- 1Department of Neurosciences, Biomedicine and Movement, University of Verona, Verona, Italy
- 2Department of Movement, Human and Health Sciences, University of Rome “Foro Italico”, Rome, Italy
- 3Division of Pediatric Gastroenterology and Nutrition, Department of Pediatrics, Massachusetts General Hospital for Children and Harvard Medical School, Boston, MA, United States
- 4Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, United States
- 5European Biomedical Research Institute of Salerno, Salerno, Italy
Introduction: The gut microbiome represents a key ecosystem influencing athletic performance through energy metabolism modulation, inflammatory response regulation, and recovery optimization in high-level athletes. However, the relationship between performance and gut microbiome composition in high-level athletes remains poorly understood.
Objectives: This systematic scoping review aims to map the current evidence on the relationship between training and gut microbiome in high-level athletes, identify specific patterns in microbial response to different training and sports, analyse the effects of nutritional interventions and highlight some methodological and knowledge gaps in the current literature.
Methodology: Following the PRISMA-ScR framework, a systematic search was conducted on PubMed, Scopus and Web of Science (2015-2025). Studies were selected according to defined criteria, including a population of high-level athletes, interventions through training and/or nutritional protocols and based on outcomes related to performance and health.
Results: Nineteen studies met the inclusion criteria, comprising 12 experimental studies and 7 systematic/narrative reviews. The analysis of the studies revealed possible sport-specific patterns in microbiome modulation, with distinctive alterations in metabolic profiles, significant correlations between microbial stability and athletic performance, synergistic effects between training and probiotic supplementation and significant impacts of nutritional strategies and hormonal contraceptives on microbiome composition. The heterogeneity in analysis methodologies and the limited duration of studies emerge as the main limitations of the present study.
Conclusions: The evidence suggests that the significant role of the gut microbiome in athletic performance optimization may be considered in the future, highlighting the importance of implementing an integrated approach between training and nutrition. Further studies are needed to define specific microbiome trends for different types of sports, competition levels and supplementation targeted at implementing performance outcomes in high-level athletes.
Systematic Review Registration: https://osf.io/yh49t, identifier YH49T.
1 Introduction
The gut microbiome is established from birth and is influenced by various factors, including the mode, time, and place of birth, the type of breastfeeding and weaning and the use of antibiotics (1–5). Some studies have confirmed that human milk oligosaccharides (HMO) positively modulate the newborn's microbiome through a prebiotic action, as does lactoferrin present in maternal serum (6–8). The structure of the intestinal microbiome remains stable in adults. Still, it differs between individuals, characterized by a specific enterotype, and during the last phase of life, it tends to undergo loss of bacterial diversity (9, 10). Additionally, it is primarily influenced by diet, particularly the amount of fibre intake, short-chain fatty acids (SCFAs) production, physical activity (PA), body composition and lifestyle (5, 11–16). The gut microbiome represents a complex ecosystem that influences human physiology through multiple mechanisms, including the modulation of energy metabolism, immune and inflammatory response and the production of bioactive metabolites (17–21). In high-level athletes, the role of the intestinal microbiome appears particularly relevant, emerging as a potential modulator of athletic performance and training adaptations. Still, in strenuous and prolonged training or training not managed appropriately, they can become harmful, generating states of chronic inflammation (12, 22–24). A properly balanced microbiome in sports activity can reduce inflammatory markers and the production of Reactive Oxygen Species (ROS), further attenuating macromolecule damage (25). The gut microbiome significantly impacts training, as suggested by a bidirectional relationship between PA and the microbial community (26). The understanding of this interaction, particularly in the context of high-level sports, is still incomplete today. A study conducted by Xu et al. highlights that elite athletes present distinctive taxonomic and functional profiles in their gut microbiome, with specific genera such as Clostridiales and Faecalibacterium being more prevalent compared to non-elite subjects (27). This finding was further supported by Barton et al., who observed that the gut microbiome of professional athletes differs significantly from that of sedentary individuals, particularly at the functional metabolic level (28). The variation in microbiota composition may also be influenced by intestinal permeability, a topic that has been thoroughly examined by Fasano, who emphasised the role of the zonulin protein in regulating intestinal tight junctions and how disruptions in this system can affect overall health and physical performance (29, 30).
Several studies have supported the metabolic advantages of specific microbiome profiles and specific intestinal bacteria. Scheiman et al. reported that elite runners possess a greater abundance of Veillonella (31). This genus has been shown to convert lactate produced during physical exercise into propionate, improving endurance performance such as running (31, 32). This is in line with the results of Manor et al., which indicate a positive correlation between Veillonella and vigorous PA, further supporting the notion that specific microbial populations can enhance athletic abilities (33). Moreover, the microbiome plays a crucial role in nutrient metabolism and immune function, which is particularly important for athletes engaged in high-intensity and strenuous training. Some authors illustrate the impact of altered intestinal permeability on the systemic inflammatory response, an important factor to consider for athletes experiencing significant physical stress (29, 30). The research conducted by Heimer et al. highlights the importance of immune modulation and gastrointestinal (GI) health in achieving optimal athletic performance (34). Furthermore, certain studies have shown that dietary choices can impact the microbiome. Murtaza et al. have pointed out that the nutritional practices of elite race walkers play a significant role in shaping their gut microbiota composition (22). This suggests that diet and physical exercise are crucial elements in modulating athletes’ microbiome and optimizing physical performance (35).
The microbiome possesses dynamic capabilities in response to training and dietary changes. Akazawa et al. found significant variations in the gut microbiota during different phases of training periodization among elite athletes (36). Research in elite volleyball athletes shows that gut microbiota maintains dynamic stability, adapting its composition in response to different phases of training, competition, and recovery periods (37). This underscores the importance of personalized, direct and indirect nutritional and training strategies to optimize microbiome composition to improve performance (38–42). Furthermore, Jäger et al. have indicated that probiotics could play a role in maintaining intestinal health and improving performance results, suggesting a potential intervention pathway in elite sports (32). However, the optimization of this dynamic relationship remains unexplored.
In summary, the gut microbiome of high-level athletes in homeostatic conditions is characterized by unique microbial profiles that can confer metabolic advantages, improve nutrient absorption, and support immune function through increased abundance of beneficial bacterial species, enhanced microbial diversity and superior efficiency of the immune system compared to the sedentary population (13, 28, 43, 44). Additionally, athletes might incur dysbiosis in overtraining and non-functional overreaching conditions. According to studies conducted on zonulin and intestinal permeability, inadequate stress could compromise the functionality of the intestinal barrier, creating a connection between overtraining, intestinal dysbiosis and potential performance decline (21). The interaction between structured physical exercise, diet and microbiome composition underscores the need for further research to explore targeted interventions that could optimize athletic performance through microbiome modulation and prevent possible states of alteration of the same.
2 Methods
This systematic scoping review was conducted following the PRISMA-ScR (Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews) methodological framework and registered with the Open Science Framework (OSF) under the following DOI: 10.17605/OSF.IO/YH49T. The protocol was developed a priori to systematically guide the research and analysis process (45).
2.1 Study design
The choice to conduct a systematic scoping review stems from the heterogeneity of methodological approaches in available studies, the complexity of the microbiome high-level athlete relationship, the need to map existing evidence, the importance of identifying significant gaps in current literature and the utility of synthesizing evidence to guide future research. The objectives of the systematic scoping review are to identify available evidence on the relationship between training and gut microbiome in high-level athletes with particular attention to performance related biomarkers. Furthermore, the review aims to identify recurring patterns in the microbiome response to various training modalities and intensities that may influence physical performance parameters, analyse direct correlations between specific microbial profiles and quantitative indicators of athletic performance such as power, endurance, recovery time, evaluate the effectiveness of nutritional interventions aimed at modulating the microbiome for the optimization of sports performance and highlight methodological and knowledge gaps that limit the understanding of the microbiome potential in current scientific literature.
