- 1Embrapa Soja, Londrina, Paraná, Brazil
- 2Embrapa Genetic Resources and Biotechnology, Brasilia, Distrito Federal, Brazil
Soybean oil is a major source of vegetable oil for human consumption. Conventional soybean oil contains high levels of polyunsaturated fatty acids, and partial hydrogenation was historically used to improve oxidative stability. However, hydrogenation generates trans fatty acids, which are harmful to cardiovascular health and have therefore been banned or restricted by health regulatory agencies in several countries. Currently, techniques such as oil blending and interesterification are employed, although these approaches increase costs and highlight the need for improved soybean oil quality. Recent advances in biotechnology have enabled the development of genetically modified soybean cultivars aimed at improving the nutritional profile of soybean oil for both human consumption and food industry applications. Key advancements include the development of soybean cultivars with high-oleic acid content, which increases oxidative stability; high-linolenic acid content, which enhances nutritional and functional properties; and low-palmitic acid content, which reduces saturated fatty acid levels and contributes to healthier oils. This review explores a range of biotechnological strategies to optimize soybean oil quality, including genetic engineering, RNA interference, and gene editing, which are employed to modify key metabolic pathways responsible for oil biosynthesis. These innovations not only enhance the health benefits of soybean oil, such as reducing cardiovascular disease risk, but also improve its oxidative stability for high-temperature cooking and extended shelf life.
1 Introduction
Soybean (Glycine max [L.] Merr.) oil ranks as the second most produced vegetable oil worldwide, with an output of 60.7 million tons, second only to palm oil (Statista, 2024). The oil fraction of soybean seeds typically averages around 20%, although this value can vary depending on genotype and environmental conditions (Priolli et al., 2015). Soybean oil is used in the food, chemical, and biodiesel industries due to its versatility (Clemente and Cahoon, 2009).
Soybean oil is composed of five major fatty acids: palmitic, stearic, oleic, linoleic, and linolenic. Due to its fatty acid profile, soybean oil has low oxidative stability, which reduces shelf life and limits its use in applications that require long-term heat (Pham et al., 2010). Saturated fatty acids such as palmitic acid have been associated with adverse health outcomes, including insulin resistance, obesity, cardiovascular disease, and hyperlipidemia (Mozaffarian et al., 2009; Sears and Perry, 2015; Kaikkonen et al., 2021). Despite this, soybean oil is generally considered healthier than other fat sources, particularly those of animal origin (Messina et al., 2021). Likewise, polyunsaturated fatty acids (PUFAs) like linoleic acid (LA) and α-linolenic acid (ALA) are essential for human health and provide recognized benefits, including roles in cardiovascular protection and metabolic regulation (Yang et al., 2025).
Historically, partial hydrogenation was used to increase the shelf life of soybean oil-derived products, but this process results in the formation of trans fatty acids (TFA), up to 40% (Steele et al., 2024). However, the intake of TFAs has been strongly associated with coronary heart disease, systemic inflammation, high LDL cholesterol, and other metabolic disorders (Mozaffarian et al., 2009; Brouwer et al., 2010). In response to growing public health concerns, many countries have restricted or banned the use of partially hydrogenated oils in food production (Anvisa, 2019; WHO, 2024). The food industry has since sought alternatives to partial hydrogenation, including oil blending and interesterification, to improve the functionality of soybean oil without producing TFAs (Memon et al., 2024). While effective, these techniques introduce additional costs and processing complexity (Costales-Rodríguez et al., 2009).
Given these challenges, there is increasing demand for soybean varieties with improved oil profiles—particularly those with high-oleic acid content and lower PUFAs—thus eliminating the need for hydrogenation, oil blending, or interesterification (Wilkes and Bringe, 2015; Hudson and Hudson, 2021). Additionally, other alternatives have focused on the development of soybean cultivars with specific oil quality traits, such as high α-linolenic acid to improve nutritional properties and low palmitic acid content to produce oils with reduced saturated fat.
To achieve these improvements, a wide array of biotechnological tools has been explored, including genetic engineering, RNA interference (RNAi), and genome editing. These technologies have enabled the development of elite soybean lines with customized oil profiles. This review aims to synthesize the current biotechnological approaches for improving soybean oil composition and functionality, highlighting cutting-edge strategies.
2 Nutritional aspects of soybean oil
A balanced diet that includes fats and oils is essential for growth and the maintenance of physiological functions (Meijaard et al., 2022). In human nutrition, oil consumption plays multiple roles: it contributes to cell construction and maintenance, provides energy, aids in the absorption of fat-soluble vitamins, serves as a precursor for steroid hormones, and helps prevent various chronic diseases (Yao et al., 2020). Soybean oil is one of the most widely consumed vegetable oils worldwide, largely due to its affordability, availability, and versatility in culinary applications. China, the United States, and Brazil are the primary consumers, with average consumption of approximately 14.7, 12.29, and 8.45 million metric tons, respectively (Reportlinker, 2023).
Its widespread use is largely attributed not only to its availability but also to its chemical composition. The lipid fraction is particularly relevant due to its fatty acid composition, which is essential for its nutritional and functional quality, flavor, and oxidative stability (Fehr, 2007). Soybean oil is composed of various fatty acids, including saturated fatty acids (SFAs) such as approximately 11% palmitic acid (16:0) and 4% stearic acid (18:0); monounsaturated fatty acids (MUFAs), with about 25% oleic acid (18:1); and polyunsaturated fatty acids (PUFAs), with approximately 55% linoleic acid (18:2) and 8% linolenic acid (18:3).
Oils with more PUFAs and fewer MUFAs tend to have lower oxidative stability (Maszewska et al., 2018). The presence of multiple double bonds in linolenic and linoleic acids increases their reactivity with oxygen, making the oil more susceptible to oxidation (Liu and White, 1992). Oxidative stability is crucial to preserve oil quality during storage and cooking, particularly at high temperatures. During thermal processing (e.g., frying at 180 °C–250 °C), PUFA-rich oils undergo rapid degradation, leading to the accumulation of polar compounds and the formation of toxic products such as aldehydes and ketones (Liu and White, 1992; Bhardwaj et al., 2011).
Partial hydrogenation of soybean oil has historically been employed by the food industry to improve oxidative stability. This process extends the shelf life and is cost-effective; however, this process generates TFAs (Steele et al., 2024). TFAs are a type of unsaturated fatty acid characterized by at least one non-conjugated double bond in the trans configuration, which confers a straighter shape. This structural rigidity reduces their fluidity and leads to a higher melting point compared to their cis isomers (Bhardwaj et al., 2011). In human diets, the main source of TFAs is industrial hydrogenation of vegetable oils used in processed foods such as snacks, margarine, fast food, and cooking fats for deep frying (Pipoyan et al., 2021).
High TFA intake is widely recognized as harmful to health, being associated with increased low-density lipoprotein (LDL) cholesterol, reduced high-density lipoprotein (HDL) cholesterol, and greater risk of cardiovascular diseases (Mozaffarian et al., 2009; Brouwer et al., 2010). The World Health Organization (WHO) has issued warnings about the health impacts of TFA consumption (WHO, 2024). Since 2003, the WHO has recommended that TFAs account for less than 1% of total energy intake. Regulatory measures have been implemented globally to reduce or eliminate TFAs from the food industry.
Since 2021, the European Union has restricted industrial TFA content in fats to a maximum of 2% (Pipoyan et al., 2021). In the United States, the Food and Drug Administration (FDA) declared in 2015 that TFAs were no longer “generally recognized as safe” and banned their use in 2018 (Anderson, 2024). In Brazil, TFA labeling has been mandatory since 2003, and in 2019, the National Health Surveillance Agency (ANVISA) limited TFA content to 2% and required the elimination of partially hydrogenated oils by 2023 (Anvisa, 2019).
One common strategy to replace partial hydrogenation is oil blending, in which soybean oil is combined with more stable oils, such as palm or canola, to achieve desired textural properties and oxidative stability (Memon et al., 2024). Another widely applied approach is chemical or enzymatic interesterification, which rearranges the fatty acids on the glycerol backbone without creating trans fatty acids (Costales-Rodríguez et al., 2009). Interesterification modifies melting behavior and crystallization properties, enabling the production of margarines, shortenings, and bakery fats with improved performance (Berry, 2009). However, both blending and interesterification add complexity and cost to oil processing, reinforcing the need for soybean cultivars with inherently improved fatty acid profiles.
