- 1The Edinburgh Cell Wall Group, Institute of Molecular Plant Sciences, School of Biological Sciences, The University of Edinburgh, Edinburgh, United Kingdom
- 2Functional Plant Biology, Department of Botany, University of Innsbruck, Innsbruck, Austria
Land plants inherited several traits from their green algal ancestors (Zygnematophyceae), including a polysaccharide-rich cell wall, which is a prerequisite for terrestrial survival. A major component of both land plant and Zygnematophyceaen cell walls is the pectin homogalacturonan (HG), and its high water holding capacity may have helped algae to colonize terrestrial habitats, characterized by water scarcity. To test this, HG was removed from the cell walls of Zygnema filaments by pectate lyase (PL), and their effective quantum yield of photosystem II (YII) as a proxy for photosynthetic performance was measured in response to desiccation stress by pulse amplitude modulation (PAM). Old filaments were found to contain more HG and are more resistant against desiccation stress but relatively lose more desiccation resistance after HG removal than young filaments. After rehydration, the photosynthetic performance recovered less efficiently in filaments with a HG content reduced by PL, independently of filament age. Immunolabeling showed that partial or un-methylesterified HG occurs throughout the longitudinal cell walls of both young and old filaments, while no labeling signal occurred when filaments were treated with PL prior labeling. This confirmed that most HG can be removed from the cell walls by PL. The initial labeling pattern was restored after ~3 days. A different form of methylesterified HG was restricted to cell poles and cross cell walls. In conclusion, it was shown that the accumulation of HG in Zygnema filaments increases their resistance against desiccation stress. This trait might have played an important role during the colonization of land by Zygnematophyceae, which founded the evolution of all land plants.
Introduction
The earliest land plant fossils date to the Mid-Ordovician (Steemans et al., 2009), and it was concluded that the algal ancestors of land plants started colonizing terrestrial habits at least ~450 Myr ago (Kenrick et al., 2012). On the other hand, analyses of oxygen and carbon isotope data indicate a first expansion of terrestrial photosynthesizing organisms long before the earliest fossils, ~850 Myr ago (Knauth and Kennedy, 2009). Indeed, molecular clock estimates suggest that about 700 Myr years ago, terrestrial habitats were characterized by a community of phototrophs including bryophytes (Heckman et al., 2001). Zygnematophyceae, members of the charophyte green algae (CGA), are considered the sole ancestors of land plants (Wickett et al., 2014) and therefore must have evolved strategies to survive under terrestrial conditions. Still today, Zygnematophyceae—including their species richest genus Zygnema—occur worldwide in terrestrial habitats, where they are frequently exposed to abiotic stress such as low water availability (Holzinger and Karsten, 2013). In order to maintain a metabolically active state and long-term population survival under harsh terrestrial conditions (Pichrtová et al., 2016a), these algae require survival strategies at the cellular level. One such key element for stress survival might be the algal cell wall and in a broader sense the whole extracellular matrix (ECM); Zygnematophyceae lack protective covering tissues typical for their land plant descendants (e.g., epidermis), which makes the cell wall the only barrier between the algal protoplasts and the environment. In the past decades, considerable effort has been made to investigate the chemical composition of CGA’s cell walls (Domozych et al., 1980; O’Rourke et al., 2015). In particular, specific cell wall probes, such as antibodies, proved to be a powerful and time efficient tool to study the polysaccharide composition of algal cell walls at different developmental stages, under stress conditions, or in a phylogenetic context (Sørensen et al., 2011; Domozych et al., 2014; Andosch et al., 2015; Herburger and Holzinger, 2015). Together with biochemical, genomic, and transcriptomic data (Wodniok et al., 2011; Timme et al., 2012; Mikkelsen et al., 2014; Rippin et al., 2017; De Vries et al., 2018; Jensen et al., 2018), it was concluded that the most important core cell wall components of land plants—including cellulose, homogalacturonan (HG), xyloglucan, mannans, and xylans—were already present in some streptophyte green algae, and that a polysaccharide rich cell wall can be considered a prerequisite for terrestrial survival (Harholt et al., 2016). In their natural habitats, vegetative cells of Zygnema filaments form specialized resistant cells termed “pre-akinets” (McLean and Pessoney, 1971; Pichrtová et al., 2014a), which differ from young cells in their accumulation of starch and lipid bodies rich in C18 fatty acids (Pichrtová et al., 2016b), a reduction of chloroplast size and cell diameter and an increased resistance against osmotic and desiccation stress (Kaplan et al., 2013; Pichrtová et al., 2014a; Herburger et al., 2015). Furthermore, pre-akinete formation involves a considerable increase of the cell wall diameter, which can be attributed to an increase of pectic material in the cell wall (Herburger et al., 2018). Biochemical and immunological evidence showed that the major pectic component of late diverged CGA is HG, while other pectin-domains such as arabinans or rhamnogalacturonan I are less abundant (Sørensen et al., 2011; O’Rourke et al., 2015). HG, which is still an important component of land plant cell walls, is a linear homopolymer consisting of α-1,4-linked
Materials and Methods
Algal Source and Material
Zygnema circumcarinatum (“Saalach,” SAG 2419) was grown in liquid media or on 1.5% agar plates prepared with Bold’s Basal Medium (BBM; Bischoff and Bold, 1963) for up to 12 months. Culture conditions were described in detail previously (Herburger et al., 2015). Pectate lyase (PL; E-PLYCJ, EC 4.2.2.2; from Cellvibrio japonicus) and endo-polygalacturonase (EPG; E-PGALUSP, EC 3.2.1.15; from Aspergillus aculeatus) were purchased from Megazyme Inc. (Wicklow, Ireland), the monoclonal Antibodies (mAbs) JIM5 and JIM7 from Plant Probes (Leeds, United Kingdom); other chemicals are mainly from Sigma-Aldrich (Steinheim, Germany).
Immunocytochemistry and Histochemical Staining
Immunolabeling with the mAbs JIM5, which recognizes partially methylesterified HG (Knox et al., 1990), and JIM7 (recognizing partially but not unesterified HG; Clausen et al., 2003), was done according to Herburger et al. (2018). Briefly, 1- or 12-month-old Zygnema filaments were fixed (2% paraformaldehyde, 1 h), blocked with 1% BSA (30 min), and incubated with JIM5 or JIM7 (1:20 in PBS, 2 h). After rinsing, filaments were blocked again (0.5% BSA, 30 min) and incubated in the secondary antibody (FITC goat anti-rat-IgG (whole molecule) (Sigma-Aldrich); 1:100 in PBS, 2 h). After washing, fluorescence was visualized with a Zeiss Pascal 5 confocal laser-scanning microscope on a Zeiss Axiovert 200 M microscope (EX 488 nm, EM 505–550 nm, false colored green and 560 nm long pass, false colored red). Images were taken with an Axiocam MRc5 camera and z-stacks generated by merging up to 40 optical sections through a filament. Corresponding bright-field images were collected in a third channel and merged with the false color red image. As a control, the primary antibody was omitted or heat-inactivated prior to use. To remove homogalacturonan from algal cell walls, some filaments were treated with PL (3 U ml−1 BBM, pH 6.9) in dark and under gentle shaking for up to 24 h. Treated filaments were then subjected to immunolabeling with JIM5. Furthermore, the re-formation of the HG matrix in the cell walls was monitored during different stages of the desiccation experiment by JIM5 immunolabeling. For pectin detection, untreated and PL-treated filaments from 1- or 12-month-old Zygnema cultures were incubated with 0.02% (w/v) ruthenium red in BBM for 20 min, and red color was visualized with a Zeiss Axiovert 200 M microscope (Chamberlain, 1933).
Cryofixation and Immunogold Labeling
Algal filaments were cryo-fixed and freeze-substituted in a Leica EMPACT high-pressure freezer (Leica Microsystems) and a Leica EM AFS (Lütz-Meindl and Aichinger, 2004), followed by embedding in LR-White (London Resin Company Ltd.). Ultrathin sections were prepared with a Leica ultramicrotome and immunogold labeling with JIM7, and control experiments were done according to Holzinger et al. (2000) with modifications. Briefly, ultrathin sections were blocked, incubated with JIM 7, washed, and stained with a 10 nm gold conjugated anti rat IgA secondary AB (Sigma-Aldrich). After rinsing, samples were investigated using a Zeiss Libra 120 transmission electron microscope (80 kV) connected to a ProScan 2 k SSCCD camera, controlled with OSIS iTEM software.
Photosynthetic Performance During Desiccation and After Rehydration
The consequence of desiccation followed by rehydration on the effective quantum yield of PSII [Y(II)] in Zygnema was determined according to Herburger et al. (2015). Desiccation kinetics were recorded for (1) untreated samples and (2) samples treated with PL (3 units ml−1 BBM, pH 6.9, up to 24 h), which were either immediately subjected to desiccation treatment or after a recovering period of 12, 24, or 72 h (Figure 1 summarizes the experimental setup). For desiccation, filaments were transferred onto GF/F glass fiber filters (Whatman, Dassel, Germany) previously soaked with BBM (~220 μl), placed in a desiccation chamber (Karsten et al., 2014) and dried at ~84% relative air humidity (RH; set with KCl; Greenspan, 1977) and ~40 μmol photons m−2 s−1 for up to 10 h (n = 6). Y(II) was determined continuously with a pulse-amplitude modulated fluorimeter (PAM 2500; Heinz Walz GmbH, Effeltrich, Germany). After 10 h or after Y(II) reached 0, filters were rehydrated with BBM for 24 h, placed in a fresh chamber filled with water, and the Y(II) was measured again.
