- 1Department of Kinesiology, California State University, Sacramento, CA, United States
- 2Department of Physical Therapy, University of Arizona, Tucson, AZ, United States
- 3Department of Physiology, University of Arizona, Tucson, AZ, United States
- 4Medical Investigations of Neurodevelopmental Disorders Institute, Sacramento, CA, United States
- 5Department of Nursing, California State University, Sacramento, CA, United States
- 6Department of Speech Language and Hearing Sciences, University of Arizona, Tucson, AZ, United States
Fragile X-associated Tremor/Ataxia Syndrome (FXTAS) is a late-onset neurodegenerative disorder characterized by progressive motor dysfunction, including cerebellar ataxia and gait instability. Although tandem walking is a sensitive clinical marker of cerebellar dysfunction, its utility in tracking longitudinal motor decline in FXTAS remains unexplored and the trajectory of motor decline in FXTAS is not well characterized. Therefore, the purpose of this case report was to determine whether tandem walking performance deteriorates over a one-year period in an individual with FXTAS. A 68-year-old male with genetically confirmed FXTAS completed a 15-second tandem walking trial at baseline and again after one year. Kinematic data were collected using a Vicon motion capture system. Step width was calculated at each heel strike as the distance between the mediolateral position of the left and right heel markers. The mean step width considerably increased from baseline tandem walking of 45.21 ± 33.47 mm (SD) compared to the 1-year follow-up trial step width of 85.79 ± 15.80 mm (SD) indicating potential progressive mediolateral instability. This case report provides preliminary evidence that step width during tandem walking may be a sensitive marker of longitudinal motor decline in FXTAS and declines in gait stability can occur within one year. Larger studies with repeated measures and additional gait metrics are warranted to validate these findings.
Introduction
Gait is often used as one of the metrics to determine the quality of life of an individual due to the importance of mobility on everyday functioning (1–3). Metrics such as gait speed, step width, step length, and cadence have been used to compare pathological to healthy controls to better understand characteristics of neurological conditions (4–7). Gait deviations have been extensively studied in populations such as stroke (8–10), ataxia (11–13), Parkinson's disease (14–16), and cerebral palsy (17–19). Perhaps more importantly than classifications of gait by neurological disorders, the progression of neurological conditions as they manifest as gait deviations have been studied and reported (20–23). A less studied neurological degenerative disorder known as Fragile X-associated Tremor/Ataxia Syndrome (FXTAS), has received less attention in the analysis of biomechanical gait studies. The prevalence of the fragile X premutation is 1 in 150–300 females and 1 in 400–850 males with majority of the males with the premutation developing FXTAS (24). FXTAS is characterized by cerebellar ataxia, intention tremor, cognitive decline, and neural changes seen on MRI (i.e., middle cerebellar peduncle sign representing white matter disease, and global brain atrophy) (24). Characterizing gait changes across the progression of neurological disorders such as FXTAS, Parkinson's disease, and cerebellar ataxias is essential, as gait impairments often reflect the underlying neurodegenerative process and may serve as early biomarkers of disease severity and progression. Understanding these temporal changes can inform clinical staging, guide intervention strategies, and improve fall risk management across disease stages.
The current understanding of walking gait in FXTAS is limited as this disease was relatively recently discovered (25). Compared to healthy controls, individuals with FXTAS exhibited significantly impaired gait across all domains, characterized by reduced stride velocity and cadence, increased gait variability, and longer double-limb support times (26). Furthermore, individuals with FXTAS exhibited a reduced stride velocity, increased stride variability and asymmetry (27). Prior work provides insights into gait deficits in individuals with FXTAS, however the progression of the disease's effect on gait remains elusive.
A common hallmark gait deviation in individuals with ataxia is an increased step width (5, 28, 29). Tandem walking in individuals with ataxia becomes an increasingly difficult task with increased step width, high variability in foot placement, and a significantly greater number of missteps compared to controls (28). As such, tandem walking is one of the most sensitive clinical measurements to detect cerebellar dysfunction (30). Furthermore, step width may be an indicator of cerebellar health during tandem walking as individuals with cerebellar disease did not differ in gait speed, step length, cadence, step height, foot angle, stance time, or swing them when compared to healthy controls (28).
