- 1The Food Processing Center, University of Nebraska-Lincoln, Lincoln, NE, United States
- 2Department of Biological Systems Engineering, University of Nebraska-Lincoln, Lincoln, NE, United States
- 3Department of Food Science, University of Arkansas System Division of Agriculture, Fayetteville, AR, United States
- 4Department of Food Science and Technology, University of Nebraska-Lincoln, Lincoln, NE, United States
Salmonella is the most common bacterial pathogen associated with product recalls and outbreaks in spices. Spices are in the top three food categories for greatest number of recalls due to microbiological contamination. Current validated microbial reduction techniques for spices are tied to human health and environment concerns or negatively affect the quality characteristics of the spice which has led to the emerging of alternative technologies such as hydrogen peroxide vapor (HPV). hydrogen peroxide vapor treatment was conducted at two different temperatures (45°C and 60°C) and two dwell times (30 and 60 min). Microbial reduction and residual hydrogen peroxide were measured at three storage times: 0 h (immediately after treatment), 24 and 48 h post-treatment. The effect of HPV on the quality of whole black peppercorn was evaluated 48 h post-treatment based on changes in piperine content, total phenolics, antioxidant activity, total volatile compounds, and color. Reduction in Salmonella population ranged from 1.41–2.83 log CFU/g. Residual hydrogen peroxide of up to 500 ppm was still detected on samples after 48 h of storage. All quality parameters except for color remained unaffected between treated and untreated whole black peppercorn. The study highlights the need to explore further process design modifications before conclusions can be made on the efficacy of HPV treatment as a means for low-moisture foods pasteurization.
1 Introduction
Low-moisture foods (LMF) such as cereals, nuts, and spices are foods with a low water activity (aw < 0.85) (FAO/WHO, 2014). Although LMF do not support the growth of pathogens, foodborne illness outbreaks associated with consumption of contaminated LMF have been recurrent in recent years. The main cause of foodborne outbreaks related to LMF are pathogenic bacteria such as Salmonella spp. that are able to survive drying processes and persist longer in LMF (Beuchat et al., 2013; Keller et al., 2013). Black pepper is consumed all over the world and is a well-known spice for seasoning foods. Unfortunately, black pepper is also known for high microbiological contamination and has been implicated in Salmonella outbreaks such as the 2010 nationwide Salmonella Montevideo outbreak linked to salami contaminated by black pepper that affected 272 individuals, and the 2008 multistate outbreak linked to consumption of white pepper contaminated with Salmonella Rissen that resulted in 85 confirmed cases (CDC, 2010; ASTA, 2017).
Raised awareness of LMF as a vector for foodborne illness has led to an increased research emphasis on LMF and novel technologies to control foodborne pathogens in these foods. Additionally, because of the Food Safety Modernization Act (FSMA) of 2011, food facilities are now 2-g required to implement validated preventative controls (FDA, 2015). There are no published parameters for target log reduction of bacteria for spices (ASTA, 2017) but the FDA considers a 5-log reduction satisfactory for some foods such as almonds (Danyluk et al., 2006). Current validated and commercially available microbial reduction techniques for spices include ethylene oxide (EtO), propylene oxide (PPO), irradiation, and steam. EtO and PPO are tied to environmental and health concerns and irradiation carries with it a negative stigma for many consumers. Steam tends to be the commonly used commercial pasteurization method for black peppercorn. However, thermal treatments such as steam may cause deterioration of quality characteristics such as aroma, flavor, and color (Tainter and Grenis, 2001). Steam treated black pepper suffered significant changes in color and reduction in piperine concentration (Waje et al., 2008). Therefore, there is a need for a potential non-thermal technology that is effective in microbial inactivation without compromising on the quality of black pepper.
Gaseous technologies have gained interest due to their non-thermal nature and high penetration and diffusion of the antimicrobial components in the food. Hydrogen peroxide vapor (HPV), is advantageous over other gaseous technologies for its low toxicity, spontaneous breakdown into harmless by-products (water and oxygen), broad-spectrum efficacy, and antimicrobial activity at low temperatures (McDonnell and Russell, 1999; Linley et al., 2012). HPV inactivates bacteria by forming free hydroxyl radicals (•OH) which attack essential membrane lipids, DNA, and other cell components (McDonnell and Russell, 1999; Block, 2001). HPV is highly successful for surface decontamination and has been applied in laboratory and medical equipment, hospital wards and pharmaceutical manufacturing facilities (Linley et al., 2012). However, to date, information regarding the effectiveness of HPV for controlling foodborne pathogens on food materials and especially LMF is limited. Data gaps exist around the understanding of how factors like HPV treatment temperature and dwell time affect the outcome specific to food materials. There is also a lack of studies documenting effects of HPV on quality attributes of the food material.
Fluidization is defined as occurring when solid particles are supported and allowed to move relative to each other as a result of vertical motion of a fluid (typically a gas or liquid) in a defined and contained volume (Grace et al., 2020). Fluidization has improved other unit operations like drying, making them more effective and time efficient. The objective of this study is to explore the use of fluidized HPV to inactivate Salmonella in black peppercorn and to determine effects on the quality attributes of black peppercorn.
2 Materials and methods
2.1 Whole black peppercorn
Three lots of commercially steam sterilized whole black peppercorns were obtained from McCormick & Company, Inc. (Hunt Valley, MD) and stored refrigerated at 4°C until use. Background microbiota population was quantified before conducting experiments. Four random 10-g samples from each lot were diluted in 90 mL of 0.1% buffered peptone water (BPW; Becton, Dickinson and Company, Sparks, MD), plated on non-selective tryptic soy agar supplemented with 0.6% (w/w) yeast extract (TSAYE; Difco, Sparks, MD), and incubated at 37°C for 24 ± 2 h.
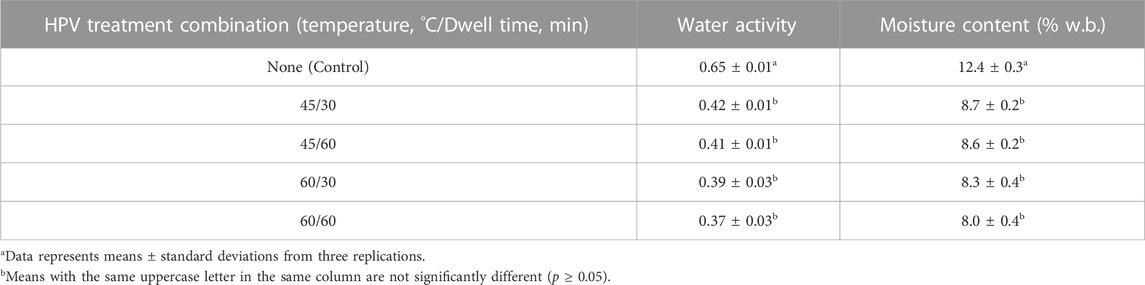
Water activity (aw) of whole black peppercorn was measured at 25°C before and after HPV treatment. Approximately 3 g of sample was placed in a disposable sample container and loaded into the test chamber of a water activity meter (Series 4 TE, Meter Group, Pullman, WA, United States). To relate the aw of the sample after treatment to the corresponding equilibrium moisture content, adsorption and desorption isotherms at 25°C were generated using a vapor sorption analyzer (VSA, Meter Group, Pullman, WA) for each black peppercorn lot. Initial moisture content used to generate the curve was measured using a halogen moisture analyzer (HR73, Mettler Toledo Laboratory and Weighing Technologies, Greifensee, Switzerland) set to 105°C. Sample was prepared by grinding whole black peppercorn in a spice grinder (WSG60, Conair Corporation, CT, United States) and passed through U.S. No. 20 sieve (0.841 mm sieve opening) to achieve a consistent particle size for moisture analysis. The final moisture content of the treated sample was estimated graphically based on the average moisture desorption isotherm (Supplementary Figure S1), using the measured water activity reading.
2.2 Salmonella inoculation of whole black peppercorn
A cocktail of five different strains of Salmonella enterica previously involved in low moisture food outbreaks and recalls was used in this study. Salmonella Agona 447,967—toasted oats (CDC, 1998), Salmonella Montevideo 488275—salami (CDC, 2010), Salmonella Mbandaka 698538—sesame paste (CDC, 2013), Salmonella Tennessee K4643—peanut butter (CDC, 2007), and Salmonella Reading Moff 180418—alfalfa sprouts (CDC, 2016). Salmonella Agona, Salmonella Montevideo, and Salmonella Mbandaka were obtained from the United States Food and Drug Administration Arkansas Regional Laboratory (Jefferson, AR). Salmonella Tennessee was obtained from Dr. Larry Beuchat at the University of Georgia, and Salmonella Reading Moff was obtained from the FDA Culture Collection (Bedford Park, IL). All the bacteria were kept in a stock culture solution of 40% sterile glycerol and stored at −80°C until use.
