- 1Rhizobiology Group, Department of Agronomic and Applied Molecular Sciences, Faculty of Agriculture and Veterinary Medicine, University of Buea, Buea, Cameroon
- 2Department of Agricultural Economics and Agribusiness, Faculty of Agriculture and Veterinary Medicine, University of Buea, Buea, Cameroon
- 3Centre for Independent Development Research (CIDR), Buea, Cameroon
- 4Farming System Group, Department of Agronomic and Applied Molecular Sciences, Faculty of Agriculture and Veterinary Medicine, University of Buea, Buea, Cameroon
- 5Department of Food Science and Technology, Faculty of Agriculture and Veterinary Medicine, University of Buea, Buea, Cameroon
Cassava (Manihot esculenta) fermentation methods can reduce the eventual fufu yield, while storage affects shelf-life and consumer acceptability. This study investigated effects of five cassava fermentation methods on fufu yield, and five storage methods on microbial load, sensory qualities and consumer acceptability of fufu. Fresh cassava roots were peeled, weighed and subjected to different fermentation methods for 5 days (e.g., tap water changed daily or every 2 days, tap water without change, borehole water without change, and tap water + bitterleaf (Vernonia amygdalina). The pH, temperature, total dissolved solids and turbidity of cassava fermentation effluent were measured daily for 5 days. Cassava fufu was stored at 0°C, 4°C, dried, in air-tight buckets and low-density polythene (LDPE) bags, and total titratable acid, pH, bacterial and fungal loads and consumer acceptability were evaluated weekly. A nine-point hedonic scale was used to assess consumer acceptability of fufu based on colour, dark spots, caking and odour. The highest fufu yield occurred in fermentation with tap water + bitterleaf (84%) and borehole water (82%) compared to when water was changed after 2 days (42%) and daily (38%). For storage at 4°C, 0°C, oven-dried, LDPE bags, and air-tight buckets in LDPE bags, respectively, bacterial load increased from 4 × 106 CFUs/mL at the start to 73, 32, 29, 145 and 85 × 106 CFUs/mL at week 4, while fungal load increased from 6 × 106 CFUs/mL at the start to 66, 32, 26, 96 and 89 × 106 CFUs/mL at week 4. Storage at 0°C and drying exhibited high consumer acceptability of cassava fufu compared to LDPE and air-tight buckets.
1 Introduction
FAO and UNICEF (2021) reported severe food insecurity in about 12% of the global population in 2020 that represents 928 million people, with increase of 148 million compared to 2019. In 2021, 278 million people in Africa were affected by hunger and 2.43 million people suffered severe food insecurity in Cameroon (FAO and UNICEF, 2022). Cassava fufu is a staple food in many African countries that faces significant challenges in processing and storage, which leads to low yield and shelf life (Chijioke et al., 2021). It is a drought resistant crop which requires little care but yields higher productivity per cultivated area than most crops (Golvalve et al., 2024). Besides addressing low cassava production in SSA, reducing post-harvest losses during fermentation to produce fufu is important for food security (Obadina A. O. et al., 2009; Njukwe et al., 2012; 2014). Local processing methods may result in qualitative and quantitative losses of cassava fufu (Madu et al., 2021), while poor storage increases microbial contamination and reduces shelf life (Sama et al., 2023).
Growth of pathogenic and spoilage microbes was reported on fermented cassava products with high microbial load that can affect human health and sustainable development (Obadina A. O. et al., 2009; Adegbehingbe et al., 2019; Sama et al., 2023). This is partly due to inappropriate cassava fufu production by unskilled processors without standard operational procedures, leading to low quantitative and qualitative outputs (Njukwe et al., 2012; Ogunyinka and Oguntuase, 2020 Addo et al., 2020). Cassava fermentation to produce fufu lasts for 4–6 days (Emmanuel, 2013), and some microbes have been identified in the process such as Baccilus subtilis, Klebsiella spp., Candida tropicalis, lactic acid bacteria and yeast (Odom et al., 2012; Obadina et al., 2006).
The quantitative and qualitative yield of cassava fufu depends on the fermentation process and storage methods (Forsythe et al., 2022), but information on these methods is scarce. However, microbial succession on cassava fufu has been reported with the starch degrading Bacillus subtilis giving way to lactic acid bacteria and yeast at the latter part of fermentation (Abriba et al., 2012). Chijioke et al. (2021) reported that ease of forming a dough and thickness of cassava fufu during cooking are quality preferences for consumers. Organic compounds produced during cassava fermentation (e.g., lactic, acetic, propanoic and butanoic acids) contribute to the characteristic flavour of the product (Abriba et al., 2012). Linamarase produced during cassava fermentation detoxifies fufu by breaking down linamarin and lotaustralin (cyanogenic glucosides) to release hydrogen cyanide (Abriba et al., 2012).
The objective of this study was to evaluate the impact of five cassava fermentation methods on the quantitative output of fufu, and to assess the effectiveness of five storage methods in maintaining fufu quality, shelf life and consumer acceptability after 4 weeks. Fermentation focused on water sources, replacement of effluent, and bitterleaf (Vernonia amygdalina) as effluent additive. Additionally, fufu quality and consumer acceptability were assessed when stored at 0°C or 4°C, oven-dried, and preserved in low-density polythene (LDPE) bags or air-tight buckets in LDPE bags. It was hypothesized that the source of fermentation water and bitterleaf additive will enhance the quantitative yield of cassava fufu, while drying storage at 4°C and storage at 0°C will boost consumer acceptability of fufu compared to LDPE bags or air-tight buckets.
2 Materials and methods
2.1 Experimental setup for fermentation process
The experiment was conducted at Rhizobiology Laboratory of the Faculty of Agriculture and Veterinary Medicine, University of Buea, Cameroon. A total of 200 kg fresh cassava roots was purchased from a local farmer in Buea and transferred to the Laboratory where they were sorted to remove rotten and smaller roots (less than 10 cm diameter and 15 cm length). For each treatment, 10 kg of fresh cassava roots were peeled using kitchen knives, roots were properly washed and rinsed with tap water. The washed cassava roots were cut into similar sizes and placed in 15L fermentation buckets. For treatment 1, the buckets were filled with tap water that was changed every 2 days. For treatment 2, the buckets were filled with tap water and the water was changed daily throughout the 5 days fermentation period. For treatment 3, water from a borehole was used for the fermentation of cassava rather than tap water. In treatment 4, tap water was used, and freshly harvested bitterleaf (V. amygdalina) leaves were placed on the surface of the fermentation bucket. The water was not changed throughout the fermentation period. This setup aimed to mimic the traditional practice of adding bitterleaf to fermentation media, as its potential role in enhancing the fermentation process remains unclear. For treatment 5, which also served as the control, tap water was used without any additive and the water was not changed throughout the fermentation period. (Table 1). All treatments were prepared in triplicates, stored in LDPE bags and allowed to ferment for 5 days.
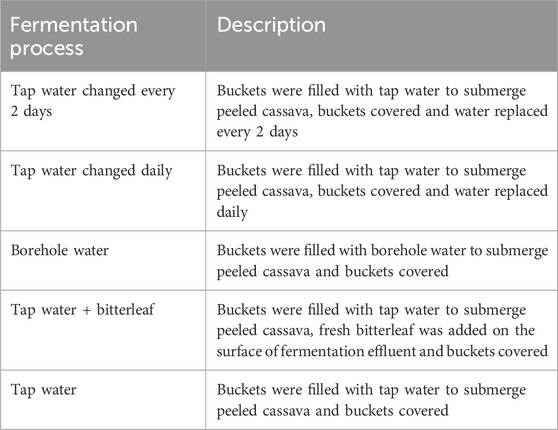
Table 1. Experimental setup with five fermentation techniques to improve quantitative yield of cassava fufu.
