlett and Anderson, 2011; Zhen et al., 2011; Guo et al., 2013), the present study aimed to identify whether video game violence can affect the capacity of empathy for pain, if so, how video game violence can affect the capacity of empathy for pain.
Materials and Methods
Participants
All the participants were recruited from the community of Southwest University (Chongqing, China). Participants were selected from approximately 200 undergraduates who completed a video game questionnaire (Anderson and Dill, 2000) that examined their previous game experience. We calculated a score representing their previous game experience and then randomly selected 20 individuals scoring above the 75th percentile and 20 individuals scoring below the 25th percentile, to comprise high and low previous-exposure groups (VG and NGs), respectively. All the participants were males aged 18 to 27 (M = 21.17, SD = 2.065). All the participants were right-handed and had normal or corrected-to-normal vision. None of them had a history of neurological or psychiatric disorders. All participants gave informed consent before scanning. After the experiment, they were paid for their participation.
Materials
Video Game Questionnaire
The video game questionnaire (Anderson and Dill, 2000) was used to select participants. Participants were asked to list three their favorite video games, indicate the number of hours they played each game in a week, and then rate the violence of their content and graphics (from 1 = not at all to 7 = extremely). Previous game experience was measured by summing the content and graphics ratings for each game, multiplying the sum of the number of hours the game was played each week, and then averaging across the three games. The questionnaire showed good reliability and validity. The internal consistency coefficient was 0.89–0.91, and the Q factor of each factor reached 0.7 (attractive factor: 0.77; violence factor: 0.90; time factor: 0.73).
Interpersonal Reactivity Index-China (IRI-C)
Interpersonal Reactivity Index-China (IRI-C) is a 22-item questionnaire for measuring trait empathy. The IRI-C is the Chinese version of IRI (Zhang et al., 2010), and four dimensions were included in this questionnaire: perspective taking, fantasy, empathic concern, and personal distress. IRI-C has been demonstrated to have satisfactory reliability and validity (Zhang et al., 2010; Jiang et al., 2014).
Stimuli in the Experiment
Eighty digital color pictures showing people’s hands, forearms, or feet in painful or non-painful situations (40 pictures each) were used as stimuli. All situations depicted familiar events that occasionally happen in our everyday life; the stimuli were similar to those used by Meng et al. (2012). Examples of painful situations depicted a hand cut by a knife and a foot stabbed by pins (Figure 1). Non-painful situations were paired with painful situations, without any nociceptive components, such as using a knife to cut cucumbers and a foot touched by an eraser. All pictures were shot from first-person perspectives and edited to the same size. Luminance, contrast, and color were matched between painful and non-painful pictures (Meng et al., 2012).
FIGURE 1
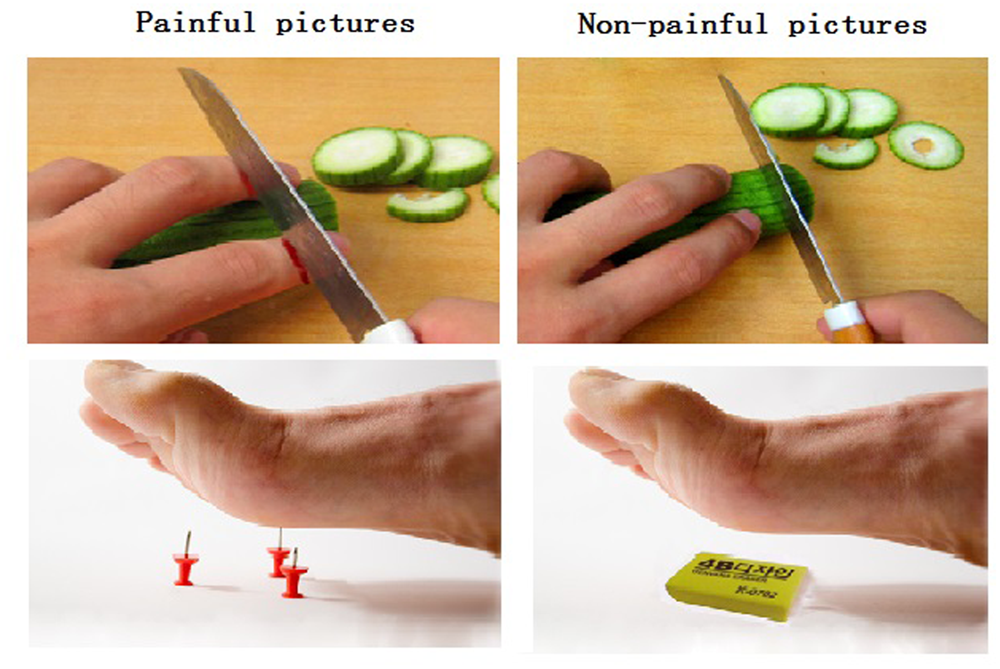
Illustration of the painful and non-painful pictures. (Left) The left panel shows examples of non-painful pictures. (Right) The right panel shows examples of painful pictures.
Procedure
First, participants were scanned to acquire high-resolution structural images. Then functional images were acquired, while the participants viewing the stimuli displayed on a gray background. The E-prime software (Psychology Software Tools, Inc., Pittsburgh, PA, USA) and a back-projection system were used for presenting the stimuli. All the pictures were randomly presented on the screen and the procedure in each run was exactly the same. Participants were asked to watch each picture carefully and to try to experience the feelings of the persons whose body parts were shown in the pictures. The oddball paradigm was used to ensure that participants viewed the pictures carefully and did not close their eyes. This entailed two kinds of trials: stimulus-only trials and stimulus–response trials.
In stimulus-only trials, each picture was presented for 2,000 ms with jittered inter-stimulus intervals (ISI, lasted for 2,000, 4,000, or 6,000ms), during which a black fixation point was presented against the gray background. Participants were instructed to view the picture carefully and just wait for the next trial. In stimulus–response trials, each picture was presented for 2,000 ms, followed by a response screen showing the following message: “painful picture: 1; non-painful picture: 4.” Participants were instructed to press “1” if they thought the picture was painful, and to press “4” if they thought the picture was non-painful. This screen remained for 2,000 ms. Jittered ISI were used as they were in the stimulus-only trials. Stimulus–response trials made up about 20 percent of all trials (16 trials) in the experiment. The two kinds of trials were randomly presented during the experiment (see Figure 2).
