- 1Key Laboratory of Biogeography and Bioresource in Arid Land, Xinjiang Institute of Ecology and Geography, Chinese Academy of Science, Urumqi, China
- 2University of Chinese Academy of Sciences, Beijing, China
RNA-sequencing has been widely used to obtain high throughput transcriptome sequences in various species, but the assembly of a full set of complete transcripts is still a significant challenge. Judging by the number of expected transcripts and assembled unigenes in a transcriptome library, we believe that some unigenes could be reassembled. In this study, using the nitrate transporter (NRT) gene family and phosphate transporter (PHT) gene family in Salicornia europaea as examples, we introduced an approach to further assemble unigenes found in transcriptome libraries which had been previously generated by Trinity. To find the unigenes of a particular transcript that contained gaps, we respectively selected 16 NRT candidate unigene pairs and 12 PHT candidate unigene pairs for which the two unigenes had the same annotations, the same expression patterns among various RNA-seq samples, and different positions of the proteins coded as mapped to a reference protein. To fill a gap between the two unigenes, PCR was performed using primers that mapped to the two unigenes and the PCR products were sequenced, which demonstrated that 5 unigene pairs of NRT and 3 unigene pairs of PHT could be reassembled when the gaps were filled using the corresponding PCR product sequences. This fast and simple method will reduce the redundancy of targeted unigenes and allow acquisition of complete coding sequences (CDS).
Introduction
Whole transcriptome sequencing (RNA-seq) with next-generation sequencing (NGS) technology has been used to uncover the complex landscape and dynamics of transcriptomes in various plant species since the success of the massively parallel pyrosequencing of the Arabidopsis transcriptome (Weber et al., 2007). Because of the great depth of sequencing it allows, RNA-seq can produce a nearly complete profile of a transcriptome, even including rare transcripts. Furthermore, RNA-seq has many advantages, such as the base-pair-level resolution, the large range of expression level, and de novo annotation (Martin and Wang, 2011). Compared with the high cost of genome sequencing, using RNA-seq, ordinary laboratories can produce transcriptome sequences for species of interest (Hamilton and Buell, 2012), and more than 50 different plant species have been sequenced using this technology (Schliesky et al., 2012).
There are two principal strategies for transcriptome assembly: de novo assembly or assembly based on a reference genome. A reference genome is not available for most species, therefore, de novo assembly of the transcriptome is commonly used for non-model species (Zhao et al., 2011). Several applications have been designed for de novo assembly, such as Multiple-k (Surget-Groba and Montoya-Burgos, 2010), Rnnotator (Martin et al., 2010), Trans-ABySS (Robertson et al., 2010), Oases (Schulz et al., 2012), and Trinity (Grabherr et al., 2011). However, regardless of which assembly application is employed, it is still difficult to reconstruct a comprehensive set of full-length transcripts, because RNA-sequence reads are often quite short and many informatics problems exist (Metzker, 2010; Martin and Wang, 2011). A Fermi estimate indicates that approximately 15,000 transcripts possibly exist in a plant transcriptome, and conceivably nearly twice as many, but not greater than 10-fold more (Schliesky et al., 2012). In fact, the number of unigenes (i.e., the final assembled sequence) often exceeds the number of expected transcripts (including isoforms), not to mention the large amount of discarded short unigenes between 0 and 200 bp in length (Schliesky et al., 2012). Therefore, some unigenes that represent the same transcript but contain a gap so that they cannot be assembled might exist in some transcriptome libraries. Finding and assembling these unigenes will allow the acquisition of full-length transcripts and further comprehensive study of the genome.
We believe that the same quantities of such unigenes were expressed in identical RNA-seq samples. RPKM (reads per kilobase of exon model per million mapped reads) is often used to quantify transcript levels in RNA-seq samples (Mortazavi et al., 2008). As shown in the schematic diagram in Figure 1, we assumed that two fragments comprised a transcript and that RT-qPCR and RNA-seq were respectively used to determine the expression of the transcript. Although, the qPCR primer pairs were designed to match different sequences in the transcript, the value obtained from Eq1 should be equal to that from Eq2. Similarly, if unigene1 and unigene2 both represent the same transcript, their expression levels as indicated by RPKM reflect the expression level of the transcript and in theory, RPKM1 should be equal to RPKM2. This should be a feasible method to distinguish the unigenes that represent the same transcript based on the expression patterns among various RNA-seq samples.
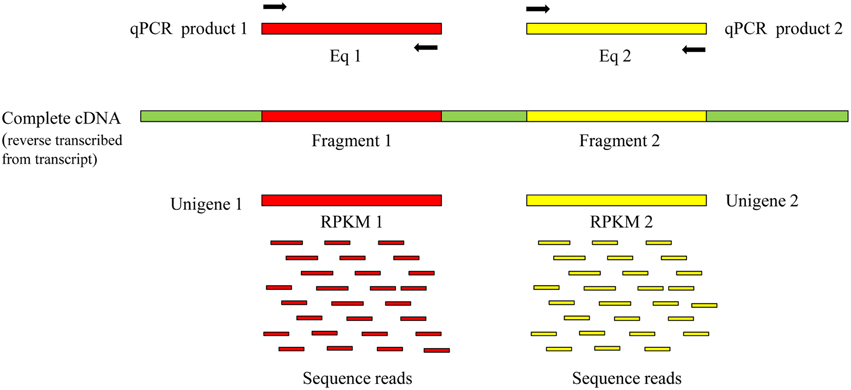
Figure 1. Schematic diagram for detecting the expression of a transcript on different fragments by RT-qPCR and RNA-seq. Eq. expression quantity. Black arrows represent primer pairs of RT-qPCR.
