- 1Department of Industrial Engineering, University of Salerno, Fisciano, Italy
- 2ProdAl Scarl, Fisciano, Italy
Starches from conventional sources such as cereal crops, pulses, and tubers have been widely utilized to produce starch-based hydrogels, which are complex networks able to absorb and retain substantial amounts of water. However, in recent years due to the increasing interest to produce these biomaterials, also starches derived from non-conventional sources have gained attention. In this study, conventional and non-conventional starches isolated from pea flour, lentil flour, unripe apples, and banana peels were used to produce starch-based hydrogels by high-pressure processing (HPP). The starch powders were isolated through traditional methods and showed high starch content (76.91%–86.56%) and minimal starch damage. According to their amylose content, ranging from 18.74% to 22.42%, these starches were classified as normal starches. Starch suspensions (25% w/w in distilled water) were treated at 600 MPa for 15 min at room temperature to enable starch gelatinization. Gel formation was assessed by analysing the gelatinization extent, structuring level, and swelling power of the samples. Furthermore, the physical appearance and flow profile of the obtained structures were evaluated. The results indicated that the starch-based hydrogels produced under these processing conditions exhibited different gel formation levels, physical appearance, and flow behaviour. These differences were attributed to the distinct properties of the recovered starches. More work is needed to assess the mechanical properties and physical stability of these structured materials during shelf life.
1 Introduction
In the last years, an increasing interest toward three-dimensional (3D) polymeric biomaterials, the so-called “natural hydrogels”, have been observed due to their potential numerous applications in different fields such as agriculture, food, pharmaceutical and cosmetic sectors (Zhao et al., 2013; Bao et al., 2019; Casadey et al., 2020; Idrees et al., 2020; Samir et al., 2022; Pires et al., 2023). Natural hydrogels can be classified as protein-based, polysaccharide-based, and decellularized tissue-derived hydrogels (Catoira et al., 2019). Considering that most biomaterials found in nature are polysaccharides, their distinctive biocompatibility, and non-toxicity paved the way for their utilization for hydrogel development (Qureshi et al., 2020). Among polysaccharide-based hydrogels, starch-based hydrogels can be considered the most desirable alternative to produce polymeric biomaterials due to their excellent biodegradability and biocompatibility (Edgar and Marks, 2020). Indeed, starch, a macronutrient found in a wide variety of foods, is the primary source of energy for humans (50%–70%), and the primary storage carbohydrate for plants (Edgar and Marks, 2020). Starch can be classified as conventional or non-conventional according to its botanical source (Santana et al., 2014). Conventional starch is typically isolated from cereals (corn, rice, wheat, etc.), legumes (beans, chickpeas, lentils, etc.), and tubers (cassava, potatoes, etc). In the last 30 years, a significant number of research activities have been performed to gelatinize or modify conventional starches, which dominate the current markets (Ahmed and Mondal, 2022). Apart from these major crops, underutilized and/or neglected starch sources have recently attracted the attention of researchers (Makroo et al., 2021; Adi Sulianto et al., 2023; Carvalho et al., 2024). In this context, starches isolated from unripe fruits, such as bananas, apples, and mango among others, have been suggested as health-promoting food additives, due to their resistance to the enzymatic action of α-amylase, inhibiting starch digestibility and consequently favourably reducing its glycaemic index (Dega and Barbhai, 2023). Considering that millions of tonnes of agri-food by-products are generated yearly from farming and agricultural processing, large amounts of polymeric components of different quality can be recovered from these costless resources, including starch (Sadh et al., 2023; Phiri et al., 2024). Starch obtained from these materials can represent a good alternative to those coming from conventional sources, provided that it is demonstrated that they possess the desired functional properties, which are critical to developing high added value products. Many authors have highlighted the possibility of recovering starch by valorizing discarded biomasses (Bello-Pérez et al., 2006; Hernández-Carmona et al., 2017; Kringel et al., 2020; Makroo et al., 2021; Showkat et al., 2021). Considering their physicochemical and structural characteristics, these starches potentially represent a good alternative to synthesize starch-based hydrogels. Chemical and physical crosslinking methods have been used to produce starch-based hydrogels (Ismail et al., 2013; Xiao, 2013). Chemically cross-linked networks have permanent junctions, while physical networks have transient junctions that arise from either polymer chain entanglements or physical interactions such as ionic interactions, hydrogen bonds, or hydrophobic interactions. However, these processing methods involve harsh chemicals and high energy use, leading to environmental concerns. As a result, there is growing interest in developing innovative and sustainable technologies for a more efficient and environmental friendly production of hydrogel. Among others high-pressure processing HPP has been proven effective in obtaining hydrogels, with reduced energy consumption, processing times, and environmental risks. High-pressure processing promotes the gelatinization and physical modification of starches, which differ from those of heat-gelatinized starches (Koshenaj and Ferrari, 2024). In the last decade, authors have produced HPP starch-based hydrogels using conventional starches, with characteristics making them suitable for different applications (Koshenaj and Ferrari, 2024). However, to the best of our knowledge, no research efforts were made to study the gelatinization under pressure of non-conventional starches. This work aimed to investigate the possibility of producing HPP hydrogels utilizing starches isolated from different sources (conventional and non-conventional), shading the light on the role of chemical-physical characteristics of starches on the gelatinization process.
2 Materials and methods
2.1 Materials
Red lentil flour (0.57% fat, 16.96% protein) and pea flour from organic farming (1.8% fat, 23% protein), purchased in a local market, were packed in plastic bags and stored at ambient temperature. Unripen apples (Annurca apple) were collected from trees grown in the garden of the University of Salerno, Italy. Bananas, imported from Maharashtra (India), were purchased in a local market. All chemicals and reagents were of analytical grade or superior.
2.2 Isolation of starch from different sources
2.2.1 Isolation of starch from pulse flour
The wet method was used to recover pea and lentil starch from the pulse flours. In brief, the flour was suspended in water (1:10 w/v), adjusting the pH to 9.5 with 1M NaOH/HCl, and the suspension was mixed using a magnetic stirrer to stabilize the protein. The mixture was allowed to settle for 3h. A centrifuge (PK121R model, ALC International, Cologno Monzese, Milan, Italy) was used to separate the liquid and solid. The supernatant was removed after centrifugation (3,360 rpm for 15 min), and the pellet was washed 4 times with distilled water. The isolated starch was dried at 35°C for 24 h.