Inoculation preparation was conducted following the Verma et al. (2021) method. A vial of each Salmonella strain frozen stock (1 mL) was thawed at room temperature for 5 min then added to 10-mL tryptic soy supplemented with 0.6% (w/w) yeast extract (TSBYE; Difco, Sparks, MD) and incubated at 37°C for 24 ± 2 h. A loopful of the incubated culture was then streaked onto TSAYE plates and incubated at 37°C for 24 ± 2 h to prepare the working plates. Working plates were sealed with parafilm and stored at 4°C for up to 1 month. A single colony from each working plate was transferred to 10-mL TSBYE and incubated at 37°C for 24 ± 2 h. A bacterial lawn was produced by spreading 0.1 mL of the overnight culture onto a TSAYE plate that was incubated at 37°C for 24 ± 2 h. The bacterial lawn was then harvested by adding 3 mL of BPW to the plate and agitating into a suspension with a sterile L-shaped spreader. The Salmonella cocktail consisted of equal amount of each Salmonella strain pipetted into a sterile 15-mL centrifuge tube and vortexed for 30 s to achieve uniform distribution of cells. One milliliter of cocktail per 50 g of sample was used to inoculate samples immediately after preparation.
Whole black peppercorn previously tempered at room temperature was aseptically transferred to a Whirl-Pak bag and sprayed with the prepared inoculum inside a biosafety cabinet. The bag was sealed, hand massaged, and then shaken for 10 min. The inoculated samples were aseptically transferred to a custom-designed humidity-controlled equilibration chamber (Lau and Subbiah, 2020) to re-equilibrate the sample to its original water activity of 0.65 ± 0.01.
2.3 Homogeneity and stability of salmonella inoculated in black peppercorn
To ensure that the bacteria was homogeneously distributed within the sample and physiologically adapted to the low water activity environment, the homogeneity and stability of Salmonella was evaluated over 15 days. Two-g samples from five random locations were removed from the humidity chamber on day 0 and three random 2-g samples were removed from the chamber on days 1, 3, 6, 9, 12, and 15. Each sample was diluted with 18 mL of BPW, serially diluted, and spread plated in duplicate onto differential media, m-TSAYE (TSAYE supplemented with 0.03 (w/v) sodium thiosulfate (Fisher Scientific, Fair Lawn, NJ) and 0.05% (w/v) ammonium iron citrate (Sigma Alrich, St. Louis, MO). Black colonies observed on m-TSAYE were considered presumptive Salmonella. The homogeneity and stability tests were repeated for all three lots of black peppercorn. Each lot was inoculated using a fresh frozen stock to produce three independent biological replicates.
2.4 HPV inactivation treatment
The treatment chamber to fluidize the black peppercorn was custom built at the University of Nebraska-Lincoln and can be seen in (Figure 1A). Polyvinyl chloride pipes (3.8 cm diameter × 24.1 cm length) were used, and the external size of the overall device was 48.3 cm by 25.4 cm. A fine mesh screen (304 stainless steel wire cloth, 80 × 80 mesh size, 0.018 cm opening size) was placed in two locations allowing the sample to stay within the treatment chamber but HPV to pass through. HPV was generated using a HPV generator (L-2, Bioquell Pharma, Andover, United Kingdom), connected to the treatment chamber, and operating in a closed-loop system (Figure 1B). Aqueous solutions of 30% hydrogen peroxide (Fisher Scientific, Fair Lawn, NJ) were used to generate HPV. To measure and record temperature, humidity, HP concentration and relative saturation the treatment chamber was instrumented with a series of probes. Temperature and humidity at the outlet of the treatment chamber (Figure 1B) were measured and recorded with data logger (GSP-6, Elitech, San Jose, CA). Hydrogen peroxide concentration (ppm), humidity, temperature and relative saturation at the inlet of the chamber (Figure 1A) were recorded and measured with the PEROXCAP® probe (HPP272, Vaisala Inc, Louisville, CO). Hydrogen peroxide concentration in the chamber was recorded using the probe provided by the manufacturer of the HPV generator (Figure 1A).
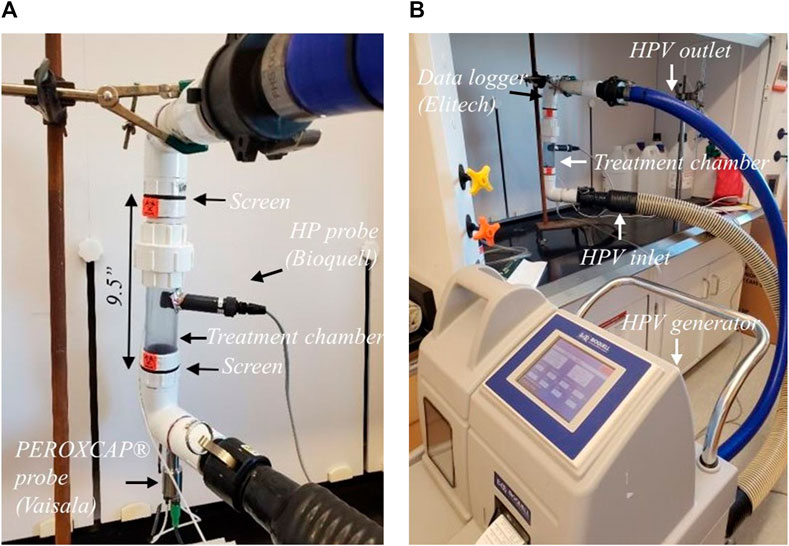
FIGURE 1. Experimental apparatus used in this study, (A) treatment chamber, (B) HPV generator connected to the treatment chamber in a closed-loop system.
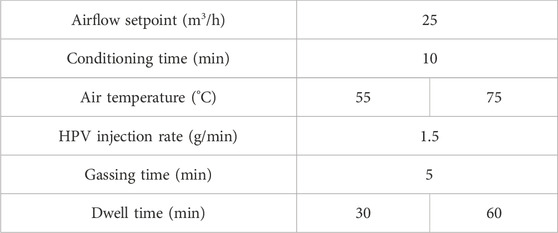
The HPV treatment process consisted of four stages: conditioning, gassing, dwell, and aeration. HPV treatment of inoculated whole black peppercorn was carried out with manipulation of the independent variables summarized in Table 1. These parameters were carefully selected based on factors influencing HPV decontamination and preliminary tests. To achieve fluidization of the black peppercorn within the treatment chamber, the terminal velocity of black peppercorns was first determined using a squirrel cage fan with attached plenum and anemometer. Based on the terminal velocity and the chamber area perpendicular to flow, an airflow rate of 25 m3/h was calculated for the airflow setpoint of air/HPV entering the chamber. Lowering the initial RH increases the amount of HPV concentration that can be generated in the decontamination chamber (Vaisala, 2021). Conditioning time of 10 min was sufficient to reduce the initial RH within the treatment chamber to <20%. Based on thermal inactivation kinetics of Salmonella in black pepper (Wei et al., 2021a), treatment temperatures of 45°C and 60°C were chosen to represent mild treatment temperatures while staying within the constraints of the equipment. Air temperature from the HPV generator was set at 55°C and 75°C to obtain treatment temperatures of 45°C and 60°C, respectively. The temperature decrease is due to heat loss in the delivery hose (Watling et al., 2002). The combination of gassing rate, gassing time, and dwell time dictated the inactivation results of the preliminary runs. HPV injection was selected at 1.5 g/min, the lowest available setting on the equipment, to minimize discoloring on the food material. A total of 7.5 g of H2O2 solution was injected per treatment. The dwell phase was done with no further HPV injection. Aeration was carried out at 40°C until the concentration of HPV dropped to a safe level of <1 ppm, as set by the Occupational Safety and Health Administration (OSHA, 2020).
Ten grams of inoculated whole black peppercorn was used per treatment. Each treatment was replicated three times; each replicate used a different production lot of whole black peppercorn. This resulted in three biological replicates per treatment scenario. Changes in Salmonella population and hydrogen peroxide residue were studied at storage times of 0, 24, and 48 h after treatment. Quality attributes were analyzed 48 h after treatment for a conservative estimate of quality loss. HPV treated samples were stored in sterile Whirl-Pak bags and held at controlled ambient conditions.
A Bioquell 6-log G. Stearothermophilus biological indicator (BI; Bioquell Pharma, Andover, United Kingdom), was included in one of the runs as a check-point for HPV treatment efficacy. The BI was placed in the treatment chamber, just below the top mesh screen, for the duration of the HPV treatment process. Once the cycle was complete, the BI was removed and promptly cultured. The carrier disc was placed in TSBYE and incubated for 7 days at 57.5°C (±4.5°C). A positive and negative control were also conducted. Test tubes were checked daily over the incubation period for evidence of growth.
