- 1School of Social Work, Columbia University, New York, NY, United States
- 2Department of Psychology, Columbia University, New York, NY, United States
- 3Division on Substance Use, Department of Psychiatry, New York State Psychiatric Institute, New York, NY, United States
- 4Department of Psychological Sciences, Swinburne University, Hawthorn, VIC, Australia
Background: Despite limited data demonstrating pronounced negative effects of prenatal cannabis exposure, popular opinion and public policies still reflect the belief that cannabis is fetotoxic.
Methods: This article provides a critical review of results from longitudinal studies examining the impact of prenatal cannabis exposure on multiple domains of cognitive functioning in individuals aged 0 to 22 years. A literature search was conducted through PsycINFO, PubMed, and Google Scholar. Articles were included if they examined the cognitive performance of offspring exposed to cannabis in utero.
Results: An examination of the total number of statistical comparisons (n = 1,001) between groups of participants that were exposed to cannabis prenatally and non-exposed controls revealed that those exposed performed differently on a minority of cognitive outcomes (worse on <3.5 percent and better in <1 percent). The clinical significance of these findings appears to be limited because cognitive performance scores of cannabis-exposed groups overwhelmingly fell within the normal range when compared against normative data adjusted for age and education.
Conclusions: The current evidence does not suggest that prenatal cannabis exposure alone is associated with clinically significant cognitive functioning impairments.
Introduction
In the United States (U.S.), and in most countries around the world, cannabis is illegal. Still, according to recent data from the U.S., more than 25 million people reported past month cannabis use, easily outpacing the number of current cocaine (2.2 million) and heroin users (494,000) (NSDUH Detailed Tables, 2017). In addition, 63.8% of respondents to a global drug survey endorsed using cannabis at least once, a rate higher than any other illicit drug (GDS, 2019). Taken together, these findings demonstrate that cannabis use persists in the U.S. as well as around the globe despite legal restrictions.
Recently, countries such as Uruguay and Canada have legalized cannabis for recreational purposes. In the U.S., 11 states have legalized adult cannabis use, while 33 states now allow medical use of the drug. As a result of these recent developments, increased concerns have been raised about cannabis use by pregnant individuals and the impact it may have on the developing fetus. Indeed, cannabis is the most frequently used illicit substance by reproductive aged women in the U.S. (van Gelder et al., 2010; NSDUH Detailed Tables, 2017; National Pregnancy Health Survey, 2019). However, reported use during pregnancy is uncommon (Ko et al., 2015; NSDUH Detailed Tables, 2017; National Pregnancy Health Survey, 2019). Even when cannabis is used by pregnant individuals, use of the drug substantially decreases as pregnancy progresses (Ko et al., 2015). Nonetheless, there remains a minority of women who consume cannabis throughout pregnancy (Ko et al., 2015).
There is a growing scientific database assessing the effects of prenatal cannabis exposure on a myriad of measures, including early physical growth. In general, when proper controls are included, no relationship between prenatal cannabis exposure and adverse physical neonatal outcomes such as birth weight and head circumference has been found (Conner et al., 2016; Grant et al., 2018). Still, a concern expressed in the scientific literature is that although cannabis may not lead to severe physical abnormalities in infants, it might cause subtle changes in the brain that later manifest as deficits in cognitive functioning.
A burgeoning number of reviews have assessed the impact of prenatal cannabis exposure on cognitive functioning (Karila et al., 2006; Wu et al., 2011; Calvigioni et al., 2014; Higuera-Matas et al., 2015). The studies reviewed show that subtle differences in the cognitive performance between children who had been exposed to the drug prenatally and controls do exist, but the conclusions drawn sometimes extend too far beyond the actual data. For example, based on these subtle differences, some researchers have suggested that children prenatally exposed to cannabis exhibit cognitive deficits and/or behavioral abnormalities (Karila et al., 2006; Wu et al., 2011; Calvigioni et al., 2014; Higuera-Matas et al., 2015). The clinical implications of these subtle differences, however, are nearly impossible to determine without knowledge of the expected range of performance for a particular group. Through the use of normative data, whereby individual or mean group scores are compared against a normative database that accounts for age, and educational level, the clinical significance of the differences can be determined. This is a core assessment principle in clinical neuropsychology but appears to be largely ignored in the literature on prenatal cannabis exposure (Harvey, 2012).
In light of the important caveat highlighted above, we felt a critical review of the empirical literature on the cognitive outcomes of children prenatally exposed to cannabis was warranted. In order to assess the clinical significance of findings from the studies reviewed, we determined whether data for cannabis-exposed groups fell outside the average range of functioning when compared against a normative database. If, study investigators did not compare their data with normative scores—this was the case for several studies—we made such comparison ourselves whenever possible. Thus, this article addresses an important gap in our scientific knowledge in that findings should shed light on the extent to which prenatal cannabis exposure produces clinical consequences on offspring. This, of course, could have important public health and policy implications.
Methods
Search Strategy
The search strategy employed aimed to identify studies that examined the cognitive effects of prenatal cannabis exposure in humans. Articles up to December 2017 were independently searched by two authors (CAT and CLH) using PsycINFO, PubMed, and Google Scholar. Search terms used keywords: cognitive, pregnancy, and marijuana. Review articles were used as an additional resource to identify studies that might have escaped detection through our initial search. Similarly, reference lists of relevant articles were reviewed for any further potentially eligible studies.
Inclusion Criteria
The inclusion criteria were: (1) full-text publication in peer-reviewed journal, (2) available in English, (3) assessed cognitive consequences of prenatal cannabis exposure in humans, and (4) provided quantitative measurement of cognitive performance. Studies were excluded if they relied exclusively on questionnaires or brain imaging data as proxies for cognitive functioning.
Data Extraction
Data was extracted regarding the University and/or research group responsible for conducting the research, cognitive domains tested, participant demographics, cannabis exposure, study findings, and caveats. Additional information was retrieved as to whether individual scores, mean scores, and/or adjusted mean scores were reported, and whether these were compared to normative databases.
Clinical Significance Assessment
Cognitive data were assessed for statistically significant differences between children exposed to cannabis prenatally and controls. We then determined whether researchers had compared individual participants' scores against normative databases for the cognitive tasks assessed. When individual scores were not reported, published prenatally exposed group means were compared against the appropriate normative databases, whenever possible. This allowed us to determine whether group means fell outside the normal range of functioning. Norms were obtained from publicly available task administration manuals.
Calculating Number of Cognitive Outcomes
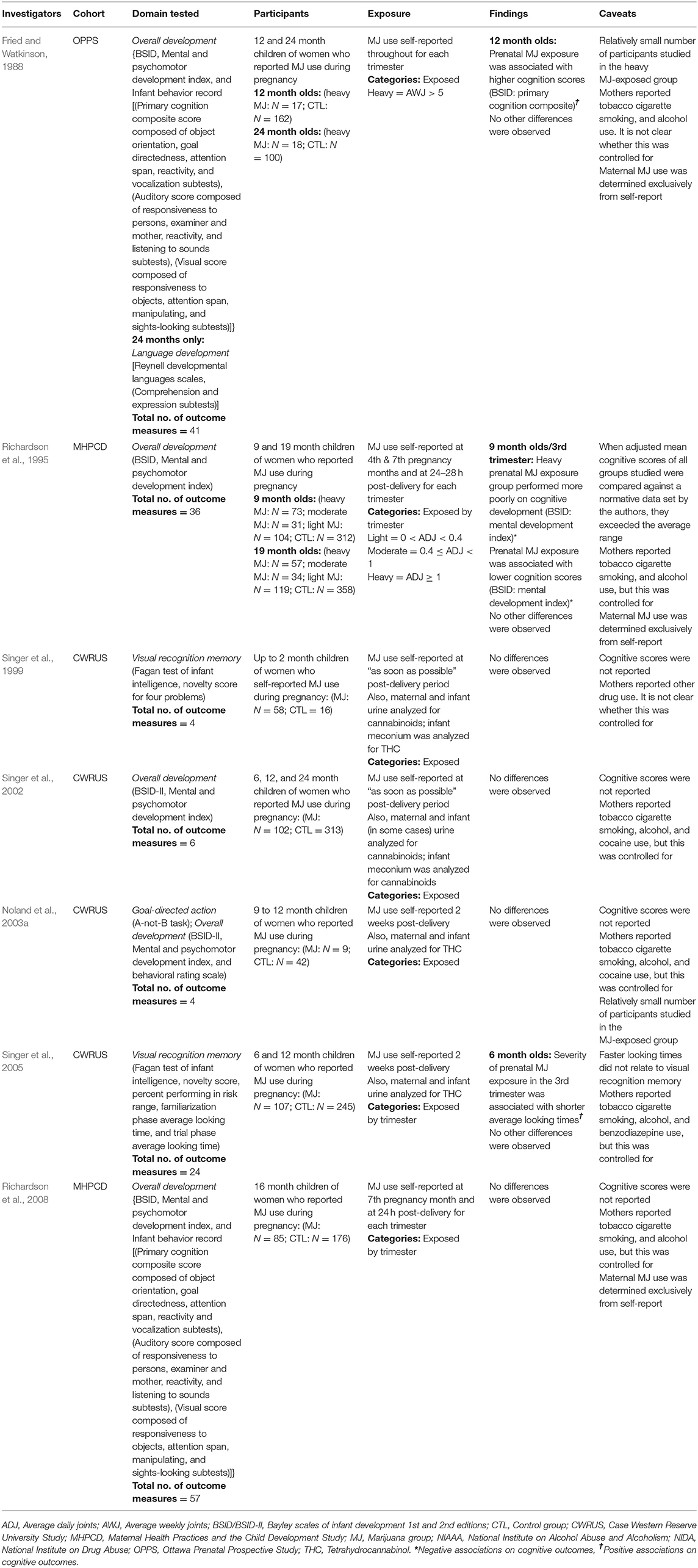
The number of cognitive outcomes per study was calculated by summing the number of tasks and/or subtests, and then multiplying by the number of study time-points, prenatal cannabis exposure levels (e.g., light, moderate, heavy), and trimester exposure categories (i.e., first, second, third). This generated the number of statistical comparisons made between different groups, per study. For example, Richardson et al. (1995) used two subtests of the Bayley Scales of Infant Development task (BSID) at two time-points (9 and 19 months) to assess children with light, moderate and heavy prenatal cannabis exposure, with levels of the exposure subdivided by trimester. Therefore, the final calculation is two (task subtests), multiplied by two (study time-points), multiplied by three (exposure levels), multiplied by three (trimesters), for a total of 36 cognitive outcomes assessed. This approach ultimately allowed us to determine the percent of cognitive outcomes on which children who were exposed to cannabis prenatally performed statistically different from controls.
