- 1Departamento de Estudios Psicológicos, Universidad Icesi, Cali, Colombia
- 2Facultad de Psicología, Universidad Pontificia Bolivariana, Palmira, Colombia
- 3Grupo Natura, Facultad de Ingeniería, Diseño y Ciencias Aplicadas, Departamento de Ciencias Farmacéuticas y Químicas, Universidad Icesi, Cali, Colombia
- 4Departamento de Ciencia Jurídica y Política, Universidad Javeriana, Cali, Colombia
- 5PhD Program of Neuroscience, Pontificia Universidad Javeriana, Hospital San Ignacio, Center for Memory and Cognition, Intellectus, Bogotá, Colombia
- 6Cognitive Neuroscience Center (CNC), Universidad de San Andrés, Buenos Aires, Argentina
Individuals who have been exposed to violence are at high risk of developing mental health problems, particularly posttraumatic stress disorder (PTSD). A prominent example is the experience of Colombia, which has suffered systemic violence for more than half a century. Subjects with trauma-related disorders have problems regulating their emotions and facial emotion recognition (FER), a phenomenon that can be explained from a biological perspective by interoception. We conducted an experimental study using the heartbeat-evoked cortical potential amplitude (HEP) to determine the differences in FER and interoceptive priming in victims of armed conflict in Colombia with PTSD, complex posttraumatic stress disorder (CPTSD), and a control group. The results of behavioral studies indicate that individuals with PTSD and CPTSD exhibit impairments in interoceptive accuracy and deficits in the FER task. Compared with those in both the control and PTSD groups, the group of CPTSD victims demonstrated a decline in FER performance following interoceptive priming relative to exteroceptive priming. At the brain level, compared with controls, individuals with CPTSD presented a reduced amplitude of the HEP in the frontocentral regions during interoceptive processing. Significant differences were observed between the CPTSD and PTSD groups in the right frontal–lateral region during interoceptive priming. Our findings suggest alterations in FER interoception and HEP attenuation in armed conflict victims with PTSD and CPTSD. These results highlight the importance of interoception tasks in understanding the neurobiological mechanisms underlying emotional regulation and recognition in populations exposed to war trauma, and they may offer potential therapeutic strategies and targets for PTSD.
1 Introduction
The violence, displacement, and war stemming from over 60 years of armed conflict in Colombia have led to widespread mental disorders affecting multiple generations (Pérez-Olmos et al., 2005; Martínez et al., 2016; Ramírez-Giraldo et al., 2017). Specifically, exposure to war has been linked to mental health issues such as PTSD (Angel, 2016). Research on the effects of war in Colombia highlighted the heightened risk of developing PTSD among victims (Campo-Arias et al., 2014; Hewitt Ramírez et al., 2014; Marroquín Rivera et al., 2020; Charles, 2022). Recently, a significant portion of war victims have presented CPTSD, a new category of PTSD from the International Classification of Disease (ICD)-11 that includes the core PTSD symptoms and three symptom clusters of (Pérez-Olmos et al., 2005) affective dysregulation, (Martínez et al., 2016) negative self-concept and (Ramírez-Giraldo et al., 2017) disturbed relationships, which collectively represent “disturbances in self-organization” (DSO) (Karatzias et al., 2023; Maercker et al., 2013; Organization WH, 2018). PTSD-related disorders are associated with difficulties in cognitive processes, including emotional regulation, FER, and interoceptive processes (which involve the brain processes that receive, process, and send information about internal body states) (Tanaka et al., 2023; Motomura et al., 2019; Terasawa et al., 2021), across different populations (Castro-Vale et al., 2020; Passardi et al., 2019; Couette et al., 2022), including war victims (Poljac et al., 2011; Gebhardt et al., 2017; Mazza et al., 2012). FER alterations are associated with interoception deficits. Interoception involves several cognitive and behavioral biological markers (Garfinkel et al., 2021; Garfinkel and Critchley, 2016; Suksasilp and Garfinkel, 2022) and is fundamental to homeostasis and allostatic processes (Santamaría-García et al., 2024). Interceptive and FER dysfunctions are strongly related and have been implicated in multiple physical and psychological disorders (Bonaz et al., 2021; Garfinkel and Critchley, 2016; Santamaría-García et al., 2024; Barrett and Simmons, 2015). Neurobiological models, including the somatic marker hypothesis (SMH) (Damsio, 1994), suggest that perceiving internal bodily signals and emotional awareness share a close physiological relationship and significantly influence decision-making processes. Previous research has associated interoception—and specifically heartbeat-evoked potentials (HEP)—with emotional awareness (Damasio, 1999) and autonomic nervous system (ANS) functioning within the framework of the SMH. Similarly, the Somatovisceral Afference Model of Emotion (SAME) (Cacioppo et al., 1992) supports this connection by proposing that emotions result from the brain’s interpretation of interoceptive signals, particularly those processed within the insular cortex. Alterations in these interoceptive processes contribute to deficits in emotional recognition and interoceptive sensitivity frequently observed in PTSD.
Interoception processes are altered in PTSD patients through two main mechanisms (Putica et al., 2024). The first involves dysregulations in physiological stress axes (Yehuda et al., 2015) and impairments in sympathetic and parasympathetic control that influence cardiac signaling related to abnormal cortical representation of cardiac interoceptive signals (Flasbeck et al., 2020; Ge et al., 2020). The second mechanism involves altered neurophysiological bases of mind–body interactions with altered functions of the insula, medial prefrontal cortex, anterior cingulate cortex and amygdala in individuals with PTSD (Fine et al., 2023; Lieberman et al., 2023; Harricharan et al., 2020; Nicholson et al., 2022; Akiki et al., 2017; McCurry et al., 2020; Nicholson et al., 2020; Nicholson et al., 2017; Beutler et al., 2022; Reinhardt et al., 2020). Electrophysiologically, HEP serves as a key interoceptive frontal cortical marker. This marker monitors cardiac activity, which is regulated by attention to the heartbeat, with peak modulation occurring between 200 and 500 ms after the R wave (Pollatos et al., 2016; Pollatos and Schandry, 2004; Pollatos and Schandry, 2008). The HEP amplitude reflects interoceptive accuracy (Pollatos and Schandry, 2004; Yuan et al., 2007) and characterizes different interoceptive deficits (Couto et al., 2015; Migeot et al., 2023; Fittipaldi et al., 2020) in various neuropsychiatric diseases (Abrevaya et al., 2020), such as the behavioral variant of frontotemporal dementia (Birba et al., 2022; Migeot et al., 2022; Salamone et al., 2021), amyotrophic lateral sclerosis (Moretta et al., 2022), autism spectrum disorder (Hatfield et al., 2019), affective disorders (Grossi et al., 2017), eating disorders (Donofry et al., 2016; Cambi et al., 2024), emotional dysregulation (Pang et al., 2019), chronic stress (Shatrova et al., 2024), PTSD and CPTSD (Simmons et al., 2009).
PTSD and CPTSD exhibit a range of interoceptive disturbances, including body dissociation, reduced interoceptive signal processing (Mueller et al., 2015; Schmitz et al., 2021), varying levels of interoceptive accuracy, from lowered to normal (Hart et al., 2013; Eggart et al., 2019), and reduced interoceptive sensitivity (Schmitz et al., 2021; Flasinski et al., 2020). To the best of our knowledge, no study has investigated the relationship between FER and interoception in war victims with PTSD and CPTSD. Against this background, this study evaluated FER through interoceptive priming tasks (Salamone et al., 2021; Schneider et al., 2020) in victims of the Colombian conflict with PTSD and CPTSD. The affect–emotion model of the interoceptive priming task assumes that the initial classification of stimuli by valence occurs prior to cognitive analysis during the early stages of perceptual processing (Barrett and Bar, 2009). Given this framework, electrophysiological correlates of this task show selective disruptions in interoceptive regions and significant differences in emotion recognition in neurodegenerative populations (Salamone et al., 2021). Studying FER and interception in victims with early exposure to war with PTSD and CPTSD is crucial for understanding the impact of war on interception and its neurophysiological mechanisms. Given that models such as the SMH and SAME underscore the relationship between interoception and emotional regulation, investigating these processes in victims of armed conflict diagnosed with PTSD and CPTSD is crucial. Such research can provide valuable insights into the neurobiological mechanisms underlying emotional regulation and interoceptive deficits among populations exposed to violence.
Considering the established connection between emotion processing and interoception (Adolfi et al., 2017) and the interoceptive and emotional dysfunctions in PTSD patients, as well as the altered emotion in victims of armed conflict in Colombia (Trujillo et al., 2019), this study could provide a relevant model for evaluating interoceptive priming in PTSD and CPTSD (Reinhardt et al., 2020; Richter and Ibáñez, 2021). We hypothesized that participants who were victims of armed conflict with PTSD and CPTSD symptoms would perform worse in FER than the control group. Additionally, it was expected that the victim group (PTSD-CPTSD) would present patterns associated with lower rates of FER and interoceptive accuracy than the control group, possibly due to alterations in interoceptive pathways. Finally, we expected victims with CPTSD to show differences in the amplitude of the HEP in frontocentral regions during interoceptive processing with higher amplitudes, unlike the control groups with lower amplitudes.
