- 1College of Sports and Health, Shandong Sport University, Jinan, China
- 2Physiotherapy Programme, Centre for Healthy Ageing and Wellness, Faculty of Health Sciences, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia
- 3Sport Science School, Beijing Sport University, Beijing, China
- 4School of Physical Education & Sports Science, South China Normal University, Guangzhou, China
Purpose: Chronic ankle instability (CAI) is characterized by a combination of peripheral dysfunction and maladaptive neuroplasticity in central nervous system, leading to persistent postural stability deficits. This study aims to investigate the effects of high-definition transcranial direct current stimulation (HD-tDCS) combined with Bosu ball training (BBT) on the static and dynamic postural stability in individuals with CAI.
Methods: A total of forty participants were randomized to receive either HD-tDCS + BBT (n = 20) or BBT (n = 20) interventions, delivered over six weeks with three 20-minute sessions per week. Static and dynamic postural stability was assessed pre- and post-intervention via single-leg stance and drop landing tests, with kinetic data captured by a force platform (1,000 Hz). Data were analyzed using two-way mixed-design ANOVA.
Results: Significant group-by-time interactions were detected in the center of pressure-root mean square (CoP_RMS) during single-leg stance (p = 0.036, η2ₚ = 0.134) and the time to stabilization (TTS) during drop landing (p = 0.007, η2ₚ = 0.209) in the mediolateral (ML) direction. Post hoc comparisons showed that the both of them were decreased after intervention, and greater decreases were observed by the intervention of HD-tDCS + BBT compared to BBT. And, a significant time main effect was observed in the CoP_RMS (p < 0.001, η2ₚ = 0.382) and the TTS (p = 0.005, η2ₚ = 0.224) in the anteroposterior direction, they both decreased after HD-tDCS + BBT and BBT interventions.
Conclusions: Both BBT alone and the combined HD-tDCS + BBT interventions enhanced static and dynamic postural stability in individuals with CAI, while the combined HD-tDCS + BBT intervention demonstrated significantly greater efficacy in improving postural stability in the ML direction compared to BBT alone.
1 Introduction
Lateral ankle sprains (LAS) represent a prevalent category among musculoskeletal injuries, constituting 10%–30% of all reported cases (1, 2). It is estimated a daily incidence exceeding 25,000 ankle sprain cases in the United States alone (3). Approximately 46% of LAS progressing to chronic ankle instability (CAI) (4), characterized by persistent symptoms such as recurrent ankle sprain, “giving way”, pain, and weakness, which may lead to long-term neuromuscular damage and an increased risk of osteoarthritis (5–7). It is estimated that approximately 2 million acute ankle sprains occur annually in the United States (8), resulting in medical costs of about $6.2 billion (9).
Individuals with CAI exhibit disruptions in sensory-motor integration, a critical process for maintaining postural stability. This impairment arises from the damage to mechanoreceptors and afferent fibers in the ankle joint due to recurrent sprains (10), and the maladaptive neuroplastic changes in sensorimotor cortical regions, particularly the primary motor cortex (M1) and primary somatosensory cortex (S1) (11). Postural stability relies on the integration of somatosensory inputs, central nervous system (CNS) processing, and motor outputs that coordinate muscle activity to regulate joint loading and balance (12). In CAI, mechanoreceptor dysfunction compromises sensory input from the ankle, while cortical reorganization in M1 and S1 alters neuromuscular control pathways (13). These combined deficits impair the CNS's capacity to modulate joint mechanics and muscle activation patterns, perpetuating postural instability and functional limitations.
Individuals with CAI demonstrate deficits in both static and dynamic postural stability, which are critical for injury prevention and functional performance. Static postural stability is commonly assessed via root mean square (RMS) of center of pressure (CoP) displacement during single-leg stance (14), which effectively predicts lower-limb injury risk and monitors rehabilitation progress (15). Compared to healthy controls, individuals with CAI exhibit greater CoP_RMS, particularly under open-eye conditions (16). Dynamic postural stability, usually evaluated through time to stabilization (TTS) during drop-landing tasks, quantifies the ability to maintain balance during high-demand activities (17). Individuals with CAI demonstrate prolonged TTS, indicating delayed neuromuscular adjustments and reduced dynamic control (18).
Conventional CAI interventions, such as sensory-targeted training (19), cryotherapy (20), ankle joint mobilization (21), and plantar massage (12), primarily target peripheral deficits (e.g., tactile sensation, proprioception, muscle strength). However, these approaches often yield limited efficacy, with persistent instability or recurrent injury in many cases (22, 23). This may reflect inadequate consideration of maladaptive CNS neuroplasticity, now recognized as a key contributor to CAI-related postural deficits (11, 15). Previous studies demonstrates reconceptualizing CAI as a global sensorimotor integration disorder rather than a localized peripheral injury, with neuroplastic maladaptations observed in cortical regions associated with postural stability (24, 25). Multimodal strategies integrating CNS interventions with peripheral therapies are needed.
Emerging evidence supports transcranial direct current stimulation (tDCS) as a promising CNS rehabilitation strategy for CAI, and a more advanced variant, high-definition tDCS (HD-tDCS), employs compact circular electrode arrays to modulate cortical excitability, enhance neuroplasticity, and improve postural stability with superior spatial specificity and prolonged physiological effects (26–28). Preliminary studies in healthy adults demonstrate that HD-tDCS enhances postural stability (29), suggesting its potential to address CNS-mediated deficits in CAI. Critical to the efficacy of HD-tDCS is the pairing of stimulation with task-specific motor training, as concurrent activation of sensorimotor networks during stimulation amplifies motor learning and skill acquisition (30). For CAI rehabilitation, progressive balance exercises—gradually increasing in complexity—may synergize with HD-tDCS by challenging sensorimotor adaptability and refining motor planning strategies, thereby enhancing postural control (31). For instance, Bosu ball training (BBT)—which creates an unstable surface environment—could serve as an effective paired task, as it demands continuous proprioceptive integration and reactive postural adjustments (30–33).
As mentioned above, postural stability plays a critical role in individuals with CAI, where postural instability serves as a key contributor to recurrent ankle sprains. While tDCS has demonstrated efficacy in improving postural stability in CAI populations, existing studies have separately investigated static and dynamic postural stability (29, 34–36). To our knowledge, no studies have investigated the effects of HD-tDCS on both static and dynamic postural stability concurrently, particularly when combined with task-specific motor training such as BBT. This study aims to investigate whether HD-tDCS paired with BBT enhances postural stability in individuals with CAI compared to BBT alone, hypothesizing that (1) Both the HD-tDCS combined with BBT intervention and the BBT alone could significantly improve static and dynamic postural stability in individuals with CAI, represented by CoP_RMS metric during single leg stance, and TTS during drop landing; (2) the combined HD-tDCS + BBT intervention demonstrates superior improvement compared to BBT alone.
