- 1Department of Food Sciences, College of Agriculture, Tikrit University, Tikrit, Iraq
- 2Department of Microbiology, College of Science, University of Manitoba, Winnipeg, MB, Canada
This study systematically evaluated the effects of thermal pasteurization (TP) and dielectric barrier discharge atmospheric cold plasma (DBD ACP) on microbial inactivation, nutritional composition, enzymatic activity, and sensory attributes of fresh mandarin juice during 18 days of storage at 4 °C. TP was applied at 95 °C for 60 s, while DBD ACP treatments were conducted for durations ranging from 30 to 120 s. TP effectively inactivated spoilage microorganisms and quality-degrading enzymes, reducing microbial counts to acceptable levels and enzymatic activities (polyphenol oxidase and pectin methylesterase) to below 20%. However, TP significantly compromised nutritional quality, with notable reductions in ascorbic acid (26%), total phenolics, and antioxidant capacity. Additionally, TP negatively impacted sensory properties, inducing heat-related pigment degradation and the development of off-flavors. In contrast, DBD ACP treatments, particularly at 30–60 s, preserved ascorbic acid, phenolic content, and antioxidant activity, maintaining values statistically similar to those of the untreated juice samples. The 120-s DBD ACP treatment achieved microbial reduction comparable to TP (total plate count: 3.30 log CFU/mL; yeast and molds: 2.40 log CFU/mL), but demonstrated limited efficacy in enzyme inactivation, with residual enzyme activities exceeding 80%. Sensory evaluation indicated that DBD ACP-preserved juice retained its color, aroma, flavor, and taste, with scores closely aligned with those of the control sample. Results suggest that while DBD ACP is effective in preserving nutritional and sensory quality, its limited ability to inactivate enzymes may necessitate a combination with other preservation methods. Overall, DBD ACP represents a promising non-thermal processing technology for the safety and preservation of fresh fruit juices.
1 Introduction
Fruit juices are widely consumed beverages, valued for their low caloric content and abundance of essential nutrients and bioactive compounds, such as polyphenols, enzymes, vitamins (notably ascorbic acid), and antioxidants, which contribute to health-promoting properties and consumer preference (Amine et al., 2023; Gupta et al., 2023). However, the high water activity and acidic nature of most fruit juices, including mandarin juice, make them highly susceptible to microbial spoilage and enzymatic degradation during the storage period (Dalvi-Isfahan and Mahmoodi-Eshkaftaki, 2024; Roobab et al., 2023; Ruxton and Myers, 2021). Thermal pasteurization (TP) remains the conventional method for ensuring microbial safety and enzymatic stability in fruit juices. Although effective in achieving microbial inactivation, TP is associated with detrimental effects on product quality, including degradation of heat-sensitive nutrients, loss of volatile aroma compounds, pigment breakdown, and degraded sensory characteristics (Ağçam et al., 2018; Umair et al., 2022). These limitations have driven interest in the development and optimization of non-thermal technologies (NTTs), such as high-pressure processing (HPP), pulsed electric fields (PEF), ultrasound (US), ozone, and atmospheric cold plasma (ACP), which aim to extend shelf life while preserving the nutritional, functional, and sensory attributes of fresh juices (Almeida et al., 2015; Pihen et al., 2024; Roobab et al., 2023). Among these technologies, ACP has emerged as a promising alternative for non-thermal pasteurization. In contrast, HPP treatment is more effective for inactivating bulk microbes, especially in liquid and semi-solid foods; however, it requires expensive equipment and pressure-resistant packaging. The PEF technology is well-suited for liquid foods, relying on electroporation to disrupt microbial cells with minimal heat generation; however, uniform treatment can be challenging (Jadhav et al., 2021). Ultrasound inactivates microorganisms through cavitation effects; however, its effectiveness is limited by the penetration depth, which depends on the size and geometry of the food product. Additionally, ultrasonic treatment may cause localized heating or mechanical damage, potentially compromising the food’s texture and quality. Ozone treatment, another non-thermal approach, is a potent oxidizing method that effectively reduces microbial loads and pesticide residues. However, its use raises safety concerns, as ozone is toxic and can induce oxidative damage if not precisely controlled during the treatment process (Yaya-González et al., 2025; Zhang et al., 2019). Therefore, ACP meets these criteria while maintaining product quality, making it a viable method for commercial application (Abbas et al., 2024; Kaur et al., 2024; Ozen and Singh, 2020). ACP is a partially ionized gas composed of electrons, ions, radicals, and neutral particles, generated at low temperatures (30 °C–60 °C) under atmospheric conditions, reducing the need for vacuum systems (Coutinho et al., 2018; Kaur et al., 2024; Yasoob et al., 2022). ACP generates a variety of reactive species, including reactive oxygen and nitrogen species (RONS), ozone, singlet oxygen, hydroxyl radicals, hydrogen peroxide, nitric oxide, and UV photons. These agents are primarily responsible for microbial inactivation by inducing oxidative damage and disrupting microbial cell membranes, proteins, and nucleic acids (Dharini et al., 2023; Illera et al., 2019). The dielectric barrier discharge (DBD) approach is one of the most commonly used techniques for generating ACP in food applications due to its ability to provide uniform plasma exposure over the treated food surface area, versatile design (planar, coaxial, or twisted electrodes), and compatibility with various generating gases (Dalvi-Isfahan and Mahmoodi-Eshkaftaki, 2024; Jiang et al., 2022). ACP can be generated through various configurations, each with distinct mechanisms and applications in the food processing industry. Corona discharge, a cold plasma system utilizing sharp electrode tips, produces non-uniform plasma and is primarily employed for treating solid food surfaces. Microwave discharge plasma yields high-density plasma but generally requires vacuum or sub-atmospheric conditions, limiting its applicability in food processing. The plasma jet system directs plasma from a chamber through a nozzle, providing precise treatment for irregular surfaces; however, uniformity may be a challenge during food treatment (Laroque et al., 2022; Vaka & Ramkumar, 2024). DBD-ACP is particularly well-suited for juice processing due to its low thermal impact, uniform plasma generation, and ability to effectively treat liquid surfaces without direct electrode contact, which helps preserve bioactive compounds, color, aroma, and vitamin content (Dalvi-Isfahan and Mahmoodi-Eshkaftaki, 2024). Therefore, voltage, treatment time, gas composition, and electrode setup are the primary factors governing the efficacy and safety of DBD-ACP. Fine-tuning these parameters is crucial for achieving high microbial inactivation while minimizing adverse effects on food quality (Vaka & Ramkumar, 2024). Recent studies have highlighted the benefits of DBD-ACP, which has demonstrated efficacy in microbial decontamination and retention of bioactive compounds in several fruit juices, including apple, blueberry, and pomegranate (Hou et al., 2019; Kovačević et al., 2016; Ozen et al., 2024). Despite the progress achieved, a gap remains in the available information, and limited data exist on the use of DBD ACP pasteurization for fresh citrus juices (including mandarin juice), which are characterized by high consumer appeal due to color, distinctive aroma, and sweet-tart flavor profile. Mundanat et al. (2025) emphasized that color, aroma, flavor, and taste are critical sensory determinants of consumer acceptance in fruit juices. Processing techniques that negatively affect these attributes can significantly reduce quality and marketability. Furthermore, enzymatic activities such as polyphenol oxidase (PPO) and pectin methyl esterase (PME) contribute to quality degradation during storage, and their stability under non-thermal treatments remains inadequately studied in fresh citrus juices in general and in fresh mandarin juice in particular. The authors also reported that color and appearance have a primary effect on perceptual quality. The authors also noted that flavor and taste play a major role in determining the acceptability and consumption of any fruit juice. This study examines the impact of DBD ACP treatment on microbial decontamination in fresh mandarin juice, compared to conventional thermal pasteurization, during a 28-day storage period at 4 °C. In addition, the study evaluates key quality parameters, including sugar content, ascorbic acid concentration, total polyphenol content, antioxidant activity, and residual enzyme activity, alongside primary sensory characteristics. These parameters have not been previously assessed in fresh mandarin juice treated with DBD ACP. The data obtained in this study will address existing knowledge gaps and evaluate the potential of DBD-ACP for industrial applications in the preservation of fresh fruit juice.
2 Materials and methods
2.1 Preparation of mandarin juice
Fresh, healthy, and undamaged mandarin fruits (Citrus reticulata L.) were manually harvested from a local orchard in northern Iraq during the 2024 growing season. After selection, the fruits were placed in a 10 kg-capacity ice-container and transported immediately to the research laboratory at the University of Tikrit. Upon arrival, the fruits were washed thoroughly with distilled water and air-dried at room temperature. The fruits were then peeled, and the juice was extracted using a cold-press juicer (Anybear Cold Press Juicer, Anybear Co., Florida, USA) to minimize thermal degradation of bioactive compounds. The extracted juice was filtered through a stainless-steel mesh filter (Fisher Scientific, USA) to remove seeds and other impurities, and subsequently stored in pre-sterilized, dry, and sealed glass containers at −5 °C in a refrigerator until further analysis.