2 Materials and methods
2.1 Participants
The study included 44 medication-free participants with a mean age of 52.82 (SD = 8.11), 31 females (70.5%) and 13 males (29.5%) with 12 years of schooling (Mean = 12.32, SD = 4.52) were distributed in 3 groups —17 healthy controls (CG), 19 victims (PTSD), and 8 victims with CPTSD—using the International Trauma Questionnaire (ITQ), which allows the identification of the distinction between PTSD and CPTSD symptomatology (Table 1 and Supplementary Table 1). The sample size was calculated with an A-priori analysis in G-Power 3.1 software. Owing to the design of this study and population, a moderate effect size of F = 0.25, a power of 0.95 and a p = 0.05 were used, and establishing a total sample of 36 subjects is adequate to determine the estimated effects. The participants were contacted through nonprofit organizations and community leaders supported by the Victims Unit of the Department of Valle del Cauca-Colombia. The victims had reported previously (Organization WH, 2018) and were extensively assessed in previous studies (Soleimani et al., 2024; Yousefi and Abdoli, 2024; Bressington et al., 2024). All participants underwent cognitive assessment using standardized instruments (Nasreddine et al., 2005; Torralva et al., 2009) and specialized questionnaires (Supplementary material 1.2, Clinical measures). The exclusion criteria included a history of mental disorders or active psychiatric illness, illiteracy, and failure to sign the informed consent form (Supplementary material 1.2, Clinical measures). The research protocol was approved by the Ethics Committee of ICESI University, and informed consent was obtained in accordance with the Declaration of Helsinki.
2.2 Measures and procedure
2.2.1 Interoceptive priming task
The interoceptive priming task consisted of two phases. The first one, the priming task, consisted of an interoceptive or exteroceptive block. In this phase, the participants were positioned in front of a 16-inch monitor screen and a keyboard. They were required to press the “Z” key while imagining/feeling their heartbeat without feedback for 2 min (interoceptive condition) or following a recorded heartbeat (exteroceptive condition), using the same procedure performed by Salamone, Legaz (Salamone et al., 2021) with a standardized heartbeat detection task (Salamone et al., 2021; Canales-Johnson et al., 2015; García-Cordero et al., 2016; Salamone et al., 2020; Salamone et al., 2018). The interoceptive condition was administered without any feedback to obtain an objective measure of interoceptive accuracy (Salamone et al., 2021; Garfinkel et al., 2015).
After the priming phase (interoceptive or exteroceptive), the participants performed the FER task. In this phase, the participants had to recognize whether the facial emotion was neutral, positive or negative. The stimuli were presented on the basis of Ekman and Friesen’s images (Ekman and Friesen, 1971) in 4 counterbalanced blocks per participant. Fifty-six faces were presented in a pseudorandom order, with 8 neutral, 16 positive (8 happiness, 8 surprise), and 32 negative faces (8 anger, 8 disgust, 8 sadness, 8 fear) (Salamone et al., 2021) (Supplementary Figure 1).
2.3 Statistical analysis
2.3.1 Behavioral analysis
FER was performed via the inverse efficiency score (IES) (Salamone et al., 2021), which combines reaction time (RT) and accuracy to analyze weighted behavioral outcomes (Jacquet and Avenanti, 2015). The IES was calculated by dividing the RT index by the percentage of correct responses to control for biases due to reaction times with low accuracy (Salamone et al., 2021; Brozzoli et al., 2008; Mevorach et al., 2006). This means that higher IES scores imply worse performance. The average IES was determined from the two FER blocks following the interoceptive condition and the two blocks following the exteroceptive condition per subject for each type of emotion in the negative, positive, and neutral conditions (Supplementary material 1.4, Statistical analysis).
2.3.2 High-density EEG (HD-EEG) preprocessing and analysis
For interoceptive priming recording, the Biosemi® Active-Two system with 128 channels at 1024 Hz + 2 external adhesive Ag/Ag-Cl electrodes placed on the left lower abdominal quadrant and under the right clavicle for ECG acquisition, as well as two electrodes placed on the left and right mastoids for reference fixation, were used (Ventura-Bort and Weymar, 2024; Migeot et al., 2023). The data sampling rate was changed to 256 Hz and filtered between 0.5–40 mV. Eye movement correction was performed via independent component analysis (Kim and Kim, 2012) and a visual inspection protocol following the parameters of previous studies (Salamone et al., 2021; García-Cordero et al., 2016; Yoris et al., 2018). The ECG signal and R waves were identified with the peakfinder function in MATLAB (Yoder, 2009). This signal was segmented for HEP analysis into epochs from −200 to 800 ms (Canales-Johnson et al., 2015; García-Cordero et al., 2016; Salamone et al., 2020; Yoris et al., 2018). HEPs were generated for each condition in an 800 ms window, delimiting EEG epochs between −200 and 500 ms close to the R-wave peak, corrected between −300 and 0 ms (Salamone et al., 2021; Salamone et al., 2018). HEPs were generated for each condition prior to FER (interoception-exteroception). Thus, to test for HEP modulations in the PTSD group, the interoception conditions were compared through a point-by-point Monte Carlo permutation test with bootstrapping (5,000 permutations, p = < 0.05) (Manly, 2018). The main HEP analyses were conducted on a frontal ROI associated with interoceptive attention modulation (Couto et al., 2015; Salamone et al., 2021; García-Cordero et al., 2016), which is composed of 11 electrodes: C9, C10, C14, C15, C18, C19, C20, C27, C28, C31 and C32. Analyses were also performed with four frontal ROIs: left-frontal: C26, C27, C28, C31, C32, D3, D4, D5, D6, and D7; central-frontal: C11, C12, C18, C19, C20, C21, C22, C23, C24, and C25; right-frontal-lateral: B17, B18, B19, B20, B21, B22, B23, B30, B1, and B2; and left-frontal-lateral: D11, D12, D13, D14, D17, D18, D19, D20, D27, and D28, to assess modulation at different locations.
3 Results
3.1 Demographics and cognitive data
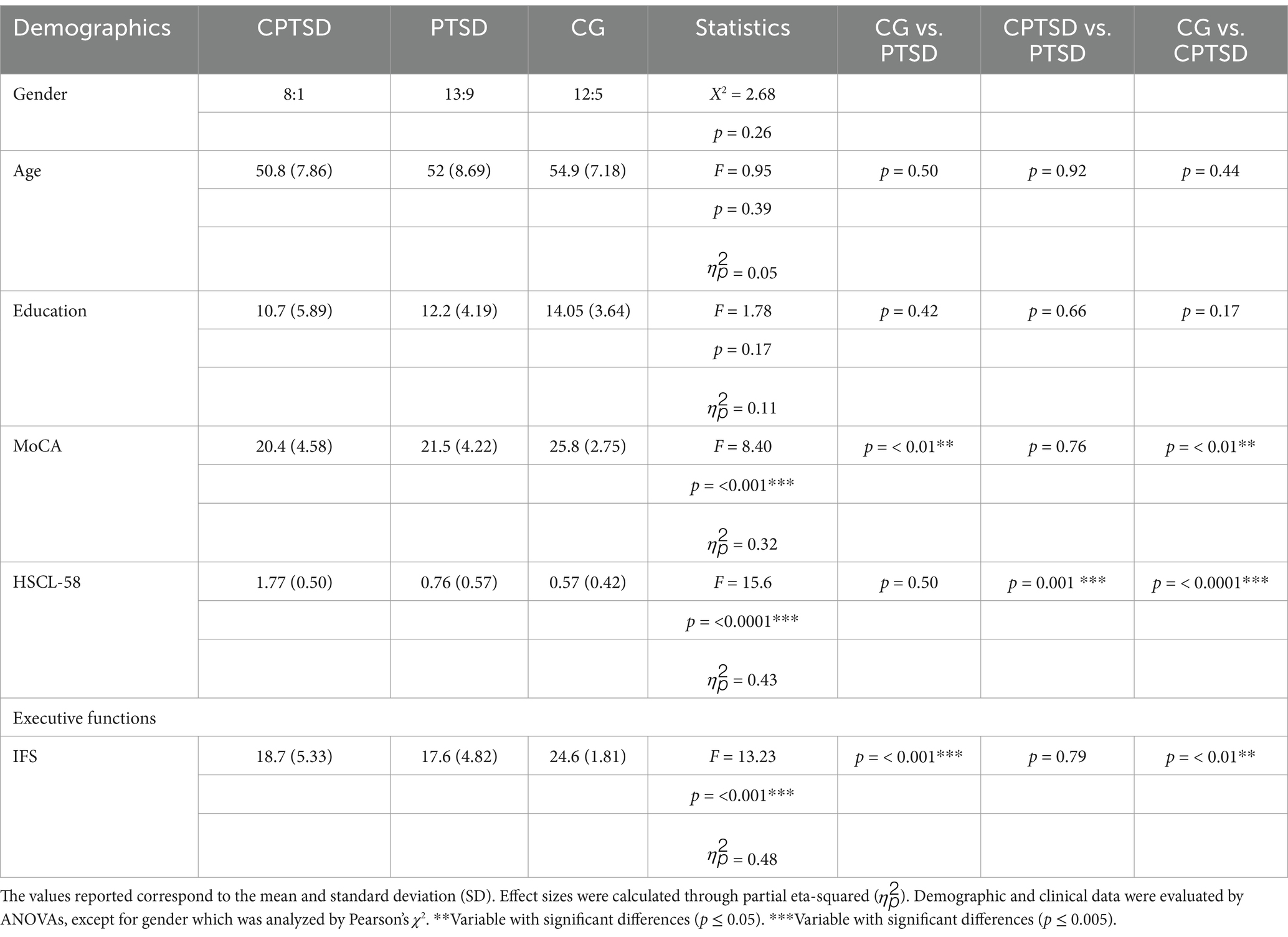
Significant differences were found in the IFS and MoCA test scores between the groups (including working memory scales) (Table 1).
