- Department of Physical Education, Tianjin University of Sport, Tianjin, China
Background: Repeated-sprint training in hypoxia (RSH) has emerged as a novel strategy to optimize repeated-sprint ability (RSA), aerobic capacity, and anaerobic performance in athletes. Although numerous studies have explored its efficacy compared with repeated-sprint training in normoxia (RSN), inconsistencies remain regarding overall benefits and modulating factors.
Objectives: This study aimed to quantify the overall effect of RSH vs. RSN on athletic performance through a systematic review and multilevel meta-analysis and to identify categorical and continuous moderators influencing intervention efficacy.
Methods: A comprehensive literature search was conducted across PubMed, Embase, Web of Science, and Cochrane Library up to January 31, 2025. Randomized controlled trials comparing RSH and RSN were included. Two reviewers independently screened, extracted, and assessed study quality. Random-effects multilevel models were used to calculate Hedges' g for overall and domain-specific outcomes (RSA, aerobic and anaerobic performance). Categorical (e.g., outcome types, intervention format, sex) and continuous moderators [e.g., inspired oxygen fraction (FiO2), intervention duration, frequency, and exercise-to-rest ratio] were tested via meta-regression. Publication bias was assessed through funnel plots and regression-based Egger tests.
Results: Eighteen studies (N = 378 participants) were included, yielding 55 independent effect sizes. RSH significantly improved performance outcomes compared with RSN (g = 0.50, 95% CI: 0.34–0.67, p < 0.001). Subgroup analyses revealed stronger effects for RSA (g = 0.61) than for aerobic (g = 0.42) or anaerobic (g = 0.39) outcomes. Moderator analyses indicated that outcome type, exercise format, and FiO2) significantly moderated the effect size, with lower FiO2 (–13%–14%) and longer training duration (weeks) associated with greater gains. No sex differences were found. Funnel plot symmetry suggested low risk of publication bias.
Conclusion: This meta-analysis confirms that RSH provides a moderate performance advantage over RSN, particularly for repeated-sprint ability. Specific implementation parameters such as moderate hypoxia intensity and sufficient training duration enhance efficacy. These findings offer evidence-based guidance for optimizing high-intensity interval training protocols under hypoxic conditions.
1 Introduction
Since the 1968 Mexico City Olympics first systematically demonstrated the regulatory potential of altitude environments on athletic performance, training strategies based on natural or simulated hypoxic conditions have emerged as key methods for optimizing competitive readiness in athletes (1). With the rapid expansion of high-altitude training centers worldwide—such as Iten in Kenya, St. Moritz in Switzerland, Haigeng in Kunming, and Duoba in Qinghai—hypoxic interventions have become deeply embedded within the periodized training frameworks of various sports (1). The theory and practice of hypoxic training have evolved continuously. In the late 1980s, Soviet scholar Streltcov, drawing from clinical medicine, introduced the concept of intermittent hypoxic training (IHT) to improve cardiopulmonary function via periodic hypoxic exposure (2). Subsequently, Kaltchinskaia extended IHT into the athletic domain, proposing the adaptive hypothesis of “hypoxic stress–exercise unit activation”, marking a paradigm shift from rehabilitation to high-performance sports (3).
However, more than three decades of accumulated evidence have yielded mixed findings regarding the efficacy of IHT. Early studies suggested that hypoxic exposure could induce skeletal muscle angiogenesis and mitochondrial biogenesis via the Hypoxia-Inducible Factor 1 Alpha (HIF-1α) pathway (4). Yet, more rigorously controlled research later indicated that passive hypoxic stimulation alone fails to elicit these adaptations (5), unless combined with high- or maximal-intensity exercise capable of activating oxygen-sensitive signaling pathways (6, 7). A systematic review found that only 52.3% of studies involving intermittent training with hypoxia reported significant performance benefits (8), while another meta-analysis on Live Low–Train High (LLTH) models showed no statistical advantage over conventional training in enhancing sea-level endurance performance (9). This inconsistency has been attributed to the intensity-dependent nature of LLTH: only when the training intensity in hypoxia exceeds the individual anaerobic threshold can it effectively overcome the oxygen transport bottleneck to improve performance (10). In response, researchers have sought to develop novel paradigms that transcend the limitations of traditional IHT. One such strategy, repeated-sprint training in hypoxia (RSH), was proposed by Faiss in 2013. RSH combines maximal-effort sprint bouts (≤30 s) with moderate hypoxic exposure (FiO2 = 14.5%–16.4%, simulating 2,000–3,000 meters altitude), thereby creating a dual stimulus of high intensity and low oxygen availability (11). The physiological advantages of RSH span three domains: (1) at the molecular level, it activates glycolytic gene expression via the AMPK/PGC-1α pathway (7); (2) at the microvascular level, it enhances muscle oxygen uptake and reperfusion kinetics (12); and (3) at the neuromuscular level, it improves type II fiber recruitment thresholds and phosphocreatine buffering capacity (13). Notably, RSH protocols maintain an incomplete recovery state by enforcing short exercise-to-rest ratios (<1:6), thereby sustaining metabolic stress and avoiding the attenuation of adaptive responses observed in traditional IHT due to excessive recovery (3).
Evidence suggests that RSH can significantly enhance repeat sprint ability (RSA) and peak anaerobic power in elite athletes (14). Moreover, since its inception, RSH has shown expanding physiological benefits, including improved muscle oxygen diffusion capacity and lactate clearance rates (11), as well as superior performance gains compared to normoxic repeated-sprint training (RSN) in team sports settings (15). Nonetheless, existing studies vary widely in terms of participant populations (e.g., adolescents vs. elite athletes), hypoxic delivery modes (e.g., terrestrial vs. simulated), training protocols (e.g., intensity, duration), and outcome measures (e.g., strength, speed, cardiorespiratory fitness). Such heterogeneity has led to ongoing debate regarding the true efficacy of RSH, with findings ranging from significant improvements to non-significant effects or even detrimental trends. These inconsistencies may be influenced by complex factors including research design, nested outcome structures, and statistical non-independence among data sources.
To address these challenges, the present study adopts a multilevel meta-analytic framework, which overcomes the limitations of traditional meta-analyses that treat each study as contributing a single effect size. By modeling nested data structures, this approach accounts for statistical dependencies among multiple outcomes, time points, or groups within a single study, thereby enhancing the robustness and interpretability of the results. Furthermore, to comprehensively characterize the impact of RSH on athletic performance, we incorporated both categorical and continuous moderators—such as training mode, oxygen concentration, and intervention duration—into a multivariate meta-regression model. This allows us to explore key determinants and boundary conditions shaping the effectiveness of RSH.
2 Methods
2.1 Literature search strategy
This meta-analysis was conducted in accordance with the PRISMA 2020 (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. A systematic search of five major databases—PubMed, Web of Science, Embase, Scopus, and ScienceDirect—was conducted up to 31 January 2025. Boolean logic was applied to construct the search strategy, using the following terms: “repeated sprint” OR “repeated sprint” OR “sprint interval” AND “hypoxia” OR “low oxygen” OR “altitude” AND “performance” OR “athletic performance” OR “endurance” OR “power” OR “VO2max”. No restrictions were placed on language or publication date to ensure comprehensiveness. In addition, the reference lists of all eligible articles and relevant reviews were manually screened to identify any potentially missing studies. All search results were managed using EndNote 20, and duplicates were removed using the built-in “Find Duplicates” function.
2.2 Eligibility criteria
Study screening was performed independently by two reviewers (MH and BL) in two distinct stages, starting with title and abstract screening, followed by full-text evaluation. Studies were eligible for inclusion if they met the following criteria: they employed a randomized controlled trial (RCT) design with parallel-group allocation; participants were trained individuals, athletes, or adults with at least moderate levels of physical activity; the intervention group received RSH; the control group performed the same RSN; at least one quantifiable performance outcome was reported, such as sprint time, peak power, RSA, maximal oxygen uptake (VO2max), cardiorespiratory function, or Wingate test results; and sufficient data were available to calculate Hedges' g, including pre- and post-intervention means, standard deviations, and sample sizes, or equivalent statistics. Studies were excluded if they were not randomized, were published as abstracts, reviews, or animal studies, involved combined interventions in which RSH effects could not be clearly isolated, or did not provide sufficient data and failed to respond to requests for additional information. Any disagreements between reviewers were resolved through discussion with a third researcher.
2.3 Risk of bias assessment
The risk of bias in the included studies was assessed using the Cochrane Risk of Bias 2.0 tool (RoB 2.0), as recommended by the Cochrane Collaboration (16). This instrument evaluates five domains of potential bias: the randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. Each domain was rated as “low risk,” “some concerns,” or “high risk,” and an overall risk-of-bias judgment was derived accordingly. Two reviewers (ML and DL) independently performed the assessment, and any discrepancies were resolved through discussion with a third reviewer.
2.4 Data extraction
A standardized data extraction form was developed prior to analysis. Extracted data included: first author, year of publication, country/region, study design, intervention duration and frequency, inspired oxygen fraction (Fio2), training modality, sample size, participant characteristics, outcome type, and pre- and post-intervention means and standard deviations. Data were independently extracted by two reviewers (ML and DL) and cross-checked upon completion. For studies lacking numerical data, outcomes were extracted from figures using GraphDigitizer (17) or obtained directly from the corresponding authors. All extracted data were entered into a structured Excel spreadsheet, and consistency was verified through cross-validation in R.
2.5 Data synthesis
Effect sizes were calculated using Hedges' g, representing the standardized mean difference between pre- and post-intervention changes in the experimental and control groups. This metric accounts for small sample bias and is widely recommended for continuous outcome measures in meta-analyses. In the present study, Hedges' g was computed based on the difference in mean change scores between groups, following the formula below (18, 19):
The correction factor , was used to adjust for small sample bias. The pooled standard deviation was calculated using the following formula:
Variance estimation followed the procedures described by Borenstein et al. (20, 21). When the standard deviation of the change score was not reported, it was estimated using the following formula, assuming a pre–post correlation of r = 0.5 (22):
Hedges' g and its corresponding variance (var_g) were ultimately used for meta-analytic modeling.
2.6 Statistical analysis
A multilevel random-effects model was employed to perform the meta-analysis, aiming to address the statistical non-independence introduced by multiple effect sizes within individual studies, such as those arising from different outcome measures or time points. The model was constructed as a two-level nested structure (study/outcome), where the observed effect size was decomposed into the overall mean effect, a study-level random effect, an outcome-level random effect, and a residual sampling error based on the known variance of each effect size. Model fitting was performed using the rma.mv() function in the metafor package in R, with parameters estimated via restricted maximum likelihood (REML). Multilevel heterogeneity indices (I²) were calculated using the i2_ml() function, allowing for decomposition into between-study and within-study components. To explore potential sources of heterogeneity, a series of multivariate meta-regression models were constructed, incorporating both continuous (e.g., FiO2 concentration, training duration, frequency) and categorical moderators (e.g., training modality, outcome type). Moderator variables were coded according to their measurement scale, with categorical variables dummy-coded accordingly. All models retained the random-effects structure to account for the nesting of effect sizes within studies. Meta-regression results are reported as regression coefficients, standard errors, 95% confidence intervals, and p-values. Model fit was evaluated using Akaike Information Criterion (AIC) and marginal R2. For continuous moderators, moderation effects were visualized using the bubble_plot() function from the orchard package, with bubble size proportional to effect size precision (1/SE). Publication bias was assessed by constructing enhanced funnel plots via the viz_funnel() function from the metaviz package (23), alongside Egger's regression test to detect small-study effects. In cases where the primary model was multilevel and the regtest() function could not be applied, linear model approximations were used instead. When publication bias was evident, the trim-and-fill method was used to adjust the overall effect size (24).
