- 1College of Life Sciences and Technology, Harbin Normal University, Harbin, China
- 2Institute of Grass Research, Heilongjiang Academy of Agricultural Sciences, Harbin, China
Cold acclimation in alfalfa (Medicago sativa L.) plays a crucial role in cold tolerance to harsh winters. To examine the cold acclimation mechanisms in freezing-tolerant alfalfa (ZD) and freezing-sensitive alfalfa (W5), holoproteins, and low-abundance proteins (after the removal of RuBisCO) from leaves were extracted to analyze differences at the protein level. A total of 84 spots were selected, and 67 spots were identified. Of these, the abundance of 49 spots and 24 spots in ZD and W5, respectively, were altered during adaptation to chilling stress. Proteomic results revealed that proteins involved in photosynthesis, protein metabolism, energy metabolism, stress and redox and other proteins were mobilized in adaptation to chilling stress. In ZD, a greater number of changes were observed in proteins, and autologous metabolism and biosynthesis were slowed in response to chilling stress, thereby reducing consumption, allowing for homeostasis. The capability for protein folding and protein biosynthesis in W5 was enhanced, which allows protection against chilling stress. The ability to perceive low temperatures was more sensitive in freezing-tolerant alfalfa compared to freezing-sensitive alfalfa. This proteomics study provides new insights into the cold acclimation mechanism in alfalfa.
Introduction
A lack of cold tolerance is a major limiting factor for crop production, especially in northern areas. Plants exposed to low but non-freezing temperatures have developed strategies for adapting to stress and enhance cold tolerance via many complex physiological and biochemical changes. Such adaptations are primarily attributed to changes in the expression of functional and regulatory genes (Gomat et al., 2011). Numerous studies have demonstrated that cold acclimation is associated with structural and compositional modifications of compatible solutes in various subcellular compartments and changes in the transcriptome and metabolome (Knaupp et al., 2011; Zuther et al., 2012; Vaclavik et al., 2013). Although cold acclimation mechanisms have been studied for many years, the molecular and genetic foundations of this adaptation in plants remain largely unknown.
Proteomics bridges the gap between gene expression and metabolism (Heazlewood, 2011; Carrol et al., 2013), promoting the study of adaptive mechanisms to chilling stress in many plants (Cui et al., 2005; Amme et al., 2006; Yan et al., 2006; Hashimoto and Komatsu, 2007; Degand et al., 2009; Gao et al., 2009; Lee et al., 2009; Balbuena et al., 2011; Rinalducci et al., 2011; Sánchez-Bel et al., 2012a,b; Uváčková et al., 2012; Takahashi et al., 2013). Changes in proteins play a vital role in cold adaptation due to their direct action on metabolism and biosynthesis pathways. Comparative proteomics were recently applied to analyze changes in cold-sensitive proteins in different cold-tolerant cultivars, such as meadow fescue (leaf), pea (leaf and chloroplast), perennial ryegrass (leaf), strawberry (crown), and winter wheat (leaf; Kosmala et al., 2009; Bocian et al., 2011; Dumont et al., 2011; Koehler et al., 2012; Grimaud et al., 2013; Xu et al., 2013). Proteins involved in energy metabolism, photosynthesis, reactive oxygen species (ROS) scavenging, storage, protection from stress, regulation of the cell cycle and plant development in wheat and barley showed differential abundance between stress-tolerant and stress-sensitive genotypes (Kosová et al., 2014). Proteomics can provide new insights into cold acclimation and improve our understanding of the genetic differences underlying cold tolerance in plants.
High-abundance proteins, such as ribulose-1, 5-bisphosphate carboxylase/oxygenase (RuBisCO), can mask low-abundance proteins of interest. High-abundance proteins reduce the dynamic resolution of proteins and affect the analysis of some functional proteins in proteomic studies. In protein extracts from Arabidopsis and rice leaf, ∼12 and 35.3% of the spots, respectively, have been identified as RuBisCO and its derivatives (Giavalisco et al., 2005; Yan et al., 2006). RuBisCO accounts for a high percentage in abundance in green leaf tissue due to protein load capacity limitations (Xi et al., 2006); co-migration with RuBisCO also masks neighboring species (Corthalis et al., 2000; Krishnan and Natarajan, 2009). To overcome this problem, a polyethylene glycol (PEG) mediated pre-fractionation method was used to remove RuBisCO; this protocol depletes most RuBisCO and their derivatives (Kim et al., 2001).
As leguminous forage, alfalfa contributes to biological nitrogen fixation and is widely planted throughout the world. In northern climates, fall dormant cultivars have a greater capability to resist harsh winters than non-dormant varieties (Timmons and Salmon, 1932; Sprague and Fuelleman, 1941; Smith et al., 1979). It has been established that freezing-tolerant and freezing-sensitive cultivars of alfalfa share similar cold regulated (COR) gene complements; differences exist in the rate and extent of the expression of these genes in response to cold. COR genes are induced by low temperature and their translation products can mechanistically protect plants against environmental stresses (Seki et al., 2004). Marked differences were found between propagated clones from genotypes by comparing their gene products, which confirmed that variations in molecular changes that occur at low temperature are under some level of genetic control (Castonguay et al., 2006). Castonguay et al. (2006) hypothesized that some of COR genes fail to be expressed in a timely manner in response to environmental cues in freezing-sensitive alfalfa. The ability to perceive and transduce external signals into a series of molecular events leading to physiological responses differs widely among genotypes within a species (Kaur and Gupta, 2005). However, differences in the cold acclimation mechanisms between freezing-tolerant and freezing-sensitive alfalfa remain unknown. In our study, holoproteins and low-abundance proteins were extracted from alfalfa, and changes in protein categories and relative abundances during chilling were analyzed. Differences in the cold acclimation mechanisms in alfalfa would be first discussed at the protein level using proteomics in our study.
Materials and Methods
Plant Material and Culture Conditions
Two alfalfa cultivars with different freezing tolerances were studied: freezing-tolerant cultivar ZhangDong (ZD), which is fall dormant alfalfa (winter resistant cultivar), and freezing-sensitive cultivar WL525HQ (W5), which is non-dormant alfalfa (winter irresistant cultivar). ZD and W5 seeds were germinated under controlled environmental conditions as follows: 280 μmol/m2/s; 16 h light/8 h dark; and a day/night temperature regime of 25/20°C. For the cold treatment, 50 days old seedlings were transferred to a cold chamber set to 4°C for 7 days under other conditions as described above.
Protein Preparation
Leaves (1 g) from both cultivars were sampled at 0 (control), 12 h, and 7 days under cold treatment and were immediately frozen in liquid nitrogen.
Holoproteins extraction
For holoproteins extraction, frozen samples were pulverized in a pre-cooled mortar with liquid nitrogen. Samples were then suspended in three volumes of pre-cooled 10% (w/v) trichloroacetic acid (TCA)/acetone with 0.07% (v/v) β-mercaptoethanol and kept at –20°C overnight. The homogenate was centrifuged at 40,000 ×g for 1 h at 4°C. The supernatant was removed, and the precipitate was resuspended in three volumes of cold acetone containing 0.07% (v/v) β-mercaptoethanol. The mixture was incubated at –20°C for at least 1 h and centrifuged at 40,000 ×g for 1 h at 4°C. The aforementioned steps were repeated until the supernatant was colorless. The vacuum-dried pellet was dissolved in 2 ml of a lysis solution containing 7 M urea, 2 M thiourea, 4% (w/v) 3-[(3-Cholamidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS), 40 mM dithiothreitol (DTT), and 1% (v/v) protease inhibitors (PMSF). The sample was shaken at 4°C for more than 1 h. The mixture was centrifuged at 100,000 ×g for 1 h at 4°C to remove any solids. The protein concentration was quantified using a 2D-Quant kit, and the protein solution was stored at –80°C.
Low-abundance proteins extraction
Low-abundance proteins were extracted with Mg/NP-40 buffer and fractionated by PEG 4000 (PEG4000), as described by Kim et al. (2001) with slight modifications. Frozen leaves were ground in a pre-cooled mortar with liquid nitrogen. Five milliliters Mg/NP-40 buffer containing 0.5 M Tris-HCl (pH 8.3), 2% (v/v) NP-40, 20 mM MgCl2, 2% (v/v) β-mercaptoethanol, 1 mM PMSF, 1 mM ethylene diamine tetraacetic acid (EDTA) and 1% (w/v) polyvinylpolypyrrolidone (PVPP) was added, and the sample was ground on ice for 15 min. After centrifugation at 12,000 ×g for 15 min at 4°C, a 50% (w/v) PEG4000 stock solution was added to the supernatant to a final PEG concentration of 17.5% (w/v). The mixture was incubated on ice for 30 min and centrifuged at 12,000 ×g for 15 min at 4°C. Three volumes of pre-cooled 10% (w/v) TCA/acetone was added to the supernatant and kept at –20°C overnight. The remaining steps were performed according to the holoproteins extraction method described above.
The quantified holoprotein and low-abundance protein samples were loaded into an SDS-PAGE gel [12.5% (w/v)] and run for ∼2.5 h. The gel was used to determine the quality of proteins and remove RuBisCO.
