Abstract
Introduction:
Self-efficacy is an important psychological factor influencing adherence to orthodontic treatment, and it is itself affected by pain. Although physical activity is associated with pain perception and oral health is closely related to orthodontic pain, the combined effects of these factors on self-efficacy and the mediating mechanism of pain remain unclear.
Methods:
295 orthodontic patients were surveyed. Physical activity level was assessed using the Physical Activity Rating Scale-3, oral health with the Oral Health Impact Profile-14, pain with the Numeric Rating Scale, and self-efficacy with the General Self-Efficacy Scale. Mediation analyses were performed with structural equation modeling to test whether pain mediated the relationships between physical activity, oral health, and self-efficacy.
Results:
Physical activity was negatively associated with pain (a = −0.018, p < 0.001), and pain was negatively associated with self-efficacy (b = −1.124, p < 0.001). The indirect effect of physical activity on self-efficacy through pain was significant (a × b = 0.020, 95% CI [0.009, 0.037]), while the direct effect was not significant, indicating full mediation. Oral health problems were positively associated with pain (a = 0.046, p < 0.001) and negatively associated with self-efficacy both directly (c′ = −0.124, p = 0.001) and indirectly through pain (a × b = −0.052, 95% CI [−0.092, −0.023]), indicating partial mediation.
Conclusion:
Pain serves as a mediator linking preoperative physical activity level and oral health status with self-efficacy. Encouraging patients to engage in regular physical activity and maintain good oral hygiene before orthodontic treatment may strengthen patients’ ability to cope with pain and improve long-term outcomes.
1 Introduction
Pain and discomfort are among the most commonly reported adverse effects associated with orthodontic treatment (Oliver and Knapman, 1985; Krishnan, 2007). When orthodontic forces are applied, tooth movement compresses blood vessels in the periodontal tissues and dental pulp, triggering inflammatory responses. On the compressed site inflammatory mediators such as prostaglandins are released, which activate nerve endings in the affected area. These signals are then transmitted via the trigeminal nerve to the brain, resulting in the perception of pain and discomfort (Long et al., 2016; Corrêa et al., 2017). Orthodontic pain typically manifests within 4 h following the placement of arch wires, reaches its peak intensity at approximately 24 h, and subsides almost entirely by the seventh day (Jones, 1984; Kartal, 2016). Orthodontic pain can interfere with patients’ ability to speak and impair sleep quality, and it is particularly aggravated during chewing, prompting changes in dietary habits. Such disruptions can significantly reduce patients’ quality of life (Gao et al., 2021). Moreover, orthodontic pain has the potential to negatively affect patient compliance, including reduced adherence to follow-up appointments and reluctance to use orthodontic appliances. In some cases, severe pain may prompt patients to request slower treatment progression or even to discontinue treatments, thereby jeopardizing the overall treatment outcomes (Roberts et al., 1994; Xie et al., 2023). Given its high prevalence and substantial impact on treatment adherence and patient well-being, the development of effective pain management strategies is of critical clinical importance. Such strategies are essential not only for enhancing the quality of life during orthodontic treatment but also for optimizing therapeutic outcomes.
Over the past several decades, non-steroidal anti-inflammatory drugs (NSAIDs) have remained the most commonly used and effective option for managing orthodontic pain (Teoh et al., 2022; Justi et al., 2025). However, NSAIDs are associated with notable side effects, particularly gastrointestinal damage caused by the inhibition of both cyclooxygenase (COX)-1 and COX-2 enzymes, which reduces the production of gastroprotective prostaglandins (Kotowska-Rodziewicz et al., 2023; Pimenta et al., 2024). Moreover, research has indicated that NSAIDs may slow the rate of tooth movement, potentially compromising treatment outcomes. To address these limitations, COX-2 selective inhibitors were developed as an alternative to conventional NSAIDs. In 1999, the U.S. Food and Drug Administration (FDA) approved rofecoxib, which has a more rapid onset of action and may be useful in treating selected cases of acute postsurgical pain (Moore and Hersh, 2001). However, reports have indicated that rofecoxib is associated with an increased risk of cardiovascular events (Mukherjee et al., 2001; Funk and FitzGerald, 2007; Ahsan and Sahu, 2025), leading to its withdrawal from the market and raising concerns regarding the safety of other COX-2 inhibitors (Stiller and Hjemdahl, 2022). In addition to pharmacological interventions, alternative methods have been introduced for managing orthodontic pain (Li et al., 2024). For instance, the application of the anesthetic gel “Oraqix” and low-intensity infrared laser therapy has demonstrated effectiveness in pain relief (Jamloo et al., 2023; Remi et al., 2023). Mechanical approaches and emerging techniques such as vibration device (Dutta et al., 2025) and gene therapy have also been explored as potential alternatives (Shukla et al., 2023). However, despite their promise, these methods still face significant limitations in terms of practicality, effectiveness, and cost, making them unlikely to replace NSAIDs as a widely used pain management strategy. Therefore, the exploration for safe, effective, and cost-efficient pain management approaches remains a crucial area of research.
Self-efficacy is defined as an individual’s belief in their ability to successfully complete specific tasks (Bandura, 1977). It is widely accepted that individuals with higher self-efficacy are more likely to actively manage postoperative discomfort, adhere to medical recommendations, and maintain optimal oral hygiene practices, all of which contribute to improved orthodontic treatment outcomes (Bandura, 1977; O'leary, 1985). Unsurprisingly, patients’ self-efficacy is negatively correlated with pain perception (Jackson et al., 2014). Postoperative pain not only induces physical discomfort but also exerts a detrimental psychological impact, leading to a reduction in self-efficacy. Consequently, patients with diminished self-efficacy may exhibit reluctance to wear orthodontic appliances or discontinue essential adjunctive treatments, thereby compromising adherence to orthodontic care and potentially undermining treatment success. However, the role of modifiable lifestyle factors, such as prior physical activity and oral health status, in shaping self-efficacy during orthodontic treatment remains unclear.