2.2.2 Isolation of starch from unripe apples
Starch was isolated from apple fruits using a method reported by Kasemsuwan (1995) with slight modification. Unripe apple fruits were sliced and comminute in a blender after adding 0.3% (w/v) of sodium metabisulphite. The obtained apple puree was filtered to remove most of the liquid, and the filtrate was centrifuged at 6,500 rpm for 40 min to precipitate the starch. Afterward, the recovered starch was washed under mechanical stirring for 1 h with 10% toluene in 0.1 M sodium chloride solution and allowed to stand for 4 h to remove protein and chlorophyll pigments. The pellet was then washed three times with distilled water and then dried at 35°C for 48 h.
2.2.3 Isolation of starch from banana peels
Starch was isolated from banana peels using the alkaline method (Yang et al., 2022) with some modifications. The banana peels were dipped in a citric acid solution (0.5%, w/v) for 10 min and drained to prevent browning. The peels were dried at 40°C ± 1°C until constant moisture content was reached and then ground to obtain a smooth powder. Afterward, a solution of 0.2% sodium metabisulphite was added to the dried banana powder with a w/v ratio of 1:2. The solution was mixed using a magnetic stirrer and allowed to settle for 4h. The pellet was then washed three times with distilled water and dried at 35°C for 48 h.
2.3 Characterization of recovered starches
2.3.1 Chemical and physical characteristics of the isolated starches
Moisture and ash content of starches were determined following the guidelines of the Association of Official Analytical Chemists (2005).
Total lipid content was determined by Soxhlet extraction followed by weight difference evaluation. Briefly, five grams of the solid sample were weighed into a thimble and extracted with 80 mL of diethyl ether for 5 h. After extraction, the solvent was evaporated using a vacuum evaporator, and the flask was dried at 104°C for 30 min until a constant weight was reached.
Crude proteins were determined by the Kjeldahl method, where the total organic nitrogen is converted to ammonium sulfate through the digestion of the sample with sulfuric acid (5 mL, 96%) in the presence of catalysts. The digestate was neutralized with alkali (30% NaOH, 50 mL) and distilled into ascorbic acid solution (2%). The borate anions formed are titrated with standardized acid (0.1 M HCl). As a result of this analysis, the crude protein content of the sample is determined. A factor of 6.25 is used to convert the percentage of nitrogen into the percentage of crude protein.
Starch, amylose content, and starch damage were determined using a rapid enzymatic assay (Megazyme International Ireland Ltd., Wicklow, Ireland). Additionally, starch particle size distribution was determined by dynamic light scattering (DLS), using a Malvern Mastersizer 2000 instrument (Malvern Instruments Ltd., Worcestershire, UK). The characteristic diameters d (0.1), d (0.5), and d (0.9) were evaluated, corresponding to the 10th, 50th (median value), and 90th percentile of the cumulative size distribution curve.
2.3.2 FTIR measurements
Fourier Transform Infrared Spectroscopy (FT-IR) was used to identify specific chemical groups present in the recovered starches from different sources. Each starch powder was analyzed using an FT/IR-400 spectrometer (Jasco Corporation, Kyoto, Japan). The FT-IR spectra of the samples were recorded at wavelengths ranging from 4,000 to 950 cm−1. The resulting starch-averaged spectrum was smoothed to remove any potential noise using a fifteen-point adaptive smoothing function. Subsequently, the baseline modification and a normalized function were applied.
2.3.3 Scanning electron microscopy
The morphology of the starch granules was analyzed by scanning electron microscopy. The starch was mounted on an aluminum stub and coated by a 10 nm thick gold-palladium alloy sputter coater before being analyzed in a high-resolution ZEISS HD15 Scanning Electron Microscope (Zeiss, Oberkochen, Germany) at ×500 magnification.
2.4 Hydrogel production
2.4.1 Sample preparation and HPP treatments
Suspensions of each recovered starch were produced adding to the powder distilled water to reach a concentration of 25% (w/w) to produce the HPP hydrogels. For each sample, the starch suspension (3g) was thoroughly mixed and vacuum-packed in flexible pouches (polymer/aluminium/polymer film OPP30-A19-LDPE70). Additionally, the packed suspensions were agitated until full homogenization to prevent particle sinking and then treated under pressure in a laboratory-scale high-pressure unit (U111, Unipress, Warsaw, Poland). The equipment, described in detail by Maresca and Ferrari (2017), can be operated at a pressure of up to 700 MPa and temperature in the range of −40°C - 100°C.
Hydrogels from different starches were produced setting the pressure to achieve the complete gelatinization of the samples at 600 MPa, the processing time at 15 min, and the temperature at 25°C. No temperature increase was detected during HPP treatment. Hydrogel samples were stored at ambient conditions until further analyses.
2.4.2 Determination of gel formation
Gel formation was assessed by determining the gelatinization extent, structuring level, and swelling power of the samples. In brief, the degree of gelatinization was evaluated by measuring the loss of birefringence of the starch granules using an optical inverted microscope (Nikon Eclipse, TE 2000S, Nikon Instruments Europe B.V., Amsterdam, Netherlands) with a polarisation filter and a ×20 objective coupled to a DS Camera Control Unit (DS-5M-L1, Nikon Instruments Europe B. V., Amsterdam, Netherlands). Before observation, a small amount of the HPP-treated sample was spotted on a microscope slide and covered with a cover glass. Additionally, the gelatinization level of the samples was determined by evaluating the efficiency index (EI) by Equation 1, as proposed by Larrea-Wachtendor et al. (2019).
The EI is a crucial parameter specifically indicating the drained weight of the structured material.
