- 1Sports Center, Federal University of Santa Catarina, University Campus, Florianópolis, Santa Catarina, Brazil
- 2Health Sciences Center, Federal University of Santa Catarina, Florianópolis, Santa Catarina, Brazil
- 3Health Sciences Center/NUPAIVA, Federal University of Santa Catarina, Florianópolis, Brazil
Background: COVID-19 can lead to reduced functional capacity, loss of muscle mass, and lasting and persistent symptoms, resulting in reduced physical activity.
Objective: To evaluate the effects of a multicomponent training on functional capacity, persistent symptoms, body composition, pulmonary function, and physical activity levels in patients significantly impaired by SARS-CoV-2.
Methods: The participants were randomly assigned (1:1) to either the intervention group (IG), which received multicomponent training (balance/aerobic/resistance), or the control group (CG). Functional capacity [6 min walk test (6MWT)—primary outcome, sit and reach, sit-to-stand, timed up and go], persistent symptoms (dyspnea, fatigue, post-COVID functional status, frailty), body composition (dual-energy x-ray absorptiometry and bioimpedance), pulmonary function, and physical activity levels (accelerometry) were evaluated at baseline and after 24 weeks. Generalized estimating equations were used, with the significance level set at α = 0.05. Outcomes were analyzed by intention-to-treat (ITT) and per-protocol (PP) approaches. Effect sizes were calculated from the mean difference between groups of changes between pre- and post-intervention.
Results: Forty participants [age = 52.00 (12.93) years, 19 women] were included. The primary outcome 6MWT showed improvement in both groups in the ITT analysis (IG: 35.5 m, 95% CI: −3.0 to 74.1; CG: 37.4 m, 95% CI: −5.26 to 80.2) and in the IG (87.6 m, 95% CI: 50.6–124.4) in the PP analysis. The IG showed a reduction in mental fatigue (−1.7 points, 95% CI: −0.5 to 3.5) and general fatigue (−6.5 points, 95% CI: −9.4 to −3.5) in our ITT analysis. The IG also revealed improvement in timed up and go test (−1.6 s, 95% CI: −2.6 to −0.6), mental fatigue (−2.0points, 95% CI: −3.6 to 0.7), general fatigue (−6.4points, 95% CI: −11.0 to −1.6), and a protective effect against increased body fat in PP analysis.
Conclusion: This program was effective in improving fatigue in patients previously significantly affected by COVID-19.
1 Introduction
The manifestation of COVID-19 symptoms is not homogeneous, with some infected individuals showing no symptoms while others rapidly progress to severe and critical cases (1). Particularly among hospitalized individuals, the disease is exacerbated by physical inactivity, leading to adverse impacts on functional capacity (2, 3) and body composition, especially in muscle quantity and function (4–6).
Following the acute phase of COVID-19, many individuals experience a syndrome known as post-COVID-19 condition, and the number of people with late sequelae remains unknown (7). This condition is characterized by substantial functional impairment (2, 3) and may encompass long-lasting symptoms such as dyspnea and fatigue, accompanied by diminished exercise tolerance and functional restrictions (8–11). Fatigue stands out as the most prevalent symptom in both hospitalized and non-hospitalized patients, persisting up to 2 years postinfection (12–15). This symptom may have as one of its origins the muscle damage caused during the infection, which may result from mitochondrial changes, inflammation, capillary injury in muscle biopsies, and reduced energy supply (16–18). The physical and functional limitations observed in post-COVID-19 condition perpetuate a vicious cycle, wherein reduced physical activity (PA) levels are associated with a lower functional capacity and so on.
In this context, exercise intolerance, primarily driven by persistent dyspnea and fatigue (16), emerges as a multifaceted phenomenon influenced by both physical and psychological factors (19). Given that reduced functional capacity is associated with a higher risk of mortality in clinical populations (20, 21) and a decline in activities of daily living among the elderly (22), there is an urgent public health need to reduce COVID-19 symptoms both in the acute phase and persistent symptoms and to enhance the functional capacity of survivors (3, 23, 24). Physical exercise rehabilitation plays a pivotal role in addressing these challenges (25, 26).
Recent studies have begun to uncover the benefits of post-COVID rehabilitation models that emphasize exercise (26–32). These models have shown promise in improving various outcomes such as fatigue, functional capacity, strength, muscle cross-sectional area, and muscle quality (27–30). A recent meta-analysis concluded that respiratory training- and exercise-based rehabilitation interventions are effective in improving functional capacity (6 min walking test, dyspnea) in post-COVID-19 conditions. However, there is moderate and low certainty of evidence, respectively. Regarding fatigue, the evidence was limited and could not be synthesized (33). Additionally, body composition is an outcome little evaluated in randomized clinical trials to date, despite its involvement in the acute phase of the disease and possibly in the post-COVID-19 condition (26, 31, 32).
In this regard, most of the evidence from randomized controlled trials to date reports brief interventions, lasting up to a maximum of 16 weeks, often delivered in alternative formats such as semi-supervised, tele-supervised, or home-based (26, 32–34). However, there remains a scarcity of randomized controlled trials investigating multicomponent training strategies—those that combine different physical abilities—to address the complex, limiting symptoms and functional limitations often observed in post-critical COVID-19 cases.
In addressing this research gap, our study examined the impact of a multicomponent training regimen on functional outcomes, body composition, and persistent symptoms among patients significantly affected by COVID-19. Additionally, we assessed physical activity levels and sedentary behavior (SB) before and after the intervention. The hypothesis is that multicomponent training will be superior to a control procedure, involving physical activity recommendations, in improving functional capacity, pulmonary function, persistent symptoms, body composition, and physical activity levels in patients post-COVID-19 infection.
2 Methods
2.1 Study design
This was a single-center, single-blinded, parallel-group, randomized, controlled trial conducted at the Federal University of Santa Catarina (UFSC), Florianópolis, Brazil, between November 2021 and April 2023. The complete protocol can be found in Delevatti et al. (35). This study was approved by the Human Research Ethics Committee of the institution of origin (Protocol 4,909,599), pre-registered in the Brazilian Registry of Clinical Trials (RBR-10y6jhrs), and is nested within a larger controlled trial, multicentric, with different training/rehabilitation programs, called Recovery Trial. The manuscript was reported according to the Consolidated Standards of Reporting Trials (CONSORT) guidelines (36). All participants provided oral and written informed consent.
2.2 Participants
Women and men (≥18 years old) who had previously been admitted to the UFSC University Hospital with moderate to critical COVID-19 (37) or who were not hospitalized but experienced chronic postinfection fatigue [score >4 on the Chalder scale (38)] participated in the study.
All eligible and interested patients who wished to participate in the study underwent a prior medical screening which included clinical evaluation by physicians. The eligibility criteria for the study were as follows: minimum age of 18 years; discharge from the hospital or completion of the acute phase of the disease at least 6 weeks prior; and general respiratory, functional, and cognitive stability. More details about the eligibility criteria can be found in Delevatti et al. (35). Individuals who had previously participated in a rehabilitation or physical exercise program or who were not available to participate in the proposed intervention were excluded from the study.
Participant selection was non-probabilistic and voluntary. The participants were recruited through medical screening at the University Hospital—UFSC or through contact through online and television advertising materials. All participants provided oral and written informed consent after the objectives, procedures, and risks of the study had been explained.
2.3 Randomization and blinding
The participants were randomized (www.randomizer.org) in permuted blocks of 4–6, stratified by sex, into either the intervention group (IG) or the control group (CG) in a 1:1 ratio. A computer-generated random number sequence was created by an independent researcher. After baseline measurements, the participants were assigned consecutive numbers, which were forwarded to an external data manager, who subsequently returned the corresponding allocation to the study’s exercise providers. Blinding of the participants and exercise providers was not possible after group allocation. However, the study's care providers (exercise and control procedure) did not participate in treatment action assessment and data analysis or interpretation.
All assessments were conducted by the same evaluators at baseline and after 24 weeks. All evaluators were experienced in collecting the outcomes and were blinded to group allocation; moreover, the intervention team did not participate in outcome assessments. Unfortunately, however, blinding of the statistical analysis was not possible.
2.4 Clinical data
For the participants who agreed to participate, a complementary medical history was administered during the medical screening, containing sociodemographic and general health information, along with a clinical history related to the period of COVID-19 infection, such as type of hospitalization, duration of hospitalization, and use or non-use of mechanical ventilation.
2.5 Intervention
The intervention was a 24-week supervised program divided into two phases. Training was performed twice a week in Phase 1 and thrice a week in Phase 2 (∼70 min/session). The 24 weeks were composed of 1 week of familiarization and two mesocycles of 5 weeks in Phase 1, 1 regenerative week, and three mesocycles of 4 weeks in Phase 2. The program had a multicomponent structure, with balance (15 min/session), resistance training (15–20 min/session), and aerobic training (25 min/session) in Phase 1 and resistance training (20–25 min/session) and aerobic training (25 min/session) in Phase 2. The progression strategies adopted in Phase 1 were an increase in volume in resistance and aerobic training and an increase in complexity in balance training. In Phase 2, the progression aimed to increase the intensity of strength and aerobic training, while balance training was discontinued.
The order of the main parts (resistance and aerobic) was alternated over the weeks with the aim of not prioritizing one of the components and to serve as a motivational factor. Balance training was discontinued in Phase 2, but if trainers identified a patient who still required this training, this was included individually at the beginning of the session. In Phase 2, resistance training consisted of training with machines and weights, while in Phase 1, it was just body weight and resistance bands. Details of the intervention, such as specific exercises and progression strategies, are presented in Figure 1.