2.5 Microbial enumeration
A sub-sample of the HPV treated sample (2 g out of 10 g) was diluted with 18 mL of BPW and homogenized for 1 min in a stomacher. The dispersion was serially diluted using 9-mL BPW tubes and spread plated in duplicate onto m-TSAYE for enumerating Salmonella. All plates were incubated at 37°C for 24 ± 2 h after which colonies were counted. For each treatment, an untreated inoculated sample (2 g), the control, was enumerated the same way to determine the initial population count before treatment. Log reduction was calculated by subtracting the log count after treatment from the log of the initial population in the control.
2.6 Residual hydrogen peroxide
Residual hydrogen peroxide was determined using the Back et al. (2014) method. Briefly, hydrogen peroxide test strips (Bartovation, NY) were used in the dilutant of the first microbial dilution that was prepared for microbial enumeration. The test strip was immersed for 1 s and read after 30 s. Three different varieties of test strips were screened: low-level (0–50 ppm), mid-level (0–400 ppm), and high-level (0-10,000 ppm). The mid-level strips were determined to be the best fit.
2.7 Quality analysis
Quality analysis of the treated whole black peppercorn was evaluated by treating uninoculated whole black peppercorn at both low and high temperature for the long dwell time of 60 min. This was done to study the effect of temperature on the quality attributes at the most severe treatment condition tested.
For the determination of piperine, total phenolics, and antioxidant activity, the sample was prepared as outlined in the Official Analytical Methods of the American Spice Trade Association (ASTA, 1985). Briefly, whole black peppercorn was ground using a spice grinder (WSG60, Conair Corporation, CT, United States) and passed through U.S. No. 20 sieve (0.841 mm sieve opening) to achieve a consistent particle size for quality analysis. 0.5 g of ground sample was mixed with 100 mL of ethanol (Decon Labs, Inc., 200 proof) and magnetically stirred overnight. The solution was filtered, and the ethanol extract was stored at 4°C under dark condition until analysis.
2.7.1 Piperine content
Piperine concentration was determined with a high-performance liquid chromatograph (1,260, Agilent Technologies, Santa Clara, CA). The column was an Agilent Poroshell 120 EC-C18 4.6 × 100 mm, 2.7 micron, with a matching 4.6 mm × 5 mm guard column. Isocratic elution was performed with 50% methanol and 50% water at 1 mL/min. Injection volume was 10 μL and the diode array detector was set to record signal at 341 nm. Standard piperine (98% purity, Alfa Aesar, Tewksbury, MA) solutions were prepared in ethanol in a range of 25–400 μg/mL to generate the standard curve (Supplementary Figure S2).
2.7.2 Total phenolics
The total phenolic content was determined using the Folin-Ciocalteu assay (Singleton et al., 1965). An aliquot (0.2 mL) of the sample extract and standard solutions of gallic acid (2, 4, 6, and 8 μg/mL) were used, with ethanol being used as blank. Folin-Ciocalteu phenol reagent (2 mL) was added to each sample tube and vortexed for 5 s. After 10 min under dark conditions at room temperature, 2 mL of Na2CO3 was added to the mixture and vortexed for 5 s. Distilled water was added to bring the total volume up to 5 mL. The samples were incubated for 2 h at room temperature under dark conditions. Absorbance against the prepared blank was measured at 765 nm with a spectrophotometer (UV-1800, Shimadzu Corp., Kyoto, Japan). A standard curve (Supplementary Figure S3) was prepared by plotting the absorbance against concentration of gallic acid solutions and the total phenolic content was reported as milligrams of gallic acid equivalents (GAE) per Gram of black pepper.
2.7.3 Antioxidant activity
Antioxidant activity is commonly determined by the 1,1-Diphenyl-2-picryl-hydrazyl (DPPH) radical scavenging assay; this method was first reported by Blois. (1958). A solution of DPPH (40 μg/mL) was prepared, and 2 mL was mixed with 0, 0.2, 0.4, 0.6, and 0.8 mL of black peppercorn ethanol extract in test tubes, and ethanol was added to make up a 5 mL solution in all the tubes. The mixtures were vortexed for 5 s and incubated at room temperature under dark conditions for 30 min. DPPH is a stable free radical showing a maximum absorbance at 517 nm. When DPPH reacts with an antioxidant compound, the radicals are scavenged and absorbance is reduced (Brand-Williams et al., 1995). This reduction was read at 517 nm with a spectrophotometer (UV-1800, Shimadzu Corp., Kyoto, Japan). Absorbance values fell within 0.221–0.698 which is said to be the correct range of accuracy for this test (Sharma and Bhat, 2009). The scavenging activity of the DPPH radical in each sample was calculated according to the following equation:
Acontrol is the absorbance of the control containing 0 mL extract and Asample are the absorbances of solutions containing black peppercorn ethanol extract.
2.7.4 Volatile compounds
Terpene extraction was performed using solid phase microextraction (SPME). The fiber used was 85 μm, CAR/PDMS, Stableflex, 24 Ga, Manual Supelco (Bellefonte, PA). A 20-mL headspace vial containing 10-mg ground black peppercorn and 1-mL ultrapure water was placed in a heat block on a stir plate with heating capability at 65°C and equilibrated for 20 min. The SPME fiber was inserted into the headspace above the sample and adsorbed for 30 min. Samples were desorbed into the injection at 250°C for 3 min.
Gas chromatography analysis was performed using a Gas Chromatograph equipped with a Flame Ionization Detector (GC-FID) (GC-2010 Plus, Shimadzu Corp., Kyoto, Japan) and a Mass Spectrometer (GC-MS) (GCMS-QP2010 SE, Shimadzu Corp., Kyoto, Japan). Samples were analyzed by both GC-FID and GC-MS and separation was performed on each using a HP-5 (30 m × 0.25 mm inner diameter, 5% phenyl-methylpolysiloxane, 1.0 µm film thickness) capillary column (Agilent, Santa Clara, CA). For both GC-MS and GC-FID analysis, the injector temperature was 250°C. Helium was used as the carrier gas and column flow rate was 1.0 mL/min. The oven temperature was programmed for 45°C–180°C at 6°C/min, then from 180°C to 250°C at 3°C/min, then from 250°C to 280°C with a 5 min hold at 280°C. The GC-FID detector temperature was 300°C and the interface temperature for the GC-MS had an ion source temperature of 230°C and an interface temperature of 250°C. GC-MS was performed in full scan mode, with a scan range of 20–300 m/z. The volatiles were identified by comparison of their mass spectra with the National Institute of Standards and Technology NIST17 spectral library, literature data, and retention indices. The retention indices were performed after running alkane standards of 5–20 carbons and online searches of similar work with HP5 or comparable columns. Calibration curves were performed for several standards, and compounds concentrations were calculated from the linear regression lines from authentic standards or quantified as equivalents of related compounds where standard was not available.
2.7.5 Color
The color of untreated and treated whole black peppercorn was measured using a colorimeter (BC-10, Minolta Co. Ltd., Osaka, Japan). The instrument was calibrated before the start of each measurement using a white calibration tile. The sample was placed in a black plastic container in lieu of a Petri dish and measurements were taken at five random locations. Color values were recorded as average L* (+: lighter; −: darker), a* (+: redder; −: greener), and b* (+: yellower; −: bluer). The total color difference (∆𝐸) value (Robertson, 1977) was calculated and used to assess the effect of the hydrogen peroxide vapor on the color of black peppercorn. The formula is as follows:
2.8 Statistical analysis
Analysis of variance (ANOVA) for the response variable (log reduction) was conducted with the SAS 9.4 software (SAS Institute., Cary, NC). Each of the different combinations of the two factors (temperature and dwell time) for multiple time points (storage times) were considered as separate treatments in the analysis. There were three replicates for each treatment. A repeated measures structure was added to the ANOVA to account for the correlation between the multiple time points. Tukey’s multiple range test was conducted in cases showing a significant difference (α = 0.05). Water activity, moisture content, and quality results were also analyzed using ANOVA with Tukey’s adjustment for the differences between before and after HPV treatment.
3 Results and discussion
3.1 Whole black peppercorn
The background microbiota population was measured as < 3 log CFU/g for all three lots, suggesting background microbiota did not interfere with pathogen recovery using differential media. Initial water activity and moisture content of black peppercorn were 0.65% ± 0.01% and 12.4% ± 0.3% wet basis (w.b.), respectively. This is slightly higher than reported aw values of 0.58–0.62 (Duncan et al., 2017; Bang et al., 2020; Wei et al., 2021b). This difference could be attributed to the product gaining moisture from the environment due to prolonged storage in a walk-in cooler (4°C) when packed in bag-in-box that might not offer sufficient moisture barrier.
