- 1Institute of Radiology, University Hospital Erlangen, Erlangen, Germany
- 2Faculty of Health, Medical and Life Sciences, University of Furtwangen, Schwenningen, Germany
- 3Department of Radiology, Chobanian & Avedisian School of Medicine, Boston University, Boston, MA, United States
Many people with osteoarthritis of the knee suffer from overweight, obesity, and cardiometabolic conditions. In the present subanalysis of a randomized controlled trial of the effect of whole-body electromyostimulation (WB-EMS) on knee osteoarthritis in overweight Caucasians, we focus on participants with Metabolic Syndrome (MetS). Based on previous research, we hypothesized that WB-EMS significantly improves the Metabolic Syndrome Z score (MetS-Z score) compared with non-training controls. Thirty-two of the initial 72 overweight adults (58 ± 6 years, body mass index: 31 ± 4 kg/m2) with knee osteoarthritis, randomly allocated to a 29-week standard WB-EMS application or to a non-exercising control group (CG) and suffering from MetS, were included. The primary outcome was the MetS-Z score, based on the criteria of the International Diabetes Federation. Secondary outcomes were MetS components, i.e., waist circumference, mean arterial blood pressure, fasting glucose, triglycerides, and HDL-cholesterol. Based on the intention-to-treat principle, analysis of covariance determines differences between the groups (i.e., “effects”). In total, three participants were lost to 29-week follow-up. The attendance rate averaged 89% ± 9% in the WB-EMS group. Adverse effects related to the intervention were not observed. WB-EMS (n = 17) induced a non-significant, medium-size effect (p = 0.061; η2 = 0.13) on the MetS-Z score compared with non-exercise CG (n = 15). In addition, no significant effects (p ≥ 0.146) were observed for MetS components. In the present study, we observed a moderate, although non-significant effect on the MetS-Z score. Given that the WB-EMS application was well-tolerated and accepted by the participants, we conclude that this exercise technology may offer (limited) benefits for MetS treatment. Nevertheless, further studies should address this issue with higher statistical power.
Introduction
There is consensus that physical activity and exercise positively impact cardiometabolic conditions (1). However, due to musculoskeletal and/or cardiovascular diseases, many people are unable or limited in their ability to conduct exercise with intensity levels adequate to address health-related outcomes (2). People with osteoarthritis (OA) might be such a cohort. Considering the close association of knee OA and overweight/obesity not only due to higher mechanical load but also to the proinflammatory effects of increased visceral adipose tissue (VAT) fraction (3), people with knee OA are at increased risk of cardiometabolic diseases (4, 5). Because of its time efficiency and joint friendliness, whole-body electromyostimulation (WB-EMS) is a safe and attractive training technology for people with limited options for conventional exercise (6). Although there is some evidence that WB-EMS provides similar positive changes on cardiometabolic outcomes as high-intensity resistance exercise (7), only a few studies have focused on WB-EMS effects on cardiometabolic outcomes (8). Recently, a systematic review and meta-analysis summarized the effect of WB-EMS on Metabolic Syndrome (MetS) (9). Although the authors observed a small but significant effect and reported low heterogeneity in trial results, cohorts and WB-EMS protocols of the individual studies vary considerably. This includes (superimposed) WB-EMS protocols not or not adequately suitable for people with OA of the lower extremities. This led us to determine the effects of WB-EMS on MetS in overweight to obese people with OA of the knee. Our primary hypothesis was that standard WB-EMS application (8) significantly improves MetS as summarized in a continuous Z score, which combined individual values and cut-offs for the five MetS components (10), compared with a non-training control group (CG) with usual (OA) care in people with knee OA and the MetS according to the International Diabetes Federation (IDF) (11).
Material and methods
Study design
The present study is part of the randomized controlled “electromyostimulation for the treatment of knee osteoarthritis (OA) (EMSOAT) study” (12), a WB-EMS trial that focuses on overweight and obese adults. However, in the present analysis, we focus on WB-EMS effects on MetS. EMSOAT was planned, initiated, and conducted by the Institute of Radiology, University Hospital Erlangen, Germany. The study was approved by the University Ethics Committee (Nr. 352_20 B) and was conducted in full adherence to the Helsinki Declaration (13). After detailed information, all study participants provided written approval. The project was registered under ClinicalTrials.gov (NCT05672264).