3 Results
3.1 Study selection and inclusion
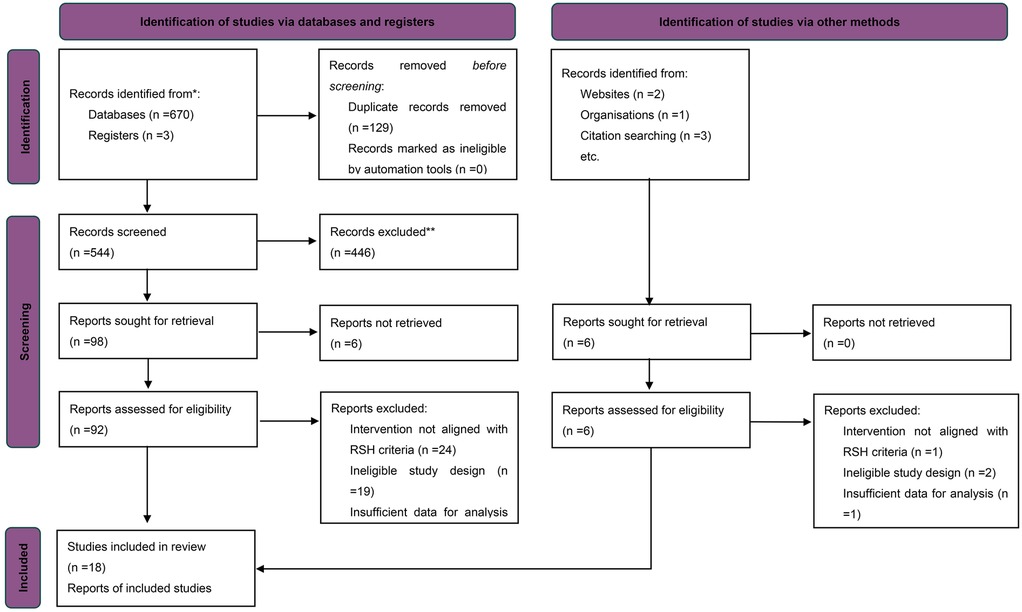
The systematic search and screening process was conducted in accordance with the PRISMA 2020 guidelines (25). A total of 478 records were initially identified. After removing duplicates using EndNote 20, 263 articles remained for title and abstract screening. During this stage, 216 studies were excluded based on the following criteria: non-athlete populations, non-interventional designs, interventions not aligned with the study objectives (e.g., absence of repeated-sprint training or lack of hypoxic exposure), or the absence of a control group. The remaining 47 articles were subjected to full-text review based on pre-specified inclusion criteria. Studies were eligible if they met the following conditions: (1) a randomized controlled trial (RCT) with a parallel-group design; (2) the intervention group performed repeated-sprint training under hypoxic conditions with inspired oxygen fraction (FiO2) below normoxic levels (<20.9%); (3) the control group underwent the same training protocol under normoxic conditions (RSN); (4) participants were healthy trained individuals or competitive athletes; (5) at least one quantifiable performance-related outcome was reported, such as RSA, VO2max, or PPO; and (6) sufficient data were available (i.e., pre- and post-intervention means, standard deviations, and sample sizes), or could be obtained from the authors upon request. Articles were excluded if they involved non-RSH interventions, inappropriate study designs, or lacked sufficient data for effect size estimation.
In total, 18 studies published between 2013 and 2025 met the inclusion criteria. All were published in English and collectively included 386 athlete participants. The study selection process is summarized in the PRISMA flow diagram (Figure 1). Overall, the screening ensured that included studies were of high methodological quality, exhibited acceptable homogeneity, and provided sufficient extractable data to support the subsequent multilevel meta-analysis.
3.2 Study characteristics
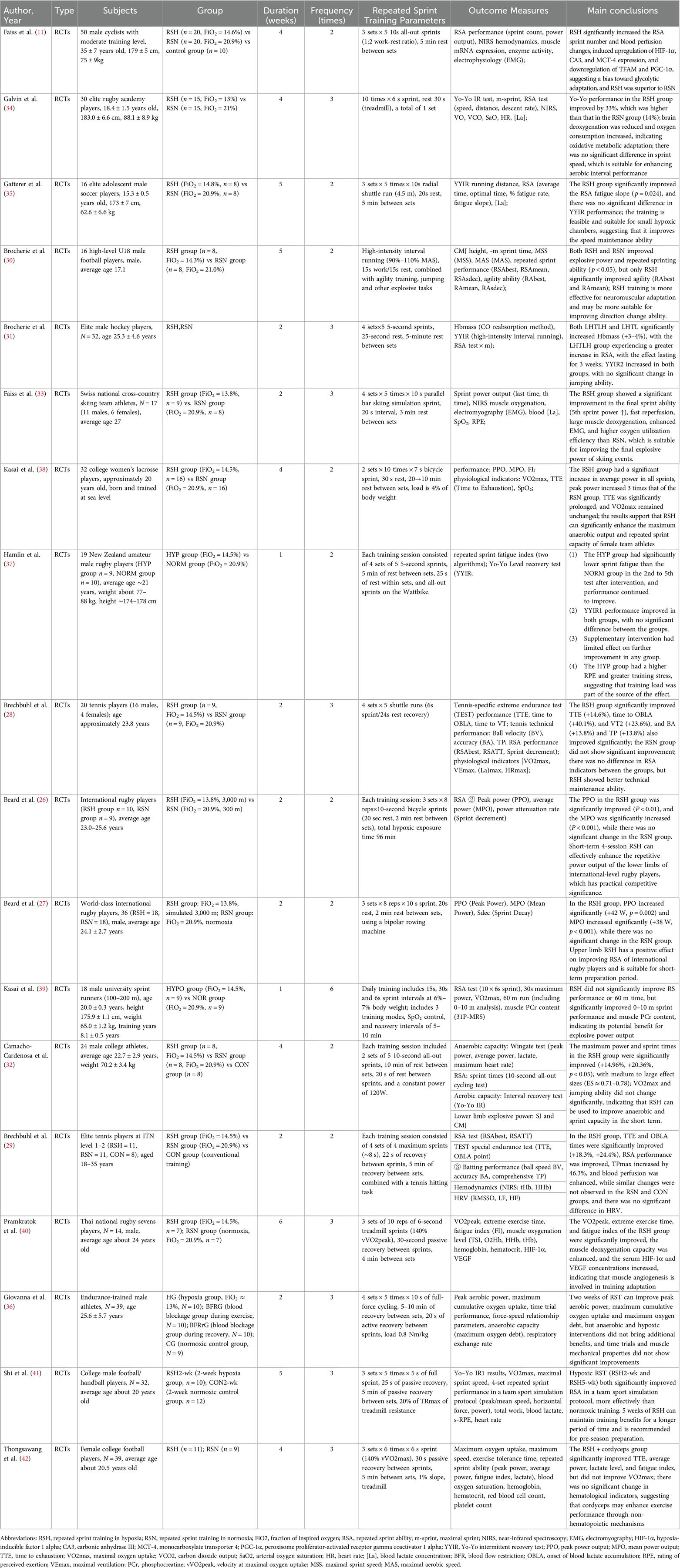
A total of 18 randomized controlled trials published between 2013 and 2024 were included in the analysis (11, 26–42). These studies were conducted across various countries and regions in Europe, Asia, and Oceania, and all involved adolescent or adult competitive athletes participating in sports such as football, rugby, tennis, and sprinting. The cumulative sample size was 370 participants, with ages predominantly ranging from 15–30 years. All studies included both a RSH group (FiO2 = 13.0%–14.8%) and RSN group (FiO2 = 20.9%). See in Table 1.
Intervention durations ranged from 1–6 weeks, with training frequencies typically set at 2–3 sessions per week. The repeated-sprint protocols were implemented in the form of running, cycling, or sport-specific exercises. A typical training session consisted of 2–6 sets, each comprising 4–10 sprints lasting 5–10 s, with 20–30 s of intra-set rest and 2–5 min of inter-set rest. Primary outcome measures included repeat sprint ability (e.g., RSAbest, RSATT), peak and mean power output (PPO, MPO), maximal oxygen uptake (VO2max), explosive performance (e.g., CMJ, SJ), and aerobic intermittent capacity (Yo-Yo IR1/IR2). Several studies also assessed physiological mechanisms such as muscle oxygenation, heart rate variability, and blood biomarkers, providing a robust dataset for subsequent meta-analytic modeling.
3.3 Risk of bias assessment in included studies
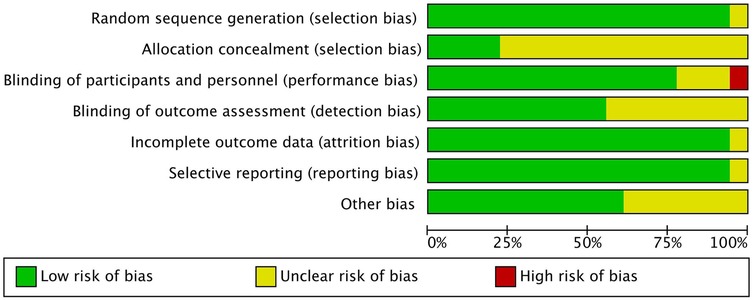
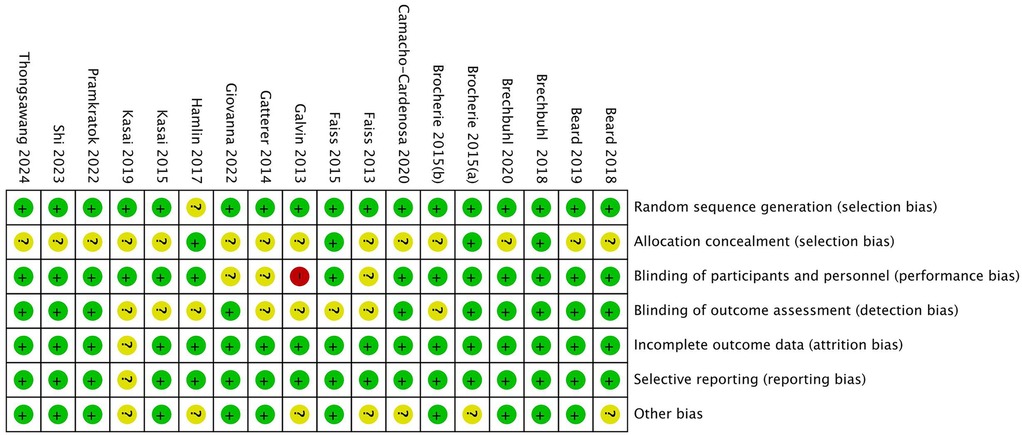
In the quality assessment of the 18 included studies, most studies showed a low risk of bias for random sequence generation (selection bias), allocation concealment (selection bias), blinding of outcome assessment (detection bias), and selective reporting (reporting bias), as indicated by the green marks. However, it is important to note that due to the nature of the intervention-based RCTs, achieving full blinding of participants and personnel (performance bias) is often not feasible. Consequently, this aspect of the risk of bias is challenging to control, with some studies showing unclear (yellow marks) or high risk (red marks) in this domain. Additionally, some studies presented unclear risks regarding incomplete outcome data (attrition bias). Nevertheless, the majority of studies demonstrated high quality, with no significant evidence of systematic bias. Future research should aim to ensure stringent blinding where possible to further minimize potential bias. See Figures 2, 3.
3.4 Overall effect estimates (main effects from multilevel modeling)
A total of 53 independent effect sizes were synthesized using a multilevel random-effects model. The results indicated that RSH produced a statistically significant advantage over RSN in enhancing athletic performance, with a pooled moderate effect size [Hedges' g = 0.35, 95% CI (0.18, 0.51), p < 0.01]. The between-study heterogeneity was low (I2 = 24.83%), suggesting good consistency across studies. The prediction interval (PI) ranged from −0.16–0.85, indicating that while future studies may observe varying effects, the overall trend remains favorable toward RSH.
Among the pre-defined outcome categories, RSH showed the most pronounced effect on RSA, with a moderate-to-large pooled effect size [ES = 0.50, 95% CI (0.12, 0.88), p < 0.01] and moderate heterogeneity (I² = 34.28%). Although the prediction interval was wide [PI = (–0.75, 1.75)], implying possible nonsignificant or even negative effects under certain conditions, the overall pattern supports the beneficial impact of RSH on RSA outcomes.
