- 1State Key Laboratory of Water Pollution Control and Green Resource Recycling, College of Environmental Science and Engineering, Tongji University, Shanghai, China
- 2State Key Laboratory of Disaster Reduction in Civil Engineering, Department of Geotechnical Engineering, College of Civil Engineering, Tongji University, Shanghai, China
High-entropy alloy nanomaterials (HEA-NMs), composed of multiple metallic elements, offer tunable electronic structures, abundant active sites, and excellent stability, making them highly promising for electrocatalysis. This mini-review summarizes their structural effects influencing electrocatalytic behavior, recent advances in synthesis strategies, and electrocatalytic applications, including the hydrogen evolution reaction (HER), oxygen evolution reaction (OER), oxygen reduction reaction (ORR), carbon dioxide reduction reaction (CO2RR), and nitrate reduction reaction (NO3RR). Challenges in synthesis scalability and mechanistic probing are discussed, along with future directions for atomic-level design and data-driven catalyst optimization. HEA-NMs offer a versatile platform for sustainable energy and environmental electrocatalysis.
1 Introduction
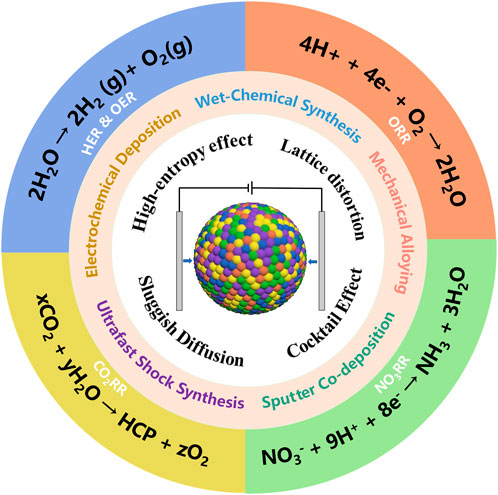
The urgent demand for sustainable energy and environmental solutions has accelerated the development of advanced electrocatalysts, which play a central role in enhancing reaction kinetics and lowering activation barriers in processes such as water splitting (Liu et al., 2025), fuel cells (Serov, 2022), and CO2 conversion (Gandionco et al., 2024). High-entropy alloys (HEAs), composed of five or more principal elements in near-equimolar ratios, have emerged as a promising class of materials due to their high configurational entropy, which stabilizes single-phase solid solutions (Yeh et al., 2004). Their unique features including lattice distortion, sluggish diffusion, and multi-element synergy contribute to enhanced structural stability and tunable catalytic properties.
The integration of HEA concepts into the nanoscale has led to the emergence of high-entropy alloy nanomaterials (Li et al., 2024a), which further benefit from increased surface-to-volume ratios, abundant active sites, and adjustable electronic structures. These attributes enhance intermediate adsorption, accelerate reaction kinetics, and improve product selectivity. Since the first synthesis of HEA nanoparticles in 2018 (Yao et al., 2018), HEA-NMs have demonstrated impressive performance in various electrochemical reactions, including the hydrogen evolution reaction (HER), oxygen evolution reaction (OER), oxygen reduction reaction (ORR), carbon dioxide reduction (CO2RR), and nitrate reduction (NO3RR).
This mini-review summarizes recent progress in HEA-NMs for electrocatalysis. We examine the mechanistic origins of their activity, current synthetic approaches for controlled nanostructure fabrication, and their applications in representative electrocatalytic reactions. Finally, we outline remaining challenges and future opportunities for advancing HEA-NMs as multifunctional electrocatalysts for next-generation energy and environmental technologies.
2 Mechanistic effects underlying the electrocatalytic behavior of HEA-NMs
2.1 High-entropy effect
The high-entropy effect, derived from the near-equiatomic incorporation of five or more elements, leads to high configurational entropy (ΔSmix ≥ 1.5 R) (Wang et al., 2024) and low Gibbs free energy of intermetallic compounds (ΔGmix = ΔHmix − TΔSmix) (Lee et al., 2023), favoring the formation of stable solid-solution phases over intermetallics. At the nanoscale, this effect is amplified by high surface energy, enabling single-phase structures and enhancing long-term stability in electrochemical environments. Homogeneous multi-element distributions further ensure consistent catalytic activity.
2.2 Lattice distortion and electronic modulation
Atomic size mismatch induces severe lattice distortion in HEA-NMs, disrupting periodic structures and modifying the local electronic environment (Li et al., 2024b). This shifts d-band centers and tunes adsorption energies of key intermediates, boosting catalytic performance. Nanoscale effects intensify distortion and promote favorable electronic interactions for reactions such as CO2 and NO3− reduction (Shaikh et al., 2024).
2.3 Sluggish diffusion and microstructural stability
The multi-element environment introduces diverse diffusion barriers, reducing atomic mobility and suppressing grain coarsening (Lee et al., 2023). This “sluggish diffusion” helps preserve the nanostructure and compositional uniformity under harsh conditions, contributing to the thermal and electrochemical durability of HEA-NMs.
2.4 Cocktail effect and synergistic active sites
The cocktail effect describes the synergistic properties from multielemental interactions that go beyond the sum of individual elements (Kamaruddin et al., 2024). These include coexisting catalytic sites, adaptive surface reconstructions, and multi-pathway reactivity, all of which enhance charge transfer and overall catalytic versatility (Liu et al., 2024).
