- 1Department of Police Tactics, Fujian Police College, Fuzhou, China
- 2Fujian Key Laboratory of Innate Immune Biology, Biomedical Research Center of South China, College of Life Sciences, Fujian Normal University Qishan Campus, Fuzhou, Fujian Province, China
- 3China Institute of Sport Science, Beijing, China
Exercise is widely recognized as a critical determinant of health, yet its effects can diverge substantially depending on intensity, duration, and individual characteristics. This review synthesizes current knowledge on the mechanisms underlying exercise-induced stress responses, outlining a sequential cascade from biomechanical signal perception, through organelle and metabolic regulation, to systemic integration via hormonal, myokine, and immune pathways. We highlight the concept of a bidirectional threshold theory, which proposes that moderate exercise promotes adaptation and health benefits, while excessive exercise may trigger maladaptive responses and pathological outcomes. At the same time, we note that significant inter-individual variability in exercise responses raises important questions regarding the generalizability of this framework. By integrating evidence across molecular, cellular, and systemic levels, this review provides a holistic perspective on the dual effects of exercise, underscores the need for improved biomarkers to monitor adaptive vs. maladaptive responses, and identifies research gaps that must be addressed to translate these mechanisms into personalized exercise strategies.
1 Introduction
Exercise is an essential component of human health, and its physiological impact has long been a central focus of biomedical research. With advancements in exercise biology, researchers have increasingly elucidated the profound effects of exercise on cellular stress responses, tissue remodeling, and systemic regulation (1–4). Beyond its well-documented role in enhancing physical performance, exercise also exerts significant influence on mental well-being, serving as a non-pharmacological strategy to alleviate psychological stress and reduce the incidence of anxiety and depression (5, 6). Understanding the biological response mechanisms associated with exercise-induced stress is therefore crucial for the rational application of exercise in health promotion and disease prevention.
The stress response elicited by exercise is a complex process involving cardiovascular adaptation, endocrine modulation, immune activation, and intracellular signaling cascades (7). These responses exhibit clear dose-dependent effects. In this context, the bidirectional threshold theory has emerged as a valuable framework: moderate exercise elicits beneficial adaptations, whereas excessive exercise can lead to pathological damage depending on the intensity and duration of the stimulus (8). For example, the production of reactive oxygen species (ROS) during moderate exercise activates adaptive signaling pathways (e.g., JNK, NF-κB), thereby enhancing antioxidant defenses and repair mechanisms (9, 10). Conversely, chronic ROS overload may trigger apoptosis, mitochondrial dysfunction, and pro-inflammatory signaling, ultimately accelerating tissue damage (10). Similarly, while mechanical loading stimulates integrin-FAK-Akt signaling to promote cell survival and differentiation, sustained or excessive activation may predispose tissues to fibrosis and maladaptive remodeling (11–14). Despite these advances, current literature is often fragmented, with a stronger emphasis on adaptive outcomes while insufficiently addressing maladaptive or pathological responses to overtraining. This imbalance hinders a comprehensive understanding of the “duality” of exercise. Moreover, many studies rely on cross-sectional or animal models, leaving gaps in longitudinal human data that could validate molecular findings in real-world training scenarios (15). Individual differences—including sex, age, genetic background, and comorbidities—further complicate the interpretation of exercise responses, yet remain underexplored (16). These limitations highlight the need for a more critical and integrative perspective that connects molecular mechanisms with clinical translation (17, 18).
To address these gaps, this review synthesizes recent findings into a unified conceptual framework of the biological response cascade to exercise-induced stress. Specifically, it progresses through three interconnected layers: (i) primary responses, including mechanical signal transduction and organelle stress; (ii) secondary regulation, emphasizing metabolic reprogramming and energy sensing; and (iii) systemic integration, involving endocrine, immune, and neuro-metabolic networks.
Within this framework, we highlight both the adaptive and maladaptive trajectories of exercise responses, aiming to establish a balanced understanding of how exercise can act as both a health-promoting stimulus and a potential pathological challenge. By critically analyzing existing evidence, identifying research limitations, and proposing future directions, this review seeks to provide a more comprehensive foundation for personalized exercise prescriptions and translational exercise medicine.
2 Primary responses to exercise-induced stress signals
Exercise triggers a diverse array of primary stress signals at the cellular level, which are first sensed through mechanical transduction and organelle responses (19, 20). These mechanisms represent the foundation of the adaptive cascade but also constitute the initial nodes where maladaptation may arise under conditions of excessive or prolonged stimulation (21, 22) (Figure 1).
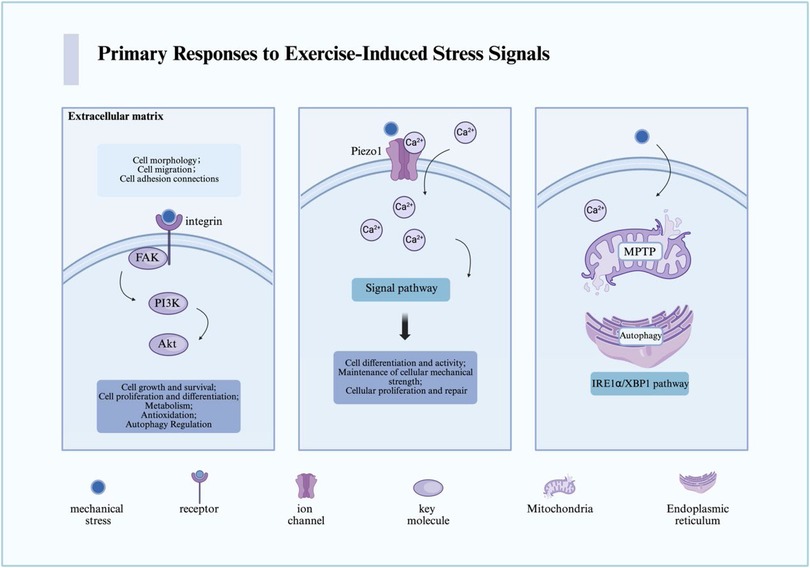
Figure 1. The primary pathways through which cells sense mechanical stress derived from exercise-induced stress are illustrated. In the figure, we present the key receptors, pathways, and cellular responses involved in the sensing and transduction of mechanical stress. For detailed descriptions, refer to the corresponding sections in the text. This figure was created using Biorender. MPTP, mitochondrial permeability transition pore; Ca, calcium; FAK, focal adhesion kinase; PI3K, phosphoinositide 3-kinase; Akt, also known as protein kinase B (PKB); Piezo1, piezo-type mechanosensitive ion channel component 1.
2.1 Mechanical signal transduction
Cells perceive external mechanical forces primarily through integrins, focal adhesion kinase (FAK), and mechanosensitive ion channels such as Piezo1. Under moderate loading, integrins activate FAK and downstream PI3K/Akt signaling, which promote survival, proliferation, and differentiation—key processes for tissue adaptation and repair (11, 14, 23). Similarly, Piezo1-mediated Ca2+ influx enhances osteoblast differentiation and vascular remodeling, supporting musculoskeletal and cardiovascular health (24–26).
However, the same pathways can contribute to pathological remodeling when excessively or persistently activated. Continuous Piezo1 activation may induce pathological Ca2+ overload, leading to mitochondrial dysfunction, inflammasome activation, and maladaptive fibrosis (27–29). Similarly, sustained FAK overexpression has been implicated in fibrosis and tumor progression, suggesting that while transient activation supports regeneration, chronic overstimulation may shift toward disease phenotypes (30, 31). These dual effects exemplify the bidirectional threshold theory, emphasizing that the biological outcome depends not only on whether these pathways are activated but also on the intensity, duration, and recovery dynamics of the stimulus (32).
The threshold for activation also differs by exercise mode. For example, endurance training typically induces moderate, repetitive integrin-FAK-Akt activation that supports angiogenesis and mitochondrial biogenesis (33), whereas resistance training imposes acute high-intensity loads that more strongly activate mTORC1-mediated anabolic pathways (34–36). Yet, excessive resistance training may surpass the adaptive threshold, leading to inflammatory microdamage and impaired recovery (37). Future research should quantify these thresholds across exercise types to define the molecular boundaries between adaptation and overtraining injury (38).
Quantitatively, in many human studies IL-6 levels have been observed to increase several-fold (e.g., ∼5-fold) within 1–3 h after a bout of endurance exercise (39). Similarly, serum BDNF concentrations are commonly reported to rise substantially (e.g., tens of percent) in the first hour following moderate exercise (40, 41). More precise quantification across various exercise modalities and populations remains a priority for future work.