Participants
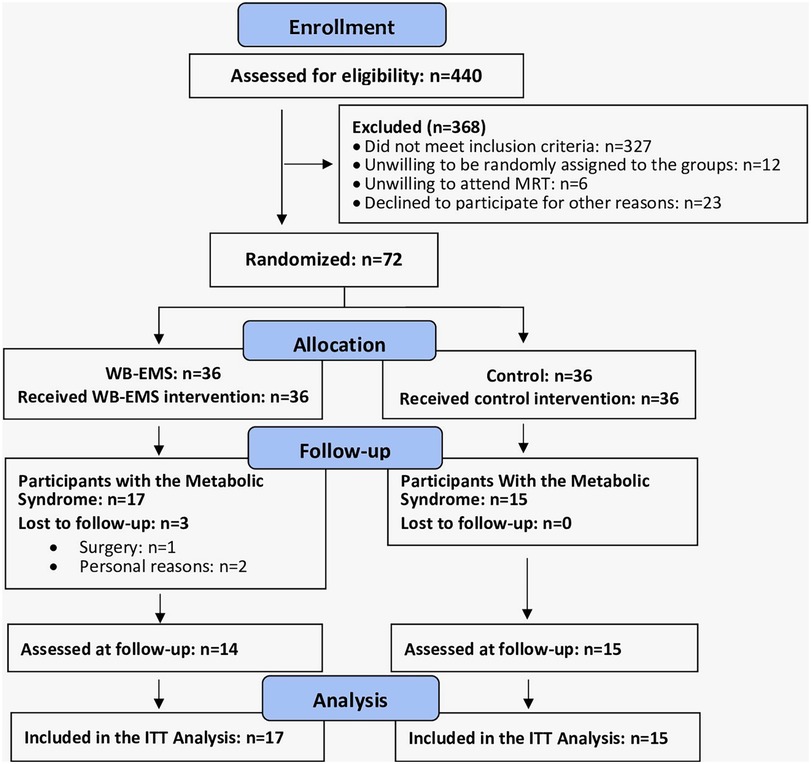
The recruitment process of EMSOAT was described in detail in another publication (12). Briefly, participants were included by the study physician if they met the following inclusion criteria (Figure 1): (a) age 40–70 years, (b) overweight/obesity [body mass index (BMI) > 25 kg/m2], (c) knee OA Kellgren–Lawrence grades 2 and 3. Exclusion criteria were (a) WB-EMS or resistance exercise ≥60 min/week in the last 12 months; (b) glucocorticoid or opioid therapy; (c) trauma of the knee joint; (d) intra-articular knee injections in the last 12 weeks; (e) conditions, diseases, and corresponding therapy with relevant impact on our study outcomes (e.g., rheumatoid arthritis, fibromyalgia); and (f) contraindications for WB-EMS (14). In summary, a total of 72 participants were eligible and willing to participate and were randomly allocated to the WB-EMS (n = 36) and CG (n = 36). After adding the eligibility criteria of MetS according to the IDF (11) for this cohort, 17 participants of the WB-EMS and 15 participants of the CG were included in the present analysis.
Randomization and blinding
Using the envelope method, lots were placed in small opaque capsules (“kinder egg”, Ferrero, Italy) and drawn from a bowl by the participants. A researcher not involved in the present project prepared the lots and supervised the procedure to realize allocation concealment. After the randomization procedure, the primary investigator (SK) enrolled participants and instructed them in detail about their study status and corresponding dos and don’ts. Outcome assessors were blinded to the participants’ group status (WB-EMS or CG) and were not allowed to ask.
Study procedures
The WB-EMS group conducted the procedure described below, while the CG received a “usual care” intervention (physiotherapy) that can be considered ineffective in the context of the present research issue. In addition, both groups were asked to conduct a self-management education program for knee OA. Of importance, adherence to the WB-EMS protocol was closely monitored by the trainers. In parallel, adherence to physiotherapy was evident from the billing of physiotherapy practices.
WB-EMS intervention
Briefly, we applied a consistently and closely supervised (one trainer—two trainees for each session) WB-EMS protocol (15) of 1.5 × 20 min/week (i.e., three sessions in 2 weeks) for 29 weeks. Both thighs and upper arms, gluteal, abdomen, chest, lower back, latissimus, and upper back were simultaneously stimulated by a miha bodytec®, Type II WB-EMS device (Gersthofen, Germany). We applied bipolar current with 85 Hz, 350 µs impulse width, and a direct impulse boost using an interval protocol with a 6-s impulse and 4-s impulse break. Impulse intensity was prescribed at “6-7” (i.e., “hard + to very hard”) on the Borg CR10 Scale. During the impulse phase, low-amplitude, low-intensity movements [e.g., low-amplitude squat with latissimus pulleys, butterfly reverse, straight pullovers with trunk flexion, one-legged stand (extended knee) with biceps curl] (16) were performed in a standing position.1 This protocol can be considered the standard protocol predominately applied in research and commercial facilities (8).
Control intervention (physiotherapy)
Based on the underlying research topic of the EMSOAT project (knee OA) and in accordance with the German S2-guideline on knee OA, the CG underwent six physiotherapy sessions. To ensure that all participants of the CG received this “usual care” standard treatment, participants received a prescription for six physiotherapy treatment sessions (20 min each), prescribed by the study physician. The six sessions were carried out once a week during the first 2–3 months of the study. The treatment was carried out individually in a diagnosis-oriented manner. In summary, techniques to reduce pain and detonate the muscle tissue were applied. Further, in many cases, the physiotherapists aimed to improve knee joint mobility and leg muscle strength. Overall, the physiotherapeutic intervention of the EMSOAT project can largely be regarded as a non-training control group in the context of the present research question, particularly due to its non-specific content.
