- Department of Applied Genetics and Cell Biology, University of Natural Resources and Applied Life Sciences, Vienna, Austria
The value of Agrobacterium tumefaciens for plant molecular biologists cannot be appreciated enough. This soil-borne pathogen has the unique capability to transfer DNA (T-DNA) into plant systems. Gene transfer involves both bacterial and host factors, and it is the orchestration of these factors that determines the success of transformation. Some plant species readily accept integration of foreign DNA, while others are recalcitrant. The timing and intensity of the microbially activated host defense repertoire sets the switch to “yes” or “no.” This repertoire is comprised of the specific induction of mitogen-activated protein kinases (MAPKs), defense gene expression, production of reactive oxygen species (ROS) and hormonal adjustments. Agrobacterium tumefaciens abuses components of the host immunity system it mimics plant protein functions and manipulates hormone levels to bypass or override plant defenses. A better understanding of the ongoing molecular battle between agrobacteria and attacked hosts paves the way toward developing transformation protocols for recalcitrant plant species. This review highlights recent findings in agrobacterial transformation research conducted in diverse plant species. Efficiency-limiting factors, both of plant and bacterial origin, are summarized and discussed in a thought-provoking manner.
Introduction
In their natural habitats, plants live in close contact with a myriad microorganisms. Plant-microbe associations can be mutually beneficial, such as the root nodule symbiosis with nitrogen-fixing bacteria or the more wide-spread association of plant roots with arbuscular mycorrhizal fungi (reviewed in Parniske, 2008; Markmann and Parniske, 2009). In contrast, pathogenic fungi or bacteria impair plant development and cause various disease symptoms in their hosts. The gram-negative Agrobacterium tumefaciens of the family Rhizobeaceae is a “special case.” It is a biotroph pathogen, which markedly alters the physiology and morphology of infected host plants. What makes Agrobacterium so special is its capability for interkingdom gene transfer. In nature, wild type A. tumefaciens (as well as A. rhizogenes and A. vitis) causes “crown gall disease,” characterized by the growth of tumor-like structures (calli) on host species. The genetic information for this anatomical reprogramming is encoded on the tumor-inducing (Ti) plasmid. The transfer DNA (T-DNA) derived from the Ti plasmid is imported into the host cell's cytoplasm and subsequently into the nucleus (Gelvin, 2003, 2005; Dafny-Yelin et al., 2008; Pitzschke and Hirt, 2010b). T-DNA transport is mediated by agrobacterial virulence factors, and—involuntarily—supported by proteins of the attacked host. Over the last decade, microbiologists and plant scientists have disclosed an impressive portfolio of agrobacterial infection strategies, some of which resemble those in other pathogen-host interactions. Plant defense mechanisms counteracting these strategies are equally diverse and impressive.
Principal Steps
The principal steps and factors involved in Agrobacterium-mediated plant transformation are comparatively well-understood, and reviews can be found in e.g., (Gelvin, 2009, 2010a,b; Pitzschke and Hirt, 2010b). Briefly, agrobacteria sense phenolic substances that are secreted by wounded plant tissue. Reception of these signals drives the expression of bacterial virulence (vir) genes. Subsequently, Vir proteins are produced, and single-stranded T-DNA molecules are synthesized from the Ti plasmid. The T-complex, i.e., T-DNA associated with certain Vir proteins, is injected into the host cytoplasm. A sophisticated network of bacterial and plant factors mediates translocation of the T-DNA to its final destination, the host cell's nucleus.
Agrobacterium inserts substrates (T-DNA and virulence proteins including VirD2, VirE2, VirE3, VirD5, and VirF) into the host cell by a type IV secretion system (Cascales and Christie, 2003). This strategy is also employed for the delivery of microbial factors by other plant pathogens, including Xanthomonas campestris (Thieme et al., 2005) and Burkholderia (Engledow et al., 2004). Likewise, mammalian pathogens including Bordetella pertussis, Legionella pneumophila, Brucella spp., and Helicobacter pylori, use type IV machineries to export effector proteins to the extracellular milieu or the cell cytosol (Christie and Vogel, 2000). Remarkably, under laboratory conditions, agrobacteria can genetically transform virtually any type of eukaryote, ranging from yeast (Bundock et al., 1995) to human cells (Kunik et al., 2001) (reviewed in Michielse et al., 2005; Lacroix et al., 2006). The T-complex, consisting of T-DNA, bacterial virulence proteins (VirE2, VirD2) and the host factor VIP1 (VirE2-interacting protein 1) is imported into the nucleus. Subsequently, the proteinaceous components are stripped off, releasing the T-DNA from the T-complex. This step relies on degradation of VirE2, VirD2, and VIP1 by the plant SCF proteasomal machinery (see below). The bacterial F-box protein VirF, which is contained in and confers substrate specificity to the SCF complex, participates in this degradation. If the T-complex disintegrates before it is in contact with the host's chromatin, the delivered transgenes are expressed for only a few days. The loss of transgene activity at later stages likely results from the T-DNA being degraded by host nucleases (Gelvin, 2003). In contrast, if the T-DNA is shielded until the T-complex is in contact with chromatin, stable transformants can be obtained. Due to its affinity for histones, VIP1 most probably guides the T-DNA to its target destination, the chromatin (Lacroix et al., 2008).
Since the discovery of the gene transfer mechanism (Schell and Van Montagu, 1977; Holsters et al., 1978), Agrobacterium strains have been converted (“disarmed”) into efficient delivery systems for the genetic manipulation of plants. While transient expression approaches can provide rapid answers on e.g., subcellular localization, protein-protein interaction and promoter/effector relationships (Andrews and Curtis, 2005; Li et al., 2009; Pitzschke, 2013b), genetic engineering requires the transgene(s) to be stably integrated in the host genome.
The so-called disarmed/non-oncogenic A. tumefaciens strains employed are deprived of their Ti properties, and the T-DNA region is used as a vehicle for the introduction of tailor-made DNA sequences. Any DNA sequence placed between T-DNA “border sequences” (Ti-plasmid-derived 25-bp direct repeats) can be transferred (Gelvin, 2012). Disarmed strains, therefore, facilitate transformation, but do not provoke callus growth or other abnormalities caused by oncogenic strains. Consequently, phenotypic abnormalities that may be exhibited by transformed plants are primarily due to the particular transgene being expressed. Furthermore, by using armed and disarmed strains side-by-side, host responses that are independent of or dependent on Ti sequences can be distinguished.
Transcriptional Re-Programming of Host Cells
The advent of full genome sequencing and microarray technologies has created the opportunity to draw a complete picture on Agrobacterium-induced changes at the transcript level. Gene expression profiling data have been generated for various plant species, and comprehensive databases (e.g., http://www.plexdb.org) and bioinformatics resources even allow comparison of transcriptional responses across multiple plant species (Dash et al., 2012). One major finding from diverse microarray studies was that agrobacteria largely modify host gene expression, particularly that of defense-related genes.
This fact had already been recognized in the “pre-microarray era.” cDNA-AFLP analysis of Ageratum conyzoides plant cell cultures enabled the identification of (non-oncogenic) Agrobacterium-induced transcripts, many of which encoded putative defense factors (Ditt et al., 2001). In a subsequent study the same research group observed an anti-correlation between Agrobacterium-mediated transformation efficiency and defense gene expression levels (Ditt et al., 2005). By the approach of suppression subtractive hybridization and DNA macroarrays, Veena Jiang et al. (2003) provided the first insight into the molecular kinetics of Agrobacterium -plant interactions. Transcriptional responses of tobacco BY-2 cell cultures to a subset of agrobacterial strains, impaired in T-DNA and/or Vir protein transfer, were monitored over a 36-h-period. All strains elicited a general defense response during early stages of infection. However, expression of defense-related genes was repressed at later stages—exclusively by the transfer-competent strains. More detailed expression profiling of selected genes furthermore disclosed the “unintentional” participation of the host cellular machinery in the transformation process (Veena Jiang et al., 2003).
Microbial Attack and Plant Defense
Microbes attempting to invade their hosts betray themselves by the presence of so-called microbe- or pathogen-associated molecular patterns (MAMPs or PAMPs). These molecules, which are recognized as “non-self” initiate the first line of defense, known as PAMP-triggered immunity (PTI) (Nurnberger et al., 2004; Sanabria et al., 2008; Boller and He, 2009) (see below). Pathogens, in turn, aim to overcome PTI activation by injecting certain effector proteins into the host cytoplasm. Perception of these pathogen-encoded effectors by cognate intracellular plant proteins raises the second line of defense, effector-triggered immunity (ETI) (Bonardi and Dangl, 2012; Gassmann and Bhattacharjee, 2012). This response is characterized by the induction of localized apoptosis (hypersensitive response, HR) and systemic defense signaling. Plants capable of activating ETI can thus not only restrict pathogen spread, but they can also fortify themselves against subsequent attacks (Shah and Zeier, 2013).
MAMPs and Their Perception
MAMPs are best described as molecular “signatures” typical of whole classes of microbes (Boller and Felix, 2009). MAMP perception through specific cell-surface-located proteins (“pattern recognition receptors”) is a conserved strategy of eukaryotic innate immune systems. Because MAMPs initiate defense responses in many plant species, they are also referred to as “general elicitors” (Nurnberger et al., 2004). Prominent examples of MAMPs include oligopeptide elicitors such as those derived from EF-Tu (elongation factor thermo unstable), flagellin, and cryptogein (a fungal sterol-scavenging protein), as well as glycol-conjugates, including bacterial lipopolysaccharides and peptidoglycan, and the fungal MAMPs beta-glucan, chitin and chitosan oligosaccharides (reviewed in Silipo et al., 2010).
The two undoubtedly best-characterized MAMP receptors in plants, FLS2 and EFR, recognize the oligopeptides flagellin and EF-Tu, respectively. Owing to their composite structure, these membrane-located leucine-rich repeat-receptor-like kinases (LRR-RLK) convert and transmit perceived “attack signals” into the interior of cells to initiate appropriate defense responses. On the contrary, the primary “aims” of pathogens are to claim nutrients from and multiply to high levels in their hosts. To avoid or block defense responses during early stages of infection, pathogens have two options: (1) evade recognition and “sneak in” or (2) “step in self-consciously” and counteract the elicited warfare attack. Biotrophs, such as Pseudomonas syringae, A. tumefaciens, Xanthomonas campestris, and Botrytis cinerea, have developed sophisticated strategies to block defense signaling in their hosts at several steps (Pitzschke et al., 2009c).