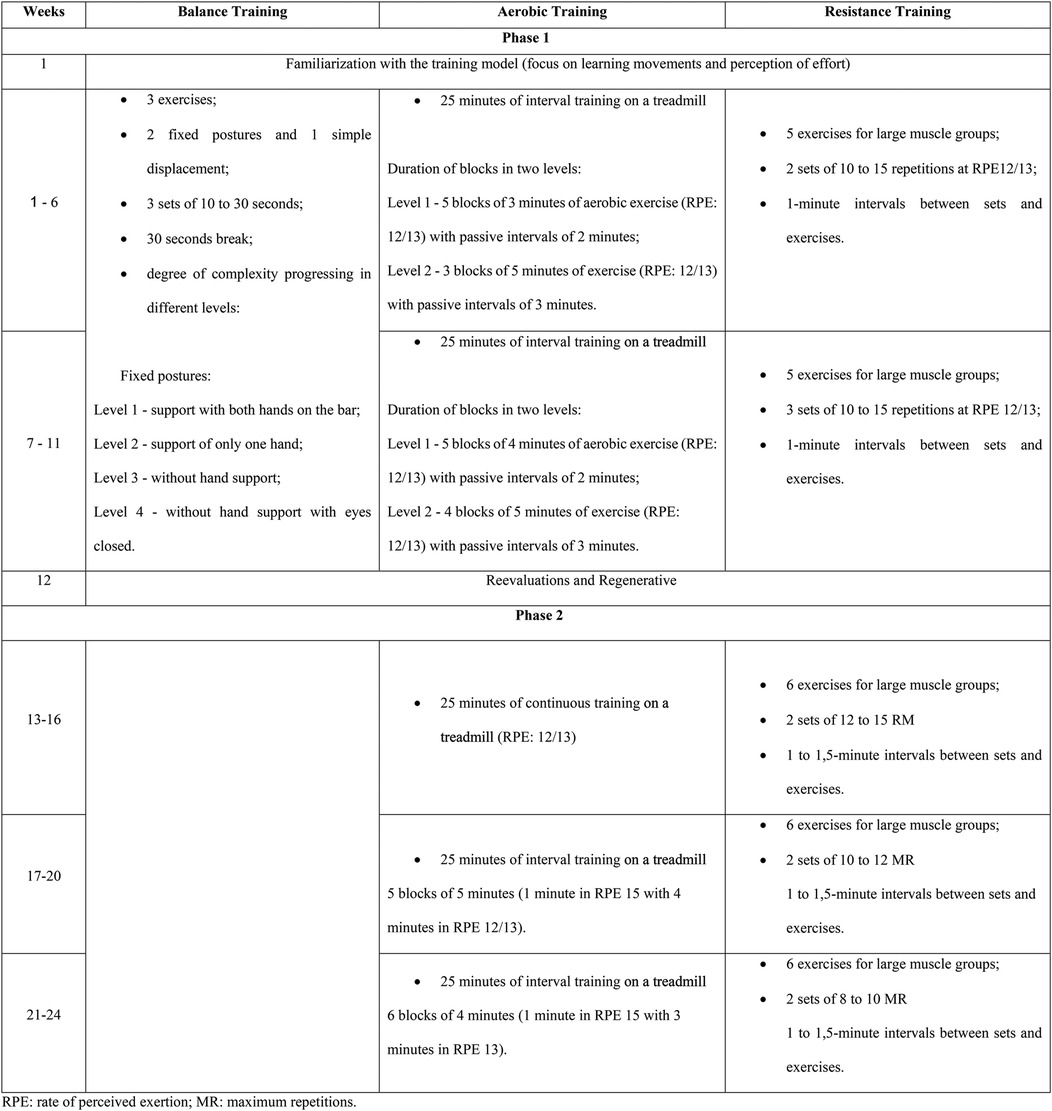
Figure 1. Structuring of training sessions throughout the rehabilitation program (CORE-study). RPE, rate of perceived exertion; MR, maximum repetitions.
The sessions happened in the Rehabilitation Center of the Sports Center, UFSC. Three instructors with previous experience in exercise prescription supervised the intervention, and physical education students helped with the general running of the sessions. The instructor–patient ratio in the sessions was 1:2.
2.6 Adherence
The adherence was assessed by the frequency of participation in the training sessions. When a participant in the intervention group had two or more absences, they were contacted to determine the reason for their absence and to offer assistance if necessary.
2.7 Control procedures
The patients allocated to the control group received recommendations and guidance on physical activity and sedentary behavior in a face-to-face meeting lasting approximately 1 h. The information was presented by one of the professors responsible for the program and taken from two chapters of the Brazilian Guide to Physical Activity (39) and was directed to the age group of each patient (adult or elderly). One of the chapters, titled “Understanding Physical Activity,” primarily focuses on understanding the domains of physical activity, intensity levels, and the physical capacities that should be prioritized. The other chapter is age-specific, presenting the main recommendations regarding the amount of weekly practice time, appropriate intensity levels, possible activities, and the physical capacities that should be prioritized—for example, aerobic and strength activities for adults and aerobic, strength, and balance activities for older adults. The patients received copies of the chapters presented and had the opportunity to clarify possible doubts about the topics addressed.
2.8 Load and safety monitoring
The external and internal training load, peripheral O2 saturation (SpO2), heart rate (HR), blood pressure, and capillary glycemia were evaluated. The internal load was assessed using the rate of perceived exertion (RPE) of the session, measured on the Borg CR10 scale adapted by Foster et al. (40). The external load from resistance training was assessed through repetitions in the sit-and-stand exercise during Phase 1 and through weights in the leg press exercise during Phase 2. For aerobic training, the external load was evaluated through distance covered and speed on the treadmill. SpO2 was evaluated before, during, and after all exercise sessions, and the participants did not start or had the session interrupted immediately if the oximetry showed values <90%. Blood pressure was also measured before all exercise sessions following the procedures described by Barroso et al. (41). Capillary blood glucose collections were performed primarily for safety, in patients with diabetes, adopting the cutoff point to start the session, with values between 100 and 250 mg/dL.
2.9 Adverse events
The patients were monitored throughout the program for the occurrence of adverse events using a standardized form. This form has questions about the general well-being of the patient, as well as symptoms, pain, and other adverse events associated with physical exercise or not. In the IG, this information was collected weekly, while for the CG, this form was completed by video call every 6 weeks and at the end of the study period.
We classified the severity of adverse events as follows: catastrophic, for events resulting in death; major, for events causing permanent harm, including loss of function; moderate, for events resulting in semipermanent harm lasting from 3 days up to 1 year; and minor, for events causing no permanent harm and lasting <3 days (42).
2.10 Outcomes
All outcomes were evaluated before the start of the intervention and after 24 weeks.
2.10.1 Functional capacity
The primary outcome is the change in the distance covered in the 6 min walk test (6MWT), as it is the most representative outcome of the general functionality. The 6MWT was performed according to the American Thoracic Society (43) in a predetermined space of 30 m. Every 2 min, the values of HR, peripheral oxygen saturation (SpO2), and perception of central and peripheral exertion by the Borg CR10 scale, adapted by Foster et al. (40), were recorded. Two attempts were made with an interval of at least 15 min, and the longest distance covered was adopted.
The sit-to-stand (STS) test was performed according to the Rikli and Jones (44) battery, measuring the number of repetitions performed in 30 s. The timed up and go (TUG) test was performed in two attempts for each of the speeds, maximum (TUG-m) and usual (TUG-u), with a 1 min interval between each attempt, adopting the shortest time for each speed (45). Flexibility was evaluated by the sit and reach test (46), using the Wells bank as an instrument, adopting the highest value achieved in two attempts.
2.10.2 Persistent symptoms
Four different scales involving functional status were applied: Modified Medical Research Council (MMRC) (47), Chalder Fatigue Scale (38), Post-COVID-19 Functional Status (PCFS) (48), and Tilburg Frailty Indicator (49).
2.10.3 Body composition, phase angle (PhA), and anthropometry
Body composition was assessed using computerized densitometry by dual-energy x-ray absorptiometry (DXA) with a Hologic® instrument (Discovery Wi Fan-beam S/N 81593, Hologic, Inc., Bedford, MA, USA) calibrated and used following the manufacturer's recommendations. PhA measurement was evaluated using multifrequency octapolar bioimpedance (InBody® 720, Biospace, Los Angeles, CA, USA). The PhA was calculated using the following equation: PhA = arctan (Xc/R) × (180 /π) (50). To determine the body mass index (BMI in kg/m2), body mass was measured using the previously mentioned bioimpedance device, and height was measured using a stadiometer (Alturaexata®, with 1 mm precision). The waist was measured using a flexible and inelastic measuring tape (Cescorf®, with a precision of 1 mm), and the waist-to-height ratio (WHR) was determined.
All measurements were always taken in the morning after at least 4 h of fasting. All participants were instructed to avoid physical exercise the day before the assessment; to abstain from alcoholic, caffeinated, or diuretic drinks in the 48 h prior to assessment; and, in the case of women, to not be menstruating. The participants were also instructed to wear gym clothes without zippers or metal; to be barefoot; to remove earrings, rings, or any type of adornment; and to urinate 30 min before assessment. After the assessments, everyone received a standardized snack consisting of 25 g of whole-grain crackers and a banana. Further details can be found in Delevatti et al (35).
2.10.4 Physical activity and sedentary behavior
Physical activity and sedentary behavior were evaluated with GT3X+ accelerometers. The accelerometer was affixed to the right side of the hip using an elastic belt, and the participants were instructed to wear it continuously for 7 consecutive days. They were asked to remove the accelerometer only during sleep, showering, or activities involving water. Data were collected at a frequency of 30 H and analyzed in 60 s epochs. We interpreted consecutive values of zero (with a tolerance of 2 min) over ≥60 min. as a period of non-use and excluded them from the analysis (51). The data were only considered valid when the participant had used the accelerometer and had accumulated a minimum number of records over 4 days of use during the week, including one weekend day (10 h/day). The mean values at each PA intensity and mean SB were calculated using the cutoff points by Freedson et al. (52), considering SB as 0–99 counts/min, light physical activity as 100–1,951 counts/min, and moderate to vigorous physical activity as ≥1,952 counts/min, using the vertical axis. These cutoff points were applied for the adult population. The data were analyzed with the ActiLife software (Actigraph v.6.12.1). All data were analyzed as minutes/day to adjust for the number of days when the device was used.