FIGURE 2
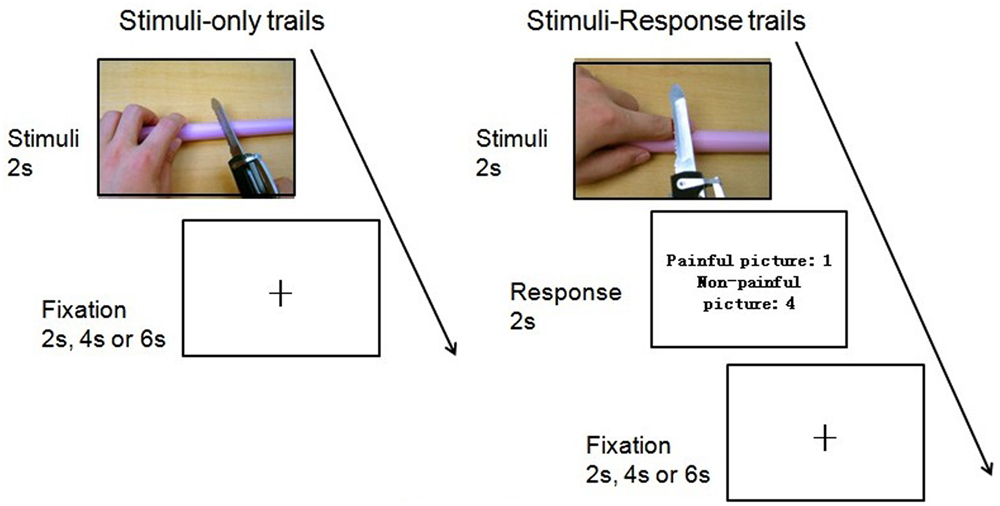
Flow paradigm of the experiment.
All the participants responded in all 17 stimulus–response trials. The mean number of correct answers of all participants was 14.64 (SD = 2.13). Participants in the two groups did not differ significantly in their mean number of correct answers (MV G = 14.57, SD = 2.31; MNG = 14.71, SD = 2.02). Therefore, both groups viewed the presented pictures equally.
fMRI Image Acquisition and Analysis
Scanning was performed with a whole-body 3T Siemens scanner (Siemens Magnetom Trio Tim, Southwest University, Chongqing, China). Functional images were acquired using an echo-planar imaging (EPI) sequence and the following parameters: slice number = 32, TR = 2000 ms, TE = 30 ms, flip angle = 90°, matrix size = 64 × 64, slice thickness = 3 mm. Images were acquired using an ascending interleaved sequence with no temporal gap between consecutive image acquisitions. There was one run of functional scanning that was approximately 9 min (270 EPI volumes). A high-resolution structural image was acquired using a T1-weighted, multiplanar reconstruction (MPR) sequence and the following parameters: TR = 1900 ms, TE = 2.52 ms, slice thickness = 1 mm, flip angle = 9°, matrix size = 256 × 256, voxel size = 3 mm × 3 mm × 3 mm.
Data preprocessing was carried out with SPM8 (Statistical Parametric Mapping, Wellcome Department of Imaging Neuroscience, London, UK) implemented in MATLAB 7.0 (The Math Works, Inc., Sherborn, MA, USA). The first five volumes were discarded to allow for T1 equilibration effects. Data preprocessing included slice-timing correction, correction for head motion (realigned to the first volume), normalization, and smoothing using a 6-mm full-width half-maximum isotropic Gaussian kernel. It should be noted that head motions in all participants were corrected and met the criteria with head motion < 3 mm. In this case, three participants were deleted from the analysis.
We then analyzed the neural responses to painful and non-painful stimuli in the VG (PVG and NVG) and in the NG (PNG and NNG). Statistical analyses were performed using the general linear model (GLM) implemented in SPM8. GLMs were estimated using a hemodynamic response function and a high pass filter of 128 Hz, as well as correction for autocorrelations. For the analysis, the six movement regressors of each subject were also included in the design matrix as covariates. The simple main effects of each subject for two types of events (P and NP) were computed by applying ‘1 -1’ contrasts. The four first-level individual contrast images (PVG, NVG, PNG, and NNG) were then analyzed at the second group level adopting the methods of independent samples t-test.
Brain activation representing the perception of painful stimuli was defined using the (PVG + PNG) – (NVG + NNG) contrast. The full factorial model was established to identify different brain regions between NG and VG [(PNG - NNG) - (PVG - NVG), (PVG - NVG) - (PNG - NNG)].
Results
Two participants in the NG did not complete our study so their data were deleted. The data of two participants in the NG and one in the VG were deleted either because of excessive head movements. Hence, the data in our final analysis were collected from 35 participants, including 18 participants in the VG and 17 participants in the NG.
Behavioral Data
An independent-sample t-test of VVG exposure in two conditions was conducted and results were found the difference of familiarity with the VG (M = 142.39, SD = 18.66) and NG (M = 25.62, SD = 6.44), t(33) = 5.78, p < 0.05, suggesting significant difference between VG and NG.
No significant difference between VG (M = 49.38, SD = 8.57), and NG (M = 47.52, SD = 10.79) in IRI-C total score, t(33) = 0.57, p > 0.05, either in each dimension [t(33) = 0.11, 0.26, 1.09, and 0.68, p > 0.05].
No significant difference between VG (M = 72.12, SD = 17.49), and NG (M = 72.66, SD = 25.19) in BPAQ total score, t(33) = -0.077, p > 0.05, either in each dimension [t(33) = -0.034, 0.365, -0.065, and -0.397, p > 0.05].
fMRI Data
As a first step, we contrasted the brain activity of the painful conditions with the non-painful conditions for all the subjects. The results of (PVG + PNG) - (NVG + NNG) showed that when viewing others in pain, regions were activated in the right supramarginal gyrus (rSMG), lateral middle occipital gyrus, lateral fusiform gyrus, right inferior occipital gyrus, inferior parietal gyrus, middle temporal gyrus, and visual related regions such as V2 (see Figure 3 and Table 1).
