- Agricultural Biotechnology Research Center, Academia Sinica, Taipei, Taiwan, Republic of China
Zinc (Zn) is an essential plant micronutrient but is toxic in excess. To cope with excess Zn, plant species possess a strict metal homeostasis mechanism. The Zn hyperaccumulator Arabidopsis halleri has developed various adaptive mechanisms involving uptake, chelation, translocation and sequestration of Zn. In this mini review, we broadly discuss the different Zn tolerance mechanisms and then focus on controlled Zn uptake in A. halleri. Members of the ZRT/IRT-like protein (ZIP) family of metal transporters are mainly regulated by Zn and are involved in Zn uptake. A few members of the ZIP family, such as IRT1 and IRT2, are regulated by iron (Fe) and can transport multi-metals, including Zn, Fe, Mn, Cd, and Co. This mini-review also discusses the differential expression of multiple metal ZIP transporters in A. halleri and A. thaliana, a non-hyperaccumulator, with Zn exposure as well as Fe deficiency and their role in controlled Zn uptake and tolerance.
Introduction
Heavy metal pollution in the soil has greatly increased over the past decades because of mining and industrial activities, overuse of chemical fertilizers, and waste-water irrigation (Nriagu and Pacyna, 1988). Metals such as cadmium (Cd), mercury (Hg), and lead (Pb) are considered non-essential because they do not have any role in any physiological process in plants. In contrast, metals such as zinc (Zn), iron (Fe), copper (Cu), manganese (Mn), molybdenum (Mo), and nickel (Ni) are essential micronutrients required for normal growth and metabolism of plants (Marschner, 1995). For example, Zn is a cofactor for many enzymes, and many proteins contain Zn-binding structural domains (Clarke and Berg, 1998; Guerinot and Eide, 1999).
Zn has an important role in several physiological and metabolic processes in plants (Ramesh et al., 2004). However, in excess, Zn can be toxic and influence the status of the other metal ions, thus resulting in severe growth defects in plants (Marschner, 1995). At toxic concentrations, Zn replaces other divalent cations such as Fe, magnesium (Mg) and Mn, which are involved in the proper functioning of a number of photosynthetic enzymes, thereby resulting in lower photosynthetic rates and photo-oxidative damage (Vanassche and Clijsters, 1986a,b, 1990). To avoid potential toxicity caused by displacement of these elements, metal ion homeostasis must be strictly controlled in plants.
Zn-tolerant and -hyperaccumulating species have various mechanisms to cope with excess Zn levels. This review focuses on the current understanding of the Zn homeostasis network in Zn hyperaccumulators and addresses Zn and Fe crosstalk in response to Zn tolerance and hyperaccumulation.
Zn Hyperaccumulators: A Model System to Understand Zn Homeostasis in Plants
Plant species that can grow at growth-limiting concentrations of metals such as Ni, Zn, Cd, Co, or Cu have naturally selected increased tolerance and are called metal-tolerant species. In addition, a few plant species, called as metal hyperaccumulators, can tolerate and also accumulate these metals in their shoot tissues at four orders of magnitude higher than those of non-hyperaccumulators (Roosens et al., 2008; Kramer, 2010). Approximately 500 plant taxa can accumulate such high concentrations of potentially toxic metals; 20 of these are Zn hyperaccumulators (Baker and Brooks, 1989; Reeves and Baker, 2000; Kramer, 2010).
Several species of the Brassicaceae family are metal hyperaccumulators. Examples are Noccaea caerulescens and Arabidopsis halleri. N. caerulescens was the first identified Zn hyperaccumulator and was reported to accumulate about 25,000 to 30,000 μg g-1 dry weight (DW) of total Zn (Brown et al., 1995; Shen et al., 1997). A. halleri is a Zn/Cd hyperaccumulator (Ernst, 1974; Kupper et al., 2000; Zhao et al., 2000, 2006; Bert et al., 2002; Cosio et al., 2004) and can accumulate >10,000 and >100 μg g-1 DW of Zn and Cd, respectively. A. halleri is closely related to A. thaliana, a Zn non-hyperaccumulator, whose genome has been thoroughly explored. Thus, A. halleri is a good model system to study Zn tolerance and Zn hyperaccumulation mechanisms. A few comparative transcriptomic studies of A. halleri have identified several key genes involved in the Zn hyperaccumulation process (Becher et al., 2004; Weber et al., 2004; Chiang et al., 2006; Talke et al., 2006).