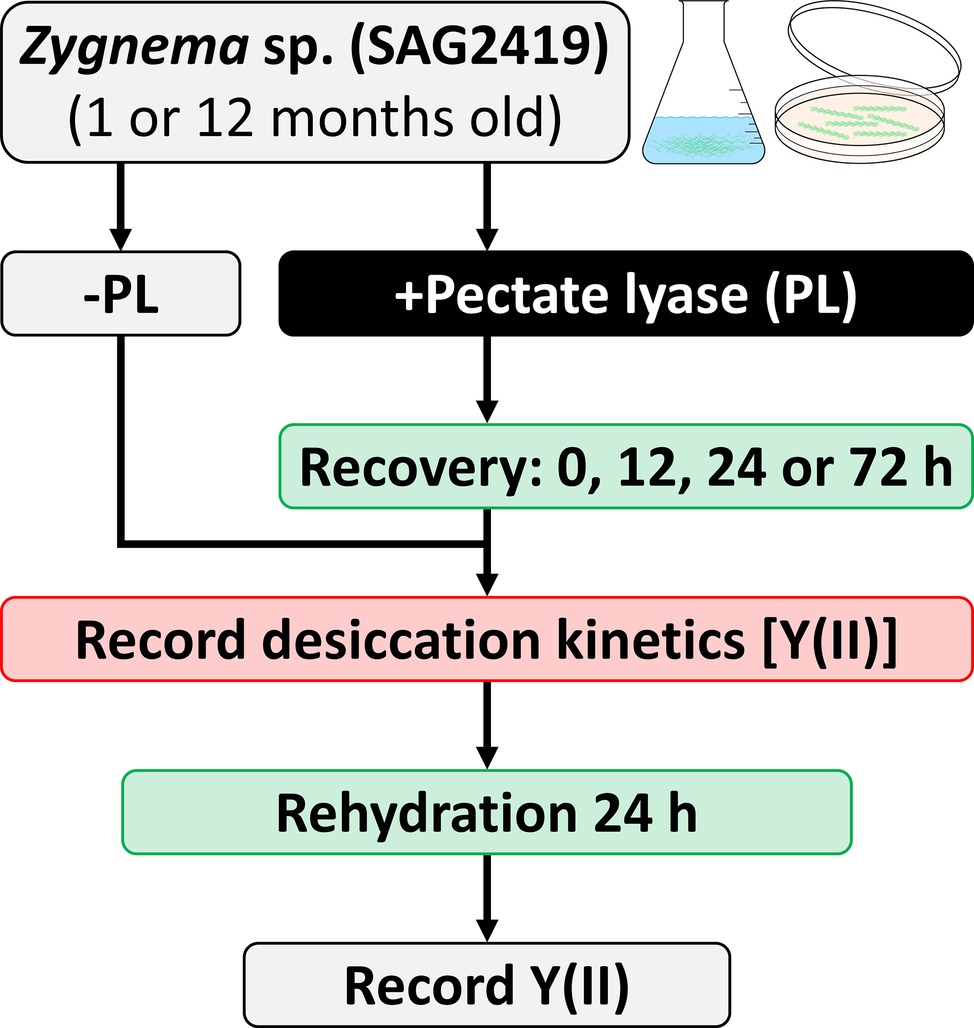
Figure 1. Experimental setup for enzymatic homogalacturonan removal and measuring the photosynthetic performance in response to desiccation treatment. Algal filaments are desiccated on glass fiber filters in a desiccation chamber.
Comparing Homogalacturonan Content in Young and Old Zygnema Cell Walls
From 1- and 12-month-old Zygnema cultures, the alcohol insoluble residue (AIR) was prepared (Foster et al., 2010); briefly, ~0.5 g fresh weight of Zygnema filaments were 70% ethanol extracted for 2 h, pelleted, and re-extracted overnight in the same solution until the supernatant was transparent. Then, the pellet was suspended in pure acetone and air dried. 5 mg of AIR were digested with 3 U/ml PL (in 200 μl 50 mM collidine buffer, pH 8.0) at 25°C for 3 days. After reaction stop, the supernatant was dried and re-dissolved in 20 μl EtOH, and digestion products were separated by thin-layer chromatography (TLC). In an additional experiment, 0.4 mg of young or old Zygnema AIR was hydrolyzed with 2 M TFA (120°C, 1 h), dried, re-dissolved in 20 μl EtOH, and analyzed by TLC. Furthermore, 1 mg of old Zygnema AIR was digested with 10 U/ml EPG [in 100 μl pyridine/acetic acid/water (1/1/98, v/v/v), pH 4.5] at 25°C for 3 days and then dried, re-dissolved in 20 μl EtOH, and subjected to TLC. Control groups lacked enzyme or TFA. TLC plates were developed in butan-1-ol:acetic acid:water (2:1:1; BAW; two ascents) on 60 F254 silica-gel plates (Merck, Darmstadt, Germany). Sugar bands were stained with thymol/H2SO4 (Jork et al., 1994) and quantified using ImageJ. Loaded markers: rhamnose (Rha), xylose (Xyl), mannose (Man), arabinose (Ara), glucose (Glc), galactose (Gal), glucuronic acid (GlcA), galacturonic acid (GalA), and PL products, GalA1–3–4,5-unsaturated 4-deoxyuronic acid (ΔUA) oligosaccharides.
Results
Pectate Lyase Removes Homogalacturonan Detectable by Immunolabeling
Pectate lyase catalyzes the endolytic and exolytic cleavage of polygalacturonic acid by β-elimination (Herron et al., 2000) and was therefore used to remove demethylesterified HG from Zygnema cell walls. Ruthenium red, which is commonly used to visualize pectins, stained both longitudinal and cross cell walls of filaments from 1-month-old cultures red (Figure 2A). Filaments treated with PL (Figure 2B), and recovering for 10 h after PL treatment (Figure 2C) showed weaker staining. Filaments from 12-month-old cultures showed strong red staining in all cell wall areas and in amorphous sheaths surrounding the filaments (Figure 2D), the latter being less visible in young filaments (Figure 2A). PL treatment of old filaments resulted in a patchy labeling pattern in longitudinal but not cross cell walls (Figure 2E). A 10 h recovering period resulted in a slightly stronger staining in outer cell walls (Figure 2F). Labeling with the mAb JIM5 showed that HG epitopes were abundant across the longitudinal cell walls of filaments from 1 month cultures before PL treatment. In contrast to Ruthenium red staining, signal was lacking in cross cell walls between individual cells and the pectic sheath layer (Figure 3A). PL treatment prior immunolabeling strongly and uniformly reduced the JIM5 signal in the cell walls (Figure 3B). A recovery period of 10 h after PL treatment only partly restored the mAb signal with a maximum in band shaped areas adjacent to cross cell walls (Figure 3C). In contrast, 72 h of recovery restored the initial signal (Figure 3D). In filaments from 12-month-old cultures (Figures 3E,F), the JIM5 labeling pattern was similar: Untreated filaments showed strong signal in longitudinal but not cross cell walls or the pectic sheath layer (Figure 3E). However, filaments treated with PL showed signal in band-shaped areas close to cross cell walls, while a patchy labeling pattern occurred in the rest of the longitudinal cell walls (Figure 3F). Ten hours of recovery after PL treatment restored the mAb signal partly and reduced the patchy labeling pattern (Figure 3G). Filaments recovering for 72 h showed full JIM5 signal restoration (Figure 3H).
