- 1Department of Neurology, Intermountain Medical Center Neurosciences Institute, Intermountain Health, Salt Lake City, UT, United States
- 2Shirley Ryan AbilityLab, Chicago, IL, United States
- 3Department Physical Medicine & Rehabilitation, Northwestern University, Chicago, IL, United States
- 4Department of Family Medicine, Intermountain Health, Salt Lake City, UT, United States
- 5Healthcare Delivery Institute, Intermountain Health, Salt Lake City, UT, United States
- 6Department of Analytics, Select Health, Salt Lake City, UT, United States
- 7Department of Physical Therapy and Human Movement Sciences, Northwestern University, Chicago, IL, United States
- 8Neurologic Physical Therapy, Intermountain Park City Hospital, Park City, UT, United States
- 9Lifestyle Medicine and Wellness Center, Intermountain Park City Hospital, Park City, UT, United States
As Parkinson's disease (PD) progresses, relatively mild symptoms advance to a major disorder that affects every organ system in the body. Current care for people with PD (PwP) reacts to rising disability. There is a missed opportunity to keep PwP as healthy as possible. In this perspective, we spell out our vision for a proactive, value-based health care model built around a patient-centered integrated practice unit (IPU) for PD. The IPU will provide integrated interdisciplinary care overseen by a specialized Parkinson's primary care physician working closely with a movement disorders neurologist. The IPU will implement an evidence-based exercise program for people early in the disease. The focus of this intervention is a heart rate driven high-intensity aerobic exercise program, which is the only treatment with evidence that it can slow disease progression. It will also include resistance exercises, flexibility exercise and balance exercise. For people whose disease is moderate or severe, the IPU will provide care curated through a network of rehabilitation providers with expertise in PD all of whom understand the exercise prescription. By integrating care, slowing disease progression, and incorporating specialized rehabilitation we anticipate improving healthspan. In creating the IPU as a fully capitated (shared-risk) model in which the IPU and the insurance company assume joint accountability for quality and cost of care we anticipate demonstrating financial sustainability of implementing the exercise prescription and providing integrated care.
1 Introduction
The dominant narrative of Parkinson's disease (PD) in the United States is one of rising disability managed reactively through a fragmented healthcare system (1). The first goal of this perspective is to propose a new narrative: one that keeps people with PD (PwP) healthy. This is accomplished through an integrated proactive model that extends healthspan. Healthspan is defined as the period of life spent with relatively good physical and mental function (2). The second goal of this perspective is to make clear that financial incentives must be realigned to support what is best for the patient, not what is best for the health system, physician, or insurance company. Without such realignment the new model of care we propose will never be financially viable.
We suggest an Integrated Practice Unit (IPU) (3, 4) as a vehicle through which financial incentives can be harmoniously aligned with best clinical practice. In an IPU the health providers and the insurance company share joint accountability for quality and cost of care. Integrated care is already a suggested best practice in PD (1, 5, 6) and there are centers across the United States providing at least partially integrated services. These include The Struthers Parkinson's Center in Minnesota and the Neuromedicine Service and Science Hub Model at the University of Florida (7). Parkinson's Foundation Centers of Excellence also provide more comprehensive care than most clinics. Yet, none of these centers exist as Integrated Practice Units in the true definition of the term because none have assumed joint accountability for quality and cost of health care (8). Through examples from our ongoing efforts to implement the Parkinson's Elevated IPU at Intermountain Health in Salt Lake City, UT we highlight real-world barriers and solutions to completely reshaping the narrative of PD from one of disability to health.
2 Current problems in the Parkinson's journey
Clinical care for PD unfolds under a chronic illness model in which the patient-physician dyad reacts to symptoms as they arise (9). We worked with our Parkinson's Patient Family Advisory Committee (PFAC) and reviewed the literature to better understand the current state of PD care in the United States. Our patients and their carers shared that many PD journeys start with a diagnostic odyssey in which the patient sees various specialists (e.g.,: orthopedist for ‘frozen shoulder,’ gastroenterologist for severe constipation, primary care for tremor) before enough motor symptoms manifest to raise the possibility of parkinsonism as a root cause for all symptoms. The subsequent delivery of the diagnosis is notoriously poor; one member of our PFAC shared that after her abrupt diagnosis, she was left so unsupported by her care team that she lost two years in unfounded despair because she thought her life was over. Her experience has been echoed in published reports (10, 11).
In those initial years after diagnosis, patients are prescribed dopamine replacement therapy and managed at ∼3–6-month intervals by neurologists. Review of the literature reveals that these patients are lucky to even see a neurologist. Wait times for neurologic care are high and according to Medicare claims data one-third of people with PD do not receive any regular neurologic care (12). PwP are generally sent to physical therapy (PT) only if they have impaired balance or gait, with utilization increasing only as the disease progresses (13). They might be told verbally that exercise is important for PD, but little is done to reinforce or support this. Referrals to sub-specialists such as sleep medicine, gastroenterology, and urology may be placed to address non-motor symptoms of the disease, but patients are left on their own to navigate between these providers (11). Patients are also expected to coordinate their care between their neurologist and their primary care physician (PCP).
It is only when disability increases and patients start falling and aspirating that services escalate: more referrals to PT/occupational therapy/speech-language pathology, more frequent primary care and neurology visits (13). Inevitably, an event such as pneumonia or hip fracture necessitates inpatient admission; the literature confirms PwP are more likely to have unplanned hospital admissions than their age-matched peers (14). From there, PwP are less likely to return to their pre-morbid place of residence and have higher in-hospital mortality than their age matched peers (15). Finally, when PwP transition to hospice they usually have no further contact with their movement physician and PCP as per hospice regulations.
3 The ideal Parkinson’s journey
After exploring problems with the current state of care, our PFAC helped us map the ideal journey (Figure 1), which we then separated into four main stages. Starting at the very earliest point, when someone is at risk for PD but has no pathology, they need Prevention or delay of disease. Once someone has clinically relevant symptoms such as rapid eye movement sleep behavior disorder (16), they need a rapid Early Diagnosis and referral to a Parkinson's IPU. There have been several very promising advances in how to detect Parkinson's earlier and with greater certainty. They include: (1) CSF biomarkers (17) (2) alpha synuclein skin biopsies (18) and (3) advanced brain imaging techniques and artificial intelligence (19). The earlier a person is diagnosed, and the earlier a person receives comprehensive healthcare, the better the prognosis. Diagnosing PD early is important because it allows initiation of disease modifying treatment as early as possible and reduces unnecessary suffering, healthcare utilization and cost.
