- 1Faculty of Biology and Medicine, University of Lausanne, Lausanne, Switzerland
- 2Sports and Exercise Medicine Center, Swiss Olympic Medical Center, Lausanne University Hospital, Lausanne, Switzerland
- 3Institute of Sports Sciences, University of Lausanne, Lausanne, Switzerland
- 4Sports Sciences Department, AudioVitality, Lausanne, Switzerland
Objective: Low-Frequency Vibration (LFV) is a type of sound therapy used for relaxation and stress management. This study investigated the effects of LFV on heart rate variability (HRV), compared to a session without any vibrations (No-vibration) in healthy male participants.
Methods: Intra-individual comparative study: participants experienced two blinded 40-minutes sessions, separated by a week of wash-out period, a LFV and a No-vibration one, in a soundproof environment. HRV temporal and frequential parameters were measured before, during, and after each session.
Results: Both sessions showed a decrease in heart rate between pre-session (64.2 ± 1.9 and 61 ± 1.9 BPM) and during intervention (58.7 ± 2.1 and 58.6 ± 1.7). Only LFV was associated with enhanced HRV variables at 30 min post-intervention compared to pre-session (78.9 ± 15.1 u.a vs. 112.6 ± 27.8 u.a). LFV significantly increased parasympathetic activity, as evidenced by higher HRV variables measures 30 min post-session, compared to the No-vibration session (p = 0.007).
Conclusion: Vagal tone was improved 30 min after a LFV session in healthy active male participants, indicating its potential utility as a recovery modality. Further research is warranted to assess long-term effects and applications in diverse populations.
1 Introduction
Vibroacoustic therapy (VAT) is a type of sound therapy that involves passing low frequency sine-wave vibrations into the body. This method aims to provide both auditory and vibratory stimuli (1). Many recent studies have analyzed the effects of VAT on pain management and on spasticity in post-stroke neurorehabilitation showing a neuromodulator and a muscle relaxation effect (2, 3). Low-frequency vibration (LFV) is a form of VAT that is emphasizes its effect on vibrations and is not sound-based. However there is significant variability in how VAT is applied across studies, including differences in frequency ranges, session durations, and exposure conditions (4). In most research, a frequency of 40 Hz is used for its potential relaxation effects after a 20 min exposure (5–7). VAT is also hypothesized to enhance cardiac vagal regulation and physical relaxation by reducing heart rate (HR) (8, 9). Indeed, it has been showed that VAT can interact with tissues through mechanotransduction via the Pacini corpuscles and Merkel cells, directly influencing the autonomic nervous system (ANS) (10). AudioVitality®, a company specialized in VAT, has developed a unique approach involving 40-minute sessions with a combination of 40–80 Hz fundamental frequencies and harmonics. Such harmonic distortions have been shown to generate specific electrical responses in the human brain (11). AudioVitality® RubesaSounds™ apply harmonic distortions in the original and unique context of continuous sounds.
Heart rate variability (HRV), a non-invasive measure of autonomic cardiac control, is increasingly used to assess stress and recovery (12–14). The utility of HRV as a broad-spectrum health indicator with possible application to both clinical and to healthy population has only begun to be explored recently. HRV is offering insight into the balance and interactions between the sympathetic and parasympathetic branches of the ANS (15). High HRV temporal metrics generally indicating greater parasympathetic activity and better recovery (16, 17). Some key HRV metrics, including the root mean square of successive beat-to-beat interval differences (RMSSD) and the high-frequency (HF) power spectrum, have been shown to reflect cardiac vagal tone, giving clinical insight on recovery status in patient (17, 18). Recent studies have shown that VAT, can positively affect the parasympathetic nervous system, particularly in post-exercise recovery (19, 20). Enhanced vagal tone, which correlates with improved well-being and performance, has been observed with multiple interventions using vibrations (21–23). Despite VAT and LFV showing promising benefits in managing stress, the number of objective research on LFV's impact on heart rate variability (HRV) is limited.
The objective of this study was to examine the effects of Low-Frequency Vibration (LFV) on short-term cardiac autonomic regulation in healthy male participants.
2 Materials and methods
2.1 Study group
Twenty-nine male participants were included in the study. Participants were recruited through posters posted on the university campus, at the sports medicine unit from Lausanne University Hospital or by direct contact with sports club. The participants had to be regularly active [Tier 1–2 of participant Classification Framework (24)], men, between the age of 18 and 40 and in good general health. The participants were excluded from the study if they already participated in a AudioVitality® or any other LFV session, suffered from any acute injury or pathology that may prevent the proper course of the study or endanger their own health. We also excluded participants with known sound hypersensibility and those not accepting to be informed of incidental findings. Every participant was contacted by one of the co-investigators for an initial screening of the inclusion and exclusion criteria. Participants signed an informed consent before the beginning of the study.
In total, 29 participants were included in the study. Among them, two were excluded from the analysis: one because he freely decided to stop after the first session and one who showed signs of arrhythmia on heart rate recordings and was referred for a cardiac check-up. A final number of 27 men were included in the analysis. The mean age of the population was 28 ± 5 years, mean height was 182.8 ± 6.7 cm, and mean weight was 79.4 ± 9.8 kg.