FIGURE 3
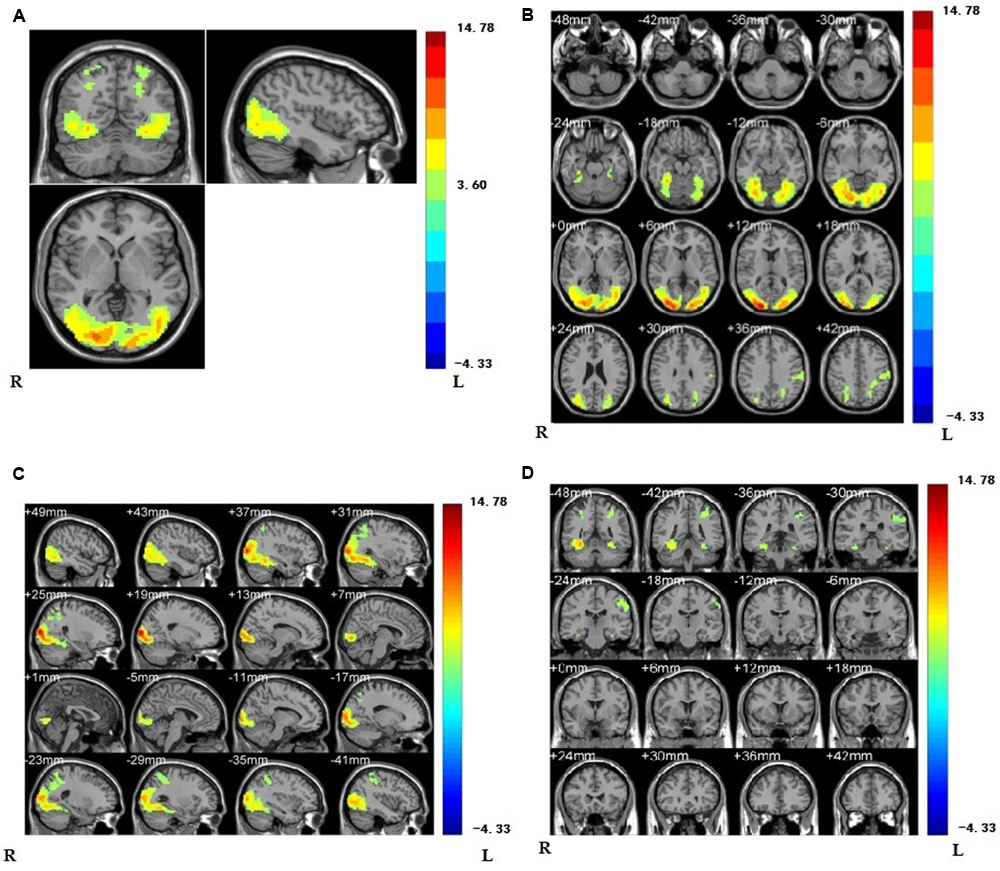
(A) Regions showing higher activation are the regions of supramarginal gyrus, lateral middle occipital gyrus, lateral fusiform gyrus, right inferior occipital gyrus, inferior parietal gyrus, middle temporal gyrus, and visual related regions such as V2 compared with non-painful stimuli (p < 0.001, Alphasim corrected; k > 1361). We have separately MR imaging at the position of oblique-axial (B) plane, oblique coronal; (C) and sagittalia; (D) in regions activated.
Table 1
| Region of activation | Lat. | MNI |
t-value | k | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Middle occipital gyrus | R | 21 | –96 | 12 | 14.78 | 483 |
| Middle occipital gyrus | L | –24 | –87 | 12 | 9.04 | 683 |
| Fusiform gyrus | R | 30 | –51 | –12 | 9.90 | 427 |
| Inferior occipital gyrus | R | 33 | –75 | –9 | 9.06 | 216 |
| Fusiform gyrus | L | –30 | –63 | –12 | 7.68 | 357 |
| Supramarginal gyrus | R | 33 | –54 | 57 | 5.18 | 130 |
| Inferior parietal gyrus | L | –27 | –51 | 48 | 4.76 | 187 |
| Secondary visual cortex (V2) | R | 36 | –87 | 9 | 11.75 | 369 |
Brain regions showing significant activation in lateral middle occipital gyrus, lateral fusiform gyrus, right inferior occipital gyrus, right supramarginal gyrus, inferior parietal gyrus, middle temporal gyrus, and visual related regions such as V2 while viewing painful stimuli compared with non-painful stimuli (p < 0.001, Alphasim corrected; k > 1361).
And one sample t-test in VG and NG was separately conducted and the results showed that, in both groups, regions in lateral occipital gyrus, lateral fusiform gyrus, right middle temporal gyrus, and the Secondary visual cortex (V2) were significantly activated (see Figures 4A,B and Table 2).
FIGURE 4
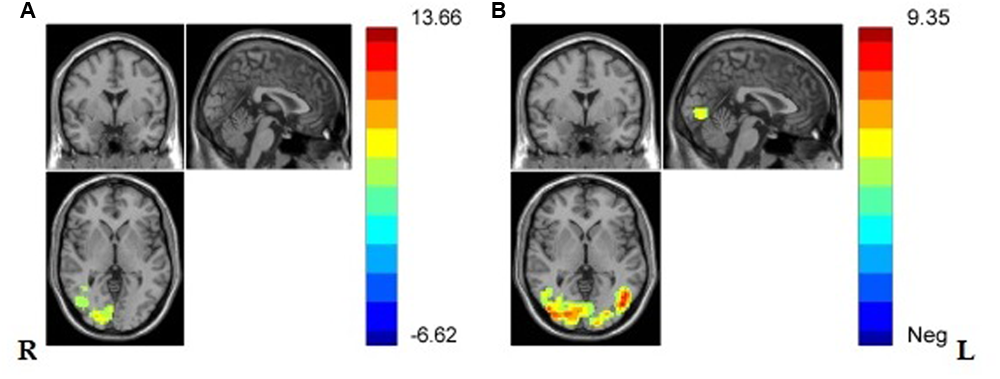
(A) Brain regions showing significant activation while viewing painful stimuli compared with non-painful stimuli in NG in lateral middle occipital gyrus, lateral V2, right middle temporal gyrus, lateral fusiform gyrus. (p < 0.001, Alphasim corrected; k > 1138). (B) Brain regions showing significant activation while viewing painful stimuli compared with non-painful stimuli in VG in lateral occipital gyrus, lateral fusiform gyrus, left V2, and right middle temporal gyrus (p < 0.001, Alphasim corrected; k > 1132).