Rationale for Not Conducting Meta-Analysis
We did not perform a meta-analysis because there were vast differences between studies in quantification of exposure, assessment time-points, location, covariates used, and cognitive outcomes measured. We have instead presented a critical review of the articles embedded within the results section, enabling the reader to be aware of the limitations in interpretation of the prenatal cannabis exposure studies included in this paper. Due to space restrictions, only those studies that reported positive and/or negatively associated cognitive outcomes are critically reviewed in the text of the results section. In the discussion we have additionally provided suggestions for how future studies may improve the reporting, interpretation and communication of their results.
Review of Results
Characteristics of Included Studies
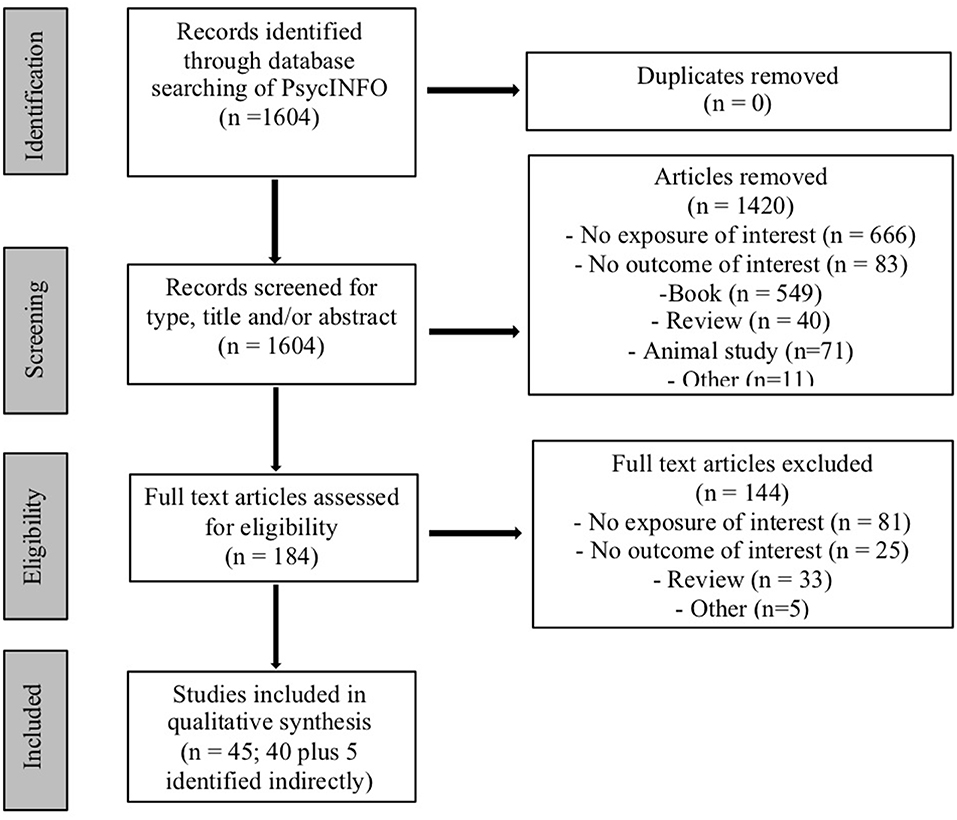
A total of 1,604 articles were identified in the initial search, of which 184 were chosen for full text review after exclusion of irrelevant studies based on title, abstract, keywords, and/or results. Of these, 144 did not meet inclusion criteria and were excluded, with 40 (and 5 additional articles identified indirectly) deemed appropriate for this review based on inclusion criteria (see Figure 1). Individual studies ranged in sample size from 9 (for cannabis-exposed children) to 538 (for control children) participants, and length of follow up ranged from 2 months to 22 years.
The majority of included articles derive from two major longitudinal studies—Ottawa Prenatal Prospective Study (OPPS) and Maternal Health Practices and the Child Development Study (MHPCD). The OPPS examined the potential relationship between prenatal cannabis, tobacco, and alcohol exposure and offspring development in a predominantly White middle-class cohort originally recruited in Ottawa, Canada (Fried and Watkinson, 1988, 1990, 2000, 2001; O'Connell and Fried, 1991; Fried et al., 1992a,b, 1997, 1998, 2003; Smith et al., 2004, 2006, 2016). The second most represented study—MHPCD—focuses primarily on low socioeconomic status African-American women and their offspring recruited from an outpatient prenatal clinic in Pittsburg, Pennsylvania (Day et al., 1994, 2011; Richardson et al., 1995, 2002, 2008, 2009, 2015; Leech et al., 1999; Goldschmidt et al., 2004, 2008, 2012; Willford et al., 2010).
The remaining articles came from seven other studies. The University of Miami's Jamaican Study (UMJS) recruited its participants in Jamaica, and is the only study where participants were recruited outside of Canada and the U.S. (Hayes et al., 1991). Another study we have labeled as the Case Western Reserve University Study (CWRUS), follows a mostly Black cohort located at the county hospital for Cleveland, Ohio (Singer et al., 1999, 2002, 2005, 2008; Noland et al., 2003a,b, 2005; Lewis et al., 2004, 2010). Others we have labeled as the Drexel and Robert Wood Johnson Universities Study (DRWJ), follows children from a predominantly African American sample in the U.S. (Bennett et al., 2008; Carmody et al., 2011) and the Boston and Harvard Universities Study (BHUS), which follows children from a mostly African-American and Caribbean cohort in Boston, Massachusetts (Frank et al., 2005; Beeghly et al., 2006; Rose-Jacobs et al., 2011, 2012). Finally, the remaining articles are from studies conducted by the University of Miami School of Medicine (UMSM) (Morrow et al., 2006), the Yale Child Study Center (YCSC) (Mayes et al., 2007), or the Children's Hospital of Philadelphia (CHP) (Hurt et al., 2005, 2009). Space constraints preclude us from detailing the methodologies in each of these studies; this information can be found in earlier reviews (Day et al., 1985; Fried, 2002; Jaddoe et al., 2012; Huizink, 2014).
Assessment Tools Used
A total of 30 studies subdivided mothers by whether they consumed cannabis or not during pregnancy (yes/no). Fifteen studies further subdivided participants by self-reported level (“low,” “moderate,” and “heavy”) of cannabis exposure.
Only a third of the studies confirmed the presence of cannabis with biological assays using urine and/or meconium. In addition, few studies (12 out of 45 studies) tested for the potential effects of prenatal cannabis exposure as a function of trimester (first, second, or third) (Table 1).
The cognitive domains tested, participant demographics, level of cannabis exposure, proposed findings, and caveats for all included studies are presented in Tables 1–4. Due to the different types of exposure categorization and cognitive measures used across studies, results were grouped by age at which the prenatally exposed children were assessed. Seven studies reported on infants and toddlers (up to 24 months); 19 reported on children (3 to 9 years); 12 reported on early adolescence (9 to 12 years), and eight reported on adolescence and early adulthood (13 to 22 years).
Tables A1–A4 in Supplementary Material outline whether each study reported individual, group mean, and adjusted group mean cognitive task scores and compared them to normative data. When cognitive task scores were reported, and normative databases were publicly available, we carried out the comparisons ourselves and report the results below.