2.10.5 Pulmonary function
Pulmonary function assessment was carried out following the guidelines established by the American Thoracic Society and the European Respiratory Society for the single-breath carbon monoxide uptake in the lung (53). The diffusion lung capacity for carbon monoxide (DLCO, mL/min/mmHg), alveolar volume (VA, L), and carbon monoxide transfer coefficient (KCO, mL/min/mmHg/L) were measured using the Vmax® system (VIASYS Respiratory Care Inc., USA). DLCO, VA, and KCO values were also expressed as percentages of the predicted values, according to Guimarães et al. (54).
2.11 Statistical analyses
Sample size was calculated a priori considering the distance covered in the 6MWT as the primary outcome based on the study of Liu et al. (55). This study showed an intragroup and intergroup change after the 50 m intervention in favor of the intervention group, which is the expected clinical difference in the study. The sample size calculation was conducted for a repeated measures ANOVA (within-between interaction), using the f effect size (value 0.35), a two-tailed α level of 0.05, a repeated measures correlation of 0.5, and a desired statistical power of 80% (β = 0.20). First, the effect size d was calculated using the means and standard deviations reported in Liu et al. (55). Then, this d value was converted into f for use in G*Power. The estimated required sample size was 22 individuals per group. However, accounting for a potential sample loss of 30%, the recruitment target was set at 30 participants per group.
The continuous sample characterization variables were tested for normality and homogeneity using the Shapiro–Wilk and Levene tests, respectively. Continuous variables are presented by mean and standard deviation or by median and interquartile range. The categorical variables characterizing the sample were presented by absolute (sampled n) and relative (%) frequencies. For comparison between groups, the independent t-test or its non-parametric corresponding test was used for continuous data and Fisher's exact test for categorical data.
Outcomes were presented by mean and standard error. Analysis by generalized estimation equations was used, adopting the Bonferroni post hoc test. The generalized estimating equation method with normal distribution, logarithmic link function, robust estimate of covariance matrix, and interdependent working correlation matrix structure was used to assess differences both between and within groups, using “group” and “time” as factors across all outcomes. Outcomes were analyzed by intention-to-treat (ITT) and per-protocol (PP) analyses. For the PP analysis, the patients who completed at least 70% of the proposed sessions in the IG and those who attended the 24-week evaluations in the CG were included. Participation was monitored through direct supervision. For ITT analysis, the pre-intervention values of all randomized patients and the post-intervention values of all patients assessed at that time were maintained. For missing post-intervention data, a maximum likelihood estimate was used for imputation. This method estimates missing data based on the distributions of observed data to predict the most likely values of the missing data. The adopted significance index was 0.05. Effect sizes (ES) were calculated from the mean difference between groups of changes between pre- and post-intervention (ΔIG − ΔCG), divided by the pooled baseline standard deviation, as described by Morris (56). Values were classified as small (0.20 ≤ d < 0.50), medium (0.50 ≤ d < 0.80), and large (d ≥ 0.80). The statistical analysis was carried out using Statistical Package for the Social Sciences (SPSS), version 22.0.
3 Results
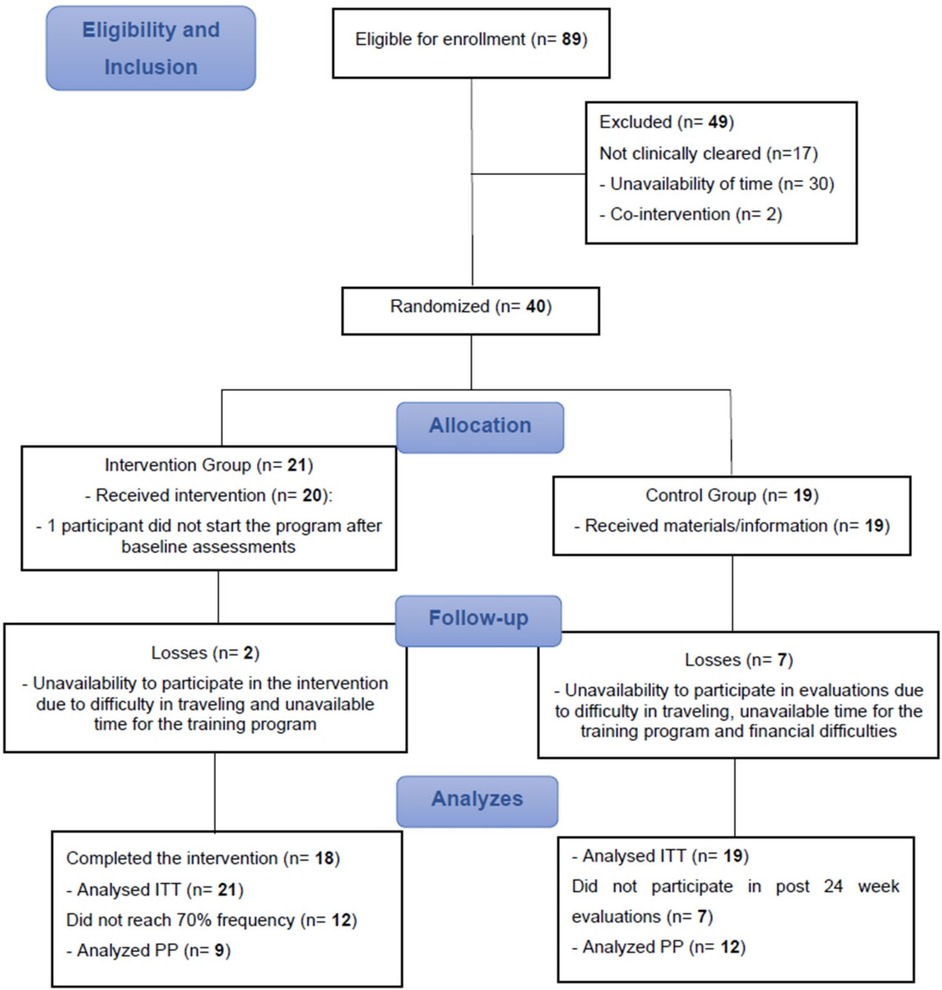
A total of 89 participants volunteered to take part in the study, of whom 49 did not meet the inclusion criteria or refused to participate after being duly informed of the objectives and procedures. Thus, 40 participants were randomized, 21 in the IG and 19 in the CG. During study follow-up, three participants withdrew from the IG, and five participants from the CG did not participate in the 24-week evaluations for personal reasons (Figure 2).
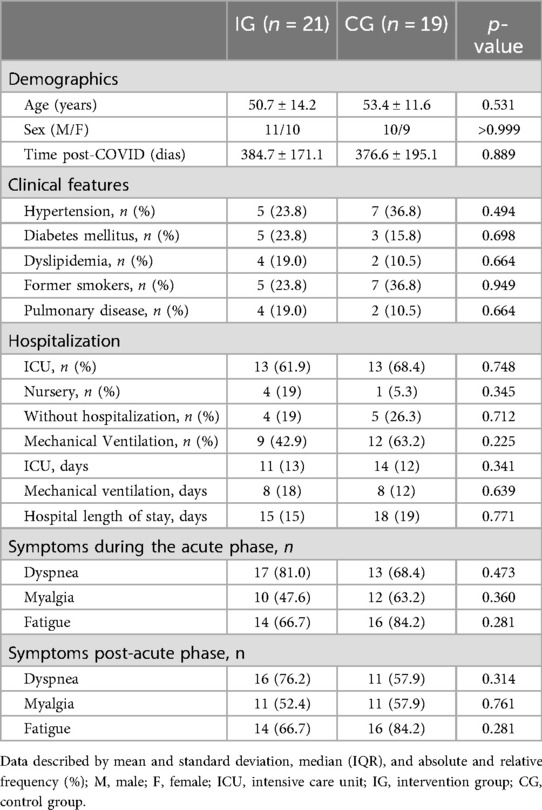
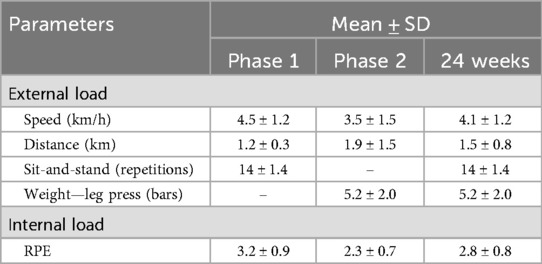
There were no between-group differences in sociodemographic data, presence of chronic diseases, types and periods of hospitalization, and symptoms. In general, of the 40 participants (21 male and 18 female), 31 (77.5%) underwent hospitalization in the acute phase of the disease, and 21 (52.50%) required mechanical ventilation. The most common symptom, both in the acute and the post-phase, was fatigue, reported by 75% of participants at both times (Table 1). The mean internal and external loads for each phase, as well as the overall 24-week period, are presented in Table 2.
3.1 Adherence to the exercise intervention
The mean adherence of the IG participants was 60.91% or 1.5 weekly sessions, with nine not reaching the 70% minimum frequency for the PP analysis. The adherence means of the IG participants who reached >70% session attendance and were included in the PP analysis was 82.38% or 2.1 sessions per week.
During the 24 weeks of the program, one participant experienced an acute myocardial infarction, but this event did not occur during or shortly after a training session. There were no serious adverse events related to the intervention.