High-oleic oils (over 70% oleic acid content), oil blending, and interesterification are employed to replace partially hydrogenated vegetable oils (Zambelli, 2021). High-oleic oils are also considered healthier dietary components, offering improved oxidative stability and longer shelf life. One example is olive oil, a key element of the Mediterranean diet, which contains approximately 70%–80% oleic acid (Riolo et al., 2022). High intake of this fatty acid has been associated with anti-inflammatory effects, reduced cardiovascular risk, improved lipid profiles, and decreased abdominal fat (Owen et al., 2000; Tutunchi et al., 2020). Moreover, global dietary guidelines recommend that MUFAs comprise 10%–25% of total energy intake, depending on national policies (Schwingshackl et al., 2022).
In parallel, although PUFA-rich oils have lower oxidative stability, other strategies have focused on increasing their levels because of their nutritional benefits. Soybean oil contains high concentrations of linoleic acid (omega-6). High PUFA intake, particularly omega-3 (α-linolenic) and omega-6 (linoleic) fatty acids, is considered essential for a healthy diet and is associated with reduced cardiovascular risk and overall mortality (Chen et al., 2021). PUFAs have also been linked to the prevention of neurodegenerative diseases, inflammation, cancer, obesity, and LDL cholesterol reduction (Nogoy et al., 2020; Kotlyarov and Kotlyarova, 2022). Another study indicates that supplementation with omega-3 fatty acids significantly reduces adverse cardiovascular events (Huang et al., 2023).
In addition to MUFAs and PUFAs, oils with a low content of saturated fatty acids (SFAs), such as palmitic (16:0) and stearic acid (18:0), also offer nutritional advantages. Palmitic acid (16:0) is the predominant saturated fatty acid (SFA) in soybean oil, accounting for approximately 10%–11% of its composition. High intake of SFAs, particularly palmitic acid, has been associated with adverse health outcomes, including increased levels of low-density lipoprotein (LDL) cholesterol, insulin resistance, central adiposity, and elevated cardiovascular risk (Briggs et al., 2017; Kaikkonen et al., 2021). Reducing SFA content in edible oils is therefore considered beneficial for cardiovascular health. According to the U.S. Dietary Guidelines for Americans, the recommended intake of saturated fats should not exceed 10% or less of total caloric consumption (Astrup et al., 2021).
Soybean oil has many nutritional benefits but also presents some challenges from an industrial perspective. To meet the diverse demands of the consumer market, biotechnological strategies can be employed to develop soybean varieties with tailored characteristics, addressing the needs of increasingly health-conscious consumers.
3 Biotechnological tools applied to plant breeding
Biotechnology provides a set of tools that can accelerate and refine plant breeding. Several methods can be applied in this context, including transgenesis, RNA interference (RNAi), and gene editing (Figure 1A). The application of these technologies aims to increase crop productivity, improve nutritional quality, and confer tolerance to biotic and abiotic stresses, in addition to meeting consumer market demands and promoting agricultural sustainability.
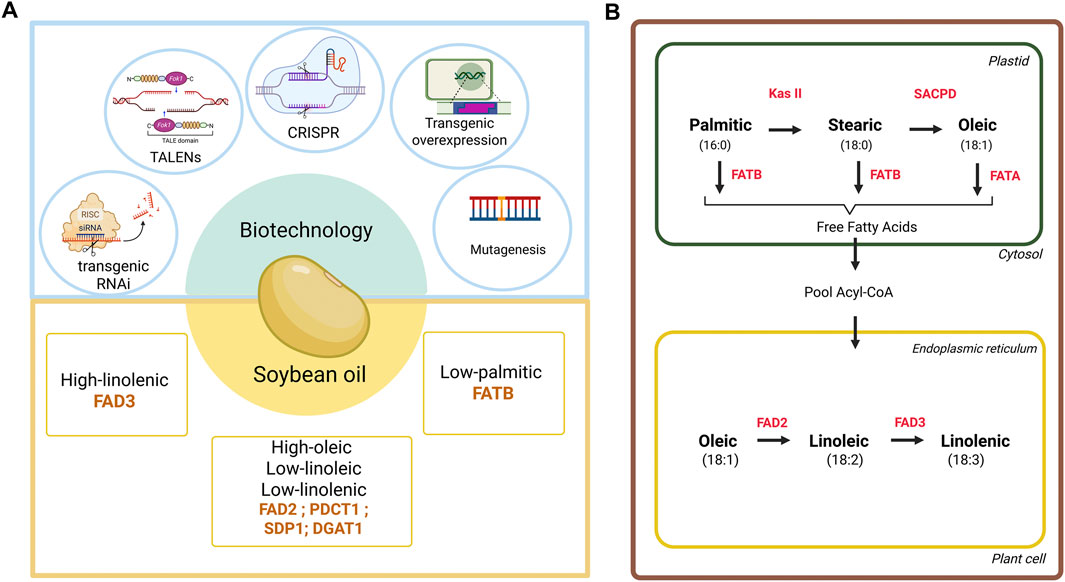
Figure 1. Biotechnological tools and metabolic pathways involved in fatty acid biosynthesis in soybean. (A) Schematic representation of biotechnological tools used to improve soybean oil profile, including transgenic RNA interference (RNAi), transcription activator-like effector nucleases (TALENs), CRISPR/Cas9, transgenic overexpression, and mutagenesis. These tools have been applied to regulate genes such as FAD2, FAD3, FATB, PDCT1, and SDP1, aiming to increase oleic acid content and reduce levels of saturated (palmitic) and polyunsaturated (linoleic, linolenic) fatty acids. (B) Fatty acid biosynthesis pathway in plant cells. In plastids, palmitic acid (16:0) is elongated to stearic (18:0) by KAS II, and subsequently SACPD the convertion to oleic acid (18:1). In endoplasmic reticulum, oleic acid can be desaturated linoleic (18:2) and linolenic acid (18:3) via FAD2 and FAD3, respectively. Genes: KAS II, 3-ketoacyl-ACP synthase II; SACPD, stearoyl-ACP desaturase; FATA/FATB, acyl-ACP thioesterases A/B; FAD2/FAD3, fatty acid desaturases 2/3. Created with BioRender.com.
Transgenic plants contain in their genome one or more genes that have been artificially inserted (Jhansi Rani and Usha, 2013). The inserted sequence, known as a transgene, may originate from a closely related species or from a completely different one (Benfey and Chua, 1989). The insertion of a transgene can confer a desirable trait that the target plant does not have, silence a gene from the same species, or even overexpress a gene of interest (Herrera-Estrella et al., 2005). Most transgenic plants used in agriculture involve the insertion of a cassette containing a gene encoding a foreign protein (Shewry et al., 2008). However, this tool can also be employed to reduce the expression of native proteins through RNA-mediated post-transcriptional gene silencing.
RNA interference (RNAi) is a defense mechanism against viruses and retrotransposons in eukaryotic organisms that mediates post-transcriptional gene silencing (Christie et al., 2011). This silencing process can be triggered by an exogenous double-stranded RNA (dsRNA) molecule or by the expression of a genetic construct that releases these molecules. Once inside the cell, the dsRNA is recognized and cleaved by the Dicer enzyme into 21 to 24 nucleotides small interfering RNAs (siRNAs). These siRNAs are incorporated into the RNA-induced silencing complex (RISC), whose main component is an Argonaute (AGO) protein (Cai et al., 2018). The RISC complex uses the guide strand of the siRNA to locate and cleave complementary messenger RNA (mRNA), which is subsequently degraded by 5′-3′ exoribonucleases and/or 3′-5′ exosome activity, leading to reduced mRNA levels and post-transcriptional gene silencing (Baulcombe, 2004; Zhang and Guo, 2017).
Another approach currently being used to develop soybean cultivars with improved oil traits is genome editing. In this context, gene editing technologies include Transcriptional Activator Effector Nucleases (TALENs) and Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) systems. These tools share two core components: a programmable endonuclease capable of inducing double-strand breaks (DSBs) in DNA and a targeting mechanism for sequence-specific recognition (Belhaj et al., 2013; Jiang et al., 2015).