2.2 Research strategy
The literature search was conducted on three databases PubMed, Scopus and Web of Science, from January 2015 to September 2025. The search string was developed using MeSH terms and keywords related to three main domains: intestinal microbiome, training/sports and athletic performance (“Gastrointestinal Microbiome” OR “Gut Microbiome” OR “Gut Microbiota” OR “Intestinal Microbiota”) AND (“Exercise” OR “Physical Activity” OR “Physical Activities” OR “Sports” OR “Athletic” OR “Athletics”) AND (“Training Program*” OR “Periodization” OR “Exercise Prescription” OR “Training Load”).
2.3 Eligibility criteria
The inclusion criteria were structured following the Population, Concept and Context (PCC) framework, including high-level or elite athletes (P), the gut microbiome, nutrition, and probiotics, prebiotics, postbiotics and symbiotic/postbiotic supplementation (C) and athletic performance (C). Eligible studies included original research, including randomized controlled trials (RCTs) and observational studies, as well as systematic and narrative reviews published in English language (46, 47). The exclusion criteria were equally precisely defined, including studies on non-high-level or non-elite athletes, studies with incomplete data on the microbiome, and studies on animal models only.
2.4 Study selection and data extraction
The selection of studies followed a three-phase process, initial screening of titles and abstracts, full-text evaluation of potentially eligible articles, and exclusion of duplicate articles. J.C. and A.F. conducted the selection process, with a third reviewer A.P. consulted to resolve any discrepancies. Data were extracted using a standardized form, including study characteristics, design, population, duration, microbiome analysis methodology, training protocols, nutritional interventions, primary and secondary outcomes, main results and limitations.
2.5 Data analysis
The analysis followed a narrative-descriptive approach, particularly identifying recurring patterns and methodological gaps. Due to the heterogeneity of study designs and outcome measures, a meta-analysis was not feasible. Data synthesis focused on thematic analysis and identification of convergent findings across studies.
2.6 Quality assessment
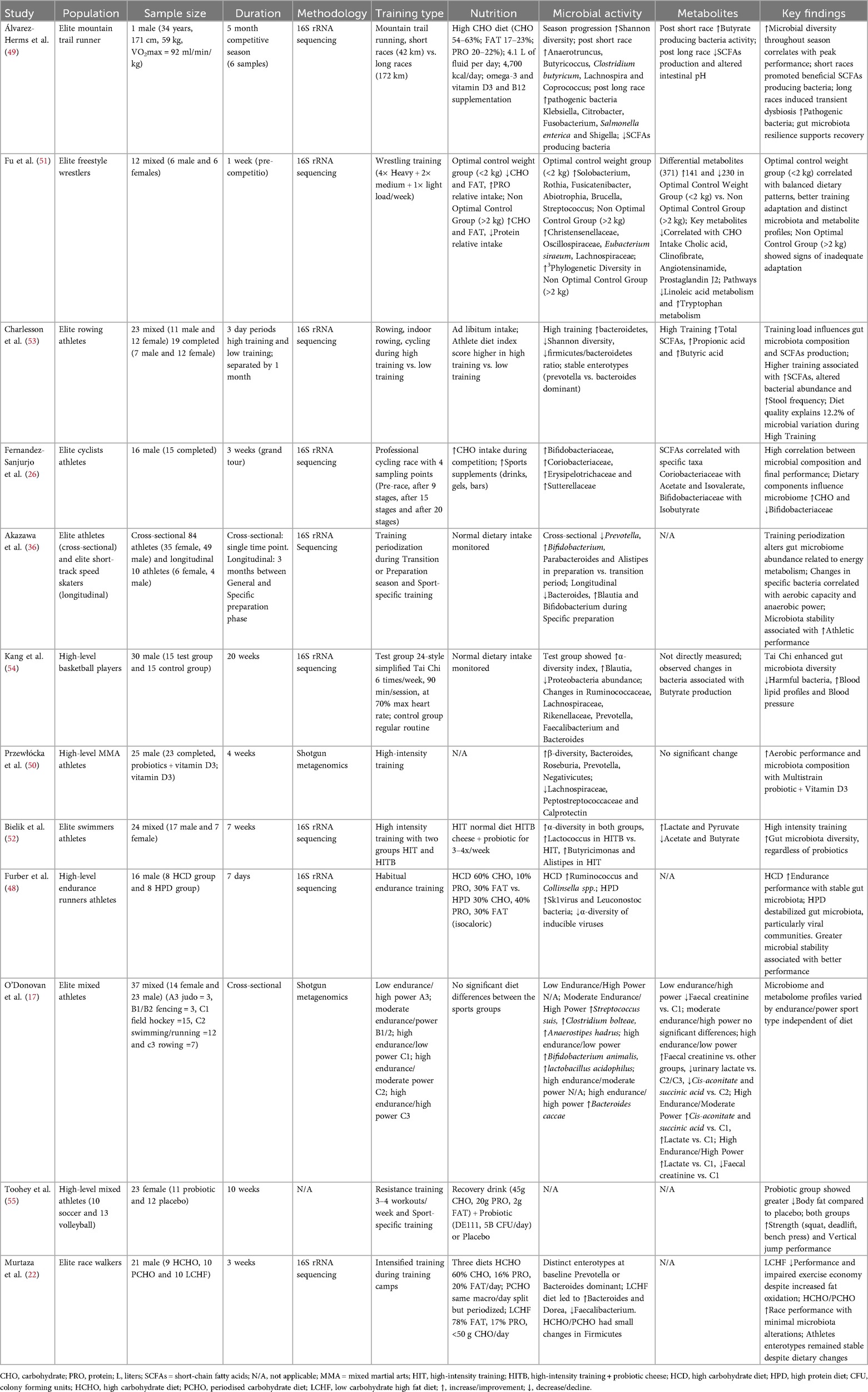
In accordance with scoping review methodology, no formal quality assessment tool was applied to individual studies, as the primary aim was to map the existing evidence and identify knowledge gaps rather than assess methodological quality (46, 47). However, study characteristics including design, population, methodology, and limitations were systematically recorded during data extraction to provide contextual information for interpretation of findings. For the 12 experimental studies included, we assessed key methodological characteristics including study design, sample size, presence of control groups, intervention duration and dropout rates (Supplementary Table S1). This evaluation revealed that 5 studies employed RCTs (48, 50, 52, 54, 55), 5 were observational/cross-sectional studies (17, 26, 36, 51, 53), 1 used a controlled intervention design (22) and 1 was a case study (49). Sample sizes ranged from 1 to 84 participants, with intervention durations varying from 7 days to 20 weeks. Key methodological challenges identified across studies included small sample sizes in most studies, short intervention durations in several studies and varied analytical approaches limiting direct comparisons. Additionally, conducting research with elite or high-level athletes may presents intrinsic logistical and ethical constraints, including limited participant availability, competition schedules and restrictions on experimental interventions during training and competition periods, which contribute to the observed methodological limitations alongside the inherent complexities of microbiome research methodologies.
