- Department of Chemistry, Faculty of Science and Technology, The University of the West Indies, St Augustine, Trinidad Tobago
The Maillard reaction not only results in the formation of flavor compounds, but also harmful by-products, including the infamous toxicant acrylamide. Dietary acrylamide cannot be eliminated, but its levels in foods can be minimized. This review discusses the mechanisms of inhibition, effectiveness under varied conditions, and limitations of the sulfur-containing compounds: thiols, sulfites, thioethers, and thiosulfinates. These compounds have proven to be effective inhibitors of acrylamide formation. Among these compounds, the thiols are deemed the most effective. However, such success is masked by their negative effects on food taste and odor.
1 Introduction
First reported in 1912 by Louis Camille Maillard, the Maillard reaction described the development of a yellow-brown color caused by a heated reaction between sugars and amino acids in water (Van Boekel, 1998). Present studies further describe the Maillard reaction as a complex series of non-enzymatic browning reactions that occur during food processing. In these reactions, amino acids, amines, and proteins undergo condensation with carbonyl sources: reducing sugars, ketones, aldehydes, oxidized lipids, and polyphenols (Bittner, 2006; Mazumder et al., 2019). Such reactions result in the formation of an unstable Schiff base which undergoes rearrangement to a more stable amadori product. The product eventually degrades, resulting in the formation of reactive carbonyl and dicarbonyl species. These compounds participate in further reactions giving rise to Strecker aldehydes, aldol condensation products, and melanoidins (Mazumder et al., 2019).
The rate at which the Maillard reaction progresses can be affected by several factors including pH, temperature, moisture, and food matrices. An increase in pH and temperature favors the progression of the Maillard reaction due to greater protein participation; proteins become more soluble at higher pH and amino acid residues become more pronounced at higher temperatures (≥55°C) due to protein denaturation. On the other hand, a decrease in moisture favors the Maillard reaction. Although moisture content encourages reactant dissolution and mobility, a further surge in the moisture level will decelerate the reaction rate. This is because high levels of moisture dilute the reactants’ concentration. Thus, the overall mobility of the system is diminished. Therefore, the maximum browning rate has been documented between a water activity range of 0.65–0.75 (Wong et al., 2015). For foods, the diverse conditions which exist in the matrices present multiple reactants, and hence, a complex array of Maillard reaction products. As such, the rate of the Maillard reaction is difficult to determine in foods. The identification and quantification of the Maillard reaction products, and the determination of their rates of formation and their rates of participation in secondary reactions are necessary for the proposal of a plausible Maillard reaction mechanism and rate equation for a particular food matrix (Lund and Ray, 2017).
While there are several pros surrounding the occurrence of the Maillard reaction, the consequences are dignified and worthy of consideration. Albeit its contribution to food flavor, aroma, and color, the Maillard reaction gives rise to some harmful products, including a process contaminant, acrylamide (ACR). ACR shows a variety of adverse effects on human health, as it is identified to be neurotoxic, cytotoxic, hepatotoxic, immunotoxic, genotoxic, mutagenic, and “possibly carcinogenic” to humans (Zamani et al., 2017; Gülcan et al., 2020). The most popular Maillard route by which ACR is formed involves a heated reaction with asparagine (ASN) and an α-hydroxycarbonyl compound such as a reducing sugar (Jin et al., 2013). This dehydration gives rise to the characteristic Schiff base, the key intermediate in ACR formation. The Schiff base is more of an efficient precursor rather than the amadori product, undergoing decarboxylation which results in the formation of azomethine ylide. ACR is formed from azomethine ylide via three pathways: 1) directly from azomethine ylide 2) through the β-elimination of the tautomer of azomethine ylide and 3) through dehydration and subsequent deamination of azomethine ylide (Figure 1). ACR could also be formed via another route involving the interaction between asparagine and α-dicarbonyl compounds (e.g., glyoxal, methylglyoxal). These compounds can be formed through sugar degradation or the degradation of the amadori product. The corresponding Schiff base formed from this alternate route gives rise to azomethine ylide; this compound yields ACR either directly, or indirectly via the formation of 3-aminopropionamide (Jin et al., 2013; Lund and Ray, 2017). Less popular routes showed ACR yield through acrylic acid (oxidized acrolein) and through ammonia and asparagine (Jin et al., 2013).