Strategies of Zn Tolerance and Hyperaccumulation: Sequestration for Detoxification
Zn hyperaccumulators prevent toxicity symptoms and cope with excess metal ions using various strategies such as effective metal uptake, increased xylem loading and increased detoxification in shoot tissues (Kramer, 2010). In recent years, many components involved in these processes have been identified and characterized (Verbruggen et al., 2009; Kramer, 2010; Deinlein et al., 2012). Zn tolerance and hyperaccumulation are better understood because of investigation of species closely related to the model A. thaliana. A. halleri and N. caerulescens share 94 and 88% nucleotide similarity, respectively, with A. thaliana. This similarity paves the way for detailed transcriptomic studies and proteomic profiling with respect to deficiency or excess Zn (Becher et al., 2004; Weber et al., 2004; Chiang et al., 2006; Talke et al., 2006; van de Mortel et al., 2006; Schneider et al., 2012). These studies have provided knowledge of Zn uptake, xylem loading and unloading in the detoxification process and have shed light on the involvement of other metal homeostasis networks in Zn uptake and tolerance mechanisms.
Zn hyperaccumulators possess effective root-to-shoot Zn translocation mechanisms through symplastic movement and effective xylem loading (Clemens, 2006; Kramer, 2010; Verbruggen et al., 2009). In recent years, several types of transporters involved in this process have been identified in Zn hyperaccumulators and thoroughly investigated. P-type ATPase (HMA) transporters are mainly involved in root-to-shoot translocation of Zn (Hussain et al., 2004; Verret et al., 2004; Hanikenne et al., 2008; Kim et al., 2009; Barabasz et al., 2010; Lochlainn et al., 2011). HMA4 is triplicated and also constitutively expressed at a high level in A. halleri, thereby mediating effective root-to-shoot translocation and resulting in Zn tolerance (Hanikenne et al., 2008). However, overexpressing AhHMA4 in A. thaliana did not considerably enhance root-to-shoot translocation of Zn and caused Zn hypersensitivity because of lack of an efficient detoxification mechanism in shoot tissues (Hanikenne et al., 2008). These observations emphasize the complexity of metal hyperaccumulation and tolerance mechanisms of metal hyperaccumulators. In addition to P-type ATPases, members of multi-drug and toxic compound extrusion transporters (MATEs) and oligopeptide transporters are highly and constitutively expressed in Zn hyperaccumulators and reported to be involved in Zn translocation (Talke et al., 2006; van de Mortel et al., 2006; Hu et al., 2012; Pineau et al., 2012). FRD3, a member of the MATE family, which functions in citrate efflux into the root vasculature and is involved in the long-distance transport of Fe, was more highly expressed in A. halleri than in A. thaliana. Recently, FRD3 was found to play a role in Zn tolerance in A. thaliana and, possibly, Zn translocation (Pineau et al., 2012). Once Zn is efficiently translocated into shoot tissues, several tonoplast transporters participate in the sequestration of Zn into shoot vacuoles. Metal tolerance protein 1, a tonoplast-localized Zn transporter, is highly expressed in both roots and shoots of A. halleri and also linked to a major quantitative trait locus (QTL) responsible for Zn tolerance (Drager et al., 2004; Kobae et al., 2004; Gustin et al., 2009; Kawachi et al., 2009; Shahzad et al., 2010; Willems et al., 2010). Some other members of HMA and ATP-binding cassette transporters are highly expressed in shoots of A. halleri, but their exact role in vacuole sequestration of Zn has not been proven by functional studies (Becher et al., 2004; Weber et al., 2004; Chiang et al., 2006).