2 Materials and methods
2.1 Sample size estimation
An a priori power analysis (G*Power 3.1) indicated that 26 participants were required to achieve 0.95 statistical power at α = 0.05, based on a previous study's group-by-time interaction effect size (η2p = 0.122 equals to effects size f = 0.372) for CoP_RMS during single-leg stance in individuals with CAI undergoing neuromuscular electrical stimulation (pre: 8.13 ± 1.07 mm vs. post: 6.60 ± 1.14 mm) (37).
2.2 Participants
Participants were recruited from August to October 2024 via university e-newsletters, posters, and direct emails. Seventy-five individuals were screened for eligibility using International Ankle Consortium guidelines and additional criteria (38), with 40 providing informed consent. Inclusion criteria were: (1) history of ≥1 ankle sprain >1 year prior with acute symptoms (pain, swelling, activity limitation >1 day); (2) age 18–25 years without athletic specialization; (3) ≥ 2 episodes of ankle instability/"giving way” in the past 6 months; (4) persistent instability/functional impairment; and (5) Cumberland Ankle Instability Tool score <24 (39). Exclusion criteria included lower-limb fractures/surgeries, acute injury within 3 months, bilateral CAI, or neurological disorders impairing motor control (e.g., cerebellar disorders, stroke) (33). The study was approved by the Shandong Sport University Ethics Committee (No. 2023036) and adhered to the Declaration of Helsinki.
2.3 Protocol
This single-blind RCT employed a computer-generated random sequence to allocate 40 participants (1:1) into two interventions: (1) HD-tDCS + BBT and (2) BBT (sham HD-tDCS + BBT). Both interventions underwent six weeks of intervention (3 sessions/week, 20 min/session), with HD-tDCS/sham and BBT administered concurrently. The protocol comprised: 10-minute warm-up, four targeted exercises (30-second each, 30-second rest intervals after each exercise, cycle repeated five times), totaling 20-minute of exercise, followed by 10-minute cooldown. Static and dynamic postural stability were assessed pre- and post-intervention, with test sequences randomized via computer-generation to minimize order effects.
2.4 High-definition transcranial direct current stimulation
HD-tDCS was delivered via a StarStim8 device (Neuroelectrics, Spain) using a 10/20 EEG-compliant montage of five 5-mm electrodes: one anode (Cz) and four cathodes (Fz, Pz, C3, C4) (40) (Figure 1). Active stimulation applied 2 mA to the anode, with return current distributed across cathodes. The protocol included a 30-second ramp-up to 2 mA, 19-minute at 2 mA, and a 30-second ramp-down. Sham stimulation mirrored this timing but delivered subthreshold currents (<0.1 mA) during the 19-minute phase to preserve blinding (41). Neuro-modeling confirmed focal targeting of sensorimotor cortices (M1/S1) corresponding to foot-ankle representations (42).
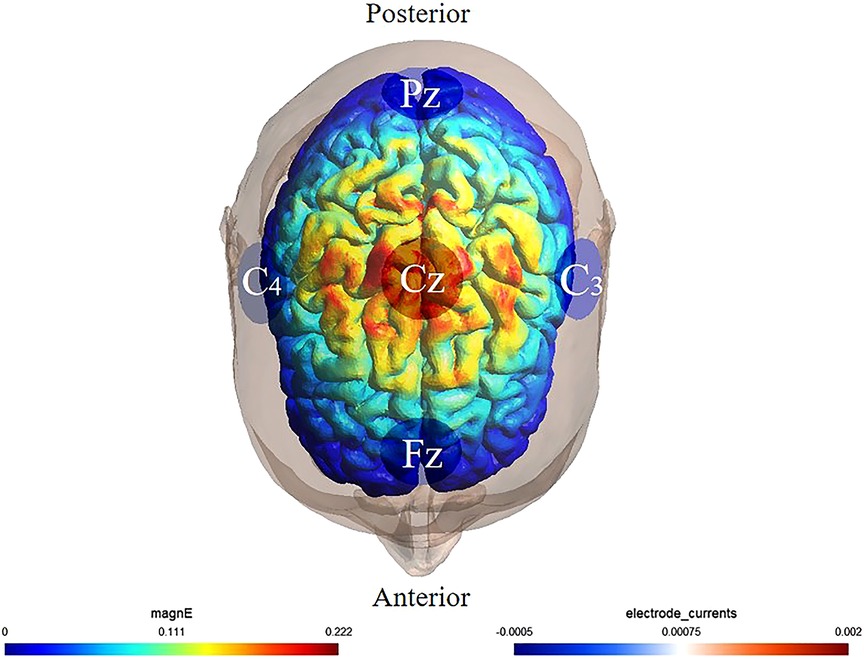
Figure 1. Illustration of HD-tDCS electrode placement. The anode was placed over Cz of the 10/20 EEG template; the four cathodes were placed over Fz, C3, Pz, and C4. Warmer and cooler colors reflect the larger and smaller modeled electric field normal component, respectively.
2.5 Bosu ball training
Under the guidance of certified instructors, participants performed Bosu ball training following a progressively intensified program. The intervention involved standing on the soft surface of the Bosu ball with the affected limb positioned superiorly and the unaffected limb adjacent. During weeks 1–2, the training progression consisted of: single-leg stance maintenance; single-leg stance with lower extremity anteroposterior swing (30°–45°); single-leg stance with lower extremity mediolateral swing (20°–30°); and single-leg squats. During weeks 3–4, the training progression consisted of: swallow balance positions; single-leg stance with anteroposterior swing (45°–60°); single-leg stance with lower extremity mediolateral swing(30°–45°); and dynamic single-leg squat take-ups. During weeks 5–6, the training progression consisted of: single-leg stance with ball-catching; single-leg stance with lower extremity anteroposterior mediolateral swing(45°–60°); single-leg stance with lower extremity mediolateral swing (30°–45°); and functional reaching tasks involving forward trunk flexion to touch edge of Bosu ball while maintaining single-leg stability(Figure 2).
