- 1Department of Horticulture, Faculty of Agriculture, Ordu University, Ordu, Turkey
- 2Department of Horticulture, Faculty of Agriculture, Ankara University, Ankara, Turkey
Shoot-tip cultures of Pyrus elaeagrifolia Pallas, an important gene source for drought and chlorosis resistance in pear rootstock breeding, were established from a wild mature tree originated from seed. Murashige and Skoog basal medium supplemented with different concentrations of benzyladenine (BA) singly or in combination with auxin was used in the study. In the initial culture, the highest percentages (>80%) of shoot proliferation were obtained in the mediums supplemented with 9.0 μM BA and 0.5 μM indole-3-acetic acid. In the subcultures, the highest shoot proliferation rates were obtained in the medium containing 4.5 and 9.0 μM BA. The shoot proliferation rates ranged from 91.1 ± 2.4 to 96.4 ± 2.0% in the second subculture and from 76.7 ± 7.8 to 89.4 ± 3.3% in the third subculture. In the second subculture, the shoots grown on 9.0 μM BA without auxin produced the best proliferation (10.6 ± 1.6). For the in vitro rooting experiments, the highest rooting rate (54.2 ± 10.4%), root length (10.5 ± 2.4 mm), and root number (2.5 ± 0.6) were obtained from 10 days dark treatment on the medium containing half strength of macronutrients supplemented with 5 μM indole-3-butyric acid (IBA). For the ex vitro rooting experiments, shoot rooting was significantly influenced by 10 mM IBA applied as quick-dip method. The percentage of rooting was 55 ± 9.6% and root number was 1.8 ± 0.3 at this concentration.
Introduction
Iron-chlorosis in pears is a widespread problem, particularly in regions with calcareous soils (Dolcet-Sanjuan et al., 2004). Pyrus elaeagrifolia Pallas is one of the gene sources used to improve rootstock tolerance to drought and chlorosis (Lombard and Westwood, 1987). Thus, Claveria et al. (2010) used interspecific crosses including Pyrus amygdaliformis, P. amygdaliformis ssp. persica, P. elaeagrifolia, and P. communis ssp. cordata in developing resistant rootstocks to iron chlorosis. According to Lombard and Westwood (1987), drought tolerance and nitrogen, phosphorus, iron, boron, and zinc uptake from soil of pear scion cultivar by P. elaeagrifolia are at high levels. Moreover, this species is resistant to black end, pear decline, and wooly pear aphid. In Turkey, P. elaeagrifolia seedlings are used as rootstock for P. communis (Lombard and Westwood, 1987; Bell et al., 1996). In general, older wild trees of the species are grafted by pear scions (Koksal et al., 2002). Thus, healthy pear trees could be grown in arid soils with pH 7.5–8. However, seedlings of this species display high genetic variation. For these reasons, vegetative clonal propagation techniques could be used for selected P. elaeagrifolia genotypes as rootstocks.
Pyrus elaeagrifolia originated from Turkey, Russia, and southeast Europe (Lombard and Westwood, 1987; Bell et al., 1996). There are many types of this species in the Anatolian region of Turkey. The selection of clonal rootstocks from these dwarf or semi-dwarf genotypes, which are tolerant to chlorosis, and drought, resistant to diseases and pests, is very important in pear breeding. Similar to most fruit trees and nut species, reproduction of uniform copies of an original P. elaeagrifolia parent plant is possible by cuttings, layering, and micro-propagation. Among these techniques, only propagation by cutting has been studied in this species. Two types of P. elaeagrifolia have been propagated by softwood cuttings: the rooting percentages were determined as 11.4 and 43.8% (Dumanoglu et al., 1999). Micro-propagation allows fruit breeders to quickly multiply a new rootstock in a short time (Webster, 1995; Hartmann et al., 1997). Axillary bud multiplication is a widely used means of mass propagation of plants that are genotypically and phenotypically the same as the original plant from which they were produced (Evans, 1990). Results on micropropagation of P. communis (Cheng, 1979; Shen and Mullins, 1984; Rodriguez et al., 1991; Al-Maarri et al., 1994; Iglesias et al., 2004), P. calleryana (Berardi et al., 1993), P. calleryana, P. betulaefolia (Yeo and Reed, 1995), and P. syriaca (Shibli et al., 1997) have been reported by several researchers (Chevreau and Bell, 2005). However, micropropagation of P. elaeagrifolia has not been studied. In this study, we report in vitro shoot proliferation and in vitro and ex vitro root formation of P. elaeagrifolia.
Materials and Methods
Plant Material
In the present study, a wild mature tree originated from the seed of P. elaeagrifolia Pallas was used as plant material, and in vitro shoot-tip cultures were established from actively growing shoots.