3.2 FER results
We found differences in FER across groups in the positive, negative, and neutral conditions (F = 5.653, p = 0.0068) (Supplementary Figure 1). Contrasts revealed that both the CPTSD group and the PTSD group performed worse than the CG did (CPTSD: mean = 1.037, SD = 0.718; PTSD: mean = 0.904, SD = 0.632; CG: mean = 0.609, SD = 0.516; η2 = 0.22). Additionally, comparisons by group and priming type did not reveal differences between the interoception and exteroception conditions (F = 0.692, p = 0.405) (Supplementary material 2.1, FER results).
3.3 Interoceptive and exteroceptive accuracy
In addition, we found differences between groups in interoceptive and exteroceptive accuracy. More specifically, the contrast showed bordering differences in the interoceptive and exteroceptive conditions between the PTSD patients and the CG, and the PTSD patients and the CG also presented significant outcomes in the exteroceptive conditions, as expected (Supplementary Table 2).
3.4 Correlations between interoceptive accuracy and emotion recognition
The correlations between interoceptive accuracy and the emotion recognition index indicated that a higher interoceptive accuracy index was associated with better performance in recognizing negative emotion in the CG (R = −0.66, p = 0.004) (Supplementary Figure 2 and Supplementary Table 3). However, this relationship was not observed in the CPTSD and PTSD groups (R = −0.4, p = 0.29; R = −0.39, p = 0.096, respectively). Furthermor, exteroceptive accuracy did not show any significant associations in any of the group (Supplementary Figure 2).
Separate correlations for positive and neutral emotions revealed no significant relationships between interoceptive accuracy and emotion recognition in any of the groups (Supplementary Figure 2 and Supplementary Table 3). When combining the CPTSD and PTSD groups, victims collectively performed better on the IES in interoceptive priming, as compared to the correlation observed between the IES and accuracy in exteroceptive priming (Supplementary Figure 2).
Moreover, interoceptive accuracy emerged as a significant predictor of high performance in the FER of negative emotions in the CG (β = −6.12, p = 0.004), accounting for 39% of the variance. Similarly, in the group of victims, interoceptive accuracy significantly influenced FER performance in response to negative emotions (β = −3.46, p = 0.008). This effect may be partially explained by the inclusion of individuals with PTSD in this group, with interoceptive accuracy accounting for 20% of the variance (R2 = 0.20).
3.5 EEG results
Compared to the CG using an unpaired t test, the CPTSD group showed significantly greater (i.e., less negative) HEP amplitudes in the interoceptive condition, particularly in the fronto-central region of interest (ROI) at 500 ms [M = −0.108, SD = 0.485 for CPTSD-Intero; M = −0.121, SD = 0.472 for CG-Intero; t(16.567) = −2.94325, p = <0.01, d = 0.022] (Figure 1A). This finding revealed differences in a second HEP component (250–600 ms), in which the CPTSD group showed higher amplitude than the CG, suggesting hyperactivity in the cognitive processing of the sensory stimulus. We also found significant differences between the CPTSD and PTSD groups in the right frontal–lateral ROI at 150 ms [M = −0.002, SD = 0.507 for CPTSD-Intero; M = −0.022, SD = 0.527 for PTSD-Intero; t(13.439) = −3.027, p = 0.0132, d = 0.040], specifically, in a first HEP component (50–250 ms). In this window, the CPTSD group again showed less negative (i.e., larger) amplitudes than the PTSD group, suggesting increased early afferent processing from the heart to the brain in CPTSD (Figure 1B).
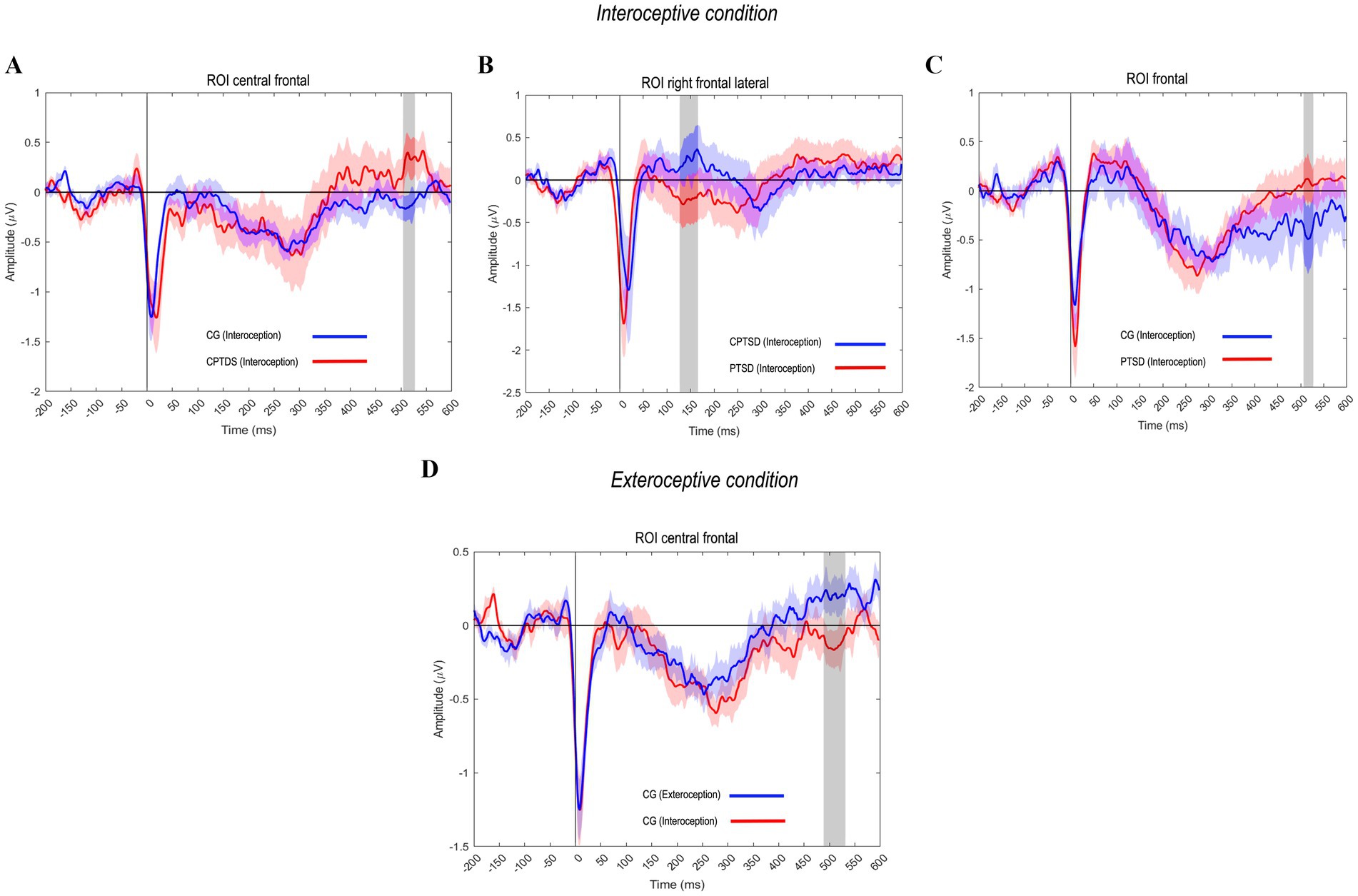
Figure 1. HEP amplitude modulations across groups and conditions. (A) The CPTSD group showed significantly greater (less negative) HEP amplitudes in the interoceptive condition at 500 ms in the fronto-central ROI, reflecting a second HEP component (250–600 ms) linked to cognitive processing of the sensory stimulus. (B) Significant differences between the CPTSD and PTSD groups were observed in the right frontal-lateral ROI at 150 ms, particularly in the first HEP component (50–250 ms), suggesting heightened early afferent processing from the heart to the brain in the victim groups. (C) The PTSD and CG groups exhibited significant differences in the second HEP component at 500 ms, with the PTSD group showing less negative HEP amplitudes. (D) Significant differences in the frontal region between the interoceptive and exteroceptive conditions in the CG at 500 ms, indicating a second HEP component, potentially associated with blood pressure waves synchronized with brain activity.