In addition to the above structural and electronic effects, the careful selection of elemental combinations is essential in the designation of HEA-NMs. Factors such as mixing enthalpy, atomic size, and electronegativity must be considered to ensure the formation of single-phase solid solution nanoalloys. Moreover, computational approaches such as density functional theory (DFT) and machine learning (ML) play an increasingly important role in elucidating the microenvironment of HEA-NMs and forecasting interfacial reactions. For instance, Roy et al. (2022) demonstrated the use of ML algorithms to screen high-entropy alloy catalysts suitable for the selective hydrogenation of CO2 to methanol. These predictive tools provide valuable insights for the rational design of HEA-NMs tailored for specific electrocatalytic applications.
3 Synthetic strategies of HEA-NMs for electrocatalysis
The synthesis of HEA-NMs requires methods that ensure compositional uniformity, structural tunability, and nanoscale stability. The main synthesis methods for HEA-NMs include five representative strategies.
3.1 Mechanical alloying
This solid-state method uses high-energy ball milling to alloy elemental powders through repeated cold welding and fracturing (Figure 1a) (Mongella et al., 2025). It enables the formation of metastable solid solutions from even immiscible elements. Post-annealing or cryomilling enhances grain refinement. Though scalable and solvent-free, it often yields irregular or aggregated particles with limited morphology control.
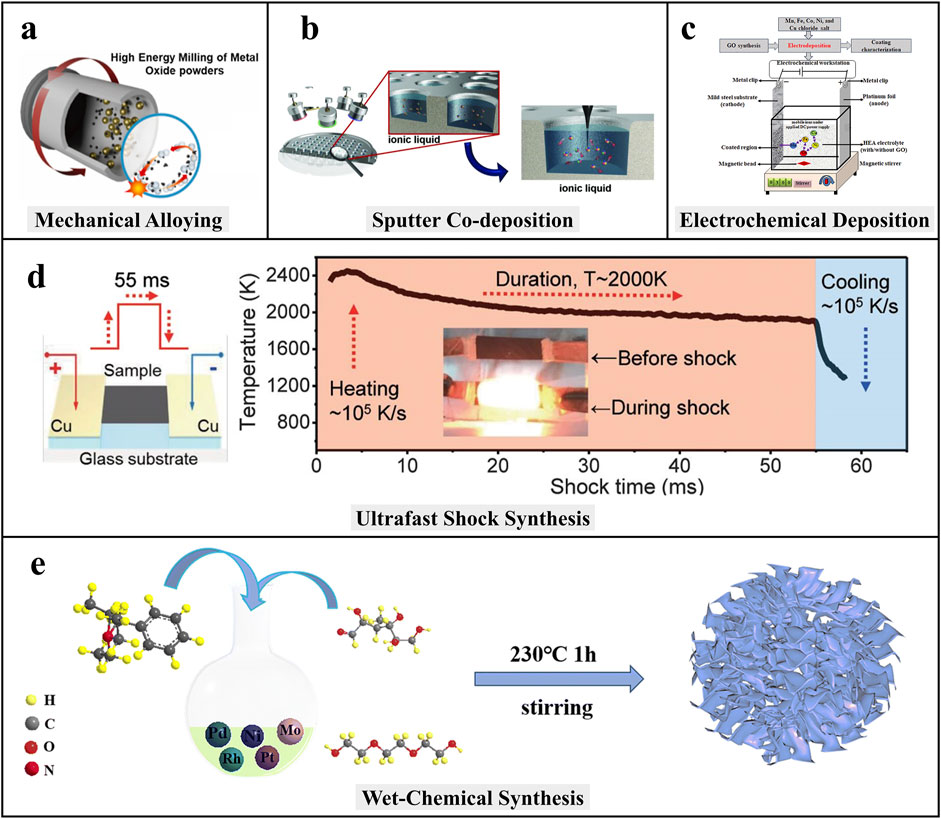
Figure 1. Five synthesis methods for HEA-NMs: (a) Mechanical alloying (Mongella et al., 2025); (b) Sputter co-deposition (Löffler et al., 2018); (c) Electrochemical deposition (Bian et al., 2023); (d) Ultrafast shock methods (Yao et al., 2018); and (e) Wet chemical synthesis (Wei et al., 2023).
3.2 Sputter co-deposition
This technique allows atomic-level control by co-depositing multiple metals onto substrates (Figure 1b). Parameters like power and deposition time tune size and composition. CrMnFeCoNi HEA-NMs synthesized via ionic liquid as substrate showed exceptionally high electrocatalytic activity and phase stability (Löffler et al., 2018). This method is ideal for fabricating model electrocatalysts for fundamental studies.
3.3 Electrochemical deposition
Electrochemical methods involve co-deposition of multiple metal ions onto conductive substrates under applied potential (Figure 1c) (Bian et al., 2023). This strategy enables the direct growth of HEA-NMs with controllable thickness and crystallinity. It is energy-efficient, room-temperature, and easily scalable. However, achieving uniform deposition of elements with vastly different reduction potentials remains a challenge, often requiring pulse techniques or complexing agents to balance deposition kinetics. These issues become more pronounced during scale-up, with reduced control over composition and particle uniformity.