Two-Dimensional Gel Electrophoresis
Quantified protein solutions were diluted in rehydration buffer containing 8 M urea, 2% (w/v) CHAPS, 0.3% (w/v) DTT, and 1% (v/v) IPG buffer. IEF strips (24 cm, linear pH 4–7) were rehydrated in 450 μl of rehydration protein solution at 20°C for 12 h in an Ettan IPGphor 3 electrophoresis system (GE Healthcare). IEF was run using the following parameters: 200 V for 2 h, 500 V for 2 h, 1000 V for 2 h, 8000 V for 3 h, and 8000 V for 65000 VH. After IEF, the strips were equilibrated twice in equilibration buffer [6 M urea, 1.5 M Tris-HCl (pH 8.8), 30% (v/v) glycerol, and 2% (w/v) SDS] with 1% (w/v) DTT for 15 min and then with 4% (w/v) iodoacetamide for 15 min. For two-dimensional electrophoresis, the strips were placed on a 12.5% (w/v) SDS-PAGE gel and run at 1 w per gel for 1 h, followed by 13 w per gel for 4.5–5 h. An Ettan DALTSix system (GE Healthcare) was used for two-dimensional electrophoresis. Subsequently, the gels were fixed in 40% (v/v) alcohol and 10% (v/v) acetic acid for 30 min, swollen in 10% (v/v) acetic acid for 20 min, and stained with Coomassie brilliant blue G-250. The gels were rinsed with 10% (v/v) acetic acid until the protein spots were distinct from the background. The gels were then stored in deionized water.
Image Acquisition, Data Analysis, and Protein Identification
Gels were scanned using an ImageScanner III (GE Healthcare), and images were analyzed using ImageMaster 2D Platinum v7.0 software (GE Healthcare). Gels of three independent biological replicates per treatment were analyzed. Spots were automatically detected and matched, and mismatched and unmatched spots were artificially modified through manual editing. The spot intensities were normalized according to total intensity of valid spots to reduce the differences in the protein loading and gel staining. Tukey’s test (P < 0.05) was applied to test the abundance change of the spots. Only spots with volume ratios over 1.5-fold (P < 0.05) were selected for MS identification.
Each marked protein was cut from the gel and cleaved with trypsin. Peptide identification was performed using a 5800 MALDI-TOF/TOF mass spectrometer (AB SCIEX) according to the protocol described by Kosová et al. (2013). The obtained peak list was used to search the databases NCBI Medicago (3967), downloaded on June 12, 2014, NCBI Viridiplantae (973373), downloaded on September 13, 2013, and Uniprot (540732), downloaded on September 3, 2013, using MASCOT V2.2 software. Database searches were conducted using the following parameters: peptide mass tolerance ± 100 ppm; fragment mass tolerance ± 0.4 Da; a maximum of one missed cleavage; cysteine carbamidomethylation allowed as a fixed modification; and oxidation of methionine allowed as a dynamical modification. Only significant hits, as defined by the MASCOT probability analysis (P < 0.05) with a protein score CI % greater than 95 and a protein score above 50, were accepted. A functional classification of proteins was performed based on the Gene Ontology database1 and the Uniprot database2.
Statistic Analysis
The data shown in image analyses (holoproteins and low-abundance) and the differential abundance of proteins in the three samples under evaluation (control, 12 h and 7 days) were both compared by analysis of variance (ANOVA) followed by Tukey’s multiple comparison test (P < 0.05). Cluster analysis was used to show identified proteins visually changed in the relative abundance using the software PermutMatrix v1.9.3 (Caraux and Pinloche, 2005). The average values of three biological replicates were used to compare the protein changes among different treatments.
Results
Image Analyses of Holoproteins and Low-Abundance Proteins of alfalfa
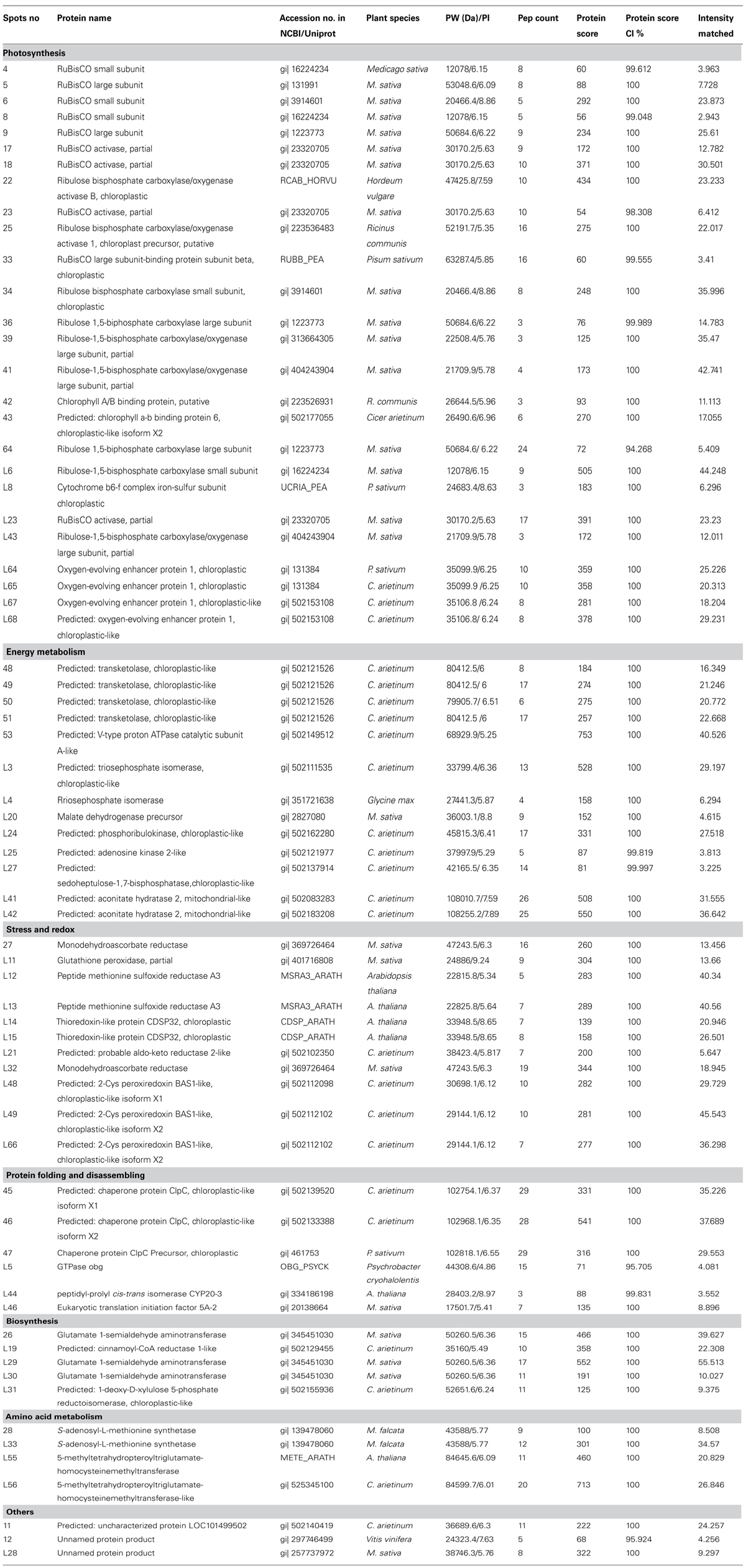
In our study, both holoproteins and low-abundance proteins of alfalfa were extracted to analyze protein changes during chilling stress. The SDS-PAGE result (Figure 1) revealed that 17.5% PEG could remove most RuBisCO large subunits; new electrophoretic bands containing low-abundance proteins were clearly detected. Image analysis revealed approximately 498 ZD holoprotein spots and 516 W5 holoprotein spots (Figure 2A). Approximately 423 spots were detected in the ZD low-abundance proteome, of which 308 spots were unique to low-abundance versus holoproteins; the ratio of low-abundance protein spots to holoprotein spots was ∼61.85%. In the W5 proteome, this ratio was 64.13%; approximately 320 of 499 spots were unique to low-abundance versus holoproteins (Figure 2B). PEG fractionation was useful for the resolution of low-abundance proteins. The detection of new low-abundance proteins aided our analysis of protein variations and adaptive mechanisms during chilling.