Explants Establishment and Shoot Proliferation Experiments
Murashige and Skoog (1962) basal medium containing 3% (w/v) sucrose and 0.7% (w/v) Difco Bacto agar was used in all experiments. The MS basal medium was supplemented with 0.3 μM gibberellic acid (GA3). For the initial culture and multiplication experiments, benzyladenine (BA; 0.0, 4.5, and 9.0 μM), in combination with indole-3-butyric acid (IBA; 0.0, 0.5, and 2.5 μM), and indole-3-acetic acid (IAA; 0.0, 0.5, and 2.5 μM) was used. All growth regulators were added to the media before autoclaving at 121°C for 20 min. The pH was adjusted to 5.7 before adding agar and autoclaving. All cultures were kept at 24 ± 1°C under a photoperiod of 16 h of cool white fluorescent light (35 μmol⋅m-2⋅s-1).
For explants establishment, shoot tips of 2 cm length were washed in running tap water for 5 min and surface sterilized in a solution of sodium hypochlorite (3% active chlorine) with 0.1% (v/v) Tween 20 for 15 min and rinsed three times with sterile distilled water. The explants of shoot tips (≈1 cm) were cultured in glass tubes (12 cm× 2.5 cm) containing 10 ml of MS medium supplemented with BA combinations of IBA and IAA. After 4 weeks in culture, the percentage of explants forming shoots and the number of shoots that were >1 cm long was recorded.
In vitro shoots to be used as explants for multiplication experiments should be in sufficient number. For this reason, the shoots (>1 cm) from the initial cultures were subcultured on MS medium supplemented with 9.0 μM BA for 4 weeks. Then, the shoots proliferated on this medium were subcultured in glass flasks (250 ml) containing 50 ml of MS medium supplemented with combinations of BA and IBA or IAA at 4 week intervals, three times. Every time, the shoots were subcultured on fresh medium of the same composition as the previous subculture. The percentage of shoot multiplication and the number of shoots (>1 cm) per proliferated shoot were only recorded at the end of the second and third subcultures on the MS medium supplemented with different BA × auxin combinations. The data of the first subculture was not evaluated since the explants used in this subculture were taken from the cultures on the MS medium supplemented with 9.0 μM BA.
The experiments with three factors (BA concentration, auxin concentration, and auxin type) were set up as a completely randomized design with factorial combinations of BA (0.0, 4.5, and 9.0 μM) and IBA (0.0, 0.5, and 2.5 μM) or IAA (0.0, 0.5, and 2.5 μM). Each treatment in the initial culture included 12 replications (tubes). The shoot multiplication experiment consisted of three replications (flasks) with five shoots in each treatment.
Rooting Experiments
The rooting experiments were conducted in vitro and ex vitro conditions with micropropagated shoots approximately 1–2 cm long obtained from the fifth subcultures. The MS basal medium containing half strength of macronutrients supplemented with 3% (w/v) sucrose and 0.7% (w/v) Difco Bacto agar was used for in vitro rooting experiment. The effects of various auxin treatments, which were basal medium with no growth regulators (control), naphthaleneacetic acid (NAA; 5 and 10 μM), and IBA (5 and 10 μM) in basal medium, 10 – second dip in IBA (10 and 20 mM) and dissolved in 50% ethanol, were tested in light or darkness for 10 days followed by light. A two-factor (auxin × darkness) experiment was set up as a completely randomized design with factorial combinations of auxin and darkness treatments. Each treatment included 24 replications (tubes).
For ex vitro rooting experiments, microcuttings were quick-dipped in solutions of 0, 10, 20, 30, or 40 mM IBA in 50% ethanol. These cuttings were inserted into plastic boxes (10 cm × 8 cm × 5 cm) containing perlite. The box was sealed with plastic film to prevent desiccation. The humidity in the boxes was gradually reduced by cracking open the seals. The experiment was a one-factor (auxin treatments) completely randomized design, consisted of four replications (boxes) with five microcuttings in each treatment. The cultures were grown at 24 ± 1°C under 16-h photoperiod with 35 μmol⋅m-2⋅s-1 supplied by cool-white fluorescent bulbs for both in vitro and ex vitro treatments.
The data was collected after 45 days for in vitro and 75 days for ex vitro experiment. The percentage of rooted shoots, the number of roots, and average root length per rooted shoot were determined. The calluses were rated on a scale of 0–4 (0 = no callus, 1 = very small, 2 = small, 3 = medium, 4 = large) per shoot.
The plantlets were transplanted to the pots containing an autoclaved mixture of peat (70%), perlite (12%), sand (12%), and orchard soil (6%) and the pots were covered by plastic film. The plastic film was particularly opened after 3 weeks in the growth room.
Statistical Analysis
Multifactorial variance analysis (ANOVA) was performed on the data by Minitab software (MINITAB Inc.). Means were compared by Duncan’s multiple range test (P < 0.05). Before the analysis, Arcsin transformations were used for the percentage data.