2.2 Experimental design
This prospective observational study focused on the state of HRV variables before, during and after a LFV session and a No-vibration session with a within-subjects analysis.
The participants only received partial information on LFV prior to the study to avoid any expectation bias and offer a semi-blinded design. Randomization in the order of the sessions was not achievable to avoid expectation bias because LFV is felts significantly by the participants. All participants went then through the No-vibration session first, and the LFV session second. The participants were told they would go through two different kinds of LFV sessions without any explanations of the No-vibration session. A detailed explanation of the entire process, including a description of both sessions and their differences was given to every participant at the end of the study.
Every session took place in AudioVitality® soundproof studios (Figure 1). The participants were lying on a bed for the whole intervention. The beginning of each AudioVitality® session is guided by a pre-recorded voice to help the participant to deeply relax before the session. This 4-minutes introduction was performed in both sessions (No-vibration and LFV). The first session consisted of a 40-minute-long silence with no frequencies emitted (referred as No-vibration in the article). HRV was monitored during the entire session. After the session, the participants stayed in the studios for another 30 min to allow for multiple post-session short-term HRV measurements. The second session took place in the same conditions and consisted of a standardized 40-minute low-frequency sound session. The experimental procedures were explained to all the participants, following the Declaration of 92 Helsinki, and approved by the local ethics committee (CER-VD #2023-01296).

Figure 1. AudioVitality® soundproof studio. Participants were lying on the bed during the entire intervention. Low-frequency sounds vibrations were emitted from the 3 bass speakers, all oriented towards the participant.
2.3 Heart rate variability
2.3.1 Acquisition
R-R intervals were collected during the entire protocol using a heart rate monitor (H10, Polar Electro Oy, Kempele, Finland) (25) and a dedicated smartphone-based application (Polar Sensor Logger app, Jukka Happonen, Finland). The measurement started 5 min before the session and lasted until 30 min post-session.
Analyses were performed in supine position on six different five-minutes samples taken at specific time point. Pre-session (pre), at 20 min during the session (during-20), at 40 min during the session (during-40), 5 min post session (post), 15 min post-session (post-15) and 30 min post-session (post-30).
2.3.2 Confounding factors
As HRV is influenced by many external factors and has high inter-individual variations, a within-subject design has been highly recommended in the literature (26). This design helps to offer optimal experimental control, contribute to the reduction of individual differences in respiratory rates, offer an increased statistical power, and reduce the impact of external factors (17). As testing occurred with 1 week in between, the time of experimentation was kept exactly the same for each individuals to avoid circadian differences in HRV (27).
We designed the study to control as much as possible external factors, including the measuring environment, which was kept as stable as possible (place, time, room temperature, air humidity, noise, devices used, persons present during the measurements). Furthermore, all electronic devices were removed from the proximity of the participant during the study.
We instructed participants to strictly control internal factors, which mainly comprise: stopping intense physical efforts 48 h before examination, stop alcohol and analgesics 12 h before, avoid wearing confining clothes, corsets or supports stockings on the day of their venue and avoid any intake of nicotine, coffee and food 3 h before examination (28). The importance of coming to both visits in similar settings was repeated to the participants.
Prior to the start of the session, participants were asked to go to the bathroom if needed and then lie down in the studio comfortably. To keep the measurements as standardized as possible, communication between the investigator and the participant was restricted to the minimum.
2.3.3 Analyses
The analysis of raw R-R intervals at the 6 time points was conducted using Kubios Premium software (Kubios, Finland). Each dataset underwent a manual and automatic review in Kubios Premium to identify and correct any artifacts or ectopic beats (29, 30). Time- and frequency-domain HRV analyses were conducted on the final 4 min of five-minute R-R interval samples in a supine position, adhering to the international standards (31, 32). Following the latest guidelines by Laborde et al. (17), HRV was assessed in three conditions: resting (measured during a five-minute supine rest prior to sessions), reactivity HRV (analyzing changes in HRV variables from baseline to during-event), and recovery HRV (evaluating changes in HRV variables from during-event to post-event measurements). This three phase experimental structure is advocated as optimal for HRV analysis (17, 33), facilitating comprehensive assessments at each phase and measuring HRV's stimulus-response capabilities. Focus was given to vagally-related HRV variables, logarithmic transformation of the Root Mean Square of Successive Differences (LnRMSSD), Heart Rate (HR) and Low-Frequency + High-Frequency divided by Heart Rate [(LF + HF)/HR]. All the variables are further described in the cited sources (34). We calculated LnRMSSD with a logarithm transformation from RMSSD creating a new variable. Applying a logarithmic transformation (Ln) to RMSSD helps normalize the data distribution, thereby reducing the impact of extreme values and improving comparability between individuals (35). Moreover, LnRMSSD has been shown to be a reliable and sensitive indicator of recovery and training load, particularly in elite athletes (36).