2.2 Interactions between organelles
Mitochondria and the endoplasmic reticulum (ER) serve as critical hubs of cellular stress responses (42, 43). Moderate exercise enhances mitochondrial oxidative capacity and transiently activates the unfolded protein response (UPR), supporting proteostasis and energy supply (44). However, under excessive exercise, pathological events emerge: persistent opening of the mitochondrial permeability transition pore (MPTP) can trigger ATP depletion and apoptosis, while unresolved ER stress can shift from adaptive UPR to pro-apoptotic signaling (via CHOP and JNK), culminating in cell death (45, 46).
Importantly, mitochondria and the ER are not isolated. They communicate through specialized structures known as mitochondria-associated membranes (MAMs), which mediate Ca2+ flux, ROS signaling, and lipid transfer (47). Exercise modulates these interactions in a bidirectional manner (43). Moderate exercise promotes Ca2+-dependent mitochondrial activation and metabolic efficiency, whereas excessive Ca2+ transfer via MAMs may lead to mitochondrial Ca2+ overload, ROS accumulation, and apoptotic signaling (48). In addition, lysosomes also participate in this crosstalk by regulating autophagy and mitophagy, processes essential for clearing damaged organelles and maintaining cellular homeostasis (49, 50). Dysregulation of these networks under chronic overtraining may therefore contribute to systemic fatigue and impaired recovery (50, 51).
In summary, primary stress responses to exercise involve finely tuned signaling through mechanical sensors and organelle networks. While these pathways underpin the health benefits of physical activity, their chronic or excessive activation can drive maladaptive remodeling and disease. This duality underscores the importance of exercise “dose” and sets the stage for secondary metabolic reprogramming, discussed in the following section.
3 Secondary regulation of exercise-induced metabolism
This cascade can be temporally framed: mechanical signals emerge within seconds to minutes, metabolic and organelle adaptations occur over hours, while systemic endocrine and immune effects manifest across days to weeks (52). Beyond the primary mechanical and organelle-level responses, exercise triggers profound changes in cellular metabolism (53). These secondary regulatory mechanisms revolve around the cell's capacity to sense and respond to energy fluctuations, reprogram metabolic networks, and establish long-term adaptations through epigenetic regulation (Figure 2).
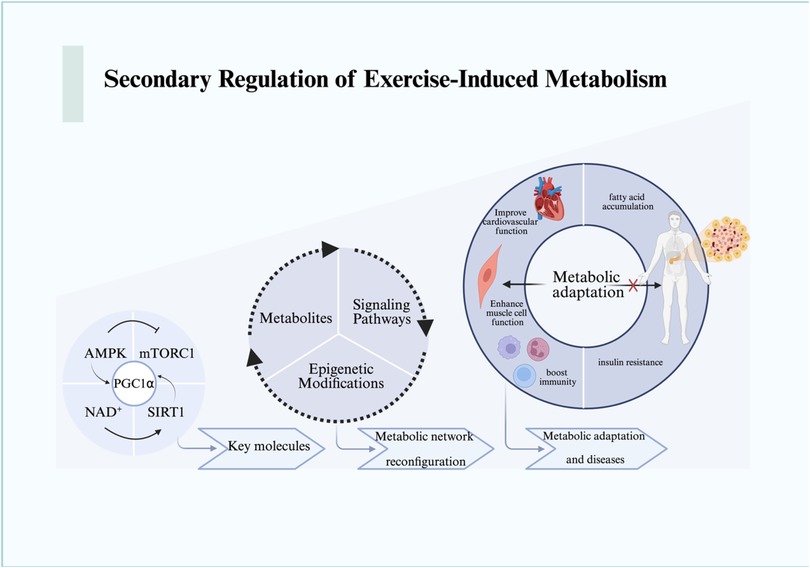
Figure 2. The metabolic responses and changes resulting from the mechanical stress signal transduction cascade are illustrated. We depict the metabolic reactions that expand from the molecular level to the cellular level and beyond, encompassing the macro processes of metabolic remodeling and adaptation. For detailed descriptions, refer to the corresponding sections in the text. This figure was created using Biorender. AMPK, AMP-activated protein kinase; mTORC1, mammalian target of rapamycin complex 1; NAD+, nicotinamide adenine dinucleotide; PGC1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; SIRT1, sirtuin 1.
3.1 Energy sensing: AMPK, mTORC1, and NAD+
Energy sensing is primarily mediated by AMP-activated protein kinase (AMPK), mechanistic target of rapamycin complex 1 (mTORC1), and the NAD+-dependent sirtuin family (54, 55). During energy deprivation, elevated AMP/ATP ratios activate AMPK, which in turn suppresses mTORC1 by phosphorylating TSC2 and Raptor, thereby inhibiting protein synthesis and promoting autophagy (56). This ensures cellular survival under energy stress while conserving resources for essential processes.
NAD+ plays a pivotal role in dynamically regulating this process. Increased NAD+ levels activate SIRT1, which deacetylates and activates PGC-1α, thereby enhancing mitochondrial biogenesis and oxidative metabolism (57, 58). Conversely, NAD+ depletion compromises sirtuin activity, attenuating the adaptive response. Importantly, AMPK and NAD+ signaling are tightly coupled: AMPK enhances NAD+ biosynthesis through upregulation of nicotinamide phosphoribosyl transferase (NAMPT), creating a feed-forward loop that integrates energy sensing with mitochondrial function (59, 60).
This dynamic interplay exemplifies how exercise fine-tunes metabolic pathways according to energetic demands. Yet, excessive exercise may overwhelm these networks: chronic AMPK overactivation has been associated with impaired anabolic signaling and fatigue, while sustained mTORC1 inhibition can lead to muscle atrophy (61, 62). Thus, the balance between AMPK and mTORC1 is essential in defining the adaptive vs. maladaptive trajectory of exercise responses.
3.2 Metabolic network reprogramming and epigenetic regulation
Exercise induces systemic metabolic reprogramming, involving glucose utilization, lipid oxidation, and ketone body metabolism (63–66). For instance, β-hydroxybutyrate (BHB), a major ketone body elevated during prolonged exercise or fasting, not only serves as an alternative fuel but also functions as a signaling molecule (67, 68). BHB directly inhibits the NLRP3 inflammasome by blocking potassium efflux and preventing ASC oligomerization, thereby exerting anti-inflammatory effects (68, 69). This mechanism highlights how metabolic intermediates act as regulators of immune-inflammatory responses during exercise.
Epigenetic regulation further extends these adaptive changes. Exercise alters DNA methylation, histone modifications, and non-coding RNA expression, reshaping transcriptional programs in muscle, adipose tissue, and immune cells (69–71). For example, exercise-induced histone acetylation at metabolic gene promoters enhances oxidative capacity (71, 72), while microRNAs (miRNAs) fine-tune pathways related to angiogenesis, mitochondrial function, and inflammation (73, 74). Notably, miR-1 and miR-133a have been implicated in regulating muscle hypertrophy, while miR-21 modulates fibrosis-related signaling (75, 76). These small RNA-mediated effects, previously discussed as independent regulatory factors, are best understood within the broader context of epigenetic reprogramming, where they contribute to the persistence of exercise-induced phenotypes (77).
Taken together, metabolic network reprogramming integrates immediate energy sensing with long-term epigenetic adaptations (78). This dual regulation enables the body to flexibly respond to diverse exercise intensities. However, unresolved or maladaptive reprogramming—such as sustained inflammatory signaling or fibrosis-related gene activation—may underlie the transition from adaptive responses to pathological remodeling under conditions of excessive exercise (79, 80).
4 Systemic integration of exercise-induced stress responses
The primary and secondary stress responses triggered by exercise ultimately converge at the systemic level, where hormones, myokines, neurotrophic factors, and immune mediators coordinate cross-tissue communication (81, 82). This integration ensures that local cellular adaptations translate into organism-wide benefits, yet it also represents the level at which excessive stress can propagate maladaptive outcomes such as chronic inflammation, neuroendocrine imbalance, or metabolic dysfunction (83, 84) (Figure 3).
