- 1Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, United States
- 2Department of Medicine, Harvard Medical School, Boston, MA, United States
- 3Department of Biomolecular Sciences, School of Pharmaceutical Sciences of Ribeirão Preto, University of São Paulo, São Paulo, Brazil
- 4Joslin Diabetes Center, Boston, MA, United States
- 5Genitourinary Oncology Program, Beth Israel Deaconess Medical Center, Boston, MA, United States
- 6Department of Imaging, Dana-Farber Cancer Institute, Boston, MA, United States
- 7Department of Radiology, Brigham and Women’s Hospital, Boston, MA, United States
- 8Department of Biology, Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA, United States
Introduction: The incidence of metastatic prostate cancer (mPCa) is increasing despite a decrease in the prevalence of prostate cancer (PCa). Androgen deprivation therapy (ADT), the mainstay of systemic treatment for mPCa, is associated with numerous side effects, including a decline in muscle mass and physical function, which lead to the exacerbation of age-related frailty and sarcopenia. Exercise plays a key role in ameliorating or preventing the progression of ADT-related side effects and in improving muscle mass, fitness, and strength. However, exercise interventions in patients with mPCa have been understudied, with a lack of studies focusing on frailty and sarcopenia and the mechanisms by which exercise could address these issues.
Purpose: Thus, we have designed the FIERCE trial to assess the effects of a 16-week multicomponent exercise intervention, encompassing resistance and aerobic training, on frailty and sarcopenic status and their potential mechanistic biomarkers, as well as on cancer cell proliferation (NCT06040125).
Methods: The FIERCE trial is a prospective study aiming to recruit 80 pre-frail/frail men with mPCa receiving ADT who will be randomized to either an exercise or an attention control group. The 16-week exercise intervention will include thrice-weekly, clinic-supervised, resistance exercise circuit training and self-directed home-based aerobic exercise. The attention control group will receive a stretching program and will be offered the exercise program following the study period. The primary outcome is frailty, measured by the Fried frailty phenotype (i.e., muscle loss, exhaustion, physical activity, gait speed, and strength) and frailty-associated biomarkers [IL-6, TNF-α, C-reactive protein (CRP)]. Secondary outcomes include sarcopenia, measured using dual-energy x-ray absorptiometry scans and sarcopenia-associated muscle biopsy-driven biomarkers (myokines and insulin pathway markers). An exploratory outcome will assess how exercising patient-derived plasma will affect the proliferation of prostate cancer cells (LNCaP cells).
Conclusion: This first-of-its-kind study targets a vulnerable, understudied population: frail men with mPCa. If successful, our findings will establish the efficacy of a multicomponent exercise intervention on frailty and sarcopenic status, providing the foundation for future larger phase II and III trials to confirm the findings and potentially establish exercise as a safe and necessary part of the standard of care for frail metastatic prostate cancer patients.
1 Introduction
Androgen deprivation therapy (ADT), the mainstay systemic treatment for men with recurrent and/or metastatic prostate cancer (mPCa), works by depriving the body of hormones such as testosterone. Given the critical role testosterone plays in activating lipolysis and promoting lean mass hypertrophy, substantial body composition changes and loss of muscle strength and physical function can occur leading to the development or exacerbation of geriatric syndromes such as frailty and sarcopenia (1). Frailty, a comprehensive loss of functional reserve in multiple physiological systems, is a common clinical concern found in 40%–90% of men with PCa on ADT and is associated with a worse prognosis (2–5). Specifically, severely frail men showed an 86% higher risk of all-cause mortality and a 44% increased risk of cancer-specific mortality compared with their non-frail counterparts (4). Similarly, sarcopenia, characterized by a decrease in muscle mass, strength, and function, occurs in >80% of men with mPCa on ADT (6–8) and is an independent risk factor of cancer-specific mortality (9). An intervention that can address frailty and sarcopenia, alongside persisting ADT-related concerns, is critical to improve the clinical care of this population.
Aerobic and resistance training is a recommended complementary therapy for PCa patients and has been shown to prevent further deterioration of ADT-related adverse effects, such as skeletal muscle mass loss, fat mass gain, and reduced cardiorespiratory fitness and muscular strength (10–13). In addition, the inclusion of functional movements (e.g., balance) can maximize the beneficial effects of exercise on physical function, muscular strength, muscular endurance, and body composition (14–19). Within the context of frailty and sarcopenia, a multicomponent exercise intervention is recommended, given the multifaceted exercise stimulus that is required of an intervention to target the various characteristics of both syndromes (20). Nevertheless, men with mPCa are largely underrepresented in previous exercise oncology research, and no studies have specifically focused on frailty and sarcopenia in this population (20–23). Thus, a rigorously designed randomized controlled trial is warranted, which examines an exercise prescription specifically targeting the frailty–sarcopenia–ADT axis in frail men with mPCa.
Understanding whether exercise prevents or reverses frailty and sarcopenia, as well as the mechanisms implicated, is critical for improving health and quality of life in men with mPCa on ADT, yet the mechanisms by which exercise improves frailty and sarcopenia, particularly in the context of ADT, are unknown (7). Proposed mechanisms are improvements in systemic inflammatory and skeletal muscle tissue-related outcomes (7). Thus, we will investigate systemic inflammatory biomarkers associated with frailty [IL-6, TNF-α, C-reactive protein (CRP)] and muscle tissue-related outcomes associated with sarcopenia (myokine release and activation of insulin pathway-related biomarkers) to aid in refining explanations surrounding the frailty–sarcopenia–ADT axis with exercise (5, 24–29). In addition, exploring whether the effects of exercise are associated with biochemical cancer progression will provide insight into understanding the roles of exercise in long-term oncologic outcomes in the context of mPCa.
The overall objective of the FIERCE trial is to evaluate the efficacy of a 16-week multicomponent exercise intervention encompassing supervised, clinic-based circuit training utilizing resistance exercises and self-directed aerobic exercise on frailty and sarcopenia with an exploration of novel biomarkers at the intersection of frailty, sarcopenia, and disease progression. The primary outcome of this study is change in frailty score, measured by the Fried frailty phenotype (i.e., muscle loss, exhaustion, physical activity, gait speed, and strength) and frailty-associated biomarkers (IL-6, TNF-α, CRP). Secondary outcomes include change in sarcopenia status, measured using a computerized tomography (CT) scan and sarcopenia-associated muscle biopsy-driven biomarkers (myokines and insulin pathway markers). An exploratory outcome will assess how plasma from patients undergoing the exercise intervention affects prostate cancer cell proliferation (LNCaP cells). We hypothesize that the exercise group, compared with the attention control group, will exhibit the following: (1) a lower proportion of frail and pre-frail scores and improved individual scores of each frailty component; (2) improvements in systemic inflammatory biomarkers; (3) improved sarcopenia status; (4) increased gene and protein expression of muscle biopsy-driven anti-inflammatory myokines and insulin pathways; and (5) suppressed biochemical progression of PCa.
2 Methods
2.1 Study design
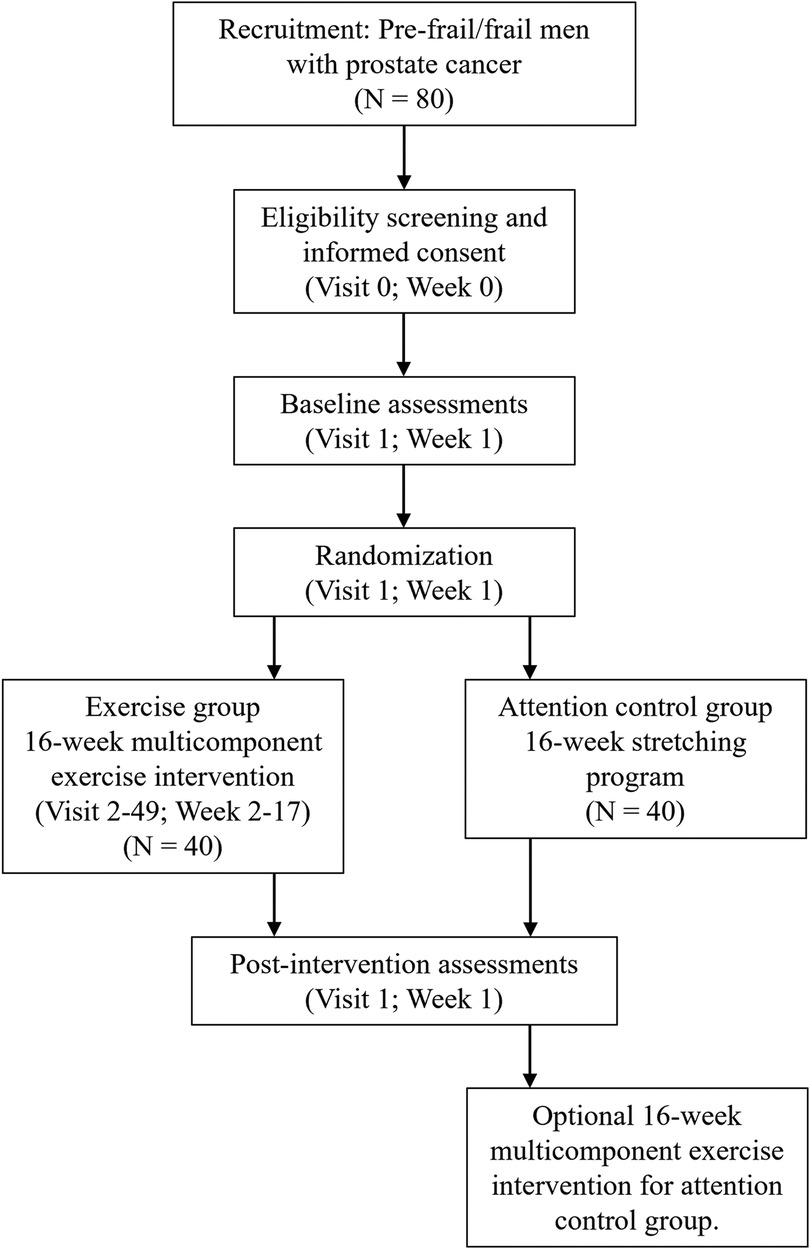
The FIERCE trial is a two-arm randomized controlled trial underway at the Dana-Farber Cancer Institute (NCT06040125). Outcomes are measured at baseline (week 1) and at post-intervention (week 18). A total of 80 men diagnosed with metastatic prostate cancer, who have a history of or are currently receiving, androgen deprivation therapy and are considered pre-frail or frail, are randomized to one of two groups: (1) intervention group, who will receive a 16-week supervised, clinic-based, multicomponent exercise intervention in conjunction with self-directed aerobic exercise or (2) attention control group, who will receive a home-based stretching program. Those randomized to the attention control group will be offered the exercise program following the initial 16-week study period (Figure 1).