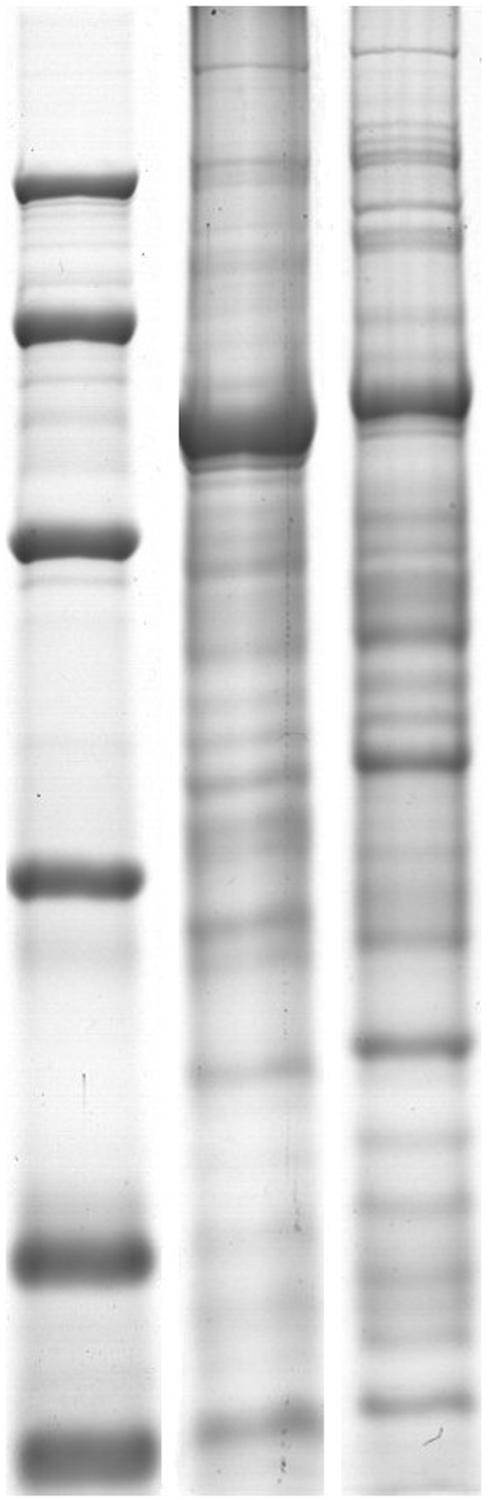
FIGURE 1. SDS-PAGE of contrast of holoproteins and low-abundant proteins. (A) Are holoproteins extracted by TCA/acetone. (B) Are low-abundant proteins extracted by PEG. 12.5% SDS-PAGE gel showing RuBisCO depletion. RuBisCO large subunit (LSU) and small subunit (SSU) are marked.
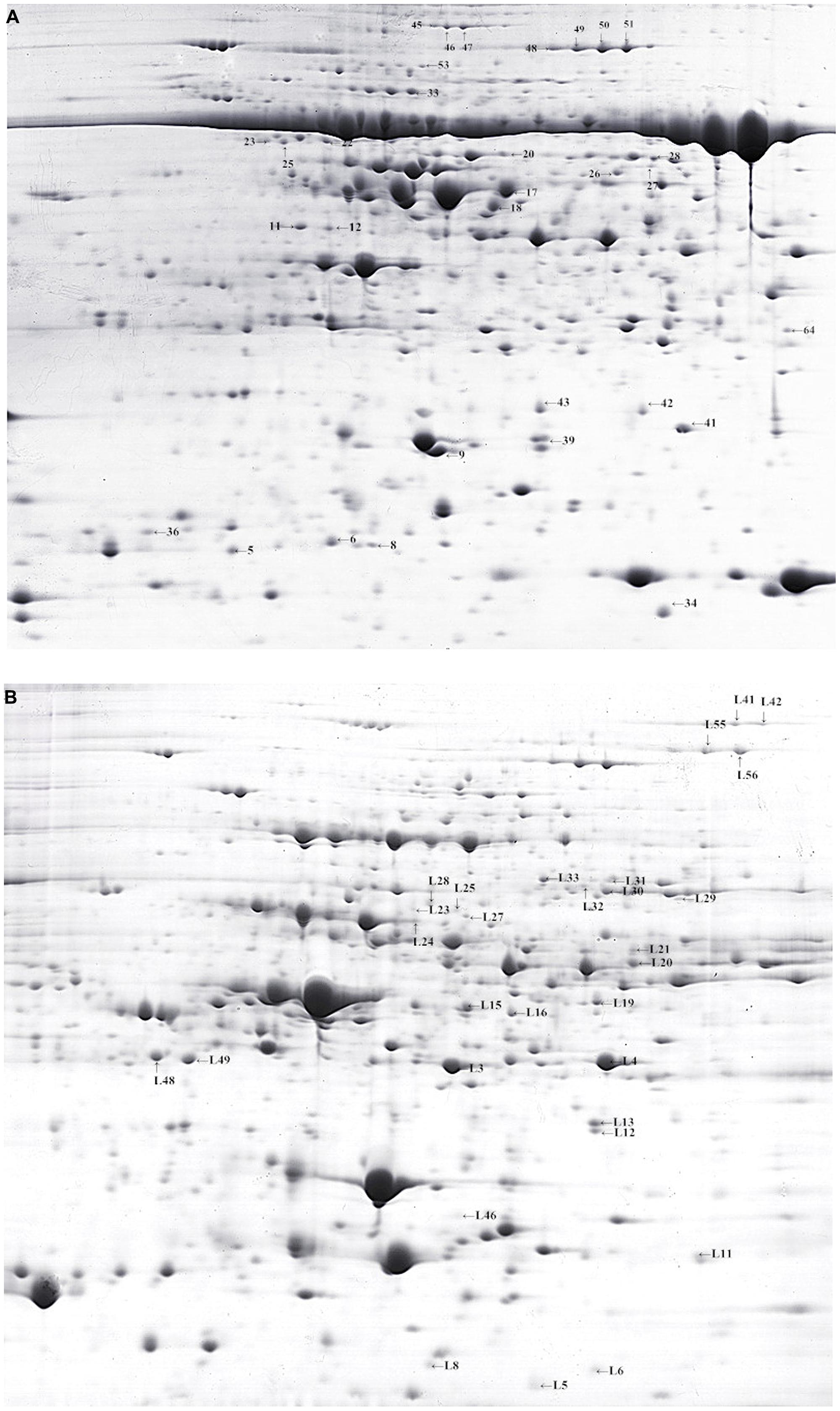
FIGURE 2. Two-dimensional gel electrophoresis of Medicago sativa leaves (control of W5). (A,B) 2-DE gels showing identified spots of holoproteins and low-abundant proteins, respectively.
Proteomic Differences in alfalfa in Response to Low Temperature
To detect differences in cold acclimation between freezing-tolerant alfalfa and freezing-sensitive alfalfa, the variety and relative abundance of proteins were analyzed in two alfalfa cultivars (ZD and W5) in three different growth conditions (control, 4°C for 12 h, and 4°(C for 7days). Holoproteins and low-abundance proteins were extracted from leaves. Two-dimensional gel electrophoresis (2-DE) revealed that a total of 84 spots were differentially expressed at ratios over 1.5-fold in relation to cold acclimation. Of these, 67 spots were successfully identified by MALDI_TOF/TOF (Table 1). Clustering analysis (Figure 3) was used to visually describe changes of 67 spots in relative abundance during chilling using PermutMatrix software v1.9.3. Compared with W5, more spots were identified in ZD as responding to low temperature: the relative abundance of 49 spots changed in ZD, whereas 24 spots changed in W5. Of these, 15 spots changed in both cultivars in response to low temperature. Compared with controls, chilling (12 h) caused a significant up-accumulation of 13 spots in ZD and six spots in W5, and 34 spots were down-accumulated in ZD (spots 17 and 28 unchanged) and 14 spots in W5 (spots 17, 28, L55 and L56 unchanged). Compared with chilling for 12 h, chilling for 7 days caused an up-accumulation of seven spots in ZD (spots 4, 18, 26, 33, L25, L28, and L46) and five spots (spots 4, 18, 26, L46 and L68), 13 spots (spots 17, 28, L3∼L4, L12∼L15, L19, L29, L33, and L55∼L56) in ZD and eight spots (spots 48∼51, L43 and L48∼L49) in W5 were down-regulated. In additional, nine unchanged spots were found in W5 with a greater relative abundance than in ZD. Chilling affected the relative abundance and variety of proteins. The number of changed proteins were more at 4°C for 12 h than for 7 days.