Table 2
| Conditions | Region of activation | Lat. | MNI |
t-value | k | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| NG | Middle occipital gyrus | R | 21 | –96 | 12 | 13.66 | 367 |
| Middle occipital gyrus | L | –42 | –87 | 0 | 6.32 | 476 | |
| Secondary visual cortex (V2) | R | 36 | –87 | 9 | 8.26 | 102 | |
| Secondary visual cortex (V2) | L | –36 | –9 | 0 | 5.92 | 116 | |
| Middle temporal gyrus | R | 51 | –69 | 12 | 4.67 | 99 | |
| Fusiform gyrus | R | 36 | –48 | –15 | 6.28 | 249 | |
| Fusiform gyrus | L | –27 | –60 | –15 | 7.53 | 199 | |
| VG | Middle occipital gyrus | L | –45 | –75 | 0 | 7.89 | 442 |
| Middle occipital gyrus | R | 45 | –81 | 0 | 7.53 | 365 | |
| Fusiform gyrus | L | –48 | –66 | 0 | 8.30 | 195 | |
| Fusiform gyrus | R | 30 | –66 | –12 | 7.66 | 362 | |
| Secondary visual cortex (V2) | L | –12 | –78 | –9 | 7.09 | 268 | |
| Middle temporal gyrus | R | 48 | –63 | –3 | 6.21 | 93 | |
Brain regions showing significant activation in lateral occipital gyrus, lateral fusiform gyrus, left V2, and right middle temporal gyrus in VG, and significant activation in lateral occipital gyrus, lateral fusiform gyrus, right lingual gyrus, left V2, and right middle temporal gyrus while viewing painful stimuli compared with non-painful stimuli (p < 0.001, Alphasim corrected; k > 1132 in VG and k > 1138 in NG).
Since the focus of our study was to explore how previous exposure to video game violence influenced participants’ empathic responses, we examined the activation of brain regions showing differences between the two groups. There is no significant difference between VG and NG (p < 0.001, Alphasim corrected).
Discussion
The goal of our study was to explore the influence of previous exposure to video game violence on neural empathic responses to the pain of others. fMRI results showed that there is significant difference between viewing painful pictures of others and viewing non-painful pictures, which has also been proved separately in VG and NG. While further examination didn’t show that the empathic neural pattern is different between groups.
Consistent with previous fMRI studies on empathy for pain (e.g., Jackson et al., 2005; Cheng et al., 2008; Nummenmaa et al., 2008; Akitsuki and Decety, 2009; Guo et al., 2012, 2013), the present study found that viewing painful pictures activated many empathy-related regions in the (PVG + PNG) - (NVG + NNG) contrast.
Unlike the linguistic function that has been generally hitherto acknowledged, the supramarginal gyrus is also closely linked to empathy. The supramarginal gyrus is part of the somatosensory association cortex, which is involved in perception of space and limbs location and a part of the mirror neuron system (Carlson, 2012). It has been proved that supramarginal gyrus, especially the right supramarginal gyrus (rSMG) is significantly associated with self-other distinction, the crucial part of the theory of mind (ToM), attributing to the self-other distinction during empathy (Hoffmann et al., 2015). Empathy involves sharing the emotional state of others and being aware of the state both of self and others (Singer and Lamm, 2009). Failure of self-other distinction during empathy results in egocentric emotional responses and deficits in ToM (Hoffmann et al., 2015). Silani et al. (2013) found that overcoming emotional egocentricity bias in empathic judgment is associated with increased activation in the rSMG. What’s more, a research conducted by Lang et al. (2011) found the same rSMG activation to emotional exclamations of others’ pain. This is consistent with what we had expected, as viewing others in pain will activate the regions related to empathy. The occipital gyrus are mainly associated with visual processing (Berlucchi, 2014). It has been proved that the inferior occipital gyrus plays an important part in identifying emotionally important visual clues, and viewing unpleasant pictures can significantly activate the left inferior occipital gyrus compared to the neutral situations (Geday et al., 2003). What’s more, the posterior fusiform and inferior occipital gyrus were assumed as the core regions in identifying emotionally important visual clues (Geday et al., 2003). The present study also found that participants viewing painful pictures had stronger activation in the inferior parietal lobule than those viewing non-painful pictures. The inferior parietal lobule has a critical function in distinguishing between self-produced actions and actions generated by others (Decety and Jackson, 2004; Lamm et al., 2008). A previous fMRI study demonstrated that higher activation of this region reflects less self-other overlap, which leads to greater accuracy during social perception (Lawrence et al., 2006). Another significantly activated region is the lateral fusiform, which is known as a key region related to facial perception. However, it has been proved that fusiform gyrus is associated with the processing of “ToM” and so is empathy (Castelli et al., 2000; Gallagher et al., 2000; Moll et al., 2002). It can be modulated by emotional valence, and it has been proved that right fusiform gyrus was more active than the left during emotional processing (Geday et al., 2003). This is consistent with our findings, which suggest that exposure to painful pictures will induce the emotional response and the unpleasant pictures are more arousing.