Salicornia europaea, one of the most salt-tolerant halophytes, can tolerate as much as 1000 mM NaCl in the soil (Flowers and Colmer, 2008; Lv et al., 2011). It also has a high capacity for the absorption of inorganic nitrogen, such as and (Webb et al., 2012). Three types of nitrate transporters, NRT1, NRT2, and NRT3 (NAR2) that aid in the transport of the principal inorganic nitrogen source have been identified in higher plants (Forde, 2000; Chen et al., 2008). In Arabidopsis, there are 53 NRT1 genes, 7 NRT2 genes and 2 NRT3 genes (Chapman and Miller, 2011; Tsay and Hsu, 2011). Some members of the NRT1 family are peptide transporters (PTR), therefore, NRT1 and PTR are usually classified into a single family known as NRT1/PTR (Tsay et al., 2007). For the absorption of inorganic phosphate (Pi), four types of phosphate transporters, PHT1, PHT2, PHT3, and PHT4, have been identified in higher plants. And there are 9 PHT1 genes, 1 PHT2 genes, 3 PHT3 genes and 6 PHT4 genes in Arabidopsis (Liu et al., 2011).
In a previous study, we subjected four samples of S. europaea to RNA-seq via Illumina HiSeq 2000 and assembled the sequence reads de novo using Trinity (Ma et al., 2013). There were 118 NRT unigenes (including 75 NRT1/PTR, 37 NRT2, and 6 NRT3) and 47 PHT unigenes (including 24 PHT1, 2 PHT2, 12 PHT3, and 9 PHT4) in the various transcriptome libraries. It is possible that some unigenes could be reassembled because Xiao et al. found that two unigenes for an ammonium transporter (AMT) in the transcriptome libraries were assembled through experimental validation (Xiao et al., 2014). However, a global analysis of all of the unigenes in the gene family with respect to the assembly of the AMT unigenes considered in this study was not performed and the expression pattern of the unigenes was not unambiguously classified. Therefore, in this study, the method was improved to systematically find and assemble putative unigenes that represented single transcripts for the entire NRT and PHT family in S. europaea. The steps of this method are shown in Figure 2.
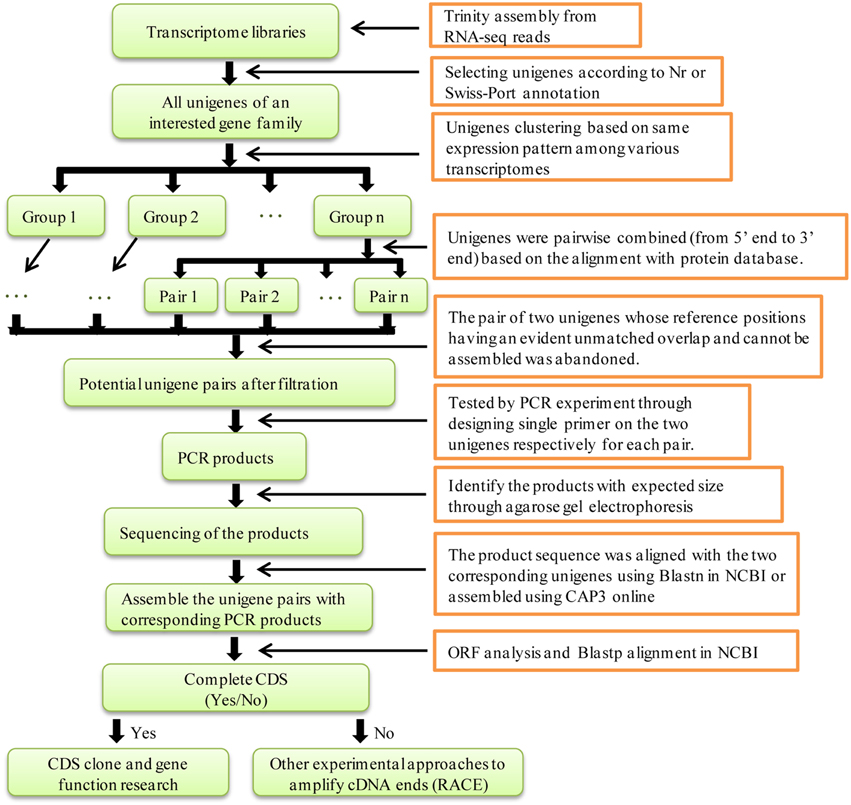
Figure 2. Flow chart for distinguishing and assembling potential unigenes for a transcript in a transcriptome library. The transcriptome library contained all of the unigenes which were assembled by Trinity from RNA-seq reads.
Materials and Methods
RNA-seq of S. europaea Samples
In a previous study, we RNA-sequenced four S. europaea samples (shoot and root, under 200 mM NaCl-treated or NaCl-free conditions, respectively) using the Illumina HiSeq 2000 (Ma et al., 2013). Numerous unigenes for each transcriptome library were generated and these unigenes could not be extended on either end as assembled by Trinity. A library named Unigene-All was clustered among the four transcriptome libraries using an application TGICL to obtain non-redundant unigenes as long as possible (Pertea et al., 2003). Functional annotation of the unigenes was accomplished using Blastx alignment (e < 0.00001) between the unigenes and protein databases including Nr, Swiss-Prot, KEGG, and COG. The proteins that with the highest sequence similarity were taken as the annotation for the unigenes. The RPKM method (Mortazavi et al., 2008), which normalizes for the effect of the gene lengths and sequencing levels in the calculation of gene expression was used to determine gene expression level under four conditions.
Collection and Clustering of NRT and PHT Unigenes
In this study, the NRT and PHT unigenes were collected from Unigene-All transcriptome library based on the Nr or Swiss-Prot annotation. The information for each unigene is comprised of the sequence length, the annotation and the RPKM values for corresponding libraries. The software Cluster 3.0 was used to group unigenes by hierarchical clustering method with log transform data according to the expression level in four libraries (de Hoon et al., 2004). The clustering results were viewed using Java TreeView (Strickler et al., 2012). Unigenes that had the same expression pattern were classified into a group.
Potential Unigenes Belonging to a Transcript after Filtration
The unigenes in each group were located in sequences from Arabidopsis thaliana using Blastx in GenBank to estimate the position of the corresponding transcript based on the alignment with a reference protein, for example, locating the 5′end but not the 3′ end, or locating the 3′end but not the 5′ end, or failing to locate either the 5′or the 3′ end. Pairwise analysis was used for the unigenes in each group and combinations in which two unigenes had an evident unmatched overlap and could not be assembled were not considered. However, combinations of unigenes that contained a gap or had not an evidence overlap were retained for further analysis.
