Abstract
At least a quarter of adult patients with severe brain injury in a disorder of consciousness may have cognitive abilities that are hidden due to motor impairment. In this case series, we developed a tool that extracted acoustic and semantic processing biomarkers from electroencephalography recorded while participants listened to a story. We tested our method on two male adolescent survivors of severe brain injury and showed evidence of acoustic and semantic processing. Our method identifies cognitive processing while obviating demands on attention, memory, and executive function. This lays a foundation for graded assessments of cognition recovery across the spectrum of covert cognition.
1 Introduction
In at least 25% of adults with severe brain injury who are diagnosed with a disorder of consciousness (DoC) such as vegetative state, functional magnetic resonance imaging (fMRI) and electroencephalography (EEG) can reveal high-level cognitive activity that is otherwise hidden due to profound motor impairment (Bodien et al., 2024).
Covert cognition is currently detected via fMRI and EEG tests that measure brain responses correlated with imagined or attempted movements to command. Brain activity following the command “move your hand” is contrasted against the command “relax”; a positive response indicates the patient's ability to hear, understand, and repeatedly perform the task without loss of attention, drowsiness, or other factors limiting sustained performance (Bodien et al., 2024). This unequivocally signals cognitive-motor dissociation (CMD).
However, the patient must cross a very high threshold of cognitive functioning to achieve a positive response in established CMD testing (Talukdar et al., 2019). Unsurprisingly, these tasks may fail to identify patients in whom cognitive abilities have only partially recovered.
Brain responses to language provide an attractive alternative window into covert cognition. Comprehension of a spoken text—in other words, integration of successive word meanings to form a context—requires attention, lexical access, syntactic parsing, working memory operations and inference, among other tightly integrated cognitive processes (Moore, 2007). As such, it entails many of the elements of cognition—just as command-following paradigms encompass a different but overlapping subset of elements (Bodien et al., 2024). EEG correlates of semantic processing are known to have prognostic value: examples include the N400 event-related potential (ERP) to incongruent sentence endings (Steppacher et al., 2013), and the rhythmic modulation of EEG responses phase-locked to modulation at word, phrasal and sentential linguistic levels (Gui et al., 2020). Unfortunately, the stimuli used by these studies are artificially manipulated and the meaning of each stimulus is disconnected from the next, which makes listening both jarring and monotonous—and hence, susceptible to inattention.
Recent approaches have begun to focus on natural-language story listening (O'Sullivan et al., 2015). EEG correlates of auditory processing, while listening to Lewis Carroll's Alice In Wonderland, were similar between healthy subjects and patients with CMD (Braiman et al., 2018). New signal-processing methods have been developed for extracting responses that are specific to semantic content in natural language—in non-brain-injured controls, these responses are exquisitely sensitive to the subject's understanding of meaning in natural speech (Broderick et al., 2018, 2022).
We apply these novel methods to two adolescents with DoC resulting from severe brain injuries sustained as children. We have previously demonstrated CMD in these subjects based on positive motor command-following responses (Kim et al., 2022b,a); we also showed supporting evidence from ERPs to classical, discrete, auditory language stimuli (Kim et al., 2022a). Here, we derive acoustic and semantic processing markers from natural-language stimuli using methods that estimate temporal response functions (TRFs) (Crosse et al., 2016). The acoustic TRF reflects perceptual and attentional aspects of auditory processing; it contains analogs of the N100 and other event-related potentials that are more familiarly seen in discrete-stimulus designs (Martin et al., 2008). The semantic TRF contains a distinctive component that is spatio-temporally similar to the N400 event-related potential—here, we will include both the N400 and its TRF analog under the umbrella term meaning-induced neural dynamics (MIND).
2 Methods
2.1 Participants
In this paper, we report the data of two adolescent participants with DoC following severe brain injury. Participant P1 had experienced a traumatic brain injury complicated by hypoxemia and associated cardiac arrest at age 9. P1 participated in the current study during a single testing session, at age 16. Participant P2 experienced anoxic brain injury secondary to cardiac arrest at age 13. P2 participated in two separate testing sessions: the discrete semantic paradigm (discussed in detail in Section 2.3.3) was collected at age 18, and the remaining paradigms (discussed in Section 2.3) were collected at age 21. Assessments with the Coma Recovery Scale-Revised (CRS-R) (Giacino et al., 2004) were consistent with vegetative state (P1's total score was 7; P2's total score was 6 in both assessments).
Both participants were previously included in two studies (Kim et al., 2022b,a); they were labeled as participants C and E in Kim et al. (2022b) and as P1 and P2 in Kim et al. (2022a). In the study Kim et al. (2022b), we reported two EEG tests as part of a larger cohort: auditory evoked potentials (AEPs) in simple auditory paradigms, and correlates of motor command-following in oscillatory EEG components (MCF-EEG). In the associated case-series publication (Kim et al., 2022a), we provided a multi-modal profile for P1 and P2, including fMRI motor command-following (MCF-fMRI), EEG correlates of discrete semantic comprehension (N400), fluorodeoxyglucose positron emission tomography, structural MRI, and clinical histories along with the previously-reported ERP and MCF-EEG findings.
All procedures were approved by the institutional review board (IRB) of Weill Cornell Medicine. Parental consents were obtained as per IRB protocols.