Summary of Results
In general, prenatal cannabis exposure was associated with few effects, negative or positive. Of the 1,004 cognitive outcomes assessed, children with prenatal cannabis exposure performed more poorly on 34 (3.4%) and better on 9 (0.9%) when compared to a control group.
Cognitive task scores for individual participants were not included or compared to normative data in any of the studies reviewed. Mean cognitive task scores were reported in 17 studies and compared against normative data by the authors on only one occasions (Rose-Jacobs et al., 2011). Adjusted group means were reported in seven studies and compared to normative data by the authors on two occasions (Richardson et al., 1995; Rose-Jacobs et al., 2011). Because the performance of children exposed to cannabis prenatally did not differ from non-exposed children on the majority of cognitive outcomes, we will discuss the studies where significant effects were found.
Studies Assessing Infants and Toddlers (0–24 Months)
Table 1 shows studies examining the association between prenatal cannabis exposure and cognitive functioning in infants and toddlers. A total of 169 cognitive outcomes were assessed. About half (55 percent) were obtained from the MHPCD, 22 percent from the OPPS, and 22 percent from CWRUS.
Generally, prenatal cannabis exposure was not associated with cognitive performance. However, prenatal cannabis exposure was associated with worse performance on two cognitive outcomes (Richardson et al., 1995) and better performance on two cognitive outcomes (Fried and Watkinson, 1988; Singer et al., 2005). Cognitive performance scores were compared to a normative database by the authors in one (Richardson et al., 1995) of the seven articles (Table A1 in Supplementary Material).
Fried and Watkinson (1988) assessed the cognitive functioning of 12- and 24-month old children whose mothers reported using over 5 joints per week during their pregnancies. Of 38 cognitive outcomes reported, one significant association was found. Children who were prenatally exposed to cannabis performed better than non-exposed children on the BSID as indicated by higher Primary Composite scores. It is important to point out that although unadjusted mean scores were reported; they were not compared to normative data. We attempted to obtain normative scores but were unable to do so, making it difficult to determine the clinical relevance of the finding. It is also important to note that there was a relatively small number (N = 17) of children in the group with prenatal cannabis exposure.
Singer et al. (2005) studied a considerably larger sample (N = 107) of infants with prenatal cannabis exposure. Of the 24 cognitive outcomes assessed, one significant positive association was found: prenatal cannabis exposure in the third trimester was associated with shorter average looking times on the Visual Recognition Memory task. However, shorter average looking times were not associated with visual recognition memory. Task scores were not reported, nor were they compared against a normative dataset making it difficult to determine the clinical relevance of the finding.
Unlike the study above, Richardson et al. (1995) compared cognitive task scores to normative data. Of the 36 cognitive outcomes assessed, two significant associations were reported. Third trimester marijuana use predicted lower scores on the BSID mental development index in 9-month old infants. Additionally, infants who were prenatally exposed to more than one joint per day in the third trimester performed more poorly than controls on the BSID mental development index. Importantly, both group's scores on this task were above the normal range when adjusted to account for confounding variables (Table A1 in Supplementary Material).
Studies Assessing Young Children (3–9 Years)
Table 2 shows studies examining the association between prenatal cannabis exposure and cognitive functioning in children aged 3 to 9 years. A total of 397 cognitive outcomes were assessed. The largest proportion of cognitive outcomes (46 percent) derived from the MHPCD. The OPPS contributed 27 percent, the UMJS 9 percent, and the CWRUS 8 percent. Another 5 percent came from the DRWJ. Finally, the remaining 10 percent came either from the BHUS, UMSM or the YCSC studies.
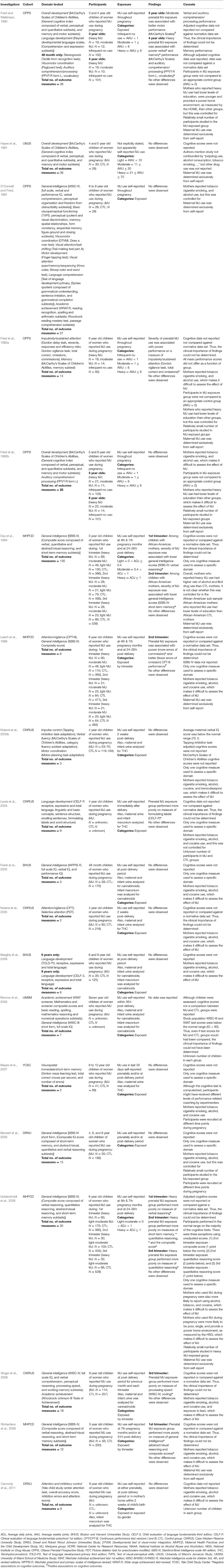
Performance on the vast majority (95.7%) of the 380 cognitive outcomes assessed was similar between the groups. Prenatal cannabis exposure was significantly associated with 17 cognitive outcomes: better performance on two (Fried and Watkinson, 1990; Leech et al., 1999) and worse performance on 15 (Fried and Watkinson, 1990; Day et al., 1994; Leech et al., 1999; Lewis et al., 2004; Goldschmidt et al., 2008; Singer et al., 2008; Richardson et al., 2009). For this age group, no group of investigators compared cognitive task scores to a normative database (Table A2 in Supplementary Material). In addition, cognitive scores were not reported for the majority of studies (68%) (Hayes et al., 1991; Day et al., 1994; Leech et al., 1999; Lewis et al., 2004; Frank et al., 2005; Noland et al., 2005; Beeghly et al., 2006; Morrow et al., 2006; Mayes et al., 2007; Bennett et al., 2008; Singer et al., 2008; Richardson et al., 2009; Carmody et al., 2011).
Leech et al. (1999) assessed the cognitive functioning of 6-year old children whose mothers reported cannabis during pregnancy (maximum N = 110). Of the 6 cognitive outcomes assessed, two significant associations were found. Prenatal cannabis exposure was associated with both better (fewer omission errors) and poorer (more commission errors) on the Continuous Performance Test—Version III (CPT-III). However, the authors did not report or compare scores to those from a normative database.
These mixed findings appear to conflict with those from a previous study. Day et al. (1994), assessed the cognitive functioning of 3-year old children with prenatal cannabis exposure (maximum N = 110). Of the 135 cognitive outcomes assessed, two significant associations were found. Among children with African-American mothers, severity of cannabis exposure during the first and second trimesters was associated with lower scores on the SBIS-IV verbal reasoning and short-term memory subscales, respectively. However, the authors did not report or compare scores to those from a normative database, again making it difficult to determine the clinical significance of the findings. Furthermore, mothers who reported heavy cannabis use also had higher rates of alcohol and illicit drug use than controls. It is unclear whether this was controlled for in the African-American subsample, which makes it difficult to isolate the potential effects of prenatal cannabis exposure from those of other drugs.
Similarly, Lewis et al. (2004), did not control for other drug use in their study of language development in 4-year old children with prenatal cannabis exposure (N = unknown). Of the nine cognitive outcomes assessed, one significant association was found. Children in the prenatal cannabis exposure group performed more poorly on the CELF-P formulating labels task. However, the authors did not report or compare scores to those from a normative database, so the clinical significance of the finding is difficult to determine.
In an attempt to minimize the impact of other drug use, Singer et al. (2008), conducted a study in which the cognitive functioning of 9-year-old children with prenatal cannabis exposure was examined (N = 114). In addition, these researchers controlled for mothers' cigarette smoking, alcohol use and cocaine use (Singer et al., 2008). Of the 18 cognitive outcomes assessed, one significant association was found. Prenatal cannabis exposure in the third trimester was associated with poor performance on a WISC-IV measure of processing speed. However, the investigators did not report individual or group scores, nor did they compare scores against a normative database.
In a study conducted by Richardson et al. (2009), the cognitive functioning of 3-year-old children who were exposed to cannabis prenatally was assessed (N = 56), and the researchers also controlled for mothers' use of other drugs. Of the 12 cognitive outcomes assessed, two significant associations were found. Children with prenatal cannabis exposure during the first trimester performed more poorly on the SBIS-IV, as evidenced by lower abstract/visual reasoning and composite scores. Again, the researchers did not report or compare scores to those from a normative database.
Fried and Watkinson (1990) conducted a study with 3 and 4-year-old children of women who reported cannabis use during pregnancy (maximum N = 19). Of the 38 cognitive outcomes assessed, 4 significant associations were found. For the 3-year-old children, prenatal cannabis exposure of 1 to 6 average weekly joints was associated with better motor performance on the McCarthy's Scales of Children's Abilities motor subtest. On the other hand, for 4-year-old children, exposure of over 6 average weekly joints was associated with poorer performance on the verbal and memory subtests, and the PPVT-R. Unlike the above studies, Fried and Watkinson (1990) reported mean cognitive task scores for the study groups. However, normative data for the tasks were not publicly available, which prevented us from comparing the mean scores to normative data ourselves. Therefore, the clinical significance of the study findings could not be determined.