3.4 Ultrafast shock synthesis
Ultrafast shock synthesis enables rapid formation of HEA-NMs under non-equilibrium conditions, typically using carbothermal shock, Joule heating, or laser ablation. These methods achieve heating rates exceeding 105 K/s, promoting atomic-scale mixing and preventing phase segregation (Figure 1d). The resulting nanoparticles are ultrasmall, often below 5 nm, providing better atomic control. For instance, PtPdRhRuCe HEA-NMs synthesized via carbothermal shock showed enhanced ammonia oxidation activity and phase stability (Yao et al., 2018).
3.5 Wet-chemical synthesis
Co-reduction or polyol processes allow controlled nucleation and morphology tuning with scalability. These cost-effective methods are surfactant-compatible but require careful optimization to prevent contamination. Wei et al. synthesized PtMoPdRhNi HEA-NMs exhibiting an ultralow overpotential of 9.7 mV at 10 mA cm−2 in the alkaline HER via this approach (Figure 1e) (Wei et al., 2023). However, during scale-up, variations in nucleation and reduction rates often lead to elemental segregation and broader particle size distributions.
Each synthesis method for HEA-NMs has specific strengths and limitations in atomic control, scalability, cost, and tunability. Mechanical alloying is inexpensive and scalable but lacks control over morphology and composition. Sputter co-deposition offers atomic precision, suited for mechanistic studies, though high cost and poor scalability limit broader use. Electrochemical deposition is energy-efficient under mild conditions, but uniform co-deposition is difficult due to reduction potential differences. Ultrafast shock synthesis rapidly produces ultrafine, mixed nanoparticles but needs specialized equipment and has limited compositional control. Wet-chemical synthesis is low-cost and flexible but prone to segregation and contamination. Thus, synthesis strategies should match the performance and scalability demands of the target application.
Despite progress, scaling HEA-NM synthesis remains difficult. During batch production, elemental segregation arises from differences in reduction kinetics and nucleation, especially in wet-chemical and electrochemical methods. These approaches also struggle to maintain uniform particle size and composition. Phase control is harder under thermal and concentration gradients at Gram scale. While mechanical alloying and ultrafast shock synthesis show gram-scale potential (e.g., Yao et al., 2018), industrial adoption is limited by reproducibility, cost, and complexity. Solving these issues is key to enabling HEA-NMs in practical electrocatalytic systems.
4 Electrocatalytic applications of HEA nanomaterials
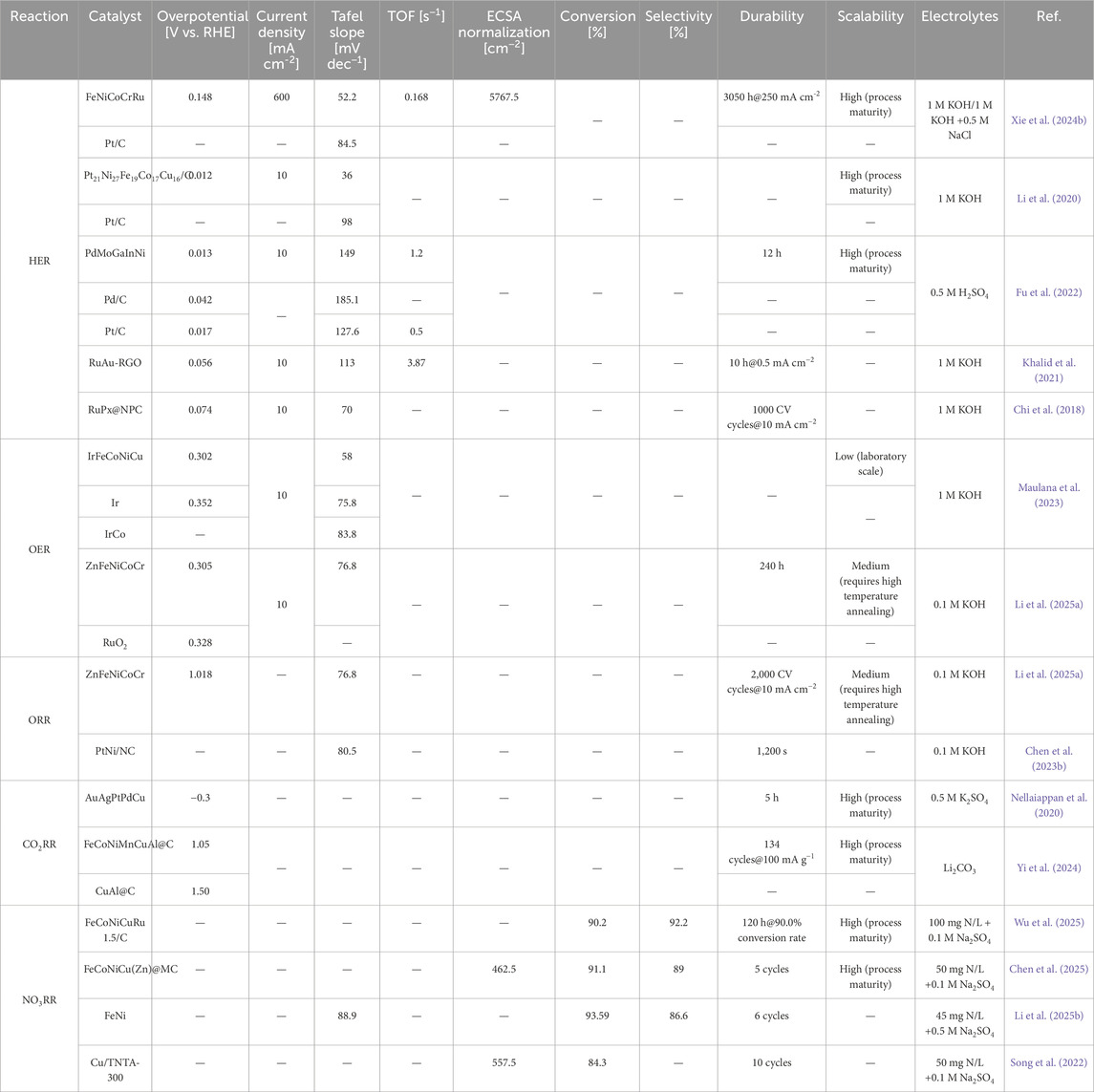
HEA-NMs, with high surface-to-volume ratios and compositional complexity, offer abundant active sites, tunable intermediate adsorption, and robust structural stability. These characteristics make them highly promising for key electrochemical reactions, including HER, OER, ORR, CO2RR, and NO3RR, where they often match or surpass the performance of state-of-the-art single or bimetallic catalysts in terms of overpotential, current density, Tafel slope, conversion, selectivity, and durability (Table 1) (Hao et al., 2022; Wang et al., 2025).