2.2 Data acquisition
EEG data were recorded with a 128-channel HydroCel Geodesic Sensor Net (EGI, Eugene, OR) (Tucker, 1993). In P1, gel-based sensors were used; in P2, saline sponge and gel-based sensors were used. The impedance of all electrodes was < 75 kΩ at the beginning of the recording as per the manufacturer's specifications. The signals were recorded at 1000 Hz. A 2-piece speaker system was located at 45° (left/right of the midline) at a distance of 57 cm to the ears. Each speech stimulus was normalized to a volume of 70 dB SPL. Stimulus presentation and data acquisition were conducted using the BCI2000 software platform (Schalk et al., 2004).
2.3 Stimuli and tasks
We estimated an acoustic and a semantic TRF from the EEG elicited by continuous natural-language listening. We compared these responses against those from the discrete ERP-based paradigms. The paradigms used in this study are described below.
2.3.1 Discrete “clicks” paradigm
For P1, our assessment of auditory processing comes from a click presentation paradigm, in which a rapid sequence of click sounds is designed to elicit an auditory evoked potential (AEP). This paradigm requires no participation beyond passive listening. The click stimuli were a train of 300 biphasic square pulses each with a duration of 1 ms, presented every 745 ms ± 15 S.D.
2.3.2 Discrete “beeps” paradigm
For P2, our assessment of auditory processing was derived from responses to “standard” stimuli in an auditory oddball paradigm (Polich, 2007). This paradigm has frequently been used in both adults and children—including children with brain injury (Kim et al., 2022b; Duszyk et al., 2019)—to assess early attentional processing. During this paradigm, the participant listens to a sequence of abrupt, frequent standard stimuli mixed in random order with rarer deviant stimuli. This task requires no participation beyond passive listening. For the purposes of the current analysis, we consider only the responses to the 270 “standard” stimuli, which comprised about 66% of the stimuli and which were square-wave beeps of 340 ms duration with a fundamental frequency of 400 Hz, repeated with a period of 1 second. Further details about the stimuli used in this paradigm can be found in Kim et al. (2022b).
2.3.3 Discrete N400 paradigm
To estimate P1 and P2's discrete semantic ERPs, we used the sentences from the classic N400 paradigm (Bloom and Fischler, 1980) that are composed of 6–8 words (Connolly and Phillips, 1994). For P1 the stimulus total duration was around 11 min and consisted of 60 congruent sentences (e.g., “Apples and cherries are a type of fruit.”) and 60 incongruent sentences (e.g., “A wild pig is called a shirt.”). For P2, the stimulus total duration was around 6 minutes and consisted of 40 congruent and 40 incongruent sentences. More details can be found in Kim et al. (2022a).
2.3.4 Continuous natural language paradigm
During this paradigm, the participants listened to continuous age-appropriate natural language stimuli, as an audiobook. For P1, we presented 5 min and 40 s of Yertle the Turtle and Other Stories by Dr. Seuss read by an adult female reader. For P2, we used around 14 min of Judy Moody Gets Famous! by Peter H. Reynolds read by an adult male reader; this was divided into four sections of equal length, with pauses of a few seconds added between sections.
2.4 Data processing
Data were processed offline using the MNE package for Python (Gramfort et al., 2013). First, the EEG signals were re-referenced to the average of the right and left mastoids and down-sampled to 250 Hz (after applying an anti-aliasing filter). The signals were then visually inspected by an expert, who marked bad channels (which were then removed and reconstructed via linear interpolation) and bad data segments (which were removed from the analysis in the case of discrete stimuli only). Afterward, the signals were high-pass filtered with a cut-off frequency of 1 Hz. Undesired artifacts from blinks, cardiac activity, and muscle contractions were removed by rejecting the corresponding sources from an independent component analysis (ICA) decomposition using the Infomax algorithm (Bell and Sejnowski, 1995) and projecting back into the sensor space. Lastly, a low-pass filter at 8 Hz was applied to the cleaned EEG signals as in Broderick et al. (2018) and O'Sullivan et al. (2015).
2.5 Discrete stimuli analysis
The discrete stimuli (clicks, beeps, and N400 paradigms), elicit an event-related potential (ERP). For this analysis, the data were segmented into epochs time-locked to the onset of the target sound stimuli. For the clicks and beeps paradigms, epochs spanned 100 ms pre-stimulus and 500 ms post-stimulus. For the N400 paradigm, epochs spanned 200 ms pre-stimulus and 800 ms post-stimulus. Each epoch was baseline-corrected by subtracting the mean voltage over the pre-stimulus interval. For the discrete acoustic paradigms (clicks and beeps), the epochs were then processed by the MNE implementation of autoreject (Jas et al., 2016, 2017) which identified and rejected bad epochs (autoreject was not used for the N400 paradigms due to the small number of epochs of this paradigm 60 congruent and 60 incongruent for P1, 40 congruent and 40 incongruent for P2). The ERP was obtained from each session by averaging across epochs at each time point.
2.6 Continuous stimuli analysis
Linear de-convolution techniques were used to estimate temporal response functions (TRFs) (Crosse et al., 2016) that reflected the brain's response to continuous stimuli. Separate analyses estimated TRFs from the acoustic and semantic features of the stimuli, with the goal of extracting evidence for both “hearing” and “understanding”. We used a Python implementation of the TRF algorithm developed in our lab and inspired by the one made public by Crosse and colleagues (Crosse et al., 2016; Steinkamp, 2023; Bialas et al., 2023).