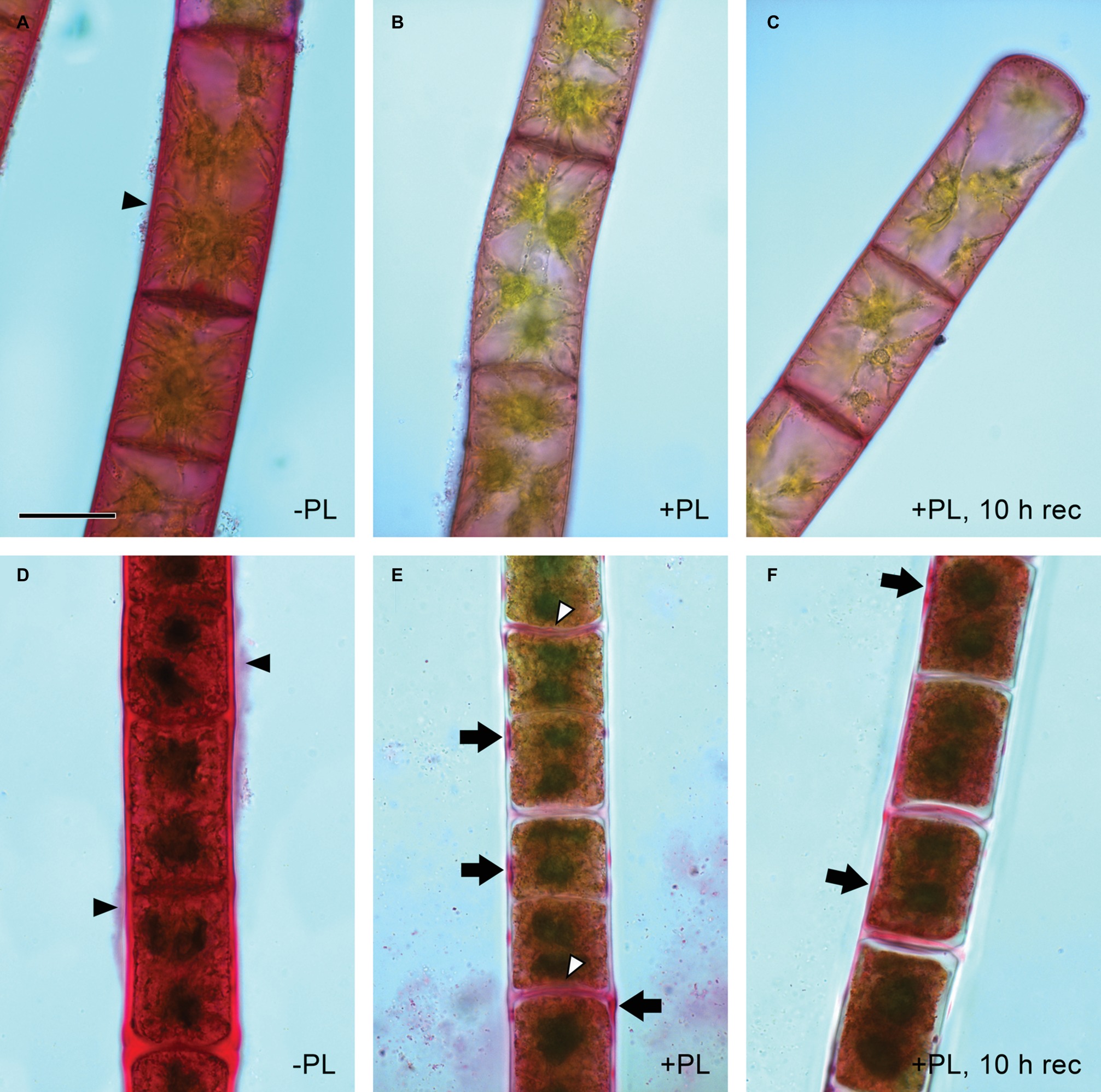
Figure 2. Detection of pectin by ruthenium red staining. Young (A–C) and old (D,E) filaments. (A,D) No pectate lyase treatment, (B,E) PL treatment and (C,F) PL treatment followed by 10 h recovery. (A,D) Red staining in cell walls and pectic sheath layer (arrowheads), (E) patchy staining in outer cell walls (arrows) and throughout most cross cell walls (arrowheads), (F) patchy staining in (E) replaced by more continuous staining (arrows). Scale bar = 20 μm.
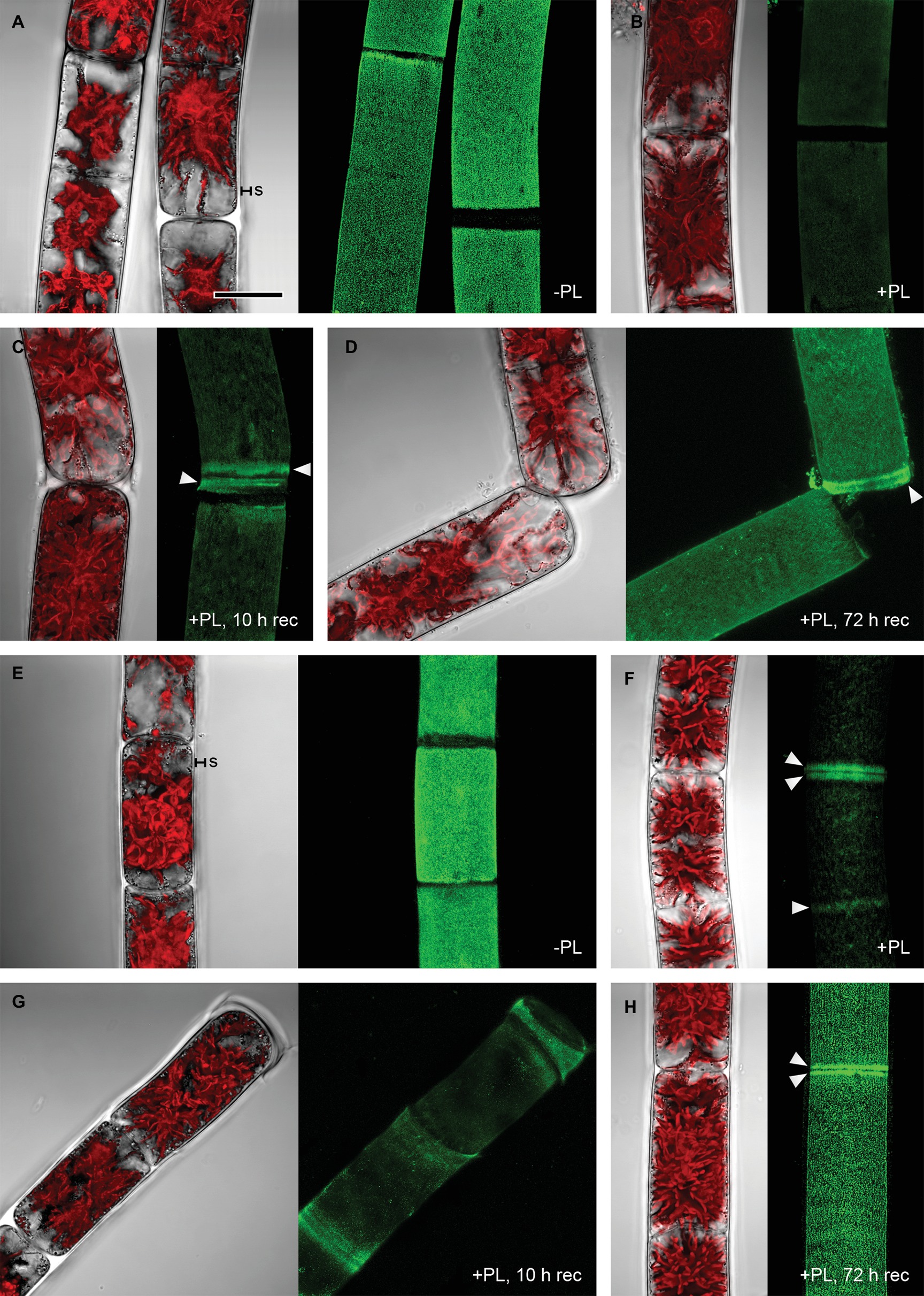
Figure 3. Detection of partially methylesterified homogalacturonan by mAb JIM5 in Zygnema filaments. Young (A–D) and old (E–H) filaments were JIM5-labeled (green, z-stacks) before pectate lyase treatment (−PL), after PL treatment (+PL) or after PL treatment followed by a recovery period (+PL, 10–12 h rec). The corresponding bright-field images include red chloroplast autofluorescence. (A,E) pectic sheath layer (s), (B,F) Labeling strongest close to cross cell walls (arrowheads), (C,G) re-formation of HG matrix next to cross cell walls (arrowheads), (D,H) labeling strongest in cross cell wall areas (arrowheads). Scale bar = 20 μm.
Another Form of Partially Methylesterified Homogalacturonan Localizes at Cell Poles and Cross Cell Walls
JIM7, recognizing partially but not unesterified HG, bound to cell wall areas close to cell poles in both young and old Zygnema filaments (Figures 4A,B). Labeling was stronger in old filaments, whereas the signal decreased gradually toward the center of the cells (Figure 4B). Furthermore, labeling occurred in cross cell walls (Figures 4C,D).