2.2 Thermal pasteurization and DBD-ACP experiments
For thermal treatment, the pasteurization system at the Department of Food Science, University of Tikrit, was used to perform thermal pasteurization, following the method described by Ferreira et al. (2022). The process involved heating juice samples to 95 °C for 60 s. Pasteurization was carried out by submerging the juice samples in a thermostatic water bath (Selecta Frigiterm 6,000,382, Barcelona, Spain). Each sample was sealed in a heat-resistant polyethylene bag (4 × 8 cm) containing 25 mL of juice. The time required to reach the target temperature was determined in advance using a K-type thermocouple connected to a digital thermometer, ensuring that the temperature was maintained within ±2 °C of the set point. Once the desired temperature was reached, the samples were held in the water bath for 30 s, then immediately transferred to an ice bath to rapidly cool and stop further thermal effects. Cold plasma experiments were conducted using a DBD-ACP system (Model 6CP120/60–7.5, Phenix Tech, USA), as illustrated in Figure 1. The procedure was based on the method described by Lou et al. (2025), with minor modifications. The apparatus consisted of two circular aluminum plate electrodes (diameter: 5 cm) and two circular quartz glass dielectric barriers (upper barrier diameter: 9 cm; lower barrier diameter: 6 cm; thickness: 1 mm). A high-voltage, high-frequency power supply (fixed frequency: 10 kHz; output voltage: 14 kV) was used to generate the plasma discharge. For the DBD-ACP treatment, 10 mL of fresh mandarin juice was placed in a sterile Petri dish (diameter, 5 cm; depth, 1 cm). The electric field strength was maintained at 14 kV/cm, with a 0.5 cm gap between the electrode and the juice surface. The distance between the upper electrode and the ground electrode was approximately 1 cm. DBD ACP exposure durations were set at 30, 60, 90, and 120 s. All experiments were performed at room temperature (24 °C ± 1 °C) using ambient air as the working gas. Following treatment, the juice samples were transferred into sterile, dry 50 mL glass tubes and stored at 4 °C in a refrigerator for subsequent analysis. The temperature of the juice was measured both before and after treatment using a non-contact infrared thermometer (Model: Omega OS423-LS, Stamford, United States). All experiments and measurements were conducted in triplicate.
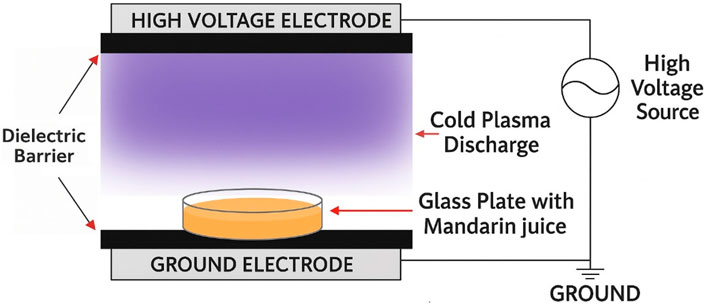
Figure 1. A schematic diagram of the DBD ACP system (Model No. 6CP120/60–7.5, Phenix Tech, France) used in this study to treat mandarin juice under atmospheric conditions.
2.3 Determination of microbial load
This study utilized the natural microbial load to reflect real-world processing and storage conditions, enabling a realistic assessment of treatment effectiveness, spoilage, shelf life, and sensory quality, especially for minimally processed products, such as fresh juice. However, a limitation is the use of high-quality, hand-selected fruits, which likely had a lower initial microbial load. In the industry, fruit quality varies, and contamination may be higher; therefore, the findings of this study may not fully capture the industrial processing challenges of fruit juices. The microbiological analysis was conducted using the method described by Umair et al. (2019) and Martínez-Hernández et al. (2017), with minor modifications. A 10 mL of mandarin juice was homogenized with 90 mL of sterile saline solution (8.5 g NaCl/L; Sigma Chemical Co., St. Louis, MO, USA) for 1 min using a stomacher (to ensure uniform mixing). From appropriate serial dilutions, 1 mL was poured-plated onto Plate Count Agar (PCA) and incubated at 30 °C for 48 h to determine the total viable microbial count. For yeast and mold enumeration (YM count), 100 μL of the selected dilution was spread-plated onto Potato Dextrose Agar (PDA) supplemented with 100 mg/L of chloramphenicol (Sigma Chemical Co., St. Louis, MO, USA) to inhibit bacterial growth. Plates were incubated at 25 °C for 6 days under aerobic conditions. All microbiological assays were conducted in triplicate, and results were expressed as log colony-forming units (CFU) per gram of fresh weight.
2.4 Determination of total sugar content
Total soluble sugar content was determined using the phenol–sulfuric acid colorimetric assay, as described by Haron et al. (2017). Juice samples (1 mL) were transferred into test tubes and mixed with 1 mL of 5% (w/v) aqueous phenol solution. Subsequently, 5 mL of concentrated sulfuric acid (H2SO4) was carefully added to each mixture. The reaction mixtures were vortexed for 1 min and then incubated at ambient temperature (∼25 °C) for 30 min to allow for color development. Absorbance was measured at 490 nm using a UV–VIS spectrophotometer (Shimadzu Scientific Instruments Co., Ltd., Beijing, China). A glucose calibration curve (ranging from 0 to 1000 μg/mL) was used to quantify the sugar concentration in the samples. Results were expressed as grams of total sugars per liter (g/L) of juice.
2.5 Determination of ascorbic acid content
The ascorbic acid (vitamin C) concentration in mandarin juice was determined using a redox titration method as described by Escudero-López et al. (2013). A 5 mL of juice sample was mixed with an equal volume of 3% (w/v) aqueous metaphosphoric acid solution, which acts as a stabilizing and precipitating agent to prevent oxidation of ascorbic acid. The mixture was then centrifuged at 4,000 × g for 10 min to remove suspended solids. The resulting supernatant (5 mL) was titrated with 2,6-dichlorophenolindophenol (DCPIP) solution, a redox indicator that becomes colorless upon reduction by ascorbic acid. The appearance of a persistent light pink color marked the endpoint of the titration, as ascorbic acid converted to dehydroascorbic acid (C6H6O6). Ascorbic acid concentration was quantified using a standard calibration curve prepared with known concentrations of ascorbic acid ranging from 0 to 600 mg/L. Results were expressed in milligrams per liter (mg/L).
2.6 Determination of total phenolic content
Total phenolic content (TPC) was determined using a modified Folin–Ciocalteu colorimetric method, as described by Escudero-López et al. (2016). Mandarin juice samples were initially diluted with distilled water at a 5:1 (v/v) ratio to ensure an appropriate concentration for spectrophotometric analysis. Then, 6 mL of the diluted sample was transferred into glass centrifuge tubes and centrifuged at 6,000 × g for 15 min to remove particulate matter. Subsequently, 20 μL of the resulting supernatant was mixed with 2 mL of deionized water and 100 μL of Folin–Ciocalteu reagent. After a 5-min reaction period at room temperature, 5 mL of 20% (w/v) sodium carbonate (Na2CO3) solution was added to create an alkaline medium, which is essential for the Folin–Ciocalteu reaction to proceed. The Folin–Ciocalteu reagent contains phosphomolybdic and phosphotungstic acids that, under alkaline conditions, are reduced by phenolic compounds. This redox reaction results in the formation of a blue-colored complex, commonly referred to as the molybdenum–tungsten blue complex (Dominguez-López et al., 2024). The reaction was then incubated in the dark at ambient temperature (∼25 °C) for 2 h to allow full color development. Absorbance was measured at 765 nm using a UV–VIS spectrophotometer (Shimadzu Scientific Instruments Co., Ltd., Beijing, China), and the total phenolic content was quantified using a gallic acid calibration curve ranging from 0 to 500 mg/L. Results were expressed as milligrams of gallic acid equivalents per liter of juice (mg GAE/L).
2.7 Determination of antioxidant capacity
The antioxidant capacity of mandarin juice was evaluated using the DPPH (2,2-diphenyl-1-picrylhydrazyl) free radical scavenging assay, as described by Kelebek and Selli (2014). Juice samples were diluted with deionized water at a 2:1 (v/v) ratio (juice to water) and then centrifuged at 5,000 × g for 15 min to obtain a clear supernatant. A DPPH solution was prepared by dissolving 0.025 g of DPPH in 50% (v/v) ethanol. Subsequently, 0.5 mL of the juice supernatant was mixed with 2.5 mL of the DPPH solution and vortexed for 1 min. The mixture was then incubated in the dark at room temperature (∼25 °C) for 30 min to prevent photodegradation. After incubation, the absorbance was measured at 515 nm using a UV–VIS spectrophotometer (Shimadzu Scientific Instruments Co., Ltd., Beijing, China). The percentage of DPPH radical scavenging activity was calculated using the following Equation 1:
2.8 Determination of residual enzyme activity
The residual activities of peroxidase (POD) and polyphenol oxidase (PPO) were determined following the method of Dars et al. (2019), with minor modifications. Mandarin juice samples were centrifuged at 8,000 rpm for 15 min at 4 °C, and the resulting supernatant was used for enzymatic assays. For POD activity, a reaction mixture containing 0.32 mL of 0.2 mol/L potassium phosphate buffer (pH 6.8), 0.32 mL of 5% (w/v) pyrogallol, and 0.6 mL of 0.147 mol/L hydrogen peroxide was prepared. The change in absorbance at 420 nm was recorded over 3 min. PPO activity was assessed by mixing 1.5 mL of the supernatant with 0.5 mL of 0.5 mol/L catechol and 3.0 mL of 0.2 mol/L potassium phosphate buffer (pH 6.8), followed by measurement of absorbance at 410 nm over 3 min. Pectin methylesterase (PME) activity was measured according to Saeeduddin et al. (2015). Briefly, 10 mL of enzyme extract was mixed with 40 mL of 1% (w/v) pectin solution containing 0.15 mol/L NaCl. The absorbance was monitored at 620 nm for 2 min. Lipoxygenase (LOX) activity was determined following the protocol described by Aguiló-Aguayo et al. (2008). LOX was extracted using a cold buffer system containing phenolic inhibitors at 2 °C, and the extract was clarified by centrifugation at 3,000 rpm for 10 min at 4 °C. The reaction was initiated with linoleic acid as the substrate, and the formation of hydroperoxides was monitored by measuring the absorbance at 234 nm for 3 min. All enzymatic activities were monitored spectrophotometrically using a UV–VIS spectrophotometer (Shimadzu Scientific Instruments Co., Ltd., Beijing, China). Residual enzyme activity (%) was calculated relative to the untreated control using the following Equation 2:
All assays were conducted in triplicate, and results were expressed as mean ± SD.