2.6.1 Acoustic TRF (A-TRF)
The acoustic TRF was obtained by relating an acoustic representation of the stimulus to the EEG signal. First, the left and right channels of the audio file were averaged to obtain one sound signal. As proposed in the mTRF Toolbox (Crosse et al., 2016, 2021), we extracted the speech envelope directly from the sound signal, after down-sampling to match the EEG sampling rate of 250 Hz, by computing the root-mean-square (RMS) over a window of samples and applying a logarithmic compression factor of 0.3 as in Crosse et al. (2021) and Biesmans et al. (2016). We report the A-TRF using the forward model, which aims to derive a linear de-convolved mapping from the stimulus to each of the EEG channels independently. Thus, one can plot the spatial representation of these models across the scalp—akin to the kinds of topographic analyses that are common in ERP research (see Crosse et al., 2016 for more details). The TRFs are then found by multiple linear regression, with a regularization parameter λ set to 105 (a value that we had been found to be a reasonable one-size-fits-all setting from similar data sets).
2.6.2 Semantic TRF (S-TRF)
The semantic TRF was obtained by relating a semantic representation of the stimulus to the EEG signal. Here, we use the lexical surprisal index from each word of the presented stimuli: each word was represented by its conditional probability of occurrence given the word sequence that preceded it, calculated using a large language model—specifically, the 12-layer, 117M parameter version of GPT-2 (Radford et al., 2019). Since brain responses tend to be larger for unexpected words compared to expected words (Kutas and Hillyard, 1984; Mesik et al., 2021), surprisal was represented as an impulse at the onset of each word, where the impulse height was equal to the negative log of the conditional word probability. This leads to less-expected (more informative) words receiving higher surprisal values (Mesik et al., 2021). For this analysis, we employed the forward modeling approach, where the spatial patterns were obtained using equation (5) from Crosse et al. (2016). The regularization parameter in the linear regression was set as λ = 105.
2.7 Spatial and temporal range of interest
For the discrete and continuous paradigms, we computed the acoustic and semantic responses from Cz and Pz, respectively, as in published studies (Kim et al., 2022b; Broderick et al., 2018; Gonzalez-Heydrich et al., 2015; Takeshita et al., 2002; Chalehchaleh et al., 2024). For the acoustic paradigms, we noted the latency of the largest negative peak within the range of 80–300 ms (Sussman et al., 2008; Lightfoot, 2016; Stapells et al., 2002). We used a wide range for identifying the auditory evoked potential since a longer-latency response (around 200 ms) is expected in children younger than 12 (Sussman et al., 2008; Lightfoot, 2016; Stapells et al., 2002) and this had previously been found to have persisted into adolescence for P1 (Kim et al., 2022a). For the semantic paradigms, we identified the MIND component as the largest negative peak within 250-500 ms, by analogy with the N400 event-related potential (Kutas and Federmeier, 2000; Kutas and Hillyard, 1980; Camarrone and Van Hulle, 2019).
2.8 Statistical analysis
2.8.1 Discrete stimuli
We performed a one-sample two-sided t-test at each time point across trials for both the “clicks” and “beeps” paradigms to identify when the ERP response was significantly different from zero. For the classic “N400” paradigm, we performed a two-sample two-sided t-test at each time point across trials to determine when the incongruent response significantly differed from the congruent response. Statistically significant time points (p < 0.05, uncorrected) are shown as black horizontal lines in Figure 1.
Figure 1
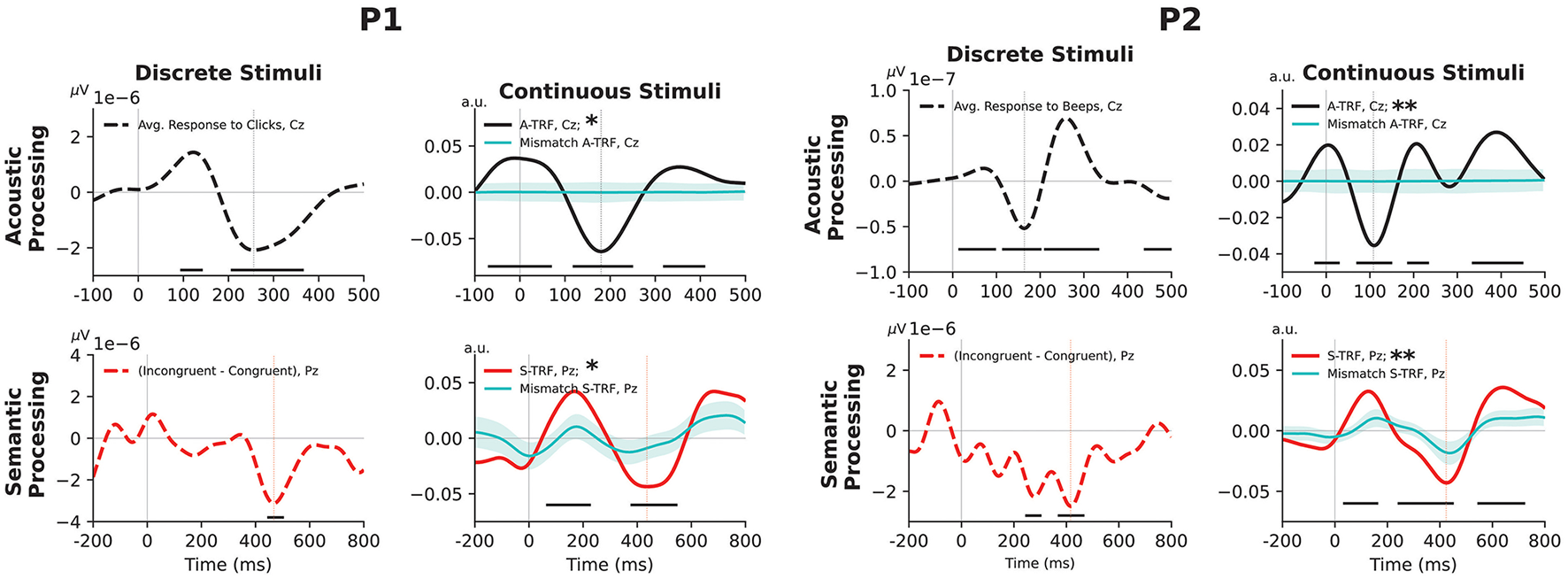
Event-related EEG markers of acoustic and semantic processing of discrete and continuous stimuli for P1 (left) and P2 (right). Top-left subplots: average auditory evoked potential recorded from channel Cz (from “click” stimuli in the case of P1, and 340 ms “beep” stimuli for P2). Top-right subplots: acoustic TRF response, estimated at channel Cz. Bottom-left subplots: average difference between responses to incongruent and congruent stimuli in the classical ‘N400' paradigm, at channel Pz. Bottom-right subplots: TRF response correlated with lexical-surprisal feature values at channel Pz. Dotted vertical lines indicate the latency of the largest negative peak (Figure 2 shows the corresponding topographic scalp maps). For discrete stimuli, time points at which event-related potentials were significantly different from zero (uncorrected p-value < 0.05) are indicated with a black horizontal line. For continuous stimuli, asterisks in legends denote the overall statistical significance level: *for p-values < 0.05 and **for p-values < 0.01. Additionally, we show the mean of the re-permuted TRFs (see Section 2.8.2) as cyan traces, and their mean ± one standard deviation as cyan-shaded regions: this is a visualization of the distribution of the TRFs under the null hypothesis that stimulus ordering does not influence the response.