Effluent temperature in fermentation tank was measured daily by dipping a 40 cm hand-held thermometer into fermentation effluent for 90 s and temperature recorded. The effluent pH was determined with a pH meter (Extech DO700) following the three points calibration (4.00pH, 7.00pH, and 10.00 pH), while total dissolved solids was measured by gravimetric analysis where water sample was filtered and evaporated through heating and the residue was weighed. Turbidity of the fermentation effluent was measured using a turbidity meter (Extech Model TB400) and expressed in nepholometric turbidity unit (NTUs). To determine the characteristic changes (temperature, pH, turbidity, and total dissolved solid), 10 mL fermentation effluent was collected at 0, 1, 2, 3, 4, and 5 days.
2.2 Production of cassava fufu
Decomposing soft cassava from each fermentation process was pulped by squashing and scrubbing on plastic sieve (0.3–0.5 cm aperture) and screened with tap water. Discarded cassava wastes that were not soft enough during fermentation were weighed to establish amount of cassava lost during processing. The wet sediment after decantation was placed in polypropylene bags, tied and pressed to dewater, about 75 kg stone was placed on the wet mash for 24 h to complete the dewatering process before shredding using a hand weaved shredder (0.2–0.5 mm aperture size). Spillage during shredding and dewatering was weighed and recorded as ratio of loss with respect to original quantity at beginning of fermentation process. Fufu was packaged in low-density polythene bags for storage, while samples were collected for further investigation of microbial quality.
2.3 Storage methods of cassava fufu
Fufu samples with the best quantitative and qualitative yields from the fermentation process were selected for storage to assess their quality and consumer acceptability. Samples were stored at room temperature, 0°C, or 4°C, or underwent oven drying (Table 2). For packaging, LDPE bags and airtight buckets were used, reflecting common market practices for fufu storage.
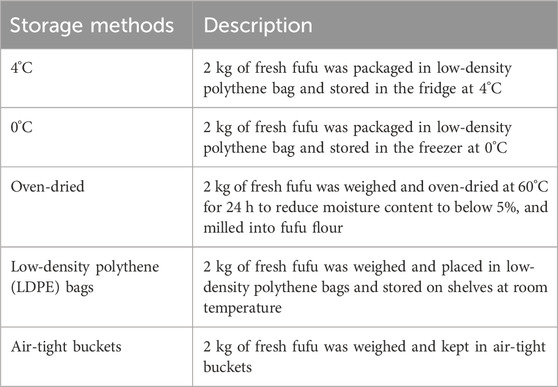
Table 2. Experimental setup with five storage methods to maintain quality and extend shelf life of cassava fufu.
2.4 Physical and chemical properties of cassava fufu and fermentation effluent
Physical and chemical properties of cassava fufu and fermentation effluent were analysed at the Agroecology laboratory, Faculty of Agriculture and Veterinary Medicine, University of Buea, Cameroon. Fufu from different fermentation processes were pulped and dewatered under aseptic conditions, and water content of fufu was determined and compared to fufu from the local market. The pH, total titratable acid, bacterial and fungal loads were determined as described below.
2.4.1 Measurement of pH
The pH or hydrogen ion concentration of each sample was measured with a standard meter (ATC, model HI-8915) according to AOAC (1990). The pH meter was standardized with standard buffers of pH four and pH 7. The pH was determined by making 10% w/v suspension of sample in distilled water. The suspension was mixed thoroughly and a pH meter probe calibrated with pH buffer seven was introduced into each sample, and read when values were stable.
2.4.2 Titratable acidity
Titratable acidity (expressed as lactic acid) was determined using AOAC (1990) method. Homogenate of the sample was prepared as that of pH determination. The slurry was filtered through Whatman No. 1 filter paper. Aliquot (10 mL) was titrated with 0.1 M NaOH using phenolphtalene as end-point indicator. Three drops of 0.1% phenolphtalene indicator was added to flask and mixed thoroughly before titrating with 0.1 M NaOH. Titration continued until a permanent pink colour was observed. Titratable acidity was expressed as lactic acid following 1 mL of 0.1 M NaOH = 0.009 g of lactic acid.
2.4.3 Moisture content
One thousand grams (1,000 g) (Wi) of each sample was weighed into a crucible and dried in the oven at 60°C for 8 h. It was cooled and reweighed (Wk). The weight was recorded and the sample was returned to the oven for further drying at 45°C for 4 h. Drying, cooling and weighing was repeatedly done until a constant weight (Wf) was obtained. The moisture content (%) was calculated as follows:
2.5 Microbial load on cassava fufu
The microbial load on cassava fufu was measured at the Rhizobiology Laboratory of the Faculty of Agriculture and Veterinary Medicine, University of Buea, Cameroon. Bacterial and fungal loads were determined after serial dilution and inoculation unto nutrient agar plates and Sabouraud dextrose agar. For serial dilution (10–1), 10 g of each fufu sample was weighed using an electronic balance (Kern 573/KB/DS version 7.5 2019–02) and dissolved in 90 mL distilled water using a flame sterilized glass rod and thoroughly mixed using a vortex (Heido Test tube shakers, Hei-Mix Reax top) to obtain a homogenous stock. A further 10-fold serial dilution was done up to 10–8. Nutrient agar was prepared by adding 14 g of the medium in 500 mL of distilled water, thoroughly mixed and autoclaved at 121°C for 15 min, and allowed to cool to 45°C. The medium was poured into petri dishes for inoculation. Sabouraud dextrose agar was prepared by dissolving 20 g of the medium in 500 mL of distilled water and autoclaved for 15 min at 121°C, allowed to cool to 50°C and poured into petri dishes. One mL of the 10–6 dilution of each sample was cultured by pour-plating in three petri dishes of nutrient agar. The nutrient agar plates for bacteria isolation were incubated aerobically at 37°C for 24–48 h in an inverted position (DNP-902 laboratory incubator). Fungal plates were incubated at 30°C for 72 h. The bacterial and fungal loads were enumerated using a colony counter (CC-J3A).
2.6 Sensory evaluation of fufu acceptability and organoleptic properties
A nine-point hedonic scale was used to evaluate the rate of spoilage and acceptability of fufu under different storage conditions for 4 weeks (Iwe, 2002; Laura et al., 2010). The hedonic scale ranges between 1–9, with one corresponding to least acceptable (extremely bad) and nine corresponding to most acceptable (extremely good) (Iwe, 2002; Laura et al., 2010). A total of 40 randomly selected panelists comprising PhD, Master degree students and staff of the University of Buea. The panelists were accustomed to consuming fufu, familiar with its sensory characteristics, and capable of detecting changes in those characteristics. This was done at the Faculty of Agriculture and Veterinary Medicine teaching and research farm of the university of Buea. The panellists were between 23 and 45 years old and generally familiar with the physical characteristics of fermented cassava fufu. Only those who gave their consents were invited to take part in the study and participants were free to withdraw at any time during the evaluation process. Confidentiality and anonymity were maintained and participants were allowed to respond only to questions relevant to the present study. Samples were presented to the panelists randomly, and each panelist evaluated fufu samples stored under different conditions, rating their perception using the hedonic scale. Perceptible characteristics of fufu such as changes in colour, appearance of black/brown spots (mould), odour (different from the characteristic smell of fufu), stickiness (gummy), and caking were considered for scoring acceptability.
