- 1Medical Psychological Center, The Second Xiangya Hospital of Central South University, Changsha, China
- 2Medical Psychological Institute of Central South University, Changsha, China
- 3China National Clinical Research Center on Mental Disorders (Xiangya), Changsha, China
- 4Department of Psychiatry, The First Affiliated Hospital of Soochow University, Suzhou, China
Background: It has been suggested that adolescents with conduct disorder (CD) may have a deficit in the affective and cognitive domains empathy, but studies exploring networks within the key brain regions of affective and cognitive empathy in adolescents with CD are lacking.
Methods: Functional connectivity (FC) analyses among key brain regions of the affective and cognitive empathy with resting-state functional magnetic resonance imaging (fMRI) were conducted in 30 adolescent boys with CD and 33 demographically matched healthy controls (HCs).
Results: Atypical FC within the key brain regions of affective empathy was not observed in CD adolescents. However, we found that CD adolescents showed decreased frontotemporal connectivity within the key brain regions of cognitive empathy in relation to HCs, that is, the FCs between right temporoparietal junction and ventromedial prefrontal cortex as well as dorsomedial prefrontal cortex.
Conclusion: These findings may provide insight into neural mechanism underlying a cognitive empathy deficiency of CD adolescents from the perspective of FC.
Introduction
Conduct disorder (CD) is defined as a repetitive and persistent pattern of antisocial behavior in which the basic rights of others or social norms are violated (APA, 2013). CD has been reported to occur in about 16% of preadolescents (Olsson, 2009; Jiang et al., 2015). It has been suggested that antisocial behavior displayed by children with CD might be a result of atypical empathic responses to others’ suffering (Blair, 2005). The ability to empathize is critical for navigating complex social interactions and developing meaningful interpersonal relationships (Hooker et al., 2008; De Waal, 2012; Zaki and Ochsner, 2012).
Empathy, which refers to one’s cognitive as well as the emotional reactions to the observed experience of others (Shamay-Tsoory, 2011), is divided into affective and cognitive domains (Shamay-Tsoory et al., 2009; Shamay-Tsoory, 2011, 2013). More specifically, the capacity to experience affective reactions to the observed experiences of others or share a “fellow feeling” has been described as affective empathy. Its underlying processes include emotional contagion, emotion recognition and affect sharing (Shamay-Tsoory, 2011). The capacity to engage in the cognitive process of adopting another’s psychological point of view has been described as cognitive empathy, and its underlying processes include perspective taking and a more rational understanding of the emotions of others (Shamay-Tsoory, 2011). The neural substrate of affective empathy is thought to include anterior insula (AI), anterior cingulate cortex (ACC), and inferior frontal gyrus (IFG); while the neural substrate of cognitive empathy is thought to include the medial prefrontal cortex (mPFC), temporo-parietal junction (TPJ) and superior temporal sulcus (STS; Shamay-Tsoory, 2011, 2013; Walter, 2012; Raz et al., 2014).
A number of studies demonstrated that CD patients had an empathy deficit (Cohen and Strayer, 1996; Wied et al., 2005; Lovett and Sheffield, 2007; Schwenck et al., 2012). Relative to control subjects, individuals with CD have been shown to exhibit atypical empathic neural responses in brain areas associated with both affective and cognitive empathy, including mainly the amygdala (Sterzer et al., 2005; Stadler et al., 2007; Decety et al., 2009; Jones et al., 2009), AI (Lockwood et al., 2013; Michalska et al., 2015), ACC (Stadler et al., 2007; Lockwood et al., 2013), IFG (Lockwood et al., 2013), and TPJ (Decety et al., 2009; Dong et al., 2016). Thus, such findings are consistent with the suggestion that CD patients may be deficient in both domains of empathy. To our knowledge, although the brain regions associated with affective and cognitive empathy have been identified, studies exploring empathy domains in CD adolescents from the perspective of functional connectivity (FC) are lacking.
If CD adolescents have aberrant interactions within the key brain regions associated with cognitive and affective empathy, such alterations may be reflected in resting-state functional magnetic resonance imaging (fMRI) studies of FC, namely studies examining connectivity patterns in brains in the absence of task demands. Resting-state FC analysis can clarify the neural basis of specific behaviors or symptoms as a complement of task-fMRI approaches (Abram et al., 2016). Resting-state connectivity, which reveal temporal interactions between proximal and distal regions (Biswal et al., 1995), has been shown to predict individual differences in neural activity induced by the task (Tavor et al., 2016). Besides, resting-state FC data can also be used to examine the neural interaction associated with mental processes (Abram et al., 2016). Moreover, the resting-state findings are not been interfered by the individual variations including the attention, effort, or comprehension, thereby making resting-state analysis an effective tool of overcoming such limitations in task-based studies (Abram et al., 2016). Given these advantages, resting-state FC analysis provides an effective way by which to investigate the neural substrate underlying empathy.
With the aim of exploring whether there were altered FCs within the key brain regions of cognitive and affective empathy in male CD adolescents in comparison with the healthy controls (HCs), we conducted a rs-fMRI study with region of interest (ROI)-based FC analysis in 30 male CD adolescents and 33 demographically matched HCs. Based on previous studies, we hypothesized that CD adolescents would exhibit altered affective and cognitive empathy network connectivity.
Materials and Methods
Participants
The CD group consisted of 30 male adolescents who were recruited from out-patient clinics affiliated with the Second Xiangya Hospital of Central South University (Changsha, Hunan, China). The HCs included 34 healthy age-, and IQ-matched boys recruited from local middle schools in the same region. The Chinese version of the Wechsler Intelligence Scale for Children (C-WISC; Gong and Cai, 1993) was applied to measure IQ. The present study was approved by each school’s administration and the Ethics Committee of the Second Xiangya Hospital of Central South University. All participants and their parents were informed of the purpose of this study and provided written informed consent to be involved in the study.
The Structured Clinical Interview for the DSM-IV-TR Axis I Disorders-Patient Edition (SCID-I/P) (First et al., 2001) was administered to all participants by two well-trained psychiatrists. If there are inconsistence between the two psychiatrists, final decision will be made by the major researcher. All participants in the CD group were confirmed to fulfill the DSM-IV-TR criteria for CD (APA, 2000). Information was collected from each patient and at least one corresponding parent to improve the reliability of the diagnostic interview. A psychiatrist made the final decision as to whether the information provided by each patient and his parents were consistent.