2.2 Participant eligibility
Men are eligible if they meet the following criteria: (1) diagnosed with metastatic prostate cancer; (2) aged >18 years; (3) have received at least 3 months of androgen deprivation therapy or are currently receiving at least 1 month of androgen deprivation therapy and are expected to remain on therapy for the duration of the study; (4) classified as pre-frail or frail as determined by the FRAIL scale (score of 1–2 = pre-frail; 3–5 = frail) (30–33); (5) medically cleared by their clinicians for exercise; (6) English-speaking; and (7) inactive (defined as engaging <60 min of moderate-to-vigorous aerobic exercise per week in the past month or <2 resistance exercise sessions per week in the past 4 months). Exclusion criteria include the following: (1) actively receiving chemotherapy or radiotherapy; (2) having unstable bone lesions; (3) having any uncontrolled comorbidity, condition, or contraindication that may prevent participation in exercise; and (4) receiving treatment for other active malignancy (except basal cell carcinoma).
2.3 Recruitment and informed consent
Participants are currently being recruited through clinician referral and screening of participant lists at the Dana-Farber Cancer Institute, Boston, MA, USA, as well as by way of advertisements in participant waiting rooms, the Partners Rally Platform, and other relevant prostate cancer community groups and events. All participants require clinician clearance before being formally screened via phone. Phone screening comprises three short questionnaires to determine eligibility: the Godin Leisure Time Questionnaire (34) to assist in determining current exercise levels, the Physical Activity Readiness Questionnaire (35) to confirm health status and how this relates to undertaking an exercise program, and the FRAIL questionnaire (30–33) to assess frailty status. Upon confirmation of eligibility and interest, participants consent via wet or electronic signature.
2.4 Ethics approval
The study has been approved by the Institutional Review Board at the Dana-Farber Cancer Institute (IRB #23-109). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
2.5 Randomization and blinding
After baseline testing, participants are randomly allocated to either the exercise or attention control group using a 1:1 ratio and a permuted blocked design with varying block sizes and stratified by frailty status as assessed by the FRAIL scale. The study biostatistician and co-investigator (HU) prepared the randomization list before study start-up, after which the investigators, who are blinded to this list creation, access it through a web-based application to conduct randomization and subsequently verbally inform the participants of their group allocation. Testers and participants are blinded to group allocation during baseline testing. Owing to the nature of the intervention and logistical limitations, participants and study staff cannot be blinded to the intervention allocation.
2.6 Measurements
2.6.1 Overview
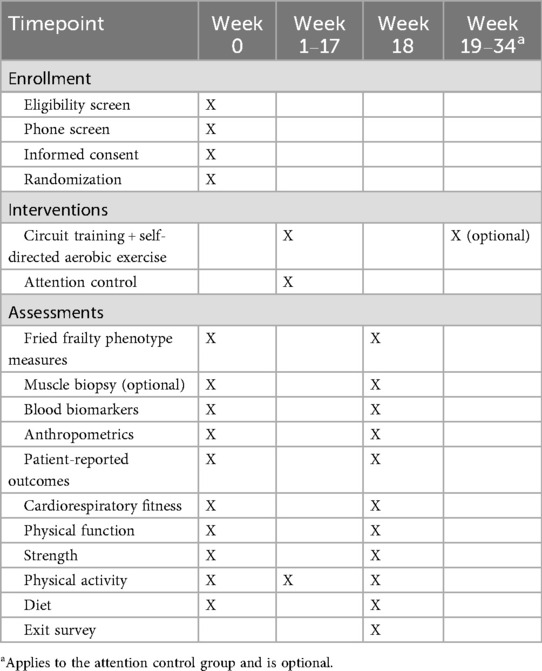
Testing is completed over 2 days at baseline and post-intervention. Order of days is divided by a collection of biospecimens (i.e., blood and muscle tissue) and non-physical tests (i.e., questionnaires and body composition) completed on 1 day, and all other physically demanding tests are completed on the other (∼2–3 days apart). This separation of measures is to ensure participants can recover from the biopsy and not complete any physically demanding activities that may influence blood or muscle biomarkers. All measures are collected at all time points by study staff members trained in exercise oncology unless otherwise specified (Table 1).
2.6.2 Primary outcomes
2.6.2.1 Fried frailty phenotype
The Fried frailty phenotype is a composite score of the five measures: muscle loss, exhaustion, physical activity, slowness, and weakness. Participants receive a score of 1 (experience muscle loss, exhaustion, etc.) or 0 (do not experience) for each characteristic, with a total score of 1–2 identified as pre-frail and ≥3 as frail (36, 37). The Fried frailty phenotype was selected as it offers the most accurate way to capture frailty status using objective measures with pre-determined cutoff points (1, 36). A modified version of the Fried frailty phenotype was selected to tailor to the known effects of ADT, e.g., muscle loss, to ensure frailty was captured in a way that was tailored to the studied population, as has been proposed by other prostate cancer studies assessing frailty (1, 36).
2.6.2.1.1 Muscle loss
A skeletal muscle index (SMI—calculated as appendicular skeletal muscle mass / height2) sarcopenia score of ≤7.26 kg/m2, as assessed by dual-energy x-ray absorptiometry (DXA) scan, is the cut point used for identifying muscle loss = 1 point (6, 38). In addition, in a subset of patients with available standard of care computed tomography (CT) scans within ∼1 month of baseline and post-intervention time points, sarcopenia will also be assessed with a skeletal muscle index of ≤52.4 cm2/m2 as the cut point used for identifying muscle loss = 1 point (39). Skeletal muscle area is measured on axial CT images at the level of the L3 vertebral body using a validated, fully automated algorithm (40). SMI is calculated as the ratio of muscle area (cm2) to squared height (m2). A CT series will be analyzed into two steps: (1) a convolutional neural network model is used to identify a slice at the L3 level, and (2) the chosen slice is passed to a segmentation model to estimate the cross-sectional areas of muscle (40).
2.6.2.1.2 Exhaustion
Exhaustion is assessed using the vitality score from the Short Form Health Survey (SF-36), a 36-item questionnaire assessing quality of life. A cut point of <50 for those 50–65 years and <40 for those >65 years is used to identify exhaustion = 1 point (36, 37, 41).
2.6.2.1.3 Low physical activity
The Community Health Activities Model Program for Seniors’ physical activity questionnaire is used to assess physical activity, with low activity defined as <383 kcals = 1 point (36, 37). This is a 41-item questionnaire asking about time spent undertaking certain physical activities (42).
2.6.2.1.4 Slowness
Usual gait speed is assessed over a 15-foot flat surface distance with an average time over two attempts of ≥7 s for participants ≥173 cm tall or ≥6 s for those <173 cm tall considered as “slow” = 1 point (36, 37). In addition, the participant is asked to walk at their “fast” pace for the 15-foot distance a further two times, but this is not utilized for the Fried frailty phenotype composite score.
2.6.2.1.5 Weakness
The chair stand test is used to assess leg strength, where a time ≥12 s to complete five chair stands is considered weaker = 1 point (36, 37). Participants are asked to sit in a chair with their hands crossed over their chest. While keeping the feet flat and back straight, participants rise to a full standing position and then sit back down, repeating five stands as fast as they can.