Ultimately, the swelling power was determined by modifying the method described by Kusumayanti et al. (2015) as reported by Larrea-Wachtendor et al. (2019). HPP-treated samples were centrifuged in a centrifuge PK130R (ALC, Winchester, Virginia, USA) at 1,351× g for 10 min and the pellet was weighed before and after drying at 105°C for 6 h. The swelling power, evaluated by Equation 2, is defined as the weight of the wet pellet over the dry weight of the starch in the hydrogel samples:
2.5 Characterization of hydrogels
2.5.1 Flow curves
Flow curves of the HPP hydrogels were obtained in a rheometer AR 2000 (TA Instruments, New Castle, Delaware, USA), equipped with a Peltier plate and a circulating water bath (DC10-Haake K10, Karlsruhe, Germany). Small samples were carefully placed on the Peltier plate surface of the rheometer. The flow curves were obtained by varying the shear rate from 0.1 to 100 s-1 at 25°C while monitoring shear rate, shear stress, and viscosity.
2.5.2 pH measurements
The pH of the hydrogels was measured using 1 g of the sample, dissolved in 10 mL of distilled water, and stirred evenly. A pH meter (pH-Metro BASIC 20+) was used to measure the pH of the solution.
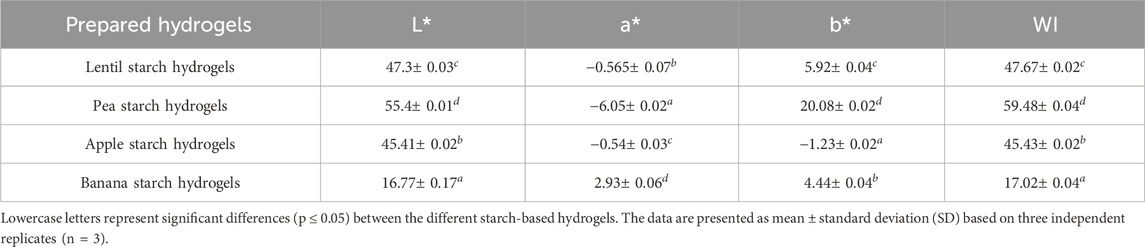
2.5.3 Colour measurements
The colour parameters in the CIELAB scale, namely the lightness (L*), redness (a*), and yellowness (b*), of the starch-based HPP hydrogels were detected using a colorimeter CR-400 (Konica Minolta Inc., Tokyo, Japan). Moreover, the whiteness index (WI) was evaluated using Equation 3, as reported by Kaur et al. (2013).
2.6 Statistical analysis
All the experiments as well as the analyses on the obtained starches and hydrogels were performed in triplicate and the results were reported as means ± standard deviations. Differences among mean values were analysed by one-way variance (ANOVA), by using SPSS 20 (SPSS IBM., Chicago, USA) statistical package. Tukey test was performed to determine statistically significant differences (p < 0.05).
3 Results and discussion
3.1 Characterization of the isolated starches
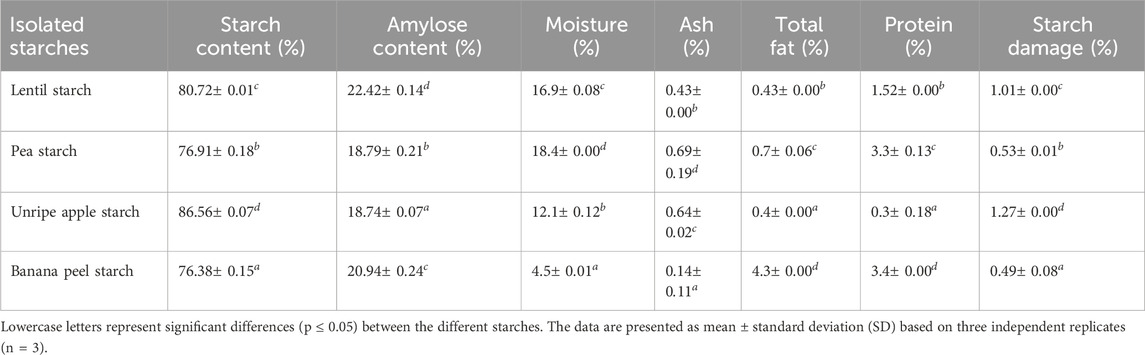
In Table 1 the chemical and physical characteristics of the starches isolated from different sources were reported. The starch isolated from unripe apples had the highest starch content, followed by starches isolated from lentil flour, pea flour, and banana peel. According to the amylose content, all isolated starches can be classified as normal starches (Koshenaj and Ferrari, 2024). Pea starch shows a higher protein content, as a higher amount of proteins was present in pea flour, while banana peel starch showed a higher total fat content. Low values of starch damage were detected, confirming that the starch isolation methods used were gentle and did not cause significant physical or mechanical damage to the starch granules. This is of utmost importance to maintain the functional properties of the starch unvaried. In fact, starch damage can affect water absorption, gelatinization, and enzymatic hydrolysis properties of starches. The low levels of damaged starch demonstrate that the starch retains its native granular structure and functional properties, making it suitable for various applications.