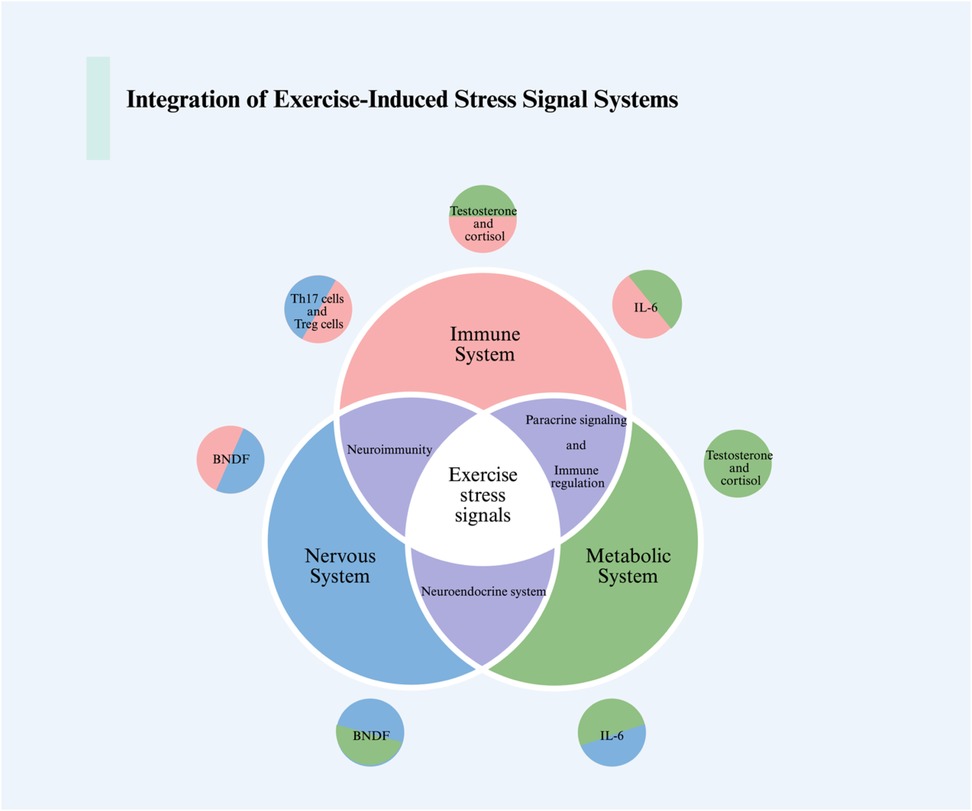
Figure 3. The integration of intercellular, intertissue, and intersystem communications in response to exercise stress signals is illustrated in the figure. We showcase the interactions between the nervous system, immune system, and metabolic system in response to exercise-induced stress. This includes the communication processes among immune cells, muscle cells, neurons, and how hormones and the endocrine system tie together this integrative communication process. For detailed descriptions, refer to the corresponding sections in the text. This figure was created using Biorender. BDNF, brain-derived neurotrophic factor; IL-6, interleukin-6; Th17, T helper 17; Treg, regulatory T cells.
4.1 Endocrine and myokine signaling
Exercise induces profound endocrine adjustments, including elevated catecholamines, cortisol, and growth hormone, which transiently mobilize energy reserves and promote tissue repair (85, 86). Beyond classical hormones, skeletal muscle acts as an endocrine organ by releasing myokines such as irisin, interleukin-6 (IL-6), and myostatin (81, 87). These factors exert diverse systemic effects, ranging from enhancing thermogenesis and lipid metabolism (irisin) to modulating immune cell activation (IL-6).
While moderate exercise-induced myokine release supports metabolic homeostasis and immune surveillance, excessive or chronic activation may shift this balance (5, 88). For example, persistently elevated IL-6 levels are associated with systemic inflammation and insulin resistance, whereas prolonged cortisol elevation can impair immunity and muscle protein synthesis (89). Thus, endocrine and myokine responses exemplify the bidirectional nature of exercise-induced systemic signaling.
4.2 Neuro-immuno-metabolic interactions: the role of BDNF
Brain-derived neurotrophic factor (BDNF) represents a critical node linking exercise-induced stress with neural plasticity and cognitive resilience. Exercise robustly enhances BDNF expression in both the hippocampus and peripheral circulation, primarily through activation of Ca2+-dependent CREB signaling and downstream PI3K/Akt and MAPK/ERK pathways (90, 91). BDNF binds to its receptor TrkB, promoting neuronal survival, dendritic growth, and synaptic plasticity (92, 93).
Importantly, BDNF also mediates cross-talk between the nervous, immune, and metabolic systems (94). By enhancing neuronal stress resistance, BDNF contributes to the attenuation of hypothalamic-pituitary-adrenal (HPA) axis hyperactivation, thereby reducing systemic stress hormone exposure (95, 96). Furthermore, exercise-induced BDNF upregulation has been linked to improved glucose metabolism and increased mitochondrial function in peripheral tissues, suggesting that BDNF acts as a systemic coordinator of neuro-immuno-metabolic interactions (97).
Conversely, inadequate recovery or chronic overtraining may blunt BDNF responses, impair synaptic resilience, and exacerbate neuroinflammation. Such alterations may contribute to fatigue, mood disturbances, and impaired cognitive performance commonly observed in overtrained athletes (98, 99).
4.3 Immune adaptation and dysregulation
Exercise exerts a dual influence on the immune system. Moderate physical activity enhances natural killer (NK) cell activity, boosts antigen presentation, and promotes anti-inflammatory cytokine profiles, thereby strengthening immune defense and surveillance against tumors and infections (100). In contrast, prolonged exhaustive exercise can suppress NK cell cytotoxicity, elevate pro-inflammatory cytokines (e.g., TNF-α, IL-1β), and increase susceptibility to infections (101–104).
At the molecular level, immune responses are tightly coupled to metabolic reprogramming. AMPK activation in T cells supports memory formation and stress tolerance, whereas excessive glycolytic reprogramming under chronic stress drives T-cell exhaustion (105, 106). This highlights the systemic feedback loop whereby metabolic and immune adaptations are intertwined in defining exercise outcomes.
5 Biomarkers and multimodal analyses of exercise-induced stress
Identifying reliable biomarkers and applying multimodal analytical approaches are critical for evaluating exercise-induced stress responses. Biomarkers provide measurable indicators of adaptive vs. maladaptive trajectories, while advanced analytical technologies allow for a systems-level understanding of complex responses.
5.1 Molecular and cellular biomarkers
Biomarkers of exercise stress span multiple categories, including mitochondrial dynamics, oxidative stress, inflammation, and cell death pathways (107). Mitochondrial fusion protein MFN2 and pyroptosis-related GSDMD have been implicated as regulators of muscular and systemic adaptation (108–111). Decreased MFN2 expression has been associated with impaired mitochondrial quality control and reduced endurance capacity (112, 113). However, current evidence is largely derived from animal and cross-sectional studies; longitudinal human cohort data are limited, and causal links to athletic performance remain speculative. Therefore, conclusions regarding MFN2 and exercise performance should be interpreted cautiously.
Oxidative stress-related biomarkers provide additional insights. Superoxide dismutase 2 (SOD2), glutathione peroxidase (GPx), and catalase represent key antioxidant defenses upregulated during moderate exercise (112, 114, 115). Conversely, excessive or exhaustive exercise often leads to their depletion alongside increased lipid peroxidation (MDA) and elevated pro-inflammatory cytokines such as TNF-α and IL-6 (116). These markers not only indicate cellular redox balance but also reflect systemic inflammation, making them valuable for assessing the transition from physiological adaptation to pathological stress.
5.2 Epigenetic and non-coding RNA biomarkers
Exercise alters the expression of various non-coding RNAs, which can serve as potential biomarkers of adaptive remodeling or pathological stress. For example, miR-1, miR-133a, and miR-206 are strongly linked to muscle hypertrophy and regeneration (117–119). In addition, miR-29b has been reported to inhibit fibrosis in certain experimental settings (120, 121). However, some studies—such as the use of nanoparticle-delivered miR-29b to inhibit fibrosis—were conducted in vitro under osteogenic conditions rather than in the context of exercise-induced cardiac fibrosis (122, 123). This discrepancy highlights the importance of contextual validation before extrapolating findings to exercise physiology.
5.3 Multimodal analytical approaches
Advances in high-throughput and single-cell technologies enable a multimodal perspective on exercise-induced stress (124). Single-cell transcriptomics, proteomics, and metabolomics provide unprecedented resolution in capturing cell-type specific responses (125). For example, single-cell sequencing has revealed exercise-induced heterogeneity in immune cell metabolic reprogramming (126). Moreover, extracellular vesicles (EVs), including exosomes, have gained attention as carriers of exercise-induced signals (127, 128). Reports suggest that EVs can transport transcriptional regulators such as PGC-1α mRNA, thereby influencing mitochondrial biogenesis (129, 130). However, most current evidence stems from neural stem cell-derived exosome studies rather than direct exercise experiments, and the causal relationship between exercise, exosomal cargo, and enhanced endurance capacity remains to be clarified (129, 131).
Therefore, while exosomes and other multimodal biomarkers hold great promise, more rigorous exercise-specific experimental validation is needed to confirm their functional relevance.
5.4 Integrative framework and limitations
Multimodal biomarker approaches must account for inter-individual variability, including sex, age, genetic background, and training status (132). These factors can significantly modulate biomarker responses, complicating the definition of universal thresholds. For example, older individuals may exhibit blunted antioxidant responses (133), while genetic polymorphisms in mitochondrial genes could influence stress resilience. Integrating multimodal datasets with clinical phenotypes is thus essential to establish robust biomarkers for guiding personalized exercise prescriptions.