Therefore, the purpose of this case report was to investigate whether tandem walking could detect progression in gait deficits after a one-year follow-up period in an individual with FXTAS. We hypothesized that the participant would exhibit a wider step width at the one-year follow-up compared to baseline during a 15-second tandem walking trial.
This investigation serves as a feasibility report to determine whether quantitative tandem gait assessment can detect longitudinal changes in motor performance in FXTAS, and to evaluate the practicality of using step width as markers of disease progression in a single-subject design. Establishing reliable markers of gait progression, such as changes in tandem walking performance, could lay the groundwork for defining distinct stages of motor decline in FXTAS and lead to larger study sizes. By complementing tandem gait assessment with standard gait metrics over time, it may be possible to develop a comprehensive, clinically relevant staging system that reflects the natural trajectory of gross motor impairment in this population.
Methods
Participant
One participant was recruited from the Medical Investigations of Neurodevelopmental Disorders (MIND) Institute and was medically confirmed through genetic testing to have the premutation of the fragile-X messenger ribonucleoprotein 1 gene (FMR1) and confirmed FXTAS. FXTAS was diagnosed genetically by the MIND Institute. At baseline, the participant was a 68 year old male, 177 cm in height, weighed 110 kg, and had a BMI of 35.1. The participant's FXTAS stage was unknown at the time of the biomechanical testing. The gait evaluation of this FXTAS case report was not part of a standard procedure at the Medical Investigation of Neurological Disorders Institute as only one individual's data was collected. We believe the individual was in the earlier stages of the disease due to independence of ambulation but this was not officially determined. Furthermore, the participant was able to drive independently to our laboratory. Gait analysis of the participant was conducted at the California State University, Sacramento in the Biomechanics Laboratory. The participant signed an informed consent that was approved by the Institutional Review Board at the California State University, Sacramento (31). Follow-up testing of gait for the FXTAS participant occurred one year later at the age of 69 years.
Instrumentation
An 8-camera Vicon motion capture system (Vicon 612, Vicon Motion Systems, Lake Forest, CA, USA) collected kinematic data at 100 Hz at the California State University, Sacramento.
Procedures
The participant had reflective markers placed bilaterally on the posterior heel on the calcaneus, in line with the Achilles tendon. The participant was instructed to perform a single 15-second tandem walking trial by taking steps and having each foot land in front of the other foot at their self-selected speed. The tandem walk was visually demonstrated by the researcher. The participant did not complete a familiarization trial, but did practice taking 2 steps for confirmation that he was performing this correctly. The 15-second data collection began when the participant took their first tandem step. The participant traversed a designated 6-meter walkway and completed a single trial, during which they took as many steps as possible within the allotted time. The trial ended after 15 s, regardless of how far the participant traversed.
Data analysis
Step width, defined as the mediolateral distance between the heels at heel strike (Figure 1), was calculated for each consecutive step throughout the 15-second tandem walking trial. While there is no universally accepted cutoff for a “failed” step during tandem walking, prior work in cerebellar ataxia suggests that step widths near 50 mm reflect instability and deviation from ideal tandem foot placement of 4 mm in healthy controls (28). In this case report, step width was treated as a continuous indicator of balance control, with larger values indicating greater mediolateral instability. All motion trajectory data were processed using a custom-written MATLAB program (MathWorks Inc., Natick, MA, USA) and low-pass filtered at 20 Hz using a second-order Butterworth filter.