Results
Shoot Proliferation
In the initial culture, the interaction effects of BA × auxin concentration and auxin type × auxin concentration on shoot proliferation rate were significant (Table 1). Almost at all concentrations of auxin, 4.5, and 9.0 μM BA produced the highest shoot proliferation rates. On the other hand, shoot proliferation increased with 0.0 and 0.5 μM concentrations of IAA and 2.5 μM concentration of IBA. In the initial culture, the highest percentages (>80%) of shoot proliferation were obtained from the medium supplemented with 9.0 μM BA and 0.5 μM IAA (Table 1) In the second and the third subcultures, the shoot multiplication rate was significantly influenced by BA concentrations with the highest obtained from 4.5 to 9.0 μM BA concentrations. The shoot multiplication rates ranged from 91.1 ± 2.4 to 96.4 ± 2.0% and from 76.7 ± 7.8 to 89.4 ± 3.3% in the second and third subculture, respectively.
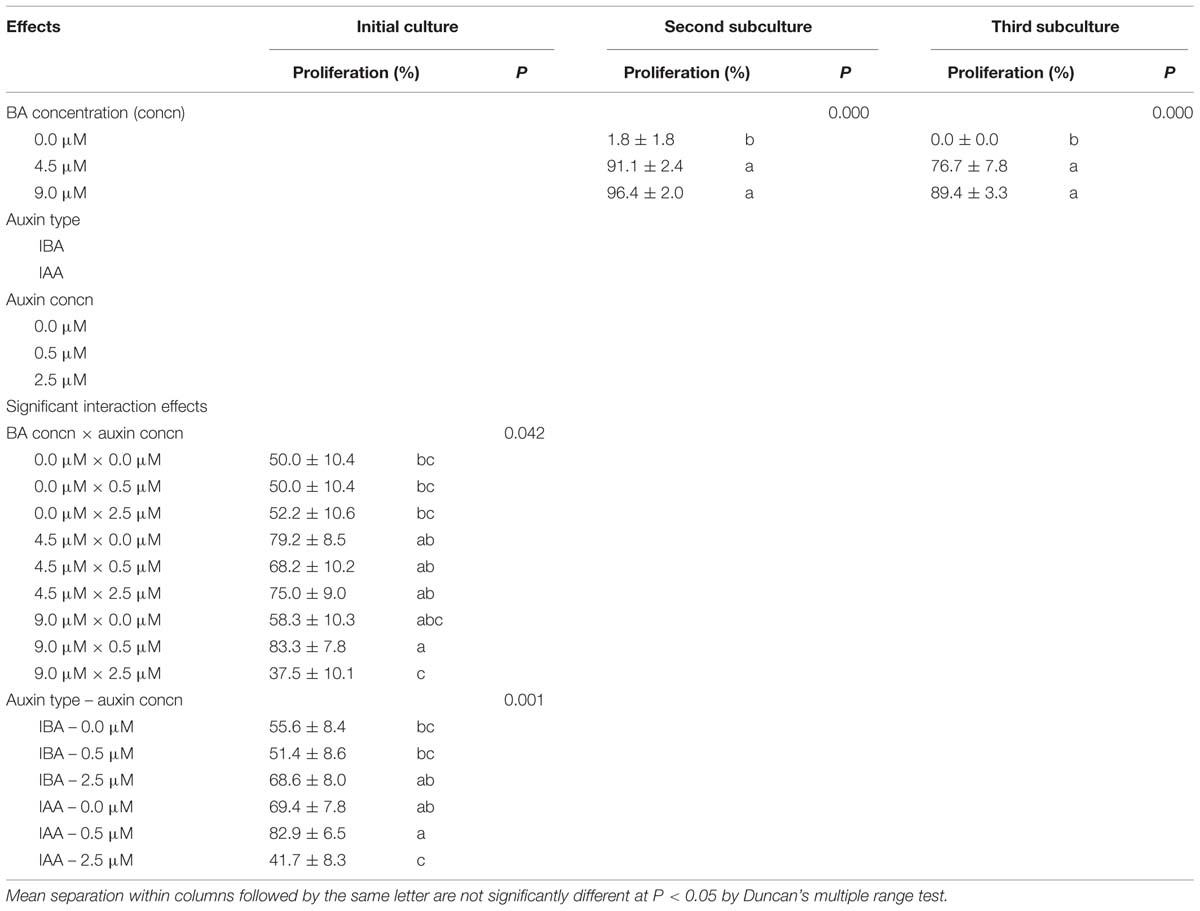
TABLE 1. Effect of benzyladenine (BA), indole-3-butyric acid (IBA), and indole-3-acetic acid (IAA) on the percentage of shoot proliferation in Pyrus elaeagrifolia Pallas.
Benzyladenine concentration × auxin type × auxin concentration interaction effect on the number of shoots per explant was significant in the initial culture (Table 2). The highest shoot proliferation (1.7 ± 0.2) was obtained from the medium containing 4.5 μM BA to 2.5 μM IBA. The mean shoot proliferation was not high for explants at this stage, whereas it highly increased in the second subculture (Table 2). The interaction of BA × auxin concentration was significant and the shoots grown on 9.0 μM BA without auxin produced the best shoot proliferation (10.6 ± 1.6). The highest number of shoots was recorded at 9.0 μM BA in the third subculture (Figure 1). Multiplication was significantly influenced by BA concentrations in this subculture.