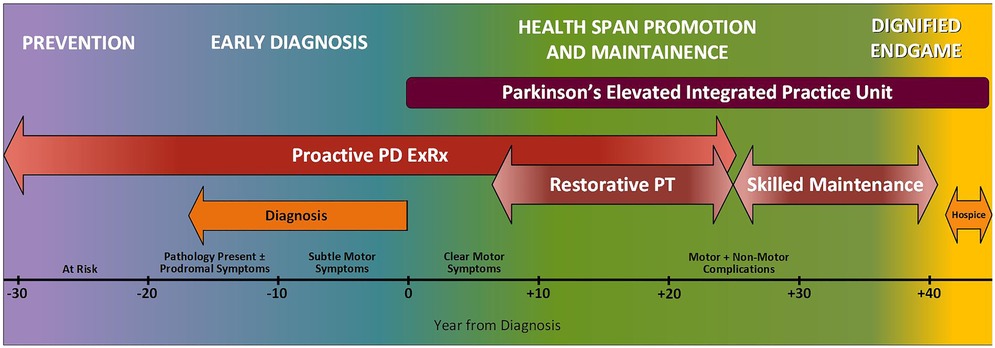
Figure 1. Ideal journey of a person with Parkinson’s disease. The ideal journey will accelerate diagnosis, extend the healthspan, and make the endgame shorter and more meaningful.
The remaining phases of a person's journey take place within the IPU: first Healthspan Promotion and Maintenance (the longest phase of the journey spanning several decades and including different forms of rehabilitation) and finally, the Dignified Endgame in which hospice care is provided as part of the IPU. We take the term ‘Endgame’ from Samuel Beckett's drama of the same name (20). Our PFAC members expressed appreciation for the use of this term because it clearly describes a phase they know is coming and should be approached with care and compassion. Such candid acknowledgment facilitates preparation for and control of their own end-of-life plans.
For the rest of this paper, the focus is exclusively on the care that unfolds within the IPU (Healthspan Promotion and Maintenance and Dignified Endgame)– the vehicle through which we propose to provide financially sustainable proactive integrated health care.
4 The integrated practice unit
First described in 2013 by Porter and Lee, an IPU provides care organized around a medical condition or set of closely related conditions that is delivered by a dedicated and connected multidisciplinary team who assume joint accountability for quality and cost of care (3, 4). In the Parkinson's Elevated IPU, we will provide integrated proactive and reactive care. Strategic, insight-driven proactive care will reduce the likelihood of needing complex, expensive reactive care. Outcomes that matter to the patient will serve as our barometer of clinical success. Existing outcome measure sets are compared elsewhere (21) but, in brief, the Parkinson's Elevated IPU will deploy fidelity measures (eg: percent of patients adhering to the PD Exercise Prescription) along with measures of quality of life such as those proposed by the International Consortium for Health Outcomes Measurement (22).
New models of care must focus on cost because the current healthcare system cost structure is not aligned to incentivize best patient care, nor is it fiscally sustainable in the long-term. The current fee-for-service (FFS) healthcare payment model supports only reactive care by paying for each service or procedure rendered individually to a patient with disease or disability. In the FFS model there are no financial incentives to provide proactive or integrated care, or care that achieves outcomes that matter to the patient. We describe in the final section of this perspective how an IPU properly aligns financial incentives through joint accountability between insurance companies and healthcare providers to ensure high quality care at a sustainable cost. Because Parkinson's Disease affects every organ system in the body, it is an ideal condition around which to pilot an IPU (1). The approach we present will reduce both disease and financial burden over the lifespan of the person with PD while simultaneously improving their quality of care and patient experience.
5 The five elements of the Parkinson’s Elevated integrated practice unit
The general tenets of an IPU are described in a “Playbook for Health Care Leaders” (4). Guided by this, we have defined 5 core clinical elements of the Parkinson's Elevated IPU.
5.1 Integrated, interdisciplinary care, including primary care
The backbone of the IPU will be integrated care that is “coordinated across professions, facilities, and support systems, continuous over time and between visits, and tailored to patient and family needs, values, and preferences” (23). To achieve this as well as to maintain control over total cost of care, a person's primary care physician (PCP) and movement neurologist must work closely, as originally proposed by Bloem, Okun, and Klein (24). But what does this look like in practice? We are currently running a healthcare delivery experiment by integrating a primary care physician (TWS) into the Intermountain movement disorders clinic to see only PwP and their carers. To date this has been met with immense satisfaction from patients and physicians. Care coordinators (Nurse, Exercise Health Coach) provide support for this integrated approach (6).
5.2 Proactive implementation of the Parkinson’s exercise prescription (PD ExRx)
One major goal of the Parkinson's Elevated IPU is to promote early and high adherence of PwP to the Parkinson's Exercise Prescription (PD ExRx) which we have detailed elsewhere (25). In short, when we refer to PD ExRx, we are referring to an exercise prescription that includes four components. The first component, PD ExRx aerobic exercise, has the most evidence to suggest positive disease modification (26–34). The other three components of the exercise prescription—PD ExRx resistance training, PD ExRx flexibility training, and PD ExRx neuromotor training—have not been shown to be directly disease modifying; however they can improve physical function (35) and are associated with better long-term motor outcomes (36). Long-term participation in these modalities can also prevent frailty and debility which if left unaddressed significantly increase morbidity and mortality (37, 38). The neuromotor component of the prescription is particularly important for improving locomotion, improving balance, and reducing falls (39).
Although the IPU will ultimately focus on implementation of all four components of the PD ExRx, we have focused our initial efforts heavily on PD ExRx aerobic exercise because a cure for PD remains elusive (40), and decades of drug trials have failed to produce a disease-modifying treatment (41, 42). Medication and surgery provide a way to mitigate disability and return some quality of life, but they do not slow disease progression. A growing body of evidence suggests that aerobic exercise may be disease-modifying when performed at high intensity (30 min three times a week at 80%–85% heart rate maximum) (26–29). Although the exact mechanism for probable disease modification is unknown, one reason why aerobic exercise is so beneficial for people with PD is that it causes positive health related benefits on the endocrine system, the inflammatory system, and also the neurotrophic system (Luthra et al. manuscript accepted pending revision). If the PD ExRx aerobic can be deployed at the earliest phases of the disease, longer-term complications will likely be delayed: the very definition of proactive care. Although there are many areas of care that can be provided to PwP proactively as detailed in the perspective by Bloem et al. (1), here, we highlight aerobic exercise specifically, because failure to routinely deploy a treatment that most likely slows disease progression is a huge missed opportunity in current PD care.