None of the HCs met the criteria for psychiatric disorders, or had a history of CD symptoms or aggression. Both the CD adolescents and HCs were excluded from the study if they reported any following exclusion criteria: a history of attention deficit-hyperactivity disorder, oppositional-defiant disorder, or any psychiatric or emotional disorder; diagnosis of any pervasive developmental or chronic neurological disorder, Tourette syndrome, post-traumatic stress disorder, or obsessive compulsive disorder; persistent headaches; head trauma; a history of alcohol or substance abuse in the past year; contraindications to magnetic imaging; or an IQ ≤ 80.
Clinical Assessments
Affective and cognitive empathy were evaluated with the Interpersonal Reactivity Index (IRI; Davis, 1980, 1983), which includes four subscales: empathic concern, perspective taking, fantasy, and personal distress. To assess the cognitive empathy we used the mean score of the perspective taking and fantasy subscales, whereas emotional empathy was assessed using the mean score of the empathic concern and personal distress subscales (Shamay-Tsoory et al., 2009). Callous unemotional (CU) trait phenotype was evaluated with the CU subscale of the Antisocial Process Screening Device (APSD; Frick and Hare, 2001; Vitacco et al., 2003). Six items were included in the callous unemotional subscale: cares about schoolwork; emotions are fake; feel bad when do something wrong; acts charming to get things; concerned about others’ feelings; hides feelings from others. The conduct problems subscale of the Strength and Difficulties Questionnaire (SDQ) was used to measure conduct problems of adolescents (Yao et al., 2009).
Data Acquisition
Imaging data were acquired on a PHILIPS Achieva 3.0-T magnetic resonance scanner at the Second Xiangya Hospital of Central South University. All participants were instructed to lie in a supine position with their eyes closed, to remain still, and to think of nothing in particular, but to avoid falling asleep. Their heads were fixed snugly with foam pads to minimize head movement. Images were acquired with an echo planar imaging sequence with the following parameters: 36 axial slices, repetition time/echo time = 2000/30 ms, 64 × 64 matrix, 90° flip angle, field of view = 240 mm × 240 mm, thickness/gap = 4.0/0 mm, and 206 volumes. The total time of resting acquisition was 6 min 52 s.
Data Processing