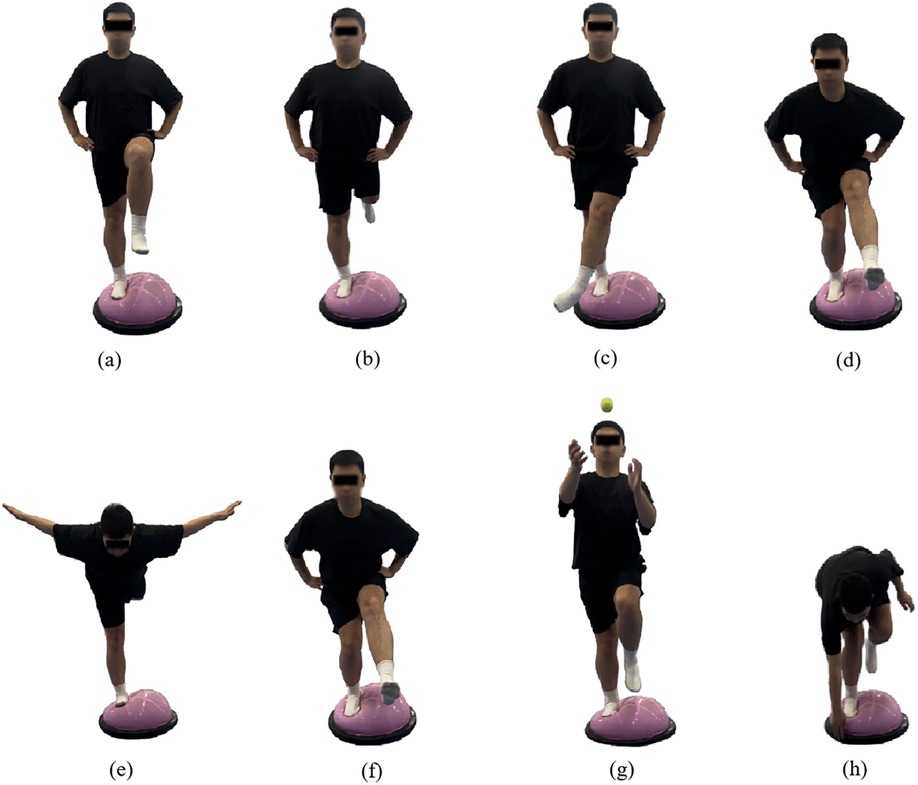
Figure 2. Illustrations of the bosu ball training movements (a) single-leg stance, (b) single-leg stance with swing forward-backward, (c) single-leg stance with swing medial-lateral, (d) single-leg squat, (e) swallow balanced stance, (f) single-legged squat and take-ups, (g) catching a ball while single-leg stance, and (h) bending over to touch the edge while single-leg stance.
2.6 Static postural stability test
Participants completed the single leg stance task to assess static postural stability. After reviewing procedures, warming up, and practicing (≥3 trials), they stood on their affected leg atop a force platform (AMTI, Watertown, MA, USA), hands on hips, gaze fixed forward. The unaffected leg was raised to calf level, with the affected foot maintaining full contact for 30 s. Trials were discarded and repeated if: (1) limbs made contact, (2) hands moved from hips, or (3) trunk/hip deviation exceeded 30. Three valid trials were averaged for analysis, with ≥1-minute rest between attempts to minimize fatigue.
2.7 Dynamic postural stability test
Participants performed a drop-landing task to assess dynamic postural stability (43). Standing on a 20 cm wooden platform in front of a force plate, they positioned feet shoulder-width apart, hands at their waist, and gaze fixed forward. Following instructions, participants stepped forward with their affected limb, dropped onto the force plate, and stabilized on the affected leg for 5 s (Figure 3) for three trials. A successful trial required landing without losing balance or corrective movements. Prior to the formal testing, participants completed three practice trials to become familiar with the procedure.
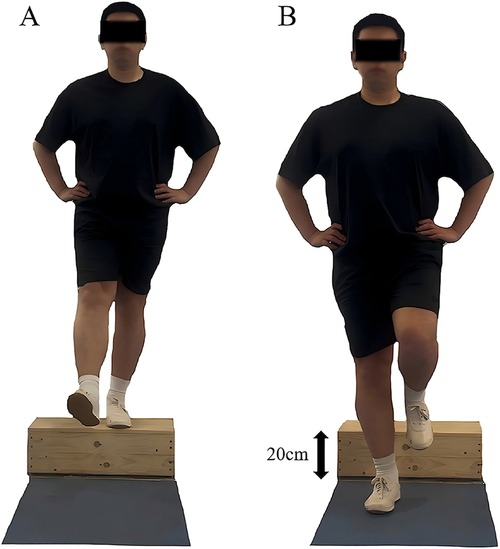
Figure 3. Illustration of the dynamic postural stability test. (A) Starting position. (B) Ending position. Right leg represents the affected side, while the left leg represents the unaffected side.
2.8 Data reduction
During static postural stability test, CoP_RMS was calculated from anteroposterior (AP) and mediolateral (ML) directions using CoP data sampled at 1,000 Hz. The raw data were filtered using a fourth-order low-pass Butterworth filter with a 12 Hz cutoff frequency (14). Filtered data were used to compute CoP_RMS (mm) for each participant using the following formulas (14):
where xi and yi represent CoP coordinates in AP and ML directions, while x bar and y bar denote their means. The denominator N−1 reflects sample-based calculation.
During dynamic postural stability test, ground reaction force (GRF) data were recorded at 1,000 Hz and filtered using a fourth-order low-pass Butterworth filter with a 12 Hz cutoff frequency (14). Filtered data from initial landing (GRF > 10 N) to 5 s post-landing were used to compute time to stabilization (TTS) through sequential average using the following formulas (44):
where Fx and Fy represent AP and ML GRF components. TTS was defined as the time from landing to when the sequential average of each component remained within ±25% of the standard deviation of the overall mean GRF for ≥1 s (45) (Figure 4).
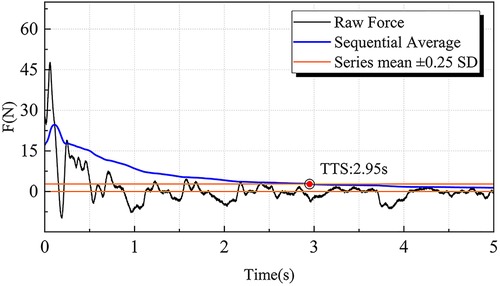
Figure 4. Illustration of the time to stabilization (TTS) calculation. The moment when the sequential average of ground reaction forces in the anteroposterior and mediolateral directions reaches and remains within the range of the series mean ± 0.25 SD is defined as the TTS. TTS, time to stabilization.