Self-management education program for knee OA
A self-management program of osteoarthritis (17) with six sessions of 60 min each was applied for the WB-EMS and CG. Briefly, the program aimed to provide education, information, and counseling to prevent the progression of OA, reduce fear and avoidance attitudes, and thus improve the participants’ quality of life and mobility.
Study outcomes
As described above, the EMSOAT study primarily concentrated on assessing outcomes linked to knee osteoarthritis. However, in this article, we focus on the MetS-Z score (10) based on the IDF-criteria (11), which includes waist circumference (WC), mean arterial blood pressure (MAP), fasting glucose (FPG), triglycerides (TG), and HDL-cholesterol (HDL-C) as components.
Metabolic syndrome Z score (MetS-Z score)
• Changes in the MetS-Z score from baseline and 29-week follow-up (FU).
Metabolic syndrome components
• Changes in waist circumference between baseline and 29-week FU.
• Changes in MAP between baseline and 29-week FU.
• Changes in FPG between baseline and 29-week FU.
• Changes in triglycerides between baseline and 29-week FU.
• Changes in HDL-cholesterol between baseline and 29-week FU.
Assessments
Metabolic syndrome-Z score
We calculated a Z score using individual subject data, IDF MetS cut-off criteria (11), and standard deviations (SD; denominators of each factor in the formula) of the given (either men or women) cohort at baseline.
For the female participants, the MetS-Z score was calculated [(50 − HDL-C)/SD − HDL-C] + [(Triglycerides − 150)/SD − TGs] + [(fasting glucose − 100)/SD − FPG] + [(waist circumference − 88)/SD − WC] + [(mean arterial blood pressure − 100)/SD − MAP].
The corresponding MetS-Z score for the male participants was calculated [(40 − HDL-C)/SD − HDL-C] + [(triglycerides − 150)/SD − TGs] + [(fasting glucose − 100)/SD − FPG] + [(waist circumference − 80)/SD − WC] + [(mean arterial blood pressure − 100)/SD − MAP].
Metabolic syndrome components
Waist circumference
Waist circumference was determined in a standing position as the minimum circumference between the distal end of the rib cage and the top of the iliac crest along the midaxillary line at the end of a normal expiration.
Mean arterial blood pressure
After 10 min of relaxation, blood pressure was determined twice on the right arm with a rest of 20 s between the samples and in a sitting position. MAP was calculated using the formula (diastolic blood pressure + diastolic blood pressure + systolic blood pressure)/3.
Glucose, triglycerides, and HDL-C
After an overnight fast, blood was consistently sampled between 7:00 and 9:00 am in a sitting position from an antecubital vein. Serum samples were centrifuged for 20 min at 3,000 RPM and immediately analyzed. All biomarkers were measured in standard clinical laboratories.
Baseline characteristics and confounding factors
Body height was measured using a Holtain stadiometer (Crymych Dyfed, UK) and body mass and body composition were determined by performing direct-segmental, multifrequency bioimpedance analysis (DSM-BIA, InBody 770, Seoul, Korea).
A standardized questionnaire gathered information on (a) demographic factors; (b) physical limitations, comorbidities, history of operations, pharmacological treatment, and dietary supplements; as well as (c) lifestyle aspects such as physical activity, exercise, and diet. After 29 weeks of intervention, participants were asked to complete the FU questionnaire that aimed to identify changes in conditions/diseases, pharmacologic and physical therapy, and exercise and diet, i.e., factors that could potentially affect the present study outcomes. Questionnaires were checked by the study physician together with the participant at 29-week FU to verify potential changes in confounding factors.
Adverse effects of the interventions
Adverse effects (AEs) were defined as any untoward medical occurrence, disease or injury, or any untoward clinical signs, including an abnormal laboratory finding. In detail, AE monitoring focused predominately on the WB-EMS intervention and was conducted by WB-EMS trainers on a weekly basis. Apart from personal interviews, we determined inflammatory markers (e.g., ultrasensitive C-reactive protein, interleukin-1β) at baseline and 29-week FU (18).
Sample size calculation
Sample size calculation [analysis of covariance (ANCOVA)] was based on the primary outcome of the EMSOAT project “pain of the knee joint” not addressed in the present contribution.
Statistical analysis
We applied the intention-to-treat (ITT) principle with multiple imputation (R Development Core Team, Vienna, Austria) together with Amelia II (19) and analyzed all the participants who were randomly assigned to the study groups at the start of the study. Linear mixed-effects ANOVA was applied to analyze the effects of continuous within- and between-subjects variables. Pearson chi-square tests were used to analyze categorical variables (Table 1). Partial eta squared (η2) were used to indicate standardized effects sizes. All tests were two-tailed, and significance was accepted at p < 0.05.