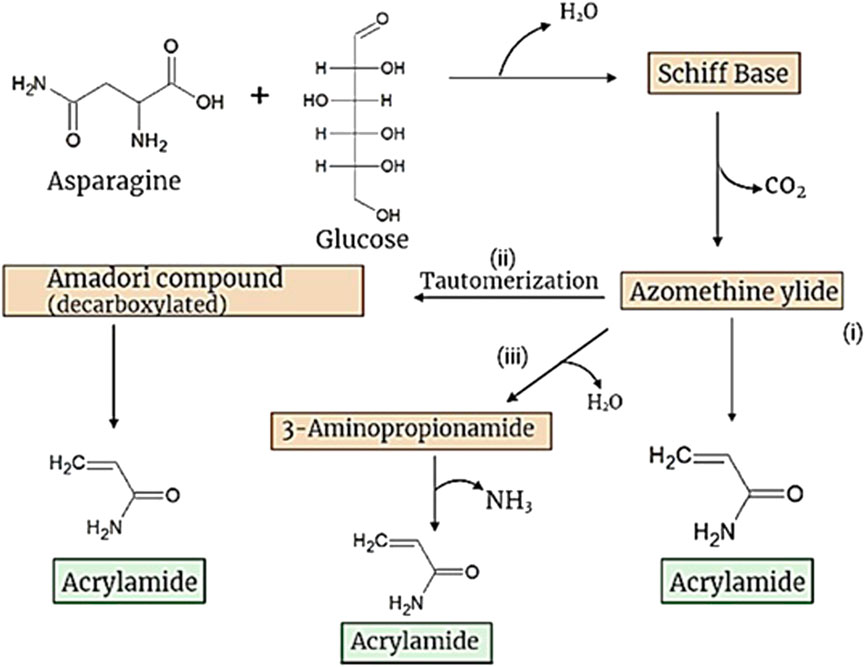
FIGURE 1. Schematic diagram of acrylamide formation via major Maillard reaction pathways (Jin et al., 2013).
Due to its unavoidable occurrence during food processing, research efforts have been geared towards the inhibition of ACR formation. In recent years, various amino acids, enzymes, antioxidants, polyphenols, and food additives have been investigated in an attempt to understand their mechanisms of inhibition (Lund and Ray, 2017). This has proven to be difficult due to the diverse physical and chemical characteristics of these compounds. Their effects on the Maillard reaction are different; hence the outcomes imposed on ACR formation vary significantly from compound to compound.
A contemporary sub-group of compounds that have garnered keen scientific interest in their roles as Maillard reaction inhibitors are sulfur-containing compounds. This is because they are excellent detoxifying agents, strong nucleophiles, excellent reducing and anti-browning agents, and scavengers of Reactive Oxygen Species (ROS) (Friedman et al., 1982; Friedman, 1994; Augustine and Bent, 2019; Augustine et al., 2021). The sulfur-containing compounds can be further divided into various classes: thiols (glutathione, cysteine), sulfites (sodium bisulfite), the thioethers (methionine), and the thiosulfinates (allicin). These compounds are major components of the diet or have been introduced during food processing as either preservatives or additives. The aim of this article is to provide an overview about the inhibition of ACR by these compounds, thus including their mechanisms of inhibition, effectivity under various conditions, and the limitations of their inhibitory roles.