In a similar study, Fried et al. (1992a) assessed the cognitive functioning of 6-year old children with prenatal cannabis exposure (maximum N = 19). Of the 14 cognitive outcomes assessed, one significant association was found. Prenatal cannabis use was associated with poorer performance on the Gordon Vigilance task. Although unadjusted mean task scores were reported, the authors did not compare them (or adjusted scores) against normative data.
Finally, for the study by Goldschmidt et al. (2008), we were able assess the clinical relevance of the study findings. Out of 30 cognitive outcomes assessed, five significant associations were found. Children with prenatal cannabis exposure (maximum N = 175) of more than one joint per day in the first and second trimesters performed worse than children of abstainers on five SBIS-IV measures: short-term memory, verbal and quantitative reasoning domains, and composite scores. Although Goldschmidt et al. (2008) reported cognitive task scores for the study groups they did not compare them to normative data. However, because the published norms for the tasks were publicly available, we were able to conduct the comparison ourselves. The composite score in the 2nd trimester exposure group and the quantitative reasoning score for the 3rd trimester exposure group fell 1 point below the normal range. Furthermore, the quantitative reasoning subscale score for the 2nd trimester exposure group fell two points below the normal range (See Table A2 in Supplementary Material). It is important to note that although mean scores fell below the normal range of performance, the task scores were unadjusted for covariates.
Studies Assessing Early Adolescents (9–12 Years)
Table 3 shows studies assessing the association between prenatal cannabis exposure and cognitive performance in early adolescents. A total of 278 cognitive outcomes were assessed. The majority (55 percent) were obtained from the OPPS. The MHPCD followed with 27 percent. Two BHUS articles yielded seven percent and the remaining 11 percent came from three other study cohorts.
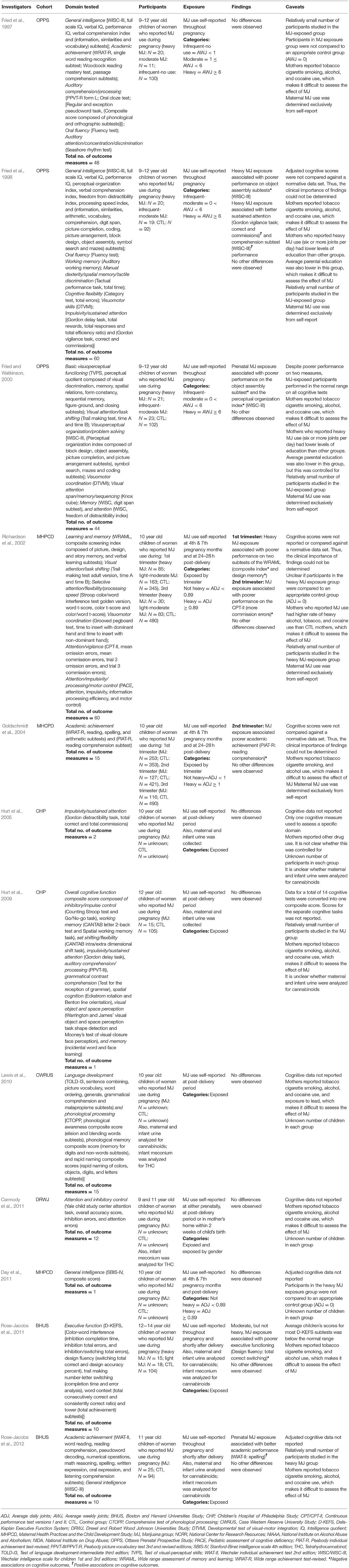
Performance on the majority of 278 cognitive outcomes (96%) was similar between the groups. Prenatal cannabis exposure was significantly associated with 12 cognitive outcomes: better performance on four (Fried et al., 1998; Rose-Jacobs et al., 2012) and worse performance on eight (Fried et al., 1998; Fried and Watkinson, 2000; Richardson et al., 2002; Goldschmidt et al., 2004; Rose-Jacobs et al., 2011). The authors compared cognitive performance scores to a normative database in one of the five studies in which a significant association was found (Rose-Jacobs et al., 2011).
Richardson et al. (2002) assessed the cognitive functioning of 10-year-old children (maximum N = 163) whose mothers reported cannabis use during pregnancy. Of the 60 cognitive outcomes assessed, three were significantly associated with prenatal cannabis exposure. Exposure to more than 0.89 average daily joints in the 1st trimester was associated with poorer performance on the composite index and design memory subtests of the Wide Range Assessment of Memory and Learning, and overall exposure during the 2nd trimester was associated with more commission errors on the Continuous Performance Test—Version II (CPT-II). Again, cognitive scores were not reported or compared against a normative.
The findings above seem to conflict with those from a subsequent study. Rose-Jacobs et al. (2012) assessed the association between cognitive functioning and prenatal cannabis exposure in 11-year old children (maximum N = 18). Of the 10 cognitive outcomes assessed, one significant association was found. Prenatal cannabis exposure was associated with better spelling scores. Although cognitive task scores were reported they were not compared to a normative database.
In a 1998 investigation, Fried and colleagues assessed the executive functioning of 9- to 12-year old participants, who were exposed to cannabis prenatally (maximum N = 20) (Fried et al., 1998). Of the 60 cognitive outcomes assessed four significant associations were found. Children whose mothers smoked over 6 joints per week during pregnancy demonstrated better sustained attention both on the Gordon Vigilance task as evidenced by more correct responses and fewer commission errors and WISC-III comprehension subtest. However, the same level of exposure was associated with lower scores on the WISC-III's object assembly subscales. Although mean task scores were reported, they were not compared against a normative database. We attempted to obtain normative scores but were unable to do so, making it difficult to determine the clinical relevance of the findings.
In a subsequent study, Fried and Watkinson (2000) examined the cognitive functioning of 9- and 12-year-old children whose mothers reported cannabis use during pregnancy (maximum N = 23). Of the 44 cognitive outcomes assessed, two significant associations with prenatal cannabis exposure were found. Children prenatally exposed to more than 6 average weekly joints performed more poorly on the object assembly subtest and the perceptual organization index. Although mean task scores were reported, they were not compared against a normative database by the authors. We obtained normative scores and conducted the appropriate comparison. We found that mean scores for the object assembly subtest and perceptual organization index were within the normal range.
Goldschmidt et al. (2004) assessed the potential association between prenatal cannabis exposure and cognitive functioning by comparing over two hundred (maximum N = 253) 10-year-old children with prenatal cannabis exposure to matched controls. Of the 15 cognitive outcomes assessed, one significant association with prenatal cannabis exposure was found. Second trimester exposure was related to lower reading comprehension and underachievement. Although mean scores were reported, they were not compared to a normative database by the researchers. We attempted to obtain normative scores but were unable to do so, making it difficult to determine the clinical relevance of the findings. Regarding the underachievement (defined as a significant disparity between a child's score on the WRAT-R and the expected level of achievement based on SBIS scores), 15 percent (19/127) of the children exposed to more than one joint per day during the second trimester were classified as underachievers, compared with eight percent (33/421) among controls.
Unlike the above studies, Rose-Jacobs et al. (2011) compared cognitive task scores to a normative database. Of the 10 cognitive outcomes assessed, one significant association was found. After adjusting for covariates such as IQ and other drug use, lighter, but not heavier, prenatal cannabis exposure was associated with worse performance on the Design Fluency task (total correct switching). Importantly, Rose-Jacobs et al. (2011) determined that the mean score for this task fell within the normal range when compared against normative data.
Studies Assessing Adolescents and Early Adults (13 to 22 Years)
Table 4 shows studies assessing the cognitive functioning of adolescents and young adults. The MHPCD provided the majority (63 percent) of the cognitive outcomes. The OPPS contributed the rest (37 percent).
Performance on the majority (94%) of the 157 cognitive outcomes assessed was similar between the groups. However, prenatal cannabis exposure was associated with better performance on one cognitive outcome (Willford et al., 2010) and worse performance on nine (Fried and Watkinson, 2001; Fried et al., 2003; Smith et al., 2004; Willford et al., 2010; Goldschmidt et al., 2012). For this age group, participant's cognitive scores were not compared to norms in any of the studies that found significant associations with prenatal cannabis exposure (Table A4 in Supplementary Material).
Willford et al. (2010) assessed the cognitive functioning of 16-year-old children whose mothers reported cannabis use during pregnancy (maximum N = 132). Of 57 cognitive outcomes assessed, four significant associations with prenatal cannabis exposure were found. Cannabis exposure in the 3rd trimester was associated with better performance on a measure of visuomotor coordination. In addition, cannabis exposure in the 1st trimester was associated with slower processing speed and with two measures of interhemispheric transfer in the 3rd trimester on the Bimanual Coordination Task (BCT). Children whose mothers smoked cannabis during the 3rd trimester of pregnancy also demonstrated better BCT performance on a measure of visuomotor coordination. It is important to point out that task scores were not reported and that normative data are not available for the tests used.
