- Division of Plant Microbe Interactions, Council of Scientific and Industrial Research-National Botanical Research Institute, Lucknow, India
Along with many adaptive strategies, dynamic changes in protein abundance seem to be the common strategy to cope up with abiotic stresses which can be best explored through proteomics. Understanding of drought response is the key to decipher regulatory mechanism of better adaptation. Rice (Oryza sativa L.) proteome represents a phenomenal source of proteins that govern traits of agronomic importance, such as drought tolerance. In this study, a comparison of root cytoplasmic proteome was done for a drought tolerant rice (Heena) cultivar in PEG induced drought conditions. A total of 510 protein spots were observed by PDQuest analysis and 125 differentially regulated spots were subjected for MALDI-TOF MS-MS analysis out of which 102 protein spots identified which further led to identification of 78 proteins with a significant score. These 78 differentially expressed proteins appeared to be involved in different biological pathways. The largest percentage of identified proteins was involved in bioenergy and metabolism (29%) and mainly consists of malate dehydrogenase, succinyl-CoA, putative acetyl-CoA synthetase, and pyruvate dehydrogenase etc. This was followed by proteins related to cell defense and rescue (22%) such as monodehydroascorbate reductase and stress-induced protein sti1, then by protein biogenesis and storage class (21%) e.g. putative thiamine biosynthesis protein, putative beta-alanine synthase, and cysteine synthase. Further, cell signaling (9%) proteins like actin and prolyl endopeptidase, and proteins with miscellaneous function (19%) like Sgt1 and some hypothetical proteins were also represented a large contribution toward drought regulatory mechanism in rice. We propose that protein biogenesis, cell defense, and superior homeostasis may render better drought-adaptation. These findings might expedite the functional determination of the drought-responsive proteins and their prioritization as potential molecular targets for perfect adaptation.
Introduction
Rice (Oryza sativa L.) is an important staple cereal crops ranked second after maize worldwide and considered as a primary source of food for more than half the world's population including Asia. India is the second largest producer of rice after China and account for about 20% of all world rice production. Rice is exclusively grown for human consumption and therefore there is immense pressure for high production to feed the largely growing world population (Ahsan et al., 2008). Climate related diverse abiotic stresses (drought, flood, salt, cold etc.) are the principal sources of risk and uncertainties in agriculture and causes wide fluctuations in agricultural output. While attempts have been made to reduce the adverse effects of weather on agriculture through scientific research and technology development, the performance of agriculture, especially in developing countries, still depends largely on the weather. Drought is the major abiotic stress limiting the productivity of rice as it hampers plant growth and development, and shrinks harvest size (Subba et al., 2013). In Asia, drought, a major abiotic stress, is responsible for affecting about 20% of the total rice-growing area (Pandey and Bhandari, 2008). Rice germplasm exhibit a tremendous genetic source for controlling agronomical importance characters such as drought tolerance. Moreover, relatively smaller genome size and well-organized database make it a choice of model plant for monocots to study the physiological, biochemical and molecular aspects during development, abiotic as well as biotic stress conditions (Atkinson and Urwin, 2012; Narsai et al., 2013). The better understanding of drought tolerance mechanism and the efficiency to develop drought tolerant varieties can be correlated with identification of trait associated genes (Tuberosa and Salvi, 2006; Lafitte et al., 2007; Sreenivasulu et al., 2007). However, it remains a challenging task due to its highly complex network of several metabolic pathways (Price et al., 2002). Understanding the mechanism of dehydration response is the key to decipher the regulatory mechanism of better adaptation (Subba et al., 2013).
Plant roots are important organs to uptake soil water and nutrients, perceiving and transducing of soil water deficit signals to shoot (Davies and Zhang, 1991; Moumeni et al., 2011) which further triggers an array of physiological, morphological and molecular responses in the whole plant. Understanding the root responses through transcriptomics with parallel insights on protein level changes has always been an area of interest and mostly relies on comparative studies of diverse genetic background under drought (Sengupta et al., 2011). Roots are supposed to be the primary site for sensing drought stress to initiate signaling cascade at molecular level in responses to drought. Plants can successfully adopt a tolerance mechanism against environmental stress by regulating gene expression through transcriptional changes in regulatory and functional proteins (Périn et al., 2007). In a study, Kawasaki et al. (2001) observed that gene expression profiling of rice under salt stress may result in up and downregulation of ~10% of the transcripts. However, the gene expression studies do not offer insights into the quantity and quality of the proteins as the amount of proteins is not always correlated to that of mRNA. The post-translational modifications like phosphorylation, glycosylation and removal of signal peptides enable proteins for activities and subcellular localization (Kawasaki et al., 2001). As a consequence, understanding the stress mechanism at protein level may provide some more useful insights to study the actual biotic stress response. In this context, evolution of proteomics is playing a crucial role as necessary and complementary approach to address such issues in the post-genomic era (Zivy and de Vienne, 2000; van Wijk, 2001). Serious effort has been made for functional identification of tissues, organs, and development specific rice gene under environmental changes like biotic and abiotic stresses using systematic studies in proteomic analysis (Khan and Komatsu, 2004; Komatsu, 2005; Ali and Komatsu, 2006) and knowledge of these proteins would help in understanding the stress tolerance related molecular mechanism in rice at the translation level.
We report here the comparative proteomic analysis of rice root for PEG simulated drought responsiveness in time dependent manner. Proteins were separated by two-dimensional gel electrophoresis (2-DE) followed by PD Quest analysis and identified by MALDI-Mass Spectrometry (MS) using available proteome databases.
Materials and Methods
Plant Material Collection
After screening of several local rice varieties, a drought tolerant variety Heena cultivated in northern Indian was selected for the present study. Three-weeks-old seedlings of rice were stressed for 7 days using 20% polyethylene glycol PEG-6000 in the nutrient solution. Exposure to PEG-6000 solutions is supposed to mimic drought stress with limited metabolic interferences. Effective screening of large sets of germplasm for drought tolerance has been carried out by PEG-based in vitro screening as a suitable method with good accuracy (Muscolo et al., 2014). Roots samples from three independent biological replicates were harvested on first, third, and seventh day and immediately flash frozen in liquid nitrogen and stored independently at −80°C for further analyses.
Extraction of Rice Root Proteins and 2-Dimensional Gel Electrophoresis (2DE) Analysis
Rice roots harvested in three replications were pooled to normalize the effect of variations in the biological replicates and used for extraction of soluble proteins. Root tissue (5 g) was pulverized to a fine powder in liquid nitrogen and suspended in 10 ml lysis buffer containing 0.5 M Tris HCl (pH 7.5), 0.1 M KCl, 50 mM EDTA (pH 8.0), 0.7 M Sucrose, 10 mM thiourea, 2 mM PMSF, and 2% β-mercaptoethenol. Suspension was mixed with 10 ml of Tris saturated phenol (pH 8.0) for 30 min with gentle shaking and centrifuge at 9000 × g for 10 min. Upper phase was transferred in separated vial and 0.1M ammonium acetate (dissolved in methanol) added in upper phase and incubated at −20°C for overnight. Next day, the solution was centrifuged again at 9000 × g and supernatant was discarded. Pellet was washed with 100% chilled acetone twice and then air dried pellet was dissolved in rehydration buffer [8 M urea, 2 M thiourea, 4% (w/v) CHAPS, 20 mMDTT, 0.5% (v/v) Pharmalyte (4–7)]. Total root protein concentration was determined by Bradford assay (Bio-Rad, USA). Isoelectric focusing was carried out with 250 μg of protein as given by Agrawal et al. (2008). Briefly, the 13-cm IEF strips (pH 4–7) were rehydrated with protein for 16 h and electrofocused using an IPGphor system (Bio-Rad, USA) at 20°C up to 25,000 Vh. This focused strips were subjected to reduction with 1% (w/v) DTT in 10 mL of equilibration buffer [6 M urea, 50 mM Tris-HCl (pH 8.8), 30% (v/v) glycerol and 2% (w/v) SDS] and followed by alkylation using 2.5% (w/v) iodoacetamide in same equilibration buffer. The strips were then loaded on top of 12.5% polyacrylamide gels for SDS-PAGE. The gels were fixed and stained with a silver stain plus kit as per protocol (Bio-Rad, USA).
Image Acquisition and Data Analysis
The silver stained gel obtained after 2DE were scanned for image acquisition using the Bio-Rad FluorS system equipped with a 12-bit camera. Images from three biological replicate 2-DE gels were taken for the data analysis in PDQuest version 8.0.1 (Bio-Rad). The parameters like protein spot quality, molecular mass, and pI of individual protein was assessed as described earlier by Agrawal et al. (2013). The spots showing reproducibility in quality and quantity in at least two of the three replicate gels were taken in consideration during analysis. A normalized spot volume for protein quantification was obtained by PDQuest software using the total spot volume normalization procedure to avoid experimental variations in gels due to protein load or staining.