2 Acrylamide inhibition
2.1 Thiols
The thiols have been highlighted for their strong nucleophilicity, scavenging abilities of ROS and effective inhibition of non-enzymatic browning (Friedman, 1994; Davis and Snyderwine, 1995; Zeng et al., 2009). These properties are dependent on the SH group which gives rise to an even more nucleophilic thiolate anion. Owing to the polarizability of the sulfur atom, and its effectiveness in charge delocalization, the thiols demonstrate greater reactivity through the formation of this anion. Thus, several mechanisms have been exhibited by the thiols, by which the accumulation of ACR is inhibited. Thiols such as cysteine and glutathione are able to compete with asparagine for α-hydroxycarbonyl compounds, thus impeding the major pathway for ACR formation (Figure 2).
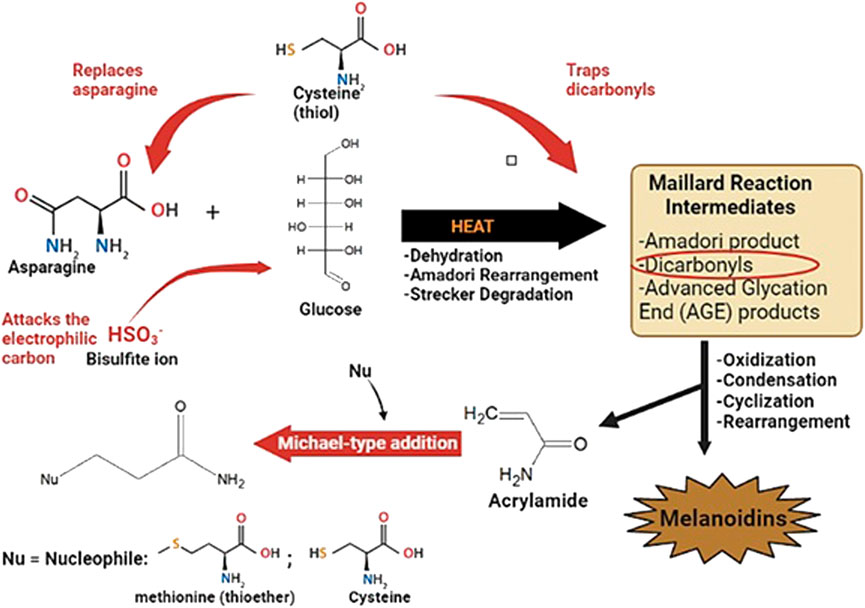
FIGURE 2. Inhibitory effects of sulfur-containing compounds (shown in red) on acrylamide formation and accumulation.
When cysteine was added to an equimolar asparagine/glucose closed model system, the ACR formation observed for cysteine (polar, sulfur-containing amino acid), was significantly reduced in comparison to lysine (polar, basic amino acid) and glutamine (polar, neutral amino acid), where an elevation in ACR concentration was observed with the latter. Furthermore, cysteine’s reducing effect decreased with increasing temperature (140°C–200°C) but increased with heating time (5–35 min) at 160°C. The minimal performance could be due to possible degradation of the amino acid at higher temperatures. This reduction effect was independent of the type of α-hydroxycarbonyl present (Claeys et al., 2005a). Another study showed similar reduction effects upon addition of cysteine to ASN/fructose and ASN/glucose model systems. The reduction effects were much more significant when compared to non-sulfur additives such as NaCl and ascorbic acid. Addition of as little as 0.2% cysteine to the ASN/fructose model system showed a 93.01% decline in ACR levels. Further addition of cysteine (1.5%) resulted in a 97.95% reduction of ACR. In an ASN/glucose system, an addition of 1.5% cysteine resulted in non-detectable levels of ACR (Claeys et al., 2005b). A complementary behavior was seen with glutathione upon addition to an ASN/glucose system. Significant inhibition of ACR formation was observed with the greater success of 38%–86% and 57%–82% demonstrated at lower temperatures: 120 and 140°C, respectively. The application of GSH achieved better inhibition of ACR formation at temperatures below 160°C (Zhu et al., 2020). Furthermore, the addition of the thiols lowered the pH of the ASN/glucose system. This condition resulted in the protonation of asparagine’s amine group, thus encouraging the inhibition of ACR formation (Zhu et al., 2020).