In terms of aerobic capacity, RSH also demonstrated a statistically significant benefit compared with RSN [ES = 0.23, 95% CI (0.02, 0.44), p = 0.03], with very low heterogeneity (I² = 3.52%), indicating a high degree of consistency among studies. The prediction interval (–0.04, 0.49) suggests that while some future findings may cluster near null effects, the general tendency favors a positive outcome.
In contrast, the evidence supporting an improvement in anaerobic performance was not statistically significant [ES = 0.11, 95% CI (–0.23, 0.45), p = 0.67], and heterogeneity was minimal (I² = 2.61%). The prediction interval (–0.23, 0.45) reflects a lack of consistency in observed effects, which may be attributed to variation in sample sizes, intervention durations, or measurement methods across studies. See in Figure 4.
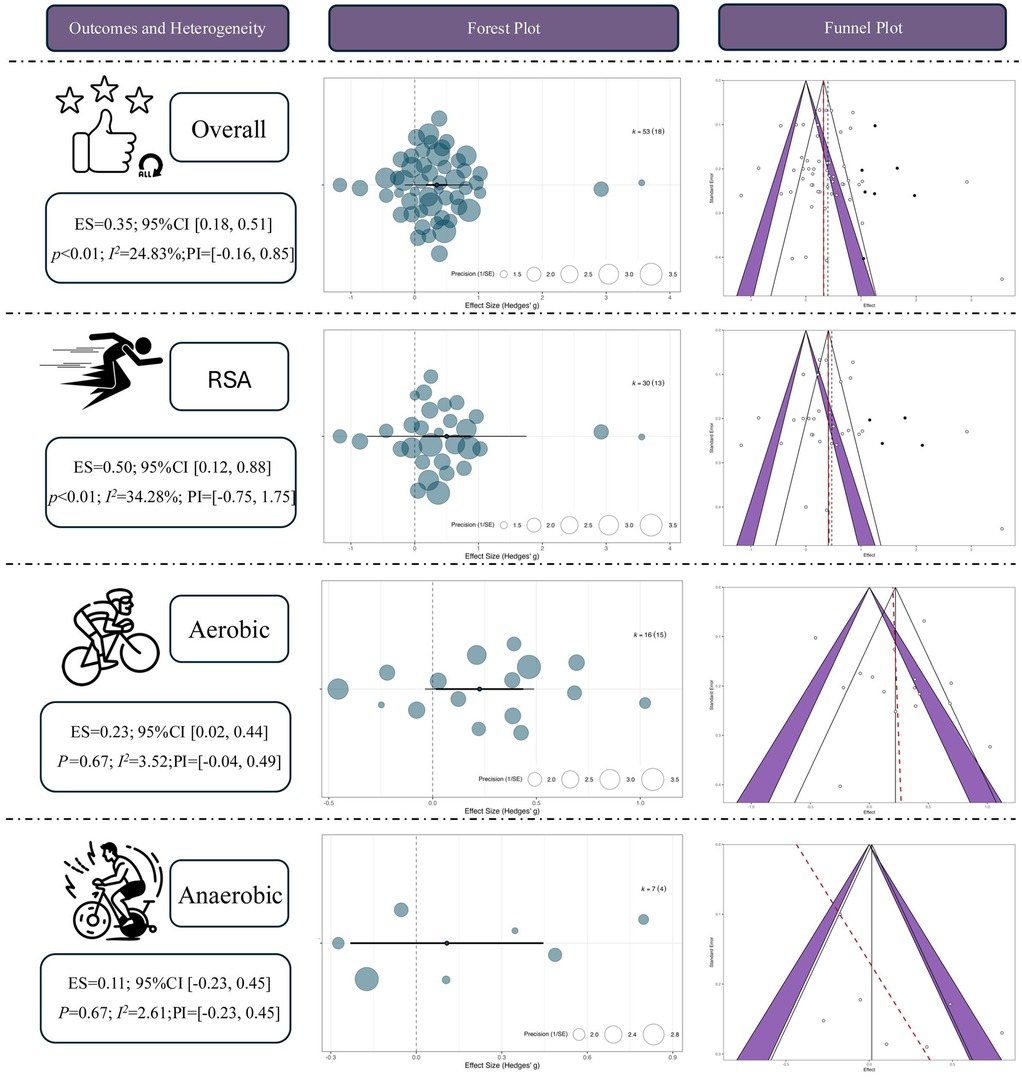
Figure 4. Overall effect of repeated sprint training in hypoxia (RSH) on athletic performance. ES, effect size; CI, confidence interval; RSA, repeated sprint ability; P, p-value; I², I-squared (heterogeneity statistic).
3.5 Moderator analysis
3.5.1 Categorical moderators
3.5.1.1 Specific outcome indicators
To examine the differential effects of RSH across various performance outcomes, subgroup analyses were conducted using outcome category as a moderator. The results showed that, within the domain of RSA, RSH produced significant moderate-to-large effects for RSA_Time [ES = 1.09, 95% CI (0.31, 1.86), p < 0.01] and RSA_N [ES = 1.00, 95% CI (0.09, 1.92), p < 0.05], suggesting that RSH can effectively improve both the total number and cumulative duration of maximal sprints. A moderate and significant effect was also observed for RSA_Sprint [ES = 0.82, 95% CI (0.08, 1.56), p < 0.05], indicating benefits in sprint speed during repeated efforts. In contrast, other RSA-related outcomes such as RSA_P (ES = 0.21, p = 0.51), RSA_DEC (ES = 0.56, p = 0.13), RSA_D (ES = 0.11, p = 0.82), and RSA_A (ES = 0.39, p = 0.18) showed positive trends but did not reach statistical significance. The effects for 10 m sprint and RSA_10 m were also nonsignificant with wide confidence intervals, indicating insufficient evidence at present.
In the aerobic capacity domain, RSH demonstrated a significant effect on the Yo-Yo Intermittent Recovery test [YYIR; ES = 0.35, 95% CI (0.00, 0.69), p < 0.05], indicating enhanced intermittent running endurance. However, the effect on VO2max was not statistically significant [ES = 0.16, 95% CI (–0.10, 0.42), p = 0.22], possibly due to short intervention durations or insufficient training load. Regarding anaerobic performance, RSH showed a moderate but non-significant effect on Wingate peak power (ES = 0.47, p = 0.11) and a small, non-significant, and negative effect on average power (ES = –0.09, p = 0.69), suggesting that short-duration high-intensity power output may not be a primary adaptation target of RSH. See in Figure 5.
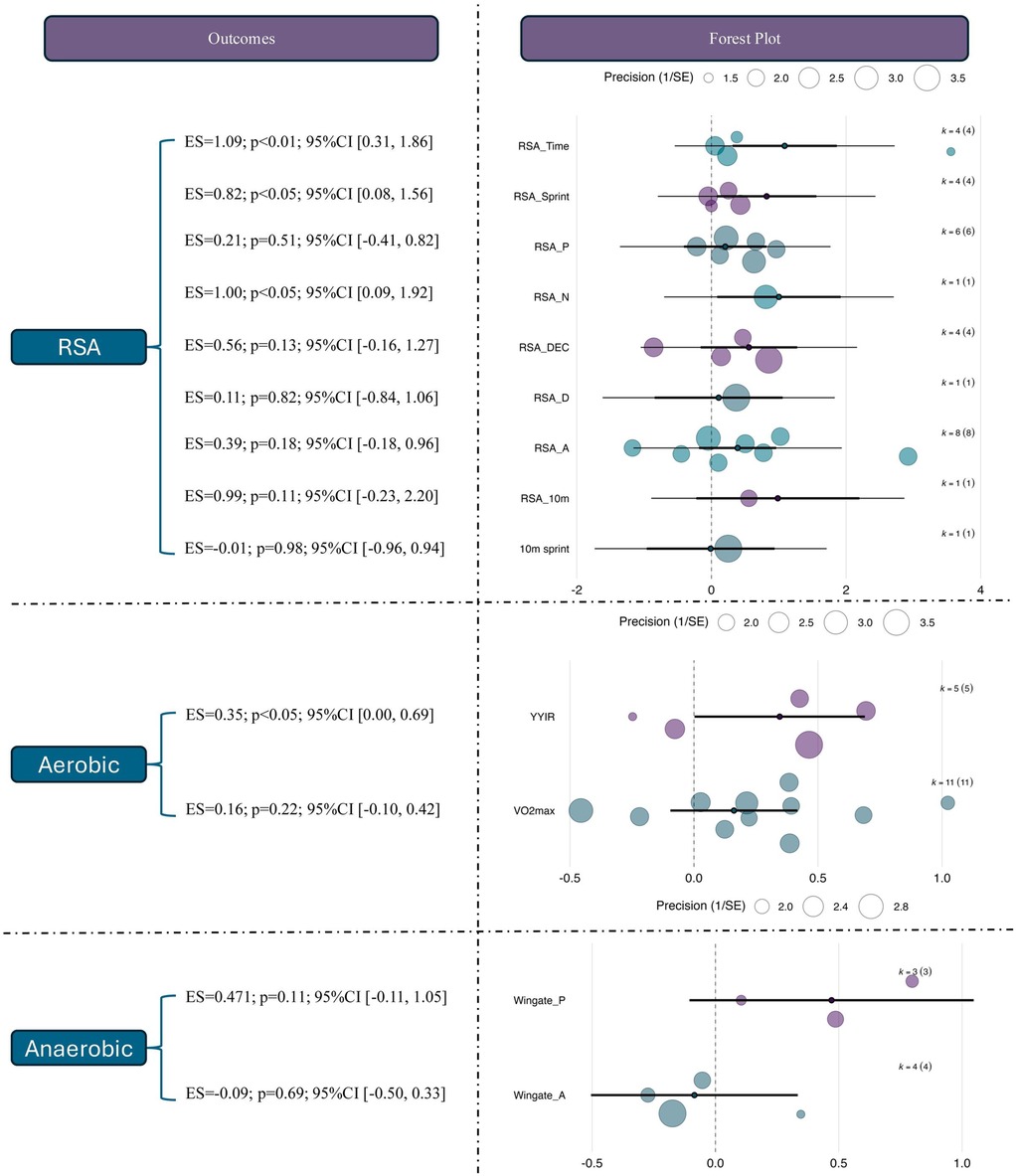
Figure 5. Effects of RSH on specific outcome measures across different performance domains. ES, effect size; CI, confidence interval; RSA, repeated sprint ability; RSA_Time, repeated sprint ability total time; RSA_Sprint, repeated sprint ability sprint time; RSA_N, repeated sprint ability number; RSA_P, repeated sprint ability peak; RSA_DEC, repeated sprint ability decay; RSA_D, repeated sprint ability distance; RSA_A, repeated sprint ability average; 10 m sprint, 10-meter sprint test; YYIR, Yo-Yo intermittent recovery test; VO2max, maximal oxygen uptake; Wingate_P, wingate peak power; 2ingate_A, Wingate average power.