Image processing was performed in Data Processing Assistant for Resting-state fMRI program [DPARSFA V2.3, Chao-Gan and Yu-Feng (2010)1] and the Resting-state fMRI Data Analysis Toolkit [REST V1.8, Song et al. (2011)]. The following steps were included: (1) the first 10 volumes were discarded to allow for signal equilibration and adaptation of participants to scanning noise; (2) slice timing with the 18th slice as a reference slice; (3) head motion correction; (4) head motion scrubbing regressor (threshold for ‘bad’ time point, frame-wise displacements > 0.5 mm as well as 1 back and 2 forward neighbors); (5) spatial normalization based on echo-planar-imaging templates and resampling (3 mm × 3 mm × 3 mm); (6) smoothing with a 6-mm full-width at half maximum Gaussian kernel; (7) de-trending and filtering data with residual signals within 0.01–0.1 Hz to discard biases from high-frequency physiological noise and low-frequency drift.
The criterion for excessive head motion criterion was translation > 2 mm in any direction or rotation > 2° around any axis in six head motion parameters. One HC subject was excluded for excessive head motion.
Statistical Analyses
Behavior
In the SPSS 18.0.0, independent two-sample t-tests were used to compare the distributions of age, IQ, and psychological profiles between the CD and HC groups.
Functional Connectivity
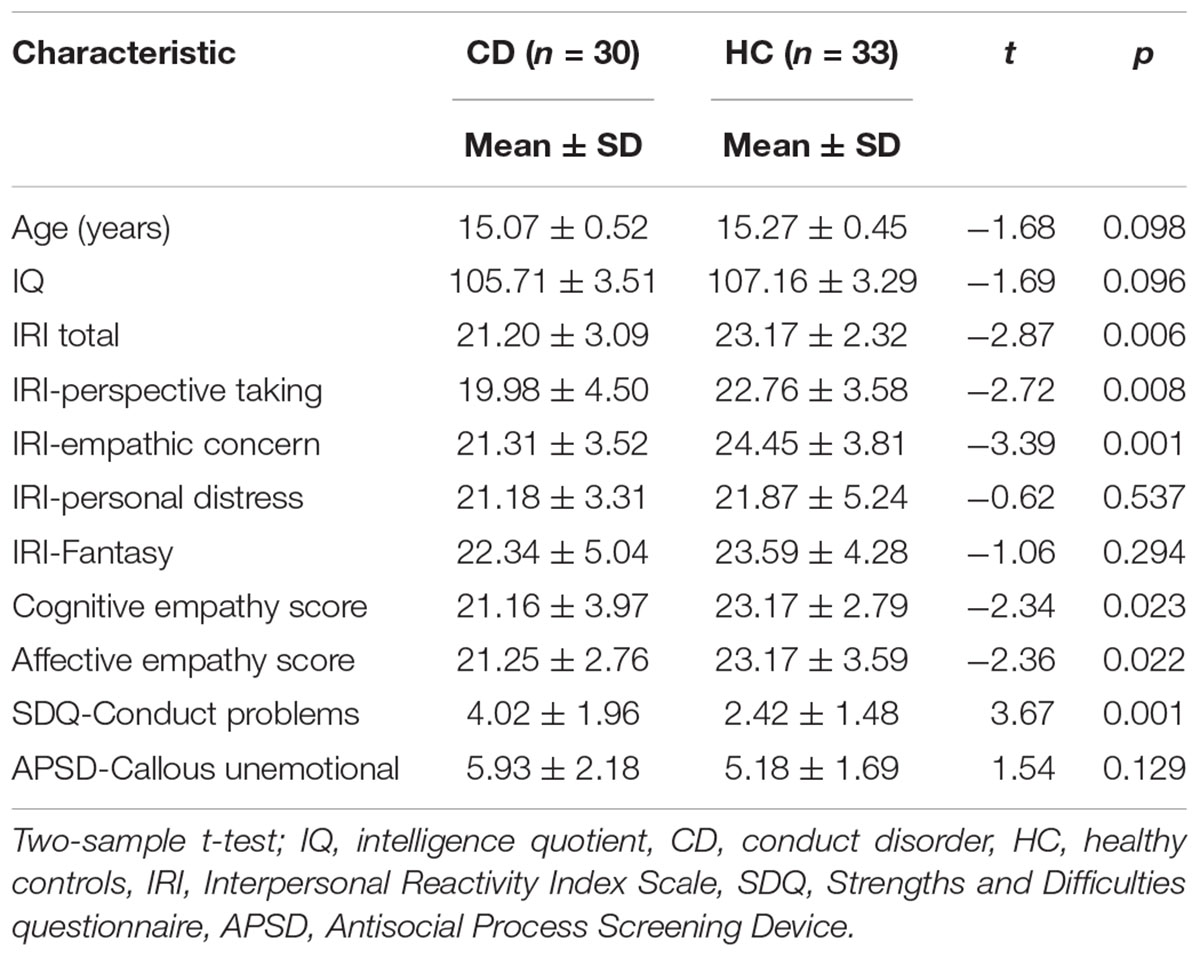
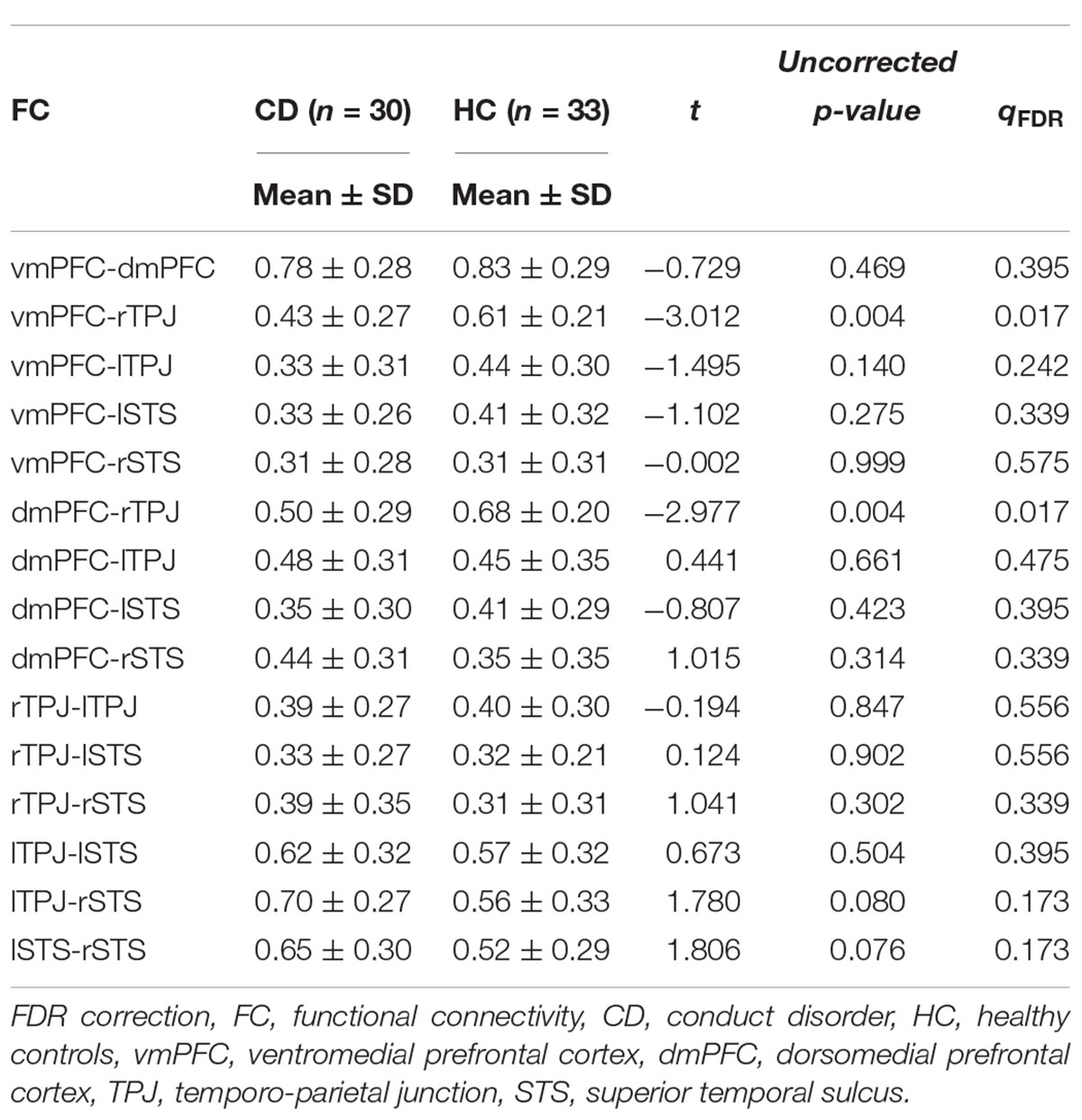
Region of interest-based FC analyses were completed in REST software. The ROIs for cognitive empathy encompassed the vmPFC, dmPFC, TPJ, and STS; and the ROIs for affective empathy encompassed the AI, ACC, IFG and supplementary motor area. A total of 12 ROIs (6, cognitive empathy, Figure 1A; 6, affective empathy, Figure 1B) were defined with a sphere with a 3-mm radius. To be specific, the definition of ROIs mainly referred the article of Raz et al. (2014), which investigated the two modes of empathy by analyzing fMRI fluctuations of network cohesion with a ROI method. The 6 ROIs of cognitive empathy in our study were defined on the basis of a fMRI item analysis in a theory of mind task (Dodell-Feder et al., 2011), which was consistent with Raz et al. (2014). The 4 ROIs of affective empathy were defined on the basis of a meta-analysis on empathy for pain (Lamm et al., 2011), which was consistent with Raz et al. (2014). Besides, two additional ROIs (right IFG and supplementary motor area) of affective empathy were defined on the basis of fMRI meta-analysis of Fan et al. (2011). The right IFG and supplementary motor area are also important in affective empathy as the theory proposed by Shamay-Tsoory (2011). Detailed descriptions of the ROIs are provided in Supplementary Table S1.