Protein Identification
The silver stained protein spots were digested for MALDI MS/MS were digested with trypsin (Promega Corporation, MA, USA) as per manufacturer instruction. The stained protein spots were excised manually, washed thoroughly with water and crushed in small pieces. These gel pieces were washed with 15 mM potassium ferricynide and 50 mM sodium thiosulfate (1:1) for destaining. Then gel pieces were dehydrated with solution A [100% Acetonitrile (ACN): 50 mM Ammonium bicarbonate (ABC) in 2:1], subsequently rehydrated with 25 mM ABC. These steps were repeated until gel pieces become white. Final supernatant was discarded and gel pieces were dried in speedvac. After this gel pieces were rehydrated with 0.2 μg/μL trypsin and 50 mM ammonium bicarbonate and incubated at 37°C overnight for digestion. Peptides were extracted from gel slices with 1% TFA in 50% ACN twice. The peptide solution was concentrated down to 5 μL by vacuum centrifuge. Peptide solution mixed with 5 mg/ml CHCA (α-Cyano-4-hydroxycinnamic acid) matrix and spotted onto MALDI plate.
MALDI-TOF-TOF Analysis
A 4800 Proteomics Analyzer (Applied Biosystems, USA) with TOF/TOF optics was used for all MALDI-MS and MS/MS applications. Samples for MALDI were prepared by adding 0.5 μl matrix solution (5 mg/mL a-Cyano-4-hydroxycinnamic acid in 50% ACN containing 0.1% TFA) to 0.5 μl trypsin digested protein sample and left for air dry at room temperature on stainless steel 384 well-target plate after spotting. The plate containing spot was inserted in the mass spectrometer and subjected to mass analysis. A mixture of four proteins angiotensin I, Glu-fibrino-peptide B, ACTH (1e17), and ACTH (18e39) was used to calibrated the mass spectrometer externally. Further, the instrument was externally calibrated with fragment of Glufibrino-peptide B for MS/MS experiments.
The monoisotopic peptide masses obtained from MALDI-TOF/TOF were analyzed by the 4000 Series Explorer software version 3.5. Spectra were collected in a data dependent mode. For each spot total 500 laser shots were accumulated to generate ions for MS analysis and 30 most abundant ions were used for MS/MS analysis. All exported spectra as Mascot Generic Format (MGF) files from MALDI platforms were acquired in linear positive mode, smoothed by the Savitzky–Golay algorithm and the baseline subtracted for further analysis. Peak detection criterion for peak to be considered was set to minimum S/N = 10. Protein identification was performed using Mascot software (http://www.matrixscience.com) NCBInr databases 20160522 (87959973 sequences; 32280238673 residues). The database search criteria were as follows: taxonomy, O. sativa (rice) (172275 sequences), peptide tolerance, ±100 ppm, MS/MS tolerance, ±0.2 Da; peptide charge +1; maximum allowed missed cleavage, 1; fixed modification, cysteine carbamidomethylation; variable modification, methionine oxidation; instrument type, MALDI-TOF/TOF. Protein scores were measured from sum of the series of peptide scores as a non-probabilistic basis for ranking protein hits. The only protein spots whose MOWSE score was above the significant threshold level (Table S3) determined by Mascot along with the number of peptides matched and % coverage of matched protein were considered to represent a positive identification. In all the protein identifications, probability scores were greater than the score fixed by Mascot as significant with a p < 0.05.
The similar expression pattern of the identified differential proteins was determined by SOTA (self-organizing tree algorithm) clustering on the log transformed fold induction expression values using Multi Experiment Viewer (MEV) software (The Institute for Genomic Research, TIGR) of rice. The clustering was performed with Pearson correlation as distance with 10 cycles and maximum cell diversity of 0.8 (Romijin et al., 2005). SOTA is a neural network that grows adopting a binary tree topology, where hierarchical cluster are obtained as result with the accuracy and robustness (Herrero et al., 2001). Each branch summarizes the patterns of all similarly expressed proteins with centroid expression profile of a group.
Expression Analysis of Some Selected Candidate Genes Using qRT PCR
Real time-PCR was performed in 20 μl for a set of selected genes using Fast SYBR Green PCR Master Mix (Agilent Technologies, USA). The list of selected genes and oligonucleotide primers (Sigma-Aldrich, USA) used for each gene are listed in Table S1. Oligonucleotide primers for vetiver ubiquitin gene were used as the internal control for establishing equal amounts of cDNA in all reactions. The reactions were performed using the following cycle conditions, an initial 94°C for 2 min, followed by 30 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s, and the final 5 min extension at 72°C. After obtaining the ct-value for each reaction, the relative expression was calculated by 2^-delta Ct method.
Result and Discussion
Dehydration-Induced Changes and 2-DE Analysis
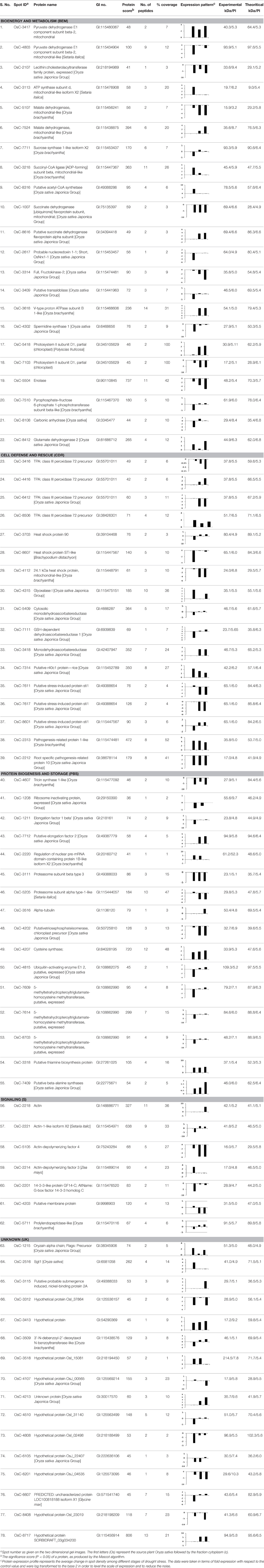
To understand the drought tolerance mechanism in rice, we carried out the comparative proteomic analysis of roots of the drought tolerance Heena cultivar of rice at various time points after drought induction in 10% PEG (Figure 1A). 2DE was performed at pH 4–7 IpG strip from 21 days old hydroponically grown rice root tissue in three replicates and images were analyzed by the PDQuest software version 8.0.1 as described above (Figure 1B). The mean value of the high-quality spots was used as the spot quantity on the master gel (Figure 1C, Table S2). For comparative proteomics study minimum 2.5 fold change in protein expression either increase or decrease at any one of the stages was considered for differential spot identification. A criterion of p < 0.01 was used to determine the significant difference for analyzing the parallel spots between genotype with analysis of one-way variance (ANOVA). A total of 125 differentially expressed drought responsive protein spots from all time points were subjected to MALDI-TOF/TOF analysis. Out of which 78 spots were identified significantly as indicated by arrows on the higher level matchset image generated by PD Quest (Figure 2) and summarized in Table S3. Some typical gel regions representing protein spots with altered expression are enlarged and shown in Figure 3. These protein spots actually account for 45 distinct proteins (Table 1), suggesting 57% unique protein identifications, while the remaining 43% of the identified differentially expressed proteins either correspond to post-translationally modified forms or may be members of multigene families. Of the 78 identified differentially expressed proteins, 30 protein spots were clearly up-regulated, and 26 were down-regulated, while 22 of the protein spots showed a mixed pattern of development stage dependent expression.
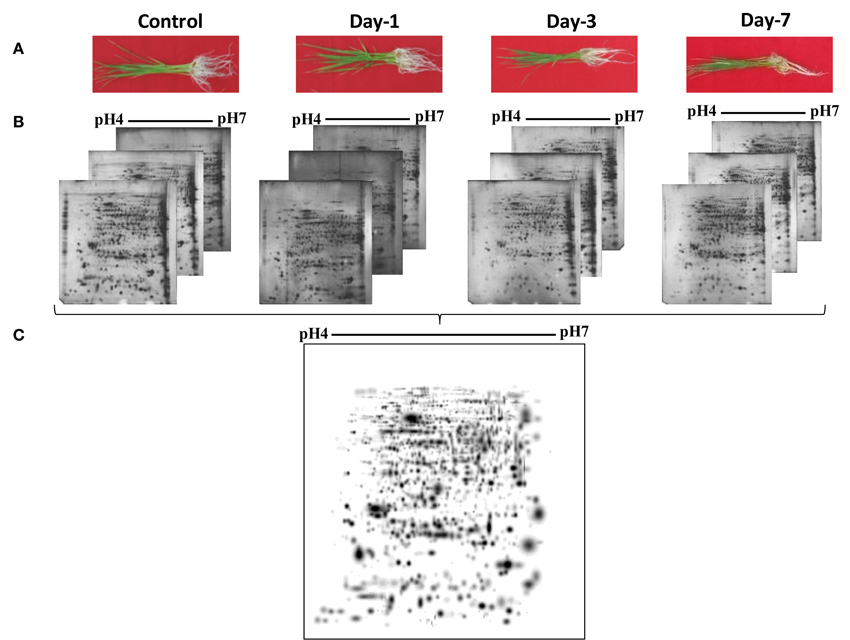
Figure 1. 2-DE analysis of root proteome of rice. (A) Rice plant showing different stages of drought. (B) Proteins were extracted from rice root tissue and equal amounts (250 μg) of proteins were separated by 2-DE as described in Materials and Methods Section. (C) Three replicate silver-stained gels for each stage were computationally combined using PDQuest software and one representative master standard gel image was generated.