2.6.2.2 Blood biomarkers
A trained phlebotomist performs a fasting blood draw, obtaining both serum-separating tubes and ethylenediaminetetraacetic acid (EDTA) samples. After being processed and aliquoted, the blood serum and plasma samples are stored in a −80°C freezer for later batched analysis. Analysis of blood through commercially available kits (Thermo Fisher Scientific) will be used for biomarkers relating to inflammation, frailty, or sarcopenia (i.e., IL-6, TNF-α). Plasma will also be used for exploratory studies assessing effects on prostate cancer cell proliferation.
2.6.3 Secondary outcomes
2.6.3.1 Sarcopenia status
As described under assessing muscle loss as part of the Fried frailty phenotype.
2.6.3.2 Muscle biopsy
Skeletal muscle biopsies of the vastus lateralis are optional, where participants are asked at the time of consent if they wish to partake. Skeletal muscle-derived biomarkers to be assessed include myokines [IL-6, IL-15, secreted protein acidic and rich in cysteine (SPARC), oncostatin M (OSM), decorin, irisin] and insulin pathway-related biomarkers (GLUT-4 receptors, IGF-1, IGFBP-3). The muscle biopsy is performed by a qualified nurse practitioner and/or physician assistant.
The local area (quadriceps thigh muscle) is anesthetized with a numbing spray, followed by an injection of lidocaine. A cannula (∼13.5-gauge) is then inserted into the vastus lateralis muscle perpendicularly, such that it pierces through the layer of fascia surrounding the muscle fibers. To obtain the biopsy, the needle is fed through the cannula, and the biopsy trigger is activated, resulting in a spring-loaded, rapid collection of ∼10–15 mg muscle tissue. The cannula remains inserted into the muscle, the needle is removed, and the procedure is repeated until an adequate sample is collected. After sampling is complete, the cannula is removed, and pressure is applied to the area to stop any potential bleeding, with a bandage placed over the biopsy site to promote healthy healing. The muscle sample is briefly washed on clean gauze and frozen using liquid nitrogen for storage at −80°C for later batched analysis. The biopsies occur following a 12 h fast. The post-intervention biopsy is performed at a distance of ∼1 cm from the first incision and 72–96 h after the last training session for participants in the exercise to reflect resting levels of mRNA expression (43).
2.6.4 Exploratory outcomes
2.6.4.1 Prostate cancer cell proliferation
The proliferation rate of LNCaP cells is assessed using plasma samples at baseline and at post-intervention. LNCaP cell line is grown in ATCC-formulated RPMI 1640 media supplemented with 5% fetal calf serum (FCS) and 1% penicillin–streptomycin (ATCC Manassas, VA, USA). LNCaP cells (100 µL) are seeded at a concentration of 50,000 mL in a 96-well plate containing either 5% FCS with or without the addition of 5% human plasma from test subjects in triplicate for 48 h to determine cell proliferation. As an alternative, patient serum collected could be used in lieu of FCS, and this will be experimentally determined. After plating the same number of cells for a defined period of time, final cell numbers will be determined by removing supernatant and fixing cells with 100 µL 4% paraformaldehyde in the plate for 20 min. Fixed cells are then incubated for an additional 20 min with 100 µL 2% crystal violet (Thermo Fisher Scientific, Edmonton, Canada) dye solution (0.1%, w/v, with ethanol 2%, v/v in 0.5 M Tris-C1, pH 7.80) (44). The stained cells are washed in tap water and solubilized with an SDS solution (0.1%, w/v, with ethanol 50%, v/v, in 0.5 M Tris-C1, pH 7.8; 100 µL per well) for 30 min. The crystal violet dye is released by the fixed cells into the supernatant, and the absorbance is measured by a Molecular Devices spectrophotometer (San Jose, CA, USA) at 600 nm.
2.6.5 Covariates
2.6.5.1 Participant characteristics, health behaviors, and medical history
Participant demographic and medical history data were collected at baseline from medical records, and participants completed questionnaires. Information collected includes age, gender, race, ethnicity, non-cancer medical history, cancer history, family cancer history, anthropometric history, smoking habits, alcohol intake, medication use, and vitamin and supplement use.
2.6.5.2 Anthropometrics
Body composition is assessed via bioelectrical impedance analysis (BIA) using a validated device (Tanita 780, Arlington Heights, IL, USA). The device estimates body fat using an algorithm based on the user’s age, sex, height, and body weight. Waist and hip circumference is also obtained using a constant-tension tape measure.
2.6.5.3 Participant-reported outcomes
Health-related quality of life is assessed using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-C30 (45). A higher functional score represents a healthier level of functioning, a higher global health status represents a higher quality of life, and a higher symptom scale represents a higher level of symptoms. Sleep quality is assessed using the Pittsburgh Sleep Quality Index (PSQI), which contains 19 questions evaluating seven domains of sleep—subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction (46, 47). The Functional Assessment of Cancer Therapy—Bone Pain (FACT-BP) is used to assess cancer-related bone pain and its effect on quality of life (48). The Brief Pain Inventory (BPI) is used to assess the impact of general pain on quality of life. The BPI includes a sensory and reactive dimension and has been previously validated in breast cancer survivors (49). The Exercise Benefits/Barriers Scale (EBBS) is used to assess barriers to exercise (50). Finally, the expanded Prostate Cancer Index Composite-26 is used to measure health-related quality of life across five prostate cancer-specific domains (51).
2.6.5.4 Cardiorespiratory fitness
To assess maximal oxygen consumption (VO2peak) and accurately prescribe the intensity for the self-directed aerobic exercise, a maximal cardiopulmonary exercise test is completed on a cycle ergometer (ErgoSelect 100; Ergoline) using an incremental ramp protocol (52). Participants complete a 3 min warm-up with no resistance and then start the ramp protocol at 10 W and proceed into an incremental ramp protocol increasing 10 W every minute until volitional fatigue. Cadence is kept between 60 and 70 revolutions per minute. Heart rate (HR; FT4; Polar, USA) and rate of perceived exertion (Borg scale 1–10) are recorded every minute and at the end of the test. Expired gas analysis (TrueOne 2400; ParvoMedics, Inc.) is used to measure VO2peak. The results of this test are used to calculate the target heart rate for the self-directed aerobic exercise.
2.6.5.5 Physical function
Functional power is measured using a stair climb test that has been successfully performed and correlated with lower-extremity power and mobility performance in older adults (53). Participants are instructed to ascend a flight of 10 stairs one step at a time as quickly as possible. Timing begins when one foot steps on the third stair and ends when one foot reaches the ninth stair. Time is recorded to the nearest 0.01 s, where three trials are completed, and the fastest is used to calculate power (54).
Mobility is assessed using the Timed Up and Go (TUG) test, which has been shown to predict immediate fall risk better than static balance tests or isometric muscle strength (55). Participants begin seated in a chair, walk to a line on the floor 3 m from the chair, turn around, and return to the same seated position as quickly and safely as possible. Scores are taken as the time to complete the task once, where an average of the time for three trials is calculated.
2.6.5.6 Muscular strength
Grip strength is measured using a handheld dynamometer on the participant's dominant hand. The subject is asked to grip the handle of the dynamometer with one hand using as much grip pressure as possible while holding for 3–5 s. The average of three attempts is calculated.
One repetition maximum (1 RM) is estimated from 10 RM muscle strength tests on two exercises: (1) leg press and (2) chest press. One RM values are calculated and reported using validated equations (56, 57). Following a warm-up, a maximum of five attempts is given to reach the final 10 RM load with a 1–2 min rest period between attempts.
2.6.5.7 Physical activity
Participants are provided an accelerometer (ActiGraph, Pensacola, FL, USA) to wear for 7 days at all times except for when swimming, bathing, and/or sleeping. The device records activity data and is electronically transferred to a computer via USB cable at the time of completion. In addition, control participants are also given weekly physical activity logs to complete over the initial 16-week intervention period.
2.6.5.8 Diet
An automated self-administered 24 h dietary assessment tool is used to assess recent dietary patterns for two weekdays and one weekend day (58). This is completed via an online portal over 3 non-consecutive days where possible.
2.6.5.9 Exit survey
A study-tailored questionnaire evaluating intervention satisfaction is provided at post-intervention testing only. This survey includes questions related to the following: (1) timing of the intervention; (2) length of sessions; (3) telephone-based approach; (4) usefulness of the session content in terms of physical activity; and (5) satisfaction with interventionists. A separate exit survey is provided to the two groups and tailored to the respective exercise or stretching intervention completed.