Additional Uptake Controls: Fe Homeostasis and Zn Tolerance
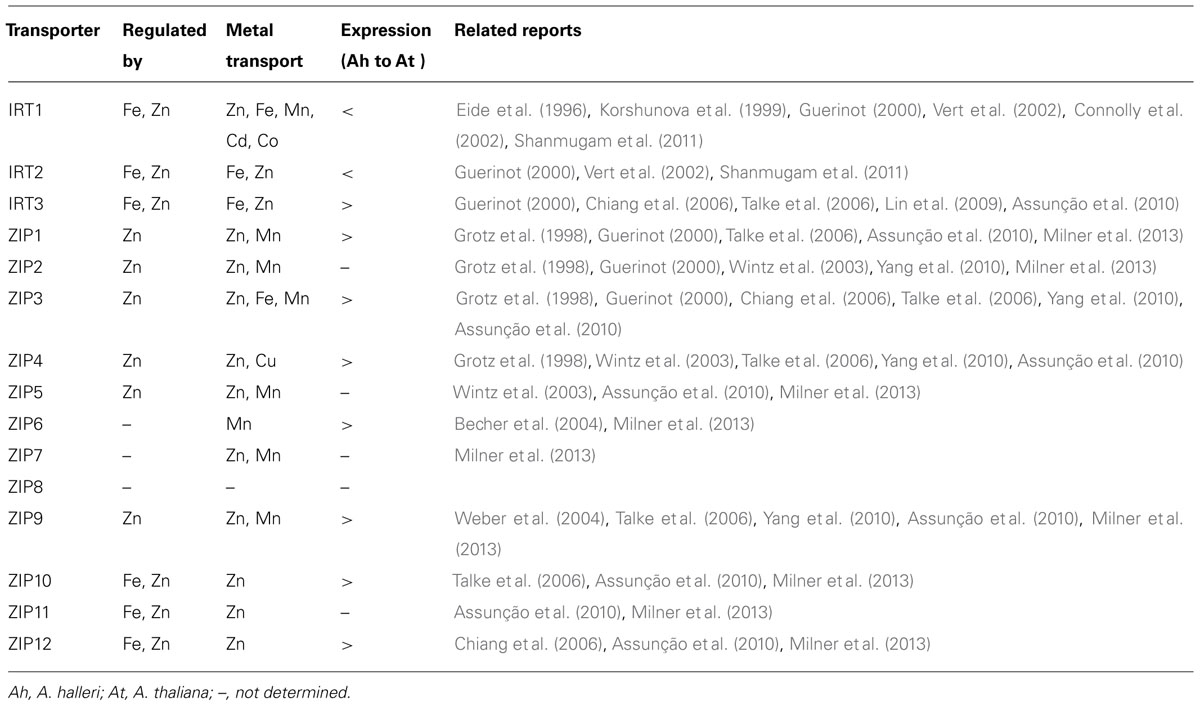
Zn enters the root system through specific membrane transporters, mainly ZRT/IRT-like protein (ZIP) transporters. The Arabidopsis genome contains 15 members of the ZIP family (Table 1). Most are located in the plasma membrane and are involved in micronutrient uptake. IRT1 is well characterized and regulated by Fe status. IRT1 can transport Fe, Mn, Co, Cd, and Zn. The knockout mutant of IRT1, irt1-1, exhibits severe growth defects, and excess supply of Fe can rescue the defective growth (Vert et al., 2002). IRT2, too, is regulated by Fe status and can transport both Fe and Zn (Vert et al., 2001, 2009). Under Fe deficiency, FIT, together with AtbHLH38 and AtbHLH39, members of the basic helix-loop-helix transcription factor family, transcriptionally regulate the expression of IRT1 and IRT2 (Yuan et al., 2008; Wu et al., 2012). Promoter regions of IRT1 and IRT2 contain the E-box motif CANNTG, a potential binding site for FIT (Colangelo and Guerinot, 2004). Apart from IRT1 and IRT2, other ZIP family members are mainly regulated by Zn status and are involved in Zn transport (Table 1). Under Zn deficiency, 2 members of the basic-region leucine-zipper family of transcription factors, bZIP19 and bZIP23, are involved in the transcriptional regulation of ZIP family transporters by binding ZDRE elements in their promoter region (Assunção,et al., 2010). In light of the ability of the ZIP family transporters to conduct multi-metal transport, their expression and regulation under excess Zn or other metal ions is likely a complex phenomenon.