The finding of better visuomotor coordination above seems to conflict with findings from an earlier study. In that study, Smith et al. (2004) employed fMRI in young adults that were (N = 16) or were not (N = 15) prenatally exposed to cannabis while they completed a Go/No-Go task. Of the six cognitive outcomes assessed, one significant association with prenatal cannabis exposure was found. Participants exposed to cannabis prenatally had significantly more errors of commission for the “Press for all letters except X” condition than did controls. Of note, the clinical significance of this finding could not be determined because task scores were not reported and norms were not available.
Fried and Watkinson (2001) assessed the cognitive functioning of children of women who reported cannabis use during pregnancy (maximum N = 26). Of 22 cognitive outcomes assessed, one significant association with prenatal cannabis exposure was found. Prenatal exposure to more than 6 average weekly joints was associated with poorer performance on the CPT stability subtest. Task scores were not reported or compared against a normative dataset making it difficult to understand the clinical relevance of the finding.
Goldschmidt et al. (2012) did report mean cognitive task scores of children of women who reported cannabis use during pregnancy (maximum N = 139). Of the 12 cognitive outcomes assessed, two significant associations with prenatal cannabis exposure were found. Children exposed to one or more joints per day during the first trimester achieved lower Wechsler Individual Achievement Test (WIAT) composite and reading scores. Although unadjusted mean cognitive task scores were reported, they were not compared against normative data. We attempted to obtain normative scores but were unable to do so, making it difficult to determine the clinical relevance of the finding. It is also important to note that it is unclear whether other illicit drug use was assessed in the study.
Fried et al. (2003) assessed cognitive functioning of 13 to 16-year-old children exposed to cannabis prenatally (maximum N = 25). The researchers also controlled for mothers' other illicit drug use. Of the 12 cognitive outcomes assessed, two significant associations with prenatal cannabis exposure were found. Prenatal exposure to more than 6 weekly joints was associated with slower reaction times on the abstract designs test and lower spelling recognition scores on the Peabody individual achievement test (PIAT). Regarding the Abstract Designs Test, task scores were not reported or compared against a normative dataset. On the other hand, task scores were reported for the PIAT. We obtained norms for the task and found that they were within the normal range.
Discussion
General Discussion of Findings
In general, the findings of this critical review indicate that prenatal cannabis exposure is associated with few effects on the cognitive functioning of offspring. Overall, we found a total of 1,001 statistical comparisons between groups of participants that were exposed to cannabis prenatally and non-exposed controls. Cognitive performance was statistically different on only 4.3% of cognitive measures—worse on 3.4% and better in 0.9%. Importantly, we found evidence for scores being below the normal range in only 0.3% of the total sample. Thus, despite analyzing studies spanning approximately three decades, we conclude the evidence does not support an association between prenatal cannabis exposure and clinically relevant cognitive deficits.
Nonetheless, the study by Goldschmidt et al. (2008) deserves special attention as it is the only article we found where the group exposed to cannabis prenatally obtained scores that fell outside the normal range of cognitive functioning. The authors concluded that prenatal cannabis exposure “has a significant effect on school-age intellectual development” and “could impair a child's academic functioning.”
The above conclusion should probably be tempered for several reasons. First, there were differences in maternal cognitive ability, poverty, and home environment. This is critical, as it has been demonstrated that poverty adversely affects children's cognitive development (Hurt and Betancourt, 2016). Although controlled for statistically, it is difficult to account for the potential effects of these covariates. Second, contributions of preschool and day-care attendance were not determined because this information was not available for all participants. This makes it difficult to determine the extent to which the current findings overlap with those of an earlier investigation by this group of researchers, which found that preschool and day-care attendance mediated the relationship between prenatal cannabis exposure and cognitive functioning (Day et al., 1994). More importantly, it precludes deeper analysis into variables that may have greater explanatory power than cannabis exposure. Third, only unadjusted scores were reported, and therefore, we were only able to compare these to a normative database. It remains to be determined if after scores are adjusted for sociodemographic and other factors they might be within the normal range.
Assessing Cognitive Function
Another limitation in the study by Goldschmidt et al. (2008), is that only one task was used to measure each domain. This limitation was observed in virtually all other studies. Performance on multiple tasks, which assess the same domain, should be evaluated in neuropsychological research because individual tasks may tap slightly different components of the domain of interest. This can also help clarify interpretations when conflicting results are obtained with only one measure.
The study by O'Connell and Fried (1991) is a good case in point. The researchers used multiple measures allowing for a more comprehensive understanding of the impact of cannabis exposure on the domains assessed. The authors compared 28 prenatally exposed children (6–9 years old) with 28 non-exposed children (O'Connell and Fried, 1991). Assessment was comprehensive, tapping multiple domains including attention, cognitive flexibility and memory for a total of 27 cognitive outcomes. Participants were matched on the basis of maternal prenatal alcohol and tobacco consumption. Mothers of children exposed prenatally had consumed on average more than 1 joint per week, but exposure ranged from one to 50 joints per week (mean = 14, SD = 15). Prenatally exposed children performed as well as controls, and no positive or negative associations were found on cognitive measures. There are now multiple studies assessing children of similar ages that agree with these results (Hayes et al., 1991; Fried et al., 1992b; Noland et al., 2003b, 2005; Frank et al., 2005; Beeghly et al., 2006; Morrow et al., 2006; Mayes et al., 2007; Bennett et al., 2008; Carmody et al., 2011). In the future, researchers should keep in mind that meaningful group differences should be observed on multiple measures of a particular cognitive domain before making assertions about the long-term impact of prenatal cannabis exposure.
Determining Clinical Significance and Language Precision
When we attempted to determine the extent to which cognitive performance was truly impaired—fell below the average range when compared against a normative database—we found evidence for this in only 3 of the 1,004 possible cognitive outcomes measured (<0.3% percent).
Despite this, there appears to be a tendency to interpret any difference as deficits representing substantial loss of function. One possible reason for confusion might be that the term “deficit” has at least two meanings and some conflate them. One is captured by the canonical situation in which one group performs statistically significantly less well on a task. However, its clinical significance, or everyday import, is difficult or impossible to determine because scores are rarely compared against a normative database. Normative data are obtained from a large, randomly selected representative sample and incorporate important variables such as age and education, and establish a baseline distribution for a measurement. Scores obtained empirically should be compared against norms to determine whether statistically significant findings are meaningful. This brings us to the second meaning of the term “deficit,” a substantial loss of function, in which performance falls outside of the normal range and bears clinical significance (Both meanings probably represent end points on a continuum). The problem in the literature on prenatal substance exposure is that in most studies, results support only the first (or difference) interpretation, but are discussed in terms of the “dysfunctional” interpretation. In essence, the English word “deficit” (or “impairment”) is ambiguous, and researchers in this field often switch meanings in moving from actual findings to discussion of the implications of such findings.
The majority of the reviewed studies did not include a comparison with normative data. In many cases, mean task scores were not reported making it impossible for others to make comparisons. Researchers should be encouraged to report data obtained for each individual participant, and use measures for which normative data has been collected and is available. This will go a long way in determining the extent to which prenatal cannabis exposure affects subsequent cognition or any other measure. Unfortunately, simply stating that there is a deficit in one group does not adequately inform future research, nor does it provide useful guidance for public policy makers, legal practitioners, or health professionals.
Previously, other researchers in this area have also cautioned against making definitive statements about “deficits” (Fried and Smith, 2001; Fried, 2002; Huizink and Mulder, 2006). Fried and Smith (2001) felt so strongly about this that they remarked, “caveats, coupled with the relatively sparse literature, are a combination that makes any definitive statement problematic, presumptuous, and foolhardy” (Fried and Smith, 2001). Unfortunately, the literature is replete with language purporting to report the “impact of prenatal cannabis exposure,” when what is actually reported is merely a correlational relationship (a positive or negative association). The inappropriate use of causal language tends to lead to premature and/or erroneous conclusions regarding the actual effects of prenatal cannabis exposure on subsequent cognitive functioning.
Examination and Reporting of Confounding Variables
A greater understanding is necessary of the fact that many children with prenatal cannabis exposure are also exposed to factors often seen in people with low socio-economic status, such as poor nutrition, parents with lower levels of education and parents who may also use other substances, including nicotine and alcohol, among a host of other confounding variables. While a select few of the studies included in this review assessed and controlled for some of these variables, the majority did not. One noteworthy example is that tobacco use frequently occurred with cannabis use, and results from several studies that have revealed prenatal tobacco exposure alone, both directly through smoking by the mother and indirectly by secondhand smoke, is associated with lower scores in several cognitive domains (Polanska et al., 2015). These observations make it particularly difficult to disentangle the effects of tobacco from those of cannabis. Nonetheless, a consequence of the current movement to liberalize cannabis laws is that there appears to be a growing number of women who use cannabis-based products—and not other substances such as tobacco and alcohol—to treat nausea and vomiting during pregnancy (Dickson et al., 2018; Young-Wolff et al., 2019). This development provides an opportunity for future studies to assess the impact of prenatal cannabis exposure alone. Additional studies should account for the many other variables that potentially influence subsequent functioning of prenatally exposed offspring.