2.9 Statistics analysis
Normality was confirmed using the Shapiro–Wilk test. A two-way mixed-design ANOVA evaluated main effects and interactions. Group (HD-tDCS + BBT vs. BBT) was specified as the between-subjects factor, and time (week0 vs. week7) as the within-subjects factor. Significant interactions were decomposed using Bonferroni-adjusted post hoc pairwise comparisons with correction for multiple testing. Effect sizes were reported as partial eta squared (η2ₚ: small = 0.01–0.06, moderate = 0.06–0.14, large > 0.14) for ANOVA results (46) and Cohen's d (trivial < 0.20, small = 0.21–0.50, medium = 0.51–0.80, large > 0.81) (47) for post hoc contrasts. Data are presented as mean ± standard deviation (SD). Significance was set at p < 0.05.
3 Results
All dependent variables were normally distributed. Forty participants were randomly assigned to HD-tDCS + BBT (n = 20) or BBT (n = 20) interventions (Figure 5). Six withdrew due to scheduling conflicts, leaving 18 (HD-tDCS + BBT: 20.1 ± 1.3 years, 175.5 ± 8.0 cm, 72.4 ± 9.6 kg) and 16 (BBT: 21.0 ± 1.8 years, 173.3 ± 12.0 cm, 68.9 ± 11.5 kg) participants in each group. No between-group differences in age, height, or body mass existed (p > 0.05).
Figure 5. Participation flow chart. Final analysis included data from 34 participants. 41 participants were excluded from the original 75 recruited due to various reasons; HD-tDCS, high-definition transcranial direct current stimulation; BBT, Bosu ball training.
Figure 6 revealed a significant group × time interaction for CoP_RMSml (p = 0.036, η2ₚ = 0.134). Post hocs showed both interventions reduced CoP_RMSml from week0 to week7 (HD-tDCS + BBT: p < 0.001, d = 1.826; BBT: p = 0.027, d = 0.765), with a greater reduction in the HD-tDCS + BBT intervention (p = 0.002, d = 1.105). CoP_RMSap exhibited a main effect of time (p < 0.001, η2ₚ = 0.382), with decreases after interventions.
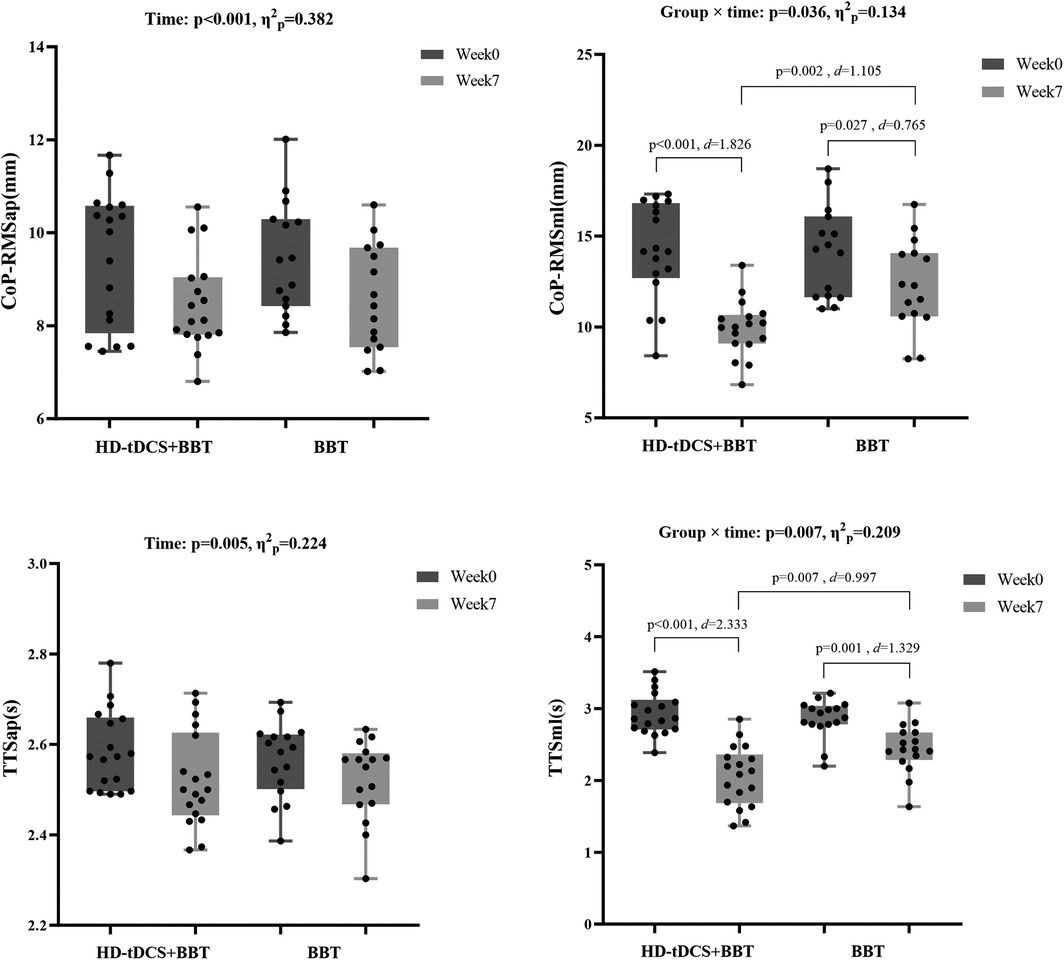
Figure 6. Static and dynamic postural stability before and after intervention. CoP_RMS, root mean square of the center of pressure; HD-tDCS, high-definition transcranial direct current stimulation; BBT, Bosu ball training; TTS, time to stabilization; ap, anterior-posterior direction; ml, medial-lateral direction.
Figure 6 showed a significant group × time interaction for TTSml (p = 0.007, η2ₚ = 0.209). Post hocs indicated reduced TTSml over time in both groups (HD-tDCS + BBT: p < 0.001, d = 2.333; BBT: p = 0.001, d = 1.329), with a larger decrease in the HD-tDCS + BBT intervention (p = 0.007, d = 0.997). TTSap demonstrated a main effect of time (p = 0.005, η2ₚ = 0.224), with reductions after interventions.
4 Discussion
This study investigated the effects of HD-tDCS combined with BBT on static and dynamic postural stability in individuals with CAI. Our results supported Hypotheses 1 and 2, demonstrating that both HD-tDCS + BBT and BBT alone significantly reduced CoP_RMS and TTS. And, HD-tDCS + BBT elicited greater improvements compared to BBT alone, suggesting enhanced efficacy of the combined intervention for postural stability.