Pain is a subjective sensation characterized by significant individual variability and influenced by numerous factors, including age, gender, personal pain thresholds, the magnitude of orthodontic force applied, the type of orthodontic appliance, emotional and mental state, cultural background, and prior pain experiences (Apkarian et al., 2005; McGrath, 1994). Among these factors, physical activity is known to influence pain perception through endogenous opioid release, improved circulation, and stress modulation (Sluka et al., 2018). Although physical activity has been established as an effective pain management strategy, postoperative exercise prescriptions are still not routinely employed for pain management in orthodontic treatment. Notably, previous studies have demonstrated an association between prior physical activity level and general pain perception. Specifically, individuals with higher weekly activity levels exhibit lower pain sensitivity to the same stimulus (Am and Ob, 2020; Årnes et al., 2023). Similarly, better oral health reduces inflammation and discomfort, potentially mitigating post-treatment pain (Shang et al., 2023; Kane, 2017). However, whether these factors collectively contribute to enhanced self-efficacy by reducing orthodontic pain has yet to be explored. Understanding this relationship provides a foundation for developing non-pharmacological pre-operative pain management strategies to enhance patient outcomes.
Therefore, this study aims to investigate whether prior physical activity level and oral health status influence self-efficacy by mediating orthodontic pain. We thus propose the following hypotheses:
H1: Preoperative physical activity level is positively associated with postoperative self-efficacy.
H2: Preoperative oral health status is negatively associated with postoperative self-efficacy.
H3: Preoperative physical activity level is negatively associated with postoperative pain.
H4: Preoperative oral health status is negatively associated with postoperative pain.
H5: Postoperative pain is negatively associated with postoperative self-efficacy.
H6: Postoperative pain mediates the relationship between preoperative physical activity level and oral health status with postoperative self-efficacy.
2 Methods and materials
2.1 Study participants and procedure
The study survey was conducted between January 1, 2024, and April 15, 2025. Participants were interviewed upon their arrival at the Affiliated Stomatological Hospital of Chongqing Medical University for orthodontic visits. The study details were explained to them, and patients who agreed to participated provided written informed consent prior to participation. Four assessment tools were used: the Physical Activity Rating Scale-3 (PARS-3), the Oral Health Impact Profile, 14-item version (OHIP-14), the General Self-Efficacy Scale (GSES), and Numerical Rating Scale-11 (NRS-11) for pain. All questionnaires were administered in paper form, taking approximately 6 minutes to complete across two sessions. The first assessment was conducted prior to orthodontic treatment, during which participants completed the PARS-3 and OHIP-14 questionnaires, analyzed as the pre-treatment data. The second assessment was conducted approximately 24 h after orthodontic treatment—when pain is considered to reach its peak (Ingersoll et al., 1996)—during which participants completed the GSES and the NRS-11 pain assessment.
Participants were included in the study if they were undergoing orthodontic treatment, were at least 18 years of age, and had no prior history of orthodontic treatment. Individuals were excluded if they had clinically confirmed neurological, rheumatological, or psychological disorders (based on psychologist’s reports), had a preexisting chronic orofacial pain condition (either odontogenic or non-odontogenic), were on continuous analgesic, steroid, or neurological therapies, or were unable to read, understand, and/or complete the questionnaire. A total of 564 patients were initially recruited, of whom 295 (58 males and 237 females) completed all assessments. Among the participants, 95 (32.2%) used Ormco Damon™ Q2, 42 (14.2%) used Damon Clear 2, and 158 (53.6%) used Invisalign aligners. Detailed demographic information, including age, height, and weight, is presented in the Results section. The study was conducted in compliance with relevant laws and the ethical guidelines of the Ethics Committee of Chongqing Medical University, and the study was registered under registration number 202404242127000599824.
2.2 Measures
2.2.1 PARS-3
The level of physical activity was assessed using the revised version of PARS-3 (Liang, 1994a), which is widely used in the Chinese population (Zou et al., 2024; Tong and Meng, 2023; Yan et al., 2024). This scale evaluates three key components of physical activity: intensity, duration, and frequency, with each component offering five response options scored from 1 to 5. The total physical activity score ranges from 0 to 100 points and is categorized into three levels: low (≤ 19 points), moderate (20–42 points), and high (≥ 43 points). The total physical activity score is calculated using the formula: physical activity score = intensity score × (duration score − 1) × frequency score. The questionnaire has demonstrated good test–retest reliability (r = 0.82) (Liang, 1994b) and has been widely used in studies assessing physical activity levels in Chinese populations (Luo et al., 2025; Liu et al., 2025; Wang et al., 2025).
2.2.2 OHIP-14
The OHIP was originally developed by Slade and Spencer (1994) and consisted of 49 items. It was designed to evaluate the negative impacts on functional, psychological, and social well-being stemming from oral health status within a given period. Widely employed in research and clinical practice to assess oral health and hygiene issues, the OHIP was subsequently adapted into a 14-item (Slade, 1997) version and translated into multiple languages (Kenig et al., 2023; Deshpande and Nawathe, 2015; Klotz et al., 2024). Each item in the OHIP-14 questionnaire is assessed using a five-point Likert scale, ranging from 0 (never) to 4 (always). The total score is calculated as the sum of all individual item scores, with a possible range of 0 to 56. Higher scores indicate a greater negative impact on the patient’s quality of life. The Chinese version of the OHIP-14 has demonstrated strong psychometric properties, with excellent internal consistency (Cronbach’s α = 0.93) and good construct validity, as indicated by corrected item–total correlations ranging from 0.53 to 0.71, and has been widely applied in Chinese populations with favorable performance (Hongxing et al., 2014; Xin and Ling, 2006; Dongxin et al., 2025).