4.1 HER and OER
HEA-NMs enhance HER and OER via d-band modulation and atomic-level disorder, promoting water dissociation and intermediate adsorption (Wei et al., 2023). In HER, tuning ΔGH* across heterogeneous sites improves both Volmer and Tafel steps (Huang et al., 2024). In OER, a wide distribution of *OOH binding energies enables overpotential reduction and activity enhancement (He et al., 2023). The entropy-stabilized structure and “cocktail effect” contribute to long-term durability. HEA catalysts also maintain stable performance over a broad range of loadings, enabling integration into thin-film and porous electrodes for practical water electrolysis applications.
4.2 ORR
HEA-NMs improve ORR activity by tuning electronic structures and *OOH adsorption through multi-element alloying (Zhao et al., 2024). The disordered surface provides a range of binding energies, promoting 4e− pathways and enhanced kinetics. Many HEAs surpass Pt/C in mass activity, half-wave potential, and durability (Chen et al., 2023a). Their corrosion resistance and composition flexibility allow operation across pH conditions and electrolytes (Xie et al., 2024a). HEA catalysts also deliver stable performance across various loadings, supporting their integration into cathodes for fuel cells and metal–air batteries (Figure 2b).
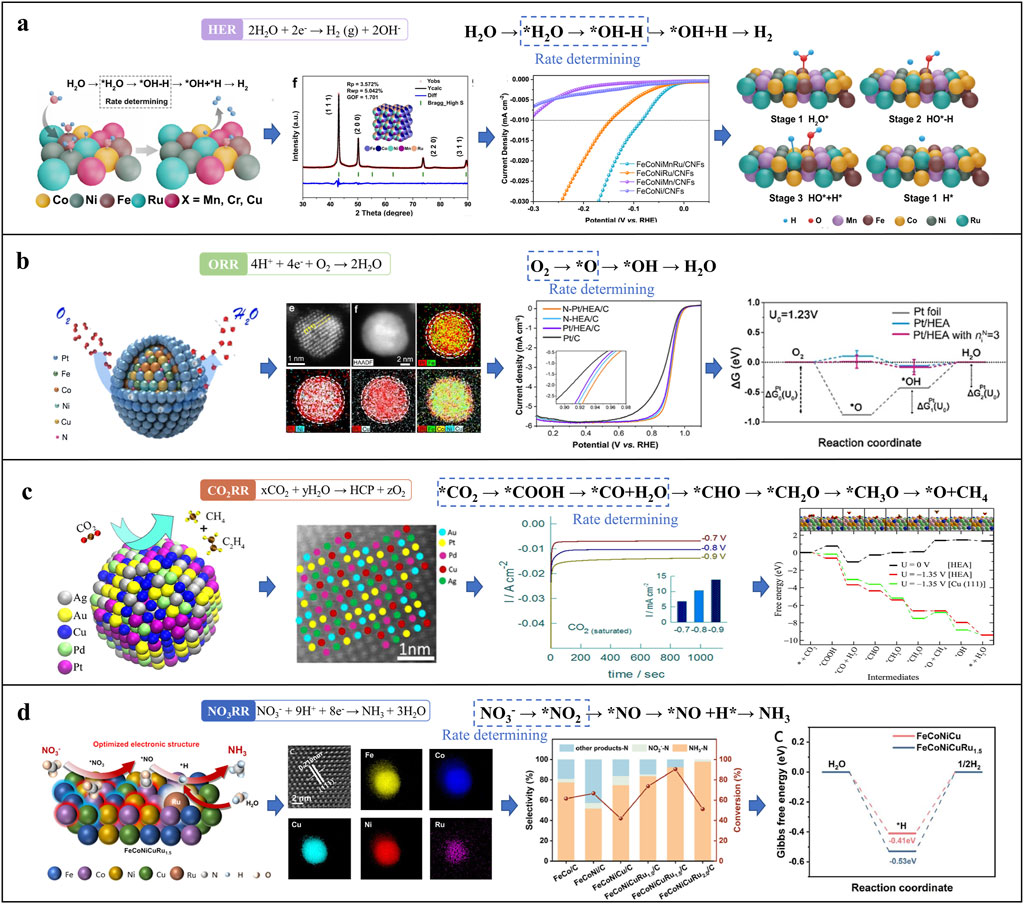
Figure 2. Applications of HEA-NMs in electrocatalysis: (a) HER (Hao et al., 2022), (b) ORR (Zhao et al., 2024; Chen et al., 2023a), (c) CO2RR (Nellaiappan et al., 2020), and (d) NO3RR (Wu et al., 2025; Chen et al., 2025).