TALEN technology introduced significant innovations by offering greater design flexibility and specificity (Boch et al., 2009). TALENs consist of a FokI nuclease domain fused to a customizable DNA-binding domain composed of transcription activator-like effectors (TALEs) derived from Xanthomonas spp. (Joung and Sander, 2013). The TALEs contain conserved 34-amino-acid repeats, with target specificity conferred by variable di-residues (RVDs) at positions 12 and 13 (Boch et al., 2009). The most common RVDs are NN, NG, HD, and NI. The TALEN system consists of two DNA-binding domains from TALEN proteins, each linked to FokI nuclease. FokI nuclease dimerizes to generate a double-strand break at the target DNA site, triggering the cellular repair machinery (Bhardwaj and Nain, 2021).
The CRISPR-Cas system is a defense mechanism of bacteria against viruses and has become a central tool in plant genome editing due to its precision and simplicity (Wiedenheft et al., 2012). Fragments of exogenous DNA are integrated into the CRISPR locus; these loci are associated with Cas genes that synthesize proteins with nuclease domains (Mojica et al., 2005). The CRISPR/Cas9 system, a class 2 type II from Streptococcus pyogenes, uses a guide RNA to direct the Cas9 endonuclease to the target sequence, promoting double-strand breaks near a PAM region (5′-NGG-3′) (Hsu et al., 2014; Jiang F and Doudna JA, 2017). Variants of the Cas12, Cas13, and Cas14 systems expand the applications for DNA and RNA editing (Liu et al., 2020), consolidating CRISPR as an essential platform in modern biotechnology.
As a biotechnological tool, CRISPR/Cas has been widely used since its first functional demonstration in eukaryotic cells (Jinek et al., 2012). Adapted for use in gene editing, the Cas endonuclease is guided by guide RNA (gRNA) that leads the enzyme to a genome-specific site, promoting cleavages that may be repaired by non-homologous end joining (NHEJ) or homologous recombination (HR) machinery (Jiang et al., 2015; Li et al., 2024). The technique allows for knockouts, insertions, gene regulation, and new methods such as base editing and prime editing (Anzalone et al., 2020). Advances in CRISPR-Cas genome editing have expanded the molecular tools for plant genome engineering to generate new traits in crop plants.
4 Biotechnological strategies to improve the quality of soybean oil for human consumption
4.1 High-oleic, low-linolenic and low-linoleic soybean oil
High-oleic soybean is a strategic biotechnological advancement for the food industry. Oleic acid provides several benefits for cardiovascular health and also confers greater oxidative stability. In this sense, biotechnology approaches have been employed to increase oleic acid in soybean oil. Key genetic modifications and their effects on fatty acid profiles in these soybean lines are summarized in Table 1.
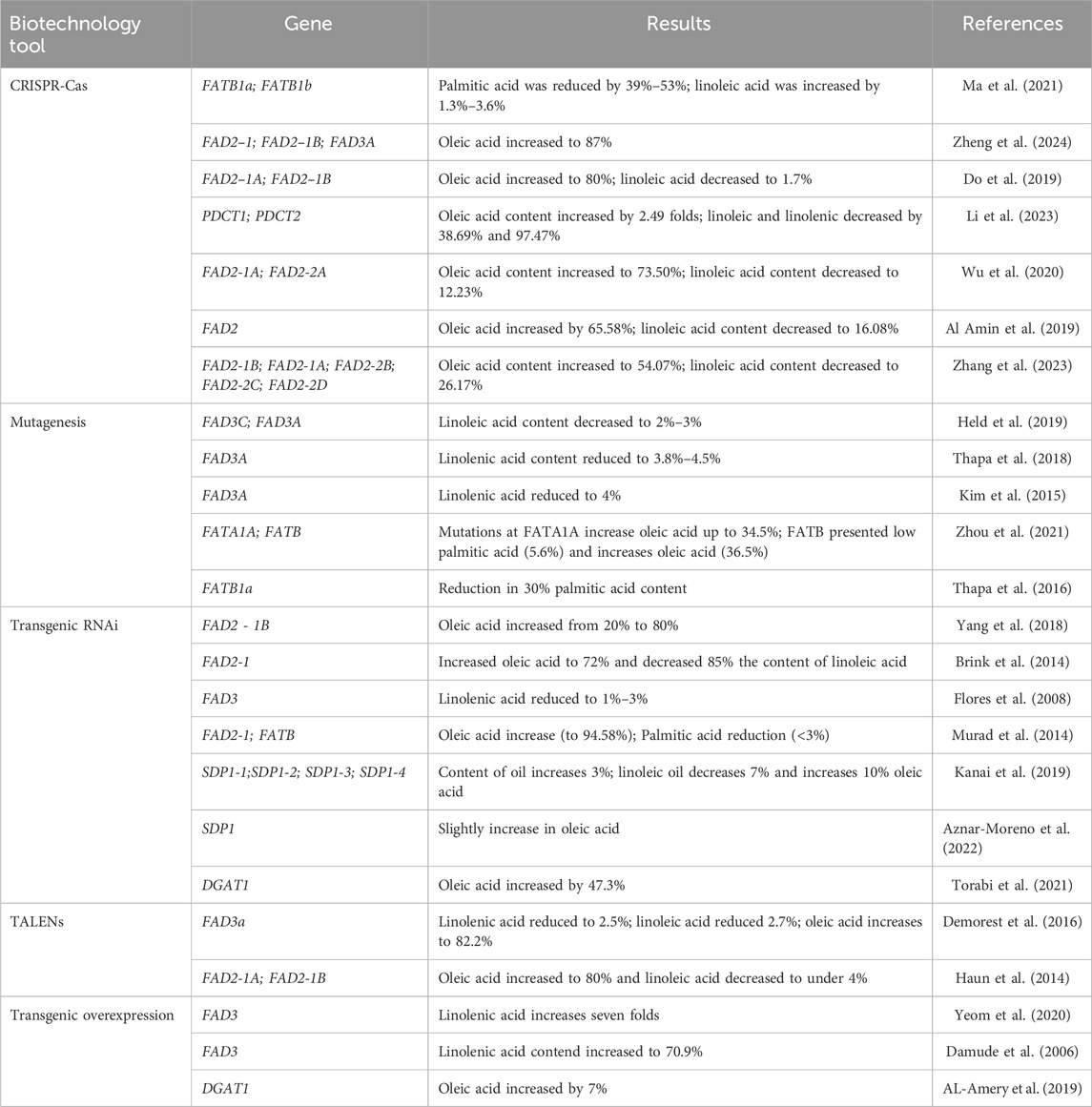
Table 1. Summary of gene targets and biotechnological tools used to modify the fatty acid composition of soybean oil.
In soybean, oleic acid levels are regulated by the fatty acid desaturase 2 enzyme (FAD2) localized in the endoplasmic reticulum. It is the main enzyme responsible for the biosynthesis of polyunsaturated fatty acids (PUFAs) in non-photosynthetic tissues, such as developing seeds of oilseed crops (Zhang et al., 2012). FAD2 introduces a double bond at the delta-12 (omega-6) position, catalyzing the conversion of oleic acid to linoleic acid, which is subsequently desaturated to linolenic acid by the FAD3 enzyme (Figure 1B). In soybean, the FAD2 gene is present in two copies: FAD2-1 and FAD2-2. Several variants for these copies have already been reported, including FAD2-1A, FAD2-1B, FAD2-2A, FAD2-2B, FAD2-2C, FAD2-2D and FAD2-2F, which differ in chromosomal location and expression patterns (Dar et al., 2017; Lakhssassi et al., 2017).
The first commercial soybean variety with high-oleic profile was Plenish®, a transgenic RNAi soybean developed by DuPont Pioneer contain approximately 75% oleic acid due to the insertion of a fragment of the FAD2-1 gene under the control of a seed-specific promoter (Brink et al., 2014). This trait was combined with mutations associated with low linolenic content, resulting in a product with less than 2% linolenic acid (EFSA GMO Panel, 2016). Another high-oleic soybean variety is Vistive® Gold, developed by Monsanto/Bayer, in which the FAD2 gene was silenced through the insertion of an RNAi cassette expressing dsRNA molecules (Eck, 2011; Knowlton, 2022). In addition, a Brazilian group developed a soybean cultivar using RNAi-mediated silencing of FAD2-1, which led to a significant increase in oleic acid, reaching levels up to 94.5% (Murad et al., 2014).