2.2.3 GSES
GSES was designed by Schwarzer and Jerusalem (1995) to assess individuals’ confidence in successfully accomplishing tasks in various contexts and has been shown to effectively predict motivation, academic and work-related performance, well-being and quality of life. The questionnaire contains 10 items scored on a 4-point Likert scale (1 = “not at all true,” 2 = “hardly true,” 3 = “moderately true,” 4 = “exactly true”). The total score ranges from 10 to 40, with higher scores indicating greater self-efficacy. In this study, we used the Chinese version of the GSES, which has demonstrated satisfactory reliability and validity in previous research (Zhang and Schwarzer, 1995).
2.2.4 NRS-11 for pain
NRS-11 is a unidimensional measure frequently employed to evaluate subjective pain intensity in adults, and it is widely utilized in assessing dental pain. Although multiple versions of the NRS exist, the 11-point scale is most used (Choi et al., 2024; Hartrick et al., 2003). Respondents select an integer from 0 to 10 that best represents their perceived pain intensity at the time of assessment, where “0” denotes “no pain” and “10” denotes “the most severe pain imaginable.” Following the placement of orthodontic appliances, pain typically peaks at approximately 24 h, and therefore, the respondents were surveyed around 24 h post-procedure.
2.3 Statistical analysis
Data were analyzed using IBM SPSS Statistics 28.0 for preliminary analyses and AMOS 28.0 for structural equation modeling. First, descriptive statistics were calculated, and independent-samples t-tests were used to examine sex differences in demographic variables, physical activity (PARS-3), oral health (OHIP-14), perceived pain (NRS-11), and self-efficacy (GSES). Pearson’s correlation analysis was then conducted to assess the direction and strength of associations among the main study variables.
To test the hypothesized mediation model, structural equation modeling with maximum likelihood estimation was performed in AMOS. Specifically, the indirect effect of perceived pain on the relationships between oral health, physical activity, and self-efficacy was evaluated. Indirect effects were tested using a non-parametric bootstrap method (5,000 resamples) with bias-corrected 95% confidence intervals (CI). A mediation effect was considered significant when the confidence interval did not include zero. The reliability of the study measures was examined using Cronbach’s α coefficients, all of which indicated good internal consistency. Statistical significance was set at p < 0.05 (two-tailed), and results are presented as mean ± standard deviation (SD).
3 Results
Table 1 summarizes the demographic and descriptive characteristics of the study sample. A total of 295 participants (58 males and 237 females) were included, with a mean age of 26.39 years (SD = 6.54). The average height, weight, and body mass index (BMI) were 164.44 cm (SD = 7.47), 62.42 kg (SD = 21.90), and 22.98 kg/m2 (SD = 7.40), respectively. For the main study variables, the mean scores were 3.55 (SD = 1.51) for pain intensity (NRS-11), 24.87 (SD = 7.14) for general self-efficacy (GSES), 26.40 (SD = 24.16) for physical activity (PARS-3), and 17.81 (SD = 10.46) for oral health-related quality of life (OHIP-14). Independent-samples t-tests indicated significant sex differences in anthropometric measures: males were taller, heavier, and had higher BMI than females (all p < 0.05). In contrast, no significant sex differences were observed for age, NRS-11, GSES, PARS-3, or OHIP-14 (all p > 0.05). Supplementary Figure 1 presents boxplots of the four primary study variables: pain, GSES, PARS-3, and OHIP-14. The distributions show clear differences in variability across measures. PAIN scores are clustered, indicating relatively low variability in postoperative discomfort. In contrast, PARS-3 displays the widest spread, reflecting substantial individual differences in preoperative physical activity levels. GSES and OHIP-14 demonstrate moderate dispersion, with both variables showing well-defined interquartile ranges and centrally positioned medians.
Table 1
| Variables | Total (n = 295) | Male (n = 58) | Female (n = 237) | t | p |
|---|---|---|---|---|---|
| Age (year) | 26.39 ± 6.54 | 26.57 ± 6.74 | 26.34 ± 6.5 | 0.237 | 0.813 |
| Height (cm) | 164.44 ± 7.47 | 175.93 ± 5.74 | 161.63 ± 4.61 | 20.149 | 0.000 |
| Weight (kg) | 62.42 ± 21.90 | 77.59 ± 30.47 | 58.71 ± 17.4 | 4.541 | 0.000 |
| BMI | 22.98 ± 7.40 | 24.91 ± 9.18 | 22.51 ± 6.83 | 2.227 | 0.027 |
| NRS-11 | 3.55 ± 1.51 | 3.72 ± 1.69 | 3.5 ± 1.47 | 1.002 | 0.317 |
| GSES | 24.87 ± 7.14 | 24.53 ± 7.65 | 24.95 ± 7.03 | −0.400 | 0.690 |
| PARS-3 | 26.4 ± 24.16 | 27.84 ± 26.08 | 26.05 ± 23.72 | 0.507 | 0.612 |
| OHIP-14 | 17.81 ± 10.46 | 18.05 ± 11.31 | 17.75 ± 10.27 | 0.199 | 0.843 |
Demographic and descriptive characteristics by sex.
BMI, body mass index; NRS-11, numerical rating scale-11 for subjective pain level. GSES, general self-efficacy scale; PARS-3, physical activity scale, 3 items; OHIP-14, oral health impact profile, 14 items. N = 295.