Verified by PCR and Sequencing of Products
The selected pairs of unigenes were subjected to PCR amplification. We assumed that the two unigenes came from a single transcript and were joined together based on the position of the 5′ or the 3′ end. Forward and reverse primers were respectively designed based on the two corresponding unigenes, and at least a 200 bp overlap between the hypothetical PCR products and each unigene was ensured.
The total RNA of S. europaea was extracted using an RNeasy Mini Kit (Qiagen) and cDNA synthesis was accomplished with a Reversed Transcription Reagent Kit (TaKaRa) following the manufacturer's instructions. The PCR reaction was performed in Bio-Rad PCR Instrument in triplicate. The PCR reaction mixture contained 10 μL of high fidelity Es Taq mix (CWBIO), 2 μL of a 10-fold dilution of cDNA, 0.8 μL of each of the forward and reverse primers (10 μM), and 6.4 μL of PCR-grade water in a final volume of 20 μL. The following reaction conditions were used: 2 min at 94°C, 30 cycles of 30 s at 94°C and 30 s at the corresponding annealing temperature and 1 min at 72°C, and a final 5 min at 72°C. PCR products were visualized with 1.2% agarose gel electrophoresis and the expected bands were collected using a Gel Extraction Kit (Omega). The products were directly sequenced (BGI, Beijing) or sequenced after insertion into a pMD20-T vector (TaKaRa) and cloning in Escherichia Coli. The PCR sequences were aligned with the two corresponding unigenes using Blastn in NCBI and assembled based on matching overlaps using CAP3 (http://doua.prabi.fr/software/cap3) (Huang and Madan, 1999).
Sequence Analysis of the Assembled Unigenes
The assembled sequences were performed Blastx alignment in nr database of Arabidopsis thaliana to determine their annotation of coding protein. Open reading frames (ORF) were analyzed on ORF Finder (http://www.ncbi.nlm.nih.gov/gorf/gorf.html) to find the biggest one and whether that had a termination codon forward the initiation codon in 5′ end. The deduced amino acid sequences were searched against the nr protein database using Blastp in GenBank to judge whether it was a complete CDS through comparing the number of amino acid with closely related organisms.
Expression Analysis of Two Fragments of a Transcript
To prove that the unigenes that comprise a single transcript had same expression levels, we randomly chose two pairs of unigenes (comprising to a transcript) and quantified the gene expression via real-time qPCR. S. europaea was exposed to NaCl stress or NaCl-free conditions, and then the shoots and roots were collected in triplicate. The total RNA was extracted using an RNeasy Mini Kit (Qiagen) and contaminating DNA was eliminated using an RNase-free DNase kit (Qiagen). An aliquot of 1 μg of the total RNA was used for cDNA synthesis at a final volume of 20 μL using a Reverse Transcription Reagent Kit (TaKaRa) following the manufacturer's instructions. Specific primer pairs for various unigenes were designed online using NCBI Primer-BLAST (Ye et al., 2012). Two reference genes (UBC and CAC) were used as an internal control for RT-qPCR under NaCl stress (Xiao et al., 2015a). RT-qPCR was performed in 96-well optical plates with a CFX96 Real-Time PCR Detection System (Bio-Rad, USA) using SYBR real-time PCR premixture (BioTeke, Beijing). The relative expression quantities of the unigenes were calculated by the 2−ΔΔCt method (Livak and Schmittgen, 2001).
Results
NRT and PHT Unigene Sets
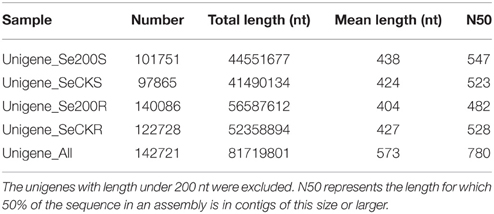
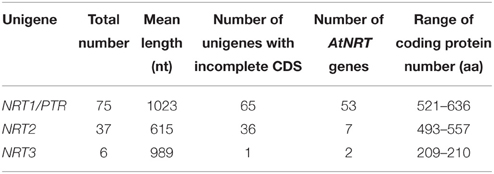
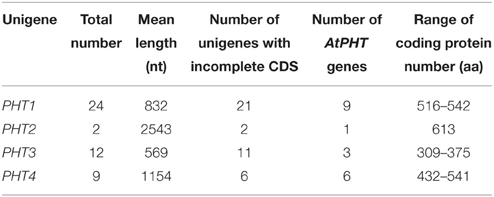
More than 140,000 high-quality unigenes in the Unigene-All transcriptome library were generated and had a mean size of 573 bp, which indicated that the transcriptomes were not completely assembled and that most unigenes did not contain a complete CDS (coding sequence) because of their short length and N50 value (Table 1). We found a total of 118 NRT unigenes, including 75 NRT1 unigenes, 37 NRT2 unigenes, and 6 NRT3 unigenes per Nr annotation or Swiss-Prot annotation, in which only 10 NRT1 unigenes, 1 NRT2 unigene and 5 NRT3 unigenes contained a complete CDS as indicated by an ORF analysis and Blastx alignment with other species in GenBank (Table 2A). NRT3 transcripts were relatively well assembled because of their short coding region (approximately 200 aa), while most unigenes for NRT1/PTR and NRT2 did not contain a complete CDS and could not be directly utilized for studies on gene function. Besides, 47 PHT unigenes were found, including 24 PHT1 unigenes, 2 PHT2 unigenes, 12 PHT3 unigenes, and 9 PHT4 unigenes, in which most did not contained a complete CDS except 3 PHT1 unigenes, 1 PHT3 unigenes, and 3 PHT4 unigenes (Table 2B).