3 Results
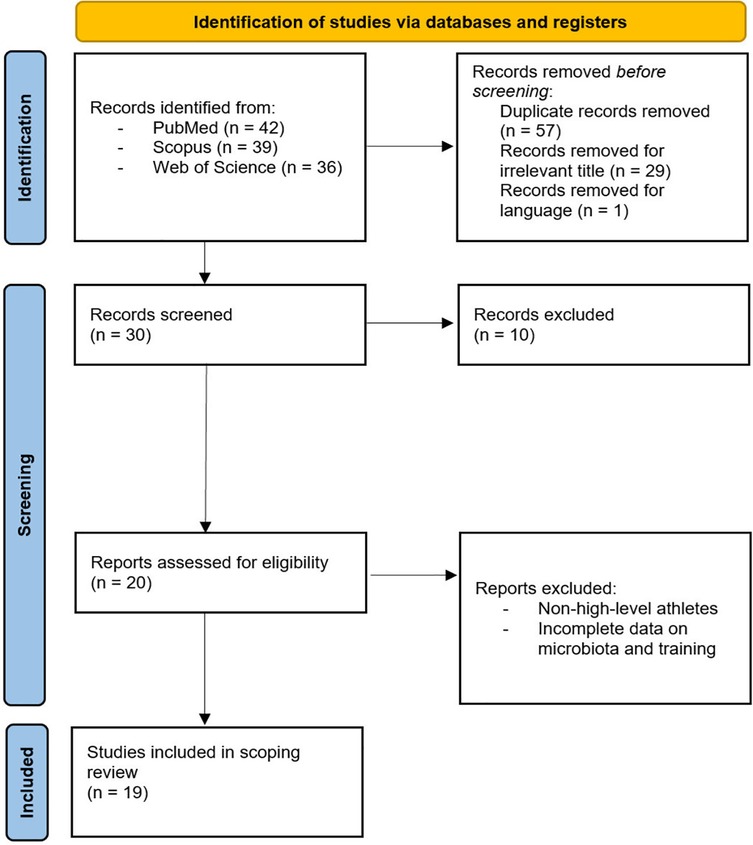
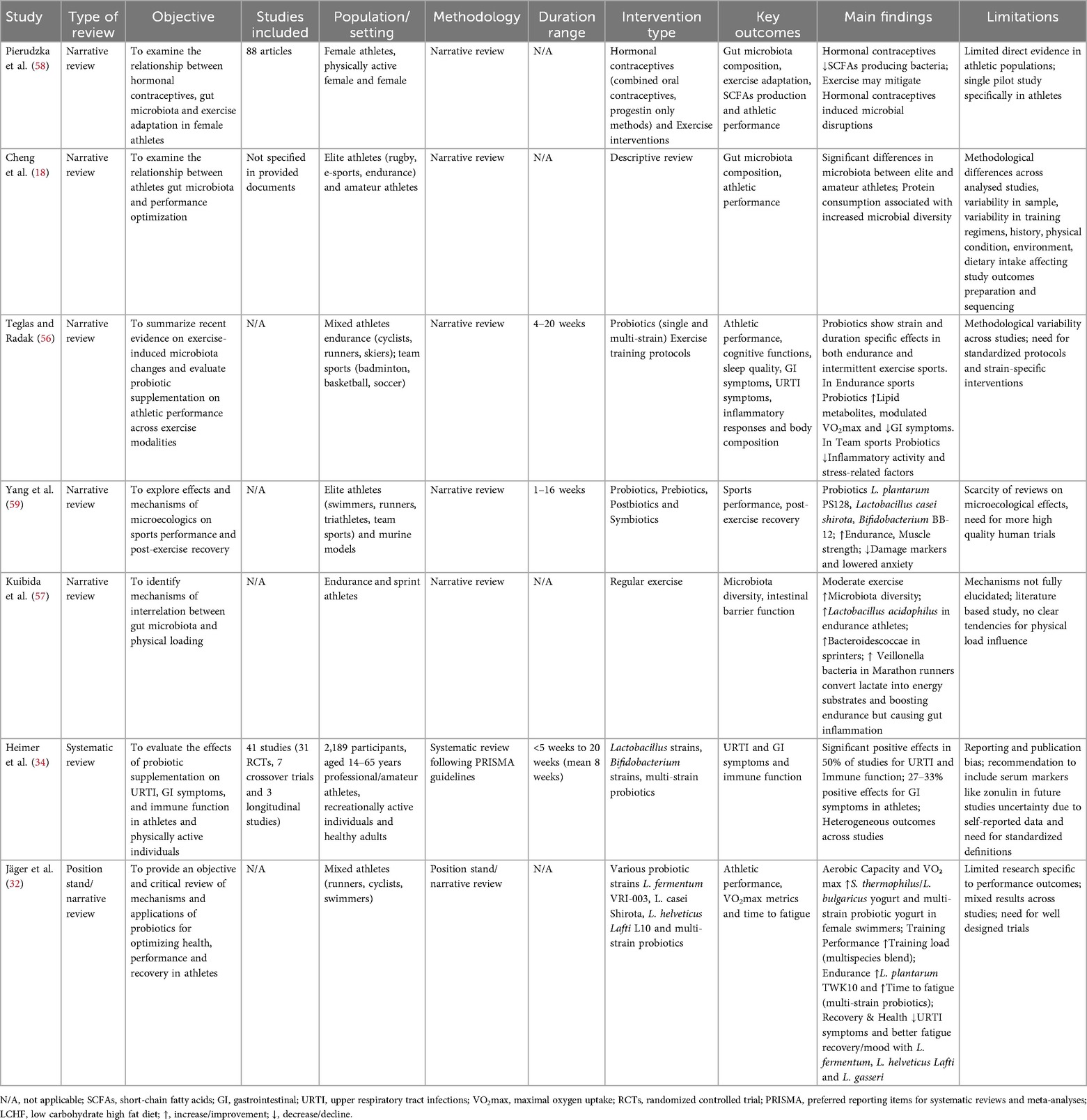
The research process initially identified 117 articles. After removing duplicates (n = 57), screening titles and abstracts (n = 29), and excluding (n = 1) non-English language article, (n = 30) articles underwent full-text screening. Of these, (n = 10) were excluded, leaving (n = 20) eligible articles. One additional article was excluded for focusing on non-elite athletes, resulting in a final inclusion of (n = 19) articles (Figure 1). Nineteen studies met the final inclusion criteria, comprising 12 experimental studies and 7 systematic/narrative reviews. The included experimental studies involved various athletic populations including Endurance sports (n = 4): Cyclists, Runners, Race Walkers, Mountain Trail Runner (22, 26, 48, 49), Power (n = 3): MMA athletes, Freestyle Wrestlers, Swimmers and Rowing (50–53), Team sports (n = 2): Basketball, Volleyball and Soccer (54, 55) and Mixed sports (n = 2): Multiple disciplines (17). The duration of interventions ranged from 4 to 20 weeks, with sample sizes between 16 and 84 participants and microbiome analysis methodologies primarily included 16S rRNA sequencing and Shotgun Metagenomics (Table 1). The systematic and narrative reviews provided analyses of the effects of probiotics (32, 34, 56), syntheses of adaptation mechanisms between the microbiome, performance and/or hormonal contraceptives (18, 57, 58) and an overview of practical applications (59) (Table 2).
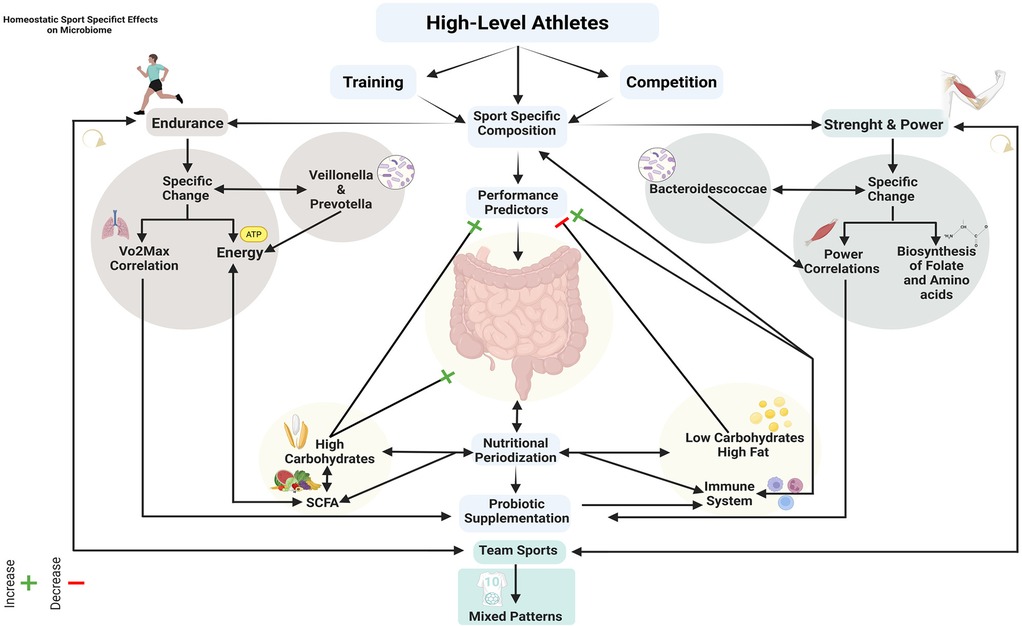
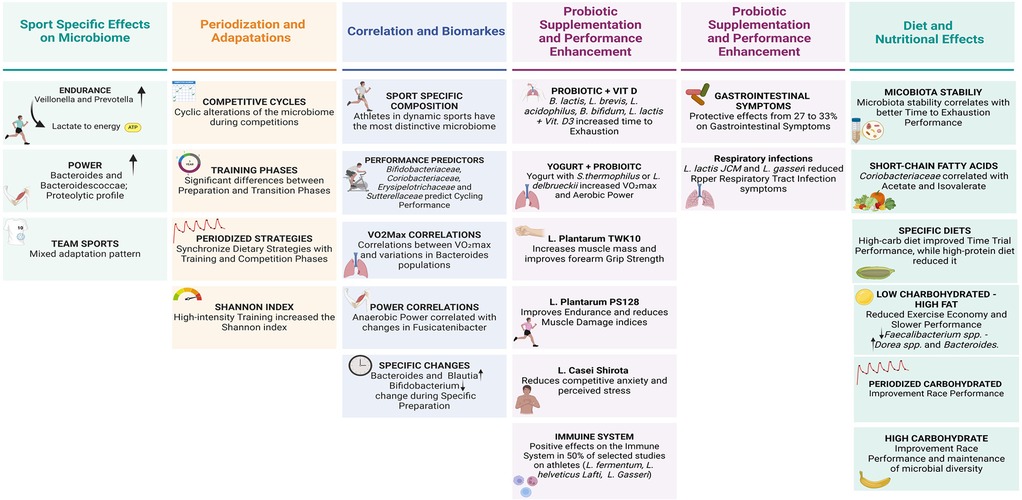
Figure 3. Integrated framework of training, nutrition and gut microbiome interactions in athletic performance optimization.