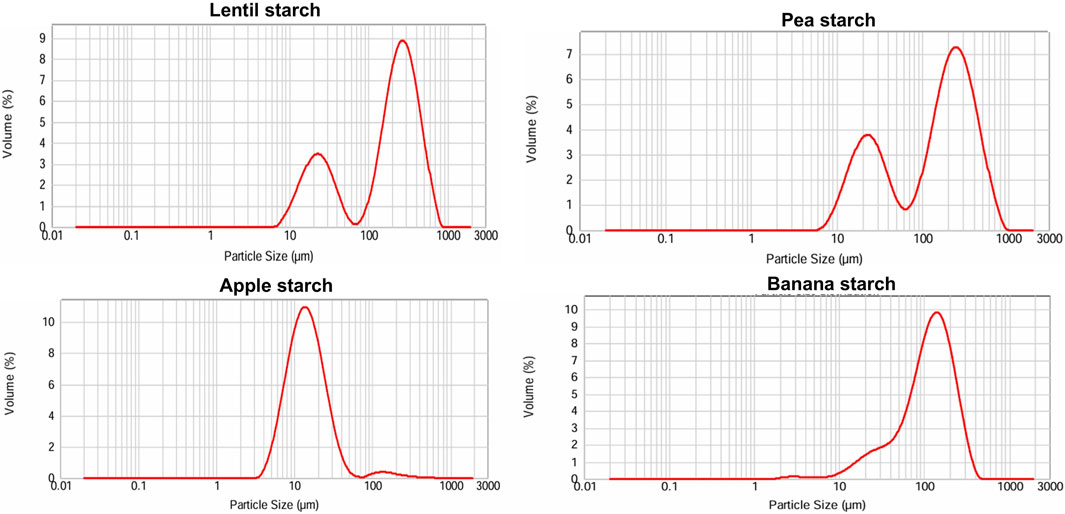
The particle size distributions of starch granules isolated from the various sources investigated in this work reveal distinct patterns (Figure 1). Starches obtained from lentil and pea flours both exhibit bimodal size distributions, indicating the presence of two different populations. For lentil starch, D10 was 18.92 µm, D50 was 208.48 µm, and D90 was 447.10 µm, while pea starch had slightly smaller particle size with a D10 of 17.90 µm, a D50 of 171.03 µm, and a D90 of 435.37 µm. In contrast, starches obtained from unripe apple and banana peel showed unimodal distributions, thus a more uniform particle size distribution. Apple starch has a smaller particle size with a D10 of 7.05 µm, a D50 of 13.85 µm, and a D90 of 28.78 µm, while banana peel starch featured larger particle size with a D10 of 31.97 µm, a D50 of 116.94 µm, and a D90 of 235.27 µm.
SEM images presented in Figure 2 showed that lentil and banana starch granules had a spherical (equant) shape. The pea starch granules showed high sphericity and apple starch granules had mostly smooth roughness shapes. Several small protuberances were observed on the surface of some of the granules which could be due to the presence of fibers, protein, fat, or other impurities remaining attached to the particle after the starch isolation process. Based on genotypes and growth conditions, starch granules from different botanical origins exhibit different sizes and shapes, which could affect their physicochemical and digestibility properties. Based on the findings of (Yang et al., 2022), indicating that smaller starch granules exhibit lower resistance to digestion, it can be concluded that the small granules found in apple starch might also show reduced digestion resistance. However, further research is needed to confirm this hypothesis and understand the specific characteristics of recovered starches.
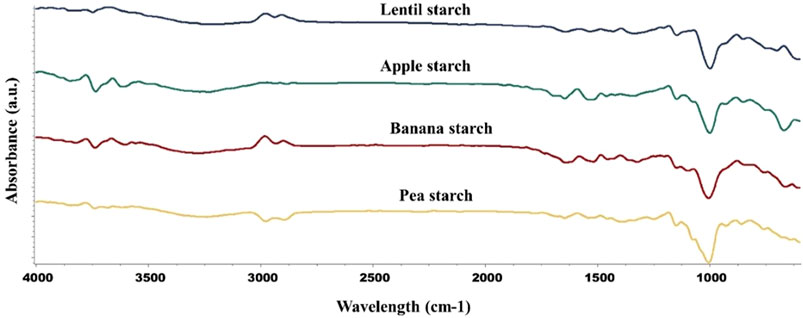
The FT-IR spectra were reported in Figure 3, determining the chemical bonds in isolated starches. Peaks around 1,000–1,150 cm−1 were detected in all samples, which are likely corresponding to the C-O and C-O-C stretching vibrations, indicating the presence of amylose and amylopectin in the samples (Yang et al., 2022). CH bending vibrations (850–900 cm−1) were also found in lentils, pea, and apple starches, indicating the CH groups in the glucose units (Adi Sulianto et al., 2024). The presence of peaks at 1,323, 1,335, 1,339, and 1,396 cm−1 in all starch samples were mainly associated with bending vibrations of CH2 groups and possibly O-H groups (Lucas-Aguirre et al., 2024). Amide bands (1,530–1,540 cm−1 and 1,640–1,650 cm−1) were present in all samples, suggesting the presence of protein residues or bound water molecules (Nandiyanto et al., 2019). C-H stretching vibrations (2,930–2,970 cm−1) were detected in all starch types and corresponded to the CH2 and CH3 groups in the starch molecules (Zhang et al., 2023). O-H stretching vibrations (3,200–3,850 cm−1) indicated hydrogen bonding and the presence of hydroxyl groups, which are common in polysaccharides due to the abundance of hydroxyl groups (Thanyapanich et al., 2021). The values and peaks observed in the FTIR spectra suggested that the isolated starches had high purity and amylose and amylopectin were the predominant compounds. The presence of consistent starch-related peaks across all samples supported this conclusion.
3.2 Determination of gel formation
Table 2 presents the results of gel formation obtained by microscopic analysis, efficiency index evaluation, and swelling power measurement.
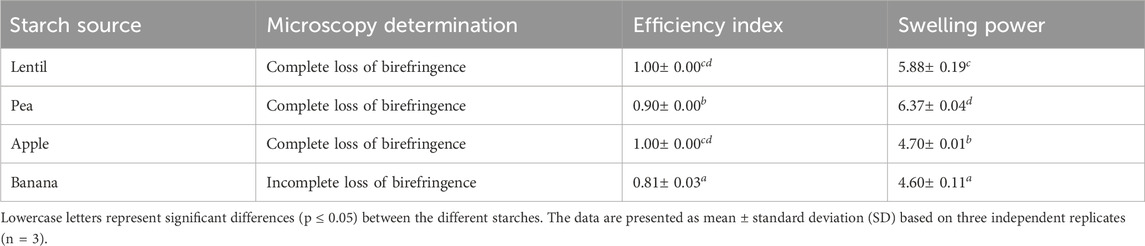
Table 2. Microscopy determination, efficiency index, and swelling power of obtained starch-based HPP hydrogels.