Expression Patterns Clustering and Unigenes Grouping
To find unigenes that had the same expression pattern, we clustered the NRT1, NRT2, NRT3, PHT1, PHT2, PHT3, and PHT4 unigenes based on gene expression under four conditions (200S, CKS, 200R, CKR). The unigenes, which are the nearest neighbors and indicate the shortest branch, have the most similar expression pattern. Based on the similarity of the unigenes expression, two to five unigenes were clustered into a group for further analysis, which were marked a box (Figures S1A, S1B). For the cluster of NRT unigenes, 18 NRT1 groups and 9 NRT2 groups were generated for further analysis (Table S1). There was no analysis for NRT3, because only 1 NRT3 unigene (Unigene60534) did not have complete CDS and could not be assembled with other 5 NRT3 unigenes that all had a complete CDS. For the cluster of PHT unigenes, 4 PHT1 groups, 1 PHT2 groups, 4 PHT3 groups, and 2 PHT4 groups were selected for next step (Table S2).
Potential Unigene Pairs Belonging to a Transcript
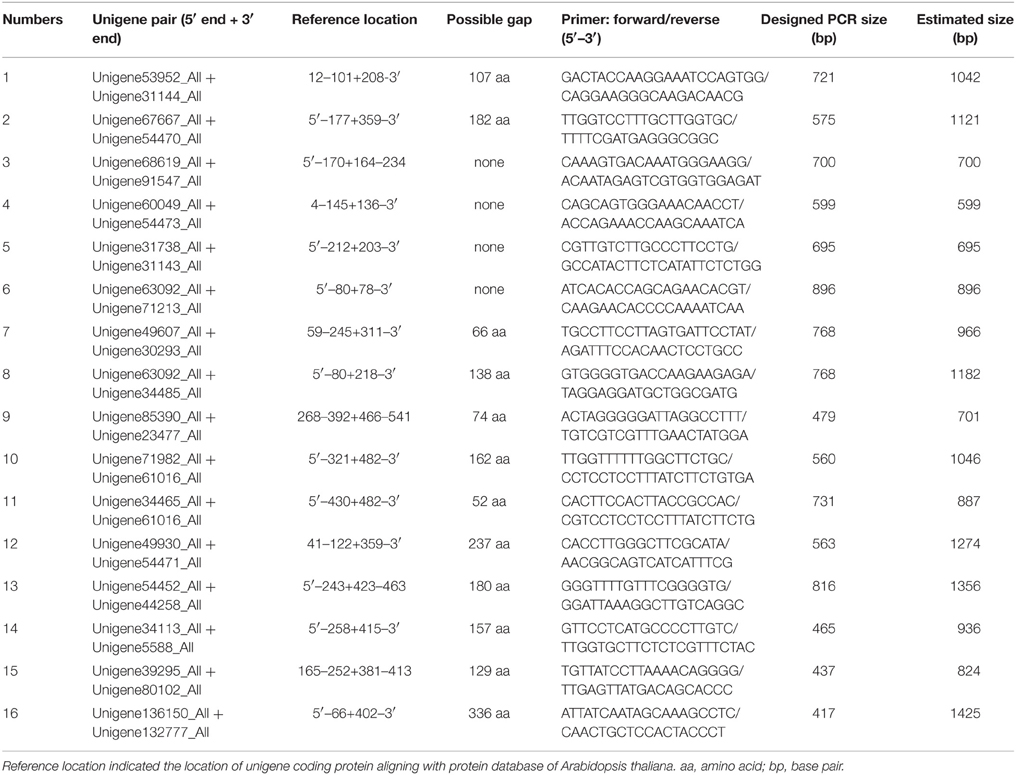
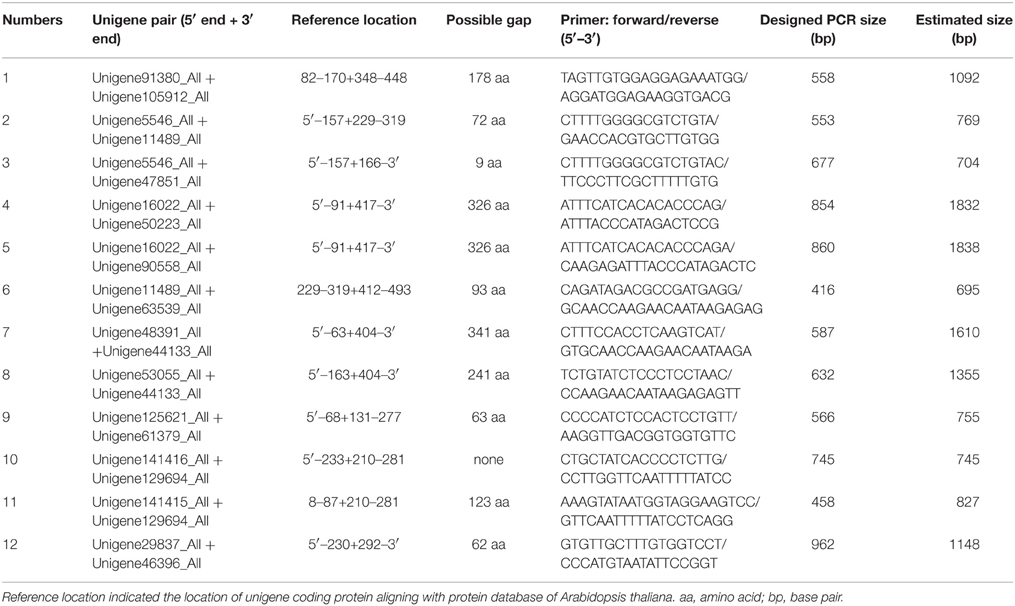
The position of a unigene was estimated according to its alignment with a reference protein using Blastx in GenBank. Unigene pairs (two unigenes), which had a gap or did not have an evident overlap were combined and generated a supposed transcript according to the position of encoding protein. As shown in Table S1, we selected 0–2 potential NRT unigene pairs for each group. In total 16 unigene pairs, including 12 NRT1 unigene pairs and 4 NRT2 unigene pairs were selected for further experimental verification (Table 3A). As shown in Table S2, we selected 0–3 potential PHT unigene pairs for each group. Twelve unigene pairs, including 8 PHT1 unigene pairs, 3 PHT3 unigene pairs, and 1 PHT4 unigene pairs were selected for next experimental verification (Table 3B).