Exposure: Timing, Amount, and Dose-Dependence
Only a limited number of studies confirmed the presence of cannabis with biological assays and none conducted quantitative analyses to confirm mother's self-reported cannabis use. In addition, few tested for the potential effects of prenatal cannabis exposure as a function of trimester. Both factors may be important for determining the extent to which prenatal cannabis exposure impacts cognitive functioning.
Another concern is the inappropriate use of the term “dose-response fashion.” This term was used by researchers to characterize that greater frequency of cannabis use was associated with a higher risk of adverse effects (Fried et al., 1992a; Fried and Watkinson, 2001). In a true dose-response study, however, all factors are held constant and experimenters systematically alter drug exposure. Increased effects with increasing dose are then a powerful demonstration that it is the drug that is causing effects. However, mothers who used more cannabis were different people—likely leading different lives—from those using less or no cannabis. Researchers made statistical corrections for as many covariates as they could, based on the measures included in the original studies, but it seems unlikely that these captured all of the complex differences expected among mothers and their children. In short, it is too strong a claim to describe results as a “dose-response” because: (1) all other factors were not held constant, and (2) the varied exposure to cannabis was not manipulated as a controlled experiment, but occurred for other, unknown, reasons.
Type I and Type II Errors
Almost all studies in this literature have set their Type I error rate to 0.05, where stated. Given the number of negative and positive associations of prenatal cannabis exposure with cognitive outcomes compared to comparison groups, there is a strong likelihood of both Type I and Type II errors. The findings of this review, namely that only 3 of the 1,004 possible cognitive outcomes measured (<0.3% percent) fell below the average range when compared against a normative database, are what we would statistically expect to find only by chance.
Limitations
The current review has at least three important limitations. First, as described, we acknowledge that normative data were unavailable for a limited number of measures on which prenatally exposed children performed significantly worse than controls. Therefore, it is possible that some of these measures were, in fact, below the normal range and might have changed our conclusions. In order to address this issue, future studies should either conduct the appropriate comparisons or report individual participants' scores and the normal range for each cognitive task so that readers can draw appropriate conclusions.
Another potential drawback is that its focus is limited to cognitive functioning. This focus was selected because of the obvious clinical implications and because it is within the realm of the authors' expertise area. We recognize, however, that there is a wide range of possible effects of prenatal cannabis exposure on the developing fetus, including effects on birth weight and emotional development. As a result, it is our hope that researchers critically review the influence of early cannabis exposure on other outcome measures.
In addition, it is possible that the cognitive tasks employed by most of the investigators of the reviewed studies are insensitive to the prenatal effects of cannabis. As such, more sensitive task batteries might result in a different pattern of findings. This possibility, along with other limitations associated with the current review, suggest that caution should be exercised when extrapolating findings from this investigation.
Implications
The current review of the literature found that there are relatively few cognitive alterations noted in offspring exposed to cannabis prenatally. It is important also to note that these results should be interpreted taking into account limitations of the current state of the literature. It is also critical to understand that subsequent studies, especially those that address the limitations point out here, may yield a more concerning pattern of effects.
Regardless, at present, we are concerned that a misunderstanding of the relationship between prenatal cannabis exposure and subsequent cognitive functioning leads to an oversimplification of the complex relationships between socioeconomic factors and functioning of the individual whether drug use is involved or not. Misinterpretations of the complex interactions of relevant factors in itself can cause harm to pregnant women and their children by leading to punitive policies and enhancing unwarranted stigma. In some cases, intense stigma has resulted in removal of children from their families, and even in maternal incarceration. The rationale for such policies is, in part, that prenatal cannabis exposure causes persistent deleterious effects, especially on cognitive functioning. Findings from this review suggest that this assumption should be reevaluated to ensure that our assumptions do not do more harm than the drug itself.
Author Contributions
CT: conceptualization, literature survey, statistical analyses, manuscript preparation and editing. CM-K and KO'M: data extraction, preparation of the tables, manuscript editing, and supporting role in conceptualization. CH: idea and conceptualization, literature survey, preparation of the first manuscript draft, manuscript editing, project leader, and corresponding author.
Funding
This work was funded by the National Institute on Drug Abuse (NIDA; Grant number DA037801 is gratefully acknowledged). KO'M was supported by an Australian Government Research Training Program Scholarship.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Drs. Jennifer Manly and Anthony Ahmed for providing access to available normative data for cognitive tests, and Simon Bartholomew Xu and Matthew Medina-Kirchner for assisting in obtaining identified articles.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyg.2020.00816/full#supplementary-material
Abbreviations
ADJ, Average daily joints; AWJ, Average weekly joints; BCT, Bimanual coordination task; BHUS, Boston and Harvard Universities Study; BSID, Bayley scales of infant development 1st edition; BSID-II, Bayley scales of infant development 2nd edition; CELF-3, Child evaluation of language fundamentals-third edition; CELF-P, Clinical evaluation of language fundamentals-preschool 1st edition; CHP, Children's Hospital of Philadelphia Study; CPT, Continuous performance test version I; CPT-II, Continuous performance test version II; CPT-III, Continuous performance test version III; CTL, Control group; CTOPP, Comprehensive test of phonological processing; CWRUS, Case Western Reserve University Study; D-KEFS, Delis-Kaplan Executive Function System; DRWJ, Drexel and Robert Wood Johnson Universities Study; DTVMI, Developmental test of visual-motor integration; fMRI, Functional magnetic resonance imaging; IQ, Intelligence quotient; MHPCD, Maternal Health Practices and the Child Development Study; MJ, Marijuana group; NCRR, National Center for Research Resources; NIAAA, National Institute on Alcohol Abuse and Alcoholism; NIDA, National Institute on Drug Abuse; OPPS, Ottawa Prenatal Prospective Study; PACE, Pediatric assessment of cognitive deficiency; PDT, Picture deletion task for preschoolers-modified; PIAT, Peabody individual achievement test; PIAT-R, Peabody individual achievement test-revised; PPVT-III, Peabody picture vocabulary test 3rd edition; PPVT-R, Peabody picture vocabulary test revised edition; SD, Standard deviation; SBIS-IV, Stanford-Binet intelligence scale 4th edition; THC, Tetrahydrocannabinol; TOLD-I3, Test of language development-intermediate third edition; TOLD-P3, Test of language development-primary third edition; TVPS, Test of visual-perceptual skills; UMJS, University of Miami's Jamaican Study; UMSM, University of Miami School of Medicine Study; U.S., United States; WAIS, Weschler adult intelligence scale; WCST-CV, Wisconsin card sorting test-computer version I; WCST-CV2, Wisconsin card sorting test-computer version II; WIAT, Wechsler individual achievement test 1st edition; WIAT-II, Wechsler individual achievement test 2nd edition; WISC, Wechsler intelligence scale for children 1st; WISC-III, Wechsler intelligence scale for children 3rd edition; WISC-IV, Wechsler intelligence scale for children 4th edition; WISC-R, Wechsler intelligence scale for children revised edition; WPPSI-R, Wechsler preschool and primary scales of intelligence-revised; WRAML, Wide range assessment of memory and learning; WRAT, Wide range achievement test; WRAT-R, Wide range achievement test-revised; YCSC, Yale Child Study Center.