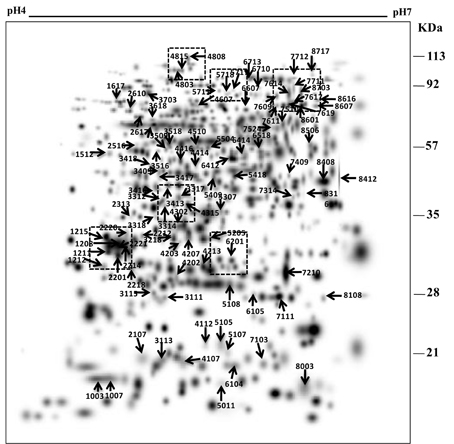
Figure 2. A higher level master image was created in silico by PDQuest from three replicate gels of each time point. The boxed areas marked with dotted lines represent the zoomed-in gel sections in Figure 3. The numbers correspond with the spot IDs listed in Table 1.
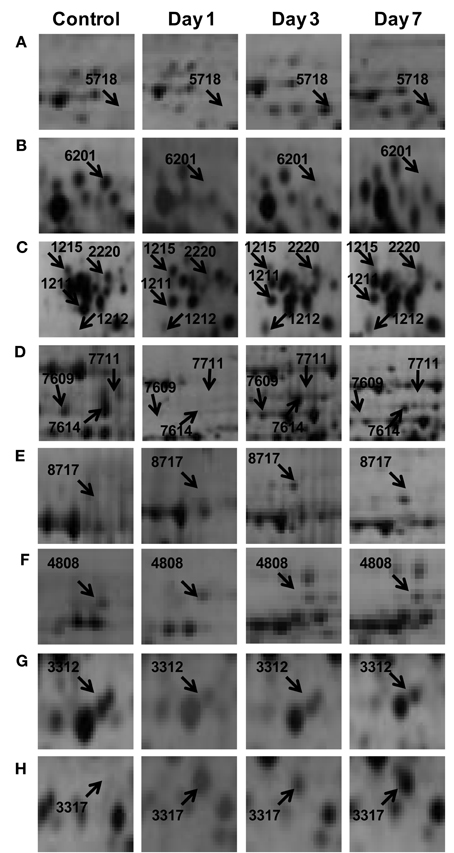
Figure 3. Some of the differentially expressed root proteins represented in the enlarged gel sections (A–H) corresponds to the marked boxed areas in Figure 2.
Functional Distribution of Dehydration Responsive Proteins
To understand the function of the proteins associated with drought induced changes in the rice roots, the differentially expressed proteins were sorted into different functional categories. Seventy-eight identified differentially expressed proteins could be assigned to five functional classes based on their putative roles in the drought-response (Figure 4, Table 1). In case of Heena the largest percentage of the identified proteins was involved in bioenergy and metabolism (29%), cell defense and rescue (22%), protein biogenesis and storage (21%), cell signaling 9%, and miscellaneous (19%; Figure 4). In a number of cases, many proteins were represented by multiple isoelectric forms (Table 1), suggesting the possible post-translational modification of the candidate protein.
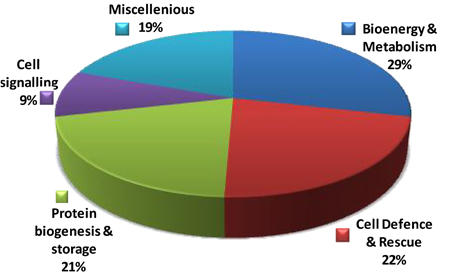
Figure 4. Distribution of 78 identified differentially expressed rice root proteins in five functional classes based on their putative functions assigned them using protein database.
Bioenergy and Metabolism (BEM)
The BEM class contained 24 proteins which appeared as differential spots. ATP synthase subunit d (OsC-3113), malate dehydrogenase, mitochondrial-like (OsC-5107), succinyl-CoA ligase [ADP-forming] subunit beta (OsC-3218), putative acetyl-CoA synthetase (OsC-8316), V-type proton ATPase subunit B 1-like (OsC-3618), glutamate dehydrogenase 2 (OsC-8412), putative succinate dehydrogenase flavoprotein alpha subunit (OsC-8616) were upregulated, while pyruvate dehydrogenase E1 component subunit beta-2 (OsC-3417), malate dehydrogenase, mitochondrial-like (OsC-7524), succinate dehydrogenase [ubiquinone] flavoprotein subunit, mitochondrial (OsC-1007), probable nucleoredoxin (OsC-2617), fructokinase-2 (OsC-3314), spermidine synthase 1(OsC-4302), pyruvate dehydrogenase E1 component (OsC-4803), lecithin:cholesterolacyl transferase family protein (OsC-2107), and enolase (OsC-5504) were downregulated. Other proteins like sucrose synthase 1-like isoform X2 (OsC-7711), putative transaldolase (OsC-3409), pyrophosphate-fructose 6-phosphate 1-phosphotransferase (OsC-7510) and carbonic anhydrase (OsC-8108) showed mixed pattern of expression.
Alteration in bioenergy metabolism under drought can be addressed by increase in abundance of enzymes involved in acetyl CoA synthesis, TCA cycle, transport proteins, synthesis of osmoprotectants, and protein metabolism. Acetyl-CoA synthetase (OsC-8316) showed a 43 fold increase in abundance on first day and 66 fold on the third day of drought induction (Table 1); however the expression remains similar as control on the 7th day. The enzyme catalyzes the formation of acetyl CoA from acetate utilizing inorganic phosphate (Lin and Oliver, 2008) and can be employed in the TCA cycle during aerobic respiration for energy production and electron carriers. It is to be noted here that drought stress resulted in downregulation of pyruvate dehydrogenase (OsC-3417), hence the upregulation of Acetyl-CoA synthetase possibly provided an alternate source of energy production (Schwer et al., 2006).
The TCA cycle enzymes like succinyl co-A ligase (OsC-3218), succinate dehydrogenase (OsC-1007) and malate dehydrogenase (OsC-7524) were upregulated. Succinyl-CoA ligase catalyzes the reversible inter conversion of succinyl-CoA to succinate (Cavalcanti et al., 2014). There was a 20 fold increase in the enzyme abundance at first day (Table 1). The result corroborates with earlier studies which reported increase in enzyme expression in wheat roots in response to aluminum stress (Drummond et al., 2001). Succinate dehydrogenase catalyzes the oxidation of succinate to form fumarate (Singer et al., 1973; Jardim-Messeder et al., 2015). The enzyme is an important component of TCA cycle as well as the electron transport chain (Popova et al., 2010; Huang and Millar, 2013). Two isozymes of succinate dehydrogenase were found as differential spots, of which one (OsC-1007) was downregulated while the other (OsC-8616) was upregulated showing 28 fold increase in activity on the third day (Table 1). The transcription of succinate dehydrogenase flavoprotein subunit has been found to be up-regulated in Ilex paraguariensis leaves in response to water deficit and ABA application (Acevedo et al., 2013). Furthermore, its increased activity results in root elongation (Huang et al., 2012), and also has a role in plant adaptation toward stress and especially by combating reactive oxygen species (Pastore et al., 2007). Of the two isozymes of malate dehydrogenase identified, one was downregulated (OsC-5107), while the other isozyme (OsC-7524) showed 5 and 11 fold increase in activity on third and seventh day of drought stress, respectively (Table 1). ATPases are integral transport proteins that help in the hydrolysis of ATP as well as movement of protons across membranes to generate electrochemical gradients (Palmgren and Harper, 1999). The action of ATPase can influence stress mechanism by changing the membrane potential and proton gradient (Elmore and Coaker, 2011). Transcript and protein levels of ATPase have been reported to increase under salt stress conditions (Batelli et al., 2007). Interestingly V-type proton ATPase subunit B 1-like protein (OsC-3618) and ATP synthase subunit d mitochondrial-like isoform X2 (OsC-3113) were upregulated. While, approximately 8 fold increases in activity of V-type proton ATPase was noted on third and seventh day; the activity of mitochondrial ATP synthase increased to approximately 33 fold in first day and 58 fold in third day (Table 1).