2.9 Sensory evaluation
A total of 40 participants were selected from a pool of 100 research scholars, comprising faculty, staff, and graduate students. The group consisted of 20 males and 20 females, aged between 25 and 50 years. All participants were non-smokers, reported no known allergies to fruit juices, and expressed a strong interest in sensory assessment. While 70% of the panelists were untrained, 30% possessed formal certification in sensory evaluation. Before testing, panelists were thoroughly briefed on the use of the sensory scorecard and the evaluation procedure. Sensory evaluation was conducted in a dedicated sensory analysis room under controlled conditions, following the triangle test method with reference to the control sample, as described by Villanueva et al. (2005). To minimize bias, samples were anonymously labeled using random three-digit codes and served in identical, neutral glass containers with plastic lids. Panelists were seated in individual sensory booths separated by partitions to prevent interaction. The order of sample presentation was random for each participant to reduce sensory adaptation and carryover effects. The test protocol was designed to help panelists detect, identify, and differentiate sensory attributes among the juice samples. A hybrid hedonic line scale, based on a standard 10-point scale, was used to record responses, where 1 = no difference and 10 = extremely different. Panelists were asked to evaluate and comment on perceptible differences in color, flavor, aroma, and taste between the juice samples.
2.10 Statistical analysis
One-way ANOVA and Fisher’s Least Significant Difference (LSD) test were performed using SAS 9.0 (Cary, NC, USA) to compare means. Experiments were done in triplicate, with significance set at p < 0.05.
3 Results and discussion
3.1 Temperature profiles during TP and DBD ACP treatments
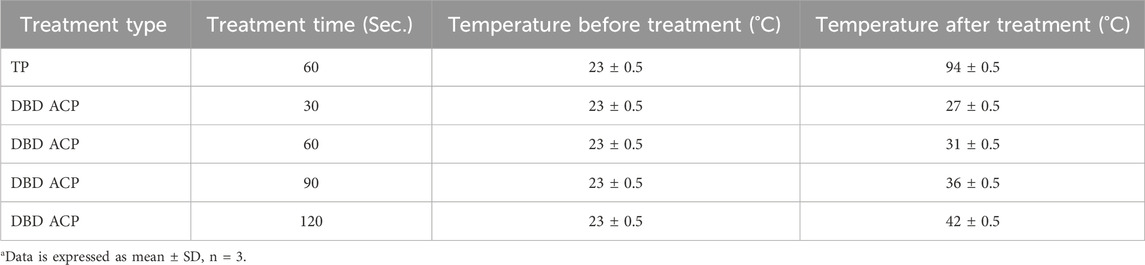
Table 1 presents the temperature variations in fresh mandarin juice before and after TP and DBD ACP treatments across varying exposure times. This parameter was measured to quantify the thermal load imposed by each processing method, as temperature is a critical factor influencing microbial inactivation, nutrient degradation, enzyme activity, and sensory attributes in juice processing (Petruzzi et al., 2017; Zia et al., 2024). TP treatment resulted in a significant rise in juice temperature, from an initial 23 °C ± 0.5 °C to 94 °C ± 0.5 °C, consistent with conventional high-temperature short-time (HTST) pasteurization protocols. This substantial thermal input is well-documented for its effectiveness in microbial inactivation and enzymatic denaturation (Umair et al., 2022), mechanisms that are further discussed in later sections. However, such elevated thermal exposure also contributes to the degradation of thermolabile compounds, particularly ascorbic acid and phenolic constituents, as previously demonstrated by Polydera et al. (2005), Patras et al. (2010), and Chen et al. (2013). The high exit temperature observed confirms the thermal stress imparted to the juice matrix, which corresponds with the observed reductions in antioxidant capacity and ascorbic acid content reported later in this study, consistent with prior findings (Nayak et al., 2020). In contrast, the DBD ACP treatments induced only moderate increases in juice temperature, ranging from 27 °C ± 0.5 °C (30 s) to a maximum of 42 °C ± 0.5 °C (120 s). These values remained well below the thresholds typically associated with thermal degradation, reaffirming the non-thermal classification of this technology. The minimal temperature rise aligns with the operational mechanism of cold plasma, which relies primarily on RONS, ultraviolet photons, and local electric fields rather than thermal energy for microbial inactivation and biochemical modulation (Mishra et al., 2025). Similar findings were reported by Misra et al. (2011) and Ziuzina et al. (2014), who documented temperature increases below 45 °C in plasma-treated juices and vegetables, confirming the mild thermal footprint of the process. The low thermal input during DBD ACP treatment accounts for the high retention of heat-sensitive nutritional compounds (e.g., ascorbic acid, total phenolics) and the limited inactivation of enzymes such as polyphenol oxidase (PPO) and pectin methyl esterase (PME), as presented later in this study. This contrast in temperature profiles between TP and DBD ACP underscores a fundamental trade-off in juice processing technologies. While TP ensures microbial and enzymatic stability through intense heat application, it compromises nutritional and sensory quality. Conversely, DBD ACP preserves nutritional and sensory attributes due to its minimal thermal impact but may require additional process optimization, such as extended treatment duration or integration with mild heat, to achieve comparable microbial and enzymatic inactivation levels (Bermúdez-Aguirre et al., 2013; Ozen and Singh, 2020; Zargarchi et al., 2024). In summary, TP treatment subjected the juice to near-boiling temperatures, resulting in significant thermal stress and associated quality degradation. In contrast, DBD ACP treatment-maintained juice temperatures within a non-thermal range even after 120 s of exposure, thereby preserving thermosensitive bioactive compounds and organoleptic properties. These findings reinforce the potential of DBD ACP as a non-thermal preservation technology for fruit juices, particularly in applications prioritizing nutrient retention and minimal processing.
3.2 Effect of TP and ACP-DBD treatments on microbial stability during storage
Table 2 presents the total plate count (TPC) and yeast and mold (YM) levels in fresh mandarin juice subjected to TP and DBD-ACP treatments at various exposure durations. Microbial stability was monitored over 18 days of storage at 4 °C. The results showed that in untreated control samples, microbial proliferation occurred rapidly. TPC increased from 3.11 ± 0.05 to 8.40 ± 0.15 log CFU/mL, while YM increased from 2.40 ± 0.03 to 5.00 ± 0.10 log CFU/mL by day 18, confirming the high perishability of fresh juice due to its nutrient-rich composition and native microflora. These trends align with previous findings for untreated citrus juices (Tiwari et al., 2009; Wibowo et al., 2015). Results indicate that the TP significantly (p ≤ 0.05) reduced both TPC and YM to non-detectable levels (ND) at day 0, with microbial regrowth delayed until after day 6. By day 18, TPC and YM levels reached 3.05 ± 0.09 and 2.31 ± 0.04 log CFU/mL, respectively. This moderate regrowth may be attributed to post-processing contamination or the recovery of sub-lethally injured microorganisms, as previously observed in TP-treated juices (Pala and Toklucu, 2013). Despite this, microbial counts remained below the spoilage threshold of 6 log CFU/mL, confirming TP’s effectiveness in extending shelf life, albeit at the expense of sensory and nutritional quality (Mandha et al., 2023). In comparison, the antimicrobial efficacy of DBD ACP was strongly dependent on treatment duration. At 30 s, DBD ACP achieved a partial reduction in TPC (2.00 ± 0.04 log CFU/mL), but microbial growth resumed rapidly, reaching 6.55 ± 0.11 log CFU/mL (TPC) and 4.90 ± 0.08 log CFU/mL (YM) by day 18, values comparable to the untreated control. This limited efficacy is consistent with earlier reports highlighting insufficient microbial suppression at shorter plasma exposure times (Luo et al., 2025; Sohbatzadeh et al., 2021). A 60-s DBD ACP treatment demonstrated improved microbial control, delaying exponential microbial growth until the mid-storage stage. However, by day 18, microbial levels (TPC: 5.70 ± 0.11; YM: 3.81 ± 0.05 log CFU/mL) remained significantly higher than those in TP-treated samples, indicating partial but insufficient inactivation. The 90-s DBD ACP treatment yielded more favorable results. No microbial growth was detected until day 3, and by day 18, TPC and YM counts remained relatively low (4.80 ± 0.09 and 2.52 ± 0.04 log CFU/mL, respectively). This trend aligns with previous findings where DBD ACP durations of 90 s or greater significantly delayed microbial regrowth and extended shelf life in various fruit juices (Dasan and Boyaci, 2018). The most pronounced antimicrobial effect was observed with 120 s of DBD ACP treatment. Both TPC and YM remained non-detectable up to day 6, with final counts by day 18 reaching 3.30 ± 0.09 (TPC) and 2.40 ± 0.03 log CFU/mL (YM). These values were comparable to those observed in TP-treated juice, confirming that extended DBD ACP treatment can achieve similar microbial suppression without the need for heat application. The observed microbial inactivation is attributed to the generation of RONS in the plasma field, which disrupts cellular membranes and damages microbial DNA and proteins (Xu and Bassi, 2025). Many studies have reported similar findings, observing microbial suppression and prolonged shelf life in ACP-treated fruit juices with longer treatment durations (Dasan and Boyaci, 2018; Starek et al., 2019; Xiang et al., 2018). Moreover, Figure 2 summarizes and compares the efficacy of TP and various DBD ACP treatments in controlling microbial load over the storage period. In untreated control samples, TPC and YM levels increased steadily from day 0, confirming the high spoilage (Wibowo et al., 2015). TP achieved complete microbial inactivation on day 0 (non-detectable), with regrowth delayed until day 9. Final counts at day 18 remained below spoilage thresholds, consistent with previously reported microbial suppression in thermally pasteurized citrus juices, albeit often accompanied by sensory and nutritional degradation (Basak et al., 2025; Vieira et al., 2018). DBD ACP demonstrated treatment time-dependent antimicrobial activity. The 30-s treatment yielded partial reductions but failed to prevent rapid microbial resurgence by day 18. A 60-s exposure delayed peak microbial growth, yet final counts remained above spoilage limits, reflecting limited long-term efficacy (Luo et al., 2025; Sohbatzadeh et al., 2021). The 90-s treatment showed enhanced suppression, with undetectable microbial counts until day 3 and lower final values compared to shorter treatments (Dasan and Boyaci, 2018). The 120-s DBD ACP treatment exhibited the most effective microbial control, delaying detectable growth until day 9 and resulting in TPC and YM levels comparable to TP by the end of storage. This finding aligns with those of Starek et al. (2019) in ACP-treated apple juice. Collectively, these results underscore the potential of DBD ACP as a non-thermal alternative to conventional pasteurization for enhancing the microbiological safety of fruit juices. However, results obtained also highlight the importance of optimizing treatment duration to achieve sufficient microbial inactivation. Additionally, microbial regrowth observed across all treatments emphasizes the critical role of aseptic packaging and hygienic post-treatment handling in ensuring product safety.