A total of 292 and 165 LRR-RLK genes were retrieved from the rice and Arabidopsis genomes, respectively (Hwang et al., 2011). These large numbers provide an idea of the versatility of LRR-RLK applications. Specific roles have been ascribed to individual family members. Studies in individual LRR-RLK mutants have contributed to our understanding of pathogen perception in general. They also demonstrate the similarity of early plant responses to agrobacteria and other microbial pathogens.
For instance, fls2 mutants fail to recognize flagellin and are more susceptible to infection by the pathogen Pseudomonas syringae (Zipfel et al., 2004). Similarly, mutants deficient in EFR, the receptor for the agrobacterial MAMP EF-Tu, are hypersensitive to Agrobacterium-mediated transformation (Zipfel et al., 2006). These examples demonstrate that “ignoring” the invader is not advisable. Instead, perception is the first and mandatory step to restrict bacterial invasion. FLS2 gene induction upon pathogen exposure or flagellin treatment (Boutrot et al., 2010), as well as EFR1 induction by EF-Tu-derived peptides (Zipfel et al., 2006) reflect additional host mechanisms to better target the suspected invaders.
MAPK Signaling
One of the early intracellular events following pathogen perception is signal transduction and amplification through mitogen-activated protein kinases (MAPKs) (Nakagami et al., 2005; Pitzschke et al., 2009c; Huang et al., 2012; Rasmussen et al., 2012). MAPK cascades are conserved eukaryotic signaling modules. Their minimal components, a MAPK kinase kinase (MAPKKK), a MAPKK and a MAPK, represent multigene families. Exogenous or developmental signals are perceived by a receptor which subsequently (directly or indirectly) initiates the MAPK cascade. Once activated, a MAPKKK phosphorylates its downstream MAPKK which in turn phosphorylates and thereby activates its downstream MAPK (Nakagami et al., 2005). MAPK-mediated phosphorylation of target proteins can alter their properties, such as subcellular location, DNA-binding specificity, enzymatic activity or stability. There is ample evidence for disturbed MAPK signaling markedly affecting biotic and abiotic stress tolerance (Rohila and Yang, 2007; Pitzschke and Hirt, 2009; Pitzschke et al., 2009a; Rodriguez et al., 2010; Sinha et al., 2011; Persak and Pitzschke, 2013; Zhang et al., 2013b). It is very likely that such a scenario will hold true in many plant species.
MAPK Signaling and the Multifunctional Protein VIP1
In the context of agrobacteria and pathogen defense, one member of the Arabidopsis MAPK family has merited special attention: MPK3. This protein is activated within few minutes upon treatment with pathogens or bacterial elicitor-derived peptides such as flg22 and elf18 (Djamei et al., 2007; Lu et al., 2009). MPK3 is an important positive regulator in defense signaling (Nakagami et al., 2005; Pitzschke et al., 2009c). From a pathogen's point of view, activation of MPK3 should be avoided to circumvent repelling. Accordingly, agrobacteria have evolved strategies to co-opt induction of this kinase. MPK3 phosphorylates the host protein VIP1 and thereby triggers cyto-nuclear translocation of this bZIP transcription factor (Djamei et al., 2007). VIP1, which enters the nucleus via interaction with importin alpha (Citovsky et al., 2004) subsequently induces expression of defense genes such as PR1 (pathogenesis-related protein 1) (Djamei et al., 2007; Pitzschke et al., 2009b; Pitzschke and Hirt, 2010a). Agrobacteria, on the other hand, hijack VIP1 as a shuttle for nuclear import of the T-complex (Citovsky et al., 2004). A number of plant species lack putative VIP1 homologs; yet these species are transformable. This apparent paradox was solved by the discovery and characterization of virulence factor VirE3. VirE3 functionally replaces the “shuttle” function of VIP1, thus ensuring nuclear import of the T-DNA (Lacroix et al., 2005). In contrast to VIP1, VirE3 is not a transcription factor and is therefore unlikely to (directly) induce defense gene expression. VirE3 may thus be an attractive target for biotechnological approaches.
VIP1 as transcriptional regulator
A random-DNA-selection-assay (RDSA) enabled the identification of putative VIP1 target sequences. The DNA consensus motif recognized by VIP1 (VRE—VIP1 response element) was found to be enriched in promoters of stress-responsive genes (Pitzschke et al., 2009b). Notably, this motif does not resemble known regulatory DNA elements. In vivo, VIP1 directly binds to VRE sites in the promoter of MYB44 (Pitzschke et al., 2009b), a stress-related transcription factor (Jung et al., 2008; Persak and Pitzschke, 2013). Importantly, this binding occurs in a stress-dependent manner that correlated with the MPK3 activation profile (Pitzschke et al., 2009b). Through binding to VRE sites, VIP1 might directly regulate expression of another stress-responsive gene, thioredoxin Trxh8. In protoplast cotransfection experiments, VIP1 triggered the expression of the pathogen-responsive PR1 gene (Djamei et al., 2007). However, this PR1 induction is likely an indirect effect. The PR1 promoter is devoid of VRE sites; and PR1 is known as a late stress-responsive gene, in contrast to the early and transient nature of MPK3 activation and VIP1 cyto-nuclear translocation. A very recent report (Lacroix and Citovsky, 2013) provides a deeper insight into the VRE-VIP1 mechanism. In agreement with the original study (Pitzschke et al., 2009b), VIP1 bound VRE in vitro, and VIP1-VRE binding strongly correlated with transcriptional activation levels in vivo. Presence of the agrobacterial F-box protein VirF did not affect VIP1-VRE binding in vitro. In contrast, coexpression of virF markedly decreased VIP1 transcriptional activation ability in vivo. The most likely explanation for this effect is that in vivo, VirF prevents VRE induction by triggering proteasomal degradation of VIP1 (Lacroix and Citovsky, 2013). In fact, agrobacteria have learned to control VIP1 abundance by abusing the host proteasome machinery (see below). Being aware of the ongoing host-pathogen arms race, it is tempting to speculate that VIP1 may not only turn on expression of host defense genes. Instead, agrobacteria may benefit from one or more VIP1-induced gene products involuntarily provided by the plant. Discovering the VIP1-targetome seems a highly rewarding undertaking. Screening of the Arabidopsis genome for promoters enriched in VRE and related motifs isolated by RDSA (Pitzschke et al., 2009b) could be a first step in that direction (Pitzschke, unpublished).
Overexpression studies in tobacco have shown that VIP1 also promotes transformation efficiency in heterologous systems (Tzfira et al., 2002). The cross-species functionality of VIP1 as transcription factor was further documented in a rather non-conventional expression system: protoplasts from red leaves of poinsettia (Euphorbia pulcherrima). Polyethylenglycol-mediated cotransfection experiments showed that VIP1 efficiently induces VRE-mediated gene expression (Pitzschke and Persak, 2012). For this transactivation to occur neither a tissue context, chloroplasts nor external stimuli are required.
In its unquestionable key role in Agrobacterium-mediated transformation, VIP1 presents an attractive target for manipulation. It appears feasible to uncouple the T-complex-vehicle from the defense-gene-inducer function. Experiments with a C-terminally truncated VIP1 variant have shown that full-length VIP1 is required for stable, but not for transient transformation (Li et al., 2005a). The transgenesis-enhancing effect most likely derives from VIP1 acting as mediator between host nucleosomes and T-DNA/VirE2 complexes. Therefore, replacing critical residues rather than deleting certain domains/peptides seems a more purposeful approach. Indeed, mutation of Lys212, located in the bZIP domain, rendered VIP1 fully incapable of transactivating the PR1 promoter or a synthetic VRE promoter (Pitzschke et al., 2009b).
The SCF Proteasomal Machinery, VirF and VBF
Many biological processes, including host-pathogen interactions, are controlled by SCF (Skp1-Cul1-F-box protein) ubiquitin ligase complexes. These complexes mediate the proteasomal degradation of specific target proteins. The F-box protein contained in SCF complexes confers substrate specificity (Lechner et al., 2006).
Although prokaryotes lack SCF complexes, F-box-encoding genes are found in some pathogenic bacteria. The translocation of F-box effectors appears to be a wide-spread “infection strategy.” Pathogens secrete F-box proteins into their hosts to abuse the SCF machinery, resulting in high infection rates. However, F-box effectors are intrinsically unstable proteins which are rapidly degraded by the host proteasome pathway (Magori and Citovsky, 2011b). The Citovsky laboratory uncovered yet another level of agrobacterial cleverness and callousness: Destabilization of the agrobacterial F-box protein VirF is counteracted by the bacterial effector, VirD5 (Magori and Citovsky, 2011a). As if this was not enough, agrobacteria also exploit additional host factors to maximize infection: Diverse pathogens, including Agrobacterium, induce expression of VBF (VIP1-binding factor), a host-encoded F-box protein. VBF can functionally replace the agrobacterial VirF in regulating VIP1 and VirE2 protein levels (Zaltsman et al., 2010b). Analogous to VirF, VBF destabilizes VirE2 and VIP1, most likely via SCF-mediated proteasomal degradation (Zaltsman et al., 2010a). A very recent study extends on this finding and highlights the importance of VBF at the final stage of T-DNA pre-integration (Zaltsman et al., 2013). As reported earlier, T-complexes can be reconstituted from ssDNA and VirE2 in vitro (Zupan et al., 1996). Its tight packaging by VirE2 molecules shields the ssDNA from the outside and makes it inaccessible to degradation by exogenously added DNAse. In the presence of extracts from wild type, but not from VBF antisense plants, this “shielding effect” was found to be rapidly lost. Thus, VBF-mediated uncoating of the T-complex indeed results in unmasking of the T-DNA (Zaltsman et al., 2013).