4.3 CO2RR
HEA-NMs offer diverse active sites for key intermediates such as *COOH, *CO, and *CHO, enabling tuning of adsorption energies and product selectivity (Ding et al., 2021; Nellaiappan et al., 2020). Stronger *CO binding favors CH4 formation, while weaker binding promotes CO evolution. Multi-metallic surfaces facilitate simultaneous stabilization of intermediates and C–C coupling (Xing et al., 2025). Nanoscale features, including high surface area and short diffusion lengths, enhance conversion efficiency. HEA catalysts demonstrate low overpotentials, high current densities, and selective production of hydrocarbons and alcohols, showing strong potential for CO2 utilization in energy devices (Figure 2c).
4.4 NO3RR
In NO3RR, product selectivity (N2, NH3, NO2−) depends on both thermodynamic and kinetic factors, including *NO, *NH2, and *N adsorption strengths (Chen et al., 2025). Strong *N binding favors NH3, while weaker adsorption promotes N2 evolution. HEA-NMs offer tunable active sites that selectively stabilize intermediates like NO2− and NH2OH, enabling controlled electron transfer and improved selectivity (Tang et al., 2024). Local pH affects *NH3 desorption and catalyst durability. Cl−-containing electrolytes can generate reactive chlorine species, further enhancing nitrogen selectivity by suppressing by-products (Tokazhanov et al., 2020). Our FeCoNiCu-based HEAs demonstrate high nitrate conversion and Faradaic efficiency (Figure 2d), confirming their promise in water treatment and ammonia synthesis (Chen et al., 2025; Wu et al., 2025).
5 Support-enhanced electrocatalysis of HEA-NMs
To enhance dispersion and prevent aggregation of high-surface-energy HEA nanoparticles, HEA-NMs are commonly integrated with conductive supports such as graphene, carbon nanotubes (CNTs), and reduced graphene oxide (rGO). These supports offer anchoring sites that stabilize nanoparticles, promote effective charge transport, and improve electrochemical accessibility. For example, FeCoNiIrRu HEAs supported on carbon nanofibers showed improved OER activity due to better dispersion and conductivity (Zhu et al., 2022). Mesoporous carbon-supported FeCoNiCu(Zn) HEAs exhibited enhanced NO3RR performance, attributed to the accelerated mass transfer and intermediate confinement enabled by the porous architecture (Chen et al., 2025).
Beyond conductivity, interfacial charge transfer also plays a crucial role. Ling et al. (2025) demonstrated that rGO anchoring induced d-band modulation in FeCoNiCuSn HEAs, facilitating NO3RR. Similarly, MXene-based Pt-doped HEAs exhibited enhanced HER activity through orbital hybridization with hydrogen (Shu et al., 2024).
Hydrophobic/hydrophilic tailoring of the support surface can further modulate gas–liquid–solid interfaces. For instance, controlling the hydrophobicity of a Co–Cu–Mo–Pd–Re catalyst layer enabled high-current HER by preventing H2 bubble accumulation (Wang et al., 2024).
6 Challenges and prospects
(1) Scalable and controllable synthesis: The scalable synthesis of phase-pure HEA-NMs remains challenging due to compositional deviation, particle size heterogeneity, and environmental risks such as metal leaching. Higher catalyst loadings can induce interfacial stress and detachment in practical electrolyzers. Recovery strategies using green separation techniques should be developed to enable sustainable application.
(2) Mechanistic understanding of catalytic processes: The complex interactions among diverse active sites and their dynamic evolution during reactions remain poorly understood. In-situ and operando techniques, such as X-ray absorption spectroscopy, Raman, and TEM, combined with density functional theory (DFT), are essential for elucidating structure–activity relationships and guiding rational catalyst design.
(3) Data-driven optimization: The vast compositional space of HEA-NMs renders traditional screening inefficient. Integrating machine learning and high-throughput computation can accelerate catalyst discovery by predicting optimal compositions and identifying key performance descriptors, enabling targeted design of HEA-NMs for specific electrochemical reactions.
(4) Sustainability and Material Lifecycle: The long-term application of HEA-NMs requires attention to sustainability. Many systems still rely on noble metals (e.g., Pt, Pd, Ru), raising cost and supply concerns. Recent efforts toward noble-metal-free HEAs using Fe, Co, Ni, or Cu show promise. End-of-life recovery, recyclability, and lifecycle impact assessments remain underexplored and should be integrated into future HEA catalyst development strategies.
7 Conclusion
This mini-review summarizes recent advances in high-entropy alloy nanomaterials (HEA-NMs), focusing on their synthesis, mechanistic effects, and electrocatalytic applications in key reactions such as HER, OER, ORR, CO2RR, and NO3RR. While the compositional complexity of HEA-NMs enables tunable catalytic properties and superior stability, it also poses challenges in achieving controlled synthesis and understanding dynamic reaction mechanisms. Future research should integrate atomic-scale structural design, in situ/operando characterization, and machine-learning-guided screening to accelerate rational catalyst discovery and advance HEA-NMs for clean energy and environmental technologies.