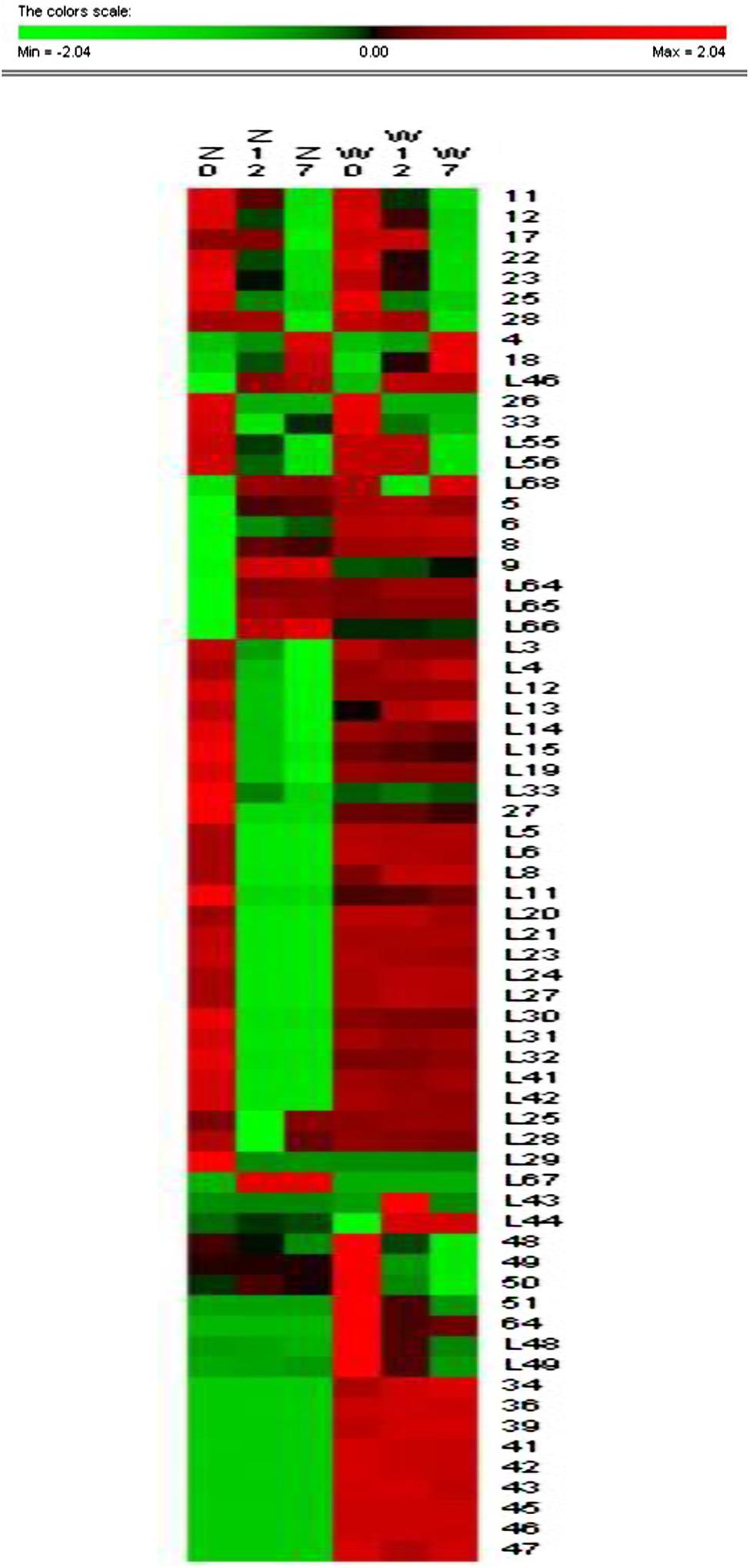
FIGURE 3. Cluster analysis of 67 identified spots. Cluster analysis reveals changes of the relative abundance of identified protein by software PermutMatrix v1.9. Normalized rows (Z scores) and Ward’s algorithm have been used to analyze data. In the figure, ZC is control of ZD, Z12 is treatment of ZD at 4°C for 12 h, Z7 is treatment of ZD at 4°C for 7 days, WC is control of W5, W12 is treatment of W5 at 4°C for 12 h, and W7 is treatment of W5 at 4°C for 7 days. Digits in the figure represent spots No.
Protein functional analysis has been carried out according to Gene Ontology database and the Uniprot database. Based on their functional features, the 67 differentially expressed proteins were classified into five categories, as follows: photosynthesis, protein metabolism, energy metabolism, stress and redox, and other functions (Figure 4 and Table 1). Among these protein spots, 11 changed in a similar manner in both ZD and W5 under cold stress. Compared with control, the relative abundance of uncharacterized protein LOC101499502 (spot 11), unnamed protein product (spot 12), and RuBisCO activase (spots 22, 23, and 25) declined at 12 h and that of RuBisCO activase (spots 17 and 28) declined at 7 days, the relative abundance of RuBisCO small subunit (spot 4), RuBisCO activase (spot 18) and eukaryotic translation initiation factor 5A-2 (eIF-5A, spot L46) continuously increased at 12 h and 7 days, and glutamate 1-semialdehyde aminotransferase (spot 26) was down-regulated at 12 h and up-regulated at 7 days. The expression patterns of RuBisCO large subunit-binding protein subunit beta (spot 33), 5-methyltetrahydropteroyltriglutamate-homocysteinemethyltransferase (spots L55 and L56), and oxygen-evolving enhancer protein (spot L68) were altered in different ways in ZD and W5 (Figure 3). During chilling, the relative abundance of the remaining 32 spots changed in ZD and remained constant in W5. Of these, RuBisCO large subunit (spots 5 and 9), RuBisCO small subunit (spots 6 and 8) and oxygen-evolving enhancer protein (spots L64∼L66) were up-regulated at 12 h, and triosephosphate isomerase (spots L3 and L4), peptide methionine sulfoxide reductase (spots L12 and L13), thioredoxin-like protein (spots L14 and L15), cinnamoyl-CoA reductase (spot L19) and S-adenosyl-L-methionine synthetase (spot L33) were continuously down-regulated at 12 h and 7 days. Monodehydroascorbate reductase (spots 27 and L32), GTPase obg (spot L5), RuBisCO small subunit (spot L6), cytochrome b6-f complex iron-sulfur subunit (spot L8), glutathione peroxidase (spot L11), malate dehydrogenase precursor (spot L20), aldo-keto reductase (spot L21), RuBisCO activase (spot L23), phosphoribulokinase (spot L24), sedoheptulose-1, 7-bisphosphatase (spot L27), glutamate 1-semialdehyde aminotransferase (spot L30), 1-deoxy-D-xylulose 5-phosphate reductoisomerase (spot L31) and aconitate hydratase 2 (spots L41 and L42) maintained the same lower relative abundance at 12 h and 7 days compared with the control. The relative abundance of adenosine kinase 2 (spot L25) and unnamed protein product (spot L28) decreased at 12 h and increased at 7 days. Two spots were expressed exclusively in ZD: the relative abundance of glutamate 1-semialdehyde aminotransferase (spot L29) decreased and that of oxygen-evolving enhancer protein (spot L67) increased. In W5, only nine proteins spots were differentially expressed and remained unchanged in ZD. RuBisCO large subunit (spot L43) and peptidyl-prolyl cis-trans isomerase (PPlase, spot L44) were up-regulated, transketolase (spots 48∼51) and 2-Cys peroxiredoxin (spots L48 and 49) were down-regulated at 12 h and 7 days, and RuBisCO large subunit (spot 64) was only down-regulated at 12 h. In additionally, RuBisCO small and large subunit (spots 34, 36, 39, and 41), chlorophyll A/B binding protein (spots 42 and 43) and chaperone protein ClpC (spots 45∼47) were only highly expressed in W5 under chilling stress.
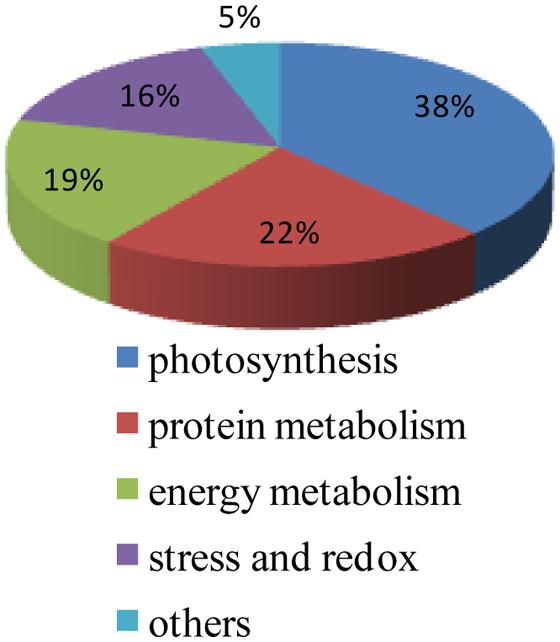
FIGURE 4. Functional classification of 67 protein spots responding to cold acclimation. The 67 indentified spots include proteins involved in photosynthesis (26 spots), protein metabolism (15 spots), energy metabolism (13 spots), stress and redox (10 spots), and other functions (three spots).
Cold acclimation in plant is a complex progress and involved in various proteins. In our study, photosynthesis related proteins were the largest category of differentially expressed in both ZD and W5. A series of changes between RuBisCO activase (spots 17, 18, 22, 23, 25, and L23) and RuBisCO large and small subunits (spots 4, 5, 6, 8, 9, 64, L6, and L43) were found in ZD and W5 in response to low temperature. Compared to freezing-sensitive alfalfa, a greater number of proteins were changed in freezing-tolerant alfalfa under chilling stress. In ZD, protein metabolism, energy metabolism and stress and redox related proteins were influenced, such as S-adenosyl-L-methionine synthetase (spot L33), GTPase obg (spot L5), glutathione peroxidase (spot L11), malate dehydrogenase precursor (spot L20), and so on. Autologous metabolism and biosynthesis were slowed to response low temperature in ZD. Increased PPIase and eIF-5A is consistent with an increased capability for protein folding and protein biosynthesis, suggesting increased protection against chilling stress in W5.