2.4 Low-frequency sounds
To accurately analyze inter-participants responses, the exact same protocol of low-frequency sounds was used for every LFVsession. It consisted of twelve, equally long, continuous sounds. Each sound followed the same cycle: 3 s of linear fade-in (from silence to “full intensity”), 3 min of sounds at full intensity (70 dB), 3 s of linear fade-out (from full intensity to silence), and finally 9 s of silence before starting the cycle again for the next sound. At “full intensity”, the sound pressure level, measured at the head was, on average, 72 dB. Each sound contains a low-frequency fundamental frequency (between 40 and 80 Hz) with several of its harmonics. The fundamental frequency was the most prominent (intensity-wise). Harmonics did not exceed 1,000 Hz. The relationship between the sounds' harmonics is a copyrighted technology AudioVitality® RubesaSounds™.
Sounds were diffused through three speakers mounted to the ceiling of the room. All speakers were focused on the center of the room, where the bed was positioned. One speaker was located above the head, and the other two towards the bottom of the bed, one bye each foot. A subwoofer was also used under the bed, pointing towards the bottom of the bed.
2.5 Statistical analysis
All data passed normality Shapiro–Wilk test and are expressed as mean ± standard deviation (SD). All data were presented as absolute and as normalized to a percentage of individual pre- values. HRV time-course analysis as a function of session (No-vibration or LFV) was analyzed using a 2 (session) × 6 (recovery time points) simple repeated-measures ANOVA. The Bonferroni correction post hoc test was applied when F was significant in the ANOVA. For all statistical analyses, an α value of 0.05 was accepted as the level of statistical significance. Statistical analyses were performed with JASP version 0.18.3.
A two-way repeated measures ANOVA with SESSION (LFV vs. No-vibration) and TIME (PRE, DURING, POST) as within-subject factors was conducted to assess the effects of the session type and measurement timing on key HRV-related variables.
In our study, all missing values were handled using the Mean Substitution method. Specifically, we replaced missing values (n = 12) with the mean of the available data for the corresponding variable. This method is widely used in scientific research as it helps maintain the overall distribution characteristics of the dataset while minimizing data loss (37). Additionally, mean substitution provides a straightforward approach to handling missing data, especially when the proportion of missing values is low, ensuring that the integrity of the dataset is preserved for subsequent analyses (38).
3 Results
3.1 Comparison between sessions in pre-session values
Pre-session HR was statistically slightly higher in LFV in comparison to No-vibration (p-value 0.023) (Figure 2). However, no significant difference was found in pre-session lnRMSSD or (LF + HF)/HR between the two sessions (p-value 0.164 and p-value 0.645 respectively) (Figure 3).
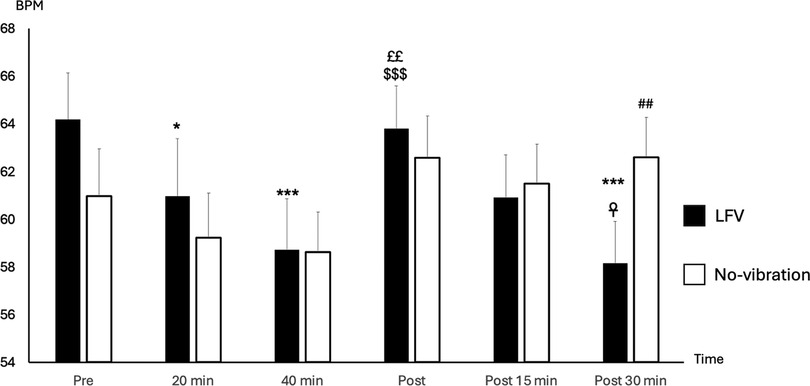
Figure 2. State of HR (BPM) according to time in No-vibration and LFV. State of heart rate (BPM during sessions phases in two tests conditions (black: LFV; white: No-vibration). *p < 0.05 significant differences with Pre; ***p < 0.001 significant differences with Pre; ££p < 0.01 significant difference with 20 min; $$$p < 0.001 significant difference with 40 min; ☥p < 0.05 significant difference with Post; ##p < 0.01 significant difference with LFV session. The analysis revealed significant main effects of SESSION [F(1,130) = 6.12, p = .0147, η2 ≈ 0.045] and TIME [F(2,130) = 5.71, p = .0042, η2 ≈ 0.042]. A highly significant SESSION × TIME interaction was also observed [F(2,130) = 36.45, p < .0001, η2 ≈ 0.22], showing a more pronounced heart rate reduction following the LFV session.