Micro-bombardment studies in N. benthamiana leaves have disclosed a cytoplasmic-nuclear distribution of VBF. In contrast, VBF/VIP1 complexes occur exclusively in the nucleus. Based on these observations, VBF may have additional functions in the cytoplasm, besides acting in T-complex disassembly in the nucleus, (Zaltsman et al., 2010b). Alternatively, VBF may re-locate upon pathogen attack (similar to VIP1). If this—currently hypothetic—scenario was true, a straight-forward question arises. Is VBF distribution phosphorylation-dependent; is it controlled by MAPKs? At least in silico, such scenario appears possible (Pitzschke, unpublished). MAPKs phosphorylate their targets at serine or threonine residues adjacent to a proline. A kinase interaction motif [KIM; R/K-x2-6-I/Lx(/L)], known to be recognized by mammalian MAPKs (Tanoue and Nishida, 2003), assists MAPK binding also in substrate proteins of plant MAPKs (Schweighofer et al., 2007). The VBF protein sequence contains one Ser-Pro dipeptide motif as well as one KIM (position 164-171) (Figure 1). Pathogen-activated MAPK(s), such as MPK3, may phosphorylate residue Ser17 and thereby initiate VBF nuclear translocation.

Figure 1. Arabidopsis VBF protein sequence. A peptide matching the consensus motif for MAPK interaction [R/K-x2-6-I/Lx(/L)], and a putative MAPK phosphorylation site are highlighted.
The Role of Plant Hormones in Transformation and Tumor Formation
A plethora of developmental and stimulus-triggered responses are signaled via phytohormones. Auxin is involved in essentially all aspects of plant growth and development (Benjamins and Scheres, 2008; Ljung, 2013). Ethylene controls fruit ripening and plant senescence. It also mediates biotic stress and numerous other environmental responses (Merchante et al., 2013). Abscisic acid controls seed germination, stomatal movement and is tightly connected with diverse abiotic and biotic stress responses (Nakashima and Yamaguchi-Shinozaki, 2013). Salicylic acid (SA), jasmonate and ethylene primarily act in biotic stress protection. There is ample evidence for the existence of substantial crosstalk between plant hormone defense pathways (De Torres Zabala et al., 2009; Robert-Seilaniantz et al., 2011a; Boatwright and Pajerowska-Mukhtar, 2013). These reports highlighted the importance of the plant's need to dynamically balance absolute and relative levels of phytohormones. A complex and comprehensive review on plant hormones and pathogen response was published very recently (Denance et al., 2013).
Agrobacteria largely shift the “hormone balance” in their infected hosts. This effect on endogenous growth regulators will ultimately lead to agrobacterium-induced tumor formation. An elaborate study provided an insight into Agrobacterium-induced phytohormonal changes, and it allowed the researchers to separate tumor-dependent and-independent host responses. Lee et al. (2009) examined the physiological changes and adaptations during tumor development provoked by an oncogenic strain (C58) or a disarmed derivate (GV3101), which only lacks the T-DNA but not the Vir factors (VirD2, VirE2, VirE3, VirF) (Holsters et al., 1980). The oncogenic strain was found to cause much stronger host responses than the disarmed strain. The authors monitored the kinetics of Agrobacterium-induced concentration changes of plant hormones, including SA, ethylene, jasmonic acid and indole-3-acetic acid (IAA, the most important auxin). In parallel, they assessed transcriptional changes, with a focus on hormone biosynthesis genes. At the early stage of infection, IAA and ethylene started to accumulate, while later, after T-DNA integration, primarily SA levels increased.
In the subsequent sections particular attention is given to the roles of auxin and SA in the agrobacterium/plant interaction.
Auxin
Auxin-controlled processes are tightly linked to the intracellular auxin gradient. As reviewed recently (Korbei and Luschnig, 2011), this asymmetric hormone distribution arises from polar deployment and intracellular trafficking of auxin carriers. The stability and activity of these auxin transport proteins, in turn, is controlled by a number of post-translational modifications (Lofke et al., 2013; Rahman, 2013).
Upon its perception by a small number of F-box proteins, auxin rapidly induces the expression of two types of transcriptional regulators, encoded by the aux/IAA and ARF (auxin response factor) gene families. In fact, each physiological response might result from the combinatorial interaction between individual members of these two families (Kim et al., 1997). ARFs directly induce or repress the transcription of their target genes that contain auxin responsive elements in the promoter. By binding to their partner ARFs, aux/IAA proteins keep ARFs in an inactive state. In the presence of auxin, this inhibition is released by degradation of the aux/IAA protein. Recent comprehensive reviews on these principles of auxin responses can e.g., be found in (Korbei and Luschnig, 2011; Lofke et al., 2013; Rahman, 2013).
Several plant pathogens interfere with auxin signaling. This interference can occur at several levels. For instance, Pseudomonas syringae was shown to alter Arabidopsis auxin physiology via its type III effector protein AvrRpt2 (Cui et al., 2013). In this scenario, AvrRpt2 promotes auxin response by stimulating the turnover of aux/IAA proteins, the key negative transcriptional regulators in auxin signaling. Furthermore, some P. syringae strains were found to produce auxin themselves (Glickmann et al., 1998).
miR393 as regulator of auxin signaling and bactericide synthesis
Agrobacteria employ an impressive strategic repertoire to manipulate host auxin levels and signal transduction. First, auxin is one of the T-DNA products introduced by oncogenic A. tumefaciens (Weiler and Schroder, 1987). Because auxin stimulates cell growth and gall formation, T-DNA-based auxin biosynthesis serves the pathogen directly in remodeling its host. Attacked host plants, on the other hand, try to evade or at least restrict this remodeling. They employ a gene silencing-based mechanism involving production of a particular micro RNA. miR393 targets three major auxin receptors (F-box proteins TIR1, AFB2, AFB3) and contributes to antibacterial resistance (Navarro et al., 2006). Increased levels of miR393 were found in C58-infiltrated zones, but not in areas infiltrated with the disarmed control (Pruss et al., 2008). miR393 appears to be a versatile instrument to keep pathogen invasion in check. miR393 expression is induced by the PAMP-derived peptide flg22 (Robert-Seilaniantz et al., 2011b). Notably, flagellin sequences from Agrobacterium (as well as Rhizobium) are exceptionally divergent from this PTI-triggering conserved 22-amino-acid motif (Felix et al., 1999). Arabidopsis plants overexpressing miR393 have a higher resistance to biotrophic pathogens (Robert-Seilaniantz et al., 2011b). The authors showed that miR393/auxin-related resistance is due to interference with another hormone pathway, SA. Generally, auxin and SA act as negative and positive regulators of plant defense, respectively (Denance et al., 2013). These opposing effects are largely due to the repressive effect of auxin on SA levels and signaling, although auxin also represses defense in an SA-pathway-independent manner (Kazan and Manners, 2009; Mutka et al., 2013). As proposed by (Robert-Seilaniantz et al., 2011b), miR393 represses auxin signaling and thereby prevents auxin from antagonizing SA signaling. Infection studies with auxin signaling mutants furthermore indicated that the auxin-regulated transcription factor ARF9 induces accumulation of camalexin, but represses accumulation of glucosinolate (Robert-Seilaniantz et al., 2011b). Compared to camalexin, glucosinolates are considered more effective protectants against biotrophic invaders. Therefore, miR393-related stabilization of ARF9 in inactive complexes may present a means to shift camalexin toward glucosinolate production. Whether miR393 synthesis upon agrobacterial attack “only” serves to repress auxin-related callus growth or whether it has additional functions in the defense remains to be established. As noticed recently, naturally high contents of glucosinolates per se are no obstacle to transformation. Tropaeolum majus, a glucosinolate-rich plant of the order Brassicales, is transformed by agro-infiltration of leaves (GV3101, disarmed strain) to high efficiency (Pitzschke, 2013b).
Besides camalexin and glucosinolates, plants produce various other secondary metabolites to defend themselves against biotrophic pathogens. Agrobacteria can defy at least one major group of bactericides. Several phenolic compounds are enzymatically converted by the agrobacterial protein VirH; and a virH2 mutant was found to be more susceptible to growth inhibition by these substances (Brencic et al., 2004).
One member of the bactericidal polyamines deserves special attention, putrescine. A recent study (Kim et al., 2013) documented that putrescine accumulation is controlled by MAPK signaling involving MPK3 and MPK6. In Arabidopsis, ADC genes, encoding key enzymes for putrescine biosynthesis, are induced by infection with P. syringae. adc-deficient mutants are impaired in P. syringae-induced PR1 expression. Disease susceptibility in these mutants can be recovered by exogenous putrescine. ADC transcript and putrescine levels are elevated in transgenic Arabidopsis plants expressing a constitutively active MAPK3/6 regulatory kinase in the wild-type background. In the mpk3 or mpk6 mutant background, however, this effect is largely reduced. An earlier study in tobacco had shown that plants accumulate putrescine derivatives also to combat agrobacterial infection. Auxin likely is involved in this response (Galis et al., 2004). It remains elusive whether P. syringae- and A. tumefaciens-induced putrescine synthesis are mediated by a common MPK3/MPK6 signaling pathway.