3.2 Homogeneity and stability of Salmonella inoculated in black peppercorn
Results from the homogeneity and stability test as seen in (Figure 2) shows counts of Salmonella inoculated in three independent lots of whole black pepper and equilibrated at 65% RH at 25°C for 15 days. The initial (Day 0) population count of Salmonella in whole black peppercorn was 7.85 ± 0.07 log CFU/g. A small standard deviation among the five subsamples from each lot of black pepper at Day 0 indicates a homogeneous distribution of inoculum throughout the sample. Salmonella population declined approximately 0.5 log over the first 3 days before stabilizing over the course of the following 12 days (∼0.6 log reduction from Day 3 to Day 15). This trend shows a good adaptive ability of Salmonella to the low aw environment.
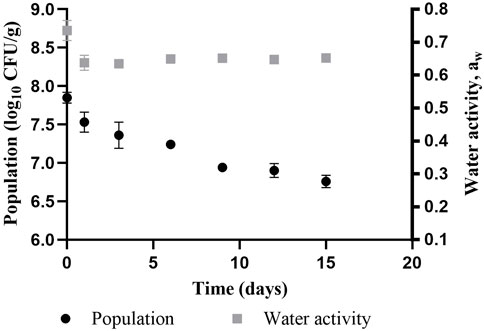
FIGURE 2. Variation in population of Salmonella cocktail inoculated in whole black peppercorn and its aw during equilibration in a controlled humidity chamber at 65% RH.
The water activity of the product was also monitored during the stability test (Figure 2). The graph shows a quick drop of aw within a day which stabilized after Day 3. The aw at the time of treatment controls the thermal inactivation of Salmonella (Smith and Marks, 2015). Therefore, it is necessary to equilibrate samples prior to microbial reduction treatments. Wet inoculation of LMF create a moisture gradient which forces the water out of the bacterial cells until a state of vapor pressure equilibrium is achieved (Dhaliwal et al., 2021). Conducting a microbial challenge study immediately after inoculation therefore is not representative of Salmonella stability in LMF and may result in higher bacterial reduction. Allowing the Salmonella cells to stabilize in the desiccated environment may offer cross-protection to osmotic stress and high temperature (Nadia et al., 2011), contributing to higher thermal resistance and a more conservative approach to microbial reduction. In this study, inactivation treatments were conducted after inoculated samples had spent between 8 and 10 days equilibrating at 25°C and 65% RH to achieve Salmonella adaptation to the environment without reaching population decline.
3.3 HPV inactivation treatment
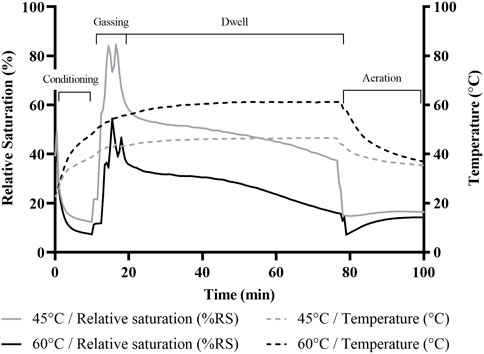
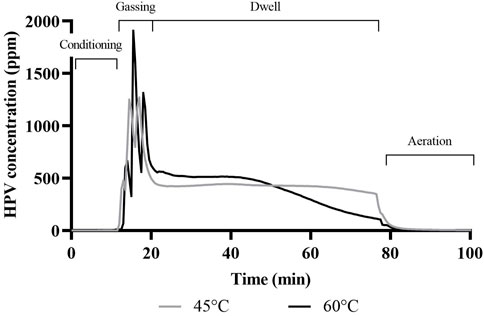
HPV treatment was carried out at 45°C and 60°C. The outcome of the different treatment temperatures on relative saturation (RS) and HPV concentration for the 60 min dwell time runs, represented as means of treatment replicates, is depicted in (Figures 3, 4), respectively. The RS value represents the saturation level of the air mixture within the chamber, reflecting both the water vapor as well as HPV in the air. It can be seen from (Figure 3) that RS never reached 100% which would have been the dew point and condensation would have appeared. However, the lower treatment temperature of 45°C resulted in a RS of almost 90% which did result in intermittent fogging of the treatment chamber during the gassing phase. Treatment temperature of 60°C resulted in lower RS and no fogging of the treatment chamber was observed during these runs. Unlike the inverse relationship between temperature and RS, HPV concentration increased with higher treatment temperature (Figure 4), resulting in a peak HPV concentration of almost 2,000 ppm for the 60°C treatment and 1,400 ppm for the 45°C treatment. Typical bio-decontamination runs range from 140–1,400 ppm (Vaisala, 2021) but the high peak HPV concentration attained in this study was likely due to the small chamber size. HPV treatment runs with shorter dwell time of 30 min (data not shown) followed the same trends with aeration commencing 30 min into dwell time resulting in an overall shorter treatment cycle.
The average log reduction of Salmonella for each treatment combination over storage time is illustrated in (Figure 5). Results range from 1.41–2.83 log CFU/g reduction, with the lowest reduction for samples treated at 45°C with 30 min of dwell time and the highest reduction in samples treated at 60°C with 60 min of dwell time after 48 h of storage at room temperature. Samples with longer dwell time (60 min) had higher log reductions of Salmonella for both treatment temperature. On the other hand, higher treatment temperature (60°C) increased log reduction at similar dwell times. For all treatments, variability among replicates, represented by the error bars in (Figure 5), range from 0.29–0.9 log CFU/g (σmin—σmax) with a high corresponding coefficient of variance (10.2%–41.5% CV). Furthermore, statistical analysis showed no significant influence of temperature, dwell time or their interactions (p-values >0.05) on the inactivation of Salmonella. Storage time was the only main effect of significance (p-value <.0001) and there is a significant difference in the reduction of Salmonella population between storage times of 24 and 48 h and between 0 and 48 h for all treatments except for samples treated at 60°C with 30 min of dwell time. No treatment combination resulted in a significant difference between storage times of 0 and 24 h.
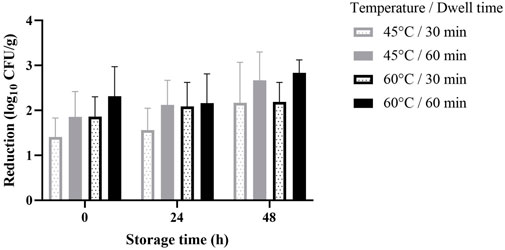
FIGURE 5. Inactivation of Salmonella cocktail with HPV at different combinations of temperature and dwell time over storage time.
The lack of statistically significant differences in Salmonella inactivation rates for the different treatment combinations seem to indicate that the lower treatment temperature/lower HPV concentration was compensated by a higher humidity environment. It is reported that the molecular deposition of water and hydrogen peroxide on the target surface represents the determining factor for microbial inactivation while HPV concentration is of secondary importance (Unger-Bimczok et al., 2008). However, the fogging of the treatment chamber at treatment temperature of 45°C was only intermittent during gassing, likely due to the constant high airflow rate that kept moving the moisture out of the chamber. While fluidizing the black peppercorn with HPV was done with the intention of increasing contact between the food material and HPV, the increased air flow rate to fluidize the black peppercorn likely resulted in the HPV travelling at a fast velocity through the system resulting in insufficient contact time with the black peppercorn. A discussion with Bioquell representatives revealed that a contact time of 2–4 min is necessary for successful inactivation (D. Root, personal communication, 21 Dec 2021). The process of hydrogen peroxide condensate deposition on a surface, or micro condensation, is said to be based on a series of successive steps starting with the adsorption of gas molecules, followed by the formation and growth of a thin film, and finally the development of droplets (Unger-Bimczok et al., 2008).
This study resulted in a ≤3 log reduction of Salmonella in black peppercorn using fluidized HPV treatment. The level of reduction is similar to results reported for Escherichia coli on apples (<2 log), Salmonella, E. coli, and Listeria on lettuce (≤3 log), and Salmonella on red pepper (<2.5 log) using HPV (Sapers et al., 2003; Back et al., 2014; Song and Kang, 2021). In contrast, a recent study with vacuumed HPV treatment resulted in a 4.52 log CFU/g reduction of S. Typhimurium in whole black peppercorn without significant color change (Song and Kang, 2021). In this study, 0.5 mL of 50% aqueous hydrogen peroxide was used to generate HPV and inactivate Salmonella inoculated in whole black pepper exposing it to the gas at 55°C for 1 min. This drastic difference in inactivation results likely stem from the vacuum (<200 Pa) environment as this study was conducted under atmospheric pressure conditions. The concept of vacuum assisted HPV treatment is not a new one and dates back to a patent issued in 1977 for cold gas sterilization where articles to be sterilized are placed in an enclosure and vacuum is drawn to vaporize the H2O2 solution (Forstrom and Wardle, 1977). Vacuum conditions have been proven to result in greater gas penetration (Fichet et al., 2007) and is reported to accelerate HPV removal (Block, 1991); this could be beneficial for complex device (or material) decontamination/sterilization (McDonnell et al., 2002) or food surfaces with crevices or cracks (Song and Kang, 2022). However, it is important to note that no initial aw values of the black peppercorn were reported, and their methods indicate that inoculated samples were dried for 1 h in a biosafety hood with no mention of the stability of the Salmonella population. Finally, heat resistance varies widely at the strain level, and differences in inactivation results between both studies could also stem from the difference in Salmonella strains.