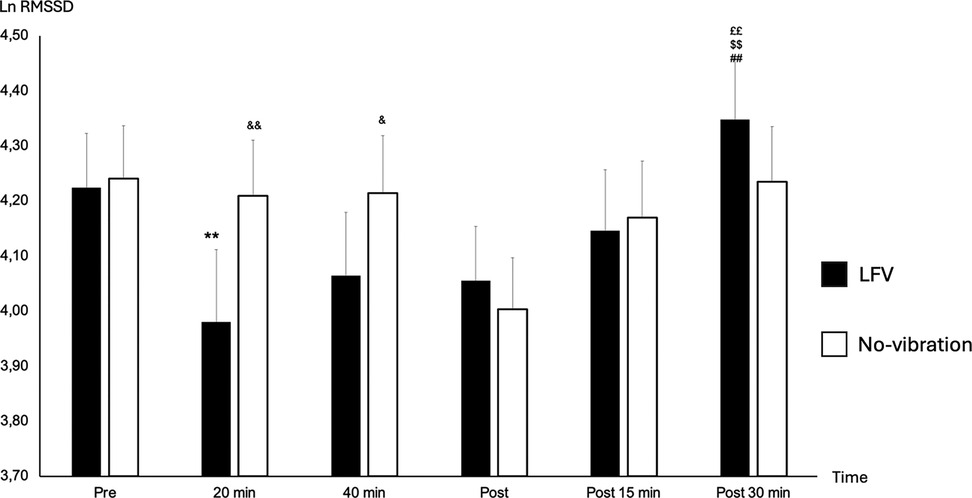
Figure 3. State of lnRMSSD according to time in No-vibration and LFV. State of Ln RMSSD during sessions phases in two tests conditions (black: LFV; white: No-vibration). **p < 0.01 significant differences with PRE; ££p < 0.01 significant differences with 20 min; $$p < 0.01 significant differences with 40 min; ##p < 0.01 significant differences with post 5 min; &p < 0.05 significant differences with LFV session; &&p < 0.01 significant differences with LFV session. A significant main effect of SESSION was found [F(1,130) = 4.61, p = .034, η2 ≈ 0.035], along with a significant main effect of TIME [F(2,130) = 6.56, p = .0019, η2 ≈ 0.048]. There was also a significant SESSION × TIME interaction [F(2,130) = 32.51, p < .0001, η2 ≈ 0.20], indicating a stronger time-dependent modulation under the LFV condition.
3.2 Reactivity HRV
The reactivity HRVs (between pre and during-20) were not different in all variables for LFV and No-vibration session (p-value >0.05). A significant drop in HR between pre- and during-40 was found in both sessions (p-value <0.001). Additionally, we found a significant difference between No-vibration and LFV at the during-20 time point showing a drop of RMSSD only in the LFV session (p-value <0.001).
3.3 Comparison between pre-session and post-30 min in both sessions
A significant time effect between pre- and post-30 was observed for HR (p-value 0.003), which decreased, and (LF + HF)/HR which increased in the LFV session only (Figure 4) (p-value 0.035). No significant difference was observed in RMSSD between pre- and post-30 in both sessions (p-value >0.05).
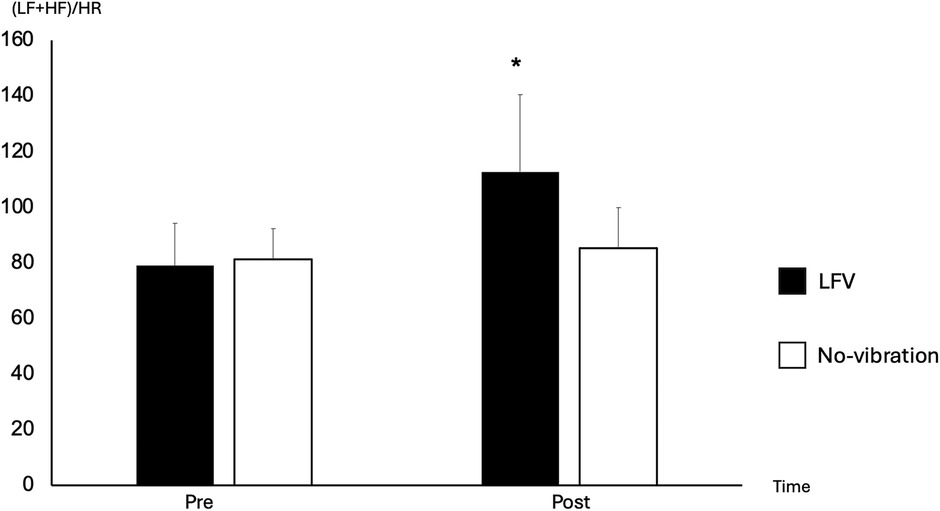
Figure 4. Comparison of (LF + HF)/HR between pre- and post-30 in No-vibration and LFV. Focus on the differences of (LF + HF)/HR between pre- and post 30 in LFV (black) and No-vibration (white), *p < 0.05 significant differences with pre-. We can see a 166% increase in (LF + HF)/HR between pre- and post-30 in the LFV session vs. 121% in the No-vibration session. A strong main effect of SESSION was observed [F(1,130) = 27.24, p < .0001, η2 ≈ 0.17], while the TIME main effect was not significant [F(2,130) = 1.67, p = .1917]. However, the SESSION × TIME interaction was significant [F(2,130) = 19.66, p < .0001, η2 ≈ 0.13], suggesting a treatment-specific effect in the modulation of the (LF + HF)/HR ratio.
3.4 Recovery HRV
The recovery HRVs (comparison between during-20 and post-30) were significant in LFV for (LF + HF)/HR (p-value 0.007) and lnRMSSD (p-value 0.015). No significant difference was seen between during-20 and post-30 in all HRV variables and HR for the No-vibration sessions (Figure 3).
3.5 Comparison between both sessions in post-30 values
Post 30 min HR and HRV variables were not significantly different in both sessions (p-value >0.05).
4 Discussion
This study aimed to investigate how HRV was modulated during and after a LFV session compared to a No-vibration session in healthy male participants. Consistent with the literature on whole-body vibrations (39, 40), our findings revealed a significant increase in some parasympathetic nervous system metrics 30 min after the LFV session.
