- 1School of Physical Education, Anhui Polytechnic University, Wuhu, Anhui, China
- 2School of Foreign Studies, Anhui Polytechnic University, Wuhu, Anhui, China
- 3Collaborative Innovation Center for Sports and Health Industry, Anhui Polytechnic University, Wuhu, Anhui, China
Background: Emotion regulation is vital for psychological well-being. Exercise can enhance regulation via attentional and cognitive control, with event-related potentials (ERPs) offering neural insights. Tai Chi benefits mood and stress, yet its neurophysiological effects remain unclear. This study examined the impact of 8-week Tai Chi training on N2 and P3 components of emotional processing in female college students using a modified oddball paradigm.
Method: Forty healthy female college students were randomly assigned to an 8-week Tai Chi group (n = 20, 20.25 ± 1.33 yrs.) or control group (n = 20, 19.65 ± 1.09 yrs.). Tai Chi participants completed thrice-weekly 50 min sessions at 60–69% HRmax, including warm-up, practice, and relaxation, while controls observed without exercising. Pre- and post-intervention, all completed a modified oddball task recording N2/P3 ERPs and reaction times, alongside emotional regulation assessments, enabling comparison of neurophysiological and behavioral responses across negative, neutral, and positive stimuli.
Results: After 8 weeks, the Tai Chi group exhibited significantly shorter reaction times compared to the control group (p < 0.001), with improvements evident only in the exercise group over time. ERP analyses revealed that Tai Chi training selectively reduced N2 amplitudes to negative stimuli, suggesting decreased early sensitivity to negative information. Additionally, the Tai Chi group showed overall shorter N2 latencies compared to the control group, indicating faster early-stage neural processing. In contrast, P3 amplitudes increased across all valence conditions in the exercise group, reflecting enhanced allocation of attentional resources during later cognitive processing. No significant effects were observed for P3 latency.
Conclusion: This study demonstrates that 8 weeks of Tai Chi training modulated both neural and behavioral responses to emotional stimuli in healthy female college students, suggesting potential benefits for attentional processes in emotional regulation, though neural-behavioral links require further investigation.
1 Introduction
Emotion regulation refers to the processes by which individuals influence the occurrence, experience, and expression of emotions (Gross, 1998). It is essential for psychological well-being and social adaptation, playing a key role in interpersonal functioning. Exercise has been identified as an effective strategy for enhancing emotion regulation (Thayer et al., 1994; Bernstein and McNally, 2017; Edwards et al., 2017), with evidence suggesting that it optimizes attention allocation and cognitive control, thereby improving the efficiency of emotional information processing (Gomez-Pinilla and Hillman, 2013). Event-related potentials (ERPs), given their high temporal resolution, provide a powerful approach for investigating the neural mechanisms underlying these effects. Previous studies have shown that acute and chronic aerobic exercise can modulate ERP responses to emotional stimuli, including reductions in early components such as N1 and N2, suggesting reduced neural sensitivity to negative information and improved emotional stability (Zhang et al., 2022; Hwang et al., 2019).
Accumulating evidence indicates that aerobic exercise exerts both immediate and long-term benefits on emotional regulation (Long et al., 2021; Mizzi et al., 2022; Kim et al., 2022; Thom et al., 2019; Ligeza et al., 2023). Acute exercise may temporarily enhance emotion regulation through dopaminergic mechanisms that adjust arousal levels (Zheng and Hasegawa, 2016). In contrast, long-term exercise fosters more sustained adaptations, including enhanced reappraisal ability and neuroplastic changes, such as strengthened hippocampal-amygdala connectivity, improved prefrontal-amygdala white matter integrity, and increased prefrontal dopamine availability (Mizzi et al., 2022; Schmitt et al., 2020; Schaeffer et al., 2014; Chen et al., 2017). These findings highlight that regular exercise can influence both behavioral and neurophysiological markers of emotion regulation.
Tai Chi, a traditional Chinese mind–body exercise integrating movement, breath control, and focused attention, has been shown to improve mood and reduce anxiety, depression, and stress (Frith et al., 2011; Wang et al., 2004; Zheng et al., 2018; Zhang et al., 2019; Xu et al., 2021). However, most studies rely on self-report measures, with limited attention to its neural mechanisms. ERPs offer an opportunity to address this gap by examining key components involved in emotional processing. Specifically, N2, typically observed over frontal-central regions, reflects conflict monitoring, cognitive control, and early attention allocation (Carretié et al., 2004; Palomba et al., 1997). while P3, maximal over parietal regions, indexes later-stage emotional evaluation and resource allocation (Huang and Luo, 2006; Patel and Azzam, 2005; Hillman et al., 2009). Changes in these components have been linked to individual differences in emotion regulation (Lewis et al., 2006; Gardener et al., 2013), yet it remains unclear whether long-term Tai Chi training modulates these ERP markers.
The present study focuses on healthy female college students for two reasons. First, women demonstrate greater sensitivity to negative emotions than men and are more prone to emotion regulation difficulties (Bradley et al., 2001; Güntekin et al., 2017; Gard and Kring, 2007). Second, college students are at a critical developmental stage, with female students particularly vulnerable to stress and anxiety, making them an important population for intervention research. To minimize gender-related variability in ERP responses and enhance interpretability, only female participants were included. Building on evidence that regular aerobic exercise exerts measurable effects on emotion regulation after approximately 8 weeks (Mizzi et al., 2022; Zhang et al., 2019; Olson et al., 2017), and that Tai Chi has been shown to enhance neuroplasticity in college populations (Cui et al., 2019). Therefore, this study employs 8 weeks of moderate-intensity Tai Chi training to explore its neurophysiological mechanisms in emotion regulation for healthy female college students.
Using a modified oddball paradigm with negative, neutral, and positive emotional images, this study examined the effects of an 8-week Tai Chi training program on ERP and behavioral indices of emotional regulation. We hypothesized that Tai Chi would modulate the N2 and P3 components during emotional processing, reflecting potential changes in attentional and cognitive control mechanisms.
2 Participants and methods
2.1 Participants
Based on our research questions and prior knowledge in the field (Henz and Schöllhorn, 2018; Li et al., 2022; Wang et al., 2024), the sample size calculation, test types, and effect size selection were determined accordingly. Using the G*Power 3.1 program for sample size estimation, the significance level was set at α = 0.05, the effect size at d = 0.8, and statistical power at 1 − β = 0.8. A priori power analysis indicated that a minimum of 20 participants per group was required to achieve adequate statistical power. To account for potential attrition and data loss, 50 participants were initially recruited. Ultimately, 40 participants were included in the final analysis. Of the excluded participants, five did not meet the minimum attendance requirement, three voluntarily withdrew during the intervention period, and two were excluded due to excessive electroencephalography (EEG) artifacts that rendered their data unusable. The study participants were female students enrolled in public physical education courses at Anhui Polytechnic University, none of whom were majoring in physical education. All participants read and signed an informed consent form and received ethical approval from the Ethics Committee of the Institute of Neuroscience and Cognitive Psychology at Anhui Polytechnic University (AHPU-PED-2022-001). All procedures involving human participants were conducted in accordance with the relevant guidelines and regulations, including the Declaration of Helsinki.
Participants were screened to meet the following inclusion criteria: (1) right-handed and no reported color blindness; (2) no history of psychiatric disorders, systemic neuromuscular diseases, cardiovascular diseases, head injuries, or medications that might affect cognition; (3) no involvement in any systematic or regular physical exercise other than normal physical education activities; and (4) no experience in any school sports team within the past 3 years. In addition, none of the participants had prior experience with Tai Chi training. All participants completed the State–Trait Anxiety Inventory (STAI) (Spielberger, 1970) and the Beck Depression Inventory (BDI-II) (Beck et al., 1996) to assess their baseline psychological status, ensuring the inclusion of healthy individuals.
2.2 Procedures
Before the formal exercise intervention, all participants completed an informed consent form and a basic information questionnaire (e.g., medical history, age, height, etc.). The consent form detailed the organization, experiment location, experiment duration, experimental procedures, participant requirements, related rewards, and contact information. Participants could only formally join the experiment after fully understanding the research details and signing the consent form.
All participants were randomly assigned to the Tai Chi exercise group or the no-intervention control group. Based on prior instructional surveys, during the exercise intervention for the Tai Chi group, complex technical movements and movements with similar training functions were excluded. The intervention focused on easy-to-learn, easy-to-practice, and effective movements from the 24-form Tai Chi, including typical movements such as Wild Horse’s Mane, Hand Strums the Lute, Lop Knee Stance, Reverse Elbow, Grasping the Sparrow’s Tail, and Golden Rooster Stands on One Leg. This ensured that the participants could smoothly connect all technical movements during their training. The training followed a progressive approach: initially, the focus was on technique instruction to cultivate interest and help participants master the basic movements; in the later stages, technical guidance was provided to help participants establish a long-term exercise habit.
The experimental procedure consisted of three phases: During the pre-test phase, conducted within 1 week prior to the start of the intervention, all participants entered the laboratory to undergo EEG testing and psychological assessments, completing the STAI and BDI-II to evaluate baseline anxiety and depression levels. The purpose of administering these questionnaires was to confirm that all participants met the study’s inclusion criteria of being emotionally healthy, and they were therefore used for baseline screening only. The intervention lasted for 8 weeks, with participants engaging in three Tai Chi sessions per week, each lasting 50 min and consisting of 10 min of warm-up, 35 min of Tai Chi practice, and 5 min of cool-down stretching. Heart rate was continuously monitored during all training sessions using a chest strap device (Magene, China) with ECG-based detection, ensuring uninterrupted measurement. Exercise intensity was maintained at 60–69% of HRmax (120–145 beats/min), verified through real-time device feedback and cross-checked with the Rating of Perceived Exertion (RPE). To promote adherence, participants attended one group session per week, while the remaining two sessions were individually scheduled. For each individual session, participants first signed in on-site and then immediately completed the training under researcher supervision. Training logs were kept for all sessions, and adherence was verified weekly by reviewing logs and check-in records. The control group did not perform Tai Chi but followed the same schedule of three weekly sessions (one group and two individual), during which they completed the same check-in procedures and remained seated at the training site to observe, thereby matching the experimental group’s exposure to the training environment and researcher contact.