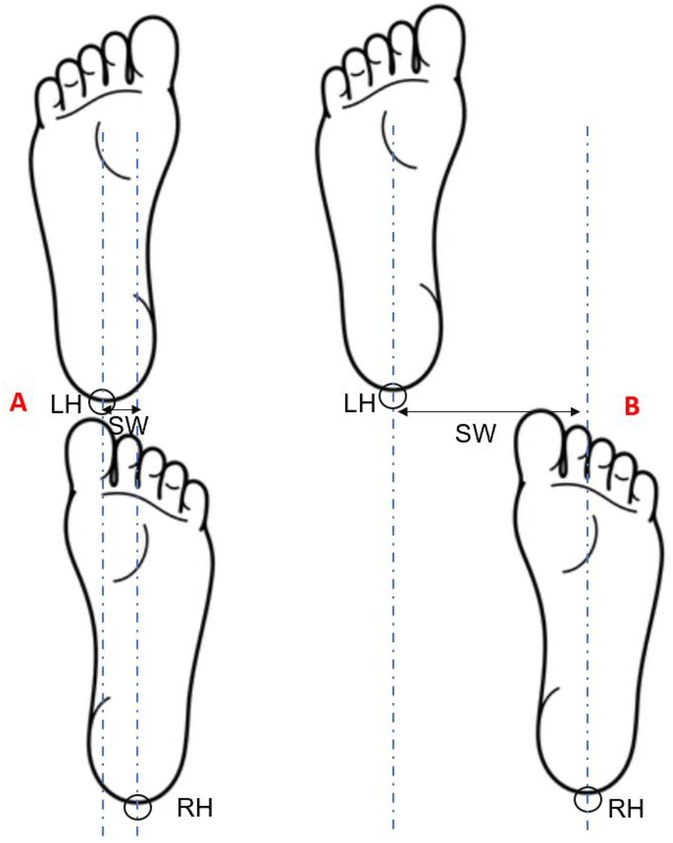
Figure 1. Schematic illustration of step width calculation during tandem walking. (A) shows a trial with a relatively narrow step width, while (B) shows a trial with a wider step width. LH, left heel marker; RH, right heel marker; vertical dashed lines represent the positions of the heel markers at heel strike; SW, step width, indicated by the double-headed arrow.
The step widths for each heel strike at baseline and at the 1-year follow-up were calculated and summarized descriptively. Because the participant completed only four full steps at baseline and two full steps at follow-up, the sample size was too small to assume normality or perform inferential testing with confidence. Therefore, statistical analyses were de-emphasized, and the focus was placed on descriptive comparisons of the trajectories, mean values, and variability of step width between the two sessions. Observed features of the trajectories (e.g., ability to achieve true tandem stance, corrective steps, and side-to-side differences) were also qualitatively interpreted to provide a more comprehensive picture of the participant's performance.
Results
The individual took four full steps in the baseline tandem 15-second walking trial and two full steps in the 1-year follow-up. The mean step width during the baseline tandem walking trial was 45.22 ± 33.47 mm (SD). The mean step width at the 1-year follow-up trial was 85.79 ± 15.80 mm (SD) (Figure 2). At the one-year follow-up, the patient was indeed incapable of achieving a true tandem position even in standing, whereas one year earlier they were able to attain a true tandem position, albeit with a corrective step. In addition to the widening of step width, the number of steps achieved within the fixed 15-second trial window decreased from four steps at baseline to two steps at follow-up.