It is well known that other interventions such as mindfulness and short sleep can positively influence HRV variables (41–43). Both sessions created relaxing environment, as participants were free from distractions during the intervention. We observed HR fluctuations during the No-vibration and the LFV session with a significant decrease between baseline and during the intervention, showing an increase in parasympathetic tone in both sessions, that could be attributed to the environment of the experiment.
We found significant reactivity HRVs (changes in HRV variables between pre- and during-20) only for the LFV session in lnRMSSD, while the No-vibration session showed no difference in HRV. Additionally, a significant difference was found at during-20 between both sessions with a lower lnRMSSD in LFV. LFV therefore seems to create an acute stress reaction on the body of the participants, showing a decrease in HRV variables during the session. It has been previously demonstrated that chronic exposure to high dose of low-frequency noise during a lifetime can induce vibroacoustic disease, showcasing the stressor effect of such frequencies on the body (44, 45).
In contrast, recovery HRVs (comparison between during-20 and post-30) were found to be statistically different in two variables (lnRMSSD and LF + HF/HR) for the LFV session only. As this increase of HRV variables was also coupled to a decrease in HR, we can assume an enhanced parasympathetic tone thus favoring recovery capacity of the body after a LFV session in comparison to the No-vibration session. When analyzing the reactivity and recovery HRV metrics during the LFV session, we observe a highly dynamic pattern, indicating a significant physiological and psychological response to the vibrations. In contrast, the No-vibration session demonstrates relatively stable HRV metrics, reflecting minimal changes in reactivity and recovery. The effect of a controlled acute stressor on the body could therefore lead to an enhanced recovery reaction of the body.
Comparing HRV variables between baseline (pre-session) and after the intervention (post-30), we found no significant difference in all HRV variables and HR in the No-vibration session between pre- and post-30 measures. In contrast, LFV is showing a relative boost (140.68%) in vagally-mediated HRV variables at 30 min post-session in (LF + HF)/HR coupled by a decrease of HR. A better understanding of the physiological process of LFV would help understanding how the recovery could be affected by this enhancement of HRV variables at 30 min post LFV.
Protocols of other studies investigating the link between LFV and HRV being widely unhomogenized especially in the type of vibrations and length of exposition, a direct comparison of our results is not possible. Most of the literature is based on shorter LFV sessions or is analyzing long-term effect of professional exposure to LFV (5–7).
Despite variations in exposure frequencies, LFV has consistently been shown to influence HRV. LFV has been shown to modulate the ANS through various physiological mechanisms, primarily via mechanotransduction and neural stimulation. Mechanotransduction, the process by which mechanical stimuli like vibration are converted into biochemical signals, occurs through specialized mechanoreceptors such as Pacinian corpuscles and Ruffini endings (46). These receptors transmit afferent input to the central nervous system, where some signals—particularly those originating from visceral or cervical regions—may engage vagal afferents, contributing to increased vagal tone and parasympathetic activation (47–49).
Additionally, vibration therapy has been linked to pain relief, supported by the Gate Control Theory of Pain, where mechanoreceptive input overrides nociceptive signals, thus reducing pain and indirectly modulating the ANS by decreasing stress (50, 51). Low-frequency vibrations influence central nervous system pathways in the brainstem, creating a positive feedback loop, leading to greater parasympathetic activation and a relaxation response (52–54). Finally, vibration affects muscle spindles and Golgi tendon organs, proprioceptive receptors that play a key role in muscle tension and tone regulation, modulating both muscular and autonomic responses (46, 55). The modulation of blood flow, facilitated by vibration-induced muscle stimulation and enhanced circulation, may further promote parasympathetic dominance, influencing blood pressure regulation and overall autonomic tone (56, 57). Collectively, these mechanisms suggest that LFV can be a valuable tool for enhancing autonomic regulation.
Our study offers a new perspective on exposure to LFV on HRV. It is important to note that AudioVitality's technology is unique and therefore making a direct comparison with previous studies impossible. Nevertheless, our study results are going in the same direction as previous literature, showing a positive effect of LFV on HRV mediated variables and ANS stimulation. Our study-design additionally offers an intra-subjects' comparison which limit the high inter-individual's differences in HRV. Further studies with a similar technology could confirm the validity of these results and put into perspectives the validity of our results.
4.1 Limitations and recommendations
In our study, HRV measurements were limited to 30 min post-intervention, raising questions about the duration of the post-treatment effects. It remains unclear whether the observed effects persist for a significant period, warranting further research into the chronic impact of LFV. Additionally, our study examined the effects of a single LFV session on HRV, while most AudioVitality protocols involve multiple sessions. Despite the short duration of our protocol, the results offer promising insights into the acute effects of LFV and highlight the need to explore its potential long-term benefits.
The study group consisted of healthy participants with no major symptoms of fatigue nor pain. The extrapolation of the results to other populations will need to be further assessed in future studies.
The study's design was constrained by the available technology, as both LFV and No-vibration sessions needed to occur in the same specialized environment. The lack of randomization possible with such technology created an inherent limitation to our study reducing internal validity. Another potential limitation of within-subject designs is participant habituation to experimental conditions (17). Nonetheless, our within-subject comparison helps mitigate high inter-individual variability, strengthening the reliability of the results despite these constraints.