The analysis of collected data highlights a significant relationship between the intestinal microbiome and athletic performance, suggesting that microbiome composition represents a determining factor for optimizing sports performance, but it is not yet fully understood. This relationship could manifest through multiple mechanisms, including modulation of energy metabolism, support for recovery processes, influence on the immune system and contribution to inflammation management. O'Donovan et al. have demonstrated that athletes present distinctive microbial compositions correlated with the type of sport practiced (17). Athletes of sports with high dynamic components possess different microbiome compositions compared to those who practice sports with both dynamic and static components (17). In particular, the correlation between specific bacterial taxa and performance parameters (17). Fernandez-Sanjurjo et al. have identified that bacterial families such as Bifidobacteriaceae, Coriobacteriaceae, Erysipelotrichaceae and Sutterellaceae are strong predictors of cycling performance, as they can have an action on inflammation management and SCFAs production (26).
3.1 Endurance sports
Endurance sports can increase Veillonella and Prevotella (22, 26). Kuibida et al. have highlighted that marathoners possess increased levels of Veillonella that convert lactate into energy substrates, enhancing aerobic resistance but can potentially incur states of intestinal inflammation (57). Moreover, O'Donovan et al. have highlighted that athletes of specialties such as race walking and prolonged running present high levels of Lactobacillus acidophilus (17). A detailed case study by Álvarez-Herms et al. provided interesting insights into the possible dynamic changes of the microbiota in an elite trail runner during an entire competitive season (49). The athlete presented maximal oxygen uptake (VO2max) levels of 92 ml/min/kg and showed a progressive increase in microbial diversity compared to peak performance (49). Furthermore, a possible distinction emerged between the effects of short (42 km) and long races (172 km) for the sport discipline, where 42 km races appeared to promote an increase in presumably beneficial bacteria and SCFAs producers, including Anaerotruncus, Butyricoccus, Clostridium butyricum and especially Lachnospira, Coprococcus, while 172 km races appeared to induce transient dysbiosis with increased opportunistic pathogenic bacteria including Klebsiella, Citrobacter, Fusobacterium, Salmonella enterica and Shigella and reduction of protective commensal bacteria (49). A positive association between microbial diversity and aerobic performance emerges from Przewłócka et al. data, which detected specific correlations between changes in VO2max and variations in Bacteroides populations. At the same time, anaerobic power was correlated with changes in Fusicatenibacter (50). Furthermore, training itself favors an increase in microbial diversity. Bielik et al. observed that high-intensity training increased the Shannon index regardless of probiotic supplementation (52).
3.2 Strength and power sports
Dedicated high-intensity training (HIT) in swimmers can generate an increase in α-diversity regardless of the use of probiotics (52). Furthermore, Kuibida et al. report that in athletes who practice power sports such as sprint specialties a prevalence of Bacteroidescoccae is observed (57). Additionally, in sports with mixed patterns between static and dynamic components, such as fencing, higher levels of Anaerostipes hadrus have been seen compared to other sports (17). Other sports with high static and high dynamic components, such as rowing, have shown pathways more expressed for folate and amino acid biosynthesis (17). Other authors, analyzing 12 elite freestyle wrestlers, highlighted how the effectiveness of pre-competition weight control may be correlated with distinct microbial patterns (51). Wrestlers with effective weight control, characterized by a difference from the target weight of less than 2 kg (<2 kg), were associated with low carbohydrate and high protein diets. Furthermore, they presented greater abundance of Solobacterium, Rothia, Fusicatenibacter, Abiotrophia, Brucella and Streptococcus, a more appropriate nutritional structure and greater adaptability to training compared to the group with less effective weight control, with a difference from the target weight greater than 2 kg (>2 kg) (51). The group with less effective weight control (>2 kg) instead showed higher levels of Christensenellaceae, Oscillospiraceae, Eubacterium siraeum and Lachnospiraceae, positively correlated with relative carbohydrate intake (51). Moreover, this group presented signs of possible inadequate adaptation to intensified training load, evidenced by the presence of leukocytes, occult blood and proteins in urine, suggesting a relationship between microbiota, metabolic adaptation and performance in wrestling (51).
3.3 Team sports
Team sports can generate a mixed pattern of microbiota adaptation. Interventions like Tai Chi could be a low-intensity strategy to integrate into training as a possible microbiota modulator, generating an increase in Bacteroidetes and a decrease in Proteobacteria (54). Moreover, team sports present high levels of dynamism, such as field hockey, which has been seen to have very high levels of Lactobacillus acidophilus (17).
3.4 SCFAs and energy metabolism
SCFAs produced by the gut microbiome emerge as key mediators in the relationship between microbial composition and athletic performance. Fernandez-Sanjurjo et al. have identified significant correlations between Coriobacteriaceae and acetate, between Coriobacteriaceae and isovalerate and between Bifidobacteriaceae and isobutyrate, highlighting the role of these bacterial metabolites in supporting energy metabolism during physical exercise (26). The same authors indicate that the relationship between microbiota and performance depends not on a single factor but on multiple factors.
The differential metabolomic analysis in the study by Fu et al. on 12 freestyle wrestlers identified 371 different metabolites between the group with effective weight control (<2 kg) and the group with ineffective weight control (>2 kg), with particular relevance for lipid and amino acid metabolism (51). Some key metabolites negatively correlated with carbohydrate intake included cholic acid, clofibrate, angiotensinamide and prostaglandin J2 (51). Functional enrichment revealed some specific patterns, including downregulation of linoleic acid metabolism and upregulation of tryptophan metabolism in the effective weight control group, suggesting a more favourable metabolic profile for inflammation management and energy regulation during the pre-competition phase (51).