2.7 Intervention
2.7.1 Exercise group
Participants randomized to this group partake in a 16-week multicomponent exercise intervention consisting of a functional exercise warm-up and a resistance exercise circuit that is supervised in a clinic-based environment 3 days per week. The supervised sessions will be one-on-one, led by a certified exercise trainer in a hospital-based gym. Participants are also encouraged to complete self-directed home-based aerobic exercise on 4 days per week. Before beginning the supervised exercise, participants undergo a screening procedure to assess their bone pain level on a scale of 0–10. In the event bone pain is described, adjustments are made to the resistance exercise for that day to ensure the participant exercises in a pain-free motion.
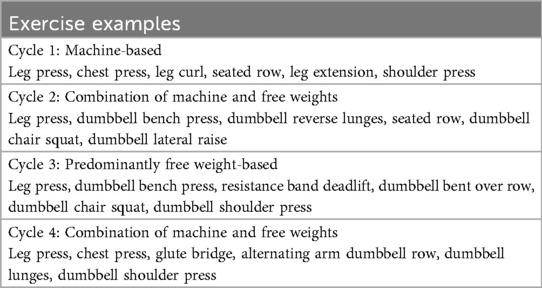
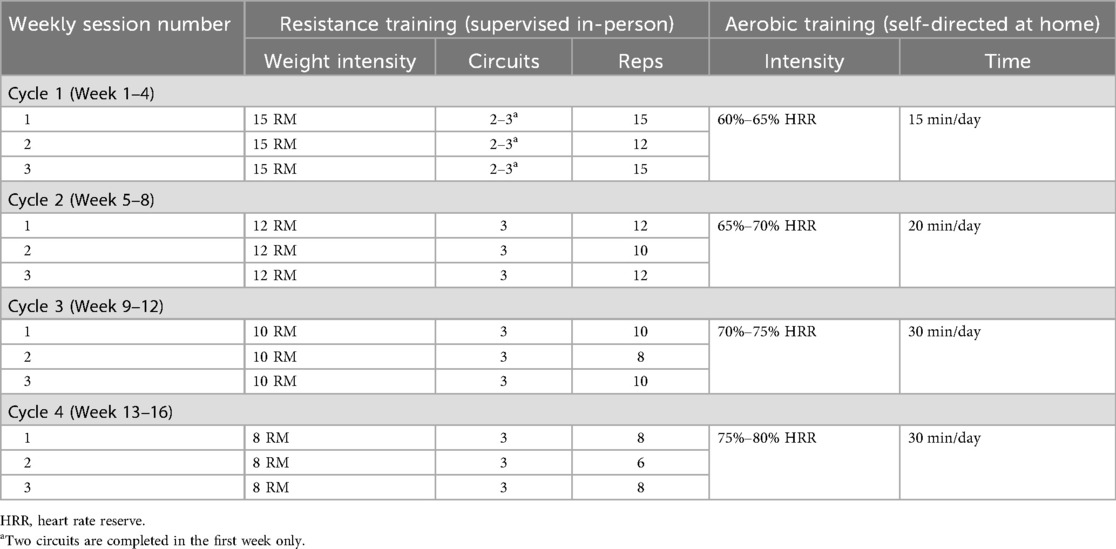
Clinic-based component: Sessions begin with a dynamic warm-up of functional exercises that target aspects of balance, coordination, agility, and functional movements associated with tasks of everyday living (example exercises include calf raises, arm swings, and wrist rotations). The warm-up is followed by a circuit-based resistance program that is performed by alternating upper and lower body exercises in a single circuit. Participants complete four cycles (one cycle = 4 weeks) over the 16-week intervention. An orientation session (counting as their first session) takes place to introduce the study and exercises to the participant and to walk them through how a session will be run and what to expect in each cycle. The first session of each new cycle involves RM testing of each exercise to be prescribed in that 4-week cycle. During any session that is not considered a de-loading session, a 5%–10% increase in weight may occur when the participant can do >3 repetitions with good technique beyond what is prescribed and reports a low rate of perceived exertion (i.e., <7); this ensures participants are consistently working at the assigned RM. In each 4-week cycle, new exercises (Table 2) are introduced to provide variety and alternative stimuli to the body, while targeting the same muscle groups each session/cycle. While in Table 2, the primary exercises are described, alternative exercises that target the same muscle group may be used, subject to participant health and abilities. With this variety in exercises also comes variety in equipment, where machine weights, dumbbells, body weight, and resistance band exercises are incorporated throughout the 16-week intervention. The exercise intervention is periodized to ensure progressive overload and training adaptations. The prescription will be at an intensity ranging from 8 to 15 RM, completing 2–3 circuits of 6–15 repetitions of each exercise (Table 3). In addition, within each week, there will be two higher intensity days and one de-load day, again to provide variety and prevent overload. Each session will conclude with a cooldown incorporating stretches.
Home-based component: Participants are offered an optional stationary bike to help complete self-directed aerobic exercise at home; however, participants may choose any form of aerobic exercise that suits their lifestyle. In addition, if they do not have one, participants are offered an activity monitor to monitor heart rate intensity. Aerobic exercise is completed on any day of the participant's choosing, 4 days per week, at an intensity of 60%–80% heart rate reserve for 15–30 min per day (Table 3). During the supervised sessions, exercise trainers record the reported home-based aerobic activity completed since the last supervised session and set goals with the participant for the upcoming week.
2.7.2 Control group
During this initial 16-week period, control participants are provided a booklet with the prescribed stretching program to be completed 7 days per week to match the prescribed frequency of exercise sessions in the exercise group, e.g., 3 days/week supervised circuit training plus 4 days/week self-directed aerobic exercise. The stretching program is self-directed home-based with instruction provided at baseline testing only to orient them with the booklet. Similar to the exercise program, the stretches change every 4 weeks, where 4–5 stretches that target both upper and lower body are prescribed. Participants in the attention control group are offered the supervised component of the 16-week intervention following the initial 16-week period.
2.8 Intervention adherence
Exercise adherence is captured for the multicomponent exercise intervention by calculating (1) the percentage of supervised circuit trainings attended (out of 48 sessions) and self-directed exercise sessions completed (out of 64 sessions) and (2) relative dose intensities of supervised circuit trainings. Reasons for missed sessions will be documented throughout the study. However, to account for any unforeseen illness, family or medical emergency, or unplanned travel, all participants may have a total of 18 weeks to complete the 48 supervised exercise sessions. Participants are considered compliant if they attend ≥70% of the total prescribed number of supervised exercise sessions (e.g., attend ≥34 out of 48 sessions), ≥70% of the self-directed exercise sessions (e.g., complete ≥45 out of 64 sessions), and complete ≥70% of the supervised resistance sessions at the prescribed intensity. Participants are provided with monetary compensation for each optional muscle biopsy and testing visit and parking validation for every visit to Dana-Farber Cancer Institute.
2.9 Adverse events
Any expected and unexpected adverse events are or will be reported to the principal investigator, who then subsequently reports to the institutional review board of the Dana-Farber Cancer Institute as per policy.
2.10 Data monitoring and management
Data are monitored internally within the Dana-Farber Cancer Institute for timeliness of submission, completeness, and adherence to protocol requirements. Monitoring begins at the time of participant registration and will continue during protocol performance and completion. The study team collects, manages, and performs quality checks of the data. Potential audits or inspections may be conducted by the principal investigator or their designated representatives. All data are stored on a secure network drive using REDCap (Research Electronic Data Capture; Vanderbilt University), a Health Insurance Portability and Accountability Act-compliant web-based application hosted by Partners HealthCare Research Computing, Enterprise Research Infrastructure & Services, on password-protected computers. Any hard-copy data are stored in locked filing cabinets in card-access facilities. The results of this study will be presented in publication, conference, and invited speaker formats.
2.11 Sample size calculation
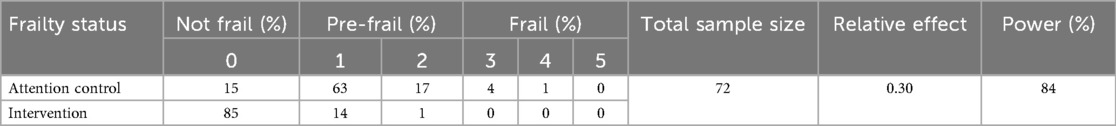
We will enrol a total of 80 eligible participants. Considering a 10% attrition rate, the sample size of the analysis population will be 72. We plan to accrue participants for 24 months. The Wilcoxon rank-sum test will be used to compare the score based on the frailty phenotype (37) between the two groups for the primary analysis. Table 4 shows the anticipated distribution of the score in each group (59). With a sample size of 72, the study will have 84% to detect the relative effect of 0.30 at a 0.05 two-sided alpha level. This power calculation was performed by the WMWssp package (60). For comparing each of the five criteria of the frailty score (binary outcomes), the sample size of 72 will provide 80% power to detect a moderate effect size (Cohen's h = 0.415) at a 0.05 two-sided alpha level (61). For each continuous outcome, two-sample t-tests will be used for the between-group comparison as the primary analysis. Log transformation may be considered, if appropriate. With a sample size of 72, the study will have 80% power to detect a moderate to large effect size (Cohen's d = 0.67) at a 0.05 two-sided alpha level.