2.8.2 Continuous stimuli
Adapting the approach of Chalehchaleh et al. (2024) and Synigal et al. (2023), we established significance in two ways:
-
(1) To test the presence/absence of a stimulus-specific response, we performed a permutation test on overall prediction accuracy: for both the acoustic and semantic paradigms, we generated 1000 re-permuted versions of the stimuli—either by randomly shifting the starting time (for acoustic features) or by shuffling the numerical values of the lexical surprisal features at the fixed time offsets of each word (for semantic features). For each re-permuted instance, we computed a TRF and calculated its cross-validated prediction accuracy (R)—this was a value ranging from -1 to 1 that estimated how well the computed TRF weights would predict unseen brain responses given corresponding stimulus information. We computed a p-value as the proportion of R values from the 1,000 re-permuted instances that exceeded the R of the original stimulus. We use asterisks in the legends of Figure 1 to denote this overall statistical significance level: *for p-values < 0.05 and **for p-values < 0.01.
-
(2) To visualize relevant parts of the waveform, we compared against the TRFs computed from re-permuted stimuli at each time-sample of the TRF waveform. Note that the TRF waveforms from the original stimuli, and from the 1,000 re-permuted stimuli, have arbitrary scaling when first computed. Therefore, each waveform was scaled so that the absolute extent of its largest positive or negative peak was equal to the respective overall R. The rescaled re-permuted TRFs were then compared time-point by time-point with the waveform from the rescaled original TRF. We use black horizontal lines in Figure 1 to indicate time periods where the original TRF significantly differed (p < 0.05, uncorrected) from the re-permuted TRFs.
3 Results
Figure 1 shows the acoustic and semantic responses to discrete stimuli and continuous stimuli for participant P1 and participant P2.
For P1, we observed a negative peak at 256 ms in the acoustic response to discrete stimuli, and at 180 ms for the acoustic TRF in response to continuous stimuli. Though the responses are not identical, they agree substantially as regards temporal response morphology, peak latency, and spatial localization. The acoustic response appears to be more characteristic of children under 12 (Sussman et al., 2008) than of P1's actual age at testing—it is possible that P1's brain injury before age 12 could have influenced the full maturation of the auditory cortex as previously noted (Kim et al., 2022b,a). P1's semantic response exhibits a negative peak at 472 ms for discrete stimuli (obtained using classical N400 paradigm) and 388 ms for continuous stimuli (obtained using semantic TRF).
For P2, we observe a negative peak at 164 ms for the discrete acoustic response and at 108 ms for the continuous acoustic response. We observe semantic responses with a latency of 416 ms for discrete stimuli and 440 ms for continuous stimuli.
For both participants, and both continuous and discrete stimuli, scalp maps (Figure 2) show dominant negative responses over central scalp for acoustic features, and over parietal scalp for semantic features. Permutation tests confirmed the overall significance of both participants' acoustic TRFs by rejecting the null hypothesis that goodness-of-fit is unaffected by the time ordering of the acoustic features, with p = 0.0055 and p = 0.0005 for the two participants, respectively. Additional permutation tests confirmed overall significance of the semantic TRFs by rejecting the null hypothesis that goodness-of-fit is unaffected by the ordering of the contextual-meaning-derived information values of the words, with p = 0.0175 and p = 0.0035 for the two participants, respectively. (Further details about the permutation tests are provided in Section 2.8.2, above; see also the caption of Figure 1).