3.5 Diet and performance
Diet is a determining factor influencing microbiota composition and consequently athletic performance. Furber et al. have demonstrated that microbiota stability is associated with better endurance performance (48). Specifically, a high-carbohydrate diet (HCD) was able to improve endurance performance, while a high-protein diet (HPD) had the opposite effect (48). Supporting this thesis, Sanjurjo et al. indicate that carbohydrates are key factors for the positive modulation of performance and the microbiota of athletes (26). Furthermore, Murtaza et al. have highlighted that a high-carbohydrate diet (HCHO) induced maintenance of microbial diversity and improvement in race performance, as did a diet with periodized carbohydrates (PCHO), concluding that a diet low in carbohydrates and high in fats (LCHF) induced negative alterations, including reduced exercise economy, slower race performance and a reduction of Faecalibacterium spp., increase Dorea spp. and Bacteroides (22). Lastly, Jäger et al. reported that diet is a key modulator of the microbiota, where nutritional variations can generate a modification in the gut microbiota (32). Whey protein intake could positively influence microbial diversity, while greater consumption of carbohydrates and fiber in athletes is associated with an increase in Prevotella (32). The effect of fats on the microbiota of athletes is still not fully explored, their quality seems to have a determining role for athletes’ health (32).
3.6 Training and nutrition periodization
The periodization of training and nutrition can generate cyclic alterations of the microbiota during competitions and significant differences can occur between preparation and transition phases (26, 50). In swimmers, decreases in training volume combined with an increase in intensity can induce an increase in α-diversity (52). In line with these results, Akazawa et al. have highlighted that in Japanese elite athletes, during the transition phase, there was a significant decrease in the genera Bifidobacterium, Parabacteroides and Alistipes and an increase in Prevotella (36). Furthermore, they highlighted a significant reduction in the genus Bacteroides and an increase in Blautia and Bifidobacterium during the specific preparation phase, with changes correlated to modifications in VO₂max and anaerobic power (36). Other authors indicate a correlation between microbial stability and the optimization of physical performance (48). Some authors have shown that intensified training periodization can influence the gut microbiota, with variations partly associated with the effectiveness of weight control (51). The functional prediction of the study revealed that the microbiota of the group with less effective weight control was more enriched in riboflavin metabolism, as it could potentially play a compensatory mechanism to maintain GI health homeostasis under intensified stress (51). This suggests that the microbiota could serve as a possible biomarker for training load adaptability and effectiveness of weight control strategies in weight category sports (51). Charlesson et al. also introduce an often overlooked aspect in athlete microbiome research, the importance of gut transit time as a gut health marker (53). Their results show that low training load periods could involve slower transit times, evidenced by reduced evacuation frequency and a greater number of participants experiencing absence of bowel movements (53). Increased α-diversity, reduced SCFAs concentrations and reduced Bacteroides abundance were observed when transit times were slower (53).
3.7 Probiotics, performance, health and recovery
Some authors support the efficacy of mixed probiotic supplementation with Bifidobacterium lactis, Levilactobacillus brevis, Lactobacillus acidophilus, Bifidobacterium bifidum and Lactococcus lactis, as they have been seen to be able to improve various aspects of athletic performance associated with the use of Vitamin D3. Przewłócka et al. reported that the combination of probiotics and vitamin D3 increased the time to exhaustion during physical effort. In contrast, no significant changes were observed in the group with vitamin D3 alone (50). Jäger et al. reported a significant increase in VO2max and aerobic power with a yogurt supplementation containing Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus for a period of 30 days (32). They also reported increased endurance performance through L. Plantarum TWK10 and a better response to training load in athletes supplemented with a multispecies probiotic blend (32). Still, other studies have not found the same effects, so the authors indicate that the regulatory mechanisms of probiotics might have an indirect action, modulating other systems (32). Lastly, the authors suggest that supplementation with multi-strain probiotics is associated with improved aerobic performance, including increased VO₂max, aerobic power and time to exhaustion (32). Yang et al. have highlighted specific effects of various probiotic strains, L. plantarum TWK10 might be able to increase muscle mass more and improve forearm grip strength, L. Plantarum PS128 has been seen to be able to improve endurance performance and reduce indices of muscle damage and L. casei Shirota might be able to improve competitive anxiety and perceived stress (59).
In the field of strength and power, Toohey et al. analyzed the effects of Bacillus subtilis in female athletes, finding significant improvements in squat 1RM, deadlift 1RM, bench press 1RM and vertical jump (55). It is necessary to indicate that these improvements were similar to the placebo group, except for the significant reduction in body fat observed only in the probiotic group. This study observed no advantages of probiotic supplementation in increasing strength and power (55). The impact of probiotics on body composition parameters and athletic performance remains uncertain, requiring further studies to clarify the role of probiotics as ergogenic supplements (32).
A crucial aspect of athletic performance, especially in intense and prolonged training contexts, is the capacity for recovery and infection resistance. Heimer et al. have highlighted that probiotics have significant positive effects on the immune system in 50% of selected studies on athletes (34). Notably, endurance athletes showed the most significant reductions in pro-inflammatory factors following single probiotic intake (34). Confirming the theory, some authors report that a high training load can compromise the immune status of athletes, thus increasing the risk of upper respiratory tract infections (URTI) (32). Furthermore, the authors have highlighted that probiotic integration benefits the prevention of URTI (32). In high-level athletes, through probiotics such as L. fermentum, L. helveticus Lafti and L. gasseri, there was a decrease in symptoms of URTI and a more excellent state of health and recovery, changes not obtained through multi-strain probiotics (32). GI problems are prevalent in endurance athletes and can compromise physical performance and nutrient absorption, generating difficulties for athletes’ health. The authors have highlighted protective effects from 27 to 33% after probiotic intake on GI symptoms, but the topic is still controversial (34). Jäger et al. also indicate that studies on the effects of probiotics in athletes show contrasting results due to methodological variability (32). However, some research reports benefits through multi-strain probiotics with B. bifidum W23, B. lactis W51, E. faecium W54, L. acidophilus W22, L. brevis W63 and L. lactis W58 on reducing zonulin levels in high-level athletes, but further studies are needed regarding the reduction of GI symptoms and probiotic intake (32).
Teglas and Radak provided a detailed overview of probiotic effects in different sports, emphasizing how supplementation effectiveness depends on multiple factors, including specific strain, colony forming units (CFU), duration and frequency of intake (56). These factors can modulate the physiological impact of probiotics both independently from training protocols and in interaction with them, producing different effects. In endurance sports, probiotic supplementation appears to positively influence lipid metabolites, including SCFAs, modulate VO2max and improve exercise duration, while in sports characterized by intermittent exercise, probiotics may reduce inflammatory process activity and improve factors related to psychological stress, such as anxiety and depression (56).
In runners, administration of Bifidobacterium animalis Lactis and Lactobacillus acidophilus (10 × 10⁹ CFU for 30 days) appeared to reduce pro-inflammatory cytokine production and maintain CD8 + cell and effector memory cell populations (56). The use of Pediococcus acidilactici and Lactobacillus plantarum (3 × 10⁹ CFU for 4 weeks) did not produce effects on GI symptoms (56). Bifidobacterium longum longum OLP-01 (1.5 × 10¹⁰ CFU for 5 weeks) showed increased distance covered and greater intestinal microbiota abundance (56). Bifidobacterium lactis, Lactobacillus brevis, Lactobacillus casei, Lactococcus lactis, Lactobacillus acidophilus, Bifidobacterium bifidum and Ligilactobacillus salivarius (2.5 × 10⁹ CFU for 12 weeks) improved VO2max, 60 s ventilation, functional capacity, respiratory reserve and exercise capacity, also reducing GI symptoms (56). Finally, Lactobacillus helveticus Lafti L10 (5 × 10⁹ CFU for 6 weeks) appeared to reduce time to exhaustion in runners (56).
In road cyclists, supplementation with a multi-strain combination of Lactobacillus and Bifidobacterium (1 × 10¹¹ CFU for periods of 4, 12 and 16 weeks) showed increased aerobic capacity, VO2max, exercise duration to exhaustion and reduced heart rate (56). Similarly to cyclists, a combination of Lactobacillus, Bifidobacterium, Enterococcus and Bacillus strains for 90 days reduced GI symptoms without generating effects on VO2max and time to exhaustion (56). In skiing, Bifidobacterium lactis BL-99 (1 × 10⁹ CFU for 8 weeks) appeared to increase levels of SCFAs and polyunsaturated fatty acids, bile salts, knee extensor strength and VO2max (56).