Figures 2A–C detail the hypothetical trajectory of healthspan for people with and without Parkinson's and demonstrate that healthspan is modifiable based on interventions such as the PD ExRx started in mid-life.
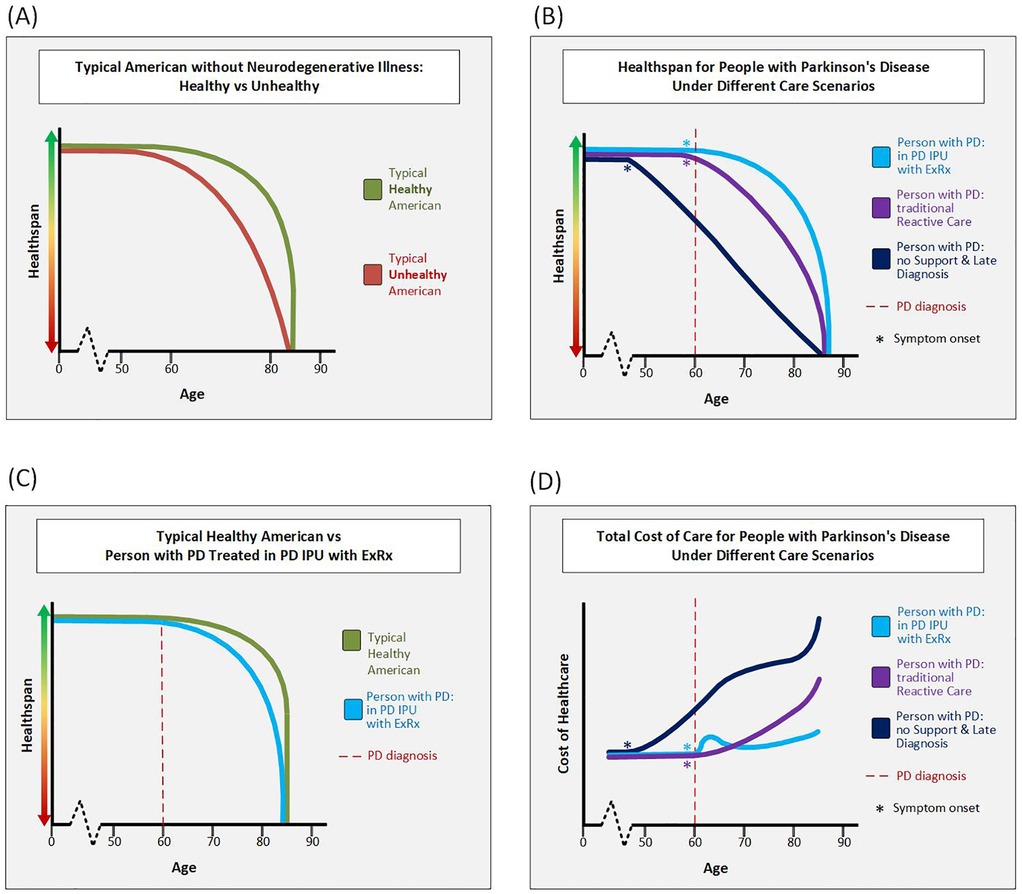
Figure 2. Hypothetical clinical and cost trajectories. (A) This figure depicts the hypothetical healthspan of typical Americans without PD or other neurodegenerative illness. A typical unhealthy American does little to promote health and may develop metabolic syndrome which increases risk of type II diabetes, heart disease, and/or stroke. These unhealthy Americans likely experience a “marginal decade” (75) or more at the end of their life in which they are alive, but have poor health and poor quality of life. In contrast, by early middle age at the latest, a typical healthy American engages in multi-modal exercise, healthy eating, quality-sleep, and care for their mental health. As long as these healthy Americans do not develop a disease out of their control such as cancer or a neurodegenerative illness, they maintain a high healthspan until their time of death. (B) This figure depicts the hypothetical healthspan of 3 PwP all diagnosed at age 60, but treated under different care scenarios. The person with PD treated in the Parkinson's Elevated IPU with the PD ExRx maintains the highest healthspan. The person with PD who receives good neurologic and family support, but is treated in the traditional reactive care model has more disability in their later decades than the person in the IPU. The third person with PD is someone without support who is diagnosed over a decade into their decline and thus starts at a much lower level of health at time of diagnosis. This person continues a precipitous decline due to continued lack of support. (C) This figure overlays the hypothetical typical healthy American and the hypothetical person with PD treated in the Parkinson's Elevated IPU model. While the healthspan of the person with PD is lower than that of the healthy person without PD, it is not that much lower. (D) This figure compares the hypothetical total cost of healthcare for PwP in different care scenarios. The lifetime cost is denoted by the area under each curve. Note that for a person with PD with late diagnosis and little support the cost of care is hypothesized to rise even before diagnosis. For a person with PD in the Parkinson's Elevated IPU, cost is hypothesized to rise at diagnosis when resources are invested in proactive care, but over time the cost curve falls below that of a person with PD treated in the traditional reactive-only care model. We anticipate that the Parkinson's Elevated IPU will reduce long-term costs through steering patients toward a more benign trajectory for both their PD and non-PD comorbidities (because exercise and care coordination help far more than just PD). In a more benign trajectory, less reactive care is needed. When reactive care is needed however, there will be reduced spend by bringing most of the care into the unit. Finally, transition to hospice at the right time will save high-but-futile spending in the last few months of life. Note that the IPU curve is the only one without steep rise in cost at end of life due to the team being able to proactively transition PwP to hospice. Abbreviations: PD, Parkinson's disease; PwP, people with Parkinson's disease; IPU, integrated practice unit.
Despite mounting evidence that the PD ExRx will meaningfully improve healthspan (29), many PwP are not routinely engaging in exercise for a variety of well-studied reasons (43–45). Certainly, the American healthcare system does not facilitate exercise adherence because financial incentives are not aligned with delivery of such a proactive intervention. In construction of our Parkinson's Elevated IPU, we are using an implementation science-informed approach to expedite the availability of this intervention to patients. So far, we have identified five key barriers to implementing the high-intensity aerobic exercise component of the Parkinson's exercise prescription (PD ExRxaerobic) at Intermountain Health (Table 1). We are piloting solutions in a single-site 48-person feasibility/efficacy/cost effectiveness study.