Starch granules show hilum-cantered birefringence, in the form of the typical “Maltese cross.” When starch granules undergo gelatinization, they lose their crystalline structure, leading to a loss of birefringence (Muñoz et al., 2015). Microscopic analyses used to determine the loss of birefringence in starch granules showed that lentil, pea, and apple starches had a complete loss of birefringence, accounting for a high gelatinization degree under the utilized HPP processing condition. Differences were observed in banana starch HPP hydrogels, where a residual birefringence was detected, indicating a lower degree of gelatinization and the presence of remaining crystalline structures. This suggests incomplete gel formation under the applied processing conditions. The swelling power, a crucial property related to the water-holding capacity of starch, was evaluated for all HPP hydrogels obtained to assess the extent of gel formation at the HPP processing conditions utilized in this work. Lentils and pea starches had higher swelling power compared to apple and banana starches. Moreover, a highly structured hydrogel was formed using lentil, pea, and apple starches, while a lower structured hydrogel was obtained with banana starch, and this finding was confirmed by the efficiency index values measured.
Based on the results obtained it can be concluded that banana starch showed a lower ability to form hydrogels under the HPP processing conditions used. The crystalline structure of banana peel starch is primarily of B-type (Kaur et al., 2022). These structures are more resistant to undergo gelation under pressure due to the fact that water was filling the channel in the cell unit of the crystallite stabilizing the structure instead of penetrating in the starch granules and causing their swelling as occurred with the other starches. The same behavior was observed for potato starch, which is also of B-type, that showed a high resistance to undergo pressure-induced gelation (Larrea-Wachtendor et al., 2019). In addition, as presented in Table 1, banana peel starch had a higher fat content, which can counteract water penetration and reduce the ability of the starch to form a gel. It is well known that amylose can form a helical structure under pressure treatments together with fat, creating some complexes that restrict the swelling of starch granules (Katopo et al., 2002).
Pea starch showed the highest swelling capacity, ability to form highly structured hydrogel and a complete loss of birefringence. The crystalline structure of pea starch is of C-type (Bogracheva et al., 1998), and is very suitable for gel formation under pressure. As shown in Table 1 the protein content of isolated pea starch is high. It is well known that the protein-starch interaction in heat-induced starch gelatinization results in an increased gelatinization temperature (Chakraborty et al., 2022). However, the pressure-induced starch gelatinization mechanism faces some differences. As shown in Table 1, pea starch granules showed low particle size thus the number of starch granules per Gram of starch in the solution is higher and they can absorb higher amounts of water. Thus, due to the low amount of residual water remaining in the solution, proteins might not be fully denatured. This hindered the capacity of proteins to act as fillers within the gel matrix and, consequently, did not affect the starch gelatinization extend (Sim and Moraru, 2020).
Figure 4 reports the pictures of the starch-based HPP hydrogels obtained. It can be observed that pea, lentil, and banana starch hydrogels showed a creamy appearance while apple hydrogels showed a gummy appearance. Despite having a lower particle size distribution, the smaller apple starch granules possess a higher total surface area, which enhances granule interactions and promotes tighter aggregation during gelation. This leads to the formation of a denser, less flexible network, resulting in the gummy consistency observed for the apple starch hydrogel.
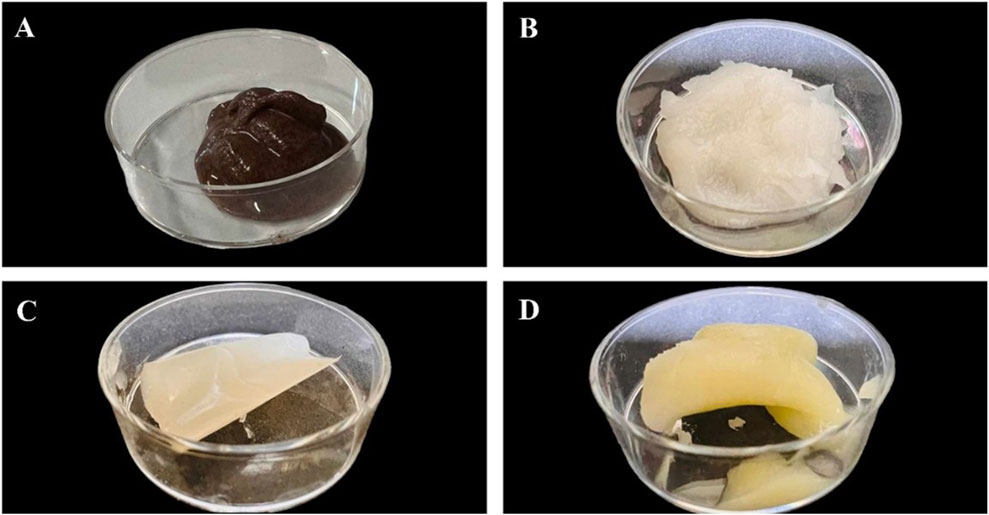
Figure 4. Picture of starch-based HPP hydrogel (A) banana starch hydrogel, (B) lentil starch hydrogel, (C) apple starch hydrogel, (D) pea starch hydrogel.
3.3 Characterization of starch-based HPP hydrogels
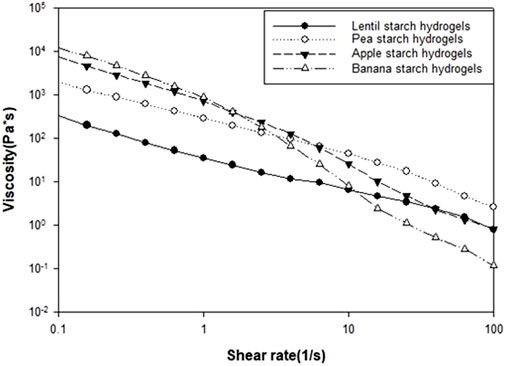
Flow curves were measured to determine the rheological properties of the hydrogels obtained in this study, particularly the correlation between the viscosity and the shear rate applied, as shown in Figure 5. As expected, the samples were characterized by a shear-thinning-Newtonian behaviour, with the viscosity values decreasing as the applied shear rate increased (Xie et al., 2009; Jiang et al., 2015).