Results
Three participants of the WB-EMS group were lost to follow-up. One participant was unable to visit the 29-week follow-up assessment due to surgery, and two participants of the WB-EMS group quit the study for personal reasons not related to the intervention (Figure 1). The attendance rate averaged 89% ± 9% in the WB-EMS group and more than 90% in the CG. No unintended adverse effects or injuries related to the WB-EMS application were observed, reported by the participants, or determined by laboratory findings (18).
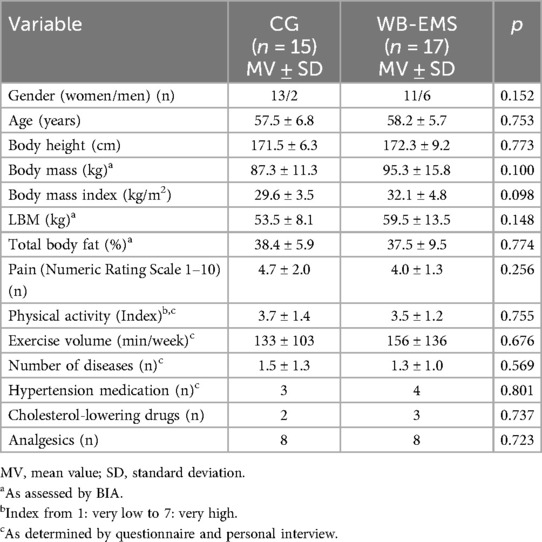
Table 1 displays baseline results of the study cohort. In summary, no significant differences were observed between the WB-EMS and the CG at baseline. Nevertheless, differences for BMI and lean body mass (LBM) were quite pronounced, while body fat (%) was comparable between the groups. Apart from seven to five participants, respectively, who took medication for hypertension or hypercholesterinemia (Table 1), no further pharmacologic therapy (e.g., GLP-1 RA) that might have relevantly affected the present outcome was reported.
Study outcomes
Table 2 shows the results for the MetS-Z score and its components at baseline along with changes after 29 weeks of intervention. Although the change in MetS-Z score did not reach statistical significance (p = 0.061), the effect size was moderate (η2 = 0.13), suggesting a potential positive trend. No significant between-group differences for prepost changes within the WB-EMS vs. CG were observed for the MetS components (Table 2). Further, significant pre- vs. postintervention changes within the groups were not observed for any of the study outcomes listed in Table 2.
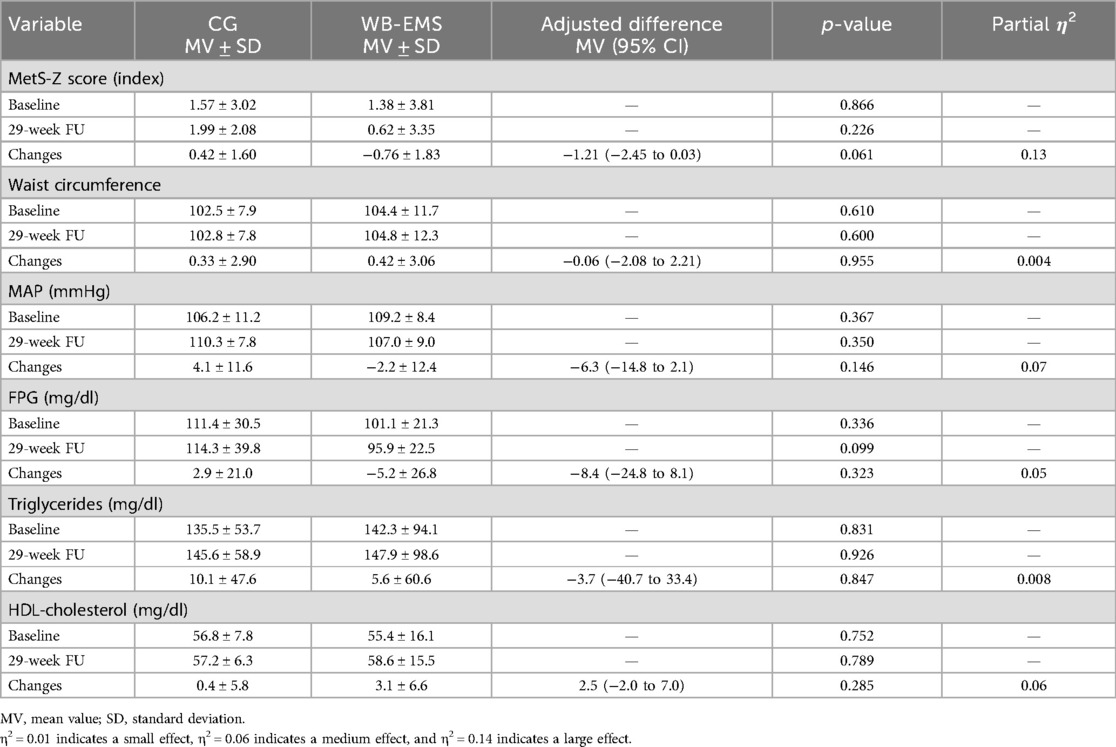
Table 2. Baseline data and changes of the MetS-Z score and its components in the CG (n = 15) and WB-EMS group (n = 17) with corresponding between-group differences (linear mixed-effects ANOVA model).