Pearson’s correlation analysis revealed significant associations among the study variables (Table 2). Pain intensity (NRS-11) was negatively correlated with self-efficacy (GSES; r = −0.326, p < 0.01) and physical activity level (PARS-3; r = −0.314, p < 0.01), and positively correlated with oral health problems (OHIP-14; r = 0.343, p < 0.01). Self-efficacy was positively correlated with physical activity (r = 0.171, p < 0.01) and negatively correlated with oral health problems (r = − 0.269, p < 0.01). Physical activity was not significantly correlated with oral health problems (r = −0.074, ns).
Table 2
| Variables | NRS-11 | GSES | PARS-3 | OHIP-14 |
|---|---|---|---|---|
| NRS-11 | 1.0 | |||
| GSES | −0.326** | 1.0 | ||
| PARS-3 | −0.314** | 0.171** | 1.0 | |
| OHIP-14 | 0.343** | −0.269** | −0.074 | 1.0 |
Pearson’s correlation analysis of each variable.
NRS-11, numerical rating scale-11 for subjective pain level. GSES, general self-efficacy scale; PARS-3, physical activity scale, 3 items; OHIP-14, oral health impact profile, 14 items. ** indicates p < 0.01. N = 295.
The hypothesized mediation model demonstrated good fit to the data, χ2(1) = 1.63, p = 0.202, CFI = 0.994, TLI = 0.964, GFI = 0.997, RMSEA = 0.046 (90% CI [0.000, 0.170], PCLOSE = 0.354), SRMR = 0.006. The predictors explained 19% of the variance in pain (R2 = 0.19) and 14% of the variance in self-efficacy (R2 = 0.14), suggesting that prior physical activity and oral health status accounted for a meaningful portion of variability in pain and, through pain, contributed modestly to explaining self-efficacy.
As shown in Table 3, physical activity (PARS3) significantly predicted lower pain (a = −0.018, SE = 0.003, p < 0.001), and pain in turn significantly predicted lower general self-efficacy (GSES; b = −1.124, SE = 0.286, p < 0.001). The direct effect of physical activity on self-efficacy was not significant (c′ = 0.025, SE = 0.017, p = 0.143), while the indirect effect through pain was significant (a × b = 0.020, 95% CI [0.009, 0.037], p < 0.01). The total effect was also significant (c = 0.045, SE = 0.017, p < 0.01), indicating that the relationship between physical activity and self-efficacy was fully mediated by pain. Similarly, oral health problems (OHIP-14) were positively associated with pain (a = 0.046, SE = 0.008, p < 0.001), and pain was negatively associated with self-efficacy (b = −1.124, SE = 0.286, p < 0.001). The direct effect of oral health on self-efficacy remained significant (c′ = −0.124, SE = 0.039, p = 0.001), and the indirect effect via pain was also significant (a × b = −0.052, 95% CI [−0.092, −0.023], p < 0.01). The total effect of oral health on self-efficacy was significant (c = −0.176, SE = 0.036, p < 0.001), consistent with partial mediation by pain. The standardized path coefficients for the mediation model are presented in Figure 1. Higher level of physical activity was indirectly associated with higher self-efficacy through reduced pain, while oral health problems exerted both direct and indirect negative effects on self-efficacy.
Table 3
| Model | Variable | Effect of X on M | Effect of M on Y | Direct effect | Indirect effect | Total effect | ||
|---|---|---|---|---|---|---|---|---|
| X (Predictor) | M (Mediator) | Y (Outcome) | a | b | c′ | (a*b) [95% CI] | c = c′ + a*b | |
| 1 | PARS | Pain | GSES | −0.018 (SE = 0.003)*** | −1.124 (SE = 0.286)*** | 0.025 (SE = 0.017), ns | 0.020 [0.009, 0.037]** | 0.045 (SE = 0.017)** |
| 2 | OHIP14 | 0.046 (SE = 0.008)*** | −0.124 (SE = 0.039)** | −0.052 [−0.092, −0.023]** | −0.176 (SE = 0.036)*** | |||
Mediation analysis of physical activity (PARS-3) and oral health (OHIP-14) on self-efficacy (GSES) through pain (NRS-11).
SE, standard error; PARS-3, physical activity rating scale, 3 items for physical activity level; OHIP-14, oral health impact profile, 14 items, for oral health-related quality of life. Indirect effect is significant if 0 value falls outside the lower bound and upper bounds of bootstrap CI. **p < 0.01, ***p < 0.001, ns, non-significant. N = 295.