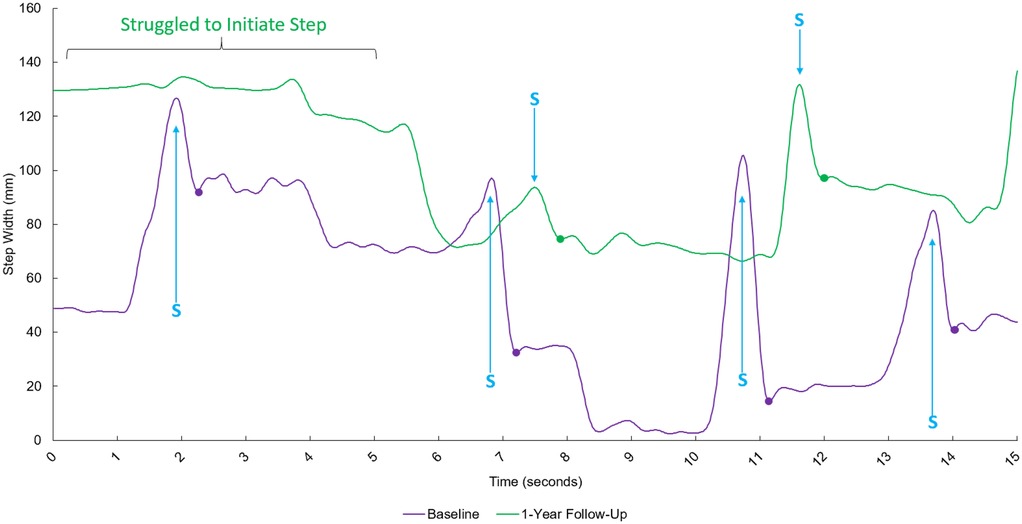
Figure 2. Time-series data exhibiting the step width between the left and right heel during tandem walking for the baseline trial (purple line) and the 1-year follow-up (green line). The x-axis represents time after starting in seconds, and the y-axis represents the step width between the left and right heel. S = Peak step width during swing phase of stepping foot. Each dot represents the measured step width at the initial foot position and subsequent heel strikes during tandem gait.
Discussion
This pilot case report investigated whether tandem walking could detect progressive gait deficits at a one-year period in an individual with FXTAS. In support with our hypothesis, we found an increase in step width during the tandem walking trial at the one-year follow-up compared to baseline.
The observed increase in step width is consistent with known gait characteristics of cerebellar ataxias and supports the notion that individuals with FXTAS exhibit progressive deterioration in mediolateral balance control over time. Tandem walking is a task that challenges dynamic postural stability by narrowing the base of support and requiring precise coordination of foot placement. The increase in step width observed in this case report may represent a compensatory strategy to maintain balance in the face of declining cerebellar control, which is a finding that aligns with prior studies in cerebellar ataxias reporting widened step width and increased gait variability as markers of progression (32, 33). Related cerebellar ataxias, such as spinocerebellar ataxia, have identified changes in tandem gait over a one year period, suggesting that FXTAS may follow a similar trajectory (34). The feasibility of tracking longitudinal changes over time have been observed in individuals with Parkinson's Disease. It is reported that individuals with Parkinson's Disease exhibited a 6%–10% increase in step width for each year that individuals were diagnosed with Parkinson's Disease (35). Lastly, it is reported that aging is significantly correlated to increases in step width during tandem gait, however healthy older adults did not exhibit greater than 50 mm (36). At baseline, our participant may have exhibited a step width that is considered healthy but exceeded the expected step width due to progression of disease.
Tandem walking is a particularly valuable task for assessing cerebellar dysfunction because it requires precise mediolateral control and challenges postural stability by narrowing the base of support. Importantly, tandem walking is already embedded in validated clinical tools such as the Scale for the Assessment and Rating of Ataxia (SARA), highlighting its established acceptability to both patients and clinicians. However, the categorical scoring used in SARA (based on the number of consecutive tandem steps achieved) may lack sensitivity to subtle longitudinal changes in performance. By quantifying kinematic features of tandem walking, such as step width, our case report demonstrates how biomechanical metrics can enhance the resolution of this established clinical task. This approach not only aligns with current clinical practice but also provides a path forward for developing more sensitive markers of progression in FXTAS.
The decline in the number of achievable tandem steps is a complementary indicator of progression. At baseline, the participant could generate four consecutive tandem steps, suggesting that despite instability he could still initiate and maintain the task. At the one-year follow-up, only two steps were possible, reflecting a diminished ability to coordinate successive foot placements and sustain the trial. Taken together with the increased step width, this reduction in step count highlights the progressive nature of mediolateral gait instability in FXTAS.