Salmonella inactivation on whole black peppercorn was also studied with radio frequency (Wei et al., 2018) and gaseous chlorine dioxide (Wei et al., 2021c) and these studies had better outcomes for microbial log reductions. The methodology for Salmonella inoculation and stabilization that was utilized in this study is identical to the work that was done with radio frequency and chlorine dioxide. It is possible that low log reductions observed in this study are attributed to the HPV treatment and interactions between HPV and Salmonella and/or interactions between HPV and the black peppercorn.
Microorganisms that use oxygen, including Salmonella, have intrinsic defense systems that can confer tolerance to H2O2 stress (McDonnell and Russell, 1999). The oxidative stress or SOS response against H2O2 cytotoxicity is done either by scavenging the oxidants with neutralizing enzymes such as catalase and glutathione or repairing DNA after it has taken place (Juven and Pierson, 1996). A study showed that H2O2 treatment induces the synthesis of 30 proteins in S. Typhimurium; five of these were heat shock proteins and due to cross-protective effects, Salmonella exposed to H2O2 exhibit increased thermal resistance (Christman et al., 1985). This may have contributed to the minimal differences in log reduction outcome between the low and high temperature treatments. Additionally, exposing Salmonella to nonlethal concentrations, or exposing the pathogen to increasing concentrations of H2O2 allows adaptation as well as resistance to the oxidant (Christman et al., 1985).
Combination technologies with HPV have been reported in recent years and these seem to achieve higher microbial inactivation. Gaseous ozone coupled with HPV reduced populations of S. Typhimurium by up to 5.2 log CFU/fruit on smooth surface of tomatoes and 4.2 log CFU/fruit on the stem scar (Fan et al., 2020). HPV treatment alone without the ozone resulted in reductions of 2.1 log CFU/fruit and 0.8 log CFU/fruit on the smooth surface and stem scar, respectively. Cold plasma activated HPV significantly improved the efficacy of HPV on fresh produce (Song and Fan, 2020). Highest reductions of S. Typhimurium were seen on smooth surface produce: tomato surface (5.28 log) and Granny Smith apple (5.50 log). Lower reductions were seen on rough surface produce: cantaloupe rind (2.61 log) and tomato-stem scar (2.35 log). These studies point to surface properties influencing the effectiveness of HPV treatment. Black peppercorn having a wrinkled rough exterior may have introduced an added barrier for microbial lethality of HPV.
Focusing in on surface properties, HPV treatment outcomes appear to differ on inert vs. biological surfaces, making it challenging to infer any successful parameters from contact surface studies. HPV is commonly used for surface decontamination and has excellent material compatibility with metals, plastics, elastomers, and typical environmental surfaces (Meszaros et al., 2005; Harris and Zanko, 2010). Verification and validation of decontamination cycles are generally done with spores of B. Stearothermophilus, reported as being the most resistant organism to HPV (Klapes and Vesley, 1990; Block, 1991; McDonnell and Russell, 1999; Harris and Zanko, 2010). The spores are inoculated onto stainless steel coupons and used as a biological indicator for the efficacy of HPV treatments. A Bioquell 6-log biological indicator was included in the high temperature quality run and our result showed a successful 6-log kill of the endospore, as indicated by a clear broth after 7 days at 57.5°C (Supplementary Figure S5). This result is contradictory to the fact that spores are more resistant to HPV than Gram-negative bacteria like Salmonella, but the spores were inoculated on a stainless steel coupon. A study examining the use of HPV for decontamination of food surfaces achieved >6 log reduction of Salmonella choleraesuis, Listeria monocytogenes, E. coli 0157:H7 and other foodborne pathogens (McDonnell et al., 2002). However, the test organisms were inoculated onto stainless steel coupons and then exposed to HPV (10- and 20-min contact time, 25°C) in an enclosure. A systematic investigation of Salmonella inactivation on inert vs. biological surfaces using HPV is necessary to draw conclusions on HPV treatment outcomes based on surface properties.
3.4 Residual hydrogen peroxide after HPV treatment
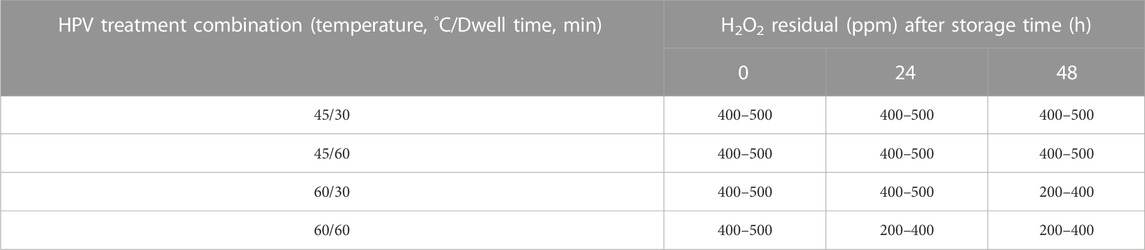
Residual hydrogen peroxide detected on whole black peppercorn after 0, 24, and 48 h of storage at ambient temperature are presented in (Table 2). Results show a high level of residue (up to 500 ppm) remain in samples treated even after 48 h of storage. Samples treated at 60°C show a small decrease in residue, measuring in the range of 200–400 ppm. This is much higher than CFR regulations which allow ≤0.5 ppm residue leached into distilled water packaged under production conditions utilizing 35% hydrogen peroxide for aseptic packaging.
There are several factors that could dictate the hydrogen peroxide residual results such as starting concentration of hydrogen peroxide, treatment time, and the food matrix. Results from other HPV studies report that residuals from lettuce treated with 1%–10% hydrogen peroxide for 10 min decreased over storage time to undetectable levels after 36 h storage (Back et al., 2014). In contrast, hydrogen peroxide residues were detected in prunes treated with HPV after 20 days of storage time (Simmons et al., 1997). Their results indicate that shorter HPV exposure time (<30 min) result in undetectable limits after 20 days but HPV exposure of 30 and 60 min declined to undetectable limits only after 90 days. It is unclear if testing was done between 0 and 20 days or between 20 and 90 days. A recent study on cold plasma activated HPV for the inactivation of S. Typhimurium on fresh produce found that the residue was dependent on the nature of the fresh produce surface as well as surface area-to-mass ratio (Song and Fan, 2021). Cantaloupe (rough surface) and lettuce (more surface area) retained higher amounts of hydrogen peroxide residue. The surface properties of whole black peppercorn may require an extended duration for the complete dissipation of hydrogen peroxide residues. A systematic future study is necessary to determine the exact length of time necessary to completely dissipate the hydrogen peroxide residual.
3.5 Final water activity and moisture content after HPV treatment
The final water activity after HPV treatment and corresponding wet basis moisture contents are summarized in (Table 3). Final moisture content was estimated from the generated desorption isotherms. Both water activity and moisture content were significantly decreased after all HPV treatment with no significant difference observed among treatments. One of the limitations of this study was that the HPV equipment required a minimum temperature of 40°C for the aeration phase. Since the aeration phase ranged from 1–2 h to reduce the HPV concentration to <1 ppm, the black peppercorns were dehydrated to a less than ideal final water activity and corresponding moisture content. Moisture loss due to treatment conditions can be compensated by increasing RH conditions (Rane et al., 2020; Wei et al., 2021a) or moisture content prior to treatment (Wei et al., 2018). In this study, treatment at a water activity of 0.8 was explored (Supplementary Figure S6) but results from the stability test indicated that Salmonella did not adapt as well to the high humidity environment. Furthermore, initial HPV treatment runs with 0.8 aw product still resulted in a final water activity of 0.48 and 0.41 for 45°C and 60°C temperature runs, respectively. These drops in water activity indicate the prolonged exposure to low moisture gas is negatively impacting the moisture content and water activity of the product. Modifications to the process design would be required to avoid or decrease the loss in moisture.
3.6 Quality analysis
Results for piperine, total phenolics, and DPPH assay for antioxidant activity are presented in (Table 4). Piperine concentration of the whole black peppercorn was derived from a standard piperine curve. The normal range for piperine is reported to range from 2%–7.4% in black pepper (Nair, 2020; Tiwari et al., 2020) and results fall within this range at ∼3%. It is not surprising that the control falls on the lower end of the spectrum as commercially steam sterilized black peppercorn was used and steam treatment has been reported to significantly reduce piperine (Waje et al., 2008). Piperine concentration was significantly different between control and 45°C HPV treatment although both fall within the normal range. Piperine, the most biologically active compound in black peppercorn has been said to protect against oxidative damage by inhibiting or quenching free radicals (Srinivasan, 2007). This may have also lowered the efficacy of hydrogen peroxide which is a strong oxidizing agent that kills bacteria by producing free hydroxyl radicals that attack essential cell components (Block, 2001).