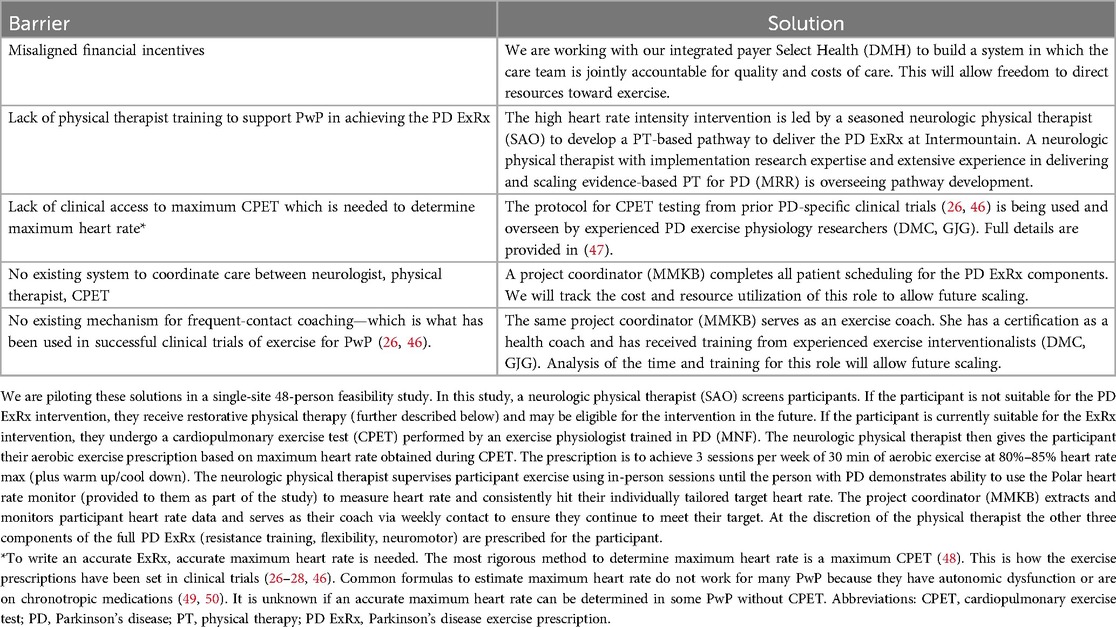
Table 1. Barriers and solutions to implementation of the Parkinson's disease exercise prescription (PD ExRx).
5.3 Specialist network for restorative and skilled maintenance therapy
Although we will always try to enroll PwP early in their disease course into all four-components of the PD ExRx, some people may not be ready for the high-intensity aerobic exercise component initially, and others may never be able to undertake such rigorous exercise because they enter the IPU late in their disease course or have non-modifiable comorbidities preventing participation. Additionally, although implementing the PD ExRx early should reduce disability, none of the treatments we deploy are curative; many people will still experience functional decline over time and will require adaptations to the ExRx. At the first sign that a person with PD could benefit from person- specific adaptations to therapy, they must be referred early and often to specialized physical therapy—which along with continued exercise and physical activity will definitively improve PD motor and non-motor symptoms and physical function (25, 29, 35, 36, 38, 51–59). When needed, PwP should also be referred to specialized occupational and speech therapy (58). This therapy should be conceptualized in two phases: restorative therapy and skilled maintenance therapy (See Figure 1).
It is important to stress that not all people with PD will respond to therapy in the same way. This is why people with PD should be treated by neuroPTs who have the skillset to prescribe appropriate person-specific therapy based on how an individual responds. It is also the case that many people with PD have a variety of comorbidities such as mobility/osteoarthritis or cardiovascular issues and cannot perform aerobic exercises at moderate to high intensity. These individuals will receive individualized exercise prescriptions.
Restorative therapy is focused on fixing or mitigating a deficit to restore function. For example: PT to improve balance, swallow therapy by a speech and language pathologist to reduce aspiration, and occupational therapy to improve hand dexterity for dressing or handwriting. Most existing physical, occupational, and speech/swallow therapy is set up as restorative therapy in which the therapist works with the patient to improve function or fix a specific deficit. However, many PwP can also benefit from skilled maintenance therapy toward the end of their life to maintain basic physical function– e.g.,: safe mobility in the home, communication, and basic activities of daily living (60). Healthspan at this late stage has clearly fallen from the pereson's prior baseline level and may not be able to be restored. However, with skilled maintenance therapy, further decline may be forestalled and some measure of health preserved to allow a higher quality last phase of life.
There are three problems with existing therapy infrastructure that limit high-quality restorative and skilled maintenance therapy. The first is that a fragmented care system does not even direct PwP to PT in appropriate numbers. In 2016–2018, only 18%–25% of participants in the Parkinson's Foundation Quality Initiative were referred to PT (13). To solve this problem PwP treated in our IPU will be referred to PT through an integrated pathway. The second problem is that, even when PwP make it to PT, they often do not receive specialized Parkinson's PT (61, 62). Although it is recommended that PwP be treated by rehabilitation therapists specialized in PD (63–65), patients frequently cannot access these specialized clinicians due to transportation barriers or lack of appropriate referrals. To solve this problem, a network modeled after Parkinson Net (66) is needed. In this Dutch network, PwP are preferentially directed to therapists who have received extra training and maintain a certain minimum volume of PwP as patients. Parkinson Net has demonstrated improved quality and reduced costs for PwP (67). This approach is becoming more recognized and available in the United States, with examples in North Carolina (68) and in California (69).
The third problem with the existing therapy infrastructure is lack of insurance funding for rehabilitation focused on “maintenance.” Many FFS insurance providers, including Medicare, base authorization and payment on the short-term achievement of functional gains rather than goals to ‘maintain’ function over the long-term. This forces therapists to discharge patients once no further ‘rehabilitation’ is possible, when in fact patients would still benefit tremendously from skilled therapists helping to maintain their function. In skilled maintenance, therapy progress should be measured by absence of decline over longer-term episodes of care.
5.4 Physician specialist care provided in the unit and through a tight network of subspecialists
All people—but especially PwP in whom every organ system is affected —need clinicians who understand the whole person and orient care around them (24). Wherever possible the Parkinson's Elevated IPU will bring the first several steps of organ-specific care into the unit. When people's needs exceed what can be provided directly by the IPU, referrals will go to specific physicians within a tight referral network. This will promote higher quality and more coordinated care because the specialists will develop PD-specific expertise and the IPU team need only coordinate with 1–2 specialists per organ-system.