Several limitations should be considered when interpreting the findings of this pilot case report. First, the case report involved only a single individual with FXTAS, limiting the generalizability of the results. While the within-subject longitudinal design allows for observation of change over time, individual variability in disease trajectory, compensatory strategies and physical condition may not reflect patterns observed in the broader FXTAS population. Second, only one 15-second tandem walking trial was conducted at each time point, which may reduce the reliability of the measured outcomes and increase the chance of random variability. Repeated trials, and increased steps such as SARA clinical guidelines, would improve measurement stability and increase the sensitivity to detect subtle changes. Additional kinematic variables such as step length variability, trunk sway, or center of mass excursions may provide a more comprehensive understanding of motor decline. Finally, potential confounding factors such as fatigue, attention (37–40), medication status (41), and motivation at the time of testing were not controlled, which could influence gait performance in a single-session assessment. Lastly, the disease stage of FXTAS in our participant was not clinically classified, and no biomechanical staging system currently exists for this condition, although the FXTAS clinical stage is determined by the severity of the motor problems and their interference with activities of daily living (42). Consequently, the baseline and follow-up assessments may have coincided with an atypical or non-representative phase of disease progression. Future studies should aim to recruit individuals with genetically confirmed FXTAS in early disease stages or FMR1 premutation carriers who have not developed FXTAS who exhibit relatively preserved gait function at baseline and track them longitudinally to enable more meaningful interpretations of functional decline and clinically relevant disease progression.
Comparative case studies in rare progressive gait disorders further highlight the utility of targeted kinematic and biomechanical analysis. For instance, Sassi et al. described altered ankle muscle activity and gait propulsion deficits in siblings with progressive pseudorheumatoid dysplasia, using EMG and kinematics to elucidate compensatory patterns during stance (43). In another case, Tedeschi et al. combined spatial-temporal parameters with plantar pressure mapping in a patient with Strumpell–Lorrain disease, identifying monopodal reliance and increased forefoot loading due to dorsiflexion constraints (44). Although our case report employs a simpler tandem walking paradigm, focusing specifically on mediolateral stability through step width, these reports highlight the depth and range of gait features that can be revealed with more comprehensive approaches. Such multi-modal assessments represent promising directions for future longitudinal gait research in FXTAS and related ataxias.
In conclusion, this feasibility case report demonstrates that step width during tandem walking increased markedly over one year in an individual with FXTAS, suggesting that this measure may be a biomarker for motor progression. Future studies should incorporate additional gait variables such as cadence and stride time variability, foot placement asymmetry, or stance to swing ratios. Most importantly, this case report provides vital feasibility data to examine the progression of FXTAS across multiple disease stages.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the California State University, Sacramento Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JL-C: Formal analysis, Writing – original draft, Investigation, Visualization, Conceptualization, Data curation, Writing – review & editing, Validation, Methodology. DB: Methodology, Conceptualization, Writing – review & editing, Funding acquisition. RH: Conceptualization, Writing – review & editing, Funding acquisition. NM: Investigation, Writing – review & editing. MK: Investigation, Writing – review & editing. RI: Funding acquisition, Supervision, Resources, Writing – review & editing, Investigation, Software, Project administration, Visualization, Methodology, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Funding was provided through NICHD grant HD036071.
