- 1Department of Biomechanics and Sports Injuries, Faculty of Physical Education and Sports Sciences, Kharazmi University, Tehran, Iran
- 2Research Unit “Sport Sciences, Health and Movement”, Higher Institute of Sports and Physical Education of Kef, University of Jendouba, Kef, Tunisia
- 3Training Department, Police College, Police Academy, Doha, Qatar
Introduction: This study aimed to quantify shoulder muscle synergies during badminton forehand overhead smash (BFOS) via non-negative matrix factorization (NMF), validate musculoskeletal (MSK) models for high-speed movements by comparing electromyography (EMG)-derived synergies with simulation results, and explore the potential of NMF-based MSK models in advancing sports science.
Methods: Twenty elite badminton players (age: 24 ± 4 years; experience: 15 ± 4 years) performed maximal-effort BFOS while EMG signals from fifteen shoulder muscles were recorded. Three-dimensional motion analysis with a ten-camera Vicon system captured kinematic data at 100 Hz. A validated OpenSim upper extremity model was implemented to simulate muscle activations via static optimization. NMF extracted synergy vectors and activation coefficients from both experimental EMG and MSK modeling data.
Results: Three muscle synergies accounted for >90% variance in both analyses with no significant differences in global VAF (p = 0.12). The first synergy (trapezius-dominant) showed 95% EMG and 97% MSK variance; the second synergy (pectoralis/anterior deltoid) exhibited 97% EMG and 94% MSK variance; the third synergy (posterior muscles) demonstrated 95% EMG and 98% MSK variance. Strong agreement between approaches was observed for both weight vectors (W1:0.81 ± 0.04, W2:0.87 ± 0.01, W3:0.88 ± 0.03) and activation coefficients (C1:0.95 ± 0.02, C2:0.98 ± 0.01, C3:0.98 ± 0.01), with differences primarily in lower trapezius activation (similarity: 0.77 ± 0.05), likely due to challenges in recording deep muscle activity through surface electromyography. These findings validate the combined experimental-computational approach for analyzing complex, high-velocity movements.
Conclusion: The strong correspondence between experimental and computational synergies validates MSK modeling for analyzing neuromuscular control during high-velocity overhead movements. The identified synergies provide a framework for understanding muscle coordination during BFOS, with potential applications in targeted training program optimization and injury prevention strategies in overhead sports.
1 Introduction
The badminton forehand overhead smash (BFOS) represents one of the most explosive strokes in racquet sports, with elite players generating shuttlecock velocities exceeding 118 m/s (1). This remarkable velocity requires exceptional neuromuscular coordination through a kinetic chain from lower extremity to racquet-shuttlecock impact (2). The repetitive and explosive nature of the BFOS places significant stress on the shoulder complex, contributing to 1%–5% of sports-related shoulder injuries attributed to badminton (2, 3).
The shoulder joint complex, with its multiple articulations and extensive musculature, transfers energy from trunk to upper extremity during overhead movements while maintaining dynamic control despite limited inherent stability (4, 5). Previous investigations have documented substantial biomechanical demands during the BFOS, with elite players generating shoulder internal rotation moments of 0.85 ± 0.12 Nm/kg and peak shoulder internal rotation velocities reaching 7148°/s (6). These velocities are comparable to baseball pitching (7200°/s) (7) and exceed tennis serving (2900°/s) (7) and volleyball spiking (2594°/s) (5, 7), highlighting the sophisticated coordination strategies required (8).
The central nervous system (CNS) faces considerable computational challenges in controlling the redundant musculoskeletal system during complex movements (9). The muscle synergy hypothesis provides a theoretical framework suggesting that the CNS simplifies this control problem by activating functional groups of muscles (synergies) rather than individual muscles independently (10). This modular organization potentially reduces the dimensionality of motor control and facilitates efficient movement execution (11). Non-negative matrix factorization (NMF) has emerged as a robust computational technique for extracting underlying muscle synergies from electromyographic (EMG) data (12). This algorithm decomposes multi-muscle activation patterns into a small set of time-invariant muscle weightings (synergy vectors) and their corresponding time-varying activation coefficients (12, 13). However, recording EMG data from all relevant muscles during high-speed actions presents significant technical challenges (7, 14), particularly for deep or inaccessible muscles that contribute substantially to shoulder function during overhead movements. Within the context of overhead sports movements, previous investigations have identified consistent synergy structures across various upper extremity tasks, including reaching (7, 15), grasping (8), and throwing (8, 15). Pale et al. (16) demonstrated that approximately three to four synergies could account for over 90% of the variance in muscle activation patterns during hand grasps, with synergy structures showing strong within-subject consistency but notable inter-subject variability. Nevertheless, the application of muscle synergy analysis to high-velocity overhead sports movements remains underexplored, particularly in badminton (17).
Recent advances in musculoskeletal (MSK) modeling have enabled comprehensive analysis of complex movements through integration of anatomical, physiological, and biomechanical principles (18). These computational approaches complement experimental measurements by providing estimates of variables that cannot be directly measured in vivo, such as individual muscle forces, joint contact forces, and moment arms (19). The OpenSim modeling framework has gained widespread adoption for biomechanical investigation, offering validated models of the upper extremity with physiologically accurate muscle parameters (20). MSK models can generate muscle activation predictions through optimization algorithms that distribute joint moments across available muscles (21). Static optimization, for example, minimizes the sum of squared muscle activations subject to moment equilibrium constraints, providing computationally efficient estimates of muscle coordination patterns (22). However, the biological validity of these computational predictions requires thorough experimental validation, particularly for complex multi-joint movements involving rapid accelerations (23). Recent studies have demonstrated that MSK modeling can effectively capture muscle synergies during dynamic movements (18). These models have successfully replicated walking and running patterns by optimizing a small number of motor control parameters (18). However, the unique demands of the BFOS, particularly on the shoulder complex, require more targeted investigations. Several investigators have compared experimentally measured EMG signals with model-predicted activations during various tasks, reporting moderate to strong correlations in controlled movements (24). Nevertheless, the accuracy of MSK model predictions during high-velocity overhead sports movements remains incompletely characterized (25), particularly regarding muscle synergy structures.
Despite extensive research on overhead throwing biomechanics, several knowledge gaps persist. First, while individual muscle activations have been characterized during badminton smashes (25, 26), the underlying synergistic control structures remain poorly understood. Second, although MSK models have been validated for various upper extremity movements (14), their applicability to high-velocity overhead sports movements requires further investigation. Third, the relationship between experimentally measured and computationally predicted muscle synergies during complex sports movements has not been thoroughly examined (17).
The objectives of this study are threefold. First, we aim to quantify shoulder muscle synergies during the forehead overhead smash via NMF. Second, we seek to validate MSK models for high-speed movements by comparing EMG-derived synergies with simulation results. Third, we aim to explore the potential of NMF-based MSK models in advancing sports science. We hypothesized that (1) three to four muscle synergies would account for >90% of the variance in muscle activation patterns during BFOS execution, (2) computational MSK modeling would produce synergy structures with strong similarity to those derived from experimental EMG recordings, and (3) key differences between experimental and computational approaches would primarily involve deep muscles that present challenges for surface EMG recording.
2 Methods
2.1 Study design
This cross-sectional, observational biomechanical study employed a repeated-measures design to analyze muscle synergies during the execution of the Badminton Forehand Overhead Smash (BFOS).
2.2 Participants
Twenty elite badminton players (all right-handed) with extensive competitive experience (15 ± 4 years) participated. All participants competed at national or international levels for a minimum of 4 years. All participants used their personal competition-grade equipment during testing. While racket specifications (weight: 85-95 g, length: 675-680 mm) and string tension (24-28 lbs) varied within tournament-legal ranges, these parameters were recorded and verified to be within ±5% variance for primary metrics. Individual technical styles were assessed by a qualified coach to ensure fundamental execution mechanics aligned with standard forehand overhead smash technique. The cohort displayed the following anthropometric characteristics: age (24 ± 4 years), height (175 ± 8.3 cm), and body mass (71 ± 15.2 kg). a priori power analysis using G*Power (version 3.1.9.7) based on previous muscle synergy investigations in comparable athletic populations (3, 21) determined that 20 participants would provide statistical power of 0.85 with an effect size of 0.65 (medium-to-large) at α=0.05 for detecting significant differences in muscle activation patterns. This sample size ensured adequate statistical power while maintaining homogeneity in the participant cohort.
Inclusion criteria encompassed: professional badminton players with ≥10 years of competitive experience, absence of shoulder or upper limb pathology within six months, active competitive participation within 12 months, and ability to execute proper maximal-effort BFOS. Exclusion criteria included musculoskeletal injuries during testing, surgical interventions affecting the upper limb within 24 months, biomechanical limitations, and concurrent participation in similar studies.