Salicylic Acid
Plants produce SA in response to pathogen attack or microbial elicitors. Mutants with constitutively elevated SA levels are generally more resistant toward biotrophic pathogens (Boatwright and Pajerowska-Mukhtar, 2013). Previously, SA was shown to attenuate A. tumefaciens-induced tumors (Yuan et al., 2007; Anand et al., 2008). Additional experimental data documented that the antagonism of auxin to SA responses (see above) is reciprocal. SA represses expression of several auxin-related genes. Moreover, by stabilizing Aux/IAA proteins, SA inhibits auxin responses (Wang et al., 2007). Elevated SA levels were observed in Arabidopsis stalks during later stages (>6 dpi) of agrobacterial infection, indicating defense activation. This response was provoked by both the oncogenic (C58) and the disarmed strain (GV3101) (Lee et al., 2009). However, Arabidopsis stems infected with C58 contained higher levels of SA, which further increased in 35-day-old tumors. The authors (Lee et al., 2009) also found that high SA levels in mutant plants (npr1, cpr5) prevented tumor development, while low levels promoted it (nahG, eds1, pad4). One specific role of SA in the Agrobacterium-plant interaction is its inhibitory effect on vir gene expression, which is accomplished by shut-down of the vir regulon (Yuan et al., 2007). What is more, SA indirectly interferes with pathogen multiplication by activating the expression of quormone-degrading enzymes (Yuan et al., 2007). In summary, SA appears to counteract agrobacterial invasion at several levels. It represses vir regulon genes (Yuan et al., 2007; Anand et al., 2008) and induces quormone-quenching genes (Yuan et al., 2007). Furthermore, SA antagonises auxin responses (Wang et al., 2007) and acts as antimicrobial agent (Gershon and Parmegiani, 1962). Interestingly, SA accumulation in Agrobacterium-infected Arabidopsis stalks was not accompanied by the induction of SA-responsive pathogenesis-related genes (3 h, 6 d, 35 dpi tested) (Lee et al., 2009). This effect is different from what is known from other plant-pathogen interactions and from pharmacological studies. Generally, in pathogen-infected plants, elevated SA synthesis triggers PR gene expression. Likewise, PR genes are induced by exogenous application of SA or its analog BTH (Lawton et al., 1996). Despite the lack of PR gene induction, SA does play a role in agrobacterial infection, as evidenced by the altered tumor size in SA-deficient/accumulating mutants (Yuan et al., 2007; Lee et al., 2009). Apparently, A. tumefaciens cannot prevent SA accumulation, but it can suppress some SA-related defense responses. As suggested by (Lee et al., 2009), abnormally high SA levels in the host may have overextended the agrobacterial control machinery.
A recent comprehensive survey of Arabidopsis transcriptome profiling data (including diverse stress treatments and biotic stress signaling mutants sid2, npr1, coi1, ein2) provided a deeper insight into the SA/PR gene relation (Gruner et al., 2013). In P syringae-treated Arabidopsis, PR1 expression fully depends on (isochorismate-synthase1) ICS1-mediated SA biosynthesis and on (non-expressor of PR1) NPR1-mediated downstream signaling. PR1 is not induced by exogenous hydrogen peroxide, abscisic acid or flg22, and it is independent of jasmonic acid and ethylene signaling (Gruner et al., 2013).
The small set of genes induced by Agrobacterium (strain C58: 35genes; strain GV3101: 28 genes) (Lee et al., 2009) is in striking contrast to the high number (948) of elicitor-responsive (EF-Tu-derived peptide elf26) transcripts. Agrobacteria clearly dampen host responses (Lee et al., 2009). This dampening is not restricted to the transcriptional level. Histological analysis (using diaminobenzidine) revealed that agrobacteria efficiently repressed H2O2 accumulation in wounded stalks over several days post-infection. The agrobacterial interference with the host's redox-regulatory machinery is also mirrored by the differential expression of several oxidative-stress-related genes (Ditt et al., 2001; Veena Jiang et al., 2003; Lee et al., 2009). By repressing H2O2 production agrobacteria may also avoid activation of ROS-dependent defense genes. Given the known sensitivity of any living cell to reactive oxygen species (ROS), the blocking of accumulation appears an agrobacterial strategy to protect both itself and its living food source, i.e., the host.
Plant Attempts to Repress Oncogene Expression
Plants exhibit an admirable perseverance in their battle against microbial manipulation. Even after unsuccessful attempts to escape Agrobacterium-induced genetic re-programming, the host cell does not surrender. Instead, transformed cells employ gene silencing mechanisms to limit the levels of T-DNA-derived transcripts. Evidence for the involvement of post-transcriptional gene silencing had been provided in a pioneering work by Dunoyer et al. (2006). Small interfering RNAs (siRNAs) directed against T-DNA oncogenes (tryptophan 2-monooxygenase and agropine synthase) were detected in Nicotiana benthamiana leaves 3 days after infiltration with virulent agrobacteria. Additional experiments in Arabidopsis further stressed the importance of gene silencing as a disease-limiting strategy. RNA interference-deficient mutant plants (rdr6, lacking a RNA-dependent RNA polymerase) were found to be hypersusceptible to agrobacterial infection, as evidenced by extensive tumor formation (Dunoyer et al., 2006). The researchers also conducted infection studies in leaves and stems of Nicotiana bethamiana carrying a post-transcriptionally-silenced reporter gene (green fluorescent protein, GFP). This approach enabled them to show that the siRNA protection strategy against T-DNA genes is efficient only at early stages of infection: Strong green fluorescence, high GFP mRNA concentrations and low siRNA concentrations were detected specifically in young tumors. Later in the infection process, the pathogen takes command. By specifically inhibiting siRNA synthesis, agrobacteria induce an anti-silencing state—thereby ensuring oncogene expression and tumor maturation (Dunoyer et al., 2006).
A more recent study furthermore documented that DNA methylation also plays a critical role in the regulation of T-DNA transcript levels (Gohlke et al., 2013). The authors compared the methylation pattern of mock- and Agrobacterium-inoculated Arabidopsis inflorescence stalks on a genome-wide level. Four-week-old tumors, arising from inoculation with the oncogenic A. tumefaciens strain C58 contained a globally hypermethylated genome. Intriguingly, a specifically low degree of methylation was observed in T-DNA-derived oncogenes (Ipt IaaH, IaaM). Data obtained from experiments with DNA methylation mutants lead to the conclusion that crown gall formation and oncogene expression correlate with the unmethylated state and, consequently that hypermethylation is a strategy to inhibit plant tumor growth.
Recalcitrance to Agrobacterium-Mediated Transformation
Agrobacterium naturally has a wide host range in plants, primarily dicot species. Driven by the demand for higher yields and improved stress tolerance the accessibility to transformation has become a prime issue in crop science. Despite intensive research it is still poorly understood why some plant species can be transformed easily, while others are recalcitrant to Agrobacterium-mediated transformation. Transformation methods of model plants and important crop species are frequently updated, documenting the striving for simpler, more robust and more efficient protocols (reviewed in e.g., Pitzschke, 2013a). These protocols primarily focus on optimizing the conditions of Agrobacterium—explant co-incubation. Here, duration, light conditions and the concentration of supplemented acetosyringone and plant hormones are key parameters.
One central message emerges from enumerable transformation studies. The outcome of co-cultivation is primarily determined by the timing and intensity at which host defense responses are activated. Understanding the molecular language of the plant—Agrobacterium dialogue is therefore of substantial interest both to basic research and agricultural science.
Studies that compare different cultivars of the same species are particularly informative, and one such study shall be mentioned here. Transformation efficiencies between rice cultivars differ greatly. The indica variety lags far behind the japonica cultivars. A comparative study of the two cultivars in transient and stable transformation assays revealed that the lower transformation efficiency in indica rice was mainly due to less-efficient T-DNA integration into the host genome (Tie et al., 2012). Microarray analyses (1, 6, 12, and 24 h post-infection) revealed major differences in the Agrobacterium-induced changes in transcriptome profiles of the two cultivars. These differences were most pronounced at the early stages of infection (within the first 6 h). The authors observed an overall stronger response in the indica cultivar (Zs), with several genes being repressed, and they postulated that some of these genes may be required for the transformation process. From this study, one may conclude that (1) although T-DNA integration represents a late step in the transformation process, the “decision” that leads to failure or success is made early. This decision is made in a narrow time window, since many Zs-specific transcripts are repressed only transiently (at the 1 OR 6 h time-point only). (2) Agrobacteria manage to actively prevent repression of integration-assisting genes in the susceptible cultivar. Among others, gene ontology (GO) annotations “stress-responsive” and “lipid transport” are overrepresented in the group of indica-specific transcripts. The lower T-DNA integration efficiency in the indica cultivar may also be attributable to the specific repression of genes related to DNA damage repair. This assumption is in good agreement with the importance of the host DNA repair machinery in T-DNA integration reported earlier (Li et al., 2005b; Citovsky et al., 2007).
The Role of Reactive Oxygen Species in Recalcitrance
A promising approach for converting hitherto non-transformable plant species is to determine the basis of this recalcitrance. Poor transformation rates can have entirely different reasons. As outlined above, bacterial and host factors contribute and need to be well-balanced. In pro- and eukaryotic organisms alike, ROS play important roles in the transmission of information. ROS- and MAPK signaling in plants is strongly inter-connected (Pitzschke and Hirt, 2009; Meng and Zhang, 2013). Because high ROS levels trigger cell death, their targeted stress-dependent production serves host organisms to restrict pathogen spread. Inappropriate ROS concentration or distribution can therefore be a barrier to successful transformation. For instance, recalcitrance in Hypericum perforatum (St. John's wart; medicinal herb), cell cultures was found to be due to an early oxidative burst, which killed 99% of the co-cultivated agrobacteria within 12 h of infection. Interestingly, the oxidative burst only affected agrobacterial viability but did not trigger plant apopotosis (Franklin et al., 2008). Antimicrobial factors likely also have a negative effect on transformation efficiency and agrobacterial viability in H. perforatum. A 12-fold increase in xanthone levels was observed in H. perforatum cells 1 day after infection. Increased xanthone levels correlated with an elevated antimicrobial and antioxidative competence. On the basis of these observations one may conclude that the plant can divert its antioxidant capacity to prevent itself, but not the invader, from oxidative damage.
One known agrobacterial factor determining oxidative resistance levels is the ferric uptake regulator Fur. A fur-deficient mutant was found to be hypersensitive to H2O2 and to have reduced catalase activity (a H2O2-detoxifying enzyme). Agrobacterial fur mutants were also compromized in tumorigenesis on tobacco leaves (Kitphati et al., 2007). Similarly, A. tumefaciens mutants in the RirA gene (rhizobial iron regulator; repressor of iron uptake) exhibited a peroxide-sensitive phenotype and were impaired in tumor formation on tobacco. In addition, induction of the virulence genes virB and virE was reduced in rirA mutants (Ngok-Ngam et al., 2009). Furthermore, A. tumefaciens mutants affected in oxidative stress tolerance have been characterized, e.g., mbfA (membrane-bound ferritin) (Ruangkiattikul et al., 2012).
