- College of Horticulture, Shenyang Agricultural University, Shenyang, China
Background: “Nine-steaming and nine-drying” is a pharmaceutical processing technique for Polygonatum sibiricum Redouté. It is an incomplete study that the effect of the method on the quality of P. sibiricum Red. is only evaluated by the changes in the contents of several components. It is necessary to explore the chemical transformations during the nine cycles of steaming and drying of P. sibiricum Red., and the relationship between the chroma and internal chemical compositions.
Methods: This study investigated the effects of the traditional nine-steaming-nine-drying method on the morphology, metabolomic profile, and medicinal components of P. sibiricum Red.
Results: Through non-targeted metabolomics analysis, 685 metabolites were identified, with 492 showing differential abundance, particularly amino acids and derivatives, lipids, carbohydrates, and phenols or flavonoids. These alterations resulted in darker coloration, as evidenced by a significant decrease in L* values, elimination of tongue-numbing effects, enhanced sweetness, and improved quality of P. sibiricum Red. The processing resulted in a significant decrease in the relative content of lipids, polysaccharides, oligosaccharides, amino acids and their derivatives, while an increase in flavonoids, saponins, polyphenols, 5-HMF and monosaccharides. The browning of P. sibiricum Red. subjected to steaming and drying mainly correlated with Maillard reaction. Fructose, erucic acid, palmitic acid, linoleic acid, pipecolic acid, L-proline, L-leucine, (5-L-Glutamyl)-L-glutamate, 5-HMF, flavonoids, polyphenols, saponins and flavor compounds, these metabolites and their derivatives can served as the main biomarkers for evaluating the quality of P. sibiricum Red. subjected to steaming and drying. However, excessive steaming and drying caused the bitter and sour flavor intensified, and the quality to deteriorate. Comprehensive analysis revealed that the quality of P. sibiricum Red. was optimal after the seventh cycle of steaming and drying, and the L* value ranged from 21.0 to 22.0, the a* value ranged from 2.0 to 5.0, the C* value ranged from 8.0 to 10.0.
Conclusion: This research elucidated the mechanisms through which steaming and drying affected the morphology and quality of P. sibiricum Red., established a theoretical foundation for quality assessment of processed P. sibiricum Red.
1 Introduction
Polygonatum sibiricum Red., commonly known as Siberian Solomon’s seal, is an important plant used in both traditional medicine and as food (Editing Committee of Chinese Flora Chinese Academy of Sciences, 1978; Chinese Pharmacopoeia Commission, 2020). It was historically documented in Li Shizhen’s Bencao Gangmu, a classical text of traditional Chinese medicine (TCM), and has been utilized both medicinally and nutritionally in ancient China. Its applications extend across East Asia and also appear in India’s Vedic medicine. P. sibiricum Red. contains numerous bioactive compounds, including polysaccharides, saponins, flavonoids, polyphenols, alkaloids, and cardiac glycosides (Sun et al., 2005; Chen et al., 2018; Tang et al., 2019; Zhang et al., 2022; Cao et al., 2024; Liu H. P. et al., 2024; Zhou et al., 2024). In TCM theory, P. sibiricum Red. is believed to tonify qi and nourish yin, thereby supporting the functions of the spleen, lung, and kidney (Chinese Pharmacopoeia Commission, 2020). Contemporary pharmacological studies have shown that P. sibiricum Red. exhibits antibacterial, anti-inflammatory, antioxidant, and antitumor properties. It also exerts beneficial effects on the cardiovascular, nervous, and immune systems, supporting its use in promoting general health (Du et al., 2016; Peng et al., 2018; Zhao et al., 2019; Wang et al., 2023; Fei et al., 2024; Jiang et al., 2024; Lai et al., 2024; Li et al., 2024; Lin et al., 2024; Pan et al., 2024; Xiao et al., 2024; Li et al., 2025; Zhao et al., 2025).
Unprocessed P. sibiricum Red. rhizomes contain mucous substances that may induce adverse reactions, including tongue numbness, throat irritation, and skin itching. To mitigate these side effects and enhance the concentration of bioactive constituents, the technique of “nine-steaming-nine-drying” is commonly employed (Yu et al., 2018; Guan et al., 2024). Many herbal monographs described the process of nine-steaming-and-nine-drying, as the Compendium of Materia Medica, however, the ancient processing took longer time and relied on experience to judge the quality of the processed products, while ancient Chinese herbal medicine monographs described the standards for nine-steaming-nine-drying, such as “honey-like sweetness, dark coloration, luster and softness”, these sensory-based criteria alone lacked the objectivity and standardization required for modern mass production.
During the processing of steaming and drying, the browning is a widespread color-changing phenomenon (Wan and Liu, 2019; Chen L. T. et al., 2024). The mechanism of food browning can be divided into two major categories: enzymatic browning and non-enzymatic browning. Enzymatic browning is caused by phenolases catalyzing the formation of quinones and their polymers from phenols, this reaction usually occurs in fresh plant tissues rapidly and requires the involvement of oxygen, and the phenolases are relatively heat-resistant, and can be inactivated at 100 °C for 2–8 min (Wang F. et al., 2025; Arnold et al., 2025). Non-enzymatic browning mainly occurs in a series of reactions when carbohydrates are subjected to heating, resulting in the production of dark-colored melanoids, volatile compounds, etc. Maillard Reaction, a complex reaction, where amino compounds (proteins, peptides, amines, and amino acids) react with carbonyl compounds (reducing sugars, lipids, aldehydes, ketones, polyphenols, ascorbic acids, and steroids) to produce various compounds, including melanoids, ketones, aldehydes and heterocyclic compounds, these compounds play an extremely important role in the formation of the quality of food, including its chemical composition, color, aroma, taste, etc., (Pan and Melton, 2007; Jiang et al., 2022; Yang et al., 2024; Wang W. et al., 2025). Without the presence of amino compounds, the aldoses or ketoses subjected to the steaming or other high-temperature processing will also undergo the caramelization reaction, resulting in the caramels, furfurals or melanins through the etherification, dehydration, cleavage and polymerization of saccharides (Taş and Gökmen, 2017; Dilara and Gökmen, 2022). In addition, the ascorbic acid reaction and oxidative polymerization of phenolic substances can cause the non-enzymatic browning. The processing of P. sibiricum Red. rhizomes inevitably alters their chemical composition and morphology. Previous studies have examined changes in polysaccharides, xanthine 5-hydroxymethylfurfural (5-HMF), and saponins content (Deng et al., 2024; Ren et al., 2025) showed that the two innovative streaming methods for Polygonatum cyrtonema rhizome resulted in the degradation of polysaccharides and the increase in the contents of 5-HMF, reducing sugar, amino acid, phenolics and diosgenin. Chemical constituent modifications under various processing methods significantly influence pharmacological effects (Ren et al., 2025). However, the chemical transformations during the nine cycles of steaming and drying of P. sibiricum Red., and the relationship between the chroma and internal chemical compositions remain unexplored.
Metabolomics, with its high sensitivity and throughput, enables comprehensive profiling of metabolites from biological samples, offering insights and facilitating differential metabolite analysis. This study employed spectrophotometry, colorimetry, and non-targeted metabolomics to investigate chemical variations during P. sibiricum Red. rhizome processing, aiming to establish a theoretical foundation for standardizing processing technology and quality evaluation of steamed and dried P. sibiricum Red.
2 Materials and methods
2.1 Preparation of samples
Three-year-old P. sibiricum Red. were collected from Yingemen Town, Qingyuan County, Fushun City, China, and identified as P. sibiricum Red. by Professor Zhongyun Piao of Shenyang Agricultural University (Voucher specimen number PE 02089832). Rhizomes of uniform size (length: 8.0 cm–20.0 cm diameter: 1.0 cm–2.0 cm) were selected, cleaned, steamed (100 °C–105 °C) and dried in a drying oven (JINGHONG DHG-9140A; Shanghai, China) at 50 °C according to Table 1 specifications, labeled S0-S9. The epidermis of the rhizomes subjected to each cycle of drying remained dry while the interior was soft and tender. During processing, partial samples were collected after each cycle of steaming and drying, dried to water content≤8%, cut into small segments, freeze-dried, ground to a powder, sifted through a 60-mesh sieve, and stored at −80 °C.
2.2 Color measurement
Color measurements of P. sibiricum Red. powder was performed using a colorimeter (Chroma Meter CR-3; Minolta Corp., NJ, United States), which recorded lightness (L*), redness (a*), and yellowness (b*) values across samples subjected to different cycles of nine-steaming-nine-processing. Dried P. sibiricum Red. powder (2.5 g) was placed in a quartz dish (diameter: 3 cm) and the colourimeter probe placed below. Calibration was performed using a white standard plate. The colorimetric value (C) was calculated using Formula 1.
2.3 Quantification of saponins, polysaccharides, flavonoids and phenolic compounds
2.3.1 Total saponins
The dried P. sibiricum Red. powder (0.5 g) was added to 20 mL of 90% ethanol to extract saponins, then the mixture was ultrasonically extracted (40 kHz, 70 W) for 30 min at 60 °C. After centrifugation, the precipitation was digested with 15 mL distilled water, and then petroleum ether was used to dehydrate it once with an equal volume. The lower layer liquid was retained, and the upper layer was extracted three times with 15 mL of water-saturated n-butanol. The upper layer was evaporated and then fixed with methanol. Finally, the absorbance values were determined at 530 nm using the vanillin-glacial acetic acidperchloric acid colorimetric method (Wan et al., 2013).
2.3.2 Total polysaccharides
The dried P. sibiricum Red. powder (0.5 g) was added to 15 mL distilled water to extract polysaccharides, then the mixture was ultrasonically extracted (40 kHz, 90 W) for 30 min at 60 °C, centrifuged (3,600 r/min, 10 min), and retained the supernatant. This process was repeated 5 times. All the supernatant was then removed proteins with a trichloromethane-n-butanol solution (4:1) with a volume ratio of 5:1, finally total polysaccharides were separated with 80% ethanol and dissolved in 50.0 mL of distilled water. The absorbance of the resulting solution was measured at 490 nm using the phenol-sulfuric acid colorimetric method.
2.3.3 Total flavonoids
The dried P. sibiricum Red. powder (0.5 g) was added to 20 mL methanol to extract flavonoids, then the mixture was ultrasonically extracted (40 kHz, 80 W) for 30 min at 60 °C. After centrifugation, the upper layer liquid was retained, then the absorbance values were determined at 510 nm using the sodium nitrite-aluminum nitrate colorimetric method (Wan et al., 2013).
2.3.4 Total phenols
The dried P. sibiricum Red. powder (0.5 g) was added to 20 mL 60% ethanol to extract phenols, then the mixture was ultrasonically extracted (40 kHz, 80 W) for 30 min at 60 °C. After centrifugation, the upper layer liquid was retained, then the absorbance values were determined at 760 nm using the Folin-Ciocalteau’s reagent colorimetric method (Li et al., 2022).
2.4 Metabolomic analysis
P. sibiricum Red. powder (20.0 mg) was accurately weighed and added to 600 µL MeOH containing 2-Amino-3-(2-chloro-phenyl)-propionic acid (4 ppm), vortexed for 30 s, followed by the addition of 100 mg glass bead. The mixture was placed in a tissue grinder for 90 s at 60 Hz, extracted for 15 min using ultrasound at room temperature, and centrifuged for 10 min at 12,000 rpm and 4 °C. The supernatant was filtered through a 0.22 µm membrane and transferred into the detection bottle for Liquid chromatography-mass spectrometry (LC-MS) analysis.