This investigation received approval from the Research Ethics Committee of Kharazmi University (Approval Code: IR.KHU.REC.1402.020) and adhered to the principles outlined in the Declaration of Helsinki (2013 revision). All participants provided written informed consent after receiving detailed explanations of the study procedures, potential risks, and benefits. Participants were informed of their right to withdraw at any time without consequence. Data confidentiality was maintained throughout the investigation with all personal identifiers removed before analysis. The protocol was preregistered in the Open Science Framework (OSF) database (osf.io/twx5h) prior to participant enrollment.
2.3 Instrumentation and data acquisition
2.3.1 Motion capture system
Kinematic data were captured using ten Vicon high-speed cameras (100 Hz) following validated methodologies for overhead sports movements (6, 8). This system has demonstrated excellent test-retest reliability (ICC > 0.95) and concurrent validity (r > 0.92) (6). Thirty-four retroreflective markers positioned at standardized anatomical landmarks enabled precise reconstruction throughout BFOS execution. System calibration was performed before each testing session using a standardized 5-point wand method achieving residual errors <0.5 mm.
2.3.2 Surface electromyography (EMG)
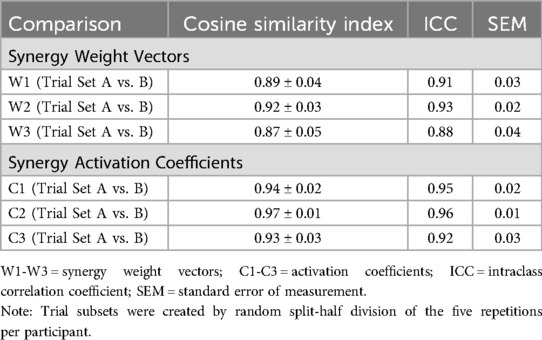
EMG signals from fifteen muscles were recorded using a Myon 320 wireless system (1000 Hz sampling frequency, CMRR > 100 dB). The muscle groups monitored included anterior, middle, and posterior deltoid; infraspinatus; upper, middle, and lower pectoralis major; latissimus dorsi; lateral and medial triceps; biceps brachii; upper, middle, and lower trapezius; and serratus anterior. Surface preparation followed standardized SENIAM protocols (15, 26), and bipolar Ag/AgCl electrodes (10 mm diameter, 20 mm inter-electrode distance) were positioned parallel to muscle fiber orientation (9, 15, 26) (Figure 1).
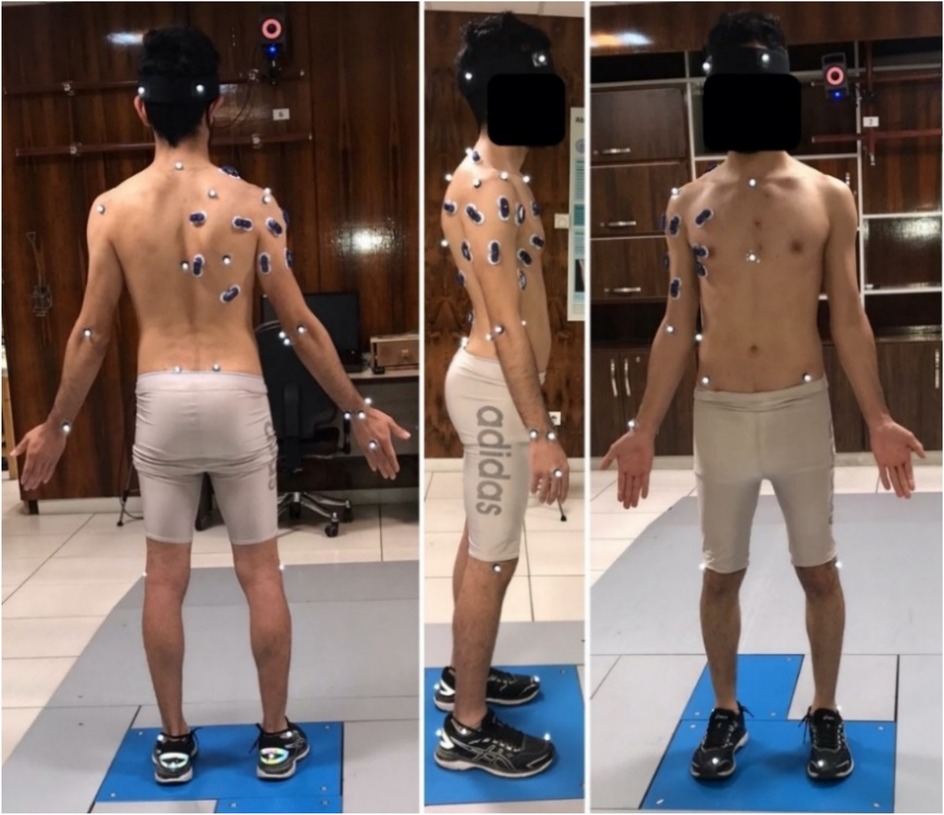
Figure 1. Experimental setup showing Vicon motion capture system with retroreflective markers and surface EMG electrodes monitoring 15 shoulder/arm muscles (anterior/middle/posterior deltoid, infraspinatus, pectoralis major, latissimus dorsi, triceps, biceps, trapezius, serratus anterior) during badminton overhead smash execution. [Reprinted from (25) with permission].
All EMG signals underwent comprehensive preprocessing following established protocols for high-velocity movements (27). Raw signals were first inspected for artifacts and quality using signal-to-noise ratio assessment (threshold >20 dB). Bandpass filtering employed a zero-lag 4th-order Butterworth filter to minimize phase distortion while preserving physiologically relevant frequency content. The 50 Hz notch filter width was set at 1 Hz bandwidth (Q-factor=50) to selectively remove power line interference while preserving adjacent signal components. Full-wave rectification preserved signal energy, followed by smoothing with a critically damped 20 Hz low-pass filter (equivalent to 25 ms moving average window) to create linear envelopes that effectively captured muscle activation dynamics without excessive signal attenuation.
2.4 Experimental protocol
2.4.1 Preparation and familiarization
Participants attended a familiarization session prior to data collection to become acquainted with procedures. On testing day, participants completed a 15-minute neuromuscular preparation protocol consistent with established racquet sports practices (28).
2.4.2 Movement task execution
Each participant performed five maximal-effort BFOS trials with 60-second recovery intervals. A standardized shuttlecock suspension system positioned the shuttle at 2.8 m height. Task execution was monitored for technique consistency by an experienced coach (Figure 2), and any trials with technical errors or excessive marker occlusion were excluded and repeated. A minimum of three valid trials per participant were required for inclusion in analysis.
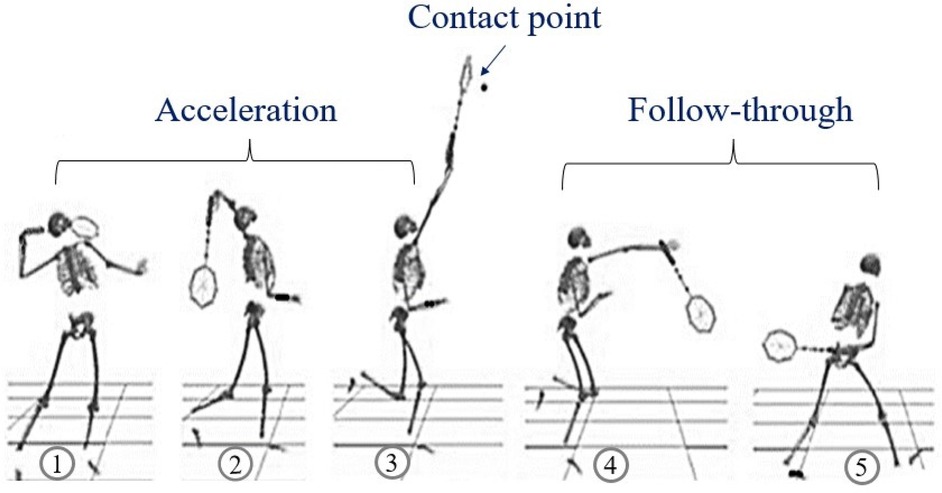
Figure 2. Sequential phases of the badminton forehand overhead smash (BFOS), preparation, acceleration, impact, and follow-through. Movement analysis was based on racquet velocity thresholds. [Reprinted from (25) under CC BY 4.0 license].