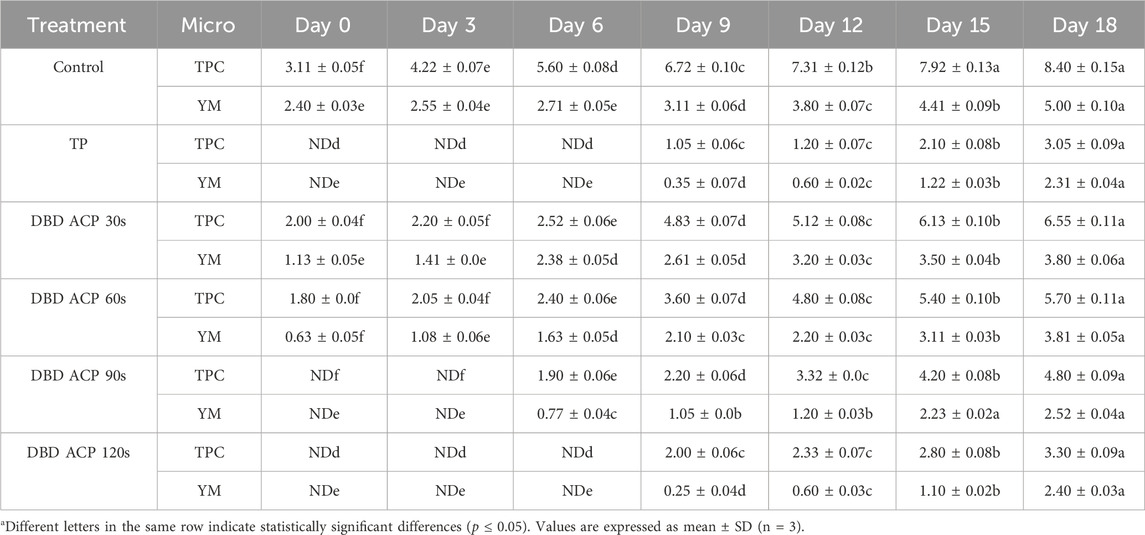
Table 2. Microbial stability in fresh mandarin juice after TP and DBD ACP treatments during storage at 4 °C (log CFU/mL).
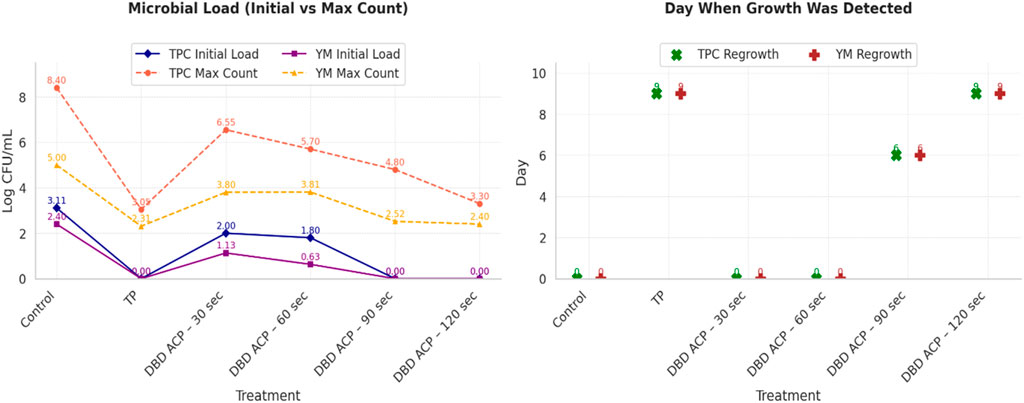
Figure 2. Comparative efficacy of TP and DBD ACP treatments on microbial reduction and regrowth in fresh mandarin juice during the storage period.
3.3 Effect of TP and ACP-DBD treatments on the chemical composition of mandarin juice
The impact of TP and DBD ACP treatments on the chemical composition and antioxidant activity of mandarin juice is presented in Table 3. The parameters evaluated include total sugar content, ascorbic acid content, total phenolics, and antioxidant capacity. Results showed that the total sugar content of untreated (control) mandarin juice was 94.67 ± 0.40 g/L. TP treatment significantly reduced the sugar content to 88.31 ± 0.42 g/L (p ≤ 0.05), likely due to thermal degradation or Maillard-type reactions. In contrast, juices treated with DBD ACP for 30–120 s retained most of their sugar content, with values ranging from 94.40 ± 0.36 to 91.87 ± 0.34 g/L. The minimal reduction observed in the DBD ACP treatments was not statistically significant for the 30- and 60-s durations, indicating that non-thermal plasma processing does not substantially impact sugar integrity. These findings are consistent with those of Umair et al. (2019), who observed minimal changes in the sugar content of carrot juice subjected to non-thermal plasma, suggesting that the short exposure time and ambient temperature of DBD ACP treatments preserve sugar stability. Authors reported that cold plasma treatment at 60 kV for 3–4 min slightly enhanced the sugar content in carrot juice compared to the untreated control. While treatment at 70 kV for 4 min yielded the highest concentrations of sucrose, fructose, and glucose, indicating improved extractability of intracellular sugars, likely due to membrane disruption without the thermal degradation associated with conventional processing. Authors also reported that treatment at 80 kV for 4 min resulted in significant reductions in sugar content, particularly in fructose and glucose. This decline may be attributed to oxidative degradation driven by the excessive generation of reactive species at longer plasma treatment duration. Regarding ascorbic acid content, results showed that the TP treatment led to a marked degradation of ascorbic acid, decreasing from 429 ± 5.2 mg/L in the control to 317 ± 5.1 mg/L, representing a 26% reduction (p ≤ 0.05). Conversely, DBD ACP treatments preserved ascorbic acid content effectively, with values ranging from 423 ± 4.7 to 414 ± 4.9 mg/L. No significant differences were found between the control and any of the DBD ACP-treated samples. This aligns with a prior study by Leite et al. (2021), which demonstrated that ACP could maintain the integrity of heat-sensitive compounds such as ascorbic acid. The degradation observed in thermally processed samples is likely due to the oxidative and thermal lability of ascorbic acid, which is well documented in thermally treated citrus juices (Polydera et al., 2005). In addition, the total phenolic content in the control juice was 615 ± 6.0 mg GAE/L. The TP treatment significantly decreased this value to 476 ± 6.2 mg GAE/L (p ≤ 0.05), indicating phenolic degradation due to heat exposure. DBD ACP treatment for 30 s resulted in a non-significant change (612 ± 5.6 mg GAE/L), whereas longer treatments (90 and 120 s) led to moderate reductions, with values of 572 ± 6.3 and 558 ± 5.8 mg GAE/L, respectively. The preservation of phenolics under ACP conditions may be attributed to the lack of thermal input and limited oxidative stress during plasma exposure. These findings are consistent with the observations reported by Illera et al. (2019) and Leite et al. (2021), who noted the relative stability of polyphenolic compounds during non-thermal plasma treatment of apple juice. In particular, Illera et al. (2019) observed a significant increase in total phenolic content (TPC) following all cold plasma treatments, with the highest increases recorded after 4 and 5 min of exposure 69% and 64%, respectively. Although TPC values declined during subsequent storage, they remained elevated compared to the untreated control even after 28 days of storage. The results of the present study align with these earlier findings. DBD-ACP treatment did not significantly reduce TPC levels for treatment durations up to 60 s. However, a slight reduction in TPC was observed at 90 and 120 s of treatment. Despite this, the TPC in these samples remained higher than that observed in the thermally pasteurized sample. These outcomes suggest that DBD-ACP treatment preserves or enhances TPC more effectively than conventional thermal methods. Nevertheless, further investigation is needed to explain the mechanisms underlying the observed variations in TPC following DBD-ACP treatment, particularly in relation to the complex reaction chemistry of phenolic compounds during plasma exposure. Moreover, results showed that the antioxidant capacity was highest in the control (52.14% ± 0.9%). TP treatment significantly decreased antioxidant activity to 39.22% ± 1.3% (p ≤ 0.05), indicating a loss of bioactive compounds. In contrast, the antioxidant values in DBD ACP-treated samples (50.05% ± 1.2% to 49.09% ± 1.0%) were statistically similar to those in the control, indicating a minimal impact on antioxidant potential. This is consistent with the retention of ascorbic acid and phenolic content in DBD ACP-treated samples, both of which contribute significantly to antioxidant activity. The present findings are in agreement with those reported by Almeida et al. (2015), who investigated the effects of both direct and indirect cold plasma treatments (70 kV) on prebiotic orange juice across various treatment durations (15, 30, 45, and 60 s). Their study demonstrated that cold plasma application had no significant impact on the antioxidant capacity of the juice, regardless of the treatment time. In short, TP treatment resulted in significant reductions in all measured quality parameters of mandarin juice, particularly ascorbic acid (26%) and antioxidant activity (24.8%). In contrast, DBD ACP treatments, particularly at shorter durations (30–60 s), effectively preserved the chemical integrity and antioxidant properties of the juice. These results support the application of DBD ACP as a promising non-thermal technology for juice preservation, capable of maintaining nutritional and functional properties while avoiding the detrimental effects associated with conventional thermal processing. On the other hand, one limitation of this study is the use of high-quality, hand-selected fruits for juice extraction, which likely resulted in a lower initial microbial load. In industrial or bulk processing, such an ideal selection is not always feasible, and microbial contamination may be significantly higher due to variable fruit quality and handling conditions. Therefore, the results observed under these controlled conditions may not fully represent the microbial challenges encountered in industrial processing. Future studies should consider simulating more realistic worst-case contamination scenarios better to assess the robustness and effectiveness of the processing techniques.