Mitochondrial pyruvate dehydrogenase (OsC-4803) is an intermediate enzyme that links glycolysis to the citric acid cycle. The enzyme was downregulated under drought stress and results substantiate the earlier reports (Simova-Stoilova et al., 2015). Fructokinase-2 (OsC-3314) is mainly involved in carbohydrate metabolism, more specifically, sucrose and fructose metabolism (Odanaka et al., 2002). Previously rice fructokinase showed elevated levels of expression during infection with the fungus, Magnapor theoryzae (Ryu et al., 2006). Sucrose synthase (OsC-7711), a major protein of energy metabolism, plays an important role in controlling the mobilization of sucrose into various pathways essential for various metabolic, structural, and other storage functions of the plant cell (Hesse and Willmitzer, 1996). The protein expression followed a miscellaneous pattern with 2.7 fold abundance on third day (Table 1), indicating an alteration in enzyme function more toward osmotic adjustment and storage compounds (Hasibeder et al., 2015).
Cell Defense and Rescue (CDR)
Seventeen differentially expressed proteins related to cell defense and rescue were identified, out of which pathogenesis-related protein 1 (PR1, OsC-2313), one isoform of TPA: class III peroxidase 72 precursor (OsC-4416), glyoxalase I (OsC-4315), putative r40c1 protein (OsC-7314), stress-induced protein sti1 (OsC-7617, OsC-8601), and monodehydroascorbate reductase (OsC-3418) were upregulated whereas root specific pathogenesis-related protein 10 (OsC-2212), 24.1 kDa heat shock protein (OsC-4112), isozyme of monodehydroascorbate reductase (OsC-5409), TPA: class III peroxidase 72 precursor (OsC-6412 and OsC-3416), isozyme of stress-induced protein sti1 (OsC-7611) and heat shock protein STI (OsC-8607) were downregulated, while heat shock protein (HSP)-90 (OsC-3703) and GSH-dependent dehydroascorbatereductase 1 (OsC-7111) showed miscellaneous pattern of expression.
The ROS scavengers provide cells with an efficient machinery for detoxifying and H2O2 and constitute the first line of defense. A considerable upregulation of these enzymes was a major tolerance mechanism adopted by the variety. The plant class III peroxidases are known to basically involved in metabolism of ROS along with some other function like auxin metabolism, lignin and suberin formation and cross-linking of cell wall components (Almagro et al., 2009). The class III peroxidase (OsC-4416) expression gradually increased up to 11 fold from first to seventh day (Table 1). Since the protein is involved in lignin biosynthesis, a gradual increase in expression suggests enhanced lignin production. Increased amount of lignin builds the mechanical strength of cell wall and thereby protects roots against the dry soil. In addition, cell wall modification is also used to minimize water loss and cell dehydration, thus helping plants to resist and recover from drought upon availability of water (Yoshimura et al., 2008). An 11 fold increase in expression was observed in glyoxalase 1 (OsC-4315), which is a component of the glyoxalase system that carries out the detoxification of methylglyoxal and the other reactive aldehydes produced as a normal part of metabolism (Vander, 1989). Glyoxalase 1 has been identified as a salt induced proteome in rice roots and plays an important role in methylglyoxal detoxification in salt tolerant rice, thus ensuring redox homeostasis (El-Shabrawi et al., 2010). Putative r40c1 protein showed increased abundance in response to drought. The exact function of the protein is unknown; however its upregulation has been correlated to drought tolerance (Kumar et al., 2015). The expression of monodehydroascorbate reductase (OsC-5409) was increased by 9 fold on seventh day. It is a critical component of the ROS scavenging system in rice, and its expression can improve drought tolerance in rice (Wang et al., 2005). Interestingly, the expression of pathogenesis related protein-1 (PR1) was 22 fold increased within 24 h of drought induction; however root specific pathogenesis related protein-10 (PR10) was down regulated in response to drought. PR1 gene expression is salicylic-acid responsive and induced in response to a variety of pathogens (Elvira et al., 2008) while PR-10 protein expression is induced through activation of jasmonic acid signaling pathway (Hashimoto et al., 2004). Thus, it might be suggested that drought stress might responsible in increase expression of SA regulated PR proteins and decreases in JA-regulated defense.
Protein Biogenesis and Storage (PBS)
A total of 15 proteins were found as differential spots, out of which nuclear pre-mRNA domain-containing protein 1B-like isoform X2 (OsC-2220), alpha-tubulin (OsC-3516), putative elongation factor 2 (OsC-7712), putative thiamine biosynthesis protein (OsC-3318), putative beta-alanine synthase (OsC-7409), and cysteine synthase (OsC-4207, OsC-4207) was upregulated; tricin synthase 1 (OsC-4607), putative triose phosphate isomerase (OsC-4202), proteasome subunit alpha type-1 (OsC-5205) was downregulated; while three isozymes of 5-methyl tetra hydropteroyl triglutamate-homocysteine methyltransferase (OsC-7609, OsC-7614, OsC-8703), elongation factor 1 beta (OsC-1211), proteasome subunit beta type 3 (OsC-3111) and ubiquitin-activating enzyme E1 2, putative (OsC-4815) showed miscellaneous pattern of expression.
Cysteine synthase is a key enzyme for mediating abiotic tolerance owing to the production of antioxidants and metal chelators, such as glutathione, metallothionein, and phytochelatin. In an earlier study cysteine synthase was found to be increased in the roots of wheat during stress (Yang et al., 2007). We also observed significantly upregulation of cysteine synthase (OsC-4207) in tolerant variety used for analysis. Tubulin class of proteins is known to play a critical role in cell division and elongation and alpha-tubulin (OsC-3516) has been specifically reported in roots of drought tolerant rice verities (Rabello et al., 2008). Elongation factor 2 (OsC-7712) which showed 24 fold increases in abundance is reportedly a stress responsive protein which is mostly downregulated in sensitive verities of rice (Chen et al., 2015). There was a decrease in abundance of proteasome subunit beta type 3 protein (OsC-5205) in response to drought while ubiquitin activating enzyme (OsC-4815), required for ubiquitination, showed a gradual increase in abundance and increased 2-fold by the end of seventh day (Table 1). The protein ubiquitination plays a central role in regulating the transcriptional changes required for adaption and survival strategies of plants to different environmental stresses (Dametto et al., 2015). Glutamate dehydrogenase expedites amino acid synthesis in drought stress conditions where nitrogen assimilation is decreased and also known to maintain homeostasis during stress (Masclaux-Daubresse et al., 2010). Present study showed a 4 fold increase in glutamate dehydrogenase (OsC-8412) expression on seventh day (Table 1). Putative thiamine biosynthesis protein (OsC-3318) showed an 18 fold increase on first day and 27 fold increase on the seventh day. Recent reports suggest that vitamin B1 (thiamine) participates in the processes underlying plant adaptations to various types stress conditions including cold, heat, drought, and other oxidative stress (Rapala-Kozik et al., 2012). A gradual increase in putative beta-alanine synthase (OsC-7409) suggests significant enrichment of beta-alanine which functions as an osmoprotectant under drought conditions in various plant systems (Ranjan et al., 2012).
Cell Signaling
The response of roots to water limiting conditions seems to be crucial to trigger drought tolerance mechanisms, since roots are one of the primary sites for stress signal perception in which a signaling mechanism initiates a cascade of gene expression responses to drought. These changes in protein profile can result in successful adaptations leading to stress tolerance by regulating protein expression and signal transduction in the stress response (regulatory proteins) or directly protecting the plant against environmental stress (functional proteins). Seven cell signaling related proteins were identified as differential spots, out of which actin (OsC-2218) and actin-1-like isoform X2 (OsC-2221) were significantly upregulated. Whilst a 20-fold increase in expression of actin-depolymerizing factor 3 (OsC-2214) was observed on third day (Table 1), a consistent decrease in the expression of actin-depolymerizing factor 4 (OsC-5105) was noticed during the study. Other proteins like the 14-3-3-like protein (OsC-2201), putative membrane protein (OsC-4203), and prolyl endopeptidase-like protein (OsC-5711) showed miscellaneous pattern of expression in response to drought. Actin depolymerizing factors (ADFs) are small actin-binding proteins and are known to confer abiotic drought tolerance (Huang et al., 2012). There was an increase in abundance of actin depolymerizing factor 3 (OsC-2214) by 8 and 20 fold, respectively, on first and third day of drought induction, however the expression came down as similar to control at seventh day, thus suggesting its regulatory role in drought tolerance. During signal transduction, 14-3-3 proteins mediate several protein-protein interactions through post-translational modification like phosphorylation which ultimately affects multiple plant functions (Chen et al., 2006). Surprisingly, a non-coherent expression of 14-3-3 protein (OsC-2201) was observed under drought stress. A decrease in prolyl endopeptidase (OsC-5711) expression was observed on first of drought induction; however an increase in its abundance was noted at third day and the abundance increased by 3.7-fold at the end of seventh day.