2.5 Musculoskeletal modeling
2.5.1 Model development and implementation
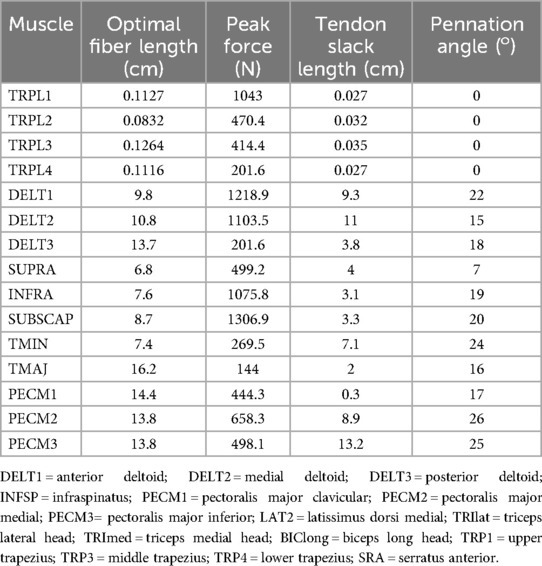
The computational framework employed the Wu upper extremity musculoskeletal model (29) implemented in OpenSim (version 4.5), incorporating three degrees of freedom at the scapulothoracic, acromioclavicular, and glenohumeral joints. The model integrates 32 Hill-type musculotendon actuators with physiologically-based parameters including optimal fiber length, tendon slack length, pennation angle, maximum isometric force, and force-length-velocity relationships (30). Subject-specific scaling was applied based on marker positions during static trials.
2.5.2 Simulation pipeline
The simulation workflow consisted of four sequential steps:
a. Inverse Kinematics: Joint angles were calculated by minimizing the weighted sum of squared differences between experimental and model markers, with convergence criteria of <0.5 cm RMS error.
b. Inverse Dynamics: Joint moments were computed using the Newton-Euler equations of motion based on joint kinematics and segment inertial properties.
c. Static Optimization: Muscle activation patterns were predicted using a cost function minimizing the sum of squared muscle activations (Equation 1) while satisfying joint moment equilibrium constraints:
Equation 1:
Subject to:
Where ai represents the activation level of muscle i, ri,j is the moment arm of muscle i about joint j, Fi is the force produced by muscle i, and Mj is the moment at joint j determined from inverse dynamics.
Static optimization was selected for its computational efficiency and established reliability in predicting muscle coordination patterns during complex movements (29). While we acknowledge the inherent limitations of this approach in capturing time-dependent muscle dynamics during explosive movements, our implementation incorporated Hill-type muscle models with force-length-velocity properties to partially account for these effects. The cost function minimizing the sum of squared muscle activations has demonstrated strong correlation with experimentally measured activation patterns in previous upper-limb studies (21).
d. Forward Dynamics Verification: Computed muscle activation patterns were validated by comparing resulting joint kinematics with experimental data. This simulation pipeline has demonstrated accuracy in predicting experimentally measured muscle activation patterns (r = 0.78-0.92) and joint kinetics (RMSE < 12%) (4, 21). To assess model generalizability to unmeasured muscles, we implemented a leave-one-out validation approach that yielded prediction accuracy of r = 0.73 ± 0.08, suggesting reasonable applicability to deep muscles operating under similar biomechanical constraints (4) (Figure 3).
Figure 3. Opensim upper extremity musculoskeletal model shown from anterior, lateral, and posterior views, featuring 32 hill-type musculotendon actuators and 15 degrees of freedom across the shoulder, elbow, and wrist joints with subject-specific scaling. The views illustrate the complete muscle representation from different anatomical perspectives.
2.6 Muscle synergy analysis
2.6.1 EMG signal processing
Raw EMG signals underwent preprocessing including bandpass filtering (20-450 Hz), notch filtering (50 Hz), full-wave rectification, and smoothing with a 20 Hz low-pass filter. Processed EMG envelopes were normalized to maximum voluntary isometric contractions (MVIC) and time-normalized to a standardized movement cycle (0%–100%) using cubic spline interpolation (31).
2.6.2 Synergy extraction
Muscle synergy analysis was performed using non-negative matrix factorization (NMF) applied to activation matrices from both experimental EMG recordings and musculoskeletal simulations. The NMF algorithm (32) decomposed the muscle activation matrix M into a synergy weight matrix W and synergy coefficient matrix C, minimizing reconstruction error (Equation 2). The optimization employed multiplicative update rules with 100 iterations and convergence tolerance of 10−6, repeated 50 times with randomized initial conditions.
Equation 2:
Where ‖·‖F represents the Frobenius norm, calculated as the square root of the sum of squared matrix elements. A smaller Frobenius norm indicates superior approximation accuracy and more precise modeling of underlying muscle synergies (33).
2.6.3 Determination of optimal synergy number
The optimal number of synergies was determined using multiple criteria: global Variance Accounted For (VAF, threshold of 90%), local VAF for individual muscles (threshold of 0.75), dimensional analysis (slope reduction <5%), and cross-validation using split-half reliability analysis (Figure 4).
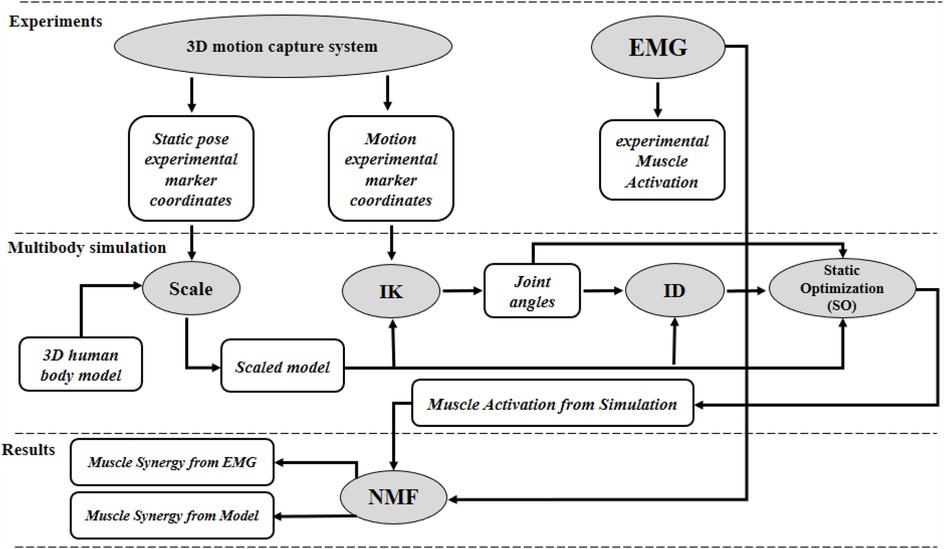
Figure 4. Methodological framework comparing muscle synergies derived from experimental EMG and musculoskeletal modeling during BFOS, including data acquisition, signal processing, modeling, non-negative matrix factorization, and quantitative comparison of synergy components.
These threshold criteria are widely established in neuromuscular research for extracting functionally relevant synergies (10, 11). The global VAF threshold of 90% has demonstrated particular validity for analyzing high-velocity movements including overhead throwing (34) and jumping (35), effectively distinguishing between structurally significant synergies and noise components. The local VAF threshold of 75% ensures that each individual muscle's activation pattern is adequately represented in the extracted synergies, preventing underrepresentation of muscles with smaller but functionally significant contributions (16).
To establish the optimal synergy number with greater statistical rigor, we implemented two complementary cross-validation approaches. First, a leave-one-out cross-validation procedure was conducted where synergies were extracted from n-1 trials and used to reconstruct the excluded trial, repeated for each trial and subject. This analysis confirmed that three synergies consistently provided reconstruction accuracy exceeding our predetermined VAF thresholds. Second, split-half reliability analysis divided trials into two independent subsets, with separate synergy extraction performed on each subset followed by quantitative comparison of the resulting synergy structures. Intraclass correlation coefficients (ICC) and cosine similarity indices were calculated to assess between-subset consistency (see Results section 3.7, Table 6).
2.7 Data analysis and outcome measures
2.7.1 Kinematic and kinetic analysis
Three-dimensional joint kinematics and kinetics were analyzed at key phases of the BFOS: preparation, acceleration, impact, and follow-through. Primary outcome measures included joint angular displacements, velocities, moments, and powers for shoulder and elbow movements, plus racquet head velocity at impact.
2.7.2 Muscle synergy quantification
Outcome measures included number of synergies required to achieve VAF thresholds, synergy weight vectors (W), synergy activation coefficients (C), and reconstruction accuracy.
2.7.3 Comparative analysis and similarity metrics
Comparison between EMG-derived and simulation-derived muscle synergies employed multiple metrics: Scalar Product Similarity Index (Equation 4) (36), Cosine Similarity, and Pearson's Correlation Coefficient.
Equation 4:
Where values approaching 1 indicate greater similarity between experimental and computational synergy vectors.
2.7.4 Reliability analysis
Movement Reliability was assessed by analyzing key kinematic parameters (shoulder angles, elbow angles, and scapular kinematics) across the five repetitions for each participant. Inter-trial consistency was quantified using coefficient of variation (CV) and ICC[3,1] to determine movement stability and reproducibility. Acceptable reliability was defined as CV < 10% and ICC > 0.80 (37).