3.5.1.2 Type of training modality
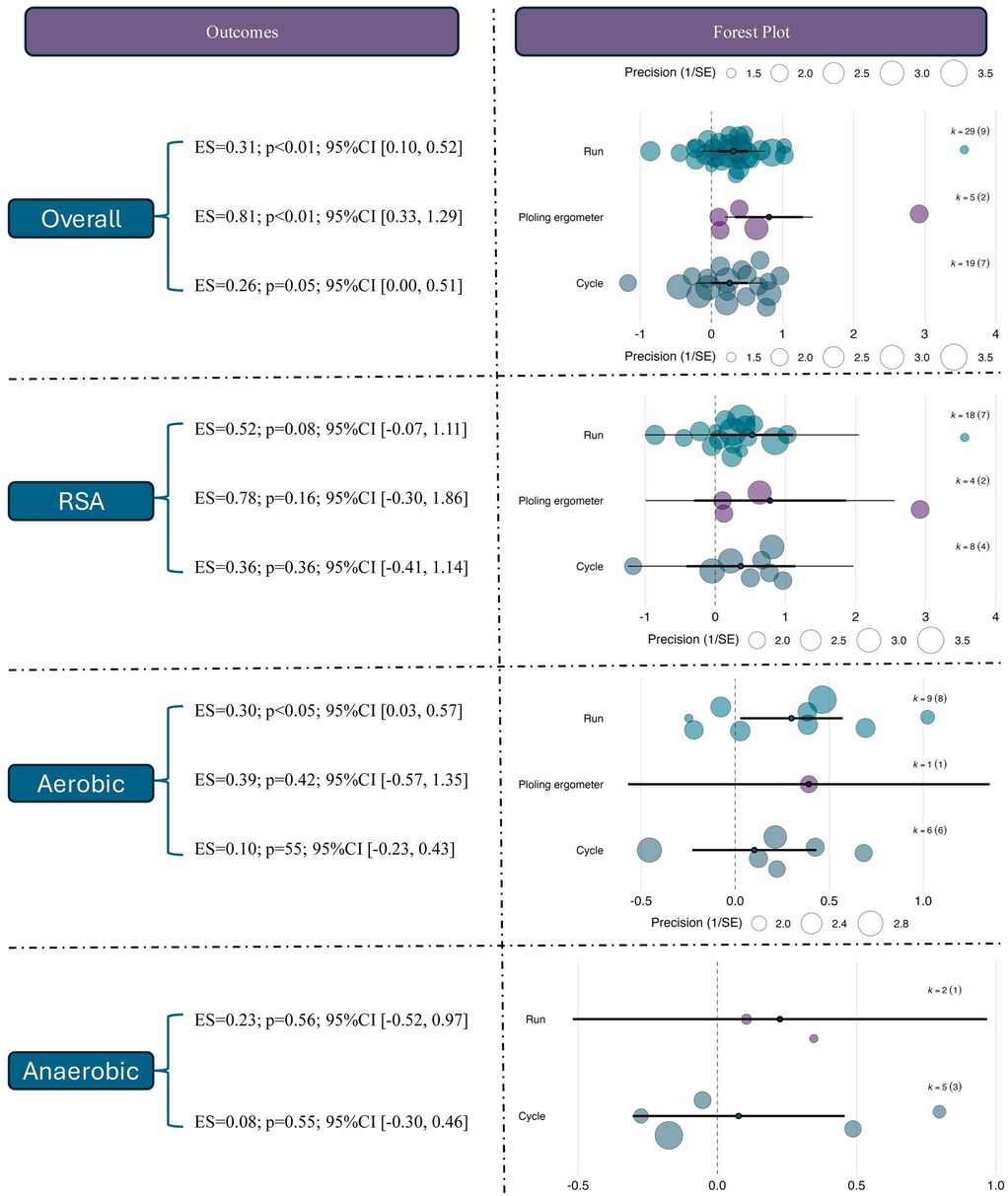
To explore the potential differences in adaptation across various exercise modalities, subgroup analyses were conducted using training mode (running, circuit-based training, or cycling-based training) as a categorical moderator. The results revealed that studies using rowing ergometer-based training observed the largest and statistically significant effect size [ES = 0.81, 95% CI (0.33, 1.29), p < 0.01], which was notably greater than those observed in running [ES = 0.31, 95% CI (0.10, 0.52), p < 0.01] and cycling interventions [ES = 0.26, 95% CI (0.00, 0.51), p = 0.05]. This suggests that under hypoxic conditions, rowing-based RSH may confer superior performance benefits, possibly due to its greater whole-body metabolic demand.
For RSA outcomes, although none of the three training modalities yielded statistically significant results, rowing-based RSH showed the largest effect size [ES = 0.78, 95% CI (–0.30, 1.86), p = 0.16], followed by running (ES = 0.52, p = 0.08) and cycling (ES = 0.36, p = 0.36), suggesting a potential advantage of rowing-based RSH in enhancing sprint capacity, despite current evidence remaining inconclusive. Regarding aerobic capacity, only the running-based intervention reached statistical significance [ES = 0.30, 95% CI (0.03, 0.57), p < 0.05], while rowing (ES = 0.39, p = 0.42) and cycling (ES = 0.10, p = 0.55) showed non-significant positive trends with wide confidence intervals. For anaerobic outcomes, none of the modalities produced statistically significant effects. Effect sizes were ES = 0.23 for running and ES = 0.08 for cycling, suggesting that anaerobic performance may not be the most responsive endpoint to RSH interventions. See in Figure 6.
3.5.1.3 Sex
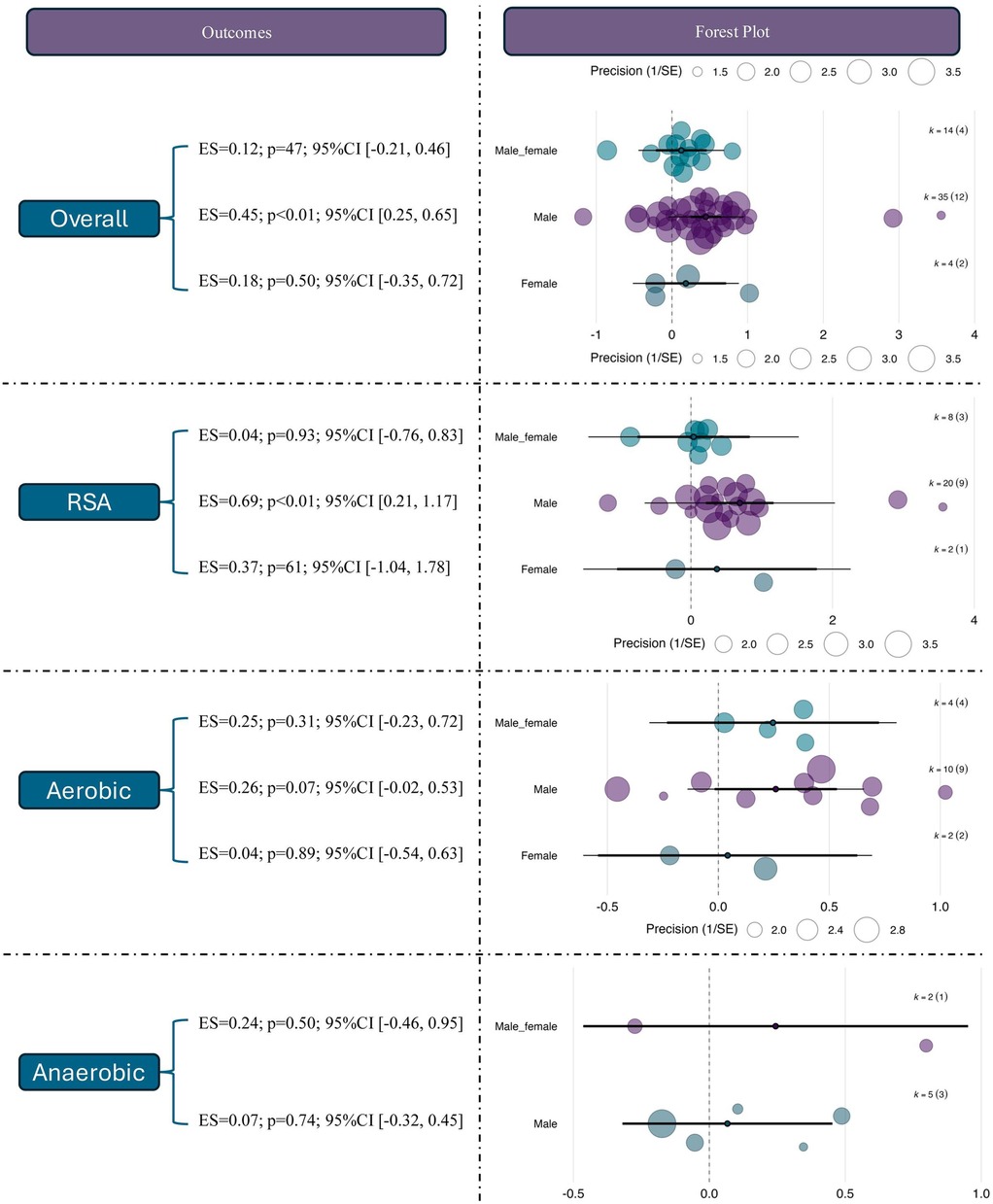
The moderating role of sex in the effects of RSH was further examined. For overall performance outcomes, studies involving male participants showed a statistically significant moderate effect [ES = 0.45, 95% CI (0.25, 0.65), p < 0.01], whereas female-only (ES = 0.18, p = 0.50) and mixed-sex groups (ES = 0.12, p = 0.47) did not reach statistical significance. This suggests that current evidence primarily reflects RSH effects in male populations.
For RSA outcomes, male participants demonstrated a significant improvement [ES = 0.69, 95% CI (0.21, 1.17), p < 0.01], while no significant effects were observed in female (ES = 0.37, p = 0.61) or mixed-sex groups (ES = –0.04, p = 0.93). Notably, the confidence interval for the female group was wide (95% CI [–1.04, 1.78]), indicating small sample sizes and result instability. In the aerobic domain, the male subgroup showed a near-significant trend (ES = 0.26, p = 0.07), while no effects were observed in the female (ES = –0.04) or mixed groups (ES = –0.25), both with p > 0.3. For anaerobic performance, none of the sex-based subgroups showed significant effects; the effect sizes were ES = –0.07 (p = 0.74) for males and ES = –0.24 (p = 0.50) for mixed-sex samples. See in Figure 7.
3.5.2 Continuous moderators
3.5.2.1 Meta-regression of continuous predictors on overall effects
To further investigate the moderating role of training parameters in RSH interventions, a multivariate meta-regression model was constructed based on 53 effect sizes. Four continuous variables were included: FiO2, ER, intervention duration (in weeks), and weekly training frequency (per). A multilevel meta-regression framework was applied to account for between-study heterogeneity.
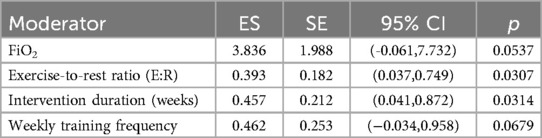
The results showed that both exercise-to-rest ratio [Estimate = 0.393, SE = 0.182, 95% CI (0.037, 0.749), p = 0.0307] and intervention duration [Estimate = 0.457, SE = 0.212, 95% CI (0.041, 0.872), p = 0.0314] had significant positive predictive effects on training outcomes. These findings suggest that higher exercise-to-rest ratios and longer intervention periods are associated with enhanced effectiveness of RSH. See in Table 2.
Although FiO2 (Estimate = 3.836, p = 0.0537) and weekly training frequency (Estimate = 0.462, p = 0.0679) did not reach statistical significance, both showed positive trends. Notably, the relatively large regression coefficient for FiO2 indicates that even small variations in oxygen concentration may exert substantial influence on training effects, highlighting its potential value as a moderator. See in Figure 8.