Figure 2
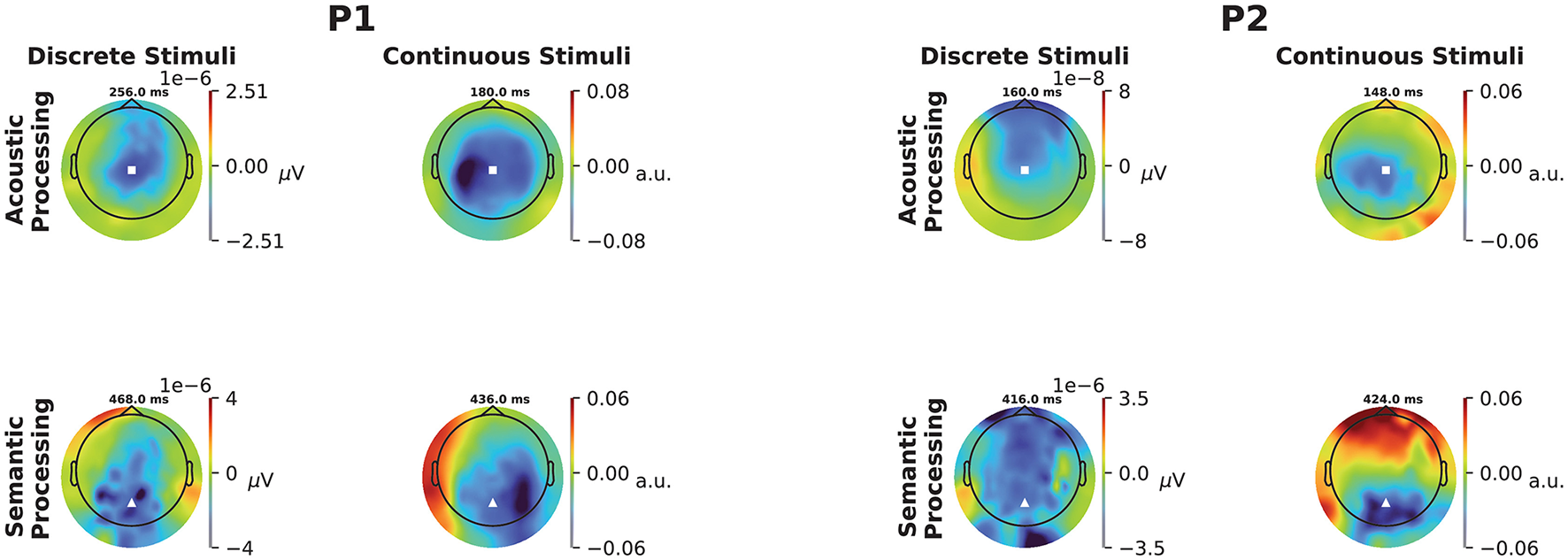
Topographic scalp maps of the ERP and TRF responses to discrete and continuous stimuli for P1 (left) and P2 (right). Each map is computed at the time lag corresponding to the largest negative peak marked by a dotted vertical line in the corresponding panel of Figure 1. The locations of Cz and Pz are marked as white squares and triangles, respectively.
4 Discussion
We have highlighted a new method for deriving correlates of acoustic processing and semantic comprehension that agree with, but go beyond, those of classical methods. In two adolescents in a disorder of consciousness following severe brain injury, we have shown evidence of preserved processing of auditory and semantic information. The acoustic and semantic results from the continuous stimuli are consistent with previous studies; displaying prominent negativity at around 100–300 ms over central scalp (Crosse et al., 2016; Sussman et al., 2008) for the former, and 400 ms over parietal scalp (Broderick et al., 2018; Kutas and Federmeier, 2000) for the latter. Consistent with what has been reported before in healthy adults (Broderick et al., 2018), there is a strong correspondence between responses from discrete and continuous stimuli.
We previously reported that the two participants in this study met diagnostic criteria for cognitive motor dissociation (CMD). This was based on their positive EEG responses to the commonly-used task of attempted motor command-following, indicating covert volitional engagement. Our current findings—evidence of covert semantic processing—enrich their CMD diagnosis by providing further supporting evidence of covert cognition from a complementary cognitive task.
The meaning-induced neural dynamics (MIND) identified in the semantic TRF (S-TRF), and our statistical tests of their overall significance, are the basis for our strongest claims of preserved cognition. Not only do these participants' N400 waveforms and analogous MIND peaks in the S-TRF qualitatively resemble those of healthy subjects (Broderick et al., 2018, 2022), they also depend specifically on the semantically-coherent ordering of the words. This is because the S-TRF is estimated by regression against surprisal, a measure of each word's informational contribution given its preceding context. Broderick et al. (2022) demonstrated experimentally that the S-TRF MIND depended on the stimulus text having coherent meaning, rather than being a non-specific word-level response: the peak deteriorated as stimulus word order was increasingly scrambled. In the current study, our permutation tests perform a similar scrambling, but on the analysis side: they simulate the null hypothesis that the same set of surprisal values, time-locked to the same set of word onset times, would produce equally well-fitting and predictive S-TRF results regardless of their ordering. We were able to reject this null hypothesis for both participants, indicating that the specific ordering of word meanings in the stimulus text was crucial for recovering the observed S-TRF from their EEG. For these participants' families, this is the strongest evidence to date that the participants' brains were capable of processing spoken word meanings in context.