Figure 1
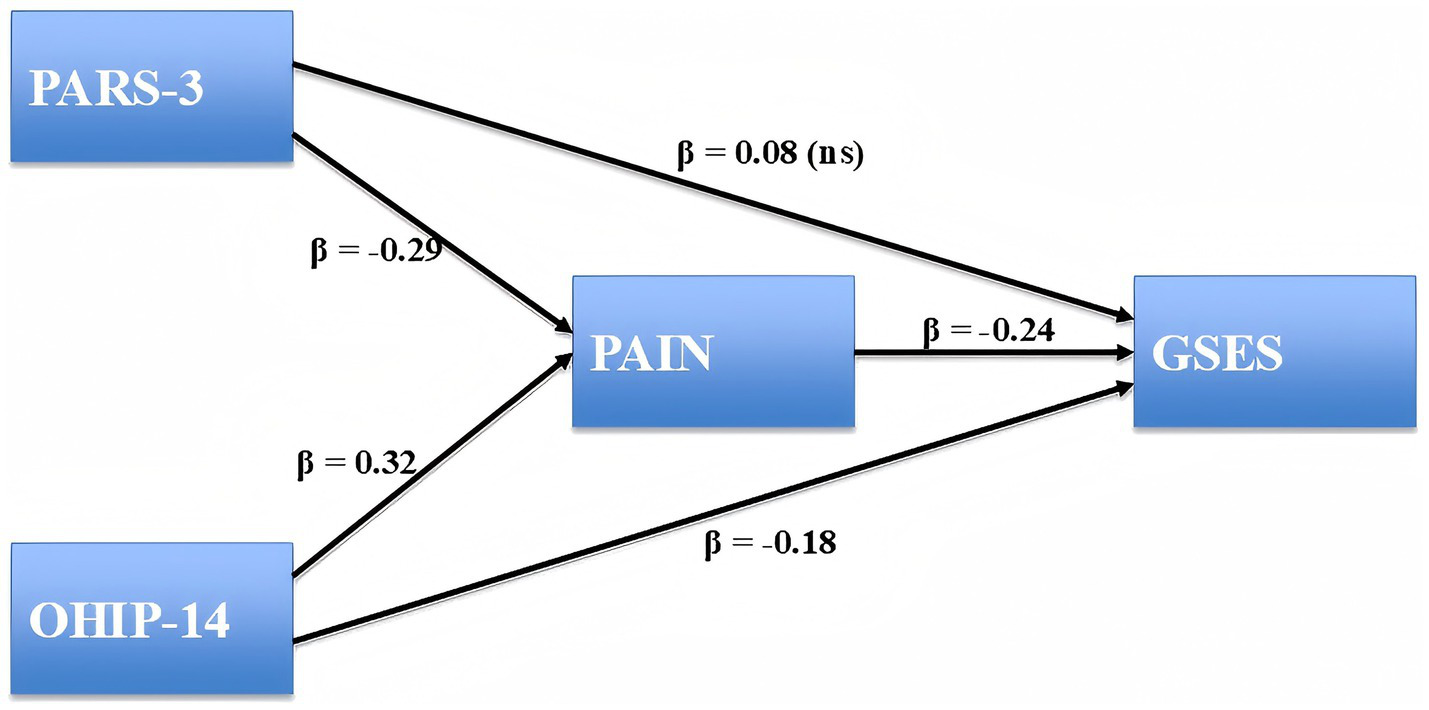
Structural equation model testing the mediating role of pain. Standardized path coefficients (β) are displayed. Nonsignificant paths are marked as “ns.” Physical Activity Rating Scale-3 (PARS-3), Oral Health Impact Profile-14 (OHIP-14), and General Self-Efficacy Scale (GSES). N = 295.
4 Discussion
The current study underscores the mediating role of subjective pain in linking preoperative physical activity level and oral health with postoperative self-efficacy among orthodontic patients. The results indicate that physical activity was indirectly associated with greater self-efficacy through its effect on pain, with no significant direct effect. By contrast, oral health influenced self-efficacy both directly and indirectly through pain. Specifically, better oral health status before orthodontic treatment was associated with lower perceived pain, which in turn contributed to greater self-efficacy. These findings align with prior research suggesting that individuals with higher levels of physical activity have lower pain sensitivity to the same stimulus (Årnes et al., 2023), reinforcing the role of exercise in pain management (Am and Ob, 2020; Årnes et al., 2023). Similarly, our results support the findings of Hadler-Olsen et al. (2024) who reported that poor periodontal health exacerbates pain perception through inflammation, whereas good oral hygiene mitigates discomfort. Additionally, the observed negative correlation between pain and self-efficacy supports psychological models suggesting that self-efficacy is influenced by negative subjective sensation (Cheng et al., 2018; Kardash et al., 2024). While previous studies have independently examined the effects of physical activity and oral health on pain perception, the present study makes a novel contribution by identifying pain as a mediating factor between these variables and self-efficacy. Specifically, the results showed that the effect of physical activity on self-efficacy was fully mediated by pain, whereas the effect of oral health was partially mediated by pain, with both direct and indirect pathways. These findings offer a more comprehensive understanding of the interrelationships among physical, behavioral, and psychological factors in orthodontic treatment outcomes.
From a clinical perspective, these findings support the incorporation of recommendations for regular physical activity and improved oral hygiene into pre-treatment orthodontic care as non-pharmacological strategies to enhance patient outcomes. Given that orthodontic treatment typically lasts 18–24 months, maintaining an active lifestyle and good oral hygiene throughout the treatment process may further strengthen patients’ self-efficacy in coping with discomfort, thereby contributing to better long-term results (Naseri et al., 2020; Szczepańska-Gieracha and Mazurek, 2020). Regular physical activity is known to increase pain tolerance through multiple physiological mechanisms, including the release of endogenous opioids, improved circulation, faster resolution of inflammation, and regulation of stress-related hormones such as cortisol (Am and Ob, 2020). Similarly, maintaining good oral hygiene reduces the accumulation of dental plaque and gingival inflammation, decreases periodontal perception, and lowers the risk of secondary infections, all of which can alleviate treatment-related discomfort (Luchian et al., 2024). Collectively, these findings highlight the importance of integrating lifestyle-based interventions into orthodontic pain management to optimize patient well-being.
This study has several limitations that should be acknowledged when interpreting the findings. First, its cross-sectional design prevents the establishment of causal relationships; future research should adopt longitudinal or interventional approaches to further verify the mediating role of pain between physical activity, oral health, and self-efficacy. Second, the levels of physical activity, oral health status, and pain were assessed using self-reported questionnaires, which may be subject to recall and social desirability biases. Objective measures such as accelerometer-based activity monitoring, clinical periodontal examinations, and biomarkers of pain should be incorporated in future studies to improve measurement accuracy. Third, psychosocial factors such as anxiety, stress, and sleep quality, which may also influence pain perception and self-efficacy, were not included in the current analysis (Sobol-Kwapinska et al., 2016). Future research should integrate these variables to provide a more comprehensive understanding of the underlying mechanisms.