FAD2-1A and FAD2-1B genes were also modified using TALENS by the Calyxt team. These alterations increased the oleic content to 80% in soybean oil (Haun et al., 2014). Subsequently, the FAD3A gene with mutations was stacked in the same cultivar, further improving the fatty acid profile, resulting in the development of the Calyno variety (Demorest et al., 2016). Calyno oil was the first gene-edited food product to be released directly to consumers, due to the deregulation of products developed using technologies that are not considered genetically modified organisms (GMO) (Knowlton, 2022).
New plants derived from gene editing have been subject to regulatory review in various countries. In most cases, small modifications derived from NHEJ process after cleavage by proteins such as TALENs or CRISPR-associated proteins have been considered non-GMO by regulatory agencies and have not required the same oversight as transgenic crops (Entine et al., 2021). This represents an advantage of using CRISPR-Cas to generate new commercial soybean varieties.
In addition to the varieties currently available on the market, CRISPR-Cas9—the most recent molecular tool for gene editing—has also emerged as a promising approach for developing high-oleic soybean varieties. Several studies have demonstrated that silencing genes from the FAD family improves soybean oil quality. The application of CRISPR-Cas9 has led to the silencing of FAD2–1A and FAD2–1B, resulting in a significant increase in oleic acid concentration to over 80%, while linoleic acid content in the seed decreased to approximately 1.7% (Do et al., 2019). Significant modulations have also been achieved through CRISPR-Cas, with reports showing oleic acid content reaching 65.58% and linoleic acid content reduced to 16.08% (Al Amin et al., 2019). Mutants with simultaneous silencing of FAD2-1A and FAD2-2A showed an increase in oleic acid content of up to 73.5%, while linoleic acid levels dropped to 12% (Wu et al., 2020). The simultaneous silencing of FAD2-1B, FAD2-1A, FAD2-2B, FAD2-2C, and FAD2-2D led to an increase in oleic acid from 18.58% to 54.07% and a reduction in linoleic acid from 57.19% to 26.17% (Zhang et al., 2023). Furthermore, the knockout of FAD2–1A, FAD2–1B, and FAD3A genes using CRISPR-Cas9 resulted in an oleic acid content increase to approximately 87% (Zheng et al., 2024).
In soybean, the omega-3 fatty acid desaturase gene (FAD3) catalyzes the conversion of linoleic acid into linolenic acid by introducing a third double bond into the carbon chain (Combs and Bilyeu, 2019). In this species, three FAD3 genes have been described: FAD3A, FAD3B, and FAD3C (Bilyeu et al., 2011). Genetic modifications through mutagenesis have successfully produced soybean lines with reduced linolenic acid content. Mutants for the FAD3A gene have shown reductions in linolenic acid to 3%–4% in soybean seeds (Kim et al., 2015; Thapa et al., 2018). Similar results were observed in another study, in which lines carrying mutant alleles of both FAD3C and FAD3A exhibited linolenic acid levels between 2% and 3% (Held et al., 2019). Additionally, the use of an RNAi cassette expressing siRNAs targeting the FAD3 gene led to extremely low linolenic acid production, with some events producing as little as 1% (Flores et al., 2008). Linolenic acid levels below 3% are a key target for improving oil functionality in the marketplace (Combs and Bilyeu, 2019).
Modifications in other genes involved in the fatty acid biosynthetic pathway have also yielded promising results in producing soybeans with an optimized fatty acid profile. The SDP1 gene (SUGAR DEPENDENT1), which is involved in TAG degradation, has been targeted through genetic manipulation to increase oleic acid content. RNAi-mediated silencing of SDP1 led to elevated oleic acid levels and reduced linoleic acid content (Kanai et al., 2019). In another study, RNAi-mediated silencing of SDP1 slightly increased oleic acid (18:1) levels and led to up to a 20% increase in total seed oil content without affecting protein levels (Aznar-Moreno et al., 2022).
The diacylglycerol acyltransferase type I (DGAT1) catalyzes the esterification of diacylglycerol with fatty acids in the final step of TAG biosynthesis. Interestingly, gene silencing of DGAT1 using trans-acting siRNA decreased total seed oil content by 8.3% but increased oleic acid levels by 47.3% (Torabi et al., 2021). On the other hand, overexpression of DGAT1 gene from Vernonia galamensis in soybean resulted in an approximately 4% increase in total seed oil and up to a 7% increase in oleic acid content (AL-Amery et al., 2019). The knockout of PDCT gene (diacylglycerol cholinephosphotransferase) using CRISPR-Cas9, resulted in increased levels of monounsaturated fatty acids, such as oleic acid, and a substantial reduction in linoleic and linolenic acids (Li et al., 2023). These studies reveal that additional genes can be explored for genetic modification to further improve soybean oil quality.
Many genetic approaches and technologies have been used to develop high-oleic, low-linoleic, and low-linolenic soybean varieties, bringing advantages not only to consumers but also to farmers. A notable example is the Plenish® soybean cultivar. Industry offers attractive premiums to farmers who grow Plenish® high-oleic soybean, ranging from US$ 1 to US$ 2 per bushel, due to their superior oil characteristics (Pioneer, 2021). In 2024, it is estimated that 1.6 million acres of this soybean variety were planted in the United States, with projections reaching 2.7 million acres by 2027 (Anderson, 2024). Although maintaining the identity of these varieties requires additional care, such as equipment cleaning and record-keeping, the economic and functional advantages have supported the expansion of their cultivation.
4.2 Low-palmitic soybean oil
Saturated fatty acids such as palmitic acid are associated with increased cardiovascular health risks (Briggs et al., 2017; Kaikkonen et al., 2021). According to health guidelines, the desirable level of saturated fatty acids in soybean oil is around 7% (Fehr, 2007; Hudson and Hudson, 2021). Two classical loci have been associated with reduced palmitic acid content in soybean: Fap1 and Fap3 (Hudson and Hudson, 2021). A mapping study revealed that the fap1 locus is linked to decreased palmitic acid levels through a mutation in the ketoacyl-ACP synthase IIIA (KASIIIA) gene. This mutation disrupts proper transcript splicing, resulting in increased gene expression (Cardinal et al., 2014). The fap3 locus contains the FATB1A gene (Cardinal et al., 2007), and several biotechnological approaches targeting this gene have reported decreased palmitic acid content. Mutations in these two genes have shown additive effects, reducing palmitic acid content to approximately 4% (Cardinal et al., 2014).
In plastids, two distinct acyl-ACP thioesterases (enzymes encoded by the paralogous genes FATA and FATB) hydrolyze acyl-ACP to produce free fatty acids (FFAs) (Zhou et al., 2021; Liao et al., 2024). These enzymes catalyze the release of fatty acids into the cytoplasm, where they are converted into acyl-CoA derivatives (Figure 1B). FATA predominantly hydrolyzes 18:1 (oleic acid) and FATB hydrolyzes saturated fatty acyl-ACPs 16:0 (palmitic acid) and 18:0 (stearic acid) (Zhou et al., 2021). In soybean, FATB1a and FATB1b alleles have been identified as having greater influence on reducing palmitic acid levels (Bachleda et al., 2016; Ma et al., 2021). As key enzymes in fatty acid biosynthesis, FAT enzymes are relevant targets for biotechnological strategies aimed at reducing palmitic acid content in the lipid profile.
Studies have shown that FATB1 mutations result in significantly lower palmitic acid levels in soybean seeds, positively impacting the nutritional profile of the oil. Using TILLING-by-Sequencing, several alleles - FATA1A, FATB1A, FATB1B, FATB2A, and FATB2B- were discovered (Zhou et al., 2021). Mutations in FATA1A were associated with elevated oleic acid content (up to 34.5%), while mutations in FATB1B led to reductions in palmitic acid of up to 50% (Zhou et al., 2021). Marker-assisted selection (MAS) can support breeding programs by accelerating selection and reducing costs. Some studies using MAS have linked the FATB1a gene to reduced palmitic acid content in soybean seeds (Bachleda et al., 2016). Through mutagenesis, soybean lines with mutations in FATB1A showed up to 30% reductions in palmitic acid content (Thapa et al., 2016).
RNAi-mediated silencing of the FATB gene in soybean resulted in a reduction of palmitic acid to 3% in seed oil content (Murad et al., 2014). Vistive® Gold variety, developed by Monsanto/Bayer, combines high-oleic levels with reduced SFA content due to the silencing of the FATB gene via RNAi (Eck, 2011; Knowlton, 2022).