2.12 Statistical analysis
This study will assess whether a 16-week, multicomponent exercise intervention will improve the frailty status of mPCa on ADT. Furthermore, we will assess the effect of the intervention on sarcopenia status, skeletal muscle-derived biomarkers, and cell proliferation rate.
A total of 80 eligible patients who complete the baseline assessment will be randomly assigned to the exercise or attention control groups in an allocation ratio of 1:1 using a permuted blocked design with varying block size (with investigators blinded to block size). The randomization will be stratified by frailty status (pre-frail vs. frail) at baseline assessment.
All analyses will be performed based on the intention-to-treat principle. We will repeat the same analyses with the subjects who complied with the protocol as secondary analyses. Baseline demographic and clinical measures, as well as baseline values of trial outcomes, will be presented by randomized intervention group; continuous measures will be summarized by mean (SD) or median (IQR) and categorical measures by frequencies (percent's). The randomized groups will be compared concerning baseline demographic and clinical variables using two-sample t-tests, Wilcoxon rank-sum tests, or Fisher's exact tests to confirm baseline comparability. These variables will be included in the adjusted analyses if clinically meaningful or statistically significant differences are identified.
The primary outcome is the score based on the Fried frailty phenotype (37), which consists of five criteria, and the score will take an integer value from 0 to 5. The Wilcoxon rank-sum test will be used as the primary analysis for comparing the score distribution between groups. Mean score and a corresponding 0.95 confidence interval (CI) will be calculated. We will also assess the intervention effect on each of the five criteria for the frailty phenotype for hypothesis generation purposes. For each, we will perform Fisher's exact test for the between-group comparison and estimate an odds ratio and a corresponding 0.95 CI. Regression analysis will be used to identify baseline factors associated with the outcomes, where proportional odds models will be used for the score as an ordered categorical variable with six categories, and logistic regression models will be used for each of the five criteria.
For other outcome continuous variables, mean, median, and corresponding 0.95 CIs will be calculated in each group. The primary comparison will be performed by two-sample t-tests. To assess the robustness of findings, Wilcoxon rank-sum tests will also be performed as secondary analyses. ANOVA models will also be used to identify baseline factors associated with the outcomes. Log transformation may be considered, if appropriate.
For longitudinal data, we will use generalized linear mixed-effect models with an appropriate link function (i.e., identity-link for continuous outcomes and logit-link for binary outcomes). The independent variables will include an indicator variable for the randomized intervention, time, and intervention-by-time interaction. Random intercepts (and random slopes) will be specified at the subject level. The within-subject correlation structure will be determined prior to evaluation of the between-group difference, using information criterion measures to determine the correlation structure providing the best model fit. In the model, the main effect of the intervention will test for group differences over the entire study period. Regarding missing values, the primary analysis will be all available data analysis, assuming that the missing mechanism is missing at random. As sensitivity analyses, we will apply multiple imputations (62).
3 Discussion
The FIERCE trial is the first exercise oncology study focusing on clinical and molecular analyses related to patients with mPCa during ADT treatment. The FIERCE trial employs a comprehensive analysis of the side effects of ADT treatment, assessing frailty and sarcopenia through questionnaires, physical evaluation, and imaging tests. In addition, muscle biopsies and plasma/serum will be used to evaluate biomarkers of the effects of exercise on frailty and sarcopenia. Potential modulating agents of physical exercise released by muscle cells will be examined on the LNCaP cell line, a human prostate cancer cell line that exhibits androgen-sensitive growth.
Men with mPCa undergoing treatment with ADT present with, among other side effects, loss of muscle mass, sarcopenia, and frailty, with a consequent increase in the risk of mortality (3, 4, 8). The reduction in muscle mass and frailty induced by ADT treatment has been an important clinical issue in patient management (63). In this regard, evidence supports the association of exercise-based interventions with global health improvements in patients undergoing ADT treatment with or without a diagnosis of metastases (10–12). Research has supported the feasibility and safety of physical exercise in men with metastatic or advanced prostate cancer (64–67). For example, in patients with advanced prostate cancer receiving ADT and patients with bone metastases, exercise training was able to increase muscle mass, strength, and aerobic capacity (67–70). Interestingly, Houben et al. (68) demonstrated that resistance exercise effectively countered ADT-induced side effects, regardless of protein supplementation combined with resistance exercise. Therefore, the beneficial effects of exercise among men with prostate cancer are mainly related to increased physical fitness (13) and reduced fatigue (71). Improvements in sexual and mental well-being (72, 73), as well as in quality of life (13, 74), have also been reported. However, some studies have shown no effect of exercise on sexual function (65) or quality of life (67, 75). Although these beneficial effects have been demonstrated across different training protocols and exercise types, multicomponent exercise-based training with a periodized prescription is promising for older adults and men with prostate cancer (69, 76).
Prior work has suggested that multicomponent exercise has significant potential to reduce ADT-induced side effects. In prostate cancer patients undergoing ADT, a combination of aerobic and resistance exercise enhances aerobic fitness and muscle strength, reduces fatigue, and preserves or increases lean mass, contributing to an increase in physical function and quality of life (69, 74, 77, 78). Furthermore, the addition of balance and flexibility exercises is particularly beneficial for older men with prostate cancer, to improve joint mobility, balance, and reduce the risk of falls (3). Above all, the maintenance or increase in muscle strength and mass, as well as the reduction in sarcopenia and frailty, is directly associated with a lower risk of mortality in men undergoing ADT treatment, regardless of cancer type (4, 7–9). Although multicomponent exercise may uniquely improve several components of health-related physical fitness in patients undergoing ADT treatment, the effect of multicomponent exercise on biochemical variables is poorly investigated. In this regard, Cormie et al. (77) observed a reduction in high-density lipoprotein (HDL), while Galvão et al. (69) observed a decrease in C-reactive protein, but in general, research has not identified changes in biomarkers of lipid and glucose metabolism (69, 77) or PSA concentrations (69, 74, 77, 78). Therefore, despite the beneficial effects of exercise training in men with prostate cancer, it remains unclear its impact on biochemical markers of metabolism or how exercise could modulate molecular pathways capable of mitigating the side effects of ADT treatment.
Potential molecular mechanisms that explain the beneficial effects of exercise on frailty and sarcopenia in men with mPCa are associated with low-grade inflammation (25). Conversely, changes in immunity favoring pro-inflammatory signaling are directly related to frailty (24). High levels of C-reactive protein have been consistently reported as biomarkers of frailty in older adults (79, 80). Corroborating, Navarro-Martínez et al. (5) showed that the concentrations of IL-6, C-reactive protein, and fibrinogen were positively associated with frailty in men with prostate cancer undergoing ADT. Interestingly, the authors reported that physical activity was also associated with frailty biomarkers. C-reactive protein and fibrinogen had higher serum concentrations in patients with moderate or light physical activity of <150 min per week (5). In addition, IL-6 was also higher in patients with lower walking speeds (5). Corroborating with frailty biomarkers, studies on the role of myokines in muscle tissue are still limited (28). Evidence has supported that myonectin and FGF21 were related to mitochondrial function and biogenesis (28, 81), SPARC, and brain-derived neurotrophic factor (BDNF) to muscle repair (82, 83), while increased muscle mass is associated with decorin, irisin, and FGF21 (84–86).
Moreover, the literature acknowledges the antitumor effects of exercise through myokines, particularly highlighting IL-6, IL-15, IL-10, SPARC, myostatin, irisin, decorin, and oncostatin M (87, 88). Kim et al. (89) demonstrated an increase in OSM but not SPARC and decorin after twelve weeks of exercise in prostate cancer patients treated with ADT. They used serum from these patients for in vitro testing, demonstrating that DU-145 prostate cancer cells had reduced proliferation when cultured with serum from trained patients (89). Similarly, using serum from prostate cancer patients who trained for 6 months reduced the proliferation of DU-145 cells. Myokine tests showed an increase in OSM and SPARC, while IGF-1 or IGFBP-3 remained unchanged after training (90). These antitumor effects have also been demonstrated using exogenous myokines. Investigation with PC-3 metastatic prostate cancer cells revealed that exogenous irisin reduced cell viability, while tumor growth and progression were reduced in vivo, suggesting that apoptotic pathways were enhanced by irisin (91). It was also demonstrated that exogenous decorin inhibited the growth of androgen-independent (PC3 and DU-145) and androgen-dependent (LNCaP) prostate cancer cells. In addition, the animal model treated with decorin showed delayed tumor development (92). However, research on the impact of myokines on prostate cancer is incipient, and the potential of myokines released from exercise with antitumor effects is still unclear. Furthermore, the molecular mechanisms by which exercise may contribute to the delay in muscle mass loss or gain related to ADT treatment or the impact of multicomponent exercise on cellular factors released by muscles still require further investigation and thus will be investigated in the FIERCE trial.