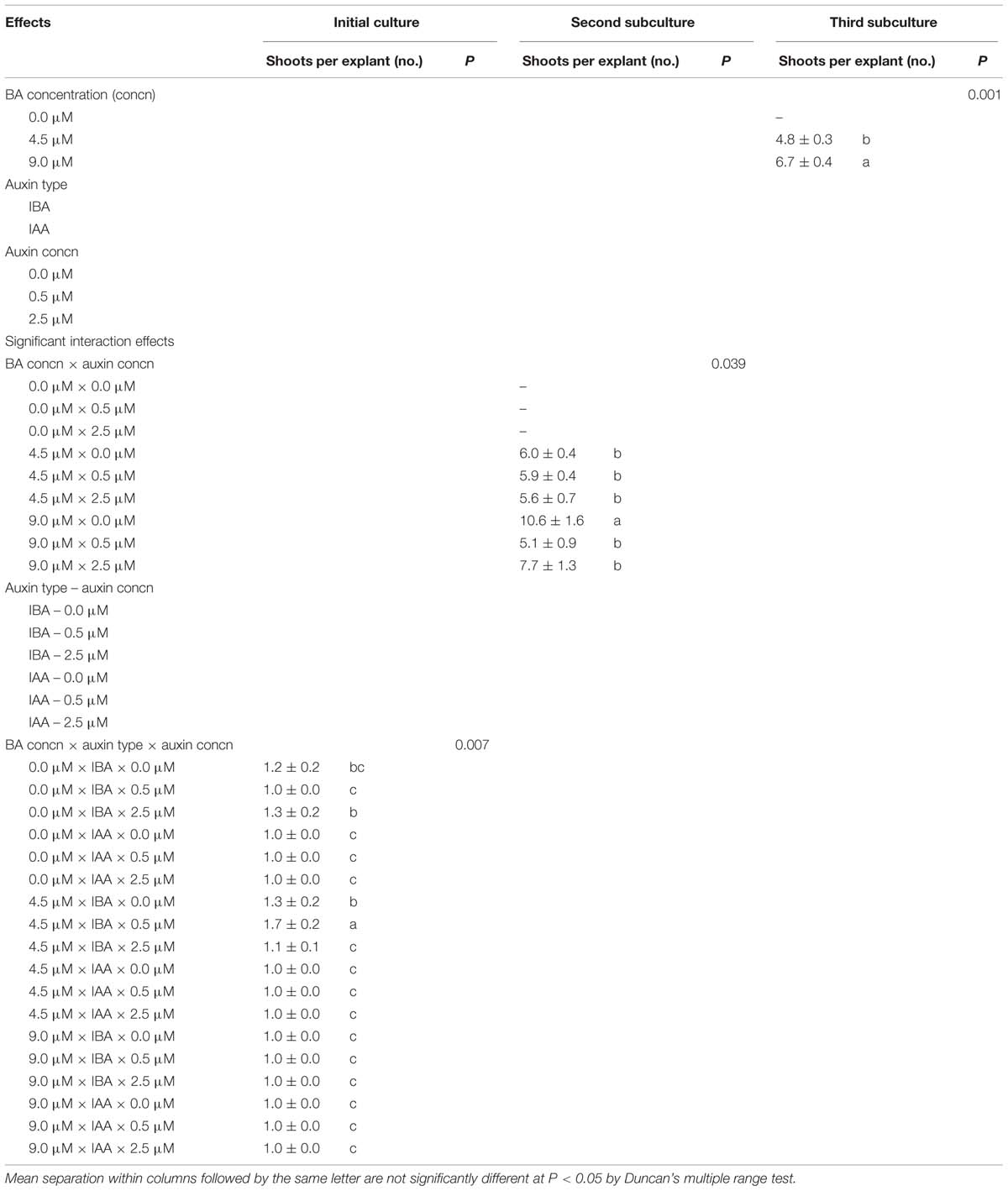
TABLE 2. Effect of benzyladenine (BA), indole-3-butyric acid (IBA), and indole-3-acetic acid (IAA) on means of shoot proliferation in Pyrus elaeagrifolia Pallas.

FIGURE 1. Shoot proliferation in Pyrus elaeagrifolia on MS medium supplemented with 4.5 μM benzyladenine (BA) and 0.3 μM gibberellic acid (GA3).