3.2 Functional outcomes
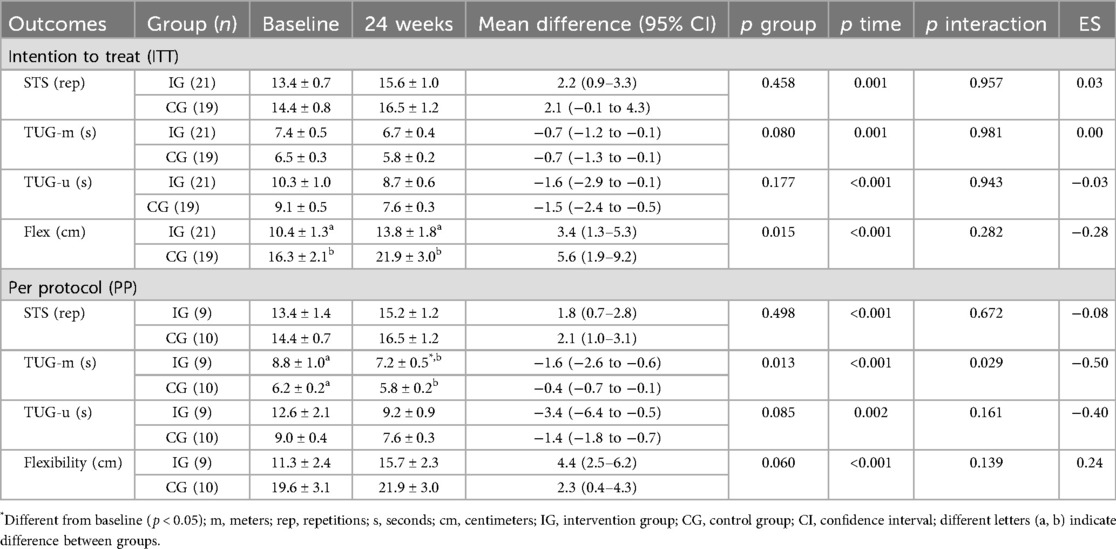
The primary outcome, distance covered in the 6MWT, improved in both groups in the ITT analysis; however, in the PP analysis, only the IG improved (ES: 0.61; 95% CI: 50.6–124.4; p = 0.001), with differences between groups at baseline showing higher values in the CG (Figure 3). The results of the secondary outcomes are presented in Table 3. Both groups improved STS, TUG-u, and flexibility test scores in both analyses (ITT and PP), with differences between groups in the flexibility test at baseline and post-intervention showing higher values in the CG for the ITT analysis. In the TUG-m, both groups showed improvements in the ITT analysis, and only the IG showed significant improvements (ES: −0.50; 95% CI: −2.6 to −0.6; p = 0.029) in PP analysis, with differences between groups at baseline showing higher values in the IG.
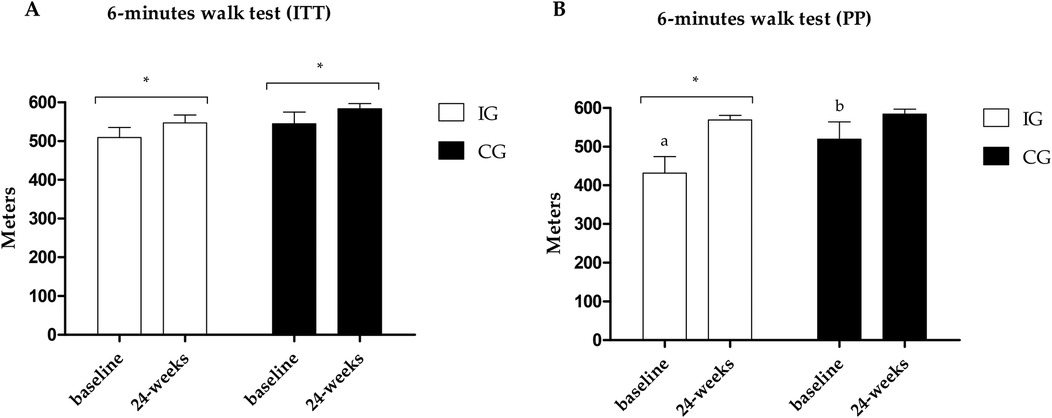
Figure 3. (A) Results of the 6MWT in the PP analysis. (B) Results of the 6MWT in the ITT analysis. *Different values between baseline and 24 weeks. Different letters indicate differences between groups at baseline.
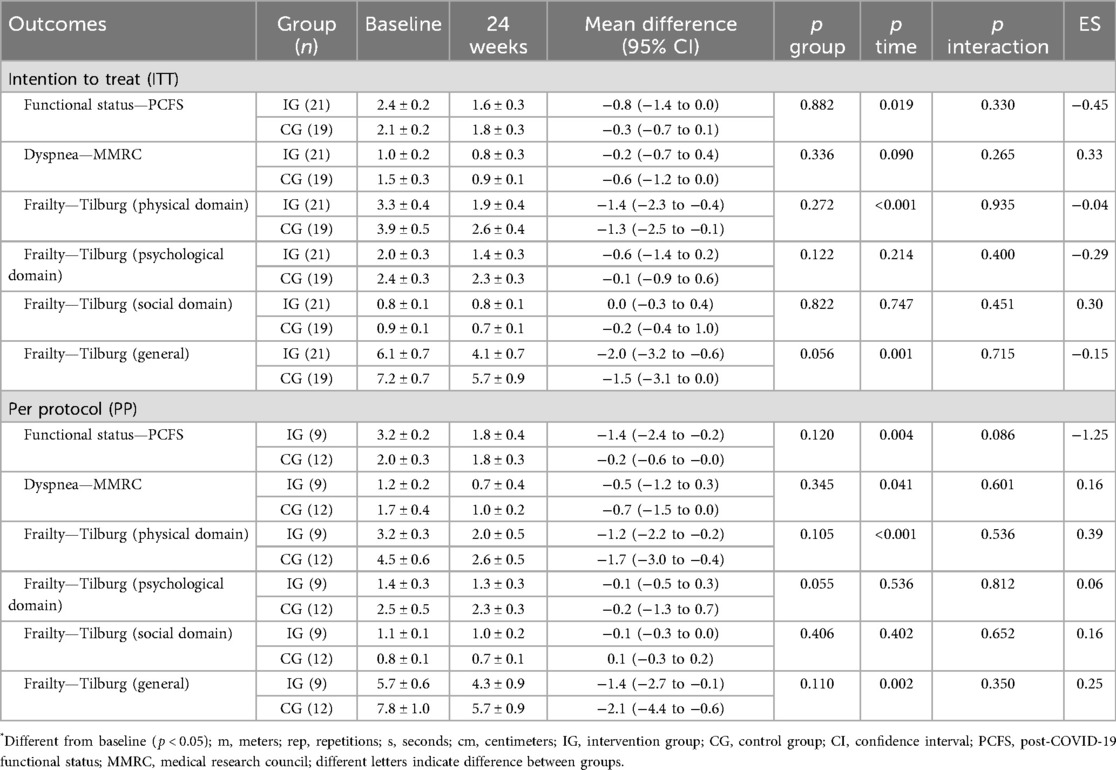
The symptoms related to functionality (PCFS, MMRC, and Tilburg scale) are presented in Table 4. The results on the PCFS and MMRC scales showed improvements in both groups in the PP analysis; however, in the ITT analysis, only PCFS showed improvements in both groups. The results in the Tilburg scale, physical and general domain, showed improvement in both groups for both analyses (ITT and PP).
Fatigue data are presented in Figures 4–6. Regarding the physical domain of the Chalder Fatigue Scale, both groups showed improvement in both the ITT and PP analyses. For the Chalder scale in the mental domain, both analyses showed improvement only in the IG (ITT: ES: −0.74; 95% CI: −0.5 to 3.5; p = 0.005/PP: ES: −0.63; 95% CI: −3.6 to 0.7; p = 0.017) with differences between groups at post-intervention showing better values in the IG. In the Chalder scale in the general domain, both analyses showed improvement only in the IG (ITT: ES: −0.70; 95% CI: −9.4 to −3.5; p = 0.026/PP: ES: −0.72; 95% CI: −11.0 to −1.6; p = 0.047) with differences between groups at post-intervention showing better values in the IG in the ITT analysis.
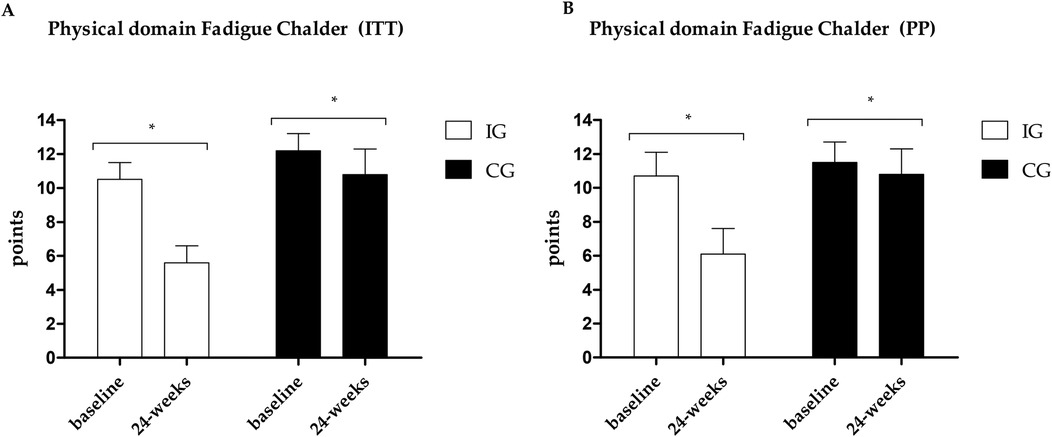
Figure 4. (A) Results of the Chalder scale—physical domain in the PP analysis. (B) Results of the Chalder scale—physical domain in the ITT analysis. *Different values between baseline and 24 weeks.
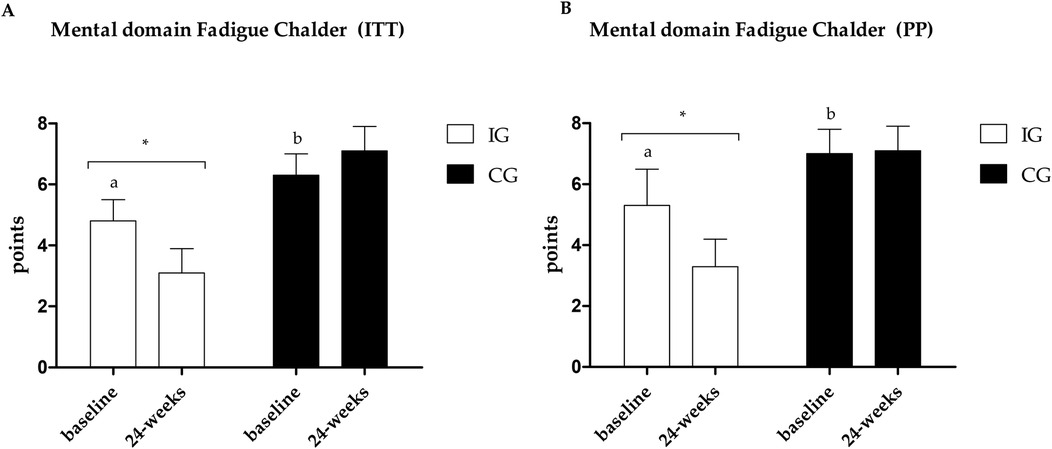
Figure 5. (A) Results of the Chalder scale—mental domain in the PP analysis. (B) Results of the Chalder scale—mental domain in the ITT analysis. *Different values between baseline and 24 weeks. Different letters indicate differences between groups at baseline.