It should be noted that there are no significant difference in the full factorial design in [(PVG - NVG) - (PNG - NNG)]. This may suggest that there is no deficit in the neural responses of empathy for pain in individuals with VVG experience, being inconsistent with some extant studies (e.g., Funk et al., 2004; Anderson et al., 2010; Strenziok et al., 2011; Zhen et al., 2011; Montag et al., 2012; Guo et al., 2013), which all suggest that long-term exposure to media violence has a desensitization effect. However, Decety et al. (2009) showed that youth with aggressive conduct disorder do not have a deficit in empathy and may have an atypical pattern of neural response while viewing others in pain. Similarly, a survey conducted by Collins and Freeman (2013) found no difference in empathy between gamers and non-gamers. This can be seen from the painful pictures and non-painful pictures contrast both in VG and NG. The brain activation in both VG and NG showed similar pattern when viewing painful pictures compared to viewing non-painful pictures. The lateral fusiform gyrus was activated in both groups, which is important during empathy.
This may indicate that long-time exposure to VVGs is not strongly associated with desensitization to violence, especially pain empathy to others. This is supported by researches conducted by Szycik et al. (2016, 2017). In their researches, the positive, negative, and neutral pictures were displayed and the fMRI data was collected. Repeated experiments proved that there was no evidence for a neural desensitization in the processing of emotionally salient stimuli, same as our research findings. Taking all the findings together, it is necessary for us to rethink the desensitization hypothesis. The catalyst model proposed by Ferguson et al. (2008) pointed that, just like competition, playing VVGs is the result of attacking intention, not the cause of it. In this case, VVGs are not significantly relevant to aggressive behaviors. At the same time, the catharsis theory of playing contends that playing VVG, especially action game, provide a way to drain the aggressive emotion and energy off, rather than increasing the aggressive belief. After enjoying themselves immersing in the games, the nervous feelings and extra energy were consumed, players are used to feeling entirely free from worry. Compared to researches based on self-report measures, neuropsychological researches are definitely more valid to testify the long-term effect.
This research was based on the comprehensive view of VVG effect without certain bias and based on what was shown in this study, it could be suggested that our research is more objective and convincing. However, our study also has some limitations and there are areas that need further exploration. The present study did not measure sensitivity to pain, so we cannot rule out the possibility that some of our findings were influenced by individual differences among the participants. On the other hand, although there were no gender-related differences on empathy shown in the present study, it still may be caused by the gender distribution. It should be noted that only males were examined in our research and the research is suitable when the participants are confined to males. Further studies should pay attention to gender distribution. Furthermore, unknown variations or inconsistencies in the functions of some brain regions and neural circuits might explain the observed activations in some brain regions. Exploring the influence of VVGs on cognitive empathy and emotional empathy separately may provide the topic with more precise findings.
Conclusion
The observation that there were no significant differences between VG and NG suggests that individuals with VVG exposure may not have a deficit in their capacity for empathy. The differences in empathy for pain between individuals with VVG experience and non-VVG experience indicated that the desensitization effect of VVGs is not significant.
Compliance With Ethical Standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained after detailed explanation of the study protocol, which was approved by the Ethics Committee of Southwest University. The Institutional Review Board at Southwest University (SWU) in Chongqing, China approved this consent procedure. Written informed consent was obtained from all participants. The Institutional Review Board at SWU approved all procedures. Informed consent was obtained from all individual participants included in the study.
Statements
Author contributions
Conceived and designed the experiments: XG and LW. Performed the experiments: XG, CL, and WP. Analyzed the data: CL, XG, WP, and MY. Wrote the paper: WP, XG, LW, and CL. Editing and Revisions: XG, AC, CL, and WP.
Funding
This study was funded by National Social Science Foundation of China (grant number 14XSH013) and Project of Research Office of Self-Supporting Personality and Community Psychology at Southwest University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1
Adolphs R. (2002). Recognizing emotion from facial expressions: psychological and neurological mechanisms.Behav. Cogn. Neurosci. Rev.121–61. 10.1177/1534582302001001003
2
Akitsuki Y. Decety J. (2009). Social context and perceived agency affects empathy for pain: an event-related fMRI investigation.Neuroimage47722–734. 10.1016/j.neuroimage.2009.04.091
3
Amodio D. M. Frith C. D. (2006). Meetings of minds: the medial frontal cortex and social cognition.Nat. Rev. Neurosci.7268–277. 10.1038/nrn1884
4
Anderson C. A. Bushman B. J. (2001). Effects of violent video games on aggressive behavior, aggressive cognition, aggressive affect, physiological arousal, and prosocial behavior: a meta-analytic review of the scientific literature.Psychol. Sci.12353–359. 10.1111/1467-9280.00366
5
Anderson C. A. Bushman B. J. (2002). Human aggression.Annu. Rev. Psychol.5327–51. 10.1146/annurev.psych.53.100901.135231
6