In A. thaliana, Zn toxicity causes reduced Fe uptake and shoot Fe accumulation, which indicates competition between Zn and Fe in root uptake (Fukao et al., 2011; Shanmugam et al., 2011). Excess Zn significantly reduces shoot Fe content and induces IRT1 and IRT2 (Fukao et al., 2011; Shanmugam et al., 2011). This response could be responsible for Zn sensitivity because IRT1 and IRT2 can also transport Zn. Zn uptake with IRT1 and IRT2 induction overloads the regular detoxification system. Interestingly, in A. thaliana, excess Fe alleviates Zn toxicity under excess Zn. Therefore, the competition between Zn and Fe plays an important role in tolerance to excess Zn (Fukao et al., 2011; Shanmugam et al., 2011; Pineau et al., 2012). As compared with A. thaliana, A. halleri shows altered expression of genes related to Fe homeostasis (Chiang et al., 2006; Shanmugam et al., 2011). The expression of the Fe-regulated ZIP transporters IRT1 and IRT2 is much lower in A. halleri than A. thaliana (Shanmugam et al., 2011). A. halleri lives in Zn-rich conditions. In A. halleri, high Zn concentration does not greatly affect shoot and root Fe accumulation, which could explain the reduced expression of IRT1 and IRT2. Therefore, Zn uptake is mainly through Zn-regulated ZIP transporters for optimal root uptake of Zn without disturbing the expression of Fe-regulated ZIP transporters.
The high expression of ZIP transporters such as IRT3, ZIP3, ZIP6, ZIP9 and ZIP12 may also have a function in Fe availability in A. halleri (Chiang et al., 2006; Talke et al., 2006; Lin et al., 2009; Willems et al., 2010). The clearest example of a ZIP transporter functioning in Fe availability is IRT3. IRT3 can transport Zn as well as Fe (Lin et al., 2009) and is also linked to a major QTL responsible for a shoot Fe accumulation phenotype in A. halleri (Willems et al., 2010). In addition, by both its expression in root stele and complementing shoot Fe content in irt1-1, IRT3 could play a role in Fe uptake and translocation in A. halleri (Lin et al., 2009; Shanmugam et al., 2011). Together, the high expression of these ZIP transporters could contribute to Fe acquisition in A. halleri and prevent loss of control of the Fe-regulated multi-metal transporters IRT1 and IRT2 under excess Zn. The major uptake of Zn through Zn-regulated ZIP transporters in coordination with a Zn detoxification mechanism helps in Zn tolerance. Thus, the balanced control of Fe- and Zn-regulated ZIP transporters could be an adaptive mechanism in metal-rich environments.
Conclusions and Future Perspectives
Our knowledge of metal tolerance and hyperaccumulation in plants has greatly improved in recent years with the identification of key genes and regulators involved in the metal homeostasis network. In addition to the proposed mechanisms for metal tolerance and hyperaccumulation, specificity in metal uptake could be a beneficial mechanism. Studies of the Zn hyperaccumulator A. halleri suggest that repression of Fe-regulated multi-metal transporters and overexpressing metal (Fe)-specific transporters may be a useful strategy for engineering plants tolerant to heavy metals. Tight control of the uptake system may also be an important strategy for tolerance of excess Zn. The function of several ZIP transporters has not been clear to date. At least, more research into the role of ZIP transporters with high expression in A. halleri will help in understanding their role in metal uptake and tolerance.
Apart from the transporters, several transcriptional regulators might be involved in the balanced Zn and Fe uptake in A. halleri, when considering the similar biological property of many ZIP transporters, but our knowledge in this area remains limited. In A. halleri, the major Fe deficiency regulator FIT was less regulated under Fe deficiency or Zn excess stress. This finding again suggests the occurrence of a complex process apart from what is already known in maintaining Fe homeostasis in a Zn-rich environment. In addition, the involvement of chelator complexes and their roles in facilitating the control of metal uptake and tolerance are not known. More research in these directions is needed.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by grants from DPIAB and Academia Sinica.
References
Assunção, A. G., Herrero, E., Lin, Y. F., Huettel, B., Talukdar, S., Smaczniak, C., et al. (2010). Arabidopsis thaliana transcription factors bZIP19 and bZIP23 regulate the adaptation to zinc deficiency. Proc. Natl. Acad. Sci. U.S.A. 107, 10296–10301. doi: 10.1073/pnas.1004788107
Baker, A. J. M., and Brooks, R. R. (1989). Terrestrial higher plants which hyperaccumulate metallic elements – a review of their distribution, ecology and phytochemistry. Biorecovery 1, 81–126.