Miscellaneous
The proteins with functions other than the defined categories were ascribed as miscellaneous proteins and constituted 22% of the differentially expressed protein. Amongst the differentially expressed miscellaneous proteins, Sgt1 (OsC-2516), submergence induced, nickel-binding protein 2A (OsC-3115), Oryzain alpha chain; Flags: Precursor [O. sativa Japonica group] (OsC-1215), hypothetical protein (OsC-3413), hypothetical protein OsI_15081 (OsC-3518), and hypothetical protein SORBIDRAFT_03g034200 (OsC-8717) were upregulated; hypothetical protein OsI_37864 (OsC-3312) was downregulated, while 3′-N-debenzoyl-2′-deoxytaxol N-benzoyltransferase (OsC-3509) and uncharacterized protein LOC100818188 (OsC-6607) showed varied expression.
SGT1 (OsC-2516) is a highly conserved protein among eukaryotes that binds specifically to the molecular chaperone, HSP90 and helps in regulation of resistance extended by many resistance proteins (Azevedo et al., 2006; Wang et al., 2010). It is interesting to note here that there was an upregulation of HSP90 (protein abundance increased to 4.6-fold) with SGT1 in response to drought in Heena roots might involve in drought tolerance. The nickel-binding protein 2A is the member of dehydration-responsive element-binding protein 2A family which acts as a transcriptional activator that binds specifically to the cis-acting dehydration-responsive element (DRE) to regulates high salinity and dehydration-inducible transcription (Dubouzet et al., 2003). A 23-fold increase in protein abundance suggests an important role of nickel-binding protein 2A (OsC-3115) in conferring drought tolerance to Heena, however its exact function as a drought resistant protein needs to be further elucidated. Another hypothetical protein SORBIDRAFT_03g034200 (OsC-8717) showed a 63-fold increase in abundance on seventh day which indicates its important role in conferring drought tolerance in later stage, however it needs further elucidation.
Cluster Analysis of Drought Responsive Protein
The unbiased hierarchical clustering method, SOTA was used to study the correlated expression pattern of the drought responsive proteins. This method allows integration of the multiple proteins showing similar expression profiles which provide comprehensive overview of the rice root protein network regulation in coordination. The data were log transformed to the base of 2 to reduce the noise and level the scale of fold-expression for SOTA clustering analysis. The analysis grouped 78 proteins into 11 distinct clusters (Figure 5), allowing a maximum diversity of 0.8 within a single cluster. Figure S1 represents the detailed information on proteins within each cluster. Only those clusters which is having n ≥ 5 (n represents number of identified proteins) were considered for coordinated expression profile of the drought responsive proteins. Analysis revealed that cluster 8 contains maximum number of proteins with similar expression followed by cluster 11. The proteins of cluster 8, displayed decreased accumulation during initial stage of drought and subsequent increase at late stages. While in cluster 1, maximum number of the proteins displayed increased accumulation during initial stage of drought and then decrease at late stage. Most of the clusters except cluster 8 showed selective representation for specific functional classes of proteins. The proteins in cluster 9, 10, and 11 were found to be over-expressed throughout drought stress in one or all stages, whereas in cluster 5 and 7 showed only down-regulated proteins. The cluster 4 which contain 7 proteins were expressed immediately in day 1 then disappear throughout suggested their role in helping in plant to tolerate drought shock. The drought responsive proteins with miscellaneous function were found to be distributed in almost all the clusters. SOTA clustering analysis may provide useful information for their putative function in drought tolerance based on the abundance in rice root.
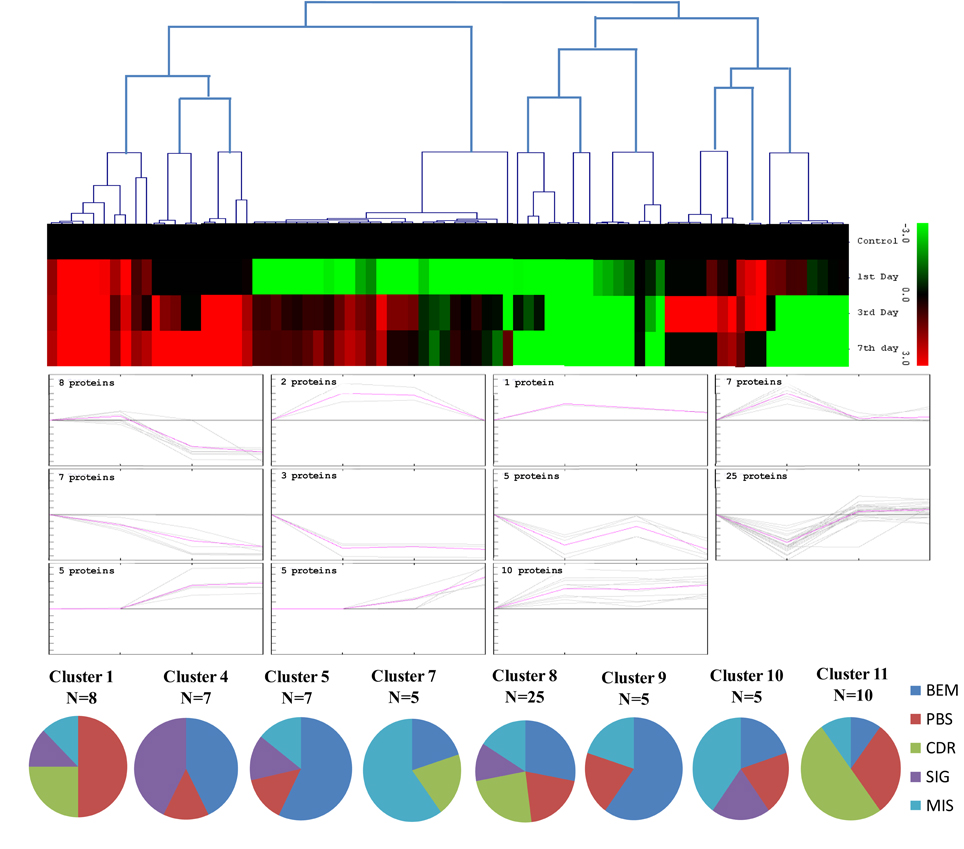
Figure 5. Expression clustering of 78 differentially expressed proteins showing 8 clusters based on their expression profiles. The gray lines represent expression profile of protein separately and the mean expression profile is indicated by pink line. Total number of proteins with same expression profile is provided in inset within the respective cluster. Detailed information on proteins within each cluster present in Figure S1.
Validation of Proteomics Data of Selected Proteins with qRT PCR
The list of putatively regulated proteins depicted in Table 1 is a snapshot of proteins from rice root. We have selected 5 genes with miscellaneous function for comparing their expression with proteome data. Among them, a highly conserved eukaryotic protein, SGT1 (OsC-2516) shows its binding specifically to the molecular chaperone to regulation of resistance extended by many resistance proteins (Wang et al., 2010). An upregulated TCA cycle enzymes succinyl co-A ligase (OsC-3218) catalyzes the reversible inter conversion of succinyl-CoA to succinate (Cavalcanti et al., 2014). Triose phosphate isomerase (OsC-4202) is required to produce ATP during glycolysis and it has been reported to have highly induced by salt or drought in rice leaves (Lee et al., 2011). Benzoyltransferase (OsC-3509) have role in final step of acylation of taxol biosynthesis pathway but its role in drought stress is hitherto unknown. Apart from these, some other unknown genes like hypothetical protein (OsC-6201), PREDICTED: uncharacterized protein (OsC-6607), hypothetical protein SORBIDRAFT_03g034200 (OsC-8717) were also taken to check their abundance. However, expression profile of these proteins were somewhat similar as determined by the 2-DE analysis (Table 1), nevertheless, there was difference in fold-induction in protein expression (Figure 6, Table 1). Since all the differentially expressed proteins were not identified, it can be assumed that some other isoforms of the same protein might be part of the root proteome. The post-translational modifications of some of the differentially expressed proteins might also affect the actual expression levels determined by two different techniques. Further study may explore such type of correlation in a better way.
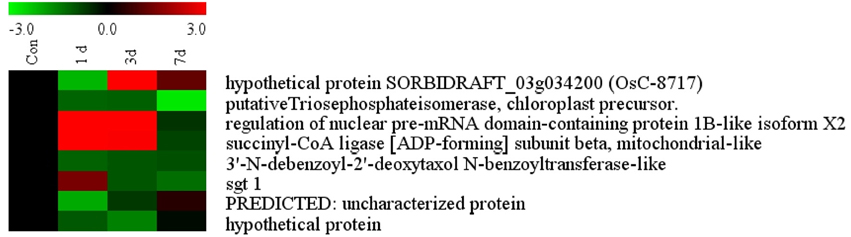
Figure 6. Quantitative validation of relative expression of selected transcripts related to different functional classes of rice root. Heat map of some differentially regulated known and hypothetical protein showing their relative expression by qRT-PCR along with their protein expression data obtained from 2DE. The signal intensity of each transcript was normalized using ubiquitin house keeping gene. The top most bar represents the fold change expression values of selected genes.