Lentil and pea starch HPP hydrogels exhibited the lowest viscosity values at low shear rates, resulting in more spreadable gels. Their high swelling power and degree of gelatinization, combined with near-neutral pH (6.82 and 7.29, respectively), contributed to a uniform gel matrix with high spreadability. However, at a shear rate of 100 s-1, these hydrogels showed the highest viscosity values, highlighting the shear-dependent viscosity of lentil and pea starch hydrogels. In contrast, apple and banana HPP hydrogels exhibited the highest viscosity values, indicating the highest resistance to flow. The lower swelling capacity of apple and banana starch granules, leading to reduced water absorption, resulted in a denser and more viscous network. Moreover, the presence of lipids and proteins in apple and banana starches also plays a significant role in the rheological behaviour of the hydrogels formed (Bravo-Núñez et al., 2019; Liu et al., 2024). Lipids interact with starch molecules to form complexes that reinforce the gel network. These complexes create a more interconnected and stable structure, which increases the gel’s thickness and resistance to flow, resulting in higher viscosity. Also, proteins can interact with starch through hydrogen bonding and hydrophobic interactions, leading to a more rigid and less spreadable gel matrix.
The differences in the pH values are mostly related to the starch isolation method utilized. Banana and apple hydrogels had an acidic pH of 4.77 and 5.58, respectively, due to the presence of sulphurous acid, formed from the hydrolysis of sodium metabisulfite in water (Ilie-Mihai et al., 2022). The alkaline conditions used for the isolation of lentil and pea starch helped to remove or neutralize acidic impurities. Consequently, the hydrogels produced with these latter starches exhibited neutral pH.
The appearance of starch-based hydrogels was also evaluated through colour measurements and the results were presented in Table 3. Lentil, pea, and apple starch hydrogels exhibited high L* and WI index values, indicating a predominance of white and light components. In contrast, banana starch hydrogel exhibits lower L* and WI values, likely enzymatic and non-enzymatic reactions occurring during the starch isolation process.
As far as the values of the parameter a* are concerned, pea hydrogels showed the highest tendency towards greenness, followed by lentil and apple starch hydrogels. Conversely, banana starch hydrogels show a tendency to redness. Pea starch hydrogels showed the highest b* values, indicating a tendency to yellowness, followed by lentil and banana starch hydrogels. Apple starch hydrogels instead exhibited negative b* values, indicating their tendency to blueness.
The starch source and the presence of specific pigments and compounds inherent to the raw materials (Subagio et al., 1996; Delgado-Pelayo et al., 2014; Thi Hanh et al., 2016; Teterycz et al., 2020), such as chlorophylls in pea and lentil starches, anthocyanins and polyphenols in apple starch, and carotenoids, including lutein, β-carotene, and α-carotene in banana starch, influenced the colour tendencies of the starch hydrogels. Additionally, the isolation process had a strong impact on the final appearance of the hydrogels, particularly for starches susceptible to browning reactions.
4 Conclusion
The isolated starches analyzed in this study exhibited variations in morphological structure and physicochemical properties, which significantly influenced not only their ability to undergo gelation under pressure but also the properties of the hydrogels formed by high-pressure processing. Pea starch showed the highest capacity to form hydrogels, while banana starch had the lowest one, as demonstrated by the lowest swelling capacity, structural integrity, and loss of birefringence of the hydrogel formed. Additionally, lentil and pea starch hydrogels showed the lowest viscosities and highest spreadability. In contrast, banana and apple starch hydrogels exhibited higher viscosity values due to the rigidity of the network formed. The physical appearance of the hydrogels also varied, with banana, lentil, and pea starch hydrogels showing a creamy texture, and apple starch hydrogels exhibiting a gummy consistency. The L*, a*, and b* values obtained from color measurements for all hydrogels were influenced by the starch source, the isolation method utilized, and the presence of various pigments in the starch sources.
Considering these findings, it can be concluded that starches isolated from both conventional and non-conventional sources can be effectively used in the preparation of high-pressure processed (HPP) hydrogels. However, more work is needed to set up proper extraction and purification methods to recover starches, particularly for those obtained from discarded biomasses. Innovative technologies and green solvents could be proposed to optimize the processes for starch recovery starches, making their production both environmentally and economically sustainable. This is particularly important when the use of by-products and agro-industrial biomasses as a source of starch is likely to be exploited. To address these challenges a multidisciplinary approach is needed, combining scientific research, engineering, and economic analysis expertise.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
KK: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review and editing. GF: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Validation, Visualization, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was financially supported by ProdAl Scarl (own funds) and the University of Salerno (Fund FARB 2020 n. 300395FRB20 FERRA), Italy.