Confounding factors
No significant changes within or between the groups were recorded for physical activity and exercise during the study period. Changes in dietary habits with lower carbohydrates/sugar and/or energy intake were reported by two participants of the WB-EMS and CG each, respectively. Antihypertensive medication was discontinued by one participant of the WB-EMS group, whereas changes in medication that focus on lipoproteins or blood lipids were not reported. In parallel, no conditions (e.g., eating disorders) or diseases (e.g., thyroid function) with a potential impact on the outcomes addressed here were recorded.
Discussion
The present study addresses WB-EMS-induced effects on MetS and its components in a cohort of overweight to obese people with knee OA and MetS according to IDF (11). In summary, we have to reject our hypothesis of significant positive effects of a standard WB-EMS protocol (8) vs. a non-training CG on the MetS-Z score that summarized MetS components to a continuous score more sensitive to metabolic changes compared with the dichotomous MetS Index used for clinical purposes (10). We observed a non-significant WB-EMS effect of moderate size for the main study outcome MetS-Z score. Significant effects or group changes were not determined for any of the five MetS components. The limited benefit on the MetS-Z score and individual MetS components along with the finding that only one participant of the WB-EMS group lost his MetS status,2 however, raises the question of the clinical relevance of our results. So far, we estimate the clinical relevance of WB-EMS as an exercise therapy to fight the MetS in overweight to obese people suffering from the MetS to be low to moderate. On the other hand, potentially more effective types of training (e.g., weight-bearing endurance exercises) are often not applicable for people with knee OA. Furthermore, the current WB-EMS approach can be considered safe, well-tolerated, and (as proved by a high attendance rate) attractive for people with MetS.
A review of the literature may provide further insight into the clinical relevance of WB-EMS in combating the MetS. In a recent meta-analysis, Guretzki et al. (9) reported low but significant effects (d` = 0.33, p = 0.013) of WB-EMS on the MetS-Z score; however, the five available trials (7, 20–23) differ considerably with respect to cohort and WB-EMS application, thus preventing a comprehensive recommendation of WB-EMS to fight the MetS. Only two trials focus on people with established MetS (21, 22). In their 12-week pilot study with obese women 18 years and older with the MetS according to NCEP-ATP II3 criteria (24), Reljic et al. (21) reported significant MetS-Z score reductions in their WB-EMS group (2 × 20 min/week, n = 15) but no significant effects vs. their non-training CG (n = 14). In parallel, the same research group (22) reported no significant effects after 12 weeks of WB-EMS (2 × 20 min/week) in their obese MetS (NCEP-ATP III) patients (n = 20) undergoing caloric restriction, compared with corresponding non-exercise controls (n = 22). In contrast, Wittmann et al. (23), who applied WB-EMS (1 × 20 min/week) and protein supplementation for 6 months in older women with sarcopenic obesity (n = 25), observed significant effects on the MetS (NCEP-ATP III) compared with non-intervention control. However, no significant effect on the MetS-Z score was determined for the isolated WB-EMS intervention (23). The two remaining WB-EMS studies either applied superimposed WB-EMS [i.e., high-intensity interval training (HIIT) superimposed by WB-EMS] for 12 weeks (20)4 or compared their 16-week WB-EMS intervention with a similar time-effective high-intensity resistance exercise training (HIT-RT) (7)5 and were thus unable to determine the proper effect of (isolated) WB-EMS on MetS. To overcome these limitations of superimposed WB-EMS application (20), exercising control (7, 20), short intervention (20–22), or non-affected cohorts (7, 20, 23), the present study compared a standard (i.e., non-superimposed) 29-week WB-EMS protocol with a non-training CG with minor physical intervention in the relevant cohort of people with the MetS.
Although predominately cohorts with low affinity to conventional exercise training were attracted due to the specific character of WB-EMS and thus a comparison of WB-EMS effects on MetS with other types of exercise might be moot, we would like to shortly address this issue. Of note, in its present application with low training volume (1-2 × 20 min week) and intermittent bouts (4–6 s) of moderate to high stimulus intensity, WB-EMS can be considered a resistance-type exercise (8). As stated above, one study that compared WB-EMS with a single set resistance exercise training to muscular failure applying intensifying [i.e., HIT-RT (25)] and similarly time-effective strategies compared with WB-EMS (30 vs. 20 min) revealed non-significantly more favorable results on the MetS-Z score in favor of the WB-EMS intervention. Addressing endurance-type exercise, in contrast to older data (26), a more recent network meta-analysis (27) reported more favorable MetS changes after resistance compared with endurance exercise. In summary, the authors suggested combined resistance and endurance exercise programs as the most effective intervention for improving MetS and cardiovascular risk factors. WB-EMS has been applied by a few intervention studies as an endurance-type exercise, i.e., with consistent or longer impulse phases and lower impulse intensity superimposed by running or cycling exercise (20, 28). However, as already mentioned, superimposed (HIIT) endurance and WB-EMS were not superior to isolated HIIT protocols, and thus the combination of conventional endurance training and WB-EMS sessions might be a more feasible concept for addressing the MetS.