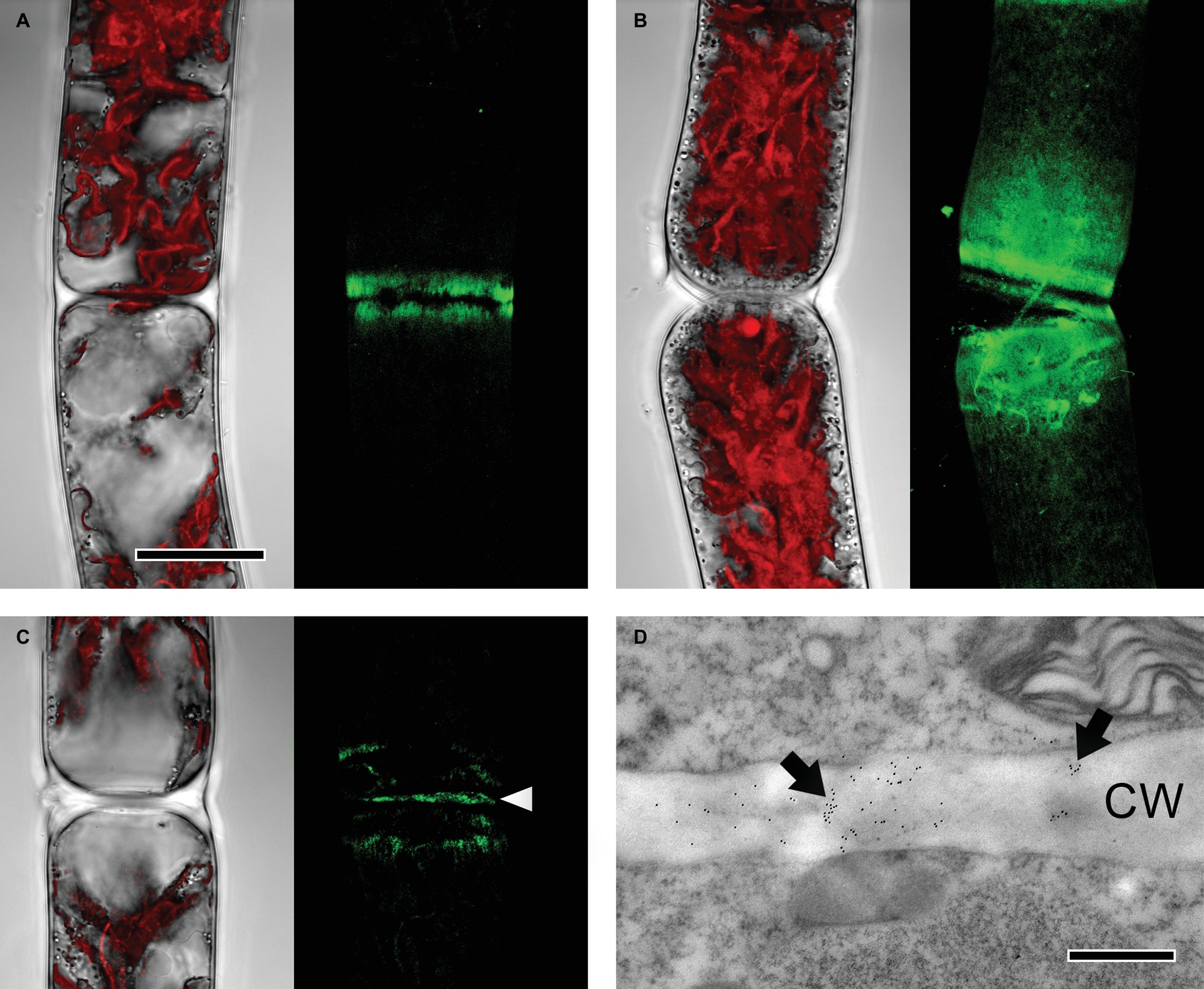
Figure 4. Detection of partially methylesterified homogalacturonan by JIM7 in Zygnema filaments. JIM7 labeling was visualized in young (A,C,D) and old (B) filaments by confocal laser scanning microscopy (A–C; green, z-stacks) and TEM (D). Corresponding bright-field images in (A–C) include red chloroplast autofluorescence. (A,B) Labeling strongest in areas close to cross cell walls, (C) signal in cross cell wall (arrowhead), (D) immunogold-labeling in cross cell wall (arrows; CW). Scale bars = 20 μm (A–C), 500 nm (D).
Removal of Homogalacturonan Reduces Resistance Against Desiccation Stress
The desiccation kinetics of untreated algal filaments and filaments treated with PL to remove homogalactuornan from the cell wall were compared to evaluate the role of HG in desiccation resistance. Algae from 1-month-old cultures and desiccated at 84% RH maintained their initial Y(II) (0.63) for ~6.5 h, before it dropped linearly to 0 within ~2 h (Figure 5A). The initial Y(II) of PL-treated algae was maintained for a shorter period (~5 h) and reached 0 after ~7.5 h of desiccation. Filaments, which were recovered after PL treatment for up to 72 h, showed similar desiccation kinetics; however, a Y(II) above 0 was maintained longer when compared with samples lacking recovery after PL treatment (Figure 5A). Desiccated filaments, which were not treated with PL, recovered their Y(II) partially after a 24 h rehydration period (~50% of the initial value; Figure 5A). Only PL treated samples, which were recovered for 72 h after desiccation stress, recovered their Y(II) to ~50% of the initial value (Figure 5A). The Y(II) of untreated algae from 12-month-old cultures did not reach 0 after 10 h of desiccation at 84% RH (Figure 5B). In contrast, PL treatment of 12 month-old samples caused a linear decline of the initial Y(II) (0.55) after ~7 h and Y(II) reached 0 after ~9.5 h. A 12 h recovering time between PL treatment and desiccation treatment produced similar results. However, longer recovering periods (24 or 72 h) prevented the Y(II) to drop to 0 even after 10 h of desiccation (Figure 5B). Rehydrating desiccated algae for 24 h allowed all samples (−PL, +PL, or +PL followed by recovering periods up to 72 h) to recover their Y(II), while untreated samples and samples recovered for 72 h after PL treatment restored their initial Y(II) (Figure 5B).
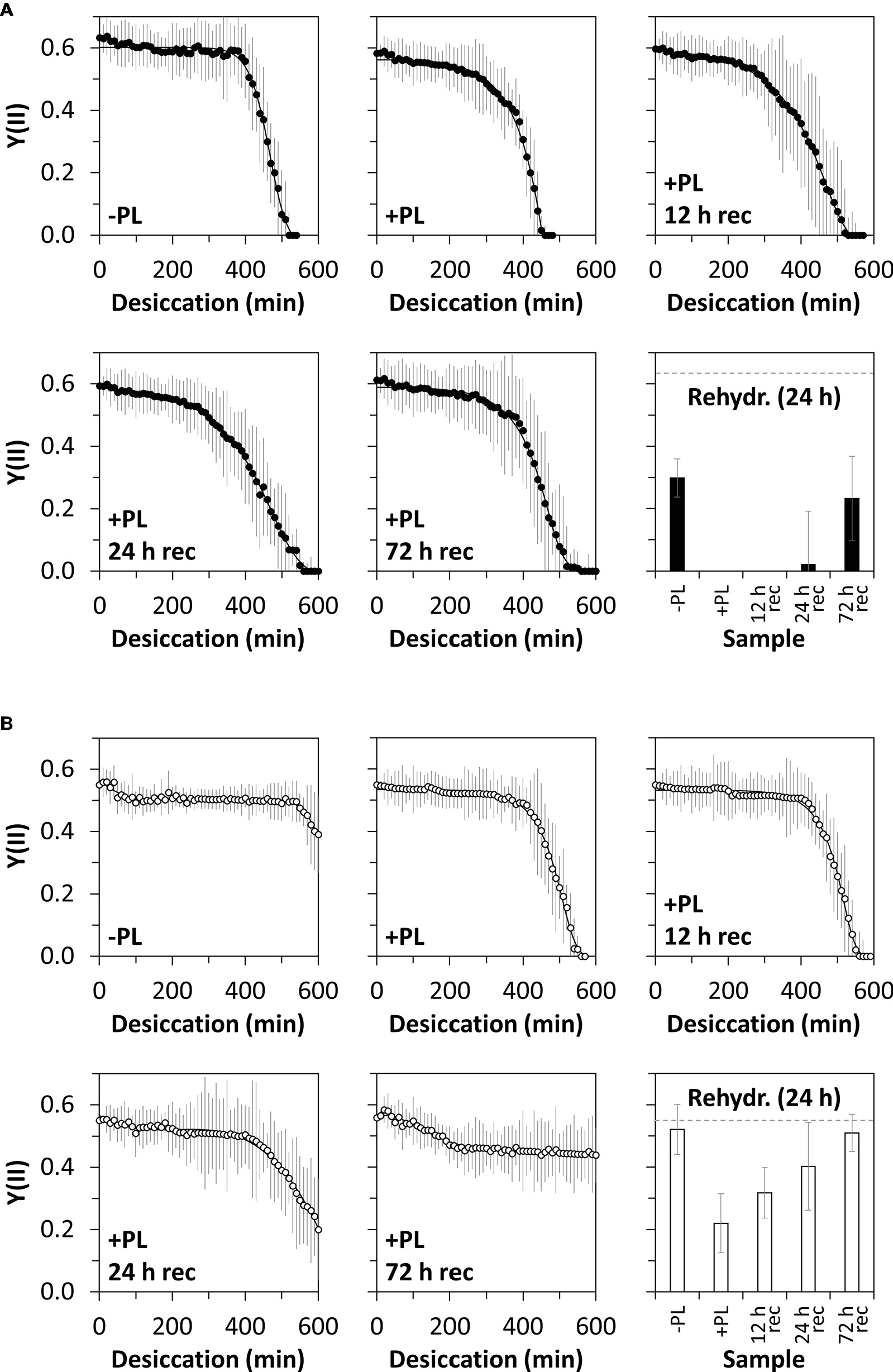
Figure 5. Effective quantum yield of PSII [Y(II)] in response to desiccation and pectate lyase (PL) treatment. Young (A) or old (B) algal filaments were desiccated at ~84% RH for up to 10 h after no PL treatment (−PL), after PL treatment (+PL) or after PL treatment followed by 12, 24, or 72 h recovery (+PL, 12 h rec–72 h rec). After recording desiccation kinetics, all samples were rehydrated for 24 h and their Y(II) measured. The dashed grey lines on the rehydration charts indicate the initial Y(II) of −PL samples. n = 6 ± SD.