6 Discussion and future directions
This review has summarized how exercise-induced stress responses progress from primary mechanical and organelle signals to secondary metabolic regulation and ultimately to systemic integration across endocrine, immune, and neural networks. By organizing these responses into a layered cascade—primary responses, secondary regulation, and systemic integration—we have highlighted the dual nature of exercise as both a health-promoting and potentially pathological stimulus. A central theme emphasized throughout this review is the bidirectional threshold theory, which provides a conceptual framework for understanding how exercise intensity and duration determine biological outcomes. While moderate exercise promotes beneficial adaptations such as mitochondrial biogenesis, enhanced antioxidant defense, and improved neuroplasticity, excessive or prolonged exercise can lead to maladaptive processes including calcium overload, mitochondrial permeability transition pore (MPTP) opening, maladaptive ER stress, chronic inflammation, and fibrosis (134, 135). However, a key limitation of the current literature is the imbalance in mechanistic evidence: adaptive responses are well characterized, but the molecular underpinnings of maladaptive trajectories remain less systematically explored. For example, while Piezo1 activation is known to facilitate vascular remodeling, its potential contribution to pathological calcium influx and tissue fibrosis under sustained activation has not been rigorously studied (136, 137). Similarly, the transition from adaptive unfolded protein response (UPR) to pro-apoptotic ER stress during exhaustive exercise requires more in vivo validation.
Another limitation lies in the translation of experimental findings to human physiology. Much of the mechanistic data derives from animal models or in vitro systems, which may not fully capture the complexity of human exercise responses. Longitudinal human cohort studies are scarce, making it difficult to establish causal links between molecular markers (e.g., MFN2, SOD2, exosomal cargo) and real-world exercise outcomes such as performance, recovery, and disease risk. Moreover, individual differences—including sex, age, training history, and genetic background—are seldom addressed in mechanistic studies, yet they critically shape exercise-induced stress responses.
While exercise is broadly beneficial, the potential for maladaptation or pathological damage cannot be overlooked, particularly in high-intensity or prolonged regimens. A balanced perspective requires integrating monitoring tools that can detect when beneficial adaptation shifts toward risk. Practical approaches include setting training intensity using relative measures such as %VO2max or %heart rate reserve (HRR) (138), tracking recovery via heart rate variability (HRV) and lactate clearance, and assessing biochemical markers such as creatine kinase (CK), interleukin-6 (IL-6), and oxidative stress indices (139–141). In addition, validated psychometric tools (e.g., RESTQ-Sport, Profile of Mood States) can identify early warning signs of overreaching or overtraining (142). These approaches should be viewed as pragmatic starting points rather than definitive guidelines. Further longitudinal clinical studies are required to validate and standardize risk-stratification strategies for different populations.
An important limitation of the bidirectional threshold framework is its sensitivity to individual-specific factors. Ageing is associated with reduced mitochondrial adaptability and a blunted antioxidant response, lowering the threshold at which maladaptive effects emerge (143, 144). Sex and hormonal status, particularly estrogen levels, modulate inflammatory and oxidative stress pathways, contributing to sex-based differences in training outcomes (145). Genetic background (e.g., polymorphisms in ACTN3, PGC-1α) further influences cardiorespiratory fitness and muscle adaptation (146). Training history also determines baseline resilience: well-trained individuals often exhibit attenuated biomarker responses compared with untrained individuals under the same workload. Finally, comorbid conditions such as diabetes, obesity, or cardiovascular disease substantially modify exercise-induced stress responses, often lowering tolerance and increasing risk for maladaptation. Collectively, these factors underscore that the “bidirectional threshold” must be interpreted flexibly rather than as a universal cut-off, highlighting the need for personalized approaches in both research and clinical translation.
From a methodological perspective, the integration of multimodal omics technologies (e.g., single-cell transcriptomics, proteomics, metabolomics) with clinical phenotyping offers a promising avenue to bridge mechanistic insights with human variability. However, technical challenges remain, such as harmonizing data across platforms, capturing transient exercise responses in real time, and distinguishing adaptive vs. maladaptive signatures within heterogeneous cell populations.
Looking forward, several areas warrant particular attention:
1. Defining molecular thresholds of adaptation vs. maladaptation across exercise intensities and modes (endurance vs. resistance), with quantitative markers to guide individualized exercise prescriptions.
2. Mechanistic studies of maladaptation, including Piezo1-mediated calcium overload, chronic FAK signaling, MPTP dysregulation, and maladaptive ER stress.
3. Validation of biomarkers in human cohorts, with longitudinal tracking to establish predictive value for performance, recovery, and disease outcomes.
4. Integration of multimodal datasets to capture the systemic nature of exercise responses, with a focus on linking molecular pathways to functional outcomes.
5. Personalized exercise medicine, leveraging genetic, epigenetic, and metabolic profiling to design tailored interventions that maximize benefits while minimizing risks.
In conclusion, the biological responses to exercise stress are not unidirectional but exist along a continuum shaped by intensity, duration, and individual context. By advancing our understanding of both adaptive and maladaptive pathways, future research can refine exercise as a precise therapeutic modality—balancing health promotion with the prevention of overtraining-related pathology.
Author contributions
JX: Writing – original draft, Funding acquisition, Resources, Formal analysis, Software, Visualization, Methodology, Data curation, Investigation. JZ: Data curation, Methodology, Writing – original draft, Formal analysis, Investigation. KS: Supervision, Writing – review & editing, Conceptualization, Formal analysis, Visualization, Validation, Project administration, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the following funding sources: University-Industry Collaborative Innovation Project (Grant No. 2023Y4020).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Priya Dharshini LC, Vishnupriya S, Sakthivel KM, Rasmi RR. Oxidative stress responsive transcription factors in cellular signalling transduction mechanisms. Cell Signal. (2020) 72:109670. doi: 10.1016/j.cellsig.2020.109670
2. He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. (2012) 481:511–5. doi: 10.1038/nature10758
3. Powers SK, Deminice R, Ozdemir M, Yoshihara T, Bomkamp MP, Hyatt H. Exercise-induced oxidative stress: friend or foe? J Sport Health Sci. (2020) 9:415–25. doi: 10.1016/j.jshs.2020.04.001
4. McGee SL, Hargreaves M. Exercise adaptations: molecular mechanisms and potential targets for therapeutic benefit. Nat Rev Endocrinol. (2020) 16:495–505. doi: 10.1038/s41574-020-0377-1