Conclusion
Crop plants including rice exhibit several adaptive and acclimatization strategies to combat environmental conditions such as drought. Such strategies include from visible phenotypic changes to complex physicochemical traits. At molecular level, these traits, often represented by differentially regulated proteins during stress and may serve as important stress tolerance markers. We report here a systematic proteomic analysis of the rice root proteins under PEG-simulated drought stress conditions. In conclusion, the root-specific comparative proteomes of rice identified a number of proteins that are putatively associated with stage specific drought tolerant. Of the 78 differentially expressed proteins, 10 were found to be differentially regulated in all the four stages during drought stress. Three proteins exclusively expressed in the control, were disappear after drought stresses. Total 8 proteins found to be newly synthesized during early or later stage of drought stress, implying their possible role in drought tolerance (Figure 7). Functional classification revealed that maximum number of proteins fall in the category of bioenergy and metabolism followed by those involved in cell defense (Figure 4). The higher number of metabolic proteins offers a unique opportunity to predict more activity toward carbon assimilation, respiration, and storage products like sucrose and starch to combat drought condition. Based on results from present study, a putative model elucidating the role of the differentially expressed proteins in different pathways like glycolysis, TCA, amino acid metabolism, ROS, and other possible mechanism(s) underlying dehydration tolerance is in Figure 8. A large number of proteins with miscellaneous functions are matter of further investigation for their putative role in drought tolerance. However, the present proteomics study could represent only a small part of the rice proteome, further investigation to assign their putative biological functions may be useful for a better understanding of complex biological traits, such as drought tolerance. Many other drought-responsive proteins still need to be identified with advancement to technology which may help in better understanding of the drought response in rice.
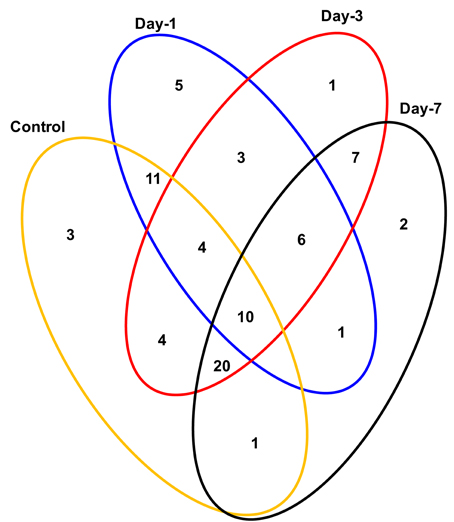
Figure 7. Venn diagram showing the distribution of differentially expressed proteins in time-specific and overlapping manner during drought stress. The areas shown in the diagram are not proportional to the number of proteins in the groups.
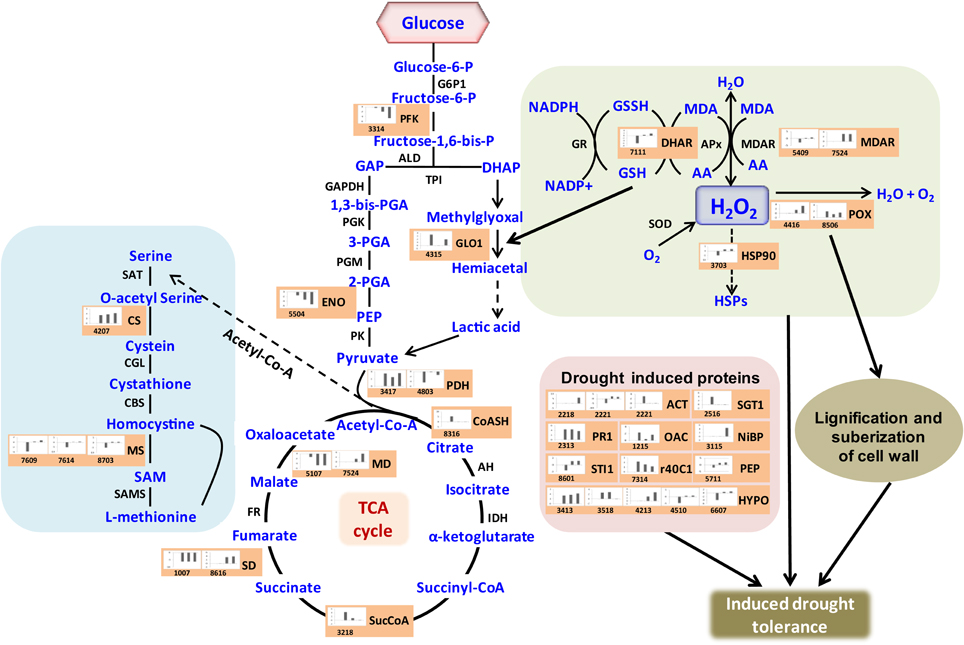
Figure 8. Illustration of role of differentially regulated proteins involved in different pathways in rice for sustaining during drought stress. Proteins engaged in carbon breakdown, protein synthesis and ROS pathway are displayed on the corresponding metabolic pathways. Graphs are the representatives of expression profile of individual protein and number given below in each graph indicates the protein identification number.
Author Contributions
CN, DC, and PC designed the experiment. LA, SG, and SM performed the experiments. LA, GP, and SK analyze the data and wrote the paper.
Funding
The study was supported by New Initiative (as a Cross Flow Technology project) “Root Biology and its Correlation to Sustainable Plant Development and Soil Fertility (BSC0204)” from Council of Scientific and Industrial Research (CSIR), New Delhi, India and JC Bose Fellowship, Science and Engineering Research Board, Department of Science and Technology, Government of India, awarded to CN.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2016.01466
Figure S1. Heat map revealing the expression of differentially regulated proteins of rice root present within each cluster.
Table S1. List of primers used for Real Time PCR.
Table S2. Reproducibility of 2-D gels.
Table S3. List of rice root proteins identified by MS/MS analysis.
Abbreviations
SAT, serine acetyl transferase; CS, cystein synthesae; CGL, Cystathionine γ-lyase; CBS, Cystathionine β-synthase; MS, methionine synthase; SAM, S-adenosylmethionine; SAMS, S-adenosylmethionine synthesae; G-6-P, Glucose 6 phosphate; PFK, pyrophosphate-dependent phosphofructokinase; ALD, aldehyde dehydrogenase; TPI, triose phosphate isomerase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; PGK, phosphoglycerate kinase; PGM, phosphoglycerate mutase; ENO, enolase; PK, pyruvate kinase; GLO1, glyoxalase 1; PDH, Pyruvate dehydrogenase; CoAS, Co-A synthesae; AH, aconitate hydratase; IDH, isocitrate dehydrogenase; SucCoA, Succinyl Co-A synthese; SD, succinate Dehydrogenase; FR, fumarase; MD, malate dehydrogenase; GR, glutathione reductase; DHAR, dehydroascorbate reductase; APx, ascorbate peroxidase; MDAR, monodehydroascorbate reductase; POX, peroxidases; SOD, superoxide dismutase; HSPs, heat shock proteins; ACT, actin; SGT1, suppressor of the G2 allele of skp1; PR, pathogenesis related protein; OAC, oryzain alpha chain; NiBP, Ni binding protein; STI1, stress inducible protein; PEP, phosphoenolpyruvate; HYPO, hypothetica.