More recently, the CRISPR/Cas9 tool has been used to edit genes in the FATB gene family, such as FATB1a, FATB1b, and FATA1. Knockout of FATB1a and FATB1b led to reductions in palmitic acid by up to 53% and stearic acid by up to 37%, with no significant changes in unsaturated fatty acids, although oleic acid content increased by 1%–3% (Ma et al., 2021). CRISPR-Cas9-generated mutants for the FATA1 gene showed increased oleic acid content, but plants exhibited growth defects during early developmental stages. Conversely, overexpression of FATA resulted in a 5.7% increase in fatty acid content in soybean leaves and a 26.9% and 23.2% increase in seed yield and seed fatty acid content, respectively (Liao et al., 2024).
4.3 High-linolenic soybean oil
Linolenic acid is an omega-3 fatty acid essential to human health and can only be obtained through the diet. Omega-3 fatty acids are well known for their role in preventing cardiovascular diseases (Chen et al., 2021). However, the linolenic acid content in conventional soybean oil is relatively low, around 8% (Fehr, 2007).
The conversion of linoleic acid (18:2) to linolenic acid (18:3) is catalyzed by the omega-3 fatty acid desaturase gene (FAD3) in the endoplasmic reticulum. In the soybean genome, three genes encode desaturase isoforms: FAD3A, FAD3B, and FAD3C (Derbyshire et al., 2023). A quantitative trait locus (QTL) associated with linolenic acid content was identified, marked by a single nucleotide polymorphism (SNP) located upstream of the FAD3 gene. This QTL explained 7% of the phenotypic variance in seed linolenic acid levels (Zhang et al., 2018), suggesting that further investigation in related genes could provide new insights into the control of this trait.
Only a few studies have explored biotechnological approaches to increase linolenic acid content in soybean. Transgenic soybean plants expressing the FAD3 gene from lesquerella (Physaria fendleri) showed a substantial enhancement in linolenic acid levels (Yeom et al., 2020). In some transformed lines, levels reached up to 52.4% of total seed oil, representing a sevenfold increase compared to non-transformed controls. In another transgenic approach, the FAD3 gene derived from the fungus F. monilifore was inserted into soybean, leading to an increase in linolenic content from 10.9% to 70.9% (Damude et al., 2006). These results demonstrate the potential of biotechnological strategies to develop high-omega-3 soybean varieties for functional food markets.
5 Conclusion and perspectives
The quality of soybean oil is a decisive factor in enhancing the product’s value in global markets. Modern biotechnology is crucial to the development of new cultivars, particularly through genome editing using CRISPR/Cas. This technology enables precise and simultaneous modifications at multiple loci, while in some cases minimizing concerns typically associated with genetically modified organisms (GMOs). The development of genome-edited soybeans can employ multiplex strategies to target multiple key genes in lipid metabolism simultaneously, thereby accelerating the generation of oil profiles optimized for different applications.
Within this context, the development of non-GMO soybean cultivars with high oleic acid content to be used by the main soybean-producing countries emerges as a strategic alternative to meet the demands of the food and biodiesel industries, as well as those of the end consumer. Furthermore, soybean cultivars with specific traits, such as high α-linolenic acid content - an essential omega-3 fatty acid - could serve the functional food and dietary supplement markets. Likewise, low-palmitic soybean oil cultivars, with reduced levels of saturated fatty acids, aim to address the demand for healthier food products. Altogether, these targeted modifications illustrate the diverse possibilities for improving soybean oil quality and creating differentiated products that increase the added value of soybean oil.
Biotechnology can also improve gene discovery related to the oil biosynthetic pathway. Although several genes associated with the lipid metabolic pathway in soybean are already known, advances in omics-based approaches–such as genomics, transcriptomics, metabolomics, epigenomics, and lipidomics–and the integration of these datasets may provide new insights into fatty acid biosynthesis, as well as reveal crucial gene interactions. Such knowledge will be essential for soybean genetic improvement.
At the same time, the food industry has been increasingly demanding oils with enhanced processing characteristics, both for industrial applications and for the consumer market. The major soybean producers worldwide–Brazil, the United States, and Argentina–must remain attentive to shifts in industry and consumer preferences. While the United States has already developed and commercialized soybean cultivars with improved lipid traits, Brazil, as the world’s leading soybean producer, must advance in the developing of the new technologies. This advancement is essential not only to maintain its leadership but also to assume a prominent role in supplying healthier food products. The lack of nationally developed cultivars may otherwise reduce Brazilian soybean to a commodity with lower added value, targeting less demanding markets.
Looking ahead, the soybean production chain must remain aligned with the evolving requirements of the consumer market. Soybean oil with enhanced functional properties and specific attributes tailored to diverse industrial and nutritional demands has the potential to consolidate soybean not merely as a commodity, but as a versatile platform for the production of specialty oils.
Author contributions
JR: Writing – original draft, Methodology; Conceptualization; Writing – review and editing. DRM: Writing – original draft. AMVS: Writing – original draft. EMGS: Writing – original draft. ELR: Funding acquisition, Writing – review and editing; ALN: Funding acquisition, Writing – review and editing; LMM-H: Funding acquisition, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. We acknowledge funding and support from Embrapa Soybean, Embrapa Genetic Resources and Biotechnology, and National Institute of S&T in Engineering Systems Biology, EngBio (processo: 408411/2024-4).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. The author(s) declare that Generative AI (ChatGPT, OpenAI, GPT-4, 2025) was used to assist in the preparation of this manuscript, including text refinement and language editing. All content was critically reviewed and approved by the authors.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Al Amin, N., Ahmad, N., Wu, N., Pu, X., Ma, T., Du, Y., et al. (2019). CRISPR-Cas9 mediated targeted disruption of FAD2-2 microsomal omega-6 desaturase in soybean (Glycine max.L). BMC Biotechnol. 19, 9–10. doi:10.1186/s12896-019-0501-2
AL-Amery, M., Downie, B., DeBolt, S., Crocker, M., Urschel, K., Goff, B., et al. (2019). Proximate composition of enhanced DGAT high oil, high protein soybeans. Biocatal. Agric. Biotechnol. 21, 101303. doi:10.1016/J.BCAB.2019.101303
Anderson, E. (2024). High oleic soybeans—still worth considering? Available online at: https://www.canr.msu.edu/news/high-oleic-soybeans-still-worth-considering.
Anvisa (2019). Resolução da Diretoria Colegiada No 332. Available online at: https://www.in.gov.br/en/web/dou/-/resolucao-rdc-n-332-de-23-de-dezembro-de-2019-235332281.