Rooting
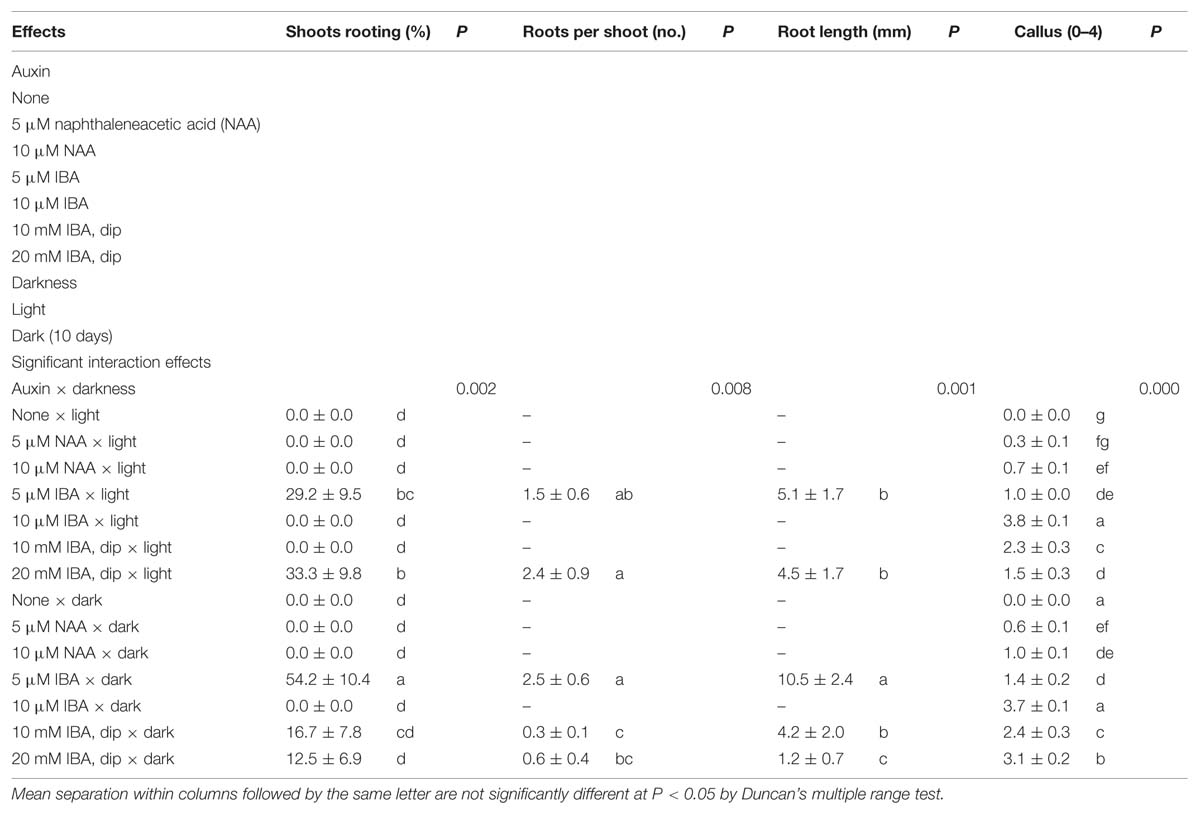
The highest rooting percentages were obtained as 54.2 ± 10.4 and 55 ± 9.6% from in vitro and ex vitro experiments, respectively. At both in vitro and ex vitro conditions, root formation was not observed in controls, the media without growth regulators. Auxin × darkness interaction was significant for in vitro rooting experiments (Table 3). The highest rooting rate (54.2 ± 10.4%) and root length (10.5 ± 2.4 mm) were obtained from 10 days dark treatment on the medium supplemented with 5 μM IBA. However, root numbers per rooted shoot on medium with 5 μM IBA were statistically similar at both light (2.4 ± 0.9) and dark (2.5 ± 0.6) treatments. NAA did not promote in vitro root formation. Generally, the shoots cultured on the medium supplemented with high levels of IBA (10 μM) or quick-dipped in 10 or 20 mM IBA solutions at both dark and light, produced larger callus than the other media. Callus degree (0–4) of in vitro micro-cuttings ranged from 0.0 to 3.8 ± 0.1 (Table 3).
In ex vitro rooting experiments, the shoot rooting was significantly influenced by 10 mM concentration of IBA in the quick-dip method (Table 4). The highest rooting percentage (55 ± 9.6%) and root numbers per rooted shoot (1.8 ± 0.3) were obtained from this concentration at the end of 75 days. The quick-dip in IBA treatments did not produce significant effect on root length and callus degree. The mean root length, measured between 17.6 ± 10.4 and 28.9 ± 7.0 mm, was higher than that obtained from in vitro experiments. One of the reasons for longer roots on micro-cuttings was the duration of the stay in perlite (75 days). Despite high concentration of IBA such as 40 mM, the callus degree of micro-cuttings ranged from 0.0 to 0.1 ± 0.03 (Table 4).