Limitations and particularities of the study
While the results of the present study provide limited support for the application of WB-EMS in people with MetS, some limitations of the present trial should be considered by the reader. The present study reported the results of a subanalysis of a larger trial with overweight to obese people with knee OA. Thus, the sample size analysis does not focus on the present study outcome and randomization did not cover the present MetS cohort. Applying the data of the meta-analysis of Guretzki et al. (9) mentioned above, the present study would be underpowered. However considering the pronounced effect-lowering methodological limitations of most of the included studies,6 we simply expect higher effects and lower variations from the present study that compared WB-EMS vs. non-training control (see below).
We applied a standard WB-EMS protocol predominately applied in the nearly 2,000 commercial WB-EMS providers in Germany (8). Briefly, this includes the application of low-volume, non-superimposed, and consistently supervised WB-EMS with low impulse frequency (85 Hz) and an intermittent impulse protocol.
The EMSOAT project implemented a “usual care” (physiotherapy) CG that focused on a few physiotherapy sessions addressing OA during the first 3 months of the 29-week intervention. Considering the low-volume, intensity, and in particular the non-specificity of this intervention in the present context of MetS, the CG can be widely considered a “non-training” control group. Another issue that frequently arises is whether the movement component of WB-EMS should not (also) be performed by a (control) group with the identical movements without EMS application. This should be indeed the case in superimposed WB-EMS protocols; however, in the present study, only slight movements (not exercises) were applied during the impulse phase of the WB-EMS application, which per se relevantly affected (at least) musculoskeletal outcomes or biomarkers (29).
We applied the MetS-Z-score for several reasons. First of all, for the assessment of an intervention effect, a continuous score is more sensitive compared with the dichotomous categorization used for clinical purposes. Weighing the changes in each component based on their variance from healthy norms enables us to effectively monitor and predict shifts in cardiometabolic health status. In addition, individual components of MetS interact in complex ways. Addressing one component may, therefore, result in improvements across multiple factors, which cumulatively leads to a greater shift in the Z score.
One may argue that changes in cardiometabolic medications might have impacted our result. However, only one participant of the WB-EMS group reported termination of antihypertensive medication, while other changes of medication related to cardiometabolic conditions were not reported. Since in parallel no relevant changes of lifestyle, including exercise habits and diet, or upcoming conditions or diseases associated with the MetS were listed, we feel that it is justified to predominately attribute the favorable effect on the MetS to the WB-EMS intervention. Nevertheless, a more rigorous tracking of confounding factors including medication, physical activity, and diet should be applied in future studies.
Because of the limited sample size of the study, the analysis was not stratified for gender or age. However, a raw comparison did not indicate relevant sex or age effects. Nevertheless, it is difficult to generalize our results to other cohorts.
Conclusion
In summary, we conclude that WB-EMS may be a viable option for those unable to engage in conventional exercise, although significant effects on cardiometabolic outcomes and more specifically, the MetS, should not necessarily be expected.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics statement
The studies involving humans were approved by Friedrich-Alexander-University Erlangen-Nürnberg, Ethics Committee (Nr. 352_20 B). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
LM: Conceptualization, Data curation, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. SK: Conceptualization, Supervision, Writing – original draft, Writing – review & editing, Resources, Validation. SvS: Conceptualization, Resources, Supervision, Writing – original draft, Writing – review & editing, Data curation, Investigation, Project administration. MK: Writing – original draft, Writing – review & editing, Formal Analysis, Methodology, Validation. FR: Methodology, Writing – original draft, Writing – review & editing, Conceptualization, Funding acquisition, Project administration. MU: Conceptualization, Funding acquisition, Project administration, Writing – original draft, Writing – review & editing, Resources. WK: Conceptualization, Funding acquisition, Project administration, Resources, Writing – original draft, Writing – review & editing, Data curation, Formal Analysis, Investigation, Methodology, Software, Supervision, Validation, Visualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was funded by the non-profit organization “Else Kröner-Fresenius-Stiftung” (2021_EKSE.22).
Acknowledgements
The present work was performed in (partial) fulfillment of the requirements for obtaining the degree “Dr. med dent.” for the first author (Leon Mendel).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. However, this had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1. ^It is important to note that these adjuvant movements per se are not intended to generate musculoskeletal or cardiometabolic effects (i.e. non-superimposed concept of WB-EMS).
2. ^However, due to the situation that only participants with the MetS were included, negative changes were not possible.