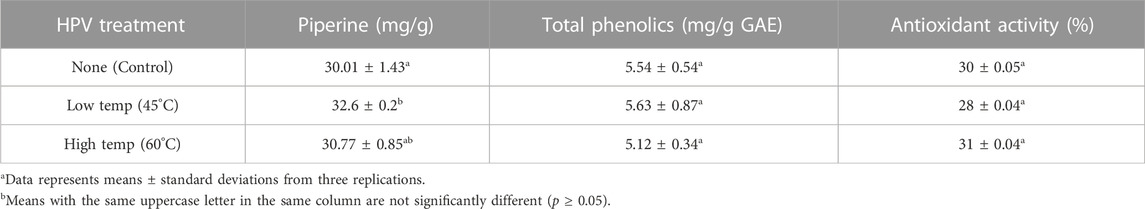
TABLE 4. Piperine content, total phenolics, and antioxidant activity of untreated and 60 min HPV treated whole black peppercorn.
Total phenolics were derived from a standard curve of gallic acid solutions and results are comparable to values reported, 2.87 mg/g GAE and 3.83 mg/g GAE (Charles, 2013; Yashin et al., 2017). Total phenolics ranged between 5.12 and 5.53 mg/g GAE with no significant difference between treated samples and control or between treatments. Antioxidant activity measured as the DPPH radical scavenging assay ranged from 28% to 31% with no significant difference between treated samples and control or between treatments. Results were measured over a range of black peppercorn extract concentrations (Supplementary Figure S4), but only one level (0.8 mL) is reported in the summary table for comparison. While the DPPH radical scavenging test is a common one for antioxidant activity and many studies have reported this assay for black peppercorn, due to differences in protocol (concentrations, reaction solvent, incubation time, reporting methods), it is not possible to compare the results. Slight increase in means of the piperine, phenolics, and DPPH test for HPV treated compared to control could be contributed to the increase of dry matter content due to the decrease in moisture content. Overall, piperine, total phenolics, and antioxidant activity were not significantly affected by HPV treatment.
More than a hundred compounds have been reported for black peppercorn volatiles; analysis performed for this research returned 110 compounds for the black peppercorn control and 91 compounds for the HPV treated samples. A summary table with 21 major components is presented in (Table 5). Major components frequently mentioned in literature attributed to black peppercorn’s characteristic flavor and aroma (α-Pinene, ß-Pinene, δ-3-Carene, Sabinene, Myrcene, Limonene, and ß-caryophyllene) are highlighted within the table. ß-caryophyllene and limonene are the top two highest components reported in literature (Kapoor et al., 2009; Dosoky et al., 2019) which is consistent with this study. Both ß-caryophyllene and limonene were not significantly affected by HPV treatment. Among all the volatile compounds, only three compounds showed significant difference after HPV treatment (Sabinene, ß-Pinene, and ρ-cymene) and these occur in small amounts (<2%) so it is highly unlikely that differences in flavor or aroma would be detected. Reported values for black pepper volatiles vary greatly due to differences in environmental factors, plant variety, cultivation practices, harvesting stage, method of extraction (distillation, simultaneous distillation-extraction (SDE), solid phase microextraction (SPME), or supercritical fluid extraction), and storage (Dosoky et al., 2019). It is interesting to note that the volatile compound, limonene, was not significantly affected by HPV treatment but was significantly affected by RF treatment of black pepper (Wei et al., 2018).
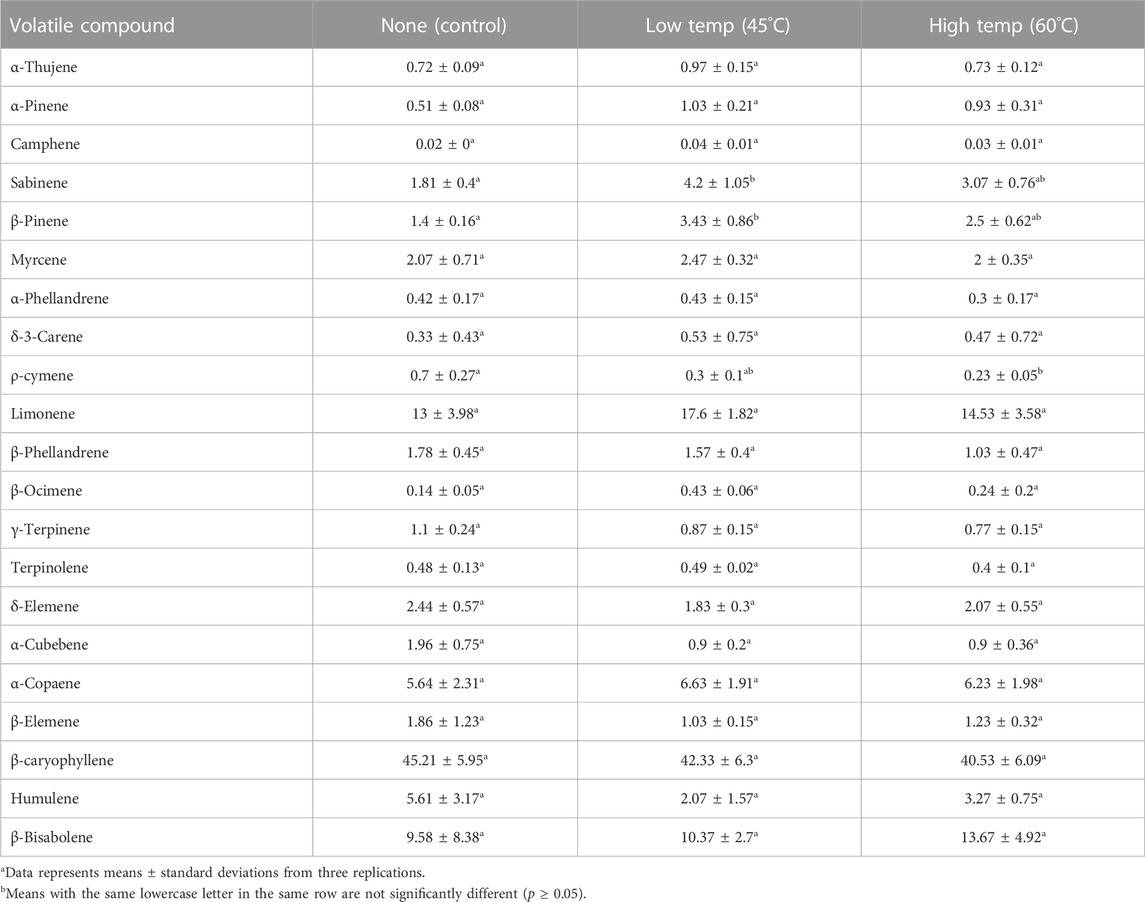
TABLE 5. Volatile oils (major components, %) of untreated and 60 min HPV treated whole black peppercorn.
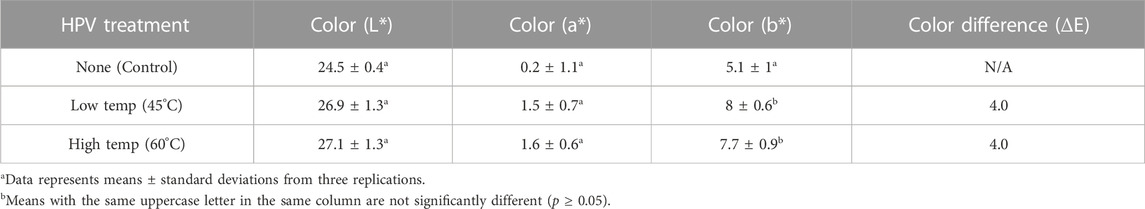
Color parameters of whole black peppercorn treated with HPV are presented in (Table 6). Differences in L*, a* and b* values were relatively small, but the combination of differences is observed when compared to the control and this is reflected in the delta E value of 4.0; a delta E color value between 3.5-5 indicates an obvious difference in color to the human eye (Mokrzycki and Tatol, 2011). Increased L* values indicate the treated samples were lighter in color and degree of yellowness increased significantly (higher b* value) after HPV treatment. Interestingly, the significant increase in b* value was also observed after processing with EtO (Duncan et al., 2017). Difference in color was visually apparent between the treated black peppercorn and control (Figure 6). Black kernels were duller and grayer after treatment and the overall yellow hue increased. Appearance in terms of color were similar for both low and high temperature treatments. The color differences may not be obvious if no comparison to a control is made.