2.8 Statistical analysis
Statistical analyses were performed using MATLAB 7.8 (MathWorks, Natick, MA) with a significance threshold established at α = 0.05. Normality of data distribution was confirmed using Shapiro–Wilk tests. All quantitative results are presented as means ± standard deviations with 95% confidence intervals where appropriate. Pearson's correlation coefficient (r) served as the primary metric for assessing similarity between muscle synergy vectors, consistent with established methodologies in previous investigations (3, 21). Correlation strength was interpreted as: weak (r < 0.3), moderate (0.3 ≤ r < 0.5), strong (0.5 ≤ r < 0.7), and very strong (r ≥ 0.7). Differences in synergy structures between EMG-derived and simulation-derived results were analyzed using multivariate analysis of variance (MANOVA) with Bonferroni post-hoc tests. Effect sizes were calculated using partial eta squared (η2p) and interpreted as: small (0.01), medium (0.06), and large (0.14). Effect sizes were interpreted following established guidelines by Cohen (38) and revised by Lakens (39) for biomechanical research.
3 Results
3.1 Global VAF analysis
The relationship between synergy number and global VAF for EMG and MSK modeling data is depicted in Figure 5. No significant differences in global VAF were observed between methods (p = 0.12, MANOVA). Following established criteria (global VAF >90%, local VAF >75%, dimensional analysis showing <5% increase with additional synergies), three muscle synergies sufficiently reconstructed the BFOS movement with high fidelity in both experimental and computational analyses.
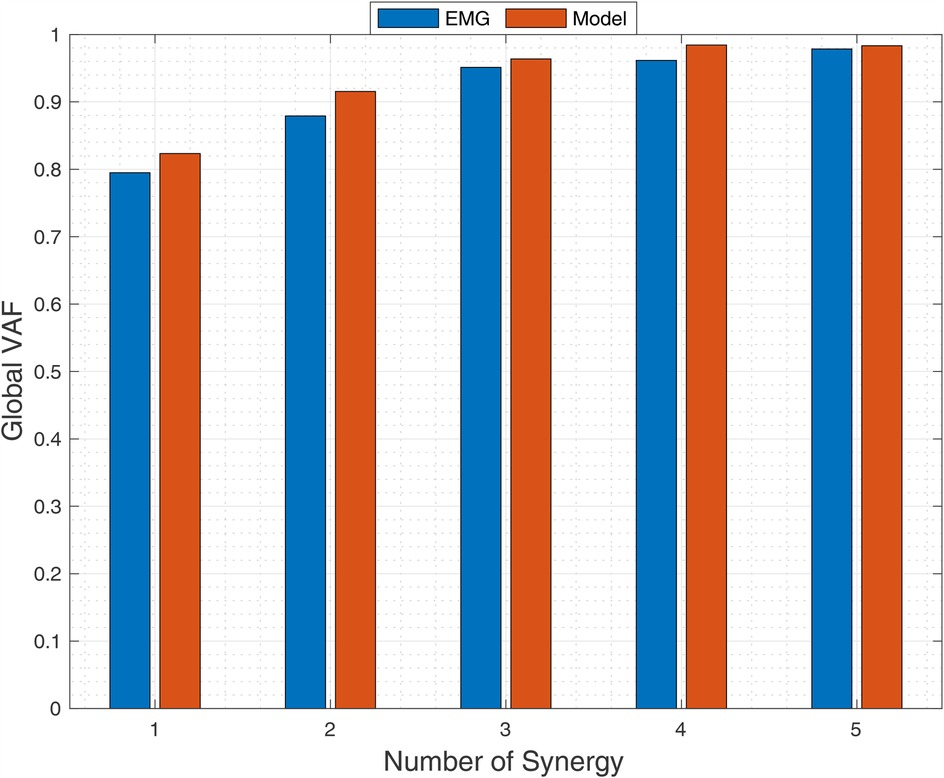
Figure 5. Relationship between synergy number and global variance accounted for (VAF) from EMG (blue) and musculoskeletal model (red) during BFOS. Three synergies were optimal based on global VAF >90%, local VAF >75%, and <5% improvement with additional synergies (p = 0.12, MANOVA).
To further validate our three-synergy solution, we conducted a leave-one-out cross-validation analysis that showed minimal reduction in reconstruction accuracy when synergies extracted from n-1 trials were used to reconstruct the excluded trial (mean ΔVAF = −3.2 ± 1.1%). This small decrease in VAF remains well above our predetermined threshold, confirming the robustness of the extracted synergy structure across different movement repetitions.
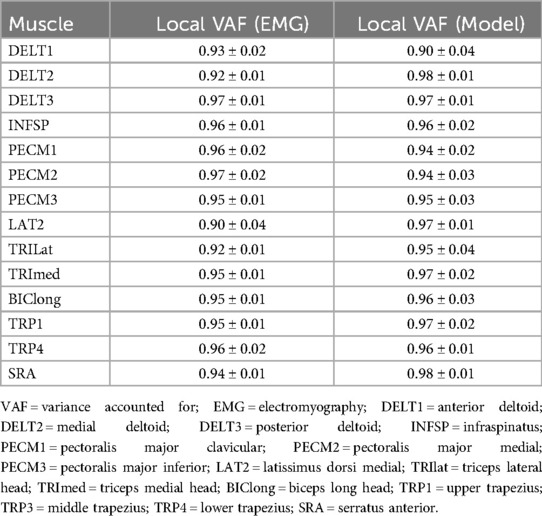
3.2 Local VAF analysis
Local VAF values confirmed precise reconstruction of each muscle for both experimental and modeling outcomes (Table 2). All muscles exhibited high local VAF values (>75%), ranging from 0.90 ± 0.04 to 0.98 ± 0.01 for modeling results and from 0.90 ± 0.04 to 0.97 ± 0.02 for experimental EMG data, robustly satisfying the threshold criterion of local VAF > 0.75 and ensuring extraction reliability of identified synergies.
3.3 Muscle synergy comparison
Analysis revealed three distinct synergies with characteristic muscle activation compositions and temporal patterns (Figure 6). The first synergy predominantly engaged the trapezius muscle group, essential for scapular rotation and stabilization. The second synergy featured significant contributions from the pectoralis muscle complex and anterior deltoid, facilitating internal rotation and shoulder flexion during acceleration phases. The third synergy showed dominance of the middle deltoid and posterior muscle groups, supporting shoulder extension and external rotation primarily during follow-through phases. This functional differentiation aligns with the biomechanical requirements of sequential BFOS execution phases. While slight discrepancies existed between EMG-derived and model-predicted synergy activation coefficients, the weighted contributions maintained physiological consistency for most muscles.
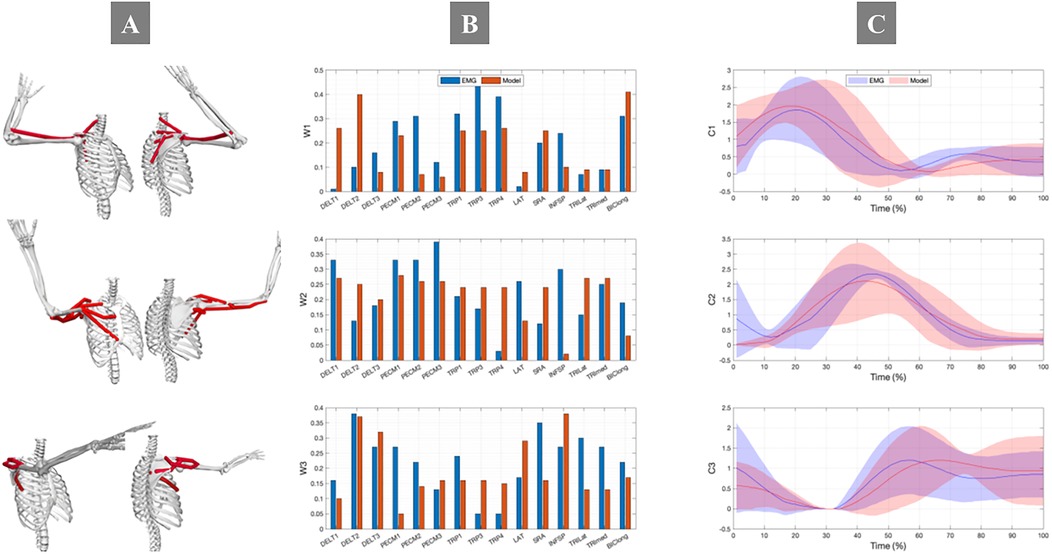
Figure 6. Comparison of (A) synergy weight vectors and (B) activation coefficients between EMG (blue) and musculoskeletal model (red) during BFOS. Three distinct functional synergies were identified: Synergy 1 (trapezius-dominant for scapular stabilization during preparation and early acceleration), Synergy 2 (pectoralis/anterior deltoid for shoulder flexion/internal rotation during late acceleration and impact), and Synergy 3 (posterior shoulder muscles for extension/external rotation during follow-through). The x-axis in panel A represents muscle weights ranging from 0-1 for each muscle (abbreviations defined in Table 2), while panel (C) shows temporal activation patterns across normalized movement cycle (0-100%). Shaded areas represent standard error (n = 20 participants).