5. Nieman DC, Wentz LM. The compelling link between physical activity and the body’s defense system. J Sport Health Sci. (2019) 8:201–17. doi: 10.1016/j.jshs.2018.09.009
6. Pedersen BK, Saltin B. Exercise as medicine—evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports. (2015) 25(Suppl 3):1–72. doi: 10.1111/sms.12581
7. Thompson PD. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease. Arterioscler Thromb Vasc Biol. (2003) 23:1319–21. doi: 10.1161/01.ATV.0000087143.33998.F2
8. Schoenfeld BJ, Ogborn D, Krieger JW. Dose-response relationship between weekly resistance training volume and increases in muscle mass: a systematic review and meta-analysis. J Sports Sci. (2017) 35:1073–82. doi: 10.1080/02640414.2016.1210197
9. Zhou Y, Zhang X, Baker JS, Davison GW, Yan X. Redox signaling and skeletal muscle adaptation during aerobic exercise. iScience. (2024) 27:109643. doi: 10.1016/j.isci.2024.109643
10. Bouviere J, Fortunato RS, Dupuy C, Werneck-de-Castro JP, Carvalho DP, Louzada RA. Exercise-stimulated ROS sensitive signaling pathways in skeletal muscle. Antioxid Basel Switz. (2021) 10:537. doi: 10.3390/antiox10040537
11. Boppart MD, Mahmassani ZS. Integrin signaling: linking mechanical stimulation to skeletal muscle hypertrophy. Am J Physiol Cell Physiol. (2019) 317:C629–41. doi: 10.1152/ajpcell.00009.2019
12. Roberts MD, McCarthy JJ, Hornberger TA, Phillips SM, Mackey AL, Nader GA, et al. Mechanisms of mechanical overload-induced skeletal muscle hypertrophy: current understanding and future directions. Physiol Rev. (2023) 103:2679–757. doi: 10.1152/physrev.00039.2022
13. Ripamonti M, Wehrle-Haller B, de Curtis I. Paxillin: a Hub for mechano-transduction from the β3 Integrin-Talin-Kindlin Axis. Front Cell Dev Biol. (2022) 10:852016. doi: 10.3389/fcell.2022.852016
14. Pang X, He X, Qiu Z, Zhang H, Xie R, Liu Z, et al. Targeting integrin pathways: mechanisms and advances in therapy. Signal Transduct Target Ther. (2023) 8:1. doi: 10.1038/s41392-022-01259-6
15. Clarkson PM. Exercise-induced muscle damage–animal and human models. Med Sci Sports Exerc. (1992) 24:510–1. doi: 10.1249/00005768-199205000-00003
16. Chrzanowski-Smith OJ, Piatrikova E, Betts JA, Williams S, Gonzalez JT. Variability in exercise physiology: can capturing intra-individual variation help better understand true inter-individual responses? Eur J Sport Sci. (2020) 20:452–60. doi: 10.1080/17461391.2019.1655100
17. Zhao Y, Wang W, Wang M, Gao F, Hu C, Cui B, et al. Personalized individual-based exercise prescriptions are effective in treating depressive symptoms of college students during the COVID-19: a randomized controlled trial in China. Front Psychiatry. (2022) 13:1015725. doi: 10.3389/fpsyt.2022.1015725
18. Sun T, Xu Y, Xie H, Ma Z, Wang Y. Intelligent personalized exercise prescription based on an eHealth promotion system to improve health outcomes of middle-aged and older adult community dwellers: pretest-posttest study. J Med Internet Res. (2021) 23:e28221. doi: 10.2196/28221
19. Bernareggi A, Bosutti A, Massaria G, Giniatullin R, Malm T, Sciancalepore M, et al. The state of the art of Piezo1 channels in skeletal muscle regeneration. Int J Mol Sci (2022) 23:6616. doi: 10.3390/ijms23126616
20. Splitt RL, DeMali KA. Metabolic reprogramming in response to cell mechanics. Biol Cell. (2023) 115:e202200108. doi: 10.1111/boc.202200108
21. Craige SM, Mammel RK, Amiri N, Willoughby OS, Drake JC. Interplay of ROS, mitochondrial quality, and exercise in aging: potential role of spatially discrete signaling. Redox Biol. (2024) 77:103371. doi: 10.1016/j.redox.2024.103371
22. Rühmkorf A, Harbauer AB. Role of mitochondria-ER contact sites in mitophagy. Biomolecules. (2023) 13:1198. doi: 10.3390/biom13081198
23. Riquelme MA, Gu S, Hua R, Jiang JX. Mechanotransduction via the coordinated actions of integrins, PI3K signaling and connexin hemichannels. Bone Res (2021) 9:8. doi: 10.1038/s41413-020-00126-w
24. Chen J, Rodriguez M, Miao J, Liao J, Jain PP, Zhao M, et al. Mechanosensitive channel Piezo1 is required for pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol. (2022) 322:L737–60. doi: 10.1152/ajplung.00447.2021
25. Liu H, Hu J, Zheng Q, Feng X, Zhan F, Wang X, et al. Piezo1 channels as force sensors in mechanical force-related chronic inflammation. Front Immunol. (2022) 13:816149. doi: 10.3389/fimmu.2022.816149
26. Mirzoev TM. The emerging role of Piezo1 channels in skeletal muscle physiology. Biophys Rev. (2023) 15:1171–84. doi: 10.1007/s12551-023-01154-6
27. Ran L, Ye T, Erbs E, Ehl S, Spassky N, Sumara I, et al. KCNN4 Links PIEZO-dependent mechanotransduction to NLRP3 inflammasome activation. Sci Immunol. (2023) 8:eadf4699. doi: 10.1126/sciimmunol.adf4699
28. Sun Y, Leng P, Song M, Li D, Guo P, Xu X, et al. Piezo1 activates the NLRP3 inflammasome in nucleus pulposus cell-mediated by Ca2+/NF-κB pathway. Int Immunopharmacol. (2020) 85:106681. doi: 10.1016/j.intimp.2020.106681
29. He J, Cheng X, Fang B, Shan S, Li Q. Mechanical stiffness promotes skin fibrosis via Piezo1-Wnt2/Wnt11-CCL24 positive feedback loop. Cell Death Dis. (2024) 15:84. doi: 10.1038/s41419-024-06466-3
30. Niu L, Cheng B, Huang G, Nan K, Han S, Ren H, et al. A positive mechanobiological feedback loop controls bistable switching of cardiac fibroblast phenotype. Cell Discov. (2022) 8:84. doi: 10.1038/s41421-022-00427-w
31. Schlaepfer DD, Ojalill M, Stupack DG. Focal adhesion kinase signaling—tumor vulnerabilities and clinical opportunities. J Cell Sci. (2024) 137:jcs261723. doi: 10.1242/jcs.261723
32. Coste B, Delmas P. PIEZO Ion channels in cardiovascular functions and diseases. Circ Res. (2024) 134:572–91. doi: 10.1161/CIRCRESAHA.123.322798
33. Ross M, Kargl CK, Ferguson R, Gavin TP, Hellsten Y. Exercise-induced skeletal muscle angiogenesis: impact of age, sex, angiocrines and cellular mediators. Eur J Appl Physiol. (2023) 123:1415–32. doi: 10.1007/s00421-022-05128-6
34. D’Hulst G, Masschelein E, De Bock K. Resistance exercise enhances long-term mTORC1 sensitivity to leucine. Mol Metab. (2022) 66:101615. doi: 10.1016/j.molmet.2022.101615
35. Schiaffino S, Reggiani C, Akimoto T, Blaauw B. Molecular mechanisms of skeletal muscle hypertrophy. J Neuromuscul Dis. (2021) 8:169–83. doi: 10.3233/JND-200568
36. Zhu WG, Thomas AC, Wilson GM, McGlory C, Hibbert JE, Flynn CG, et al. Identification of a resistance-exercise-specific signalling pathway that drives skeletal muscle growth. Nat Metab. (2025) 7:1404–23. doi: 10.1038/s42255-025-01298-7
37. Armstrong LE, Bergeron MF, Lee EC, Mershon JE, Armstrong EM. Overtraining syndrome as a Complex systems phenomenon. Front Netw Physiol. (2021) 1:794392. doi: 10.3389/fnetp.2021.794392
38. Egan B, Sharples AP. Molecular responses to acute exercise and their relevance for adaptations in skeletal muscle to exercise training. Physiol Rev. (2023) 103:2057–170. doi: 10.1152/physrev.00054.2021
39. Robson-Ansley P, Cockburn E, Walshe I, Stevenson E, Nimmo M. The effect of exercise on plasma soluble IL-6 receptor concentration: a dichotomous response. Exerc Immunol Rev. (2010) 16:56–76.20839491
40. Ferris LT, Williams JS, Shen C-L. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med Sci Sports Exerc. (2007) 39:728–34. doi: 10.1249/mss.0b013e31802f04c7
41. Hung C-L, Tseng J-W, Chao H-H, Hung T-M, Wang H-S. Effect of acute exercise mode on serum brain-derived neurotrophic factor (BDNF) and task switching performance. J Clin Med. (2018) 7:301. doi: 10.3390/jcm7100301
42. Wang L, Zhang S. Investigating the causal effects of exercise-induced genes on sarcopenia. Int J Mol Sci (2024) 25:10773. doi: 10.3390/ijms251910773