Additionally, PTSD and CG groups showed significant differences in a second HEP component at 500 ms (Figure 1C), with the PTSD group exhibiting less negative HEP amplitudes than the CG [M = −0.121, SD = 0.696 for PTSD-Intero; M = −0.178, SD = 0.626 for CG-Intero; t(30.936) = 2.392, p = < 0.02, d = 0.086], indicating stronger interoceptive cortical responses in the PTSD group. Finally, as anticipated, significant differences were found in the paired t test in the frontal region between the interoceptive and exteroceptive conditions in the CG at 500 ms [M = −0.121, SD = 0.472 for CG-Intero; M = −0.062, SD = 0.507 for CG-Extero; t(16) = −1.910, p = 0.015, d = 0.119]. This difference likely reflects a second HEP component, potentially related to blood pressure waves synchronized with brain activity, with less negative and wide amplitudes related to the exteroceptive event, in contrast to what we found with the group of victims (CPTSD-PTSD) (Figure 1D).
4 Discussion
To our knowledge, this is the first study to investigate FER using interoceptive priming tasks and cardiac interoceptive signals measured by HEP amplitudes in victims of armed conflict in Colombia with PTSD symptoms and healthy controls. Behaviorally, the observed FER impairments indicate that the PTSD and CPTSD groups exhibited deficits in FER. Specifically, compared with those in the CG and PTSD, the performance of CPTSD victims in FER decreased after receiving interoceptive priming versus exteroceptive priming. Compared with the controls, the subjects with CPTSD exhibited a reduced amplitude of the HEP in the frontocentral regions during interoceptive processing. Significant differences were observed between the CPTSD and PTSD groups in the right frontal–lateral region during interoceptive priming. Finally, the associations between interoceptive-exteroceptive accuracy and the IES revealed that the CG improved the FER accuracy of negative emotions in interoceptive priming, and we observed this same result in the correlations with grouped victims. Despite this, victims did not increase their accuracy in exteroceptive priming.
4.1 Interoceptive priming on emotion recognition
The performance of healthy controls with respect to negative emotions was lower during interoceptive priming. This finding could be linked to the influence of embodied congruency, described in studies that included heart rate and demonstrated a facilitating effect on the emotional processing of negative emotions such as fearful faces, but no such effect was observed for disgusted or neutral faces (Pezzulo et al., 2018). This approach suggests that a physiological condition such as a heartbeat is an interoceptive element that allows the simulation of certain emotions, but favors the exteroceptive processing of emotional stimuli (Yu et al., 2021) and possibly interferes with FER. Despite this support, it is unclear how interoceptive priming could induce feelings that are not congruent with negative stimuli presented in the task. In addition, we found deficits in FER general conditions in PTSD and CPTSD victims compared with controls, as expected in previous PTSD studies (Castro-Vale et al., 2020; Williams et al., 2018). After interoceptive priming, the FER of negative emotions did not improve in participants with CPTSD, whereas victims with PTSD showed better performance according to the IES index. These findings suggest that interoceptive priming differentially affects the FER of negative emotions in the presence of PTSD or CPTSD. These results indicate that alterations in interoceptive predictive coding mechanisms are linked to abnormal emotional processing and basic emotions in PTSD (Passardi et al., 2019; Couette et al., 2022; Wang et al., 2016; Couette et al., 2020). Additionally, these observed differences could be due to criteria related to the diagnosis of CPTSD, which has a set of symptoms called disturbances in self-organization (DSO) (Fernández-Fillol et al., 2021; Ford, 2021). These symptoms include affective dysregulation; therefore, it is plausible that interoceptive priming improved FER performance in the PTSD group, but did not favor the CPTSD group because of the nature of the symptoms. Moreover, trauma-related symptoms impact the recognition of basic emotions, as exposure to violence affects emotional processing in adulthood (Umiltà et al., 2013). The observed differences between the PTSD and CPTSD groups may suggest that changes in self-organization are linked to significant and sustained exposure to traumatic events and interpersonal challenges during early development (Hyland et al., 2017). An example of this is intergenerational exposure to the conflict in Colombia, which has lasted for over six decades. We interpret these findings within the SMH (Damsio, 1994; Damasio, 1999) frameworks and the SAME (Cacioppo et al., 1992), suggesting that emotions depend on how the brain interprets interoceptive signals. Therefore, disruptions in these interoceptive signals may significantly impair emotional regulation, as occurs in disorders such as PTSD. Theoretically, this is consistent with the idea that incongruence between internal and external states may hinder the value assigned to cognitive or emotional actions (Ochsner and Gross, 2014). Disruptions between physiological and affective components and exaggerated threat detection may impair emotion identification (Brown et al., 2020). Recent proposals (Silvanto and Nagai, 2025) have suggested a relationship between interoception and mental imagery, emphasizing the critical role of the insular cortex and anterior cingulate cortex (ACC) in integrating bodily and emotional processes. Specifically, the ACC serves as an integrative hub that combines sensory, emotional, and cognitive inputs during perception. This integration is particularly relevant in PTSD, where flashbacks represent a core symptom. Given this association, future research should investigate how interoceptive activation influences mental imagery and subsequently impacts FER in populations with PTSD and CPTSD. The connection with internal sensations increases CPTSD symptoms, which can be explained by generative models that combine exteroceptive “threat” and interoceptive “allostasis” priors (Krupnik, 2020; Wilkinson et al., 2017). In this sense, functions may be more affected in victims with CPTSD than in victims with PTSD because allostatic-interoceptive prediction requires regulating the body’s internal environment to predict and meet the needs generated by environmental demands before they arise (Migeot et al., 2022). This hypothesis should be explored in future studies.
According to evidence from a neurobiological model, these deficits could be explained by hyperactivation of the amygdala and insula in CPTSD (Suarez-Jimenez et al., 2020; Ressler et al., 2022) and deficits in the recognition of negative emotions (Passardi et al., 2019; Greene et al., 2020). Conversely, interoceptive accuracy was negatively correlated with the IES for negative emotion recognition in subjects not exposed to conflict and with the CPTSD score. Enhanced interoceptive ability improves emotion recognition, highlighting that interoceptive accuracy is closely related to the peripheral processing of emotional stimuli (Calì et al., 2015; Herbert et al., 2007; Pollatos et al., 2007). This evidence suggests that central and peripheral pathways are affected in CPTSD and are affected by exposure to violence. This hypothesis supports the idea that interoceptive accuracy increases sensitivity to emotional recognition of negative stimuli in healthy controls (Salamone et al., 2021; Critchley and Garfinkel, 2017; Terasawa et al., 2014) and in populations with emotion recognition deficits, such as the PTSD group.
4.2 Electrophysiological markers: HEP
With respect to electrophysiological markers, we found that, in contrast with controls, PTSD and CPTSD victims presented attenuated negative amplitudes in HEPs in frontocentral regions during interoceptive processing. This result contrasts with a previous study that did not show differences in HEP amplitudes between PTSD patients and controls (Schmitz et al., 2021). This finding suggests that this condition is more typical of neuropsychiatric disorders, as demonstrated in other studies (Flasbeck et al., 2020; Pang et al., 2019; García-Cordero et al., 2016) and in older adults (Kamp et al., 2021). Additionally, attenuated HEP amplitudes are typical of disorders with high prevalence rates of early-life maltreatment (Schmitz et al., 2020; Mueller et al., 2015). This may be the case for the older adult community we examined, who have been subjected to repeated conflicts over an extended period of time, resulting in CPTSD symptoms. Future studies should examine the effects of early-life maltreatment on interoception in victims of violence, as prior research suggests that such maltreatment may increase the risk of stress-induced interoceptive dysfunction (Schaan et al., 2019; Schulz et al., 2022).
4.3 Limitations and future directions
The present study has several limitations. First, the sample size was small, particularly in the CPTSD group, with the majority of participants being women meticulously matched with healthy controls. Although previous studies have acknowledged these factors, the low proportion of men and the small sample size (Gómez et al., 2024; Gómez et al., 2022) may partly explain these findings. The study employed a rigorous selection process with strict inclusion and exclusion criteria, ensuring the absence of neurological and psychiatric disorders, which are common in individuals affected by Colombian violence. Most participants were female survivors of violence, with men having the highest mortality rates (Urdinola et al., 2017). Research has shown that victims experience profound and lasting psychiatric and neurological difficulties as a result of the war. Another potential limitation relates to the age distribution of the subjects. Although the variations observed in interoception, FER, and HEP are not explained by the low performance in cognitive assessments (e.g., MOCA and IFS) in PTSD and CPTSD, caution should be exercised when extrapolating the results of the present study. Many variables related to biological and social factors may influence the context of violence to which the Colombian population has been exposed in recent decades. Future studies should include a larger number of subjects and consider the possibility of including other mental disorders, as these could also result from violence. Additionally, incorporating assessments of mental imagery would further clarify its role in emotional recognition and interoceptive mechanisms among populations with PTSD and CPTSD complex PTSD, given that interoceptive processing and mental imagery share common neural substrates, particularly the insular cortex. Investigating the relationships among aging, potential cognitive disorders, and social determinants associated with violence is crucial. In addition, research should include younger subjects to study the effects of violence on interoception and social cognition.