LC-MS analyses were performed using an ultra-high performance liquid chromatography (UPLC) system (Vanquish, Thermo Fisher Scientific, United States) with an ACQUITY UPLC® HSS T3 (2.1 × 150 mm, 1.8 µm) (Waters, Milford, MA, United States) column connected to Thermo Orbitrap Exploris 120 mass spectrometry detector (Thermo Fisher Scientific, United States). The column was maintained at 40 °C, with a flow rate of 0.25 mL/min and an injection volume of 2 μL. For LC-electrospray ionization (ESI) (+)-MS analysis, the mobile phases consisted of (B2) 0.1% formic acid in acetonitrile (v/v) and (A2) 0.1% formic acid in water (v/v). Separation was conducted under the following gradient: 0–1 min, 2% B2; 1–9 min, 2%–50% B2; 9–12 min, 50%–98% B2; 12–13.5 min, 98% B2; 13.5–14 min, 98%–2% B2; 14–20 min, 2% B2. For LC-ESI (−)-MS analysis, the analytes were separated using (B3) acetonitrile and (A3) ammonium formate (5 mM). Separation was conducted under the following gradient: 0–1 min, 2% B3; 1–9 min, 2%–50% B3; 9–12 min, 50%–98% B3; 12–13.5 min, 98% B3; 13.5–14 min, 98%–2% B3; 14–17 min, 2% B3. The ESI source conditions were set as follows: 30 arb of sheath gas, 10 arb of auxiliary gas, capillary temperature 325 °C, spray voltage 3.50 kV (positive) or −2.50 kV (negative), full MS resolution of 60, 000 with a primary ion scanning range of 100–1,000 m/z, secondary resolution of 15,000.
The filtered data were analyzed using Microsoft Excel 2019, SPSS 19.0, and SIMCA-P software for statistical tests. The heatmap was generated using Multiple Experiment Viewer (4.9.0). Differentially abundant metabolites (DAMs) were identified based on criteria of VIP ≥1, P value <0.05, and |log2 (fold change (FC))| ≥1.
2.5 Statistical analysis
The quality of P. sibiricum Red. subjected to nine cycles of steaming and drying was evaluated using PCA. The membership function value U(Xij) of each individual index was calculated according to Formula 2, and the correlation and statistical frequency distribution among indices were determined for PCA. The weight (Wi) of each comprehensive index and the D-value (comprehensive evaluation value) were calculated according to Formulas 3, 4, respectively, to evaluate the quality of P. sibiricum Red. subjected to different cycles of steaming and drying.
The experiments were conducted in triplicates and expressed and mean ± SD. Statistical analyses of experimental data were performed using IBM SPSS statistical software. Duncan’s tests were conducted to evaluate significant differences at the 5% level. Graphical representations were generated using Origin Lab software (Origin Lab, MA, United States).
3 Results and discussions
3.1 The effects of steaming and drying on the morphology of Polygonatum sibiricum Red. rhizomes
The traditional Chinese medicinal technique, known as “nine-steaming-nine-drying”, is commonly employed to mitigate adverse reactions, increase bioactive components, and enhance pharmaceutical effects of P. sibiricum Red. (Yu et al., 2018; Guan et al., 2024). However, this process alters the external appearance (e.g., color, luster) and internal texture (e.g., hardness, moisture content) of the rhizomes, posing challenges to standardization (Liu et al., 2020; Lu, 2021; Yu et al., 2023). In this study, unprocessed P. sibiricum Red. (S0) exhibited a yellowish-white color with tongue-numbing properties, color, as illustrated in Figure 1, after the third steaming (S3), the rate of color change decreased. Following the fourth steaming (S4), the tongue-numbing sensation dissipated, and the sweetness intensified. After the seventh steaming (S7), bitter and sour flavor notes began to develop. Previous studies found that the tongue-numbing sensation might be related to the mucilage, positive hexanal, camphene, saponins and calcium oxalate needle crystals, and polysaccharides are the main components of mucilage, therefore, in this study, the disappeared tongue-numbing sensation might be related to the decreased polysaccharides (Figure 8A) and the cracking of the calcium oxalate needle crystals dispersing within the mucous cells (Chen, 2023). The bitter flavor is usually related to the alkaloids, terpenoids, glycosides, amino acids and peptides (Germán, 2024; Luo et al., 2025; Bureau et al., 2025). As shown in Figure 2A, this investigation revealed that during processing, total content of the main bitter substances including alkaloids, glycosides, some bitter amino acids and peptides exhibited an increasing trend. The sour flavor might be related to the organic acids, as the gluconic acid, and the increasing trend was also observed for sour substances in processed P. sibiricum Red. as the cycles of steaming and drying increased (Figure 2B; Yan et al., 2018; Yan et al., 2024). These findings suggest that moderate processing can effectively ensure the quality and flavor of P. sibiricum Red.

Figure 1. Differences in the morphology of Polygonatum sibiricum Red. subjected to nine cycles of steaming and drying. S0 refers to the raw Polygonatum sibiricum Red. samples. S1-S9 refer to the nine cycles of steamed and dried Polygonatum sibiricum Red. samples.
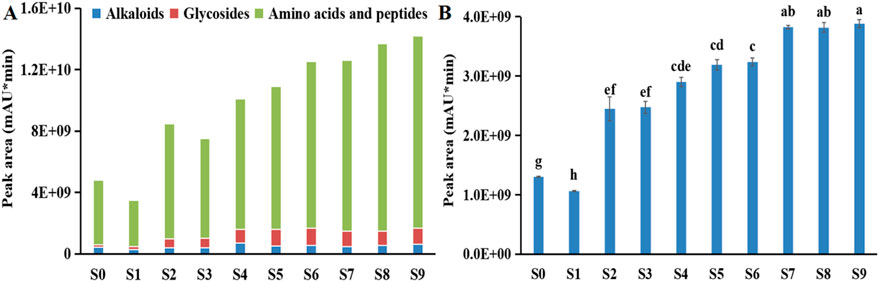
Figure 2. Change of contents of (A) main bitter substances and (B) organic acids during processing of Polygonatum sibiricum Red.
To quantify color changes during processing, a colorimeter was utilized to measure color changes in P. sibiricum Red. rhizomes subjected to different cycles of steaming and drying. L* represents color lightness, ranging from black (L* = 0) to white (L* = 100). The degree of blackening increased as the L* value decreased rapidly before the fourth steaming (S4), as shown in Figure 3A, after S4, the L* value stabilized between 20 and 24, indicating that five-steaming-five-drying maximally reduced product brightness. Positive and negative a* values indicated red and green, while positive and negative b* values denoted yellow and blue, respectively. The a* values of S0-S9 (Figure 3C), ranging from 36.13 to −14.08, peaked at the fifth steaming (S5), with browning intensity increasing as a* values rose, followed by a sharp decline. The elevated a* value may be attributed to browning during processing (Pan and D. Melton, 2007), the negative a* value revealed the red component had faded away, and the color had been changed to dark black. The b* values, ranging from 47.72 to −10.15 (Figure 3D), reached a maximum at the second steaming (S2), with color intensity increasing as b* values rose, then declined sharply, likely due to P. sibiricum Red.’ gradual transition from yellow to black. The C value, represents the purity or saturation of a color, it indicates the proportion of spectral colors, the higher the C value, the higher the purity or saturation of the color. The C value of S0-S5, as shown in Figure 3B, ranged from 50.54 to 30.69, then declined sharply, indicated that reasonable steaming and drying potentially maintained the product’s color saturation to the greatest extent. These findings offer a quantitative basis for implementing precise quality control during processing, improving upon empirical judgment by addressing the challenge of distinguishing appearance changes between consecutive steaming and drying cycles with the naked eye (Liu et al., 2020; Guan et al., 2024).
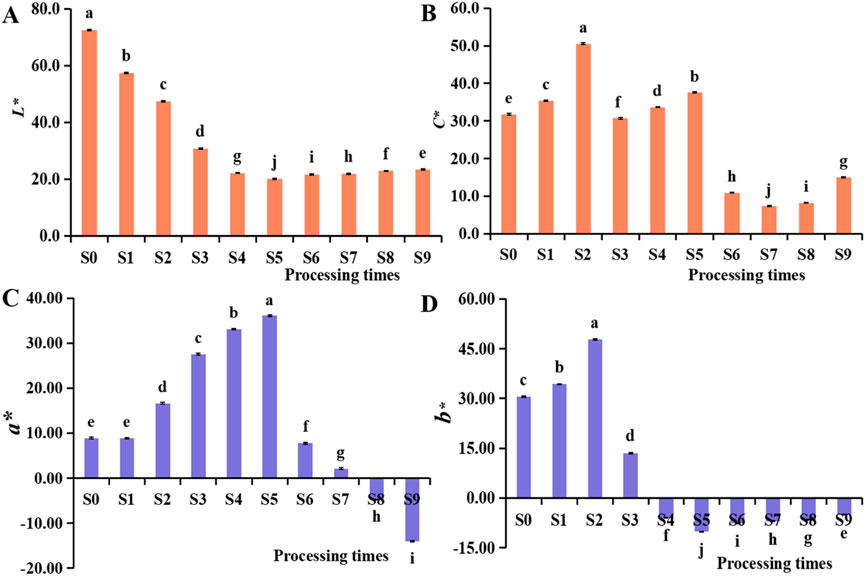
Figure 3. Differences in the colour (A–D) of Polygonatum sibiricum Red. subjected to nine cycles of steaming and drying. a-j refer to the signiffcant differences among the different steamed and dried Polygonatum sibiricum Red. samples (P < 0.05).
3.2 Effect of steaming and drying on the metabolic phenotype of Polygonatum sibiricum Red.
3.2.1 Metabolite overview of steamed and dried Polygonatum sibiricum Red.
Different steaming and drying methods can induce complex compositional changes and significant alterations in pharmacological action (Guan et al., 2024; Deng et al., 2024). To elucidate the mechanisms underlying quality differences among processed P. sibiricum Red., a non-targeted metabolism study examined metabolite alterations in samples treated with different steaming and drying cycles. The analysis detected 685 metabolites categorized into 42 classes, with the most prevalent being ‘lipids and lipid-like molecules’ (14.01%), ‘Carbohydrates’ (14.31%), and ‘Amino acids, peptides, and analogs’ (18.54%) (Figure 4). Principal component analysis (PCA) was conducted to evaluate metabolite profile variations across the nine processed samples (Figure 5). During processing, sample color darkened progressively, with notable differences observed between unprocessed P. sibiricum Red. and the first five processing cycles. In contrast, S6, S7, S8, and S9 showed overlapping PCA distributions, suggesting comparable metabolite profiles, which was further confirmed by hierarchical cluster analysis (Figure 6).
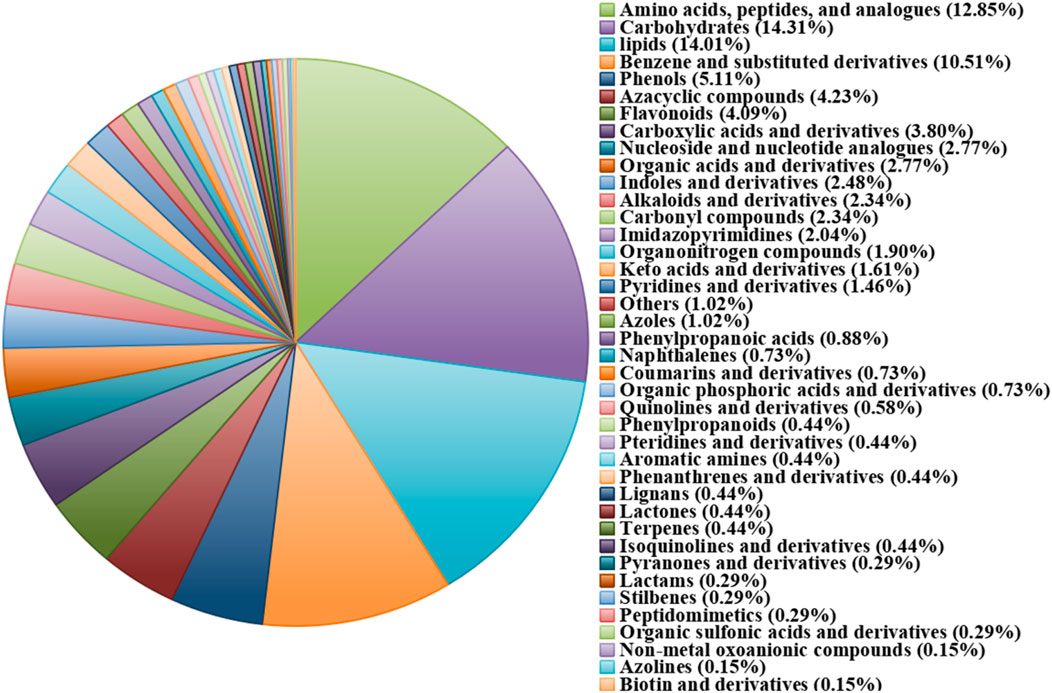
Figure 4. Pie plot of metabolite classiffcation and proportion of Polygonatum sibiricum Red. subjected to nine cycles of steaming and drying.