Anderson C. A. Carnagey N. L. Flanagan M. Benjamin A. J. Eubanks J. Valentine J. C. (2004). Violent video games: specific effects of violent content on aggressive thoughts and behavior.Adv. Exp. Soc. Psychol.36199–249. 10.1016/S0065-2601(04)36004-1
7
Anderson C. A. Dill K. E. (2000). Video games and aggressive thoughts, feelings, and behavior in the laboratory and in life.J. Pers. Soc. Psychol.78772–790. 10.1037/0022-3514.78.4.772
8
Anderson C. A. Sakamoto A. Gentile D. A. Ihori N. Shibuya A. Yukawa S. (2008). Longitudinal effects of violent video games on aggression in Japan and the United States.Pediatrics1221067–1072. 10.1542/peds.2008-1425
9
Anderson C. A. Shibuya A. Ihori N. Swing E. L. Bushman B. J. Sakamoto A. et al (2010). Violent video game effects on aggression, empathy, and prosocial behavior in eastern and western countries: a meta-analytic review.Psychol. Bull.136151–173. 10.1037/a0018251
10
Avenanti A. Bueti D. Galati G. Aglioti S. M. (2005). Transcranial magnetic stimulation highlights the sensorimotor side of empathy for pain.Nat. Neurosci.8955–960. 10.1038/nn1481
11
Barlett C. P. Anderson C. A. (2011). Re-appraising the situation and its impact on aggressive behavior.Pers. Soc. Psychol. Bull.371564–1573. 10.1177/0146167211423671
12
Bartholow B. D. Sestir M. A. Davis E. B. (2005). Correlates and consequences of exposure to video game violence: hostile personality, empathy, and aggressive behavior.Pers. Soc. Psychol. Bull.311573–1586. 10.1177/0146167205277205
13
Berlucchi G. (2014). Visual interhemispheric communication and callosal connections of the occipital lobes.Cortex561–13. 10.1016/j.cortex.2013.02.001
14
Bernhardt B. C. Singer T. (2012). The neural basis of empathy.Annu. Rev. Neurosci.351–23. 10.1146/annurev-neuro-062111-150536
15
Bufalari I. Ionta S. (2013). The social and personality neuroscience of empathy for pain and touch.Front. Hum. Neurosci.7:393. 10.3389/fnhum.2013.00393
16
Bushman B. J. Anderson C. A. (2001). Media violence and the American public: scientific facts versus media misinformation.Am. Psychol.56477–489. 10.1037/0003-066X.56.6-7.477
17
Bushman B. J. Anderson C. A. (2009). Desensitizing effects of violent media on helping others.Psychol. Sci.20273–277. 10.1111/j.1467-9280.2009.02287.x
18
Bushman B. J. Huesmann L. R. (2006). Short-term and long-term effects of violent media on aggression in children and adults.Arch. Pediatr. Adolesc. Med.160348–352. 10.1001/archpedi.160.4.348
19
Bushnell M. C. Duncan G. H. Hofbauer R. K. Ha B. Chen J. I. Carrier B. (1999). Pain perception: is there a role for primary somatosensory cortex?Proc. Natl. Acad. Sci. U.S.A.967705–7709. 10.1073/pnas.96.14.7705
20
Carlson N. R. (2012). Physiology of Behavior11th Edn.London: Pearson.
21
Carnagey N. L. Anderson C. A. Bushman B. J. (2007). The effect of video game violence on physiological desensitization to real-life violence.J. Exp. Soc. Psychol.43489–496. 10.1007/s10964-014-0202-z
22
Castelli F. Happe F. Frith U. Frith C. (2000). Movement and mind: a functional imaging study of perception and interpretation of complex intentional movement patterns.Neuroimage12314–325. 10.1006/nimg.2000.0612
23
Cheng Y. Yang C. Y. Lin C. P. Lee P. L. Decety J. (2008). Expertise modulates the perception of pain in others.Neuroimage401833–1840. 10.1016/j.neuroimage.2008.01.064
24
Collins E. Freeman J. (2013). Do problematic and non-problematic video game players differ in extraversion, trait empathy, social capital and prosocial tendencies?Comput. Hum. Behav.291933–1940. 10.1016/j.chb.2013.03.002
25
Danziger N. Prkachin K. M. Willer J. C. (2006). Is pain the price of empathy? The perception of others’ pain in patients with congenital insensitivity to pain.Brain1292494–2507. 10.1093/brain/awl155
26
Davidson R. J. Putnam K. M. Larson C. L. (2000). Dysfunction in the neural circuitry of emotion regulation—A possible prelude to violence.Science289591–594. 10.1126/science.289.5479.591
27
Decety J. (2011). The neuroevolution of empathy.Ann. N. Y. Acad. Sci.123135–45. 10.1111/j.1749-6632.2011.06027.x
28
Decety J. Jackson P. L. (2004). The functional architecture of human empathy.Behav. Cogn. Neurosci. Rev.371–100. 10.1177/1534582304267187
29
Decety J. Michalska K. J. Akitsuki Y. Lahey B. B. (2009). Atypical empathic responses in adolescents with aggressive conduct disorder: a functional MRI investigation.Biol. Psychol.80203–211. 10.1016/j.biopsycho.2008.09.004
30
Desai R. A. Krishnan-Sarin S. Cavallo D. Potenza M. N. (2010). Video-gaming among high school students: health correlates, gender differences, and problematic gaming.Pediatrics1261414–1424. 10.1542/peds.2009-2706
31
Elson M. Ferguson C. J. (2014). Twenty-five years of research on violence in digital games and aggression: empirical evidence, perspectives, and a debate gone astray.Eur. Psychol.1933–46. 10.1027/1016-9040/a000147
32
Fan Y. Duncan N. W. de Greck M. Northoff G. (2011). Is there a core neural network in empathy? An fMRI based quantitative meta-analysis.Neurosci. Biobehav. Rev.35903–911. 10.1016/j.neubiorev.2010.10.009
33
Fan Y. T. Chen C. Y. Chen C. H. Decety J. Cheng Y. W. (2014). Empathic arousal and social understanding in individuals with autism: evidence from fMRI and ERP measurements.Soc. Cogn. Affect. Neurosci.91203–1213. 10.1093/scan/nst101
34
Ferguson C. J. Kilburn J. (2009). The public health risks of media violence: a meta-analytic review.J. Pediatr.154759–763. 10.1016/j.jpeds.2008.11.033
35
Ferguson C. J. Kilburn J. (2010). Much ado about nothing: the misestimation and overinterpretation of violent video game effects in Eastern and Western nations: comment on Anderson et al. (2010). Psychol. Bull.136174–178. 10.1037/a0018566
36
Ferguson C. J. Rueda S. M. Cruz A. M. Ferguson D. E. Fritz S. Smith S. M. (2008). Violent video games and aggression: causal relationship or byproduct of family violence and intrinsic violence motivation?Crim. Justice Behav.35311–332. 10.1002/ab.20329
37