5 Conclusion
These findings highlight the critical role of pre-treatment physical activity and oral health in enhancing self-efficacy among orthodontic patients through the reduction of pain. The results suggest that incorporating non-pharmacological strategies—such as promoting regular physical activity and maintaining optimal oral hygiene—into orthodontic care before treatment may strengthen patients’ ability to cope with discomfort, thereby improving treatment adherence and long-term outcomes.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Chongqing Medical University. The study was registered under registration number 202404242127000599824. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
CZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. ZS: Formal analysis, Writing – review & editing. YG: Conceptualization, Formal analysis, Writing – original draft. TC: Investigation, Validation, Writing – review & editing. YZ: Data curation, Investigation, Writing – review & editing. YW: Data curation, Writing – review & editing. ZL: Conceptualization, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This study was supported by the Fundamental Research Funds for the Central Universities of Chongqing University, China (2023CDSKXYTY003 to ZL), the China Postdoctoral Science Foundation (2023M236864 to ZL), and the Jiangxi Provincial Higher Education Humanities and Social Sciences Research Project (TY22117 to YW). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that Generative AI was used in the creation of this manuscript. During the preparation of this work the author(s) used ChatGPT 5o in order to improve readability. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyg.2025.1745635/full#supplementary-material
SUPPLEMENTARY FIGURE 1Boxplots of the primary study variables. This figure displays the distribution of postoperative pain (PAIN), self-efficacy (GSES), physical activity level (PARS-3), and oral health impact (OHIP-14). The boxplots present medians, interquartile ranges, and overall score dispersion, offering a visual summary of the variability across the key study measures.
References
1
Ahsan A. Sahu M. A. (2025). A literature review assessing whether the use of non-steroidal anti-inflammatory drugs (NSAIDs) increases the risk of cardiovascular events. Cureus17:e92361. doi: 10.7759/cureus.92361
2
Am Z.-O. Ob P.-N. (2020). Influence of physical exercise in pain threshold in human: a systematic review. Integr. Clin. Med.4, 1–6. doi: 10.15761/ICM.1000184
3
Apkarian A. V. Bushnell M. C. Treede R.-D. Zubieta J.-K. (2005). Human brain mechanisms of pain perception and regulation in health and disease. Eur. J. Pain9, 463–484. doi: 10.1016/j.ejpain.2004.11.001,
4
Årnes A. P. Nielsen C. S. Stubhaug A. Fjeld M. K. Johansen A. Morseth B. et al . (2023). Longitudinal relationships between habitual physical activity and pain tolerance in the general population. PLoS One18:e0285041. doi: 10.1371/journal.pone.0285041,
5
Bandura A. (1977). Self-efficacy: toward a unifying theory of behavioral change. Psychol. Rev.84, 191–215. doi: 10.1037/0033-295X.84.2.191,
6
Cheng S.-T. Leung C. M. C. Chan K. L. Chen P. P. Chow Y. F. Chung J. W. Y. et al . (2018). The relationship of self-efficacy to catastrophizing and depressive symptoms in community-dwelling older adults with chronic pain: A moderated mediation model. PLoS One13:e0203964. doi: 10.1371/journal.pone.0203964,
7
Choi S. Yoon S. H. Lee H. J. (2024). Beyond measurement: a deep dive into the commonly used pain scales for postoperative pain assessment. Korean J. Pain37, 188–200. doi: 10.3344/kjp.24069,
8
Corrêa A. S. Almeida V. L. Lopes B. M. V. Franco A. Matos F. R. Quintans-Júnior L. J. et al . (2017). The influence of non-steroidal anti-inflammatory drugs and paracetamol used for pain control of orthodontic tooth movement: a systematic review. An. Acad. Bras. Cienc.89, 2851–2863. doi: 10.1590/0001-3765201720160865,
9
Deshpande N. C. Nawathe A. A. (2015). Translation and validation of Hindi version of Oral health impact Profile-14. J. Indian Soc. Periodontol.19, 208–210. doi: 10.4103/0972-124X.145806,
10
Dongxin D. Jiang Y. Jin Y. Huning W. Ying Z. Suyu G. et al . (2025). Frailty and oral-health related quality of life. Int. Dent. J.75:104497. doi: 10.1016/j.identj.2025.104497
11
Dutta S. Patil A. S. Sharma S. Deshmukh S. (2025). Utilizing vibration as a method for accelerating tooth movement during orthodontic treatment: a systematic review and meta-analysis. Clin. Invest. Orthod.84, 77–92. doi: 10.1080/27705781.2025.2487295
12
Funk C. D. Fitzgerald G. A. (2007). COX-2 inhibitors and cardiovascular risk. J. Cardiovasc. Pharmacol.50, 470–479. doi: 10.1097/FJC.0b013e318157f72d,
13
Gao M. Yan X. Zhao R. Shan Y. Chen Y. Jian F. et al . (2021). Comparison of pain perception, anxiety, and impacts on oral health-related quality of life between patients receiving clear aligners and fixed appliances during the initial stage of orthodontic treatment. Eur. J. Orthod.43, 353–359. doi: 10.1093/ejo/cjaa037,
14
Hadler-Olsen E. Petrenya N. Jönsson B. Steingrímsdóttir Ó. A. Nielsen C. S. (2024). Periodontitis is associated with decreased experimental pressure pain tolerance: the Tromsø study 2015–2016. J. Clin. Periodontol.51, 874–883. doi: 10.1111/jcpe.13968
15
Hartrick C. T. Kovan J. P. Shapiro S. (2003). The numeric rating scale for clinical pain measurement: a ratio measure?Pain Pract.3, 310–316. doi: 10.1111/j.1530-7085.2003.03034.x,
16
Hongxing L. List T. Nilsson I. M. Johansson A. Astrøm A. N. (2014). Validity and reliability of OIDP and OHIP-14: a survey of Chinese high school students. BMC Oral Health14:158. doi: 10.1186/1472-6831-14-158,
17
Ingersoll G. L. Schultz A. W. Hoffart N. Ryan S. A. (1996). The effect of a professional practice model on staff nurse perception of work groups and nurse leaders. J. Nurs. Adm.26, 52–60. doi: 10.1097/00005110-199605000-00011,
18
Jackson T. Wang Y. Wang Y. Fan H. (2014). Self-efficacy and chronic pain outcomes: A Meta-analytic review. J. Pain15, 800–814. doi: 10.1016/j.jpain.2014.05.002,
19
Jamloo H. Farjaminejad R. Afrasiabi M. Zarezadeh M. Mafi R. Jamilian A. (2023). The effectiveness of low level laser therapy and ibuprofen in the management of pain during orthodontics treatments: a meta-analysis of meta-analyses. Lasers Dent. Sci.8:2. doi: 10.1007/s41547-023-00207-z
20
Jones M. L. (1984). An investigation into the initial discomfort caused by placement of an archwire. Eur. J. Orthod.6, 48–54. doi: 10.1093/ejo/6.1.48,
21
Justi L. H. Z. Silva J. F. Santana M. S. Laureano H. A. Pereira M. E. Oliveira C. S. et al . (2025). Non-steroidal anti-inflammatory drugs and oxidative stress biomarkers in fish: a meta-analytic review. Toxicol. Rep.14:101910. doi: 10.1016/j.toxrep.2025.101910,
22
Kane S. F. (2017). The effects of oral health on systemic health. Gen. Dent.65, 30–34.