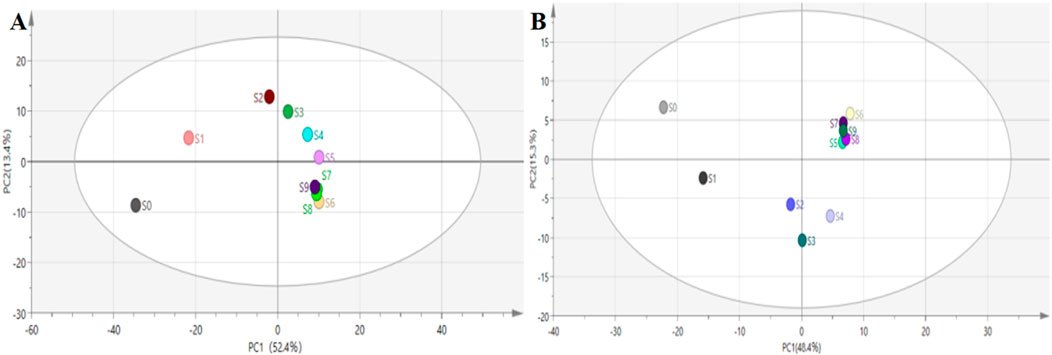
Figure 5. Metabolite overview of Polygonatum sibiricum Red. subjected to nine cycles of steaming and drying. (A) Principal component analysis (PCA) of the LC–MS identified metabolites in positive ion mode; (B) Principal component analysis (PCA) of the LC–MS identified metabolites in negative ion mode.
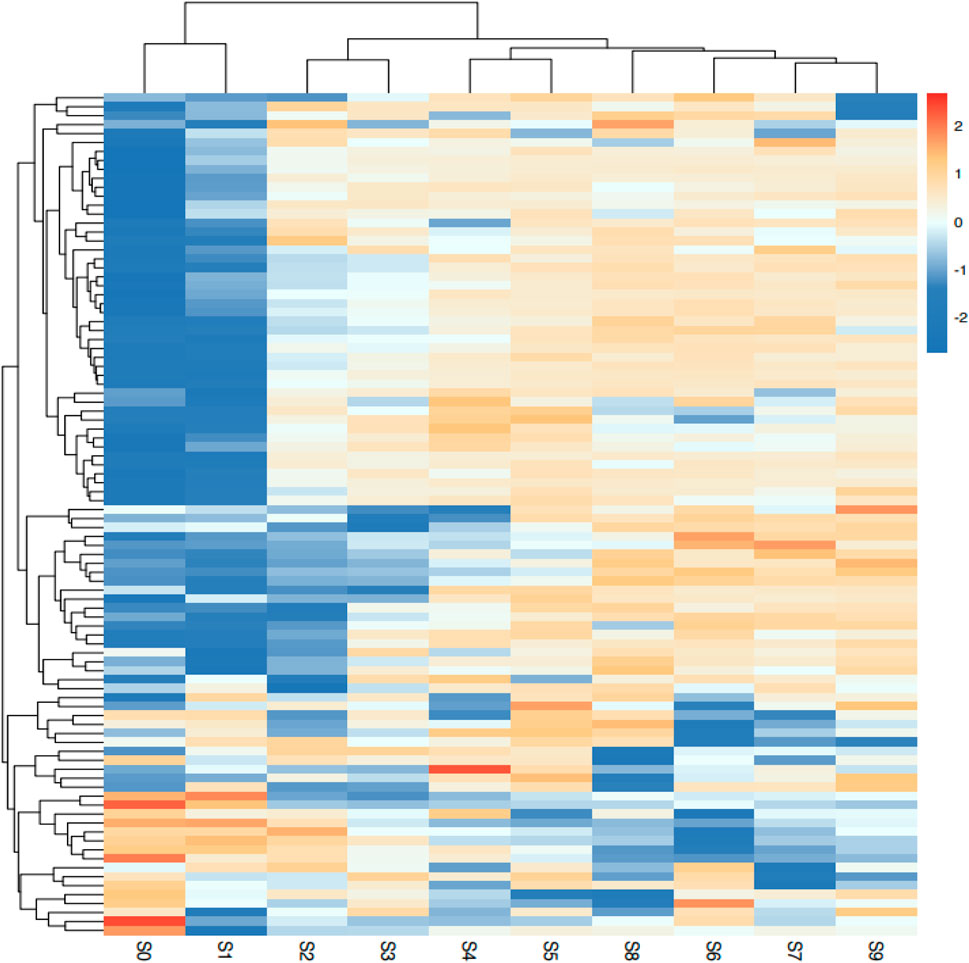
Figure 6. Clustering heatmap of the levels of all the identified metabolites of Polygonatum sibiricum Red. subjected to nine cycles of steaming and drying.
3.2.2 Differentially abundant metabolites
Variations in the relative abundance of 14 metabolite classes in P. sibiricum Red. subjected to nine cycles of steaming and drying were analyzed (Figure 7A). Lipid content peaked at the two-steaming-two-drying stage (S2) before decreasing significantly. The relative content of phenols, carbohydrates, carboxylic acids, and derivatives demonstrated an initial increase followed by a decrease, while the relative content of keto acids and derivatives increased gradually. After the fifth steaming (S5), the tongue-numbing sensation disappeared, sweetness became more pronounced, and bitterness and sourness began to emerge after the seventh steaming (S7). Compared to the S0, the relative content of 6 classes of metabolites in S7, including lipids, organic acids and derivatives, imidazopyrimidines, amino acids, peptides, and analogs, carboxylic acids and derivatives, organonitrogen compounds alkaloids and derivatives, decreased significantly. Conversely, carbohydrates, nucleosides, and nucleotide analogs increased significantly, and the color progressively darkened.
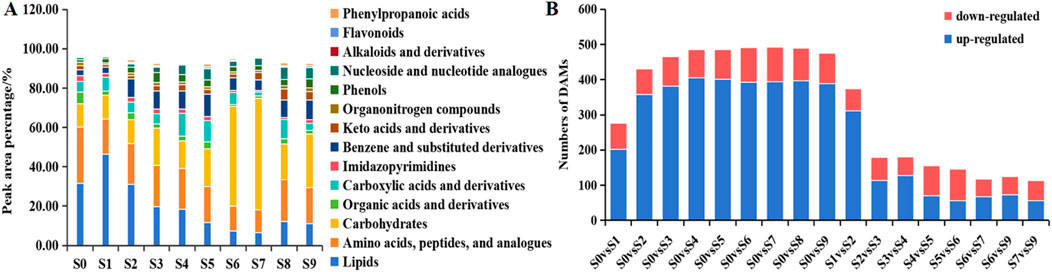
Figure 7. Differentially abundant metabolites (DAMs) in Polygonatum sibiricum Red. subjected to nine cycles of steaming and drying. (A) the variation in relative content of 14 classes of metabolites, (B) the number of DAMs.
To determine metabolite differences among the nine cycles of steaming and drying P. sibiricum Red., metabolite abundance was compared pairwise among the seventeen sample groups. Metabolites with VIP ≥1, P value <0.05, and |log2 (fold change (FC))| ≥1 were designated as differentially abundant metabolites (DAMs). As shown in Figure 7B and Supplementary Tables S1–S17 DAMs were identified across various comparisons: 276 in S0vsS1, 430 in S0vsS2, 466 in S0vsS3, 485 in S0vsS4, 486 in S0vsS5, 491 in S0vsS6, 492 in S0vsS7, 490 in S0vsS8, 476 in S0vsS9, 375 in S1vsS2, 179 in S2vsS3, 181 in S3vsS4, 156 in S4vsS5, 146 in S5vsS6, 118 in S6vsS7, 125 in S6vsS9, and 114 in S7vsS9. More abundant differences were observed in the first five cycles of steaming and drying samples, suggesting that seven-steaming-seven-drying may effectively produce abundant metabolites in P. sibiricum Red.
The top 10 DAMs showing the highest variations were selected as biomarkers to distinguish P. sibiricum Red. processed through different steaming and drying cycles (Figures 8A–F). The DAMs which appeared in the negative side of the axes meant the upregulated metabolites in the former sample compared with the latter, in contrast, those who appeared on the positive side meant the downregulated metabolites. As shown in Figure 8A, compared with S0, gentiobiose and other 9 DAMs downregulated significantly in S1, sphinganine and other DAMs. Compared with S1 (Figure 8B), two flavonoids, hesperidin and orientin upregulated significantly in S2, N-acetylaspartylglutamate, cellopentaose and other DAMs downregulated significantly. These DAMs showing the highest variations, representing the main downregulated or upregulated metabolites across all samples, mainly belonged to the lipids, amino acids, peptides, analogs, carbohydrates, carbonyl compounds, phenols or flavonoids, indicating their significance as the characteristic metabolites. These results provide clear insights into the differential metabolites after steaming and drying. The variations in metabolite accumulation and degradation among differently processed P. sibiricum Red. samples may correlate with steaming temperature, steaming time, drying temperature, and drying time.
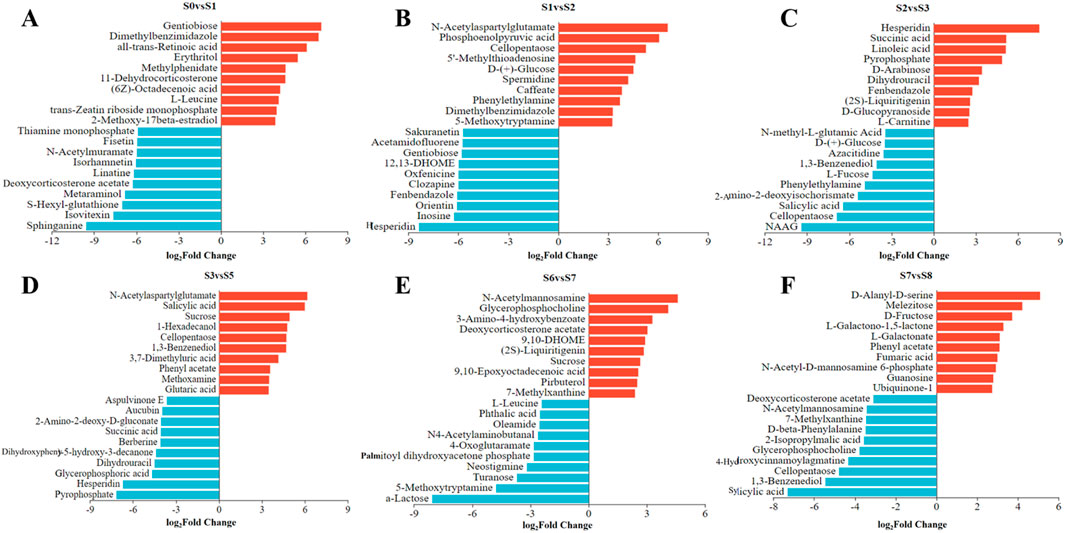
Figure 8. The plus and minus coordinate bar chart analysis of the top10 DAMs in Polygonatum sibiricum Red. subjected to nine cycles of steaming and drying (A-F).