We implemented strict standardization in measures, including pre-test instructions and consistent measurement protocols. However, some variations were still observed, such as a higher resting heart rate in the LFV session. This suggests that stricter control of external factors affecting HRV could be beneficial to reduce variability and enhance the reliability of future studies on LFV.
5 Conclusion
This study aimed to assess the impact of LFV on the ANS by measuring heart rate variability in a healthy active male cohort. Our findings demonstrated a significant increase in some vagal tone markers post-intervention in the LFV session compared to the No-vibration session. Although our technology differs from those commonly cited in the literature, the results align with other. Enhancing vagal tone through LFV could introduce a novel approach to recovery technologies, with potential applications extending to stress management. Further research on LFV is needed to validate these findings and explore broader applications.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Ethics statement
The study involving humans was approved by Commission cantonale d'éthique de la recherche sur l'être humain (CER-VD). The study was conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
RH: Investigation, Visualization, Writing – original draft. CB: Formal analysis, Writing – review & editing. FD: Data curation, Writing – review & editing. VG: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article. This study has been conducted without any external funding. The material was put to disposition by AudioVitality. The cost of the ethics committee was covered by AudioVitality. AudioVitality was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of interest
FD is the head of the sports sciences department at AudioVitality and contributed to the writing of this article. FD did not intervene in data collection, participants recruitments nor HRV analyses.
The remaining author(s) declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Møller H, Pedersen CS. Hearing at low and infrasonic frequencies. Noise Health. (2004) 6(23):37–57.
2. Kantor J, Campbell EA, Kantorová L, Marečková J, Regec V, Karasová K, et al. Exploring vibroacoustic therapy in adults experiencing pain: a scoping review. BMJ Open. (2022) 12(4):e046591. doi: 10.1136/bmjopen-2020-046591
3. Kantor J, Kantorová L, Marečková J, Peng D, Vilímek Z. Potential of vibroacoustic therapy in persons with cerebral palsy: an advanced narrative review. Int J Environ Res Public Health. (2019) 16(20):3940. doi: 10.3390/ijerph16203940
4. Punkanen M, Ala-Ruona E. Contemporary vibroacoustic therapy: perspectives on clinical practice, research, and training. Music Med. (2012) 4(3):128–35. doi: 10.47513/mmd.v4i3.316
5. Campbell EA, Hynynen J, Burger B, Ala-Ruona E. Exploring the use of vibroacoustic treatment for managing chronic pain and comorbid mood disorders: a mixed methods study. Nord J Music Ther. (2019) 28(4):291–314. doi: 10.1080/08098131.2019.1604565
6. Campbell EA, Hynynen J, Ala-Ruona E. Vibroacoustic treatment for chronic pain and mood disorders in a specialized healthcare setting. Music Med. (2017) 9(3):187–97. doi: 10.47513/mmd.v9i3.540
7. Rüütel E, Vinkel I, Eelmäe P. The effects of short-term vibroacoustic treatment on spasticity and perceived health conditions of patients with spinal cord and brain injuries. Music Med. (2017) 9(3):202–8. doi: 10.47513/mmd.v9i3.541
8. Bartel L, Mosabbir A. Possible mechanisms for the effects of sound vibration on human health. Healthcare. (2021) 9(5):597. doi: 10.3390/healthcare9050597
9. Delmastro F, Martino FD, Dolciotti C. Physiological impact of vibro-acoustic therapy on stress and emotions through wearable sensors. 2018 IEEE International Conference on Pervasive Computing and Communications Workshops (PerCom Workshops) (2018). p. 621–26
10. Piccinin MA, Miao JH, Schwartz J. Histology, meissner corpuscle. In: StatPearls. Treasure Island (FL): StatPearls Publishing (2025).