Discussion
The Removal of “Overabundant” Proteins
RuBisCO exists in most green plants and comprises 30∼60% of the total protein. RuBisCO is an important photosynthesis enzyme that fixes CO2 in the Calvin cycle (Ellis, 1979; Voet and Voet, 1995; Whitney and Andrews, 2001; Parry et al., 2003; Giavalisco et al., 2005). As an “overabundant” protein, RuBisCO influences the detection of some low-abundance proteins. PEG, at a proper concentration, effectively removes most large subunits of RuBisCO and improves the dynamic resolution of low-abundance proteins in different plant species. In Arabidopsis thaliana, 16% PEG was applied to extract low-abundance proteins using a fractionation method; 80% more spots were revealed compared to a holoprotein sample extracted using a TCA/acetone method (Xi et al., 2006). Lee et al. (2007) suggested that 15% PEG could be used for the effective extraction of low-abundance protein to analyze protein changes in rice leaves under cold stress. In our study, 17.5% PEG significantly reduced the masking effect of RuBisCO, and new proteins were detected in both ZD and W5. Thirty-five out of 67 spots were low-abundance proteins in response to chilling stress (Table 1). These data could help us to better understand cold adaptation mechanisms in alfalfa.
Proteins Changes in Proteome
Differentially expressed photosynthetic proteins
Proteomic analysis revealed that different cold acclimation mechanisms exist in ZD and W5. In some cases, the same protein exhibited differential accumulation patterns in alfalfa cultivars with different tolerance to cold stress. In our study, the largest category of proteins that responded to chilling in both ZD and W5 is photosynthesis-related proteins. It has been reported the interaction between RuBisCO activase and RuBisCO large and small subunits (Portis, 1990; Spreitzer and Salvucci, 2002). When RdreB1BI transgenic strawberries are exposed to low temperature, RuBisCO activase reactivates RuBisCO to fix any remaining CO2 and protect the dissipation of energy from the photo respiratory oxygenase reaction (Haupt-Herting et al., 2001; Gua et al., 2013). In alfalfa, RuBisCO activase (spot 18) increased in both ZD and W5; the expression of this protein was higher in ZD than that in W5 at 4°C for 7 days. A greater number of up-regulation of spots corresponding to the large and small subunits of RuBisCO (spots 4, 5, 6, 8, and 9) were found in ZD at 12 h, compared to W5 (spots 4 and L43). The abundance of the RuBisCO large subunit-binding protein subunit β (spot 33) decreased for the correct assembly of the RuBisCO holoenzyme in W5; a similar result was observed in pea (Barraclough and Ellis, 1980; Grimaud et al., 2013). Chilling stress also affected the relative abundance of RuBisCO activase (spots 17, 22, 23, 25, and L23). The relative abundance of the RuBisCO small subunit (spot L6) decreased in ZD, and RuBisCO large subunit (spot 64) decreased in W5. The disassembly of RuBisCO may reduce the photosynthetic rate under cold stress (Yan et al., 2006; An et al., 2011; Zhang et al., 2012). The series of changes between RuBisCO activase and RuBisCO large and small subunits could make the active site more accessible for carbamylation, thereby enhancing CO2 fixation to resist cold stress (Portis et al., 2008). The relative abundance of the cytochrome b6-f complex iron-sulfur subunit (spot L8) declined in ZD as in Thellungiella rosette and in a cold-tolerant Champagne cultivar of pea, which affected electron transport from PSII to PSI during cold acclimation (Gao et al., 2009; Grimaud et al., 2013); whereas its expression remained unchanged in W5. Oxygen-evolving enhancer protein 1 (spots L64, L65, L67, and L68) increased in ZD, which stabilizes the tetranuclear manganese (Mn) center that is the location of photoinhibition in PSII (Sarvikas et al., 2006). In addition to L68, abundance of the other three oxygen-tetranuclear Mn center proteins (L64, L65, and L67) was unchanged in W5. As in a freeze-tolerant genotype of Festuca pratensis, oxygen-evolving enhancer protein 1 (L68) in W5 was degraded at 12 h and accumulated at 7 days. Kosmala et al. (2009) postulated that this change may contribute to additional metabolic disturbances rather than lower susceptibility to photoinhibition.
Compared to W5, more proteins were mobilized in ZD during adaptation to low-temperature stress. The relative abundance of photosynthesis-related proteins in ZD was altered in a complex manner to relieve the influence of chilling stress on photosynthesis. However, the abundance of these proteins remained relatively stable in W5. The RuBisCO small subunit (spot 34) and large subunit (spots 36, 39, and 41) were only highly expressed in W5. The greater relative abundance of chlorophyll A/B binding protein (spots 42 and 43), a component of the light-harvesting complex, allows for the absorption of more light by chlorophyll excitation, and the transfer of energy to the photochemical reaction centers allows for adaptation to chilling stress in W5 (Green and Durnford, 1996).
Differentially expressed metabolism proteins
The category of protein metabolism involved proteins related folding and disassembling, biosynthesis and amino acid metabolism. Chilling induced eIF-5A (spot L 46) accumulation in both ZD and W5 as in wheat and Thellungiella rosette (Gao et al., 2009; Vítamìvasì et al., 2012; Kosová et al., 2013). EIF-5A is mainly involved in RNA metabolism, protein translation and the regulation of the cell cycle (Schatz et al., 1998; Thompson et al., 2004; Jao and Chen, 2006; Feng et al., 2007). Kosová et al. (2013) indicated that these changes were associated with protein expression and protein regulation and reflect more profound changes at the regulatory level in cold-treated plants. Cold affected the S-adenosylmethionine (SAM) synthetic pathway in both ZD and W5. In this pathway, methionine production is catalyzed by methionine synthase with assistance of the coenzyme 5-methyltetrahydropteroyltriglutamate-homocysteine methyltransferase (spots L55 and L56). S-adenosyl-L-methionine synthetase (spots 28 and L33) then transfers adenosine to catalyze SAM. Proteomic studies have demonstrated that SAM plays an important role in cold stress resistance (Cui et al., 2005; Kosová et al., 2013). The observed reduction in the relative abundance of spots L55 and L56 began at 12 h in ZD and at 7 days in W5. These findings suggest that cold stress affects the activity of 5-methyltetrahydropteroyltriglutamate-homocysteine methyltransferase, as reported in rice seedlings (Hashimoto and Komatsu, 2007). S-adenosyl-L-methionine synthetase was consumed to produce SAM, preventing cold injury in alfalfa, although the reduction in the coenzyme led to an insufficient supply of substrate. Chilling had a clear influence on amino acid metabolism in alfalfa, leading to a reduction in amino acid synthesis for energy conservation during low temperature adaptation.
GTPase obg (spot L5) is a conserved GTPase binding protein mainly involved in cell proliferation, cell development, signal transduction, and protein translation (Bourne et al., 1990; Kaziro et al., 1991). Through an analysis of obgc mutants in Arabidopsis and rice, Bang et al. (2012) found that plastid rRNA processing is defective, indicating that obgc functions primarily in plastid ribosome biogenesis during chloroplast development. In ZD, it was speculated that the decreased relative abundance of GTPase obg during chilling might impede the synthesis of a series of related proteins. Biosynthesis-related enzymes were also significantly influenced by cold stress. Glutamate 1-semialdehyde aminotransferase (spots 26, L29, and L30) catalyzes the conversion of glutamate-1-semialdehyde to aminolevulinic acid, which is a step in the assembly of chlorophyll, coenzyme B12, heme, and other tetrapyrrolic proteins (Jordan and Shemin, 1972; Gough et al., 2001). Cinnamoyl-CoA reductase (CCR, spot L19) catalyzes the first step in monolignol biosynthesis and plays a key role in synthesis of lignin (Zhou et al., 2010). The protein 1-deoxy-D-xylulose 5-phosphate reductoisomerase (spot L31) is a key rate-limiting enzyme and the regulatory site of terpenoid synthesis (Takahashi et al., 1998). The relative abundance of these spots decreased in ZD, and with the exception of spot 26, the expression of these spots remained unchanged in W5. Chilling stress was clearly more severe in ZD than in W5, as three biosynthetic pathways were disrupted in ZD.
In W5, the expression of PPlase (spot L44) increased under cold stress. This is similar to a finding in Populus cathayana males (Zhang et al., 2012), indicating that these proteins are involved in protein folding to overcome stress (Budiman et al., 2011). Additionally, high expression of the chaperone protein ClpC (spots 45, 46, and 47) was only observed in W5, which regulated protein metabolism and kept homeostasis. As a protein chaperone, ClpC is mainly involved in regulating the structure and function of many polypeptides and hydrolyzes irreversibly damaged proteins to prevent the accumulation of potentially cytotoxic polypeptides (Parsell and Lindquist, 1993).