During the post-test phase, conducted within 1 week after completion of the 8-week intervention, all participants returned to the laboratory to undergo the same tests as in the pre-test phase, including EEG recordings and psychological assessments, to evaluate the effects of the exercise intervention. The control group maintained their usual daily lifestyle without any additional physical exercise throughout the experiment. During each training session, control group members remained seated at the training site, did not participate in any physical exercises, and only observed the sessions.
2.3 Stimuli and task
The experiment employed a modified oddball paradigm, in which each block consisted of 100 trials (70 frequent stimuli and 30 infrequent stimuli), and participants were required to respond to every stimulus using a two-choice categorization task. The standard stimulus was an image of a vase, while the deviant stimuli consisted of 10 negative, 10 neutral, and 10 positive pictures selected from the Chinese Affective Picture System (CAPS) (Lu et al., 2005). Negative images had valence ratings (M = 2.64, SD = 0.15, range = 2.37 ~ 2.88) and arousal ratings (M = 4.94, SD = 0.20, range = 4.68 ~ 5.25). Neutral images had valence ratings (M = 5.40, SD = 0.48, range = 4.63 ~ 6.04) and arousal ratings (M = 4.76, SD = 0.56, range = 4.00 ~ 6.04). Positive images had valence ratings (M = 7.25, SD = 0.11, range = 7.11 ~ 7.46) and arousal ratings (M = 6.33, SD = 0.36, range = 5.62 ~ 6.99). Ratings were assessed on a 9-point scale, with 1 indicating the least pleasant/lowest arousal and 9 indicating the most pleasant/highest arousal. Stimuli were chosen to represent prototypical examples of negative, neutral, and positive categories, with non-overlapping valence distributions to ensure clear emotional differentiation across conditions. To minimize order effects, the presentation order within each block was randomized, with standard stimuli presented at a rate of 70% and deviant stimuli at 30%. A 2 min break was provided between blocks.
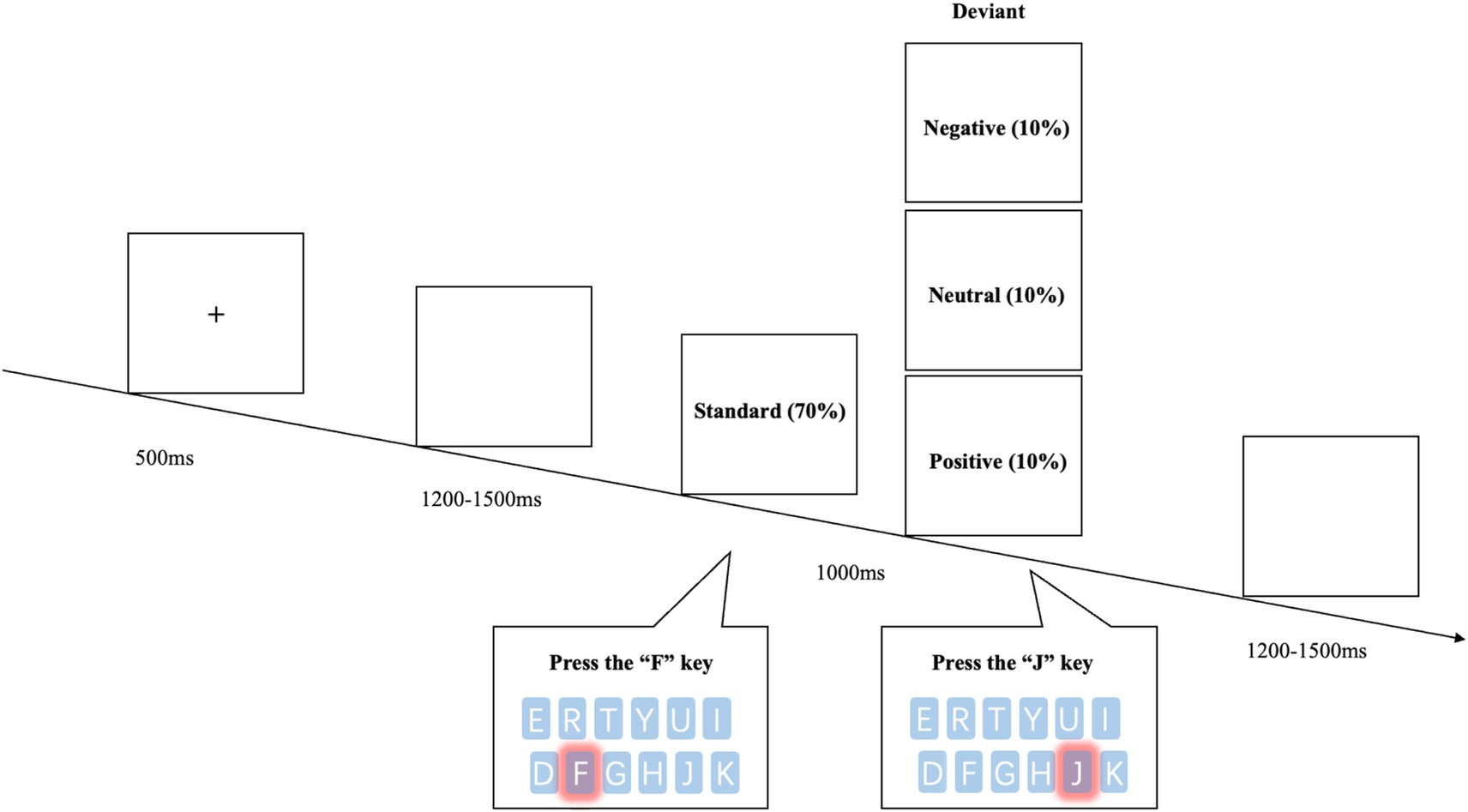
The experiment was programmed using E-prime version 2.0 (Psychology Software Tools Inc., Pittsburgh, PA). The experimental task consisted of two parts: practice and formal experiment. The practice session included 10 trials, while the formal experiment consisted of 3 blocks, each containing 100 trials. Each trial began with a fixation mark “+” displayed on the computer screen for 500 ms (Figure 1). This was followed by a blank screen for 1,200 ~ 1,500 ms, after which the stimulus image appeared. The stimulus image was displayed for 1,000 ms, and participants were required to respond within this time. When a standard image appeared, participants were instructed to press the “F” key on the keyboard as quickly as possible, and when a deviant image appeared, they were instructed to press the “J” key. Reaction times (RTs) were recorded only for responses to deviant stimuli, as they were the primary focus of emotional processing in this study. Unlike the traditional oddball paradigm, in which participants typically respond only to deviant stimuli, the modified oddball paradigm adopted here required responses to both standard and deviant stimuli. This design helped sustain continuous attentional engagement, reduced response bias. Throughout the experiment, participants remained in a quiet, comfortable environment, with the computer monitor placed 70 cm from the participant.
2.4 ERP recording and analysis
EEG data were acquired from a non-invasive, 32-channel, gel-based mBrainTrain Smarting Pro (mBrainTrain LLC., Belgrade, Serbia). According to the international 10/20 system, the recording sites were Fp1, Fp2, F3, F4, C3, C4, P3, P4, O1, O2, F7, F8, T7, T8, TP9, P7, P8, Fz, Cz, Pz, POz, FC1, FC2, CP1, CP2, FC5, FC6, CP5, CP6, TP10, FT9, and FT10. The TP9 and TP10 electrodes were placed on the left and right mastoids, respectively, and their signals were used as offline references. Horizontal and vertical electrooculogram (EOG) activity was recorded from electrodes placed on both eyes. EEG and EOG activity were recorded with a sampling rate of 1,000 Hz using an online amplifier, and all impedance levels were kept below 10kΩ.
Offline analysis of the EEG data was performed using the open-source EEGLAB (2024.2) toolbox in MATLAB (R2021a, MathWorks Inc., United States) (Delorme and Makeig, 2004) and ERPLAB (12.00) (Lopez-Calderon and Luck, 2014). During offline analysis, EEG data were down sampled to a 500 Hz sampling rate and band-pass filtered using a finite impulse response (FIR) filter with a low-pass frequency of 30 Hz and a high-pass frequency of 0.1 Hz. The data were manually checked and artifacts related to muscle activity and eye movements were rejected. The specific steps for artefact correction and rejection were as follows: First, independent component analysis (ICA, binICA; EEGLAB) was applied to the continuous EEG data, including the EOG channels Fp1 and Fp2. Then, independent components (ICs) were classified using ICLabel (EEGLAB plugin) to identify potential artefact components. If a component had more than 80% eye movement-related activity and less than 10% brain-related activity, it was marked as a potential artefact and excluded (Sanada and Katayama, 2024). For each experimental condition, EEG activity was averaged independently, and baseline correction and feature extraction were performed within the time window of 200 ms before to 1,000 ms after stimulus presentation. EEG data were then time-locked to the onset of deviant stimuli, and epochs belonging to the same experimental condition were averaged to generate the average ERP waveform for each participant under the Negative, Neutral, and Positive stimulus conditions. Consistent with previous ERP studies (Hwang et al., 2019; Qiu et al., 2019; Yuan et al., 2008; Moser et al., 2006; Buodo et al., 2017), visual inspection of the grand-averaged ERP waveforms elicited by the stimuli indicated that the N2 component emerged in the 210–290 ms time window at frontal-central scalp sites (i.e., Fz, Cz, FC1, and FC2 electrodes), and the P3 component emerged in the 300–400 ms time window at parietal scalp sites (i.e., P3, Pz, and P4 electrodes). To illustrate the spatial distributions of these components, scalp topographies of N2 (210–290ms) and P3 (300–400ms) at post-test for the Tai Chi and control groups under negative, neutral, and positive emotional conditions are provided in Supplementary Figure 1. For statistical analysis, Fz and Pz were chosen as representative sites, as they showed the most pronounced amplitudes of N2 and P3, respectively.