3. ^NCEP-ATP II: National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III).
4. ^When comparing the HIIT+WB-EMS (n = 19) with the isolated HIIT group (n = 18) to determine the isolated effect of WB-EMS, no significant effects were observed.
5. ^The authors reported non-significantly more favorable effects (p = 0.096) on the MetS-Z score (IDF-based) for the WB-EMS (n = 23) compared with the HIT-RT group (n = 23).
6. ^This refers in particular to the comparisons with exercising control groups.
References
1. Belanger MJ, Rao P, Robbins JM. Exercise, physical activity, and cardiometabolic health: pathophysiologic insights. Cardiol Rev. (2022) 30:134–44. doi: 10.1097/CRD.0000000000000417
2. Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. (2011) 43:1334–59. doi: 10.1249/MSS.0b013e318213fefb
3. Kawai T, Autieri MV, Scalia R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am J Physiol Cell Physiol. (2021) 320:C375–91. doi: 10.1152/ajpcell.00379.2020
4. Park D, Park YM, Ko SH, Choi YH, Min DU, Ahn JH, et al. Association between knee osteoarthritis and the risk of cardiovascular disease and the synergistic adverse effects of lack of exercise. Sci Rep. (2023) 13:2777. doi: 10.1038/s41598-023-29581-1
5. Piva SR, Susko AM, Khoja SS, Josbeno DA, Fitzgerald GK, Toledo FG. Links between osteoarthritis and diabetes: implications for management from a physical activity perspective. Clin Geriatr Med. (2015) 31:67–87, viii. doi: 10.1016/j.cger.2014.08.019
6. Kemmler W, Kleinoder H, Fröhlich M. Editorial: whole-body electromyostimulation: a training technology to improve health and performance in humans? Front Physiol. (2020) 11:523. doi: 10.3389/fphys.2020.00523
7. Kemmler W, Kohl M, von Stengel S. Effects of high intensity resistance training versus whole-body electromyostimulation on cardiometabolic risk factors in untrained middle aged males. A randomized controlled trial. J Sports Res. (2016) 3:44–55. doi: 10.18488/journal.90/2016.3.2/90.2.44.55
8. Kemmler W, Fröhlich M, Eifler C. Whole-Body Electromyostimulation. Effects, Limitations, Perspectives of an Innovative Training Method. Cham, Switzerland: Springer (2024).
9. Guretzki E, Kohl M, von Stengel S, Uder M, Kemmler W. Effects on whole-body electromyostimulation on the metabolic syndrome in adults at moderate to high cardiometabolic risk—a brief review and meta-analysis. Sensors. (2024) 24:6788. doi: 10.3390/s24216788
10. Johnson JL, Slentz CA, Houmard JA, Samsa GP, Duscha BD, Aiken LB, et al. Exercise training amount and intensity effects on metabolic syndrome (from studies of a targeted risk reduction intervention through defined exercise). Am J Cardiol. (2007) 100:1759–66. doi: 10.1016/j.amjcard.2007.07.027
11. Alberti KG, Zimmet P, Shaw J. Metabolic syndrome—a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med. (2006) 23:469–80. doi: 10.1111/j.1464-5491.2006.01858.x
12. Kast S, Kemmler W, Roemer FW, Kohl M, Culvenor AG, Mobasheri A, et al. Effectiveness of whole-body electromyostimulation on knee pain and physical function in knee osteoarthritis: a randomized controlled trial. Sci Rep. (2024) 14:20804. doi: 10.1038/s41598-024-71552-7
13. WHO. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. (2013) 310:291–4. doi: 10.1001/jama.2013.281053
14. Kemmler W, Weissenfels A, Willert S, Fröhlich M, Ludwig O, Berger J, et al. Recommended contraindications for the use of non-medical WB-electromyostimulation. Dtsch Z Sportmed. (2019) 70:278–81. doi: 10.5960/dzsm.2019.401
15. Kemmler W, Fröhlich M, Ludwig O, Eifler C, von Stengel S, Willert S, et al. Position statement and updated international guideline for safe and effective whole-body electromyostimulation training-the need for common sense in WB-EMS application. Front Physiol. (2023) 14:1174103. doi: 10.3389/fphys.2023.1174103
16. Weissenfels A, Teschler M, Willert S, Hettchen M, Fröhlich M, Kleinöder H, et al. Effects of whole-body electromyostimulation on chronic nonspecific low back pain in adults: a randomized controlled study. J Pain Res. (2018) 11:1949–57. doi: 10.2147/JPR.S164904
17. Nelson AE, Allen KD, Golightly YM, Goode AP, Jordan JM. A systematic review of recommendations and guidelines for the management of osteoarthritis: the chronic osteoarthritis management initiative of the U.S. Bone and Joint Initiative. Semin Arthritis Rheum. (2014) 43:701–12. doi: 10.1016/j.semarthrit.2013.11.012