Higher Amount Gala in Old Zygnema AIR After TFA Hydrolysis
Digestion of commercial HG with PL produced the oligosaccharides GalA1–3–ΔUA but not the monosaccharide GalA, which we added additionally to our TLC marker mixtures (Figure 6A, second row from left and third and fourth rows from right). When incubated with PL, AIR of both young and old Zygnema filaments yielded a number of oligosaccharides and monosaccharides (Figure 6A). However, control groups lacking PL showed a similar oligosaccharide pattern upon TLC separation as PL containing samples (Figure 6A). Nevertheless, old filaments’ AIR consistently yielded more GalA2–ΔUA than young filaments; other oligosaccharide products diagnostic for PL digestion of HG (GalA–ΔUA, GalA3–ΔUA) were not found (Figure 6A). However, GalA, which indicates the presence of HG, was detected in all samples, and the amount was higher in old AIR (Figure 6A). To further validate that Zygnema cell walls contain HG releasable by enzymatic treatment, old Zygnema AIR was subjected to digestion by EPG, which cleaves the α-1,4-
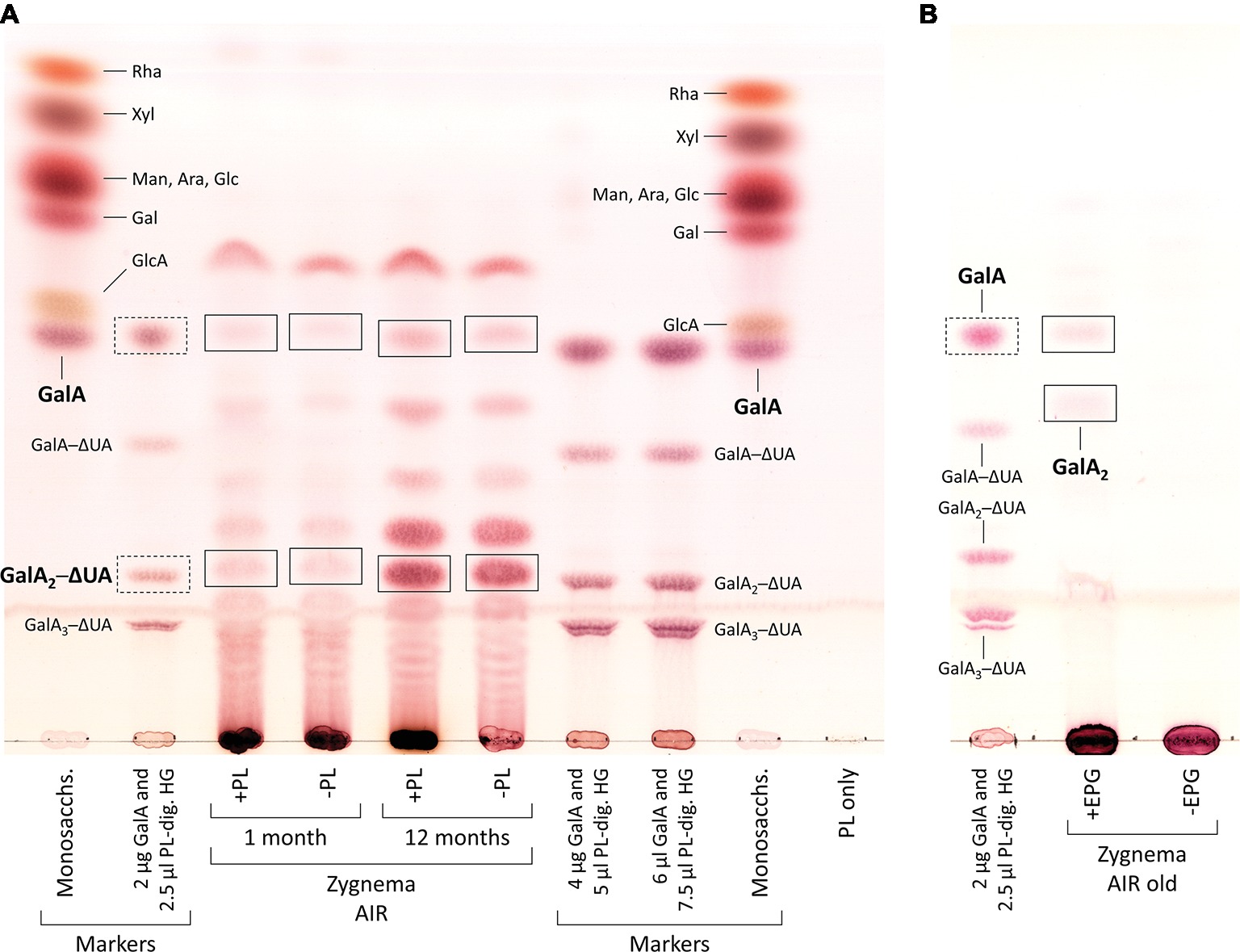
Figure 6. Enzymatic release of HG-derived oligosaccharides from cell walls of young and old Zygnema filaments. (A) AIR samples were either treated with PL (+PL) or buffer only (−PL, controls) and digestion products separated by thin-layer chromatography (TLC). (B) Old Zygnema AIR treated with EPG (+EPG) or buffer (−EPG) and product separation by TLC. Monosaccharide and galacturonic acid oligosaccharides (GalA(1–3)–ΔUA, as concentration gradient) markers are shown in separate tracks. Expected HG oligosaccharide and GalA bands in Zygnema AIR (rectangles) and GalA1–3–ΔUA marker bands (rectangles with dashed lines).
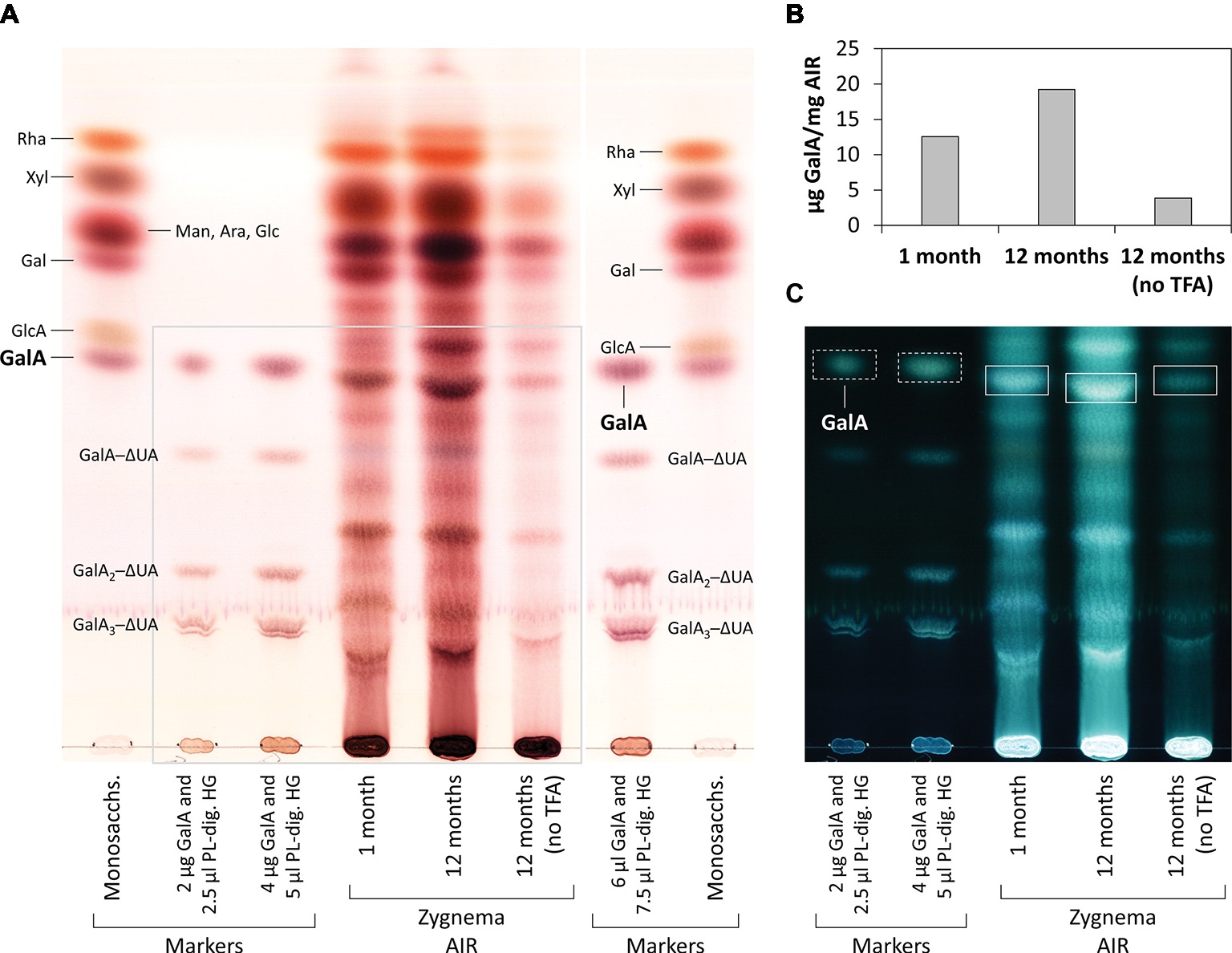
Figure 7. Monosaccharide and oligosaccharide composition of young and old Zygnema AIR. (A) Samples were TFA-hydrolyzed or left in water and hydrolysis products separated by thin-layer chromatography (TLC). TLC plate area shown in (C) is marked with a grey line. (B) Quantification of GalA in AIR samples treated or not treated with TFA. (C) Same plate as in (A), but shown with inverted colors. TLC marker bands as in Figure 6. GalA bands in Zygnema AIR used for quantification (rectangles) and GalA marker bands (rectangles with dashed lines).