11. Leino S, Brattico E, Tervaniemi M, Vuust P. Representation of harmony rules in the human brain: further evidence from event-related potentials. Brain Res. (2007) 1142:169–77. doi: 10.1016/j.brainres.2007.01.049
12. Grossman P, Taylor EW. Toward understanding respiratory sinus arrhythmia: relations to cardiac vagal tone, evolution and biobehavioral functions. Biol Psychol. (2007) 74(2):263–85. doi: 10.1016/j.biopsycho.2005.11.014
13. Porges SW. The polyvagal theory: new insights into adaptive reactions of the autonomic nervous system. Clevel Clin J Med. (2009) 76(Suppl 2):S86–90. doi: 10.3949/ccjm.76.s2.17
14. Thayer JF, Lane RD. Claude bernard and the heart-brain connection: further elaboration of a model of neurovisceral integration. Neurosci Biobehav Rev. (2009) 33(2):81–8. doi: 10.1016/j.neubiorev.2008.08.004
15. Drury RL, Porges S, Thayer J, Ginsberg JP. Editorial: heart rate variability, health and well-being: a systems perspective. Front Public Health. (2019) 7:323. doi: 10.3389/fpubh.2019.00323
16. Taskforce. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task force of the European society of cardiology and the north American society of pacing and electrophysiology. Eur Heart J. (1996) 17(3):354–81. doi: 10.1093/oxfordjournals.eurheartj.a014868
17. Laborde S, Mosley E, Thayer JF. Heart rate variability and cardiac vagal tone in psychophysiological research – recommendations for experiment planning, data analysis, and data reporting. Front Psychol. (2017) 8:213. doi: 10.3389/fpsyg.2017.00213
18. Shaffer F, McCraty R, Zerr CL. A healthy heart is not a metronome: an integrative review of the heart’s anatomy and heart rate variability. Front Psychol. (2014) 5:1040. doi: 10.3389/fpsyg.2014.01040
19. Licurci MDGB, de Almeida Fagundes A, Arisawa EALS. Acute effects of whole body vibration on heart rate variability in elderly people. J Bodyw Mov Ther. (2018) 22(3):618–21. doi: 10.1016/j.jbmt.2017.10.004
20. Wong A, Figueroa A. Effects of whole-body vibration on heart rate variability: acute responses and training adaptations. Clin Physiol Funct Imaging. (2019) 39(2):115–21. doi: 10.1111/cpf.12524
21. Pham T, Lau ZJ, Chen SHA, Makowski D. Heart rate variability in psychology: a review of HRV indices and an analysis tutorial. Sensors. (2021) 21(12):3998. doi: 10.3390/s21123998
22. Dong J-G. The role of heart rate variability in sports physiology. Exp Ther Med. (2016) 11(5):1531–36. doi: 10.3892/etm.2016.3104
23. Aubert AE, Seps B, Beckers F. Heart rate variability in athletes. Sports Med. (2003) 33(12):889–919. doi: 10.2165/00007256-200333120-00003
24. McKay AKA, Stellingwerff T, Smith ES, Martin DT, Mujika I, Goosey-Tolfrey VL, et al. Defining training and performance caliber: a participant classification framework. Int J Sports Physiol Perform. (2022) 17(2):317–31. doi: 10.1123/ijspp.2021-0451
25. Schaffarczyk M, Rogers B, Reer R, Gronwald T. Validity of the polar H10 sensor for heart rate variability analysis during resting state and incremental exercise in recreational men and women. Sensors. (2022) 22(17):6536. doi: 10.3390/s22176536
26. Quintana DS, Heathers JAJ. Considerations in the assessment of heart rate variability in biobehavioral research. Front Psychol. (2014) 5:805. doi: 10.3389/fpsyg.2014.00805
27. van Eekelen AP, Houtveen JH, Kerkhof GA. Circadian variation in cardiac autonomic activity: reactivity measurements to different types of stressors. Chronobiol Int. (2004) 21(1):107–29. doi: 10.1081/cbi-120027983
28. Ziemssen T, Siepmann T. The investigation of the cardiovascular and sudomotor autonomic nervous system-a review. Front Neurol. (2019) 10:53. doi: 10.3389/fneur.2019.00053
29. Tarvainen MP, Niskanen JP, Lipponen JA, Ranta-Aho PO, Karjalainen PA. Kubios HRV–heart rate variability analysis software. Comput Methods Programs Biomed. (2014) 113(1):210–20. doi: 10.1016/j.cmpb.2013.07.024
30. Lipponen JA, Tarvainen MP. A robust algorithm for heart rate variability time series artefact correction using novel beat classification. J Med Eng Technol. (2019) 43(3):173–81. doi: 10.1080/03091902.2019.1640306
31. Taskforce. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task force of the European society of cardiology and the north American society of pacing and electrophysiology. Circulation. (1996) 93(5):1043–65. doi: 10.1161/01.CIR.93.5.1043
32. Bourdillon N, Schmitt L, Yazdani S, Vesin J-M, Millet GP. Minimal window duration for accurate HRV recording in athletes. Front Neurosci. (2017) 11:456. doi: 10.3389/fnins.2017.00456
33. Berna G, Ott L, Nandrino J-L. Effects of emotion regulation difficulties on the tonic and phasic cardiac autonomic response. PLoS One. (2014) 9(7):e102971. doi: 10.1371/journal.pone.0102971
34. Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health. (2017) 5:258. doi: 10.3389/fpubh.2017.00258
35. Buchheit M. Monitoring training status with HR measures: do all roads lead to Rome? Front Physiol. (2014) 5:73. doi: 10.3389/fphys.2014.00073
36. Esco MR, Flatt AA. Ultra-short-term heart rate variability indexes at rest and post-exercise in athletes: evaluating the agreement with accepted recommendations. J Sports Sci Med. (2014) 13(3):535–41.25177179