In this regard, the strengths of our study include the randomized controlled design and a comprehensive set of frailty and sarcopenia measures, including the utilization of muscle tissue biopsies to assess the impact of multicomponent exercise on molecular markers associated with sarcopenia. We also want to acknowledge that our study has several limitations. While the intention is to recruit frail and pre-frail participants as defined by the FRAIL questionnaire, this questionnaire is self-reported by the participant and may lead to misclassification. Although our intervention period allows us to determine changes in frailty and sarcopenia, this period would not permit us to determine if our intervention may be associated with reductions in comorbidities, metastatic modulation, or mortality. The partial clinic-based setting of the intervention may not be replicable in other environments, e.g., community programs and telehealth.
4 Conclusion
The FIERCE trial will evaluate the efficacy of a 16-week multicomponent exercise intervention encompassing supervised, clinic-based circuit training utilizing resistance exercises and self-directed aerobic exercise on frailty and sarcopenia, with an exploration of novel biomarkers at the intersection of frailty, sarcopenia, and disease progression. We hypothesize that multicomponent exercise will be effective in reducing the progression of frailty and sarcopenia, with an impact on molecular mechanisms related to the immune response and myokines, capable of improving patients' health and inhibiting prostate cancer cell development in vitro. Moreover, the exercise intervention includes multicomponent exercise periodization, which can enhance exercise prescription for men with mPCa by integration of various exercise modalities with structured progressions over time. The results of this study may corroborate the understanding of the antitumor effects of exercise on prostate cancer cells, in addition to the impact of periodization training to reduce sarcopenia and frailty in patients with mPCa, corroborating the development of future exercise guidelines for prostate cancer survivorship.
Author contributions
RW: Methodology, Conceptualization, Funding acquisition, Writing – review & editing, Writing – original draft. AV: Writing – original draft, Writing – review & editing. AM: Conceptualization, Funding acquisition, Writing – review & editing. MN: Writing – review & editing, Project administration. JG: Writing – review & editing. JV: Writing – review & editing. MVam: Writing – review & editing. DE: Writing – review & editing. HU: Conceptualization, Funding acquisition, Writing – review & editing. MR: Writing – review & editing, Funding acquisition, Conceptualization. MVan: Funding acquisition, Writing – review & editing, Conceptualization. CD-C: Funding acquisition, Conceptualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Prostate Cancer Foundation Challenge Award (#22CHAL04) 2022.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence, and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bylow K, Mohile SG, Stadler WM, Dale W. Does androgen-deprivation therapy accelerate the development of frailty in older men with prostate cancer? A conceptual review. Cancer. (2007) 110(12):2604–13. doi: 10.1002/cncr.23084
2. Momota M, Hatakeyama S, Soma O, Tanaka T, Hamano I, Fujita N, et al. Geriatric 8 screening of frailty in patients with prostate cancer. Int J Urol. (2020) 27(8):642–8. doi: 10.1111/iju.14256
3. Winters-Stone KM, Moe E, Graff JN, Dieckmann NF, Stoyles S, Borsch C, et al. Falls and frailty in prostate cancer survivors: current, past, and never users of androgen deprivation therapy. J Am Geriatr Soc. (2017) 65(7):1414–9. doi: 10.1111/jgs.14795
4. Pan YY, Meng LC, Chen HM, Chen LK, Hsiao FY. Impact of frailty on survivals of prostate cancer patients treated with radiotherapy. Arch Gerontol Geriatr. (2022) 100:104651. doi: 10.1016/j.archger.2022.104651
5. Navarro-Martínez R, Serrano-Carrascosa M, Buigues C, Fernández-Garrido J, Sánchez-Martínez V, Castelló-Domenech AB, et al. Frailty syndrome is associated with changes in peripheral inflammatory markers in prostate cancer patients undergoing androgen deprivation therapy. Urol Oncol. (2019) 37(12):976–87. doi: 10.1016/j.urolonc.2019.08.005
6. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48(1):16–31. doi: 10.1093/ageing/afy169
7. Coletta AM, Sayegh N, Agarwal N. Body composition and metastatic prostate cancer survivorship. Cancer Treat Res Commun. (2021) 27:100322. doi: 10.1016/j.ctarc.2021.100322
8. Chiang PK, Tsai WK, Chiu AW, Lin JB, Yang FY, Lee J. Muscle loss during androgen deprivation therapy is associated with higher risk of non-cancer mortality in high-risk prostate cancer. Front Oncol. (2021) 11:722652. doi: 10.3389/fonc.2021.722652
9. Ikeda T, Ishihara H, Iizuka J, Hashimoto Y, Yoshida K, Kakuta Y, et al. Prognostic impact of sarcopenia in patients with metastatic hormone-sensitive prostate cancer. Jpn J Clin Oncol. (2020) 50(8):933–9. doi: 10.1093/jjco/hyaa045
10. Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. (2019) 51(11):2375–90. doi: 10.1249/MSS.0000000000002116
11. Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. (2010) 42(7):1409–26. doi: 10.1249/MSS.0b013e3181e0c112
12. Campbell KL, Cormie P, Weller S, Alibhai SMH, Bolam KA, Campbell A, et al. Exercise recommendation for people with bone metastases: expert consensus for health care providers and exercise professionals. JCO Oncol Pract. (2022) 18(5):e697–709. doi: 10.1200/OP.21.00454
13. Cormie P, Zopf EM. Exercise medicine for the management of androgen deprivation therapy-related side effects in prostate cancer. Urol Oncol. (2020) 38(2):62–70. doi: 10.1016/j.urolonc.2018.10.008
14. Tarazona-Santabalbina FJ, Gómez-Cabrera MC, Pérez-Ros P, Martínez-Arnau FM, Cabo H, Tsaparas K, et al. A multicomponent exercise intervention that reverses frailty and improves cognition, emotion, and social networking in the community-dwelling frail elderly: a randomized clinical trial. J Am Med Dir Assoc. (2016) 17(5):426–33. doi: 10.1016/j.jamda.2016.01.019
15. Ramos-Campo DJ, Andreu Caravaca L, Martínez-Rodríguez A, Rubio-Arias J. Effects of resistance circuit-based training on body composition, strength and cardiorespiratory fitness: a systematic review and meta-analysis. Biology. (2021) 10(5):377. doi: 10.3390/biology10050377
16. Jones LM, Stoner L, Baldi JC, McLaren B. Circuit resistance training and cardiovascular health in breast cancer survivors. Eur J Cancer Care. (2020) 29(4):e13231. doi: 10.1111/ecc.13231
17. Almstedt HC, Grote S, Korte JR, Perez Beaudion S, Shoepe TC, Strand S, et al. Combined aerobic and resistance training improves bone health of female cancer survivors. Bone Rep. (2016) 5:274–9. doi: 10.1016/j.bonr.2016.09.003
18. Giné-Garriga M, Guerra M, Pagès E, Manini TM, Jiménez R, Unnithan VB. The effect of functional circuit training on physical frailty in frail older adults: a randomized controlled trial. J Aging Phys Act. (2010) 18(4):401–24. doi: 10.1123/japa.18.4.401
19. Dun Y, Hu P, Ripley-Gonzalez JW, Zhou N, Li H, Zhang W, et al. Effectiveness of a multicomponent exercise program to reverse pre-frailty in community-dwelling Chinese older adults: a randomised controlled trial. Age Ageing. (2022) 51(3):afac026. doi: 10.1093/ageing/afac026
20. Nascimento CM, Ingles M, Salvador-Pascual A, Cominetti MR, Gomez-Cabrera MC, Viña J. Sarcopenia, frailty and their prevention by exercise. Free Radic Biol Med. (2019) 132:42–9. doi: 10.1016/j.freeradbiomed.2018.08.035
21. Norris MK, Bell GJ, North S, Courneya KS. Effects of resistance training frequency on physical functioning and quality of life in prostate cancer survivors: a pilot randomized controlled trial. Prostate Cancer Prostatic Dis. (2015) 18(3):281–7. doi: 10.1038/pcan.2015.28
22. National Institute of Health. ClinicalTrials.gov 2022. Available online at: https://www.clinicaltrials.gov/ct2/home (Accessed February 01, 2025).