23
Kardash L. Wall C. L. Flack M. Searle A. (2024). The role of pain self-efficacy and pain catastrophising in the relationship between chronic pain and depression: A moderated mediation model. PLoS One19:e0303775. doi: 10.1371/journal.pone.0303775,
24
Kartal Y. P. O. O. (2016). Insight into orthodontic appliance induced pain: mechanism, duration and management. World J. Anesthesiol.5, 28–35. doi: 10.5313/wja.v5.i1.28
25
Kenig N. Sotiroska Ivanoska K. Nikolovska J. (2023). Psychometric properties of the 14 items Oral health impact profile questionnaire translated into the Macedonian language. Acta Stomatol. Croat.57, 145–154. doi: 10.15644/asc57/2/5,
26
Klotz A. L. Prager D. Rammelsberg P. Hassel A. J. Zenthöfer A. (2024). A German version of the Oral impacts of daily performances-reliability and validity. Clin. Oral Investig.28:73. doi: 10.1007/s00784-023-05437-w,
27
Kotowska-Rodziewicz A. Zalewska A. Maciejczyk M. (2023). A review of preclinical and clinical studies in support of the role of non-steroidal anti-inflammatory drugs in dentistry. Med. Sci. Monit.29:e940635. doi: 10.12659/MSM.940635,
28
Krishnan V. (2007). Orthodontic pain: from causes to management—a review. Eur. J. Orthod.29, 170–179. doi: 10.1093/ejo/cjl081,
29
Li J. Li S. Chen H. Feng J. Qiu Y. Li L. (2024). The effect of physical interventions on pain control after orthodontic treatment: a systematic review and network meta-analysis. PLoS One19:e0297783. doi: 10.1371/journal.pone.0297783,
30
Liang D. (1994a). Stress level and its relation with physical activity in higher education. Chin. Ment. Health J.8, 5–6.
31
Liang D. (1994b). Stress level of college students and its relationship with physical exercise. Chin. Ment. Health J.8:2.
32
Liu Q. Zhu W.-D. Lou H. Zhang D.-Y. Mu F.-Z. Zhang X.-Y. et al . (2025). The influence of physical activity on emotional management ability in college students: a chain mediating role of psychological resilience and health literacy. BMC Public Health25:2878. doi: 10.1186/s12889-025-24252-4,
33
Long H. Wang Y. Jian F. Liao L.-N. Yang X. Lai W.-L. (2016). Current advances in orthodontic pain. Int. J. Oral Sci.8, 67–75. doi: 10.1038/ijos.2016.24,
34
Luchian I. Surlari Z. Goriuc A. Ioanid N. Zetu I. Butnaru O. et al . (2024). The influence of orthodontic treatment on periodontal health between challenge and synergy: a narrative review. Dent. J.12:112. doi: 10.3390/dj12040112,
35
Luo X. Liu H. Sun Z. Wei Q. Zhang J. Zhang T. et al . (2025). Gender moderates the mediating effect of psychological capital between physical activity and depressive symptoms among adolescents. Sci. Rep.15:10868. doi: 10.1038/s41598-025-95186-5,
36
McGrath P. A. (1994). Psychological aspects of pain perception. Arch. Oral Biol.39, S55–S62. doi: 10.1016/0003-9969(94)90189-9,
37
Moore P. A. Hersh E. V. (2001). Celecoxib and rofecoxib: the role of COX-2 inhibitors in dental practice. J. Am. Dent. Assoc.132, 451–456. doi: 10.14219/jada.archive.2001.0207,
38
Mukherjee D. Nissen S. E. Topol E. J. (2001). Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA286, 954–959. doi: 10.1001/jama.286.8.954,
39
Naseri N. Baherimoghadam T. Bassagh N. Hamedani S. Bassagh E. Hashemi Z. (2020). The impact of general self-efficacy and the severity of malocclusion on acceptance of removable orthodontic appliances in 10- to 12-year-old patients. BMC Oral Health20:344. doi: 10.1186/s12903-020-01293-2,
40
O'leary A. (1985). Self-efficacy and health. Behav. Res. Ther.23, 437–451. doi: 10.1016/0005-7967(85)90172-X,
41
Oliver R. G. Knapman Y. M. (1985). Attitudes to orthodontic treatment. Br. J. Orthod.12, 179–188. doi: 10.1179/bjo.12.4.179,
42
Pimenta R. P. Takahashi C. M. Barberato-Filho S. Mcclung D. C. F. Moraes F. D. S. De Souza I. M. et al . (2024). Preemptive use of anti-inflammatories and analgesics in oral surgery: a review of systematic reviews. Front. Pharmacol.14:382. doi: 10.3389/fphar.2023.1303382,
43
Remi R. V. Anantharaj A. Praveen P. Prathibha R. S. Sudhir R. (2023). Advances in pediatric dentistry: new approaches to pain control and anxiety reduction in children - a narrative review. J. Dent. Anesth. Pain Med.23, 303–315. doi: 10.17245/jdapm.2023.23.6.303,
44
Roberts E. E. Kassab J. Y. Sandham J. S. Willmot D. R. (1994). Non-completion of active orthodontic treatment. Br. J. Orthod.21, 275–278. doi: 10.1179/bjo.21.3.275,
45
Schwarzer R. Jerusalem M. (1995). General self-efficacy scale. Windsor: NFER-NELSON.