3.4 Similarity coefficient analysis
Agreement between experimental and modeling results was evaluated using scalar product similarity indices (Table 3). Similarity coefficients demonstrated substantial agreement, ranging from 0.72 ± 0.04 (infraspinatus) to 0.98 ± 0.01 (trapezius), indicating strong correspondence between experimental and computational approaches. Synergy weight vectors exhibited high similarity (W1: 0.81 ± 0.04, W2: 0.87 ± 0.01, W3: 0.88 ± 0.03), while activation coefficients showed even stronger agreement (C1: 0.95 ± 0.02, C2: 0.98 ± 0.01, C3: 0.98 ± 0.01). The trapezius muscle demonstrated nearly perfect agreement (similarity coefficient: 0.98 ± 0.01, ICC: 0.95, SEM: 0.02). Pearson's correlation coefficients between EMG-derived and simulation-derived muscle activation patterns further confirmed these findings, with very strong correlations (r ≥ 0.7) for most muscles.
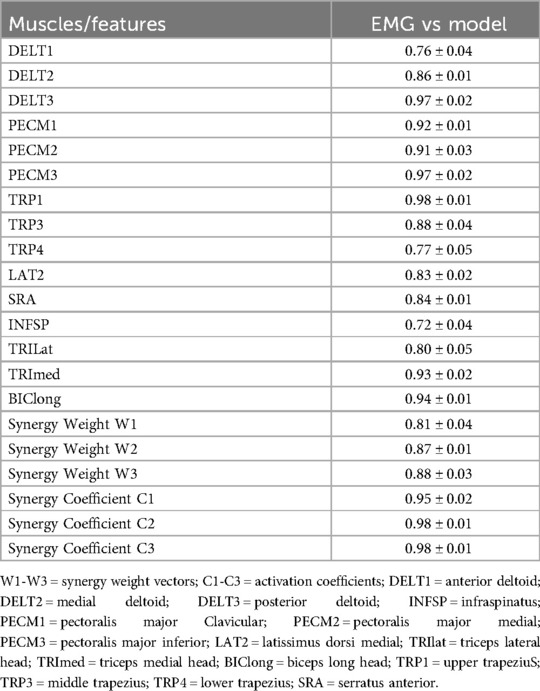
Table 3. Comparison of the mean and standard deviation of similarity coefficient for each muscle and vector of first to third synergies and for activation coefficient of first to third synergies between experimental and modeling results.
3.5 Reliability of key kinematic variables
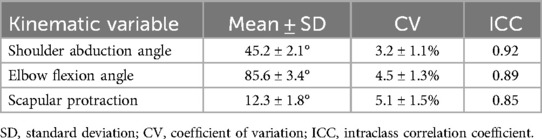
Analysis of key kinematic variables revealed excellent consistency for shoulder abduction (CV = 3.2% ± 1.1%, ICC = 0.92), good reliability for elbow flexion (CV = 4.5% ± 1.3%, ICC = 0.89), and moderate to high reliability for scapular protraction (CV = 5.1% ± 1.5%, ICC = 0.85). According to predetermined reliability criteria (CV < 10% and ICC > 0.80), these results demonstrate excellent to good movement consistency across trials, supporting the reliability of the muscle synergy analysis.
3.6 Statistical analysis of EMG-model comparison
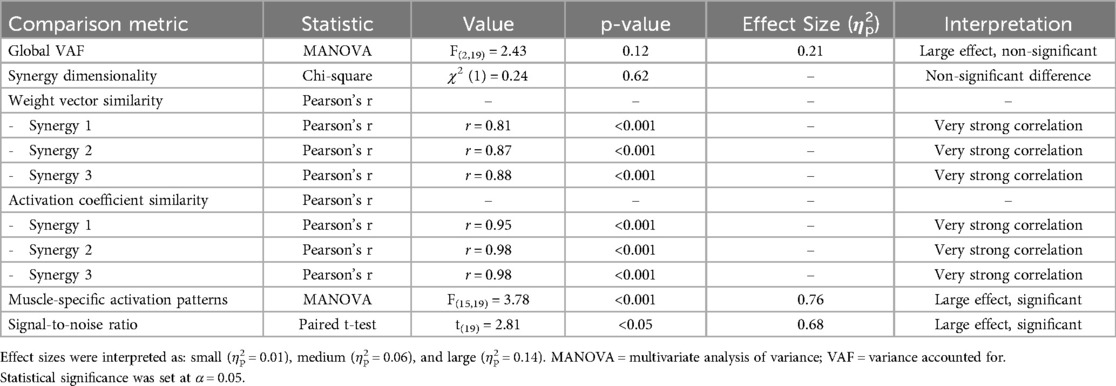
Statistical analysis comparing EMG-derived and model-predicted synergies (Table 5) revealed no significant differences in global VAF between methods (F₍₂,₁₉₎ = 2.43, p = 0.12), though a large effect size (η2p = 0.21) suggested potential practical significance. Consistency in synergy dimensionality across both methods (χ2₍₁₎ = 0.24, p = 0.62) further supported the robustness of the three-synergy solution. Weight vector similarities ranged from r = 0.81 to r = 0.88 (p < 0.001), while activation coefficient similarities exhibited even stronger correlations (r = 0.95 to r = 0.98, p < 0.001). Muscle-specific activation patterns showed significant differences between methods (F₍₁₅,₁₉₎ = 3.78, p < 0.001, η2p = 0.76), indicating that despite overall synergistic similarity, individual muscle contributions exhibited method-specific variations.
3.7 Cross-Validation of synergy structure
Cross-validation analyses between independent trial subsets (Table 6) demonstrated high consistency in both synergy weight vectors and activation coefficients. Weight vectors showed cosine similarity indices ranging from 0.87 ± 0.05 (W3) to 0.92 ± 0.03 (W2), with corresponding ICC values of 0.88 to 0.93. Activation coefficients exhibited even higher consistency, with similarity indices from 0.93 ± 0.03 (C3) to 0.97 ± 0.01 (C2) and ICC values of 0.92 to 0.96. Low standard error of measurement (0.01-0.04) across all synergy components confirmed the stability of extracted synergies across multiple movement repetitions.
4 Discussion
This investigation provides comprehensive insights into muscle coordination strategies during the badminton forehand overhead smash through integration of experimental electromyography and computational musculoskeletal modeling. Our findings revealed three distinct muscle synergies that collectively account for over 90% of the variance in muscle activation patterns during BFOS execution.
The strong agreement between EMG-derived and model-predicted synergies (similarity coefficients ranging from 0.81 to 0.88 for weight vectors and 0.95 to 0.98 for activation coefficients) suggests that MSK modeling effectively captures many aspects of neuromuscular control during high-velocity overhead movements. However, this agreement does not necessarily validate the assumption that the central nervous system optimizes the same cost function used in our static optimization approach. Rather, it suggests that biomechanical constraints may substantially influence muscle coordination patterns regardless of the specific neural control strategy employed (40). This alignment between experimental and computational approaches supports the theory that biomechanical constraints—such as joint coordination and muscle-tendon dynamics—may fundamentally drive synergy patterns (41). Similar findings in studies of gait and upper-limb coordination further reinforce this perspective (37, 42).
The three identified synergies demonstrated clear functional roles corresponding to specific BFOS phases. The first synergy, dominated by trapezius activation with contributions from upper pectoralis major and anterior deltoid, primarily facilitated scapular movement during the acceleration phase. Kinematic data revealed shoulder abduction and horizontal flexion during this phase, with the upper trapezius contributing to shoulder elevation, the lower trapezius to depression, and the middle trapezius to scapular protraction and retraction. This synergy establishes a stable platform for efficient energy transfer, aligning with Kibler et al. (43), who emphasized the critical role of scapular control in overhead movements.
The second synergy, characterized by substantial contributions from pectoralis and anterior deltoid muscles, governed internal shoulder rotation and flexion during the impact phase. This synergy also incorporated triceps activation for elbow extension, reflecting the coordinated effort between shoulder and elbow joints to accelerate and strike the shuttlecock. Previous research by Ramasamy et al. (7) demonstrated that these muscles generate peak moments of 0.85 ± 0.12 Nm/kg during smash execution, underscoring their importance in power generation.