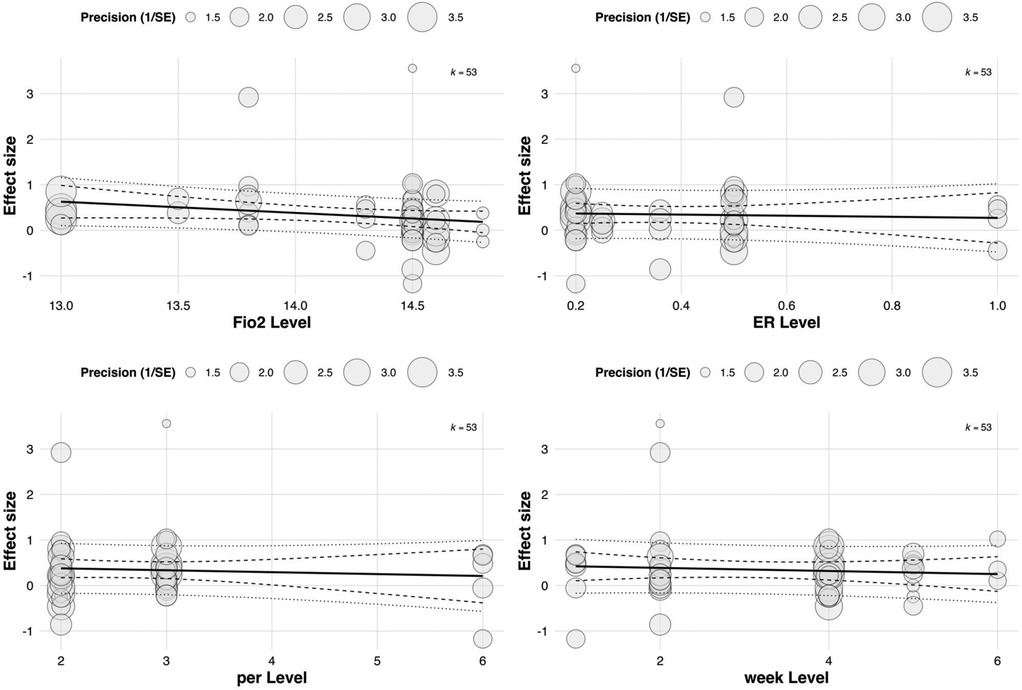
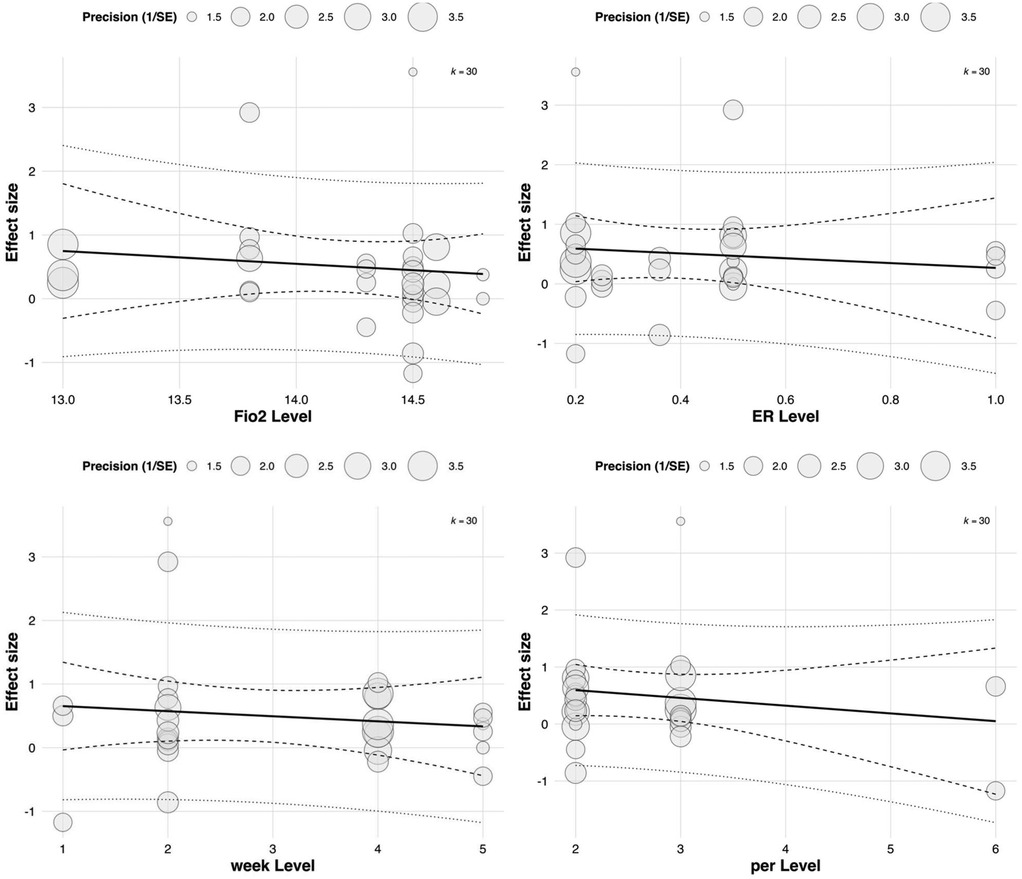
Figure 8. Meta-regression of continuous predictors on the overall effect of RSH on athletic performance.
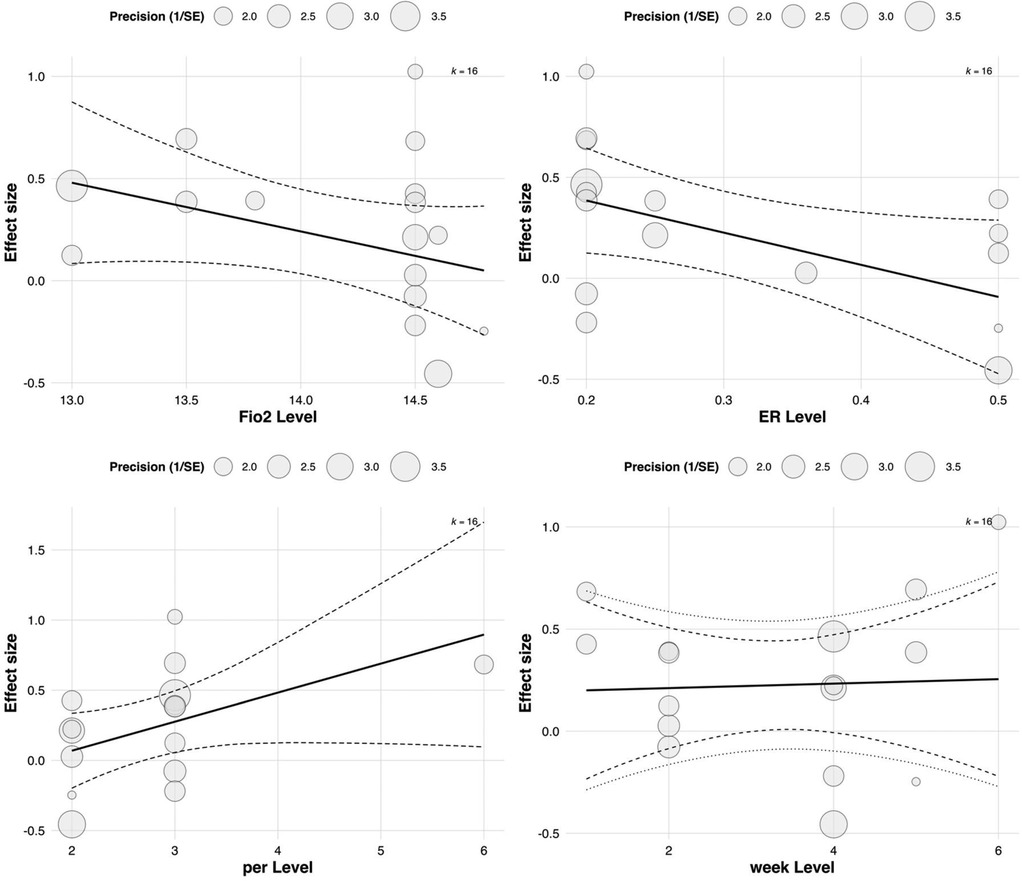
3.5.2.2 Meta-regression of continuous predictors on RSA effects
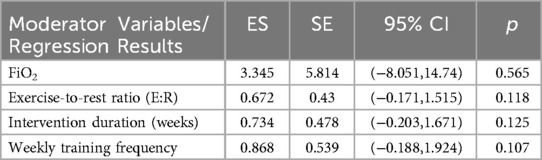
To further explore sources of heterogeneity in the effects of RSH on RSA, a multivariate meta-regression model was constructed including four continuous moderators: FiO2, exercise-to-rest ratio, intervention duration (in weeks), and weekly training frequency. The estimated regression coefficients for RSA effect sizes were as follows: FiO2 [Estimate = 3.345, SE = 5.814, 95% CI (–8.051, 14.740), p = 0.565], exercise-to-rest ratio [Estimate = 0.672, SE = 0.430, 95% CI (–0.171, 1.515), p = 0.118], intervention duration [Estimate = 0.734, SE = 0.478, 95% CI (–0.203, 1.671), p = 0.125], and training frequency [Estimate = 0.868, SE = 0.539, 95% CI (–0.188, 1.924), p = 0.107]. See in Table 3.
Although all regression coefficients were positive—suggesting that increases in these parameters may theoretically enhance RSA outcomes—none reached statistical significance. These findings indicate that current evidence is insufficient to confirm the independent moderating effects of these training parameters on RSA improvement (all p > 0.05). See in Figure 9.
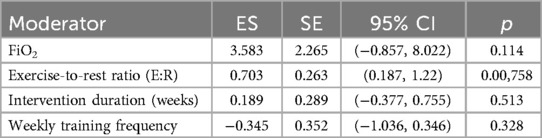
3.5.2.3 Meta-regression of continuous predictors on aerobic capacity effects
In the subgroup analysis focusing on aerobic capacity outcomes, meta-regression was conducted to assess the influence of four continuous variables: FiO2, exercise-to-rest ratio, intervention duration, and weekly training frequency. The results showed a significant moderating effect of exercise-to-rest ratio [Estimate = 0.703, SE = 0.263, 95% CI (0.187, 1.220), p = 0.00758], suggesting that higher training intensity (i.e., shorter rest intervals) may be more beneficial for improving aerobic performance. The corresponding regression plot also indicated a clear upward trend in effect sizes with increasing exercise-to-rest ratios.
The regression coefficient for FiO2 was 3.583 [SE = 2.265, 95% CI (–0.857, 8.022), p = 0.114], which did not reach statistical significance. However, the regression plot revealed a slight downward trend in effect sizes with increasing FiO2 concentration, suggesting that excessively high hypoxic intensity may not confer additional aerobic benefits and that the level of oxygen pressure should be carefully calibrated. See in Table 4.
Neither intervention duration (Estimate = 0.189, p = 0.513) nor weekly frequency (Estimate = –0.345, p = 0.328) showed statistically significant moderating effects. Notably, the plot indicated a negative correlation between training frequency and aerobic adaptation, potentially implying that excessively frequent training could induce fatigue or adaptive plateaus. These findings underscore the need for carefully periodized training strategies to optimize outcomes. See in Figure 10.