5.5 A dignified endgame: hospice provided in the IPU
A Dignified Endgame involves a smooth and supported transition to hospice in which the care team that has followed the person with PD through their Health Promotion and Maintenance phase continues to be involved in their care. This continued involvement preserves a multi-year relationship in addition to facilitating the ongoing technical management of a disease that renders many palliative pharmacologic agents contraindicated. It also promotes a proactive transition to hospice because the patient knows they will continue with the care team they trust. Such an arrangement is not possible in a FFS system: in that system, once a patient enrolls in hospice, insurance will no longer pay for them to see any clinicians outside the hospice team. Many of our patients have cited this as the main reason they do not wish to enroll in hospice at all. We are piloting a program with Intermountain hospice in which the movement neurologist serves as attending physician when a patient transitions to hospice. To-date this partnership has been met with high patient and family satisfaction as well as ongoing support from Intermountain Hospice leadership.
6 How will we pay for this: joint accountability for quality and cost of care
Programs that provide proactive integrated care likely produce the best health outcomes; yet existing financial incentives are not aligned to support these programs (70). Rather, in our current FFS system, care is provided in medical departments only after the problem has occurred. Because such reactive care is the only thing insurance will pay for, it has become the only type of care clinicians are set up to provide. Care providers financially benefit when the number of billable services are maximized (71). Insurance companies financially benefit by denying services or delaying care. When care of a person is reduced to discrete reactive care snapshots like this, patients suffer while care providers and insurance companies fight over the “necessary” and “clinically justifiable” dollars and cents of reactive care.
The Parkinson's Elevated IPU will solve these problems through a value-based care model (72) in which financial incentives are aligned with clinical best practices including proactive care. Specifically, we are planning a capitated (risk-sharing) model in which the insurance company and healthcare provider in close conjunction with the clinical care team jointly assume responsibility for quality and cost of care for all patients in the IPU. In such a model, the IPU will be paid a set amount of reimbursement per patient per year. The IPU will then be responsible for using that money to effectively manage all the care (not just Parkinson's specific care) for all the patients attributed to the IPU. If the average cost of care falls below the annual set amount, cost savings will be shared by both healthcare provider and insurance company. (Note—to prevent unplanned catastrophically high costs from crippling the program, “stop-loss” insurance will be in place.) In such an arrangement, because we will be able to re-direct funds towards services not traditionally covered in a FFS system, we will be able to implement evidence-based mechanisms of care such as the proactive PD ExRx. Success will be measured not by volume of billable services rendered but rather by outcomes that matter to the patient weighed against total cost of care. Figure 2D demonstrates the theoretical life-time cost of care for PwP in different care scenarios.
7 Conclusion
The ideal journey of a PwP is one of early, compassionate and optimistic diagnosis followed by referral to a Parkinson's Disease Integrated Practice Unit. Our proposal for the Parkinson's Elevated IPU at Intermountain Health uses integrated care to deliver a heavy dose of sustainable proactive care while still providing any reactive care needed. The five core elements of the Parkinson's Elevated IPU are: 1- Integrated, Interdisciplinary Care, including Primary Care, 2-Proactive Implementation of the Parkinson's Disease Exercise Prescription (PD ExRx), 3-Specialist Network for Restorative and Skilled Maintenance Therapy, 4-Physician Specialist Care Provided in the Unit and through a Tight Network of Subspecialists, and 5- A Dignified Endgame: Hospice Provided in the IPU. To ensure financial sustainability, the healthcare providers in the IPU will be jointly accountable with insurance companies for the quality and total cost of care delivered to PwP in the IPU.
As the national debt continues to increase (currently $35.91 Tr in late 2024) (73), the age of the population continues to increase, and healthcare expenditures continue to increase ($4.8 Tr in 2023) (74), there has never been a more urgent time to improve the quality and decrease the cost of healthcare in the US. The Parkinson's Elevated IPU has the potential to greatly improve the healthspan of PwP while reducing the overall cost of care over the course of the disease. Creation of objective healthspan indices as well as the ability to measure cost over a lifetime in people with and without PD as they age with and without proactive measures are needed to prove the value of this conceptualization of treating PwP. However, there is enough circumstantial evidence supporting the hypothetical healthspan and cost curve trajectories we have proposed (Figure 2), that we are planning to implement the Parkinson's Elevated IPU now, rather than wait for more data.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
KM: Conceptualization, Funding acquisition, Supervision, Writing – original draft, Writing – review & editing. MR: Conceptualization, Writing – review & editing. TS: Conceptualization, Writing – review & editing. DH: Conceptualization, Writing – review & editing. GG: Writing – review & editing. MB: Project administration, Visualization, Writing – review & editing. SO: Writing – review & editing. MF: Writing – review & editing. DC: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The research reported in this publication was supported by a generous philanthropic gift from the JCS Family Foundation and Robert and Mitra Ginsburg. It was also supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Numbers U01NS113851. Research is also supported by the National Institutes of Health’s National Center for Advancing Translational Sciences, Grant Number UL1TR001422. It is also supported by a generous philanthropic gift in honor of Howard Gilbert. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Acknowledgments
The authors thank the Intermountain Health Parkinson's Patient and Family Advisory Committee members for their collaboration in crafting this vision. We also acknowledge numerous Intermountain Health colleagues for their support of the Parkinson's Elevated vision: Meredyth Armitage, David Dirks, Carter Dredge, Spencer Hawkes, Robert Hoesch, Janene Holmberg, Kamie Champlin, Curt Howell, Jeffrey McNally, Kate Minick, Thomas Lombardi, Jimmy Martello, Marilyn McKasson, Tess Pendery, Patrick Porter, Adrian Puttgen, Renee Scheidell, Jill Seifert, Rajendu Srivastava, Geoff Swanson, Jason Terry, Massimo Testa, Kristy Veale, Julienne West, Timothy West, the Healthcare Delivery Institute, the Edwards Internal Medicine Clinic, and the Primary Care Service Line Leadership.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bloem BR, Henderson EJ, Dorsey ER, Okun MS, Okubadejo N, Chan P, et al. Integrated and patient-centred management of Parkinson's Disease: a network model for reshaping chronic neurological care. Lancet Neurol. (2020) 19:623–34. doi: 10.1016/S1474-4422(20)30064-8