The third synergy, featuring middle and posterior deltoid, infraspinatus, latissimus dorsi, and serratus anterior muscles, primarily controlled the deceleration phase of the BFOS. This phase involves a braking motion where antagonist muscles engage with agonists to stabilize joints and prevent injury (44). The paradoxical activation of abductor and external rotator muscles during a movement involving flexion and internal rotation highlights the complex coordination required for safely decelerating high-velocity movements (45).
The statistical analysis revealed excellent consistency of synergy structures across trial subsets, with cosine similarity indices ranging from 0.87 ± 0.05 to 0.92 ± 0.03 for weight vectors and from 0.93 ± 0.03 to 0.97 ± 0.01 for activation coefficients. These robust reliability metrics indicate that the identified three-synergy structure represents consistent neuromuscular control strategies rather than analytical artifacts. Furthermore, kinematic reliability analysis demonstrated excellent consistency for key joint angles (shoulder abduction: CV = 3.2%±1.1%, ICC = 0.92; elbow flexion: CV = 4.5%±1.3%, ICC = 0.89), supporting the stability of the extracted muscle synergies.
While our study identified consistent synergy structures across participants, we acknowledge that individual technical styles and equipment parameters (racket weight, string tension) could influence specific muscle activation patterns. Previous research indicates that racket properties can alter upper limb loading patterns by 8-12% (7). Similarly, technique variations developing from years of competitive play might produce individualized muscle coordination strategies. Though our selection criteria ensured fundamental technique consistency, subtle variations likely contributed to inter-subject variability observed in model predictions. These considerations highlight the complex interplay between equipment, technique, and neuromuscular control that future studies should explore more systematically.
Despite overall strong agreement, significant differences in muscle-specific activation patterns [F(15,19) = 3.78, p < 0.001, η2p = 0.76] were observed, particularly for the lower trapezius muscle. These discrepancies likely stem from challenges in accurately measuring and modeling this deep muscle during dynamic, high-velocity movements. Our statistical approach revealed a non-significant difference with large effect size for global VAF comparison, suggesting the study may have been underpowered to detect subtle but potentially meaningful differences between experimental and computational approaches. Future investigations should consider larger sample sizes or repeated-measures designs to better address this limitation. Additionally, our musculoskeletal model employed simplified tendon properties that do not fully capture the elastic energy storage and release during explosive movements like the BFOS. This simplification likely contributed to observed muscle-specific activation differences, particularly for muscles with substantial tendon components such as the lower trapezius. Surface EMG struggles to capture consistent signals during rapid scapular rotations, while static optimization in MSK models may oversimplify the muscle's role in scapular stabilization (46). Additionally, inter-subject variability contributes to reduced similarity between synergy vectors, as individual differences in muscle strength, joint flexibility, and activation patterns affect synergy alignment (47).
Our statistical analysis revealed an interesting pattern regarding global VAF differences between EMG-derived and model-predicted synergies. Despite not reaching statistical significance (p = 0.12), the large effect size (η2p = 0.21) suggests potential practical significance that may represent a Type II error due to limited statistical power. This highlights the importance of considering both statistical significance and effect sizes when evaluating methodological approaches (39). Furthermore, significant differences in muscle-specific activation patterns (p < 0.001, η2p = 0.76) likely reflect multiple factors, including model simplifications. Particularly relevant is the omission of tendon elasticity in our implementation, which affects force transmission dynamics during rapid movements. Tendons function as series elastic elements that can store and release energy, with studies demonstrating that neglecting this property can produce activation timing discrepancies of 30–50 ms during ballistic movements (30). This limitation particularly affects bi-articular muscles and those with long tendons, potentially explaining some of the muscle-specific differences observed in our analysis.
The model's predictive capacity for unmeasured deep muscles, particularly the rotator cuff group, warrants consideration. While direct validation through intramuscular EMG was beyond our scope, biomechanical constraints substantially limit the feasible activation space of these muscles during dynamic movements. The consistency between model predictions and surface EMG for accessible muscles with similar functions provides indirect validity evidence, though unique activation strategies for glenohumeral stabilization may not be fully captured (48). From a theoretical perspective, our findings support the muscle synergy hypothesis as a framework for understanding neuromuscular control during complex sporting movements. The consistent extraction of three functionally relevant synergies suggests that the central nervous system employs modular control strategies to simplify the coordination of multiple muscles during the BFOS. This modularity potentially represents an efficient solution to the degrees-of-freedom problem articulated by Bernstein (49), allowing effective control of the highly redundant musculoskeletal system through feedforward mechanisms, where the neuromuscular system predefines muscle activation patterns to achieve specific outcomes (50).
The validated musculoskeletal model provides a valuable tool for investigating aspects of the BFOS that are difficult to measure experimentally. The fact that muscle activations were estimated via static optimization without explicitly considering muscle synergies or intermuscular relationships—yet still produced synergy structures closely resembling experimental patterns—further strengthens the theory that biomechanical constraints fundamentally shape coordination patterns (51). This finding has significant implications for future research where experimental EMG data may be unavailable or impractical to collect. It is important to acknowledge that static optimization, which minimizes instantaneous muscle activation squared, may not fully capture the time-dependent aspects of neural control during dynamic movements. Dynamic optimization approaches that consider the entire movement trajectory might provide different muscle activation predictions (52). Despite implementing Hill-type muscle models with force-length-velocity properties, static optimization cannot fully account for dynamic inertial effects and history-dependent phenomena during explosive movements like the BFOS (21, 22). This limitation likely contributes to discrepancies observed in certain muscles, particularly those with complex activation dynamics during rapid acceleration and deceleration phases. Future studies should directly compare static and dynamic optimization approaches during high-velocity overhead movements to quantify these differences systematically (53). Additionally, future investigations should systematically examine how equipment parameters and technical style variations influence muscle synergy structures, using stratified designs that control for racket specifications, string tension, and technique variants. Nevertheless, the strong concordance between our static optimization results and experimental EMG suggests that this computationally efficient approach can provide valuable insights into muscle coordination during rapid overhead movements.
4.1 Practical recommendations
Based on our findings, several practical recommendations emerge for coaches, athletic trainers, and rehabilitation specialists working with badminton players. Training programs should emphasize coordinated activation of functional muscle groups rather than isolated strengthening exercises, focusing on the sequential activation of identified synergies—starting with scapular stabilizers (trapezius), progressing to power generators (pectoralis/anterior deltoid), and concluding with deceleration controllers (posterior muscles). Technical coaching should optimize the kinematic sequence to maximize energy transfer through the kinetic chain, with particular attention to scapular positioning. Rehabilitation protocols following shoulder injuries should progressively incorporate synergy-based training, beginning with controlled activation of the first synergy before advancing to power-generating movements. Targeted strength training for the trapezius, pectoralis, and deltoid muscles can improve stroke efficiency and shoulder stability (54). Finally, regular biomechanical screening using simplified kinematic measures may help identify movement pattern alterations that could predispose athletes to injury or compromise performance. Individual variations in synergy structures should be considered when developing personalized training programs. While our results showed consistent three-synergy patterns across participants, subtle differences in muscle weightings and activation timing may reflect individual movement strategies or adaptations (55). These variations could potentially relate to performance levels, injury history, or anatomical differences (44, 56). Future research should explore whether specific synergy characteristics correlate with performance metrics such as shuttlecock velocity or placement accuracy, as well as their relationship to injury risk profiles (57).
Despite its contributions, this study has several limitations. First, our static optimization approach may not fully capture the time-dependent dynamics of muscle activation during explosive movements, as it minimizes instantaneous muscle activation without considering temporal optimization across the entire movement trajectory. Second, our analysis focused exclusively on elite badminton players, potentially limiting generalizability to recreational players or those with different technical approaches. Third, while surface EMG provides valuable insights into superficial muscle activity, it cannot reliably capture deep muscle activations, particularly for muscles critical to shoulder function such as the rotator cuff group. Fourth, individual variations in technique and equipment specifications (racket properties, string tension) may have influenced muscle activation patterns, though we attempted to control for these factors through stringent inclusion criteria. Fifth, our computational model incorporated simplified tendon properties that may not fully represent elastic energy storage and release during rapid movements, potentially affecting muscle-specific activation predictions. Finally, while our sample size was adequate for detecting moderate to large effects, subtle differences between experimental and computational approaches may have been missed due to limited statistical power. Future studies should address these limitations through longitudinal designs, inclusion of diverse skill levels, and implementation of dynamic optimization algorithms that better account for time-dependent muscle dynamics.
We propose synergy-specific training interventions:
- Synergy 1 (trapezius-dominant): Progressive scapular stabilization exercises from static to dynamic conditions with proprioceptive elements.
- Synergy 2 (pectoralis/anterior deltoid): Power development using sport-specific exercises emphasizing rapid force production (40-60% 1RM).
- Synergy 3 (posterior muscles): Eccentric-focused training targeting deceleration capabilities.