Besides competing with asparagine, the thiols inhibit ACR formation by trapping dicarbonyl intermediates; thus they are considered “trapping agents” (Figure 2). Although the amount of ACR generated by α-dicarbonyls is quite marginal (0.2–0.5 mmol/mol) in comparison to α-hydroxycarbonyls (2.22–3.97 mmol/mol) (Blank et al., 2005), this method of inhibition is still noteworthy. Such trapping may result in the prevention of browning since carbonyl and dicarbonyl compounds contribute to color formation (Lund and Ray, 2017). The accumulation of generated ACR can be further eliminated by interaction of the SH group with the ACR molecules. The SH group reacts with ACR via a Michael-type addition, resulting in the formation of a Michael-type adduct, S-β-propionamide (Figure 2). The SH groups have shown reactivity of 100–300 times greater with vinyl compounds such as ACR, in comparison to NH2 groups (Claeys et al., 2005a).
Further investigations of the thiols were conducted in the presence of a food matrix. Glutathione was added to cookies in the range: 0.005–0.20 lb/kg for an investigation of its inhibition effect; results showed significant reduction in ACR levels. ACR declination was not directly correlated to glutathione concentration but might have been influenced by the presence of additives (NaCl, sucrose, baking powder) and the food matrix (wheat flour, milk). Nevertheless, the color difference observed among the cookies was directly correlated to glutathione concentration. Glutathione resulted in a slight increase in lightness and surface yellowing, and a slight decrease in surface redness (Claeys et al., 2005b).
2.2 Sulfites
The conventional use of sulfites during food processing can be attributed to their role as food preservatives. Sulfites, such as sodium bisulfite (NaHSO3), are useful in preventing oxidation and destroying bacteria. The inhibition effect of sulfites may be a result of the addition of HSO3− to the carbonyl group of the reducing sugar, followed by the sugar’s condensation with the amino compound (Figure 2). Formation of the Schiff base is inhibited along with the subsequent progression of the Maillard reaction. The sulfites are similar to the thiols in terms of their inhibition effect on ACR. An addition of 0.2% NaHSO3 to an ASN/fructose model system resulted in a 98.11% reduction of ACR. Further addition of 0.8% resulted in complete inhibition of ACR. In an ASN/glucose model system, reduction rates ranged from 78.47% to 96.32% with increasing amounts of NaHSO3 (Yuan et al., 2011).
Sodium Bisulfite showed greater reactivity in the ASN/fructose model system vs. the ASN/glucose model system. This can be alluded to the greater reactivity of glucose, a stronger nucleophile than HSO3−, with the amino group of ASN (Yuan et al., 2011). Furthermore, the ACR inhibition effect of the sulfites varies based on the concentration and food matrix; to further illustrate, potato chips soaked in 0.1%–0.5% NaHSO3 showed an ACR reduction of 47.4% (Ou et al., 2010). In black olives, however, 1.5 mM NaHSO3 had no effect on ACR formation in comparison to its absence, but a concentration of 25 mM resulted in 100% ACR reduction (Casado et al., 2010).