43. Lv Y, Cheng L, Peng F. Compositions and functions of mitochondria-associated endoplasmic reticulum membranes and their contribution to cardioprotection by exercise preconditioning. Front Physiol. (2022) 13:910452. doi: 10.3389/fphys.2022.910452
44. Gaspar RS, Katashima CK, Crisol BM, Carneiro FS, Sampaio I, dos Reis Silveira L, et al. Physical exercise elicits UPRmt in the skeletal muscle: the role of c-Jun N-terminal kinase. Mol Metab. (2023) 78:101816. doi: 10.1016/j.molmet.2023.101816
45. Endlicher R, Drahota Z, Štefková K, Červinková Z, Kučera O. The mitochondrial permeability transition pore-current knowledge of its structure, function, and regulation, and optimized methods for evaluating its functional state. Cells. (2023) 12:1273. doi: 10.3390/cells12091273
46. Marafon BB, Pinto AP, Ropelle ER, de Moura LP, Cintra DE, Pauli JR, et al. Muscle endoplasmic reticulum stress in exercise. Acta Physiol Oxf Engl. (2022) 235:e13799. doi: 10.1111/apha.13799
47. Barazzuol L, Giamogante F, Calì T. Mitochondria associated membranes (MAMs): architecture and physiopathological role. Cell Calcium. (2021) 94:102343. doi: 10.1016/j.ceca.2020.102343
48. Loncke J, Kaasik A, Bezprozvanny I, Parys JB, Kerkhofs M, Bultynck G. Balancing ER-mitochondrial Ca2+ fluxes in health and disease. Trends Cell Biol. (2021) 31:598–612. doi: 10.1016/j.tcb.2021.02.003
49. Moradi N, Sanfrancesco VC, Champsi S, Hood DA. Regulation of lysosomes in skeletal muscle during exercise, disuse and aging. Free Radic Biol Med. (2024) 225:323–32. doi: 10.1016/j.freeradbiomed.2024.09.028
50. Jia Z, Li H, Xu K, Li R, Yang S, Chen L, et al. MAM-mediated mitophagy and endoplasmic reticulum stress: the hidden regulators of ischemic stroke. Front Cell Neurosci. (2024) 18:1470144. doi: 10.3389/fncel.2024.1470144
51. Shi M, Dong Z, Zhao K, He X, Sun Y, Ren J, et al. Novel insights into exhaustive exercise-induced myocardial injury: focusing on mitochondrial quality control. Front Cardiovasc Med. (2022) 9:1015639. doi: 10.3389/fcvm.2022.1015639
52. Furrer R, Hawley JA, Handschin C. The molecular athlete: exercise physiology from mechanisms to medals. Physiol Rev. (2023) 103:1693–787. doi: 10.1152/physrev.00017.2022
53. Hargreaves M, Spriet LL. Skeletal muscle energy metabolism during exercise. Nat Metab. (2020) 2:817–28. doi: 10.1038/s42255-020-0251-4
54. Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. (2018) 19:121–35. doi: 10.1038/nrm.2017.95
55. Cantó C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol (2009) 20:98–105. doi: 10.1097/MOL.0b013e328328d0a4
56. Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, et al. TSC2 Integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. (2006) 126:955–68. doi: 10.1016/j.cell.2006.06.055
57. Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, et al. AMPK Regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. (2009) 458:1056–60. doi: 10.1038/nature07813
58. Cantó C, Menzies KJ, Auwerx J. NAD(+) metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metab. (2015) 22:31–53. doi: 10.1016/j.cmet.2015.05.023
59. Garten A, Schuster S, Penke M, Gorski T, De Giorgis T, Kiess W. Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat Rev Endocrinol. (2015) 11:535–46. doi: 10.1038/nrendo.2015.117
60. Deng Y, Duan R, Ding W, Gu Q, Liu M, Zhou J, et al. Astrocyte-derived exosomal nicotinamide phosphoribosyltransferase (Nampt) ameliorates ischemic stroke injury by targeting AMPK/mTOR signaling to induce autophagy. Cell Death Dis. (2022) 13:1057. doi: 10.1038/s41419-022-05454-9
61. Thomson DM. The role of AMPK in the regulation of skeletal muscle size, hypertrophy, and regeneration. Int J Mol Sci. (2018) 19:3125. doi: 10.3390/ijms19103125
62. Ham AS, Chojnowska K, Tintignac LA, Lin S, Schmidt A, Ham DJ, et al. mTORC1 signalling is not essential for the maintenance of muscle mass and function in adult sedentary mice. J Cachexia Sarcopenia Muscle. (2020) 11:259–73. doi: 10.1002/jcsm.12505
63. Puchalska P, Crawford PA. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. (2017) 25:262–84. doi: 10.1016/j.cmet.2016.12.022
64. Williams AS, Crown SB, Lyons SP, Koves TR, Wilson RJ, Johnson JM, et al. Ketone flux through BDH1 supports metabolic remodeling of skeletal and cardiac muscles in response to intermittent time-restricted feeding. Cell Metab. (2024) 36:422–437.e8. doi: 10.1016/j.cmet.2024.01.007
65. Xie Y, Zhao H, Zhao M, Huang H, Liu C, Huang F, et al. Effects of resistance exercise on blood glucose level and pregnancy outcome in patients with gestational diabetes mellitus: a randomized controlled trial. BMJ Open Diabetes Res Care. (2022) 10:e002622. doi: 10.1136/bmjdrc-2021-002622
66. Brouwers B, Hesselink MKC, Schrauwen P, Schrauwen-Hinderling VB. Effects of exercise training on intrahepatic lipid content in humans. Diabetologia. (2016) 59:2068–79. doi: 10.1007/s00125-016-4037-x
67. Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. (2015) 21:263–9. doi: 10.1038/nm.3804
68. Qi J, Gan L, Fang J, Zhang J, Yu X, Guo H, et al. Beta-Hydroxybutyrate: a dual function molecular and immunological barrier function regulator. Front Immunol. (2022) 13:805881. doi: 10.3389/fimmu.2022.805881
69. Hirata Y, Shimazaki S, Suzuki S, Henmi Y, Komiyama H, Kuwayama T, et al. β-hydroxybutyrate suppresses NLRP3 inflammasome-mediated placental inflammation and lipopolysaccharide-induced fetal absorption. J Reprod Immunol. (2021) 148:103433. doi: 10.1016/j.jri.2021.103433
70. Ehlert T, Simon P, Moser DA. Epigenetics in sports. Sports Med Auckl NZ. (2013) 43:93–110. doi: 10.1007/s40279-012-0012-y
71. Smith JAB, Murach KA, Dyar KA, Zierath JR. Exercise metabolism and adaptation in skeletal muscle. Nat Rev Mol Cell Biol. (2023) 24:607–32. doi: 10.1038/s41580-023-00606-x
72. Seaborne RA, Sharples AP. The interplay between exercise metabolism, epigenetics, and skeletal muscle remodeling. Exerc Sport Sci Rev. (2020) 48:188–200. doi: 10.1249/JES.0000000000000227
73. Sanchis-Gomar F, Arnau-Moyano M, Daimiel L, Lippi G, Leischik R, Vallecillo N, et al. Circulating microRNAs fluctuations in exercise-induced cardiac remodeling: a systematic review. Am J Transl Res. (2021) 13:13298–309.35035676
74. Estébanez B, Jiménez-Pavón D, Huang C-J, Cuevas MJ, González-Gallego J. Effects of exercise on exosome release and cargo in in vivo and ex vivo models: a systematic review. J Cell Physiol. (2021) 236:3336–53. doi: 10.1002/jcp.30094
76. Torma F, Gombos Z, Jokai M, Berkes I, Takeda M, Mimura T, et al. The roles of microRNA in redox metabolism and exercise-mediated adaptation. J Sport Health Sci. (2020) 9:405–14. doi: 10.1016/j.jshs.2020.03.004
77. Widmann M, Nieß AM, Munz B. Physical exercise and epigenetic modifications in skeletal muscle. Sports Med Auckl NZ. (2019) 49:509–23. doi: 10.1007/s40279-019-01070-4
78. Fan W, Evans RM. Exercise mimetics: impact on health and performance. Cell Metab. (2017) 25:242–7. doi: 10.1016/j.cmet.2016.10.022
79. Palumbo P, Cannizzaro E, Di Cesare A, Bruno F, Schicchi N, Giovagnoni A, et al. Cardiac magnetic resonance in arrhythmogenic cardiomyopathies. Radiol Med (Torino). (2020) 125:1087–101. doi: 10.1007/s11547-020-01289-6
80. Jetté M, Sidney K, Blümchen G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin Cardiol. (1990) 13:555–65. doi: 10.1002/clc.4960130809
81. Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. (2012) 8:457–65. doi: 10.1038/nrendo.2012.49
82. Yi J, Chen J, Yao X, Zhao Z, Niu X, Li X, Sun J, et al. Myokine-mediated muscle-organ interactions: molecular mechanisms and clinical significance. Biochem Pharmacol. (2025) 242:117326. doi: 10.1016/j.bcp.2025.117326
83. Cho JM, Vu K, Park SK, Zhu E, Li YR, Zhao P, et al. Habitual exercise modulates neuroimmune interaction to mitigate aortic stiffness. Circ Res. (2025) 136:1579–94. doi: 10.1161/CIRCRESAHA.124.325656