4 Conclusion
In conclusion, this study indicates that fluidization with HPV for the inactivation of Salmonella in black peppercorn achieved microbial reductions between 1.41–2.83 log CFU/g which are below those required to achieve pasteurization levels but may be sufficient for decontamination. Biological compounds contributing to flavor and aroma were not significantly affected by HPV but there was obvious color change, moisture loss, and high levels of hydrogen peroxide in the spice after 48 h of storage. The study highlights the need to explore further process design modifications before conclusions can be made on the efficacy of HPV treatment as a means for LMF pasteurization. Further research is needed to conclusively report if interactions between HPV and Salmonella or between HPV and the product matrix contribute to lower microbial reductions. Combination technologies or hurdle methods could be explored to increase the rate of microbial inactivation and modifications such as a larger chamber and vacuum attachment may help reduce color changes and residuals.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
All authors contributed to the conception and design of the study. ES performed the experiments and wrote the first draft of the manuscript. SW assisted with experiments. All authors contributed to the article and approved the submitted version.
Funding
This project is supported by funding from United States Department of Agriculture (USDA)—National Institute of Food and Agriculture (NIFA) Agriculture and Food Research Initiative (AFRI) grants 2015-68003-23415 and 2020-67017-33256.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer MH declared a shared affiliation with the authors ES and RV-R to the editor at the time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frfst.2023.1119715/full#supplementary-material
References
ASTA (2017). Clean, safe, spices guidance document. Avaliable At: https://www.astaspice.org/food-safety-technical-guidance/best-practices-and-guidance/clean-safe-spices-guidance-document/ (Accessed Mar 19, 2022).
ASTA (1985). Official analytical methods of the American spice trade association. Englewood Cliffs, NJ: The Association.
Back, K., Ha, J., and Kang, D. (2014). Effect of hydrogen peroxide vapor treatment for inactivating Salmonella typhimurium, Escherichia coli O157:H7 and Listeria monocytogenes on organic fresh lettuce. Food control. 44, 78–85. doi:10.1016/j.foodcont.2014.03.046
Bang, I. H., Kim, Y. E., Lee, S. Y., and Min, S. C. (2020). Microbial decontamination of black peppercorns by simultaneous treatment with cold plasma and ultraviolet C. Innov. Food Sci. Emerg. 63, 102392. doi:10.1016/j.ifset.2020.102392
Beuchat, L. R., Komitopoulou, E., Beckers, H., Betts, R. P., Bourdichon, F., Fanning, S., et al. (2013). Low-water activity foods: Increased concern as vehicles of foodborne pathogens. J.Food Prot. 76 (1), 150–172. doi:10.4315/0362-028X.JFP-12-211
Block, S. S. (1991). “Peroxygen compounds,” in Disinfection, sterilization, and preservation (Philadelphia, PA: Lea & Febiger), 167–181.
Block, S. S. (2001). “Peroxygen compounds,” in Disinfection, sterilization, and preservation (Philadelphia, PA: Lippincott Williams & Wilkins), 185–204.
Blois, M. S. (1958). Antioxidant determinations by the use of a stable free radical. Nat. Lond. 181 (4617), 1199–1200. doi:10.1038/1811199a0
Brand-Williams, W., Cuvelier, M. E., and Berset, C. (1995). Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 28 (1), 25–30. doi:10.1016/S0023-6438(95)80008-5
CDC (1998). Multistate outbreak of Salmonella serotype agona Infections linked to toasted oats cereal — United States, april–may, 1998. MMWR Morb.Mortal.Wkly.Rep. 47 (22), 462–464.
CDC (2007). Multistate outbreak of Salmonella serotype Tennessee Infections associated with peanut butter — United States, 2006–2007. MMWR Morb.Mortal.Wkly.Rep. 56 (21), 521–524.
CDC (2013). Salmonella Montevideo and Salmonella Mbandaka Infections linked to tahini sesame paste. Avaliable At: https://www.cdc.gov/salmonella/montevideo-tahini-05-13/index.html (Accessed Apr 13, 2022).
CDC (2010). Salmonella Montevideo Infections associated with salami products made with contaminated imported black and red pepper --- United States, july 2009-april 2010. MMWR Morb.Mortal.Wkly.Rep. 59 (50), 1647–1650.
CDC (2016). Multistate Outbreak ofSalmonella Reading and Salmonella Abony Infections Linked to Alfalfa Sprouts. Avaliable At: https://www.cdc.gov/salmonella/reading-08-16/index.html.
Charles, D. J. (2013). Antioxidant properties of spices, herbs and other sources. New York, NY: Springer.
Christman, M. F., Morgan, R. W., Jacobson, F. S., and Ames, B. N. (1985). Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell. 41 (3), 753–762. doi:10.1016/S0092-8674(85)80056-8
Danyluk, M. D., Harris, L. J., and Schaffner, D. W. (2006). Monte Carlo simulations assessing the risk of salmonellosis from consumption of almonds. J.Food Prot. 69 (7), 1594–1599. doi:10.4315/0362-028x-69.7.1594
Dhaliwal, H. K., Gänzle, M., and Roopesh, M. S. (2021). Influence of drying conditions, food composition, and water activity on the thermal resistance of Salmonella enterica. Food Res. Int. 147, 110548. doi:10.1016/j.foodres.2021.110548
Dosoky, N. S., Satyal, P., Barata, L. M., Joyce Kelly, R. d. S., and Setzer, W. N. (2019). Volatiles of black pepper fruits (piper nigrum L). Mol. (Basel, Switz. 24 (23), 4244. doi:10.3390/molecules24234244
Duncan, S. E., Moberg, K., Amin, K. N., Wright, M., Newkirk, J. J., Ponder, M. A., et al. (2017). Processes to preserve spice and herb quality and sensory integrity during pathogen inactivation: Processes to preserve spice quality. J.Food Sci. 82 (5), 1208–1215. doi:10.1111/1750-3841.13702
Fan, X., Sokorai, K. J. B., and Gurtler, J. B. (2020). Advanced oxidation process for the inactivation of Salmonella typhimurium on tomatoes by combination of gaseous ozone and aerosolized hydrogen peroxide. Int.J.Food Microbiol. 312, 108387. doi:10.1016/j.ijfoodmicro.2019.108387
FAO/WHO (2014). Ranking of low moisture foods in support of microbiological risk management. Avaliable At: https://ucfoodsafety.ucdavis.edu/sites/g/files/dgvnsk7366/files/inline-files/209893.pdf.
FDA (2015). FSMA final rule for preventive controls for human food. Avaliable At: https://www.fda.gov/food/food-safety-modernization-act-fsma/fsma-final-rule-preventive-controls-human-food (Accessed Mar 6, 2022).
Fichet, G., Antloga, K., Comoy, E., Deslys, J. P., and McDonnell, G. (2007). Prion inactivation using a new gaseous hydrogen peroxide sterilisation process. J. Hosp. Infect. 67 (3), 278–286. doi:10.1016/j.jhin.2007.08.020
Grace, J. R., Bi, X., and Ellis, N. (2020). Essentials of fluidization. Weinheim, Germany: Wiley VCH.
Harris, P., and Zanko, L. (2010). Vaporized hydrogen peroxide (VHP®) gaseous decontamination: 'GREEN' technology for the highest level of technology technology for the highest highest level of microbial control within a pharmaceutical facility. Avaliable At: https://ispeboston.org/download/educational_presentations/2010/2010-02-23-Microbial-Control-PHarris-and-LZanko.pdf.
Juven, B. J., and Pierson, M. D. (1996). Antibacterial effects of hydrogen peroxide and methods for its detection and quantitation. J.Food Prot. 59 (11), 1233–1241. doi:10.4315/0362-028X-59.11.1233
Kapoor, I. P. S., Singh, B., Singh, G., De Heluani, C. S., De Lampasona, M. P., and Catalan, C. A. N. (2009). Chemistry and in vitro antioxidant activity of volatile oil and oleoresins of black pepper (piper nigrum). J.Agric.Food Chem. 57 (12), 5358–5364. doi:10.1021/jf900642x
Keller, S. E., VanDoren, J. M., Grasso, E. M., and Halik, L. A. (2013). Growth and survival of Salmonella in ground black pepper (piper nigrum). Food Microbiol. 34 (1), 182–188. doi:10.1016/j.fm.2012.12.002
Klapes, N. A., and Vesley, D. (1990). Vapor-phase hydrogen peroxide as a surface decontaminant and sterilant. Appl. Environ. Microb. 56 (2), 503–506. doi:10.1128/aem.56.2.503-506.1990
Lau, S. K., and Subbiah, J. (2020). HumidOSH: A self-contained environmental chamber with controls for relative humidity and fan speed. HardwareX 8, e00141. doi:10.1016/j.ohx.2020.e00141
Linley, E., Denyer, S. P., McDonnell, G., Simons, C., and Maillard, J. (2012). Use of hydrogen peroxide as a biocide: New consideration of its mechanisms of biocidal action. J. Antimicrob. Chemother. 67 (7), 1589–1596. doi:10.1093/jac/dks129
McDonnell, G., Grignol, G., and Antloga, K. (2002). Vapor-phase hydrogen peroxide decontamination of food contact surfaces. Dairy, Food Environ. Sanitation. 22, 868–873.