2.5 Statistical analyses
All statistical analyses were conducted in IBM SPSS version 27 (IBM Corp., Armonk, NY). RTs were averaged across trials for each emotional condition (negative, neutral, and positive) and participant. Group differences at baseline were first assessed using independent-samples t-tests. Subsequently, a 2 (Group: exercise vs. non-exercise) × 2 (Time: pre- vs. post-intervention) × 3 (Emotional Valence: negative vs. neutral vs. positive) mixed-design repeated-measures ANOVA (RM-ANOVA) was performed on mean RTs, with Group as the between-subjects factor and Time and Emotional Valence as within-subjects factors. For each participant, mean amplitude and peak latency were extracted for N2 (210–290ms, Fz electrode) and P3 (300–400ms, Pz electrode). The same 2 × 2 × 3 RM-ANOVA design was applied separately for N2 and P3 mean amplitude and latency. Significant main effects and interactions were followed up with Bonferroni-corrected pairwise comparisons. For significant interactions, simple main effects analyses were conducted. When sphericity assumptions were violated, Greenhouse–Geisser corrections were applied (Jennings and Wood, 1976). Effect sizes were reported as partial eta squared (ηp2), and significance was set at p < 0.05.
3 Results
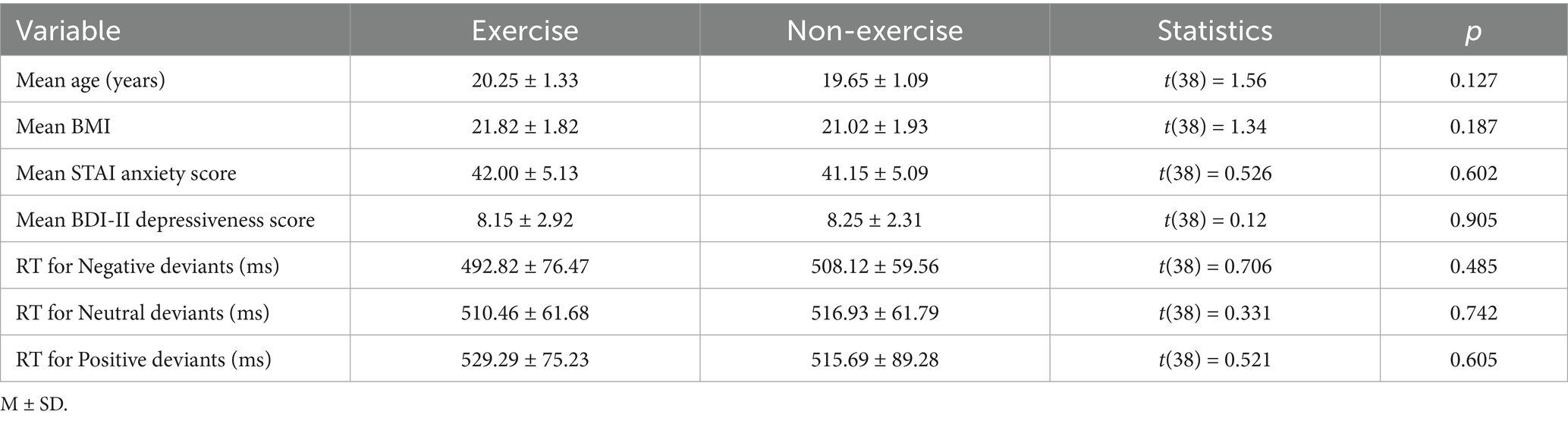
As shown in Table 1, baseline characteristics measured during the pre-test were compared between the exercise and non-exercise groups. Independent samples t-tests revealed no significant differences between the exercise group and the non-exercise group in age, BMI, STAI, BDI-II, or reaction times to the three types of deviant stimuli (p > 0.05).
3.1 Reaction times
A two-way (valence × group) ANOVA on the RTs to deviant emotional stimuli revealed a significant main effect of group after 8 weeks, F(1, 38) = 18.761, p < 0.001, η2 = 0.331, indicating that the exercise group of female college students exhibited significantly shorter RTs compared to the non-exercise group (468.51 ± 27.20 ms vs. 514.06 ± 38.36 ms). The main effect of valence was not significant, F(2, 76) = 0.949, p = 0.392. The interaction effect between valence and group was also not significant, F(2, 76) = 0.26, p = 0.772 (see Table 2; Figure 2). Therefore, regardless of the valence of the stimulus, female college students in the exercise group responded more quickly to deviant stimuli than those in the non-exercise group.
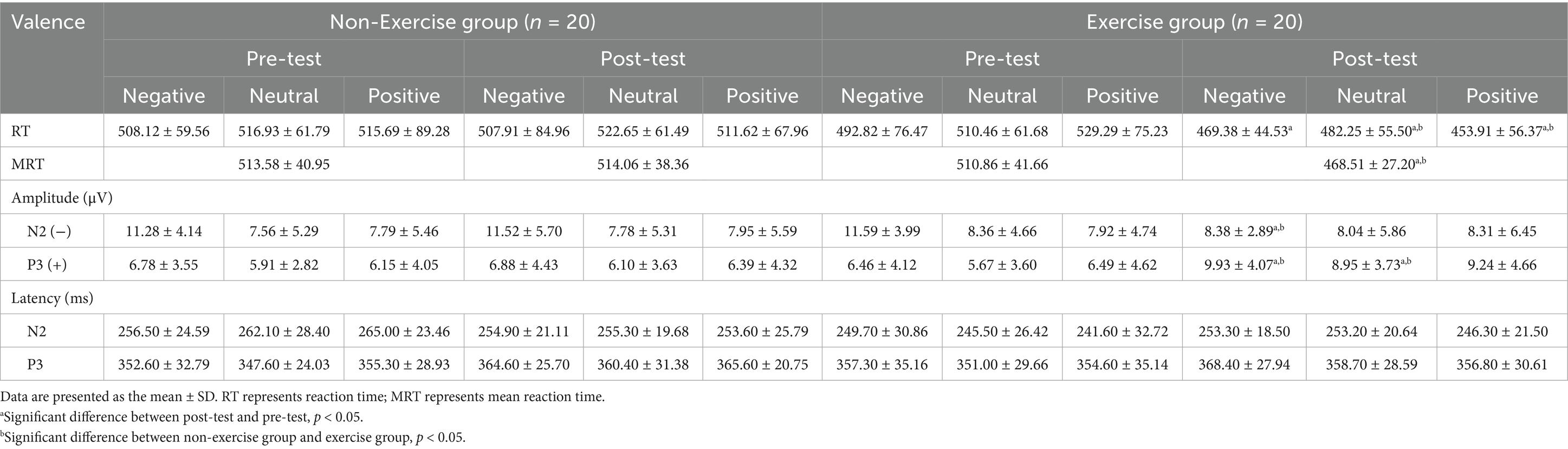
Table 2. Comparison of emotional valence, reaction times, amplitudes, and latencies across exercise and non-exercise groups.
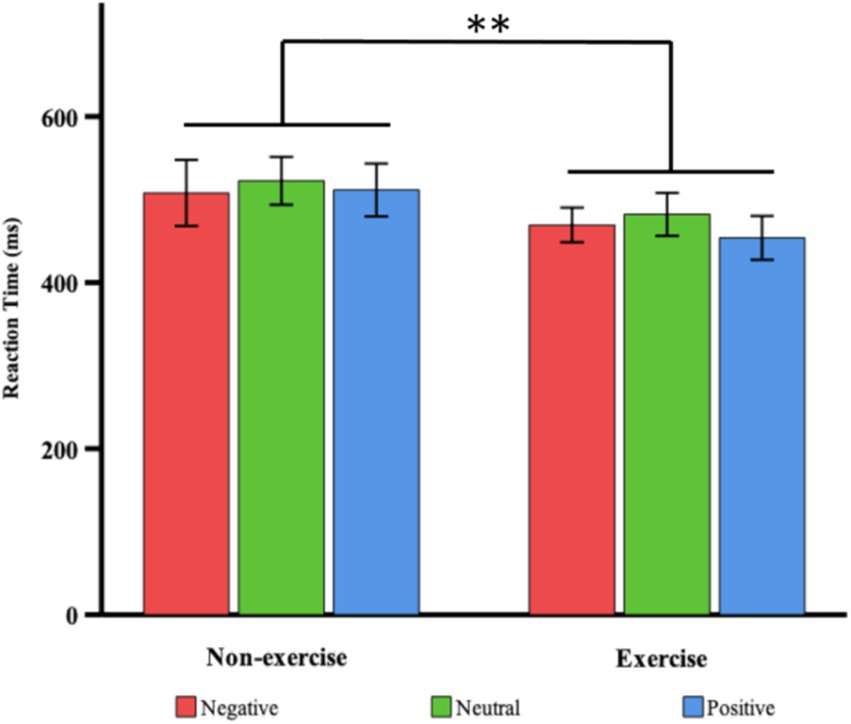
Figure 2. Comparisons of RTs in response to deviant stimuli, by valence type, between the exercise and non-exercise groups. Relative to the non-exercise group, the exercise group had faster RTs for negative (red), neutral (green) and positive (blue) deviant stimuli. Means are shown with standard errors; ** p < 0.01.
The results on reaction time revealed a significant main effect of time, F(1, 38) = 9.628, p = 0.004, η2 = 0.202. The main effect of group was also significant, F(1, 38) = 6.126, p = 0.018, η2 = 0.139. The interaction effect between time and group was significant as well, F(1, 38) = 10.078, p = 0.003, η2 = 0.21. Further simple effect analysis indicated that there was no significant change in RTs between pre-test and post-test measurements for the non-exercise group, F(1, 38) = 0.003, p = 0.96. However, the exercise group exhibited significantly lower RTs at the post-test compared to the pre-test, F(1, 38) = 19.704, p < 0.001, η2 = 0.341 (Figure 3).