5 Conclusion
The study demonstrated that interoception increases the ability to discern negative emotions in individuals with PTSD but not in those with CPTSD. Additionally, interoception enhanced the perception of neutral emotions across all groups. Electroencephalographic (EEG) analysis revealed theta-band oscillations (HEPs), particularly in frontal and frontocentral regions. These results highlight the importance of interoceptive training in enhancing emotion identification, especially in individuals with PTSD. The potential implications of these findings for treating PTSD and CPTSD are substantial. Future research should explore the role of DSO in FER and interoception among individuals affected by armed conflict with PTSD and PTSD-CPTSD. Ultimately, this improved understanding will facilitate the development of more effective treatments. The findings of this study are particularly relevant, as it is among the first conducted within the Colombian population, offering valuable insights into how prolonged exposure to armed conflict affects interoceptive processing and emotional recognition in individuals diagnosed with PTSD.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Comité de Ética de Investigación Humana. Universidad Icesi. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
EH: Conceptualization, Funding acquisition, Investigation, Methodology, Supervision, Writing – original draft. DG-S: Conceptualization, Formal analysis, Investigation, Methodology, Writing – review & editing. AB-O: Methodology, Writing – review & editing. JJ: Formal analysis, Methodology, Writing – review & editing. HS-G: Conceptualization, Methodology, Writing – review & editing. AB: Conceptualization, Formal analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by a grant from Minciencias, Colombia. Code: 0864-937-105906. Contract: 038-202 and Universidad Icesi – Convocatoria Interfacultades (grant nos. CA0313271 and CA03130118, Colombia).
Acknowledgments
We express our gratitude to the individuals who took part in the project, as well as to the institutions, including hospitals and the Victims Unit, that provided support and collaboration to make this initiative a reality.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that Gen AI was used in the creation of this manuscript. Artificial intelligence (AI) was used for English language revision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyg.2025.1567574/full#supplementary-material
References
Abrevaya, S., Fittipaldi, S., García, A. M., Dottori, M., Santamaria-Garcia, H., Birba, A., et al. (2020). At the heart of neurological dimensionality: cross-nosological and multimodal cardiac interoceptive deficits. Psychosom. Med. 82, 850–861. doi: 10.1097/PSY.0000000000000868
Adolfi, F., Couto, B., Richter, F., Decety, J., Lopez, J., Sigman, M., et al. (2017). Convergence of interoception, emotion, and social cognition: a twofold fMRI meta-analysis and lesion approach. Cortex 88, 124–142. doi: 10.1016/j.cortex.2016.12.019
Akiki, T. J., Averill, C. L., and Abdallah, C. G. (2017). A network-based neurobiological model of PTSD: evidence from structural and functional neuroimaging studies. Curr. Psychiatry Rep. 19, 1–10. doi: 10.1007/s11920-017-0840-4
Angel, C. M. (2016). Resilience, post-traumatic stress, and posttraumatic growth: Veterans' and active duty military members' coping trajectories following traumatic event exposure. J. Nedt. 47, 57–60. doi: 10.1016/j.nedt.2016.04.001
Barrett, L. F., and Bar, M. (2009). See it with feeling: affective predictions during object perception. Philos. Trans. Royal Soc. Biol. Sci. 364, 1325–1334. doi: 10.1098/rstb.2008.0312
Barrett, L. F., and Simmons, W. K. (2015). Interoceptive predictions in the brain. Nat. Rev. Neurosci. 16, 419–429. doi: 10.1038/nrn3950
Beutler, S., Mertens, Y. L., Ladner, L., Schellong, J., Croy, I., and Daniels, J. K. (2022). Trauma-related dissociation and the autonomic nervous system: a systematic literature review of psychophysiological correlates of dissociative experiencing in PTSD patients. Eur. J. Psychotraumatol. 13:2132599. doi: 10.1080/20008066.2022.2132599
Birba, A., Santamaría-García, H., Prado, P., Cruzat, J., Ballesteros, A. S., Legaz, A., et al. (2022). Allostatic-interoceptive overload in frontotemporal dementia. Biol. Psychiatry 92, 54–67. doi: 10.1016/j.biopsych.2022.02.955
Bonaz, B., Lane, R. D., Oshinsky, M. L., Kenny, P. J., Sinha, R., Mayer, E. A., et al. (2021). Diseases, disorders, and comorbidities of interoception. Trends Neurosci. 44, 39–51. doi: 10.1016/j.tins.2020.09.009
Bressington, D., Hyland, P., Steele, H., Byrne, M., Mitchell, D., Keane, C., et al. (2024). ICD-11 post-traumatic stress disorder and complex post-traumatic stress disorder in mental health support-seeking former-serving Australian defence force veterans. Austr. New Zealand J. Psychiatr. 58, 416–424. doi: 10.1177/00048674241230197
Brown, C. L., Van Doren, N., Ford, B. Q., Mauss, I. B., Sze, J. W., and Levenson, R. W. (2020). Coherence between subjective experience and physiology in emotion: individual differences and implications for well-being. Emotion 20, 818–829. doi: 10.1037/emo0000579
Brozzoli, C., Ishihara, M., Göbel, S. M., Salemme, R., Rossetti, Y., and Farnè, A. (2008). Touch perception reveals the dominance of spatial over digital representation of numbers. Proc. Natl. Acad. Sci. 105, 5644–5648. doi: 10.1073/pnas.0708414105
Cacioppo, J. T., Berntson, G. G., and Klein, D. J. (1992). “What is an emotion? The role of somatovisceral afference, with special emphasis on somatovisceral illusions” in Emotion and Social Behavior. ed. M. S. Clark (London: Sage Publications, Inc.), 63–98.
Calì, G., Ambrosini, E., Picconi, L., Mehling, W. E., and Committeri, G. (2015). Investigating the relationship between interoceptive accuracy, interoceptive awareness, and emotional susceptibility. Front. Psychol. 6:1202. doi: 10.3389/fpsyg.2015.01202
Cambi, S., Solcà, M., Micali, N., and Berchio, C. (2024). Cardiac interoception in anorexia nervosa: a resting-state heartbeat-evoked potential study. Eur. Eat. Disord. Rev. 32, 417–430. doi: 10.1002/erv.3049
Campo-Arias, A., Oviedo, H. C., and Herazo, E. (2014). Prevalencia de síntomas, posibles casos y trastornos mentales en víctimas del conflicto armado interno en situación de desplazamiento en Colombia: una revisión sistemática. Rev. Colomb. Psiquiatr. 43, 177–185. doi: 10.1016/j.rcp.2014.07.003
Canales-Johnson, A., Silva, C., Huepe, D., Rivera-Rei, Á., Noreika, V., Garcia, M. C., et al. (2015). Auditory feedback differentially modulates behavioral and neural markers of objective and subjective performance when tapping to your heartbeat. Cereb. Cortex 25, 4490–4503. doi: 10.1093/cercor/bhv076
Castro-Vale, I., Severo, M., and Carvalho, D. (2020). Lifetime PTSD is associated with impaired emotion recognition in veterans and their offspring. Psychiatry Res. 284:112666. doi: 10.1016/j.psychres.2019.112666
Charles, M. (2022). Re-thinking trauma: local journalism, peace-building and continuous traumatic stress (CTS) on the violent margins of Colombia. Media, War Conflict. 15, 202–220. doi: 10.1177/1750635220939121
Couette, M., Mouchabac, S., Adrien, V., Cagnone, V., Bourla, A., and Ferreri, F. (2022). Functional neuro-anatomy of social cognition in posttraumatic stress disorder: a systematic review. Psychiatry Res. 315:114729. doi: 10.1016/j.psychres.2022.114729
Couette, M., Mouchabac, S., Bourla, A., Nuss, P., and Ferreri, F. (2020). Social cognition in post-traumatic stress disorder: a systematic review. Br. J. Clin. Psychol. 59, 117–138. doi: 10.1111/bjc.12238
Couto, B., Adolfi, F., Velasquez, M., Mesow, M., Feinstein, J., Canales-Johnson, A., et al. (2015). Heart evoked potential triggers brain responses to natural affective scenes: a preliminary study. Auton. Neurosci. 193, 132–137. doi: 10.1016/j.autneu.2015.06.006
Critchley, H. D., and Garfinkel, S. N. (2017). Interoception and emotion. Curr. Opin. Psychol. 17, 7–14. doi: 10.1016/j.copsyc.2017.04.020
Damasio, A. R. (1999). The feeling of what happens: Body and emotion in the making of consciousness. New York, NY: Houghton Mifflin Harcourt.
Damsio, A. (1994). Descartes' error: Emotion, reason, and the human brain. New York, NY: Avon Books.