For evaluation of organoleptic properties of fufu, samples stored at 0°C and samples that were oven-dried which showed an acceptability score of five and above were reconstituted and cooked for 30 min while continuously stirring with a wooden spatula to obtain a uniform texture. The cooked fufu was allowed to cool and served for consumption. A panel of 10 consumers evaluated the organoleptic properties (colour, odour, and texture) based on a nine-point hedonic scale (Iwe, 2002). Panellists rinsed their mouth with water and waited for 20 min before tasting the next fufu.
2.7 Data analyses
The Statistical Package for Social Sciences (SPSS) Version 25 was used to analyse all data. Data were subjected to Kolmogorov-Smirnov and Shapiro-Wilk test to assess normality. Interactions of fermentation methods and daily temperature, pH, turbidity, total dissolved solids in fermentation effluents, and interactions of storage methods on duration of storage, pH, total dissolved solids, bacterial and fungal loads were tested by multivariate analysis (P < 0.05). Where significance was observed, Analysis of Variance (ANOVA) was performed and followed by Tukey’s HSD (P < 0.05). Data from hedonic scores on effect of storage methods on sensory properties of stored cassava fufu (e.g., odour, colour, mouldiness, stickiness, and organoleptic properties) that were not normally distributed were further analysed by Kruskal Wallis non-parametric test to determine if there were significant differences in mean acceptability of fufu stored under different conditions over time (Kutner et al., 2005). The data collected for organoleptic properties was also not normally distributed. Kruskal Wallis test was performed to determine if there were significant differences in mean acceptability of fufu that were oven-dry and the fufu samples stored in the freezer at 0°C based on taste. Colour and stickiness.
3 Results
3.1 Quantitative yield of fufu under different cassava fermentation methods
Total fufu output differed significantly across fermentation methods (F = 4,701.980, df = 4, P < 0.01). For treatment one where water was changed every 2 days during fermentation and treatment two where water was changed daily, some cassava roots were not sufficiently soft to be processed during pulping and dewatering. Out of the 10 kg cassava roots initially used for fermentation, 5.8 kg was not sufficiently soft after 5 days when water was changed every 2 days, while 6.2 kg was not sufficiently soft when water was changed daily (Table 3). The mean fufu yield when water was changed every 2 days was 42% while only 38% yield was obtained from treatment two when water was changed daily. There was a significant difference where tap water + bitterleaf, tap water without replacement, and borehole water were used. For fermentation with borehole, fufu output was 82% with 18% loss. There was 84% fufu yield where tap water + bitterleaf was used for fermentation (Table 3). Where only tap water was used without replacements, 79% of fufu yield was obtained (Table 3).
3.2 Effect of cassava fermentation methods on effluent properties
3.2.1 Temperature of fermentation effluent
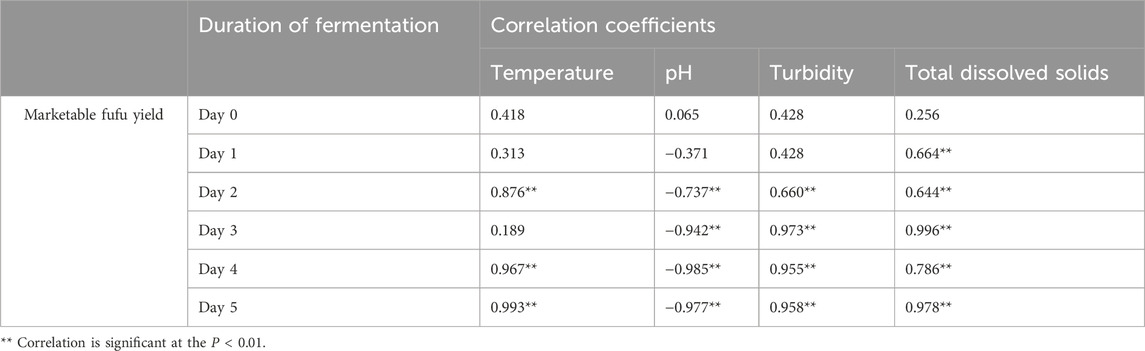
The effluent temperature after 5 days of fermentation differed significantly (P < 0.05; Figure 1A) across treatments, with the highest in tap water + bitterleaf (26.7°C), followed by bore hole water (25.93°C) and tap water (25.7), and the lowest when tap water was changed daily (22.1°C) or every 2 days (22.3°C). Pearson correlation revealed strong positive (r = 0.993, P < 0.01) relationship between temperature and marketable fufu yield (Table 4). Meanwhile, consistent increase in temperature occurred across 5 days of fermentation (Figure 1A). Temperature increased significantly (F = 30.667, df = 5, P < 0.001) from 23°C on the first day to 26°C by the fifth day. Temperature did not differ significantly (F = 0.331, df = 5 P < 0.884) for cassava fermentation where tap water was changed every 2 days. For cassava fermented with borehole water, temperature increased significantly (F = 1.327, df = 5, P < 0.001) from 22°C on first day to 26°C by the fifth day. Temperature increased significantly for cassava fermented with tap water + bitterleaf (F = 151.851, df = 5, P < 0.001) and tap water only (F = 36.541, df = 5, P < 0.001) from 22.1°C to 22°C–26.2°C and 26.2°C, respectively.

Figure 1. Variation in temperature (A) and acidity (B) of cassava fermentation effluent during 5 days of fermentation.
3.2.2 PH during fermentation
Acidity of the effluent after 5 days of fermentation differed significantly (P < 0.05; Figure 1B) across treatments, with the highest (lowest pH) in tap water + bitterleaf (4.63), followed by tap water (4.63) and bore hole water (4.77), which were lower than when tap water was changed every 2 days (5.93) or daily (6.00). Pearson correlation revealed strong negative (r = −0.977, P < 0.01) relationship between marketable fufu yield and pH (Table 4). Meanwhile, consistent decrease in effluent pH occurred across 5 days of fermentation (Figure 1B). For cassava fermented with tap water and changed every 2 days, pH dropped significantly (F = 58.733, df = 5, P < 0.001) from 6.96 on first day to 5.93 by fifth day. For cassava fermentation with daily change of tap water, pH dropped significantly (F = 28.123, df = 5 P < 0.001) from 6.93 on first day to 5.93 by fifth day. For cassava fermented with borehole water, pH dropped significantly (F = 136.943, df = 5, P < 0.001) from 6.96 on first day to 4.76 by fifth day. The pH decreased significantly for cassava fermented with tap water + bitterleaf (F = 281.547, df = 5, P < 0.001) and tap water only (F = 196.200, df = 5, P < 0.001), from 6.96 to 6.93 on first day to 4.63 and 4.66 by fifth day, respectively.
3.2.3 Turbidity during fermentation
Turbidity after 5 days of fermentation differed significantly (P < 0.05; Figure 2A) across treatments, with the highest in borehole water (536 NTUs), followed by tap water (424 NTUs) and tap water + bitterleaf (421.3 NTUs), tap water changed every 2 days (191.46 NTUs), and tap water changed daily (156.66 NTUs) as the lowest. Pearson correlation revealed strong positive (r = 0.958, P < 0.01) relationship between turbidity during fermentation and marketable fufu yield (Table 4). For cassava fermented with change of tap water every 2 days, turbidity increased significantly (F = 1857.820, df = 5, P < 0.01) from 2.7 NTUs on first day to 191.7 NTUs by fifth day. For cassava fermentation with daily tap water change, turbidity increased significantly (F = 794.327, df = 5, P < 0.01) from 2 NTUs on first day to 156.7 NTUs by fifth day. For cassava fermented with borehole, turbidity increased significantly (F = 2052.92, df = 5, P < 0.01) from 39.9 NTUs on first day to 536 NTU on fifth day. For fermentation with tap water + bitterleaf, turbidity increased significantly (F = 4,878.189, df = 5, P < 0.001) from 2.3 NTUs at on first day to 424.0 NTUs by fifth day. Where only tap water was used, turbidity increased significantly (F = 2012.545, df = 5, P < 0.01) from 3.3 NTUs at on first day to 421.3 NTUs on fifth day.