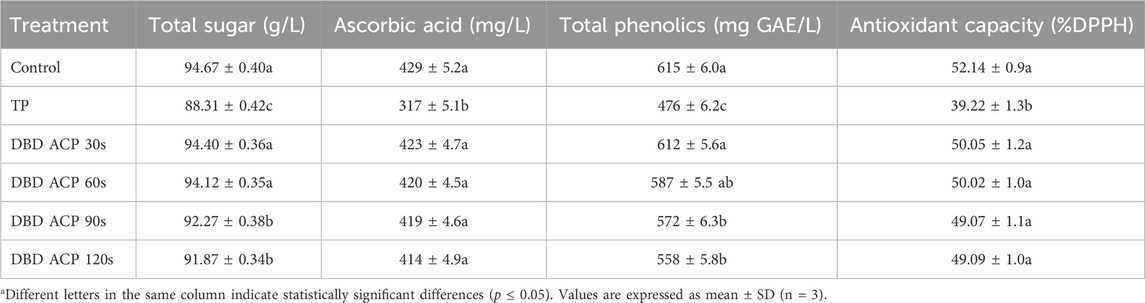
Table 3. The effect of TP and DBD ACP treatments on the chemical composition of fresh mandarin juice.
3.4 Effect of TP and DBD ACP treatments on the enzyme inactivation of fresh mandarin juice
The enzymatic stability of fresh mandarin juice subjected to TP and DBD ACP treatments was assessed by measuring the residual activity of four key enzymes: PPO, POD, PME, and LOX. These enzymes are commonly associated with quality degradation in fruit juices, including browning, the development of off-flavors, and changes in texture (Temiz and Ayhan, 2017). Results showed (Table 4) that the TP treatment resulted in a significant (p ≤ 0.05) reduction in enzyme activity across all four enzymes: PPO, POD, PME, and LOX, which were reduced to 13.70%, 17.55%, 11.30%, and 14.10%, respectively, indicating over 80% inactivation. These results are consistent with a previous study, which indicates that high-temperature treatments are effective in denaturing protein structures and inactivating enzymes (Nonglait et al., 2022). TP disrupts the tertiary and quaternary structures of enzymes, leading to a loss of their catalytic activity. However, while effective, thermal processes can also cause adverse effects on sensory and nutritional qualities (Shourove et al., 2020). In contrast, DBD ACP treatments resulted in a time-dependent but less pronounced reduction in enzyme activity. With increased exposure time, residual enzyme activities progressively significantly (p ≤ 0.05) decreased for PPO, from 86.45% to 70.30%, POD, from 85.20% to 76.15%, PME, from 84.20% to 72.40%, and LOX, from 87.20% to 73.70%, over the 30-s to 120-s time range. Although DBD ACP did not reduce enzymatic activities as drastically as thermal treatment, the trends indicate a moderate but significant (p ≤ 0.05) inactivation, which can be attributed to oxidative stress caused by RONS generated during plasma discharge. These species likely alter side chains and active sites of enzymes, leading to partial deactivation (Zhang et al., 2022). DBD ACP treatments, while less effective, preserved more enzymatic activity, which may be beneficial or detrimental depending on the application. For example, PPO and POD are undesirable due to their role in browning and off-flavor development, PME is involved in pectin degradation, which affects texture and stability, and LOX contributes to oxidative spoilage and off-flavors (Temiz and Ayhan, 2017). In addition, it is suggested that DBD ACP has the potential to inhibit the formation of alkaline compounds from protein decomposition during storage by decreasing microbial growth and retarding endogenous protease activity (Umair et al., 2019). Finally, DBD ACP shows potential as a milder, non-thermal alternative, retaining significant enzymatic activity even after 120 s of treatment. Further research should explore combinatory technologies or longer plasma exposures, enzyme-specific sensitivities, and the effects on sensory/nutritional properties for a holistic assessment.
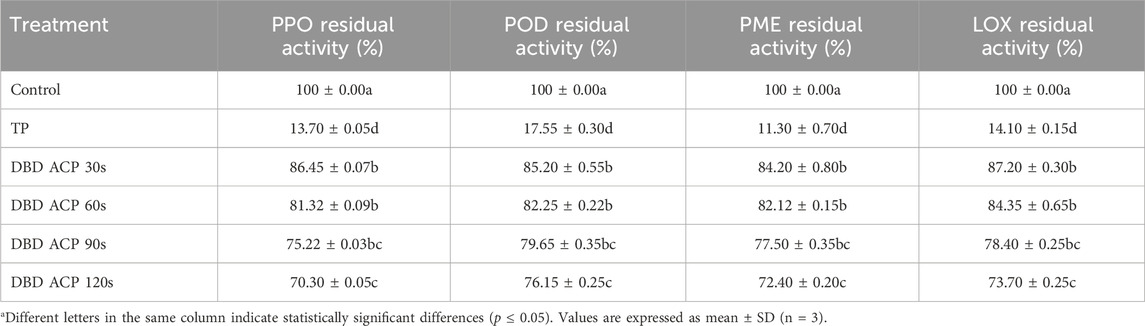
Table 4. The effect of TP and DBD ACP treatments on the enzyme inactivation of fresh mandarin juice.