References
Acevedo, R. M., Maiale, S. J., Pessino, S. C., Bottini, R., Ruiz, O. A., and Sansberro, P. A. (2013). A succinate dehydrogenase flavoprotein subunit-like transcript is up-regulated in Ilex paraguariensis leaves in response to water deficit and abscisic acid. Plant Physiol. Biochem. 65, 48–54. doi: 10.1016/j.plaphy.2012.12.016
Agrawal, L., Chakraborty, S., Jaiswal, D. K., Gupta, S., Datta, A., and Chakraborty, N. (2008). Comparative proteomics of tuber induction, development and maturation reveal the complexity of tuberization process in potato (Solanum tuberosum L.). J. Proteome Res. 7, 3803–3817. doi: 10.1021/pr8000755
Agrawal, L., Narula, K., Basu, S., Shekhar, S., Ghosh, S., Datta, A., et al. (2013). Comparative proteomics reveals a role for seed storage protein AmA1 in cellular growth, development, and nutrient accumulation. J. Proteome Res. 12, 4904–4930. doi: 10.1021/pr4007987
Ahsan, N., Lee, D. G., Alam, I., Kim, P. J., Lee, J. J., Ahn, Y. O., et al. (2008). Comparative proteomic study of arsenic-induced differentially expressed proteins in rice roots reveals glutathione plays a central role during As stress. Proteomics 8, 3561–3576. doi: 10.1002/pmic.200701189
Ali, G. M., and Komatsu, S. (2006). Proteomic analysis of rice leaf sheath during drought stress. J. Proteome Res. 5, 396–403. doi: 10.1021/pr050291g
Almagro, L., Gómez Ros, L. V., Belchi-Navarro, S., Bru, R., Ros Barceló, A., and Pedreño, M. A. (2009). Class III peroxidases in plant defence reactions. J. Exp. Bot. 60, 377–390. doi: 10.1093/jxb/ern277
Atkinson, N. J., and Urwin, P. E. (2012). The interaction of plant biotic and abiotic stresses: from genes to the field. J. Exp. Bot. 63, 3523–3543. doi: 10.1093/jxb/ers100
Azevedo, C., Betsuyaku, S., Peart, J., Takahashi, A., Noël, L., Sadanandom, A., et al. (2006). Role of SGT1 in resistance protein accumulation in plant immunity. EMBO J. 25, 2007–2016. doi: 10.1038/sj.emboj.7601084
Batelli, G., Verslues, P. E., Agius, F., Qiu, Q., Fujii, H., Pan, S., et al. (2007). SOS2 promotes salt tolerance in part by interacting with the vacuolar H+-ATPase and upregulating its transport activity. Mol. Cell. Biol. 27, 7781–7790. doi: 10.1128/MCB.00430-07
Cavalcanti, J. H. F., Esteves-Ferreira, A. A., Quinhones, C. G. S., Pereira-Lima, I. A., Nunes-Nesi, A., Fernie, A. R., et al. (2014). Evolution and functional implications of the Tricarboxylic Acid Cycle as revealed by phylogenetic analysis. Genome Biol. Evol. 6, 2830–2848. doi: 10.1093/gbe/evu221
Chen, C., Song, Y., Zhuang, K., Li, L., Xia, Y., and Shen, Z. (2015). Proteomic analysis of copper-binding proteins in excess copper-stressed roots of two rice (Oryza sativa L.) varieties with different Cu tolerances. PLoS ONE 10:e0125367. doi: 10.1371/journal.pone.0125367
Chen, F., Li, Q., Sun, L., and He, Z. (2006). The Rice 14-3-3 gene family and its involvement in responses to biotic and abiotic stress. DNA Res. 13, 53–63. doi: 10.1093/dnares/dsl001
Dametto, A., Buffon, G., Dos Reis Blasi, É. A., and Sperotto, R. A. (2015). Ubiquitination pathway as a target to develop abiotic stress tolerance in rice. Plant Signal. Behav. 10:e1057369. doi: 10.1080/15592324.2015.1057369
Davies, W. J., and Zhang, J. (1991). Root signals and the regulation of growth and development of plant in dry soil. Ann. Rev. Plant Physiol. Plant Mol. Biol. 42, 55–76. doi: 10.1146/annurev.pp.42.060191.000415
Drummond, R. D., Guimaraes, C. T., Felix, J., Ninamango-Cardenas, F. E., Carneiro, N. P., Paiva, E., et al. (2001). Prospecting sugarcane genes involved in aluminum tolerance. Genet. Mol. Biol. 24, 221–230. doi: 10.1590/S1415-47572001000100029
Dubouzet, J. G., Sakuma, Y., Ito, Y., Kasuga, M., Dubouzet, E. G., Miura, S., et al. (2003). OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-salt- and cold-responsive gene expression. Plant J. 33, 751–763. doi: 10.1046/j.1365-313X.2003.01661.x
Elmore, J. M., and Coaker, G. (2011). The role of the plasma membrane H+-ATPase in plant-microbe interactions. Mol. Plant 4, 416–427. doi: 10.1093/mp/ssq083
El-Shabrawi, H., Kumar, B., Kaul, T., Reddy, M. K., Singla-Pareek, S. L., and Sopory, S. K. (2010). Redox homeostasis, antioxidant defense, and methylglyoxal detoxification as markers for salt tolerance in Pokkali rice. Protoplasma 245, 85–96. doi: 10.1007/s00709-010-0144-6
Elvira, M. I., Galdeano, M. M., Gilardi, P., García-Luque, I., and Serra, M. T. (2008). Proteomic analysis of pathogenesis-related proteins (PRs) induced by compatible and incompatible interactions of pepper mild mottle virus (PMMoV) in Capsicum chinense L3 plants. J. Exp. Bot. 59, 1253–1265. doi: 10.1093/jxb/ern032
Hashimoto, M., Kisseleva, L., Sawa, S., Furukawa, T., Komatsu, S., and Koshiba, T. (2004). A novel rice PR10 protein, RSOsPR10, specifically induced in roots by biotic and abiotic stresses, possibly via the jasmonic acid signaling pathway. Plant Cell Physiol. 45, 550–559. doi: 10.1093/pcp/pch063
Hasibeder, R., Fuchslueger, L., Richter, A., and Bahn, M. (2015). Summer drought alters carbon allocation to roots and root respiration in mountain grassland. New Phytol. 205, 1117–1127. doi: 10.1111/nph.13146
Herrero, J., Valencia, A., and Dopazo, J. (2001). A hierarchical unsupervised growing neural network for clustering gene expression patterns. Bioinformatics 17, 126–136. doi: 10.1093/bioinformatics/17.2.126
Hesse, H., and Willmitzer, L. (1996). Expression analysis of a sucrose synthase gene from sugar beet (Beta vulgaris L.). Plant Mol. Biol. 30, 863–872. doi: 10.1007/BF00020799
Huang, S., and Millar, A. H. (2013). Succinate dehydrogenase: the complex roles of a simple enzyme. Curr. Opin. Plant Biol. 16, 344–349. doi: 10.1016/j.pbi.2013.02.007
Huang, S., Taylor, N. L., Ströher, E., Fenske, R. A., and Millar, R. A. (2012). Succinate dehydrogenase assembly factor 2 is needed for assembly and activity of mitochondrial complex II and for normal root elongation in Arabidopsis. Plant J. 73, 429–441. doi: 10.1111/tpj.12041
Jardim-Messeder, D., Caverzan, A., Rauber, R., de Souza, F. E., Margis-Pinheiro, M., and Galina, A. (2015). Succinate dehydrogenase (mitochondrial complex II) is a source of reactive oxygen species in plants and regulates development and stress responses. New Phytol. 208, 776–789. doi: 10.1111/nph.13515
Kawasaki, S., Borchert, C., Deyholos, M., Wang, H., Brazille, S., Kawai, K., et al. (2001). Gene expression profiles during the initial phase of salt stress in rice. Plant Cell 13, 889–905. doi: 10.1105/tpc.13.4.889
Khan, M., and Komatsu, S. (2004). Rice proteomics: recent developments and analysis of nuclear proteins. Phytochemistry 65, 1671–1681. doi: 10.1016/j.phytochem.2004.04.012
Komatsu, S. (2005). Rice Proteome Database: a step toward functional analysis of the rice genome. Plant Mol. Biol. 59, 179–190. doi: 10.1007/s11103-005-2160-z
Kumar, A., Bimolata, W., Kannan, M., Kirti, P. B., Qureshi, I. A., and Ghazi, I. A. (2015). Comparative proteomics reveals differential induction of both biotic and abiotic stress response associated proteins in rice during Xanthomonas oryzae pv. oryzae infection. Funct. Integr. Genomics 15, 425–437. doi: 10.1007/s10142-014-0431-y
Lafitte, H. R., Yongsheng, G., Yan, S., and Li, Z. K. (2007). Whole plant responses, key processes, and adaptation to drought stress: the case of rice. J. Exp. Bot. 58, 169–175. doi: 10.1093/jxb/erl101
Lee, D. G., Park, K. W., An, J. Y., Sohn, Y. G., Ha, J. K., Kim, H. Y., et al. (2011). Proteomics analysis of salt-induced leaf proteins in two rice germplasms with different salt sensitivity. Can. J. Plant Sci. 91, 337–349. doi: 10.4141/CJPS10022
Lin, M., and Oliver, D. J. (2008). The role of acetyl-coenzyme A synthetase in Arabidopsis. Plant Physiol. 147, 1822–1829. doi: 10.1104/pp.108.121269
Masclaux-Daubresse, C., Daniel-Vedele, F., Dechorgnat, J., Chardon, F., Gaufichon, L., and Suzuki, A. (2010). Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture. Ann. Bot. 105, 1141–1157. doi: 10.1093/aob/mcq028
Moumeni, A., Satoh, K., Kondoh, H., Asano, T., Hosaka, A., Venuprasad, R., et al. (2011). Comparative analysis of root transcriptome profiles of two pairs of drought-tolerant and susceptible rice near-isogenic lines under different drought stress. BMC Plant Biol. 11:174. doi: 10.1186/1471-2229-11-174
Muscolo, A., Sidari, M., Anastasi, U., Santonoceto, C., and Maggio, A. (2014). Effect of PEG-induced drought stress on seed germination of four lentil genotypes. J. Plant Interact. 9, 354–363. doi: 10.1080/17429145.2013.835880
Narsai, R., Devenish, J., Castleden, I., Narsai, K., Xu, L., Shou, H., et al. (2013). Rice DB: an Oryza information portal linking annotation, subcellular location, function, expression, regulation, and evolutionary information for rice and Arabidopsis. Plant J. 76, 1057–1073. doi: 10.1111/tpj.12357
Odanaka, S., Bennett, A. B., and Kanayama, Y. (2002). Distinct physiological roles of fructokinase isozymes revealed by gene-specific suppression of Frk1 and Frk2 expression in tomato. Plant Physiol. 129, 1119–1126. doi: 10.1104/pp.000703
Palmgren, M. G., and Harper, J. F. (1999). Pumping with plant P-type ATPases. J. Exp. Bot. 50, 883–893. doi: 10.1093/jxb/50.Special_Issue.883
Pandey, S., and Bhandari, H. (2008). “Drought: economics costs and research implications,” in Drought Frontiers in Rice: Crop Improvement for Increased Rainfed Production, eds R. Serraj, J. Bennett, and B. Hardy (Singapore: IRRI; World Scientific Publishing), 3–17.