3.3 Effect of steaming and drying on the saccharides of Polygonatum sibiricum Red.
Saccharides, including polysaccharides, oligosaccharides and monosaccharides, are the most important constituents of P. sibiricum Red. (Wang et al., 2017; Zhao et al., 2020; Zhao et al., 2020b). Polysaccharides exhibit blood sugar regulation and anti-cancer properties, representing significant medicinal components of P. sibiricum Red. (Jin et al., 2018; Chinese Pharmacopoeia Commission, 2020; Xiao et al., 2024; Wu et al., 2024). Research indicates that steaming treatment significantly affects both polysaccharides and their antioxidant properties (Fan et al., 2020; Wu et al., 2024; Zhang Q. H. et al., 2024). During processing, the polysaccharides showed a decreasing trend (Figure 9A). The content of total polysaccharides decreased significantly in S1−S3, decreased slowly in S4−S7, compared to the S0, the content of polysaccharides in S9 decreased by 47.38%.
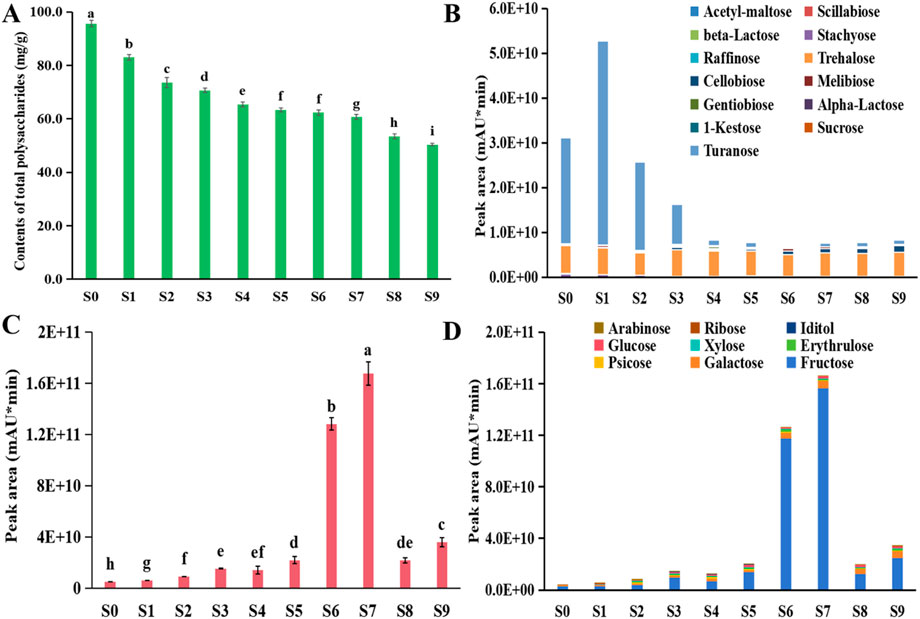
Figure 9. Change of contents of (A) total polysaccharides, (B) thirteen main oligosaccharides, (C) total monosaccharides, and (D) nine main monosaccharides during processing of Polygonatum sibiricum Red.
The content of thirteen main oligosaccharides showed dynamic changes (Figure 9B), decreased slowly in S2, S4−S8, compared to the S0, whereas had no significant decreasing in S1, S3 S9; among the oligosaccharides, turanose rapidly degraded, the relative content decreased from 76.15% to 11.18% of total oligosaccharides, the stachyose decreased from 2.37% to 0.37%, while the trehalose increased from 20.09% to 65.66%, the cellobiose increased from 0.06% to 17.95%, these suggest that the turanose in the pcocessed P. sibiricum Red. can transform to smaller monosaccharides, or participate in other reactions. Meanwhile, fructose, galactose, and glucose emerge as the primary monosaccharides, with their contents increasing substantially to comprise 93.11%, 3.51%, and 1.10% of total monosaccharides, respectively, and among this monosaccharides, only the fructose had the greatest decline, this suggests that the changes that occur in the later processing may be related to fructose (Figures 9C,D). Given the noticeable increase in sweetness after processing, fructose serves as a key biomarker for measuring sweetness in processed P. sibiricum Red., which is consistent with previous studies (Jin et al., 2018; Zhang Q. H. et al., 2024; Shi et al., 2025). After processing, the reduction in the content of total polysaccharides and oligosaccharides, increased relative content of certain monosaccharides suggests the transformation of large molecular sugars into smaller monosaccharides. However, excessive steaming may intensify bitterness. This indicates that steaming and drying are beneficial for the conversion of the saccharides in P. sibiricum Red.
3.4 Effect of steaming and drying on amino acids metabolites of Polygonatum sibiricum Red.
Amino acids and analogs represent key functional components that provide tonifying and strengthening effects while enhancing immune function. They serve as essential precursors for protein synthesis, enzyme formation, antibody production, and the formation of certain hormonal compounds in the body (Yu et al., 2019; Park et al., 2020). The analysis identified 88 amino acids, peptides, and analogs in unprocessed, steamed, and dried P. sibiricum Red., representing the most diverse group among all metabolites. As illustrated in Figure 10A, the identification revealed 29, 43, 26, 20, 12, 16, 12, 7, 10, and 51 DAMs of amino acids, peptides, and analogs in S0vsS1, S1vsS2, S2vsS3, S3vsS4, S4vsS5, S5vsS6, S6vsS7, S7vsS8, S8vsS9, S0vsS7, respectively. These data suggest a gradual reduction in changes, with more substantial differences observed in the initial five cycles of steaming and drying samples. Leucine, glutamic acid, proline, tyrosine, and valine have been identified as markers of unprocessed P. sibiricum Red. Post-processing analysis revealed amino acids predominantly existing as dipeptides or polypeptides, as demonstrated in Figures 10B–F, particularly in S0vsS7, where 47 amino acids increased and 4 decreased significantly. The metabolites showing the most substantial increase included S-Hexyl-glutathione, linatine, N-Acetylornithine, N2-Succinyl-L-glutamic acid 5-semialdehyde, tyrosine methylester, L-arogenate, (5-L-glutamyl)-L-glutamate, cyclopeptine, L-dopa, and gamma-glutamyltyramine, and others, primarily comprising dipeptides or polypeptides. Additionally, S7 exhibited elevated levels of pyroglutamic acid, leucine, tyrosine, proline, valine, glutamic acid, and arginine, confirming their status as distinguishing metabolites in the processed rhizomes. The most notable reductions occurred in L-serine, while other groups showed decreases in L-leucine, N-acetylaspartylglutamate, N-methyl-L-glutamic acid, saccharopine, N-acetyl-alpha-D-glucosamine, N-acetylleucine, L-glutamine, L-arogenate, N-a-acetylcitrulline, 2-amino-2-deoxy-D-gluconate, pyroglutamic acid, L-leucine, gamma-glutamyltyramine, predominantly comprising serine, leucine, glutamic acid, and their derivatives. These findings partially align with previous research (Park et al., 2020; Li et al., 2024). The results provide comprehensive insights into the differentiation of amino acids and their derivatives following steaming and drying processes. The variations in amino acid metabolite accumulation and degradation across different cycles of steamed and dried Polygonatum sibicum may correlate with steaming temperature, duration, drying temperature, and time (Chen N. Y. et al., 2024; Guan et al., 2024).
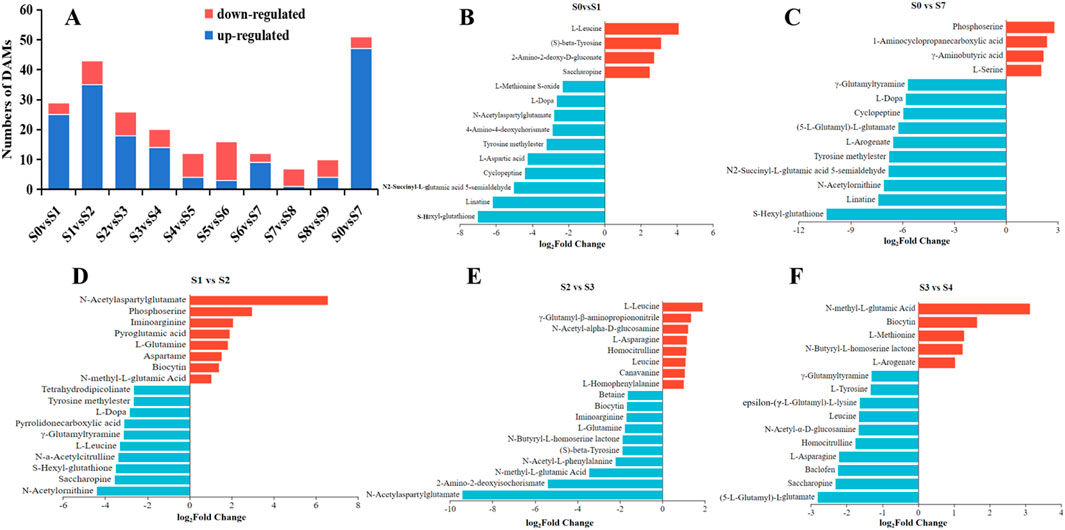
Figure 10. Differentially abundant metabolites (DAMs) of amino acids, peptides, and analogues in Polygonatum sibiricum Red. subjected to nine cycles of steaming and drying. (A) the number of DAMs of 10 groups, (B–F) plus and minus coordinate bar chart analysis of the top10 DAMs.
3.5 Effect of steaming and drying on total flavonoids, total polyphenols, and total saponins of Polygonatum sibiricum Red.
Flavonoids and polyphenols constitute essential active compounds with extensive applications in pharmaceuticals and functional foods (Wojdyło et al., 2009; Devkota et al., 2022; Zhang et al., 2022). Research has demonstrated that steaming treatments significantly enhance both the quantity of bioactive compounds, including flavonoids and polyphenols, and their antioxidant properties (Musilová et al., 2024; Ma et al., 2025; Ciudad Mulero et al., 2025). This investigation revealed that during processing, total flavonoid content exhibited an increasing trend (Figure 11A), peaking in S7, followed by a gradual decrease. A similar trend was observed for polyphenols (Figure 11B); compared to the S0, the content of polyphenols in S7 increased by 5.62 times. These findings suggest that processing causes a breakdown in the structural characteristics of some flavonoids and polyphenols, thereby increasing their accessibility for detection (Collins et al., 2024). Additionally, repeated high-temperature steaming may facilitate tissue cell fragmentation and covalent bond breakage, promote increased phenolic substance release (Mudgal and Singh, 2024; Ren et al., 2025). Furthermore, elevated temperatures can inactivate certain endogenous enzymes and prevent polyphenol oxidation, result in increased polyphenol content after steaming (Wojdyło et al., 2009; Liu R. L. et al., 2024; Xu L. et al., 2025; Xu N. et al., 2025).
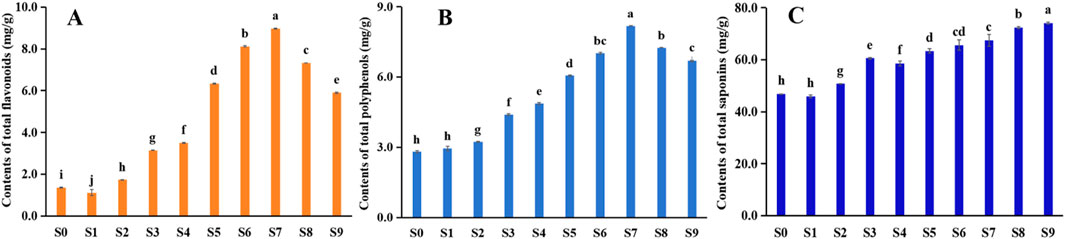
Figure 11. Change of contents of (A) total flavonoids, (B) total polyphenols, and (C) total saponins during processing of Polygonatum sibiricum Red.