2.3 Thioethers
Thioethers (thiol ethers), particularly methionine, have been recently included in various disciplines due to their unique chemical reactivity. Methionine participates in many detoxifying processes. Its excellent oxygen radical-scavenging abilities aid in the retardation of cellular aging. Additionally, methionine is excellent in absorbing selenium and zinc, thus improving bioavailability, and chelates heavy metals such as Mercury and lead (Deming, 2017; Augustine et al., 2021). In the area of food processing, this sulphur-containing amino acid is recognized as a major component of various ingredients such as herbs and seasonings. Studies have shown that the addition of methionine to foods affects neither the taste nor quality (Maleki and Djazayeri, 1968; Augustine and Bent, 2019; Augustine et al., 2021). Nevertheless, limited studies have emerged, investigating methionine’s use as an inhibitor of ACR formation. This is because former studies showed that small amounts of ACR can be formed from a reaction between methionine and reducing sugars (Zyzak et al., 2003). Recent studies, however, have provided a more in-depth account of the elimination effect of methionine on ACR levels (Figure 2). Among ten amino acids which were tested for the removal of acrylamide residues upon heating at 160°C for 15 min, methionine was included in the top four most effective amino acids, performing at pH 7; Michael-type adducts were identified for each amino acid. However, methionine was outperformed by cysteine, lysine, and glycine. (Yu et al., 2013). Furthermore, a recent study has shown that the addition of methionine (as low as 0.2 mg) to 5 mg ACR resulted in significant ACR reduction upon heating at 160°C (Augustine et al., 2021).
2.4 Thiosulfinates
Thiosulfinates are reactive species formed from the condensation of two sulfenic acids, or oxidation of disulfides. They are very reactive, thus undergoing reactions with thiols, and hydrolysis via various mechanistic pathways. Also, they are biologically relevant, acting as intermediates in numerous enzymatic reactions (Horn et al., 2001). Classified as antioxidants, these compounds inhibit ROS and break “off” a chain of reactions including mitochondrial membrane depolarization, cytochrome c release, and caspase-3-activation. These species also demonstrate antibacterial, antifungal, antithrombic, and cholesterol-lowering properties (Ríos, 2015). Natural, therapeutic antioxidants are considered attractive candidates for the advancement of inhibitors of ACR formation during food processing. Therefore, recent studies were conducted on a common thiosulfinate, allicin, and its inhibitory effects. Allicin, found in garlic, is the principal biologically active compound. When chopped or crushed, the enzyme allinase activates, thus producing allicin from alliin, which is found in intact garlic (Bayan et al., 2014).
Albeit an incomplete elucidation of allicin’s mechanism of inhibition, its effect on ACR formation in various model systems has been studied. A similar inhibition effect was seen when allicin was added to ASN/fructose and ASN/glucose model systems. Increasing concentrations of allicin from 0.0375% resulted in a decline in ACR reduction from 51.51% to 23.16%. At 0.075% allicin, only a 23.51% reduction rate in ACR formation was observed. At a concentration of about 0.5625% allicin, a promotion effect was observed (Yuan et al., 2011).
3 Limitations and recommendations
The use of sulfur-containing compounds for the inhibition of ACR formation is gaining momentum because of their affordability, dietary availability, antioxidant effects and therapeutic benefits. Hence, the following limitations need to be considered for the advancement of future work.
3.1 Unpleasant taste and odor
In addition to strong inhibition effects, the thiols are beneficial in the production of bakery goods as they improve pastries by acting as ‘softening’ agents; this is due to an interchange with gluten proteins involving thiols and disulfides (Zhu et al., 2020). Nevertheless, as much as the thiols promote the quality and safety of foods, their negative effects on food taste and aroma unequivocally compete with this benefit (Casado et al., 2010). Molecules with an SH group and another chemical function (alcohol, carbonyl, ester) are known to generate unpleasant odors and can impart rather unpleasant flavors to foods (Chenot et al., 2019). Due to this downfall, methionine may hold a competitive advantage over cysteine. Efforts to strike a balance between food taste and quality, and ACR inhibition should be made as far as the thiols are concerned. A wide range of reaction conditions, food preparation techniques, and food matrices should be investigated for the optimum blend that permits dual benefits.