Program implementation should follow a 3:2:2 ratio of training volume between synergies 1, 2, and 3 during initial phases, shifting toward 2:3:3 as players advance, reflecting the greater importance of power generation and deceleration control at higher performance levels. This synergy-based approach fundamentally differs from traditional muscle-isolated training by emphasizing coordinated activation patterns rather than individual muscle development (43).
5 Conclusions
This investigation successfully quantified muscle synergies during the badminton forehand overhead smash through non-negative matrix factorization of EMG data and MSK modeling, providing novel insights into the neuromuscular control strategies underlying this complex, high-velocity movement. Three distinct synergies were identified: (1) trapezius-dominant synergy for scapular stabilization during acceleration, (2) pectoralis/anterior deltoid synergy for internal rotation and flexion during impact, and (3) posterior muscle synergy for controlled deceleration. The computational musculoskeletal model demonstrated strong agreement with experimental EMG data for most muscles, with notable exception of the lower trapezius. These findings highlight the integration of EMG and MSK modeling as a robust approach for analyzing neuromuscular control strategies in high-velocity overhead movements. Further research should expand to other overhead sports, explore alternative algorithms such as ICA or PCA, employ advanced EMG techniques for deep muscles, and conduct longitudinal studies examining how training or rehabilitation alters synergy structures, thereby enhancing applications in both athletic performance optimization and injury prevention. Future research should expand methodological approaches to include dynamic optimization algorithms that better account for force-velocity relationships and inertial effects during explosive movements. Such approaches may improve model predictions for muscles exhibiting complex activation patterns during rapid acceleration and deceleration phases.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: N/A.
Ethics statement
The studies involving humans were approved by this investigation received approval from the Research Ethics Committee of Kharazmi University (Approval Code: IR.KHU.REC.1402.020) and adhered to the principles outlined in the Declaration of Helsinki (2013 revision). All participants provided written informed consent after receiving detailed explanations of the study procedures, potential risks, and benefits. Participants were informed of their right to withdraw at any time without consequence. Data confidentiality was maintained throughout the investigation with all personal identifiers removed before analysis. The protocol was preregistered in the Open Science Framework (OSF) database (osf.io/twx5h) prior to participant enrollment. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
RT: Conceptualization, Data curation, Methodology, Validation, Writing – original draft, Writing – review & editing. WD: Conceptualization, Data curation, Formal analysis, Resources, Supervision, Writing – original draft, Writing – review & editing. HF: Methodology, Visualization, Writing – original draft, Writing – review & editing. RM: Conceptualization, Investigation, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors thank all the experts, doctors and officers of the Police Academy for their participation in this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Liao K, Bian C, Chen Z, Yuan Z, Bishop C, Han M, et al. Repetition velocity as a measure of loading intensity in the free weight and Smith machine Bulgarian split squat. PeerJ. (2023):11. doi: 10.7717/peerj.15863
2. Phomsoupha M, Laffaye G. Injuries in badminton: a review. Sci Sports. (2020) 35(4):189–99. doi: 10.1016/j.scispo.2020.01.002
3. Steele KM, Tresch MC, Perreault EJ. The number and choice of muscles impact the results of muscle synergy analyses. Front Comput Neurosci. (2013):7. doi: 10.3389/fncom.2013.00105
4. Valero-Cuevas FJ, Cohn BA, Yngvason HF, Lawrence EL. Exploring the high-dimensional structure of muscle redundancy via subject-specific and generic musculoskeletal models. J Biomech. (2015) 48(11):2887–96. doi: 10.1016/j.jbiomech.2015.04.026
5. Blache Y, Martinez R, Dumas R, Begon M, Hagemeister N, Duprey S. Chapter 20 - motion analysis and modeling of the shoulder: challenges and potential applications. In: Scataglini S, Paul G, editors. DHM and Posturography. Cambridge, MA: Academic Press (2019). p. 261–71. doi: 10.1016/B978-0-12-816713-7.00020-9
6. Rusdiana A. 3D Kinematics analysis of overhead backhand and forehand smash techniques in badminton. Ann Appli Sport Sci. (2021) 9(3):1–9.
7. Ramasamy Y, Usman J, Razman R, Wei YM, Towler H, King M. A systematic review of the biomechanical studies on shoulder kinematics in overhead sporting motions: types of analysis and approaches. Appl Sci. (2023) 13(16):9463. doi: 10.3390/app13169463
8. Panariello D, Grazioso S, Caporaso T, Palomba A, Di Gironimo G, Lanzotti A. Biomechanical analysis of the upper body during overhead industrial tasks using electromyography and motion capture integrated with digital human models. Int J Interac Des Manufact (IJIDeM). (2022) 16(2):733–52. doi: 10.1007/s12008-022-00862-9
9. Aoi S, Ohashi T, Bamba R, Fujiki S, Tamura D, Funato T, et al. Neuromusculoskeletal model that walks and runs across a speed range with a few motor control parameter changes based on the muscle synergy hypothesis. Sci Rep. (2019) 9(1):369. doi: 10.1038/s41598-018-37460-3
10. Tresch MC, Jarc A. The case for and against muscle synergies. Curr Opin Neurobiol. (2009) 19(6):601–7. doi: 10.1016/j.conb.2009.09.002
11. Ting LH, Chiel HJ, Trumbower RD, Allen JL, McKay JL, Hackney ME, et al. Neuromechanical principles underlying movement modularity and their implications for rehabilitation. Neuron. (2015) 86(1):38–54. doi: 10.1016/j.neuron.2015.02.042
12. Rabbi MF, Pizzolato C, Lloyd DG, Carty CP, Devaprakash D, Diamond LE. Non-negative matrix factorisation is the most appropriate method for extraction of muscle synergies in walking and running. Sci Rep. (2020) 10(1):8266. doi: 10.1038/s41598-020-65257-w
13. d'Avella A. Muscle synergies. In: Binder MD, Hirokawa N, Windhorst U, editors. Encyclopedia of Neuroscience. Berlin, Heidelberg: Springer Berlin Heidelberg (2024). p. 2509–12. doi: 10.1007/978-3-540-29678-2_3678
14. Brambilla C, Atzori M, Müller H, d'Avella A, Scano A. Spatial and temporal muscle synergies provide a dual characterization of low-dimensional and intermittent control of upper-limb movements. Neuroscience. (2023) 514:100–22. doi: 10.1016/j.neuroscience.2023.01.017
15. Owens LP, Khaiyat O, Coyles G. Muscle activations of the upper extremity and core during elevation and rotational movements in overhead throwing athletes. Int J Sports Phys Ther. (2024) 19(4):466–76. doi: 10.26603/001c.94604
16. Pale U, Atzori M, Müller H, Scano A. Variability of muscle synergies in hand grasps: analysis of intra- and inter-session data. Sensors (Basel). (2020) 20(15). doi: 10.3390/s20154297
17. Scano A, Lanzani V, Brambilla C. How recent findings in electromyographic analysis and synergistic control can impact on new directions for muscle synergy assessment in sports. Appl Sci. (2024) 14(23):11360. doi: 10.3390/app142311360
18. Abdullah M, Hulleck AA, Katmah R, Khalaf K, El-Rich M. Multibody dynamics-based musculoskeletal modeling for gait analysis: a systematic review. J Neuroeng Rehabil. (2024) 21(1):178. doi: 10.1186/s12984-024-01458-y
19. Maniar N, Cole MH, Bryant AL, Opar DA. Muscle force contributions to anterior cruciate ligament loading. Sports Med (Auckland, NZ). (2022) 52(8):1737–50. doi: 10.1007/s40279-022-01674-3
20. McConnochie G, Fox A, Bellenger C, Thewlis D. LiDAR-based scaling of OpenSim musculoskeletal human models is a viable alternative to marker-based approaches — a preliminary study. J Biomech. (2025) 179:112439. doi: 10.1016/j.jbiomech.2024.112439
21. Ao D, Fregly BJ. Comparison of synergy extrapolation and static optimization for estimating multiple unmeasured muscle activations during walking. J Neuroeng Rehabil. (2024) 21(1):194. doi: 10.1186/s12984-024-01490-y
22. Di A, Benjamin JF. Comparison of synergy extrapolation and static optimization for estimating multiple unmeasured muscle activations during walking. bioRxiv. (2024). doi: 10.1101/2024.03.03.583228
23. Sitole SP, Sup FC. Continuous prediction of human joint mechanics using emg signals: a review of model-based and model-free approaches. IEEE Trans Med Robot Bionics. (2023) 5(3):528–46. doi: 10.1109/TMRB.2023.3292451
24. Jessup LN, Kelly LA, Cresswell AG, Lichtwark GA. Validation of a musculoskeletal model for simulating muscle mechanics and energetics during diverse human hopping tasks. R Soc Open Sci. (2023) 10(10):230393. doi: 10.1098/rsos.230393
25. Barnamehei H, Tabatabai Ghomsheh F, Safar Cherati A, Pouladian M. Muscle and joint force dependence of scaling and skill level of athletes in high-speed overhead task: musculoskeletal simulation study. Inform Med Unlocked. (2020) 20:100415. doi: 10.1016/j.imu.2020.100415
26. Sakurai S, Ohtsuki T. Muscle activity and accuracy of performance of the smash stroke in badminton with reference to skill and practice. J Sports Sci. (2000) 18(11):901–14. doi: 10.1080/026404100750017832
27. Mokri C, Bamdad M, Abolghasemi V. Muscle force estimation from lower limb EMG signals using novel optimised machine learning techniques. Med Biol Eng Comput. (2022) 60(3):683–99. doi: 10.1007/s11517-021-02466-z
28. Xiong J, Li S, Cao A, Qian L, Peng B, Xiao D. Effects of integrative neuromuscular training intervention on physical performance in elite female table tennis players: a randomized controlled trial. PLoS One. (2022) 17(1). doi: 10.1371/journal.pone.0262775
29. Seth A, Sherman M, Reinbolt JA, Delp SL. Opensim: a musculoskeletal modeling and simulation framework for in silico investigations and exchange. Procedia IUTAM. (2011) 2:212–32. doi: 10.1016/j.piutam.2011.04.021
30. Millard M, Uchida T, Seth A, Delp SL. Flexing computational muscle: modeling and simulation of musculotendon dynamics. J Biomech Eng. (2013) 135(2):021005. doi: 10.1115/1.4023390
31. Esposito D, Centracchio J, Bifulco P, Andreozzi E. A smart approach to EMG envelope extraction and powerful denoising for human-machine interfaces. Sci Rep. (2023) 13(1):7768. doi: 10.1038/s41598-023-33319-4
32. Russo M, Scano A, Brambilla C, d'Avella A. Synergyanalyzer: a matlab toolbox implementing mixed-matrix factorization to identify kinematic-muscular synergies. Comput Methods Programs Biomed. (2024) 251:108217. doi: 10.1016/j.cmpb.2024.108217
33. Chen Y, Wang Y, Gao F, Dong B, Chen W, Liu R. A computational method to identify optimal functional muscle synergies from estimated muscle activations. IEEE Transac Instr Measur. (2024).