Anzalone, A. V., Koblan, L. W., and Liu, D. R. (2020). Genome editing with CRISPR-Cas nucleases, base editors, transposases and prime editors. Nat. Biotechnol. 38, 824–844. doi:10.1038/s41587-020-0561-9
Astrup, A., Teicholz, N., Magkos, F., Bier, D. M., Thomas Brenna, J., King, J. C., et al. (2021). Dietary saturated fats and health: are the u.s. guidelines evidence-based? Nutrients 13, 3305. doi:10.3390/nu13103305
Aznar-Moreno, J. A., Mukherjee, T., Morley, S. A., Duressa, D., Kambhampati, S., Chu, K. L., et al. (2022). Suppression of SDP1 improves soybean seed composition by increasing oil and reducing undigestible oligosaccharides. Front. Plant Sci. 13, 863254. doi:10.3389/fpls.2022.863254
Bachleda, N., Pham, A., and Li, Z. (2016). Identifying FATB1a deletion that causes reduced palmitic acid content in soybean N87-2122-4 to develop a functional marker for marker-assisted selection. Mol. Breed. 36, 45–49. doi:10.1007/s11032-016-0468-9
Belhaj, K., Chaparro-Garcia, A., Kamoun, S., and Nekrasov, V. (2013). Plant genome editing made easy: targeted mutagenesis in model and crop plants using the CRISPR/Cas system. Plant Methods 9, 39–10. doi:10.1186/1746-4811-9-39
Benfey, P. N., and Chua, N.-H. (1989). Regulated genes in transgenic plants. Science 244, 174–181. doi:10.1126/science.244.4901.174
Berry, S. E. E. (2009). Triacylglycerol structure and interesterification of palmitic and stearic acid-rich fats: an overview and implications for cardiovascular disease. Nutr. Res. Rev. 22, 3–17. doi:10.1017/S0954422409369267
Bhardwaj, A., and Nain, V. (2021). TALENs—an indispensable tool in the era of CRISPR: a mini review. J. Genet. Eng. Biotechnol. 19, 125. doi:10.1186/S43141-021-00225-Z
Bhardwaj, S., Passi, S. J., and Misra, A. (2011). Overview of trans fatty acids: biochemistry and health effects. Diabetes Metab. Syndr. Clin. Res. Rev. 5, 161–164. doi:10.1016/J.DSX.2012.03.002
Bilyeu, K., Gillman, J. D., and LeRoy, A. R. (2011). Novel FAD3 mutant allele combinations produce soybeans containing 1% linolenic acid in the seed oil. Crop Sci. 51, 259–264. doi:10.2135/cropsci2010.01.0044
Boch, J., Scholze, H., Schornack, S., Landgraf, A., Hahn, S., Kay, S., et al. (2009). Breaking the code of DNA binding specificity of TAL-type III effectors. Science 326, 1509–1512. doi:10.1126/science.1178811
Briggs, M. A., Petersen, K. S., and Kris-Etherton, P. M. (2017). Saturated fatty acids and cardiovascular disease: replacements for saturated fat to reduce cardiovascular risk. Healthc 5, 29. doi:10.3390/HEALTHCARE5020029
Brink, K., Chui, C. F., Cressman, R. F., Garcia, P., Henderson, N., Hong, B., et al. (2014). Molecular characterization, compositional analysis, and germination evaluation of a high-oleic soybean generated by the suppression of FAD2-1 expression. Crop Sci. 54, 2160–2174. doi:10.2135/CROPSCI2012.06.0377
Brouwer, I. A., Wanders, A. J., and Katan, M. B. (2010). Effect of animal and industrial trans fatty acids on HDL and LDL cholesterol levels in humans - a quantitative review. PLoS One 5, e9434–10. doi:10.1371/journal.pone.0009434
Cai, Q., He, B., Kogel, K. H., and Jin, H. (2018). Cross-kingdom RNA trafficking and environmental RNAi — nature’s blueprint for modern crop protection strategies. Curr. Opin. Microbiol. 46, 58–64. doi:10.1016/j.mib.2018.02.003
Cardinal, A. J., Burton, J. W., Camacho-Roger, A. M., Yang, J. H., Wilson, R. F., and Dewey, R. E. (2007). Molecular analysis of soybean lines with low palmitic acid content in the seed oil. Crop Sci. 47, 304–310. doi:10.2135/cropsci2006.04.0272
Cardinal, A. J., Whetten, R., Wang, S., Auclair, J., Hyten, D., Cregan, P., et al. (2014). Mapping the low palmitate fap1 mutation and validation of its effects in soybean oil and agronomic traits in three soybean populations. Theor. Appl. Genet. 127, 97–111. doi:10.1007/s00122-013-2204-8
Chen, L. H., Hu, Q., Li, G., Zhang, L., Qin, L. Q., Zuo, H., et al. (2021). Dietary intake and biomarkers of α-Linolenic acid and mortality: a meta-analysis of prospective cohort studies. Front. Nutr. 8, 743852–167. doi:10.3389/fnut.2021.743852
Christie, M., Croft, L. J., and Carroll, B. J. (2011). Intron splicing suppresses RNA silencing in arabidopsis. Plant J. 68, 159–167. doi:10.1111/j.1365-313X.2011.04676.x
Clemente, T. E., and Cahoon, E. B. (2009). Soybean oil: genetic approaches for modification of functionality and total content. Plant Physiol. 151, 1030–1040. doi:10.1104/pp.109.146282
Combs, R., and Bilyeu, K. (2019). Novel alleles of FAD2-1A induce high levels of oleic acid in soybean oil. Mol. Breed. 39, 79–11. doi:10.1007/s11032-019-0972-9
Costales-Rodríguez, R., Gibon, V., Verhé, R., and De Greyt, W. (2009). Chemical and enzymatic interesterification of a blend of palm stearin: soybean oil for low trans-margarine formulation. J. Am. Oil Chem. Soc. 86, 681–697. doi:10.1007/S11746-009-1395-2
Damude, H. G., Zhang, H., Farrall, L., Ripp, K. G., Tomb, J. F., Hollerbach, D., et al. (2006). Identification of bifunctional delta12/omega3 fatty acid desaturases for improving the ratio of omega3 to omega6 fatty acids in microbes and plants. Proc. Natl. Acad. Sci. U. S. A. 103, 9446–9451. doi:10.1073/pnas.0511079103
Dar, A. A., Choudhury, A. R., Kancharla, P. K., and Arumugam, N. (2017). The FAD2 gene in plants: occurrence, regulation, and role. Front. Plant Sci. 8, 1789. doi:10.3389/fpls.2017.01789
Demorest, Z. L., Coffman, A., Baltes, N. J., Stoddard, T. J., Clasen, B. M., Luo, S., et al. (2016). Direct stacking of sequence-specific nuclease-induced mutations to produce high oleic and low linolenic soybean oil. BMC Plant Biol. 16, 225–228. doi:10.1186/s12870-016-0906-1
Derbyshire, M. C., Marsh, J., Tirnaz, S., Nguyen, H. T., Batley, J., Bayer, P. E., et al. (2023). Diversity of fatty acid biosynthesis genes across the soybean pangenome. Plant Genome 16, e20334–18. doi:10.1002/tpg2.20334
Do, P. T., Nguyen, C. X., Bui, H. T., Tran, L. T. N., Stacey, G., Gillman, J. D., et al. (2019). Demonstration of highly efficient dual gRNA CRISPR/Cas9 editing of the homeologous GmFAD2-1A and GmFAD2-1B genes to yield a high oleic, low linoleic and α-linolenic acid phenotype in soybean. BMC Plant Biol. 19, 311–314. doi:10.1186/s12870-019-1906-8
EFSA GMO Panel (2016). Growth of spoilage bacteria during storage and transport of meat. EFSA J. 14, e04523. doi:10.2903/j.efsa.2016.4523
Entine, J., Felipe, M. S. S., Groenewald, J. H., Kershen, D. L., Lema, M., McHughen, A., et al. (2021). Regulatory approaches for genome edited agricultural plants in select countries and jurisdictions around the world. Transgenic Res. 30, 551–584. doi:10.1007/s11248-021-00257-8
Fehr, W. R. (2007). Breeding for modified fatty acid composition in soybean. Crop Sci. 47. doi:10.2135/cropsci2007.04.0004IPBS
Flores, T., Karpova, O., Su, X., Zeng, P., Bilyeu, K., Sleper, D. A., et al. (2008). Silencing of GmFAD3 gene by siRNA leads to low α-linolenic acids (18:3) of fad3-mutant phenotype in soybean [Glycine max (Merr.)]. Transgenic Res. 17, 839–850. doi:10.1007/s11248-008-9167-6
Haun, W., Coffman, A., Clasen, B. M., Demorest, Z. L., Lowy, A., Ray, E., et al. (2014). Improved soybean oil quality by targeted mutagenesis of the fatty acid desaturase 2 gene family. Plant Biotechnol. J. 12, 934–940. doi:10.1111/pbi.12201
Held, J. P., Carrero-Colón, M., and Hudson, K. A. (2019). Combination of novel mutation in FAD3C and FAD3A for low linolenic acid soybean. Agrosystems, Geosci. Environ. 2, 1–4. doi:10.2134/age2019.01.0006