Validation of Unigene Pairs by PCR and Sequencing
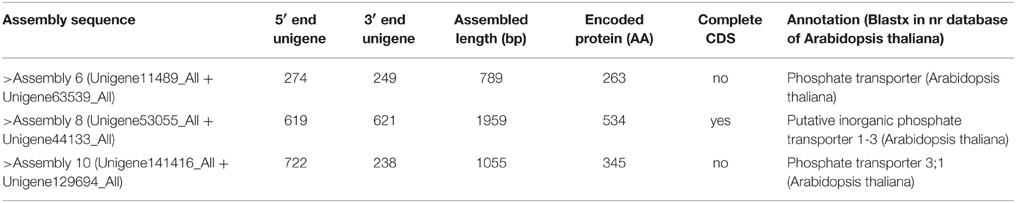
PCR products would be generated through the polymerase chain reaction using corresponding primers, if two unigenes represent a single transcript. Figure 3 shows an electrophoretic analysis of the PCR products amplified using corresponding primer pairs. One or more bands can be found in lanes 3–8, 11, 14 in Figure 3A (NRT) and lanes 6–8, 10–12 in Figure 3B (PHT). From each lane we extracted and sequenced bands (marked arrows) whose size was the size expected from the designed primers plus the size of supposed gap. We found that 10 NRT unigenes (5 unigene pairs: Unigene68619_All + Unigene91547_All; Unigene60049_All + Unigene54473_All; Unigene49607_All + Unigene30293_All; Unigene34465_Al + Unigene61016_All; Unigene34113_All + Unigene5588_All) and 6 PHT unigenes (3 unigene pairs: Unigene11489_All + Unigene63539_All; Unigene53055_All + Unigene44133_All; Unigene141416_All + Unigene129694_All) could be assembled with corresponding PCR products (Figures S2A, S2B; Data Sheets 1, 2).
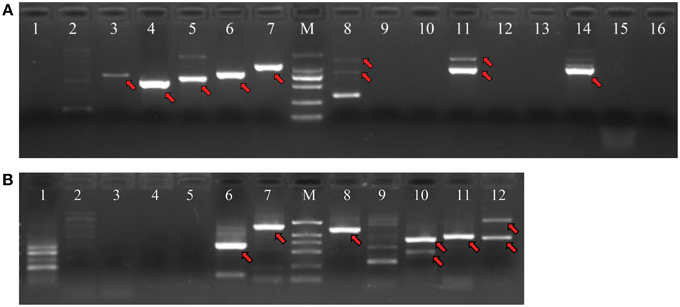
Figure 3. Agarose gel electrophoresis of PCR products for unigene pairs. PCR reactions were performed with corresponding primer pairs and a pooled cDNA template. Amplified fragments were separated by 1.2% agarose gel electrophoresis. (A) PCR products for NRT unigenes. (B) PCR products for PHT unigenes. M: DNA marker with bands of 2000 bp, 1000 bp, 750 bp, 500 bp, 250 bp, 100 bp from high to low, respectively. The serial numbers represent corresponding PCR reactions for various unigene pairs, which are coincident with the number in Tables 3A,B.
Sequence Information of the Assembled Unigenes
According to the best alignment results against the nr protein database of Arabidopsis thaliana, all 5 NRT assembled sequences were annotated nitrate transporter (Table 4A) and all 3 PHT assembled sequences were annotated phosphate transporter (Table 4B). Two of NRT assembled sequences and one of PHT assembled sequences contained complete CDS as indicated by an ORF analysis and a Blastp alignment in GenBank, which could presumably be used for further research on gene function.
Two Fragments of a Transcript have Same Expression Quantities
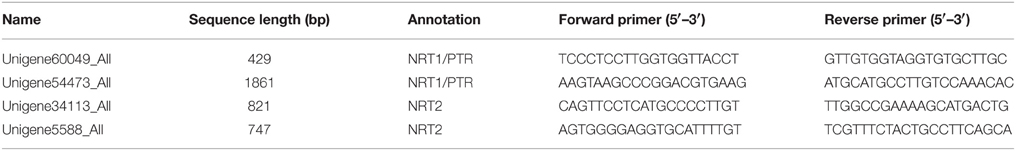
We quantified the expression levels of two pairs of unigenes (Unigene60049_All and Unigene54473_All; Unigene34113_All and Unigene5588_All) under various NaCl stress conditions via real-time qPCR technology (Table 5). There was no significant difference between the two unigenes for a single transcript in terms of expression level under the various conditions investigated (Figure 4). Furthermore, we compared the RPKM value between the two unigenes in each pair (comprising a transcript) for four RNA-seq samples (Figure 5) and found that the values were basically consistent. Although, the RPKM values of the unigene pairs (Unigene68619_All + Unigene91547_All) were not equal under the different conditions because of the bias of the RNA-seq method (Mortazavi et al., 2008), the trends of change were identical among the various samples. RPKM is a common approach for calculating the amount of the expression of many unigenes via RNA-seq, and this study indicated that the unigenes representing a single transcript had the same expression patterns based on clustering analysis of RPKM. It is an effective method to distinguish the unigenes representing a single transcript over a large range of expression levels (i.e., an RPKM range from zero to tens of thousands or higher). Moreover, the greater number of RNA-seq samples one obtains, the more expression patterns are available to single out unigenes, making this method more effective to find the unigenes for a single transcript. Unigenes that have the same annotation and same expression pattern in multiple samples are very likely to arise from the same transcript.