Discussion
The present study has shown that removing HG from the cell walls of the aero-terrestrial green alga Zygnema reduces its photosynthetic efficiency when exposed to water scarcity. The effect is the strongest in 12-month-old filaments, which exhibit a higher HG content than young filaments. This suggests that the pectin fraction in the cell wall helps algae to remain photosynthetically active in habitats with periods of low water availability. We thus conclude that the water holing capacities of HGs are crucial for survival in terrestrial habitats.
HG Accumulation in Cell Walls of Old Filaments Might Be an Adaptation to Water Scarcity
Desiccating algal filaments with an intact HG matrix, i.e., without PL treatment, gave similar results as observed in previous desiccation studies on Zygnema (Kaplan et al., 2013; Pichrtová et al., 2014a,b; Herburger et al., 2015): (1) pre-akinete-rich old filaments maintained a higher photosynthetic performance during desiccation than young filaments and (2) full recovery of photosynthesis after rehydration only occurred in old filaments. Treatment of young filaments with PL slightly reduced their desiccation resistance, while the Y(II) reached 0 about 1 h earlier than in untreated filaments. This indicates that the HG matrix influences the water holding capacity of young filaments during desiccation. Furthermore, an intact HG matrix appears to be important for recovering photosynthesis upon rehydration: only untreated filaments and filaments which were allowed to recover their HG matrix restored their Y(II) partially after a 24 h rehydration period. Algae might prioritize the allocation of metabolic energy to the re-synthesis and incorporation of HG into the cell wall, and building HG-containing layers on top of the cell walls before the photosynthetic apparatus is repaired. In contrast to young filaments, HG removal in old filaments resulted in a drastic change of the desiccation kinetics: While untreated cells maintained a high Y(II) throughout the desiccation period (10 h), Y (II) and thus photosynthesis were inhibited in PL-treated filaments after ~9.5 h. Thus, PL-treated old filaments only exhibited a slightly higher desiccation resistance when compared with unt`reated young filaments, indicating that the age-dependent HG accumulation is important for coping with water scarcity. Cellular water loss results into dramatic morphological changes in Zygnema (Herburger et al., 2015; Lajos et al., 2016), where the longitudinal cell walls expand and show convolutions along the filament’s axis. In contrast, the cross cell walls remain their shape and stay connected to the protoplasts. Regular cell wall deformation patters are also considered to contribute to the desiccation tolerance of resurrection plants, where arabinose-rich components might mediate cell wall flexibility, allowing a controlled shrinkage during water loss (Moore et al., 2013). Although algae’s morphology after desiccation stress was not re-investigated in the present study, strong damage to the cell wall of PL-treated filaments can be excluded, because a recovering period allowed to restore the photosynthetic performance. This suggests that HG is not crucial for the cell wall’s structural integrity, even though it is a structural cell wall component (see below). Recovery of old filament’s HG matrix was accompanied by a gradual recovery of photosynthesis, resulting into restoration of the initial performance after 72 h. In contrast to young filaments, old filaments were able to restore their Y(II) partially, even when they were not allowed to recover after PL treatment. This indicates the acquaintance of further desiccation resistance mechanisms resulting from an age-dependent acclimation to desiccating conditions when grown on solid media (Rippin et al., 2017). For example, old filaments exhibit a lower osmotic potential inside cells (Kaplan et al., 2013) and a lower degree of vacuolization (Herburger et al., 2015), and the accumulation of lipid bodies might help maintaining the structural integrity of the protoplast during cellular water loss.
HG Matrix Occurs Throughout Zygnema Cell Walls
Old Zygnema filaments are predominately comprised of pre-akinetes, which are considered to be crucial for long term population survival (Pichrtová et al., 2016a). As shown recently, the cell walls of pre-akinetes possess a higher pectin content than the walls of young vegetative cells, while the cellulose and hemicellulose contents are similar (Herburger et al., 2018). HG is the predominant polysaccharide in the pectin fraction of late diverged CGA (Sørensen et al., 2011), and correspondingly, we found GalA2─ΔUA oligosaccharides released by PL from Zygnema AIR. Surprisingly, high amounts of GalA2–ΔUA were also released in control samples (no PL) in a number of independent experiments; this might be caused by degradation of HG by β-elimination due to the relatively alkaline volatile buffer system used (pH 8; Renard and Thibault, 1996). Accordingly, acidic conditions (pH 4.5)—as used in the control samples in the EPG digestion experiment—did not yield any products, while adding EPG released GalA and (putatively) GalA2 from Zygnema AIR. TFA hydrolysis of Zygnema AIR also released GalA, which most likely resulted mainly from HG; although cell walls of Zygnema (Figure 7) and other CGA (Popper and Fry, 2003; O’Rourke et al., 2015) contain both GalA and rhamnose, only small amounts of them are considered to be a part of rhamnogalacturonan I (Sørensen et al., 2011). The release of both GalA2–ΔUA and GalA was higher in old filaments. Abundant occurrence of HG in Zygnema was further supported by strong binding of the mAb JIM5 (recognizing partially methyl- and unesterified HG) in both young and old Zygnema filaments. Epitopes were distributed homogeneously throughout the longitudinal cell walls. HG being a structural part of the algal cell wall—as observed in land plants—is supported by the observation that PL treatment increases the binding of Abs recognizing xyloglucan and mannan epitopes, suggesting that HG is tightly associated to cell wall hemicelluloses (Herburger et al., 2018). In the Zygnematophyceae Netrium digitus, JIM5 also gave a general cell wall staining and additionally stained the mucilage vesicles strongly, while the HG probe JIM7 (recognizing partially methyl-esterified HG) stained the growing zones of the cell center strongly (Eder and Lütz-Meindl, 2010). In Micrasterias denticulata, JIM5 and JIM7 mAbs gave labeling of the young primary cell wall but did not stain the secondary cell walls (Eder and Lütz-Meindl, 2008). JIM7 produced a stronger signal, while JIM5 labeling predominated in the nongrowing cell wall areas. Furthermore, a strong cross reactivity of both mAbs to the extracted mucilaginous fraction was observed (Eder and Lütz-Meindl, 2008). Also in Desmidium swartzii, JIM5 and JIM7 staining was observed in the developing septum (Andosch et al., 2015).
However, we did not observe JIM5 binding in the amorphous pectic sheath layer, which surrounds Zygnema filaments. Nevertheless, the sheaths are stained by ruthenium red, suggesting the presence of negatively charged compounds other than partially or unesterified HG. These sheaths might exhibit a chemical and functional similarity to the mucilage layers secreted by, for example, the unicellular CGA Micrasterias, which are rich in acidic polysaccharides (Menge, 1976; Brosch-Salomon et al., 1998; Eder et al., 2008). Pectic sheath layers were also found in Zygnema irregulare upon pre-akinete formation under desiccating conditions (Fuller, 2013). Z. irregulare belongs to the same major genus subclade as Zygnema circumcarinatum investigated in the present study; however, the pectic sheath layers stainable by ruthenium red observed in this study were considerably thinner and often did not cover the entire surface of filaments. These differences might be caused by different growth conditions, while Zygnema irregulare experienced continuous desiccation stress but Zygnema circumcarinatum did not. We found pectic sheath in both young and old filaments and—in addition to HG in the cell wall—these mucilaginous layers might contribute to desiccation resistance by binding water in close proximity to the cell surface. PL treatment removed the majority of HG epitopes recognizable by JIM5. A similar effect was observed in Penium, where PL treatment was allowed to remove the HG-rich lattice attached to the cell surface (Domozych et al., 2014). PL-treated Zygnema filaments, which were allowed to recover for 10 h, showed JIM5 binding in areas close to cross cell walls, while after 3 days, HG was detectable again throughout the longitudinal cell walls in both young and old filaments. This indicates that HG is initially secreted at the poles of individual Zygnema cells—likely in methyl-esterified from—and as the cells grow, HG might become de-methylesterified by PMEs. This is supported by the observation that JIM7 (recognizing partially methyl-esterified but not un-esterified HG) only binds to cell wall areas at cell poles, while JIM5, which can detect not only methyl but also unesterified HG, stains the cell wall from cell pole to cell pole. As shown by Domozych et al. (2014), the HG-rich lattice, which is part of the outer cell wall layer of Penium cells, is formed by secretion of highly methylesterified HG at an isthmus zone, which separates two semicells; during cell growth, PMEs remove methyl groups from HG, resulting into a HG lattice that is predominated by HG with a low DE distal from the isthmus (Domozych et al., 2014). The authors emphasize similarities between the algal lattice and the HG-rich middle lamella of land plants (localization, formation pattern, and relevance for cell adhesion), and this might also partially apply to Zygnema cell wall areas stainable by JIM5 and JIM7. The relatively fast re-formation of JIM5 epitopes upon PL treatment suggests that in addition to its function as a structural cell wall component, HG might be also secreted to the outer cell wall surface, where it increases filaments water holding capacity and adhesion to the substrate and neighboring cells/filaments. The ability of Zygnema to form dense mats improves the water holding capacity at the population level and protects individual filaments from harmful irradiation (Holzinger and Karsten, 2013). Adhesive compounds such as HG and AGPs (as recently demonstrated in Zygnema by Palacio-López et al., 2019, this issue) on the cell surface might help algae to form these mats.