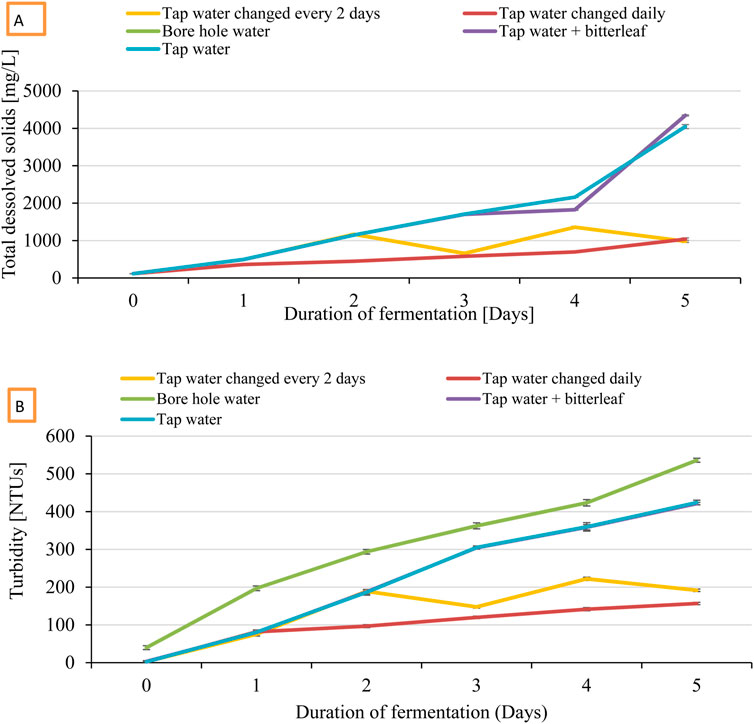
Figure 2. Variation in total dissolved solids (A) and turbidity (B) of cassava fermentation effluent during 5 days of fermentation.
3.2.4 Total dissolved solids during fermentation
Total dissolved solids after 5 days of fermentation differed significantly (P < 0.05; Figure 2B) across treatments, with the highest in bore hole water (4,348.6 mg/L) and tap water + bitterleaf (4345 mg/L) compared to tap water only, and the lowest when water was changed daily (1,038) or every 2 days (980.33 mg/L). Pearson correlation revealed strong positive (r = 0.978, P < 0.01) relationship between changes in total dissolved solids during fermentation and marketable fufu yield (Table 4). Meanwhile, consistent increase in total dissolved solids occurred across 5 days of fermentation (Figure 2B). For cassava fermented with change of tap water every 2 days, total dissolved solids increased significantly (F = 16.379, df = 5, P < 0.001) from 116 mg/L on first day to 980 mg/L on fifth day. For cassava fermentation with daily tap water change, total dissolved solids increased significantly (F = 1,457.034, df = 5, P < 0.001) from 114 mg/L on first day to 1,038 mg/L on fifth day (Supplementary Figure S2). For cassava fermentation with borehole water (F = 124.400, df = 5, P < 0.05), tap water (F = 123.346, df = 5, P < 0.001), and tap water + bitterleaf (F = 164.400, df = 5, P < 0.001), total dissolved solids increased significantly from 116, 117, and 114 mg/L on first day to 4,349, 4,345 and 4,047 mg/L on fifth day, respectively.
3.3 Effect of storage methods on cassava fufu properties
3.3.1 Total titratable acids
There was consistent decrease in total titratable acids for different storage conditions across 4 weeks. For fufu stored at 4°C, there was significant reduction in total titratable acids (F = 12.740, df = 3, P < 0.05) from 1.10 in first week to 0.30 by fourth week. There was significant decrease in total titratable acids for samples stored at 0°C (F = 30.504, df = 3, P < 0.05) from 1.03 in first week to 0.34 in fourth week. For fufu that was oven-dried or stored in LDPE bags, total titratable acids decreased significantly (F = 39.502, df = 3, P < 0.001) from 1.06 in first week to 0.28 in fourth week (Figure 3A). Similarly, fufu stored in LDPE bags (F = 14.133, df = 3, P < 0.001) and air-tight buckets experienced significant decrease in total titratable acid from 1.06 to 1.04 to 0.35 and 0.36 (F = 12.740, df = 3, P < 0.01), respectively (Figure 3A). There was no significant difference in total titratable acids when compared within weeks (Figure 2A). Multivariate analysis on total titratable acids revealed significant effect of duration of storage (F = 361.981, df = 4, P < 0.001; Table 5), but no significant effects of treatments (F = 0.454, df = 4, P > 0.05; Table 5) or interaction of fermentation method and duration of fermentation (F = 0.398, df = 3, P > 0.05; Table 5).

Figure 3. Variations in (A) Total titratable acids, (B) Acidity, (C) Bacterial counts and (D) Fungal counts of fufu stored under different conditions within 4 weeks.
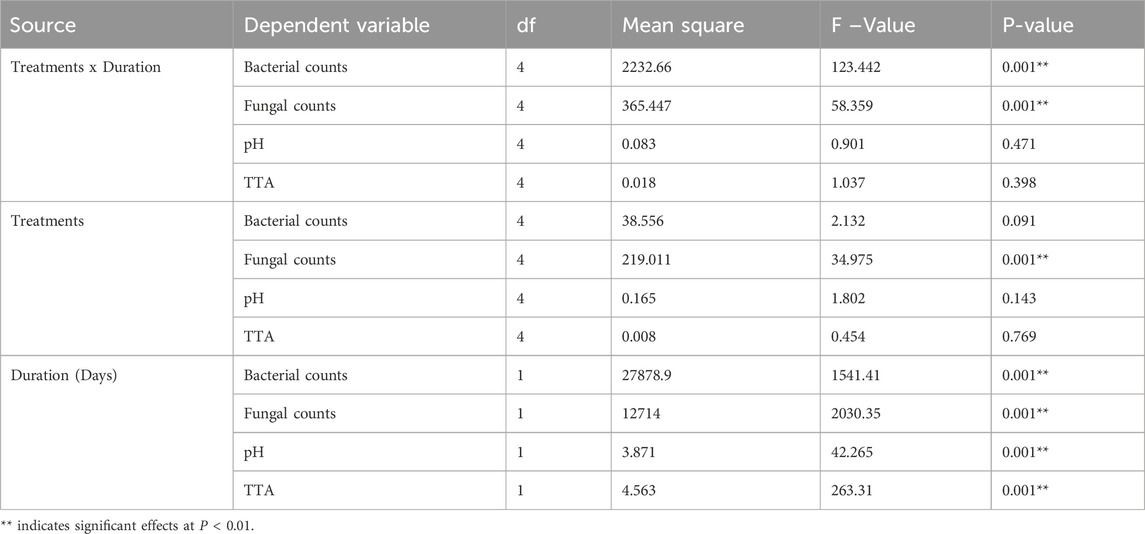
Table 5. Multivariate analysis showing significant effects of storage methods on total bacterial and fungal loads, pH and total titratable acids in stored fufu over time (P < 0.05).