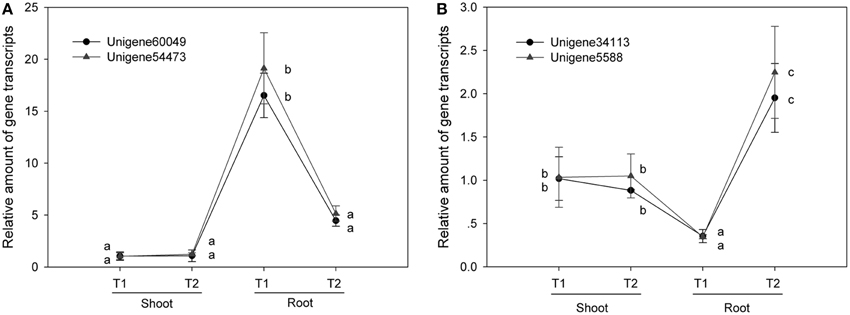
Figure 4. RT-qPCR shows that unigenes for the same transcript have the same expression levels. The same letter indicates no significant difference. T1, T2: the treatment under NaCl stress or NaCl-free conditions. (A) Unigenes pair 4 (NRT). (B) Unigenes pair 14 of (NRT).
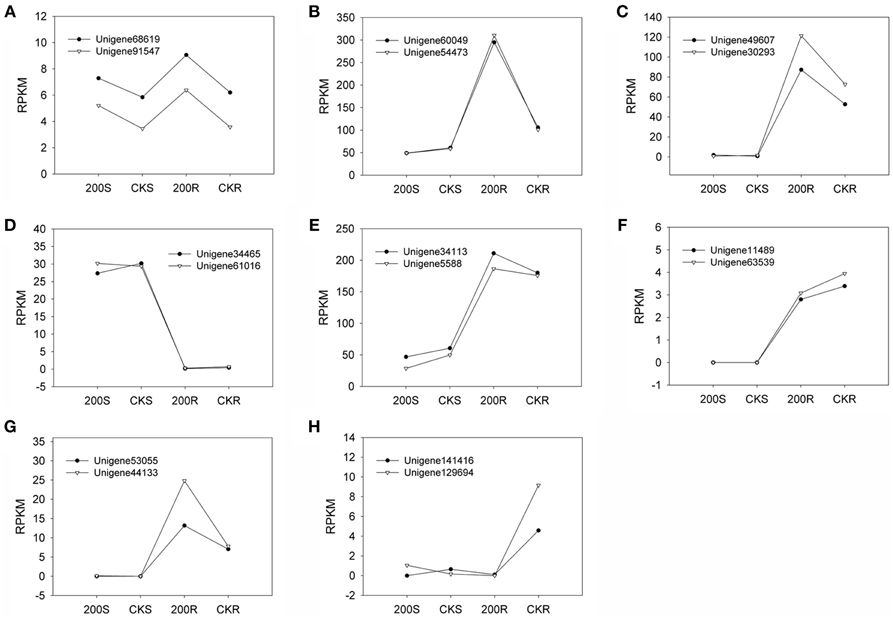
Figure 5. Expression pattern for 8 pairs of unigenes belonging to a transcript. (A–E): NRT unigene pairs. (F–H): PHT unigene pairs. RPKM (reads per kilobase of exon model per million mapped reads): a method for indicating the expression level of a unigene. Four transcriptome libraries (200S, CKS, 200R, CKR) provided samples for RNA-seq.
Discussion
RNA-seq has been widely applied to obtain transcriptome data because of its great advantages (Van Verk et al., 2013). Identifying a full set of transcripts assembled from huge amounts of raw reads is an essential requirement for the deep study of a species. However, at present, no assembly application can generate a complete transcriptome containing full-length transcripts (Steijger et al., 2013). The number of unigenes is far higher than the estimated number of transcripts and most unigenes are not full length (Schliesky et al., 2012), which indicates that two or more unigenes belong to the same transcript but exist separately in the transcriptome library. Therefore, some of these unigenes could be reassembled.
To find the unigenes that belong to a particular transcript is not easy in a transcriptome library that contains more than a 100,000 unigenes. Unigenes that have different annotations are unlikely to represent the same transcript, therefore limiting the analysis to a gene family or subfamily (i.e., one in which the unigenes that have same Nr or Swiss-Prot annotation) of interest will greatly reduce the difficulty of finding the unigenes of a single transcript. The number of unigenes for a gene family, which usually ranges from several to hundreds, depends on the size of the member genes and the quality of the assembly. In a S. europaea transcriptome library, we found 118 NRT unigenes, which could be further divided into 75 NRT1 unigenes, 37 NRT2 unigenes and 6 NRT3 unigenes. It was more practical to purposefully analyze a small number of unigenes by excluding many irrelevant unigenes, and validate the results of the analysis experimentally.
It has been shown that different unigenes for the same transcript have same expression level under identical conditions per RT-qPCR (Figure 4). Attempting to find the unigenes for a transcript based on same expression pattern under various conditions is a novel approach. RT-qPCR is a powerful tool for detecting gene expression because of its accuracy and sensitivity, but the number of detecting genes is limited (Xiao et al., 2015b), however, we can generate expression profiles for thousands of genes via RNA-seq, using a method such as RPKM, calculated based on the rate of randomly-picked reads and sequence lengths (Grabherr et al., 2011). Although, the unigenes, belonging to a given transcript might not show identical RPKM values because of a difference in the bias of the RNA-seq outcome (Wang et al., 2009), the values are approximate and the changes in the expression trends proved to be consistent among various RNA-seq samples (Figure 5). The unigenes were clustered based on their expression patterns, therefore, the more RNA-seq libraries analyzed, the more likely that the unigenes of a given transcript could be distinguished from unrelated unigenes. Clustering unigenes that have same expression pattern greatly reduces the combinations of unigenes that must be reassembled. Adding more unigenes to a group will yield more combinations so the loss of possible unigene pairs can be avoided, but the workload is greatly increased. In this study, 2–5 unigenes with similar expression patterns were grouped according the clustering results and we found that 8 pairs of unigenes could be reassembled from 118 NRT unigenes and 47 PHT unigenes.
To further decrease invalid experimental validation, impossible combinations of two unigenes in a group were excluded if their positions of the proteins coded conflicted with each other with respect to the protein sequence of Arabidopsis thaliana. We assumed that if the protein coded by two unigenes overlapped but the overlapping sequences were not identical, the unigenes must belong to a different transcript and were unlikely to be assembled. Although, the RNA-seq reads were assembled using the Trinity application, there were still some unigenes for particular transcripts that could not be assembled because of gaps. This study introduced a method to find these unigenes and fill the gaps.