Funk J. B. Baldacci H. B. Pasold T. Baumgardner J. (2004). Violence exposure in real-life, video games, television, movies, and the internet: is there desensitization?J. Adolesc.2723–39. 10.1016/j.adolescence.2003.10.005
38
Gallagher H. L. Happe F. Brunswick N. Fletcher P. C. Frith U. Frith C. D. (2000). Reading the mind in cartoons and stories: an fMRI study of ‘theory of mind’ in verbal and nonverbal tasks.Neuropsychologia3811–21. 10.1016/S0028-3932(99)00053-6
39
Geday J. Gjedde A. Boldsen A. S. Kupers R. (2003). Emotional valence modulates activity in the posterior fusiform gyrus and inferior medial prefrontal cortex in social perception.Neuroimage18675–684. 10.1016/S1053-8119(02)00038-1
40
Gentile D. A. Lynch P. J. Linder J. L. Walsh D. A. (2004). The effects of violent video game habits on adolescent hostility, aggressive behaviors, and school performance.J. Adolesc.275–22. 10.1016/j.adolescence.2003.10.002
41
Gentile D. A. Swing E. L. Anderson C. A. Rinker D. Thomas K. M. (2016). Differential neural recruitment during violent video game play in violent-and nonviolent-game players.Psychol. Pop. Media Cult.539–51. 10.1037/ppm0000009
42
Goubert L. Craig K. D. Vervoort T. Morley S. Sullivan M. J. de C Williams A. C. et al (2005). Facing others in pain: the effects of empathy.Pain118285–288. 10.1016/j.pain.2005.10.025
43
Green C. S. Bavelier D. (2006). Enumeration versus multiple object tracking: the case of action video game players.Cognition101217–245. 10.1016/j.cognition.2005.10.004
44
Green C. S. Bavelier D. (2007). Action-video-game experience alters the spatial resolution of vision.Psychol. Sci.1888–94. 10.1111/j.1467-9280.2007.01853.x
45
Gu X. Han S. (2007). Neural substrates underlying evaluation of pain in actions depicted in words.Behav. Brain Res.181218–223. 10.1016/j.bbr.2007.04.008
46
Gunter W. D. Daly K. (2012). Casual or spurious: using propensity score matching to detangle the relationship between violent video games and violent behavior.Comput. Hum. Behav.281348–1355. 10.1016/j.chb.2012.02.020
47
Guo X. Zheng L. Wang H. Zhu L. Li J. Wang Q. et al (2013). Exposure to violence reduces empathetic responses to other’s pain.Brain Cogn.82187–191. 10.1016/j.bandc.2013.04.005
48
Guo X. Zheng L. Zhang W. Zhu L. Li J. Wang Q. et al (2012). Empathic neural responses to others’ pain depend on monetary reward.Soc. Cogn. Affect. Neurosci.7535–541. 10.1093/scan/nsr034
49
Hein G. Singer T. (2008). I feel how you feel but not always: the empathic brain and its modulation.Curr. Opin. Neurobiol.18153–158. 10.1016/j.conb.2008.07.012
50
Hoffman M. L. (2008). “Empathy and Prosocial Behavior,” inHandbook of Emotions3rd EdnedsLewisM.Haviland-JonesJ. M.BarrettL. F. (New York, NY: Guilford Publications) 440–455.
51
Hoffmann F. Koehne S. Steinbeis N. Dziobek I. Singer T. (2015). Preserved self-other distinction during empathy in autism is linked to network integrity of right supramarginal gyrus.J. Autism Dev. Disord.46637–648. 10.1007/s10803-015-2609-0
52
Jackson P. L. Meltzoff A. N. Decety J. (2005). How do we perceive the pain of others? A window into the neural processes involved in empathy.Neuroimage24771–779. 10.1016/j.neuroimage.2004.09.006
53
Jerabeck J. M. Ferguson C. J. (2013). The influence of solitary and cooperative violent video game play on aggressive and prosocial behavior.Comput. Hum. Behav.292573–2578. 10.1016/j.chb.2013.06.034
54
Jiang Y. B. Hu Y. Wang Y. Zhou N. Zhu L. Wang K. (2014). Empathy and emotion recognition in patients with idiopathic generalized epilepsy.Epilepsy Behav.37139–144. 10.1016/j.yebeh.2014.06.005
55
Krahé B. Möller I. (2010). Longitudinal effects of media violence on aggression and empathy among German adolescents.J. Appl. Dev. Psychol.31401–409. 10.1016/j.appdev.2010.07.003
56
Kühn S. Romanowski A. Schilling C. Lorenz R. Seiferth N. Banaschewski T. et al (2011). The neural basis of video gaming.Transl. Psychiatry1:e53.10.1038/tp.2011.53
57
Lamm C. Batson C. D. Decety J. (2007). The neural substrate of human empathy: effects of perspective-taking and cognitive appraisal.J. Cogn. Neurosci.1942–58. 10.1162/jocn.2007.19.1.42
58
Lamm C. Decety J. Singer T. (2011). Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain.Neuroimage542492–2502. 10.1016/j.neuroimage.2010.10.014
59
Lamm C. Porges E. C. Cacioppo J. T. Decety J. (2008). Perspective taking is associated with specific facial responses during empathy for pain.Brain Res.1227153–161. 10.1016/j.brainres.2008.06.066
60
Lang S. Yu T. Markl A. Müller F. Kotchoubey B. (2011). Hearing others’ pain: neural activity related to empathy.Cogn. Affect. Behav. Neurosci.11386–395. 10.3758/s13415-011-0035-0
61
Lawrence E. J. Shaw P. Giampietro V. P. Surguladze S. Brammer M. J. David A. S. (2006). The role of “shared representations” in social perception and empathy: an fMRI study.Neuroimage291173–1184. 10.1016/j.neuroimage.2005.09.001
62
Mathews A. MacLeod C. (2005). Cognitive vulnerability to emotional disorders.Annu. Rev. Clin. Psychol.1167–195. 10.1146/annurev.clinpsy.1.102803.143916
63
Mathiak K. Weber R. (2006). Toward brain correlates of natural behavior: fMRI during violent video games.Hum. Brain Mapp.27948–956. 10.1002/hbm.20234
64
Meng J. Hu L. Shen L. Yang Z. Chen H. Huang X. et al (2012). Emotional primes modulate the responses to others’ pain: an ERP study.Exp. Brain Res.220277–286. 10.1007/s00221-012-3136-2
65
Molenberghs P. Ogilvie C. Louis W. R. Decety J. Bagnall J. Bain P. G. (2015). The neural correlates of justified and unjustified killing: an fMRI study.Soc. Cogn. Affect. Neurosci.101397–1404. 10.1093/scan/nsv027
66
Moll J. de Oliveira-Souza R. (2007). Moral judgments, emotions and the utilitarian brain.Trends Cogn. Sci.11319–321. 10.1016/j.tics.2007.06.001
67
Moll J. Oliveira-Souza R. Eslinger P. J. Bramati I. E. Mourao-Miranda J. Andreiuolo P. A. et al (2002). The neural correlates of moral sensitivity: a functional magnetic resonance imaging investigation of basic and moral emotions.J. Neurosci.222730–2736.