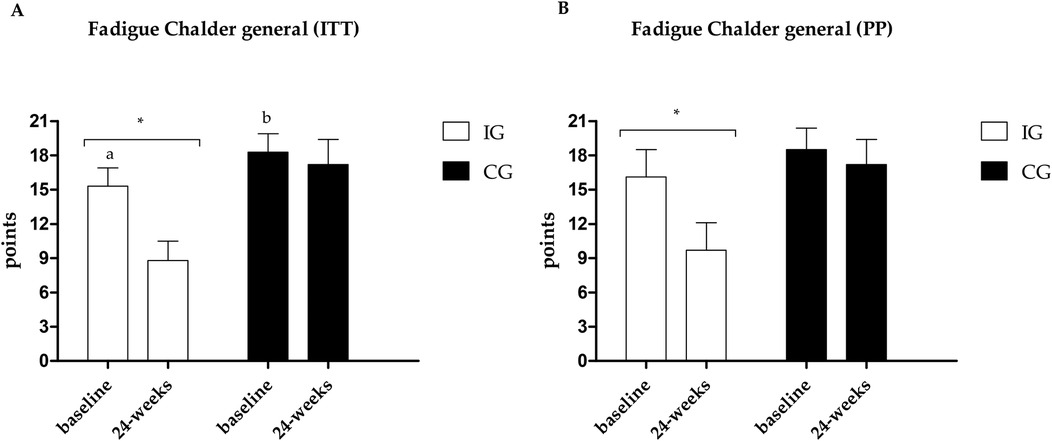
Figure 6. (A) Results of the Chalder scale—general fatigue in the PP analysis. (B) Results of the Chalder scale—general fatigue in the ITT analysis. *Different values between baseline and 24 weeks. Different letters indicate differences between groups at baseline.
3.3 Body composition outcomes
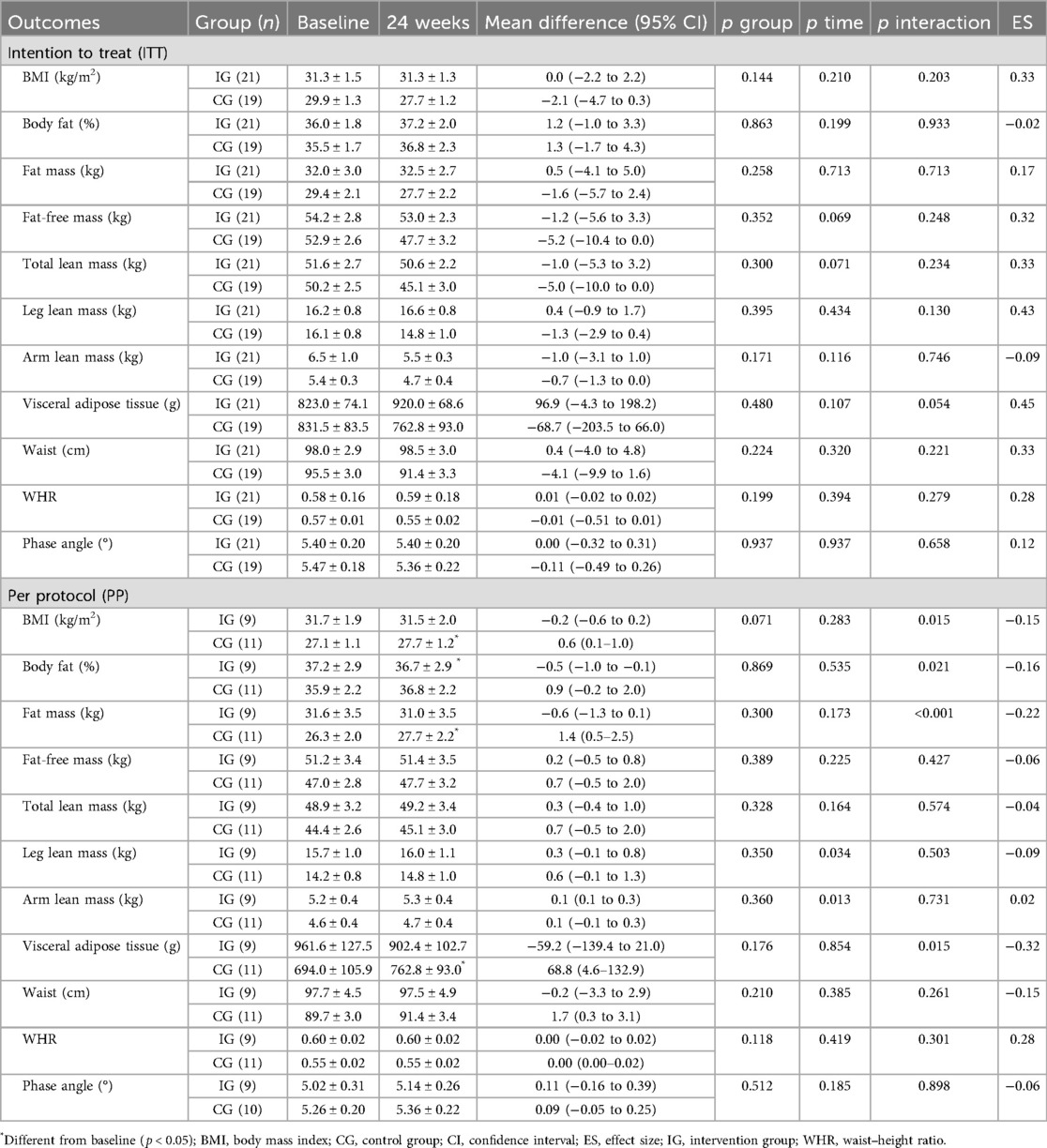
The results regarding anthropometric, body composition, and PhA outcomes are presented in Table 5. In the ITT analysis, no significant changes were identified (all p > 0.05).
The PP analysis identified that the CG increased the BMI (mean difference = 0.6 kg/m2; p = 0.018), fat mass (mean difference = 1.4 kg; p = 0.004), and the amount of visceral adipose tissue (mean difference = 68.8 g; p = 0.036). For the fat percentage, the IG showed a reduction (mean difference = −0.5%; p = 0.030). Both groups showed increased leg and arm lean mass in the PP analysis.
3.4 Physical activity and sedentary behavior
There were no significant differences between baseline and post-intervention in the minutes spent in light physical activity per week (IG: pre 2,056.2 ± 128.1; post 1,991.6 ± 164.0; CG: pre 2,303.9 ± 182.2; post 2,299.6 ± 174.9; p = 0.811) and moderate/vigorous physical activity (IG: pre 171.4 ± 26.5; post 152.3 ± 36.1; CG: pre 133.5 ± 28.1; post 112.5 ± 36.5; p = 0.970), nor in the minutes spent in sedentary time (IG: pre 4,646.2 ± 340.7; post 4,744.5 ± 286.6; CG: pre 4,089.8 ± 156.9; post 4,307.7 ± 204.4; p = 0.671).
3.6 Pulmonary function
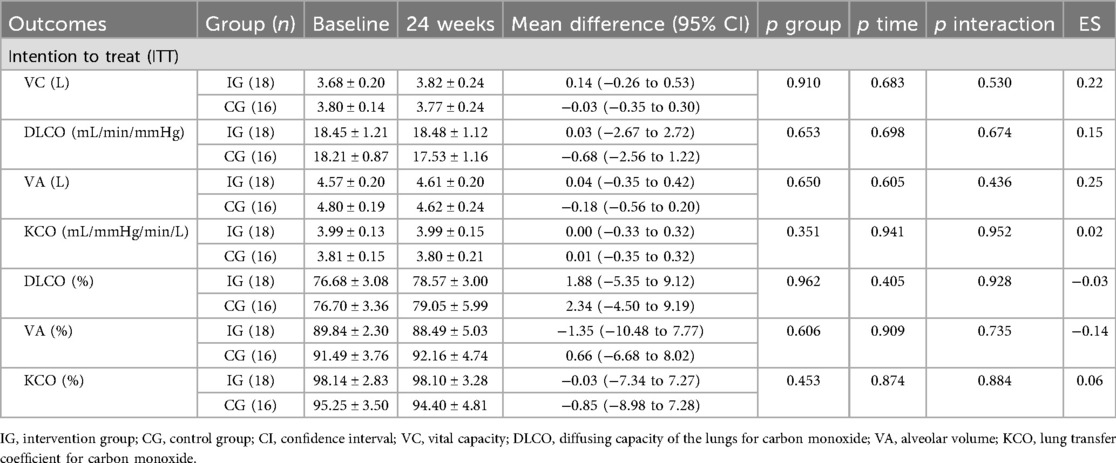
The pulmonary function results are summarized in Table 6. The ITT analysis revealed no significant differences between baseline and post-intervention values (all p > 0.05). The PP analysis could not be performed due to a substantial loss of participants in the post-intervention pulmonary function assessments.
4 Discussion
To the best of our knowledge, this is the first randomized controlled clinical trial to examine the effects of a supervised multicomponent training program, encompassing balance, aerobic, and strength exercises, lasting 24 weeks in critically debilitated patients with COVID-19. Our results indicate the non-superiority of the IG in the intention-to-treat analysis concerning the primary outcome; however, the IG demonstrated superiority in mental and general fatigue. In the per-protocol analysis, the IG showed superiority in several outcomes, including the primary outcome and body composition outcomes, demonstrating that high adherence to the program brought greater benefits to the participants. No significant differences were observed in physical activity levels or sedentary behavior and pulmonary function.