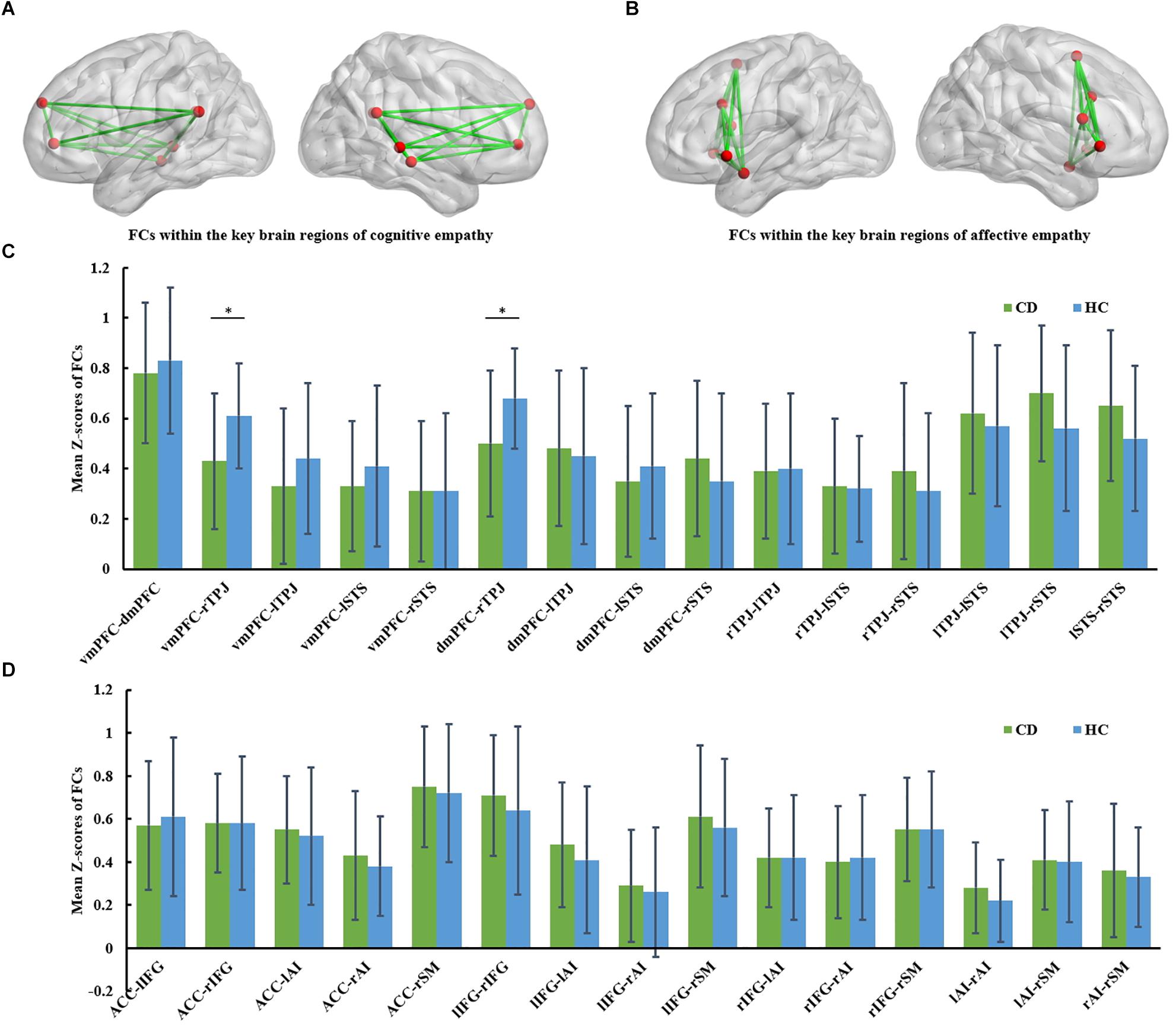
Figure 1. (A) FCs within the key brain regions of cognitive empathy. The cognitive empathy has 6 key brain regions including the ventromedial prefrontal cortex, dorsomedial prefrontal cortex, temporoparietal junction and superior temporal sulcus. The red spheres represent network nodes. The green edges represent inter-nodal functional connectivity (FC). (B) FCs within the key brain regions of affective empathy. The affective empathy has 6 key brain regions including the anterior cingulate, anterior insula, inferior frontal cortex and supplementary motor area. The red spheres represent network nodes. (C) Group differences between the conduct disorder (CD) group and healthy control (HC) group within the key brain regions of cognitive empathy. The CD group showed significantly reduced rTPJ-vmPFC and rTPJ-dmPFC FC relative to the HC group. ∗FDR corrected, p < 0.05. HC, healthy controls; CD, conduct disorder; vmPFC, ventromedial prefrontal cortex, dmPFC; dorsomedial prefrontal cortex; TPJ, temporoparietal junction; STS, superior temporal sulcus. (D) Group differences between the conduct disorder (CD) group and healthy control (HC) group within the key brain regions of affective empathy. No significant differences were observed between the CD group and HC group in the affective empathy network. ACC, anterior cingulate cortex; AI, anterior insula; IFG, inferior frontal cortex; SM, supplementary motor cortex.
We used the FC tool in REST software to extract correlation coefficients of each ROI pair; these values reflect the degree of temporal connectivity between ROI pairs (Wisner et al., 2013). The correlation coefficients were transformed into z-scores by Fisher’s z transformation method and then were exported into SPSS 18.0. Independent two-sample t-tests were used to detect group differences in FC. After that, false discovery rate (FDR, p < 0.05) corrections for multiple hypotheses testing were applied.
Brain-Behavior Analysis
Pearson correlation analyses were conducted to detect the correlation between z-scores of atypical FCs in CD adolescents within each network and corresponding empathy score. Pearson correlation analyses were performed using SPSS 18.0.0 in CD and HC group separately. False discovery rate corrections (FDR, p < 0.05) for multiple hypotheses testing were applied.
Results
Demographic and Behavioral Data
The demographic and psychiatric characteristics of the CD and HC groups are reported in Table 1. Age and IQ did not differ significantly between the two groups (both p > 0.05). Relative to the HC group, the CD group had a significant lower cognitive empathy score (t = -2.34, p = 0.023), a significant lower affective empathy score (t = -2.36, p = 0.022), and a significantly higher conduct problems trait score (t = 3.67, p = 0.001).