Barabasz, A., Kramer, U., Hanikenne, M., Rudzka, J., and Antosiewicz, D. M. (2010). Metal accumulation in tobacco expressing Arabidopsis halleri metal hyperaccumulation gene depends on external supply. J. Exp. Bot. 61, 3057–3067. doi: 10.1093/jxb/erq129
Becher, M., Talke, I. N., Krall, L., and Kramer, U. (2004). Cross-species microarray transcript profiling reveals high constitutive expression of metal homeostasis genes in shoots of the zinc hyperaccumulator Arabidopsis halleri. Plant J. 37, 251–268. doi: 10.1046/j.1365-313X.2003.01959.x
Bert, V., Bonnin, I., Saumitou-Laprade, P., De Laguerie, P., and Petit, D. (2002). Do Arabidopsis halleri from nonmetallicolous populations accumulate zinc and cadmium more effectively than those from metallicolous populations? New Phytol. 155, 47–57. doi: 10.1046/j.1469-8137.2002.00432.x
Brown, S. L., Chaney, R. L., Angle, J. S., and Baker, A. J. M. (1995). Zinc and cadmium uptake by hyperaccumulator Thlaspi caerulescens grown in nutrient solution. Soil Sci. Soc. Am. J. 59, 125–133. doi: 10.2136/sssaj1995.03615995005900010020x
Chiang, H. C., Lo, J. C., and Yeh, K. C. (2006). Genes associated with heavy metal tolerance and accumulation in Zn/Cd hyperaccumulator Arabidopsis halleri: a genomic survey with cDNA microarray. Environ. Sci. Technol. 40, 6792–6798. doi: 10.1021/es061432y
Clarke, N. D., and Berg, J. M. (1998). Zinc fingers in Caenorhabditis elegans: finding families and probing pathways. Science 282, 2018–2022. doi: 10.1126/science.282.5396.2018
Clemens, S. (2006). Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 88, 1707–1719. doi: 10.1016/j.biochi.2006.07.003
Colangelo, E. P., and Guerinot, M. L. (2004). The essential basic helix-loop-helix protein FIT1 is required for the iron deficiency response. Plant Cell 16, 3400–3412. doi: 10.1105/tpc.104.024315
Connolly, E. L., Fett, J. P., and Guerinot, M. L. (2002). Expression of the IRT1 metal transporter is controlled by metals at the levels of transcript and protein accumulation. Plant Cell 14, 1347–1357. doi: 10.1105/tpc.001263
Cosio, C., Martinoia, E., and Keller, C. (2004). Hyperaccumulation of cadmium and zinc in Thlaspi caerulescens and Arabidopsis halleri at the leaf cellular level. Plant Physiol. 134, 716–725. doi: 10.1104/pp.103.031948
Deinlein, U., Weber, M., Schmidt, H., Rensch, S., Trampczynska, A., Hansen, T. H., et al. (2012). Elevated nicotianamine levels in Arabidopsis halleri roots play a key role in zinc hyperaccumulation. Plant Cell 24, 708–723. doi: 10.1105/tpc.111.095000
Drager, D. B., Desbrosses-Fonrouge, A. G., Krach, C., Chardonnens, A. N., Meyer, R. C., Saumitou-Laprade, P., et al. (2004). Two genes encoding Arabidopsis halleri MTP1 metal transport proteins co-segregate with zinc tolerance and account for high MTP1 transcript levels. Plant J. 39, 425–439. doi: 10.1111/j.1365-313X.2004.02143.x
Eide, D., Broderius, M., Fett, J., and Guerinot, M. L. (1996). A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc. Natl. Acad. Sci. U.S.A. 93, 5624–5628.