Conflict of interest
JL-C is a Commissioner on Disability Issues, appointed by the City of Tucson, the Vice Chair of Research for ASTM F13.40, and majority owner of a forensics firm Verum Biomechanics.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Park J, Kim TH. The effects of balance and gait function on quality of life of stroke patients. NeuroRehabilitation. (2019) 44:37–41. doi: 10.3233/NRE-182467
2. Moore O, Peretz C, Giladi N. Freezing of gait affects quality of life of peoples with Parkinson’s disease beyond its relationships with mobility and gait. Mov Disord. (2007) 22:2192–5. doi: 10.1002/mds.21659
3. Brandes M, Schomaker R, Möllenhoff G, Rosenbaum D. Quantity versus quality of gait and quality of life in patients with osteoarthritis. Gait Posture. (2008) 28:74–9. doi: 10.1016/j.gaitpost.2007.10.004
4. Paker N, Bugdayci D, Goksenoglu G, Demircioğlu DT, Kesiktas N, Ince N. Gait speed and related factors in Parkinson’s disease. J Phys Ther Sci. (2015) 27:3675–9. doi: 10.1589/jpts.27.3675
5. Buckley E, Mazzà C, McNeill A. A systematic review of the gait characteristics associated with cerebellar ataxia. Gait Posture. (2018) 60:154–63. doi: 10.1016/j.gaitpost.2017.11.024
6. Stimpson KH, Heitkamp LN, Embry AE, Dean JC. Post-stroke deficits in the step-by-step control of paretic step width. Gait Posture. (2019) 70:136–40. doi: 10.1016/j.gaitpost.2019.03.003
7. Paraschiv-Ionescu A, Newman CJ, Carcreff L, Gerber CN, Armand S, Aminian K. Correction: locomotion and cadence detection using a single trunk-fixed accelerometer: validity for children with cerebral palsy in daily life-like conditions (journal of NeuroEngineering and rehabilitation DOI: 10.1186/s12984-019-0494-z). J Neuroeng Rehabil. (2019) 16:1–11. doi: 10.1186/s12984-019-0498-8
8. Jasper AM, Lazaro RT, Mehta SP, Perry LA, Swanson K, Reedy K, et al. Predictors of gait speed post-stroke: a systematic review and meta-analysis. Gait Posture. (2025) 121:70–7. doi: 10.1016/j.gaitpost.2025.04.029
9. Vive S, Elam C, Bunketorp-Käll L. Comfortable and maximum gait speed in individuals with chronic stroke and community-dwelling controls. J Stroke Cerebrovasc Dis. (2021) 30:1–9. doi: 10.1016/j.jstrokecerebrovasdis.2021.106023
10. Chow JW, Stokic DS. The contribution of walking speed versus recent stroke to temporospatial gait variability. Gait Posture. (2023) 100:216–21. doi: 10.1016/j.gaitpost.2022.12.017
11. Ilg W, Milne S, Schmitz-Hübsch T, Alcock L, Beichert L, Bertini E, et al. Quantitative gait and balance outcomes for ataxia trials: consensus recommendations by the ataxia global initiative working group on digital-motor biomarkers. Cerebellum. (2024) 23:1566–92. doi: 10.1007/s12311-023-01625-2
12. Shah VV, Rodriguez-Labrada R, Horak FB, McNames J, Casey H, Hansson Floyd K, et al. Gait variability in spinocerebellar ataxia assessed using wearable inertial sensors. Mov Disord. (2021) 36:2922–31. doi: 10.1002/mds.28740
13. Camargo CHF, Ferreira-Peruzzo SA, Ribas DIR, Franklin GL, Teive HAG. Imbalance and gait impairment in Parkinson’s disease: discussing postural instability and ataxia. Neurol Sci. (2024) 45:1377–88. doi: 10.1007/s10072-023-07205-w
14. Zanardi APJ, da Silva ES, Costa RR, Passos-Monteiro E, dos Santos IO, Kruel LFM, et al. Gait parameters of Parkinson’s disease compared with healthy controls: a systematic review and meta-analysis. Sci Rep. (2021) 11:1–13. doi: 10.1038/s41598-020-80768-2