3.5 Effect of TP and DBD ACP treatments on sensory characteristics of fresh mandarin juice
Sensory evaluation plays a pivotal role in assessing the quality and consumer acceptance of fruit juices, providing direct insight into the organoleptic attributes of color, flavor, aroma, and taste. These parameters are essential in determining product desirability and often reflect subtle physicochemical changes that may not be fully captured through instrumental analysis (Zia et al., 2024). As shown in Table 5, TP caused a significant (p ≤ 0.05) degradation of color, resulting in a darker appearance of the juice compared to the untreated control. Panelists consistently noted that TP-treated samples had reduced visual appeal. This change is attributed to heat-induced non-enzymatic browning reactions, primarily Maillard reactions and pigment oxidation, leading to the breakdown of carotenoids such as β-cryptoxanthin, lutein, and violaxanthin (Aghajanzadeh et al., 2023). DBD ACP treatment, in contrast, exhibited a time-dependent effect on color retention. Shorter treatments (30 and 60 s) preserved color scores statistically similar to the control, while longer exposures (90 and 120 s) induced slight but significant (p ≤ 0.05) darkening. These changes are likely due to oxidative degradation of chromophoric compounds by plasma-generated RONS, especially under extended exposure times (Bayati et al., 2024). However, optimized DBD ACP conditions resulted in only minor and acceptable modifications, consistent with previous findings in kiwi and other fruit juices (Kaur et al., 2024; Mundanat et al., 2025; Pipliya et al., 2023; Zhu et al., 2023). Flavor, aroma, and taste were also significantly affected by processing methods. TP markedly degraded these sensory attributes (p ≤ 0.05), primarily due to the formation of off-flavor compounds such as α-terpineol and the loss of key citrus volatiles, including d-limonene, through Maillard browning, terpene hydration, and thermal degradation (Mundanat et al., 2025). In contrast, DBD ACP treatments at all tested durations preserved these attributes, with sensory scores statistically comparable to the fresh control. The non-thermal nature of DBD ACP minimizes the formation of undesirable volatiles and protects native flavor compounds, maintaining the integrity of citrus aldehydes and esters (Maia et al., 2023; Pipliya et al., 2023; Rodrigues and Fernandes, 2023). Moreover, taste retention under DBD ACP was also attributed to the minimal thermal stress, which preserves the natural balance of sugars and acids, thereby avoiding bitterness and astringency. On the other hand, Lipoxygenase (LOX) is a key enzyme responsible for generating off-flavours in fruit and vegetable juices. It catalyzes the oxidation of polyunsaturated fatty acids, primarily linoleic and linolenic acids, into hydroperoxides, which are further converted into volatile compounds such as hexanal, (E)-2-hexenal, and nonanal. These compounds are associated with “green”, “grassy”, or even “rancid” aromas, especially at higher concentrations. While low levels of these volatiles can contribute to freshness, excessive LOX activity, particularly after juice extraction, processing, or during storage, leads to rapid flavor degradation and reduces consumer acceptability (Baysal and Demirdöven, 2007). TP denatures the enzyme’s protein structure, typically reducing LOX activity and thereby limiting the production of off-flavour volatiles. However, TP can also degrade desirable aroma compounds, negatively affecting the nutritional quality of the juice. In contrast, DBD-ACP treatment is generally less effective at completely inactivating LOX. A study on carrot juice has shown reductions in LOX activity of around 30%–60% after DBD-ACP treatment, leaving a significant portion of the enzyme active (Umair et al., 2019). As a result, juices treated with DBD-ACP may still develop off-flavours over time due to ongoing enzymatic oxidation. In summary, while DBD-ACP helps retain freshness and aroma in the short term, it may not sufficiently inhibit LOX to prevent off-flavour formation during storage. Therefore, balancing LOX inactivation with sensory and nutritional preservation remains a key challenge when selecting juice processing methods. Combining DBD-ACP with LOX inhibitors may enhance flavour stability. These findings highlight the advantage of DBD ACP in maintaining both flavor intensity and overall sensory quality in heat-sensitive juice matrices (Mundanat et al., 2025). In summary, DBD ACP proved superior to TP in preserving the sensory characteristics of mandarin juice. It maintained color vibrancy, retained native flavor and aroma compounds, and prevented the development of off-notes, even at extended treatment durations. These results reinforce the potential of DBD ACP as a non-thermal alternative for producing high-quality, minimally processed fruit juices. Future studies should focus on optimizing plasma parameters, specifically frequency, treatment time, and input power, to balance microbial inactivation efficacy with the preservation of pigments, volatile compounds, and overall sensory appeal.
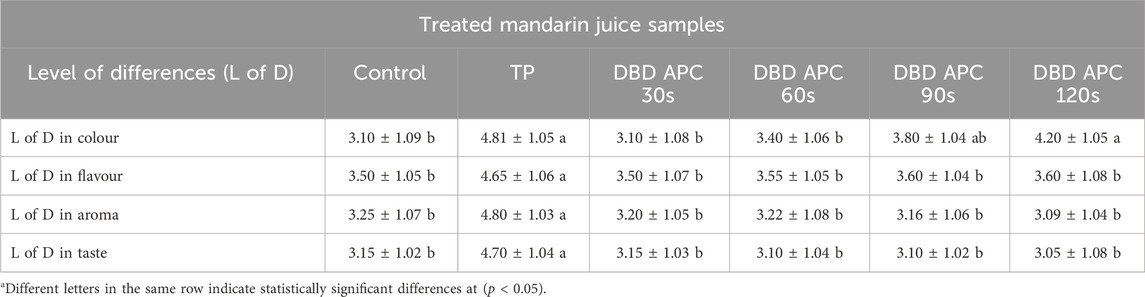
Table 5. Effect of TP and DBD APC treatments on sensory characteristics of fresh mandarin juice using a difference from control. Data expressed as mean ± SD for sensory evaluators, n = 40.
4 Conclusion
This study comprehensively evaluated the impact of TP and DBD ACP on microbial safety, nutritional quality, enzymatic activity, and sensory characteristics of fresh mandarin juice during storage. TP effectively inactivated spoilage microorganisms and key quality-degrading enzymes, including PPO, POD, PME, and LOX, thereby demonstrating its efficacy in extending shelf life. However, the thermal load significantly compromised nutritional and sensory quality, with marked reductions in ascorbic acid, total phenolics, and antioxidant capacity, alongside perceptible degradation in color, aroma, flavor, and taste due to pigment breakdown and the generation of off-flavors. In contrast, DBD ACP treatments maintained a non-thermal profile, with only minor increases in juice temperature even at extended exposure times. This enabled superior retention of ascorbic acid, phenolics, and antioxidant activity, as well as sensory attributes, particularly at shorter durations (30–60 s). Microbial suppression by DBD ACP was strongly time-dependent, with the 120-s treatment achieving microbial loads comparable to TP by the end of storage. However, enzymatic inactivation remained limited across all durations, likely due to the structural resilience of target enzymes and the absence of heat-induced denaturation. Overall, DBD ACP demonstrates strong potential as a non-thermal preservation technology for minimally processed juices, offering effective microbial control and excellent preservation of nutritional and sensory qualities. Nevertheless, its limited enzymatic inactivation suggests that DBD ACP may require integration with complementary strategies, such as mild heat treatment or an enzymatic inhibitor, to ensure complete product stability. Future research should focus on optimizing plasma parameters and exploring synergistic hurdle approaches to enhance enzyme deactivation while maintaining product quality. Moreover, for the successful scale-up of DBD-ACP technology, key factors such as treatment uniformity, exposure time, energy efficiency, equipment design, and cost-effectiveness at industrial volumes must be carefully considered. Addressing these aspects would significantly enhance the practical applicability and commercial potential of the DBD-ACP technique in the juice processing industry.
DBD-ACP showed a promising result regarding the natural microbial load; however, this is not the case in industrial or bulk processing settings. Thus, it is always mandatory to consider the worst-case scenario during research to obtain maximum efficiency during processing. Therefore, testing natural microbial data would be only sufficient to a certain extent in specific conditions, and this is one of the main limitations of this study.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
AA-G: Methodology, Writing – original draft, Visualization. BI: Supervision, Software, Writing – review and editing. YA-H: Writing – original draft, Investigation, Formal Analysis. SA: Validation, Writing – review and editing, Software, Methodology.
Funding
The authors declare that no financial support was received for the research and/or publication of this article.
Acknowledgements
The authors sincerely thank the faculty and staff of the Food Science Department at Tikrit University for their valuable support and contributions to this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbas, H. M., Altamim, E. A., Salama, M., Fouad, M. T., and Zahran, H. A. (2024). Cold plasma technology: a sustainable approach to milk preservation by reducing pathogens and enhancing oxidative stability. Sustainability 16 (20), 8754. doi:10.3390/su16208754
Ağçam, E., and Akyıldız, A. (2014). A study on the quality criteria of some Mandarin varieties and their suitability for juice processing. J. Food Process. 2014 (1), 982721. doi:10.1155/2014/982721
Ağçam, E., Akyıldız, A., and Dündar, B. (2018). “Thermal pasteurization and microbial inactivation of fruit juices,” in Fruit juices (Academic Press), 309–339.