23. Barnes O, Wilson RL, Gonzalo-Encabo P, Kang D-W, Christopher CN, Bentley T, et al. The effect of exercise and nutritional interventions on body composition in patients with advanced or metastatic cancer: a systematic review. Nutrients. (2022) 14(10):2110. doi: 10.3390/nu14102110
24. Yao X, Li H, Leng SX. Inflammation and immune system alterations in frailty. Clin Geriatr Med. (2011) 27(1):79–87. doi: 10.1016/j.cger.2010.08.002
25. Petersen AMW, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. (2005) 98(4):1154–62. doi: 10.1152/japplphysiol.00164.2004
26. Goncalves CAM, Dantas PMS, Dos Santos IK, Dantas M, da Silva DCP, Cabral B, et al. Effect of acute and chronic aerobic exercise on immunological markers: a systematic review. Front Physiol. (2019) 10:1602. doi: 10.3389/fphys.2019.01602
27. Flores-Opazo M, McGee SL, Hargreaves M. Exercise and GLUT4. Exerc Sport Sci Rev. (2020) 48(3):110–8. doi: 10.1249/JES.0000000000000224
28. Lee JH, Jun HS. Role of myokines in regulating skeletal muscle mass and function. Front Physiol. (2019) 10:42. doi: 10.3389/fphys.2019.00042
29. Coletta AM, Brewster AM, Chen M, Li Y, Bevers TB, Basen-Engquist K, et al. High-intensity interval training is feasible in women at high risk for breast cancer. Med Sci Sports Exerc. (2019) 51(11):2193–200. doi: 10.1249/MSS.0000000000002048
30. Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging. (2012) 16(7):601–8. doi: 10.1007/s12603-012-0084-2
31. Nozaki K, Kamiya K, Hamazaki N, Saito H, Saito K, Ogasahara Y, et al. Validity and utility of the questionnaire-based FRAIL scale in older patients with heart failure: findings from the FRAGILE-HF. J Am Med Dir Assoc. (2021) 22(8):1621–6.e2. doi: 10.1016/j.jamda.2021.02.025
32. Aprahamian I, Cezar NOC, Izbicki R, Lin SM, Paulo DLV, Fattori A, et al. Screening for frailty with the FRAIL scale: a comparison with the phenotype criteria. J Am Med Dir Assoc. (2017) 18(7):592–6. doi: 10.1016/j.jamda.2017.01.009
33. Sukkriang N, Punsawad C. Comparison of geriatric assessment tools for frailty among community elderly. Heliyon. (2020) 6(9):e04797. doi: 10.1016/j.heliyon.2020.e04797
34. Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. (1985) 10(3):141–6.4053261
35. Bredin SS, Gledhill N, Jamnik VK, Warburton DE. PAR-Q+ and ePARmed-X+: new risk stratification and physical activity clearance strategy for physicians and patients alike. Can Fam Physician. (2013) 59(3):273–7.23486800
36. Winters-Stone KM, Li F, Horak F, Dieckmann N, Hung A, Amling C, et al. Protocol for GET FIT prostate: a randomized, controlled trial of group exercise training for fall prevention and functional improvements during and after treatment for prostate cancer. Trials. (2021) 22(1):775. doi: 10.1186/s13063-021-05687-7
37. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. (2001) 56(3):M146–56. doi: 10.1093/gerona/56.3.M146
38. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. (2010) 39(4):412–23. doi: 10.1093/ageing/afq034
39. Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. (2008) 9(7):629–35. doi: 10.1016/S1470-2045(08)70153-0
40. Magudia K, Bridge CP, Bay CP, Babic A, Fintelmann FJ, Troschel FM, et al. Population-scale CT-based body composition analysis of a large outpatient population using deep learning to derive age-, sex-, and race-specific reference curves. Radiology. (2021) 298(2):319–29. doi: 10.1148/radiol.2020201640
41. McHorney CA, Ware JE Jr, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. (1994) 32(1):40–66. doi: 10.1097/00005650-199401000-00004
42. Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. (2001) 33(7):1126–41. doi: 10.1097/00005768-200107000-00010
43. Hulmi JJ, Kovanen V, Selänne H, Kraemer WJ, Häkkinen K, Mero AA. Acute and long-term effects of resistance exercise with or without protein ingestion on muscle hypertrophy and gene expression. Amino Acids. (2009) 37(2):297–308. doi: 10.1007/s00726-008-0150-6
44. Rundqvist H, Augsten M, Stromberg A, Rullman E, Mijwel S, Kharaziha P, et al. Effect of acute exercise on prostate cancer cell growth. PLoS One. (2013) 8(7):e67579. doi: 10.1371/journal.pone.0067579
45. Aaronson N, Ahmedzia S, Bergman B, Bergman B, Bullinger M, Cull A, et al. The European Organization for Research and Treatment for Cancer QLQ C-30: a quality of life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. (1993) 85:365–76. doi: 10.1093/jnci/85.5.365
46. Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28(2):193–213. doi: 10.1016/0165-1781(89)90047-4
47. Akman T, Yavuzsen T, Sevgen Z, Ellidokuz H, Yilmaz AU. Evaluation of sleep disorders in cancer patients based on Pittsburgh sleep quality index. Eur J Cancer Care. (2015) 24(4):553–9. doi: 10.1111/ecc.12296
48. Broom R, Du H, Clemons M, Eton D, Dranitsaris G, Simmons C, et al. Switching breast cancer patients with progressive bone metastases to third-generation bisphosphonates: measuring impact using the functional assessment of cancer therapy-bone pain. J Pain Symptom Manage. (2009) 38(2):244–57. doi: 10.1016/j.jpainsymman.2008.08.005
49. Holen JC, Lydersen S, Klepstad P, Loge JH, Kaasa S. The brief pain inventory: pain’s interference with functions is different in cancer pain compared with noncancer chronic pain. Clin J Pain. (2008) 24(3):219–25. doi: 10.1097/AJP.0b013e31815ec22a
50. Kibler JL, Brisco K. Evaluation of a brief questionnaire for assessing barriers to research participation. Ethn Dis. (2006) 16(2):547–50.17682261
51. Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. (2000) 56(6):899–905. doi: 10.1016/S0090-4295(00)00858-X
52. Jones LW, Eves ND, Mackey JR, Peddle CJ, Haykowsky M, Joy AA, et al. Safety and feasibility of cardiopulmonary exercise testing in patients with advanced cancer. Lung Cancer. (2007) 55(2):225–32. doi: 10.1016/j.lungcan.2006.10.006
53. Bean JF, Kiely DK, LaRose S, Alian J, Frontera WR. Is stair climb power a clinically relevant measure of leg power impairments in at-risk older adults? Arch Phys Med Rehabil. (2007) 88(5):604–9. doi: 10.1016/j.apmr.2007.02.004
54. Rigamonti AE, De Col A, Tamini S, Cicolini S, Caroli D, De Micheli R, et al. Multidisciplinary integrated metabolic rehabilitation in elderly obese patients: effects on cardiovascular risk factors, fatigue and muscle performance. Nutrients. (2019) 11(6):1240. doi: 10.3390/nu11061240
55. Bhatt T, Espy D, Yang F, Pai YC. Dynamic gait stability, clinical correlates, and prognosis of falls among community-dwelling older adults. Arch Phys Med Rehabil. (2011) 92(5):799–805. doi: 10.1016/j.apmr.2010.12.032
56. Reynolds JM, Gordon TJ, Robergs RA. Prediction of one repetition maximum strength from multiple repetition maximum testing and anthropometry. J Strength Cond Res. (2006) 20(3):584–92. doi: 10.1519/R-15304.1
57. Knutzen KM, Brilla LR, Caine D. Validity of 1RM prediction equations for older adults. J Strength Cond Res. (1999) 13(3):242–6.
58. Dieli-Conwright CM, Mortimer JE, Schroeder ET, Courneya K, Demark-Wahnefried W, Buchanan TA, et al. Randomized controlled trial to evaluate the effects of combined progressive exercise on metabolic syndrome in breast cancer survivors: rationale, design, and methods. BMC Cancer. (2014) 14:238. doi: 10.1186/1471-2407-14-238
59. Chen R, Wu Q, Wang D, Li Z, Liu H, Liu G, et al. Effects of elastic band exercise on the frailty states in pre-frail elderly people. Physiother Theory Pract. (2020) 36(9):1000–8. doi: 10.1080/09593985.2018.1548673
60. Happ M, Bathke AC, Brunner E. Optimal sample size planning for the Wilcoxon-Mann-Whitney test. Stat Med. (2019) 38(3):363–75. doi: 10.1002/sim.7983