The above examples document the vital importance of ROS balancing for both invader and invaded cell. It is tempting to speculate that, the reduced tumor formation in the fur/tobacco and rirA/tobacco interaction is caused by the poor viability of agrobacteria in a ROS-rich environment of infected host cells. Such a scenario would be in analogy to the situation in H. perforatum (Franklin et al., 2008), At this point, concerted efforts of microbiologists and plant biologists are needed to systematically define the proportion and identity of ROS-related agrobacterial factors playing a limiting role in plant transformation.
Another recalcitrant species of agricultural importance that has attracted attention is grapevine (Vitis vinifera). Proteomic profiling in grapevine calli grown in the absence or presence of agrobacteria allowed identification of 38 differentially expressed proteins (Zhao et al., 2011). ROS scavenging enyzmes were down-regulated in co-cultivated cells (ascorbate peroxidase, tocopherol cyclase). The authors concluded that low transformation rates and extensive necrosis in A. tumefaciens-treated grapevine derive from an impaired ROS scavenging system and an over-activation of apoptotic/hypersensitive response pathways.
Approaches to Overcome Recalcitrance
Because strong and prolonged host defense responses generally correlate with reduced transformation success (Figure 2), external attenuation of these responses may be a means to improve transformation efficiencies. The experimental approaches that can be taken to manipulate host defenses are as manifold as the defense strategies themselves. The problem can be tackled from different sides: (1) by using modified agrobacterial strains that elicit a weaker defense, as e.g., shown in a study on potato (Vences-Guzman et al., 2013); (2) by modifying the composition of plant media and/or growth conditions to keep defense levels low, e.g., Zhang et al. (2013a); (3) by transient and targeted manipulation of the plants non-self-recognition machinery (see below); (4) by counteracting the effect of antimicrobial substances. This strategy proved successful in tea, where L-glutamine was found to overcome the bactericidity of polyphenols (Sandal et al., 2007).
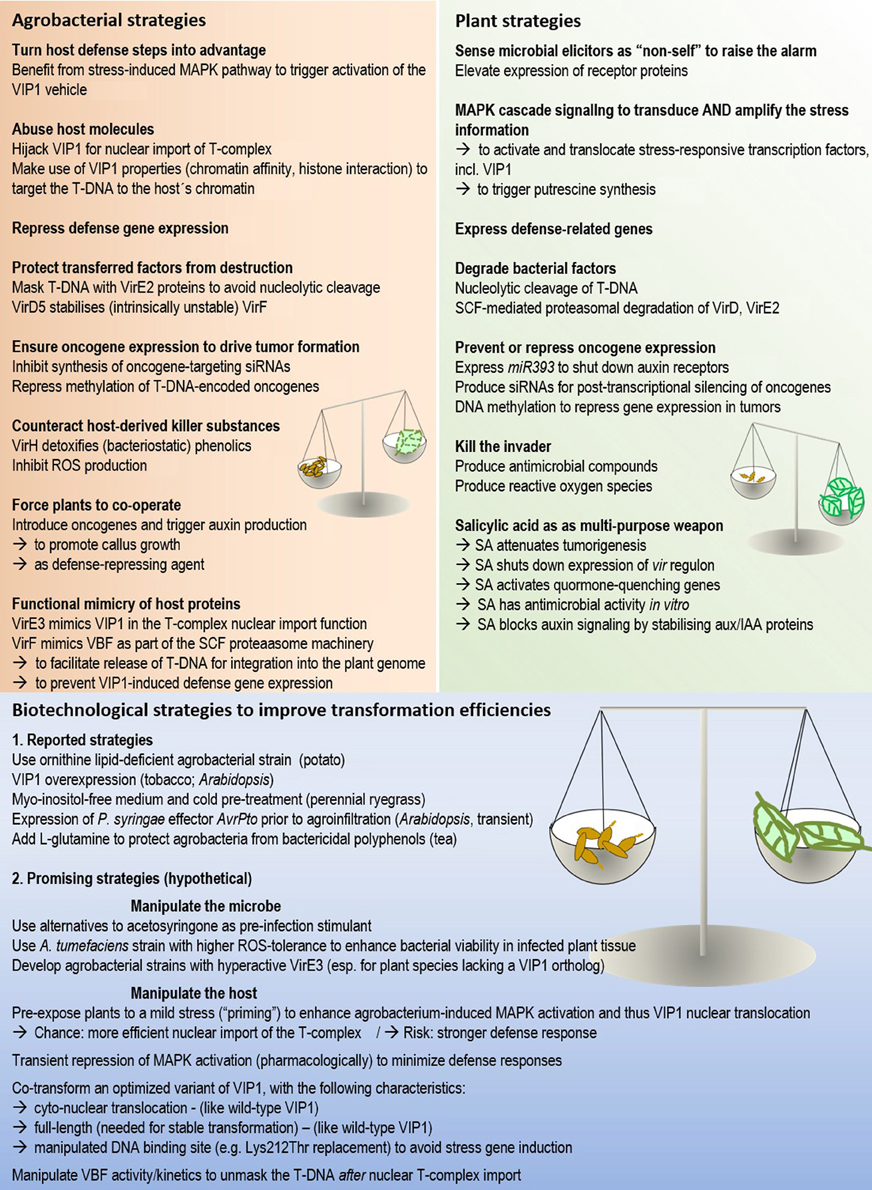
Figure 2. The molecular arms race between host and microbe in Agrobacterium-mediated plant transformation. The activities of both partners need to be well-balanced for successful transformation. Numbers in brackets refer to the corresponding sections in the manuscript.
In an innovative study Tsuda and colleagues demonstrated how detailed knowledge on plant-microbe interactions can be employed for successful transformation. AvrPto encodes an effector protein from the bacterial plant pathogen Pseudomonas syringae. The protein suppresses plant immunity by interfering with plant immune receptors. The AvrPto gene was placed under the control of a dexamethasone-inducible promoter. In transgenic Arabidopsis plants carrying the inducible construct, dexamethasone pre-treatment largely improved transformation in agro-infiltrated leaves (Tsuda et al., 2012).
An entirely different “pre-treatment strategy” proved successful in perennial ryegrass (Lolium perenne L.) (Zhang et al., 2013a). Stable transformants were obtained at an impressively high rate (84%), and 60% of the transgenic calli were regenerated into green plantlets. This was achieved by combining two strategies, while either treatment alone had little effect (10–20% transformation efficiency): (1) Myo-inositol, a component of many standard media, was removed from the callus culture medium. (2) A cold shock pre-treatment was applied prior to agrobacterial infection.
Myo-inositol levels in plants are primarily controlled by a specific oxygenase, which catalyses the first step in the conversion of this sugar into plant cell wall polysaccharides (Endres and Tenhaken, 2009). The basis of the effect observed by Zhang and colleagues is still largely elusive. It appears that myo-inositol acts in different ways and at multiple levels: omission of myo-inositol promoted Agrobacterium binding to the cell surface. It also repressed H2O2 production in infected tissue. One indirect consequence of ROS production, callus browning, could furthermore be suppressed when including the cold pre-treatment (Zhang et al., 2013a). Worthwhile questions are: Does growth of cold-pre-treated calli on myo-inositol-free medium alter cell wall composition to support agrobacterial attraction, invasion and/or survival in L. perenne cells? If so, what is the critical difference? Can such favorable cell wall characteristics be imitated to facilitate agrobacterial transformation of other recalcitrant species?
Conclusions
The molecular battle between agrobacteria and plants is impressive, instructive and challenging (Figure 2). Impressive, because the arms race takes so many forms. Instructive, because discoveries from Agrobacterium-plant interaction studies may drive progress in other fields of microbe-host association research. Challenging, because the external conditions that permit or prohibit transformation including transgene expression are diverse, and the balance needs to be determined empirically. The current state of research provides substantial breeding ground for plant scientists to search for this balance in their favorite species in a more targeted manner.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
I gratefully acknowledge the Austrian Science Foundation for financial support (FWF Elise-Richter project V167-B09). I thank Helene Persak, Jürgen Kleine-Vehn and Barbara Korbei for critical reading of the manuscript. Constructive and valuable comments of two anonymous reviewers helped to improve the manuscript.
References
Anand, A., Uppalapati, S. R., Ryu, C. M., Allen, S. N., Kang, L., Tang, Y., et al. (2008). Salicylic acid and systemic acquired resistance play a role in attenuating crown gall disease caused by Agrobacterium tumefaciens. Plant Physiol. 146, 703–715. doi: 10.1104/pp.107.111302
Andrews, L. B., and Curtis, W. R. (2005). Comparison of transient protein expression in tobacco leaves and plant suspension culture. Biotechnol. Prog. 21, 946–952. doi: 10.1021/bp049569k
Benjamins, R., and Scheres, B. (2008). Auxin: the looping star in plant development. Annu. Rev. Plant Biol. 59, 443–465. doi: 10.1146/annurev.arplant.58.032806.103805
Boatwright, J. L., and Pajerowska-Mukhtar, K. (2013). Salicylic acid: an old hormone up to new tricks. Mol. Plant Pathol. 14, 623–634. doi: 10.1111/mpp.12035
Boller, T., and Felix, G. (2009). A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 60, 379–406. doi: 10.1146/annurev.arplant.57.032905.105346
Boller, T., and He, S. Y. (2009). Innate immunity in plants: an arms race between pattern recognition receptors in plants and effectors in microbial pathogens. Science 324, 742–744. doi: 10.1126/science.1171647
Bonardi, V., and Dangl, J. L. (2012). How complex are intracellular immune receptor signaling complexes? Front. Plant Sci. 3:237. doi: 10.3389/fpls.2012.00237
Boutrot, F., Segonzac, C., Chang, K. N., Qiao, H., Ecker, J. R., Zipfel, C., et al. (2010). Direct transcriptional control of the Arabidopsis immune receptor FLS2 by the ethylene-dependent transcription factors EIN3 and EIL1. Proc. Natl. Acad. Sci. U.S.A. 107, 14502–14507. doi: 10.1073/pnas.1003347107
Brencic, A., Eberhard, A., and Winans, S. C. (2004). Signal quenching, detoxification and mineralization of vir gene-inducing phenolics by the VirH2 protein of Agrobacterium tumefaciens. Mol. Microbiol. 51, 1103–1115. doi: 10.1046/j.1365-2958.2003.03887.x
Bundock, P., Den Dulk-Ras, A., Beijersbergen, A., and Hooykaas, P. J. (1995). Trans-kingdom T-DNA transfer from Agrobacterium tumefaciens to saccharomyces cerevisiae. EMBO J. 14, 3206–3214.