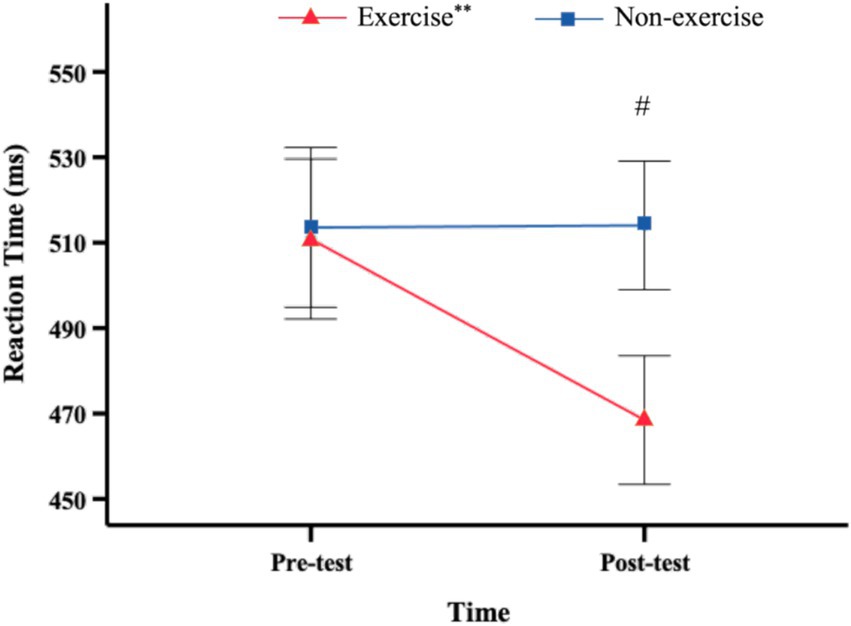
Figure 3. Reaction times (mean and SEM) as a function of time point. ** Significant difference between pre-test and post-test for this group, p < 0.01. # Significant difference between groups, p < 0.05.
3.2 ERP results
The group-level ERP waveforms, along with the mean amplitudes and peak latencies of N2 and P3 responses to deviant emotional stimuli, are presented in Figures 4, 5 and summarized in Table 2. The results of the repeated-measures ANOVAs for N2 and P3 amplitude and latency are summarized in Table 3. To further support the observed spatial distribution patterns, scalp topographies of N2 (210–290ms) and P3 (300–400ms) components at post-test for both groups are provided in Supplementary Figure 1.
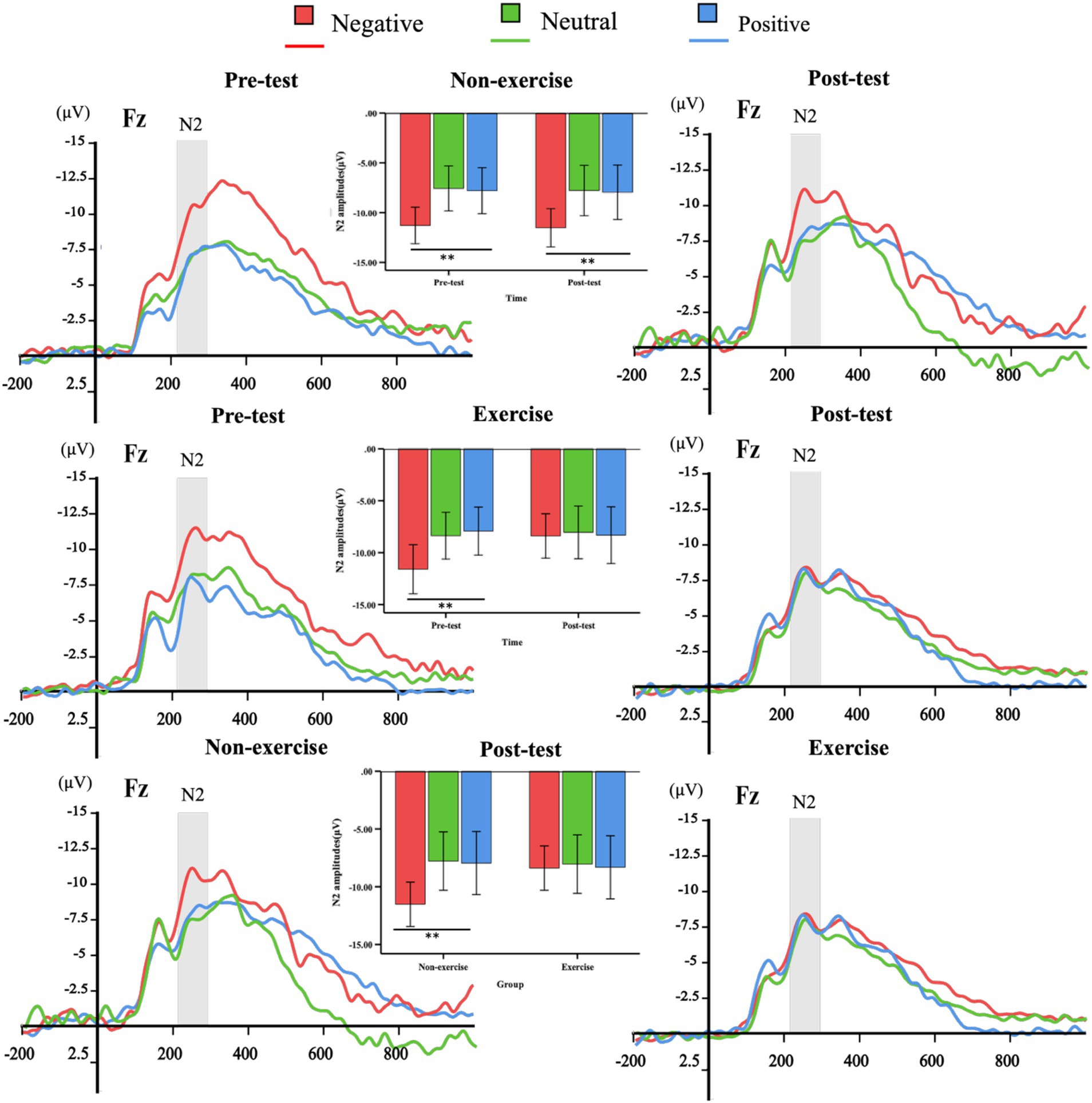
Figure 4. The overall averages of ERPs induced by deviant stimuli in the exercise and non-exercise groups. ERP waveforms triggered by negative (red), neutral (green), and positive (blue) deviant stimuli were measured at the representative frontal electrode (Fz). Additionally, N2 (210–290ms) amplitude plots for both pre-test and post-test within-group measurements, as well as between-group post-test comparisons, are presented.
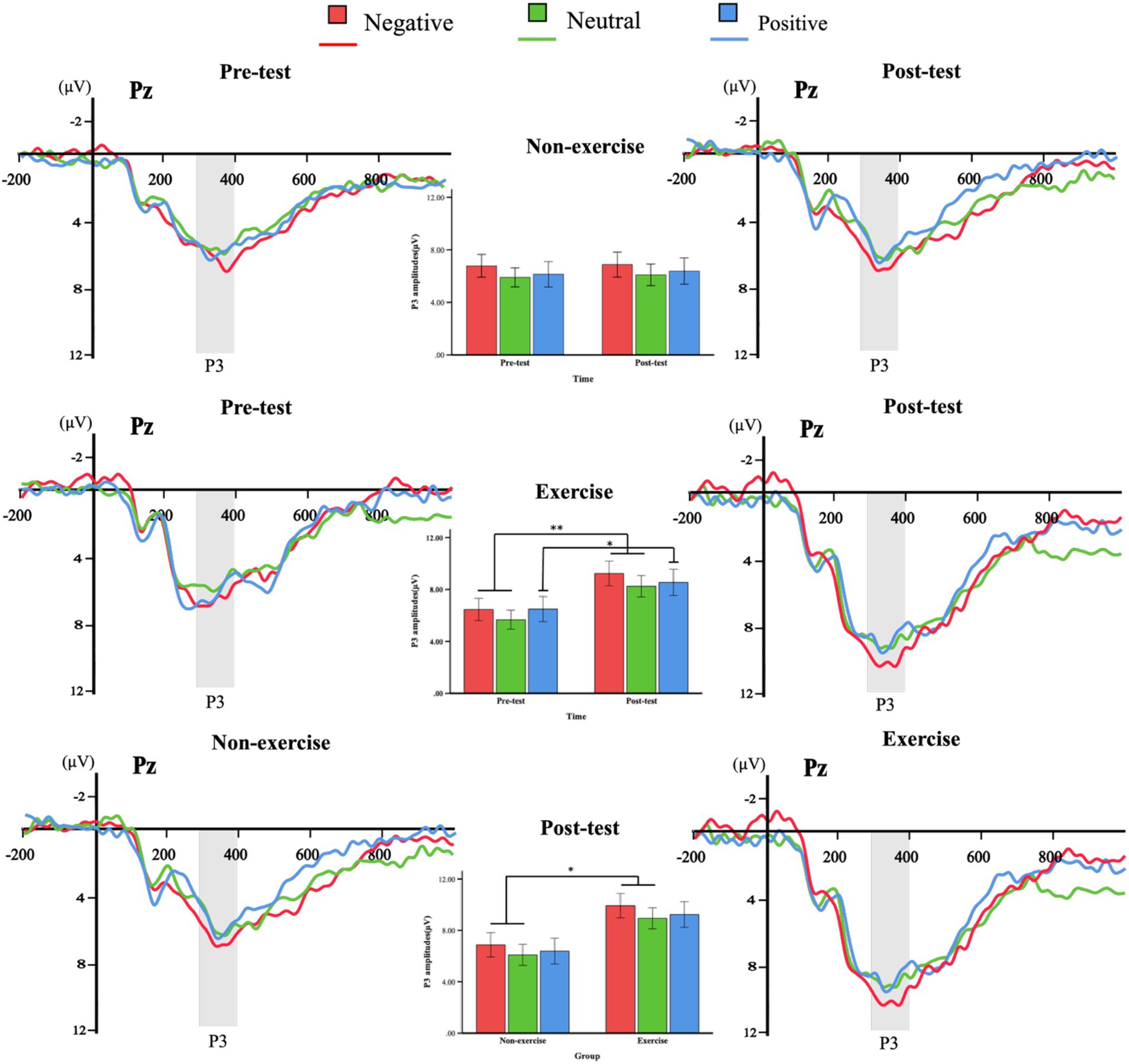
Figure 5. Grand averages of ERPs induced by deviant stimuli in the exercise and non-exercise groups. ERP waveforms evoked by negative (red), neutral (green), and positive (blue) deviant stimuli were measured at the representative parietal electrode (Pz), along with the P3 (300–400ms) amplitude plots for both groups at pre-test, post-test, and between-group comparisons at post-test.