3.2 Reaction conditions
Generally, an increase in concentration and temperature increases the rate of a chemical reaction. However, this effect is not consistent for the compounds, particularly allicin and the thiols, respectively. Furthermore, pH significantly influences the inhibition or elimination effects of amino acids, including methionine and cysteine. The effect of pH is considered relevant because of the differing pKa values of the amino acids. To further illustrate, at the pKa value of the amino group, the protonated form, NH3+, is depicted in half the number of molecules present. This renders the amino group non-functional for further participation in the Maillard reaction (Claeys et al., 2005a). Therefore, an amino acid may outperform another at a particular pH, but this may not be the case if the pH changes (Yu et al., 2013). A thorough study of the reaction conditions required for optimum inhibitory performance of these compounds is recommended, especially for the amino acids. Optimum conditions which lead to the full participation of the two nucleophilic centers of methionine and cysteine, would be a useful study towards a better understanding of their inhibitory effects on ACR formation and accumulation (Augustine et al., 2021).
3.3 Matrix effects
The effectivity of inhibition can be impacted by the immediate chemical environment. The water content, as well as the presence and consistencies of other components or additives within the matrix, may affect the mobility of the sulfur inhibitors. Additionally, the rate of diffusion of the reacting species could be affected by a high concentration of polymers (Mustapha et al., 1998). The sulfites displayed inconsistent inhibition effects in the presence of various matrices, while the thiols demonstrated a non-correlative outcome. For other compounds such as allicin and methionine, inadequate studies have been conducted on the matrix effect. Hence, further exploration is necessary to elucidate the effect of various food matrices on the inhibitory effects of these compounds.
4 Conclusion
Although the occurrence of the Maillard reaction is beneficial for food quality, taste, and aroma, the process results in the formation of unavoidable food contaminants, such as ACR. The quest for therapeutic antioxidants and effective anti-browning agents for ACR inhibition is presently ongoing. As a result, sulfur-containing compounds are promising candidates for the reduction of ACR during food processing. The thiols could inhibit ACR by competing with asparagine for α-hydroxycarbonyls, and by the trapping of α-dicarbonyls. The optimum performance of the thiols is influenced by temperature and the heat duration. The sulfites function effectively as the thiols, inhibiting ACR formation through interaction of HSO3− to the carbonyl group of the reducing sugar, thus impeding the progression of the Maillard reaction. ACR formation could be inhibited in the presence of thiosulfinates, like allicin, at low concentrations. A promotion effect on ACR levels was observed with increasing amounts. Furthermore, sulfur-containing compounds such as cysteine and methionine could reduce ACR accumulation through an elimination reaction involving the conjugation of ACR to the nucleophilic centers in a Michael-type addition. The effective use of these compounds as inhibitors will ensure minimal ACR exposure of consumers. Therefore, further research is needed to explore the inhibition effects of these compounds in the presence of various food matrices, as well as under altering conditions of temperature, pH, concentrations, and heating times. Additional insight into the inhibition mechanism of methionine on ACR formation is also necessary for an in-depth understanding of its inhibitory effect.
Author contributions
Author DAA wrote the manuscript which was read and edited by author G-AB. Both authors agreed on the submission of this work for publication.
Funding
The Funding was provided by the School of Graduate Studies and Research and the Department of Chemistry, The University of the West Indies, St. Augustine Campus, Trinidad and Tobago.
Acknowledgments
The authors thank the Department of Chemistry, The University of the West Indies for the permission to produce this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Augustine, D. A., Bent, G-A., and Nelson, P. N. (2021). Mechanistic evidence for the effect of sulphur-based additive: methionine, on acrylamide reduction. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 38 (8), 1324–1331. doi:10.1080/19440049.2021.1925166
Augustine, D. A., and Bent, G-A. (2019). Reducing acrylamide exposure: A review of the application of sulfur-containing compounds - A caribbean outlook. Eur. J. Nutr. Food Saf. 9 (3), 192–209. doi:10.9734/ejnfs/2019/v9i330058
Bayan, L., Koulivand, P. H., and Gorji, A. (2014). Garlic: a review of potential therapeutic effects. Avicenna J. Phytomed. 4 (1), 1–14.