84. Silverman MN, Deuster PA. Biological mechanisms underlying the role of physical fitness in health and resilience. Interface Focus. (2014) 4:20140040. doi: 10.1098/rsfs.2014.0040
85. Nolan D, McNulty KL, Manninen M, Egan B. The effect of hormonal contraceptive use on skeletal muscle hypertrophy, power and strength adaptations to resistance exercise training: a systematic review and multilevel meta-analysis. Sports Med Auckl NZ. (2024) 54:105–25. doi: 10.1007/s40279-023-01911-3
86. Kraemer WJ, Ratamess NA. Hormonal responses and adaptations to resistance exercise and training. Sports Med Auckl NZ. (2005) 35:339–61. doi: 10.2165/00007256-200535040-00004
87. Powers SK, Goldstein E, Lategan-Potgieter R, Schrager M, Skelton M, Demirel H. Health benefits of physical activity: what role does skeletal muscle-organ crosstalk play? Sports Med Health Sci. (2025) 7:329–40. doi: 10.1016/j.smhs.2025.02.010
88. Cadegiani FA, Kater CE. Hormonal aspects of overtraining syndrome: a systematic review. BMC Sports Sci Med Rehabil. (2017) 9:14. doi: 10.1186/s13102-017-0079-8
89. Dennis RA, Trappe TA, Simpson P, Carroll C, Emma Huang B, Nagarajan R, et al. Interleukin-1 polymorphisms are associated with the inflammatory response in human muscle to acute resistance exercise. J Physiol. (2004) 560:617–26. doi: 10.1113/jphysiol.2004.067876
90. Zhou LJ, Zhong Y, Ren WJ, Li YY, Zhang T, Liu XG. BDNF Induces late-phase LTP of C-fiber evoked field potentials in rat spinal dorsal horn. Exp Neurol. (2008) 212:507–14. doi: 10.1016/j.expneurol.2008.04.034
91. Alqarni SS, Afzal M, Alharbi KS, Alenezi SK, Alsahli TG, Zaidi S, et al. Rosiridin protects against aluminum chloride-induced memory impairment via modulation of BDNF/NFκB/PI3K/akt pathway in rats. Medicina (Kaunas). (2024) 60(11):1812. doi: 10.3390/medicina60111812
92. Di Liegro CM, Schiera G, Proia P, Di Liegro I. Physical activity and brain health. Genes (Basel). (2019) 10:720. doi: 10.3390/genes10090720
93. Jin W. Regulation of BDNF-TrkB signaling and potential therapeutic strategies for Parkinson’s disease. J Clin Med. (2020) 9:257. doi: 10.3390/jcm9010257
94. Choi SH, Bylykbashi E, Chatila ZK, Lee SW, Pulli B, Clemenson GD, et al. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science. (2018) 361:eaan8821. doi: 10.1126/science.aan8821
95. Chen Y, Tian P, Wang Z, Pan R, Shang K, Wang G, et al. Indole acetic acid exerts anti-depressive effects on an animal model of chronic mild stress. Nutrients. (2022) 14:5019. doi: 10.3390/nu14235019
96. Wincewicz D, Juchniewicz A, Waszkiewicz N, Braszko JJ. Angiotensin II type 1 receptor blockade by telmisartan prevents stress-induced impairment of memory via HPA axis deactivation and up-regulation of brain-derived neurotrophic factor gene expression. Pharmacol Biochem Behav. (2016) 148:108–18. doi: 10.1016/j.pbb.2016.06.010
97. Kong J, Xie Y, Fan R, Wang Q, Luo Y, Dong P. Exercise orchestrates systemic metabolic and neuroimmune homeostasis via the brain-muscle-liver axis to slow down aging and neurodegeneration: a narrative review. Eur J Med Res. (2025) 30:475. doi: 10.1186/s40001-025-02751-9
98. Buzdagli Y, Ozan M, Baygutalp N, Oget F, Karayigit R, Yuce N, et al. The effect of high-intensity intermittent and moderate-intensity continuous exercises on neurobiological markers and cognitive performance. BMC Sports Sci Med Rehabil. (2024) 16:39. doi: 10.1186/s13102-024-00831-7
99. Piotrowicz Z, Chalimoniuk M, Płoszczyca K, Czuba M, Langfort J. Exercise-Induced elevated BDNF level does not prevent cognitive impairment due to acute exposure to moderate hypoxia in well-trained athletes. Int J Mol Sci (2020) 21:5569. doi: 10.3390/ijms21155569
100. Rumpf C, Proschinger S, Schenk A, Bloch W, Lampit A, Javelle F, et al. The effect of acute physical exercise on NK-cell cytolytic activity: a systematic review and meta-analysis. Sports Med Auckl NZ. (2021) 51:519–30. doi: 10.1007/s40279-020-01402-9
101. Simpson RJ, Campbell JP, Gleeson M, Krüger K, Nieman DC, Pyne DB, et al. Can exercise affect immune function to increase susceptibility to infection? Exerc Immunol Rev. (2020) 26:8–22.32139352
102. Valenzuela PL, Saco-Ledo G, Santos-Lozano A, Morales JS, Castillo-Garcia A, Simpson RJ, et al. Exercise training and natural killer cells in cancer survivors: current evidence and research gaps based on a systematic review and meta-analysis. Sports Med—Open. (2022) 8:36. doi: 10.1186/s40798-022-00419-w
103. Chen C, Zhang D, Ye M, You Y, Song Y, Chen X. Effects of various exercise types on inflammatory response in individuals with overweight and obesity: a systematic review and network meta-analysis of randomized controlled trials. Int J Obes. (2025) 49:214–25. doi: 10.1038/s41366-024-01649-6
104. Bettariga F, Taaffe DR, Galvao DA, Lopez P, Bishop C, Markarian AM, et al. Exercise training mode effects on myokine expression in healthy adults: a systematic review with meta-analysis. J Sport Health Sci. (2024) 13:764–79. doi: 10.1016/j.jshs.2024.04.005
105. Ma EH, Poffenberger MC, Wong AH-T, Jones RG. The role of AMPK in T cell metabolism and function. Curr Opin Immunol. (2017) 46:45–52. doi: 10.1016/j.coi.2017.04.004
106. He J, Shangguan X, Zhou W, Cao Y, Zheng Q, Tu J, et al. Glucose limitation activates AMPK coupled SENP1-Sirt3 signalling in mitochondria for T cell memory development. Nat Commun. (2021) 12:4371. doi: 10.1038/s41467-021-24619-2
107. Arazi H, Eghbali E, Suzuki K. Creatine supplementation, physical exercise and oxidative stress markers: a review of the mechanisms and effectiveness. Nutrients. (2021) 13:869. doi: 10.3390/nu13030869
108. Zorzano A. Regulation of mitofusin-2 expression in skeletal muscle. Appl Physiol Nutr Metab Physiol Appl Nutr Metab. (2009) 34:433–9. doi: 10.1139/H09-049
109. Cefis M, Dargegen M, Marcangeli V, Taherkhani S, Dulac M, Leduc-Gaudet JP, et al. MFN2 Overexpression in skeletal muscles of young and old mice causes a mild hypertrophy without altering mitochondrial respiration and H2O2 emission. Acta Physiol Oxf Engl. (2024) 240:e14119. doi: 10.1111/apha.14119
110. Wei H, Cui D. Pyroptosis and insulin resistance in metabolic organs. Int J Mol Sci (2022) 23:11638. doi: 10.3390/ijms231911638
111. Yan X, Fu P, Zhang Y, Ling D, Reynolds L, Hua W, et al. MCC950 Ameliorates diabetic muscle atrophy in mice by inhibition of pyroptosis and its synergistic effect with aerobic exercise. Molecules. (2024) 29(3):712. doi: 10.3390/molecules29030712
112. Xie Y, Gu Y, Li Z, Zhang L, Hei Y. Effects of exercise on different antioxidant enzymes and related indicators: a systematic review and meta-analysis of randomized controlled trials. Sci Rep. (2025) 15:12518. doi: 10.1038/s41598-025-97101-4
113. Molina AJ, Bharadwaj MS, Van Horn C, Nicklas BJ, Lyles MF, Eggebeen J, et al. Skeletal muscle mitochondrial content, oxidative capacity, and Mfn2 expression are reduced in older patients with heart failure and preserved ejection fraction and are related to exercise intolerance. JACC Heart Fail (2016) 4:636–45. doi: 10.1016/j.jchf.2016.03.011
114. Powers SK, Goldstein E, Schrager M, Ji LL. Exercise training and skeletal muscle antioxidant enzymes: an update. Antioxid Basel Switz. (2022) 12:39. doi: 10.3390/antiox12010039
115. Ogino S, Ogino N, Tomizuka K, Eitoku M, Okada Y, Tanaka Y, et al. SOD2 mRNA as a potential biomarker for exercise: interventional and cross-sectional research in healthy subjects. J Clin Biochem Nutr. (2021) 69:137–44. doi: 10.3164/jcbn.21-24
116. Reihmane D, Jurka A, Tretjakovs P, Dela F. Increase in IL-6, TNF-α, and MMP-9, but not sICAM-1, concentrations depends on exercise duration. Eur J Appl Physiol. (2013) 113:851–8. doi: 10.1007/s00421-012-2491-9