Fukao, Y., Ferjani, A., Tomioka, R., Nagasaki, N., Kurata, R., Nishimori, Y., et al. (2011). iTRAQ analysis reveals mechanisms of growth defects due to excess zinc in Arabidopsis. Plant Physiol. 155, 1893–1907. doi: 10.1104/pp.110.169730
Grotz, N., Fox, T., Connolly, E., Park, W., Guerinot, M. L., and Eide, D. (1998). Identification of a family of zinc transporter genes from Arabidopsis that respond to zinc deficiency. Proc. Natl. Acad. Sci. U.S.A. 95, 7220–7224. doi: 10.1073/pnas.95.12.7220
Guerinot, M. L. (2000). The ZIP family of metal transporters. Biochim. Biophys. Acta 1465, 190–198. doi: 10.1016/S0005-2736(00)00138-3
Guerinot, M. L., and Eide, D. (1999). Zeroing in on zinc uptake in yeast and plants. Curr. Opin. Plant Biol. 2, 244–249. doi: 10.1016/S1369-5266(99)80042-9
Gustin, J. L., Loureiro, M. E., Kim, D., Na, G., Tikhonova, M., and Salt, D. E. (2009). MTP1-dependent Zn sequestration into shoot vacuoles suggests dual roles in Zn tolerance and accumulation in Zn-hyperaccumulating plants. Plant J. 57, 1116–1127. doi: 10.1111/j.1365-313X.2008.03754.x
Hanikenne, M., Talke, I. N., Haydon, M. J., Lanz, C., Nolte, A., Motte, P., et al. (2008). Evolution of metal hyperaccumulation required cis-regulatory changes and triplication of HMA4. Nature 453, 391–395. doi: 10.1038/nature06877
Hu, Y. T., Ming, F., Chen, W. W., Yan, J. Y., Xu, Z. Y., Li, G. X., et al. (2012). TcOPT3, a member of oligopeptide transporters from the hyperaccumulator Thlaspi caerulescens, is a novel Fe/Zn/Cd/Cu transporter. PLoS ONE 7:e38535. doi: 10.1371/journal.pone.0038535
Hussain, D., Haydon, M. J., Wang, Y., Wong, E., Sherson, S. M., Young, J., et al. (2004). P-type ATPase heavy metal transporters with roles in essential zinc homeostasis in Arabidopsis. Plant Cell 16, 1327–1339. doi: 10.1105/tpc.020487
Kawachi, M., Kobae, Y., Mori, H., Tomioka, R., Lee, Y., and Maeshima, M. (2009). A mutant strain Arabidopsis thaliana that lacks vacuolar membrane zinc transporter MTP1 revealed the latent tolerance to excessive zinc. Plant Cell Physiol. 50, 1156–1170. doi: 10.1093/pcp/pcp067
Kim, Y. Y., Choi, H., Segami, S., Cho, H. T., Martinoia, E., Maeshima, M., et al. (2009). AtHMA1 contributes to the detoxification of excess Zn(II) in Arabidopsis. Plant J. 58, 737–753. doi: 10.1111/j.1365-313X.2009.03818.x
Kobae, Y., Uemura, T., Sato, M. H., Ohnishi, M., Mimura, T., Nakagawa, T., et al. (2004). Zinc transporter of Arabidopsis thaliana AtMTP1 is localized to vacuolar membranes and implicated in zinc homeostasis. Plant Cell Physiol. 45, 1749–1758. doi: 10.1093/pcp/pci015
Korshunova, Y. O., Eide, D., Clark, W. G., Guerinot, M. L., and Pakrasi, H. B. (1999). The IRT1 protein from Arabidopsis thaliana is a metal transporter with a broad substrate range. Plant Mol. Biol. 40, 37–44. doi: 10.1023/A:1026438615520
Kramer, U. (2010). Metal hyperaccumulation in plants. Annu. Rev. Plant Biol. 61, 517–534. doi: 10.1146/annurev-arplant-042809-112156
Kupper, H., Lombi, E., Zhao, F. J., and Mcgrath, S. P. (2000). Cellular compartmentation of cadmium and zinc in relation to other elements in the hyperaccumulator Arabidopsis halleri. Planta 212, 75–84. doi: 10.1007/s004250000366
Lin, Y. F., Liang, H. M., Yang, S. Y., Boch, A., Clemens, S., Chen, C. C., et al. (2009). Arabidopsis IRT3 is a zinc-regulated and plasma membrane localized zinc/iron transporter. New Phytol. 182, 392–404. doi: 10.1111/j.1469-8137.2009.02766.x
Lochlainn, S. O., Bowen, H. C., Fray, R. G., Hammond, J. P., King, G. J., White, P. J., et al. (2011). Tandem quadruplication of HMA4 in the zinc (Zn) and cadmium (Cd) hyperaccumulator Noccaea caerulescens. PLoS ONE 6:e17814. doi: 10.1371/journal.pone.0017814
Milner, M. J., Seamon, J., Craft, E., and Kochian, L. V. (2013). Transport properties of members of the ZIP family in plants and their role in Zn and Mn homeostasis. J. Exp. Bot. 64, 369–381. doi: 10.1093/jxb/ers315
Nriagu, J. O., and Pacyna, J. M. (1988). Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature 333, 134–139. doi: 10.1038/333134a0
Pineau, C., Loubet, S., Lefoulon, C., Chalies, C., Fizames, C., Lacombe, B., et al. (2012). Natural variation at the FRD3 MATE transporter locus reveals cross-talk between Fe homeostasis and Zn tolerance in Arabidopsis thaliana. PLoS Genet. 8:e1003120. doi: 10.1371/journal.pgen.1003120
Ramesh, S. A., Choimes, S., and Schachtman, D. P. (2004). Overexpression of an Arabidopsis zinc transporter in Hordeum vulgare increases short-term zinc uptake after zinc deprivation and seed zinc content. Plant Mol. Biol. 54, 373–385. doi: 10.1023/B:PLAN.0000036370.70912.34
Reeves, R. D., and Baker, A. J. M. (2000). “Metal-accumulating plants,” in Phytoremediation of Toxic Metals: Using Plants to Clean up the Environment, ed. E. B. Raskin I. (New York: John Wiley and Sons), 193–229.