References
Beeghly, M., Martin, B., Rose-Jacobs, R., Cabral, H., Heeren, T., Augustyn, M., et al. (2006). Prenatal cocaine exposure and children's language functioning at 6 and 9.5 years: moderating effects of child age, birthweight, and gender. J. Pediatr. Psychol. 31, 98–115. doi: 10.1093/jpepsy/jsj028
Bennett, D. S., Bendersky, M., and Lewis, M. (2008). Children's cognitive ability from 4 to 9 years old as a function of prenatal cocaine exposure, environmental risk, and maternal verbal intelligence. Dev. Psychol.44:919. doi: 10.1037/0012-1649.44.4.919
Calvigioni, D., Hurd, Y. L., Harkany, T., and Keimpema, E. (2014). Neuronal substrates and functional consequences of prenatal cannabis exposure. Eur. Child Adolesc. Psychiatry 23, 931–941. doi: 10.1007/s00787-014-0550-y
Carmody, D. P., Bennett, D. S., and Lewis, M. (2011). The effects of prenatal cocaine exposure and gender on inhibitory control and attention. Neurotoxicol. Teratol. 33, 61–68. doi: 10.1016/j.ntt.2010.07.004
Conner, S., Bedell, V., Lipsey, K., Macones, G., Cahill, A., and Tuuli, M. (2016). Maternal marijuana use and adverse neonatal outcomes: a systematic review and meta-analysis. Obstet. Gynecol. 128, 713–723. doi: 10.1097/AOG.0000000000001649
Day, N. L., Leech, S. L., and Goldschmidt, L. (2011). The effects of prenatal marijuana exposure on delinquent behaviors are mediated by measures of neurocognitive functioning. Neurotoxicol. Teratol. 33, 129–136. doi: 10.1016/j.ntt.2010.07.006
Day, N. L., Richardson, G. A., Goldschmidt, L., Robles, N., Taylor, P. M., Stoffer, D. S., et al. (1994). Effect of prenatal marijuana exposure on the cognitive development of offspring at age three. Neurotoxicol. Teratol. 16, 169–175. doi: 10.1016/0892-0362(94)90114-7
Day, N. L., Wagener, D. K., and Taylor, P. M. (1985). Measurement of substance use during pregnancy: methodologic issues. NIDA Res. Monogr. 59, 36–47. doi: 10.1037/e496932006-004
Dickson, B., Mansfield, C., Guiahi, M., Allshouse, A. A., Borgelt, L. M., Sheeder, J., et al. (2018). Recommendations from cannabis dispensaries about first-trimester cannabis use. Obstet. Gynecol. 131, 1031–1038. doi: 10.1097/AOG.0000000000002619
Frank, D. A., Rose-Jacobs, R., Beeghly, M., Wilbur, M., Bellinger, D., and Cabral, H. (2005). Level of prenatal cocaine exposure and 48-month IQ: importance of preschool enrichment. Neurotoxicol. Teratol. 27, 15–28. doi: 10.1016/j.ntt.2004.09.003
Fried, P., and Watkinson, B. (1990). 36– and 48-month neurobehavioral follow-up of children prenatally exposed to marijuana, cigarettes, and alcohol. J. Dev. Behav. Pediatr. 11, 49–58. doi: 10.1097/00004703-199004000-00003
Fried, P. A. (2002). Conceptual issues in behavioral teratology and their application in determining long-term sequelae of prenatal marihuana exposure. J. Child Psychol. Psychiatry 43, 81–102. doi: 10.1111/1469-7610.00005
Fried, P. A., O'Connell, C. M., and Watkinson, B. (1992b). 60- and 72-month follow-up of children prenatally exposed to marijuana, cigarettes, and alcohol: cognitive and language assessment. J. Dev. Behav. Pediatr. 13, 383–391. doi: 10.1097/00004703-199212000-00001
Fried, P. A., and Smith, A. M. (2001). A literature review of the consequences of prenatal marihuana exposure: an emerging theme of a deficiency in aspects of executive function. Neurotoxicol. Teratol. 23, 1–11. doi: 10.1016/S0892-0362(00)00119-7
Fried, P. A., and Watkinson, B. (1988). 12- and 24-month neurobehavioural follow-up of children prenatally exposed to marihuana, cigarettes and alcohol. Neurotoxicol. Teratol. 10, 305–313. doi: 10.1016/0892-0362(88)90032-3
Fried, P. A., and Watkinson, B. (2000). Visuoperceptual functioning differs in 9- to 12-year olds prenatally exposed to cigarettes and marihuana. Neurotoxicol. Teratol. 22, 11–20. doi: 10.1016/S0892-0362(99)00046-X
Fried, P. A., and Watkinson, B. (2001). Differential effects on facets of attention in adolescents prenatally exposed to cigarettes and marihuana. Neurotoxicol. Teratol. 23, 421–430. doi: 10.1016/S0892-0362(01)00160-X
Fried, P. A., Watkinson, B., and Gray, R. (1992a). A follow-up study of attentional behavior in 6-year-old children exposed prenatally to marihuana, cigarettes, and alcohol. Neurotoxicol. Teratol. 14, 299–311. doi: 10.1016/0892-0362(92)90036-A
Fried, P. A., Watkinson, B., and Gray, R. (1998). Differential effects on cognitive functioning in 9- to 12-year olds prenatally exposed to cigarettes and marihuana. Neurotoxicol. Teratol. 20, 293–306. doi: 10.1016/S0892-0362(97)00091-3
Fried, P. A., Watkinson, B., and Gray, R. (2003). Differential effects on cognitive functioning in 13- to 16-year-olds prenatally exposed to cigarettes and marihuana. Neurotoxicol. Teratol. 25, 427–436. doi: 10.1016/S0892-0362(03)00029-1
Fried, P. A., Watkinson, B., and Siegel, L. S. (1997). Reading and language in 9- to 12-year olds prenatally exposed to cigarettes and marijuana. Neurotoxicol. Teratol. 19, 171–183. doi: 10.1016/S0892-0362(97)00015-9
GDS (2019). Global Drug Survey [Internet]. Available online at: https://www.globaldrugsurvey.com/gds-2019/ (accessed July 9, 2019).
Goldschmidt, L., Richardson, G. A., Cornelius, M. D., and Day, N. L. (2004). Prenatal marijuana and alcohol exposure and academic achievement at age 10. Neurotoxicol. Teratol. 26, 521–532. doi: 10.1016/j.ntt.2004.04.003
Goldschmidt, L., Richardson, G. A., Willford, J., and Day, N. L. (2008). Prenatal marijuana exposure and intelligence test performance at age 6. J. Am. Acad. Child Adolesc. Psychiatry 47, 254–263. doi: 10.1097/CHI.0b013e318160b3f0
Goldschmidt, L., Richardson, G. A., Willford, J. A., Severtson, S. G., and Day, N. L. (2012). School achievement in 14-year-old youths prenatally exposed to marijuana. Neurotoxicol. Teratol. 34, 161–167. doi: 10.1016/j.ntt.2011.08.009
Grant, K. S., Petroff, R., Isoherranen, N., Stella, N., and Burbacher, T. M. (2018). Cannabis use during pregnancy: pharmacokinetics and effects on child development. Pharmacol. Ther. 182, 133–151. doi: 10.1016/j.pharmthera.2017.08.014
Harvey, P. D. (2012). Clinical applications of neuropsychological assessment. Dialogues Clin. Neurosci. 14, 91–99.
Hayes, J. S., Lampart, R., Dreher, M. C., and Morgan, L. (1991). Five-year follow-up of rural Jamaican children whose mothers used marijuana during pregnancy. West Indian Med. J. 40, 120–123.
Higuera-Matas, A., Ucha, M., and Ambrosio, E. (2015). Long-term consequences of perinatal and adolescent cannabinoid exposure on neural and psychological processes. Neurosci. Biobehav. Rev. 55, 119–146. doi: 10.1016/j.neubiorev.2015.04.020
Huizink, A. C. (2014). Prenatal cannabis exposure and infant outcomes: overview of studies. Prog. Neuro Psychopharmacol. Biol. Psychiatry 52, 45–52. doi: 10.1016/j.pnpbp.2013.09.014
Huizink, A. C., and Mulder, E. J. H. (2006). Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neurosci. Biobehav. Revi. 30, 24–41. doi: 10.1016/j.neubiorev.2005.04.005
Hurt, H., and Betancourt, L. M. (2016). Effect of socioeconomic status disparity on child language and neural outcome: how early is early? Pediatr. Res. 79, 148–158. doi: 10.1038/pr.2015.202
Hurt, H., Betancourt, L. M., Malmud, E. K., Shera, D. M., Giannetta, J. M., Brodsky, N. L., et al. (2009). Children with and without gestational cocaine exposure: a neurocognitive systems analysis. Neurotoxicol. Teratol. 31, 334–341. doi: 10.1016/j.ntt.2009.08.002
Hurt, H., Brodsky, N. L., Roth, H., Malmud, E., and Giannetta, J. M. (2005). School performance of children with gestational cocaine exposure. Neurotoxicol. Teratol. 27, 203–211. doi: 10.1016/j.ntt.2004.10.006
Jaddoe, V. W. V., van Duijn, C. M., Franco, O. H., van der Heijden, A. J., van IIzendoorn, M. H., de Jongste, J. C., et al. (2012). The Generation R Study: design and cohort update 2012. Eur. J. Epidemiol. 27, 739–756. doi: 10.1007/s10654-012-9735-1
Karila, L., Cazas, O., Danel, T., and Reynaud, M. (2006). Short- and long-term consequences of prenatal exposure to cannabis. J. Gynecol. Obstet. Biol. Reprod. 35, 62–70. doi: 10.1016/s0368-2315(06)76373-6