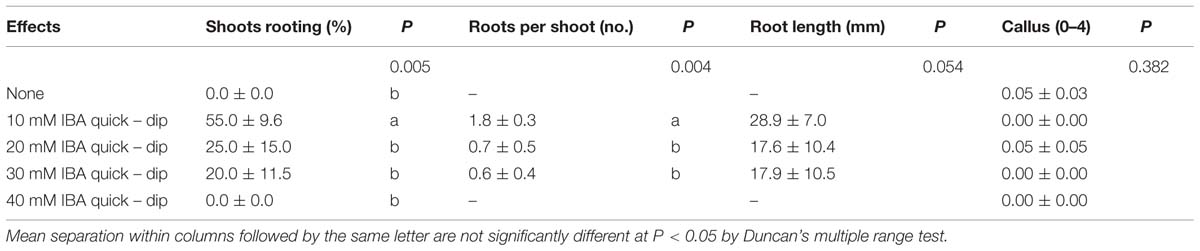
TABLE 4. Effect of quick-dip treatment in solutions of indole-3-butyric acid (IBA) on ex vitro rooting of shoots in Pyrus elaeagrifolia Pallas.
Discussion
In the present study, the shoot tip-culture from a mature tree of P. elaeagrifolia was successfully established on MS medium supplemented with some growth regulators. Tissue contamination and browning were not observed during the initial cultures and subcultures. In vitro shoot proliferation of P. elaeagrifolia was affected by BA concentrations (4.5 and 9.0 μM) in both initial cultures and subcultures. It was reported that BA concentrations of 3.3 to 20 μM produced successful multiplication of P. communis (Singha, 1982; Shen and Mullins, 1984; Baviera et al., 1989; Dolcet-Sanjuan et al., 1990; Moretti et al., 1991; Rodriguez et al., 1991; Yeo and Reed, 1995; Iglesias et al., 2004), P. calleryana (Dolcet-Sanjuan et al., 1990; Rossi et al., 1991; Berardi et al., 1993; Yeo and Reed, 1995), P. amygdaliformis (Dolcet-Sanjuan et al., 1990), P. betulifolia (Dolcet-Sanjuan et al., 1990; Yeo and Reed, 1995), and P. syrica (Shibli et al., 1997). Generally, researchers used BA alone in micro-propagation of Pyrus species and very little information is available about the effect of auxins on multiplication in this genus. Yeo and Reed (1995) examined the combinations of BA with IBA and NAA in multiplication of three rootstock selections of P. calleryana, P. betulifolia, and P. communis. The results revealed that IBA and NAA concentrations of >0.5 or 1 μM inhibited shoot multiplication in all three genotypes. Bhojwani et al. (1984) reported that shoots of P. pyrifolia were multiplied on MS medium supplemented with 6.7 μM BA and 0.1 μM NAA. Our results on IBA are in agreement with those of Yeo and Reed (1995). In the present study, a combination of high concentrations of BA (9.0 μM) and auxin (2.5 μM) inhibited shoot proliferation of P. elaeagrifolia in the initial culture. In addition, heavy callusing of the basal end and hyperhydricity of the in vitro shoots occurred at >9.0 μM BA and >2.5 μM IAA or IBA (data not shown). There is no information on the effects of IAA in Pyrus species. Our study indicated that IAA at 0.5 μM produced the highest percentage of shoot proliferation in the initial culture. According to this result, IAA is important for shoot proliferation in P. elaeagrifolia.
Both in vitro and ex vitro rooting of micro-propagated shoots of P. elaeagrifolia were only accomplished by auxin treatments. Such results were found in some other Pyrus species and woody plants (Bhojwani et al., 1984; Shen and Mullins, 1984; Baviera et al., 1989; McClelland et al., 1990; Berardi et al., 1991, 1993; Dolcet-Sanjuan et al., 1991; Moretti et al., 1991; Rodriguez et al., 1991; Rossi et al., 1991; Al-Maarri et al., 1994; Reed, 1995; Yeo and Reed, 1995; Shibli et al., 1997; Iglesias et al., 2004; Barros et al., 2005). In the current study, IBA at low concentration (5 μM) with dark treatment (10 days) stimulated in vitro root formation in micro-cuttings of P. elaeagrifolia. This result agrees with the fact that a dilute mineral medium with 0.1–10 μM IBA and NAA and an initial period of 7–10 days of dark incubation, improves rooting of pear in vitro shoots (Chevreau and Bell, 2005). We also used MS basal medium with half strength of macronutrients in the rooting experiment, but the root formation was not obtained on medium supplemented with NAA. However, Al-Maarri et al. (1994) reported that the best exogenous auxin for in vitro rooting of cultivars ‘Passe Crassane’ and ‘Williams’ pear seedlings was NAA at 1 μM. It was reported that root formation of the Pyrus species (P. betulaefolia, P. communis, P. calleryana, and P. amygdaliformis) was stimulated by exposure of shoots to high levels (10 or 32 μM) of IBA for 7 days followed by a passage on auxin-free medium (Dolcet-Sanjuan et al., 1990). Our result showed that IBA is effective auxin type for rooting of micro-cuttings in P. elaeagrifolia. Similarly, especially for recalcitrant Pyrus genotypes, treatment with 10 μM IBA during 1 or 3 weeks in darkness was suggested (Reed, 1995; Yeo and Reed, 1995), but IBA concentrations lower than 10 μM were not tested in the studies. Berardi et al. (1993) found that in vitro rooting in P. calleryana was promoted by auxins, mainly by NAA (2.7 μM). Shibli et al. (1997) reported that IBA, IAA, and NAA induced in vitro rooting of P. syrica and a maximum of 72% rooting was achieved at 17 μM IAA. For in vitro rooting of Conference pear cultivar, IAA at 2.7 μM was found to be most appropriate (Baviera et al., 1989). But, Al-Maarri et al. (1994) reported that IAA was unfavorable for rooting in pear shoots.