3.2.1 N2 amplitude and latency
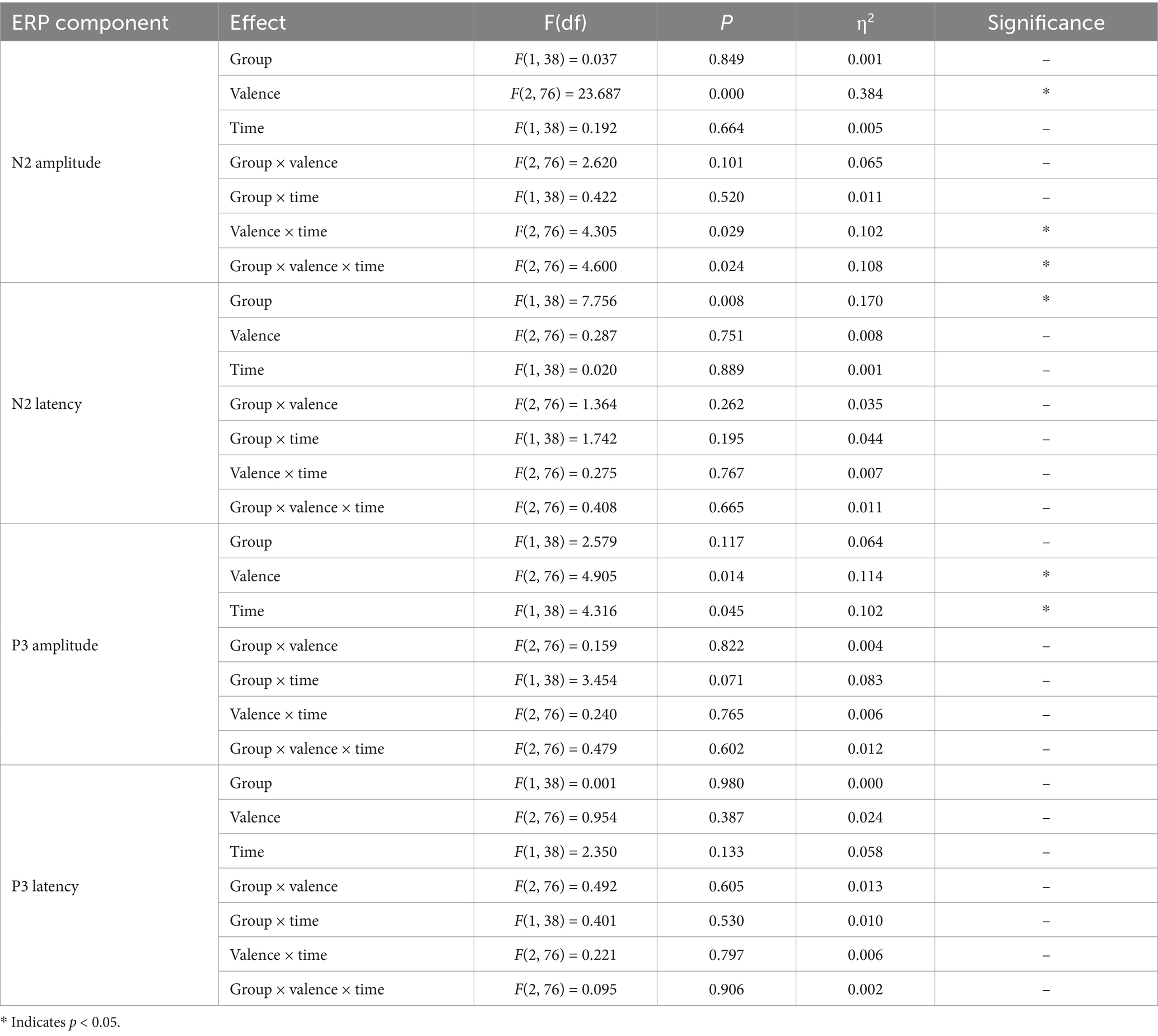
A three-way (valence × group × time) repeated-measures ANOVA conducted on the N2 amplitude induced by deviant emotional stimuli revealed a significant main effect of valence, F(2, 76) = 23.687, p < 0.001, η2 = 0.384, indicating that the N2 amplitude induced by negative stimuli was significantly higher than that induced by neutral and positive stimuli. Furthermore, the group × time interaction effect was significant, F(2, 76) = 4.305, p = 0.029, η2 = 0.102, with the N2 amplitude of the exercise group being significantly lower at post-test compared to pre-test. Additionally, a significant valence × group × time interaction was observed, F(2, 76) = 4.60, p = 0.024, η2 = 0.108.
To explore further, simple main effects analyses were conducted. In the non-exercise group, a significant valence × time interaction effect was found (pre-test: F(2, 37) = 12.934, p < 0.001, η2 = 0.411; post-test: F(2, 37) = 7.437, p = 0.002, η2 = 0.287). The N2 amplitude induced by negative stimuli was significantly higher than that induced by neutral (p < 0.01) and positive (p < 0.01) stimuli in both pre-test and post-test. However, there were no significant differences between neutral and positive stimuli in either pre-test or post-test (p > 0.05).
In the exercise group, a significant valence × time interaction effect was also found, F(2, 37) = 11.095, p < 0.001, η2 = 0.375. The N2 amplitude induced by negative stimuli was significantly higher than that induced by neutral (p < 0.01) and positive (p < 0.01) stimuli in the pre-test, but no significant differences were observed among the valence conditions in the post-test (p > 0.05).
At the post-test, there was a significant difference in the N2 amplitude between the exercise and non-exercise groups, F(1, 38) = 5.426, p = 0.025, η2 = 0.125. Further analysis revealed that, under the negative stimulus condition, the N2 amplitude of the exercise group was significantly lower than that of the non-exercise group (p = 0.025), whereas no significant differences were observed between the two groups under neutral and positive stimulus conditions (p > 0.05). These results suggest that the 8-week exercise intervention primarily influenced the processing of negative emotional stimuli. The three-way repeated-measures ANOVA (valence × group × time) for N2 latency revealed a significant main effect of group (F(1, 38) = 7.756, p = 0.008, η2 = 0.170), indicating that the Tai Chi group exhibited overall shorter N2 latencies compared to the control group. However, no significant main effects of valence or time, and no significant interactions were observed (all p > 0.05).
3.2.2 P3 amplitude and latency
A three-way (group × valence × time) repeated measures ANOVA analysis of P3 amplitude revealed a significant main effect of valence, F(2, 76) = 4.905, p = 0.014, η2 = 0.114, indicating significant differences in P3 amplitude across different emotional stimulus valence conditions. Post-hoc tests further showed that the P3 amplitude induced by negative stimuli was significantly higher than that induced by neutral stimuli (p = 0.02), while the differences between negative and positive stimuli, as well as neutral and positive stimuli, were not significant (p > 0.05). The main effect of time was also significant, F(1, 38) = 4.316, p = 0.045, η2 = 0.102, with post-test P3 amplitude being significantly higher than pre-test, suggesting an independent enhancement of attentional allocation over time. The main effect of group was not significant, F(1, 38) = 2.579, p = 0.117, η2 = 0.064. None of the interactions reached conventional significance, although the group × time interaction was marginal, F(1, 38) = 3.454, p = 0.071, η2 = 0.083. Although the main effect of group was non-significant, this marginal interaction justified exploratory post-hoc analyses.
Exploratory pairwise comparisons revealed no significant differences in P3 amplitude across valence conditions in the non-exercise group (p > 0.05). In the exercise group, post-test amplitudes were significantly higher than pre-test for negative (p = 0.007), neutral (p = 0.005), and positive stimuli (p = 0.036), indicating enhanced late-stage attentional processing following Tai Chi training. Between-group comparisons at post-test showed higher P3 amplitudes in the exercise group for negative (p = 0.029) and neutral stimuli (p = 0.019), while the difference for positive stimuli did not reach significance (p = 0.052). These between-group comparisons are considered exploratory due to the marginal group × time interaction.
The three-way repeated measures ANOVA (valence × group × time) for P300 latency under emotional stimuli showed no significant main effects (all p > 0.05) and no significant interaction (F(2, 76) = 0.095, p = 0.906).