15. Guo Y, Yang J, Liu Y, Chen X, Yang GZ. Detection and assessment of Parkinson’s disease based on gait analysis: a survey. Front Aging Neurosci. (2022) 14:916971. doi: 10.3389/fnagi.2022.916971
16. Atrsaei A, Corrà MF, Dadashi F, Vila-Chã N, Maia L, Mariani B, et al. Gait speed in clinical and daily living assessments in Parkinson’s disease patients: performance versus capacity. NPJ Park Dis. (2021) 7:1–11. doi: 10.1038/s41531-021-00171-0
17. Dussault-Picard C, Mohammadyari SG, Arvisais D, Robert MT, Dixon PC. Gait adaptations of individuals with cerebral palsy on irregular surfaces: a scoping review. Gait Posture. (2022) 96:35–46. doi: 10.1016/j.gaitpost.2022.05.011
18. Rethwilm R, Böhm H, Haase M, Perchthaler D, Dussa CU, Federolf P. Dynamic stability in cerebral palsy during walking and running: predictors and regulation strategies. Gait Posture. (2021) 84:329–34. doi: 10.1016/j.gaitpost.2020.12.031
19. MacCarthy M, Heyn P, Tagawa A, Carollo J. Walking speed and patient-reported outcomes in young adults with cerebral palsy. Dev Med Child Neurol. (2022) 64:1281–8. doi: 10.1111/dmcn.15225
20. Vila MH, Pérez R, Mollinedo I, Cancela JM. Analysis of gait for disease stage in patients with Parkinson’s disease. Int J Environ Res Public Health. (2021) 18:1–10. doi: 10.3390/ijerph18020720
21. Rastegari E, Marmelat V, Najjar L, Bastola D, Ali HH. Using gait parameters to recognize various stages of Parkinson’s disease. Proc - 2017 IEEE Int Conf Bioinforma Biomed BIBM 2017; 2017-January (2017). p. 1647–51. doi: 10.1109/BIBM.2017.8217906
22. Serrao M, Chini G, Casali C, Conte C, Rinaldi M, Ranavolo A, et al. Progression of gait ataxia in patients with degenerative cerebellar disorders: a 4-year follow-up study. Cerebellum. (2017) 16:629–37. doi: 10.1007/s12311-016-0837-2
23. Seemann J, Daghsen L, Cazier M, Lamy JC, Welter ML, Giese MA, et al. Digital gait measures capture 1-year progression in early-stage spinocerebellar ataxia type 2. Mov Disord. (2024) 39:788–97. doi: 10.1002/mds.29757
24. Hagerman RJ, Hagerman P. Fragile x-associated tremor/ataxia syndrome-features, mechanisms and management. Nat Rev Neurol. (2016) 12:403–12. doi: 10.1038/nrneurol.2016.82
25. Hagerman PJ, Hagerman RJ. Fragile x-associated, tremor/ataxia syndrome (FXTAS). Ment Retard Dev Disabil Res Rev. (2004) 10:25–30. doi: 10.1002/mrdd.20005
26. O’Keefe JA, Robertson-Dick EE, Hall DA, Berry-Kravis E. Gait and functional mobility deficits in fragile x-associated tremor/ataxia syndrome. Cerebellum. (2016) 15:475–82. doi: 10.1007/s12311-015-0714-4
27. Robertson-Dick EE, Timm EC, Pal G, Ouyang B, Liu Y, Berry-Kravis E, et al. Digital gait markers to potentially distinguish fragile x-associated tremor/ataxia syndrome, Parkinson’s disease, and essential tremor. Front Neurol. (2023) 14:1–12. doi: 10.3389/fneur.2023.1308698
28. Stolze H, Klebe S, Petersen G, Raethjen J, Wenzelburger R, Witt K, et al. Typical features of cerebellar ataxic gait. J Neurol Neurosurg Psychiatry. (2002) 73:310–2. doi: 10.1136/jnnp.73.3.310
29. Conte C, Serrao M, Casali C, Ranavolo A, Mari S, Draicchio F, et al. Planned gait termination in cerebellar ataxias. Cerebellum. (2012) 11:896–904. doi: 10.1007/s12311-011-0348-0
30. Cabaraux P, Agrawal SK, Cai H, Calabro RS, Casali C, Damm L, et al. Consensus paper: ataxic gait. Cerebellum. (2023) 22:394–430. doi: 10.1007/s12311-022-01373-9
31. Lee JS. Biomechanical gait assessment on a patient with fragile x-associated tremor/ataxia syndrome (FXTAS): a case study (2014).