4.1 Bosu ball training improved static and dynamic postural stability
This study demonstrates that both active and sham HD-tDCS, when combined with BBT, significantly improved static and dynamic postural stability in individuals with CAI in both AP and ML directions, underscoring the effectiveness of BBT as a rehabilitative intervention. These findings align with prior research: one study reported that unstable surface training enhances postural stability compared to stable surfaces by increasing neuromuscular demands and sensory integration (32), while another showed that such training elevates muscle activation and proprioceptive feedback to optimize joint stabilization (48). The observed improvements in postural stability following BBT in CAI may stem from its dual mechanisms of enhanced sensory input and neuromuscular adaptation. The compliant surface of the Bosu ball increases sensory stimulation by altering foot-support contact and pressure distribution, which amplifies proprioceptive input (49, 50). A meta-analysis confirmed that augmented sensory input significantly enhances postural stability in CAI populations (51), aligning with evidence of a strong correlation between proprioceptive acuity and postural control in this cohort (52). The inherent instability of the Bosu ball introduces controlled postural perturbations, promoting sensory reweighting—a CNS process that recalibrates reliance on visual, vestibular, and somatosensory inputs to compensate for instability (53). This adaptive mechanism synergizes with neuromuscular demands, as maintaining balance on an unstable surface requires dynamic adjustments to the center of gravity within functional limits (54, 55), thereby counteracting CAI-related deficits in lower limb strength and postural instability (56). Notably, a 4-week unstable surface training protocol improved both muscular strength and postural stability in CAI (48), supporting the results that compliant surfaces elevate neuromuscular demands, fostering enhanced muscle activation and force generation (57). Consequently, these neuromuscular adaptations contribute to improved postural stability in individuals with CAI.
4.2 Superior effects of combined HD-tDCS and Bosu ball training over Bosu ball training alone
Our findings demonstrate that HD-tDCS combined with BBT exhibited superior efficacy compared to BBT alone in enhancing static and dynamic postural stability among individuals with CAI, these results align with previous studies showing that HD-tDCS paired with foot-core exercises improves proprioception and static balance in healthy adults (36), and that targeting M1/S1 with HD-tDCS during short-foot exercises enhances proprioception and dynamic balance in CAI populations (42). Additionally, evidence that anodal tDCS over M1, when coupled with targeted muscular attention, augments motor cortex plasticity—evidenced by increased motor evoked potentials and reduced short-interval intracortical inhibition—further supports the synergistic effects of HD-tDCS and sensorimotor training on postural stability and motor learning (58, 59).
The superior efficacy of the combined intervention may be attributed to enhanced somatosensory integration, facilitated by HD-tDCS-induced neuromodulation of sensorimotor networks. Primary, HD-tDCS over S1 and M1 likely optimizes cortical excitability, improving sensory processing and motor output during BBT. This aligns with prior work demonstrating that tDCS enhances peripheral somatosensory acuity—evidenced by reduced vibration detection thresholds at the plantar surface and improved hallux sensitivity—thereby refining foot-ankle sensorimotor integration and postural control (60, 61). Such effects may stem from tDCS-mediated modulation of S1 excitability, which could synergize with proprioceptive training to enhance dynamic stability (62). Furthermore, tDCS applied over adjacent temporal-parietal regions has shown benefits for vestibulo-perceptual function (63), suggesting that stimulation effects may extend beyond targeted areas (M1/S1) to interconnected cortical networks involved in multisensory integration, collectively contributing to improved postural outcomes. Secondary, HD-tDCS may enhance postural stability via M1-mediated modulation of lower limb motor output. Individuals with CAI exhibit reduced M1 excitability projecting to the peroneus longus compared to controls (57, 64). Anodal tDCS increases M1 excitability by decreasing resting membrane potential in targeted regions, thereby augmenting corticospinal drive (34). This neuromodulation persists post-stimulation, reducing short-interval intracortical inhibition and enhancing voluntary muscle activation, which strengthens peroneal muscle contributions to postural control (59, 65).
4.3 HD-tDCS induced additional improvement of the postural stability in the ML direction
Our results indicate that the additional benefits of HD-tDCS occur specifically in the ML direction. This finding is partially supported by a previous study, which demonstrated that compared to a 4-week foot core training program, HD-tDCS improved passive kinesthesia thresholds for ankle inversion and eversion in healthy individuals, but had limited effects on ankle proprioception for plantarflexion and dorsiflexion (36). Neuromuscular control systems exhibit direction-dependent modulation in postural compensation responses. During perturbations in AP direction, postural stability is maintained through coordinated limb swing patterns and compensatory foot displacement (66). Conversely, perturbations in the ML direction may present greater neuromuscular challenges due to anatomical constraints in lateral limb repositioning (66). The stabilization response in the ML direction initiates with activation of the ankle eversion muscles to counteract inversion stresses (67, 68), a mechanism potentially compromised in individuals with CAI, thereby increasing the risk of sprain recurrence. This directional situation shows a higher correlation between ML stability deficits and fall risk compared to AP instability (69).
5 Limitations
There are several limitations to this study. Firstly, the study compared the effects of active vs. sham HD-tDCS combined with BBT on improving postural stability in individuals with CAI. However, the isolated effects of HD-tDCS remain undetermined. Despite this, our findings demonstrate that cortical stimulation significantly enhances the efficacy of BBT, establishing a clinically relevant physical therapeutic paradigm. Secondly, the study focused on the outcomes of a six-week intervention without evaluating long-term efficacy, which limits conclusions about the sustained effects of the intervention. Nevertheless, the study demonstrated clear improvements in postural stability, suggesting the potential value of prolonged intervention.
6 Conclusion
Both HD-tDCS + BBT and BBT alone significantly improves static and dynamic postural stability in individuals with CAI, while the combination of HD-tDCS and BBT is more effective than BBT alone, particularly in the ML direction. These findings highlight the potential of combining CNS interventions with peripheral therapies to improve postural instability for individuals with CAI.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Shandong Sport University Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YG: Data curation, Methodology, Writing – original draft. HG: Writing – review & editing, Data curation. XH: Writing – review & editing. XL: Data curation, Writing – review & editing. YL: Writing – review & editing, Methodology, Data curation. DW: Data curation, Methodology, Writing – review & editing. PS: Writing – review & editing, Methodology. LG: Writing – review & editing, Methodology. QS: Methodology, Supervision, Writing – review & editing, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the General Administration of Sport of China [grant number 23QN009], and the National Natural Science Foundation of China [grant number No.12102235].