As another treatment, Reed (1995) recommended that IBA or NAA dip treatment can be used for rooting pear genotypes of unknown rooting potential. But, the effect of 10 and 20 mM IBA dip procedures on in vitro rooting in P. elaeagrifolia was not as high as that of medium supplemented with 5 μM IBA in dark. However, Rodriguez et al. (1991) reported that in vitro rooting without callus formation in P. communis was achieved by immersing the basal end in 5 μM IBA solution for 1 min.
Successful ex vitro rooting can save time and reduce costs (Yeo and Reed, 1995). In our study, 10 mM IBA quick-dip treatment in ex vitro conditions stimulated rooting, but higher concentrations of IBA prevented root formation from micro-cuttings. However, Yeo and Reed (1995) reported that neither rooting nor survival occurred from ex vitro rooting treatment in any Pyrus rootstocks. In addition, ex vitro rooting was found to be unsuccessful for P. syrica when treating micro-cuttings with 0–100 μM IBA, IAA, or NAA for 1 h (Shibli et al., 1997). Al-Maarri et al. (1994) reported that ex vitro rooting was induced after cold storage pretreatment for 1 month followed by immersion for 1 h in NAA solutions (0.05–0.5 mM).
Conclusion
We reported here an effective clonal propagation protocol by shoot-tip culture from a wild mature tree of P. elaeagrifolia. The combination of 9.0 μM BA and 0.5 μM IAA and 4.5, or 9.0 μM BA without auxin is recommended for the initial cultures and subcultures, respectively. Rooting of micro-cuttings was good on MS medium modified with half-strength of macronutrients with 5 μM IBA and 10 days dark treatment for in vitro or 10 mM IBA quick – dip treatment for ex vitro. Acclimatization of plantlets was achieved in the growth room for 3 weeks. Currently, we are using this protocol successfully for other in vitro studies on P. elaeagrifolia.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Al-Maarri, K., Arnaud, Y., and Miginiac, E. (1994). Micropropagation of Pyrus communis cultivar “Passe Crassane” seedlings and cultivar “Williams”: factors affecting root formation in vitro and ex vitro. Sci. Hortic. 58, 207–214. doi: 10.1016/0304-4238(94)90152-X
Barros, M. T. F., Hipolito, C. I., and Baptista, C. G. M. (2005). In vitro rooting of portuguese pear cultivars (Pyrus communis) in response to changes in auxin induction and dark period treatments. Acta Hortic. 671, 631–636.
Baviera, J. A., Garcia, J. L., and Ibarra, M. (1989). Commercial in vitro micropropagation of pear cv. conference. Acta Hortic. 256, 63–68.
Bell, R. L., Quamme, H. A., Layne, R. E. C., and Skirvin, R. M. (1996). “Pears,” in Fruit Breeding, Tree and Tropical Fruits, eds J. Janick and J. N. Moore (New York, NY: John Wiley and Sons, Inc.), 441–514.
Berardi, G., Infante, R., and Neri, D. (1993). Micropropagation of Pyrus calleryana Dcn. from seedlings. Sci. Hortic. 53, 157–165. doi: 10.1016/0304-4238(93)90146-H
Berardi, G., Neri, D., Majorino, A., and Adversi, R. (1991). In vitro rooting of Pyrus calleryana. Acta Hortic. 300, 181–188.
Bhojwani, S. S., Mullins, K., and Cohen, D. (1984). In vitro propagation of Pyrus pyrifolia. Sci. Hortic. 23, 247–254. doi: 10.1016/0304-4238(84)90068-2
Cheng, T. Y. (1979). Micropropagation of clonal fruit tree rootstocks. Compact Fruit Trees 12, 127–137.
Chevreau, E., and Bell, R. (2005). “Pyrus spp. pear and Cydonia spp. quince,” in Biotechnology of Fruit and Nut Crops, ed. R. E. Litz (Trowbridge: CABI Publishing), 543–565. doi: 10.1079/9780851996622.0543
Claveria, E., Asin, L., Iglesias, I., Vilardell, P., Bonany, J., Simard, M. H., et al. (2010). In vitro screening for tolerance to iron chlorosis as a reliable selection tool in appear rootstocks breeding program. Acta Hortic. 935, 199–206.
Dolcet-Sanjuan, R., Claveria, E., Bonany, J., Iglesias, I., Asin, L., and Simard, M., H. (2004). Selection for new pear rootstocks: in vitro screening and field evaluation for tolerance to iron chlorosis. Acta Hortic. 658, 463–468.