In team sports, probiotic effects are equally varied. Lactobacillus casei Shirota (3 × 10¹⁰ CFU for 8 weeks) appeared to improve reaction time in cognitive tests of soccer players, while a blend of Lactobacilli, Bifidobacteria and Streptococcus strains (4.5 × 10¹¹ CFU for 4 weeks) showed no effects on VO2max (56). Moreover, Lactobacillus casei, Bifidobacterium lactis V9 and Lactobacillus plantarum P-8 (≥6–8 × 10⁹ CFU for 6 weeks) showed URTI reduction, increased Secretory Immunoglobulin A levels, decreased inflammatory factors, reduced maximum heart rate and lactate elimination rate (56). Administration of Bifidobacterium lactis CBP-001010, Lactobacillus rhamnosus CNCM I-4036, and Bifidobacterium longum ES1 (≥1 × 10⁹ CFU for 1 month) reported reduced stress, anxiety, and depression and increased post-exercise dopamine (56). Administration of Lactobacillus casei Shirota (3 × 10¹⁰ CFU for 6 weeks) in badminton players showed stress reduction and increased aerobic capacity, while Bifidobacteria and Lactobacilli strains (≥1.25 × 10¹⁰ CFU for 23 days) in basketball players appeared to show reduction of inflammatory processes and apoptosis of peripheral lymphocytes (56).
Pierudzka et al. highlighted an important gap in the scientific literature regarding the interaction between hormonal contraceptives, gut microbiota and exercise adaptations in female athletes (58). Only one pilot study appears to have directly investigated this relationship, showing that hormonal contraceptive use in physically active women appears to be associated with a reduction in SCFAs producing taxa, suggesting potential implications for energy metabolism and exercise adaptations (58). This complex interaction requires further research to develop personalized nutritional and training strategies for high-level female athletes using hormonal contraceptives.
4 Discussion
To date, we know that each athlete's gut microbiome possesses unique characteristics, and athletes in specific sports disciplines may exhibit similar trends. Moreover, the microbiome likely plays a role in optimizing physical performance. However, we still do not fully understand its effective impact on a large scale, nor the real differences in terms of its variation throughout a competitive year. The analysis of included studies has highlighted three fundamental aspects of the relationship between training and gut microbiome in athletes.
The results demonstrate distinctive microbiota adaptation patterns in relation to sport type and training load. Endurance athletes show a significant increase in Veillonella and Prevotella, which is associated with lactate metabolism and energy substrate utilization (31). In confirmation, O'Donovan et al. have demonstrated that sports with a high dynamic component generate metabolites such as lactate, succinic acid and cis-aconitic acid capable of generating specific patterns of the microbiome (17). In power sports, a prevalence of Bacteroides is observed, potentially correlated with more significant protein metabolism (52). These sport-specific adaptations suggest a functional plasticity of the microbiome in response to different metabolic demands (37).
4.1 Role of periodization
Training periodization emerges as a key factor in microbiome modulation. Longitudinal studies have highlighted cyclic alterations in microbial composition during different preparation phases, with significant variations between load and recovery periods (26, 50, 52). Charlesson et al. provided new considerations on the impact of training load on intestinal health markers in elite athletes (53). Their longitudinal study on elite rowers showed that high training load periods, compared to low training load periods, can modify SCFAs concentrations, reduce evacuation frequency, increase Bacteroides abundance and decrease α-diversity, with a lower Firmicutes/Bacteroides ratio (53). The authors also identified that training load independently influences microbiome composition even when controlling for changes in diet quality, evacuation frequency and sex (53). In particular, diet quality explains part of the microbial variation during high training load periods, while during low training load periods the training stress score appears to contribute more to the observable microbial variation (53) (Figure 2).
4.2 Interaction of nutrition, probiotics and microbiome
Nutritional interventions show a significant impact on microbiome composition and consequently on performance. Probiotic supplementation with multi-strains or single strains has been shown to generate benefits on aerobic physical performance and intestinal health, while on strength performance, at the moment, no significant benefits have been measured (32, 50, 55). High carbohydrate diet strategies favour microbial profiles associated with better endurance performance, while low carbohydrate diets can negatively alter the microbial ecosystem, as can high protein diets change the diversity of the microbiota in athletes (18, 22) (Figure 2).
4.3 Strengths and limitations
This systematic scoping review was conducted following the rigorous PRISMA-ScR methodological framework and was registered a priori with the Open Science Framework. The literature search was comprehensive, utilizing three major databases: PubMed, Scopus and Web of Science with a well defined search strategy. The inclusion of both experimental studies and systematic/narrative reviews provided a comprehensive overview of the current evidence. The narrative/descriptive analysis approach was appropriate for identifying patterns and knowledge gaps in this emerging field. Despite the robust methodology employed in this systematic scoping review, it is essential to highlight some significant methodological limitations that characterize this field of research. The variability in microbiome analysis methodologies across different studies limits the direct comparability of results, and the often reduced sample sizes of studies decrease the statistical power and generalizability of results (60). Furthermore, most studies have included male athletes. The interactions between diet, training, microbiome and performance make it difficult to isolate the specific contribution of single factors. The high variability in microbiome composition between individuals, moreover, represents a confounding factor that complicates the interpretation of results. The variability in intervention durations and training protocols is another limitation. Finally, there is an evident lack of standardization in outcome measures across studies.
4.4 Practical applications and future developments
Despite significant advances in gut microbiome research, science still faces the challenge of fully understanding the nuances of individualized responses to interventions, species redundancy, the importance of strain-specific variations, uncharacterizable microorganisms, host microbiome interactions and the long-term effects of microbial manipulations. Although these knowledge gaps, the collected evidence suggests several practical applications and directions for future research, but the fundamental pillar for microbiome modulation remains the nutritional approach (61). Yang et al. emphasize the need to consider individual differences in athletes and precision nutrition as a crucial element to optimize the effects of the microbiome (59). Probiotic supplementation is highly variable in effects but also essential and reasoning with personalized approaches with particular attention to strain specificity based on the athlete's individual profile, considering the sports discipline practiced, baseline microbiome characteristics, specific physical performance objectives and the athlete's health status, could in the future play a significant role in athletic performance. Some authors provide evidence supporting the need to synchronize dietary strategies with training and competition phases to maintain an optimal microbiota state (26, 48). Supporting these considerations, Jarret et al. report that current evidence indicates gut microbiome modulation by means of probiotic supplementation can have ergogenic benefits on endurance performance, while preliminary findings also suggest potential benefits for strength and power performance, although further research is required (62). The mechanisms are not fully understood, but may involve improved exercise recovery, immune function, nutrient absorption and alleviation of GI symptoms (62). These findings reinforce the importance of the previously mentioned synchronization approach, where it would be essential to consider diets according to the training period, adapting the nutritional approach to optimize microbial composition based on the specific needs of different phases of training periodization. The results support the utility of microbiome monitoring as a potential biomarker of training, recovery and health status in high-level athletes, integrating microbiome analysis in training periodization. Moreover, standardization in processual methodologies is necessary since, today it remains a very complex topic (63).