Acknowledgments
The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The authors thank the participants for their commitment during the research period.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fong DT, Hong Y, Chan LK, Yung PS, Chan KM. A systematic review on ankle injury and ankle sprain in sports. Sports Med. (2007) 37(1):73–94. doi: 10.2165/00007256-200737010-00006
2. Zhu X, Wei F, Li S, Zhang T, Shen P, Fong DT, et al. Toe-out landing reduces anterior talofibular ligament strain while maintaining calcaneofibular ligament strain in individuals with chronic ankle instability. J Sport Health Sci. (2025):101035. doi: 10.1016/j.jshs.2025.101035
3. Yoon SJ, Kim JB, Jung KJ, Chang HJ, Hong YC, Hong CH, et al. Evaluation of the quality of information available on the internet regarding chronic ankle instability. Medicina. (2022) 58(10):1315. doi: 10.3390/medicina58101315
4. Lin CI, Houtenbos S, Lu YH, Mayer F, Wippert PM. The epidemiology of chronic ankle instability with perceived ankle instability- a systematic review. J Foot Ankle Res. (2021) 14(1):41. doi: 10.1186/s13047-021-00480-w
5. Hertel J, Corbett RO. An updated model of chronic ankle instability. J Athl Train. (2019) 54(6):572–88. doi: 10.4085/1062-6050-344-18
6. Ma T, Liu C, Li H, Xu X, Wang Y, Tao W, et al. Rehabilitation increases cortical activation during single-leg stance in patients with chronic ankle instability. Asia-Pac J Sports Med Arthrosc Rehabil Technol. (2024) 35:65–70. doi: 10.1016/j.asmart.2023.11.008
7. Zhang J, Yang K, Wang C, Gu W, Li X, Fu S, et al. Risk factors for chronic ankle instability after first episode of lateral ankle sprain: a retrospective analysis of 362 cases. J Sport Health Sci. (2023) 12(5):606–12. doi: 10.1016/j.jshs.2023.03.005
8. Herzog MM, Kerr ZY, Marshall SW, Wikstrom EA. Epidemiology of ankle sprains and chronic ankle instability. J Athl Train. (2019) 54(6):603–10. doi: 10.4085/1062-6050-447-17
9. Gribble PA, Bleakley CM, Caulfield BM, Docherty CL, Fourchet F, Fong DT, et al. Evidence review for the 2016 international ankle consortium consensus statement on the prevalence, impact and long-term consequences of lateral ankle sprains. Br J Sports Med. (2016) 50(24):1496–505. doi: 10.1136/bjsports-2016-096189
10. Xue X, Ma T, Li Q, Song Y, Hua Y. Chronic ankle instability is associated with proprioception deficits: a systematic review and meta-analysis. J Sport Health Sci. (2021) 10(2):182–91. doi: 10.1016/j.jshs.2020.09.014
11. Needle AR, Lepley AS, Grooms DR. Central nervous system adaptation after ligamentous injury: a summary of theories, evidence, and clinical interpretation. Sports Med. (2017) 47(7):1271–88. doi: 10.1007/s40279-016-0666-y
12. Wikstrom EA, Song K, Lea A, Brown N. Comparative effectiveness of plantar-massage techniques on postural control in those with chronic ankle instability. J Athl Train. (2017) 52(7):629–35. doi: 10.4085/1062-6050-52.4.02
13. Kim KM, Kim JS, Needle AR. Soleus arthrogenic muscle inhibition following acute lateral ankle sprain correlates with symptoms and ankle disability but not with postural control. J Sport Health Sci. (2024) 13(4):559–68. doi: 10.1016/j.jshs.2024.02.005
14. Song Q, Zhang X, Mao M, Sun W, Zhang C, Chen Y, et al. Relationship of proprioception, cutaneous sensitivity, and muscle strength with the balance control among older adults. J Sport Health Sci. (2021) 10(5):585–93. doi: 10.1016/j.jshs.2021.07.005
15. McKeon PO, Hertel J. Systematic review of postural control and lateral ankle instability, part I: can deficits be detected with instrumented testing. J Athl Train. (2008) 43(3):293–304. doi: 10.4085/1062-6050-43.3.293
16. Xue X, Wang Y, Xu X, Li H, Li Q, Na Y, et al. Postural control deficits during static single-leg stance in chronic ankle instability: a systematic review and meta-analysis. Sports Health. (2024) 16(1):29–37. doi: 10.1177/19417381231152490
17. Liu Y, Dong S, Wang Q, Liu Z, Song Q, Shen P. Deficits in proprioception and strength may contribute to the impaired postural stability among individuals with functional ankle instability. Front Physiol. (2024) 15:1342636. doi: 10.3389/fphys.2024.1342636
18. Wright CJ, Arnold BL, Ross SE. Altered kinematics and time to stabilization during drop-jump landings in individuals with or without functional ankle instability. J Athl Train. (2016) 51(1):5–15. doi: 10.4085/1062-6050-51.2.10
19. McKeon PO, Wikstrom EA. The effect of sensory-targeted ankle rehabilitation strategies on single-leg center of pressure elements in those with chronic ankle instability: a randomized clinical trial. J Sci Med Sport. (2019) 22(3):288–93. doi: 10.1016/j.jsams.2018.08.017
20. Kim KM, Hart JM, Saliba SA, Hertel J. Effects of focal ankle joint cooling on unipedal static balance in individuals with and without chronic ankle instability. Gait Posture. (2015) 41(1):282–7. doi: 10.1016/j.gaitpost.2014.10.017
21. Hoch MC, Mullineaux DR, Andreatta RD, English RA, Medina-McKeon JM, Mattacola CG, et al. Effect of a 2-week joint mobilization intervention on single-limb balance and ankle arthrokinematics in those with chronic ankle instability. J Sport Rehabil. (2014) 23(1):18–26. doi: 10.1123/jsr.2012-0125
22. McKeon PO, Wikstrom EA. Sensory-targeted ankle rehabilitation strategies for chronic ankle instability. Med Sci Sports Exercise. (2016) 48(5):776–84. doi: 10.1249/mss.0000000000000859
23. Wright CJ, Linens SW, Cain MS. A randomized controlled trial comparing rehabilitation efficacy in chronic ankle instability. J Sport Rehabil. (2017) 26(4):238–49. doi: 10.1123/jsr.2015-0189
24. Maricot A, Dick E, Walravens A, Pluym B, Lathouwers E, De Pauw K, et al. Brain neuroplasticity related to lateral ankle ligamentous injuries: a systematic review. Sports Med. (2023) 53(7):1423–43. doi: 10.1007/s40279-023-01834-z