32. Ilg W, Seemann J, Giese M, Traschütz A, Schöls L, Timmann D, et al. Real-life gait assessment in degenerative cerebellar ataxia: toward ecologically valid biomarkers. Neurology. (2020) 95:E1199–210. doi: 10.1212/WNL.0000000000010176
33. Ilg W, Golla H, Thier P, Giese MA. Specific influences of cerebellar dysfunctions on gait. Brain. (2007) 130:786–98. doi: 10.1093/brain/awl376
34. Ilg W, Müller B, Faber J, van Gaalen J, Hengel H, Vogt IR, et al. Digital gait biomarkers allow to capture 1-year longitudinal change in spinocerebellar ataxia type 3. Mov Disord. (2022) 37:2295–301. doi: 10.1002/mds.29206
35. Sharma R, Pillai L, Glover A, Virmani T. Objective impairment of tandem gait in Parkinson’s disease patients increases with disease severity. Park Relat Disord. (2019) 68:33–9. doi: 10.1016/j.parkreldis.2019.09.023
36. Virmani T, Gupta H, Shah J, Larson-Prior L. Objective measures of gait and balance in healthy non-falling adults as a function of age. Gait Posture. (2018) 65:100–5. doi: 10.1016/j.gaitpost.2018.07.167
37. O’Keefe JA, Robertson EE, Ouyang B, Carns D, McAsey A, Liu Y, et al. Cognitive function impacts gait, functional mobility and falls in fragile X-associated tremor/ataxia syndrome. Gait Posture. (2018) 66:288–93. doi: 10.1016/j.gaitpost.2018.09.005
38. O’Keefe JA, Guan J, Robertson E, Biskis A, Joyce J, Ouyang B, et al. The effects of dual task cognitive interference and fast-paced walking on gait, turns, and falls in men and women with FXTAS. Cerebellum. (2021) 20:212–21. doi: 10.1007/s12311-020-01199-3
39. Annam Z. The impact of cognition on balance and gait in fragile x-associated tremor/ataxia syndrome (MS Thesis). Rush University (2024).
40. Timm EC, Purcell NL, Ouyang B, Berry-Kravis E, Hall DA, O’Keefe JA. Potential prodromal digital postural sway markers for fragile x-associated tremor/ataxia syndrome (FXTAS) detected via dual-tasking and sensory manipulation. Sensors. (2024) 24:2586. doi: 10.3390/s24082586
41. Hagerman RJ, Hall DA, Coffey S, Leehey M, Bourgeois J, Gould J, et al. Treatment of fragile x-associated tremor ataxia syndrome (FXTAS) and related neurological problems. Clin Interv Aging. (2008) 3:251–62. doi: 10.2147/cia.s1794
42. Bacalman S, Farzin F, Bourgeois JA, Cogswell J, Goodlin-Jones BL, Gane LW, et al. Psychiatric phenotype of the fragile x-associated tremor/ataxia syndrome (FXTAS) in males: newly described fronto-subcortical dementia. J Clin Psychiatry. (2006) 67:87–94. doi: 10.4088/JCP.v67n0112
43. Sassi S, Faccioli S, Farella GM, Tedeschi R, Garavelli L, Benedetti MG. Gait alterations in two young siblings with progressive pseudorheumatoid dysplasia. Children. (2022) 9:1–9. doi: 10.3390/children9121982
Keywords: fragile x-associated tremor/ataxia syndrome (FXTAS), gait, step width, tandem walking, longitudinal, follow-up, variability, balance impairment
Citation: Lee-Confer J, Baker D, Hagerman R, Maltman N, Kobel M and Imamura R (2025) Case Report: A 1-year progression of mediolateral gait instability during tandem walking in FXTAS. Front. Sports Act. Living 7:1637831. doi: 10.3389/fspor.2025.1637831
Received: 30 May 2025; Accepted: 6 October 2025;
Published: 21 October 2025.
Edited by:
Ivanna Kristianti Timotius, Satya Wacana Christian University, IndonesiaReviewed by:
Roberto Tedeschi, Independent Researcher, Bologna, ItalyEllen Buckley, The University of Sheffield, United Kingdom
Copyright: © 2025 Lee-Confer, Baker, Hagerman, Maltman, Kobel and Imamura. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jonathan Lee-Confer, bGVlY29uZmVyQGFyaXpvbmEuZWR1
 Jonathan Lee-Confer
Jonathan Lee-Confer Dian Baker4,5
Dian Baker4,5 Randi Hagerman
Randi Hagerman Nell Maltman
Nell Maltman Megan Kobel
Megan Kobel