4 Discussion
This study utilized ERP technology combined with behavioral measures to investigate the effects of 8 weeks of Tai Chi training on emotional regulation ability in healthy female college students. The results indicated that the RTs in a modified oddball task were significantly shortened in the training group, suggesting that individuals’ information processing efficiency during emotional processing tasks was enhanced. Additionally, ERP data revealed a significant decrease in the N2 amplitude under negative emotional stimuli in the training group, indicating a reduced sensitivity to negative information and a subsequent decrease in emotional conflict. For P3 amplitude, significant main effects of valence and time were observed, while the group main effect was non-significant and the group × time interaction was marginal, indicating overall enhancement of attentional allocation over time. Exploratory analyses showed that P3 amplitude increased across all valence conditions in the training group but not in the non-exercise group, suggesting that Tai Chi selectively enhances late-stage attentional processing. Given the marginal interaction, between-group differences should be interpreted cautiously. These findings highlight the beneficial effects of Tai Chi training on emotional regulation, although the observed improvements may also reflect general benefits of aerobic exercise rather than mechanisms unique to Tai Chi.
Specifically, the significantly shorter RTs observed in the training group relative to the control group suggest that Tai Chi training enhanced participants’ attentional control and cognitive flexibility, thereby facilitating faster processing of target stimuli. This finding is consistent with prior research showing that aerobic exercise can improve executive functions and increase the efficiency of cognitive information processing and visual target detection (Chen and Ringenbach, 2016; Fu and Yang, 2024; Gao et al., 2020; Wang et al., 2014; Komiyama et al., 2023). In parallel with the behavioral evidence, the Tai Chi group also exhibited significantly shorter N2 latencies compared to the control group, indicating faster neural responses during emotional stimulus processing. Shorter N2 latencies are generally interpreted as reflecting enhanced efficiency in early-stage conflict monitoring and cognitive control mechanisms (Schubert et al., 2023). This neural acceleration suggests that long-term Tai Chi practice may optimize the timing of attentional engagement, enabling participants to detect and evaluate emotional stimuli more rapidly. Taken together, the shortened RTs and N2 latencies provide converging behavioral and neural evidence that Tai Chi facilitates a more efficient coordination between early neural processing and subsequent behavioral responses during emotional regulation tasks. Importantly, these behavioral enhancements can also be interpreted through the lens of Tai Chi as an action-based mindfulness practice that cultivates a calm-energetic state. With repeated practice, participants may develop a tonic component of sustained calm readiness (Schumann et al., 2022), reducing susceptibility to distraction, alongside a phasic component that allows flexible entry into a focused, energetically engaged state when required (Steghaus and Poth, 2024). This dual mechanism provides a plausible explanation for the observed improvements in attentional control, as reflected in shorter RTs. While Tai Chi emphasizes coordinated movement, breathing control, and attentional focus, which may provide additional cognitive benefits (Li et al., 2022), the current study cannot definitively determine whether these effects are unique to Tai Chi or reflect broader exercise-related mechanisms.
Moreover, the observed reduction in N2 amplitude further underscores the beneficial effect of Tai Chi training on emotional regulation. N2, a neurophysiological indicator of emotional sensitivity (Yang et al., 2018; Li et al., 2008; Yuan et al., 2009), reflects early processing of emotional stimuli and is typically associated with cognitive control, conflict monitoring, and the allocation of attention resources (Botvinick et al., 2001; Folstein and Van Petten, 2008; Kumar et al., 2009). In this study, after 8 weeks of Tai Chi training, the training group showed a significant decrease in the average N2 amplitude under negative emotional stimuli compared to pre-training measurements. This result may be associated with reduced sensitivity to negative information, thereby lowering the vigilance and processing demands for negative stimuli during the early attention allocation phase and freeing up more resources for subsequent cognitive processes. Notably, this modulation may correspond to enhanced tonic calm readiness developed through Tai Chi practice (Schumann et al., 2022), enabling participants to reduce early conflict monitoring demands and reallocate cognitive resources more efficiently, consistent with the theoretical framework that Tai Chi promotes sustained attention and energetic engagement. Behavioral data further support this interpretation, as the training group demonstrated significantly shorter RTs in the modified oddball task, indicating more efficient emotional information processing. In line with these findings, Wang et al. (2023) demonstrated that Tai Chi practice improved individuals’ visual working memory capacity and narrowed emotional fluctuations between positive and negative stimuli, suggesting enhanced emotion regulation through attentional control mechanisms. Their results provide converging evidence that Tai Chi’s attentional demands and posture-memory coordination strengthen both cognitive capacity and emotion regulation. This perspective supports the interpretation that reduced N2 amplitudes in the present study reflect improved early attentional filtering of negative stimuli, consistent with Tai Chi’s facilitative effects on working memory and emotional stability.
When considered alongside the P3 findings, the decrease in N2 amplitude appears to facilitate more efficient late-stage attentional processing, suggesting that participants experienced reduced early interference from negative stimuli and could subsequently allocate attentional resources more effectively during target evaluation. The potential mechanism underlying the changes in N2 due to exercise may involve the modulation effect of the prefrontal-limbic system. Previous studies have shown that acute aerobic exercise can enhance the regulation of emotional processing by the dorsolateral prefrontal cortex (DLPFC) and reduce amygdala hyper-reactivity, thereby reducing excessive attention to negative stimuli (Wang et al., 2024). Over time, consistent engagement in physical training may induce steady-state adaptations and neuroplastic changes that strengthen emotion-regulatory control (Weng et al., 2016). Moreover, regular exercise can modulate the sympathetic nervous system and the hypothalamic–pituitary–adrenal (HPA) axis, attenuating physiological stress responses to negative emotions—a mechanism that may further support emotion regulation (Mikkelsen et al., 2017). Interestingly, our study found that the training group did not exhibit similar changes in N2 amplitude under neutral emotional stimuli, and no significant modulation was observed for positive emotional stimuli. This pattern aligns with previous research (Zhang et al., 2022), indicating that Tai Chi training may have exert a selective regulatory effect on negative emotional processing, with relatively limited impact on neutral or positive emotions.
The P3 component, a well-established marker of late-stage cognitive processing, reflects the allocation of attentional and cognitive resources during the evaluation of emotional stimuli (Huang and Luo, 2006; Gardener et al., 2013; Hajcak et al., 2010). In the present study, a significant main effect of valence and time was observed, while group-related effects did not reach significance. This indicates that emotional content and measurement time independently influenced P3 amplitude, with a general increase from pre- to post-test across all participants. Although the group × time interaction was only marginally significant (p = 0.071), exploratory analyses suggested a tendency for larger post-test P3 amplitudes in the Tai Chi training group, particularly for negative and neutral stimuli. This trend may imply enhanced attentional engagement and improved cognitive resource allocation following the intervention. Consistent with prior evidence linking larger P3 amplitudes to heightened attention and more efficient resource distribution (Donchin and Coles, 1988; Hillman et al., 2012), these findings suggest that Tai Chi may facilitate a state of adaptive attentional readiness rather than produce a statistically robust group effect. When considered alongside the shorter RTs and reduced N2 amplitudes, this pattern implies indicate that Tai Chi may optimize both early and late stages of emotional information processing by reducing initial conflict monitoring demands and enhancing attentional resource allocation during later cognitive operations.
Importantly, the observed time-dependent increase in P3 amplitude—regardless of group—indicates improved attentional efficiency over repeated testing, potentially reflecting learning or task-familiarity effects rather than intervention-specific outcomes. Nevertheless, the directional trend toward higher P3 amplitudes in the Tai Chi group aligns with literature demonstrating that regular physical activity enhances top-down attentional control and emotion regulation (Hillman et al., 2008; Kamijo and Takeda, 2010). The absence of an active exercise control group, however, limits our ability to distinguish Tai Chi-specific effects from the broader cognitive benefits of moderate-intensity aerobic exercise. Future research incorporating exercise-based control conditions and longitudinal neuroimaging approaches will be critical for delineating Tai Chi’s unique contributions to emotional and cognitive regulation.
Despite the valuable insights provided by this study on the effects of Tai Chi training on emotional regulation in female college students using ERP measures, several limitations should be acknowledged. First, the relatively small sample size (about 20 participants per group) reduces statistical power, particularly for higher-order interactions such as Group × Valence, which typically require 30–35 participants or more per group for stable and replicable estimates. Although the observed ERP and behavioral effects were significant and consistent with prior research, small samples may inflate effect sizes and limit generalizability. Second, the pre–post design over an eight-week period captures only short-term effects, leaving open the question of whether the observed benefits are sustained. Longitudinal studies with follow-up assessments are needed to determine the durability of Tai Chi’s effects and their neural underpinnings. Third, the study focused exclusively on N2 and P3 components, omitting other potentially informative electrophysiological or physiological indicators (e.g., P1, N1, HRV). Additionally, while the study included state–trait anxiety and depression measures, these instruments do not fully capture the experiential aspects of Tai Chi, such as momentary vitality, relaxation, and bodily ease. Tai Chi, as an action-based mindfulness practice, may modulate energetic and tense arousal in ways not reflected by traditional anxiety or affective scales. Future research could adopt circumplex-based state measures to better situate ERP and behavioral effects within participants’ lived experiences. Fourth, RT data for standard stimuli were not preserved, which restricts the ability to fully rule out general motor effects. Fifth, the absence of an active exercise control group limits interpretability; it remains unclear whether the observed improvements are specific to Tai Chi or reflect general benefits of moderate-intensity aerobic exercise. Future work should incorporate appropriate exercise control conditions to disentangle these effects. Moreover, despite controlling for intervention duration and intensity, potential confounding factors such as psychological states, lifestyle habits, and individual differences (e.g., sleep quality, social support) may have influenced outcomes, warranting stricter control in future studies. Finally, the study used a modified oddball paradigm (70–30 distribution) requiring responses to both frequent and infrequent stimuli. Unlike the classical oddball that captures unexpected rare events, this design reflects frequency discrimination, so results should be interpreted within this context.