34. Roh J, Rymer WZ, Beer RF. Evidence for altered upper extremity muscle synergies in chronic stroke survivors with mild and moderate impairment. Front Hum Neurosci. (2015) 9:6. doi: 10.3389/fnhum.2015.00006
35. Ortega-Auriol P, Besier T, McMorland AJC. Effect of surface electromyography normalisation methods over gait muscle synergies. J Electromyogr Kinesiol. (2025) 80:102968. doi: 10.1016/j.jelekin.2024.102968
36. Feldotto B, Soare C, Knoll A, Sriya P, Astill S, de Kamps M, et al. Evaluating muscle synergies with EMG data and physics simulation in the neurorobotics platform. Front Neurorobot. (2022) 16:856797. doi: 10.3389/fnbot.2022.856797
37. Dhahbi W, Zouita A, Salah FB, Chaouachi A, Chamari K, Cheze L. Reference database of the gait cycle for young healthy Tunisian adults. IRBM. (2014) 35(1):46–52. doi: 10.1016/j.irbm.2013.12.005
38. Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York, NY: Routledge (2013).
39. Lakens D. Sample size justification. Collabra: Psychology. (2022) 8(1):33267. doi: 10.1525/collabra.33267
40. Kimura A, Yokozawa T, Ozaki H. Clarifying the biomechanical concept of coordination through comparison with coordination in motor control. Front Sports Act Living. (2021) 3:753062. doi: 10.3389/fspor.2021.753062
41. Sohn MH, Ting LH. Suboptimal muscle synergy activation patterns generalize their motor function across postures. Front Comput Neurosci. (2016) 10:7. doi: 10.3389/fncom.2016.00007
42. Song C, Wang L, Ding J, Xu C, Yang H, Mao Y. Effect of upper limb repetitive facilitative exercise on gait of stroke patients based on artificial intelligence and computer vision evaluation. J Musculoskelet Neuronal Interact. (2024) 24(3):301–9. Available at: https://pubmed.ncbi.nlm.nih.gov/3921932839219328
43. Kibler WB, Sciascia A. Evaluation and management of scapular dyskinesis in overhead athletes. Curr Rev Musculoskelet Med. (2019) 12(4):515–26. doi: 10.1007/s12178-019-09591-1
44. Dhahbi W. Editorial: advancing biomechanics: enhancing sports performance, mitigating injury risks, and optimizing athlete rehabilitation. Front Sports Act Living. (2025) 7:1556024. doi: 10.3389/fspor.2025.1556024
45. Trasolini NA, Nicholson KF, Mylott J, Bullock GS, Hulburt TC, Waterman BR. Biomechanical analysis of the throwing athlete and its impact on return to sport. Arthrosc Sports Med Rehabil. (2022) 4(1):e83–91. doi: 10.1016/j.asmr.2021.09.027
46. Arghadeh R, Alizadeh MH, Minoonejad H, Sheikhhoseini R, Asgari M, Jaitner T. Electromyography of scapular stabilizers in people without scapular dyskinesis during push-ups: a systematic review and meta-analysis. Front Physiol. (2023) 14:1296279. doi: 10.3389/fphys.2023.1296279
47. Dhahbi W, Chamari K, Chèze L, Behm DG, Chaouachi A. External responsiveness and intrasession reliability of the rope-climbing test. J Strength Cond Res. (2016) 30(10):2952–8. doi: 10.1519/jsc.0000000000001367
48. Boettcher CE, Cathers I, Ginn KA. The role of shoulder muscles is task specific. J Sci Med Sport. (2010) 13(6):651–6. doi: 10.1016/j.jsams.2010.03.008
50. Doyon J, Gabitov E, Vahdat S, Lungu O, Boutin A. Current issues related to motor sequence learning in humans. Curr Opin Behav Sci. (2018) 20:89–97. doi: 10.1016/j.cobeha.2017.11.012
51. Ortega-Auriol P, Byblow WD, Ren AX, Besier T, McMorland AJC. The role of muscle synergies and task constraints on upper limb motor impairment after stroke. Exp Brain Res. (2025) 243(1):40. doi: 10.1007/s00221-024-06953-1
52. Xia N, He C, Li YA, Gu M, Chen Z, Wei X, et al. Startle increases the incidence of anticipatory muscle activations but does not change the task-specific muscle onset for patients after subacute stroke. Front Neurol. (2021) 12:789176. doi: 10.3389/fneur.2021.789176
53. Xu D, Zhou H, Quan W, Ma X, Chon TE, Fernandez J, et al. New insights optimize landing strategies to reduce lower limb injury risk. Cyborg Bionic Syst. (2024) 5:0126. doi: 10.34133/cbsystems.0126
54. Melo ASC, Moreira JS, Afreixo V, Moreira-Gonçalves D, Donato H, Cruz EB, et al. Effectiveness of specific scapular therapeutic exercises in patients with shoulder pain: a systematic review with meta-analysis. JSES Rev Rep Techniq. (2024) 4(2):161–74. doi: 10.1016/j.xrrt.2023.12.006
55. Acosta-Sojo Y, Stirling L. Individuals differ in muscle activation patterns during early adaptation to a powered ankle exoskeleton. Appl Ergon. (2022) 98:103593. doi: 10.1016/j.apergo.2021.103593
56. Dhahbi W, Ben Saad H, Dergaa I, Souaifi M, Chamari K. Injury profiling in male police cadets during initial training phase: a retrospective cohort study. Am J Mens Health. (2024) 18(6):15579883241304584. doi: 10.1177/15579883241304584
Keywords: biomechanical phenomena, elite athletes, joint instability, motor control, movement disorders, neuromuscular coordination, rotator cuff
Citation: Tajik R, Dhahbi W, Fadaei H and Mimar R (2025) Muscle synergy analysis during badminton forehand overhead smash: integrating electromyography and musculoskeletal modeling. Front. Sports Act. Living 7:1596670. doi: 10.3389/fspor.2025.1596670
Received: 20 March 2025; Accepted: 19 May 2025;
Published: 3 June 2025.
Edited by:
Christian Maurer-Grubinger, University Hospital Frankfurt, GermanyReviewed by:
Datao Xu, Ningbo University, ChinaAna Conceição, Polytechnic Institute of Santarém, Portugal
Copyright: © 2025 Tajik, Dhahbi, Fadaei and Mimar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wissem Dhahbi, d2lzc2VtLmRoYWhiaUBnbWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
 Raheleh Tajik
Raheleh Tajik Wissem Dhahbi
Wissem Dhahbi Hamed Fadaei
Hamed Fadaei Raghad Mimar
Raghad Mimar