Some unigenes might belong to a different transcript even though they have the same expression pattern. Therefore, putative unigene pairs should be validated by PCR technology (Saiki et al., 1988). The primer pairs contain forward and reverse primer sequences which were designed by mapping two different unigenes. If the two unigenes belong to the same transcript, the PCR product will be amplified exponentially, otherwise, no bands (except nonspecific amplification bands) can be found in agarose gels after electrophoresis. We validated 5 pairs of NRT unigenes from 16 pairs of putative unigenes and 3 pairs of PHT unigenes from 12 pairs of putative unigenes to comprise transcripts. The success rate of assembly can reach 31% and 25% respectively. Thus, we have discovered a feasible method for the further assembly of target unigenes.
Conclusion
Unigenes for a particular transcript that remain unassembled in transcriptome libraries after performing RNA-seq assembly using present assemblers still exist. Unigenes having a similar annotation and the same expression pattern in multiple samples are very likely to represent the same transcript. We introduced a novel approach to distinguish these putative unigenes and to further assemble a few unigenes of a gene family after experimental verification, which is a quick and useful way to extend incomplete cDNA sequences or obtain complete coding regions. This method was validated by assembling unigenes from NRT and PHT gene families and successfully produced 8 assembled sequences. Moreover, because it involves common bioinformatics tools available online and routine molecular biological techniques, biologists can easily grasp the method. We suggest that this method be given priority for use to assemble unigenes of interest when extending incomplete CDS before using rapid-amplification of cDNA ends (RACE) technology, because it assembles more unigenes more cheaply and is easy to operate without a complicated bioinformatics analysis.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the National Nature Science Foundation of China (Grant No. 31270660), the Outstanding Youth Talent Foundation for Science and Technology in Xinjiang Uygur Autonomous Region of China (No. 2013711018) and the Program of Western Doctoral Talent (No. XBBS 201201).
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2015.00843
Figure S1A. Hierarchical clustering of expression pattern for the three types of NRT unigenes. The unigenes on the closer branch had more similar expression pattern, which were grouped and marked a red box. The number represents the base pairs number of corresponding unigene.
Figure S1B. Hierarchical clustering of expression pattern for the four types of PHT unigenes. The unigenes on the closer branch had more similar expression pattern, which were grouped and marked a red box.
Figure S2A. Alignment between a PCR product and corresponding NRT unigene pairs using Blastn in NCBI. A PCR product served as the Query sequence.
Figure S2B. Alignment between a PCR product and corresponding PHT unigene pairs using Blastn in NCBI. A PCR product served as the Query sequence.
Table S1. Information regarding 27 groups of NRT unigenes. The unigenes were grouped according to the clustering of the expression patterns. The reference location indicated the location of unigene coding protein aligned with the protein database of Arabidopsis thaliana.
Table S2. Information regarding 11 groups of PHT unigenes. The unigenes were grouped according to the clustering of the expression patterns. The reference location indicated the location of unigene coding protein aligned with the protein database of Arabidopsis thaliana.
Data Sheet 1. NRT sequences of Salicornia europaea. The doc file contains the sequences of 16 unigenes pairs, 10 sequences of PCR products, and 5 assembled sequences.
Data Sheet 2. PHT sequences of Salicornia europaea. The doc file contains the sequences of 12 unigenes pairs, 8 sequences of PCR products, and 3 assembled sequences.
References
Chen, Y. F., Wang, Y., and Wu, W. H. (2008). Membrane transporters for nitrogen, phosphate and potassium uptake in plants. J. Integr. Plant Biol. 50, 835–848. doi: 10.1111/j.1744-7909.2008.00707.x
de Hoon, M. J. L., Imoto, S., Nolan, J., and Miyano, S. (2004). Open source clustering software. Bioinformatics 20, 1453–1454. doi: 10.1093/bioinformatics/bth078
Flowers, T. J., and Colmer, T. D. (2008). Salinity tolerance in halophytes. New Phytol. 179, 945–963. doi: 10.1111/j.1469-8137.2008.02531.x
Forde, B. G. (2000). Nitrate transporters in plants: structure, function and regulation. Biochimica Et Biophysica Acta Biomembranes 1465, 219–235. doi: 10.1016/S0005-2736(00)00140-1
Grabherr, M. G., Haas, B. J., Yassour, M., Levin, J. Z., Thompson, D. A., Amit, I., et al. (2011). Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 29, 644–652. doi: 10.1038/nbt.1883
Hamilton, J. P., and Buell, C. R. (2012). Advances in plant genome sequencing. Plant J. 70, 177–190. doi: 10.1111/j.1365-313X.2012.04894.x
Huang, X., and Madan, A. (1999). CAP3: a DNA sequence assembly program. Genome Res. 9, 868–877. doi: 10.1101/gr.9.9.868
Liu, F., Chang, X.-J., Ye, Y., Xie, W.-B., Wu, P., and Lian, X.-M. (2011). Comprehensive sequence and whole-life-cycle expression profile analysis of the phosphate transporter gene family in rice. Mol. Plant 4, 1105–1122. doi: 10.1093/mp/ssr058