3.3.2 Variations of pH
The pH increased (acidity decreased) steadily for all storage conditions throughout the investigation. The pH of fufu stored at 4°C increased significantly (F = 146.654, df = 3, P < 0.01) from 3.4 in first week to 4.2 in fourth week (Figure 3B). The pH of fufu stored at 0°C increased significantly (F = 59.037, df = 3, P < 0.01) from 3.5 in first week to 4.4 in week 4. There was significant increase in pH for oven-dried fufu (F = 77.563, df = 3, P < 0.01) while pH of fufu stored in LDPE bags (F = 31.567, df = 3, P < 0.01) and in air-tight buckets (F = 146.654, df = 3, P < 0.01) increased significantly from 3.5 to 3.7 in first week to 4.23 and 4.4 in fourth week, respectively (Figure 3B). In first week of storage, pH of samples stored at 4°C was significantly lower than those stored in air-tight buckets, but there was no significant difference between fufu stored at 0°C, oven-dried, and stored LDPE bags (Figure 2B). In week 2, pH of fufu stored in air-tight buckets was significantly lower than those stored at 0°C while there was no significant difference in other samples. In week 3, pH of samples stored at 0°C and LDPE bags were significantly lower than those stored at 4°C while samples stored in air-tight buckets and oven-dried samples had significantly lower pH compared to other samples. In week 4, pH of fufu stored in air-tight buckets was significantly higher than those stored at 4°C, LDPE bags and oven-dried (Figure 3B). Multivariate analysis on pH of stored fufu revealed significant effect of duration of storage (F = 42.265, df = 4, P < 0.001; Table 5), but no significant effect of storage methods (P > 0.05; Table 5) or interaction of treatment and duration of storage (P > 0.05; Table 5).
3.4 Effect of storage methods on microbial load of cassava fufu
3.4.1 Bacteria
There was consistent growth of bacterial viable counts across 4 weeks of storage. For fufu stored at 4°C, bacterial colonies increased significantly (F = 114.585, df = 3, P < 0.01) from 24.3 CFUs in first week to 73 CFUs in fourth week. For fufu stored at 0°C, the bacterial load increased significantly (F = 59.657, df = 3, P < 0.01) from 6.0 CFUs in first week to 32.7 CFUs in fourth week. For oven-dried fufu, bacterial load increased significantly (F = 62.201, df = 3, P < 0.01) from 7.0 CFUs in first week to 29.7 CFUs in fourth week. For fufu stored LDPE bags, bacterial load increased significantly (F = 737.356, df = 3, P < 0.01) from 31.0 CFUs in first week to 145.7 CFUs in fourth week. For samples stored in air-tight buckets, bacterial load increased significantly (F = 114.585, df = 3, P < 0.01) from 18.0 CFUs to 85.7 CFUs in fourth week. There was no significant difference between bacterial load on fufu stored at 0°C, oven-dried fufu and that stored in LDPE bags across storage. However, bacterial loads were significantly higher for fufu stored in LDPE bags, 4°C, and air-tight buckets than those stored at 0°C and oven-dried (Figure 3C). Bacterial load on fufu stored in LDPE bags at room temperature (average of 26°C) was significantly higher (P < 0.01) than those stored at 4°C and air-tight buckets (Figure 3C). Multivariate analysis on bacterial loads in fufu revealed significant effect of duration of storage (F = 1,541.405, df = 1, P < 0.01; Table 5) and interaction of storage method and duration (F = 123.442, df = 4, P < 0.01; Table 5), no significant effect of storage methods (P > 0.05; Table 5).
3.4.2 Fungi
There was a consistent fungal growth throughout the 4 weeks of storage. For samples stored at 4°C, fungal colony forming units increased significantly (F = 161.754, df = 3 P < 0.01) from 25 CFUs in first week to 65.7 CFUs in fourth week. For fufu stored at 0°C, fungal load increased significantly (F = 161.744, df = 3, P < 0.01) from 6.4 CFUs in first week to 32 CFUs in fourth week. For fufu that was oven-dried, fungal load increased significantly (F = 150.553, df = 3, P < 0.001) from 2.4 CFUs in first week to 26.4 CFUs in fourth week. For fufu stored in LDPE bags, fungal load increased significantly (F = 1,394, df = 3, P < 0.01) from 35 CFUs in first week to 90.4 CFUs in fourth week (Figure 3D). For fufu stored in air-tight buckets, fungal load also increased significantly (F = 221.134, df = 3, P < 0.01) from 33 CFUs to 88.7 CFUs in fourth week. There was no significant difference between fungal load on fufu stored in LDPE bags and air-tight buckets across storage (Supplementary Figure S3). Fungal load in oven-dried fufu stored in LDPE bag and 0°C were significantly lower than those stored at 4°C, LDPE bags, and air-tight buckets (P < 0.01; Figure 3D). Multivariate analysis of fungal loads in fufu during storage revealed significant effects of storage methods (F = 34.975, df = 4, P < 0.01; Table 5), duration of storage (F = 2030.347, df = 1, P < 0.01; Table 5), and interaction of storage methods and duration (F = 58.359, df = 4, P < 0.01; Table 5).
3.5 Effect of storage methods on sensory properties of stored cassava fufu
3.5.1 Odour
After the first week of storage, acceptability of fufu based on odour was scored at nine for all samples under different storage conditions. By second and third weeks of storage, acceptability based on odour of fufu in LDPE bags dropped to 8.06 and 6.4, respectively. Acceptability score based on odour for fufu stored in LDPE bags dropped to 3.3 after the fourth week, indicating that it was not acceptable. Fufu stored in air-tight buckets had similar rate of change in acceptability based on odour as LDPE bags. In first week, hedonic score for acceptability based on odour was nine and was 8.4 in second week. By fourth week, acceptability of fufu stored in air-tight buckets based on odour was 4.2, implying that it was not acceptable based on deviation from its characteristic smell. For fufu stored at 4°C, the acceptability score dropped from nine in first week to eight in second week, 7.9 in third week and 4.9 in fourth week. Change from characteristic fufu smell was very slow for 0°C and oven-dried storage. After 4 weeks of storage, hedonic score for odour of oven-dried fufu was 8.5 and 7.9 for 0°C storage, indicating that fufu stored at 0°C and oven-dried were the most acceptable after 4 weeks (Figure 4A). The hedonic score for acceptability based on development of non-characteristic smell of fufu under different storage conditions indicates that acceptability was significantly higher (H = 12.341, df = 3, P < 0.05) at 0°C and oven-dried samples compared to LDPE bags, air-tight buckets, and 4°C.