Acknowledgments
The authors would like to express their gratitude to Francesco Siano for his assistance with the chemical characterization (total fat and protein analysis), Luigi Esposito for his support with the physical characterization (DLS), and Mariarosa Scognamiglio for her help with the SEM analyses.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adi Sulianto, A., Adiyaksa, I. P., Wibisono, Y., Khan, E., Ivanov, A., Drannikov, A., et al. (2023). From fruit waste to hydrogels for agricultural applications. Clean. Technol. 6, 1–17. doi:10.3390/cleantechnol6010001
Ahmed, Q. F., and Mondal, I. H. (2022). An overview on starch - based sustainable hydrogels: potential applications and aspects. Springer US. doi:10.1007/s10924-021-02180-9
Bao, Z., Xian, C., Yuan, Q., Liu, G., and Wu, J. (2019). Natural polymer-based hydrogels with enhanced mechanical performances: preparation, structure, and property. Adv. Healthc. Mater 8, e1900670. doi:10.1002/adhm.201900670
Bello-Pérez, L. A., García-Suárez, F. J., Méndez-Montealvo, G., Do Nascimento, J. R. O., Lajolo, F. M., and Cordenunsi, B. R. (2006). Isolation and characterization of starch from seeds of Araucaria brasiliensis: a novel starch for application in food industry. Starch/Staerke 58, 283–291. doi:10.1002/star.200500455
Bogracheva, T. Y., Morris, V. J., Ring, S. G., and Hedley, C. L. (1998). The granular structure of C-type pea starch and its role in gelatinization. Biopolymers 45, 323–332. doi:10.1002/(sici)1097-0282(19980405)45:4<323::aid-bip6>3.0.co;2-n
Bravo-Núñez, Á., Garzón, R., Rosell, C. M., and Gómez, M. (2019). Evaluation of starch-protein interactions as a function of pH. Foods 8, 155. doi:10.3390/foods8050155
Carvalho, H. J. M., Barcia, M. T., and Schmiele, M. (2024). Non-conventional starches: properties and potential applications in food and non-food products. Macromol 4, 886–909. doi:10.3390/macromol4040052
Casadey, R., Broglia, M., Barbero, C., Criado, S., and Rivarola, C. (2020). Controlled release systems of natural phenolic antioxidants encapsulated inside biocompatible hydrogels. React. Funct. Polym. 156, 104729. doi:10.1016/j.reactfunctpolym.2020.104729
Catoira, M. C., Fusaro, L., Di Francesco, D., Ramella, M., and Boccafoschi, F. (2019). Overview of natural hydrogels for regenerative medicine applications. J. Mater Sci. Mater Med. 30, 115. doi:10.1007/s10856-019-6318-7
Chakraborty, I., N, P., Mal, S. S., Paul, U. C., Rahman, M. H., and Mazumder, N. (2022). An insight into the gelatinization properties influencing the modified starches used in food industry: a review. Food Bioproc Tech. 15, 1195–1223. doi:10.1007/s11947-022-02761-z
Dega, V., and Barbhai, M. D. (2023). Exploring the underutilized novel foods and starches for formulation of low glycemic therapeutic foods: a review. Front. Nutr. 10, 1162462. doi:10.3389/fnut.2023.1162462
Delgado-Pelayo, R., Gallardo-Guerrero, L., and Hornero-Méndez, D. (2014). Chlorophyll and carotenoid pigments in the peel and flesh of commercial apple fruit varieties. Food Res. Int. 65, 272–281. doi:10.1016/j.foodres.2014.03.025
Edgar, K. J., and Marks, J. A. (2020). “Green hydrogels based on starch: preparation methods for biomedical applications,” in ACS symposium series, (American chemical society), 173–196. doi:10.1021/bk-2020-1372.ch01
Hernández-Carmona, F., Morales-Matos, Y., Lambis-Miranda, H., and Pasqualino, J. (2017). Starch extraction potential from plantain peel wastes. J. Environ. Chem. Eng. 5, 4980–4985. doi:10.1016/j.jece.2017.09.034
Idrees, H., Zaidi, S. Z. J., Sabir, A., Khan, R. U., Zhang, X., and Hassan, S. U. (2020). A review of biodegradable natural polymer-based nanoparticles for drug delivery applications. Nanomaterials 10, 1970–22. doi:10.3390/nano10101970
Ilie-Mihai, R. M., Ion, B. C., and van Staden, J. (2022). Sodium metabisulfite in food and biological samples: a rapid and ultra-sensitive electrochemical detection method. Micromachines (Basel) 13, 1707. doi:10.3390/mi13101707
Ismail, H., Irani, M., and Ahmad, Z. (2013). Starch-based hydrogels: present status and applications. Int. J. Polym. Mater. Polym. Biomaterials 62, 411–420. doi:10.1080/00914037.2012.719141
Jiang, B., Li, W., Shen, Q., Hu, X., Wu, J., Jiang, B., et al. (2015). Effects of high hydrostatic pressure on rheological properties of rice starch. Int. J. Food Prop. 18, 1334–1344. doi:10.1080/10942912.2012.709209
Katopo, H., Song, Y., and Jane, J.-L. (2002). Effect and mechanism of ultrahigh hydrostatic pressure on the structure and properties of starches q. Available online at: www.elsevier.com/locate/carbpol.
Kaur, B., Venkatrao, K. B., Panesar, P. S., Chopra, H. K., and Anal, A. K. (2022). Optimization of ultrasound-assisted enzymatic extraction of resistant starch from green banana peels and its structural characterization. J. Food Sci. Technol. 59, 4663–4672. doi:10.1007/s13197-022-05546-6
Kaur, B. P., Kaushik, N., Rao, P. S., and Chauhan, O. P. (2013). Effect of high-pressure processing on physical, biochemical, and microbiological characteristics of black tiger shrimp (Penaeus monodon): high-pressure processing of shrimp. Food Bioproc Tech. 6, 1390–1400. doi:10.1007/s11947-012-0870-1
Koshenaj, K., and Ferrari, G. (2024). A comprehensive review on starch-based hydrogels: from tradition to innovation, opportunities, and drawbacks. Polym. (Basel) 16, 1991. doi:10.3390/polym16141991
Kringel, D. H., Dias, A. R. G., Zavareze, E. da R., and Gandra, E. A. (2020). Fruit wastes as promising sources of starch: extraction, properties, and applications. Starch/Staerke 72. doi:10.1002/star.201900200
Kusumayanti, H., Handayani, N. A., and Santosa, H. (2015). Swelling power and water solubility of cassava and sweet potatoes flour. Procedia Environ. Sci. 23, 164–167. doi:10.1016/j.proenv.2015.01.025
Larrea-Wachtendor, D., Tabilo-munizaga, G., and Ferrari, G. (2019). Potato starch hydrogels produced by high hydrostatic pressure (HHP): a first approach.