Aghajanzadeh, S., Ziaiifar, A. M., and Verkerk, R. (2023). Effect of thermal and non-thermal treatments on the color of citrus juice: a review. Food Rev. Int. 39 (6), 3555–3577. doi:10.1080/87559129.2021.2012799
Aguiló-Aguayo, I., Sobrino-López, Á., Soliva-Fortuny, R., and Martín-Belloso, O. (2008). Influence of high-intensity pulsed electric field processing on lipoxygenase and β-glucosidase activities in strawberry juice. Innovative Food Sci. and Emerg. Technol. 9 (4), 455–462. doi:10.1016/j.ifset.2007.12.007
Almeida, F. D. L., Cavalcante, R. S., Cullen, P. J., Frias, J. M., Bourke, P., Fernandes, F. A., et al. (2015). Effects of atmospheric cold plasma and ozone on prebiotic orange juice. Innovative Food Sci. and Emerg. Technol. 32, 127–135. doi:10.1016/j.ifset.2015.09.001
Amine, B., Nadir, A., Nouara, K. O., Akila, A. T., Kamelia, K., and Ghania, K. B. (2023). Impact of thermal and non-thermal pasteurization on the microbial inactivation of fruit juice: review. J. Food Microb. Saf. Hyg. 8, 198. doi:10.35248/2476-2059.23.8.198
Basak, S., Thakur, P., and Chakraborty, S. (2025). Pasteurization of Mandarin juice by ohmic heating and evaluation of its shelf-life under refrigerated and ambient conditions. Sustain. Food Technol. 3 (1), 239–252. doi:10.1039/d4fb00267a
Bayati, M., Lund, M. N., Tiwari, B. K., and Poojary, M. M. (2024). Chemical and physical changes induced by cold plasma treatment of foods: a critical review. Compr. Rev. Food Sci. Food Saf. 23 (4), e13376. doi:10.1111/1541-4337.13376
Baysal, T., and Demirdöven, A. (2007). Lipoxygenase in fruits and vegetables: a review. Enzyme Microb. Technol. 40 (4), 491–496. doi:10.1016/j.enzmictec.2006.11.025
Bermúdez-Aguirre, D., Wemlinger, E., Pedrow, P., Barbosa-Cánovas, G., and Garcia-Perez, M. (2013). Effect of atmospheric pressure cold plasma (APCP) on the inactivation of Escherichia coli in fresh produce. Food control. 34 (1), 149–157. doi:10.1016/j.foodcont.2013.04.022
Chen, Y., Yu, L. J., and Rupasinghe, H. V. (2013). Effect of thermal and non-thermal pasteurisation on the microbial inactivation and phenolic degradation in fruit juice: a mini-review. J. Sci. Food Agric. 93 (5), 981–986. doi:10.1002/jsfa.5989
Coutinho, N. M., Silveira, M. R., Rocha, R. S., Moraes, J., Vinicius, M., Ferreira, S., et al. (2018). Cold plasma processing of milk and dairy products. Trends Food Sci. and Technol. 74, 56–68. doi:10.1016/j.tifs.2018.02.008
Dalvi-Isfahan, M., and Mahmoodi-Eshkaftaki, M. (2024). Potential applications of atmospheric-pressure dielectric barrier discharge cold plasma for fruit preservation: advantages, effects on quality characteristics, and limitations. Innovative Food Sci. and Emerg. Technol. 94, 103675. doi:10.1016/j.ifset.2024.103675
Dars, A. G., Hu, K., Liu, Q., Abbas, A., Xie, B., and Sun, Z. (2019). Effect of thermo-sonication and ultra-high pressure on the quality and phenolic profile of mango juice. Foods 8 (8), 298. doi:10.3390/foods8080298
Dasan, B. G., and Boyaci, I. H. (2018). Effect of cold atmospheric plasma on inactivation of Escherichia coli and physicochemical properties of apple, orange, tomato juices, and sour cherry nectar. Food Bioprocess Technol. 11 (2), 334–343. doi:10.1007/s11947-017-2014-0
Dharini, M., Jaspin, S., and Mahendran, R. (2023). Cold plasma reactive species: generation, properties, and interaction with food biomolecules. Food Chem. 405, 134746. doi:10.1016/j.foodchem.2022.134746
Dominguez-López, I., Pérez, M., and Lamuela-Raventós, R. M. (2024). Total (poly) phenol analysis by the folin-ciocalteu assay as an anti-inflammatory biomarker in biological samples. Crit. Rev. Food Sci. Nutr. 64 (27), 10048–10054. doi:10.1080/10408398.2023.2220031
Escudero-López, B., Cerrillo, I., Herrero-Martín, G., Hornero-Méndez, D., Gil-Izquierdo, A., Medina, S., et al. (2013). Fermented orange juice: source of higher carotenoid and flavanone contents. J. Agric. Food Chem. 61 (37), 8773–8782. doi:10.1021/jf401240p
Escudero-López, B., Cerrillo, I., Gil-Izquierdo, Á., Hornero-Méndez, D., Herrero-Martín, G., Berná, G., et al. (2016). Effect of thermal processing on the profile of bioactive compounds and antioxidant capacity of fermented orange juice. Int. J. Food Sci. Nutr. 67 (7), 779–788. doi:10.1080/09637486.2016.1204428
Ferreira, R. M., Amaral, R. A., Silva, A. M., Cardoso, S. M., and Saraiva, J. A. (2022). Effect of high-pressure and thermal pasteurization on microbial and physico-chemical properties of Opuntia ficus-indica juices. Beverages 8 (4), 84. doi:10.3390/beverages8040084
Gupta, A., Sanwal, N., Bareen, M. A., Barua, S., Sharma, N., Olatunji, O. J., et al. (2023). Trends in functional beverages: functional ingredients, processing technologies, stability, health benefits, and consumer perspective. Food Res. Int. 170, 113046. doi:10.1016/j.foodres.2023.113046
Haron, H., Hassan, S., and Keng, C. B. (2017). Evaluation of total phenolic content, antioxidant activities and sugar content of fresh mixed fruit and vegetables juices. J. Sains Kesihat. Malays. 15 (2), 53–58. doi:10.17576/jskm-2017-1502-07
Hou, Y., Wang, R., Gan, Z., Shao, T., Zhang, X., He, M., et al. (2019). Effect of cold plasma on blueberry juice quality. Food Chem. 290, 79–86. doi:10.1016/j.foodchem.2019.03.123
Illera, A. E., Chaple, S., Sanz, M. T., Ng, S., Lu, P., Jones, J., et al. (2019). Effect of cold plasma on polyphenol oxidase inactivation in cloudy apple juice and on the quality parameters of the juice during storage. Food Chem. X 3, 100049. doi:10.1016/j.fochx.2019.100049
Jadhav, H. B., Annapure, U. S., and Deshmukh, R. R. (2021). Non-thermal technologies for food processing. Front. Nutr. 8, 657090. doi:10.3389/fnut.2021.657090
Jiang, H., Lin, Q., Shi, W., Yu, X., and Wang, S. (2022). Food preservation by cold plasma from dielectric barrier discharges in agri-food industries. Front. Nutr. 9, 1015980. doi:10.3389/fnut.2022.1015980
Kaur, S., Kumar, Y., Singh, V., Kaur, J., and Panesar, P. S. (2024). Cold plasma technology: reshaping food preservation and safety. Food Control. 163, 110537. doi:10.1016/j.foodcont.2024.110537
Kelebek, H., and Selli, S. (2014). Identification of phenolic compositions and the antioxidant capacity of mandarin juices and wines. J. Food Sci. Technol. 51, 1094–1101. doi:10.1007/s13197-011-0606-7
Kovačević, D. B., Putnik, P., Dragović-Uzelac, V., Pedisić, S., Jambrak, A. R., and Herceg, Z. (2016). Effects of cold atmospheric gas phase plasma on anthocyanins and color in pomegranate juice. Food Chem. 190, 317–323. doi:10.1016/j.foodchem.2015.05.099
Laroque, D. A., Seó, S. T., Valencia, G. A., Laurindo, J. B., and Carciofi, B. A. M. (2022). Cold plasma in food processing: design, mechanisms, and application. J. Food Eng. 312, 110748. doi:10.1016/j.jfoodeng.2021.110748
Leite, A. K., Fonteles, T. V., Miguel, T. B., da Silva, G. S., de Brito, E. S., Alves Filho, E. G., et al. (2021). Atmospheric cold plasma frequency imparts changes on cashew apple juice composition and improves vitamin C bioaccessibility. Food Res. Int. 147, 110479. doi:10.1016/j.foodres.2021.110479
Luo, X., Ma, Y., Dong, S., Li, X., and Xiang, Q. (2025). Effect of dielectric barrier discharge (DBD) treatment on microbial inactivation and quality attributes of fresh-cut apples. J. Food Meas. Charact. 19, 7165–7176. doi:10.1007/s11694-025-03460-x
Maia, D. L., Rodrigues, S., and Fernandes, F. A. (2023). Influence of glow discharge plasma treatment on cashew apple juice’s aroma profile and volatile compounds. J. Food Process. Preserv. 2023 (1), 1–13. doi:10.1155/2023/7740638
Martínez-Hernández, G. B., Amodio, M. L., and Colelli, G. (2017). Carvacrol-loaded chitosan nanoparticles maintain quality of fresh-cut carrots. Innovative Food Sci. and Emerg. Technol. 41, 56–63. doi:10.1016/j.ifset.2017.02.005
Mishra, R., Chhabra, M., and Prakash, R. (2025). Non-equilibrium cold plasmas and their impacts on physico-chemical properties of food items. Rev. Mod. Plasma Phys. 9 (1), 16. doi:10.1007/s41614-025-00192-9
Misra, N. N., Tiwari, B. K., Raghavarao, K. S. M. S., and Cullen, P. J. (2011). Nonthermal plasma inactivation of food-borne pathogens. Food Eng. Rev. 3 (3), 159–170. doi:10.1007/s12393-011-9041-9
Mundanat, A. S., Singh, V., Talniya, N. C., and Rana, S. S. (2025). Plasma modification in fruit juices: changes in structure, colour, rheological parameters and sensory properties. Food Chem. X 27, 102445. doi:10.1016/j.fochx.2025.102445
Nayak, P. K., Basumatary, B., Chandrasekar, C. M., Seth, D., and Kesavan, R. K. (2020). Impact of thermosonication and pasteurization on total phenolic contents, total flavonoid contents, antioxidant activity, and vitamin C levels of elephant apple (Dillenia indica) juice. J. Food Process Eng. 43 (8), e13447. doi:10.1111/jfpe.13447
Nonglait, D. L., Chukkan, S. M., Arya, S. S., Bhat, M. S., and Waghmare, R. (2022). Emerging non-thermal technologies for enhanced quality and safety of fruit juices. Int. J. Food Sci. Technol. 57 (10), 6368–6377. doi:10.1111/ijfs.16017