Roosens, N. H., Willems, G., and Saumitou-Laprade, P. (2008). Using Arabidopsis to explore zinc tolerance and hyperaccumulation. Trends Plant Sci. 13, 208–215. doi: 10.1016/j.tplants.2008.02.006
Schneider, T., Persson, D. P., Husted, S., Schellenberg, M., Gehrig, P., Lee, Y., et al. (2012). A proteomics approach to investigate the process of Zn hyperaccumulation in Noccaea caerulescens (J & C. Presl) F. K. Meyer. Plant J. 73, 131–142. doi: 10.1111/tpj.12022
Shahzad, Z., Gosti, F., Frerot, H., Lacombe, E., Roosens, N., Saumitou-Laprade, P., et al. (2010). The five AhMTP1 zinc transporters undergo different evolutionary fates towards adaptive evolution to zinc tolerance in Arabidopsis halleri. PLoS Genet. 6:e1000911. doi: 10.1371/journal.pgen.1000911
Shanmugam, V., Lo, J. C., Wu, C. L., Wang, S. L., Lai, C. C., Connolly, E. L., et al. (2011). Differential expression and regulation of iron-regulated metal transporters in Arabidopsis halleri and Arabidopsis thaliana – the role in zinc tolerance. New Phytol. 190, 125–137. doi: 10.1111/j.1469-8137.2010.03606.x
Shen, Z. G., Zhao, F. J., and McGrath, S. P. (1997). Uptake and transport of zinc in the hyperaccumulator Thlaspi caerulescens and the non-hyperaccumulator Thlaspi ochroleucum. Plant Cell Environ. 20, 898–906. doi: 10.1046/j.1365-3040.1997.d01-134.x
Talke, I. N., Hanikenne, M., and Kramer, U. (2006). Zinc-dependent global transcriptional control, transcriptional deregulation, and higher gene copy number for genes in metal homeostasis of the hyperaccumulator Arabidopsis halleri. Plant Physiol. 142, 148–167. doi: 10.1104/pp.105.076232
Vanassche, F., and Clijsters, H. (1986a). Inhibition of photosynthesis in Phaseolus Vulgaris by treatment with toxic concentration of zinc – effect on ribulose-1,5-bisphosphate carboxylase oxygenase. J. Plant Physiol. 125, 355–360. doi: 10.1016/S0176-1617(86)80157-2
Vanassche, F., and Clijsters, H. (1986b). Inhibition of photosynthesis in Phaseolus Vulgaris by treatment with toxic concentrations of zinc – effects on electron-transport and photophosphorylation. Physiol. Plant. 66, 717–721. doi: 10.1111/j.1399-3054.1986.tb05605.x
Vanassche, F., and Clijsters, H. (1990). Effects of metals on enzyme-activity in plants. Plant Cell Environ. 13, 195–206. doi: 10.1111/j.1365-3040.1990.tb01304.x
van de Mortel, J. E., Aarts, M. G. M., Villanueva, L. A., Schat, H., Kwekkeboom, J., Coughlan, S., et al. (2006). Large expression differences in genes for iron and zinc homeostasis, stress response, and lignin biosynthesis distinguish roots of Arabidopsis thaliana and the related metal hyperaccumulator Thlaspi caerulescens. Plant Physiol. 142, 1127–1147. doi: 10.1104/pp.106.082073
Verbruggen, N., Hermans, C., and Schat, H. (2009). Molecular mechanisms of metal hyperaccumulation in plants. New Phytol. 181, 759–776. doi: 10.1111/j.1469-8137.2008.02748.x
Verret, F., Gravot, A., Auroy, P., Leonhardt, N., David, P., Nussaume, L., et al. (2004). Overexpression of AtHMA4 enhances root-to-shoot translocation of zinc and cadmium and plant metal tolerance. FEBS Lett. 576, 306–312. doi: 10.1016/j.febslet.2004.09.023