Using the brain's response to continuous natural language stimuli as a proxy measurement of cognitive function offers many benefits. First, it offers a feasible method for deriving correlates of sensory and semantic processing in patients remaining in a DoC, requiring low effort from the patient—i.e., only the effort of listening to a story in contrast to the sustained attention and arousal control that is required in mental-imagery tasks, or in listening to monotonous, meaningless stimuli. This new method is no less feasible compared to traditional methods in that it does not require longer sessions. In fact, a semantic response can be estimated from a single minute of continuous data alone (O'Sullivan et al., 2015; Broderick et al., 2018), offering the potential for more-granular analyses that track variations in attention and arousal through the session. Second, because of the limitless corpus of potential stimulus material, multiple repeats of this paradigm can be employed without repeating the stimulus; in people recovering from brain injury, tracking residual and emerging functions can therefore be conducted while keeping the test engaging. Third, this method has been used in adult non-brain-injured controls to detect attention in the presence of competing stimuli (O'Sullivan et al., 2015); when employed in this manner, it has the potential to assess higher-order cognitive abilities, e.g. executive attention. Fourth, this method can be employed in very young children for whom methods requiring more cognitive resources such as motor command-following may be infeasible (Souto et al., 2020). Fifth, the complexity and genre of the material can be tailored according to the patient's age and interests; this not only allows accommodation of a wide range of situations but has the potential to allow complexity to be varied to gauge the degree of language recovery. Lastly and most importantly, assessing the brain's response while hearing a story has the potential of identifying cognitive recovery across a continuum beginning with sensory awareness of sounds through higher-order processing of semantics. This is expected to identify patients who have recovered some, but not all, cognitive abilities. This may include, but may not necessarily be limited to, patients identified as having metabolic preservation in the fronto-parietal network, who are referred to as MCS* (Thibaut et al., 2021).
In conclusion, while studies have reported on discordance between bedside function and covert-command-following, there is no knowledge of the range of cognitive function that may be hidden by motor impairment. Our new tool offers a way to assess a non-verbal person's speech processing abilities hierarchically (from lower-level sensory responses to processing of higher-level semantic features), and to grade their comprehension (by comparing responses to material of varying complexity). As such, we expect this method can be applied more generally to identify and characterize covert cognition both within and beyond the context of cognitive-motor dissociation.
5 Limitations
While some normative EEG data from adults are available and relevant to our participants, who were in late adolescence to early adulthood, the lack of age-matched healthy pediatric controls limits our ability to fully contextualize the observed neural responses. The discrete N400 does not differ drastically between adolescents and adults (Cummings et al., 2008)—though we might expect the same to be true of the analogous MIND in the semantic TRF, this remains to be confirmed. Future research should aim to develop normative TRF datasets in healthy children and adolescents to strengthen interpretability in clinical populations.
Statements
Data availability statement
Data and stimuli from this study are available from the corresponding author upon reasonable request, subject to data sharing agreements that comply with institutional requirements and the consent of participating families.
Ethics statement
The studies involving humans were approved by Weill Cornell Medicine Institutional Review Board (WCM-IRB). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
LA: Conceptualization, Methodology, Software, Validation, Data curation, Investigation, Writing – review & editing, Formal analysis, Writing – original draft, Visualization. JO'S: Methodology, Formal analysis, Validation, Writing – original draft, Data curation. GS: Writing – original draft, Investigation. JD: Investigation, Writing – original draft. JA: Writing – original draft, Investigation. LH: Investigation, Writing – original draft. AP: Investigation, Writing – original draft. AR: Writing – original draft, Investigation. WW: Writing – original draft, Investigation. EL: Funding acquisition, Supervision, Writing – original draft. NS: Writing – original draft, Investigation. NH: Software, Visualization, Conceptualization, Methodology, Validation, Formal analysis, Supervision, Writing – original draft, Writing – review & editing. SS: Visualization, Investigation, Conceptualization, Project administration, Validation, Funding acquisition, Supervision, Methodology, Writing – review & editing, Writing – original draft, Formal analysis.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. NH acknowledges funding by NIH (NIBIB) P41 EB018783 and the Stratton VA Medical Center. EL acknowledges support from an NSF CAREER award and the Del Monte Institute for Neuroscience at the University of Rochester. SS and NH acknowledge funding by the Blythedale Children's Hospital.
Acknowledgments
The authors gratefully acknowledge the research support of Nayoung Kim, Ryan J. Lowder, and Jacob Garetti. They especially thank the participants and families.