Ko, J. Y., Farr, S. L., Tong, V. T., Creanga, A. A., and Callaghan, W. M. (2015). Prevalence and patterns of marijuana use among pregnant and nonpregnant women of reproductive age. Am. J. Obstet. Gynecol. 213, 201.e1–e10. doi: 10.1016/j.ajog.2015.03.021
Leech, S. L., Richardson, G. A., Goldschmidt, L., and Day, N. L. (1999). Prenatal substance exposure: effects on attention and impulsivity of 6-year-olds. Neurotoxicol. Teratol. 21, 109–118. doi: 10.1016/S0892-0362(98)00042-7
Lewis, B. A., Minnes, S., Short, E. J., Weishampel, P., Satayathum, S., Min, M. O., et al. (2010). The effects of prenatal cocaine on language development at 10 years of age. Neurotoxicol. Teratol. 3, 17–24. doi: 10.1016/j.ntt.2010.06.006
Lewis, B. A., Singer, L. T., Short, E. J., Minnes, S., Arendt, R., Weishampel, P., et al. (2004). Four-year language outcomes of children exposed to cocaine in utero. Neurotoxicol. Teratol. 26, 617–627. doi: 10.1016/j.ntt.2004.06.007
Mayes, L., Snyder, P. J., Langlois, E., and Hunter, N. (2007). Visuospatial working memory in school-aged children exposed in utero to cocaine. Child Neuropsychol. 13, 205–218. doi: 10.1080/09297040600888753
Morrow, C. E., Culbertson, J. L., Accornero, V. H., Xue, L., Anthony, J. C., and Bandstra, E. S. (2006). Learning disabilities and intellectual functioning in school-aged children with prenatal cocaine exposure. Dev. Neuropsychol. 30, 905–931. doi: 10.1207/s15326942dn3003_8
National Pregnancy and Health Survey (2019). National Pregnancy and Health Survey: Drug Use Among Women Delivering Live Births (NPHS-1992-DS0001). Available online at: https://www.datafiles.samhsa.gov/study-dataset/national-pregnancy-and-health-survey-drug-use-among-women-delivering-live-births-1992 (accessed) July 9, 2019)
Noland, J. S., Singer, L. T., Arendt, R. E., Minnes, S., Short, E. J., and Bearer, C. F. (2003a). Executive functioning in preschool-age children prenatally exposed to alcohol, cocaine, and marijuana. Alcohol. Clin. Exp. Res. 27, 647–656. doi: 10.1111/j.1530-0277.2003.tb04401.x
Noland, J. S., Singer, L. T., Mehta, S. K., and Super, D. M. (2003b). Prenatal cocaine/polydrug exposure and infant performance on an executive functioning task. Dev. Neuropsychol. 24, 499–517. doi: 10.1207/S15326942DN2401_05
Noland, J. S., Singer, L. T., Short, E. J., Minnes, S., Arendt, R. E., Lester Kirchner, H., et al. (2005). Prenatal drug exposure and selective attention in preschoolers. Neurotoxicol. Teratol. 27, 429–438. doi: 10.1016/j.ntt.2005.02.001
NSDUH Detailed Tables (2017). CBHSQ [Internet]. Available online at: https://www.samhsa.gov/data/report/2017-nsduh-detailed-tables (accessed July 9, 2019)
O'Connell, C. M., and Fried, P. A. (1991). Prenatal exposure to cannabis: a preliminary report of postnatal consequences in school-age children. Neurotoxicol. Teratol. 13, 631–639. doi: 10.1016/0892-0362(91)90047-Z
Polanska, K., Jurewicz, J., and Hanke, W. (2015). Smoking and alcohol drinking during pregnancy as the risk factors for poor child neurodevelopment - a review of epidemiological studies. Int. J. Occup. Med. Environ. Health 28, 419–443. doi: 10.13075/ijomeh.1896.00424
Richardson, G. A., Day, N. L., and Goldschmidt, L. (1995). Prenatal alcohol, marijuana, and tobacco use: infant mental and motor development. Neurotoxicol. Teratol. 17, 479–487. doi: 10.1016/0892-0362(95)00006-D
Richardson, G. A., Goldschmidt, L., Larkby, C., and Day, N. L. (2015). Effects of prenatal cocaine exposure on adolescent development. Neurotoxicol. Teratol. 49, 41–48. doi: 10.1016/j.ntt.2015.03.002
Richardson, G. A., Goldschmidt, L., and Willford, J. (2008). The effects of prenatal cocaine use on infant development. Neurotoxicol. Teratol. 30, 96–106. doi: 10.1016/j.ntt.2007.12.006
Richardson, G. A., Goldschmidt, L., and Willford, J. (2009). Continued effects of prenatal cocaine use: preschool development. Neurotoxicol. Teratol. 31, 325–333. doi: 10.1016/j.ntt.2009.08.004
Richardson, G. A., Ryan, C., Willford, J., Day, N. L., and Goldschmidt, L. (2002). Prenatal alcohol and marijuana exposure: effects on neuropsychological outcomes at 10 years. Neurotoxicol. Teratol. 24, 309–320. doi: 10.1016/S0892-0362(02)00193-9
Rose-Jacobs, R., Augustyn, M., Beeghly, M., Martin, B., Cabral, H. J., Heeren, T. C., et al. (2012). Intrauterine substance exposures and Wechsler Individual Achievement Test-II scores at 11 years of age. Vulnerable Child. Youth Stud. 7, 186–197. doi: 10.1080/17450128.2011.648967
Rose-Jacobs, R., Soenksen, S., Appugliese, D. P., Cabral, H. J., Richardson, M. A., Beeghly, M., et al. (2011). Early adolescent executive functioning, intrauterine exposures and own drug use. Neurotoxicol. Teratol. 33, 379–392. doi: 10.1016/j.ntt.2011.02.013
Singer, L. T., Arendt, R., Fagan, J., Minnes, S., Salvator, A., Bolek, T., et al. (1999). Neonatal visual information processing in cocaine-exposed and non-exposed infants. Infant Behav. Dev. 22, 1–15. doi: 10.1016/S0163-6383(99)80002-2
Singer, L. T., Arendt, R., Minnes, S., Farkas, K., Salvator, A., Kirchner, H. L., et al. (2002). Cognitive and motor outcomes of cocaine-exposed infants. JAMA 287, 1952–1960. doi: 10.1001/jama.287.15.1952
Singer, L. T., Eisengart, L. J., Minnes, S., Noland, J., Jey, A., Lane, C., et al. (2005). Prenatal cocaine exposure and infant cognition. Infant Behav. Dev. 28, 431–444. doi: 10.1016/j.infbeh.2005.03.002
Singer, L. T., Nelson, S., Short, E., Min, M. O., Lewis, B., Russ, S., et al. (2008). Prenatal cocaine exposure: drug and environmental effects at 9 years. J. Pediatr. 153, 105–111.e1. doi: 10.1016/j.jpeds.2008.01.001
Smith, A. M., Fried, P. A., Hogan, M. J., and Cameron, I. (2004). Effects of prenatal marijuana on response inhibition: an fMRI study of young adults. Neurotoxicol. Teratol. 26, 533–542. doi: 10.1016/j.ntt.2004.04.004
Smith, A. M., Fried, P. A., Hogan, M. J., and Cameron, I. (2006). Effects of prenatal marijuana on visuospatial working memory: an fMRI study in young adults. Neurotoxicol. Teratol. 28, 286–295. doi: 10.1016/j.ntt.2005.12.008
Smith, A. M., Mioduszewski, O., Hatchard, T., Byron-Alhassan, A., Fall, C., and Fried, P. A. (2016). Prenatal marijuana exposure impacts executive functioning into young adulthood: an fMRI study. Neurotoxicol. Teratol. 58, 53–59. doi: 10.1016/j.ntt.2016.05.010
van Gelder, M. M., Reefhuis, J., Caton, A. R., Werler, M. M., Druschel, C. M., and Roeleveld, N. (2010). Characteristics of pregnant illicit drug users and associations between cannabis use and perinatal outcome in a population-based study. Drug Alcohol Depend. 109, 243–247. doi: 10.1016/j.drugalcdep.2010.01.007
Willford, J. A., Chandler, L. S., Goldschmidt, L., and Day, N. L. (2010). Effects of prenatal tobacco, alcohol and marijuana exposure on processing speed, visual-motor coordination, and interhemispheric transfer. Neurotoxicol. Teratol. 32, 580–588. doi: 10.1016/j.ntt.2010.06.004
Wu, C.-S., Jew, C. P., and Lu, H.-C. (2011). Lasting impacts of prenatal cannabis exposure and the role of endogenous cannabinoids in the developing brain. Future Neurol. 6, 459–480. doi: 10.2217/fnl.11.27
Keywords: marijuana, prenatal, cognition, impairment, normative data
Citation: Torres CA, Medina-Kirchner C, O'Malley KY and Hart CL (2020) Totality of the Evidence Suggests Prenatal Cannabis Exposure Does Not Lead to Cognitive Impairments: A Systematic and Critical Review. Front. Psychol. 11:816. doi: 10.3389/fpsyg.2020.00816
Received: 04 December 2019; Accepted: 02 April 2020;
Published: 08 May 2020.
Edited by:
Suzanne Wood, University of Toronto, CanadaReviewed by:
David Nutt, Imperial College London, United KingdomGiulia D'Aurizio, University of L'Aquila, Italy
Copyright © 2020 Torres, Medina-Kirchner, O'Malley and Hart. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carl L. Hart, Y2xoNDJAY29sdW1iaWEuZWR1
 Ciara A. Torres1,2
Ciara A. Torres1,2 Carl L. Hart
Carl L. Hart