2. Kaeberlein M. How healthy is the healthspan concept? Geroscience. (2018) 40:361–4. doi: 10.1007/s11357-018-0036-9
3. Porter ME, Lee TH. The Strategy That Will Fix Health Care. Brighton, MA: Harvard Buisness Review (2013).
4. Porter ME, Lee TH. Integrated practice units: a playbook for health care leaders. NEJM Catal Innov Care Deliv. (2021) 2(1). doi: 10.1056/CAT.20.0237
5. Goldman JG, Volpe D, Ellis TD, Hirsch MA, Johnson J, Wood J, et al. Delivering multidisciplinary rehabilitation care in Parkinson’s disease: an international consensus statement. J Parkinsons Dis. (2024) 14:135–66. doi: 10.3233/JPD-230117
6. Rajan R, Brennan L, Bloem BR, Dahodwala N, Gardner J, Goldman JG, et al. Integrated care in Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. (2020) 35:1509–31. doi: 10.1002/mds.28097
7. Okun MS, Ramirez-Zamora A, Foote KD. Neuromedicine service and science hub model. JAMA Neurol. (2018) 75:271–2. doi: 10.1001/jamaneurol.2017.3976
8. Wiersema JE, Bresser CC, van der Nat PB. Organizing care around conditions: an expanded model of value-based health care. NEJM Catalyst. (2023) 4(12):CAT-23. doi: 10.1056/CAT.23.0180
9. Lanoix M. Palliative care and Parkinson’s disease: managing the chronic-palliative interface. Chronic Illn. (2009) 5:46–55. doi: 10.1177/1742395309102819
10. Phillips LJ. Dropping the bomb: the experience of being diagnosed with Parkinson’s disease. Geriatr Nurs. (2006) 27:362–9. doi: 10.1016/j.gerinurse.2006.10.012
11. Boersma I, Jones J, Carter J, Bekelman D, Miyasaki J, Kutner J, et al. Parkinson disease patients’ perspectives on palliative care needs: what are they telling us? Neurol Clin Pract. (2016) 6:209–19. doi: 10.1212/CPJ.0000000000000233
12. Fullard ME, Thibault DP, Hill A, Fox J, Bhatti DE, Burack MA, et al. Utilization of rehabilitation therapy services in Parkinson disease in the United States. Neurology. (2017) 89:1162–9. doi: 10.1212/WNL.0000000000004355
13. Roberts AC, Rafferty MR, Wu SS, Miao G, Cubillos F, Simuni T, et al. Patterns and predictors of referrals to allied health services for individuals with Parkinson’s disease: a Parkinson’s foundation (PF) QII study. Parkinsonism Relat Disord. (2021) 83:115–22. doi: 10.1016/j.parkreldis.2020.11.024
14. Hassan A, Wu SS, Schmidt P, Dai Y, Simuni T, Giladi N, et al. High rates and the risk factors for emergency room visits and hospitalization in Parkinson’s disease. Parkinsonism Relat Disord. (2013) 19:949–54. doi: 10.1016/j.parkreldis.2013.06.006
15. Low V, Ben-Shlomo Y, Coward E, Fletcher S, Walker R, Clarke CE. Measuring the burden and mortality of hospitalisation in Parkinson’s disease: a cross-sectional analysis of the english hospital episodes statistics database 2009–2013. Parkinsonism Relat Disord. (2015) 21:449–54. doi: 10.1016/j.parkreldis.2015.01.017
16. Summers RLS, Rafferty MR, Howell MJ, Mackinnon CD. Motor dysfunction in REM sleep behavior disorder: a rehabilitation framework for prodromal synucleinopathy. Neurorehabil Neural Repair. (2021) 35:611–21. doi: 10.1177/15459683211011238
17. Dasari M, Medapati RV. Cerebrospinal fluid biomarkers for diagnosis of Parkinson’s disease: a systematic review. Cureus. (2025) 17(2):e79386. doi: 10.7759/cureus.79386
18. Isaacson JR, Freeman R, Gibbons CH. Clinical utility of synuclein skin biopsy in the diagnosis and evaluation of synucleinopathies. Front Neurol. (2024) 15:1510796. doi: 10.3389/fneur.2024.1510796
19. Vaillancourt DE, Barmpoutis A, Wu SS, Desimone JC, Schauder M, Chen R, et al. Automated imaging differentiation for parkinsonism. JAMA Neurol. (2025) 82(5):495–505. doi: 10.1001/jamaneurol.2025.0112
20. Beckett S. Endgame: A Play in One Act; Followed by, Act Without Words I: A Mime for One Player. New York, NY: Grove Press (1958).
22. De Roos P, Bloem BR, Kelley TA, Antonini A, Dodel R, Hagell P, et al. A consensus set of outcomes for Parkinson’s disease from the international consortium for health outcomes measurement. J Parkinsons Dis. (2017) 7:533–43. doi: 10.3233/JPD-161055
23. Singer SJ, Burgers J, Friedberg M, Rosenthal MB, Leape L, Schneider E. Defining and measuring integrated patient care: promoting the next frontier in health care delivery. Med Care Res Rev. (2011) 68:112–27. doi: 10.1177/1077558710371485
24. Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet. (2021) 397:2284–303. doi: 10.1016/S0140-6736(21)00218-X
25. Corcos DM, Lamotte G, Luthra NS, Mckee KE. Advice to people with Parkinson’s in my clinic: exercise. J Parkinsons Dis. (2024) 14:609–17. doi: 10.3233/JPD-230277
26. Schenkman M, Moore CG, Kohrt WM, Hall DA, Delitto A, Comella CL, et al. Effect of high-intensity treadmill exercise on motor symptoms in patients with de novo Parkinson disease: a phase 2 randomized clinical trial. JAMA Neurol. (2018) 75:219–26. doi: 10.1001/jamaneurol.2017.3517