Cascales, E., and Christie, P. J. (2003). The versatile bacterial type IV secretion systems. Nat. Rev. Microbiol. 1, 137–149. doi: 10.1038/nrmicro753
Christie, P. J., and Vogel, J. P. (2000). Bacterial type IV secretion: conjugation systems adapted to deliver effector molecules to host cells. Trends Microbiol. 8, 354–360. doi: 10.1016/S0966-842X(00)01792-3
Citovsky, V., Kapelnikov, A., Oliel, S., Zakai, N., Rojas, M. R., Gilbertson, R. L., et al. (2004). Protein interactions involved in nuclear import of the Agrobacterium VirE2 protein in vivo and in vitro. J. Biol. Chem. 279, 29528–29533. doi: 10.1074/jbc.M403159200
Citovsky, V., Kozlovsky, S. V., Lacroix, B., Zaltsman, A., Dafny-Yelin, M., Vyas, S., et al. (2007). Biological systems of the host cell involved in Agrobacterium infection. Cell. Microbiol. 9, 9–20. doi: 10.1111/j.1462-5822.2006.00830.x
Cui, F., Wu, S., Sun, W., Coaker, G., Kunkel, B., He, P., et al. (2013). The Pseudomonas syringae type III effector AvrRpt2 promotes pathogen virulence via stimulating Arabidopsis auxin/indole acetic acid protein turnover. Plant Physiol. 162, 1018–1029. doi: 10.1104/pp.113.219659
Dafny-Yelin, M., Levy, A., and Tzfira, T. (2008). The ongoing saga of Agrobacterium-host interactions. Trends Plant Sci. 13, 102–105. doi: 10.1016/j.tplants.2008.01.001
Dash, S., Van Hemert, J., Hong, L., Wise, R. P., and Dickerson, J. A. (2012). PLEXdb: gene expression resources for plants and plant pathogens. Nucleic Acids Res. 40, D1194–D1201. doi: 10.1093/nar/gkr938
Denance, N., Sanchez-Vallet, A., Goffner, D., and Molina, A. (2013). Disease resistance or growth: the role of plant hormones in balancing immune responses and fitness costs. Front. Plant Sci. 4:155. doi: 10.3389/fpls.2013.00155
De Torres Zabala, M., Bennett, M. H., Truman, W. H., and Grant, M. R. (2009). Antagonism between salicylic and abscisic acid reflects early host-pathogen conflict and moulds plant defence responses. Plant J. 59, 375–386. doi: 10.1111/j.1365-313X.2009.03875.x
Ditt, R. F., Nester, E., and Comai, L. (2005). The plant cell defense and Agrobacterium tumefaciens. FEMS Microbiol. Lett. 247, 207–213. doi: 10.1016/j.femsle.2005.05.010
Ditt, R. F., Nester, E. W., and Comai, L. (2001). Plant gene expression response to Agrobacterium tumefaciens. Proc. Natl. Acad. Sci. U.S.A. 98, 10954–10959. doi: 10.1073/pnas.191383498
Djamei, A., Pitzschke, A., Nakagami, H., Rajh, I., and Hirt, H. (2007). Trojan horse strategy in Agrobacterium transformation: abusing MAPK defense signaling. Science 318, 453–456. doi: 10.1126/science.1148110
Dunoyer, P., Himber, C., and Voinnet, O. (2006). Induction, suppression and requirement of RNA silencing pathways in virulent Agrobacterium tumefaciens infections. Nat. Genet. 38, 258–263. doi: 10.1038/ng1722
Endres, S., and Tenhaken, R. (2009). Myoinositol oxygenase controls the level of myoinositol in Arabidopsis, but does not increase ascorbic acid. Plant Physiol. 149, 1042–1049. doi: 10.1104/pp.108.130948
Engledow, A. S., Medrano, E. G., Mahenthiralingam, E., Lipuma, J. J., and Gonzalez, C. F. (2004). Involvement of a plasmid-encoded type IV secretion system in the plant tissue watersoaking phenotype of Burkholderia cenocepacia. J. Bacteriol. 186, 6015–6024. doi: 10.1128/JB.186.18.6015-6024.2004
Felix, G., Duran, J. D., Volko, S., and Boller, T. (1999). Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 18, 265–276. doi: 10.1046/j.1365-313X.1999.00265.x
Franklin, G., Conceicao, L. F., Kombrink, E., and Dias, A. C. (2008). Hypericum perforatum plant cells reduce Agrobacterium viability during co-cultivation. Planta 227, 1401–1408. doi: 10.1007/s00425-008-0691-7
Galis, I., Kakiuchi, Y., Simek, P., and Wabiko, H. (2004). Agrobacterium tumefaciens AK-6b gene modulates phenolic compound metabolism in tobacco. Phytochemistry 65, 169–179. doi: 10.1016/j.phytochem.2003.10.015
Gassmann, W., and Bhattacharjee, S. (2012). Effector-triggered immunity signaling: from gene-for-gene pathways to protein-protein interaction networks. Mol. Plant Microbe Interact. 25, 862–868. doi: 10.1094/MPMI-01-12-0024-IA
Gelvin, S. B. (2003). Agrobacterium-mediated plant transformation: the biology behind the “gene-jockeying” tool. Microbiol. Mol. Biol. Rev. 67, 16–37. doi: 10.1128/MMBR.67.1.16-37.2003
Gelvin, S. B. (2005). Agricultural biotechnology: gene exchange by design. Nature 433, 583–584. doi: 10.1038/433583a
Gelvin, S. B. (2009). Agrobacterium in the genomics age. Plant Physiol. 150, 1665–1676. doi: 10.1104/pp.109.139873
Gelvin, S. B. (2010a). Finding a way to the nucleus. Curr. Opin. Microbiol. 13, 53–58. doi: 10.1016/j.mib.2009.11.003
Gelvin, S. B. (2010b). Plant proteins involved in Agrobacterium-mediated genetic transformation. Annu. Rev. Phytopathol. 48, 45–68. doi: 10.1146/annurev-phyto-080508-081852
Gelvin, S. B. (2012). Traversing the cell: Agrobacterium T-DNA's journey to the host genome. Front. Plant Sci. 3:52. doi: 10.3389/fpls.2012.00052
Gershon, H., and Parmegiani, R. (1962). Antimicrobial activity of 8-quinolinols, salicylic acids, hydroxynaphthoic acids, and salts of selected quinolinols with selected hydroxy-acids. Appl. Microbiol. 10, 348–353.
Glickmann, E., Gardan, L., Jacquet, S., Hussain, S., Elasri, M., Petit, A., et al. (1998). Auxin production is a common feature of most pathovars of Pseudomonas syringae. Mol. Plant Microbe Interact. 11, 156–162. doi: 10.1094/MPMI.1998.11.2.156
Gohlke, J., Scholz, C. J., Kneitz, S., Weber, D., Fuchs, J., Hedrich, R., et al. (2013). DNA methylation mediated control of gene expression is critical for development of crown gall tumors. PLoS ONE Genet. 9:e1003267. doi: 10.1371/journal.pgen.1003267
Gruner, K., Griebel, T., Navarova, H., Attaran, E., and Zeier, J. (2013). Reprogramming of plants during systemic acquired resistance. Front. Plant Sci. 4:252. doi: 10.3389/fpls.2013.00252
Holsters, M., Silva, B., Van Vliet, F., Genetello, C., De Block, M., Dhaese, P., et al. (1980). The functional organization of the nopaline a tumefaciens plasmid pTiC58. Plasmid 3, 212–230. doi: 10.1016/0147-619X(80)90110-9
Holsters, M., Silva, B., Van Vliet, F., Hernalsteens, J. P., Genetello, C., Van Montagu, M., et al. (1978). In vivo transfer of the ti-plasmid of Agrobacterium tumefaciens to Escherichia coli. Mol. Gen. Genet. 163, 335–338. doi: 10.1007/BF00271963
Huang, G. T., Ma, S. L., Bai, L. P., Zhang, L., Ma, H., Jia, P., et al. (2012). Signal transduction during cold, salt, and drought stresses in plants. Mol. Biol. Rep. 39, 969–987. doi: 10.1007/s11033-011-0823-1
Hwang, S. G., Kim, D. S., and Jang, C. S. (2011). Comparative analysis of evolutionary dynamics of genes encoding leucine-rich repeat receptor-like kinase between rice and Arabidopsis. Genetica 139, 1023–1032. doi: 10.1007/s10709-011-9604-y
Jung, C., Seo, J. S., Han, S. W., Koo, Y. J., Kim, C. H., Song, S. I., et al. (2008). Overexpression of AtMYB44 enhances stomatal closure to confer abiotic stress tolerance in transgenic Arabidopsis. Plant Physiol. 146, 623–635. doi: 10.1104/pp.107.110981
Kazan, K., and Manners, J. M. (2009). Linking development to defense: auxin in plant-pathogen interactions. Trends Plant Sci. 14, 373–382. doi: 10.1016/j.tplants.2009.04.005
Kim, J., Harter, K., and Theologis, A. (1997). Protein-protein interactions among the Aux/IAA proteins. Proc. Natl. Acad. Sci. U.S.A. 94, 11786–11791. doi: 10.1073/pnas.94.22.11786
Kim, S. H., Yoo, S. J., Min, K. H., Nam, S. H., Cho, B. H., and Yang, K. Y. (2013). Putrescine regulating by stress-responsive MAPK cascade contributes to bacterial pathogen defense in Arabidopsis. Biochem. Biophys. Res. Commun. 437, 502–508. doi: 10.1016/j.bbrc.2013.06.080
Kitphati, W., Ngok-Ngam, P., Suwanmaneerat, S., Sukchawalit, R., and Mongkolsuk, S. (2007). Agrobacterium tumefaciens fur has important physiological roles in iron and manganese homeostasis, the oxidative stress response, and full virulence. Appl. Environ. Microbiol. 73, 4760–4768. doi: 10.1128/AEM.00531-07
Korbei, B., and Luschnig, C. (2011). Cell polarity: PIN it down! Curr. Biol. 21, R197–R199. doi: 10.1016/j.cub.2011.01.062
Kunik, T., Tzfira, T., Kapulnik, Y., Gafni, Y., Dingwall, C., and Citovsky, V. (2001). Genetic transformation of HeLa cells by Agrobacterium. Proc. Natl. Acad. Sci. U.S.A. 98, 1871–1876. doi: 10.1073/pnas.98.4.1871
Lacroix, B., and Citovsky, V. (2013). Characterization of VIP1 activity as a transcriptional regulator in vitro and in planta. Sci. Rep. 3:2440. doi: 10.1038/srep02440
Lacroix, B., Li, J., Tzfira, T., and Citovsky, V. (2006). Will you let me use your nucleus? how Agrobacterium gets its T-DNA expressed in the host plant cell. Can. J. Physiol. Pharmacol. 84, 333–345. doi: 10.1139/y05-108
Lacroix, B., Loyter, A., and Citovsky, V. (2008). Association of the Agrobacterium T-DNA-protein complex with plant nucleosomes. Proc. Natl. Acad. Sci. U.S.A. 105, 15429–15434. doi: 10.1073/pnas.0805641105
Lacroix, B., Vaidya, M., Tzfira, T., and Citovsky, V. (2005). The VirE3 protein of Agrobacterium mimics a host cell function required for plant genetic transformation. EMBO J. 24, 428–437. doi: 10.1038/sj.emboj.7600524