Conclusion
Zygnema, the species-richest genus of the algal ancestor group of all land plants (Zygnematophyceae), shows an age-depend accumulation of HG in the cell wall. Older filaments exhibit a higher resistance against desiccation stress, as previously shown by different approaches (Kaplan et al., 2013; Herburger et al., 2015; Rippin et al., 2017). We suggest that a higher HG content of the ECM allows the filaments to bind more water, which gives them their typical slimy appearance and delays a harmful cellular water loss. It is likely that this adaptation supported algae in colonizing land and helps recent Zygnema populations to survive in harsh aero-terrestrial habitats throughout the world. With this increased water holding capacity, a longer productivity range is expected, leading to massive amounts of biomass created even under partially desiccating scenarios (Holzinger and Pichrtová, 2016).
Author Contributions
KH and AH planned and designed the study during KH’s PhD appointment at the University of Innsbruck. KH performed most of the experiments and analyzed the data. AH conducted immuno-TEM and prepared AIR samples. AX performed the HG quantification assays. KH prepared the figures and wrote the manuscript draft. All authors edited the final manuscript.
Funding
The work was supported by the Austrian Science Fund (FWF) grants P 24242-B16 and I 1951-B16 to AH and in part by the Villum Foundation project TIPorNOT (00023089) to KH.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Beatrix Jungwirth, University of Innsbruck, is kindly acknowledged for excellent technical assistance in algal cultivation and TEM sectioning. We would like to thank Prof. Ursula Lütz-Meindl, University of Salzburg, for the possibility to perform the HPF/FS substitution at here Leica High Pressure freezing device. We would like to thank Ancuela Andosch from the same institution for technical assistance. We would also like to thank Prof. Stephen C. Fry, University of Edinburgh, for helpful discussions and providing TLC consumables and Thurayya Al Hinai, University of Edinburgh, for sharing GalA1–3–ΔUA marker mixtures.
References
Andosch, A., Höftberger, M., Lütz, C., and Lütz-Meindl, U. (2015). Subcellular sequestration and impact of heavy metals on the ultrastructure and physiology of the multicellular freshwater alga Desmidium swartzii. Int. J. Mol. Sci. 16, 10389–10410. doi: 10.3390/ijms160510389
Bischoff, H.W., and Bold, H.C. (1963). Phycological studies IV. Some soil algae from Enchanted Rock and related algal species. (Austin: Univiversity of Texas Publications).
Brosch-Salomon, S., Höftberger, M., Holzinger, A., and Lütz-Meindl, U. (1998). Ultrastructural localization of polysaccharides and N-acetyl-D-galactosamine in the secretory pathway of green algae (Desmidiaceae). J. Exp. Bot. 49, 145–153.
Clausen, M. H., Willats, W. G., and Knox, J. P. (2003). Synthetic methyl hexagalacturonate hapten inhibitors of anti-homogalacturonan monoclonal antibodies LM7, JIM5 and JIM7. Carbohydr. Res. 338, 1797–1800. doi: 10.1016/S0008-6215(03)00272-6
De Vries, J., Curtis, B. A., Gould, S. B., and Archibald, J. M. (2018). Embryophyte stress signaling evolved in the algal progenitors of land plants. Proc. Nat. Acad. Sci. 115, E3471–E3480. doi: 10.1073/pnas.1719230115
Domozych, D. S., Sørensen, I., Popper, Z. A., Ochs, J., Andreas, A., Fangel, J. U., et al. (2014). Pectin metabolism and assembly in the cell wall of the charophyte green alga Penium margaritaceum. Plant Physiol. 165, 105–118. doi: 10.1104/pp.114.236257
Domozych, D. S., Stewart, K. D., and Mattox, K. R. (1980). The comparative aspects of cell wall chemistry in the green algae (Chlorophyta). J. Mol. Evol. 15, 1–12. doi: 10.1007/BF01732578
Eder, M., and Lütz-Meindl, U. (2008). Pectin-like carbohydrates in the green alga Micrasterias characterized by cytochemical analysis and energy filtering TEM. J. Microsc. 231, 201–214. doi: 10.1111/j.1365-2818.2008.02036.x
Eder, M., and Lütz-Meindl, U. (2010). Analyses and localization of pectin-like carbohydrates in cell wall and mucilage of the green alga Netrium digitus. Protoplasma 234, 25–38. doi: 10.1007/s00709-009-0040-0
Eder, M., Tenhaken, R., Driouich, A., and Lütz-Meindl, U. (2008). Occurrence and characterization of arabinogalactan-like proteins and hemicelluloses in Micrasterias (streptophyta). J. Phycol. 44, 1221–1234. doi: 10.1111/j.1529-8817.2008.00576.x
Ettl, H., and Gärtner, G. (1995). Syllabus der Boden-, Luft- und Flechtenalgen. (Stuttgart: Gustav Fischer).
Foster, C. E., Martin, T. M., and Pauly, M. (2010). Comprehensive compositional analysis of plant cell walls (lignocellulosic biomass) part II: carbohydrates. J. Vis. Exp. 37:1837. doi: 10.3791/1837
Fuller, C.L. (2013). Examining morphological and physiological changes in Zygnema irregulare during a desiccation and recovery period. master’s thesis. (San Marcos (CA): California State University).
Greenspan, L. (1977). Humidity fixed points of binary saturated aqueous solutions. J. Res. Nat. Bur. Stand. Sect. A 81, 89–96. doi: 10.6028/jres.081A.011
Harholt, J., Moestrup, Ø., and Ulvskov, P. (2016). Why plants were terrestrial from the beginning. Trends Plant Sci. 21, 96–101. doi: 10.1016/j.tplants.2015.11.010
Harholt, J., Suttangkakul, A., and Scheller, H. V. (2010). Biosynthesis of pectin. Plant Physiol. 153, 384–395. doi: 10.1104/pp.110.156588
Heckman, D. S., Geiser, D. M., Eidell, B. R., Stauffer, R. L., Kardos, N. L., and Hedges, S. B. (2001). Molecular evidence for the early colonization of land by fungi and plants. Science 293, 1129–1133. doi: 10.1126/science.1061457
Herburger, K., and Holzinger, A. (2015). Localization and quantification of callose in the streptophyte green algae Zygnema and Klebsormidium: correlation with desiccation tolerance. Plant Cell Physiol. 56, 2259–2270. doi: 10.1093/pcp/pcv139
Herburger, K., Lewis, L. A., and Holzinger, A. (2015). Photosynthetic efficiency, desiccation tolerance and ultrastructure in two phylogenetically distinct strains of alpine Zygnema sp. (Zygnematophyceae, Streptophyta): role of pre-akinete formation. Protoplasma 252, 571–589. doi: 10.1007/s00709-014-0703-3
Herburger, K., Ryan, L. M., Popper, Z. A., and Holzinger, A. (2018). Localisation and substrate specificities of transglycanases in charophyte algae relate to development and morphology. J. Cell Sci. 131:jcs203208. doi: 10.1242/jcs.203208
Herron, S. R., Benen, J. A., Scavetta, R. D., Visser, J., and Jurnak, F. (2000). Structure and function of pectic enzymes: virulence factors of plant pathogens. Proc. Natl. Acad. Sci. USA 97, 8762–8769.
Holzinger, A., and Karsten, U. (2013). Desiccation stress and tolerance in green algae: consequences for ultrastructure, physiological and molecular mechanisms. Front. Plant Sci. 4:327. doi: 10.3389/fpls.2013.00327
Holzinger, A., and Pichrtová, M. (2016). Abiotic stress tolerance of charophyte green algae: new challenges for omics techniques. Front. Plant Sci. 7:678. doi: 10.3389/fpls.2016.00678
Holzinger, A., Valenta, R., and Lütz-Meindl, U. (2000). Profilin is localized in the nucleus-associated microtubule and actin system and is evenly distributed in the cytoplasm of the green alga Micrasterias denticulata. Protoplasma 212, 197–205. doi: 10.1007/BF01282920
Jensen, J. K., Busse-Wicher, M., Poulsen, C. P., Fangel, J. U., Smith, P. J., and Yang, J. Y. (2018). Identification of an algal xylan synthase indicates that there is functional orthology between algal and plant cell wall biosynthesis. New Phytol. 218, 1049–1060. doi: 10.1111/nph.15050
Jork, H., Funk, W., Fischer, W., and Wimmer, H. (1994). Thin layer chromatography: Reagents and detection methods. Volume 1b, physical and chemical detection methods: Activation reactions, reagent sequences, reagents II. (Weinheim: VCH).