27. Van Der Kolk NM, De Vries NM, Kessels RPC, Joosten H, Zwinderman AH, Post B, et al. Effectiveness of home-based and remotely supervised aerobic exercise in Parkinson’s disease: a double-blind, randomised controlled trial. Lancet Neurol. (2019) 18:998–1008. doi: 10.1016/S1474-4422(19)30285-6
28. Mak MK, Wong-Yu IS. Six-Month community-based brisk walking and balance exercise alleviates motor symptoms and promotes functions in people with Parkinson’s disease: a randomized controlled trial. J Parkinsons Dis. (2021) 11:1431–41. doi: 10.3233/JPD-202503
29. Langeskov-Christensen M, Franzén E, Grøndahl Hvid L, Dalgas U. Exercise as medicine in Parkinson’s disease. J Neurol Neurosurg Psychiatry. (2024) 95:1077–88. doi: 10.1136/jnnp-2023-332974
30. Zhang M, Li F, Wang D, Ba X, Liu Z. Exercise sustains motor function in Parkinson’s disease: evidence from 109 randomized controlled trials on over 4,600 patients. Front Aging Neurosci. (2023) 15:1071803. doi: 10.3389/fnagi.2023.1071803
31. De Almeida FO, Santana V, Corcos DM, Ugrinowitsch C, Silva-Batista C. Effects of endurance training on motor signs of Parkinson’s disease: a systematic review and meta-analysis. Sports Med. (2022) 52:1789–815. doi: 10.1007/s40279-022-01650-x
32. Crotty GF, Schwarzschild MA. Chasing protection in Parkinson’s disease: does exercise reduce risk and progression? Front Aging Neurosci. (2020) 12:186. doi: 10.3389/fnagi.2020.00186
33. Ishaq S, Shah IA, Lee SD, Wu BT. Effects of exercise training on nigrostriatal neuroprotection in Parkinson’s disease: a systematic review. Front Neurosci. (2024) 18:1464168. doi: 10.3389/fnins.2024.1464168
34. Tsukita K, Sakamaki-Tsukita H, Takahashi R. Long-term effect of regular physical activity and exercise habits in patients with early Parkinson disease. Neurology. (2022) 98:e859–71. doi: 10.1212/WNL.0000000000013218
35. Osborne JA, Botkin R, Colon-Semenza C, Deangelis TR, Gallardo OG, Kosakowski H, et al. Physical therapist management of Parkinson disease: a clinical practice guideline from the American physical therapy association. Phys Ther. (2022) 102(4):pzab302. doi: 10.1093/ptj/pzab302. Erratum in: Phys Ther. (2022) 102(8):pzac098. doi: 10.1093/ptj/pzac09834963139
36. Corcos DM, Robichaud JA, David FJ, Leurgans SE, Vaillancourt DE, Poon C, et al. A two-year randomized controlled trial of progressive resistance exercise for Parkinson’s disease. Mov Disord. (2013) 28:1230–40. doi: 10.1002/mds.25380
37. Corcos DM. Importance of upper and lower body resistance exercise for preventing and reversing sarcopenia in Parkinson’s disease. Parkinsonism Relat Disord. (2024) 123:106104. doi: 10.1016/j.parkreldis.2024.106104
38. Mak MK, Wong-Yu IS, Shen X, Chung CL. Long-term effects of exercise and physical therapy in people with Parkinson disease. Nat Rev Neurol. (2017) 13:689–703. doi: 10.1038/nrneurol.2017.128
39. Wallin A, Franzén E, Studsgaard J, Hansen MB, Johansson S, Brincks JK. Balance exercise interventions in Parkinson’s disease: a systematic mapping review of components, progression, and intensity. Parkinsonism Relat Disord. (2025) 133:107310. doi: 10.1016/j.parkreldis.2025.107310
40. Blakemore E. What is Parkinson’s Disease—and Why is it so Hard to Diagnose? Washington, DC: National Geographic (2024). Available at: https://www.nationalgeographic.com/science/article/parkinsons-disease-cause-symptoms-diagnosis (Accessed May 18, 2025).
41. Wolff A, Schumacher NU, Purner D, Machetanz G, Demleitner AF, Feneberg E, et al. Parkinson’s disease therapy: what lies ahead? J Neural Transm (Vienna). (2023) 130:793–820. doi: 10.1007/s00702-023-02641-6
42. Racette BA, Willis AW. Time to change the blind men and the elephant approach to Parkinson disease? Neurology. (2015) 85:190–6. doi: 10.1212/WNL.0000000000001739
43. Schootemeijer S, Van Der Kolk NM, Ellis T, Mirelman A, Nieuwboer A, Nieuwhof F, et al. Barriers and motivators to engage in exercise for persons with Parkinson’s disease. J Parkinsons Dis. (2020) 10:1293–9. doi: 10.3233/JPD-202247
44. Ellis T, Boudreau JK, Deangelis TR, Brown LE, Cavanaugh JT, Earhart GM, et al. Barriers to exercise in people with Parkinson disease. Phys Ther. (2013) 93:628–36. doi: 10.2522/ptj.20120279
45. Afshari M, Yang A, Bega D. Motivators and barriers to exercise in Parkinson’s disease. J Parkinsons Dis. (2017) 7:703–11. doi: 10.3233/JPD-171173
46. Patterson CG, Joslin E, Gil AB, Spigle W, Nemet T, Chahine L, et al. Study in Parkinson’s disease of exercise phase 3 (SPARX3): study protocol for a randomized controlled trial. Trials. (2022) 23:855. doi: 10.1186/s13063-022-06703-0
47. Griffith GJ, Mckee KE, Lamotte G, Luthra NS, Corcos DM. Advice to people with Parkinson’s in my clinic: get a cardiopulmonary exercise test. J Parkinsons Dis. (2025). doi: 10.1177/1877718X251330814