62. Little RJ, Rubin DB. Statistical Analysis With Missing Data. Hoboken, NJ: John Wiley & Sons (2019).
63. Mohile SG, Mustian K, Bylow K, Hall W, Dale W. Management of complications of androgen deprivation therapy in the older man. Crit Rev Oncol Hematol. (2009) 70(3):235–55. doi: 10.1016/j.critrevonc.2008.09.004
64. Hart NH, Galvão DA, Newton RU. Exercise medicine for advanced prostate cancer. Curr Opin Support Palliat Care. (2017) 11(3):247–57. doi: 10.1097/SPC.0000000000000276
65. Galvão DA, Taaffe DR, Chambers SK, Fairman CM, Spry N, Joseph D, et al. Exercise intervention and sexual function in advanced prostate cancer: a randomised controlled trial. BMJ Support Palliat Care. (2022) 12(1):29–32. doi: 10.1136/bmjspcare-2020-002706
66. Kenfield SA, Van Blarigan EL, Panchal N, Bang A, Zhang L, Graff RE, et al. Feasibility, safety, and acceptability of a remotely monitored exercise pilot CHAMP: a clinical trial of high-intensity aerobic and resistance exercise for metastatic castrate-resistant prostate cancer. Cancer Med. (2021) 10(22):8058–70. doi: 10.1002/cam4.4324
67. Cormie P, Newton RU, Spry N, Joseph D, Taaffe DR, Galvão DA. Safety and efficacy of resistance exercise in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis. (2013) 16(4):328–35. doi: 10.1038/pcan.2013.22
68. Houben LHP, Overkamp M, Van Kraaij P, Trommelen J, Van Roermund JGH, De Vries P, et al. Resistance exercise training increases muscle mass and strength in prostate cancer patients on androgen deprivation therapy. Med Sci Sports Exerc. (2023) 55(4):614–24. doi: 10.1249/MSS.0000000000003095
69. Galvão DA, Taaffe DR, Spry N, Joseph D, Newton RU. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol. (2010) 28(2):340–7. doi: 10.1200/JCO.2009.23.2488
70. Galvao DA, Taaffe DR, Spry N, Cormie P, Joseph D, Chambers SK, et al. Exercise preserves physical function in prostate cancer patients with bone metastases. Med Sci Sports Exerc. (2018) 50(3):393–9. doi: 10.1249/MSS.0000000000001454
71. Taaffe DR, Newton RU, Spry N, Joseph D, Chambers SK, Gardiner RA, et al. Effects of different exercise modalities on fatigue in prostate cancer patients undergoing androgen deprivation therapy: a year-long randomised controlled trial. Eur Urol. (2017) 72(2):293–9. doi: 10.1016/j.eururo.2017.02.019
72. Hamilton K, Chambers SK, Legg M, Oliffe JL, Cormie P. Sexuality and exercise in men undergoing androgen deprivation therapy for prostate cancer. Support Care Cancer. (2015) 23(1):133–42. doi: 10.1007/s00520-014-2327-8
73. Bourke L, Sohanpal R, Nanton V, Crank H, Rosario DJ, Saxton JM. A qualitative study evaluating experiences of a lifestyle intervention in men with prostate cancer undergoing androgen suppression therapy. Trials. (2012) 13:208. doi: 10.1186/1745-6215-13-208
74. Bourke L, Gilbert S, Hooper R, Steed LA, Joshi M, Catto JW, et al. Lifestyle changes for improving disease-specific quality of life in sedentary men on long-term androgen-deprivation therapy for advanced prostate cancer: a randomised controlled trial. Eur Urol. (2014) 65(5):865–72. doi: 10.1016/j.eururo.2013.09.040
75. Sheill G, Brady L, Hayes B, Baird AM, Guinan E, Vishwakarma R, et al. ExPeCT: a randomised trial examining the impact of exercise on quality of life in men with metastatic prostate cancer. Support Care Cancer. (2023) 31(5):292. doi: 10.1007/s00520-023-07740-4
76. Labata-Lezaun N, González-Rueda V, Llurda-Almuzara L, López-de-Celis C, Rodríguez-Sanz J, Bosch J, et al. Effectiveness of multicomponent training on physical performance in older adults: a systematic review and meta-analysis. Arch Gerontol Geriatr. (2023) 104:104838. doi: 10.1016/j.archger.2022.104838
77. Cormie P, Galvão DA, Spry N, Joseph D, Chee R, Taaffe DR, et al. Can supervised exercise prevent treatment toxicity in patients with prostate cancer initiating androgen-deprivation therapy: a randomised controlled trial. BJU Int. (2015) 115(2):256–66. doi: 10.1111/bju.12646
78. Bourke L, Doll H, Crank H, Daley A, Rosario D, Saxton JM. Lifestyle intervention in men with advanced prostate cancer receiving androgen suppression therapy: a feasibility study. Cancer Epidemiol Biomarkers Prev. (2011) 20(4):647–57. doi: 10.1158/1055-9965.EPI-10-1143
79. Puts MT, Visser M, Twisk JW, Deeg DJ, Lips P. Endocrine and inflammatory markers as predictors of frailty. Clin Endocrinol. (2005) 63(4):403–11. doi: 10.1111/j.1365-2265.2005.02355.x
80. Walston J, McBurnie MA, Newman A, Tracy RP, Kop WJ, Hirsch CH, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. (2002) 162(20):2333–41. doi: 10.1001/archinte.162.20.2333
81. Kim KH, Jeong YT, Oh H, Kim SH, Cho JM, Kim YN, et al. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat Med. (2013) 19(1):83–92. doi: 10.1038/nm.3014
82. Petersson SJ, Jørgensen LH, Andersen DC, Nørgaard RC, Jensen CH, Schrøder HD. SPARC is up-regulated during skeletal muscle regeneration and inhibits myoblast differentiation. Histol Histopathol. (2013) 28(11):1451–60. doi: 10.14670/HH-28.1451
83. Clow C, Jasmin BJ. Brain-derived neurotrophic factor regulates satellite cell differentiation and skeletal muscle regeneration. Mol Biol Cell. (2010) 21(13):2182–90. doi: 10.1091/mbc.e10-02-0154
84. El Shafey N, Guesnon M, Simon F, Deprez E, Cosette J, Stockholm D, et al. Inhibition of the myostatin/Smad signaling pathway by short decorin-derived peptides. Exp Cell Res. (2016) 341(2):187–95. doi: 10.1016/j.yexcr.2016.01.019
85. Reza MM, Subramaniyam N, Sim CM, Ge X, Sathiakumar D, McFarlane C, et al. Irisin is a pro-myogenic factor that induces skeletal muscle hypertrophy and rescues denervation-induced atrophy. Nat Commun. (2017) 8(1):1104. doi: 10.1038/s41467-017-01131-0
86. Izumiya Y, Bina HA, Ouchi N, Akasaki Y, Kharitonenkov A, Walsh K. FGF21 is an Akt-regulated myokine. FEBS Lett. (2008) 582(27):3805–10. doi: 10.1016/j.febslet.2008.10.021
87. Kim JS, Galvão DA, Newton RU, Gray E, Taaffe DR. Exercise-induced myokines and their effect on prostate cancer. Nat Rev Urol. (2021) 18(9):519–42. doi: 10.1038/s41585-021-00476-y
88. Huang Q, Wu M, Wu X, Zhang Y, Xia Y. Muscle-to-tumor crosstalk: the effect of exercise-induced myokine on cancer progression. Biochim Biophys Acta Rev Cancer. (2022) 1877(5):188761. doi: 10.1016/j.bbcan.2022.188761
89. Kim JS, Wilson RL, Taaffe DR, Galvão DA, Gray E, Newton RU. Myokine expression and tumor-suppressive effect of serum after 12 wk of exercise in prostate cancer patients on ADT. Med Sci Sports Exerc. (2022) 54(2):197–205. doi: 10.1249/MSS.0000000000002783
90. Kim JS, Taaffe DR, Galvão DA, Hart NH, Gray E, Ryan CJ, et al. Exercise in advanced prostate cancer elevates myokine levels and suppresses in-vitro cell growth. Prostate Cancer Prostatic Dis. (2022) 25(1):86–92. doi: 10.1038/s41391-022-00504-x
91. Alshanqiti KH, Alomar SF, Alzoman N, Almomen A. Irisin induces apoptosis in metastatic prostate cancer cells and inhibits tumor growth in vivo. Cancers. (2023) 15(15):4000. doi: 10.3390/cancers15154000
Keywords: prostate cancer, exercise, frailty, sarcopenia, androgen deprivation therapy
Citation: Wilson RL, Vulczak A, Morgans AK, Norris M, Greer J, Votta J, Vamvini M, Einstein DJ, Uno H, Rosenthal M, Vander Heiden MG and Dieli-Conwright CM (2025) Design of debunking the frailty–sarcopenia–ADT axis in metastatic prostate cancer with multicomponent exercise: the FIERCE trial protocol. Front. Sports Act. Living 7:1602123. doi: 10.3389/fspor.2025.1602123
Received: 3 April 2025; Accepted: 23 September 2025;
Published: 23 October 2025.
Edited by:
Ciaran Fairman, University of South Carolina, United StatesReviewed by:
Oleksandr P. Romanchuk, Lesya Ukrainka Volyn National University, UkraineGiuseppe D’Antona, University of Pavia, Italy
Copyright: © 2025 Wilson, Vulczak, Morgans, Norris, Greer, Votta, Vamvini, Einstein, Uno, Rosenthal, Vander Heiden and Dieli-Conwright. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christina M. Dieli-Conwright, Y2hyaXN0aW5hbV9kaWVsaS1jb253cmlnaHRAZGZjaS5oYXJ2YXJkLmVkdQ==
 Rebekah L. Wilson
Rebekah L. Wilson Anderson Vulczak
Anderson Vulczak Alicia K. Morgans1,2
Alicia K. Morgans1,2 Mary Norris
Mary Norris Maria Vamvini
Maria Vamvini David J. Einstein
David J. Einstein Michael Rosenthal
Michael Rosenthal Christina M. Dieli-Conwright
Christina M. Dieli-Conwright