Conflict of interest
The new method is the subject of two submitted patent applications, and also intersects with one issued patent, by some of the authors: (i) SS, NH, EL, and NS: System and method for evaluating the brain's response to spoken language in ADRD (application submitted 2024: PCT/US2024/31951). (ii) SS, NH, EL, JO'S, and NS System and method for evaluating the brain's response to spoken language (application submitted 2022: PCT/US2022/81445; US from PCT 18/718,132; EP 22847505.9; World: WO/2023/114767). (iii) NS, C. Braiman, and C. Reichenbach: A sensory evoked diagnostic and brain-computer interface for the assessment of brain function in brain-injured patients (US Patent No. 11,759,146 issued 2023; EP3589188 issued 2024). Between initial submission and final revision of this article, authors SS, NH, EL, and NS co-founded Cognitive Signals, Inc., a company engaged in the development of technologies related to the subject matter of this manuscript. Potential conflicts of interest arising from this affiliation have been disclosed and are being managed in accordance with the policies of the authors' respective institutions. The authors declare no further conflict of interest. All authors have approved the manuscript and agree with its submission.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Bell A. J. Sejnowski T. J. (1995). An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 7, 1129–1159. 10.1162/neco.1995.7.6.1129
2
Bialas O. Dou J. Lalor E. C. (2023). mtrfpy: A python package for temporal response function analysis. J. Open Source Softw. 8:5657. 10.21105/joss.05657
3
Biesmans W. Das N. Francart T. Bertrand A. (2016). Auditory-inspired speech envelope extraction methods for improved EEG-based auditory attention detection in a cocktail party scenario. IEEE trans. Neural Syst. Rehabilitat. Eng. 25, 402–412. 10.1109/TNSRE.2016.2571900
4
Bloom P. A. Fischler I. (1980). Completion norms for 329 sentence contexts. Memory Cognit. 8:631–642. 10.3758/BF03213783
5
Bodien Y. G. Allanson J. Cardone P. Bonhomme A. Carmona J. Chatelle C. et al . (2024). Cognitive motor dissociation in disorders of consciousness. New Engl. J. Med. 391, 598–608. 10.1056/NEJMoa2400645
6
Braiman C. Fridman E. A. Conte M. M. Voss H. U. Reichenbach C. S. Reichenbach T. et al . (2018). Cortical response to the natural speech envelope correlates with neuroimaging evidence of cognition in severe brain injury. Curr. Biol. 28, 3833–3839. 10.1016/j.cub.2018.10.057
7
Broderick M. P. Anderson A. J. Di Liberto G. M. Crosse M. J. Lalor E. C. (2018). Electrophysiological correlates of semantic dissimilarity reflect the comprehension of natural, narrative speech. Curr. Biol. 28, 803–809. 10.1016/j.cub.2018.01.080
8
Broderick M. P. Zuk N. J. Anderson A. J. Lalor E. C. (2022). More than words: Neurophysiological correlates of semantic dissimilarity depend on comprehension of the speech narrative. Eur. J. Neurosci. 56, 5201–5214. 10.1111/ejn.15805
9
Camarrone F. Van Hulle M. M. (2019). Measuring brand association strength with eeg: a single-trial N400 ERP study. PLoS ONE14:e0217125. 10.1371/journal.pone.0217125
10
Chalehchaleh A. Winchester M. M. Di Liberto G. (2024). Robust assessment of the cortical encoding of word-level expectations using the temporal response function. bioRxiv. 10.1101/2024.04.03.587931
11
Connolly J. F. Phillips N. A. (1994). Event-related potential components reflect phonological and semantic processing of the terminal word of spoken sentences. J. Cogn. Neurosci. 6, 256–266. 10.1162/jocn.1994.6.3.256
12
Crosse M. J. Di Liberto G. M. Bednar A. Lalor E. C. (2016). The multivariate temporal response function (MTRF) toolbox: a matlab toolbox for relating neural signals to continuous stimuli. Front. Hum. Neurosci. 10:604. 10.3389/fnhum.2016.00604
13
Crosse M. J. Zuk N. J. Di Liberto G. M. Nidiffer A. R. Molholm S. Lalor E. C. (2021). Linear modeling of neurophysiological responses to speech and other continuous stimuli: methodological considerations for applied research. Front. Neurosci. 15:705621. 10.3389/fnins.2021.705621
14
Cummings A. Čeponiene R. Dick F. Saygin A. P. Townsend J. (2008). A developmental erp study of verbal and non-verbal semantic processing. Brain Res. 1208:137–149. 10.1016/j.brainres.2008.02.015
15
Duszyk A. Dovgialo M. Pietrzak M. Zieleniewska M. Durka P. (2019). Event-related potentials in the odd-ball paradigm and behavioral scales for the assessment of children and adolescents with disorders of consciousness: a proof of concept study. Clin. Neuropsychol. 33, 419–437. 10.1080/13854046.2018.1555282
16
Giacino J. T. Kalmar K. Whyte J. (2004). The jfk coma recovery scale-revised: measurement characteristics and diagnostic utility. Arch. Phys. Med. Rehabil. 85, 2020–2029. 10.1016/j.apmr.2004.02.033
17
Gonzalez-Heydrich J. Enlow M. B. D'Angelo E. Seidman L. J. Gumlak S. Kim A. et al . (2015). Early auditory processing evoked potentials (N100) show a continuum of blunting from clinical high risk to psychosis in a pediatric sample. Schizophr. Res. 169, 340–345. 10.1016/j.schres.2015.10.037
18
Gramfort A. Luessi M. Larson E. Engemann D. A. Strohmeier D. Brodbeck C. et al . (2013). MEG and EEG data analysis with mne-python. Front. Neuroinform. 7:267. 10.3389/fnins.2013.00267
19
Gui P. Jiang Y. Zang D. Qi Z. Tan J. Tanigawa H. et al . (2020). Assessing the depth of language processing in patients with disorders of consciousness. Nat. Neurosci. 23, 761–770. 10.1038/s41593-020-0639-1
20
Jas M. Engemann D. Raimondo F. Bekhti Y. Gramfort A. (2016). “Automated rejection and repair of bad trials in MEG/EEG,” in 2016 International Workshop on Pattern Recognition in Neuroimaging (PRNI) (Trento: IEEE), 1–4.