Donofry, S. D., Roecklein, K. A., Wildes, J. E., Miller, M. A., and Erickson, K. I. (2016). Alterations in emotion generation and regulation neurocircuitry in depression and eating disorders: a comparative review of structural and functional neuroimaging studies. Neurosci. Biobehav. Rev. 68, 911–927. doi: 10.1016/j.neubiorev.2016.07.011
Eggart, M., Lange, A., Binser, M. J., Queri, S., and Müller-Oerlinghausen, B. (2019). Major depressive disorder is associated with impaired interoceptive accuracy: a systematic review. Brain Sci. 9:131. doi: 10.3390/brainsci9060131
Ekman, P., and Friesen, W. V. (1971). Constants across cultures in the face and emotion. J. Pers. Soc. Psychol. 17, 124–129. doi: 10.1037/h0030377
Fernández-Fillol, C., Pitsiakou, C., Perez-Garcia, M., Teva, I., and Hidalgo-Ruzzante, N. (2021). Complex PTSD in survivors of intimate partner violence: risk factors related to symptoms and diagnoses. Eur. J. Psychotraumatol. 12:2003616. doi: 10.1080/20008198.2021.2003616
Fine, N. B., Ben-Zion, Z., Biran, I., and Hendler, T. (2023). Neuroscientific account of guilt-and shame-driven PTSD phenotypes. Eur. J. Psychotraumatol. 14:2202060. doi: 10.1080/20008066.2023.2202060
Fittipaldi, S., Abrevaya, S., de la Fuente, A., Pascariello, G. O., Hesse, E., Birba, A., et al. (2020). A multidimensional and multi-feature framework for cardiac interoception. NeuroImage 212:116677. doi: 10.1016/j.neuroimage.2020.116677
Flasbeck, V., Popkirov, S., Ebert, A., and Brüne, M. (2020). Altered interoception in patients with borderline personality disorder: a study using heartbeat-evoked potentials. Borderline Person. Disorder Emot. Dysregulation. 7, 1–13. doi: 10.1186/s40479-020-00139-1
Flasinski, T., Dierolf, A. M., Rost, S., Lutz, A. P., Voderholzer, U., Koch, S., et al. (2020). Altered interoceptive awareness in high habitual symptom reporters and patients with somatoform disorders. Front. Psychol. 11:1859. doi: 10.3389/fpsyg.2020.01859
Ford, J. D. (2021). Progress and limitations in the treatment of complex PTSD and developmental trauma disorder. Curr. Treat. Options Psychiatry 8, 1–17. doi: 10.1007/s40501-020-00236-6
García-Cordero, I., Sedeño, L., De La Fuente, L., Slachevsky, A., Forno, G., Klein, F., et al. (2016). Feeling, learning from and being aware of inner states: interoceptive dimensions in neurodegeneration and stroke. Philos. Trans. Royal Soc. B Biol. Sci. 371:20160006. doi: 10.1098/rstb.2016.0006
Garfinkel, S. N., and Critchley, H. D. (2016). Threat and the body: how the heart supports fear processing. Trends Cogn. Sci. 20, 34–46. doi: 10.1016/j.tics.2015.10.005
Garfinkel, S. N., Gould van Praag, C. D., Engels, M., Watson, D., Silva, M., Evans, S. L., et al. (2021). Interoceptive cardiac signals selectively enhance fear memories. J. Exp. Psychol. Gen. 150, 1165–1176. doi: 10.1037/xge0000967
Garfinkel, S. N., Seth, A. K., Barrett, A. B., Suzuki, K., and Critchley, H. D. (2015). Knowing your own heart: distinguishing interoceptive accuracy from interoceptive awareness. Biol. Psychol. 104, 65–74. doi: 10.1016/j.biopsycho.2014.11.004
Ge, F., Yuan, M., Li, Y., and Zhang, W. (2020). Posttraumatic stress disorder and alterations in resting heart rate variability: a systematic review and meta-analysis. Psychiatry Investig. 17, 9–20. doi: 10.30773/pi.2019.0112
Gebhardt, C., Alliger-Horn, C., Mitte, K., and Glaesmer, H. (2017). All-or-nothing thinking: the processing of emotional expressions in traumatized post-deployment soldiers. J. Anxiety Disord. 47, 69–74. doi: 10.1016/j.janxdis.2016.12.004
Gómez, D., López, J. D., Giraldo, L. S., Huepe, D. A., García, A. M., and Trujillo, N. (2024). Exposure to armed conflict and monitoring as predictors of aggression in a population immersed in a long-term conflict. Polit. Psychol. 45, 827–839. doi: 10.1111/pops.12972
Gómez, D., López Hincapié, J. D., Cardona, L. S. G., Ugarriza, J. E., Herrera, E., and Trujillo, N. (2022). Structural analysis of the reactive-proactive aggression questionnaire in population exposed to armed conflicts. Peace Conflict J. Peace Psychol. 28, 34–43. doi: 10.1037/pac0000555
Greene, T., Gelkopf, M., Fried, E. I., Robinaugh, D. J., and Lapid, P. L. (2020). Dynamic network analysis of negative emotions and DSM-5 posttraumatic stress disorder symptom clusters during conflict. J. Trauma. Stress. 33, 72–83. doi: 10.1002/jts.22433
Grossi, D., Longarzo, M., Quarantelli, M., Salvatore, E., Cavaliere, C., De Luca, P., et al. (2017). Altered functional connectivity of interoception in illness anxiety disorder. Cortex 86, 22–32. doi: 10.1016/j.cortex.2016.10.018
Harricharan, S., Nicholson, A. A., Thome, J., Densmore, M., McKinnon, M. C., Théberge, J., et al. (2020). PTSD and its dissociative subtype through the lens of the insula: anterior and posterior insula resting-state functional connectivity and its predictive validity using machine learning. Psychophysiology 57:e13472. doi: 10.1111/psyp.13472
Hart, N., McGowan, J., Minati, L., and Critchley, H. D. (2013). Emotional regulation and bodily sensation: interoceptive awareness is intact in borderline personality disorder. J. Personal. Disord. 27, 506–518. doi: 10.1521/pedi_2012_26_049
Hatfield, T. R., Brown, R. F., Giummarra, M. J., and Lenggenhager, B. (2019). Autism spectrum disorder and interoception: abnormalities in global integration? Autism 23, 212–222. doi: 10.1177/1362361317738392
Herbert, B. M., Pollatos, O., and Schandry, R. (2007). Interoceptive sensitivity and emotion processing: an EEG study. Int. J. Psychophysiol. 65, 214–227. doi: 10.1016/j.ijpsycho.2007.04.007
Hewitt Ramírez, N., Gantiva Díaz, C. A., Vera Maldonado, A., Cuervo Rodríguez, M. P., Nelly Liliam, H. O., Juárez, F., et al. (2014). Afectaciones psicológicas de niños y adolescentes expuestos al conflicto armado en una zona rural de Colombia. Acta Colombiana Psicol. 17, 79–89. doi: 10.14718/ACP.2014.17.1.9
Hyland, P., Shevlin, M., Brewin, C. R., Cloitre, M., Downes, A., Jumbe, S., et al. (2017). Validation of post-traumatic stress disorder (PTSD) and complex PTSD using the international trauma questionnaire. Acta Psychiatr. Scand. 136, 313–322. doi: 10.1111/acps.12771
Jacquet, P. O., and Avenanti, A. (2015). Perturbing the action observation network during perception and categorization of actions' goals and grips: state-dependency and virtual lesion TMS effects. Cereb. Cortex 25, 598–608. doi: 10.1093/cercor/bht242
Kamp, S.-M., Schulz, A., Forester, G., and Domes, G. (2021). Older adults show a higher heartbeat-evoked potential than young adults and a negative association with everyday metacognition. Brain Res. 1752:147238. doi: 10.1016/j.brainres.2020.147238
Karatzias, T., Shevlin, M., Ben-Ezra, M., McElroy, E., Redican, E., Vang, M. L., et al. (2023). War exposure, posttraumatic stress disorder, and complex posttraumatic stress disorder among parents living in Ukraine during the Russian war. Acta Psychiatr. Scand. 147, 276–285. doi: 10.1111/acps.13529
Kim, D., and Kim, S.-K. (2012). Comparing patterns of component loadings: principal component analysis (PCA) versus independent component analysis (ICA) in analyzing multivariate non-normal data. Behav. Res. Methods 44, 1239–1243. doi: 10.3758/s13428-012-0193-1
Krupnik, V. (2020). Trauma or drama: a predictive processing perspective on the continuum of stress. Front. Psychol. 11:1248. doi: 10.3389/fpsyg.2020.01248
Lieberman, J. M., Rabellino, D., Densmore, M., Frewen, P. A., Steyrl, D., Scharnowski, F., et al. (2023). Posterior cingulate cortex targeted real-time fMRI neurofeedback recalibrates functional connectivity with the amygdala, posterior insula, and default-mode network in PTSD. Brain Behav. 13:e2883. doi: 10.1002/brb3.2883
Maercker, A., Brewin, C. R., Bryant, R. A., Cloitre, M., van Ommeren, M., Jones, L. M., et al. (2013). Diagnosis and classification of disorders specifically associated with stress: proposals for ICD-11. World Psychiatry 12, 198–206. doi: 10.1002/wps.20057
Manly, B. F. (2018). Randomization, bootstrap and Monte Carlo methods in biology. London: Chapman and Hall/CRC.