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Lv, S., Jiang, P., Chen, X., Fan, P., Wang, X., and Li, Y. (2011). Multiple compartmentalization of sodium conferred salt tolerance in Salicornia europaea. Plant Physiol. Biochem. 51, 47–52. doi: 10.1016/j.plaphy.2011.10.015
Ma, J., Zhang, M., Xiao, X., You, J., Wang, J., Wang, T., et al. (2013). Global transcriptome profiling of Salicornia europaea L. Shoots under NaCl Treatment. PLoS ONE 8:e65877. doi: 10.1371/journal.pone.0065877
Martin, J. A., and Wang, Z. (2011). Next-generation transcriptome assembly. Nat. Rev. Genet. 12, 671–682. doi: 10.1038/nrg3068
Martin, J., Bruno, V. M., Fang, Z., Meng, X., Blow, M., Zhang, T., et al. (2010). Rnnotator: an automated de novo transcriptome assembly pipeline from stranded RNA-Seq reads. BMC Genomics 11:663. doi: 10.1186/1471-2164-11-663
Metzker, M. L. (2010). Sequencing technologies - the next generation. Nat. Rev. Genet. 11, 31–46. doi: 10.1038/nrg2626
Mortazavi, A., Williams, B. A., Mccue, K., Schaeffer, L., and Wold, B. (2008). Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 5, 621–628. doi: 10.1038/nmeth.1226
Pertea, G., Huang, X. Q., Liang, F., Antonescu, V., Sultana, R., Karamycheva, S., et al. (2003). TIGR Gene Indices clustering tools (TGICL): a software system for fast clustering of large EST datasets. Bioinformatics 19, 651–652. doi: 10.1093/bioinformatics/btg034
Robertson, G., Schein, J., Chiu, R., Corbett, R., Field, M., Jackman, S. D., et al. (2010). De novo assembly and analysis of RNA-seq data. Nat. Methods 7, 909–912. doi: 10.1038/nmeth.1517
Saiki, R. K., Gelfand, D. H., Stoffel, S., Scharf, S. J., Higuchi, R., Horn, G. T., et al. (1988). Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 239, 487–491. doi: 10.1126/science.2448875
Schliesky, S., Gowik, U., Weber, A. P. M., and Bräutigam, A. (2012). RNA-seq assembly - are we there yet? Front. Plant Sci. 3:220. doi: 10.3389/fpls.2012.00220
Schulz, M. H., Zerbino, D. R., Vingron, M., and Birney, E. (2012). Oases: robust de novo RNA-seq assembly across the dynamic range of expression levels. Bioinformatics 28, 1086–1092. doi: 10.1093/bioinformatics/bts094
Steijger, T., Abril, J. F., Engstrom, P. G., Kokocinski, F., Hubbard, T. J., Guigo, R., et al. (2013). Assessment of transcript reconstruction methods for RNA-seq. Nat. Methods 10, 1177–1184. doi: 10.1038/nmeth.2714
Strickler, S. R., Bombarely, A., and Mueller, L. A. (2012). Designing a transcriptome next-generation sequencing project for a nonmodel plant species. Am. J. Bot. 99, 257–266. doi: 10.3732/ajb.1100292
Surget-Groba, Y., and Montoya-Burgos, J. I. (2010). Optimization of de novo transcriptome assembly from next-generation sequencing data. Genome Res. 20, 1432–1440. doi: 10.1101/gr.103846.109
Tsay, Y. F., Chiu, C. C., Tsai, C. B., Ho, C. H., and Hsu, P. K. (2007). Nitrate transporters and peptide transporters. FEBS Lett. 581, 2290–2300. doi: 10.1016/j.febslet.2007.04.047
Tsay, Y., and Hsu, P. (2011). The Role of Plasma Membrane Nitrogen Transporters in Nitrogen Acquisition and Utilization. Berlin: Springer.
Van Verk, M. C., Hickman, R., Pieterse, C. M. J., and Van Wees, S. C. M. (2013). RNA-Seq: revelation of the messengers. Trends Plant Sci. 18, 175–179. doi: 10.1016/j.tplants.2013.02.001
Wang, Z., Gerstein, M., and Snyder, M. (2009). RNA-Seq: a revolutionary tool for transcriptomics. Nat. Rev. Genet. 10, 57–63. doi: 10.1038/nrg2484
Webb, J. M., Quintã, R., Papadimitriou, S., Norman, L., Rigby, M., Thomas, D. N., et al. (2012). Halophyte filter beds for treatment of saline wastewater from aquaculture. Water Res. 46, 5102–5114. doi: 10.1016/j.watres.2012.06.034
Weber, A. P., Weber, K. L., Carr, K., Wilkerson, C., and Ohlrogge, J. B. (2007). Sampling the Arabidopsis transcriptome with massively parallel pyrosequencing. Plant Physiol. 144, 32–42. doi: 10.1104/pp.107.096677
Xiao, X., Ma, J., Wang, J., Wu, X., Li, P., and Yao, Y. (2015a). Validation of suitable reference genes for gene expression analysis in the halophyte Salicornia europaea by real-time quantitative PCR. Front. Plant Sci. 5:788. doi: 10.3389/fpls.2014.00788
Xiao, X., Wu, X., Ma, J., Li, P., Li, T., and Yao, Y. (2015b). Systematic assessment of reference genes for RT-qPCR across plant species under salt stress and drought stress. Acta Physiol. Plant. 37, 186. doi: 10.1007/s11738-015-1922-8
Xiao, X., Zhang, X., Wu, X., Ma, J., and Yao, Y. A. (2014). Assembling of an ammonium transporter gene in Salicornia europaea by expression pattern analysis of Unigene in transcriptome. Chin. J. Biotechnol. 30, 1763–1773. doi: 10.13345/j.cjb.140116
Ye, J., Coulouris, G., Zaretskaya, I., Cutcutache, I., Rozen, S., and Madden, T. L. (2012). Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformat. 13:134. doi: 10.1186/1471-2105-13-134
Keywords: RNA-seq, unigene assembly, Salicornia europaea, nitrate transporter gene, phosphate transporter gene, expression pattern, RPKM
Citation: Xiao X, Ma J, Sun Y and Yao Y (2015) A method for the further assembly of targeted unigenes in a transcriptome after assembly by Trinity. Front. Plant Sci. 6:843. doi: 10.3389/fpls.2015.00843
Received: 27 July 2015; Accepted: 25 September 2015;
Published: 14 October 2015.
Edited by:
Agnieszka Ludwików, Adam Mickiewicz University, PolandReviewed by:
Lei Song, National Cancer Institute, USAAgata Ciesla, Adam Mickiewicz University, Poland
Copyright © 2015 Xiao, Ma, Sun and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yinan Yao, eWFveWFAbXMueGpiLmFjLmNu
 Xinlong Xiao
Xinlong Xiao Jinbiao Ma2
Jinbiao Ma2 Yinan Yao
Yinan Yao