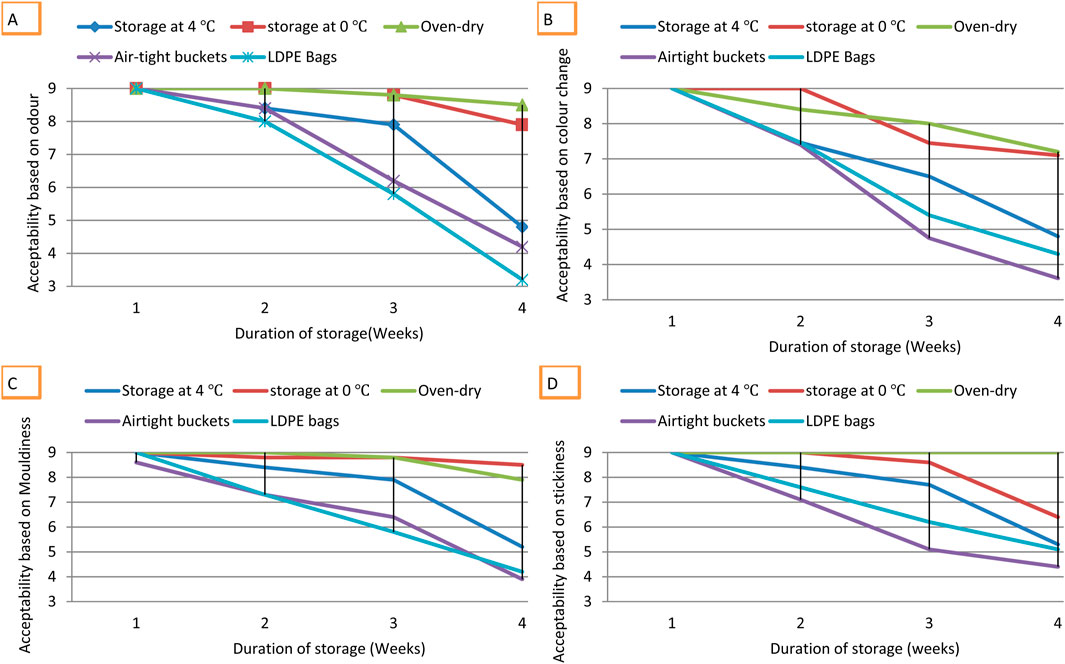
Figure 4. Acceptability of fufu based on (A) Odour (B) Colour change (C) Mouldiness and (D) stickiness in fufu under different storage conditions for 4 weeks.
3.5.2 Colour
Fufu stored in LDPE bags had the fastest colour change from white through cream white to yellow/brown. After first week of storage, acceptability based on colour was 9, indicating that it was highly liked. By second week, acceptability dropped to 7.46, and 4.75 by third week that is below the acceptable margin of 5, implying that fufu stored in LDPE bags was no more acceptable after 3 weeks. For fufu stored at 4°C, colour change caused decrease in acceptability from nine in first week to 7.46 in second, 6.14 in third week and 4.25 in fourth that is below the acceptable limit of 5. Fufu stored in air-tight buckets had slower colour change with acceptability score of nine in first week, 8.05 in second, and 5.12 by fourth week, implying that it was still slightly acceptable. Colour change was very slow in fufu stored at 0°C and oven-dried (Figure 3), with 7.1 score for fufu that was oven-dried or stored in LDPE bags after 4 weeks, while fufu stored at 0°C had 7.3, indicating the most acceptable after 4 weeks (Figure 4B). Hedonic score for acceptability based on colour change in stored fufu under different conditions indicates that acceptability was significantly higher (H = 15.350, df = 3, P < 0.05) for fufu stored at 0°C and oven-dried compared to those stored in LDPE bags, air-tight buckets, and 4°C (Figure 4B).
3.5.3 Acceptability of fufu based on mouldiness
Acceptability of fufu based on appearance of black spots (moulds) on decreased with time across storage period. The highest amount of mouldy appearance was observed in fufu stored in LDPE bags. Hedonic score for acceptability of fufu stored in LDPE bags dropped from 8.6 in first week to 4.2 in fourth week, with similar trend for fufu stored in air-tight buckets and 4°C (Figure 3C). Apart from fufu stored at 0°C, oven-dried and fufu stored in LDPE bags, the others scored below five by the fourth week. Kruskal Wallis test revealed significantly higher (H = 8.716, df = 3, P < 0.05) acceptability score for oven-dried or 0°C storage based on mouldiness as compared to fufu stored in LDPE bags, air-tight buckets, and 4°C (Figure 3C).
3.5.4 Acceptability of fufu based on stickiness (texture)
Fufu stored at 0°C and oven-dried had score of 8.9 and 9, respectively, throughout storage period, implying that they were the most acceptable storage methods (Figure 3D). Fufu stored in air-tight buckets and at 4°C were barely acceptable after 4 weeks and fufu stored in LDPE bags was not acceptable after 4 weeks (Figure 3D). Fufu stored at 0°C and oven-dried showed significantly higher acceptability based on stickiness storage in LDPE bags, air-tight buckets, and 4°C (H = 10.749, df = 3, P < 0.05).
3.6 Effect of storage methods on organoleptic properties of fufu
The acceptability of cooked fufu after 4 weeks of storage at 0°C or oven-dried were compared on the basis of taste, colour and stickiness (texture). Based on taste, fufu stored at 0°C had high hedonic scores of 8.6 and 8.0 for oven-dried fufu, indicating that both were liked and very acceptable. Based on colour, cooked fufu after storage at 0°C maintained its original white colour with acceptability score of 8.9 while oven-dried fufu exhibited slight colour change from characteristic white to cream white/brownish colour with 7.0 acceptability score (Figure 5). Oven-dried fufu was more acceptable with a score of 8.5, while the fufu stored at 0°C had an acceptability score of 7.9 based on stickiness. Kruskal wallis test showed significantly higher acceptability of fufu stored in the freezer at 0°C than fufu that were over dried.
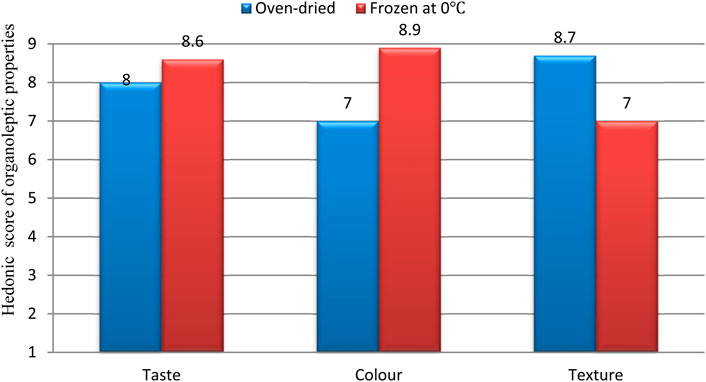
Figure 5. Acceptability of fufu based on organoleptic properties (e.g., taste, colour and stickiness).
4 Discussion
4.1 Effects of cassava fermentation methods on fufu yield
The results obtained strongly support the hypothesis that source of fermentation water and bitterleaf additive will enhance the quantitative yield of cassava fufu. The consistent decrease in pH (increase acidity) throughout cassava fermentation period indicates that fufu fermentation reduces pH of the effluent and final fufu product. The increase in acidity (decreased pH), temperature, turbidity and total dissolved solid of the fermentation effluent could be due to the effects of inherent microbes (Onwurafor et al., 2014). As fermentation progresses, production of lactic and acetic acids increases, resulting in direct acidification of the effluent and fufu product (Halake and Chinthapalli, 2020; Onwurafor et al., 2014). This is in line with Izah et al. (2016) where fermentation for ogi production and pH of the product reduced from 6.7 to 4. Oyewole and Ogundele (2001) also reported reduced pH from 6.8 to 3.8 in fufu production in Nigeria. Oyewole and Ogundele (2001) reported that fermentation was characterized by increased production of acids. The activity of lactic acid bacteria on carbohydrates of cassava roots was attributed to acid production during cassava fermentation (Shittu and Adedokun, 2010). This may be due to several microbes and enzymes such as polygalacturonase, pectinase and cellulase with tissue degrading activities and softening of products (Shittu and Adedokun, 2010).