Marroquín Rivera, A., Rincón Rodríguez, C. J., Padilla-Muñoz, A., and Gómez-Restrepo, C. (2020). Mental health in adolescents displaced by the armed conflict: findings from the Colombian national mental health survey. Child Adolesc. Psychiatry Ment. Health 14, 1–8. doi: 10.1186/s13034-020-00327-5
Martínez, N. T., Rodríguez, C. J. R., de Santacruz, C., Bautista, N. B., Collazos, J., and Gómez-Restrepo, C. (2016). Problemas mentales, trastornos del afecto y de ansiedad en la población desplazada por la violencia en Colombia, resultados de la Encuesta Nacional de Salud Mental 2015. Rev. Colomb. Psiquiatr. 45, 113–118. doi: 10.1016/j.rcp.2016.09.004
Mazza, M., Giusti, L., Albanese, A., Mariano, M., Pino, M. C., and Roncone, R. (2012). Social cognition disorders in military police officers affected by posttraumatic stress disorder after the attack of an-Nasiriyah in Iraq 2006. Psychiatry Res. 198, 248–252. doi: 10.1016/j.psychres.2011.11.027
McCurry, K. L., Frueh, B. C., Chiu, P. H., and King-Casas, B. (2020). Opponent effects of hyperarousal and re-experiencing on affective habituation in posttraumatic stress disorder. Biol. Psychiatr. Cognit. Neurosci. Neuroimag. 5, 203–212. doi: 10.1016/j.bpsc.2019.09.006
Mevorach, C., Humphreys, G. W., and Shalev, L. (2006). Opposite biases in salience-based selection for the left and right posterior parietal cortex. Nat. Neurosci. 9, 740–742. doi: 10.1038/nn1709
Migeot, J. A., Duran-Aniotz, C. A., Signorelli, C. M., Piguet, O., and Ibáñez, A. (2022). A predictive coding framework of allostatic–interoceptive overload in frontotemporal dementia. Trends Neurosci. 45, 838–853. doi: 10.1016/j.tins.2022.08.005
Migeot, J., Hesse, E., Fittipaldi, S., Mejía, J., Fraile, M., García, A. M., et al. (2023). Allostatic-interoceptive anticipation of social rejection. Neuro Image. 276:120200. doi: 10.1016/j.neuroimage.2023.120200
Moretta, P., Spisto, M., Ausiello, F. P., Iodice, R., De Lucia, N., Santangelo, G., et al. (2022). Alteration of interoceptive sensitivity: expanding the spectrum of behavioural disorders in amyotrophic lateral sclerosis. Neurol. Sci. 43, 5403–5410. doi: 10.1007/s10072-022-06231-4
Motomura, K., Terasawa, Y., Natsume, A., Iijima, K., Chalise, L., Sugiura, J., et al. (2019). Anterior insular cortex stimulation and its effects on emotion recognition. Brain Struct. Funct. 224, 2167–2181. doi: 10.1007/s00429-019-01895-9
Mueller, L. E., Schulz, A., Andermann, M., Gäbel, A., Gescher, D. M., Spohn, A., et al. (2015). Cortical representation of afferent bodily signals in borderline personality disorder: neural correlates and relationship to emotional dysregulation. JAMA Psychiatry 72, 1077–1086. doi: 10.1001/jamapsychiatry.2015.1252
Nasreddine, Z. S., Phillips, N. A., Bédirian, V., Charbonneau, S., Whitehead, V., Collin, I., et al. (2005). The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 53, 695–699. doi: 10.1111/j.1532-5415.2005.53221.x
Nicholson, A. A., Harricharan, S., Densmore, M., Neufeld, R. W., Ros, T., McKinnon, M. C., et al. (2020). Classifying heterogeneous presentations of PTSD via the default mode, central executive, and salience networks with machine learning. Neuro Image Clin. 27:102262. doi: 10.1016/j.nicl.2020.102262
Nicholson, A. A., Rabellino, D., Densmore, M., Frewen, P. A., Paret, C., Kluetsch, R., et al. (2017). The neurobiology of emotion regulation in posttraumatic stress disorder: amygdala downregulation via real-time fMRI neurofeedback. Hum. Brain Mapp. 38, 541–560. doi: 10.1002/hbm.23402
Nicholson, A. A., Rabellino, D., Densmore, M., Frewen, P. A., Steyrl, D., Scharnowski, F., et al. (2022). Differential mechanisms of posterior cingulate cortex downregulation and symptom decreases in posttraumatic stress disorder and healthy individuals using real-time fMRI neurofeedback. Brain Behav. 12:e2441. doi: 10.1002/brb3.2441
Ochsner, K. N., and Gross, J. J. (2014). The neural bases of emotion and emotion regulation: A valuation perspective. London: Guilford Publications.
Organization WH (2018). International classification of diseases for mortality and morbidity statistics (11th revision). Geneva: WHO.
Pang, J., Tang, X., Li, H., Hu, Q., Cui, H., Zhang, L., et al. (2019). Altered interoceptive processing in generalized anxiety disorder—a heartbeat-evoked potential research. Front. Psych. 10:616. doi: 10.3389/fpsyt.2019.00616
Passardi, S., Peyk, P., Rufer, M., Wingenbach, T. S., and Pfaltz, M. C. (2019). Facial mimicry, facial emotion recognition and alexithymia in post-traumatic stress disorder. Behav. Res. Ther. 122:103436. doi: 10.1016/j.brat.2019.103436
Pérez-Olmos, I., Fernández-Piñeres, P. E., and Rodado-Fuentes, S. (2005). The prevalence of war-related post-traumatic stress disorder in children from Cundinamarca, Colombia. Revista de salud Publica. 7, 268–280. doi: 10.1590/S0124-00642005000300003
Pezzulo, G., Iodice, P., Barca, L., Chausse, P., Monceau, S., and Mermillod, M. (2018). Increased heart rate after exercise facilitates the processing of fearful but not disgusted faces. Sci. Rep. 8:398. doi: 10.1038/s41598-017-18761-5
Poljac, E., Montagne, B., and de Haan, E. H. (2011). Reduced recognition of fear and sadness in post-traumatic stress disorder. Cortex 47, 974–980. doi: 10.1016/j.cortex.2010.10.002
Pollatos, O., Herbert, B. M., Mai, S., and Kammer, T. (2016). Changes in interoceptive processes following brain stimulation. Philos. Trans. Royal Soc. B Biol. Sci. 371:20160016. doi: 10.1098/rstb.2016.0016
Pollatos, O., Herbert, B. M., Matthias, E., and Schandry, R. (2007). Heart rate response after emotional picture presentation is modulated by interoceptive awareness. Int. J. Psychophysiol. 63, 117–124. doi: 10.1016/j.ijpsycho.2006.09.003
Pollatos, O., and Schandry, R. (2004). Accuracy of heartbeat perception is reflected in the amplitude of the heartbeat-evoked brain potential. Psychophysiology 41, 476–482. doi: 10.1111/1469-8986.2004.00170.x
Pollatos, O., and Schandry, R. (2008). Emotional processing and emotional memory are modulated by interoceptive awareness. Cognit. Emot. 22, 272–287. doi: 10.1080/02699930701357535
Putica, A., Argus, A., Khanna, R., Nursey, J., and Varker, T. (2024). Interoceptive interventions for posttraumatic stress: a systematic review of treatment and interoception outcomes. Traumatology 22:507. doi: 10.1037/trm0000507
Ramírez-Giraldo, A., Hernández-Bustamante, O., Romero-Acosta, K., and Porras-Mendoza, E. (2017). Estado de salud mental de personas víctimas del conflicto armado en Chengue. Psicología desde el Caribe. 34, 49–70. Available at: https://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0123-417X2017000100049
Reinhardt, K. M., Zerubavel, N., Young, A. S., Gallo, M., Ramakrishnan, N., Henry, A., et al. (2020). A multi-method assessment of interoception among sexual trauma survivors. Physiol. Behav. 226:113108. doi: 10.1016/j.physbeh.2020.113108
Ressler, K. J., Berretta, S., Bolshakov, V. Y., Rosso, I. M., Meloni, E. G., Rauch, S. L., et al. (2022). Post-traumatic stress disorder: clinical and translational neuroscience from cells to circuits. Nat. Rev. Neurol. 18, 273–288. doi: 10.1038/s41582-022-00635-8
Richter, F., and Ibáñez, A. (2021). Time is body: multimodal evidence of crosstalk between interoception and time estimation. Biol. Psychol. 159:108017. doi: 10.1016/j.biopsycho.2021.108017
Salamone, P. C., Esteves, S., Sinay, V. J., García-Cordero, I., Abrevaya, S., Couto, B., et al. (2018). Altered neural signatures of interoception in multiple sclerosis. Hum. Brain Mapp. 39, 4743–4754. doi: 10.1002/hbm.24319
Salamone, P. C., Legaz, A., Sedeño, L., Moguilner, S., Fraile-Vazquez, M., Campo, C. G., et al. (2021). Interoception primes emotional processing: multimodal evidence from neurodegeneration. J. Neurosci. 41, 4276–4292. doi: 10.1523/JNEUROSCI.2578-20.2021
Salamone, P. C., Sedeño, L., Legaz, A., Bekinschtein, T., Martorell, M., Adolfi, F., et al. (2020). Dynamic neurocognitive changes in interoception after heart transplant. Brain Commun. 2:fcaa095. doi: 10.1093/braincomms/fcaa095