Lawton, K. A., Friedrich, L., Hunt, M., Weymann, K., Delaney, T., Kessmann, H., et al. (1996). Benzothiadiazole induces disease resistance in Arabidopsis by activation of the systemic acquired resistance signal transduction pathway. Plant J. 10, 71–82. doi: 10.1046/j.1365-313X.1996.10010071.x
Lechner, E., Achard, P., Vansiri, A., Potuschak, T., and Genschik, P. (2006). F-box proteins everywhere. Curr. Opin. Plant Biol. 9, 631–638. doi: 10.1016/j.pbi.2006.09.003
Lee, C. W., Efetova, M., Engelmann, J. C., Kramell, R., Wasternack, C., Ludwig-Muller, J., et al. (2009). Agrobacterium tumefaciens promotes tumor induction by modulating pathogen defense in Arabidopsis thaliana. Plant Cell 21, 2948–2962. doi: 10.1105/tpc.108.064576
Li, J. F., Park, E., Von Arnim, A. G., and Nebenfuhr, A. (2009). The FAST technique: a simplified Agrobacterium-based transformation method for transient gene expression analysis in seedlings of Arabidopsis and other plant species. Plant Methods 5:6. doi: 10.1186/1746-4811-5-6
Li, J., Krichevsky, A., Vaidya, M., Tzfira, T., and Citovsky, V. (2005a). Uncoupling of the functions of the Arabidopsis VIP1 protein in transient and stable plant genetic transformation by Agrobacterium. Proc. Natl. Acad. Sci. U.S.A. 102, 5733–5738. doi: 10.1073/pnas.0404118102
Li, J., Vaidya, M., White, C., Vainstein, A., Citovsky, V., and Tzfira, T. (2005b). Involvement of KU80 in T-DNA integration in plant cells. Proc. Natl. Acad. Sci. U.S.A. 102, 19231–19236. doi: 10.1073/pnas.0506437103
Ljung, K. (2013). Auxin metabolism and homeostasis during plant development. Development 140, 943–950. doi: 10.1242/dev.086363
Lofke, C., Luschnig, C., and Kleine-Vehn, J. (2013). Posttranslational modification and trafficking of PIN auxin efflux carriers. Mech. Dev. 130, 82–94. doi: 10.1016/j.mod.2012.02.003
Lu, X., Tintor, N., Mentzel, T., Kombrink, E., Boller, T., Robatzek, S., et al. (2009). Uncoupling of sustained MAMP receptor signaling from early outputs in an Arabidopsis endoplasmic reticulum glucosidase II allele. Proc. Natl. Acad. Sci. U.S.A. 106, 22522–22527. doi: 10.1073/pnas.0907711106
Magori, S., and Citovsky, V. (2011a). Agrobacterium counteracts host-induced degradation of its effector F-box protein. Sci. Signal. 4, ra69. doi: 10.1126/scisignal.2002124
Magori, S., and Citovsky, V. (2011b). Hijacking of the host SCF ubiquitin ligase machinery by plant pathogens. Front. Plant Sci. 2:87. doi: 10.3389/fpls.2011.00087
Markmann, K., and Parniske, M. (2009). Evolution of root endosymbiosis with bacteria: How novel are nodules? Trends Plant Sci. 14, 77–86. doi: 10.1016/j.tplants.2008.11.009
Meng, X., and Zhang, S. (2013). MAPK cascades in plant disease resistance signaling. Annu. Rev. Phytopathol. 51, 245–266. doi: 10.1146/annurev-phyto-082712-102314
Merchante, C., Alonso, J. M., and Stepanova, A. N. (2013). Ethylene signaling: simple ligand, complex regulation. Curr. Opin. Plant Biol. 16, 554–560. doi: 10.1016/j.pbi.2013.08.001
Michielse, C. B., Hooykaas, P. J., Van Den Hondel, C. A., and Ram, A. F. (2005). Agrobacterium-mediated transformation as a tool for functional genomics in fungi. Curr. Genet. 48, 1–17. doi: 10.1007/s00294-005-0578-0
Mutka, A. M., Fawley, S., Tsao, T., and Kunkel, B. N. (2013). Auxin promotes susceptibility to Pseudomonas syringae via a mechanism independent of suppression of salicylic acid-mediated defenses. Plant J. 74, 746–754. doi: 10.1111/tpj.12157
Nakagami, H., Pitzschke, A., and Hirt, H. (2005). Emerging MAP kinase pathways in plant stress signalling. Trends Plant Sci. 10, 339–346. doi: 10.1016/j.tplants.2005.05.009
Nakashima, K., and Yamaguchi-Shinozaki, K. (2013). ABA signaling in stress-response and seed development. Plant Cell Rep. 32, 959–970. doi: 10.1007/s00299-013-1418-1
Navarro, L., Dunoyer, P., Jay, F., Arnold, B., Dharmasiri, N., Estelle, M., et al. (2006). A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science 312, 436–439. doi: 10.1126/science.1126088
Ngok-Ngam, P., Ruangkiattikul, N., Mahavihakanont, A., Virgem, S. S., Sukchawalit, R., and Mongkolsuk, S. (2009). Roles of Agrobacterium tumefaciens RirA in iron regulation, oxidative stress response, and virulence. J. Bacteriol. 191, 2083–2090. doi: 10.1128/JB.01380-08
Nurnberger, T., Brunner, F., Kemmerling, B., and Piater, L. (2004). Innate immunity in plants and animals: striking similarities and obvious differences. Immunol. Rev. 198, 249–266. doi: 10.1111/j.0105-2896.2004.0119.x
Parniske, M. (2008). Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nat. Rev. Microbiol. 6, 763–775. doi: 10.1038/nrmicro1987
Persak, H., and Pitzschke, A. (2013). Tight interconnection and multi-level control of Arabidopsis MYB44 in MAPK cascade signalling. PLoS ONE 8:e57547. doi: 10.1371/journal.pone.0057547
Pitzschke, A. (2013a). From bench to barn: plant model research and its applications in agriculture. Adv. Genet. Eng. 2, 1–9. doi: 10.4172/2169-0111.1000110
Pitzschke, A. (2013b). Tropaeolum tops tobacco - simple and efficient transgene expression in the order brassicales. PLoS ONE 8:e73355. doi: 10.1371/journal.pone.0073355
Pitzschke, A., and Hirt, H. (2009). Disentangling the complexity of mitogen-activated protein kinases and reactive oxygen species signaling. Plant Physiol. 149, 606–615. doi: 10.1104/pp.108.131557
Pitzschke, A., and Hirt, H. (2010a). Mechanism of MAPK-targeted gene expression unraveled in plants. Cell Cycle 9, 18–19. doi: 10.4161/cc.9.1.10329
Pitzschke, A., and Hirt, H. (2010b). New insights into an old story: Agrobacterium-induced tumour formation in plants by plant transformation. EMBO J. 29, 1021–1032. doi: 10.1038/emboj.2010.8
Pitzschke, A., and Persak, H. (2012). Poinsettia protoplasts - a simple, robust and efficient system for transient gene expression studies. Plant Methods 8, 14. doi: 10.1186/1746-4811-8-14
Pitzschke, A., Djamei, A., Bitton, F., and Hirt, H. (2009a). A major role of the MEKK1-MKK1/2-MPK4 pathway in ROS signalling. Mol. Plant 2, 120–137. doi: 10.1093/mp/ssn079
Pitzschke, A., Djamei, A., Teige, M., and Hirt, H. (2009b). VIP1 response elements mediate mitogen-activated protein kinase 3-induced stress gene expression. Proc. Natl. Acad. Sci. U.S.A. 106, 18414–18419. doi: 10.1073/pnas.0905599106
Pitzschke, A., Schikora, A., and Hirt, H. (2009c). MAPK cascade signalling networks in plant defence. Curr. Opin. Plant Biol. 12, 421–426. doi: 10.1016/j.pbi.2009.06.008
Pruss, G. J., Nester, E. W., and Vance, V. (2008). Infiltration with Agrobacterium tumefaciens induces host defense and development-dependent responses in the infiltrated zone. Mol. Plant Microbe Interact. 21, 1528–1538. doi: 10.1094/MPMI-21-12-1528
Rahman, A. (2013). Auxin: a regulator of cold stress response. Physiol. Plant. 147, 28–35. doi: 10.1111/j.1399-3054.2012.01617.x
Rasmussen, M. W., Roux, M., Petersen, M., and Mundy, J. (2012). MAP kinase cascades in Arabidopsis innate immunity. Front. Plant Sci. 3:169. doi: 10.3389/fpls.2012.00169
Robert-Seilaniantz, A., Grant, M., and Jones, J. D. (2011a). Hormone crosstalk in plant disease and defense: more than just jasmonate-salicylate antagonism. Annu. Rev. Phytopathol. 49, 317–343. doi: 10.1146/annurev-phyto-073009-114447
Robert-Seilaniantz, A., Maclean, D., Jikumaru, Y., Hill, L., Yamaguchi, S., Kamiya, Y., et al. (2011b). The microRNA miR393 re-directs secondary metabolite biosynthesis away from camalexin and towards glucosinolates. Plant J. 67, 218–231. doi: 10.1111/j.1365-313X.2011.04591.x
Rodriguez, M. C., Petersen, M., and Mundy, J. (2010). Mitogen-activated protein kinase signaling in plants. Annu. Rev. Plant Biol. 61, 621–649. doi: 10.1146/annurev-arplant-042809-112252
Rohila, J. S., and Yang, Y. (2007). Rice mitogen-activated protein kinase gene family and its role in biotic and abiotic stress response. J. Integr. Plant Biol. 49, 751–759. doi: 10.1111/j.1744-7909.2007.00501.x
Ruangkiattikul, N., Bhubhanil, S., Chamsing, J., Niamyim, P., Sukchawalit, R., and Mongkolsuk, S. (2012). Agrobacterium tumefaciens membrane-bound ferritin plays a role in protection against hydrogen peroxide toxicity and is negatively regulated by the iron response regulator. FEMS Microbiol. Lett. 329, 87–92. doi: 10.1111/j.1574-6968.2012.02509.x
Sanabria, N., Goring, D., Nurnberger, T., and Dubery, I. (2008). Self/nonself perception and recognition mechanisms in plants: a comparison of self-incompatibility and innate immunity. New Phytol. 178, 503–514. doi: 10.1111/j.1469-8137.2008.02403.x
Sandal, I., Saini, U., Lacroix, B., Bhattacharya, A., Ahuja, P. S., and Citovsky, V. (2007). Agrobacterium-mediated genetic transformation of tea leaf explants: effects of counteracting bactericidity of leaf polyphenols without loss of bacterial virulence. Plant Cell Rep. 26, 169–176. doi: 10.1007/s00299-006-0211-9
Schell, J., and Van Montagu, M. (1977). Transfer, maintenance, and expression of bacterial Ti-plasmid DNA in plant cells transformed with A. tumefaciens. Brookhaven Symp. Biol. 36–49.