The primary outcome, 6MWT, showed an improvement in both groups in the ITT analysis (IG: 35 m; CG: 32 m), but in the PP analysis, only the IG had a significant change (IG: 88 m; CG: 16 m). Despite the difference between the groups at the baseline of the PP analysis, the moderate effect size (ES: 0.61) indicates superiority of the IG and significant clinical relevance of using multicomponent training in post-COVID rehabilitation. Our findings reinforce the extant literature of evidence indicating functional improvements, especially using the 6MWT, after different physical training models in the post-COVID-19 population (57–59).
In the context of the 6MWT, it's worth mentioning that the difference between pre- and post-intervention aligns with a study by McDonald et al. (59), which indicates an approximate 30 m difference as protective for various comorbidities. Particularly in the results of the PP analysis of the present study, an improvement of approximately 50 m is observed at the lower limit of the CI (95%).
The improvement found in the CG in functional capacity outcomes may possibly be explained by the cumulative effect of the natural course of post-illness recovery, even up to a year after infection. However, when we examined only the patients who adhered to the training in the IG, we found significant results in the 6MWT and TUG-m speed in the IG, with moderate effect sizes (0.61 and −0.51, respectively), demonstrating enhanced functional rehabilitation through the practice of supervised physical exercises. However, adherence to the intervention is required.
The improvement in the IG in TUG-m speed in PP analysis can be explained by some factors, such as the specific balance training carried out in Phase 1 of the program. The best values for this outcome may imply a better quality of life, as worse TUG scores are associated with a higher BMI, more comorbidities, and worse perception of physical health (60).
The positive results in post-COVID functional status (PCFS), mainly in PP analyses, with a large ES (1.25), reinforce the positive effects of multicomponent training in recovering the functional capacity of individuals after COVID-19 infection. In the MMRC, both groups showed improvements in the PP analysis. Dyspnea is one of the most reported persistent symptoms during and after COVID-19 infection (61).
In the Tilburg scale, improvements were found in the physical and general domain in both groups in the ITT and PP analysis, and even in the case of adults and elderly people (average age: 52.25 ± 13.00 years), both groups started participation in the study with values >5 in the general domain, indicating a state of fragility (62). Positively, after 24 weeks, in both analyses, the IG left the frailty zone, indicating scores <5, which did not occur in the CG, which continued in the frailty zone with scores >5 despite the improvement.
Fatigue is one of the most prevalent symptoms in acute post-COVID-19 or long-term COVID patients (8–10), and it's the most related symptom in the sample during the post-acute phase. An improvement was observed only in the IG in both analyses (ITT and PP) in the mental and general domains. In the physical domain, the ITT analysis shows improvement in both groups with a moderate ES between groups (ES: −0.74), and the PP analysis brings a large ES (ES: −0.89). Fatigue is associated with a decline in quality of life, a reduction in the ability to perform activities of daily living, and a reduction in the ability to produce maximum strength or power (63); therefore, reducing the levels of fatigue found can directly help in improving fatigue, quality of life, and functional capacity of individuals.
The evidence indicates that muscle damage resulting from mitochondrial changes, inflammation, and capillary injury in muscle biopsies could be one of the possible causes of post-COVID-19 (16–18), so we could expect the recovery of both to occur in parallel. However, we did not present results for body composition, possibly due to the multifactorial nature that fatigue presents in COVID-19 survivors, and especially due to the possible contribution of psychological factors that seem to have a great influence in longer periods after the acute phase of COVID-19 (64). Additionally, 1 year after hospital discharge, it was found that overweight and obese individuals admitted to the ICU had increased lean body mass, but 62% of these patients had fatigue (65).
In addition to the prior recovery of lean mass that could possibly have already occurred with the participants, according to the previously mentioned study, the low weekly training volume, an important factor for the hypertrophy process (66, 67), could possibly explain the lack of results, in general, in the ITT analysis for body composition parameters. When we adjusted the analysis to only individuals who participated in >70% of the training sessions, we could see some small changes, mainly regarding protection against increased body fat (ES: −0.22) and visceral adipose tissue (ES: −0.32), which becomes important as evidence shows that there is a trend toward an increase in body fat post-COVID-19 (68–70). It is also important to highlight that the CG did not follow a traditional control, as there was a meeting with exercise professionals in which the benefits of physical activity, the domains of physical activity, and recommendations for weekly practice were discussed, in addition to the delivery of two chapters of the Brazilian Guide of Physical Activity (39).
This study found no significant differences in lung function parameters between baseline and post-intervention in the ITT analysis. Notably, both groups presented DLCO mean values <80% of the predicted values at both assessments, reflecting persistent impairment in pulmonary diffusion capacity. These findings are consistent with prior systematic reviews and meta-analyses indicating that impaired DLCO is the most frequently observed abnormality in pulmonary function tests between 6 and 12 months after recovery from COVID-19 (71), with a reported prevalence of 43% (95% CI: 22%–65%) beyond 6 months post-hospital discharge (72). Unfortunately, the considerable loss of participants in post-intervention lung function assessments precluded the performance of the PP analyses, preventing a conclusive evaluation of the effects of supervised exercise on pulmonary outcomes.
As strengths of this study, it's worth noting that the program demonstrated safety, a currently debated aspect in randomized controlled trials of post-COVID-19 rehabilitation programs (33). The program proved to be well tolerated, with few sample losses (n = 8), and a reasonable average participation (60.9%). Despite the low number of individuals from the IG included in the per-protocol analysis (n = 9), the high adherence among these patients stands out, with a frequency of 81.61%. Additionally, a decrease in internal load and an increase in external load over time demonstrate a possible adaptation to training and improvement in exercise tolerance.
Another point is that there is no study to date that has reported outcomes resulting from a 24-week intervention. Furthermore, the program was administered approximately a year after participants contracted COVID-19, and most intervention studies took place over a period of no more than 6 months. Therefore, the positive changes observed, particularly in the per-protocol analysis, underscore the significance of continued efforts to improve functional outcomes and body composition even a year after infection.
The study presents some limitations, such as the limited sample size in the per-protocol analysis, where a significant number of participants did not reach the 70% session attendance frequency. To mitigate these limitations, in addition to monitoring attendance and frequently contacting absent participants, instructors offered the opportunity for makeup training sessions in Phase 1 of the study, as well as alternative scheduling options for sessions, in cases requested by participants.
As clinical applications, this study presents a low-cost and easy-to-apply training protocol that can be easily replicated in different scenarios. In addition, it differs from most investigations with the post-COVID-19 infection population, mainly in methodological rigor, both in clinical methodological aspects such as randomization, blinding, and control, and in the control of the training prescription that combined individualized prescriptions in three different physical qualities and requires a minimum use of equipment, in a frequency of just two weekly sessions.
5 Conclusion
We concluded that the 24-week multicomponent physical training is effective in reducing one of the most prevalent persistent symptoms in the post-COVID condition, fatigue and mental fatigue, in individuals infected with SARS-CoV-2. However, the results are not superior to the recommendations for structured physical activity in outcomes related to functional capacity, body composition, and physical activity levels among individuals who did not reach the 70% session attendance frequency.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Comitê de Ética em Pesquisa com Seres Humanos (CEPSH)/Universidade Federal de Santa Catarina. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AD: Formal analysis, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. MD: Data curation, Formal analysis, Investigation, Writing – original draft. CdS: Formal analysis, Investigation, Methodology, Writing – review & editing. MS: Investigation, Methodology, Supervision, Writing – review & editing. PdM: Investigation, Project administration, Supervision, Writing – review & editing. MC: Investigation, Project administration, Supervision, Writing – review & editing. FH: Formal analysis, Investigation, Supervision, Writing – review & editing. AG: Conceptualization, Investigation, Project administration, Supervision, Visualization, Writing – review & editing. CF: Investigation, Methodology, Project administration, Supervision, Visualization, Writing – review & editing. CR: Formal analysis, Investigation, Methodology, Writing – review & editing. FF: Investigation, Supervision, Formal analysis, Methodology, Writing – review & editing. RM: Data curation, Investigation, Project administration, Supervision, Writing – review & editing. RD: Conceptualization, Investigation, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Financial support was provided under the UNIEDU/FUMDES (Santa Catarina's University Scholarship Program), and the Coordination for the Improvement of Higher Education Personnel (CAPES) (Announcement Impacts of Pandemic #0057/2022).
Acknowledgments
The authors would like to thank the food company LeveCroc for providing the whole-grain crackers consumed by the participants. LeveCroc only contributed the biscuits provided to the participants after the assessments that required fasting beforehand, they were not involved in the study design, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence, and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. World Health Organization (WHO). Clinical Management of COVID-19: Living Guideline. Geneva: World Health Organization (2022).