Differentially expressed energy metabolism proteins
The third functional category of proteins that respond to low temperatures in alfalfa involve energy metabolism such as the Calvin cycle, Kreb’s cycle, and glycolysis pathways. Chilling had a large effect on carbon metabolism (phosphoribulokinase, spot L24, and sedoheptulose-1, 7-bisphosphatase, spot L27), ATP production (malate dehydrogenase precursor, spot L20, and aconitate hydratase 2, spots L41 and L42) and glycolysis (triosephosphate isomerase, spots L3 and L4) in ZD. Transketolase (spots 48∼51), which is involved in the Calvin cycle were down-regulated in W5. Unlike cold-tolerant pea, transketolase was more highly expressed in W5 compared to ZD, whereas this protein was constitutively expressed in ZD under cold stress (Grimaud et al., 2013). In addition, a reduction in the abundance of adenosine kinase 2 (spot L25) was shown to disturb the regulation of energy metabolism and the balance of ATP, ADT, and AMP at the beginning of chilling in ZD (Veuthey and Stucki, 1987; Zeleznikar et al., 1995). The increase in adenosine kinase 2 expression allowed for recovered activity and the maintenance of the energy balance in the cell at 7 days. We deduced that a decrease in the abundance of several enzymes leads to slowed energy metabolism as energy is stored for maintaining body balance in alfalfa.
Differentially expressed stress and redox proteins
In plants, cellular redox homeostasis can be disturbed by cold stress. Such disturbances result in the production of ROS, which stimulate oxidative damage in the organism. Therefore, certain ROS-scavenging enzymes play an important role in the scavenging of ROS and maintaining iron balance. Glutathione peroxidase (spot L11; Liu et al., 2014), thioredoxin-like protein (spots L14 and L15; Zhang et al., 2012; Kosová et al., 2013), probable aldo-keto reductase 2 (spot L21; Gao et al., 2009; Koehler et al., 2012) and 2-Cys peroxiredoxin (spots L48, L49, and L66; Yan et al., 2006; Gao et al., 2009) were up-regulated under cold stress. Their functions are mainly involved in detoxication, protection from oxidative damage, preventing the membrane lipid from peroxidation and enhancing tolerance to ROS (Bartels, 2001; Kim et al., 2011). Monodehydroascorbate reductase (spot 27 and L32) is an important enzyme involved in the regeneration of ascorbic acid for scavenging hydrogen peroxide (Arrigoni et al., 1981), and peptide methionine sulfoxide reductase A3 (spots L12 and L13) may repair oxidatively damaged proteins in vivo (Brot et al., 1982a,b). To resist cold stress, ZD consumes abundant ROS-scavenging enzymes to maintain cellular redox homeostasis and protect the body. The relative abundance of 2-Cys peroxiredoxin (spot L66) increased; this protein catalyzes the reduction of various hydroperoxide to the corresponding alcohol or water and detoxifies alkyl hydroperoxides and peroxinitrite (Dietz et al., 2006; Kim et al., 2011). The relative abundance of 2-Cys peroxiredoxin (spots L48 and L49) declined in W5, whereas other ROS-scavenging enzymes were not significantly affected by low temperature. It may be inferred that ZD is more sensitive to chilling stress than W5 in spite of its freeze-tolerant genotype. When chilling (4°C) occurs over a short period (such as 12 h), ZD consumes enzymes to resist stress. The enzyme expression profile then changed to maintain homeostasis, as indicated by a low relative abundance observed at 7 days. The observed response to chilling in ZD was more drastic and complex than in W5.
As a freezing-tolerant genotype, ZD has stronger cold tolerance than the freezing-sensitive cultivar W5. However, ZD is more sensitive to chilling than W5, and a greater number of changes were observed in proteins involved in photosynthesis, energy metabolism, stress and redox, biosynthesis metabolism and amino acid metabolism in ZD under chilling stress. On one hand, more enzymes were consumed to produce proteins for regulating metabolism and maintaining homeostasis in ZD. On the other hand, low temperature influenced enzymatic activity and altered the metabolic and synthesis pathways. In W5, the expression of many proteins remained unchanged. However, protein metabolism-related proteins were more active in W5 compared to ZD. In conclusion, ZD mobilizes a large number of proteins to adapt low temperature, and autologous metabolism and biosynthesis are slowed to reduce consumption for homeostasis. W5 enhances its capability for protein folding and protein biosynthesis to overcome chilling stress. The perception of low temperature is more sensitive in freezing-tolerant alfalfa than in freezing-sensitive alfalfa. Proteomics provides new insight into cold acclimation mechanisms in alfalfa.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The research was supported by special postdoctoral funding from Heilongjiang (LBH-TZ1209), the National Natural Science Foundation of Heilongjiang (C201313), and the 12th 5-year-plan scheme (2011BAD17B04-2).
Footnotes
References
Amme, S., Matros, A., Schlesier, B., and Mock, H. P. (2006). Proteome analysis of cold stress response in Arabidopsis thaliana using DIGE-technology. J. Exp. Bot. 57, 1537–1546. doi: 10.1093/jxb/erj129
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
An, B. Y., Liu, X. Y., Tan, H., Lin, W. H., and Sun, L. W. (2011). Comparative profile of RuBisCO-interacting proteins from Arabidopsis: photosynthesis under cold conditions. Prog. Biochem. Biophys. 38, 455–463. doi: 10.3724/SP.J.1206.2011.000009
Arrigoni, O., Dipierro, S., and Borraccino, G. (1981). Ascorbate free radical reductase, a key enzyme of the ascorbic acid system. FEBS Lett. 125, 242–245. doi: 10.1016/0014-5793(81)80729-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Balbuena, T. S., Salas, J. J., Martínez-Force, E., Garcés, R., and Thelen, J. J. (2011). Proteome analysis of cold acclimation in sunflower. J. Proteome Res. 10, 2330–2346. doi: 10.1021/pr101137q
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bang, W. Y., Chen, J., Jeong, I. S., Kim, S. W., Kim, C. W., and Jung, H. S.,et al. (2012). Functional characterization of ObgC in ribosome biogenesis during chloroplast development. Plant J. 71, 122–134. doi: 10.1111/j.1365-313X.2012.04976.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Barraclough, R., and Ellis, R. J. (1980). Protein synthesis in chloroplasts IX. Assembly of newly-synthesized large subunits into ribulose bishopshate carboxylase in isolated intact pea chloroplasts. Biochim. Biophys. Acta 608, 19–31. doi: 10.1016/0005-2787(80)90129-X
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bartels, D. (2001). Targeting detoxification pathways: an efficient approach to obtain plants with multiple stress tolerance. Trends Plant Sci. 6, 284–286. doi: 10.1016/S1360-1385(01)01983-5
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bocian, A., Kosmala, A., Rapacz, M., Jurczyk, B., Marczak,Ł., and Zwierzykowski, Z. (2011). Differences in leaf proteome response to cold acclimation between Lolium perenne plants with distinct levels of frost tolerance. J. Plant Physiol. 168, 1271–1279. doi: 10.1016/j.jplph.2011.01.029
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bourne, H. R., Sanders, D. A., and McCormick, F. (1990). The GTPase superfamily: a conserved switch for diverse cell functions. Nature 348, 125–132. doi: 10.1038/348125a0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Brot, N., Weissbach, L., Werth, J., and Weissbach, H. (1982a). The biochemistry of methionine sulfoxide residues in proteins. Biofactor 3, 91–96. doi: 10.1016/0968-0004(82)90204-3
Brot, N., Weissbach, L., Werth, J., and Weissbach, H. (1982b). Reduction of N-acetyl methionine sulfoxide: a simple assay for peptide methionine sulfoxide reductase. Anal. Biochem. 122, 291–294. doi: 10.1016/0003-2697(82)90283-4
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Budiman, C., Koga, Y., Takano, K., and Kanaya, S. (2011). FK506-binding protein 22 from a psychrophilic bacterium, a cold shock-inducible peptidyl prolyl isomerase with the ability to assist in protein folding. Int. J. Mol. Sci. 12, 5261–5284. doi: 10.3390/ijms12085261
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Caraux, G., and Pinloche, S. (2005). PermutMatrix: a graphical environment to arrange gene expression profiles in optimal linear order. Bioinformatics 21, 1280–1281. doi: 10.1093/bioinformatics/bti141
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Carrol, A. W., Joshi, H. J., and Heazlewood, J. L. (2013). Managing the green proteomes for the next decade of plant research. Front. Plant Sci. 4:501. doi: 10.3389/fpls.2013.00501
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Castonguay, Y., Laberge, S., Brummer, C. E., and Volenec, J. J. (2006). Alfalfa winter hardiness: a research retrospective and integrated perspective. Adv. Agron. 90, 203–265. doi: 10.1016/S0065-2113(06)90006-6
Corthalis, G. L., Wasinger, V. C., Hochstrasser, D. F., and Sanchez, J. C. (2000). The dynamic range of protein expression: a challenge for proteomic research. Electrophoresis 21, 1104–1115. doi: 10.1002/(SICI)1522-2683(20000401)21:6<1104::AID-ELPS1104>3.3.CO;2-3
Cui, S. X., Huang, F., Wang, J., Ma, X., Cheng, Y. S., and Liu, J. Y. (2005). A proteomic analysis of cold stress responses in rice seedlings. Proteomics 5, 3162–3172. doi: 10.1002/pmic.200401148
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Degand, H., Faber, A. M., Dauchot, N., Mingeot, D., Watillon, B., Cutsemet, P. V.,et al. (2009). Proteomic analysis of chicory root identifies proteins typically involved in cold acclimation. Proteomics 9, 2903–2907. doi: 10.1002/pmic.200800744
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Dietz, K. J., Jacob, S., Oelze, M. L., Laxa, M., Tognetti, V., De Miranda, S. M. N.,et al. (2006). The function of peroxiredoxins in plant organelle redox metabolism. J. Exp. Bot. 57, 1697–1709. doi: 10.1093/jxb/erj160
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Dumont, E., Bahrman, N. R., Goulas, E., Valot, B., Sellier, H., Hilbert, J. L.,et al. (2011). A proteomic approach to decipher chilling response from cold acclimation in pea (Pisum sativum L.). Plant Sci. 180, 86–98. doi: 10.1016/j.plantsci.2010.09.006