Functional Connectivities Within the Key Brain Regions of Cognitive Empathy
The group comparison results of analyses of covariance in FC involving the cognitive empathy network (vmPFC-dmPFC, vmPFC-rTPJ, vmPFC-lTPJ, vmPFC-lSTS, vmPFC-rSTS, dmPFC-rTPJ, dmPFC-lTPJ, dmPFC-lSTS, dmPFC-rSTS, rTPJ-lTPJ, rTPJ-lSTS, rTPJ-rSTS, lTPJ-lSTS, lTPJ-rSTS and lSTS-rSTS) are reported in Table 2 and Figure 1C. Notably, relative to the HC group, the CD group was found to have significantly weaker vmPFC-rTPJ (t61 = -3.012, qFDR = 0.017 ), and dmPFC-rTPJ FCs (t61 = -2.977, qFDR = 0.017).
Functional Connectivities Within the Key Brain Regions of Affective Empathy
The group comparison results of analyses of covariance involving the affective empathy network (ACC-lIFG, ACC-rIFG, ACC-lAI, ACC-rAI, ACC-SM, lIFG-rIFG, lIFG-lAI, lIFG-rAI, lIFG-SM, rIFG-lAI, rIFG-rAI, rIFG-SM, lAI-rAI, lAI-SM, rAI-SM) are reported in Supplementary Table S2 and Figure 1D. There were no significant differences between the CD group and HC group in FCs within the affective empathy network.
Brain-Behavior Analyses
There were no significant correlations between the z-scores of altered FCs within the key brain regions of cognitive empathy (vmPFC-rTPJ, p = 0.094; dmPFC-rTPJ, p = 0.151) and the behavioral score of cognitive empathy in CD adolescents. Besides, there were no significant correlations (vmPFC-rTPJ, p = 0.794; dmPFC-rTPJ, p = 0.876) were observed within the altered FCs and the cognitive empathy score.
Discussion
The present study was the first to employ resting-state fMRI to explore FCs underlying affective and cognitive empathy in adolescents with CD. The behavioral analyses demonstrated that the CD adolescents had an affective and cognitive empathy deficit. In terms of the FC analyses, we found that CD adolescents exhibited decreased frontotemporal connectivity within the key brain regions of cognitive empathy, specifically, the vmPFC-rTPJ and dmPFC-rTPJ FCs. However, we did not find any evidence of an atypical FC within the key brain regions of affective empathy.
Our findings reflected that the CD adolescents had decreased FC between the mPFC and TPJ. The activity of both regions have been frequently reported in the empathic neural responses induced by observing painful pictures (Lamm et al., 2007, 2011). The TPJ is an association cortex that integrates input from the lateral and posterior thalamus, as well as from visual, auditory, somatosensory, and limbic areas (Decety and Lamm, 2007). The mPFC is a highly interconnected brain region with notable afferents from the dorsal-lateral prefrontal cortex, anterior STS, TPJ, and other brain regions (Van Overwalle, 2009). The considerable neural input that the mPFC receives may contribute to its capacity for abstract inference making (Leslie et al., 2004; Amodio and Frith, 2006; Van Overwalle, 2009). Generally, the TPJ has been linked with transient detection and evaluation of information of another’s state at a relatively perceptual level, whereas the mPFC has been associated with longer lasting processing of information related to the self and more abstract cognition (Gallagher and Frith, 2003; Shamay-Tsoory et al., 2009; Van Overwalle, 2009; Van Overwalle and Baetens, 2009; Raz et al., 2014). Appropriate medial-fronto-temporal communication is thought to facilitate the use of external social cues from temporal regions when deducing the internal emotional states of others in the mPFC (Abram et al., 2016). Given the mPFC, especially the vmPFC, is a crucial and specific brain region of the cognitive empathy (Shamay-Tsoory et al., 2006, 2009; Shamay-Tsoory and Aharon-Peretz, 2007), reduced connectivity between vmPFC and right TPJ in CD may reflect reduced access to external social cues in relation to cognitive empathy. Such a phenomenon may explain why adolescents with CD have poor empathic accuracy (Martin-Key et al., 2016).
Notably, we found that the z-scores of altered FCs within the key brain regions of cognitive empathy are not significantly positively associated with the cognitive empathy score measured by the IRI scale. Several possible reasons may contribute to these. On the one hand, distinct empathic neural responses may not necessarily result in different conscious subjective ratings indicated by the measures used in the present study. This might reflect the atypical FCs observed here was unconscious. Similar phenomenon has also been found in the task-fMRI studies which explored the empathy (Xu et al., 2009; Dong et al., 2016). On the other hand, the adolescents are prone to have some bias in fill out the self-report measures. Herpertz et al. (2010) also found that the neuroimaging data were not consistently with the self-report measures in CD adolescents, which may reflect that CD boy’s self-image of being “cool” rather than their real emotional experience. Overall, the empathy related behavior-brain correlations still need further explanations in the future studies.
Interestingly, we observed reduced vmPFC-TPJ FC only in the right hemisphere. The right TPJ has been described as playing a pivotal role in self-other discrimination (Decety and Lamm, 2006). Uddin et al. (2006) demonstrated selective impairment of self-other distinction when repetitive transcranial magnetic stimulation was applied over the right TPJ of participants performing a perceptual task involving discrimination between images of one’s own face and other familiar faces, thereby providing direct evidence for a casual role for this region in self-other discrimination. The findings of Decety and Sommerville (2003) further indicated that the right TPJ within the right fronto-parietal network played a pivotal role in distinguishing self from other, and that the prefrontal cortex was integral to coordinate and contrast cognitive representation of self and other. Hence, atypical FCs between the right TPJ and mPFC suggest that adolescents with CD may have a deficiency in self-other discrimination, a crucial component of cognitive empathy (de Waal, 2008; Singer and Lamm, 2009; Shamay-Tsoory, 2011).