Vert, G., Barberon, M., Zelazny, E., Seguela, M., Briat, J. F., and Curie, C. (2009). Arabidopsis IRT2 cooperates with the high-affinity iron uptake system to maintain iron homeostasis in root epidermal cells. Planta 229, 1171–1179. doi: 10.1007/s00425-009-0904-8
Vert, G., Briat, J. F., and Curie, C. (2001). Arabidopsis IRT2 gene encodes a root-periphery iron transporter. Plant J. 26, 181–189. doi: 10.1046/j.1365-313x.2001.01018.x
Vert, G., Grotz, N., Dedaldechamp, F., Gaymard, F., Guerinot, M. L., Briat, J. F., et al. (2002). IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 14, 1223–1233. doi: 10.1105/tpc.001388
Weber, M., Harada, E., Vess, C., Roepenack-Lahaye, E., and Clemens, S. (2004). Comparative microarray analysis of Arabidopsis thaliana and Arabidopsis halleri roots identifies nicotianamine synthase, a ZIP transporter and other genes as potential metal hyperaccumulation factors. Plant J. 37, 269–281. doi: 10.1046/j.1365-313X.2003.01960.x
Willems, G., Frerot, H., Gennen, J., Salis, P., Saumitou-Laprade, P., and Verbruggen, N. (2010). Quantitative trait loci analysis of mineral element concentrations in an Arabidopsis halleri × Arabidopsis lyrata petraea F2 progeny grown on cadmium-contaminated soil. New Phytol. 187, 368–379. doi: 10.1111/j.1469-8137.2010.03294.x
Wintz, H., Fox, T., Wu, Y. Y., Feng, V., Chen, W., Chang, H. S., et al. (2003). Expression profiles of Arabidopsis thaliana in mineral deficiencies reveal novel transporters involved in metal homeostasis. J. Biol. Chem. 278, 47644–47653. doi: 10.1074/jbc.M309338200
Wu, H., Chen, C., Du, J., Liu, H., Cui, Y., Zhang, Y., et al. (2012). Co-overexpression FIT with AtbHLH38 or AtbHLH39 in Arabidopsis-enhanced cadmium tolerance via increased cadmium sequestration in roots and improved iron homeostasis of shoots. Plant Physiol. 158, 790–800. doi: 10.1104/pp.111.190983
Yang, T. J., Lin, W. D., and Schmidt, W. (2010). Transcriptional profiling of the Arabidopsis iron deficiency response reveals conserved transition metal homeostasis networks. Plant Physiol. 152, 2130–2141. doi: 10.1104/pp.109.152728
Yuan, Y., Wu, H., Wang, N., Li, J., Zhao, W., Du, J., et al. (2008). FIT interacts with AtbHLH38 and AtbHLH39 in regulating iron uptake gene expression for iron homeostasis in Arabidopsis. Cell Res. 18, 385–397. doi: 10.1038/cr.2008.26
Zhao, F., Lombi, E., Breedon, T., and Mcgrath, S. (2000). Zinc hyperaccumulation and cellular distribution in Arabidopsis halleri. Plant Cell Environ. 23, 507–514. doi: 10.1046/j.1365-3040.2000.00569.x
Keywords: Arabidopsis halleri, hyperaccumulators, ZIP transporters, Fe homeostasis, Zn tolerance
Citation: Shanmugam V, Lo J-C and Yeh K-C (2013) Control of Zn uptake in Arabidopsis halleri: a balance between Zn and Fe. Front. Plant Sci. 4:281. doi: 10.3389/fpls.2013.00281
Received: 29 April 2013; Accepted: 09 July 2013;
Published online: 31 July 2013.
Edited by:
Mark G. M. Aarts, Wageningen University, NetherlandsReviewed by:
Henk Schat, Vrije Universiteit Amsterdam, NetherlandsVasileios Fotopoulos, Cyprus University of Technology, Cyprus
Copyright: © 2013 Shanmugam, Lo and Yeh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kuo-Chen Yeh, Agricultural Biotechnology Research Center, Academia Sinica, 128 Academia Road Section 2, Taipei, Taiwan 11529, Republic of China e-mail:a2N5ZWhAZ2F0ZS5zaW5pY2EuZWR1LnR3