Herrera-Estrella, L., Simpson, J., and Martínez-Trujillo, M. (2005). Transgenic plants: an historical perspective. Methods Mol. Biol. 286, 681–694. doi:10.1201/9781003055211-66
Hsu, P. D., Lander, E. S., and Zhang, F. (2014). Development and applications of CRISPR-Cas9 for genome engineering. Public Health Rep. 157, 1262–1278. doi:10.1016/j.cell.2014.05.010
Huang, L., Zhang, F., Xu, P., Zhou, Y., Liu, Y., Zhang, H., et al. (2023). Effect of Omega-3 polyunsaturated fatty acids on cardiovascular outcomes in patients with diabetes: a meta-analysis of randomized controlled trials. Adv. Nutr. 14, 629–636. doi:10.1016/J.ADVNUT.2023.04.009
Hudson, K. A., and Hudson, M. E. (2021). Genetic variation for seed oil biosynthesis in soybean. Plant Mol. Biol. Rep. 39, 700–709. doi:10.1007/s11105-020-01276-1
Jhansi Rani, S., and Usha, R. (2013). Transgenic plants: types, benefits, public concerns and future. J. Pharm. Res. 6, 879–883. doi:10.1016/J.JOPR.2013.08.008
Jiang, F., and Doudna, J. A. (2017). CRISPR-Cas9 structures and mechanisms. Annu. Rev. Biophys. 46, 505–529. doi:10.1146/annurev-biophys-062215-010822
Jiang, F., Zhou, K., Ma, L., Gressel, S., and Doudna, J. A. (2015). STRUCTURAL BIOLOGY. A Cas9-guide RNA complex preorganized for target DNA recognition. Sci. 348, 1477–1481. doi:10.1126/science.aab1452
Jinek, M., Chylinski, K., Fonfara, I., Hauer, M., Doudna, J. A., and Charpentier, E. (2012). A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Sci. 337, 816–821. doi:10.1126/science.1225829
Joung, J. K., and Sander, J. D. (2013). TALENs: a widely applicable technology for targeted genome editing. Nat. Rev. Mol. Cell Biol. 14, 49–55. doi:10.1038/nrm3486
Kaikkonen, J. E., Jula, A., Viikari, J. S. A., Juonala, M., Hutri-Kähönen, N., Kähönen, M., et al. (2021). Associations of serum fatty acid proportions with obesity, insulin resistance, blood pressure, and fatty liver: the cardiovascular risk in young finns study. J. Nutr. 151, 970–978. doi:10.1093/jn/nxaa409
Kanai, M., Yamada, T., Hayashi, M., Mano, S., and Nishimura, M. (2019). Soybean (glycine max L.) triacylglycerol lipase GmSDP1 regulates the quality and quantity of seed oil. Sci. Rep. 9, 8924–10. doi:10.1038/s41598-019-45331-8
Kim, M., Song, J. T., Bilyeu, K. D., and Lee, J. D. (2015). A new low linolenic acid allele of GmFAD3A gene in soybean PE1690. Mol. Breed. 35, 155–156. doi:10.1007/s11032-015-0352-z
Kotlyarov, S., and Kotlyarova, A. (2022). Clinical significance of polyunsaturated fatty acids in the prevention of cardiovascular diseases. Front. Nutr. 9, 998291. doi:10.3389/fnut.2022.998291
Lakhssassi, N., Zhou, Z., Liu, S., Colantonio, V., AbuGhazaleh, A., and Meksem, K. (2017). Characterization of the FAD2 gene family in soybean reveals the limitations of gel-based TILLING in genes with high copy number. Front. Plant Sci. 8, 324. doi:10.3389/fpls.2017.00324
Li, H., Zhou, R., Liu, P., Yang, M., Xin, D., Liu, C., et al. (2023). Design of high-monounsaturated fatty acid soybean seed oil using GmPDCTs knockout via a CRISPR-Cas9 system. Plant Biotechnol. J. 21, 1317–1319. doi:10.1111/pbi.14060
Li, L., Zhang, D., Zhang, Z., and Zhang, B. (2024). CRISPR/Cas: a powerful tool for designing and improving oil crops. Trends Biotechnol. xx, 773–789. doi:10.1016/j.tibtech.2024.09.007
Liao, W., Guo, R., Qian, K., Shi, W., Whelan, J., and Shou, H. (2024). The acyl–acyl carrier protein thioesterases GmFATA1 and GmFATA2 are essential for fatty acid accumulation and growth in soybean. Plant J. 118, 823–838. doi:10.1111/TPJ.16638
Liu, H. R., and White, P. J. (1992). Oxidative stability of soybean oils with altered fatty acid compositions. J. Am. Oil Chem. Soc. 69, 528–532. doi:10.1007/BF02636103
Liu, Z., Dong, H., Cui, Y., Cong, L., and Zhang, D. (2020). Application of different types of CRISPR/Cas-based systems in bacteria. Microb. Cell Fact. 19, 172–14. doi:10.1186/s12934-020-01431-z
Ma, J., Sun, S., Whelan, J., and Shou, H. (2021). CRISPR/Cas9-mediated knockout of GmFATB1 significantly reduced the amount of saturated fatty acids in soybean seeds. Int. J. Mol. Sci. 22, 3877–14. doi:10.3390/ijms22083877
Maszewska, M., Florowska, A., Dłuzewska, E., Wroniak, M., Marciniak-Lukasiak, K., and Zbikowska, A. (2018). Oxidative stability of selected edible oils. Mol. A J. Synth. Chem. Nat. Prod. Chem. 23, 1746. doi:10.3390/MOLECULES23071746
Meijaard, E., Abrams, J. F., Slavin, J. L., and Sheil, D. (2022). Dietary fats, human nutrition and the environment: balance and sustainability. Front. Nutr. 9, 878644–878647. doi:10.3389/fnut.2022.878644
Memon, H. D., Mahesar, S. A., Sirajuddin, , Kara, H., Sherazi, S. T. H., and Talpur, M. Y. (2024). A review: health benefits and physicochemical characteristics of blended vegetable oils. Grain Oil Sci. Technol. 7, 113–123. doi:10.1016/J.GAOST.2024.05.001
Messina, M., Shearer, G., and Petersen, K. (2021). Soybean oil lowers circulating cholesterol levels and coronary heart disease risk, and has no effect on markers of inflammation and oxidation. Nutrition 89, 111343. doi:10.1016/j.nut.2021.111343
Mojica, F. J. M., Díez-Villaseñor, C., García-Martínez, J., and Soria, E. (2005). Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J. Mol. Evol. 60, 174–182. doi:10.1007/s00239-004-0046-3
Mozaffarian, D., Aro, A., and Willett, W. C. (2009). Health effects of trans-fatty acids: experimental and observational evidence. Eur. J. Clin. Nutr. 63, S5–S21. doi:10.1038/sj.ejcn.1602973
Murad, A. M., Vianna, G. R., Machado, A. M., Da Cunha, N. B., Coelho, C. M., Lacerda, V. A. M., et al. (2014). Mass spectrometry characterisation of fatty acids from metabolically engineered soybean seeds. Anal. Bioanal. Chem. 406, 2873–2883. doi:10.1007/s00216-014-7709-8
Nogoy, K. M. C., Kim, H. J., Lee, Y., Zhang, Y., Yu, J., Lee, D. H., et al. (2020). High dietary oleic acid in olive oil-supplemented diet enhanced omega-3 fatty acid in blood plasma of rats. Food Sci. Nutr. 8, 3617–3625. doi:10.1002/FSN3.1644
Owen, R. W., Mier, W., Giacosa, A., Hull, W. E., Spiegelhalder, B., and Bartsch, H. (2000). Phenolic compounds and squalene in olive oils: the concentration and antioxidant potential of total phenols, simple phenols, secoiridoids, lignansand squalene. Food Chem. Toxicol. 38, 647–659. doi:10.1016/S0278-6915(00)00061-2
Pham, A. T., Lee, J. D., Shannon, J. G., and Bilyeu, K. D. (2010). Mutant alleles of FAD2-1A and FAD2-1B combine to produce soybeans with the high oleic acid seed oil trait. BMC Plant Biol. 10, 195. doi:10.1186/1471-2229-10-195
Pioneer (2021). Processor premiums for pioneer® brand plenish® high oleic soybeans to increase in 2022, with cargill delivery locations added. Available online at: https://www.pioneer.com/us/news-and-events/news/media-release/plenish-soybean-premiums.html (Accessed June 29, 2025).
Pipoyan, D., Stepanyan, S., Stepanyan, S., Beglaryan, M., Costantini, L., Molinari, R., et al. (2021). The effect of trans fatty acids on human health: regulation and consumption patterns. Foods 10, 2452. doi:10.3390/FOODS10102452
Priolli, R. H. G., Campos, J. B., Stabellini, N. S., Pinheiro, J. B., and Vello, N. A. (2015). Association mapping of oil content and fatty acid components in soybean. Euphytica 203, 83–96. doi:10.1007/s10681-014-1264-4
Reportlinker (2023). Global soybean oil domestic consumption by country. Available online at: https://www.reportlinker.com/dataset/6dcebbfd6c71642d04df0b89ebd65644e412fca5 (Accessed June 26, 2025).