With regard to the affective empathy, we found that the CD adolescents had lower affective empathy score, suggesting that the CD adolescents had a deficiency of affective empathy from the behavioral level. Previous task-fMRI studies (Decety et al., 2009; Lockwood et al., 2013; Schwenck et al., 2016) also found children with conduct problems exhibited atypical empathic neural responses in brain regions associated with affective empathy. Interestingly, we did not observe any atypical resting FC within the key brain regions of affective empathy in CD adolescents. Several reasons may account for this result. On the one hand, previous articles (Lamm et al., 2007; Bernhardt and Singer, 2012; Vera-Estay et al., 2016; Lockwood et al., 2017) have documented that the empathy is heavily context-dependent, being crucially affected by the agent’s motivation as well as the various social factors. Therefore, atypical affective neural responses by adolescents with CD, reported by the previous studies might have something to do with who they are feeling with and for, not the general malfunctioning. On the other hand, since many studies indicated that CU trait is an important factor influencing the affective empathy (Cheng et al., 2012; Schwenck et al., 2012), the matched-CU trait between the CD adolescents and HCs may also be the potential influential factor contributing to this result. Overall, further investigations, which explore the network alterations of affective empathy in CD adolescents with a task fMRI method and bigger sample, are necessary.
There are several limitations of this work that should be mentioned. First, the limited sample size can easily lead to false positives and inflated effected sizes (Yarkoni, 2010). Although the sample size in the present study is not less than similar studies typically done in this domain (Zhang et al., 2014; Jiang et al., 2016; Sun et al., 2018), the results in the present study still need a further verification with a bigger sample. Second, although ROI-based method has been frequently used in previous studies (Lockwood et al., 2013; Raz et al., 2014), it has an inherent methodological constraint: subjective ROIs selection. Besides, our study was only on the basis of resting fMRI data. Overall, the conclusion in the present study still needs further confirmation and replication of the task-based and experimental studies. Specifically, exploring the whole-brain FCs seeding in the crucial regions of cognitive and affective empathy is extremely meaningful whiling analyzing the empathy-related task-data. Third, the generalization of our result is still under restriction to some extent as we control the effect of CU trait. Therefore, our results still need further extensions in CD adolescents with and without CU trait.
Conclusion
In conclusion, the present study represents the first attempt to investigate FCs related to affective and cognitive empathy in adolescents with CD. Reduced frontotemporal FCs (vmPFC/dmPFC-rTPJ) within the key brain regions of cognitive empathy were observed in adolescents with CD. Atypical FC within the key brain regions of affective empathy network was not observed in the CD group. Given that the vmPFC is a core region of social inference, and the TPJ is a primary region integrating the perceptual input, the observed decoupling between vmPFC/dmPFC and rTPJ in CD adolescents may reveal an insufficient use of external input and less involvement of social inference while empathizing with others. The present study may partly uncover the neural mechanism underlying the cognitive empathy deficiency in CD adolescents.
Author Contributions
SY and XW designed the study. YJ, YG, QM, and DD performed the study. DD analyzed the data and wrote the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (81471384 to SY), the National Key Technologies R&D Program in China’s 11th 5-year plan (2009BAI77B02 to SY), and Specialized Research Fund for the Doctoral Program of Higher Education (20130162110043 to SY).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyg.2018.02778/full#supplementary-material
Footnotes
References
Abram, S. V., Wisner, K. M., Fox, J. M., Barch, D. M., Wang, L., Csernansky, J. G., et al. (2016). Fronto-temporal connectivity predicts cognitive empathy deficits and experiential negative symptoms in schizophrenia. Hum. Brain Mapp. 38, 1111–1124. doi: 10.1002/hbm.23439
Amodio, D. M., and Frith, C. D. (2006). Meeting of minds: the medial frontal cortex and social cognition. Nat. Rev. Neurosci. 7, 268–277. doi: 10.1038/nrn1884
APA (2000). Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR. 4th edn. Washington, DC: American Psychiatric Publishing.
APA (2013). Diagnostic and Statistical Manual of Mental Disorders, 5th edn. Washington, DC: American Psychiatric publishing.
Bernhardt, B. C., and Singer, T. (2012). The neural basis of empathy. Annu. Rev. Neurosci. 35, 1–23. doi: 10.1146/annurev-neuro-062111-150536
Biswal, B., Yetkin, F. Z., Haughton, V. M., and Hyde, J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 34, 537–541.
Blair, R. J. R. (2005). Responding to the emotions of others: dissociating forms of empathy through the study of typical and psychiatric populations. Conscious. Cogn. 14, 698–718.
Chao-Gan, Y., and Yu-Feng, Z. (2010). DPARSF: a matlab toolbox for “pipeline” data analysis of resting-state fMRI. Front. Syst. Neurosci. 4:13. doi: 10.3389/fnsys.2010.00013
Cheng, Y., Hung, A. Y., and Decety, J. (2012). Dissociation between affective sharing and emotion understanding in juvenile psychopaths. Dev. Psychopathol. 24, 623–636. doi: 10.1017/S095457941200020X
Cohen, D., and Strayer, J. (1996). Empathy in conduct-disordered and comparison youth. Dev. Psychol. 32, 988–998. doi: 10.1037/0012-1649.32.6.988
Davis, M. H. (1980). A multidimensional approach to individual differences in empathy. JSAS Catalog Sel. Doc. Psychol. 10:85.
Davis, M. H. (1983). Measuring individual differences in empathy: evidence for a multidimensional approach. J. Pers. Soc. Psychol. 44, 113–126. doi: 10.1037/0022-3514.44.1.113
de Waal, F. B. (2008). Putting the altruism back into altruism: the evolution of empathy. Annu. Rev. Psychol. 59, 279–300.
Decety, J., and Lamm, C. (2006). Human empathy through the lens of social neuroscience. Sci. World J. 6, 1146–1163. doi: 10.1100/tsw.2006.221
Decety, J., and Lamm, C. (2007). The role of the right temporoparietal junction in social interaction: how low-level computational processes contribute to meta-cognition. Neuroscientist 13, 580–593. doi: 10.1177/1073858407304654
Decety, J., Michalska, K. J., Akitsuki, Y., and Lahey, B. B. (2009). Atypical empathic responses in adolescents with aggressive conduct disorder: a functional MRI investigation. Biol. Psychol. 80, 203–211. doi: 10.1016/j.biopsycho.2008.09.004
Decety, J., and Sommerville, J. A. (2003). Shared representations between self and other: a social cognitive neuroscience view. Trends Cogn. Sci. 7, 527–533.