Dolcet-Sanjuan, R., Mok, D. W. S., and Mok, M. C. (1990). Micropropagation of Pyrus and Cydonia and their responses to fe-limiting conditions. Plant Cell Tissue Organ Cult. 21, 191–199. doi: 10.1007/BF00047611
Dolcet-Sanjuan, R., Mok, D. W. S., and Mok, M. C. (1991). In vitro manipulations of Pyrus species and Cydonia oblonga. Acta Hortic. 300, 45–50.
Dumanoglu, H., Aygun, A., Alay, A., Gunes, N. T., and Ozkaya, M. T. (1999). Effects of timing, IBA and putrescine on rooting and shooting in Pyrus elaegrifolia pall. softwood cuttings. Turk. J. Agric. For. 23, 559–565.
Evans, N. E. (1990). “Micropropagation,” in Methods in Molecular Biology. Plant Cell and Tissue Culture, eds J. W. Pollard and J. M. Walker (New Jersey: Humana Press), 93–103. doi: 10.1385/0-89603-161-6:93
Hartmann, H. T., Kester, D. E., Davies, F. T., and Geneve, R. L. (1997). Plant Propagation: Principles and Practices, 6th Edn. New Jersey: Prentice-Hall, Inc.
Iglesias, I., Vilardell, P., Bonany, J., Claveria, E., and Dolcet-Sanjuan, R. (2004). Micropropagation and field evaluation of the pear (Pyrus communis L.) “IGE 2002,” a new selection of the cultivar Dr. Jules Guyot. J. Am. Soc. Hort. Sci. 129, 389–393.
Koksal, A. I., Dumanoglu, H., and Gunes, N. (2002). “Ankara” Pear Cultivar. Ankara: Ajans-Turk Press.
Lombard, P. B., and Westwood, M. N. (1987). “Pear rootstocks,” in Rootstocks for Fruit Crops, eds R. C. Rom and R. F. Carlson (New York, NY: John Wiley and Sons, Inc.), 145–183.
McClelland, M. T., Smith, M. A. L., and Carothers, Z. B. (1990). The effects of in vitro and ex vitro root initiation on subsequent microcutting root quality in three woody plants. Plant Cell Tissue Organ Cult. 23, 115–123. doi: 10.1007/BF00035831
Moretti, C., Scozzoli, A., Pasini, D., and Paganelli, F. (1991). In vitro propagation of pear cultivars. Acta Hortic. 300, 115–117.
Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15, 473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x
Reed, B. M. (1995). Screening Pyrus germplasm for in vitro rooting response. HortScience 30, 1292–1294.
Rodriguez, R., Sala, C. D., Cuozzo, L., and Ancora, G. (1991). Pear in vitro propagation using a double-phase culture system. HortScience 26, 62–64.
Rossi, V., Paoli, G. D., and Pozzo, F. D. (1991). Propagation of Pyrus calleryana sel. D6 by in vitro culture. Acta Hort. 300, 145–148.
Shen, X. S., and Mullins, M. G. (1984). Propagation in vitro of pear, Pyrus communis L., cultivars “William’s Bon Chretien,” “Packham’s Triumph” and “Beurre Bosc.” Sci. Hortic. 23, 51–57. doi: 10.1016/0304-4238(84)90044-X
Shibli, R. A., Ajlouni, M. M., Jaradat, A., Aljanabi, S., and Shatnawi, M. (1997). Micropropagation in wild pear (Pyrus syrica). Sci. Hortic. 68, 237–242. doi: 10.1016/S0304-4238(96)00972-7
Singha, S. (1982). Influence of agar concentration on in vitro shoot proliferation of Malus sp. “Almey” and Pyrus communis “Seckel.” J. Am. Soc. Hort. Sci. 107, 657–660.
Webster, A. D. (1995). Temperate fruit tree rootstock propagation. N. Z. J. Crop Hortic. Sci. 23, 355–372. doi: 10.1080/01140671.1995.9513912
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keywords: micropropagation, Pyrus elaeagrifolia, tissue culture, wild pear
Citation: Aygun A and Dumanoglu H (2015) In vitro shoot proliferation and in vitro and ex vitro root formation of Pyrus elaeagrifolia Pallas. Front. Plant Sci. 6:225. doi: 10.3389/fpls.2015.00225
Received: 22 January 2015; Accepted: 22 March 2015;
Published online: 31 March 2015.
Edited by:
Andrea Polle, Georg-August-Universität Göttingen, GermanyReviewed by:
Thomas Teichmann, Georg-August-Universität Göttingen, GermanyBekir San, Suleyman Demirel University, Turkey
Copyright © 2015 Aygun and Dumanoglu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahmet Aygun, Department of Horticulture, Faculty of Agriculture, Ordu University, Ordu, TurkeyYXlndW5hNzBAeWFob28uY29t
 Ahmet Aygun
Ahmet Aygun Hatice Dumanoglu2
Hatice Dumanoglu2