Author contributions
JC: Investigation, Writing – original draft. AW: Writing – original draft, Investigation. YZ: Investigation, Writing – original draft. YX: Investigation, Writing – original draft. HZ: Writing – review and editing, Project administration, Funding acquisition. WT: Writing – review and editing, Conceptualization, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by National Key Research and Development Program of China (2023YFC3700051), the Shanghai Science and Technology Plan Project (No. 23ZR1467000), Basic Research Project of Tongji University (No. 22120240354), and National Natural Science Foundation of China (No. 21976134).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bian, H. W., Wang, R., Zhang, K. Z., Zheng, H. L., Wen, M. J., Li, Z. M., et al. (2023). Facile electrodeposition synthesis and super performance of nano-porous Ni-Fe-Cu-Co-W high entropy alloy electrocatalyst. Surf. Coatings Technol. 459, 129407. doi:10.1016/j.surfcoat.2023.129407
Chen, J. Y., Wu, A. N., Hong, C. Y., Zhang, R. Y., Zheng, H., and Teng, W. (2025). Medium entropy alloy nanoparticles incorporated in ordered mesoporous carbons for enhanced electrocatalytic nitrate-to-ammonia conversion. J. Environ. Chem. Eng. 13 (1), 115047. doi:10.1016/j.jece.2024.115047
Chen, T., Qiu, C. Y., Zhang, X. K., Wang, H. C., Song, J., Zhang, K., et al. (2023a). An ultrasmall ordered high-entropy intermetallic with multiple active sites for the oxygen reduction reaction. J. Am. Chem. Soc. 146 (1), 1174–1184. doi:10.1021/jacs.3c12649
Chen, X., Guo, J., Liu, J., Luo, Z., Zhang, X., Qian, D., et al. (2023b). Nanostructure engineering and electronic modulation of a PtNi alloy catalyst for enhanced oxygen reduction electrocatalysis in Zinc–air batteries. J. Phys. Chem. Lett. 14 (7), 1740–1747. doi:10.1021/acs.jpclett.2c03835
Chi, J.-Q., Lin, W.-K., Dong, J.-H., Yan, B., Qin, K.-L., Liu, J.-F., et al. (2018). Hydrogen evolution activity of ruthenium phosphides encapsulated in nitrogen- and phosphorous-codoped hollow carbon nanospheres. ChemSusChem 11 (4), 743–752. doi:10.1002/cssc.201702010
Ding, T. L., Liu, Z., Chen, T., Zhang, T., Shen, W., Liu, X., et al. (2021). Atomically precise dinuclear site active toward electrocatalytic CO2 reduction. J. Am. Chem. Soc. 143 (30), 11317–11324. doi:10.1021/jacs.1c05754
Fu, X., Zhang, J., Xia, S., Wang, F., Ma, C., Yue, D., et al. (2022). High-entropy alloy nanosheets for fine-tuning hydrogen evolution. ACS Catal. 12 (19), 11955–11959. doi:10.1021/acscatal.2c02778
Gandionco, K. A., Kim, J., Bekaert, L., Hubin, A., and Lim, J. (2024). Single-atom catalysts for the electrochemical reduction of carbon dioxide into hydrocarbons and oxygenates. Carbon Energy 6 (3). doi:10.1002/cey2.410
Hao, J. C., Zhuang, Z. C., Cao, K. C., Gao, G. H., Wang, C., Lai, F. L., et al. (2022). Unraveling the electronegativity-dominated intermediate adsorption on high-entropy alloy electrocatalysts. Nat. Commun. 13 (1), 2662. doi:10.1038/s41467-022-30379-4
He, R., Yang, L. L., Zhang, Y., Jiang, D., Lee, S., Horta, S., et al. (2023). A 3d-4d-5d high entropy alloy as a bifunctional oxygen catalyst for robust aqueous zinc-air batteries. Adv. Mater. 35 (46), e2303719. doi:10.1002/adma.202303719
Huang, K., Cao, X., Lu, Y., Xiu, M. Z., Cui, K., Zhang, B. W., et al. (2024). Lattice-disordered high-entropy alloy engineered by thermal dezincification for improved catalytic hydrogen evolution reaction. Adv. Mater. 36 (32), e2304867. doi:10.1002/adma.202304867
Kamaruddin, H., Zhang, J. H., Liang, Y., Wei, Y. F., and Huang, Y. Z. (2024). A review of noble metal-free high entropy alloys for water splitting applications. J. Mater. Chem. A 12 (17), 9933–9961. doi:10.1039/d4ta00690a
Khalid, M., Zarate, X., Saavedra-Torres, M., Schott, E., Maria Borges Honorato, A., Rafe Hatshan, M., et al. (2021). Electro-reduced graphene oxide nanosheets coupled with RuAu bimetallic nanoparticles for efficient hydrogen evolution electrocatalysis. Chem. Eng. J. 421, 129987. doi:10.1016/j.cej.2021.129987
Lee, S. A., Bu, J. W., Lee, J. W., and Jang, H. W. (2023). High-entropy nanomaterials for advanced electrocatalysis. Small Sci. 3 (5), 2200109. doi:10.1002/smsc.202200109