5 Conclusion
This study demonstrated that 8 weeks of Tai Chi training modulated both neural (N2, P3) and behavioral (RT) responses to emotional stimuli in healthy female college students, suggesting potential benefits for attentional and cognitive processes in emotional regulation. However, as the study did not directly test the associations between ERP changes and behavioral outcomes, interpretations regarding more “effective” attentional allocation should be made with caution. Future research should examine these neural-behavioral links to strengthen the evidence base.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The study was approved by the Ethics Committee of the Institute of Neuroscience and Cognitive Psychology at Anhui Polytechnic University (AHPU-PED-2022-001). Informed written consent was obtained from all participants prior to their involvement in the study. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JY: Data curation, Formal analysis, Investigation, Software, Visualization, Writing – original draft, Writing – review & editing. QZ: Data curation, Formal analysis, Investigation, Validation, Writing – review & editing. DF: Data curation, Investigation, Methodology, Writing – review & editing. YW: Funding acquisition, Investigation, Methodology, Supervision, Writing – review & editing. HL: Investigation, Methodology, Writing – review & editing. ZC: Conceptualization, Investigation, Writing – review & editing. JX: Conceptualization, Investigation, Writing – review & editing. AW: Investigation, Methodology, Validation, Writing – review & editing. JL: Investigation, Methodology, Validation, Writing – review & editing. YZ: Funding acquisition, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Key Project of Humanities and Social Sciences in Anhui Province Universities (2024AH052247).
Acknowledgments
We thank all the participants.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyg.2025.1620704/full#supplementary-material
References
Beck, A. T., Steer, R. A., and Brown, G. K. (1996). Beck depression inventory: BDI-II: manual. San Antonio, Texas, USA: Psychological Corporation.
Bernstein, E. E., and McNally, R. J. (2017). Acute aerobic exercise helps overcome emotion regulation deficits. Cogn. Emot. 31, 834–843. doi: 10.1080/02699931.2016.1168284
Botvinick, M. M., Braver, T. S., Barch, D. M., Carter, C. S., and Cohen, J. D. (2001). Conflict monitoring and cognitive control. Psychol. Rev. 108, 624–652. doi: 10.1037/0033-295X.108.3.624
Bradley, M. M., Codispoti, M., Sabatinelli, D., and Lang, P. J. (2001). Emotion and motivation part II: sex differences in picture processing. Emotion 1, 300–319. doi: 10.1037//1528-3542.1.3.300-319
Buodo, G., Sarlo, M., Mento, G., Messerotti Benvenuti, S., and Palomba, D. (2017). Unpleasant stimuli differentially modulate inhibitory processes in an emotional go/nogo task: an event-related potential study. Cogn. Emot. 31, 127–138. doi: 10.1080/02699931.2015.1089842
Carretié, L., Hinojosa, J. A., Martín-Loeches, M., Mercado, F., and Tapia, M. (2004). Automatic attention to emotional stimuli: neural correlates. Hum. Brain Mapp. 22, 290–299. doi: 10.1002/hbm.20037
Chen, C., Nakagawa, S., An, Y., Ito, K., Kitaichi, Y., and Kusumi, I. (2017). The exercise-glucocorticoid paradox: how exercise is beneficial to cognition, mood, and the brain while increasing glucocorticoid levels. Front. Neuroendocrinol. 44, 83–102. doi: 10.1016/j.yfrne.2016.12.001
Chen, C. C., and Ringenbach, S. (2016). Dose–response relationship between intensity of exercise and cognitive performance in individuals with down syndrome: a preliminary study. J. Intellect. Disabil. Res. 60, 606–614. doi: 10.1111/jir.12258
Cui, L., Yin, H., Lyu, S., Shen, Q., Wang, Y., Li, X., et al. (2019). Tai Chi Chuan vs general aerobic exercise in brain plasticity: a multimodal MRI study. Sci. Rep. 9:17264. doi: 10.1038/s41598-019-53731-z
Delorme, A., and Makeig, S. (2004). EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 134, 9–21. doi: 10.1016/j.jneumeth.2003.10.009
Donchin, E., and Coles, M. G. (1988). Is the P300 component a manifestation of context updating? Behav. Brain Sci. 11, 357–374. doi: 10.1017/S0140525X00058027
Edwards, M. K., Rhodes, R. E., and Loprinzi, P. D. (2017). A randomized control intervention investigating the effects of acute exercise on emotional regulation. Am. J. Health Behav. 41, 534–543. doi: 10.5993/AJHB.41.5.2
Folstein, J. R., and Van Petten, C. (2008). Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology 45, 152–170. doi: 10.1111/j.1469-8986.2007.00602.x
Frith, J. J., Kerr, J. H., and Wilson, G. V. (2011). Immediate improvements in emotion and stress following participation in Aerobics, Circuit Training and Tai Chi. Int. J. Sport Psychol. 42:480. doi: 10.5555/20123028203
Fu, H.-L., and Yang, C.-T. (2024). Exploring the influence of a 4-week aerobic exercise intervention on cognitive control processes in young adults: An SFT and DDM study. Prog. Brain Res. 283, 193–229. doi: 10.1016/bs.pbr.2023.12.003
Gao, L., Zhu, L., Hu, L., Hu, H., Wang, S., Bezerianos, A., et al. (2020). Mid-task physical exercise keeps your mind vigilant: evidences from behavioral performance and EEG functional connectivity. IEEE Trans. Neural Syst. Rehabil. Eng. 29, 31–40. doi: 10.1109/TNSRE.2020.3030106
Gard, M. G., and Kring, A. M. (2007). Sex differences in the time course of emotion. Emotion 7, 429–437. doi: 10.1037/1528-3542.7.2.429
Gardener, E. K., Carr, A. R., MacGregor, A., and Felmingham, K. L. (2013). Sex differences and emotion regulation: an event-related potential study. PLoS One 8:e73475. doi: 10.1371/journal.pone.0073475
Gomez-Pinilla, F., and Hillman, C. (2013). The influence of exercise on cognitive abilities. Compr. Physiol. 3:403. doi: 10.1002/cphy.c110063
Gross, J. J. (1998). Antecedent-and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. J. Pers. Soc. Psychol. 74, 224–237. doi: 10.1037/0022-3514.74.1.224
Güntekin, B., Femir, B., Gölbaşı, B. T., Tülay, E., and Başar, E. (2017). Affective pictures processing is reflected by an increased long-distance EEG connectivity. Cogn. Neurodyn. 11, 355–367. doi: 10.1007/s11571-017-9439-z
Hajcak, G., MacNamara, A., and Olvet, D. M. (2010). Event-related potentials, emotion, and emotion regulation: an integrative review. Dev. Neuropsychol. 35, 129–155. doi: 10.1080/87565640903526504
Henz, D., and Schöllhorn, W. I. (2018). Temporal courses in EEG theta and alpha activity in the dynamic health qigong techniques Wu Qin xi and Liu Zi Jue. Front. Psychol. 8:2291. doi: 10.3389/fpsyg.2017.02291
Hillman, C. H., Buck, S. M., Themanson, J. R., Pontifex, M. B., and Castelli, D. M. (2009). Aerobic fitness and cognitive development: event-related brain potential and task performance indices of executive control in preadolescent children. Dev. Psychol. 45, 114–129. doi: 10.1037/a0014437
Hillman, C. H., Erickson, K. I., and Kramer, A. F. (2008). Be smart, exercise your heart: exercise effects on brain and cognition. Nat. Rev. Neurosci. 9, 58–65. doi: 10.1038/nrn2298
Hillman, C. H., Kamijo, K., and Pontifex, M. B. (2012). “The relation of ERP indices of exercise to brain health and cognition” In: eds. Henning Boecker, Charles H. Hillman, Lukas Scheef, Heiko K. Strüder, Functional neuroimaging in exercise and sport sciences (New York, NY, USA: Springer), 419–446.
Huang, Y.-X., and Luo, Y.-J. (2006). Temporal course of emotional negativity bias: an ERP study. Neurosci. Lett. 398, 91–96. doi: 10.1016/j.neulet.2005.12.074
Hwang, R.-J., Chen, H.-J., Guo, Z.-X., Lee, Y.-S., and Liu, T.-Y. (2019). Effects of aerobic exercise on sad emotion regulation in young women: an electroencephalograph study. Cogn. Neurodyn. 13, 33–43. doi: 10.1007/s11571-018-9511-3
Jennings, J. R., and Wood, C. C. (1976). The e-adjustment procedure for repeated-measures analyses of variance. Psychophysiology 13, 277–278. doi: 10.1111/j.1469-8986.1976.tb00116.x
Kamijo, K., and Takeda, Y. (2010). Regular physical activity improves executive function during task switching in young adults. Int. J. Psychophysiol. 75, 304–311. doi: 10.1016/j.ijpsycho.2010.01.002
Kim, H., Kim, J., Woo, M., and Kim, T. (2022). Changes in inhibitory control, craving and affect after yoga vs. aerobic exercise among smokers with nicotine dependence. Front. Psych. 13:940415. doi: 10.3389/fpsyt.2022.940415
Komiyama, T., Takedomi, H., Aoyama, C., Goya, R., and Shimegi, S. (2023). Acute exercise has specific effects on the formation process and pathway of visual perception in healthy young men. Eur. J. Neurosci. 58, 3239–3252. doi: 10.1111/ejn.16082
Kumar, S., Soto, D., and Humphreys, G. W. (2009). Electrophysiological evidence for attentional guidance by the contents of working memory. Eur. J. Neurosci. 30, 307–317. doi: 10.1111/j.1460-9568.2009.06805.x
Lewis, M. D., Lamm, C., Segalowitz, S. J., Stieben, J., and Zelazo, P. D. (2006). Neurophysiological correlates of emotion regulation in children and adolescents. J. Cogn. Neurosci. 18, 430–443. doi: 10.1162/089892906775990633
Li, X., Geng, J., Du, X., Si, H., and Wang, Z. (2022). Relationship between the practice of tai chi for more than 6 months with mental health and brain in university students: an exploratory study. Front. Hum. Neurosci. 16:912276. doi: 10.3389/fnhum.2022.912276
Li, H., Yuan, J., and Lin, C. (2008). The neural mechanism underlying the female advantage in identifying negative emotions: an event-related potential study. NeuroImage 40, 1921–1929. doi: 10.1016/j.neuroimage.2008.01.033
Ligeza, T. S., Maciejczyk, M., Wyczesany, M., and Junghofer, M. (2023). The effects of a single aerobic exercise session on mood and neural emotional reactivity in depressed and healthy young adults: a late positive potential study. Psychophysiology 60:e14137. doi: 10.1111/psyp.14137
Long, Z., Liu, G., Xiao, Z., and Gao, P. (2021). Improvement of emotional response to negative stimulations with moderate-intensity physical exercise. Front. Psychol. 12:656598. doi: 10.3389/fpsyg.2021.656598
Lopez-Calderon, J., and Luck, S. J. (2014). ERPLAB: an open-source toolbox for the analysis of event-related potentials. Front. Hum. Neurosci. 8:213. doi: 10.3389/fnhum.2014.00213
Lu, B., Hui, M., and Yu-Xia, H. (2005). The development of native Chinese affective picture system--a pretest in 46 college students. Chin. Ment. Health J. 11, 4–7.