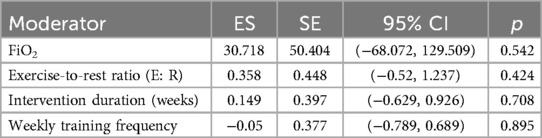
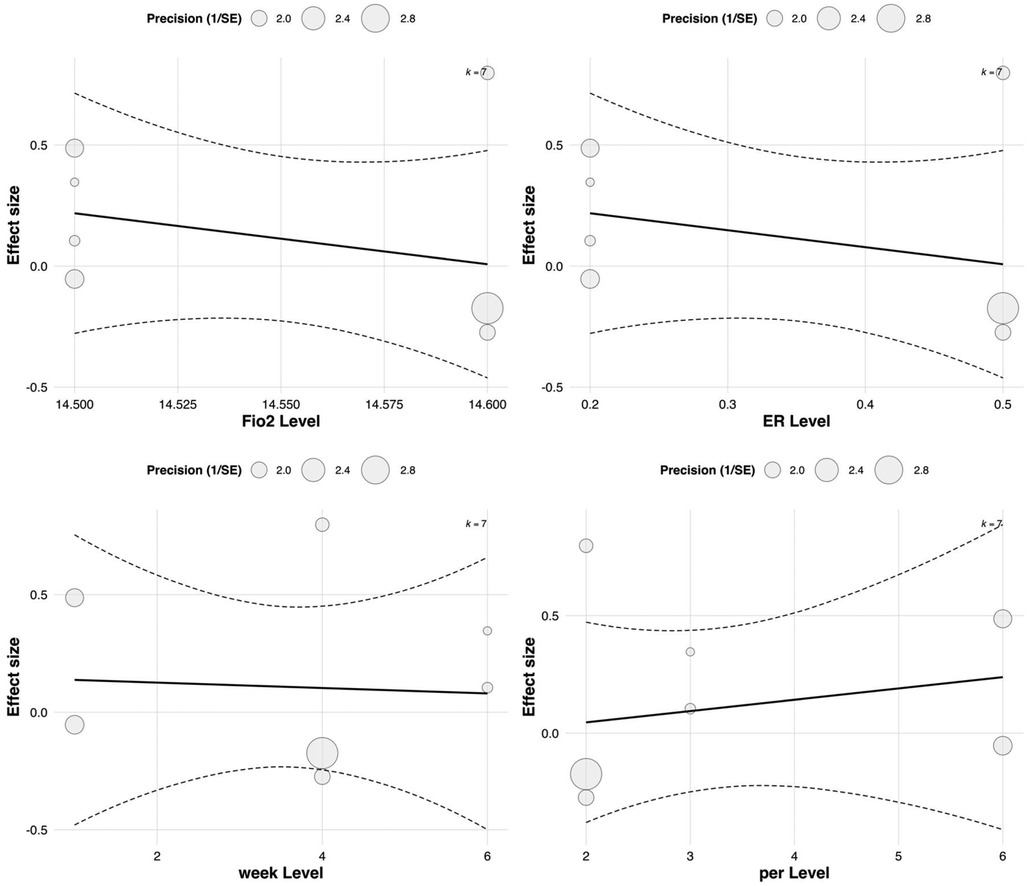
3.5.2.4 Meta-regression of continuous predictors on anaerobic performance effects
In the multilevel meta-regression analysis of anaerobic performance outcomes, none of the continuous moderators demonstrated statistically significant effects. Specifically, the regression coefficient for FiO2 was ES = 30.72 with a standard error of 50.40 [95% CI (–68.07, 129.51), p = 0.542], indicating a non-significant relationship between oxygen concentration and anaerobic performance improvements. The regression coefficient for exercise-to-rest ratio was ES = 0.36 [SE = 0.45, 95% CI (–0.52, 1.24), p = 0.424], also lacking statistical significance. See in Table 5.
Similarly, no significant effects were observed for intervention duration [ES = 0.15, SE = 0.40, 95% CI (–0.63, 0.93), p = 0.708] or training frequency [ES = –0.05, SE = 0.38, 95% CI (–0.79, 0.69), p = 0.895]. The corresponding bubble plots showed largely flat regression lines with wide confidence bands, further supporting the limited moderating role of these continuous variables in explaining the variability in anaerobic performance outcomes. See in Figure 11.
3.6 Publication bias assessment
To assess the robustness of the main model, a sensitivity analysis was first conducted by sequentially excluding each study and re-estimating the model. The results showed that no single study substantially altered the overall effect size, indicating high stability of the findings. Additionally, a PI was used to evaluate the generalizability of the results; the PI for the overall effect was (–0.16, 0.85), suggesting that despite some heterogeneity across studies, the estimated effect remains sufficiently robust and generalizable.
Publication bias was evaluated using contour-enhanced funnel plots combined with Egger's regression test. The funnel plots for the overall model and the RSA subgroup were largely symmetrical, with no obvious signs of asymmetry, indicating a low risk of publication bias. In the aerobic and anaerobic subgroups, the data points appeared slightly skewed; however, the Egger regression lines did not significantly deviate from the vertical axis, and no statistical significance was detected. Further adjustments using the trim-and-fill method revealed only minor changes in the overall effect estimates after imputing potentially missing studies, supporting the reliability of the original results. See in Figure 4.
4 Discussion
4.1 Effects of RSH on athletic performance
This study systematically evaluated the effects of repeated-sprint training in hypoxia (RSH) on multiple dimensions of athletic performance using a multilevel meta-analytic approach. The results demonstrated that RSH had a significant moderate effect on improving repeated sprint ability (RSA), a small but statistically significant advantage for sport-specific aerobic capacity, and no clear effect on anaerobic capacity due to substantial heterogeneity and inconsistent findings.
RSA reflects an athlete's ability to perform multiple sprints over a short period while maintaining sprint quality with limited recovery, and it is widely used to predict performance in team sports. The meta-analysis revealed a pooled effect size of ES = 0.50 [95% CI (0.12, 0.88), p < 0.01], representing the most prominent effect across all outcome types. Several original studies have confirmed the robustness of this finding. For instance, Faiss et al. (11) reported that RSH upregulated key molecular markers such as carbonic anhydrase and MCT transporters, enhancing lactate clearance and proton transport efficiency during sprints. Gatterer et al. (35) observed a significant reduction in RSA fatigue slope among adolescent football players, suggesting improved fatigue resistance. Brocherie et al. (30) further demonstrated that performance gains persisted for up to three weeks post-intervention in elite field hockey players, with total sprint time reduced by 3.5% (p < 0.01), indicating potential medium-term retention effects. On the other hand, some studies have expressed caution regarding RSH efficacy, especially when discrepancies exist between the training and testing modalities, such as running vs. cycling, linear vs. change-of-direction movement, or mismatched loading protocols (43, 44). These findings suggest that training–testing specificity is a critical moderator of RSH effectiveness.
Regarding aerobic capacity, the pooled effect size was ES = 0.23 [95% CI (0.02, 0.44), p = 0.03], representing a small but statistically significant effect. Pramkratok et al. (40) reported a 7.5% increase in VO2max after six weeks of RSH, along with significant upregulation of the HIF-1α and VEGF pathways, suggesting that the aerobic benefits of RSH may be mediated by hypoxia-induced angiogenesis and mitochondrial biogenesis. However, Kasai et al. (38) and Brocherie et al. (14) failed to observe meaningful changes in VO2max among elite athletes, and the pooled confidence interval crossed the null, indicating that VO2max responses may depend on training dose, baseline fitness, and measurement sensitivity. It is noteworthy that RSH produced more consistent improvements in sport-specific aerobic indicators such as the Yo-Yo Intermittent Recovery (YYIR) test. For example, Galvin et al. (34) and Hamlin et al. (37) both reported superior improvements in YYIR1 performance in the RSH group compared with RSN, possibly due to enhanced activation of peripheral metabolic pathways (e.g., muscle oxygen uptake and lactate clearance) rather than central cardiovascular adaptations. This suggests that the aerobic improvements from RSH may reflect “specific adaptation” rather than “maximal physiological enhancement.”.
For anaerobic capacity, the meta-analysis did not reveal statistically significant effects [ES = 0.11, 95% CI (–0.23, 0.45), p = 0.67]. While some studies, such as Gutknecht et al. (45) and Woorons et al. (46), reported increases in Wingate peak and mean power (+11.9% and +6.2%, respectively, both p < 0.05), most studies found no systematic advantage of RSH over RSN. For instance, Kasai et al. (38) and Gatterer et al. (47) reported no significant between-group differences in peak or mean power output. Potential explanations include insufficient stimulation of anaerobic pathways by short-duration training and the limited sensitivity of the Wingate test in detecting subtle metabolic adaptations.
In summary, RSH exerts a consistent and significant positive effect on RSA and shows modest benefits for sport-specific aerobic performance, particularly in high-intensity intermittent sports. However, its impact on VO2max and anaerobic capacity appears limited and may depend on a combination of training dose, modality, and individual baseline characteristics.
4.2 Moderating variables
To further explore how study characteristics influence the effects of RSH, a series of categorical and continuous moderators were tested using a multilevel meta-regression framework. The results showed that these moderators played an important role in explaining between-study heterogeneity, suggesting that the efficacy of RSH may be shaped by training dose parameters, physiological background, and implementation strategies. Among the categorical variables, outcome type emerged as a significant moderator. RSH produced the largest and most consistent effects on RSA outcomes [e.g., sprint count, fatigue index; ES = 0.50, 95% CI (0.12, 0.88), p < 0.01], whereas the effects on VO2max (ES = 0.23, p = 0.67) and anaerobic performance (ES = 0.11, p = 0.67) were less stable. These findings suggest that RSH may be more suited for improving intermittent performance rather than eliciting substantial gains in cardiorespiratory endurance or anaerobic power output. Training modality also significantly moderated the effects, with running-based RSH interventions yielding greater benefits than cycling or rowing ergometer protocols. This may be due to training–testing specificity: the closer the match between training and testing modalities, the more pronounced the adaptation (48).
Subgroup analysis by sex revealed no statistically significant differences between groups; however, most included studies predominantly involved male athletes, which inherently limits the generalizability of sex-based findings. Although slightly larger effect sizes were observed in mixed-sex or female subgroups, these results should be interpreted with caution due to the small number of female-specific studies and limited statistical power. This gender imbalance underscores a critical gap in the current RSH literature and highlights the need for future trials to systematically examine sex-specific responses to hypoxic sprint training. Among the continuous moderators, inspired oxygen fraction (FiO₂), exercise-to-rest ratio (E:R), intervention duration (in weeks), and weekly training frequency were examined within a multivariate regression framework. Among these, FiO₂ demonstrated a marginally significant association with training outcomes (ES = 3.84, SE = 1.99, p = 0.0537), suggesting a potential—but not conclusive—moderating role. Given the proximity of this p-value to the conventional significance threshold, caution is warranted in interpreting this result as evidence of a reliable effect. Rather than inferring causality, these findings should be viewed as hypothesis-generating and indicative of trends that merit further investigation. Notably, prior literature has suggested a nonlinear dose–response relationship between inspired oxygen levels and performance outcomes (49, 50). Within this context, moderate hypoxic exposures (FiO₂ = 14.0%–14.5%) are often hypothesized to balance sufficient hypoxic stress with tolerable physiological demand. However, the present data do not permit firm conclusions regarding an optimal FiO₂ range. Levels below 14% have been associated in some studies with central nervous system suppression and impaired motor output, while more severe hypoxia (FiO₂ ≤ 12%) may exacerbate metabolic stress without concomitant performance benefits. These insights, although mechanistically plausible, remain speculative in the absence of statistically robust moderator effects and should be interpreted with due methodological restraint.
Regarding the exercise-to-rest ratio, results suggested that relatively longer rest intervals (i.e., lower E: R values) may enhance power output and metabolic recovery (ES = 0.393, p = 0.0307), a finding consistent with studies by Raberin et al. (51, 52) and Faiss et al. (3). Longer inter-set recovery may promote phosphocreatine resynthesis and oxygenation, facilitating peripheral adaptation and improving muscular oxygen delivery. Additionally, it has been shown that nitric oxide production and vasodilation are enhanced under hypoxic conditions, particularly in “all-out sprint + incomplete recovery” protocols, where RSH may stimulate greater muscle reperfusion and fast-twitch fiber recruitment (11, 53).
Training frequency and intervention duration also showed marginally significant trends. In endurance-related outcomes such as VO2max, a nonlinear pattern was observed, where longer interventions (≥4 weeks) and moderate weekly frequency (3–4 sessions/week) were more effective for promoting mitochondrial biogenesis and metabolic remodeling (40, 54). However, no consistent patterns were found for anaerobic or explosive performance outcomes.
It is worth noting that baseline training status may also influence RSH outcomes. Participants with lower training backgrounds (e.g., sedentary females or recreationally active youth) often exhibit greater adaptive potential in VO2max and aerobic capacity (55, 56), whereas elite athletes may require more potent stimuli to elicit further adaptations due to approaching physiological ceilings (57). Although sex did not significantly moderate overall effects, minor fluctuations in female performance may occur across menstrual cycle phases. Nonetheless, existing studies support the safety and adaptability of RSH across both sexes (38, 58).