37. Little RJA, Rubin DB. Statistical Analysis With Missing Data, 3rd ed. Hoboken, NJ: John Wiley & Sons, Inc (n.d.).
39. Jalilian H, Zamanian Z, Gorjizadeh O, Riaei S, Monazzam MR, Abdoli-Eramaki M. Autonomic nervous system responses to whole-body vibration and mental workload: a pilot study. Int J Occup Environ Med. (2019) 10(4):174–84. doi: 10.15171/ijoem.2019.1688
40. Choi W, Mizukami K. The effect of whole body vibration by sonic waves on mood, the autonomic nervous system, and brain function in elderly. Nihon Ronen Igakkai Zasshi. (2020) 57(4):441–9. doi: 10.3143/geriatrics.57.441
41. Bernardi L, Sleight P, Bandinelli G, Cencetti S, Fattorini L, Wdowczyc-Szulc J, et al. Effect of rosary prayer and yoga mantras on autonomic cardiovascular rhythms: comparative study. Br Med J. (2001) 323(7327):1446–9. doi: 10.1136/bmj.323.7327.1446
42. Laborde S, Allen MS, Borges U, Dosseville F, Hosang TJ, Iskra M, et al. Effects of voluntary slow breathing on heart rate and heart rate variability: a systematic review and a meta-analysis. Neurosci Biobehav Rev. (2022) 138:104711. doi: 10.1016/j.neubiorev.2022.104711
43. Besson C, De Stefani G, Baggish AL, Schmitt L, Millet G, Gremeaux V. Comparison of 1-hour floatation-REST versus conventional napping on heart rate variability in active individuals. BMJ Open Sport Exerc Med. (2024) 11(1):e002292. doi: 10.1136/bmjsem-2024-002292
44. Alves-Pereira M, Castelo Branco NA. Vibroacoustic disease: biological effects of infrasound and low-frequency noise explained by mechanotransduction cellular signalling. Prog Biophys Mol Biol. (2007) 93(1–3):256–79. doi: 10.1016/j.pbiomolbio.2006.07.011
46. Proske U, Gandevia SC. The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force. Physiol Rev. (2012) 92(4):1651–97. doi: 10.1152/physrev.00048.2011
47. Lehrer P, Kaur K, Sharma A, Shah K, Huseby R, Bhavsar J, et al. Heart rate variability biofeedback improves emotional and physical health and performance: a systematic review and meta analysis. Appl Psychophysiol Biofeedback. (2020) 45(3):109–29. doi: 10.1007/s10484-020-09466-z
48. Lundeberg T. Long-term results of vibratory stimulation as a pain relieving measure for chronic pain. Pain. (1984) 20(1):13–23. doi: 10.1016/0304-3959(84)90807-8
49. Eshuis TAH, Stuijt PJC, Timmerman H, Nielsen PML, Wolff AP, Soer R. Music and low-frequency vibrations for the treatment of chronic musculoskeletal pain in elderly: a pilot study. PLoS One. (2021) 16(11):e0259394. doi: 10.1371/journal.pone.0259394
50. Melzack R, Wall PD. Pain mechanisms: a new theory. Science. (1965) 150(3699):971–9. doi: 10.1126/science.150.3699.971
51. Casale R, Hansson P. The analgesic effect of localized vibration: a systematic review. Part 1: the neurophysiological basis. Eur J Phys Rehabil Med. (2022) 58(2):306–15. doi: 10.23736/S1973-9087.22.07415-9
52. Wall PD, Cronly-Dillon JR. Pain, itch, and vibration. Arch Neurol. (1960) 2:365–75. doi: 10.1001/archneur.1960.03840100003002
53. Doi A, Sakasaki J, Tokunaga C, Sugita F, Kasae S, Nishimura K, et al. Both ipsilateral and contralateral localized vibratory stimulations modulated pain-related sensory thresholds on the foot in mice and humans. J Pain Res. (2018) 11:1645–57. doi: 10.2147/JPR.S162379
54. Pantaleo T, Duranti R, Bellini F. Effects of vibratory stimulation on muscular pain threshold and blink response in human subjects. Pain. (1986) 24(2):239–50. doi: 10.1016/0304-3959(86)90046-1
55. Yoon J-Y, Kang S-R, Kim H-S, Won YH, Park S-H, Seo J-H, et al. Effects of low-frequency whole-body vibration on muscle activation, fatigue, and oxygen consumption in healthy young adults: a single-group repeated-measures controlled trial. J Sport Rehabil. (2022) 31(8):984–92. doi: 10.1123/jsr.2021-0170
56. Lohman EB 3rd, Bains GS, Lohman T, DeLeon M, Petrofsky JS. A comparison of the effect of a variety of thermal and vibratory modalities on skin temperature and blood flow in healthy volunteers. Med Sci Monit. (2011) 17(9):MT72–81. doi: 10.12659/MSM.881921
Keywords: heart rate variability, autonomic nervous system, whole-body vibration, low-frequency sound, recovery, sport performance
Citation: Hauser R, Besson C, Degache F and Gremeaux V (2025) Heart rate variability response to low-frequency sounds vibrations in regularly active male subjects. Front. Sports Act. Living 7:1573660. doi: 10.3389/fspor.2025.1573660
Received: 9 February 2025; Accepted: 26 May 2025;
Published: 27 June 2025.
Edited by:
Lei Zhang, First Affiliated Hospital of Wenzhou Medical University, ChinaReviewed by:
Robet L. Drury, ReThink Health, United StatesJan Gajewski, Józef Piłsudski University of Physical Education in Warsaw, Poland
Copyright: © 2025 Hauser, Besson, Degache and Gremeaux. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rafaël Hauser, cmFmYWVsLmhhdXNlckB1bmlsLmNo
†These authors share last authorship
 Rafaël Hauser
Rafaël Hauser Cyril Besson
Cyril Besson Francis Degache
Francis Degache Vincent Gremeaux
Vincent Gremeaux