5 Conclusions
To date, we cannot yet claim to have a clear vision on the topic of microbiome and sport. This systematic scoping review provides a mapping of available evidence on the relationship between training and gut microbiome in high-level athletes. The analysis of included studies has allowed the identification of initial but not exhaustive recurring patterns in microbiome adaptations in response to training, highlighting specificities related to the type of sport and training load. The results support a significant but still premature role of the microbiome in optimizing athletic performance through sport-specific adaptations in microbial composition, modulation of energy metabolism and inflammatory response, interaction with nutritional interventions and probiotic supplementation and a correlation with performance and recovery markers. The gut microbiome emerges as a potentially determining factor for optimizing athletic performance and health, offering new possible perspectives for personalized interventions, as well as integrated nutrition and training strategies. To date, we know that the main modulator for microbiome composition is nutrition (Figure 3).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
JC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. AP: Methodology, Supervision, Validation, Visualization, Writing – review & editing. AF: Conceptualization, Methodology, Project administration, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank the ‘Egidio Meneghetti’ Library of the University of Verona for their assistance in developing the search string used in this study, which included MeSH terms and keywords related to gut microbiome, exercise and athletic performance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issue please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fspor.2025.1641923/full#supplementary-material
References
1. Cho CE, Norman M. Cesarean section and development of the immune system in the offspring. Am J Obstet Gynecol. (2013) 208(4):249–54. doi: 10.1016/j.ajog.2012.08.009
2. Sevelsted A, Stokholm J, Bønnelykke K, Bisgaard H. Cesarean section and chronic immune disorders. Pediatrics. (2015) 135(1):e92–8. doi: 10.1542/peds.2014-0596
3. Schommer NN, Gallo RL. Structure and function of the human skin microbiome. Trends Microbiol. (2013) 21(12):660–8. doi: 10.1016/j.tim.2013.10.001
4. Gregory KE, Walker WA. Immunologic factors in human milk and disease prevention in the preterm infant. Curr Pediatr Rep. (2013) 1(4):1–4. doi: 10.1007/s40124-013-0028-2
5. De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. (2010) 107(33):14691–6. doi: 10.1073/pnas.1005963107
6. Underwood MA, Gaerlan S, De Leoz ML, Dimapasoc L, Kalanetra KM, Lemay DG, et al. Human milk oligosaccharides in premature infants: absorption, excretion, and influence on the intestinal microbiota. Pediatr Res. (2015) 78(6):670–7. doi: 10.1038/pr.2015.162
7. Walker AW, Ince J, Duncan SH, Webster LM, Holtrop G, Ze X, et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. (2011) 5(2):220–30. doi: 10.1038/ismej.2010.118
8. Flint HJ, Scott KP, Louis P, Duncan SH. The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol. (2012) 9(10):577–89. doi: 10.1038/nrgastro.2012.156
9. Gillings MR, Paulsen IT, Tetu SG. Ecology and evolution of the human microbiota: fire, farming and antibiotics. Genes (Basel). (2015) 6(3):841–57. doi: 10.3390/genes6030841
10. Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A, et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. (2019) 7(1):14. doi: 10.3390/microorganisms7010014
11. Singh RK, Chang HW, Yan D, Lee KM, Ucmak D, Wong K, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. (2017) 15(1):73. doi: 10.1186/s12967-017-1175-y
12. da Rocha AL, Pinto AP, Kohama EB, Pauli JR, de Moura LP, Cintra DE, et al. The proinflammatory effects of chronic excessive exercise. Cytokine. (2019) 119:57–61. doi: 10.1016/j.cyto.2019.02.016
13. Mohr AE, Jäger R, Carpenter KC, Kerksick CM, Purpura M, Townsend JR, et al. The athletic gut microbiota. J Int Soc Sports Nutr. (2020) 17(1):24. doi: 10.1186/s12970-020-00353-w
14. Den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. (2013) 54(9):2325–40. doi: 10.1194/jlr.R036012
15. Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol. (2015) 21(29):8787–803. doi: 10.3748/wjg.v21.i29.8787
16. Mancin L, Burke LM, Rollo I. Fibre: the forgotten carbohydrate in sports nutrition recommendations. Sports Med. (2025) 55(5):1067–83. doi: 10.1007/s40279-024-02167-1
17. O'Donovan CM, Madigan SM, Garcia-Perez I, Rankin A, O’ Sullivan O, Cotter PD. Distinct microbiome composition and metabolome exists across subgroups of elite Irish athletes. J Sci Med Sport. (2020) 23(1):63–8. doi: 10.1016/j.jsams.2019.08.290
18. Cheng SC, Chang C, Chen YC, Gojobori T, Chiu PK. Human gut microbiome determining athletes’ performance: an insight from genomic analysis. Ecol Genet Genom. (2025) 34:100327. doi: 10.1016/j.egg.2025.100327
19. Ursell LK, Metcalf JL, Parfrey LW, Knight R. Defining the human microbiome. Nutr Rev. (2012) 70(Suppl 1):S38–44. doi: 10.1111/j.1753-4887.2012.00493.x
20. Arumugam M, Raes J, Pelletier E, Paslier DL, Yamada T, Mende DR, et al. Enterotypes of the human gut microbiome. Nature. (2011) 473:174–80. doi: 10.1038/nature09944
21. Fasano A. All disease begins in the (leaky) gut: role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Res. (2020) 9:F1000. doi: 10.12688/f1000research.20510.1
22. Murtaza N, Burke LM, Vlahovich N, Charlesson B, O'Neill H, Ross ML, et al. The effects of dietary pattern during intensified training on stool microbiota of elite race walkers. Nutrients. (2019) 11(2):261. doi: 10.3390/nu11020261
23. Hughes RL. A review of the role of the gut microbiome in personalized sports nutrition. Front Nutr. (2020) 6:191. doi: 10.3389/fnut.2019.00191
24. Mach N, Fuster-Botella D. Endurance exercise and gut microbiota: a review. J Sport Health Sci. (2017) 6(2):179–97. doi: 10.1016/j.jshs.2016.05.001
25. Przewłócka K, Folwarski M, Kaźmierczak-Siedlecka K, Skonieczna-Żydecka K, Kaczor JJ. Gut-muscle AxisExists and may affect skeletal muscle adaptation to training. Nutrients. (2020) 12(5):1451. doi: 10.3390/nu12051451
26. Fernandez-Sanjurjo M, Fernandez J, Martinez-Camblor P, Rodriguez-Alonso M, Ortolano-Rios R, Pinto-Hernandez P, et al. Dynamics of gut microbiota and short-chain fatty acids during a cycling grand tour are related to exercise performance and modulated by dietary intake. Nutrients. (2024) 16(5):661. doi: 10.3390/nu16050661
27. Xu Y, Zhong F, Zheng X, Lai HY, Wu C, Huang C. Disparity of gut microbiota composition among elite athletes and young adults with different physical activity independent of dietary Status: a matching study. Front Nutr. (2022) 9:843076. doi: 10.3389/fnut.2022.843076
28. Barton W, Penney NC, Cronin O, Garcia-Perez I, Molloy MG, Holmes E, et al. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut. (2018) 67(4):625–33. doi: 10.1136/gutjnl-2016-313627
29. Fasano A. Zonulin, regulation of tight junctions, and autoimmune diseases. Ann N Y Acad Sci. (2012) 1258(1):25–33. doi: 10.1111/j.1749-6632.2012.06538.x
30. Fasano A. Intestinal permeability and its regulation by Zonulin: diagnostic and therapeutic implications. Clin Gastroenterol Hepatol. (2012) 10(10):1096–100. doi: 10.1016/j.cgh.2012.08.012
31. Scheiman J, Luber JM, Chavkin TA, MacDonald T, Tung A, Pham LD, et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat Med. (2019) 25(7):1104–9. doi: 10.1038/s41591-019-0485-4
32. Jäger R, Mohr AE, Carpenter KC, Kerksick CM, Purpura M, Moussa A, et al. International society of sports nutrition position stand: probiotics. J Int Soc Sports Nutr. (2019) 16(1):62. doi: 10.1186/s12970-019-0329-0