2. Debeaumont D, Boujibar F, Ferrand-Devouge E, Artaud-Macari E, Tamion F, Gravier FE, et al. Cardiopulmonary exercise testing to assess persistent symptoms at 6 months in people with COVID-19 who survived hospitalization: a pilot study. Phys Ther. (2021) 101:pzab099. doi: 10.1093/ptj/pzab099
3. COVID-19 Rapid Guideline: Managing the Long-term Effects of COVID-19. London: National Institute for Health and Care Excellence (NICE) (2020). (NICE Guideline, N.188). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK567261/7
4. Welch C, Greig C, Masud T, Wilson D, Jackson TA. COVID-19 and acute sarcopenia. Aging Dis. (2020) 11(6):1345–51. doi: 10.14336/AD.2020.1014
5. de Moura PH, de Souza H, Brandão DC, Barros C, Correia M, Reinaux C, et al. Mapping peripheral and abdominal sarcopenia acquired in the acute phase of COVID-19 during 7 days of mechanical ventilation. Sci Rep. (2023) 13(1):29807. doi: 10.1038/s41598-023-29807-2
6. Andrade-Junior MCD, Salles ICDD, de Brito CMM, Pastore-Junior L, Righetti RF, Yamaguti WP. Skeletal muscle wasting and function impairment in intensive care patients with severe COVID-19. Front Physiol. (2021) 12:640973. doi: 10.3389/fphys.2021.640973
7. Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV. A clinical case definition of post-COVID-19 condition by a delphi consensus. Lancet Infect Dis. (2022) 22(4):e102–7. doi: 10.1016/S1473-3099(21)00703-9
8. Carfi A, Bernabe R, Landi F. Post-acute care study group. Persistent symptoms in patients after acute COVID-19. JAMA. (2020) 324(6):603–5. doi: 10.1001/jama.2020.12603
9. Halpin SJ, McIvor C, Whyatt G, Adams A, Harvey O, McLean L, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol. (2021) 93(2):1013–22. doi: 10.1002/jmv.26368
10. Kamal M, Abo Omirah M, Hussein A, Saeed H. Assessment and characterisation of post-COVID-19 manifestations. Int J Clin Pract. (2021) 75(3):e13746. doi: 10.1111/ijcp.13746
11. D'Cruz RF, Waller MD, Perrin F, Periselneris J, Norton S, Smith LJ, et al. Chest radiography is a poor predictor of respiratory symptoms and functional impairment in survivors of severe COVID-19 pneumonia. ERJ Open Res. (2021) 7(1):00096–2020. doi: 10.1183/23120541.00655-2020
12. Fernandez-de-Las-Penas C, Rodríguez-Jiménez J, Cancela-Cilleruelo I, Guerrero-Peral A, Martín-Guerrero JD, García-Azorín D, et al. Post–COVID-19 symptoms 2 years after SARS-CoV-2 infection among hospitalized vs nonhospitalized patients. JAMA Netw Open. (2022) 5(11):e2242106. doi: 10.1001/jamanetworkopen.2022.42106
13. Alkodaymi MS, Omrani OA, Ashraf N, Abou Shaar B, Almamlouk R, Riaz M, et al. Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: a systematic review and meta-analysis. Clin Microbiol Infect. (2022) 28(5):657–66. doi: 10.1016/j.cmi.2022.01.014
14. Zeng N, Zhao YM, Yan W, Li C, Lu QD, Liu L, et al. A systematic review and meta-analysis of long term physical and mental sequelae of COVID-19 pandemic: call for research priority and action. Mol Psychiatry. (2023) 28(1):423–33. doi: 10.1038/s41380-022-01614-7
15. Yang T, Yan MZ, Li X, Lau EH. Sequelae of COVID-19 among previously hospitalized patients up to 1 year after discharge: a systematic review and meta-analysis. Infection. (2022) 50(5):1067–109. doi: 10.1007/s15010-022-01862-3
16. Silva CC, Bichara CNC, Carneiro FRO, Palacios VRDCM, Berg AVSV, Quaresma JAS, et al. Muscle dysfunction in the long coronavirus disease 2019 syndrome: pathogenesis and clinical approach. Rev Med Virol. (2022) 32(6):e2355. doi: 10.1002/rmv.2355
17. Hejbøl EK, Harbo T, Agergaard J, Madsen LB, Pedersen TH, Østergaard LJ, et al. Myopathy as a cause of fatigue in long-term post-COVID-19 symptoms: evidence of skeletal muscle histopathology. Eur J Neurol. (2022) 29(9):2832–41. doi: 10.1111/ene.15435
18. Gil S, de Oliveira Junior GN, Sarti FM, Jacob Filho W, Longobardi I, Turri JAO, et al. Acute muscle mass loss predicts long-term fatigue, myalgia, and health care costs in COVID-19 survivors. J Am Med Dir Assoc. (2023) 24(1):10–6. doi: 10.1016/j.jamda.2022.11.013
19. Soares MN, Eggelbusch M, Naddaf E, Gerrits KH, van der Schaaf M, van den Borst B, et al. Skeletal muscle alterations in patients with acute COVID-19 and post-acute sequelae of COVID-19. J Cachexia Sarcopenia Muscle. (2022) 13(1):11–22. doi: 10.1002/jcsm.12896
20. Fuentes-Abolafio IJ, Stubbs B, Pérez-Belmonte LM, Bernal-López MR, Gómez-Huelgas R, Cuesta-Vargas AI. Physical functional performance and prognosis in patients with heart failure: a systematic review and meta-analysis. BMC Cardiovasc Disord. (2020) 20(1):512. doi: 10.1186/s12872-020-01725-5
21. Yang L, He Y, Li X. Physical function and all-cause mortality in patients with chronic kidney disease and end-stage renal disease: a systematic review and meta-analysis. Int Urol Nephrol. (2023) 55(5):1219–28. doi: 10.1007/s11255-022-03397-w
22. Wang DXM, Yao J, Zirek Y, Reijnierse EM, Maier AB. Muscle mass, strength, and physical performance predicting activities of daily living: a meta-analysis. J Cachexia Sarcopenia Muscle. (2020) 11(1):3–25. doi: 10.1002/jcsm.12502
23. Zhu Y, Wang Z, Zhou Y, Onoda K, Maruyama H, Hu C, et al. Summary of respiratory rehabilitation and physical therapy guidelines for patients with COVID-19 based on recommendations of world confederation for physical therapy and national association of physical therapy. J Phys Ther Sci. (2020) 32(8):545–9. doi: 10.1589/jpts.32.545
24. Grabowski DC, Maddox KE. Postacute care preparedness for COVID-19: thinking ahead. JAMA. (2020) 323(20):2007–8. doi: 10.1001/jama.2020.4686
25. Jimeno-Almazán A, Pallarés JG, Buendía-Romero Á, Martínez-Cava A, Franco-López F, Sánchez-Alcaraz Martínez BJ, et al. Post-COVID-19 syndrome and the potential benefits of exercise. Int J Environ Res Public Health. (2021) 18(10):5329. doi: 10.3390/ijerph18105329
26. Longobardi I, Goessler K, de Oliveira Júnior GN, do Prado DML, Santos JVP, Meletti MM, et al. Effects of a 16-week home-based exercise training programme on health-related quality of life, functional capacity, and persistent symptoms in survivors of severe/critical COVID-19: a randomised controlled trial. Br J Sports Med. (2023) 57(20):1295–303. doi: 10.1136/bjsports-2022-106681
27. Teixeira do Amaral V, Viana AP, Heubel AD, Linares SN, Martinelli B, Witzler PH, et al. Cardiovascular, respiratory, and functional effects of home-based exercise training after COVID-19 hospitalization. Med Sci Sports Exerc. (2022) 54(11):1795–803. doi: 10.1249/MSS.0000000000002977
28. Daynes E, Gerlis C, Chaplin E, Gardiner N, Singh SJ. Early experiences of rehabilitation for individuals post-COVID to improve fatigue, breathlessness, exercise capacity and cognition–a cohort study. Chronic Respir Dis. (2021) 18:14799731211015691. doi: 10.1177/14799731211015691
29. Imamura M, Mirisola AR, Ribeiro FDQ, De Pretto LR, Alfieri FM, Delgado VR, et al. Rehabilitation of patients after COVID-19 recovery: an experience at the physical and rehabilitation medicine institute and lucy montoro rehabilitation institute. Clinics. (2021) 76:e2804. doi: 10.6061/clinics/2021/e2804
30. Gonzalez-Gerez JJ, Saavedra-Hernandez M, Anarte-Lazo E, Bernal-Utrera C, Perez-Ale M, Rodriguez-Blanco C. Short-term effects of a respiratory telerehabilitation program in confined COVID-19 patients in the acute phase: a pilot study. Int J Environ Res Public Health. (2021) 18(14):7511. doi: 10.3390/ijerph18147511
31. Nambi G, Abdelbasset WK, Alrawaili SM, Elsayed SH, Verma A, Vellaiyan A, et al. Comparative effectiveness study of low versus high-intensity aerobic training with resistance training in community-dwelling older men with post-COVID-19 sarcopenia: a randomized controlled trial. Clin Rehabil. (2022) 36(1):59–68. doi: 10.1177/02692155211036956
32. Jimeno-Almazán A, Franco-López F, Buendía-Romero Á, Martínez-Cava A, Sánchez-Agar JA, Sánchez-Alcaraz Martínez BJ, et al. Rehabilitation for post-COVID-19 condition through a supervised exercise intervention: a randomized controlled trial. Scand J Med Sci Sports. (2022) 32(12):1791–801. doi: 10.1111/sms.14240
33. Pouliopoulou DV, Macdermid JC, Saunders E, Peters S, Brunton L, Miller E, et al. Rehabilitation interventions for physical capacity and quality of life in adults with post–COVID-19 condition: a systematic review and meta-analysis. JAMA Netw Open. (2023) 6(9):e2333838. doi: 10.1001/jamanetworkopen.2023.33838
34. do Amaral VT, Viana AA, Heubel AD, Linares SN, Martinelli B, Camprigher Witzler PH, et al. Cardiovascular, respiratory and functional effects of tele-supervised home-based exercise training in individuals recovering from COVID-19 hospitalization: a randomized clinical trial. medRxiv (2022) 2022.01. doi: 10.1101/2022.01.24.22269745
35. Delevatti RS, Danielevicz A, Sirydakis ME, de Melo PUG, de la Rocha Freitas C, Rech CR, et al. Effects of physical training on functional, clinical, morphological, behavioural and psychosocial outcomes in post-COVID-19 infection: cOVID-19 and REhabilitation study (CORE-study)—a study protocol for a randomised controlled clinical trial. Trials. (2023) 24(1):39. doi: 10.1186/s13063-022-07055-5
36. Deak AD, Alves CE, Costa CDF, Queiroz CN, Nader EM, Yoshinaga F, et al. CONSORT – Checklist Para Relatar Ensaios Clínicos. Estudantes Para Melhores Evidências. Cochrane (2022). Available online at: https://eme.cochrane.org/consort-checklist-para-relatar-um-ensaio-clinico/ (Accessed August 28, 2025).
38. Jackson C. The Chalder fatigue scale (CFQ 11). Occup Med. (2015) 65(1):86–86. doi: 10.1093/occmed/kqu168
39. Ministério da Saúde. Secretaria de Atenção Primária à Saúde. Departamento de Promoção da Saúde. Guia de Atividade Física para a População Brasileira [recurso Eletrônico]/Ministério da Saúde, Secretaria de Atenção Primária à Saúde, Departamento de Promoção da Saúde. Brasília: Ministério da Saúde (2021). p. 54.