Bittner, S. (2006). When quinones meet amino acids: chemical, physical and biological consequences. Amino Acids 30 (3), 205–224. doi:10.1007/s00726-005-0298-2
Blank, I., Robert, F., Goldmann, T., Pollien, P., Varga, N., Devaud, S., et al. (2005). Mechanisms of acrylamide formation: Maillard-induced transformation of asparagine. Adv. Exp. Med. Biol. 561, 171–189. doi:10.1007/0-387-24980-X_14
Casado, F. J., Antonio, H. S., and Alfredo, M. (2010). Reduction of acrylamide content of ripe olives by selected additives. Food Chem. x. 119 (1), 161–166. doi:10.1016/j.foodchem.2009.06.009
Chenot, C., Robiette, R., and Collin, S. (2019). First evidence of the cysteine and glutathione conjugates of 3-sulfanylpentan-1-ol in hop (Humulus lupulus L.). J. Agric. Food Chem. 67 (14), 4002–4010. doi:10.1021/acs.jafc.9b00225
Claeys, W. L., De Vleeschouwer, K., and Hendrickx, M. E. (2005a). Effect of amino acids on acrylamide formation and elimination kinetics. Biotechnol. Prog. 21 (5), 1525–1530. doi:10.1021/bp050194s
Claeys, W. L., De Vleeschouwer, K., and Hendrickx, M. E. (2005b). Kinetics of acrylamide formation and elimination during heating of an asparagine− sugar model system. J. Agric. Food Chem. 53 (26), 9999–10005. doi:10.1021/jf051197n
Davis, C. D., and Snyderwine, E. G. (1995). Protective effect of N-acetylcysteine against heterocyclic amine-induced cardiotoxicity in cultured myocytes and in rats. Food Chem. Toxicol. 33 (8), 641–651. doi:10.1016/0278-6915(95)00033-x
Deming, T. J. (2017). Functional modification of thioether groups in peptides, polypeptides, and proteins. Bioconjug. Chem. 28 (3), 691–700. doi:10.1021/acs.bioconjchem.6b00696
Friedman, M. (1994). Mechanisms of beneficial effects of sulfur amino acids. Washington: ACS Publications.
Friedman, M., Wehr, C. M., Schade, J. E., and MacGregor, J. T. (1982). Inactivation of aflatoxin B1 mutagenicity by thiols. Food Chem. Toxicol. 20, 887–892. doi:10.1016/s0015-6264(82)80223-x
Gülcan, Ü., Uslu, C. C., Mutlu, C., Arslan-Tontul, S., and Erbaş, M. (2020). Impact of inert and inhibitor baking atmosphere on HMF and acrylamide formation in bread. Food Chem. 332, 127434. doi:10.1016/j.foodchem.2020.127434
Horn, V., Sujata, L., Thedford, A. K., and Baltes, A. M. Y. (2001). “Other dietary components and cardiovascular risk,” in Nutrition in the prevention and treatment of disease. Editors Ann M. Coulston, Cheryl L. Rock, and Elaine R. Monsen (San Diego: Academic Press), 291–302.