117. Li H, Kang L, Wu R, Li C, Zhang Q, Zhong R, et al. miR-378-mediated glycolytic metabolism enriches the Pax7Hi subpopulation of satellite cells. Cell Regen Lond Engl. (2022) 11:11. doi: 10.1186/s13619-022-00112-z
118. Gasparini P, Ferrari A, Casanova M, Limido F, Massimino M, Sozzi G, et al. MiRNAs as players in rhabdomyosarcoma development. Int J Mol Sci (2019) 20:5818. doi: 10.3390/ijms20225818
119. Shintani-Ishida K, Tsurumi R, Ikegaya H. Decrease in the expression of muscle-specific miRNAs, miR-133a and miR-1, in myoblasts with replicative senescence. PLoS One. (2023) 18:e0280527. doi: 10.1371/journal.pone.0280527
120. Lv X, Lu P, Hu Y, Xu T. Overexpression of MiR-29b-3p inhibits atrial remodeling in rats by targeting PDGF-B signaling pathway. Oxid Med Cell Longev. (2021) 2021:3763529. doi: 10.1155/2021/3763529
121. Wang M, Huo Z, He X, Liu F, Liang J, Wu L, et al. The role of MiR-29 in the mechanism of fibrosis. Mini Rev Med Chem. (2023) 23:1846–58. doi: 10.2174/1389557523666230328125031
122. Qin H, Ji Y, Li G, Xu X, Zhang C, Zhong W, et al. MicroRNA-29b/graphene oxide-polyethyleneglycol-polyethylenimine complex incorporated within chitosan hydrogel promotes osteogenesis. Front Chem. (2022) 10:958561. doi: 10.3389/fchem.2022.958561
123. Wang J, Cui Y, Liu H, Li S, Sun S, Xu H, et al. MicroRNA-loaded biomaterials for osteogenesis. Front Bioeng Biotechnol. (2022) 10:952670. doi: 10.3389/fbioe.2022.952670
124. Lovrić A, Rassolie A, Alam S, Mandić M, Saini A, Altun M, et al. Single-cell sequencing deconvolutes cellular responses to exercise in human skeletal muscle. Commun Biol (2022) 5:1121. doi: 10.1038/s42003-022-04088-z
125. Bhattachan P, Jeschke MG. Single-cell transcriptome analysis in health and disease. Shock Augusta Ga. (2024) 61:19–27. doi: 10.1097/SHK.0000000000002274
126. Yu Y, Zhang X, Chen Y, Li Y, Bian S, Yang Y, et al. Single-cell sequencing of immune cells after marathon and symptom-limited cardiopulmonary exercise. iScience. (2023) 26:106532. doi: 10.1016/j.isci.2023.106532
127. Nederveen JP, Warnier G, Di Carlo A, Nilsson MI, Tarnopolsky MA. Extracellular vesicles and exosomes: insights from exercise science. Front Physiol. (2021) 11:604274. doi: 10.3389/fphys.2020.604274
128. Fischetti F, Poli L, De Tommaso M, Paolicelli D, Greco G, Cataldi S. The role of exercise parameters on small extracellular vesicles and microRNAs cargo in preventing neurodegenerative diseases. Front Physiol. (2023) 14:1241010. doi: 10.3389/fphys.2023.1241010
129. Li B, Chen Y, Zhou Y, Feng X, Gu G, Han S, et al. Neural stem cell-derived exosomes promote mitochondrial biogenesis and restore abnormal protein distribution in a mouse model of Alzheimer’s disease. Neural Regen Res. (2024) 19:1593–601. doi: 10.4103/1673-5374.385839
130. Guo J, Zhou F, Liu Z, Cao Y, Zhao W, Zhang Z, et al. Exosome-shuttled mitochondrial transcription factor A mRNA promotes the osteogenesis of dental pulp stem cells through mitochondrial oxidative phosphorylation activation. Cell Prolif. (2022) 55:e13324. doi: 10.1111/cpr.13324
131. Llorente A, Brokāne A, Mlynska A, Puurand M, Sagini K, Folkmane S, et al. From sweat to hope: the role of exercise-induced extracellular vesicles in cancer prevention and treatment. J Extracell Vesicles. (2024) 13:e12500. doi: 10.1002/jev2.12500
132. Noone J, Mucinski JM, DeLany JP, Sparks LM, Goodpaster BH. Understanding the variation in exercise responses to guide personalized physical activity prescriptions. Cell Metab. (2024) 36:702–24. doi: 10.1016/j.cmet.2023.12.025
133. El Assar M, Álvarez-Bustos A, Sosa P, Angulo J, Rodríguez-Mañas L. Effect of physical activity/exercise on oxidative stress and inflammation in muscle and vascular aging. Int J Mol Sci. (2022) 23:8713. doi: 10.3390/ijms23158713
134. Cardinale DA, Gejl KD, Petersen KG, Nielsen J, Ørtenblad N, Larsen FJ. Short-term intensified training temporarily impairs mitochondrial respiratory capacity in elite endurance athletes. J Appl Physiol. (2021) 131:388–400. doi: 10.1152/japplphysiol.00829.2020
135. Pereira BC, Da Rocha AL, Pinto AP, Pauli JR, De Souza CT, Cintra DE, et al. Excessive eccentric exercise-induced overtraining model leads to endoplasmic reticulum stress in mice skeletal muscles. Life Sci. (2016) 145:144–51. doi: 10.1016/j.lfs.2015.12.037
136. Wang JY, Li BH, Liu CY, Wang QH, Wang J, Guo MJ, et al. Piezo1 in heart failure: a new perspective from cytomechanical sensing to diverse cellular pathways. Mol Biol Rep. (2025) 52:862. doi: 10.1007/s11033-025-10969-3
137. Fang XZ, Li M, Wang YX, Zhang P, Sun MM, Xu JX, et al. Mechanosensitive ion channel Piezo1 mediates mechanical ventilation-exacerbated ARDS-associated pulmonary fibrosis. J Adv Res. (2023) 53:175–86. doi: 10.1016/j.jare.2022.12.006
138. Granero-Gallegos A, González-Quílez A, Plews D, Carrasco-Poyatos M. HRV-Based Training for improving VO2max in endurance athletes. A systematic review with meta-analysis. Int J Environ Res Public Health. (2020) 17:7999. doi: 10.3390/ijerph17217999
139. Cipryan L. IL-6, antioxidant capacity and muscle damage markers following high-intensity interval training protocols. J Hum Kinet. (2017) 56:139–48. doi: 10.1515/hukin-2017-0031
140. Tajra V, Tibana RA, Vieira DC, de Farias DL, Teixeira TG, Funghetto SS, et al. Identification of high responders for interleukin-6 and creatine kinase following acute eccentric resistance exercise in elderly obese women. J Sci Med Sport. (2014) 17:662–6. doi: 10.1016/j.jsams.2013.09.012
141. de Sousa Neto IV, da Cunha Nascimento D, Prestes J, da Fonseca EF, Celes RS, Rolnick N, et al. Initial muscle quality affects individual responsiveness of interleukin-6 and creatine kinase following acute eccentric exercise in sedentary obese older women. Biology (Basel). (2022) 11:537. doi: 10.3390/biology11040537
142. Qian H, Lee S. A multidimensional prediction model for overtraining risk in youth soccer players: integrating physiological and psychological markers. J Sports Sci. (2025) 43:1819–34. doi: 10.1080/02640414.2025.2521211
143. Ringholm S, Gudiksen A, Frey Halling J, Qoqaj A, Meizner Rasmussen P, Prats C, et al. Impact of aging and lifelong exercise training on mitochondrial function and network connectivity in human skeletal muscle. J Gerontol A Biol Sci Med Sci (2023) 78:373–83. doi: 10.1093/gerona/glac164
144. Zhu Y, Zhou X, Zhu A, Xiong S, Xie J, Bai Z. Advances in exercise to alleviate sarcopenia in older adults by improving mitochondrial dysfunction. Front Physiol. (2023) 14:1196426. doi: 10.3389/fphys.2023.1196426
145. Bellanti F, Matteo M, Rollo T, De Rosario F, Greco P, Vendemiale G, et al. Sex hormones modulate circulating antioxidant enzymes: impact of estrogen therapy. Redox Biol. (2013) 1:340–6. doi: 10.1016/j.redox.2013.05.003
Keywords: exercise-induced stress, bidirectional threshold, biological response cascade, adaptation and maladaptation, exercise variability
Citation: Xu J, Zhang J and Sang K (2025) Mechanisms of the biological response cascade to exercise-induced stress: a comprehensive review. Front. Sports Act. Living 7:1691779. doi: 10.3389/fspor.2025.1691779
Received: 24 August 2025; Accepted: 21 October 2025;
Published: 13 November 2025.
Edited by:
Haibo Wang, Anhui Science and Technology University, ChinaReviewed by:
Liang Guo, Shanghai University of Sport, ChinaNavid Abedpoor, Islamic Azad University, Iran
Yike Wu, Shenzhen Second People's Hospital, China
Copyright: © 2025 Xu, Zhang and Sang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kai Sang, c2FuZ2syMDIzQDE2My5jb20=
 Jing Xu1
Jing Xu1 Kai Sang
Kai Sang