Saponins demonstrate antibacterial, antitumor, and other therapeutic effects (Pan et al., 2024; Zhang Q. H. et al., 2024; Song et al., 2024). Analysis of saponin content in P. sibiricum Red. processed through multiple steaming and drying cycles reveals, as shown in Figure 11C, minimal variation during the initial two cycles. A significant increase occurred in S3 (P < 0.05), followed by gradual increases after S4. The mass fraction in S9 reached 74.06 ± 3.38 mg/g, representing a 1.58-fold increase compared to S0. Research suggests that steaming facilitates the conversion of steroidal saponins to glycosides and secondary glycosides, thereby elevated saponin content (Guan et al., 2024; Wang et al., 2024).
3.6 Effect of steaming and drying on non-enzymatic browning of Polygonatum sibiricum Red.
Previous studies have established that heat treatment affected sample color and processing product quality through enzymatic reactions and non-enzymatic reactions (Li et al., 2018; Suri et al., 2019; Ren et al., 2025). However, the steaming process for a long time (95 °C ∼ 105 °C) can cause the deactivation of polyphenol oxidase to inhibit the enzymatic browning. Therefore, it can be inferred that non-enzymatic browning was the main reason of the color change in P. sibiricum Red. subjected to nine cycles of steaming and drying. In this study, the content of polysaccharides and oligosaccharides decreased continuously (Figures 9A,B), the monosaccharides, especially the fructose, with their contents increased substantially in S1-S7, then rapidly decreased in S8 and S9 (Figures 9C,D), alongside an increase in nine main lipids in S3-S5 and decrease in S6-S9 (Figure 12A), significant decrease in 1-aminocyclopropanecarboxylic acid, gamma-aminobutyric acid, L-proline and L-leucine (Figure 12B), these findings indicate that steaming and drying induced the Maillard Reaction, where amino compounds (proteins, peptides, amines, and amino acids) reacted with carbonyl compounds (reducing sugars, lipids, aldehydes, ketones, polyphenols, ascorbic acids, and steroids) to produce various compounds including melanoids, ketones, aldehydes and heterocyclic compounds (Li et al., 2018; Li et al., 2018; Suri et al., 2019; Wan and Liu, 2019; Zhang Q. H. et al., 2024; Chen, 2023). 5-HMF, an intermediate product of the Maillard reaction, commonly occurs in traditional Chinese medicinal products and undergoes notable content changes during processing, storage, and compatibility. It demonstrates pharmacological effects, including antioxidation, improved learning and memory, antiallergy, neuroprotection, and anti-inflammation, while also finding applications in agricultural plant growth regulation and as a spice (Uchida et al., 2020; Joachim et al., 2022; Mahon et al., 2024).
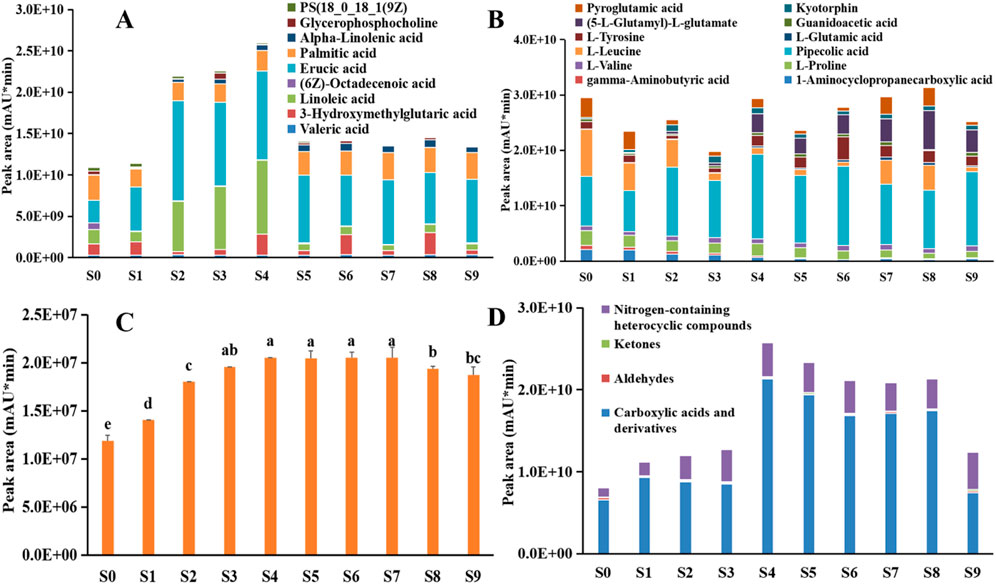
Figure 12. The variation in the relative content of lipids (A), amino acids (B), 5-HMF (C) and carbonyl compounds (D) in Polygonatum sibiricum Red. subjected to nine cycles of steaming and drying.
During steaming and drying, 5-HMF content increased progressively until the seventh steaming (Figure 12C), then gradually decreased, where amino acids condense with reducing sugars to form Amadori/Heynes rearrangased after reaching its peak, suggesting that steaming and drying may promote the Maillard reactionement products, which further decompose into flavor compounds such as furfural and reduced ketones, as shown in Figure 12D, the content of aldehydes, ketones, carboxylic acids and derivatives increased significantly in S4-S8 compared to S0, nitrogen-containing heterocyclic compounds content increased progressively until the fourth steaming (Huang et al., 2024; Wang et al., 2024b). In this study, the caramelization reaction can not occur in P. sibiricum Red. subjected to nine cycles of steaming and drying because of the presence of amino acids, furthermore, only a small amount of ascorbic acid was detected in the processed P. sibiricum Red., but the dehydroascorbic acids or 2,3-Diketogulonic acid, as the intermediate products of ascorbic acid degradation, were not detected, in conclusion, it can be concluded that the browning mainly correlated with Maillard reaction.
3.7 Correlation analysis between 31 morphology and quality traits
Pearson’s correlation analysis finds widespread application across disciplines, facilitating the identification of crucial evaluation indicators in comprehensive assessments. This method enables the selection of metrics highly relevant to evaluation objectives, thereby streamlining evaluation models and enhancing assessment accuracy and efficiency (Zhang X. Y. et al., 2024; Mitiku et al., 2025). The analysis revealed relationships between 27 primary quality traits and 4 morphological traits (Figure 13). The bigger the circle, the stronger the correlation. As shown by the color bar, from the deepest red equals to the strongest positive correlation to the deepest blue equal to the strongest negative correlation. The parameter L* showed positive correlations with amino acids, peptides, and analogs, C*, xylose, imidazopyrimidines, organic acids and derivatives, arabinose, lipids, b*, and polysaccharides, with particularly strong positive correlations with the latter six components. Negative correlations were observed with carboxylic acids and derivatives, total monosaccharides, fructose, keto acids and derivatives, flavonoids, polyphenols, saponins, nucleoside and nucleotide analogs, and benzene and substituted derivatives, with notably strong negative correlations with the latter five components. 5-HMF demonstrated positive correlations with 23 parameters, particularly strong with iditol, imidazopyrimidines, glucose, erythrulose, and C*, while showing negative correlations with carboxylic acids and derivatives, total monosaccharides, and fructose. This investigation specifically illuminates the relationship between chroma and internal chemical composition, offering novel insights into how steaming and drying processes affect P. sibiricum Red. ’s flavor and quality.
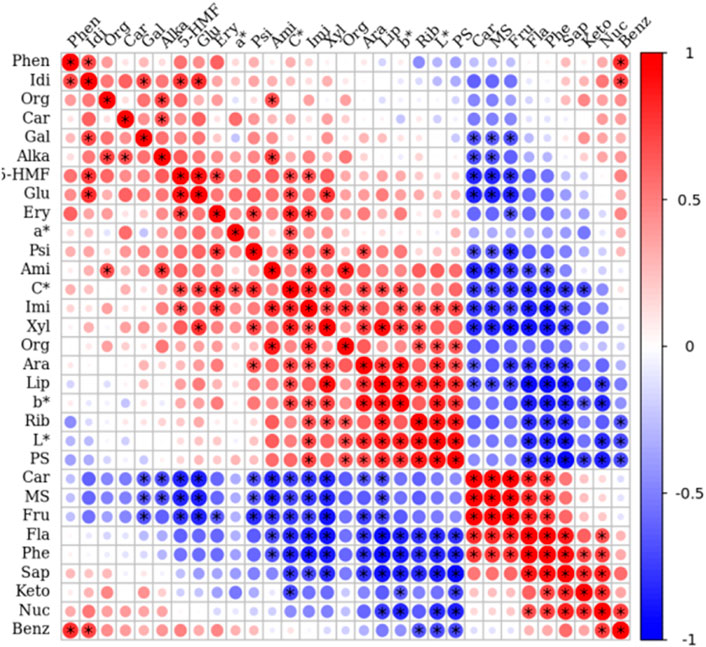
Figure 13. Pearson’s correlation coefficients of 31 morphology and quality traits of the raw rhizomes and nine samples under different cycles of steaming and drying of Polygonatum sibiricum Red. Fla: Flavonoids; Phe: Polyphenols; Sap: Saponins; PS: Polysaccharides; Fru: Fructose; Rib: Ribose; Gal: Galactose; Psi: Psicose; Ery: Erythrulose; Xyl:Xylose; Glu: Glucose; Idi: Iditol; Ara: Arabinose; MS: Total monosaccharides; Lip: Lipids; Ami: Amino acids, peptides, and analogues; Carb: Carbohydrates; Orga: Organic acids and derivatives; Car: Carboxylic acids and derivatives; Imi: Imidazopyrimidines; Benz: Benzene and substituted derivatives; Keto: Keto acids and derivatives; Orgn: Organonitrogen compounds; Nuc: Nucleoside and nucleotide analogues; Alka: Alkaloids and derivatives; Phen: Phenylpropanoic acids.
3.8 PCA and comprehensive evaluation based on 27 quality traits
Nine-steaming-nine-drying represents a TCM processing technique that alters medicinal materials by reducing toxicity, enhancing efficacy, improving absorption, and adjusting medicinal properties (Yu et al., 2018; Guan et al., 2024). The quality evaluation of processed P. sibiricum Red. rhizomes requires multiple parameters for accurate assessment. A comprehensive evaluation methodology incorporating PCA, membership function analysis, and cluster analysis encompasses multiple evaluation indicators, thereby avoids the limitations of single-indicator assessment and enables objective quality evaluation of medicinal materials (Chen W. M. et al., 2024; Zhang Y. Q. et al., 2024). The quality analysis of P. sibiricum Red. processed through nine cycles of steaming and drying utilized PCA. This analysis categorized the quality traits of P. sibiricum Red. into six main components (Table 2). The first principal component, comprising saponins, nucleoside and nucleotide analogs, flavonoids, polyphenols, and benzene and substituted derivatives, demonstrated a load score exceeding 0.90 with a contribution rate of 53.769%. The second principal component, based on carboxylic acids and derivatives, showed a maximum load score of 0.63 with a contribution rate of 13.221%. The third principal component indicators, including organic acids and derivatives, amino acids, peptides and analogs, and arabinose, exhibited load scores above 0.60 with a variance contribution rate of 7.901%. The fourth principal component presented an eigenvalue of 1.759 and a variance contribution of 6.514%. The fifth and sixth principal components showed variance contributions of 5.393% and 4.628%, respectively, with imidazopyrimidines and carboxylic acids and derivatives demonstrating a maximum positive impact on these components.