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ellis, R. J. (1979). The most abundant protein in the world. Trends Biochem. Sci. 4, 241–244. doi: 10.1016/0968-0004(79)90212-3
Feng, H., Chen, Q., Feng, J., Zhang, J., Yang, X., and Zuo, J. (2007). Functional characterization of the Arabidopsis eukaryotic translation initiation factor 5A-2 that plays a crucial role in plant growth and development by regulating cell division, cell growth, and cell death. Plant Physiol. 144, 1531–1545. doi: 10.1104/pp.107.098079
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gao, F., Zhou, Y. J., Zhu, W. P., Li, X. F., Fan, L. M., and Zhang, G. F. (2009). Proteomic analysis of cold stress-responsive proteins in Thellungiella rosette leaves. Planta 230, 1033–1046. doi: 10.1007/s00425-009-1003-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Giavalisco, P., Nordhoff, E., Kreitler, T., Kloppel, K. D., Lehrach, H., Klose, J.,et al. (2005). Proteome analysis of Arabidopsis thaliana by two-dimensional gel electrophoresis and matrix-assisted laser desorption/ionisation-time of flight mass spectrometry. Proteomics 5, 1902–1913. doi: 10.1002/pmic.200401062
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gomat, H. Y., Deleporte, P., Moukini, R., Mialounguila, G., Ognouabi, N., Saya, A. R.et al. (2011). What factors influence the stem taper of eucalyptus: growth, environmental conditions, or genetics? Ann. For. Sci. 68, 109–120. doi: 10.1007/s13595-011-0012-3
Gough, K. C., Hawes, W. S., Kilpatrick, J., and Whitelam, G. C. (2001). Cyanobacterial GR6 glutamate-1-semialdehyde aminotransferase: a novel enzymebased selectable marker for plant transformation. Plant Cell Rep. 20, 296–300. doi: 10.1007/s002990100337
Green, B. R., and Durnford, D. G. (1996). The chlorophyll-carotenoid proteins of oxygenic photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47, 685–714. doi: 10.1146/annurev.arplant.47.1.685
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Grimaud, F., Renaut, J., Dumont, E., Sergeant, K., Lucau-Danila, A., and Blervacq, A. S. (2013). Exploring chloroplastic changes related to chilling and freezing tolerance during cold acclimation of pea (Pisum sativum L.). J. Proteomics 80, 145–159. doi: 10.1016/j.jprot.2012.12.030
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gua, X. B., Gao, Z. H., Zhuang, W. B., Qiao, Y. S., Wang, X. Y., and Mi, L. (2013). Comparative proteomic analysis of rd29A: RdreB1BI transgenic and non-transgenic strawberries exposed to low temperature. J. Plant Physiol. 170, 696–706. doi: 10.1016/j.jplph.2012.12.012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hashimoto, M., and Komatsu, S. (2007). Proteomic analysis of rice seedlings during cold stress. Proteomics 7, 1293–1302. doi: 10.1002/pmic.200600921
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Haupt-Herting, S., Klug, K., and Fock, H. P. (2001). A new approach to measure gross CO2 fluxes in leaves. Gross CO2 assimilation, photorespiration, and mitochondrial respiration in the light in tomato under drought stress. Plant Physiol. 126, 388–396. doi: 10.1104/pp.126.1.388
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Heazlewood, J. L. (2011). The green proteome: challenges in plant proteomics. Front. Plant Sci. 2:6. doi: 10.3389/fpls.2011.00006
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Jao, D. L. E., and Chen, K. Y. (2006). Tandem affinity purification revealed the hypusine-dependent binding of eukaryotic initiation factor 5A to the translating 80S ribosomal complex. J. Cell. Biochem. 97, 583–598. doi: 10.1002/jcb.20658
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Jordan, P. M., and Shemin, D. (1972). Aminolevulinic Acid Aynthetase, in the Enzymes. New York: Academic Press.
Kaur, N., and Gupta, A. K. (2005). Signal transduction pathways under abiotic stresses in plants. Curr. Sci. 88, 1771–1780.
Kaziro, Y., Itoh, H., Kozasa, T., Nakafuku, M., and Satoh, T. (1991). Structure and function of signal-transducing GTP-binding proteins. Annu. Rev. Biochem. 60, 349–400. doi: 10.1146/annurev.bi.60.070191.002025
Kim, M. D., Kim, Y. H., Kwon, S. Y., Jang, B. Y., Lee, S. Y., Yun, D. J.,et al. (2011). Overexpression of 2-cysteine peroxiredoxin enhances tolerance to methyl viologen-mediated oxidative stress and high temperature in potato plants. Plant Physiol. Biochem. 49, 891–897. doi: 10.1016/j.plaphy.2011.04.001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kim, S. T., Cho, K. S., Jang, Y. S., and Kang, K. Y. (2001). Two-dimensional electrophoretic analysis of rice proteins by polyethylene glycol fractionation for protein arrays. Electrophoresis 22, 2103–2109. doi: 10.1002/1522-2683(200106)22:10<2103::AID-ELPS2103>3.0.CO;2-W
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Knaupp, M., Mishra, K. B., Nedbal, L., and Heyer, AG. (2011). Evidence for a role of raffinose in stabilizing photosystem II during freeze-thaw cycles. Planta 234, 477–486. doi: 10.1007/s00425-011-1413-0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Koehler, G., Wilson, R. C., Goodpaster, J. V., Sønsteby, A., Lai, X. Y., and Witzmann, F. A. (2012). Proteomic study of low-temperature responses in strawberry cultivars (Fragaria × ananassa) that differ in cold tolerance. Plant Physiol. 159, 1787–1805. doi: 10.1104/pp.112.198267
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kosmala, A., Bocian, A., Rapacz, M., Jurczyk, B., and Zwierzykowski, Z. (2009). Identification of leaf proteins differentially accumulated during cold acclimation between Festuca pratensis plants with distinct levels of frost tolerance. J. Exp. Bot. 60, 3595–3609. doi: 10.1093/jxb/erp205
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kosová, K., Vítámvás, P., Planchon, S., Renaut, J., Vankova, R., and Prášil, I. T. (2013). Proteome analysis of cold response in spring and winter wheat (Triticum aestivum) crowns reveals similarities in stress adaptation and differences in regulatory processes between the growth habits. J. Proteome Res. 18, 4830–4845. doi: 10.1021/pr400600g
Kosová, K., Vítámvás, P., and Prášil, I. T. (2014). Proteomics of stress responses in wheat and barley – search for potential protein markers of stress tolerance. Front. Plant Sci. 5:711. doi: 10.3389/fpls.2014.00711
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Krishnan, H. B., and Natarajan, S. S. (2009). A rapid method for depletion of RuBisCO from soybean (Glycine max) leaf for proteomic analysis of lower abundance proteins. Phytochemistry 70, 1958–1964. doi: 10.1016/j.phytochem.2009.08.020
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lee, D. G., Ahsan, N., Lee, S. H., Kang, K. Y., Lee, J. J., and Lee, B. H. (2007). An approach to identify cold-induced low-abundant proteins in rice leaf. C. R. Biol. 330, 215–225. doi: 10.1016/j.crvi.2007.01.001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lee, D. G., Ahsan, N., Lee, S. H., Lee, J. J., Bahk, J. D., Kang, K. Y.,et al. (2009). Chilling stress-induced proteomic changes in rice roots. J. Plant Physiol. 166, 1–11. doi: 10.1016/j.jplph.2008.02.001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Liu, H. M., Fang, L., Che, Y. S., Wu, F. Z., and Yang, C. P. (2014). Protein expression patterns in two Spiraea species in response to cold treatment. Mol. Biol. Rep. 41, 4533–4547. doi: 10.1007/s11033-014-3324-1
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Parry, M. A. J., Andralojc, P. J., Mitchell, R. A. C., Madgwick, P. J., and Keys, A. J. (2003). Manipulation of RuBisCO: the amount, activity, function and regulation. J. Exp. Bot. 54, 1321–1333. doi: 10.1093/jxb/erg141
Parsell, D. A., and Lindquist, S. (1993). The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu. Rev. Genet. 27, 437–496. doi: 10.1146/annurev.ge.27.120193.002253
Portis, A. R. (1990). RuBisCO activase. Biochim. Biophys. Acta 1015, 15–28. doi: 10.1016/0005-2728(90)90211-L
Portis, A. R., Li, C., Wang, D., and Salvucci, M. E. (2008). Regulation of RuBisCO activase and its interaction with RuBisCO. J. Exp. Bot. 59, 1597–1604. doi: 10.1093/jxb/erm240
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Rinalducci, S., Egidi, M. G., Karimzadeh, G., Jazii, F. R., and Zolla, L. (2011). Proteomic analysis of a spring wheat cultivar in response to prolonged cold stress. Electrophoresis 32, 1807–1818. doi: 10.1002/elps.201000663
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sánchez-Bel, P., Egea, I., Sánchez-Ballesta, M. T., Sevillano, L., Del Carmen Bolarin, M., and Flores F. B. (2012a). Proteome changes in tomato fruits prior to visible symptoms. Plant Cell Physiol. 53, 470–484. doi: 10.1093/pcp/pcr191
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sánchez-Bel, P., Egea, I., Sánchez-Ballesta, M. T., Martinez-Madrid, C., Fernandez-Garcia, N., and Romojaro, F.et al. (2012b). Understanding the mechanisms of chilling injury in bell pepper fruits using the proteomic approach. J. Proteomics 75, 5463–5478. doi: 10.1016/j.jprot.2012.06.029
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sarvikas, P., Hakala, M., Pätsikkä, E., Tyystjärvi, T., and Tyystjärvi, E. (2006). Action spectrum of photoinhibition in leaves of wild type and npq1-2 and npq4-1 mutants of Arabidopsis thaliana. Plant Cell Physiol. 473, 391–400. doi: 10.1093/pcp/pcj006
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Schatz, O., Oft, M., Dascher, C., Schebesta, M., Rosorius, O., and Jaksche, H.et al. (1998). Interaction of the HIV-1 Rev cofactor eukaryotic initiation factor 5A with ribosomal protein L5. Proc. Natl. Acad. Sci.U.S.A. 95, 1607–1612. doi: 10.1073/pnas.95.4.1607
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Seki, M., Satou, M., Sakurai, T., Akiyama, K., Iida, K., Ishida, J.,et al. (2004). RIKEN Arabidopsis full length (RAFL) cDNA and its applications for expression profiling under abiotic stress conditions. J. Exp. Bot. 55, 213–223. doi: 10.1093/jxb/erh007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Smith, D. M., Stucker, R. E., and Elling, L. J. (1979). “Fall dormancy in alfalfa: a valuable predictive tool,” in Report of the 26th Improvement Conference. Agriculture Review and Manuals. Science and Education Administration, (Saint Paul, MN: USDA-SEA), 34.