33. Manor O, Dai CL, Kornilov SA, Smith B, Price ND, Lovejoy JC, et al. Health and disease markers correlate with gut microbiome composition across thousands of people. Nat Commun. (2020) 11(1):5206. doi: 10.1038/s41467-020-18871-1
34. Heimer M, Teschler M, Schmitz B, Mooren FC. Corrigendum: health benefits of probiotics in sport and exercise-non-existent or a matter of heterogeneity? A systematic review. Front Nutr. (2022) 9:1051918. doi: 10.3389/fnut.2022.1051918
35. Donati Zeppa S, Agostini D, Gervasi M, Annibalini G, Amatori S, Ferrini F, et al. Mutual interactions among exercise, sport supplements and microbiota. Nutrients. (2019) 12(1):17. doi: 10.3390/nu12010017
36. Akazawa N, Nakamura M, Eda N, Murakami H, Nakagata T, Nanri H, et al. Gut microbiota alternation with training periodization and physical fitness in Japanese elite athletes. Front Sports Act Living. (2023) 5:1219345. doi: 10.3389/fspor.2023.1219345
37. Carlone J, Giampaoli S, Alladio E, Rosellini G, Barni F, Salata E, et al. Dynamic stability of gut microbiota in elite volleyball athletes: microbial adaptations during training, competition and recovery. Front Sports Act Living. (2025) 7:1662964. doi: 10.3389/fspor.2025.1662964
38. Marchesi JR, Adams DH, Fava F, Hermes GD, Hirschfield GM, Hold G, et al. The gut microbiota and host health: a new clinical frontier. Gut. (2016) 65(2):330–9. doi: 10.1136/gutjnl-2015-309990
39. Dallas DC, Sanctuary MR, Qu Y, Khajavi SH, Van Zandt AE, Dyandra M, et al. Personalizing protein nourishment. Crit Rev Food Sci Nutr. (2017) 57(15):3313–31. doi: 10.1080/10408398.2015.1117412
40. Proia P, Rossi C, Alioto A, Amato A, Polizzotto C, Pagliaro A, et al. MiRNAs expression modulates osteogenesis in response to exercise and nutrition. Genes (Basel). (2023) 14(9):1667. doi: 10.3390/genes14091667
41. Lukic-Sarkanovic M, Roklicer R, Trivic T, Manojlovic M, Gilic B, Milovancev A, et al. Acute muscle damage as a metabolic response to rapid weight loss in wrestlers. Biomed Hum Kinet. (2024) 16:99–105. doi: 10.2478/bhk-2024-0010
42. Carlone J, Lista M, Romagnoli R, Sgrò P, Piacentini MF, Di Luigi L. The role of the hormonal profile of constitutional biotypes in the training process. Medicina Dello Sport. (2023) 76:343–52. doi: 10.23736/S0025-7826.23.04346-6
43. Petersen LM, Bautista EJ, Nguyen H, Hanson BM, Chen L, Lek SH, et al. Community characteristics of the gut microbiomes of competitive cyclists. Microbiome. (2017) 5(1):98. doi: 10.1186/s40168-017-0320-4
44. Clarke SF, Murphy EF, O'Sullivan O, Lucey AJ, Humphreys M, Hogan A, et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. (2014) 63(12):1913–20. doi: 10.1136/gutjnl-2013-306541
45. Mak S, Thomas A. Steps for conducting a scoping review. J Grad Med Educ. (2022) 14(5):565–7. doi: 10.4300/JGME-D-22-00621.1
46. Peters MDJ, Marnie C, Tricco AC, Pollock D, Munn Z, Alexander L, et al. Updated methodological guidance for the conduct of scoping reviews. JBI Evid Synth. (2020) 18(10):2119–26. doi: 10.11124/JBIES-20-00167
47. Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. (2005) 8(1):19–32. doi: 10.1080/1364557032000119616
48. Furber MJW, Young GR, Holt GS, Pyle S, Davison G, Roberts MG, et al. Gut microbial stability is associated with greater endurance performance in athletes undertaking dietary periodization. mSystems. (2022) 7(3):e0012922. doi: 10.1128/msystems.00129-22
49. Álvarez-Herms J, Burtscher M, González-Benito A, Corbi F, Odriozola-Martínez A. The gut microbiota characterization of a world-class mountain trail runner during a complete competition season: a case report. J Athl Train. (2025) 60(3):252–8. doi: 10.4085/1062-6050-0143.24
50. Przewlócka K, Folwarski M, Kaczmarczyk M, Skonieczna-Żydecka K, Palma J, Bytowska ZK, et al. Combined probiotics with vitamin D3 supplementation improved aerobic performance and gut microbiome composition in mixed martial arts athletes. Front Nutr. (2023) 10:1256226. doi: 10.3389/fnut.2023.1256226
51. Fu P, Wang C, Zheng S, Gong L. Differences in gut microbiota and metabolites between wrestlers with varying precompetition weight control effect. Physiol Genomics. (2024) 56(12):845–54. doi: 10.1152/physiolgenomics.00026.2024
52. Bielik V, Hric I, Ugrayová S, Kubánová L, Putala M, Grznár L, et al. Effect of high-intensity training and probiotics on gut microbiota diversity in competitive swimmers: randomized controlled trial. Sports Med Open. (2022) 8(1):64. doi: 10.1186/s40798-022-00453-8
53. Charlesson B, Jones J, Abbiss C, Peeling P, Watts S, Christophersen CT. Training load influences gut microbiome of highly trained rowing athletes. J Int Soc Sports Nutr. (2025) 22(1):2507952. doi: 10.1080/15502783.2025.2507952
54. Kang D, Wang X, Wang J. Intervention study of tai chi training on the intestinal flora of college student basketball players. Medicine (Baltimore). (2023) 102(36):e35044. doi: 10.1097/MD.0000000000035044
55. Toohey JC, Townsend JR, Johnson SB, Toy AM, Vantrease WC, Bender D, et al. Effects of probiotic (Bacillus subtilis) supplementation during offseason resistance training in female division I athletes. J Strength Cond Res. (2020) 34(11):3173–81. doi: 10.1519/JSC.0000000000002675
56. Teglas T, Radak Z. Probiotic supplementation for optimizing athletic performance: current evidence and future perspectives for microbiome-based strategies. Front Nutr. (2025) 12:1572687. doi: 10.3389/fnut.2025.1572687
57. Kuibida V, Kokhanets P, Lopatynska V. Coadaptation mechanism of the gut microbiota and human organism to physical loading. Regul Mech Biosyst. (2023) 14:213–9. doi: 10.15421/022332
58. Pierudzka W, Slawatycki J, Klemenska P, Warczak K, Wasilewska P, Horwat P, et al. Hormonal contraceptives and the gut microbiome in female athletes: implications for health, performance, and exercise-related physiological adjustments. Cureus. (2025) 17(7):e88789. doi: 10.7759/cureus.88789
59. Yang K, Chen Y, Wang M, Zhang Y, Yuan Y, Hou H, et al. The improvement and related mechanism of microecologics on the sports performance and post-exercise recovery of athletes: a narrative review. Nutrients. (2024) 16(11):1602. doi: 10.3390/nu16111602
60. Mancin L, Paoli A, Berry S, Gonzalez JT, Collins AJ, Lizarraga MA, et al. Standardization of gut microbiome analysis in sports. Cell Rep Med. (2024) 5(10):101759. doi: 10.1016/j.xcrm.2024.101759
61. Fasano A. The physiology of hunger. N Engl J Med. (2025) 392(4):372–81. doi: 10.1056/NEJMra2402679
62. Jarrett H, Medlin S, Morehen JC. The role of the gut microbiome and probiotics in sports performance: a narrative review update. Nutrients. (2025) 17(4):690. doi: 10.3390/nu17040690
Keywords: gut microbiome, gut microbiota, athletic performance, high-level athletes, sports training, sports nutrition
Citation: Carlone J, Parisi A and Fasano A (2025) The performance gut: a key to optimizing performance in high-level athletes: a systematic scoping review. Front. Sports Act. Living 7:1641923. doi: 10.3389/fspor.2025.1641923
Received: 5 June 2025; Accepted: 22 September 2025;
Published: 20 October 2025.
Edited by:
Rubén Maneiro, University of Vigo, SpainReviewed by:
Stefano Raimondi, University of Modena and Reggio Emilia, ItalyQi Han, National Institute of Sports Medicine, China
Copyright: © 2025 Carlone, Parisi and Fasano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junior Carlone, anVuaW9yLmNhcmxvbmVAdW5pdnIuaXQ=
†ORCID:
Junior Carlone
orcid.org/0009-0008-4042-6109
Attilio Parisi
orcid.org/0000-0003-2648-8406
Alessio Fasano
orcid.org/0000-0002-2134-0261
 Junior Carlone
Junior Carlone Attilio Parisi
Attilio Parisi Alessio Fasano
Alessio Fasano