Dodell-Feder, D., Koster-Hale, J., Bedny, M., and Saxe, R. (2011). fMRI item analysis in a theory of mind task. Neuroimage 55, 705–712. doi: 10.1016/j.neuroimage.2010.12.040
Dong, D., Ming, Q., Wang, X., Yu, W., Jiang, Y., Wu, Q., et al. (2016). Temporoparietal junction hypoactivity during pain-related empathy processing in adolescents with conduct disorder. Front. Psychol. 7:2085. doi: 10.3389/fpsyg.2016.02085
Fan, Y., Duncan, N. W., De Greck, M., and Northoff, G. (2011). Is there a core neural network in empathy? an fMRI based quantitative meta-analysis. Neurosci. Biobehav. Rev. 35, 903–911. doi: 10.1016/j.neubiorev.2010.10.009
First, M. B., Spitzer, R. L., Gibbon, M., and Williams, J. B. (2001). Structured Clinical Interview for DSM-IV-TR Axis I Disorders—Non-Patient Edition. New York, NY: New York State Psychiatric Institute.
Frick, P., and Hare, R. (2001). Antisocial Process Screening Device: APSD. Toronto: Multi-Health Systems.
Gallagher, H. L., and Frith, C. D. (2003). Functional imaging of ‘theory of mind’. Trends Cogn. Sci. 7, 77–83. doi: 10.1016/S1364-6613(02)00025-6
Gong, Y., and Cai, T. (1993). Wechsler Intelligence Scale for Children, Chinese Revision (C-WISC). Hunan: Map Press.
Herpertz, S. C., Thomas, H., Ivo, M., Vloet, T. D., Fink, G. R., Tony, S., et al. (2010). Emotional processing in male adolescents with childhood-onset conduct disorder. J. Child Psychol. Psychiatry 49, 781–791. doi: 10.1111/j.1469-7610.2008.01905.x
Hooker, C. I., Verosky, S. C., Germine, L. T., Knight, R. T., and D’Esposito, M. (2008). Mentalizing about emotion and its relationship to empathy. Soc. Cogn. Affect. Neurosci. 3, 204–217. doi: 10.1093/scan/nsn019
Jiang, Y., Guo, X., Zhang, J., Gao, J., Wang, X., Situ, W., et al. (2015). Abnormalities of cortical structures in adolescent-onset conduct disorder. Psychol. Med. 45, 3467–3479. doi: 10.1017/S0033291715001361
Jiang, Y., Liu, W., Ming, Q., Gao, Y., Ma, R., Zhang, X., et al. (2016). Disrupted Topological Patterns of Large-Scale Network in Conduct Disorder. Sci. Rep. 6:37053. doi: 10.1038/srep37053
Jones, A. P., Laurens, K. R., Herba, C. M., Barker, G. J., and Viding, E. (2009). Amygdala hypoactivity to fearful faces in boys with conduct problems and callous-unemotional traits. Am. J. Psychiatry 166, 95–102. doi: 10.1176/appi.ajp.2008.07071050
Lamm, C., Decety, J., and Singer, T. (2011). Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage 54, 2492–2502. doi: 10.1016/j.neuroimage.2010.10.014
Lamm, C., Nusbaum, H. C., Meltzoff, A. N., and Decety, J. (2007). What are you feeling? using functional magnetic resonance imaging to assess the modulation of sensory and affective responses during empathy for pain. Plos. One 2:e1292. doi: 10.1371/journal.pone.0001292
Leslie, A. M., Friedman, O., and German, T. P. (2004). Core mechanisms in “theory of mind”. Trends Cogn. Sci. 8, 528–533.
Lockwood, P. L., Ang, Y. S., Husain, M., and Crockett, M. J. (2017). Individual differences in empathy are associated with apathy-motivation. Sci. Rep. 7:17293. doi: 10.1038/s41598-017-17415-w
Lockwood, P. L., Sebastian, C. L., McCrory, E. J., Hyde, Z. H., Gu, X., De Brito, S. A., et al. (2013). Association of callous traits with reduced neural response to others’ pain in children with conduct problems. Curr. Biol. 23, 901–905. doi: 10.1016/j.cub.2013.04.018
Lovett, B. J., and Sheffield, R. A. (2007). Affective empathy deficits in aggressive children and adolescents: a critical review. Clin. Psychol. Rev. 27, 1–13.
Martin-Key, N., Brown, T., and Fairchild, G. (2016). Empathic accuracy in male adolescents with conduct disorder and higher versus lower levels of callous-unemotional traits. J. Abnorm. Child Psychol. 45, 1385–1397. doi: 10.1007/s10802-016-0243-8
Michalska, K. J., Zeffiro, T. A., and Decety, J. (2015). Brain response to viewing others being harmed in children with conduct disorder symptoms. J. Child Psychol. Psychiatry 57, 510–519. doi: 10.1111/jcpp.12474
Olsson, M. (2009). DSM diagnosis of conduct disorder (CD)—a review. Nord. J. Psychiatry 63, 102–112. doi: 10.1080/08039480802626939
Raz, G., Jacob, Y., Gonen, T., Winetraub, Y., Flash, T., Soreq, E., et al. (2014). Cry for her or cry with her: context-dependent dissociation of two modes of cinematic empathy reflected in network cohesion dynamics. Soc. Cogn. Affect. Neurosci. 9, 30–38. doi: 10.1093/scan/nst052
Schwenck, C., Ciaramidaro, A., Selivanova, M., Tournay, J., Freitag, C. M., and Siniatchkin, M. (2016). Neural correlates of affective empathy and reinforcement learning in boys with conduct problems: fMRI evidence from a gambling task. Behav. Brain Res. 320, 75–84. doi: 10.1016/j.bbr.2016.11.037
Schwenck, C., Mergenthaler, J., Keller, K., Zech, J., Salehi, S., Taurines, R., et al. (2012). Empathy in children with autism and conduct disorder: group-specific profiles and developmental aspects. J. Child. Psychol. Psychiatry 53, 651–659. doi: 10.1111/j.1469-7610.2011.02499.x
Shamay-Tsoory, S. G. (2011). The neural bases for empathy. Neuroscientist 17, 18–24. doi: 10.1177/1073858410379268
Shamay-Tsoory, S. G. (2013). Dynamic functional integration of distinct neural empathy systems. Soc. Cogn. Affect. Neurosci. 9, 1–2. doi: 10.1093/scan/nst107
Shamay-Tsoory, S. G., and Aharon-Peretz, J. (2007). Dissociable prefrontal networks for cognitive and affective theory of mind: a lesion study. Neuropsychologia 45, 3054–3067.