Li, H., Han, Y., Qi, W., Zhang, D., Yu, Y., Cai, W., et al. (2020). Fast site-to-site electron transfer of high-entropy alloy nanocatalyst driving redox electrocatalysis. Nat. Commun. 11 (1), 5437. doi:10.1038/s41467-020-19277-9
Li, J., Li, P.-T., Zhang, N., and Shang, H.-S. (2025a). A ZnFeNiCoCr high-entropy alloy for efficient bifunctional oxygen electrocatalysis. Rare Met. 44 (3), 1789–1799. doi:10.1007/s12598-024-03079-9
Li, L., Guo, P., Zhang, Q., Mao, D., Sun, Q., Liu, M., et al. (2025b). In situ formation of FeNi nanoparticles on polypyrrole hydrogel for efficient electrocatalytic nitrate reduction to ammonia. Molecules 30 (6), 1271. doi:10.3390/molecules30061271
Li, M. G., Lin, F. X., Zhang, S. P., Zhao, R., Tao, L., Li, L., et al. (2024a). High-entropy alloy electrocatalysts go to (sub-)nanoscale. Sci. Adv. 10 (23), eadn2877. doi:10.1126/sciadv.adn2877
Li, Y. J., Yao, Z. P., Gao, W. P., Shang, W., Deng, T., and Wu, J. B. (2024b). Nanoscale design for high entropy alloy electrocatalysts. Small 20 (21), e2310006. doi:10.1002/smll.202310006
Ling, Y., Zheng, , Su, X., Zhang, H., Zou, Y., Liu, Z., et al. (2025). Microwave-assisted fabrication of a self-supported graphene-based high-entropy alloy electrode for efficient and stable electrocatalytic nitrate reduction to ammonia. Inorg. Chem. Front. 12 (2), 682–691. doi:10.1039/d4qi02881c
Liu, S. J., Wei, Y., Wang, M. K., and Shen, Y. (2025). The future of alkaline water splitting from the perspective of electrocatalysts-seizing today's opportunities. Coord. Chem. Rev. 522, 216190. doi:10.1016/j.ccr.2024.216190
Liu, Y. X., Ding, W. H., Liu, J. X., Zhao, G. Z., Li, W. Y., and Liu, Y. Q. (2024). Electrochemical synthesis of wormcast-like Pd-based polycrystalline high entropy aggregates for methanol water co-electrocatalysis. J. Mater. Chem. A 12 (44), 30757–30767. doi:10.1039/d4ta06304j
Löffler, T., Meyer, H., Savan, A., Wilde, P., Manjón, A. G., Chen, Y.-T., et al. (2018). Discovery of a multinary noble metal–free oxygen reduction catalyst. Adv. Energy Mater. 8 (34), 1802269. doi:10.1002/aenm.201802269
Maulana, A. L., Chen, P.-C., Yang, Z., Lizandara-Pueyo, Y., Seeler, C., Abruña, F., et al. (2023). Understanding the structural evolution of IrFeCoNiCu high-entropy alloy nanoparticles under the acidic oxygen evolution reaction. Nano Lett. 23 (14), 6637–6644. doi:10.1021/acs.nanolett.3c01831
Mongella, G. M., Im, T., Kim, M., and Lee, C. S. (2025). Reaction kinetics study of hydrogen reduction of high energy milled CoCrFeNi high-entropy alloy (HEA). J. Mater. Res. Technol. 35, 5591–5599. doi:10.1016/j.jmrt.2025.02.196
Nellaiappan, S., Katiyar, N. K., Kumar, R., Parui, A., Malviya, K. D., Pradeep, K. G., et al. (2020). High-entropy alloys as catalysts for the CO2 and CO reduction reactions: experimental realization. Acs Catal. 10 (6), 3658–3663. doi:10.1021/acscatal.9b04302
Roy, D., Mandal, S. C., and Pathak, B. (2022). Machine learning assisted exploration of high entropy alloy-based catalysts for selective CO2 reduction to methanol. J. Phys. Chem. Lett. 13 (25), 5991–6002. doi:10.1021/acs.jpclett.2c00929
Serov, A. (2022). Nickel catalysts for affordable fuel cells. Nat. Catal. 5 (11), 971–972. doi:10.1038/s41929-022-00872-6
Shaikh, J. S., Rittiruam, M., Saelee, T., Márquez, V., Shaikh, N. S., Khajondetchairit, P., et al. (2024). First-principles and experimental insight of high-entropy materials as electrocatalysts for energy-related applications: hydrogen evolution, oxygen evolution, and oxygen reduction reactions. Mater. Sci. Eng. R-Reports 160, 100813. doi:10.1016/j.mser.2024.100813
Shu, Z., Shi, Z., Ng, M.-F., Tan, T. L., and Cai, Y. (2024). Unveiling the effect of solvent for hydrogen evolution in Pt-doped MXenes and corresponding high-entropy phase. Mater. Today Sustain. 26, 100808. doi:10.1016/j.mtsust.2024.100808
Song, Q., Zhang, S., Hou, X., Li, J., Yang, L., Liu, X., et al. (2022). Efficient electrocatalytic nitrate reduction via boosting oxygen vacancies of TiO2 nanotube array by highly dispersed trace Cu doping. J. Hazard. Mater. 438, 129455. doi:10.1016/j.jhazmat.2022.129455