25. Hass CJ, Bishop MD, Doidge D, Wikstrom EA. Chronic ankle instability alters central organization of movement. Am J Sports Med. (2010) 38(4):829–34. doi: 10.1177/0363546509351562
26. Geiger M, Supiot A, Zory R, Aegerter P, Pradon D, Roche N. The effect of transcranial direct current stimulation (tDCS) on locomotion and balance in patients with chronic stroke: study protocol for a randomised controlled trial. Trials. (2017) 18(1):492. doi: 10.1186/s13063-017-2219-6
27. Kuo HI, Bikson M, Datta A, Minhas P, Paulus W, Kuo MF, et al. Comparing cortical plasticity induced by conventional and high-definition 4x1 ring tDCS: a neurophysiological study. Brain Stimul. (2013) 6(4):644–8. doi: 10.1016/j.brs.2012.09.010
28. Datta A, Bansal V, Diaz J, Patel J, Reato D, Bikson M. Gyri-precise head model of transcranial direct current stimulation: improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul. (2009) 2(4):201–7.e1. doi: 10.1016/j.brs.2009.03.005
29. Wang B, Shen B, Xiao S, Zhou J, Fu W. Effects of four weeks intervention combining high-definition transcranial direct current stimulation and foot core exercise on dynamic postural stability. J Biomech. (2024) 177:112418. doi: 10.1016/j.jbiomech.2024.112418
30. Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist. (2011) 17(1):37–53. doi: 10.1177/1073858410386614
31. Burcal CJ, Jeon H, Gonzales JM, Faust ME, Thomas AC, Hubbard-Turner TJ, et al. Cortical measures of motor planning and balance training in patients with chronic ankle instability. J Athl Train. (2019) 54(6):727–36. doi: 10.4085/1062-6050-450-17
32. Ha SY, Han JH, Sung YH. Effects of ankle strengthening exercise program on an unstable supporting surface on proprioception and balance in adults with functional ankle instability. J Exerc Rehabil. (2018) 14(2):301–5. doi: 10.12965/jer.1836082.041
33. Huang X, Gao H, Fu H. Effects of transcranial direct current stimulation combined with Bosu ball training on the injury potential during drop landing in people with chronic ankle instability. Front Physiol. (2024) 15:1451556. doi: 10.3389/fphys.2024.1451556
34. Bruce AS, Howard JS, VAN Werkhoven H, McBride JM, Needle AR. The effects of transcranial direct current stimulation on chronic ankle instability. Med Sci Sports Exercise. (2020) 52(2):335–44. doi: 10.1249/mss.0000000000002129
35. Xiao S, Wang B, Zhang X, Zhou J, Fu W. Acute effects of high-definition transcranial direct current stimulation on foot muscle strength, passive ankle kinesthesia, and static balance: a pilot study. Brain Sci. (2020) 10(4):246. doi: 10.3390/brainsci10040246
36. Xiao S, Wang B, Zhang X, Zhou J, Fu W. Effects of 4 weeks of high-definition transcranial direct stimulation and foot core exercise on foot sensorimotor function and postural control. Front Bioeng Biotechnol. (2022) 10:894131. doi: 10.3389/fbioe.2022.894131
37. Wang Y, Zheng H, Wang J, Xu P, Sun W. Neuromuscular electrical stimulation of peroneal Longus improve balance control ability in young adults with chronic ankle instability: a randomized controlled trial. Am J Phys Med Rehabil. (2024) 103(12):1088–93. doi: 10.1097/phm.0000000000002510
38. Gribble PA, Delahunt E, Bleakley C, Caulfield B, Docherty CL, Fourchet F, et al. Selection criteria for patients with chronic ankle instability in controlled research: a position statement of the international ankle consortium. J Orthop Sports Phys Ther. (2013) 43(8):585–91. doi: 10.2519/jospt.2013.0303
39. Hiller CE, Refshauge KM, Bundy AC, Herbert RD, Kilbreath SL. The cumberland ankle instability tool: a report of validity and reliability testing. Arch Phys Med Rehabil. (2006) 87(9):1235–41. doi: 10.1016/j.apmr.2006.05.022
40. Villamar MF, Volz MS, Bikson M, Datta A, Dasilva AF, Fregni F. Technique and considerations in the use of 4 × 1 ring high-definition transcranial direct current stimulation (HD-tDCS). J Vis Exp. (2013) (77):e50309. doi: 10.3791/50309
41. Palm U, Reisinger E, Keeser D, Kuo MF, Pogarell O, Leicht G, et al. Evaluation of sham transcranial direct current stimulation for randomized, placebo-controlled clinical trials. Brain Stimul. (2013) 6(4):690–5. doi: 10.1016/j.brs.2013.01.005
42. Ma Y, Yin K, Zhuang W, Zhang C, Jiang Y, Huang J, et al. Effects of combining high-definition transcranial direct current stimulation with short-foot exercise on chronic ankle instability: a pilot randomized and double-blinded study. Brain Sci. (2020) 10(10):749. doi: 10.3390/brainsci10100749
43. Mao M, Yin Y, Luo D, Liu H, Yu B. Evaluation of dynamic postural control during single-leg landing tasks using initial impact force, landing leg stiffness and time to stabilisation. Sports Biomech. (2024) 23(2):182–95. doi: 10.1080/14763141.2020.1833969
44. Fransz DP, Huurnink A, de Boode VA, Kingma I, van Dieën JH. Time to stabilization in single leg drop jump landings: an examination of calculation methods and assessment of differences in sample rate, filter settings and trial length on outcome values. Gait Posture. (2015) 41(1):63–9. doi: 10.1016/j.gaitpost.2014.08.018
45. Fransz DP, Huurnink A, de Boode VA, Kingma I, van Dieën JH. The effect of the stability threshold on time to stabilization and its reliability following a single leg drop jump landing. J Biomech. (2016) 49(3):496–501. doi: 10.1016/j.jbiomech.2015.12.048
46. Pierce CA, Block RA, Aguinis H. Cautionary note on reporting eta-squared values from multifactor ANOVA designs. Educ. Psychol. Meas. (2004) 64(6):916–24. doi: 10.1177/0013164404264848