Ozen, E., and Singh, R. K. (2020). Atmospheric cold plasma treatment of fruit juices: a review. Trends Food Sci. and Technol. 103, 144–151. doi:10.1016/j.tifs.2020.07.020
Ozen, E., Adhikari, K., and Singh, R. K. (2024). Effect of atmospheric cold plasma on the physicochemical properties and volatile compounds of apple and cantaloupe juices. Food Bioprocess Technol. 17 (12), 5372–5384. doi:10.1007/s11947-024-03458-1
Pala, Ç. U., and Toklucu, A. K. (2013). Microbial, physicochemical and sensory properties of UV-C processed orange juice and its microbial stability during refrigerated storage. LWT-Food Sci. Technol. 50 (2), 426–431. doi:10.1016/j.lwt.2012.09.001
Patras, A., Brunton, N. P., O'Donnell, C., and Tiwari, B. K. (2010). Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci. and Technol. 21 (1), 3–11. doi:10.1016/j.tifs.2009.07.004
Petruzzi, L., Campaniello, D., Speranza, B., Corbo, M. R., Sinigaglia, M., and Bevilacqua, A. (2017). Thermal treatments for fruit and vegetable juices and beverages: a literature overview. Compr. Rev. Food Sci. Food Saf. 16 (4), 668–691. doi:10.1111/1541-4337.12270
Pihen, C., López-Malo, A., and Ramírez-Corona, N. (2024). Effect of UV LED and pulsed light treatments on polyphenol oxidase activity and Escherichia coli inactivation in apple juice. J. Agric. Food Chem. 72 (25), 14294–14301. doi:10.1021/acs.jafc.3c08888
Pipliya, S., Kumar, S., and Srivastav, P. P. (2023). Effect of dielectric barrier discharge nonthermal plasma treatment on physicochemical, nutritional, and phytochemical quality attributes of pineapple (Ananas comosus (L.)) juice. J. Food Sci. 88 (11), 4403–4423. doi:10.1111/1750-3841.16767
Polydera, A. C., Stoforos, N. G., and Taoukis, P. S. (2005). Quality degradation kinetics of pasteurised and high pressure processed fresh navel orange juice: nutritional parameters and shelf life. Innovative Food Sci. and Emerg. Technol. 6 (1), 1–9. doi:10.1016/j.ifset.2004.10.004
Rodrigues, S., and Fernandes, F. A. (2023). Effect of dielectric barrier discharge plasma treatment in pasteurized orange juice: changes in volatile composition, aroma, and mitigation of off-flavors. Food Bioprocess Technol. 16 (4), 930–939. doi:10.1007/s11947-022-02976-0
Roobab, U., Abida, A., Madni, G. M., Ranjha, M. M. A. N., Zeng, X. A., Khaneghah, A. M., et al. (2023). An updated overview of ultrasound-based interventions on bioactive compounds and quality of fruit juices. J. Agric. Food Res. 14, 100864. doi:10.1016/j.jafr.2023.100864
Ruxton, C. H., and Myers, M. (2021). Fruit juices: are they helpful or harmful? An evidence review. Nutrients 13 (6), 1815. doi:10.3390/nu13061815
Saeeduddin, M., Abid, M., Jabbar, S., Wu, T., Hashim, M. M., Awad, F. N., et al. (2015). Quality assessment of pear juice under ultrasound and commercial pasteurization processing conditions. LWT-Food Sci. Technol. 64 (1), 452–458. doi:10.1016/j.lwt.2015.05.005
Shourove, J. H., Zzaman, W., Chowdhury, R. S., and Hoque, M. M. (2020). Effect of thermal treatment on physicochemical stability and antioxidant properties of locally available underutilized star fruit juice. Asian Food Sci. J. 14 (3), 41–53. doi:10.9734/afsj/2020/v14i330133
Sohbatzadeh, F., Yazdanshenas, H., Soltani, A. H., and Shabannejad, A. (2021). An innovative strategy to rapidly inactivate 8.2-log Enterococcus faecalis in fresh pineapple juice using cold atmospheric plasma. Sci. Rep. 11 (1), 16010. doi:10.1038/s41598-021-95452-2
Starek, A., Pawłat, J., Chudzik, B., Kwiatkowski, M., Terebun, P., Sagan, A., et al. (2019). Evaluation of selected microbial and physicochemical parameters of fresh tomato juice after cold atmospheric pressure plasma treatment during refrigerated storage. Sci. Rep. 9 (1), 8407. doi:10.1038/s41598-019-44946-1
Temiz, A., and Ayhan, D. K. (2017). “Enzymes in minimally processed fruits and vegetables,” in Minimally processed refrigerated fruits and vegetables (Boston, MA: Springer US), 93–151.
Tiwari, B. K., O’Donnell, C. P., Muthukumarappan, K., and Cullen, P. J. (2009). Effect of sonication on orange juice quality parameters during storage. Int. J. Food Sci. Technol. 44 (3), 586–595. doi:10.1111/j.1365-2621.2008.01858
Umair, M., Jabbar, S., Nasiru, M. M., Sultana, T., Senan, A. M., Awad, F. N., et al. (2019). Exploring the potential of high-voltage electric field cold plasma (HVCP) using a dielectric barrier discharge (DBD) as a plasma source on the quality parameters of carrot juice. Antibiotics 8 (4), 235. doi:10.3390/antibiotics8040235
Umair, M., Jabeen, S., Ke, Z., Jabbar, S., Javed, F., Abid, M., et al. (2022). Thermal treatment alternatives for enzymes inactivation in fruit juices: recent breakthroughs and advancements. Ultrason. Sonochemistry 86, 105999. doi:10.1016/j.ultsonch.2022.105999
Vaka, U., and Ramkumar, M. C. (2024). Application of non-thermal plasma technology for enhancing food processing and storage: a review. Food Chem. Adv. 5, 100788. doi:10.1016/j.focha.2024.100788
Vieira, F. N., Lourenço, S., Fidalgo, L. G., Santos, S. A., Silvestre, A. J., Jerónimo, E., et al. (2018). Long-term effect on bioactive components and antioxidant activity of thermal and high-pressure pasteurization of orange juice. Molecules 23 (10), 2706. doi:10.3390/molecules23102706
Villanueva, N. D. M., Petenate, A. J., and Silva, M. (2005). Performance of the hybrid hedonic scale as compared to the traditional hedonic, self-adjusting, and ranking scales, 16, 691–703.
Wibowo, S., Grauwet, T., Santiago, J. S., Tomic, J., Vervoort, L., Hendrickx, M., et al. (2015). Quality changes of pasteurised orange juice during storage: a kinetic study of specific parameters and their relation to colour instability. Food Chem. 187, 140–151. doi:10.1016/j.foodchem.2015.03.131
Xiang, Q., Liu, X., Li, J., Liu, S., Zhang, H., and Bai, Y. (2018). Effects of dielectric barrier discharge plasma on the inactivation of Zygosaccharomyces rouxii and quality of apple juice. Food Chem. 254, 201–207. doi:10.1016/j.foodchem.2018.02.008
Xu, Y., and Bassi, A. (2025). Non-thermal plasma decontamination of microbes: a state of the art. Biotechnol. Prog. 41 (2), e3511. doi:10.1002/btpr.3511
Yasoob, A. N., Abdalameer, N. K., and Mohammed, A. Q. (2022). Plasma production and applications: a review. Int. J. Nanosci. 21 (06), 2230003. doi:10.1142/s0219581x22300036
Yaya-González, A., Laika, J., and Peralta-Ruiz, Y. (2025). Emerging nonthermal technologies for food safety: trends, limitations, and future research. Curr. Opin. Chem. Eng. 49, 101178. doi:10.1016/j.coche.2025.101178
Zargarchi, S., Hornbacher, J., Afifi, S. M., Saremnezhad, S., Günal-Köroğlu, D., Capanoglu, E., et al. (2024). Exploring the impact of cold plasma treatment on the antioxidant capacity, ascorbic acid, phenolic profile, and bioaccessibility of fruits and fruit juices. Food Front. 5 (3), 1108–1125. doi:10.1002/fft2.372
Zhang, Z. H., Wang, L. H., Zeng, X. A., Han, Z., and Brennan, C. S. (2019). Non-thermal technologies and its current and future application in the food industry: a review. Int. J. Food Sci. Technol. 54 (1), 1–13. doi:10.1111/ijfs.13903
Zhang, B., Tan, C., Zou, F., Sun, Y., Shang, N., and Wu, W. (2022). Impacts of cold plasma technology on sensory, nutritional and safety quality of food: a review. Foods 11 (18), 2818. doi:10.3390/foods11182818
Zhu, Y., Zhang, M., Mujumdar, A. S., and Liu, Y. (2023). Application advantages of new non-thermal technology in juice browning control: a comprehensive review. Food Rev. Int. 39 (7), 4102–4123. doi:10.1080/87559129.2021.2021419
Zia, H., Slatnar, A., Košmerl, T., and Korošec, M. (2024). A review study on the effects of thermal and non-thermal processing techniques on the sensory properties of fruit juices and beverages. Front. Food Sci. Technol. 4, 1405384. doi:10.3389/frfst.2024.1405384
Keywords: mandarin juice, dielectric barrier discharge, thermal pasteurization, microbial inactivation, sensory attributes
Citation: Abdul-Ghaffar AN, Iqdiam BM, Al-Hadidy YI and Abed SA (2025) Comparative effects of dielectric barrier discharge atmospheric cold plasma and thermal pasteurization on mandarin juice safety and quality during storage. Front. Food Sci. Technol. 5:1692060. doi: 10.3389/frfst.2025.1692060
Received: 25 August 2025; Accepted: 29 October 2025;
Published: 14 November 2025.
Edited by:
Francesco Donsi’, University of Salerno, ItalyReviewed by:
Shima Saffarionpour, University of Toronto, CanadaDharini Manoharan, University of Wisconsin–Platteville, United States
Copyright © 2025 Abdul-Ghaffar, Iqdiam, Al-Hadidy and Abed. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Basheer M. Iqdiam, YmFzaGlyLm1AdHUuZWR1Lmlx
 Ali Nazar Abdul-Ghaffar1
Ali Nazar Abdul-Ghaffar1 Basheer M. Iqdiam
Basheer M. Iqdiam Yasmeen I. Al-Hadidy
Yasmeen I. Al-Hadidy