Kaplan, F., Lewis, L. A., Herburger, K., and Holzinger, A. (2013). Osmotic stress in Arctic and Antarctic strains of the green alga Zygnema (Zygnematales, Streptophyta): effects on photosynthesis and ultrastructure. Micron 44, 317–330. doi: 10.1016/j.micron.2012.08.004
Karsten, U., Herburger, K., and Holzinger, A. (2014). Dehydration, temperature, and light tolerance in members of the aeroterrestrial green algal genus Interfilum (Streptophyta) from biogeographically different temperate soils. J. Phycol. 50, 804–816. doi: 10.1111/jpy.12210
Kenrick, P., Wellman, C. H., Schneider, H., and Edgecombe, G. D. (2012). A timeline for terrestrialization: consequences for the carbon cycle in the Palaeozoic. Philos. Trans. R. Soc. Lond. B 367, 519–536. doi: 10.1098/rstb.2011.0271
Knauth, L. P., and Kennedy, M. J. (2009). The late Precambrian greening of the earth. Nature 460, 728–732. doi: 10.1038/nature08213
Knox, J. P., Linstead, P. J., King, J., Cooper, C., and Roberts, K. (1990). Pectin esterification is spatially regulated both within cell walls and between developing tissues of root apices. Planta 181, 512–521. doi: 10.1007/BF00193004
Lajos, K., Mayr, S., Buchner, O., Blaas, K., and Holzinger, A. (2016). A new microscopic method to analyse desiccation-induced volume changes in aeroterrestrial green algae. J. Microsc. 263, 192–199. doi: 10.1111/jmi.12409
Lütz-Meindl, U., and Aichinger, N. (2004). Use of energy-filtering transmission electron microscopy for routine ultrastructural analysis of high-pressure-frozen or chemically fixed plant cells. Protoplasma 223, 155–162. doi: 10.1007/s00709-003-0033-3
McLean, R. J., and Pessoney, G. F. (1971). “Formation and resistance of akinetes of Zygnema” in Contributions in phycology. eds. B. C. Parker, and R. M. Brown Jr. (Lawrence, KS: Allen), 145–152.
Menge, U. (1976). Ultracytochemische Untersuchungen an Micrasterias denticulata Bréb. Protoplasma 88, 287–303. doi: 10.1007/BF01283253
Mikkelsen, M. D., Harholt, J., Ulvskov, P., Johansen, I. E., Fangel, J. U., Doblin, M. S., et al. (2014). Evidence for land plant cell wall biosynthetic mechanisms in charophyte green algae. Ann. Bot. 114, 1217–1236. doi: 10.1093/aob/mcu171
Moore, J. P., Nguema-Ona, E. E., Vicré-Gibouin, M., Sørensen, I., Willats, W. G., Driouich, A., et al. (2013). Arabinose-rich polymers as an evolutionary strategy to plasticize resurrection plant cell walls against desiccation. Planta 237, 739–754. doi: 10.1007/s00425-012-1785-9
Morris, E. R., Powell, D. A., Gidley, M. J., and Rees, D. A. (1982). Conformations and interactions of pectins: I. Polymorphism between gel and solid states of calcium polygalacturonate. J. Mol. Biol. 155, 507–516. doi: 10.1016/0022-2836(82)90484-3
O’Rourke, C., Gregson, T., Murray, L., Sadler, I. H., and Fry, S. C. (2015). Sugar composition of the pectic polysaccharides of charophytes, the closest algal relatives of land-plants: presence of 3-O-methyl-
Palacio-López, K., Tinaz, B., Holzinger, A., and Domozych, D. S. (2019). Arabinogalactan proteins and the extracellular matrix of charophytes: a sticky business. Front. Plant Sci. 10:447. doi: 10.3389/fpls.2019.00447
Pichrtová, M., Arc, E., Stöggl, W., Kranner, I., Hájek, T., Hackl, H., et al. (2016b). Formation of lipid bodies and changes in fatty acid composition upon pre-akinete formation in Arctic and Antarctic Zygnema (Zygnematophyceae, Streptophyta) strains. FEMS Microbiol. Ecol. 92:fiw096. doi: 10.1093/femsec/fiw096
Pichrtová, M., Hájek, T., and Elster, J. (2016a). Annual development of mat-forming conjugating green algae Zygnema spp. in hydro-terrestrial habitats in the Arctic. Polar Biol. 39, 1653–1662. doi: 10.1007/s00300-016-1889-y
Pichrtová, M., Hájek, T., and Elster, J. (2014a). Osmotic stress and recovery in field populations of Zygnema sp.(Zygnematophyceae, Streptophyta) on Svalbard (High Arctic) subjected to natural desiccation. FEMS Microb. Ecol. 89, 270–280. doi: 10.1111/1574-6941.12288
Pichrtová, M., Kulichová, J., and Holzinger, A. (2014b). Nitrogen limitation and slow drying induce desiccation tolerance in conjugating green algae (Zygnematophyceae, Streptophyta) from polar habitats. PLoS One 9:e113137. doi: 10.1371/journal.pone.0113137
Popper, Z. A., and Fry, S. C. (2003). Primary cell wall composition of bryophytes and charophytes. Ann. Bot. 91, 1–12. doi: 10.1093/aob/mcg013
Renard, C. M. G. C., and Thibault, J. F. (1996). Pectins in mild alkaline conditions: β-elimination and kinetics of demethylation. Prog. Biotechnol. 14, 603–608.
Rippin, M., Becker, B., and Holzinger, A. (2017). Enhanced desiccation tolerance in mature cultures of the streptophytic green alga Zygnema circumcarinatum revealed by transcriptomics. Plant Cell Physiol. 58, 2067–2084. doi: 10.1093/pcp/pcx136
Sørensen, I., Pettolino, F. A., Bacic, A., Ralph, J., Lu, F., O’Neill, M. A., et al. (2011). The charophycean green algae provide insights into the early origins of plant cell walls. Plant J. 68, 201–211. doi: 10.1111/j.1365-313X.2011.04686.x
Steemans, P., Le Hérissé, A., Melvin, J., Miller, M. A., Paris, F., Verniers, J., et al. (2009). Origin and radiation of the earliest vascular land plants. Science 324:353. doi: 10.1126/science.1169659
Timme, R. E., Bachvaroff, T. R., and Delwiche, C. F. (2012). Broad phylogenomic sampling and the sister lineage of land plants. PLoS One 7:e29696. doi: 10.1371/journal.pone.0029696
Wickett, N. J., Mirarab, S., Nguyen, N., Warnow, T., Carpenter, E., Matasci, N., et al. (2014). Phylotranscriptomic analysis of the origin and early diversification of land plants. Proc. Natl. Acad. Sci. USA 111, E4859–E4868. doi: 10.1073/pnas.1323926111
Willats, W. G., Orfila, C., Limberg, G., Buchholt, H. C., van Alebeek, G. J. W., Voragen, A. G., et al. (2001). Modulation of the degree and pattern of methyl esterification of pectic homogalacturonan in plant cell walls: implications for pectin methyl esterase action, matrix properties and cell adhesion. J. Biol. Chem. 276, 19404–19413. doi: 10.1074/jbc.M011242200
Wodniok, S., Brinkmann, H., Glöckner, G., Heidel, A. J., Philippe, H., Melkonian, M., et al. (2011). Origin of land plants: do conjugating green algae hold the key? BMC Evol. Biol. 11:104. doi: 10.1186/1471-2148-11-104
Wolf, S., and Greiner, S. (2012). Growth control by cell wall pectins. Protoplasma 249, 169–175. doi: 10.1007/s00709-011-0371-5
Keywords: cell wall, desiccation, green algae, homogalacturonan, pectin, photosynthesis, terrestrialization, Zygnematophyceae
Citation: Herburger K, Xin A and Holzinger A (2019) Homogalacturonan Accumulation in Cell Walls of the Green Alga Zygnema sp. (Charophyta) Increases Desiccation Resistance. Front. Plant Sci. 10:540. doi: 10.3389/fpls.2019.00540
Edited by:
Zoë A. Popper, National University of Ireland Galway, IrelandReviewed by:
Peter Ulvskov, University of Copenhagen, DenmarkJesper Harholt, University of Copenhagen, Denmark
Copyright © 2019 Herburger, Xin and Holzinger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andreas Holzinger, YW5kcmVhcy5ob2x6aW5nZXJAdWliay5hYy5hdA==
†Present address: Klaus Herburger, Department of Plant and Environmental Sciences, University of Copenhagen, Copenhagen, Denmark
 Klaus Herburger
Klaus Herburger Anzhou Xin
Anzhou Xin Andreas Holzinger
Andreas Holzinger