48. ACSM. ACSM’S Guidelines for Exercise Testing and Prescription. Philadelphia: Wolters Kluwer (2022).
49. Griffith G, Lamotte G, Mehta N, Fan P, Nikolich J, Springman V, et al. Chronotropic incompetence during exercise testing as a marker of autonomic dysfunction in individuals with early Parkinson’s disease. J Parkinsons Dis. (2024) 14:121–33. doi: 10.3233/JPD-230006
50. Lamotte G, Mckee KE, Luthra NS, Corcos DM. Advice to people with Parkinson’s in my clinic: orthostatic hypotension. J Parkinsons Dis. (2024) 14:1139–46. doi: 10.3233/JPD-240149
51. Prodoehl J, Rafferty MR, David FJ, Poon C, Vaillancourt DE, Comella CL, et al. Two-year exercise program improves physical function in Parkinson’s disease: the PRET-PD randomized clinical trial. Neurorehabil Neural Repair. (2015) 29:112–22. doi: 10.1177/1545968314539732
52. Ellis TD, Colon-Semenza C, Deangelis TR, Thomas CA, Hilaire MS, Earhart GM, et al. Evidence for early and regular physical therapy and exercise in Parkinson’s disease. Semin Neurol. (2021) 41:189–205. doi: 10.1055/s-0041-1725133
53. Altmann LJ, Stegemoller E, Hazamy AA, Wilson JP, Bowers D, Okun MS, et al. Aerobic exercise improves mood, cognition, and language function in Parkinson’s disease: results of a controlled study. J Int Neuropsychol Soc. (2016) 22:878–89. doi: 10.1017/S135561771600076X
54. David FJ, Robichaud JA, Leurgans SE, Poon C, Kohrt WM, Goldman JG, et al. Exercise improves cognition in Parkinson’s disease: the PRET-PD randomized, clinical trial. Mov Disord. (2015) 30:1657–63. doi: 10.1002/mds.26291
55. Mirelman A, Bonato P, Camicioli R, Ellis TD, Giladi N, Hamilton JL, et al. Gait impairments in Parkinson’s disease. Lancet Neurol. (2019) 18:697–708. doi: 10.1016/S1474-4422(19)30044-4
56. Silva-Batista C, De Lima-Pardini AC, Nucci MP, Coelho DB, Batista A, Piemonte MEP, et al. A randomized, controlled trial of exercise for parkinsonian individuals with freezing of gait. Mov Disord. (2020) 35:1607–17. doi: 10.1002/mds.28128
57. Allen NE, Canning CG, Almeida LRS, Bloem BR, Keus SH, Lofgren N, et al. Interventions for preventing falls in Parkinson’s disease. Cochrane Database Syst Rev. (2022) 6:CD011574.35665915
58. Rafferty MR, Foster ER, Roberts AC, Smaller KA, Johnson LL, Lawson RA. Stemming the tide: the proactive role of allied health therapy in Parkinson’s disease. J Parkinsons Dis. (2024) 14:S7–S19. doi: 10.3233/JPD-230267
59. Rafferty MR, Prodoehl J, Robichaud JA, David FJ, Poon C, Goelz LC, et al. Effects of 2 years of exercise on gait impairment in people with Parkinson disease: the PRET-PD randomized trial. J Neurol Phys Ther. (2017) 41:21–30. doi: 10.1097/NPT.0000000000000163
60. Rafferty MR, Nettnin E, Goldman JG, Macdonald J. Frameworks for Parkinson’s disease rehabilitation addressing when, what, and how. Curr Neurol Neurosci Rep. (2021) 21:12. doi: 10.1007/s11910-021-01096-0
61. Nijkrake MJ, Keus SH, Oostendorp RA, Overeem S, Mulleners W, Bloem BR, et al. Allied health care in Parkinson’s disease: referral, consultation, and professional expertise. Mov Disord. (2009) 24:282–6. doi: 10.1002/mds.22377
62. Clarke CE, Patel S, Ives N, Rick CE, Woolley R, Wheatley K, et al. Clinical effectiveness and cost-effectiveness of physiotherapy and occupational therapy versus no therapy in mild to moderate Parkinson’s disease: a large pragmatic randomised controlled trial (PD REHAB). Health Technol Assess. (2016) 20:1–96. doi: 10.3310/hta20630
63. Farbman ES. The case for subspecialization in neurology: movement disorders. Front Neurol. (2011) 2:22. doi: 10.3389/fneur.2011.00022
64. Sturkenboom I, Talebi AH, Maas BR, De Vries NM, Darweesh SKL, Kalf JG. Specialized allied health care for Parkinson’s disease: state of the art and future directions. J Parkinsons Dis. (2024) 14:S193–s207. doi: 10.3233/JPD-230307
65. Talebi AH, Ypinga JHL, De Vries NM, Nonnekes J, Munneke M, Bloem BR, et al. Specialized versus generic allied health therapy and the risk of Parkinson’s disease complications. Mov Disord. (2023) 38:223–31. doi: 10.1002/mds.29274
66. Gray B, Sarnak DO, Tanke M. ParkinsonNet: An Innovative Dutch Approach to Patient-Centered Care for a Degenerative Disease (2016). The Commonwealth Fund. Available at: https://www.commonwealthfund.org/publications/case-study/2016/dec/parkinsonnet-innovative-dutch-approach-patient-centered-care (Accessed November 8, 2024).
67. Ypinga JHL, De Vries NM, Boonen L, Koolman X, Munneke M, Zwinderman AH, et al. Effectiveness and costs of specialised physiotherapy given via ParkinsonNet: a retrospective analysis of medical claims data. Lancet Neurol. (2018) 17:153–61. doi: 10.1016/S1474-4422(17)30406-4
68. UNC-Movement-Disorders-Division. Park NC. Available at: http://www.parknc.org (Accessed November 15, 2024).
69. Rompen L, De Vries NM, Munneke M, Neff C, Sachs T, Cedrone S, et al. Introduction of network-based healthcare at kaiser permanente. J Parkinsons Dis. (2020) 10:207–12. doi: 10.3233/JPD-191620
70. Yordanov D, Oxholm AS, Prætorius T, Kristensen SR. Financial incentives for integrated care: a scoping review and lessons for evidence-based design. Health Policy. (2024) 141:104995. doi: 10.1016/j.healthpol.2024.104995
71. Hunter K, Kendall D, Ahmadi L. The Case Against Fee-for-Service Health Care. Washington, DC: Third Way (2021). Available at: https://thirdway.imgix.net/pdfs/the-case-against-fee-for-service-health-care.pdf (Accessed May 18, 2025).
72. Lewis C, Horstman C, Blumenthal D, Abrams MK. Value-Based Care: What It Is, and Why It’s Needed. The Commonwealth Fund (2023). Available at: https://www.commonwealthfund.org/publications/explainer/2023/feb/value-based-care-what-it-is-why-its-needed (Accessed December 19, 2024).
73. Fiscaldata.Tresury.gov. What is the National Debt? (2024). Available at: https://fiscaldata.treasury.gov/americas-finance-guide/national-debt/ (Accessed November 8, 2024).
74. Fiore JA, Madison AJ, Poisal JA, Cuckler GA, Smith SD, Sisko AM, et al. National health expenditure projections, 2023–32: payer trends diverge as pandemic-related policies fade. Health Aff (Millwood). (2024) 43:910–21. doi: 10.1377/hlthaff.2024.00469
Keywords: Parkinson's disease, healthspan, integrated practice unit, proactive care, high-intensity aerobic exercise
Citation: McKee KE, Rafferty MR, Sakata TW, Hedges DM, Griffith GJ, Bingham MMK, Obradovich SA, Francis MN and Corcos DM (2025) Parkinson's Elevated: improving healthspan. Front. Sports Act. Living 7:1529075. doi: 10.3389/fspor.2025.1529075
Received: 20 November 2024; Accepted: 12 May 2025;
Published: 2 June 2025.
Edited by:
David Broom, Coventry University, United KingdomReviewed by:
David Arthur Hart, University of Calgary, CanadaCopyright: © 2025 McKee, Rafferty, Sakata, Hedges, Griffith, Bingham, Obradovich, Francis and Corcos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kathleen E. McKee, a2F0aGxlZW4ubWNrZWVAaW1haWwub3Jn
†These authors have contributed equally to this work
 Kathleen E. McKee
Kathleen E. McKee Miriam R. Rafferty
Miriam R. Rafferty Theadora W. Sakata1,4,5,†
Theadora W. Sakata1,4,5,† Garett J. Griffith
Garett J. Griffith Daniel M. Corcos
Daniel M. Corcos