The consistent increase in turbidity and total dissolved solids during fermentation could be due to cassava tissues breakdown and changes in physical and chemical characteristics of fermentation effluent (Okowa et al., 2016). This progressive increase in turbidity and total dissolved solids have been reported. Kigigha and Kombo (2016) reported that total dissolve solids and turbidity increased and pH decreased during fermentation as guinea corn, with increased total dissolved solids from 107.53 mg/L at the start of fermentation to 1,320.22 mg/L after 72 h. Okowa et al. (2016) also reported a similar trend during maize fermentation. The increased pH, temperature turbidity and total dissolved solids with corresponding increase in quantitative output of fufu in some of the effluents could be attributed to build-up of tissue-degrading enzymes and lactic acid bacterial (Shittu and Adedokun, 2010). Although the exact role played by bitterleaf in cassava fermentation is unknown, Udensi et al. (2019) reported that scented leaf (Occimum viridis) enhanced break down of tissue in cassava fermentation process.
The significantly high cassava waste after fermentation due to insufficient softness where fermentation effluent was changed every 2 days or daily could be attributed to the fact that effluent change stops or slows down microbial and enzyme activities involved in tissue breakdown. Changing water reduces effluent temperature and breaks the microbial build up process. Shittu and Adedokun (2010) reported that cassava softening during fermentation is due to enzymes and microbes that breakdown pectin and cellulose, and interrupting these activities slows the breakdown process. Meanwhile, tap water is usually treated with chemicals to reduce microbial growth and activities, which affects microbial build up during fermentation as compared to untreated borehole water with potentially more microbial communities and functions that may boost cassava fermentation. Also, bitterleaf additive may play a similar role played by scented leaf in cassava breakdown as reported by Udensi et al. (2019) who reported that scented leaf (Occimum viridis) enhanced break down of tissue in cassava fermentation process.
4.2 Effects of storage methods on acceptability of cassava fufu
The observed increase in microbial load on samples stores in LDPE bags, 4°C and air-tight buckets could be attributed to the fact that fungi and bacteria significantly reduce in storage at temperature below 4°C compared to 25°C (Abdul et al., 2018). Wang et al. (2023) reported that chilling food at 0°C and below could significantly reduce microbial load and render it safe for consumption. This higher microbial load associated with high temperature reported in this study is in line with Sama et al. (2023) who reported consistent bacterial and fungal growth on fufu stored in LDPE bags in Buea.
Significantly low microbial load on oven-dried fufu can be attributed to unfavourable environmental conditions (absence of moisture) that limited microbial growth (Coradi et al., 2020). Similarly, low microbial growth on samples stored at 0°C could be attributed to extremely low temperature that hinders microbial growth because few bacterial and fungal species can survive (Abdul et al., 2018). Coradi et al. (2020) reported that high temperature reduced microbial growth and spoilage in stored maize while Abdul et al. (2018) reported refrigeration as the best way to control microbes in stored products. Reduced moisture reportedly retard microbial growth (Padonou et al., 2009). The absence of moisture in dried fufu samples offered an unconducive environment for bacteria and fungi to thrive, which is consistent with Pellisssery et al. (2020) who recommended chilling temperatures for storage to reduce microbial growth. These results support our hypotheses that storing fufu at 0°C or oven-dried will improve consumer acceptability as compared to LDPE bags or air-tight buckets.
5 Conclusion
Low quantitative fufu yield when water was changed daily or every 2 days during fermentation resulted from insufficient softness of cassava for pulping that led to high wastes, which strongly discourages water change during fermentation. The use of borehole water or tap water + bitterleaf resulted in relatively higher quantitative outputs that should be encouraged in cassava fermentation to produce fufu. The significantly low bacterial and fungal load on fufu stored at 0°C or oven-dried highlights their ability to maintain fufu quality and boost storability. Fufu stored at 0°C or oven-dried had significantly higher consumer acceptability in terms of colour change, odour, mouldiness and texture when compared to the others. These results demonstrate the potential for long-term storage of cassava fufu through drying, especially where refrigeration is not available or too costly.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the studies involving humans because the study did not involve any vulnerable persons and all participants were above 18 years of age and gave their verbal/written informed consent to willingly participate in the study. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
VS: Conceptualization, Formal Analysis, Methodology, Writing – original draft, Writing – review and editing. EM: Supervision, Writing – original draft, Writing – review and editing. RN: Conceptualization, Supervision, Writing – original draft, Writing – review and editing. CN: Investigation, Methodology, Resources, Supervision, Validation, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We sincerely acknowledge the support of Miss Olougou Marie Noela Enyoe, Miss Ndakwe Abigail Tifu and all the members of the Rhizobiology Laboratory of the Faculty of Agriculture and Veterinary Medicine of the University of Buea.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abriba, C., Heshaw, E. E., Lenox, J., Eja, M., Okpoh, S. I., and Agbor, E. A. (2012). Microbial succession and odour reduction during controlled fermentation of cassava tubers for the production of foofoo, a staple food cunsumed popularly in Nigeria. J. Microbiol. Biotechnol. Res. 2 (4), 500–506. Available online at: www.scholarsresearchlibrary.com.
Addo, G. M., Mutala, A. H., and Badu, K. (2020). Comparison of microbiological and sensory qualities of ‘fufu’ processed from grinding machines and the traditional method at ayigya in the kumasi metropolis, Ghana. Int. J. Microbiol. Res. 30 (5), 20–26. doi:10.9734/MRJI/2020/v30i530216
Adegbehingbe, K., Fakoya, S., Marcus, B., Adeleke, B., Fagbohun, O., and Adejoro, D. (2019). Antibacterial properties of the predominant microorganisms isolated from fermenting cassava tubers during fufu production against selected enteropathogenic bacteria. Eur. J. Nutr. Food Saf. 9 (3), 287–296. doi:10.9734/ejnfs/2019/v9i330068
Buke, G. D., Anselimo, O. M., Onyango, A. N., Mathara, J. M., and Badaka, Q. D. (2021). Effect of temperature, storage containers and improved hygiene on the microbial safety and chemical quality of traditional meat products. Eur. J. Agric. Food Sci. 3 (4), 21–28.
Chijioke, U., Madu, T., Okoye, B., Ogunka, A. P., Ejechi, M., Ofoeze, M., et al. (2021). Quality attributes of fufu in south-East Nigeria: guide for cassava breeders. Int. J. Food Sci. Technol. 56, 1247–1257. doi:10.1111/ijfs.14875
Coradi, P. C., Maldener, V., and Lutz, E. (2020). Influences of drying temperature and storage conditions for preserving the quality of maize postharvest on laboratory and field scale. Sscience Rep. 10 (1), 18–25.
Emmanuel, T. (2013). “Enhancing cassava marketing and processing in Cameroon: drivers, constraints, and prospects of the value chain,” in Rebuilding West africa’s food potential. Editor A. Elbehri (FAO/IFAD).
Fao, IFAD, and Unicef, W. F. P. and W. H. O. (2021). The State of Food Security and Nutrition in the World 2021. Transforming food systems for food security, improved nutrition and affordable healthy diets for all. Rome: FAO. doi:10.4060/cb4474en
Fao, IFAD, and Unicef, W. F. P. and W. H. O. (2022). The State of Food Security and Nutrition in the World 2022. Repurposing food and agricultural policies to make healthy diets more affordable. Rome, FAO. doi:10.4060/cc0639en
Forsythe, L., Tufan, H., Bouniol, A., Kleih, U., and Fliedel, G. (2022). Quality attributes of fufu in South-East Nigeria: guide for cassava breeders. Int. J. Food Sci. Technol. 56, 1115–1123. doi:10.1111/ijfs.14680
Golvalve, L. de O., Julio, S. N., Zago, L., and Santana, I. (2024). Cassava, an illustrious (un)known: consumption of recipes with the root and its derived products. Int. J. Gastron. Food Sci. 35 (1), 100812. doi:10.1016/j.ijgfs.2023.100812
Halake, N. H., and Chinthapalli, B. R. (2020). Fermentation of traditional african cassava based foods: microorganisms role in nutritional and safety value. J. Exp. Agric. Int. 42 (9), 56–65. doi:10.9734/jeai/2020/v42i930587
Izah, S. C., Kigigha, L. T., and Okowa, I. P. (2016). Microbial quality assessment of fermented maize Ogi (a cereal product) and options for overcoming constraints in production. Biotechnol. Res. 2 (2), 81–93.