Sprague, M. A., and Fuelleman, R. F. (1941). Measurements of recovery after cutting and fall dormancy of varieties and strains of alfalfa, Medicago sativa. Agron. J. 33, 437–447. doi: 10.2134/agronj1941.00021962003300050006x
Spreitzer, R. J., and Salvucci, M. E. (2002). RuBisCO: structure, regulatory interactions, and possibilities for a better enzyme. Annu. Rev. Plant Biol. 53, 449–475. doi: 10.1146/annurev.arplant.53.100301.135233
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Takahashi, D., Kawamura, Y., and Uemura, M. (2013). Changes of detergent-resistant plasma membrane proteins in oat and rye during cold acclimation: association with differential freezing tolerance. J. Proteome Res. 12, 4998–5011. doi: 10.1021/pr400750g
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Takahashi, S., Kuzuyama, T., Watanabe, H., and Seto, H. (1998). A 1-deoxy-D-xylulose 5-phosphate reductoisomerase catalyzing the formation of 2-C-methyl-D-erythritol 4-phosphate in an alternative nonmevalonate pathway for terpenoid biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 95, 9879–9884. doi: 10.1073/pnas.95.17.9879
Thompson, J. E., Hopkins, M. T., Taylor, C., and Wang, T. W. (2004). Regulation of senescence by eukaryotic translation initiation factor 5A: implications for plant growth and development. Trends Plant Sci. 9, 174–179. doi: 10.1016/j.tplants.2004.02.008
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Timmons, F. L., and Salmon, S. C. (1932). The resistance of certain varieties and regional strains of alfalfa to controlled low temperatures. Agron. J. 24, 642–655. doi: 10.2134/agronj1932.00021962002400080007x
Uváčková, L’., Takáčb, T., Boehm, N., Obert, B., and Šamaj, J. (2012). Proteomic and biochemical analysis of maize anthers after cold pretreatment and induction of androgenesis reveals an important role of anti-oxidative enzymes. J. Proteomics 75, 1886–1894. doi: 10.1016/j.jprot.2011.12.033
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Vaclavik, L., Mishra, A., Mishra, K. B., and Hajslova, J. (2013). Mass spectrometry-based metabolomic fingerprinting for screening cold tolerance in Arabidopsis thaliana accessions. Anal. Bioanal. Chem. 405, 2671–2683. doi: 10.1007/s00216-012-6692-1
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Veuthey, A. L., and Stucki, J. (1987). The adenylate kinase reaction acts as a frequency filter towards fluctuations of ATP utilization in the cell. Biophys. Chem. 26, 19–28. doi: 10.1016/0301-4622(87)80003-0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Vítamìvasì, P., Prasíil, I. T., Kosová, K., Planchon, S., and Renaut, J. (2012). Analysis of proteome and frost tolerance in chromosome 5A and 5B reciprocal substitution lines between two winter wheats during long-term cold acclimation. Proteomics 12, 68–85. doi: 10.1002/pmic.201000779
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Whitney, S. M., and Andrews, T. J. (2001). Plastome-encoded bacterial ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) supports photosynthesis and growth in tobacco. Proc. Natl. Acad. Sci. U.S.A. 98, 14738–14743. doi: 10.1073/pnas.261417298
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Xi, J., Wang, X., Li, S., Zhou, X., Yue, L., Fan, J.,et al. (2006). Polyethylene glycol fractionation improved detection of low-abundant proteins by two-dimensional electrophoresis analysis of plant proteome. Phytochemistry 67, 2341–2348. doi: 10.1016/j.phytochem.2006.08.005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Xu, J., Li, Y., Sun, J., Du, L., Zhang, Y., Yu, Q.,et al. (2013). Comparative physiological and proteomic response to abrupt low temperature stress between two winter wheat cultivars differing in low temperature tolerance. Plant Biol. 15, 292–303. doi: 10.1111/j.1438-8677.2012.00639.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Yan, S. P., Zhang, Q. Y., Tang, Z. C., Su, W. A., and Sun, W. N. (2006). Comparative proteomic analysis provides new insights into chilling stress responses in rice. Mol. Cell. Proteomics 5, 484–496. doi: 10.1074/mcp.M500251-MCP200
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Zeleznikar, R. J., Dzeja, P. P., and Goldberg, N. D. (1995). Adenylate kinase-eatalyzed phosphoryl transfer couples ATP utilization with its generation by glycolysis in intact muscle. J. Biol. Chem. 270, 7311–7319. doi: 10.1074/jbc.270.13.7311
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Zhang, S., Feng, L. H., Jiang, H., Ma, W. J., Korpelainen, H., and Li, C. Y. (2012). Biochemical and proteomic analyses reveal that Populus cathayana males and females have different metabolic activities under chilling stress. J. Proteome Res. 11, 5815–5826. doi: 10.1021/pr3005953
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Zhou, R., Jackson, L., Shadle, G., Nakashima, J., Temple, S., Chen, F.,et al. (2010). Distinct cinnamoyl CoA reductases involved in parallel routes to lignin in Medicago truncatula. Proc. Natl. Acad. Sci. U.S.A. 107, 17803–17808. doi: 10.1073/pnas.1012900107
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Zuther, E., Schulz, E., Childs, L. H., and Hinch, D. K. (2012). Clinal variation in the non-acclimated and cold-acclimated freezing tolerance of Arabidopsis thaliana accessions. Plant Cell Environ. 35, 1860–1878. doi: 10.1111/j.1365-3040. 2012.02522
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: proteomics, cold acclimation, RuBisCO, metabolism, homeostasis, alfalfa
Citation: Chen J, Han G, Shang C, Li J, Zhang H, Liu F, Wang J, Liu H and Zhang Y (2015) Proteomic analyses reveal differences in cold acclimation mechanisms in freezing-tolerant and freezing-sensitive cultivars of alfalfa. Front. Plant Sci. 6:105. doi: 10.3389/fpls.2015.00105
Received: 22 December 2014; Paper pending published: 16 January 2015;
Accepted: 09 February 2015; Published online: 27 February 2015.
Edited by:
Silvia Mazzuca, Università della Calabria, ItalyReviewed by:
Jill Christine Preston, University of Vermont, USAPingfang Yang, Wuhan Botanical Garden – Chinese Academy of Sciences, China
Copyright © 2015 Chen, Han, Shang, Li, Zhang, Liu, Wang, Liu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guiqing Han, College of Life Sciences and Technology, Harbin Normal University, South of Shida Road No. 1, Harbin 150050, China e-mail:Y2N5ajE1QDE2My5jb20=
 Jing Chen
Jing Chen Guiqing Han1,2*
Guiqing Han1,2* Jikai Li
Jikai Li Hailing Zhang
Hailing Zhang Fengqi Liu
Fengqi Liu Jianli Wang
Jianli Wang