18. Kelmendi B, Kast S, von Stengel S, Kohl M, Roemer F, Uder M, et al. The effect of whole-body electromyostimulation on inflammatory biomarkers and adipokines in overweight to obese adults with knee osteoarthritis: a randomized controlled study. Dtsch Z Sportmed. (2024) 75:57–63. doi: 10.5960/dzsm.2024.591
19. Honaker J, King G, Blackwell M. Amelia II: a program for missing data. JSS. (2011) 45:1–47. doi: 10.18637/jss.v045.i07
20. Amaro-Gahete FJ, De-la OA, Jurado-Fasoli L, Martinez-Tellez B, Ruiz JR, Castillo MJ. Exercise training as a treatment for cardiometabolic risk in sedentary adults: are physical activity guidelines the best way to improve cardiometabolic health? The FIT-AGEING randomized controlled trial. J Clin Med. (2019) 8(12):2097. doi: 10.3390/jcm8122097
21. Reljic D, Konturek PC, Herrmann HJ, Neurath MF, Zopf Y. Effects of whole-body electromyostimulation exercise and caloric restriction on cardiometabolic risk profile and muscle strength in obese women with the metabolic syndrome: a pilot study. J Physiol Pharmacol. (2020) 71:89–98. doi: 10.26402/jpp.2020.1.08
22. Reljic D, Dieterich W, Herrmann HJ, Neurath MF, Zopf Y. “HIIT the inflammation”: comparative effects of low-volume interval training and resistance exercises on inflammatory indices in obese metabolic syndrome patients undergoing caloric restriction. Nutrients. (2002) 14: 1996. doi: 10.3390/nu14101996
23. Wittmann K, Sieber C, von Stengel S, Kohl M, Freiberger E, Jakob F, et al. Impact of whole body electromyostimulation on cardiometabolic risk factors in older women with sarcopenic obesity: the randomized controlled FORMOsA-sarcopenic obesity study. Clin Interv Aging. (2016) 11:1697–706. doi: 10.2147/CIA.S116430
24. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. (2001) 285:2486–97. doi: 10.1001/jama.285.19.2486
26. Wewege MA, Thom JM, Rye KA, Parmenter BJ. Aerobic, resistance or combined training: a systematic review and meta-analysis of exercise to reduce cardiovascular risk in adults with metabolic syndrome. Atherosclerosis. (2018) 274:162–71. doi: 10.1016/j.atherosclerosis.2018.05.002
27. Liang M, Pan Y, Zhong T, Zeng Y, Cheng ASK. Effects of aerobic, resistance, and combined exercise on metabolic syndrome parameters and cardiovascular risk factors: a systematic review and network meta-analysis. Rev Cardiovasc Med. (2021) 22:1523–33. doi: 10.31083/j.rcm2204156
28. Amaro-Gahete FJ, De-la OA, Sanchez-Delgado G, Robles-Gonzalez L, Jurado-Fasoli L, Ruiz JR, et al. Whole-body electromyostimulation improves performance-related parameters in runners. Front Physiol. (2018) 9:1576. doi: 10.3389/fphys.2018.01576
29. Kemmler W, von Stengel S. Muskuläre Belastung unterschiedlicher Ganzkörper-Elektromyostimulations-(WB-EMS) Protokolle—eine Crossover-Untersuchung mit vortrainierten Personen ohne WB-EMS Erfahrung [Muscular effects of different whole-body electromyostimulation (WB-EMS) protocols—a crossover study with pre-trained individuals without WB-EMS experience]. Physikalische Medizin. (2020) 30:146–54. doi: 10.1055/a-1019-7894
Keywords: metabolic syndrome, overweight, obesity, whole-body electromyostimulation, cardiometabolic risk factors
Citation: Mendel L, Kast S, von Stengel S, Kohl M, Roemer FW, Uder M and Kemmler W (2025) The effect of whole-body electromyostimulation on metabolic syndrome in affected adults: A subanalysis of a randomized controlled trial. Front. Sports Act. Living 7:1585579. doi: 10.3389/fspor.2025.1585579
Received: 28 February 2025; Accepted: 6 June 2025;
Published: 1 July 2025.
Edited by:
Jacob Allen Goldsmith, United States Department of Veterans Affairs, United StatesReviewed by:
Christopher Matthew Cirnigliaro, United States Department of Veterans Affairs, United StatesShane J. T. Balthazaar, University of Birmingham, United Kingdom
Tommy Sutor, United States Department of Veterans Affairs, United States
Copyright: © 2025 Mendel, Kast, von Stengel, Kohl, Roemer, Uder and Kemmler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wolfgang Kemmler, d29sZmdhbmcua2VtbWxlckBmYXUuZGU=
†ORCID:
Wolfgang Kemmler
orcid.org/0000-0003-3515-0669
 Leon Mendel1
Leon Mendel1 Matthias Kohl
Matthias Kohl Frank W. Roemer
Frank W. Roemer Wolfgang Kemmler
Wolfgang Kemmler