In summary, the training effects of RSH are influenced by multiple interacting factors. Optimal adaptations appear to be achieved under moderate hypoxic doses (FiO2 = 14%), with a balanced exercise-to-rest ratio, intervention durations of at least four weeks, and individualized training frequency.
4.3 Potential mechanisms
Repeated-sprint training in hypoxia (RSH) induces specific physiological adaptations by combining maximal sprint bouts with incomplete recovery under reduced oxygen conditions. Its efficacy is underpinned by three key mechanisms: improved oxygen delivery, enhanced phosphagen system function, and glycolytic remodeling.
First, the combined hypoxic and high-intensity load preferentially recruits fast-twitch (FT) muscle fibers, which are particularly sensitive to oxygen availability. RSH promotes vasodilation via nitric oxide (NO) signaling, enhancing perfusion and oxygen uptake in FT-dominant regions, thereby improving sprint capacity (59). This response is mediated by shear stress-induced activation of endothelial and neuronal NO synthase (60). Second, RSH enhances energy supply by accelerating phosphocreatine (PCr) resynthesis and increasing PCr and glycogen stores, enabling sustained ATP regeneration (61). Short-term interventions have demonstrated 14%–18% increases in glycogen and 21% in PCr after RSH (39). In high-intensity, short-rest settings, PCr recovery is more strongly associated with sprint performance than acid–base buffering (62). Third, RSH activates hypoxia-inducible factor-1α (HIF-1α), which upregulates glycolytic and buffering proteins such as monocarboxylate transporter 4 (MCT4) and carbonic anhydrase III (CA3), improving lactate clearance and pH stability (63). RSH enhances MCT4 expression in FT fibers (±20%) and increases CA3 activity more than normoxic training (11, 64). Conversely, mitochondrial oxidative phosphorylation markers (e.g., COX IV, SDHB) are unchanged or downregulated (65), indicating a shift toward glycolytic metabolism.
Together, these mechanisms enhance repeated-sprint ability by improving FT fiber oxygenation, anaerobic energy regeneration, and metabolic resilience under hypoxic stress.
4.4 Practical recommendations for intervention design
Given the diverse physiological demands across sports, RSH protocols should be tailored to specific athletic contexts to enhance transferability and training efficacy. For team sports (e.g., football, rugby), where repeated sprint ability (RSA) and recovery under fatigue are critical, sessions should include 2–3 weekly bouts of 5–10 s maximal sprints with short recovery intervals (1:2–1:4), under moderate hypoxia (FiO₂ = 14.0%–14.5%). Sport-specific drills such as shuttle runs or change-of-direction sprints are recommended to maximize ecological validity. For individual sprint or power athletes (e.g., track sprinters, cyclists), emphasis should be placed on neuromuscular quality. Use fewer repetitions with full recovery between sets (e.g., 3–5 min), gradually progressing from FiO₂ 15.5%–14.0% to elevate metabolic load without impairing technique. For endurance athletes (e.g., rowers, middle-distance runners), RSH may complement aerobic base training by improving buffering capacity. Sessions can include 20–30 s efforts at near-maximal intensity, with incomplete recovery and FiO₂ between 13.5%–14.5%. Across all sports, careful monitoring of training load via heart rate variability, RPE, and oxygen saturation is advised to avoid non-functional overreaching. RSH should be periodized within mesocycles and supported with appropriate recovery strategies (e.g., sleep, nutrition). These targeted guidelines provide a practical framework for integrating RSH across diverse training environments, maximizing its applied value in sport-specific performance enhancement.
4.5 Research limitations
Despite the overall robustness of the present meta-analysis, several limitations warrant consideration. First, although funnel plot symmetry and Egger's test suggested a low risk of publication bias, subtle small-study effects or selective reporting cannot be entirely ruled out—especially in outcome domains with fewer effect sizes, such as anaerobic performance or female-only samples. The concentration of studies within a few research groups may also introduce potential investigator or institutional bias. Second, most included interventions were of short duration (1–6 weeks), limiting the ability to assess whether observed gains in performance reflect transient physiological responses or sustainable adaptations. Long-term effects on training retention, detraining resistance, and competition-season performance remain unknown. Third, the lack of follow-up assessments precluded insight into the persistence of RSH-induced adaptations. Although moderate hypoxic doses (∼14.0% FiO₂) and adequate training duration (≥4 weeks) appeared beneficial, current evidence offers limited understanding of the dose–response dynamics or potential maladaptations associated with chronic hypoxic exposure. These limitations highlight the need for well-powered, longitudinal trials incorporating extended follow-up, diverse populations, and rigorous reporting standards. Future research should aim to clarify optimal hypoxic dosing strategies, characterize individualized response patterns, and evaluate the long-term efficacy and safety of RSH in both elite and sub-elite athletic settings.
5 Conclusion
This systematic review and multilevel meta-analysis comprehensively evaluated the effects of RSH on athletic performance. The findings indicate that RSH can significantly enhance overall performance, with a clear advantage over normoxic training particularly in improving RSA. Moderate-to-small effects were observed for aerobic and anaerobic outcomes, accompanied by considerable heterogeneity. Moderator analyses revealed that training effects varied significantly across sport modalities, sex composition, and outcome types. More pronounced benefits were observed when training and testing modalities matched (e.g., running-based protocols), in predominantly male samples, and when specific outcome measures such as RSA-Time and YYIR were employed. Among continuous moderators, higher exercise-to-rest ratios and moderate intervention durations were significant positive predictors of training efficacy, underscoring the importance of scientifically informed parameter design to maximize training gains. From a mechanistic perspective, RSH offers distinct advantages over normoxic training due to its ability to simultaneously activate multiple adaptation pathways under hypoxia–high-intensity coupling. These include intensity-dependent vasodilation mediated by nitric oxide (NO), optimized phosphocreatine (PCr) resynthesis kinetics, and upregulation of glycolytic and buffering systems driven by hypoxia-inducible factor 1-alpha (HIF-1α).
Taken together, RSH represents a physiologically targeted training modality that is particularly suitable for competitive sports requiring repeated high-intensity efforts. However, its effectiveness is moderated by several factors, including oxygen pressure, training structure, individual fitness level, and training status. Future research should aim to refine optimal dosing strategies, clarify the mechanisms underlying improvements in different performance domains, and characterize individualized response patterns. Expanding the application of RSH to female athletes, youth populations, and varying levels of athletic expertise will also be essential to advance the precision and efficiency of hypoxia-based training interventions.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
MH: Conceptualization, Investigation, Methodology, Software, Supervision, Writing – original draft, Writing – review & editing, Data curation, Formal analysis, Funding acquisition, Project administration, Resources, Validation, Visualization. BL: Conceptualization, Data curation, Formal analysis, Resources, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. FZ232001, Research on the path to improve the physical health level of college students based on functional training.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wilber RL. Historical Development of Altitude Training and Hypoxic Conditioning. London, UK: Routledge (2024).
2. Serebrovska T, Serebrovska Z, Egorov E. Fitness and therapeutic potential of intermittent hypoxia training: a matter of dose. Fiziol Zh. (2016) 62:78–91. doi: 10.15407/fz62.03.078
3. Faiss R, Raberin A, Brocherie F, Millet GP. Repeated-sprint training in hypoxia: a review with 10 years of perspective. J Sports Sci. (2024):1–15. doi: 10.1080/02640414.2024.2416821
4. Hoppeler H, Vogt M, Weibel ER, Flück M. Response of skeletal muscle mitochondria to hypoxia. Exp Physiol. (2003) 88:109–19. doi: 10.1113/eph8802513
5. Lundby C, Calbet JA, Robach P. The response of human skeletal muscle tissue to hypoxia. Cell Mol Life Sci. (2009) 66:3615–23. doi: 10.1007/s00018-009-0146-8
6. Hoppeler H, Vogt M. Muscle tissue adaptations to hypoxia. J Exp Biol. (2001) 204:3133–9. doi: 10.1242/jeb.204.18.3133
7. Zoll J, Ponsot E, Dufour S, Doutreleau S, Ventura-Clapier R, Vogt M, et al. Exercise training in normobaric hypoxia in endurance runners. III. Muscular adjustments of selected gene transcripts. J Appl Physiol. (2006) 100:1258–66. doi: 10.1152/japplphysiol.00359.2005
8. Millet GP, Roels B, Schmitt L, Woorons X, Richalet J-P. Combining hypoxic methods for peak performance. Sports Med. (2010) 40:1–25. doi: 10.2165/11317920-000000000-00000
9. Seitz H, Preißler E, Catalá-Lehnen P, Weitl M. Effects of the ‘live low-train high’ method on variables of endurance capacity: a systematic review. German J Sports Med. (2020) 71(2):43–50. doi: 10.5960/dzsm.2019.413
10. Girard O, Brocherie F, Millet GP. Effects of altitude/hypoxia on single-and multiple-sprint performance: a comprehensive review. Sports Med. (2017) 47:1931–49. doi: 10.1007/s40279-017-0733-z
11. Faiss R, Léger B, Vesin J-M, Fournier P-E, Eggel Y, Dériaz O, et al. Significant molecular and systemic adaptations after repeated sprint training in hypoxia. PLoS One. (2013) 8:e56522. doi: 10.1371/journal.pone.0056522
12. Brocherie F, Racinais S, Cocking S, Townsend N, Couderc A, Piscione J, et al. Repeated-Sprint training at 5000-m simulated altitude in preparation for the world rugby women’s sevens series: too high? Med Sci Sports Exerc. (2023a) 55:1923–32. doi: 10.1249/MSS.0000000000003226
13. Alvarez L. Fatigue neuromusculaire en répétition de sprint avec différents niveaux d'hypoxie et d'occlusion vasculaire. Université de lausanne, faculté des sciences sociales et politiques (Master’s thesis). Université de Lausanne, Faculté des sciences sociales et politiques (2016).
14. Brocherie F, Girard O, Faiss R, Millet GP. Effects of repeated-sprint training in hypoxia on sea-level performance: a meta-analysis. Sports Med. (2017) 47:1651–60. doi: 10.1007/s40279-017-0685-3
15. Zelenovic M, Kontro T, Stojanovic T, Alexe DI, Bozic D, Aksovic N, et al. Effects of repeated sprint training in hypoxia on physical performance among athletes: a systematic review. Int J Morphol. (2021) 39:1625–34. doi: 10.4067/S0717-95022021000601625
16. Eldridge S, Campbell M, Campbell M, Dahota A, Giraudeau B, Higgins J, et al. Revised Cochrane risk of bias tool for randomized trials (RoB 2.0): additional considerations for cluster-randomized trials. Cochrane Methods Cochrane Database Syst Rev. (2016) 10.
17. Lee J, Lee W, Kim J. MatGD: materials graph digitizer. ACS Appl Mater Interfaces. (2023) 16:723–30. doi: 10.1021/acsami.3c14781
18. Hedges LV, Tipton E. Meta-analysis. In: Steptoe A, editor. Handbook of Behavioral Medicine: Methods and Applications. New York, NY: Springer (2010). p. 551–65.
19. Hamman EA, Pappalardo P, Bence JR, Peacor SD, Osenberg CW. Bias in meta-analyses using Hedges’d. Ecosphere. (2018) 9:e02419. doi: 10.1002/ecs2.2419
20. Borenstein M, Cooper H, Hedges L, Valentine J. Effect sizes for continuous data. Handbook Res Synth Meta-anal. (2009) 2:221–35.
21. Borenstein M. Comprehensive meta-analysis software. In: Egger M, Higgins JPT, Smith GD, editors. Systematic Reviews in Health Research: Meta-Analysis in Context, 3rd ed. Hoboken, NJ: John Wiley & Sons Ltd. (2022). p. 535–48.
22. Koesters M. Every effect size has its place: a commentary on the avoidance of pre–post effect sizes. Epidemiol Psychiatr Sci. (2017) 26:369–70. doi: 10.1017/S204579601700004X
23. Wagner J, Chelaru F, Kancherla J, Paulson JN, Zhang A, Felix V, et al. Metaviz: interactive statistical and visual analysis of metagenomic data. Nucleic Acids Res. (2018) 46:2777–87. doi: 10.1093/nar/gky136
24. Duval S. The trim and fill method. In: Rothstein HR, Sutton AJ, Borenstein M, editors. Publication Bias in Meta-analysis: Prevention, Assessment and Adjustments. Chichester, UK: Wiley (2005). p. 127–44.
25. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. doi: 10.1136/bmj.n71
26. Beard A, Ashby J, Chambers R, Brocherie F, Millet GP. Repeated-sprint training in hypoxia in international rugby union players. Int J Sports Physiol Perform. (2019a) 14:850–4. doi: 10.1123/ijspp.2018-0170
27. Beard A, Ashby J, Kilgallon M, Brocherie F, Millet GP. Upper-body repeated-sprint training in hypoxia in international rugby union players. Eur J Sport Sci. (2019b) 19:1175–83. doi: 10.1080/17461391.2019.1587521
28. Brechbuhl C, Brocherie F, Millet GP, Schmitt L. Effects of repeated-sprint training in hypoxia on tennis-specific performance in well-trained players. Sports Med Int Open. (2018) 2:E123–32. doi: 10.1055/a-0719-4797
29. Brechbuhl C, Brocherie F, Willis SJ, Blokker T, Montalvan B, Girard O, et al. On the use of the repeated-sprint training in hypoxia in tennis. Front Physiol. (2020) 11:588821. doi: 10.3389/fphys.2020.588821
30. Brocherie F, Girard O, Faiss R, Millet GP. High-intensity intermittent training in hypoxia: a double-blinded, placebo-controlled field study in youth football players. J Strength Cond Res. (2015) 29:226–37. doi: 10.1519/JSC.0000000000000590
31. Brocherie F, Millet GP, Hauser A, Steiner T, Rysman J, Wehrlin JP, et al. Live high-train low and high” hypoxic training improves team-sport performance. Med Sci Sports Exerc. (2015) 47:2140–9. doi: 10.1249/MSS.0000000000000630
32. Camacho-Cardenosa A, Camacho-Cardenosa M, Martínez-Guardado I, Brazo-Sayavera J, Timon R, Olcina G. Effects of repeated-sprint training in hypoxia on physical performance of team sports players. Rev Bras Med Esporte. (2020) 26:153–7. doi: 10.1590/1517-869220202602188454
33. Faiss R, Willis S, Born D-P, Sperlich B, Vesin J-M, Holmberg H-C, et al. Repeated double-poling sprint training in hypoxia by competitive cross-country skiers. Med Sci Sports Exerc. (2015) 47:809–17. doi: 10.1249/MSS.0000000000000464
34. Galvin HM, Cooke K, Sumners DP, Mileva KN, Bowtell JL. Repeated sprint training in normobaric hypoxia. Br J Sports Med. (2013) 47:i74–9. doi: 10.1136/bjsports-2013-092826
35. Gatterer H, Philippe M, Menz V, Mosbach F, Faulhaber M, Burtscher M. Shuttle-run sprint training in hypoxia for youth elite soccer players: a pilot study. J Sports Sci Med. (2014) 13:731.25435763
36. Giovanna M, Solsona R, Sanchez AM, Borrani F. Effects of short-term repeated sprint training in hypoxia or with blood flow restriction on response to exercise. J Physiol Anthropol. (2022) 41:32. doi: 10.1186/s40101-022-00304-1
37. Hamlin MJ, Olsen PD, Marshall HC, Lizamore CA, Elliot CA. Hypoxic repeat sprint training improves rugby player’s repeated sprint but not endurance performance. Front Physiol. (2017) 8:24. doi: 10.3389/fphys.2017.00024
38. Kasai N, Mizuno S, Ishimoto S, Sakamoto E, Maruta M, Goto K. Effect of training in hypoxia on repeated sprint performance in female athletes. Springerplus. (2015) 4:1–7. doi: 10.1186/s40064-015-1041-4
39. Kasai N, Mizuno S, Ishimoto S, Sakamoto E, Maruta M, Kurihara T, et al. Impact of six consecutive days of sprint training in hypoxia on performance in competitive sprint runners. J Strength Cond Res. (2019) 33:36–43. doi: 10.1519/JSC.0000000000001954
40. Pramkratok W, Songsupap T, Yimlamai T. Repeated sprint training under hypoxia improves aerobic performance and repeated sprint ability by enhancing muscle deoxygenation and markers of angiogenesis in rugby sevens. Eur J Appl Physiol. (2022) 122:611–22. doi: 10.1007/s00421-021-04861-8
41. Shi Q, Tong TK, Nie J, Tao D, Zhang H, Tan X, et al. Repeated-sprint training in hypoxia boosts up team-sport-specific repeated-sprint ability: 2-week vs 5-week training regimen. Eur J Appl Physiol. (2023) 123:2699–710. doi: 10.1007/s00421-023-05252-x
42. Thongsawang S, Chorcharoenying S, Yimlamai T. Effect of Cordyceps Sinensis on repeated-sprint performance following repeated-sprint training in hypoxia in female soccer players. Journal of Physical Education and Sport. (2024) 24:2170–8.
43. Goods PS, Dawson B, Landers GJ, Gore CJ, Peeling P. No additional benefit of repeat-sprint training in hypoxia than in normoxia on sea-level repeat-sprint ability. J Sports Sci Med. (2015) 14:681.26336357
44. Montero D, Lundby C. Repeated sprint training in hypoxia versus normoxia does not improve performance: a double-blind and cross-over study. Int J Sports Physiol Perform. (2017) 12:161–7. doi: 10.1123/ijspp.2015-0691
45. Gutknecht AP, Gonzalez-Figueres M, Brioche T, Maurelli O, Perrey S, Favier FB. Maximizing anaerobic performance with repeated-sprint training in hypoxia: in search of an optimal altitude based on pulse oxygen saturation monitoring. Front Physiol. (2022) 13:1010086. doi: 10.3389/fphys.2022.1010086
46. Woorons X, Dupuy O, Mucci P, Millet GP, Pichon A. Cerebral and muscle oxygenation during repeated shuttle run sprints with hypoventilation. Int J Sports Med. (2019) 40:376–84. doi: 10.1055/a-0836-9011
47. Gatterer H, Menz V, Salazar-Martinez E, Sumbalova Z, Garcia-Souza LF, Velika B, et al. Exercise performance, muscle oxygen extraction and blood cell mitochondrial respiration after repeated-sprint and sprint interval training in hypoxia: a pilot study. J Sports Sci Med. (2018) 17:339.30116106
48. Girard O, Brocherie F, Millet GP. On the use of mobile inflatable hypoxic marquees for sport-specific altitude training in team sports. Br J Sports Med. (2013) 47:i121–3. doi: 10.1136/bjsports-2013-092794
49. Bowtell JL, Cooke K, Turner R, Mileva KN, Sumners DP. Acute physiological and performance responses to repeated sprints in varying degrees of hypoxia. J Sci Med Sport. (2014) 17:399–403. doi: 10.1016/j.jsams.2013.05.016
50. Brocherie F, Racinais S, Couderc A, Piscione J, Girard O. Four sessions of repeated-sprint cycling training with or without severe hypoxia do not modify overground running sprint force–velocity profile. Int J Sports Physiol Perform. (2023b) 19:80–3. doi: 10.1123/ijspp.2023-0112
51. Raberin A, Elmer J, Willis S, Richard T, Vernillo G, Iaia M, et al. The oxidative-glycolytic balance influenced by sprint duration is key during repeated sprints in hypoxia. Med Sci Sports Exercise. (2022).
52. Raberin A, Willis SJ, Richard T, Elmer J, Vernillo G, Iaia FM, et al. Hypoxia does not change performance and psychophysiological responses during repeated cycling sprints to exhaustion with short exercise-to-rest ratio. Int J Sports Physiol Perform. (2023) 18:213–7. doi: 10.1123/ijspp.2022-0234
53. Casey DP, Joyner MJ. Compensatory vasodilatation during hypoxic exercise: mechanisms responsible for matching oxygen supply to demand. J Physiol (Lond). (2012) 590:6321–6. doi: 10.1113/jphysiol.2012.242396
54. Granata C. Effects of different exercise intensity and volume on markers of mitochondrial biogenesis in human skeletal muscle (Doctoral dissertation). Victoria University (2015).
55. Kong Z, Lei OK, Sun S, Li L, Shi Q, Zhang H, et al. Hypoxic repeated sprint interval training improves cardiorespiratory fitness in sedentary young women. J Exerc Sci Fitness. (2022) 20:100–7. doi: 10.1016/j.jesf.2022.01.005
56. Piperi A, Warnier G, DE Ten Ryen S, Benoit N, Antoine N, Copine S, et al. Repeated sprint training in hypoxia improves repeated sprint ability to exhaustion similarly in active males and females. Med Sci Sports Exerc. (2024) 56(10):1988–99. doi: 10.1249/MSS.0000000000003485
57. Stray-Gundersen J, Chapman RF, Levine BD. Living high-training low” altitude training improves sea level performance in male and female elite runners. J Appl Physiol. (2001) 91:1113–20. doi: 10.1152/jappl.2001.91.3.1113
58. Tounsi M, Jaafar H, Aloui A, Souissi N. Soccer-related performance in eumenorrheic Tunisian high-level soccer players: effects of menstrual cycle phase and moment of day. J Sports Med Phys Fitness. (2017) 58:497–502.28222573
59. Mcdonough P, Behnke BJ, Padilla DJ, Musch TI, Poole DC. Control of microvascular oxygen pressures in rat muscles comprised of different fibre types. J Physiol (Lond). (2005) 563:903–13. doi: 10.1113/jphysiol.2004.079533
60. Ferguson SK, Hirai DM, Copp SW, Holdsworth CT, Allen JD, Jones AM, et al. Impact of dietary nitrate supplementation via beetroot juice on exercising muscle vascular control in rats. J Physiol (Lond). (2013) 591:547–57. doi: 10.1113/jphysiol.2012.243121
61. Haseler LJ, Hogan MC, Richardson RS. Skeletal muscle phosphocreatine recovery in exercise-trained humans is dependent on O2availability. J Appl Physiol. (1999) 86:2013–8. doi: 10.1152/jappl.1999.86.6.2013
62. Mendez-Villanueva A, Edge J, Suriano R, Hamer P, Bishop D. The recovery of repeated-sprint exercise is associated with PCr resynthesis, while muscle pH and EMG amplitude remain depressed. PLoS One. (2012) 7:e51977. doi: 10.1371/journal.pone.0051977
63. De Smet S, Van Herpt P, D'Hulst G, Van Thienen R, Van Leemputte M, Hespel P. Physiological adaptations to hypoxic vs. normoxic training during intermittent living high. Front Physiol. (2017) 8:347. doi: 10.3389/fphys.2017.00347
64. Feng H-Z, Jin J-P. Carbonic anhydrase III is expressed in mouse skeletal muscles independent of fiber type-specific myofilament protein isoforms and plays a role in fatigue resistance. Front Physiol. (2016) 7:597. doi: 10.3389/fphys.2016.00597
Keywords: repeated-sprint training, hypoxia, athletic performance, meta-analysis, repeated-sprint ability, aerobic capacity, anaerobic power, training adaptation
Citation: Han M and Liu B (2025) A multilevel meta-analysis of the effects of repeated sprint training in hypoxia on athletic performance. Front. Sports Act. Living 7:1641379. doi: 10.3389/fspor.2025.1641379
Received: 18 June 2025; Accepted: 15 July 2025;
Published: 21 August 2025.
Edited by:
Emiliano Cè, University of Milan, ItalyReviewed by:
Raphael Faiss, Université de Lausanne, SwitzerlandCesar Osorio, Metropolitan University of Educational Sciences, Chile
Copyright: © 2025 Han and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meng Han, MTk4NjE4MTE3NzZAMTYzLmNvbQ==
 Meng Han
Meng Han Binglin Liu
Binglin Liu