Schweighofer, A., Kazanaviciute, V., Scheikl, E., Teige, M., Doczi, R., Hirt, H., et al. (2007). The PP2C-type phosphatase AP2C1, which negatively regulates MPK4 and MPK6, modulates innate immunity, jasmonic acid, and ethylene levels in Arabidopsis. Plant Cell 19, 2213–2224. doi: 10.1105/tpc.106.049585
Shah, J., and Zeier, J. (2013). Long-distance communication and signal amplification in systemic acquired resistance. Front. Plant Sci. 4:30. doi: 10.3389/fpls.2013.00030
Silipo, A., Erbs, G., Shinya, T., Dow, J. M., Parrilli, M., Lanzetta, R., et al. (2010). Glyco-conjugates as elicitors or suppressors of plant innate immunity. Glycobiology 20, 406–419. doi: 10.1093/glycob/cwp201
Sinha, A. K., Jaggi, M., Raghuram, B., and Tuteja, N. (2011). Mitogen-activated protein kinase signaling in plants under abiotic stress. Plant Signal. Behav. 6, 196–203. doi: 10.4161/psb.6.2.14701
Tanoue, T., and Nishida, E. (2003). Molecular recognitions in the MAP kinase cascades. Cell. Signal. 15, 455–462. doi: 10.1016/S0898-6568(02)00112-2
Thieme, F., Koebnik, R., Bekel, T., Berger, C., Boch, J., Buttner, D., et al. (2005). Insights into genome plasticity and pathogenicity of the plant pathogenic bacterium Xanthomonas campestris pv. vesicatoria revealed by the complete genome sequence. J. Bacteriol. 187, 7254–7266. doi: 10.1128/JB.187.21.7254-7266.2005
Tie, W., Zhou, F., Wang, L., Xie, W., Chen, H., Li, X., et al. (2012). Reasons for lower transformation efficiency in indica rice using Agrobacterium tumefaciens-mediated transformation: lessons from transformation assays and genome-wide expression profiling. Plant Mol. Biol. 78, 1–18. doi: 10.1007/s11103-011-9842-5
Tsuda, K., Qi, Y., Nguyen, L. V., Bethke, G., Tsuda, Y., Glazebrook, J., et al. (2012). An efficient Agrobacterium-mediated transient transformation of Arabidopsis. Plant J. 69, 713–719. doi: 10.1111/j.1365-313X.2011.04819.x
Tzfira, T., Vaidya, M., and Citovsky, V. (2002). Increasing plant susceptibility to Agrobacterium infection by overexpression of the Arabidopsis nuclear protein VIP1. Proc. Natl. Acad. Sci. U.S.A. 99, 10435–10440. doi: 10.1073/pnas.162304099
Veena Jiang, H., Doerge, R. W., and Gelvin, S. B. (2003). Transfer of T-DNA and Vir proteins to plant cells by Agrobacterium tumefaciens induces expression of host genes involved in mediating transformation and suppresses host defense gene expression. Plant J. 35, 219–236. doi: 10.1046/j.1365-313X.2003.01796.x
Vences-Guzman, M. A., Guan, Z., Bermudez-Barrientos, J. R., Geiger, O., and Sohlenkamp, C. (2013). Agrobacteria lacking ornithine lipids induce more rapid tumour formation. Environ. Microbiol. 15, 895–906. doi: 10.1111/j.1462-2920.2012.02867.x
Wang, D., Pajerowska-Mukhtar, K., Culler, A. H., and Dong, X. (2007). Salicylic acid inhibits pathogen growth in plants through repression of the auxin signaling pathway. Curr. Biol. 17, 1784–1790. doi: 10.1016/j.cub.2007.09.025
Weiler, E. W., and Schroder, J. (1987). Hormone genes and crown gall disease. Trends Biochem. Sci. 12, 271–275. doi: 10.1016/0968-0004(87)90133-2
Yuan, Z. C., Edlind, M. P., Liu, P., Saenkham, P., Banta, L. M., Wise, A. A., et al. (2007). The plant signal salicylic acid shuts down expression of the vir regulon and activates quormone-quenching genes in Agrobacterium. Proc. Natl. Acad. Sci. U.S.A. 104, 11790–11795. doi: 10.1073/pnas.0704866104
Zaltsman, A., Krichevsky, A., Kozlovsky, S. V., Yasmin, F., and Citovsky, V. (2010a). Plant defense pathways subverted by Agrobacterium for genetic transformation. Plant Signal. Behav. 5, 1245–1248. doi: 10.4161/psb.5.10.12947
Zaltsman, A., Krichevsky, A., Loyter, A., and Citovsky, V. (2010b). Agrobacterium induces expression of a host F-box protein required for tumorigenicity. Cell Host Microbe. 7, 197–209. doi: 10.1016/j.chom.2010.02.009
Zaltsman, A., Lacroix, B., Gafni, Y., and Citovsky, V. (2013). Disassembly of synthetic Agrobacterium T-DNA-protein complexes via the host SCF(VBF) ubiquitin-ligase complex pathway. Proc. Natl. Acad. Sci. U.S.A. 110, 169–174. doi: 10.1073/pnas.1210921110
Zhang, W. J., Dewey, R. E., Boss, W., Phillippy, B. Q., and Qu, R. (2013a). Enhanced Agrobacterium-mediated transformation efficiencies in monocot cells is associated with attenuated defense responses. Plant Mol. Biol. 81, 273–286. doi: 10.1007/s11103-012-9997-8
Zhang, X., Cheng, T., Wang, G., Yan, Y., and Xia, Q. (2013b). Cloning and evolutionary analysis of tobacco MAPK gene family. Mol. Biol. Rep. 40, 1407–1415. doi: 10.1007/s11033-012-2184-9
Zhao, F., Chen, L., Perl, A., Chen, S., and Ma, H. (2011). Proteomic changes in grape embryogenic callus in response to Agrobacterium tumefaciens-mediated transformation. Plant Sci. 181, 485–495. doi: 10.1016/j.plantsci.2011.07.016
Zipfel, C., Kunze, G., Chinchilla, D., Caniard, A., Jones, J. D., Boller, T., et al. (2006). Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 125, 749–760. doi: 10.1016/j.cell.2006.03.037
Zipfel, C., Robatzek, S., Navarro, L., Oakeley, E. J., Jones, J. D., Felix, G., et al. (2004). Bacterial disease resistance in Arabidopsis through flagellin perception. Nature 428, 764–767. doi: 10.1038/nature02485
Keywords: Agrobacterium tumefaciens, transformation, plant defense, reactive oxygen species, VIP1
Citation: Pitzschke A (2013) Agrobacterium infection and plant defense—transformation success hangs by a thread. Front. Plant Sci. 4:519. doi: 10.3389/fpls.2013.00519
Received: 27 September 2013; Accepted: 02 December 2013;
Published online: 18 December 2013.
Edited by:
Stanton B. Gelvin, Purdue University, USAReviewed by:
Lois Banta, Williams College, USASaikat Bhattacharjee, Regional Centre for Biotechnology, India
Copyright © 2013 Pitzschke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea Pitzschke, Department of Applied Genetics and Cell Biology, University of Natural Resources and Applied Life Sciences, Muthgasse 18, Vienna A-1190, Austria e-mail:YW5kcmVhLnBpdHpzY2hrZUBib2t1LmFjLmF0
 Andrea Pitzschke
Andrea Pitzschke