Liu, Q., Guan, H., Guo, Y., Wang, D., Yang, Y., Ji, H., et al. (2024). Structure and in vitro digestibility of amylose–lipid complexes formed by an extrusion-debranching-complexing strategy. Food Chem. 437, 137950. doi:10.1016/j.foodchem.2023.137950
Lucas-Aguirre, J. C., Quintero-Castaño, V. D., Beltrán-Bueno, M., and Rodríguez-García, M. E. (2024). Study of the changes on the physicochemical properties of isolated lentil starch during germination. Int. J. Biol. Macromol. 267, 131468. doi:10.1016/j.ijbiomac.2024.131468
Makroo, H. A., Naqash, S., Saxena, J., Sharma, S., Majid, D., and Dar, B. N. (2021). Recovery and characteristics of starches from unconventional sources and their potential applications: a review. Appl. Food Res. 1, 100001. doi:10.1016/j.afres.2021.100001
Maresca, P., and Ferrari, G. (2017). Modeling of the microbial inactivation by high hydrostatic pressure freezing. Food control. 73, 8–17. doi:10.1016/j.foodcont.2016.05.047
Muñoz, L. A., Pedreschi, F., Leiva, A., and Aguilera, J. M. (2015). Loss of birefringence and swelling behavior in native starch granules: microstructural and thermal properties. J. Food Eng. 152, 65–71. doi:10.1016/j.jfoodeng.2014.11.017
Nandiyanto, A. B. D., Oktiani, R., and Ragadhita, R. (2019). How to read and interpret ftir spectroscope of organic material. Indonesian J. Sci. Technol. 4, 97–118. doi:10.17509/ijost.v4i1.15806
Phiri, R., Mavinkere Rangappa, S., and Siengchin, S. (2024). Agro-waste for renewable and sustainable green production: a review. J. Clean. Prod. 434, 139989. doi:10.1016/j.jclepro.2023.139989
Pires, P. C., Mascarenhas-Melo, F., Pedrosa, K., Lopes, D., Lopes, J., Macário-Soares, A., et al. (2023). Polymer-based biomaterials for pharmaceutical and biomedical applications: a focus on topical drug administration. Eur. Polym. J. 187, 111868. doi:10.1016/j.eurpolymj.2023.111868
Qureshi, M. A., Nishat, N., Jadoun, S., and Ansari, M. Z. (2020). Polysaccharide based superabsorbent hydrogels and their methods of synthesis: a review. Carbohydr. Polym. Technol. Appl. 1, 100014. doi:10.1016/j.carpta.2020.100014
Sadh, P. K., Chawla, P., Kumar, S., Das, A., Kumar, R., Bains, A., et al. (2023). Recovery of agricultural waste biomass: a path for circular bioeconomy. Sci. Total Environ. 870, 161904. doi:10.1016/j.scitotenv.2023.161904
Samir, A., Ashour, F. H., Hakim, A. A. A., and Bassyouni, M. (2022). Recent advances in biodegradable polymers for sustainable applications. Npj Mater Degrad. 6, 68. doi:10.1038/s41529-022-00277-7
Santana, L., Angela, A., and Meireles, M. (2014). New starches are the trend for industry applications: a review. Food Public Health 4, 229–241. doi:10.5923/j.fph.20140405.04
Showkat, Q. A., Rather, J. A., Abida, J., Dar, B. N., Makroo, H. A., and Majid, D. (2021). Bioactive components, physicochemical and starch characteristics of different parts of lotus (Nelumbo nucifera Gaertn.) plant: a review. Int. J. Food Sci. Technol. 56, 2205–2214. doi:10.1111/ijfs.14863
Sim, S. Y. J., and Moraru, C. I. (2020). High-pressure processing of pea protein–starch mixed systems: effect of starch on structure formation. J. Food Process Eng. 43. doi:10.1111/jfpe.13352
Subagio, A., Morita, N., and Sawada, S. (1996). Carotenoids and their fatty-acid esters in banana peel. J. Nutr. Sci. Vitaminol. 42, 553–566. doi:10.3177/jnsv.42.553
Teterycz, D., Sobota, A., Zarzycki, P., and Latoch, A. (2020). Legume flour as a natural colouring component in pasta production. J. Food Sci. Technol. 57, 301–309. doi:10.1007/s13197-019-04061-5
Thanyapanich, N., Jimtaisong, A., and Rawdkuen, S. (2021). Functional properties of banana starch (Musa spp.) and its utilization in cosmetics. Molecules 26, 3637. doi:10.3390/molecules26123637
Thi Hanh, N., Vinh Hoang, N., and Thi Phuong Thao, P. (2016). Change of chlorophyl and vitamin C in green peas (Pisum sativum) during thermal treatments. Vietnam J. Agri. Sci. 14 (7), 1068–1074. Available online at: https://www.semanticscholar.org/paper/CHANGE-OF-CHLOROPHYLL-AND-VITAMIN-C-IN-GREEN-PEAS-Hanh-Hoang/cd7ffbaa37bfc561baa2e7d0bf5379c6a6e96233.
Xiao, C. (2013). Current advances of chemical and physical starch-based hydrogels. Starch/Staerke 65, 82–88. doi:10.1002/star.201200113
Xie, F., Yu, L., Su, B., Liu, P., Wang, J., Liu, H., et al. (2009). Rheological properties of starches with different amylose/amylopectin ratios. J. Cereal Sci. 49, 371–377. doi:10.1016/j.jcs.2009.01.002
Yang, M., Chang, L., Jiang, F., Zhao, N., Zheng, P., Simbo, J., et al. (2022). Structural, physicochemical and rheological properties of starches isolated from banana varieties (Musa spp.). Food Chem. X 16, 100473. doi:10.1016/j.fochx.2022.100473
Zhang, L., Apea-Bah, F. B., Chen, X., Hornung, P. S., Malunga, L. N., and Beta, T. (2023). The physicochemical and structural properties and in vitro digestibility of pea starch isolated from flour ground by milling and air classification. Food Chem. 419, 136086. doi:10.1016/j.foodchem.2023.136086
Keywords: starch, conventional sources, non-conventional sources, natural hydrogels, starch-based hydrogels, high-pressure processing
Citation: Koshenaj K and Ferrari G (2025) Production of HPP natural hydrogels from conventional and non-conventional starch sources. Front. Food Sci. Technol. 5:1629161. doi: 10.3389/frfst.2025.1629161
Received: 15 May 2025; Accepted: 09 July 2025;
Published: 17 July 2025.
Edited by:
Robert Sevenich, Technical University of Berlin, GermanyReviewed by:
Sunil C. K., National Institute of Food Technology, Entrepreneurship and Management, Thanjavur (NIFTEM-T), IndiaDennis Valino Cantre, University of the Philippines Los Baños, Philippines
Copyright © 2025 Koshenaj and Ferrari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giovanna Ferrari, Z2ZlcnJhcmlAdW5pc2EuaXQ=
 Katerina Koshenaj
Katerina Koshenaj Giovanna Ferrari
Giovanna Ferrari