Jin, C., Wu, X., and Zhang, Y. (2013). Relationship between antioxidants and acrylamide formation: A review. J. Agric. Food Chem., 611–620. doi:10.1021/acs.jafc.7b00882
Lund, M. N., and Ray, C. A. (2017). Control of Maillard reactions in foods: Strategies and chemical mechanisms. J. Agri. Food. Chem. 65 (23), 4537–4552. doi:10.1021/acs.jafc.7b00882
Maleki, M., and Djazayeri, A. (1968). Effect of baking and amino acid supplementation on the protein quality of Arabic bread. J. Sci. Food Agric. 19 (8), 449–451. doi:10.1002/jsfa.2740190807
Mazumder, M. R., Hongsprabhas, P., and Vasudevan, R. T. (2019). In vitro and in vivo inhibition of maillard reaction products using amino acids, modified proteins, vitamins, and genistein: A review. J. Food Biochem. 43 (12), e13089. doi:10.1111/jfbc.13089
Mustapha, W. A. W., Hill, S. E., Blanshard, J. M. V., and Derbyshire, W. (1998). Maillard reactions: do the properties of liquid matrices matter. Food Chem. x. 62 (4), 441–449. doi:10.1016/s0308-8146(98)00087-9
Ou, S., Shi, J., Huang, C., Zhang, G., Teng, J., Jiang, Y., et al. (2010). Effect of antioxidants on elimination and formation of acrylamide in model reaction systems. J. Hazard. Mat. 182 (1), 863–868. doi:10.1016/j.jhazmat.2010.06.124
Ríos, J-L. (2015). in Apoptotic activities of mediterranean plant species. Editors Victor R. Preedy, and Ronald Ross Watson (San Diego: Academic Press), 611–620.
Van Boekel, M. A. J. S. (1998). Effect of heating on Maillard reactions in milk. Food Chem. x. 62 (4), 403–414. doi:10.1016/S0308-8146(98)00075-2
Wong, C. W., Wijayanti, H. B., and Bhandari, B. R. (2015). Maillard reaction in limited moisture and low water activity environment, 41-63. New York, NY: Springer.
Yu, M., Ou, S., Liumengzi, D., Huang, C., and Zhang, G. (2013). Effect of ten amino acids on elimination of acrylamide in a model reaction system. Afr. J. Food Sci. 7 (9), 329–333. doi:10.5897/ajfs2013.1031
Yuan, Y., Shu, C., Zhou, B., Qi, X. L., and Xiang, J. G. (2011). Impact of selected additives on acrylamide formation in asparagine/sugar Maillard model systems. Food Res. Int. 44, 449–455. doi:10.1016/j.foodres.2010.09.025
Zamani, E., Shokrzadeh, M., Fallah, M., and Shaki, F. (2017). A review of acrylamide toxicity and its mechanism. Mazums-pbr. 3 (1), 1–7. doi:10.18869/acadpub.pbr.3.1.1
Zeng, X., Ka-Wing, C., Yue, J., Zhi-Xiu, L., Jian-Jun, S., Shi-Yi, O., et al. (2009). Inhibition of acrylamide formation by vitamins in model reactions and fried potato strips. Food Chem. x. 116 (1), 34–39. doi:10.1016/j.foodchem.2009.01.093
Zhu, Y., Luo, Y., Sun, G., Wang, P., Hu, X., and Chen, F. (2020). Inhibition of acrylamide by glutathione in asparagine/glucose model systems and cookies. Food Chem. 329, 127171. doi:10.1016/j.foodchem.2020.127171
Keywords: acrylamide, glutathione, thiols, maillard reaction, inhibition, allicin, sulfur compunds
Citation: Augustine DA and Bent G-A (2022) Acrylamide, a toxic maillard by-product and its inhibition by sulfur-containing compounds: A mini review. Front. Food. Sci. Technol. 2:1072675. doi: 10.3389/frfst.2022.1072675
Received: 17 October 2022; Accepted: 17 November 2022;
Published: 01 December 2022.
Edited by:
Majid Nooshkam, Ferdowsi University of Mashhad, IranReviewed by:
Zahra Zareie, Gorgan University of Agricultural Sciences and Natural Resources, IranMoein Bashash, Ferdowsi University of Mashhad, Iran
Copyright © 2022 Augustine and Bent. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Grace-Anne Bent, R3JhY2UtQW5uZS5CZW50QHN0YS51d2kuZWR1
 Dahryn Andilla Augustine
Dahryn Andilla Augustine Grace-Anne Bent
Grace-Anne Bent