Shamay-Tsoory, S. G., Aharon-Peretz, J., and Perry, D. (2009). Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain 132, 617–627. doi: 10.1093/brain/awn279
Shamay-Tsoory, S. G., Tibi-Elhanany, Y., and Aharon-Peretz, J. (2006). The ventromedial prefrontal cortex is involved in understanding affective but not cognitive theory of mind stories. Soc. Neurosci. 1, 149–166. doi: 10.1080/17470910600985589
Singer, T., and Lamm, C. (2009). The social neuroscience of empathy. Ann. N. Y. Acad. Sci. 1156, 81–96. doi: 10.1111/j.1749-6632.2009.04418.x
Song, X. W., Dong, Z. Y., Long, X. Y., Li, S. F., Zuo, X. N., Zhu, C. Z., et al. (2011). REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS. One 6:e25031. doi: 10.1371/journal.pone.0025031
Stadler, C., Sterzer, P., Schmeck, K., Krebs, A., Kleinschmidt, A., and Poustka, F. (2007). Reduced anterior cingulate activation in aggressive children and adolescents during affective stimulation: association with temperament traits. J. Psychiatr. Res. 41, 410–417.
Sterzer, P., Stadler, C., Krebs, A., Kleinschmidt, A., and Poustka, F. (2005). Abnormal neural responses to emotional visual stimuli in adolescents with conduct disorder. Biol. Psychiatry 57, 7–15. doi: 10.1016/j.biopsych.2004.10.008
Sun, X., Ma, R., Jiang, Y., Gao, Y., Ming, Q., Wu, Q., et al. (2018). MAOA genotype influences neural response during an inhibitory task in adolescents with conduct disorder. Eur. Child Adolesc. Psychiatry 9, 1159–1169. doi: 10.1007/s00787-018-1170-8
Tavor, I., Parker Jones, O., Mars, R. B., Smith, S. M., Behrens, T. E., and Jbabdi, S. (2016). Task-free MRI predicts individual differences in brain activity during task performance. Science 352, 216–220. doi: 10.1126/science.aad8127
Uddin, L. Q., Molnar-Szakacs, I., Zaidel, E., and Iacoboni, M. (2006). rTMS to the right inferior parietal lobule disrupts self-other discrimination. Soc. Cogn. Affect. Neurosci. 1, 65–71. doi: 10.1093/scan/nsl003
Van Overwalle, F. (2009). Social cognition and the brain: a meta-analysis. Hum. Brain Mapp. 30, 829–858. doi: 10.1002/hbm.20547
Van Overwalle, F., and Baetens, K. (2009). Understanding others’ actions and goals by mirror and mentalizing systems: a meta-analysis. Neuroimage 48, 564–584. doi: 10.1016/j.neuroimage.2009.06.009
Vera-Estay, E., Seni, A. G., Champagne, C., and Beauchamp, M. H. (2016). All for one: contributions of age, socioeconomic factors, executive functioning, and social cognition to moral reasoning in childhood. Front. Psychol. 7:277. doi: 10.3389/fpsyg.2016.00227
Vitacco, M. J., Rogers, R., and Neumann, C. S. (2003). The antisocial process screening device: an examination of its construct and criterion-related validity. Assessment 10, 143–150.
Walter, H. (2012). Social cognitive neuroscience of empathy: concepts, circuits, and genes. Emot. Rev. 4, 9–17. doi: 10.1177/1754073911421379
Wied, M., Goudena, P. P., and Matthys, W. (2005). Empathy in boys with disruptive behavior disorders. J. Child Psychol. Psychiatry 46, 867–880.
Wisner, K. M., Patzelt, E. H., Lim, K. O., and MacDonald, A. W. 3rd (2013). An intrinsic connectivity network approach to insula-derived dysfunctions among cocaine users. Am. J. Drug Alcohol Abuse 39, 403–413. doi: 10.3109/00952990.2013.848211
Xu, X., Zuo, X., Wang, X., and Han, S. (2009). Do you feel my pain? racial group membership modulates empathic neural responses. J. Neurosci. 29, 8525–8529. doi: 10.1523/JNEUROSCI.2418-09.2009
Yao, S., Zhang, C., Zhu, X., Jing, X., McWhinnie, C. M., and Abela, J. R. (2009). Measuring adolescent psychopathology: psychometric properties of the self-report strengths and difficulties questionnaire in a sample of Chinese adolescents. J. Adolesc. Health 45, 55–62. doi: 10.1016/j.jadohealth.2008.11.006
Yarkoni, T. (2010). Big correlations in little studies: inflated fMRI correlations reflect low statistical power—commentary on Vul et al. (2009). Perspect. Psychol. Sci. 4, 294–298. doi: 10.1111/j.1745-6924.2009.01127.x
Zaki, J., and Ochsner, K. N. (2012). The neuroscience of empathy: progress, pitfalls and promise. Nat. Neurosci. 15, 675–680. doi: 10.1038/nn.3085
Keywords: conduct disorder, cognitive empathy, affective empathy, functional connectivity, adolescent
Citation: Dong D, Jiang Y, Gao Y, Ming Q, Wang X and Yao S (2019) Atypical Frontotemporal Connectivity of Cognitive Empathy in Male Adolescents With Conduct Disorder. Front. Psychol. 9:2778. doi: 10.3389/fpsyg.2018.02778
Received: 31 August 2018; Accepted: 27 December 2018;
Published: 11 January 2019.
Edited by:
Roumen Kirov, Institute of Neurobiology (BAS), BulgariaReviewed by:
Drozdstoy Stoyanov Stoyanov, Plovdiv Medical University, BulgariaWoo-Young Ahn, Seoul National University, South Korea
Xiaolin Zhou, Peking University, China
Copyright © 2019 Dong, Jiang, Gao, Ming, Wang and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuqiao Yao, c2h1cWlhb3lhb0Bjc3UuZWR1LmNu; c2h1cWlhb3lhb0AxNjMuY29t
 Daifeng Dong1,2,3
Daifeng Dong1,2,3 Qingsen Ming
Qingsen Ming Shuqiao Yao
Shuqiao Yao