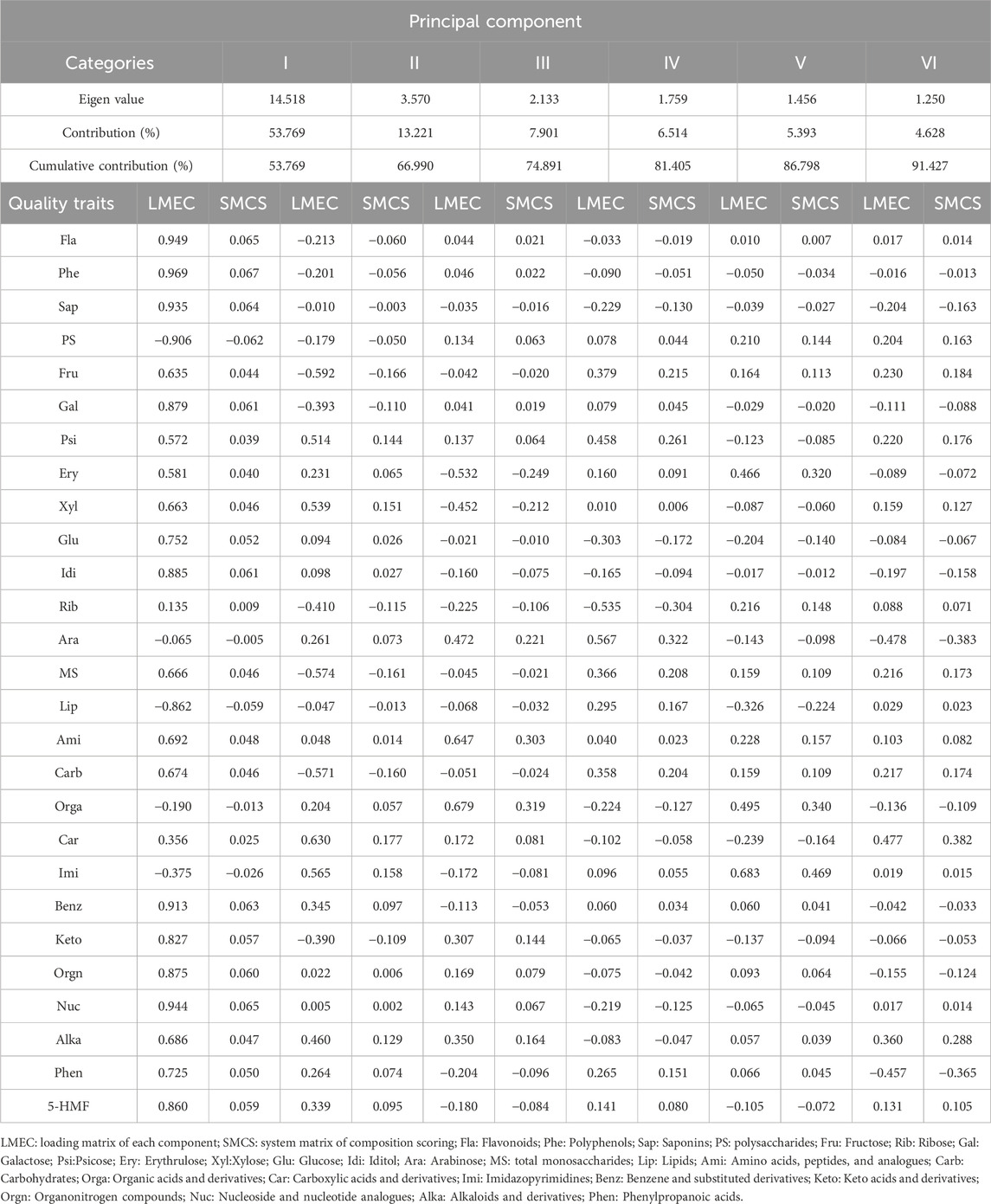
Table 2. Eigen values and correlation contribution, index weight of each comprehensive indexes, loading matrix of each component, and system matrix of composition scoring.
Based on the U-value and index weight of comprehensive indices for each sample, the D-value comprehensive quality evaluation was conducted for P. sibiricum Red. materials subjected to different steaming and drying cycles (Table 3). Higher D-value indicates superior quality of processed P. sibiricum Red. S1 and S0 exhibited lower D-values, while increasing steaming and drying cycles corresponded to gradual D-value increases, reaching peak quality at S7 before the gentle decline. These findings suggest that moderate steaming and drying effectively reduces P. sibiricum Red. irritability while enhances beneficial properties. Analysis of primary active ingredient changes in P. sibiricum Red., particularly those associated with pharmacological activity, establishes optimal processing parameters: L* value below 30, colorimetric value (C value) below 10, and a value approximately 2, conditions under which P. sibiricum Red. main active ingredient content remained elevated. Thus, these comprehensive evaluation methods effectively reflect TCM quality and provide an objective assessment of Chinese medicinal materials and herbal slices, establishing a robust foundation for selecting optimal therapeutic processing methods.
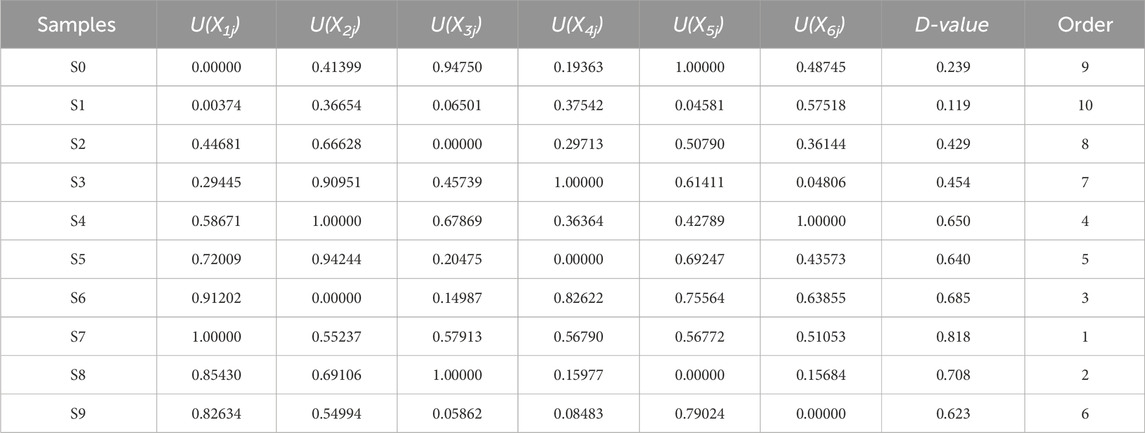
Table 3. Comprehensive index value, U(X) value and D-value of nine-steamed-nine-dried Polygonatum sibiricum Red. materials.
4 Conclusion
The steaming and drying processes significantly influenced the morphology, metabolomic profile, and medicinal components of P. sibiricum Red. With increasing cycles of steaming and drying, the rhizomes darkened progressively, as evidenced by a significant decrease in L* values. The tongue-numbing sensation diminished, and after the seventh steaming and drying cycle, bitter and sour tastes gradually emerged. Non-targeted metabolomics analysis detected 685 metabolites in P. sibiricum Red. The browning of P. sibiricum Red. subjected to steaming and drying mainly correlated with Maillard reaction. The steaming and drying procedure mediates metabolic pathways such as saccharides metabolism, amino acids metabolism, thus affecting the composition of metabolites (such as fructose, erucic acid, palmitic acid, linoleic acid, pipecolic acid, L-proline, L-leucine, (5-L-Glutamyl)-L-glutamate, 5-HMF, flavonoids, polyphenols, saponins and flavor compounds) in P. sibiricum Red. These metabolites and their derivatives serve as the main biomarkers for evaluate the quality of P. sibiricum Red. subjected to steaming and drying. Pearson’s correlation analysis demonstrated relationships between 27 main quality traits associated with pharmacological activity and 4 morphology traits. Combined with comprehensive evaluation results, the optimal preparation process can be assessed more objectively using L* value, C value, and a value compared to empirical judgment alone. This study provides comprehensive insights into the mechanisms by which steaming and drying affects the morphology and quality of P. sibiricum Red., establishing a theoretical foundation for quality assessment of processed P. sibiricum Red.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
RW: Data curation, Writing – original draft. PL: Formal Analysis, Investigation, Methodology, Writing – original draft. SC: Data curation, Investigation, Writing – original draft. JG: Investigation, Methodology, Writing – original draft. YL: Investigation, Writing – original draft. HS: Data curation, Investigation, Writing – original draft. SW: Data curation, Investigation, Writing – original draft. HL: Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The research was supported by grants from State Administration of Traditional Chinese Medicine of the People’s Republic of China (No. Z20220087).
Acknowledgments
This work is supported by the State Administration of Traditional Chinese Medicine of the People’s Republic of China (No. Z20220087). The authors would like to thank Suzhou PANOMIX Biomedical Tech Co., Ltd. (https://www.panomix.com/) for their support in the extraction and detection of the samples.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frfst.2025.1666149/full#supplementary-material
References
Arnold, M., Suliburska, J., Świeca, M., Wojdyło, A., and Michałowska, A. G. (2025). Storage stability of bioactive-enriched freeze-dried gala apples: functional properties, sensory evaluation, and enzymatic browning. Food Bioprocess Technol., 1–19. doi:10.1007/s11947-025-03975-7
Bureau, S., Leca, A., Gouble, B., Garcia, C., Danelski, W., Hallmann, E., et al. (2025). Impact of conventional and innovative processing conditions on organoleptic and nutritional properties of applesauce from organic and conventional production systems. Food Chem. 467, 142346. doi:10.1016/j.foodchem.2024.142346
Cao, R., Fang, X. Y., Li, Z. Y., Li, S. J., Guo, Q. Q., and Chai, Y. Y. (2024). Effect of Polygonatum sibiricum saponins on gut microbiota of mice with ulcerative colitis. Fitoterapia 174, 105855. doi:10.1016/j.fitote.2024.105855
Chen, W. (2023). Study on morphological characteristics of calcium oxalate needle crystal and digestion method of polygonatum stinged tongue component (Taian: Shandong Agricultural University). Master’s Degree Thesis.
Chen, H., Li, Y. J., Li, X. F., Sun, Y. J., Li, H. W., Su, F. Y., et al. (2018). Homoisoflavanones with estrogenic activity from the rhizomes of Polygonatum sibiricum. J. Asian Nat. Prod. Res. 20 (1), 92–100. doi:10.1080/10286020.2017.1343821
Chen, N. Y., Ding, Y. Z., Li, X., Li, J., Cheng, Y. X., Tian, Y., et al. (2024). Chemical structures and immunomodulatory activities of polysaccharides from Polygonatum kingianum. Int. J. Biol. Macromol. 279 (3), 135406. doi:10.1016/j.ijbiomac.2024.135406
Chen, L. T., Chen, R., Elsayed, M. A., Mabrouk, M., Jiang, H. Y., Mou, X. W., et al. (2024). Nutritional quality assessment of miscellaneous cassava tubers using principal component analysis and cluster analysis. Foods Basel, Switz. 13 (12), 1861. doi:10.3390/foods13121861
Chen, W. M., Wang, Y., Wang, X. M., Shao, Y. H., Tu, Z. C., and Liu, J. (2024). Effect of superheated steam on maillard reaction products, digestibility, and antioxidant activity in β-Lactoglobulin-glucose system. Int. J. Biol. Macromol. 287, 138514. doi:10.1016/j.ijbiomac.2024.138514
Chinese Pharmacopoeia Commission (2020). Pharmacopoeia of the people’s Republic of China, 1. Beijing: China Medical Science Press, 319.