Riolo, R., De Rosa, R., Simonetta, I., and Tuttolomondo, A. (2022). Olive oil in the mediterranean diet and its biochemical and molecular effects on cardiovascular health through an analysis of genetics and epigenetics. Int. J. Mol. Sci. 23, 16002. doi:10.3390/ijms232416002
Schwingshackl, L., Heseker, H., Kiesswetter, E., and Koletzko, B. (2022). Dietary fat and fatty foods in the prevention of non-communicable diseases: a review of the evidence. Trends Food Sci. Technol. 128, 173–184. doi:10.1016/J.TIFS.2022.08.002
Sears, B., and Perry, M. (2015). The role of fatty acids in insulin resistance. Lipids Health Dis. 14, 121–129. doi:10.1186/s12944-015-0123-1
Shewry, P. R., Jones, H. D., and Halford, N. G. (2008). “Plant biotechnology: transgenic crops,” in Advances in biochemical engineering/biotechnology (Berlin: Springer), 149–186. doi:10.1007/10_2008_095
Statista (2024). Consumption of vegetable oils worldwide from 2013/14 to 2023/2024, by oil type. Available online at: https://www.statista.com/statistics/263937/vegetab.
Steele, L., Drummond, E., Nishida, C., Yamamoto, R., Branca, F., Parsons Perez, C., et al. (2024). Ending trans fat—The First-ever global elimination program for a noncommunicable disease risk factor: JACC international. J. Am. Coll. Cardiol. 84, 663–674. doi:10.1016/j.jacc.2024.04.067
Thapa, R., Carrero-Colón, M., and Hudson, K. A. (2016). New alleles of fatb1a to reduce palmitic acid levels in soybean. Crop Sci. 56, 1076–1080. doi:10.2135/cropsci2015.09.0597
Thapa, R., Carrero-Colón, M., Addo-Quaye, C., Held, J., Dilkes, B., and Hudson, K. A. (2018). New alleles of FAD3A lower the linolenic acid content of soybean seeds. Crop Sci. 58, 713–718. doi:10.2135/cropsci2017.08.0490
Torabi, S., Sukumaran, A., Dhaubhadel, S., Johnson, S. E., LaFayette, P., Parrott, W. A., et al. (2021). Effects of type I diacylglycerol O-acyltransferase (DGAT1) genes on soybean (Glycine max L.) seed composition. Sci. Rep. 11, 2556–14. doi:10.1038/s41598-021-82131-5
Tutunchi, H., Ostadrahimi, A., and Saghafi-Asl, M. (2020). The effects of diets enriched in monounsaturated oleic acid on the management and prevention of obesity: a systematic review of human intervention studies. Adv. Nutr. 11, 864–877. doi:10.1093/advances/nmaa013
WHO (2024). Trans fat. 1. Available online at: https://www.who.int/news-room/fact-sheets/detail/trans-fat.
Wiedenheft, B., Sternberg, S. H., and Doudna, J. A. (2012). RNA-Guided genetic silencing systems in bacteria and archaea. Nature 482, 331–338. doi:10.1038/nature10886
Wilkes, R. S., and Bringe, N. A. (2015). Applications of trait enhanced soybean oils. Trait. Oils Foods, 71–92. doi:10.1002/9781118961117.ch5
Wu, N., Lu, Q., Wang, P., Zhang, Q., Zhang, J., Qu, J., et al. (2020). Construction and analysis of GmFAD2-1A and GmFAD2-2A soybean fatty acid desaturase mutants based on CRISPR/Cas9 technology. Int. J. Mol. Sci. 21, 1104. doi:10.3390/IJMS21031104
Yang, J., Xing, G., Niu, L., He, H., Guo, D., Du, Q., et al. (2018). Improved oil quality in transgenic soybean seeds by RNAi-mediated knockdown of GmFAD2-1B. Transgenic Res. 27, 155–166. doi:10.1007/s11248-018-0063-4
Yang, Z., Chen, Y., Ma, S., Zhang, M., Tang, T., and Du, C. (2025). Bioengineering of long-chain polyunsaturated fatty acids in oilseed crops. Prog. Lipid Res. 99, 101333. doi:10.1016/J.PLIPRES.2025.101333
Yao, Y., Ding, L., and Huang, X. (2020). Diverse functions of lipids and lipid metabolism in development. Small Methods 4, 1900564–18. doi:10.1002/smtd.201900564
Yeom, W. W., Kim, H. J., Lee, K. R., Cho, H. S., Kim, J. Y., Jung, H. W., et al. (2020). Increased production of α-Linolenic acid in soybean seeds by overexpression of lesquerella FAD3-1. Front. Plant Sci. 10, 1812. doi:10.3389/fpls.2019.01812
Zambelli, A. (2021). Current status of high oleic seed oils in food processing. JAOCS, J. Am. Oil Chem. Soc. 98, 129–137. doi:10.1002/aocs.12450
Zhang, X., and Guo, H. (2017). mRNA decay in plants: both quantity and quality matter. Curr. Opin. Plant Biol. 35, 138–144. doi:10.1016/j.pbi.2016.12.003
Zhang, J., Wang, X., Lu, Y., Bhusal, S. J., Song, Q., Cregan, P. B., et al. (2018). Genome-wide scan for seed composition provides insights into soybean quality improvement and the impacts of domestication and breeding. Mol. Plant 11, 460–472. doi:10.1016/j.molp.2017.12.016
Zhang, J., Liu, H., Sun, J., Li, B., Zhu, Q., Chen, S., et al. (2012). Arabidopsis fatty acid desaturase FAD2 is required for salt tolerance during seed germination and early seedling growth. PLoS One 7, e30355. doi:10.1371/journal.pone.0030355
Zhang, Q., Liu, L., Xiao, Z., Sun, Y., Xi, Y., Sun, T., et al. (2023). Construction and functional evaluation of CRISPR/Cas9 multiple knockout vectors of the FAD2 gene family. Agronomy 13, 1737. doi:10.3390/agronomy13071737
Zheng, Y., Guo, T., Xia, T., Guo, S., Chen, M., Ye, S., et al. (2024). Utility of arabidopsis KASII promoter in development of an effective CRISPR/Cas9 system for soybean genome editing and its application in engineering of soybean seeds producing super-high oleic and low saturated oils. J. Agric. Food Chem. 72, 21720–21730. doi:10.1021/acs.jafc.4c05840
Zhou, Z., Lakhssassi, N., Knizia, D., Cullen, M. A., El Baz, A., Embaby, M. G., et al. (2021). Genome-wide identification and analysis of soybean acyl-ACP thioesterase gene family reveals the role of GmFAT to improve fatty acid composition in soybean seed. Theor. Appl. Genet. 134, 3611–3623. doi:10.1007/s00122-021-03917-9
Keywords: high-oleic soybean, CRISPR-Cas, oxidative stability, trans fatty acid, high-linolenic, low-palmitic
Citation: Da Rosa J, Marin DR, Vieira da Silva AM, De Souza EMG, Rech EL, Nepomuceno AL and Mertz-Henning LM (2025) Biotechnological advances in enhancing soybean oil quality for human health and food industry applications. Front. Food Sci. Technol. 5:1658589. doi: 10.3389/frfst.2025.1658589
Received: 02 July 2025; Accepted: 29 September 2025;
Published: 16 October 2025.
Edited by:
Dalia Z. Alomari, Hashemite University, JordanReviewed by:
Chang Du, South China Normal University, ChinaKayla Flyckt McReynolds, Corteva Agriscience, United States
Copyright © 2025 Da Rosa, Marin, Vieira da Silva, De Souza, Rech, Nepomuceno and Mertz-Henning. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juliana Da Rosa, anVfbGlhbmFyb3NhQGhvdG1haWwuY29t; Liliane Marcia Mertz-Henning, bGlsaWFuZS5oZW5uaW5nQGVtYnJhcGEuYnI=
 Juliana Da Rosa
Juliana Da Rosa Daniel Rockenbach Marin
Daniel Rockenbach Marin Angela Maria Vieira da Silva
Angela Maria Vieira da Silva Enya Maria Gois De Souza
Enya Maria Gois De Souza Elibio Leopoldo Rech
Elibio Leopoldo Rech Alexandre Lima Nepomuceno
Alexandre Lima Nepomuceno Liliane Marcia Mertz-Henning
Liliane Marcia Mertz-Henning