Mikkelsen, K., Stojanovska, L., Polenakovic, M., Bosevski, M., and Apostolopoulos, V. (2017). Exercise and mental health. Maturitas 106, 48–56. doi: 10.1016/j.maturitas.2017.09.003
Mizzi, A. L., McKinnon, M. C., and Becker, S. (2022). The impact of aerobic exercise on mood symptoms in trauma-exposed young adults: a pilot study. Front. Behav. Neurosci. 16:829571. doi: 10.3389/fnbeh.2022.829571
Moser, J. S., Hajcak, G., Bukay, E., and Simons, R. F. (2006). Intentional modulation of emotional responding to unpleasant pictures: An ERP study. Psychophysiology 43, 292–296. doi: 10.1111/j.1469-8986.2006.00402.x
Olson, R. L., Brush, C. J., Ehmann, P. J., and Alderman, B. L. (2017). A randomized trial of aerobic exercise on cognitive control in major depression. Clin. Neurophysiol. 128, 903–913. doi: 10.1016/j.clinph.2017.01.023
Palomba, D., Angrilli, A., and Mini, A. (1997). Visual evoked potentials, heart rate responses and memory to emotional pictorial stimuli. Int. J. Psychophysiol. 27, 55–67. doi: 10.1016/S0167-8760(97)00751-4
Patel, S. H., and Azzam, P. N. (2005). Characterization of N200 and P300: selected studies of the event-related potential. Int. J. Med. Sci. 2, 147–154. doi: 10.7150/ijms.2.147
Qiu, F., Peng, W., Li, M., Zhang, L., Zhu, H., Tan, X., et al. (2019). Effects of physical exercise on negative emotional susceptibility in young adult females: an event-related potential study. Brain Res. 1722:146382. doi: 10.1016/j.brainres.2019.146382
Sanada, M., and Katayama, J. (2024). Unpleasant emotion inhibits attentional focus toward a peripheral target in a visual search: an ERP study. Exp. Brain Res. 242, 1399–1409. doi: 10.1007/s00221-024-06796-w
Schaeffer, D. J., Krafft, C. E., Schwarz, N. F., Chi, L., Rodrigue, A. L., Pierce, J. E., et al. (2014). An 8-month exercise intervention alters frontotemporal white matter integrity in overweight children. Psychophysiology 51, 728–733. doi: 10.1111/psyp.12227
Schmitt, A., Upadhyay, N., Martin, J. A., Rojas Vega, S., Strüder, H. K., and Boecker, H. (2020). Affective modulation after high-intensity exercise is associated with prolonged amygdalar-insular functional connectivity increase. Neural Plast. 2020:7905387. doi: 10.1155/2020/7905387
Schubert, A. L., Löffler, C., Hagemann, D., and Sadus, K. (2023). How robust is the relationship between neural processing speed and cognitive abilities? Psychophysiology 60:e14165. doi: 10.1111/psyp.14165
Schumann, F., Steinborn, M. B., Kürten, J., Cao, L., Händel, B. F., and Huestegge, L. (2022). Restoration of attention by rest in a multitasking world: theory, methodology, and empirical evidence. Front. Psychol. 13:867978. doi: 10.3389/fpsyg.2022.867978
Spielberger, C. D. Manual for the state-trait anxiety inventory (self-evaluation questionnaire). (1970). Palo Alto, CA: Consulting Psychologists Press.
Steghaus, S., and Poth, C. H. (2024). Feeling tired versus feeling relaxed: two faces of low physiological arousal. PLoS One 19:e0310034. doi: 10.1371/journal.pone.0310034
Thayer, R. E., Newman, J. R., and McClain, T. M. (1994). Self-regulation of mood: strategies for changing a bad mood, raising energy, and reducing tension. J. Pers. Soc. Psychol. 67, 910–925. doi: 10.1037/0022-3514.67.5.910
Thom, N. J., O’Connor, P. J., Clementz, B. A., and Dishman, R. K. (2019). Acute exercise prevents angry mood induction but does not change angry emotions. Med. Sci. Sports Exerc. 51, 1451–1459. doi: 10.1249/MSS.0000000000001922
Wang, M., Chi, S., Wang, X., and Wang, T. (2024). Effects of tai Chi on anxiety and theta oscillation power in college students during the COVID-19 pandemic: a randomized controlled trial. PLoS One 19:e0312804. doi: 10.1371/journal.pone.0312804
Wang, X., Liu, T., Jin, X., and Zhou, C. (2024). Aerobic exercise promotes emotion regulation: a narrative review. Exp. Brain Res. 242, 783–796. doi: 10.1007/s00221-024-06791-1
Wang, Y. T., Taylor, L., Pearl, M., and Chang, L.-S. (2004). Effects of tai Chi exercise on physical and mental health of college students. Am. J. Chin. Med. 32, 453–459. doi: 10.1142/S0192415X04002107
Wang, Y., Tian, J., and Yang, Q. (2023). Tai Chi exercise improves working memory capacity and emotion regulation ability. Front. Psychol. 14:1047544. doi: 10.3389/fpsyg.2023.1047544
Wang, C.-H., Tsai, C.-L., Tseng, P., Yang, A. C., Lo, M.-T., Peng, C.-K., et al. (2014). The association of physical activity to neural adaptability during visuo-spatial processing in healthy elderly adults: a multiscale entropy analysis. Brain Cogn. 92, 73–83. doi: 10.1016/j.bandc.2014.10.006
Weng, T. B., Pierce, G. L., Darling, W. G., Falk, D., Magnotta, V. A., and Voss, M. W. (2016). The acute effects of aerobic exercise on the functional connectivity of human brain networks. Brain Plast. 2, 171–190. doi: 10.3233/BPL-160039
Xu, S., Baker, J. S., and Ren, F. (2021). The positive role of tai chi in responding to the covid-19 pandemic. Int. J. Environ. Res. Public Health 18:7479. doi: 10.3390/ijerph18147479
Yang, J., Zhang, S., Lou, Y., Long, Q., Liang, Y., Xie, S., et al. (2018). The increased sex differences in susceptibility to emotional stimuli during adolescence: an event-related potential study. Front. Hum. Neurosci. 11:660. doi: 10.3389/fnhum.2017.00660
Yuan, J., Luo, Y., Yan, J. H., Meng, X., Yu, F., and Li, H. (2009). Neural correlates of the females' susceptibility to negative emotions: An insight into gender-related prevalence of affective disturbances. Hum. Brain Mapp. 30, 3676–3686. doi: 10.1002/hbm.20796
Yuan, J., Yang, J., Meng, X., Yu, F., and Li, H. (2008). The valence strength of negative stimuli modulates visual novelty processing: electrophysiological evidence from an event-related potential study. Neuroscience 157, 524–531. doi: 10.1016/j.neuroscience.2008.09.023
Zhang, Y., Fu, R., Sun, L., Gong, Y., and Tang, D. (2019). How does exercise improve implicit emotion regulation ability: preliminary evidence of mind-body exercise intervention combined with aerobic jogging and mindfulness-based yoga. Front. Psychol. 10:1888. doi: 10.3389/fpsyg.2019.01888
Zhang, Y., Li, Y., Shi, Z., and Franz, E. (2022). Does acute exercise benefit emotion regulation? Electrophysiological evidence from affective ratings and implicit emotional effects on cognition. Biol. Psychol. 172:108375. doi: 10.1016/j.biopsycho.2022.108375
Zheng, X., and Hasegawa, H. (2016). Central dopaminergic neurotransmission plays an important role in thermoregulation and performance during endurance exercise. Eur. J. Sport Sci. 16, 818–828. doi: 10.1080/17461391.2015.1111938
Keywords: Tai Chi, emotion regulation, event-related potential (ERP), aerobic exercise, cognitive control
Citation: Yuan J, Zeng Q, Feng D, Wang Y, Li H, Cong Z, Xu J, Wang A, Li J and Zhang Y (2025) The effect of 8-week Tai Chi training on emotional regulation in female college students: an ERP study of N2 and P3 under a modified oddball paradigm. Front. Psychol. 16:1620704. doi: 10.3389/fpsyg.2025.1620704
Edited by:
Bernadette Ann Murphy, Ontario Tech University, CanadaReviewed by:
Hsueh-Sheng Chiang, Beth Israel Deaconess Medical Center and Harvard Medical School, United StatesMichael B. Steinborn, Julius Maximilian University of Würzburg, Germany
Yi Wang, Weinan Normal University, China
Copyright © 2025 Yuan, Zeng, Feng, Wang, Li, Cong, Xu, Wang, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Zhang, emhhbmd5b25nQGFocHUuZWR1LmNu
 Jin Yuan
Jin Yuan Quanwen Zeng1
Quanwen Zeng1 Huimin Li
Huimin Li Anjie Wang
Anjie Wang Jun Li
Jun Li Yong Zhang
Yong Zhang