Santamaría-García, H., Migeot, J., Medel, V., Hazelton, J. L., Teckentrup, V., Romero-Ortuno, R., et al. (2024). Allostatic interoceptive overload across psychiatric and neurological conditions. Biol. Psychiatry 97, 28–40. doi: 10.1016/j.biopsych.2024.06.024
Schaan, V. K., Schulz, A., Rubel, J. A., Bernstein, M., Domes, G., Schächinger, H., et al. (2019). Childhood trauma affects stress-related interoceptive accuracy. Front. Psych. 10:473251. doi: 10.3389/fpsyt.2019.00750
Schmitz, M., Bertsch, K., Löffler, A., Steinmann, S., Herpertz, S. C., and Bekrater-Bodmann, R. (2021). Body connection mediates the relationship between traumatic childhood experiences and impaired emotion regulation in borderline personality disorder. Borderline Person. Disorder Emot. Dysreg. 8, 1–13. doi: 10.1186/s40479-021-00157-7
Schmitz, M., Müller, L. E., Schulz, A., Kleindienst, N., Herpertz, S. C., and Bertsch, K. (2020). Heart and brain: cortical representation of cardiac signals is disturbed in borderline personality disorder, but unaffected by oxytocin administration. J. Affect. Disord. 264, 24–28. doi: 10.1016/j.jad.2019.11.139
Schmitz, M., Müller, L. E., Seitz, K. I., Schulz, A., Steinmann, S., Herpertz, S. C., et al. (2021). Heartbeat evoked potentials in patients with post-traumatic stress disorder: an unaltered neurobiological regulation system? Eur. J. Psychotraumatol. 12:1987686. doi: 10.1080/20008198.2021.1987686
Schneider, I., Bertsch, K., Izurieta Hidalgo, N. A., Müller, L. E., Defiebre, N., and Herpertz, S. C. (2020). The sound and face of others: vocal priming effects on facial emotion processing in posttraumatic stress disorder. Psychopathology 52, 283–293. doi: 10.1159/000503584
Schulz, A., Deuter, C. E., Breden, I.-H., Vögele, C., Wingenfeld, K., Otte, C., et al. (2022). Noradrenergic activation induced by yohimbine decreases interoceptive accuracy in healthy individuals with childhood adversity. Dev. Psychopathol. 34, 1013–1024. doi: 10.1017/S0954579420001613
Shatrova, D., Cáncer, P. F., and Caperos, J. M. (2024). The role of interoception in reducing trauma-associated distress: a feasibility study. Eur. J. Psychotraumatol. 15:2306747. doi: 10.1080/20008066.2024.2306747
Silvanto, J., and Nagai, Y. (2025). How interoception and the insula shape mental imagery and aphantasia. Brain Topogr. 38, 1–8. doi: 10.1007/s10548-025-01101-6
Simmons, A., Strigo, I. A., Matthews, S. C., Paulus, M. P., and Stein, M. B. (2009). Initial evidence of a failure to activate right anterior insula during affective set shifting in posttraumatic stress disorder. Psychosom. Med. 71, 373–377. doi: 10.1097/PSY.0b013e3181a56ed8
Soleimani, A., Akbarizadeh, F., Kachooei, M., and Farahani, H. (2024). Psychometric validation of the international trauma questionnaire (ITQ-18) in middle-aged Iranian women in 2023. Int. J. Behav. Sci. 17, 209–216. doi: 10.30491/ijbs.2024.414330.2024
Suarez-Jimenez, B., Albajes-Eizagirre, A., Lazarov, A., Zhu, X., Harrison, B. J., Radua, J., et al. (2020). Neural signatures of conditioning, extinction learning, and extinction recall in posttraumatic stress disorder: a meta-analysis of functional magnetic resonance imaging studies. Psychol. Med. 50, 1442–1451. doi: 10.1017/S0033291719001387
Suksasilp, C., and Garfinkel, S. N. (2022). Towards a comprehensive assessment of interoception in a multi-dimensional framework. Biol. Psychol. 168:108262. doi: 10.1016/j.biopsycho.2022.108262
Tanaka, Y., Terasawa, Y., and Umeda, S. (2023). Heartbeat-evoked potentials reflect the relationship between interoception and cognitive functions. Autonomic Nerv. Syst. 60, 92–96. doi: 10.32272/ans.60.2_92
Terasawa, Y., Moriguchi, Y., Tochizawa, S., and Umeda, S. (2014). Interoceptive sensitivity predicts sensitivity to the emotions of others. Cognit. Emot. 28, 1435–1448. doi: 10.1080/02699931.2014.888988
Terasawa, Y., Motomura, K., Natsume, A., Iijima, K., Chalise, L., Sugiura, J., et al. (2021). Effects of insular resection on interactions between cardiac interoception and emotion recognition. Cortex 137, 271–281. doi: 10.1016/j.cortex.2021.01.011
Torralva, T., Roca, M., Gleichgerrcht, E., Lopez, P., and Manes, F. (2009). INECO frontal screening (IFS): a brief, sensitive, and specific tool to assess executive functions in dementia–CORRECTED VERSION. J. Int. Neuropsychol. Soc. 15, 777–786. doi: 10.1017/S1355617709990415
Trujillo, S., Trujillo, N., Valencia, S., Ugarriza, J. E., and Acosta, M. A. (2019). Executive and behavioral characterization of chronic exposure to armed conflict among war victims and veterans. Peace Conflict J. Peace Psychol. 25, 312–324. doi: 10.1037/pac0000408
Umiltà, M. A., Wood, R., Loffredo, F., Ravera, R., and Gallese, V. (2013). Impact of civil war on emotion recognition: the denial of sadness in Sierra Leone. Front. Psychol. 4:523. doi: 10.3389/fpsyg.2013.00523
Urdinola, B. P., Torres Avilés, F., and Velasco, J. A. (2017). The homicide atlas in Colombia: contagion and under-registration for small areas. Cuadernos de Geografía: Rev. Colombiana Geografía. 26, 101–118. doi: 10.15446/rcdg.v26n1.55429
Ventura-Bort, C., and Weymar, M. (2024). Transcutaneous auricular vagus nerve stimulation modulates the processing of interoceptive prediction error signals and their role in allostatic regulation. Hum. Brain Mapp. 45:e26613. doi: 10.1002/hbm.26613
Wang, X., Xie, H., Cotton, A. S., Duval, E. R., Tamburrino, M. B., Brickman, K. R., et al. (2016). Preliminary study of acute changes in emotion processing in trauma survivors with PTSD symptoms. PLoS One 11:e0159065. doi: 10.1371/journal.pone.0159065
Wilkinson, S., Dodgson, G., and Meares, K. (2017). Predictive processing and the varieties of psychological trauma. Front. Psychol. 8:1840. doi: 10.3389/fpsyg.2017.01840
Williams, C. L., Milanak, M. E., Judah, M. R., and Berenbaum, H. (2018). The association between PTSD and facial affect recognition. Psychiatry Res. 265, 298–302. doi: 10.1016/j.psychres.2018.04.055
Yehuda, R., Hoge, C. W., McFarlane, A. C., Vermetten, E., Lanius, R. A., Nievergelt, C. M., et al. (2015). Post-traumatic stress disorder. Nat. Rev. Dis. Primers 1, 1–22. doi: 10.1038/nrdp.2015.57
Yoris, A., Abrevaya, S., Esteves, S., Salamone, P., Lori, N., Martorell, M., et al. (2018). Multilevel convergence of interoceptive impairments in hypertension: new evidence of disrupted body–brain interactions. Hum. Brain Mapp. 39, 1563–1581. doi: 10.1002/hbm.23933
Yousefi, S., and Abdoli, F. (2024). Assessing the Persian international trauma questionnaire: a psychometric study. Eur. J. Trauma Dissoc. 8:100404. doi: 10.1016/j.ejtd.2024.100404
Yu, A. N. C., Iodice, P., Pezzulo, G., and Barca, L. (2021). Bodily information and top-down affective priming jointly affect the processing of fearful faces. Front. Psychol. 12:625986. doi: 10.3389/fpsyg.2021.625986
Keywords: interoception, violence, posttraumatic stress disorder, emotion recognition, heartbeat evoked cortical potential amplitude
Citation: Herrera E, Gutierrez-Sterling D, Barrera-Ocampo A, Jaramillo JO, Santamaría-García H and Birba A (2025) Impaired interoception in Colombian victims of armed conflict with PTSD: a preliminary HEP study. Front. Psychol. 16:1567574. doi: 10.3389/fpsyg.2025.1567574
Edited by:
Michiel M. Spapé, University of Helsinki, FinlandReviewed by:
Gabriella Bottini, University of Pavia, ItalyJuha Silvanto, University of Surrey, United Kingdom
Copyright © 2025 Herrera, Gutierrez-Sterling, Barrera-Ocampo, Jaramillo, Santamaría-García and Birba. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eduar Herrera, ZWhlcnJlcmFAaWNlc2kuZWR1LmNv; Agustina Birba, YWd1c3RpbmEuYmlyYmFAZ21haWwuY29t
†These authors have contributed equally to this work and share first authorship
 Eduar Herrera
Eduar Herrera Daniela Gutierrez-Sterling1,2†
Daniela Gutierrez-Sterling1,2† Alvaro Barrera-Ocampo
Alvaro Barrera-Ocampo Hernando Santamaría-García
Hernando Santamaría-García Agustina Birba
Agustina Birba