Tang, S. S., Xie, M. H., Yu, S. R., Zhan, X., Wei, R. L., Wang, M. Y., et al. (2024). General synthesis of high-entropy single-atom nanocages for electrosynthesis of ammonia from nitrate. Nat. Commun. 15 (1), 6932. doi:10.1038/s41467-024-51112-3
Tokazhanov, G., Ramazanova, E., Hamid, S., Bae, S., and Lee, W. (2020). Advances in the catalytic reduction of nitrate by metallic catalysts for high efficiency and N2 selectivity: a review. Chem. Eng. J. 384, 123252. doi:10.1016/j.cej.2019.123252
Wang, C., Wang, Y., Xiong, Y., Hao, F., Liu, F., Guo, L., et al. (2025). Tailored high-entropy alloy nanomaterials for electrocatalytic applications. EnergyChem 7 (3), 100155. doi:10.1016/j.enchem.2025.100155
Wang, K. X., Wang, G. Q., Liu, Y., Cai, Q. Q., Chen, X., Zhang, L., et al. (2024). Regulation of electrocatalytic properties of high entropy alloy electrocatalysts for the oxygen evolution reaction. J. Mater. Chem. A 12 (43), 29311–29334. doi:10.1039/d4ta04984e
Wang, X., Cechanaviciute, I. A., Banko, L., Pokharel, S., Quast, T., Ludwig, A., et al. (2024). From quinary Co–Cu–Mo–Pd–Re materials libraries to gas diffusion electrodes for alkaline hydrogen evolution. Adv. Funct. Mater. 34 (33), 2400180. doi:10.1002/adfm.202400180
Wei, M., Sun, Y. Y., Zhang, J. Y., Ai, F., Xi, S. B., and Wang, J. K. (2023). High-entropy alloy nanocrystal assembled by nanosheets with d-d electron interaction for hydrogen evolution reaction. Energy Environ. Sci. 16 (9), 4009–4019. doi:10.1039/d3ee01929b
Wu, A., Hong, C., Zheng, H., and Teng, W. (2025). Multi-site synergistic relay electrocatalysis with high-entropy nanoalloys for effective nitrate reduction to ammonia. Chin. Chem. Lett., 111066. doi:10.1016/j.cclet.2025.111066
Xie, M. K., Xiao, X., Wu, D. J., Zhen, C., Wu, C. S., Wang, W. J., et al. (2024a). MOF-mediated synthesis of novel PtFeCoNiMn high-entropy nano-alloy as bifunctional oxygen electrocatalysts for zinc-air battery. Nano Res. 17 (6), 5288–5297. doi:10.1007/s12274-024-6526-4
Xie, Y. C., Xu, S. C., Meng, A. C., Zheng, B. J. D., Chen, Z. R., Tour, J. M., et al. (2024b). Laser-induced high-entropy alloys as long-duration bifunctional electrocatalysts for seawater splitting. Energy Environ. Sci. 17 (22), 8670–8682. doi:10.1039/d4ee01093k
Xing, R., Wang, X., Wang, G., Lu, Z., Yang, X., Wang, H., et al. (2025). Non-precious metal high-entropy alloys for CO2 electroreduction. Nanoscale 17 (15), 9374–9379. doi:10.1039/D5NR00260E
Yao, Y. G., Huang, Z. N., Xie, P. F., Lacey, S. D., Jacob, R. J., Xie, H., et al. (2018). Carbothermal shock synthesis of high-entropy-alloy nanoparticles. Science 359 (6383), 1489–1494. doi:10.1126/science.aan5412
Yeh, J.-W., Chen, S.-K., Lin, S.-J., Gan, J.-Y., Chin, T.-S., Shun, T.-T., et al. (2004). Nanostructured high-entropy alloys with multiple principal elements: novel alloy design concepts and outcomes. Adv. Eng. Mater. 6 (5), 299–303. doi:10.1002/adem.200300567
Yi, J., Deng, Q., Cheng, H., Zhu, D., Zhang, K., and Yang, Y. (2024). Unique hierarchically structured high-entropy alloys with multiple adsorption sites for rechargeable Li–CO2 batteries with high capacity. Small 20, 2401146. doi:10.1002/smll.202401146
Zhao, X. R., Cheng, H., Chen, X. B., Zhang, Q., Li, C. Z., Xie, J., et al. (2024). Multiple metal-nitrogen bonds synergistically boosting the activity and durability of high-entropy alloy electrocatalysts. J. Am. Chem. Soc. 146 (5), 3010–3022. doi:10.1021/jacs.3c08177
Keywords: high-entropy alloy nanomaterials, electrocatalysis, fundamental mechanisms, synthesis strategies, catalytic performance
Citation: Chen J, Wu A, Zhang Y, Xie Y, Zheng H and Teng W (2025) A mini-review on high-entropy alloy nanomaterials for electrocatalysis: advances and prospects. Front. Mater. 12:1613997. doi: 10.3389/fmats.2025.1613997
Received: 18 April 2025; Accepted: 20 May 2025;
Published: 02 June 2025.
Edited by:
Kunal Mondal, Oak Ridge National Laboratory (DOE), United StatesReviewed by:
Arnab Bose, Boehringer Ingelheim, United StatesDebabrata Moitra, The University of Tennessee, Knoxville, United States
Copyright © 2025 Chen, Wu, Zhang, Xie, Zheng and Teng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Teng, d3RlbmdAdG9uZ2ppLmVkdS5jbg==
 Jiayu Chen
Jiayu Chen Anni Wu2
Anni Wu2 Hu Zheng
Hu Zheng Wei Teng
Wei Teng