Ciudad Mulero, M., Vega, E. N., Herrera, P. G., Fernández Tomé, S., Pedrosa, M. M., Arribas, C., et al. (2025). New gluten-free extruded snack-type products based on rice and chickpea and fortified with passion fruit skin: extrusion cooking effect on phenolic composition, non-nutritional factors, and antioxidant properties. Molecules 30 (6), 1225. doi:10.3390/molecules30061225
Collins, A., Santhakumar, A., Latif, S., Chinkwo, K., Francis, N., and Blanchard, C. (2024). Impact of processing on the phenolic content and antioxidant activity of Sorghum bicolor L. moench. Mol. Basel, Switz. 29 (15), 3626. doi:10.3390/molecules29153626
Deng, Z. Y., Yao, X. J., Li, C. X., Zhang, B., Zhong, R. M., and Li, H. Y. (2024). Comparison with Polygonatum cyrtonema hua steaming with huangjiu or honey based on UPLC-Q-Exactive MS/MS analysis combined with multi-component variation. Food Biosci. 61, 104854. doi:10.1016/j.fbio.2024.104854
Devkota, H. P., Paudel, K. R., Lall, N., Tomczyk, M., and Atanasov, A. G. (2022). Editorial: pharmacology of plant polyphenols in human health and diseases. Front. Pharmacol. 13, 945033. doi:10.3389/fphar.2022.945033
Dilara, Ş., and Gökmen, V. (2022). Kinetic modeling of maillard and caramelization reactions in sucrose-rich and low moisture foods applied for roasted nuts and seeds. Food Chem. 395, 133583. doi:10.1016/j.foodchem.2022.133583
Du, L., Nong, M. N., Zhao, J. M., Peng, X. M., Zong, S. H., and Zeng, G. F. (2016). Polygonatum sibiricum polysaccharide inhibits osteoporosis by promoting osteoblast formation and blocking osteoclastogenesis through Wnt/β-catenin signalling pathway. Sci. Rep. 6 (1), 32261. doi:10.1038/srep32261
Editing Committee of Chinese Flora, Chinese Academy of Sciences (1978). Flora of China, 15. Beijing: Science Press, 78.
Fan, B., Wei, G., Gan, X., Li, T., Qu, Z., Xu, S., et al. (2020). Study on the varied content of Polygonatum cyrtonema polysaccharides in the processing of steaming and shining for nine times based on HPLC-MS/MS and chemometrics. Microchem. J. 159, 105352. doi:10.1016/j.microc.2020.105352
Fei, P., Sun, Z. Y., Liu, X. Y., Jiang, P. Y., Feng, H. X., Chen, X., et al. (2024). Antibacterial activity and mechanism of Polygonatum sibiricum extract against bacillus cereus and its application in pasteurized milk. Foodborne pathogens Dis. 3, 160–167. doi:10.1089/fpd.2023.0110
Germán, Z. (2024). Potential of bitter medicinal plants: a review of flavor physiology. Pharmaceuticals 17 (6), 722. doi:10.3390/ph17060722
Guan, Y. H., Liang, Z. W., Li, R. Y., Guo, Y. J., Dang, L. J., Gong, F. M., et al. (2024). Chemical composition and antioxidant activity of Polygonatum kingianum processed by the traditional method of “Nine Cycles of Steaming and Sun-Drying”. Food Chem. X 22, 101292. doi:10.1016/j.fochx.2024.101292
Huang, Y. Y., Sun, Y., Arshad, M., Lu, T. T., and Chen, X. M. (2024). Unraveling the temporal changes of maillard reaction products and aroma profile in coffee leaves during hot-air drying. J. Food Compos. Analysis 128, 106055. doi:10.1016/j.jfca.2024.106055
Jiang, T., Wu, T., Gao, P., Wang, L., Yang, X., Chen, X., et al. (2022). Research on processing-induced chemical variations in Polygonatum cyrtonema rhizome by integrating metabolomics and glycomics. Molecules 27 (18), 5869. doi:10.3390/molecules27185869
Jiang, X., Wang, Y. M., Lin, Z. C., Li, C., Wang, Q., Zhang, J. Y., et al. (2024). Polygonatum sibiricum polysaccharides: a promising strategy in the treatment of neurodegenerative disease. Neurochem. Int. 181, 105902. doi:10.1016/j.neuint.2024.105902
Jin, J., Lao, J., Zhou, R., He, W., Qin, Y., Zhong, C., et al. (2018). Simultaneous identification and dynamic analysis of saccharides during steam processing of rhizomes of Polygonatum cyrtonema by HPLC⁻QTOF⁻MS/MS. Molecules 23, 2855–2867. doi:10.3390/molecules23112855
Joachim, G., Ralf, H., Mehtap, K., Naime, B., Georg, F., Philipp, S., et al. (2022). Alpha-ketoglutarate or 5-HMF: single compounds effectively eliminate leukemia cells via Caspase-3 apoptosis and antioxidative pathways. Int. J. Mol. Sci. 16, 9034. doi:10.3390/IJMS23169034
Lai, W. W., Ning, Q., Wang, G. H., Gao, Y., Liao, S. X., and Tang, S. S. (2024). Antitumor activity of Polygonatum sibiricum polysaccharides. Archives Pharmacal Res. 47 (8-9), 696–708. doi:10.1007/s12272-024-01511-3
Li, J. H., Liu, Y. J., Huang, Y. L., Yu, Z. J., Hu, M. B., Huang, R. Z., et al. (2018). Investigation of maillard reaction involvement in the steam processing of Panax notoginseng root. Trop. J. Pharm. Res. 2, 299. doi:10.4314/tjpr.v17i2.15
Li, H. Y., Fan, C. X., Liu, J. S., Wang, B., and Li, H. B. (2022). Integration of full-length transcriptomes and anthocyanin metabolite analysis for understanding fruit coloration mechanism in Schisandra chinensis. Physiology Mol. Biol. Plant 28, 921–933. doi:10.1007/s12298-022-01179-3
Li, X. L., Ma, R. H., Ni, Z. J., Wang, W., Thakur, K., Zhang, J. G., et al. (2024). Dioscin from Polygonatum sibiricum induces apoptosis and autophagy in Ishikawa human endometrial cancer cell and in vivo. Food Sci. Hum. Wellness 13 (5), 2601–2616. doi:10.26599/fshw.2022.9250209
Li, Y., Liu, Z. Y., Yan, H. Y., Zhou, T. L., Zheng, L. M., Wen, F., et al. (2025). Polygonatum sibiricum polysaccharide ameliorates skeletal muscle aging and mitochondrial dysfunction via PI3K/Akt/mTOR signaling pathway. Phytomedicine 136, 156316. doi:10.1016/j.phymed.2024.156316
Lin, H. Y., Wang, W. H., Peng, M. Q., Kong, Y. F., Zhang, X. W., Wei, X. H., et al. (2024). Pharmacological properties of polygonatum and its active ingredients for the prevention and treatment of cardiovascular diseases. Chin. Med. 19 (1), 1. doi:10.1186/s13020-023-00871-0
Liu, D. P., Wang, Y., Wang, G. Y., Gu, X. Z., Liu, T. L., Zhang, C., et al. (2020). Correlation analysis of color change and maillard reaction during processing of gardeniae fructus praeparatus. China J. Chin. materia medica 45 (10), 2382–2388. doi:10.19540/j.cnki.cjcmm.20200221.305
Liu, H. P., Zhang, H., Geng, M. T., Shi, D. X., Liu, D. S., Jiao, Y. X., et al. (2024). The impact of cooking on antioxidant and enzyme activities in ruichang yam polyphenols. Foods 14 (1), 14. doi:10.3390/foods14010014
Liu, R. L., Zhang, X. L., Cai, Y. H., Xu, S., Xu, Q., Ling, C. L., et al. (2024). Research progress on medicinal components and pharmacological activities of Polygonatum sibiricum. J. Ethnopharmacol. 328, 118024. doi:10.1016/j.jep.2024.118024
Lu, S. L. (2021). Scientific research on the nine steaming and nine sun-drying processing of Rehmannia glutinosa based on its properties and chemical composition. Taiyuan: Shanxi university.
Luo, T., He, Y., Jiang, L., Yang, L., Hou, X., Zhang, G. S. Z., et al. (2025). Flavor perception and biological activities of bitter compounds in food. Food Chem. 477, 143532. doi:10.1016/j.foodchem.2025.143532
Ma, S. T., Tian, F., Liang, H. Y., Zhang, Y., Cao, H., Wu, M. H., et al. (2025). Study of the changes of 10 flavonoids in crude and processed aurantii fructus (zhiqiao) by ultrahigh-performance liquid chromatography and high-resolution tandem mass spectrometry. J. Food Compos. Analysis 140, 107238. doi:10.1016/j.jfca.2025.107238
Mahon, R. T., Ciarlone, G. E., Roney, N. G., and Swift, J. M. (2024). Cardiovascular parameters in a swine model of normobaric hypoxia treated with 5-Hydroxymethyl-2-Furfural (5-HMF). Front. Physiology 10, 10395. doi:10.3389/fphys.2019.00395
Mitiku, A., Gudina, G., Yasin, A. B., and Abdu, M. (2025). Comprehensive assessment of genetic variability, association analysis, and elucidation of direct and indirect effects of yield and yield contributing traits from diverse exogenous soybean (glycine max L.) genotypes in jimma district, southwest Ethiopia. Adv. Agric. 1, 2865503. doi:10.1155/AIA/2865503
Mudgal, S., and Singh, N. (2024). Effect of parboiling treatment times on the physicochemical, cooking, textural, and pasting properties and amino acid, phenolic, and sugar profiles of germinated paddy rice from different rice varieties. J. Food Sci. 89 (6), 3208–3229. doi:10.1111/1750-3841.17048
Musilová, J., Franková, H., Fedorková, S., Lidiková, J., Vollmannová, A., Sulírová, K., et al. (2024). Comparison of polyphenols, phenolic acids, and antioxidant activity in sweet potato (Ipomoea batatas L.) tubers after heat treatments. J. Agric. Food Res. 18, 101271. doi:10.1016/j.jafr.2024.101271
Pan, G. G., and Melton, L. D. (2007). Nonenzymatic browning of lactose and caseinate during dry heating at different relative humidities. J. Agric. Food Chem. 24, 10036–10042. doi:10.1021/jf072257n
Pan, J., Ni, Z. J., Thakur, K., Khan, M. R., Zhang, J. G., and Wei, Z. J. (2024). Bioactivity and application potential of O/W emulsions derived from carboxylic acid-based NADES-Extracted total saponins from Polygonatum cyrtonema hua. Food Chem. 463 (Pt 3), 141363. doi:10.1016/j.foodchem.2024.141363
Park, N. Y., Jeong, Y. J., Lee, G. D., and Kwon, J. H. (2020). Monitoring of maillard reaction characteristics under various roasting conditions of Polygonatum odoratum root. J. Korean Soc. Food Sci. Nutr. 4, 647–654.