68
Montag C. Weberb B. Trautner P. Newportb B. Marketta S. Walter N. T. (2012). Does excessive play of violent first-person-shooter-video-games dampen brain activity in response to emotional stimuli?Biol. Psychol.89107–111. 10.1016/j.biopsycho.2011.09.014
69
Nummenmaa L. Hirvonen L. Parkkola R. Hietanen J. K. (2008). Is emotional contagion special? An fMRI study on neural systems for affective and cognitive empathy.Neuroimage43571–580. 10.1016/j.neuroimage.2008.08.014
70
Phelps E. A. O’Connor K. J. Gatenby J. C. Gore J. C. Grillon C. Davis M. (2001). Activation of the left amygdala to a cognitive representation of fear.Nat. Neurosci.4437–441. 10.1038/86110
71
Silani G. Lamm C. Ruff C. C. Singer T. (2013). Right supramarginal gyrus is crucial to overcome emotional egocentricity bias in social judgments.J. Neurosci.3315466–15476. 10.1523/JNEUROSCI.1488-13.2013
72
Singer T. Lamm C. (2009). The social neuroscience of empathy.Ann. N. Y. Acad. Sci.115681–96. 10.1111/j.1749-6632.2009.04418.x
73
Singer T. Seymour B. O’Doherty J. Kaube H. Dolan R. J. Frith C. D. (2004). Empathy for pain involves the affective but not the sensory components of pain.Science3031157–1161. 10.1126/science.1093535
74
Singer T. Seymour B. O’Doherty J. P. Stephan K. E. Dolan R. J. Frith C. D. (2006). Empathic neural responses are modulated by the perceived fairness of others.Nature439466–469. 10.1038/nature04271
75
Strenziok M. Krueger F. Deshpande G. Lenroot R. K. van der Meer E. Grafman J. (2011). Fronto-parietal regulation of media violence exposure in adolescents: a multi-method study.Soc. Cogn. Affect. Neurosci.6537–547. 10.1093/scan/nsq079
76
Szycik G. R. Mohammadi B. Hake M. (2016). Excessive users of violent video games do not show emotional desensitization: an fMRI study.Brain Imaging Behav.10.1007/s11682-016-9549-y[Epub ahead of print].
77
Szycik G. R. Mohammadi B. Münte T. F. Wildt B. T. (2017). Lack of evidence that neural empathic responses are blunted in excessive users of violent video games: an fMRI study.Front. Psychol.8:174. 10.3389/fpsyg.2017.00174
78
Tear M. J. Nielsen M. (2014). Video games and prosocial behavior: a study of the effects of non-violent, violent and ultra-violent gameplay.Comput. Hum. Behav.418–13. 10.1177/0146167213520459
79
Valeriani M. Betti V. Le P. D. De A. L. Miliucci R. Restuccia D. et al (2008). Seeing the pain of others while being in pain: a laser-evoked potentials study.Neuroimage401419–1428. 10.1016/j.neuroimage.2007.12.056
80
Walter H. Adenzato M. Ciaramidaro A. Enrici I. Bara B. G. (2004). Understanding intentions in social interaction: the role of the anterior paracingulate cortex.J. Cogn. Neurosci.161854–1863. 10.1162/0898929042947838
81
Wang Y. Mathews V. P. Kalnin A. J. Mosier K. M. Dunn D. W. Saykin A. J. (2009). Short term exposure to a violent video game induces changes in frontolimbic circuitry in adolescents.Brain Imaging Behav.338–50. 10.1007/s11682-008-9058-8
82
Winston J. S. Vuilleumier P. Dolan R. J. (2003). Effects of low-spatial frequency components of fearful faces on fusiform cortex activity.Curr. Biol.131824–1829. 10.1016/j.cub.2003.09.038
83
Yoon J. S. Somers C. L. (2003). Aggressive content of high school students’. TV viewing.Psychol. Rep.93949–953. 10.2466/pr0.2003.93.3.949
84
Zhang F. B. Dong Y. Wang L. Chan C. Y. Xie L. F. (2010). Reliability and validity of the Chinese version of the Interpersonal Reactivity Index-C.Chin. J. Clin. Psychol.2155–157.
85
Zhen S. J. Xie H. L. Zhang W. Wang S. J. Li D. P. (2011). Exposure to violent computer games and Chinese adolescents’ physical aggression: the role of beliefs about aggression, hostile expectations, and empathy.Comput. Hum. Behav.271675–1687. 10.1016/j.chb.2011.02.006
Summary
Keywords
violent video games, violence, empathy, empathy for pain, fMRI
Citation
Gao X, Pan W, Li C, Weng L, Yao M and Chen A (2017) Long-Time Exposure to Violent Video Games Does Not Show Desensitization on Empathy for Pain: An fMRI Study. Front. Psychol. 8:650. doi: 10.3389/fpsyg.2017.00650
Received
21 November 2016
Accepted
11 April 2017
Published
02 May 2017
Volume
8 - 2017
Edited by
Jintao Zhang, Beijing Normal University, China
Reviewed by
Kai Yuan, Xidian University, China; Sheng Zhang, Yale University, USA
Updates
Copyright
© 2017 Gao, Pan, Li, Weng, Yao and Chen.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuemei Gao, 38153531@qq.com Antao Chen, 375273948@qq.com
† These authors are co-first authors.
This article was submitted to Psychopathology, a section of the journal Frontiers in Psychology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.