Kigigha, L. T., and Kombo, N. (2016). Changes in Microbial density and in-situ water quality parameters during fermentation of Guinea corn (Sorgum bicolor) medium. ASIO J. Microbiol. Food Sci. Biotechnol. Innovations 3 (1), 41–45.
Kutner, M. H., Nachtsheim, C. N., Neter, J., and Li, W. (2005). Applied linear statistical models. fourth ed. Mc Graw-Hill.
Laura, N., Coline, M., and Michael, O. (2010). The 9-point hedonic scale: are words and numbers compatible? Food Qual. Prefer. 21 (8), 1008–1015. doi:10.1016/j.foodqual.2010.05.017
Madu, T., Okoye, B., Onyemauwa, N., Bukeje, B., and Ofoeze, M. (2021). Consumer testing of fufu in rural and urban areas in Nigeria, in understanding the drivers of trait preferences and the development of multi-user RTB product profiles, WP1, step 4. RTBfoods Field Sci. Rep. Umudike, Niger., 20. doi:10.18167/agritrop/.00637
Njukwe, E., Hanna, R., Sarr, P. S., Shigeru, A., Kirscht, H., Mbairanodji, A., et al. (2014). Cassava value chain development through partnership and stakeholders’ platform in Cameroon. Int. J. Agric. Policy Res. 2 (11), 383–392. doi:10.15739/IJAPR.011
Njukwe, E., Nguenkam, A., Mbairanodji, A., Ngue-Bissa, T., and Hanna, R. (2012). “Improving food security and income and enhancing farmers’ livelihoods in Cameroon through the introduction and promotion of improved cassava germplasm,” in Proceedings of the 11th triennial symposium of the ISTRC-AB, kinshasa, DRC. Editors R. U. Okechukwu, and P. Ntawuruhunga, 289–292.
Nweke, F. I., Dunstan, S. C., Spencer, S., and Lynam, J. K. (2002). The cassava transformation africa’s best kept secret. Michigan State University Press, 60–65.
Obadina, A. O., Oyewole, O. B., and Odusami, A. O. (2009a). Microbiological safety and quality assessment of some fermented products (lafun, fufu, garri). Sci. Res. Essays 4 (5), 432–435. Available online at: http://wwwacademicjournals.org/SRE.
Obadina, A. O., Oyewole, O. B., and Odusami, T. (2009b). Microbiological safety and quality assessment of some fermented products (lafun, fufu, garri). Sci. Res. Essays 4 (5), 432–435. Available online at: http://wwwacademicjournals.org/SRE.
Obadina, A. O., Oyewole, O. B., Sanni, L. O., and Keith, T. I. (2006). Biopreservative activities of L. plantarum strains in Fermenting Cassava ‘fufu. Afr. J. Biotechnol. 5 (8), 620–623.
Odom, T. C., Udensi, E. A., and Nwanekenze, E. C. (2012). Microbiological quality of hawked retted cassava fufu in Aba Metropolis of Aba state. Niger. Food J. 30 (1), 53–58.
Ogiehor, I. S., and Ikenebomeh, M. J. (2005). Extension of shelf life of garri by hygienic handling and sodium benzoate treatment. Afr. J. Biotechnol. 4 (7), 744–748. doi:10.5897/ajb2005.000-3135
Ogunyinka, O., and Oguntuase, A. (2020). Analysis of cassava production and processing by various groups in support of cassava value chain in the south west of Nigeria. ISABB J. Food Agric. Sci. 9, 11–19. doi:10.5897/ISABB-JFAS2020.0113
Okowa, l. P., Kigigha, L. T., and Izah, S. C. (2016). Variation in physic0-chemical water quality during fermentation of maize for ogi production. Biotechnol. Res. 2 (3), 125–131.
Onwurafor, E. U., Onweluzo, J. C., and Ezeoke, A. M. (2014). Effect of fermentation methods on chemical and microbial properties of mung bean (vigna radiata) flour. J. Niger. Inst. Food Sci. Technol. 32 (1), 89–96. doi:10.1016/s0189-7241(15)30100-4
Oyewole, O. B., and Ogundele, S. L. (2001). Effect of length of fermentation on the functional characteristics of fermented cassava 'fufu'. J. Food Technol. Afr. 6 (2), 38–40. doi:10.4314/jfta.v6i2.19283
Padonou, S. W., Hounhouigan, J. D., and Nago, M. C. (2009). Physical, Chemical and Microbial characreristics of lafun produced in Benin. Afr. J. Biotechnol. 8 (2), 124–129.
Pellisssery, A. J., Vina yamohan, P. G., Amalaradjou, M. A. R., and Venkitanayaranan, K. (2020). “Chapter 17 – spoilage bacteria and meat quality,” in Meat quality analysis. Editors A. K. Biswas, and P. k. Mandal (Cambridge MA: Academic Press), 307–334.
Sama, V., Molua, E. L., Nkongho, R. N., and Ngosong, C. (2023). Potential of sodium benzoate additive to control food-borne pathogens and spoilage microbes on cassava (Manihot esculenta Crantz) fufu and shelf-life extension. J. Agric. Food Res. 11 (1), 100521–100529. doi:10.1016/j.jafr.2023.100521
Shittu, T. A., and Adedokun, I. I. (2010). Comparative evaluation of the functional and sensory characteristics of three tradition fermented cassava products. J. Nat. Sci. Eng. Technol. 9 (2), 106–116.
Udensi, J. U., Ebe, T., Ugochukqu, G. M., Awurum, I. N., Mgbemena, I. C., Aroh, K., et al. (2019). Enhancement of cassava fermentation using nail and scent leaf (Occimum viridis). Int. J. Adv. Res. 7 (10), 1128–1136. doi:10.21474/ijar01/9933
Keywords: acceptability, cassava, fermentation, fufu, storage
Citation: Sama V, Molua EL, Nkongho RN and Ngosong C (2025) Cassava (Manihot esculenta) fermentation methods affected fufu yield, while drying and cold storage extended the shelf-life and consumer acceptability. Front. Food Sci. Technol. 5:1400395. doi: 10.3389/frfst.2025.1400395
Received: 17 April 2024; Accepted: 17 April 2025;
Published: 30 April 2025.
Edited by:
Serap Cosansu, Sakarya University, TürkiyeReviewed by:
George Abong, University of Nairobi, KenyaNnan Diby, Universite Peleforo Gon Coulibaly, Côte d'Ivoire
Copyright © 2025 Sama, Molua, Nkongho and Ngosong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valentine Sama, c2FtYXZhbGVudGluZTM2QGdtYWlsLmNvbQ==
 Valentine Sama
Valentine Sama Ernest L. Molua
Ernest L. Molua Raymond Ndip Nkongho
Raymond Ndip Nkongho Christopher Ngosong
Christopher Ngosong