40. Foster C, Florhaug JA, Franklin J, Gottschall L, Hrovatin LA, Parker S, et al. A new approach to monitoring exercise training. J Strength Cond Res. (2001) 15(1):109–15.11708692
41. Barroso BIDL, Souza MBCAD, Bregalda MM, Lancman S, Costa V. Worker health in COVID-19 times: reflections on health, safety, and occupational therapy. Cad Bras Ter Ocup. (2020) 28:1093–102. doi: 10.4322/2526-8910.ctoARF2091
42. World Alliance for Patient Safety. WHO draft Guidelines for Adverse Event Reporting and Learning Systems: From Information to Action. Geneva: World Health Organization (2005).
43. Crapo RO, Casaburi R, Coates AL, Enright PL, MacIntyre NR, McKay RT, et al. American Thoracic society ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. (2002) 166:111–7. doi: 10.1164/ajrccm.166.1.at1102
45. Podsiadlo D, Richardson S. The timed “up & go": a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. (1991) 39(2):142–8. doi: 10.1111/j.1532-5415.1991.tb01616.x
46. Wells KF, Dillon EK. The sit and reach—a test of back and leg flexibility. Res Q Exerc Sport. (1952) 23(1):115–8.
47. Launois C, Barbe C, Bertin E, Nardi J, Perotin JM, Dury S, et al. The modified medical research council scale for the assessment of dyspnea in daily living in obesity: a pilot study. BMC Pulm Med. (2012) 12:61. doi: 10.1186/1471-2466-12-61
48. Klok FA, Boon GJ, Barco S, Endres M, Geelhoed JM, Knauss S, et al. The post-COVID-19 functional Status scale: a tool to measure functional status over time after COVID-19. Eur Respir J. (2020) 56(1):2001494. doi: 10.1183/13993003.01494-2020
49. Gobbens RJ, van Assen MA, Luijkx KG, Wijnen-Sponselee MT, Schols JM. The tilburg frailty indicator: psychometric properties. J Am Med Dir Assoc. (2010) 11(5):344–55. doi: 10.1016/j.jamda.2009.11.003
50. Norman K, Stobäus N, Pirlich M, Bosy-Westphal A. Bioelectrical phase angle and impedance vector analysis–clinical relevance and applicability of impedance parameters. Clin Nutr. (2012) 31(6):854–61. doi: 10.1016/j.clnu.2012.05.008
51. Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc. (2011) 43(2):357–64. doi: 10.1249/MSS.0b013e3181ed61a3
52. Freedson P, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. (1998) 30:777–81. doi: 10.1097/00005768-199805000-00021
53. Graham BL, Brusasco V, Burgos F, Cooper BG, Jensen R, Kendrick A, et al. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J. (2017) 49(1):1600016. doi: 10.1183/13993003.00016-2016
54. Guimarães VP, de Miranda DM, Reis MAS, Andrade TL, Matos RL, Soares MR, et al. Valores de referência para a difusão do monóxido de carbono (fator de transferência) em uma amostra brasileira da raça branca. J Bras Pneumol. (2019) 45(5):e20180262. doi: 10.1590/1806-3713/e20180262
55. Liu K, Zhang W, Yang Y, Zhang J, Li Y, Chen Y. Respiratory rehabilitation in elderly patients with COVID-19: a randomized controlled study. Complement Ther Clin Pract. (2020) 39:101166. doi: 10.1016/j.ctcp.2020.101166
56. Morris SB. Estimating effect sizes from pretest-posttest-control group designs. Organ Res Methods. (2008) 11(2):364–86. doi: 10.1177/1094428106291059
57. Stavrou VT, Tourlakopoulos KN, Vavougios GD, Papayianni E, Kiribesi K, Maggoutas S, et al. Eight weeks unsupervised pulmonary rehabilitation in previously hospitalized patients with SARS-CoV-2 infection. J Pers Med. (2021) 11(8):806. doi: 10.3390/jpm11080806
58. Curci C, Negrini F, Ferrillo M, Bergonzi R, Bonacci E, Camozzi DM, et al. Functional outcome after inpatient rehabilitation in post-intensive care unit COVID-19 patients: findings and clinical implications from a real-practice retrospective study. Eur J Phys Rehabil Med. (2021) 57(3):443–50. doi: 10.23736/S1973-9087.20.06660-5
59. McDonald CM, Henricson EK, Abresch RT, Florence J, Eagle M, Gappmaier E, et al. The 6-minute walk test and other clinical endpoints in duchenne muscular dystrophy: reliability, concurrent validity, and minimal clinically important differences from a multicenter study. Muscle Nerve. (2013) 48(3):357–68. doi: 10.1002/mus.23905
60. Kear BM, Guck TP, McGaha AL. Timed up and go (TUG) test: normative reference values for ages 20 to 59 years and relationships with physical and mental health risk factors. J Prim Care Community Health. (2017) 8(1):9–13. doi: 10.1177/2150131916659282
61. Laviolette L, Laveneziana P, ERS research seminar faculty. Dyspnoea: a multidimensional and multidisciplinary approach. Eur Respir J. (2014) 43(6):1750–62. doi: 10.1183/09031936.00092613
62. Andreasen J, Lund H, Aadahl M, Gobbens RJ, Sorensen EE. Content validation of the tilburg frailty indicator from the perspective of frail elderly: a qualitative explorative study. Arch Gerontol Geriatr. (2015) 61(3):392–9. doi: 10.1016/j.archger.2015.08.017
63. Twomey R, Aboodarda SJ, Kruger R, Culos-Reed SN, Temesi J, Millet GY. Neuromuscular fatigue during exercise: methodological considerations, etiology and potential role in chronic fatigue. Neurophysiol Clin. (2017) 47(2):95–110. doi: 10.1016/j.neucli.2017.03.002
64. Poole-Wright K, Guennouni I, Sterry O, Evans RA, Gaughran F, Chalder T. Fatigue outcomes following COVID-19: a systematic review and meta-analysis. BMJ Open. (2023) 13(4):e063969. doi: 10.1136/bmjopen-2022-063969
65. Perli VAS, Sordi AF, Lemos MM, Fernandes JSA, Capucho VBN, Silva BF, et al. Body composition and cardiorespiratory fitness of overweight COVID-19 survivors in different severity degrees: a cohort study. Sci Rep. (2023) 13(1):17615. doi: 10.1038/s41598-023-44738-8
66. Schoenfeld BJ, Ogborn D, Krieger JW. Dose-response relationship between weekly resistance training volume and increases in muscle mass: a systematic review and meta-analysis. J Sports Sci. (2017) 35(11):1073–82. doi: 10.1080/02640414.2016.1210197
67. Figueiredo VC, de Salles BF, Trajano GS. Volume for muscle hypertrophy and health outcomes: the most effective variable in resistance training. Sports Med. (2018) 48(3):499–505. doi: 10.1007/s40279-017-0793-0
68. Di Filippo L, De Lorenzo R, D'Amico M, Sofia V, Roveri L, Mele R, et al. COVID-19 is associated with clinically significant weight loss and risk of malnutrition, independent of hospitalisation: a post-hoc analysis of a prospective cohort study. Clin Nutr. (2021) 40(4):2420–6. doi: 10.1016/j.clnu.2020.10.043
69. Joris M, Minguet P, Colson C, Joris J, Fadeur M, Minguet G, et al. Cardiopulmonary exercise testing in critically ill coronavirus disease 2019 survivors: evidence of a sustained exercise intolerance and hypermetabolism. Crit Care Explor. (2021) 3(7):e0491. doi: 10.1097/CCE.0000000000000491
70. Peball M, Rass V, Valent D, Beer R, Schiefecker AJ, Limmert V, et al. Body composition and physical performance 1 year after COVID-19. Am J Phys Med Rehabil. (2024) 103(2):124–33. doi: 10.1097/PHM.0000000000002314
71. Lee JH, Yim JJ, Park J. Pulmonary function and chest computed tomography abnormalities 6–12 months after recovery from COVID-19: a systematic review and meta-analysis. Respir Res. (2022) 23(1):233. doi: 10.1186/s12931-022-02163-x
Keywords: rehabilitation, physical exercise, COVID-19, physical capacity, SARS-CoV-2
Citation: Danielevicz A, Diesel M, dos Santos CES, Sirydakis MEdM, de Melo PUG, Constantini MI, Hansen F, Gerage AM, Freitas CdlR, Rech CR, Fonseca FR, Maurici R and Delevatti RS (2025) Effects of a 24-week multicomponent training program on functional capacity, persistent symptoms, body composition, and physical activity in patients significantly affected by COVID-19: the COVID-19 and REhabilitation study (CORE-study)—randomized clinical trial. Front. Sports Act. Living 7:1549132. doi: 10.3389/fspor.2025.1549132
Received: 20 December 2024; Accepted: 26 August 2025;
Published: 22 September 2025.
Edited by:
Christina May Moran de Brito, Hospital Sirio Libanes, BrazilReviewed by:
Renato Fraga Righetti, Hospital Sirio Libanes, BrazilElnara Marcia Negri, University of São Paulo, Brazil
Jesús González-Moreno, Universitat de València, Spain
Copyright: © 2025 Danielevicz, Diesel, dos Santos, Sirydakis, de Melo, Constantini, Hansen, Gerage, Freitas, Rech, Fonseca, Maurici and Delevatti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Angelica Danielevicz, YW5nZWxpY2FfZGFuaWVsZXZpY3pAaG90bWFpbC5jb20=
 Angelica Danielevicz
Angelica Danielevicz Mabel Diesel
Mabel Diesel Carla Elane Silva dos Santos
Carla Elane Silva dos Santos Maria Eduarda de Moraes Sirydakis1
Maria Eduarda de Moraes Sirydakis1 Aline Mendes Gerage
Aline Mendes Gerage Cintia de la Rocha Freitas
Cintia de la Rocha Freitas Rodrigo Sudatti Delevatti
Rodrigo Sudatti Delevatti