McDonnell, G., and Russell, A. D. (1999). Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 12 (1), 147–179. doi:10.1128/CMR.12.1.147
Meszaros, J. E., Antloga, K., Justi, C., Plesnicher, C., and McDonnell, G. (2005). Area fumigation with hydrogen peroxide vapor. Appl. Biosaf. 10 (2), 91–100. doi:10.1177/153567600501000206
Mokrzycki, W. S., and Tatol, M. (2011). Colour difference de—a survey. Mach. Graph. Vis. 20, 383–412.
Nadia, G., Riky, P., and Shlomo, S. (2011). Effect of desiccation on tolerance of Salmonella enterica to multiple stresses. Appl. Environ. Microbiol. 77 (5), 1667–1673. doi:10.1128/AEM.02156-10
Nair, K. P. (2020). The geography of black pepper (piper nigrum) the "king" of spices – volume 1. Cham, Switzerland: Springer International Publishing.
OSHA (2020). OSHA occupational chemical database | hydrogen peroxide. Avaliable At: https://www.osha.gov/chemicaldata/630.
Rane, B., Bridges, D. F., and Wu, V. C. H. (2020). Gaseous antimicrobial treatments to control foodborne pathogens on almond kernels and whole black peppercorns. Food Microbiol. 92, 103576. doi:10.1016/j.fm.2020.103576
Robertson, A. R. (1977). The CIE 1976 color-difference formulae. Color Res. Appl. 2 (1), 7–11. doi:10.1002/j.1520-6378.1977.tb00104.x
Sapers, G. M., Walker, P. N., Sites, J. E., Annous, B. A., and Eblen, D. R. (2003). Vapor-phase decontamination of apples inoculated with Escherichia coli. J. Food Sci. 68 (3), 1003–1007. doi:10.1111/j.1365-2621.2003.tb08278.x
Sharma, O. P., and Bhat, T. K. (2009). DPPH antioxidant assay revisited. Food Chem. 113 (4), 1202–1205. doi:10.1016/j.foodchem.2008.08.008
Simmons, G. F., Smilanick, J. L., John, S., and Margosan, D. A. (1997). Reduction of microbial populations on prunes by vapor-phase hydrogen peroxide. J.Food Prot. 60 (2), 188–191. doi:10.4315/0362-028X-60.2.188
Singleton, V. L., Joseph, A., and Rossi, J. (1965). Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 16 (3), 144–158. doi:10.5344/ajev.1965.16.3.144
Smith, D. F., and Marks, B. P. (2015). Effect of rapid product desiccation or hydration on thermal resistance of Salmonella enterica serovar enteritidis PT 30 in wheat flour. J.Food Prot. 78 (2), 281–286. doi:10.4315/0362-028X.JFP-14-403
Song, W., and Kang, D. (2021). Inactivation of Escherichia coli O157:H7 and Salmonella typhimurium in black and red pepper by vacuumed hydrogen peroxide vapour. Lett. Appl. Microbiol. 132, 290–297. doi:10.1111/jam.15230
Song, W., and Kang, D. (2022). Inactivation of Salmonella typhimurium, Escherichia coli O157:H7 and Listeria monocytogenes on alfalfa seeds by the combination treatment of vacuumed hydrogen peroxide vapour and vacuumed dry heat. Lett. Appl. Microbiol. 74 (6), 909–915. doi:10.1111/lam.13678
Song, Y., and Fan, X. (2020). Cold plasma enhances the efficacy of aerosolized hydrogen peroxide in reducing populations of Salmonella typhimurium and Listeria innocua on grape tomatoes, apples, cantaloupe and romaine lettuce. Food Microbiol. 87, 103391. doi:10.1016/j.fm.2019.103391
Song, Y., and Fan, X. (2021). Hydrogen peroxide residue on tomato, apple, cantaloupe, and romaine lettuce after treatments with cold plasma activated hydrogen peroxide aerosols. J. Food Prot. 84 (8), 1304–1308. doi:10.4315/JFP-21-051
Srinivasan, K. (2007). Black pepper and its pungent principle-piperine: A review of diverse physiological effects. Crit.Rev.Food Sci.Nutr. 47 (8), 735–748. doi:10.1080/10408390601062054
Tainter, D. R., and Grenis, A. T. (2001). Spices and seasonings: A food technology handbook. Weinheim, Germany: Wiley VCH.
Tiwari, A., Mahadik, K. R., and Gabhe, S. Y. (2020). Piperine: A comprehensive review of methods of isolation, purification, and biological properties. Med. Drug Discov. 7, 100027. doi:10.1016/j.medidd.2020.100027
Unger-Bimczok, B., Kottke, V., Hertel, C., and Rauschnabel, J. (2008). The influence of humidity, hydrogen peroxide concentration, and condensation on the inactivation of geobacillus stearothermophilus spores with hydrogen peroxide vapor. J. Pharm. Innov. 3 (2), 123–133. doi:10.1007/s12247-008-9027-1
Vaisala (2021). Considering condensation: Influences in hydrogen peroxide vapor bio-decontamination. Avaliable At: https://www.vaisala.com/en/lp/white-paper-influences-condensation-hydrogen-peroxide-vapor-bio-decontamination.
Verma, T., Chaves, B. D., Howell, T., and Subbiah, J. (2021). Thermal inactivation kinetics of Salmonella and Enterococcus faecium NRRL B-2354 on dried basil leaves. Food Microbiol. 96, 103710. doi:10.1016/j.fm.2020.103710
Waje, C. K., Kim, H., Kim, K., Todoriki, S., and Kwon, J. (2008). Physicochemical and microbiological qualities of steamed and irradiated ground black pepper (piper nigrum L). J. Agr. Food Chem. 56 (12), 4592–4596. doi:10.1021/jf8002015
Watling, D., Ryle, C., Parks, M., and Christopher, M. (2002). Theoretical analysis of the condensation of hydrogen peroxide gas and water vapour as used in surface decontamination. PDA J.Pharm.Sci.Technol. 56 (6), 291–299.
Wei, X., Chen, L., Chaves, B. D., Ponder, M. A., and Subbiah, J. (2021c). Modeling the effect of temperature and relative humidity on the ethylene oxide fumigation of Salmonella and Enterococcus faecium in whole black peppercorn. Food Sci. Technol. 140, 110742. doi:10.1016/j.lwt.2020.110742
Wei, X., Lau, S. K., Stratton, J., Irmak, S., Bianchini, A., and Subbiah, J. (2018). Radio-frequency processing for inactivation of Salmonella enterica and Enterococcus faecium NRRL B-2354 in black peppercorn. J.Food Prot. 81 (10), 1685–1695. doi:10.4315/0362-028X.JFP-18-080
Wei, X., Vasquez, S., Thippareddi, H., and Subbiah, J. (2021a). Evaluation of Enterococcus faecium NRRL B-2354 as a surrogate for Salmonella in ground black pepper at different water activities. Int. J. Food Microbiol. 344, 109114. doi:10.1016/j.ijfoodmicro.2021.109114
Wei, X., Verma, T., Danao, M. C., Ponder, M. A., and Subbiah, J. (2021b). Gaseous chlorine dioxide technology for improving microbial safety of spices. Innovative Food Sci. Emerg. Technol. 73, 102783. doi:10.1016/j.ifset.2021.102783
Keywords: hydrogen peroxide vapor (HPV), fluidization, low-moisture foods, black peppercorn, Salmonella
Citation: Summers E, Wason S, Subbiah J and Villa-Rojas R (2023) Inactivation of Salmonella enterica in black peppercorn by fluidization with hydrogen peroxide vapor. Front. Food. Sci. Technol. 3:1119715. doi: 10.3389/frfst.2023.1119715
Received: 09 December 2022; Accepted: 12 May 2023;
Published: 30 May 2023.
Edited by:
Xinyao Wei, Fuzhou University, ChinaReviewed by:
Manuela Hernandez-Herrero, Autonomous University of Barcelona, SpainManirul Haque, University of Nebraska-Lincoln, United States
Aubrey Francis Mendonca, Iowa State University, United States
Copyright © 2023 Summers, Wason, Subbiah and Villa-Rojas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rossana Villa-Rojas, cnZpbGxhcm9qYXMyQHVubC5lZHU=
 Edel Summers
Edel Summers Surabhi Wason
Surabhi Wason Jeyamkondan Subbiah3
Jeyamkondan Subbiah3 Rossana Villa-Rojas
Rossana Villa-Rojas