21
Jas M. Engemann D. A. Bekhti Y. Raimondo F. Gramfort A. (2017). Autoreject: automated artifact rejection for meg and eeg data. Neuroimage159, 417–429. 10.1016/j.neuroimage.2017.06.030
22
Kim N. O'Sullivan J. Olafson E. Caliendo E. Nowak S. Voss H. U. et al . (2022a). Cognitive-motor dissociation following pediatric brain injury: what about the children? Neurol.: Clini. Pract. 12, 248–257. 10.1212/CPJ.0000000000001169
23
Kim N. Watson W. Caliendo E. Nowak S. Schiff N. D. Shah S. A. et al . (2022b). Objective neurophysiologic markers of cognition after pediatric brain injury. Neurol.: Clini. Pract. 12, 352–364. 10.1212/CPJ.0000000000200066
24
Kutas M. Federmeier K. D. (2000). Electrophysiology reveals semantic memory use in language comprehension. Trends Cogn. Sci. 4, 463–470. 10.1016/S1364-6613(00)01560-6
25
Kutas M. Hillyard S. A. (1980). Reading senseless sentences: Brain potentials reflect semantic incongruity. Science207, 203–205. 10.1126/science.7350657
26
Kutas M. Hillyard S. A. (1984). Brain potentials during reading reflect word expectancy and semantic association. Nature307, 161–163. 10.1038/307161a0
27
Lightfoot G. (2016). Summary of the N1-P2 cortical auditory evoked potential to estimate the auditory threshold in adults. Seminars Hear. 37, 001–008. 10.1055/s-0035-1570334
28
Martin B. A. Tremblay K. L. Korczak P. (2008). Speech evoked potentials: from the laboratory to the clinic. Ear Hear. 29, 285–313. 10.1097/AUD.0b013e3181662c0e
29
Mesik J. Ray L. Wojtczak M. (2021). Effects of age on cortical tracking of word-level features of continuous competing speech. Front. Neurosci. 15:635126. 10.3389/fnins.2021.635126
30
Moore R. K. (2007). Spoken language processing: piecing together the puzzle. Speech Commun. 49, 418–435. 10.1016/j.specom.2007.01.011
31
O'Sullivan J. A. Power A. J. Mesgarani N. Rajaram S. Foxe J. J. Shinn-Cunningham B. G. et al . (2015). Attentional selection in a cocktail party environment can be decoded from single-trial eeg. Cereb. Cortex25, 1697–1706. 10.1093/cercor/bht355
32
Polich J. (2007). Updating p300: an integrative theory of p3a and p3b. Clini. Neurophysiol. 118, 2128–2148. 10.1016/j.clinph.2007.04.019
33
Radford A. Wu J. Child R. Luan D. Amodei D. Sutskever I. et al . (2019). Language models are unsupervised multitask learners. OpenAI Blog1:9.
34
Schalk G. McFarland D. J. Hinterberger T. Birbaumer N. Wolpaw J. R. (2004). Bci2000: a general-purpose brain-computer interface (BCI) system. IEEE Trans. Biomed. Eng. 51, 1034–1043. 10.1109/TBME.2004.827072
35
Souto D. O. Cruz T. K. F. Fontes P. L. B. Batista R. C. Haase V. G. (2020). Motor imagery development in children: changes in speed and accuracy with increasing age. Front. Pediat. 8:100. 10.3389/fped.2020.00100
36
Stapells D. R. (2002). “Cortical event-related potentials to auditory stimuli,” in Handbook of Clinical Audiology (Baltimore: Lippingcott, Williams & Wilkins), 378–406.
37
Steinkamp S. R. (2023). pymtrf: Python-Based Multivariate Temporal Response Function (MTRF) Implementation. Available online at: https://github.com/SRSteinkamp/pymtrf (accessed May 10, 2025).
38
Steppacher I. Eickhoff S. Jordanov T. Kaps M. Witzke W. Kissler J. (2013). N400 predicts recovery from disorders of consciousness. Ann. Neurol. 73, 594–602. 10.1002/ana.23835
39
Sussman E. Steinschneider M. Gumenyuk V. Grushko J. Lawson K. (2008). The maturation of human evoked brain potentials to sounds presented at different stimulus rates. Hear. Res. 236, 61–79. 10.1016/j.heares.2007.12.001
40
Synigal S. R. Anderson A. J. Lalor E. C. (2023). Electrophysiological indices of hierarchical speech processing differentially reflect the comprehension of speech in noise. bioRxiv. 10.1101/2023.03.30.534927
41
Takeshita K. Nagamine T. Thuy D. Satow T. Matsuhashi M. Yamamoto J. et al . (2002). Maturational change of parallel auditory processing in school-aged children revealed by simultaneous recording of magnetic and electric cortical responses. Clini. Neurophysiol. 113, 1470–1484. 10.1016/S1388-2457(02)00202-X
42
Talukdar U. Hazarika S. M. Gan J. Q. (2019). Motor imagery and mental fatigue: inter-relationship and eeg based estimation. J. Comput. Neurosci. 46, 55–76. 10.1007/s10827-018-0701-0
43
Thibaut A. Panda R. Annen J. Sanz L. R. Naccache L. Martial C. et al . (2021). Preservation of brain activity in unresponsive patients identifies mcs star. Ann. Neurol. 90, 89–100. 10.1002/ana.26095
44
Tucker D. M. (1993). Spatial sampling of head electrical fields: the geodesic sensor net. Electroencephalogr. Clin. Neurophysiol. 87, 154–163. 10.1016/0013-4694(93)90121-B
Summary
Keywords
natural language, temporal response function (TRF), pediatric, disorder of consciousness, cognitive function
Citation
Alkhoury L, O'Sullivan J, Scanavini G, Dou J, Arora J, Hamill L, Patchell A, Radanovic A, Watson WD, Lalor EC, Schiff ND, Hill NJ and Shah SA (2025) Leveraging meaning-induced neural dynamics to detect covert cognition via EEG during natural language listening—a case series. Front. Psychol. 16:1616963. doi: 10.3389/fpsyg.2025.1616963
Received
23 April 2025
Accepted
06 June 2025
Published
08 July 2025
Volume
16 - 2025
Edited by
Rajanikant Panda, University of California, San Francisco, United States
Reviewed by
Sibapriya Chaudhuri, University of California, San Francisco, United States
Rohini Surve, National Institute of Mental Health and Neurosciences (NIMHANS), India
Updates
Copyright
© 2025 Alkhoury, O'Sullivan, Scanavini, Dou, Arora, Hamill, Patchell, Radanovic, Watson, Lalor, Schiff, Hill and Shah.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sudhin A. Shah sut2006@med.cornell.edu
†These authors have contributed equally to this work and share senior authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.