48. Cuğ M, Duncan A, Wikstrom E. Comparative effects of different balance-training-progression styles on postural control and ankle force production: a randomized controlled trial. J Athl Train. (2016) 51(2):101–10. doi: 10.4085/1062-6050-51.2.08
49. Alizamani S, Ghasemi G, Lenjan Nejadian S. Effects of eight week core stability training on stable- and unstable-surface on ankle muscular strength, proprioception, and dorsiflexion in athletes with chronic ankle instability. J Bodyw Mov Ther. (2023) 34:6–12. doi: 10.1016/j.jbmt.2023.04.005
50. Viseux F, Lemaire A, Barbier F, Charpentier P, Leteneur S, Villeneuve P. How can the stimulation of plantar cutaneous receptors improve postural control? Review and clinical commentary. Neurophysiol Clin. (2019) 49(3):263–8. doi: 10.1016/j.neucli.2018.12.006
51. Hu X, Liao J, Hu X, Zeng Z, Wang L. Effects of plantar-sensory treatments on postural control in chronic ankle instability: a systematic review and meta-analysis. PLoS One. (2023) 18(6):e0287689. doi: 10.1371/journal.pone.0287689
52. Liu Y, Song Q, Liu Z, Dong S, Hiller C, Fong DTP, et al. Correlations of postural stability to proprioception, tactile sensation, and strength among people with chronic ankle instability. Motor Control. (2024) 28(4):464–79. doi: 10.1123/mc.2023-0084
53. Mademli L, Mavridi D, Bohm S, Patikas DA, Santuz A, Arampatzis A. Standing on unstable surface challenges postural control of tracking tasks and modulates neuromuscular adjustments specific to task complexity. Sci Rep. (2021) 11(1):6122. doi: 10.1038/s41598-021-84899-y
54. Hof AL. The equations of motion for a standing human reveal three mechanisms for balance. J Biomech. (2007) 40(2):451–7. doi: 10.1016/j.jbiomech.2005.12.016
55. Behm DG, Muehlbauer T, Kibele A, Granacher U. Effects of strength training using unstable surfaces on strength, power and balance performance across the lifespan: a systematic review and meta-analysis. Sports Med. (2015) 45(12):1645–69. doi: 10.1007/s40279-015-0384-x
56. Dong S, Liu Y, Liu Z, Shen P, Sun H, Zhang P, et al. Can arthrogenic muscle inhibition exist in peroneal muscles among people with chronic ankle instability? A cross-sectional study. Sports Med Open. (2024) 10(1):35. doi: 10.1186/s40798-024-00710-y
57. Nepocatych S, Ketcham CJ, Vallabhajosula S, Balilionis G. The effects of unstable surface balance training on postural sway, stability, functional ability and flexibility in women. J Sports Med Phys Fitness. (2018) 58(1-2):27–34. doi: 10.23736/s0022-4707.16.06797-9
58. Yamaguchi T, Moriya K, Tanabe S, Kondo K, Otaka Y, Tanaka S. Transcranial direct-current stimulation combined with attention increases cortical excitability and improves motor learning in healthy volunteers. J Neuroeng Rehabil. (2020) 17(1):23. doi: 10.1186/s12984-020-00665-7
59. Weier AT, Pearce AJ, Kidgell DJ. Strength training reduces intracortical inhibition. Acta Physiol. (2012) 206(2):109–19. doi: 10.1111/j.1748-1716.2012.02454.x
60. Yamamoto S, Ishii D, Ichiba N, Yozu A, Kohno Y. Cathodal tDCS on the motor area decreases the tactile threshold of the distal pulp of the hallux. Neurosci Lett. (2020) 719:133887. doi: 10.1016/j.neulet.2018.10.032
61. Zhou J, Lo OY, Lipsitz LA, Zhang J, Fang J, Manor B. Transcranial direct current stimulation enhances foot sole somatosensation when standing in older adults. Exp Brain Res. (2018) 236(3):795–802. doi: 10.1007/s00221-018-5178-6
62. de Moura M, Hazime FA, Marotti Aparicio LV, Grecco LAC, Brunoni AR, Hasue RH. Effects of transcranial direct current stimulation (tDCS) on balance improvement: a systematic review and meta-analysis. Somatosens Mot Res. (2019) 36(2):122–35. doi: 10.1080/08990220.2019.1624517
63. Kyriakareli A, Cousins S, Pettorossi VE, Bronstein AM. Effect of transcranial direct current stimulation on vestibular-ocular and vestibulo-perceptual thresholds. Neuroreport. (2013) 24(14):808–12. doi: 10.1097/WNR.0b013e3283646e65
64. Pietrosimone BG, Gribble PA. Chronic ankle instability and corticomotor excitability of the fibularis longus muscle. J Athl Train. (2012) 47(6):621–6. doi: 10.4085/1062-6050-47.6.11
65. Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. (2001) 57(10):1899–901. doi: 10.1212/wnl.57.10.1899
66. Hu S, Ma X, Ma X, Sun W, Zhou Z, Chen Y, et al. Relationship of strength, joint kinesthesia, and plantar tactile sensation to dynamic and static postural stability among patients with anterior cruciate ligament reconstruction. Front Physiol. (2023) 14:1112708. doi: 10.3389/fphys.2023.1112708
67. Hertel J. Functional anatomy, pathomechanics, and pathophysiology of lateral ankle instability. J Athl Train. (2002) 37(4):364–75.12937557
68. Menacho Mde O, Pereira HM, Oliveira BI, Chagas LM, Toyohara MT, Cardoso JR. The peroneus reaction time during sudden inversion test: systematic review. J Electromyogr Kinesiol. (2010) 20(4):559–65. doi: 10.1016/j.jelekin.2009.11.007
Keywords: ankle sprains, neuromuscular control, non-invasive brain stimulation, postural control, unstable surface training
Citation: Ge Y, Gao H, Huang X, Luo X, Liu Y, Wang D, Shen P, Guo L and Song Q (2025) Effects of HD-tDCS combined with Bosu ball training on static and dynamic postural stability among individuals with chronic ankle instability. Front. Sports Act. Living 7:1618683. doi: 10.3389/fspor.2025.1618683
Received: 26 April 2025; Accepted: 28 May 2025;
Published: 25 June 2025.
Edited by:
Jia Han, Shanghai University of Medicine and Health Sciences, ChinaCopyright: © 2025 Ge, Gao, Huang, Luo, Liu, Wang, Shen, Guo and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qipeng Song, c29uZ3FpcGVuZ0BzZHBlaS5lZHUuY24=
 Yubin Ge
Yubin Ge He Gao
He Gao Xueke Huang
Xueke Huang Xin Luo
Xin Luo Yanhao Liu
Yanhao Liu Dongmei Wang
Dongmei Wang Peixin Shen
Peixin Shen Liang Guo
Liang Guo Qipeng Song
Qipeng Song