Peng, X. M., He, J. C., Zhao, J. M., Wu, Y. L., Shi, X. Z., Du, L., et al. (2018). Polygonatum sibiricum polysaccharide promotes osteoblastic differentiation through the ERK/GSK-3β/β-Catenin signaling pathway in vitro. Rejuvenation Res. 21 (1), 44–52. doi:10.1089/rej.2017.1956
Ren, W., Wu, M., Wang, B., Xu, H., Wei, W., and Sun, D. (2025). Constant temperature and humidity combined with vacuum-steam pulsed steaming of Polygonatum cyrtonema rhizome: quality attribute and browning mechanism. Food Chem. 463 (P4), 141472. doi:10.1016/j.foodchem.2024.141472
Shi, S., Zhang, J., Zhang, J., Ma, S., Hu, Y., Zhu, H., et al. (2025). Structural characterization of raw and wine-steamed Polygonatum cyrtonema hua oligosaccharides and their bioactivity on immune regulation via modifying the gut microbiota. Int. Immunopharmacol. 153, 114468. doi:10.1016/j.intimp.2025.114468
Song, Q. Y., Chen, Y. W., Shao, Y., Pu, W. T., Ye, B. H., Shi, X. X., et al. (2024). Investigation of the functional components in health beverages made from Polygonatum cyrtonema rhizomes provides primary evidence to support their claimed health benefits. Metabolites 7, 376. doi:10.3390/metabo14070376
Sun, L. R., Li, X., and Wang, S. X. (2005). Two new alkaloids from the rhizome of Polygonatum sibiricum. J. Asian Nat. Prod. Res. 7 (2), 127–130. doi:10.1080/10286020310001625157
Suri, K., Singh, B., Kaur, A., Yadav, M. P., and Narpinder Singh, N. (2019). Impact of infrared and dry air roasting on the oxidative stability, fatty acid composition, maillard reaction products and other chemical properties of black cumin (Nigella sativa L.) seed oil. Food Chem. 295, 537–547. doi:10.1016/j.foodchem.2019.05.140
Tang, C., Yu, Y. M., Qi, Q. L., Wu, X. D., Wang, J., and Tang, S. A. (2019). Steroidal saponins from the rhizome of Polygonatum sibiricum. J. Asian Nat. Prod. Res. 21 (3), 197–206. doi:10.1080/10286020.2018.1478815
Taş, N. G., and Gökmen, V. (2017). Maillard reaction and caramelization during hazelnut roasting: a multiresponse kinetic study. Food Chem. 221, 1911–1922. doi:10.1016/j.foodchem.2016.11.159
Uchida, R., Kato, M., Hattori, Y., Kikuchi, H., Watanabe, E., Kobayashi, K., et al. (2020). Identification of 5-Hydroxymethylfurfural (5-HMF) as an active component citrus jabara that suppresses FcεRI-mediated mast cell activation. Int. J. Mol. Sci. 7, 2472. doi:10.3390/ijms21072472
Wan, X. Y., and Liu, Z. L. (2019). Comparative analysis of maillard reaction products and anti-oxidant activity of dichloromethane extraction from polygonati rhizoma after processing. Chin. Traditional Herb. Drugs 50 (03), 604–610.
Wan, R. L., Li, H. Y., Zhang, W. Q., and Piao, Z. Y. (2013). Effects of combined application of nitrogen, phosphorus and potassium on yield, quality and nutrient absorption and distribution of Pulsatilla sinensis. Chin. Med. 36 (11), 1721–1726.
Wang, S., Wang, B., Hua, W., Niu, J., Dang, K., Yi, Q., et al. (2017). De novo Assembly and Analysis of Polygonatum sibiricum Transcriptome and Identification of Genes Involved in Polysaccharide Biosynthesis. Int. J. Mol. Sci. 18 (9), 1950. doi:10.3390/ijms18091950
Wang, R. N., Li, R. Y., Zheng, P., Yang, Z. C., Qian, C., Wang, Z., et al. (2023). Silver nanoparticles modified with Polygonatum sibiricum polysaccharide improve biocompatibility and infected wound bacteriostasis. J. Microbiol. (Seoul, Korea) 61 (5), 543–558. doi:10.1007/s12275-023-00042-8
Wang, M., Ye, C., Wang, Q., Ting, H. E., Shenggao, Y. I. N., and Zhou, G. (2024). Fingerprint study of polygonati rhizoma with steaming and exposing to the sun alternatively for different times. Med. Plant 15 (2), 18–20. doi:10.19601/J.CNKI.ISSN2152-3924.2024.02.005
Wang, R. C., Zhai, X. Y., Hartel, R. W., Chang, Y. W., Pang, W. W., Han, W., et al. (2024b). Effects of saccharide type and extended heating on the maillard reaction and physicochemical properties of high-solid gelatin gels. Food Chem. 459, 140249. doi:10.1016/j.foodchem.2024.140249
Wang, F., Wang, S., Qin, C., Li, J., Zhang, Z., Ji, L., et al. (2025). Preparation, flavor compounds, and antioxidant activity of hawthorn fruit-derived maillard reaction products. Appl. Biol. Chem. 68 (1), 49. doi:10.1186/s13765-025-01022-9
Wang, W., Hu, J., Xu, Y., Li, X., and Bi, J. (2025). Insights into the structural alterations of different pectin fractions and their impact on enzymatic browning during Apple pulp processing. Food Chem. 492 (Pt 3), 145660. doi:10.1016/j.foodchem.2025.145660
Wojdyło, A., Figiel, A., and Oszmiański, J. (2009). Effect of drying methods with the application of vacuum microwaves on the bioactive compounds, color, and antioxidant activity of strawberry fruits. J. Agric. Food Chem. 57 (4), 1337–1343. doi:10.1021/jf802507j
Wu, W. J., Wang, Y. L., Yi, P., Su, X. F., Mi, Y., Wu, L. L., et al. (2024). Various steaming durations alterdigestion, absorption, andfermentation by human gutmicrobiota outcomes of Polygonatum cyrtonema huapolysaccharides. Front. Nutr. 11, 1466781. doi:10.3389/fnut.2024.1466781
Xiao, L. X., Ping, Y. N., Sun, S. S., Xu, R., Zhou, X. R., Wu, H. Y., et al. (2024). TMT-Based quantitative proteomics unveils the protective mechanism of Polygonatum sibiricum polysaccharides on septic acute liver injury. J. proteomics 310, 105331. doi:10.1016/j.jprot.2024.105331
Xu, N., Lei, J., Xu, X., Hu, R., Liu, M., Jia, B., et al. (2025). Integrative transcriptomics and metabolomics analyses reveals maillard reaction and antioxidant capacity-mediated leaf browning during the flue-curing process in Nicotiana tabacum L. BMC Plant Biol. 25 (1), 1126. doi:10.1186/s12870-025-07150-0
Xu, L., Song, X. J., Yao, D., Wang, C. Y., Yao, X. M., and Li, Z. J. (2025). Dynamic migration of phenolics in microwaved combined cooked sorghum: focus on the polyphenols interact with starch/protein. Food Chem. X 27, 102342. doi:10.1016/j.fochx.2025.102342
Yan, Z., Zheng, L., Nie, J., Li, Z., and Cheng, Y. (2018). Evaluation indices of sour flavor for Apple fruit and grading standards. J. Integr. Agric. 17 (5), 994–1002. doi:10.1016/s2095-3119(17)61795-7
Yan, Y., Zou, M., Tang, C., Ao, H., He, L., Qiu, S., et al. (2024). The insights into sour flavor and organic acids in alcoholic beverages. Food Chem. 460 (P3), 140676. doi:10.1016/j.foodchem.2024.140676
Yang, J., Xue, C., Zhang, L., Meng, N., Yang, J., Cui, Y., et al. (2024). Exploration on the mechanism of “black as lacquer and sweetness as candy” based on the reactions of “crosslinking coloring and Maillard” and “oligosaccharide hydrolysis” during the processing of radix rehmanniae. Arabian J. Chem. 17 (9), 105917. doi:10.1016/j.arabjc.2024.105917
Yu, M., He, S., Tang, M., Zhang, Z., Zhu, Y., and Sun, H. (2018). Antioxidant activity and sensory characteristics of maillard reaction products derived from different peptide fractions of soybean meal hydrolysate. Food Chem. 243, 249–257. doi:10.1016/j.foodchem.2017.09.139
Yu, Y., Li, Z. M., Cao, G. T., Li, S. L., and Yang, H. S. (2019). Effects of ball milling micronization on amino acids profile and antioxidant activities of Polygonatum cyrtonema hua tuber powder. J. Food Meas. Charact. 13 (3), 2106–2117. doi:10.1007/s11694-019-00131-6
Yu, M., Dai, Y., Liu, T. T., Xiao, Y. Q., and Li, L. (2023). Correlation analysis of the material basis and color characteristics of Polygonum multiflorum decoction pieces during the ancient classic nine steaming and nine sun-drying processing. Chin. J. Exp. Pharmacol. 29 (16), 178–187. doi:10.13422/j.cnki.syfjx.20230661
Zhang, H. L., Hao, F. L., Yao, Z. F., Zhu, J. L., Jing, X., and Wang, X. W. (2022). Efficient extraction of flavonoids from Polygonatum sibiricum using a deep eutectic solvent as a green extraction solvent. Microchem. J. 175, 107168. doi:10.1016/j.microc.2021.107168
Zhang, X. Y., Wu, G. F., Wu, Y. H., Tang, N., Huang, L., Dai, D. Q., et al. (2024). Diversity analysis and comprehensive evaluation of 101 soybean (glycine max L.) germplasms based on sprout quality characteristics. Foods 13 (21), 3524. doi:10.3390/foods13213524
Zhang, Y. Q., Zhang, D. D., Wu, Y. F., Yang, X. G., Li, H. Y., Wei, Q. Z., et al. (2024). Evaluation of the effects of acetic acid and ozone treatment on the quality of fresh-cut lotus root based on grey relational analysis. Sci. Rep. 14 (1), 26466. doi:10.1038/s41598-024-77902-9
Zhang, Q. H., Lin, X. Y., and Su, W. K. (2024). Study on the components changes of polysaccharides and saponins during nine steaming and drying of Polygonatum sibiricum. J. Sci. food Agric. 11, 6862–6874. doi:10.1002/JSFA.13516
Zhao, H., Wang, Q. L., Hou, S. B., and Chen, G. (2019). Chemical constituents from the rhizomes of Polygonatum sibiricum red. and anti-inflammatory activity in RAW264.7 macrophage cells. Nat. Prod. Res. 33 (16), 2359–2362. doi:10.1080/14786419.2018.1440220
Zhao, P., Li, X., Wang, Y., Yan, L., Guo, L., Huang, L., et al. (2020). Characterisation and saccharide mapping of polysaccharides from four common polygonatum spp. Carbohydr. Polym. 233, 115836. doi:10.1016/j.carbpol.2020.115836
Zhao, P., Li, X., Wang, Y., Zhang, X., Jia, H., Guo, L., et al. (2020b). Comparative studies on characterization, saccharide mapping and antiglycation activity of polysaccharides from different polygonatum ssp. J. Pharm. Biomed. Analysis 186 (prepublish), 113243. doi:10.1016/j.jpba.2020.113243
Zhao, G. B., ZhouY, T. Y. H., Abbas, M., Dong, S. W., Zhao, X. Y., Liu, X., et al. (2025). Potential antitumor effect of polysaccharides extracted from Polygonatum sibiricum on human prostate cancer PC3 cells. Oncol. Lett. 29 (1), 28. doi:10.3892/OL.2024.14774
Keywords: Polygonatum sibiricum Red., nine-steaming-nine-drying, morphology, metabolomic analysis, active ingredients
Citation: Wang R, Li P, Chen S, Guo J, Liu Y, Shen H, Wang S and Li H (2025) Elucidating the effects of nine-steaming-nine-drying on the morphology, metabolomic profile, and active ingredients of Polygonatum sibiricum Redouté. Front. Food Sci. Technol. 5:1666149. doi: 10.3389/frfst.2025.1666149
Received: 15 July 2025; Accepted: 30 September 2025;
Published: 22 October 2025.
Edited by:
Digambar Kavitake, National Institute of Nutrition (ICMR), IndiaReviewed by:
Djiazet Steve, UMR7274 Laboratoire Réactions et Génie des Procédés (LRGP), FranceMichael Villacis, Escuela Politécnica Nacional, Ecuador
Copyright © 2025 Wang, Li, Chen, Guo, Liu, Shen, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haiyan Li, bGloYWl5YW5Ac3lhdS5lZHUuY24=
 Rui Wang
Rui Wang Jinchao Guo
Jinchao Guo Haiyan Li
Haiyan Li