Pastore, D., Trono, D., Laus, M. N., di Fonzo, N., and Flagella, Z. (2007). Possible plant mitochondria involvement in cell adaptation to drought stress. A case study: durum wheat mitochondria. J. Exp. Bot. 58, 195–210. doi: 10.1093/jxb/erl273
Périn, C., Rebouillat, J., Brasileiro, A. C. M., Diévart, A., Gantet, P., Breitler, J. C., et al. (2007). “Novel insights into the genomics of rice root adaptive development,” in Rice Genetics, eds D. S. Brar, D. J. Mackill, B. Hardy (Londres; Singapore: World Scientific; IRRI), 117–141.
Popova, V. N., Eprintseva, A. T., Fedorina, D. N., and Igamberdiev, A. U. (2010). Succinate dehydrogenase in Arabidopsis thaliana is regulated by light via phytochrome A. FEBS Lett. 584, 199–202. doi: 10.1016/j.febslet.2009.11.057
Price, A. H., Cairns, J. E., Horton, P., Jones, H. G., and Griffiths, H. (2002). Linking drought-resistance mechanisms to drought avoidance in upland rice using a QTL approach: progress and new opportunities to integrate stomatal and mesophyll responses. J. Exp. Bot. 53, 989–1004. doi: 10.1093/jexbot/53.371.989
Rabello, A. R., Guimarães, C. M., Rangel, P. H. N., da Silva, F. R., Seixas, D., de Souza, E., et al. (2008). Identification of drought-responsive genes in roots of upland rice (Oryza sativa L). BMC Genomics 9:485. doi: 10.1186/1471-2164-9-485
Ranjan, A., Pandey, N., Lakhwani, D., Dubey, N. K., Pathre, U. V., and Sawant, S. V. (2012). Comparative transcriptomic analysis of roots of contrasting Gossypium herbaceum genotypes revealing adaptation to drought. BMC Genomics 13:680. doi: 10.1186/1471-2164-13-680
Rapala-Kozik, M., Wolak, N., Kudja, M., and Banas, A. K. (2012). The upregulation of thiamine (vitamin B1) biosynthesis in Arabidopsis thaliana seedlings under salt and osmotic stress conditions is mediated by abscisic acid at the early stages of this stress response. BMC Plant Biol. 12:2. doi: 10.1186/1471-2229-12-2
Romijin, E. P., Christis, C., Wieffer, M., Gouw, W. J., Fullaondo, A., Sluijs, P., et al. (2005). Expression clustering reveals detailed co-expression patterns of functionally related proteins during b cell differentiation. A proteomic study using a combination of one-dimensional gel electrophoresis, LC-MS/MS, and stable isotope labeling by amino acids in cell culture (SILAC). Mol. Cell Proteomics 4, 1297–1310. doi: 10.1074/mcp.M500123-MCP200
Ryu, H. S., Han, M., Lee, S. K., Cho, J. I., Ryoo, N., Heu, S., et al. (2006). A comprehensive expression analysis of the WRKY gene superfamily in rice plants during defense response. Plant Cell Rep. 25, 836–847. doi: 10.1007/s00299-006-0138-1
Schwer, B., Bunkenborg, J., Verdin, R., Andersen, J., and Verdin, E. (2006). Reversible lysine acetylation controls the activity of the mitochondrial enzyme acetyl-CoA synthetase. Proc. Natl. Acad. Sci. U.S.A. 103, 10224–10229. doi: 10.1073/pnas.0603968103
Sengupta, D., Kannan, M., and Reddy, A. R. (2011). A root proteomics-based insight reveals dynamic regulation of root proteins under progressive drought stress and recovery in Vigna radiata (L.) Wilczek. Planta 233, 1111–1127. doi: 10.1007/s00425-011-1365-4
Simova-Stoilova, L. P., Romero-Rodríguez, M. C., Sánchez-Lucas, R., Navarro-Cerrillo, R. M. J., Medina-Aunon, J. A., and Jorrín-Novo, J. V. (2015). 2-DE proteomics analysis of drought treated seedlings of Quercus ilex supports a root active strategy for metabolic adaptation in response to water shortage. Front. Plant Sci. 6:627. doi: 10.3389/fpls.2015.00627
Singer, T. P., Oestreicher, G., and Hogue, P. (1973). Regulation of succinate dehyrogenase in higher plants: I. Some general characteristics of the membrane-bound enzyme. Plant Physiol. 52, 616–621. doi: 10.1104/pp.52.6.616
Sreenivasulu, N., Sopory, S. K., and Kavi Kishor, P. B. (2007). Deciphering the regulatory mechanisms of abiotic stress tolerance in plants by genomic approaches. Gene 388, 1–13. doi: 10.1016/j.gene.2006.10.009
Subba, P., Kumar, R., Gayali, S., Shekhar, S., Parveen, S., Pandey, A., et al. (2013). Characterisation of the nuclear proteome of a dehydration-sensitive cultivar of chickpea and comparative proteomic analysis with a tolerant cultivar. Proteomics 13, 1973–1992. doi: 10.1002/pmic.201200380
Tuberosa, R., and Salvi, S. (2006). Genomics-based approaches to improve drought tolerance of crops. Trends Plant Sci. 11, 405–412. doi: 10.1016/j.tplants.2006.06.003
van Wijk, K. J. (2001). Challenges and prospects of plant proteomics. Plant Physiol. 126, 501–508. doi: 10.1104/pp.126.2.501
Vander, J. D. (1989). “The glyoxalase system,” in Glutathione: Chemical, Biochemical, and Medical Aspects, eds D. Dolphin, R. Poulson, and O. Avramović (New York, NY: John Wiley & Sons Ltd.), 597–641.
Wang, F. Z., Wang, Q. B., Kwon, S. Y., Kwak, S. S., and Su, W. A. (2005). Enhanced drought tolerance of transgenic rice plants expressing a pea manganese superoxide dismutase. J. Plant Physiol. 162, 465–472. doi: 10.1016/j.jplph.2004.09.009
Wang, K., Uppalapati, S. R., Zhu, X., Dinesh-Kumar, S. P., and Mysore, K. S. (2010). SGT1 positively regulates the process of plant cell death during both compatible and incompatible plant-pathogen interactions. Mol. Plant Pathol. 11, 597–611. doi: 10.1111/j.1364-3703.2010.00631.x
Yang, Q., Wang, Y., Zhang, J., Shi, W., Qian, C., and Peng, X. (2007). Identification of aluminum-responsive proteins in rice roots by a proteomic approach: cysteine synthase as a key player in Al response. Proteomics 7, 737–749. doi: 10.1002/pmic.200600703
Yoshimura, K., Masuda, A., Kuwano, M., Yokota, A., and Akashi, K. (2008). Programmed proteome response for drought avoidance/tolerance in the root of a C3 xerophyte (wild watermelon) under water deficits. Plant Cell Physiol. 49, 226–241. doi: 10.1093/pcp/pcm180
Keywords: drought, proteomics, rice root, tolerant cultivar, 2-DE, MALDI-MS/MS, SOTA analysis
Citation: Agrawal L, Gupta S, Mishra SK, Pandey G, Kumar S, Chauhan PS, Chakrabarty D and Nautiyal CS (2016) Elucidation of Complex Nature of PEG Induced Drought-Stress Response in Rice Root Using Comparative Proteomics Approach. Front. Plant Sci. 7:1466. doi: 10.3389/fpls.2016.01466
Received: 23 July 2016; Accepted: 14 September 2016;
Published: 29 September 2016.
Edited by:
Paul Christiaan Struik, Wageningen University and Research Centre, NetherlandsReviewed by:
Abu Hena Mostafa Kamal, University of Texas at Arlington, USASanjay K. Gupta, University of Minnesota, USA
Supratim Basu, New Mexico Consortium, USA
Copyright © 2016 Agrawal, Gupta, Mishra, Pandey, Kumar, Chauhan, Chakrabarty and Nautiyal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chandra S. Nautiyal, bmF1dGl5YWxuYnJpQGx5Y29zLmNvbQ==
†These authors have contributed equally to this work.
 Lalit Agrawal
Lalit Agrawal Swati Gupta
Swati Gupta Shashank K. Mishra
Shashank K. Mishra Garima Pandey
Garima Pandey Susheel Kumar
Susheel Kumar Puneet S. Chauhan
Puneet S. Chauhan Debasis Chakrabarty
Debasis Chakrabarty Chandra S. Nautiyal*
Chandra S. Nautiyal*