46
Shang J. Liu H. Zheng Y. Zhang Z. (2023). Role of oxidative stress in the relationship between periodontitis and systemic diseases. Front. Physiol.14:1210449. doi: 10.3389/fphys.2023.1210449,
47
Shukla M. Jain S. Reddy C. M. Raghav P. Amit K. Yangzom R. et al . (2023). Gene therapy: a step toward advanced orthodontics – a narrative review. SRM Journal of Research in Dental Sciences14, 144–149. doi: 10.4103/srmjrds.srmjrds_86_23
48
Slade G. D. (1997). Derivation and validation of a short-form oral health impact profile. Community Dent. Oral Epidemiol.25, 284–290. doi: 10.1111/j.1600-0528.1997.tb00941.x,
49
Slade G. D. Spencer A. J. (1994). Development and evaluation of the oral health impact profile. Community Dent. Health11, 3–11.
50
Sluka K. A. Frey-Law L. Hoeger Bement M. (2018). Exercise-induced pain and analgesia? Underlying mechanisms and clinical translation. Pain159, S91–S97. doi: 10.1097/j.pain.0000000000001235,
51
Sobol-Kwapinska M. Bąbel P. Plotek W. Stelcer B. (2016). Psychological correlates of acute postsurgical pain: A systematic review and meta-analysis. Eur. J. Pain20, 1573–1586. doi: 10.1002/ejp.886,
52
Stiller C. O. Hjemdahl P. (2022). Lessons from 20 years with COX-2 inhibitors: importance of dose–response considerations and fair play in comparative trials. J. Intern. Med.292, 557–574. doi: 10.1111/joim.13505,
53
Szczepańska-Gieracha J. Mazurek J. (2020). The role of self-efficacy in the recovery process of stroke survivors. Psychol. Res. Behav. Manag.13, 897–906. doi: 10.2147/PRBM.S273009
54
Teoh L. Mccullough M. Taing M.-W. (2022). Efficacy of oxycodone for postoperative dental pain: A systematic review and meta-analysis. J. Dent.125:104254. doi: 10.1016/j.jdent.2022.104254,
55
Tong W. Meng S. (2023). Effects of physical activity on Mobile phone addiction among college students: the chain-based mediating role of negative emotion and E-health literacy. Psychol. Res. Behav. Manag.16, 3647–3657. doi: 10.2147/PRBM.S419799,
56
Wang T. Nie Y. Yao X. Zhang J. Li Y. Sun H. et al . (2025). The chain mediating role of emotion regulation and stress perception in physical activity alleviating college students’ health anxiety. Sci. Rep.15:29189. doi: 10.1038/s41598-025-14481-3,
57
Xie L. Ma Y. Sun X. Yu Z. (2023). The effect of orthodontic pain on dental anxiety: a review. J. Clin. Pediatr. Dent.47, 32–36. doi: 10.22514/jocpd.2023.051,
58
Xin W. N. Ling J. Q. (2006). Validation of a Chinese version of the oral health impact profile-14. Zhonghua Kou Qiang Yi Xue Za Zhi41, 242–245. doi: 10.3760/j.issn:1002-0098.2006.04.016,
59
Yan H.-M. Huang P. Chen R. Wang Y.-C. (2024). The relationship between physical activity and mental health of middle school students: the chain mediating role of negative emotions and self-efficacy. Front. Psychol.15:448. doi: 10.3389/fpsyg.2024.1415448,
60
Zhang J. X. Schwarzer R. (1995). Measuring optimistic self-beliefs: a Chinese adaptation of the general self-efficacy scale. Psychologia38, 174–181.
61
Zou R. Wang K. Li D. Liu Y. Zhang T. Wei X. (2024). Study on the relationship and related factors between physical fitness and health behavior of preschool children in Southwest China. BMC Public Health24:1759. doi: 10.1186/s12889-024-19269-0,
Summary
Keywords
oral health, orthodontic pain, pain management, physical activity, self-efficacy
Citation
Zhao C, Sun Z, Gong Y, Cao T, Zhao Y, Wen Y and Li Z (2026) Exercise before braces: the mediating effect of pain on the association between physical activity and self-efficacy. Front. Psychol. 16:1745635. doi: 10.3389/fpsyg.2025.1745635
Received
13 November 2025
Revised
12 December 2025
Accepted
16 December 2025
Published
12 January 2026
Volume
16 - 2025
Edited by
Lingxiao He, Xiamen University, China
Reviewed by
Xiyao Shan, Shanghai Jiao Tong University, China
Pengwei Song, Guangxi Science and Technology Normal University, China
Updates
Copyright
© 2026 Zhao, Sun, Gong, Cao, Zhao, Wen and Li.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhencheng Li, zcli@cqu.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.