Abstract
Genome editing technologies enable precise modifications of DNA sequences in vivo and offer a great promise for harnessing plant genes in crop improvement. The precise manipulation of plant genomes relies on the induction of DNA double-strand breaks by sequence-specific nucleases (SSNs) to initiate DNA repair reactions that are based on either non-homologous end joining (NHEJ) or homology-directed repair (HDR). While complete knock-outs and loss-of-function mutations generated by NHEJ are very valuable in defining gene functions, their applications in crop improvement are somewhat limited because many agriculturally important traits are conferred by random point mutations or indels at specific loci in either the genes’ encoding or promoter regions. Therefore, genome modification through SSNs-mediated HDR for gene targeting (GT) that enables either gene replacement or knock-in will provide an unprecedented ability to facilitate plant breeding by allowing introduction of precise point mutations and new gene functions, or integration of foreign genes at specific and desired “safe” harbor in a predefined manner. The emergence of three programmable SSNs, such as zinc finger nucleases, transcriptional activator-like effector nucleases, and the clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated protein 9 (Cas9) systems has revolutionized genome modification in plants in a more controlled manner. However, while targeted mutagenesis is becoming routine in plants, the potential of GT technology has not been well realized for traits improvement in crops, mainly due to the fact that NHEJ predominates DNA repair process in somatic cells and competes with the HDR pathway, and thus HDR-mediated GT is a relative rare event in plants. Here, we review recent research findings mainly focusing on development and applications of precise GT in plants using three SSNs systems described above, and the potential mechanisms underlying HDR events in plant cells. We then address the challenges and propose future perspectives in order to facilitate the implementation of precise genome modification through SSNs-mediated GT for crop improvement in a global context.
Introduction
Genome editing has become a powerful tool for functional genomics research in plants and genetic improvement of agricultural crops through precise manipulation of plant genomes. This relies on the creation of targeted DNA double strand breaks (DSBs) by sequence-specific nucleases (SSNs) at specified genomic locations, which will stimulate the cell’s DNA repair machinery. To date, four classes of SSNs, meganucleases/homing endonucleases which in general refer to I-SceI or I-CreI, zinc finger nucleases (ZFNs), transcriptional activator-like effector nucleases (TALENs), and the clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated protein 9 (Cas9), have been developed to cleave target genes of interest. Upon induction of a DNA DSB, the subsequent repair process in eukaryotic cells predominantly goes through the error-prone non-homologous end joining (NHEJ) pathway to create random indels, leading to frameshift mutations and target gene knockout. The presence of a donor DNA containing sequences homologous to those flanking the DSB site can greatly increase the chance of a precise DSB repair through the homology-directed repair (HDR) pathway, leading to gene replacement or foreign gene cassette knock-in as intended (Puchta, 2005; Puchta and Fauser, 2014; Voytas and Gao, 2014; Baltes and Voytas, 2015). Whereas complete knock-outs and loss-of-function mutations are very valuable in defining gene functions, deciphering complex metabolite pathways and becoming routine in plants (Lawrenson et al., 2015; Ma et al., 2015), their applications in crop improvement are somewhat limited because many agriculturally important traits are conferred by the random point mutations or indels at specific loci in either the genes’ encoding or promoter regions, change of gene expression levels or insertion of a new gene (Kumar et al., 2016; Sun et al., 2016). So far, target gene replacement or gene targeting (GT) has not yet been well established as a feasible technique in higher plants. The primary barrier is the high frequency of illegitimate recombination by which DNA integrates at non-homologous sites (Steinert et al., 2016). NHEJ is the primary pathway involved in DNA repair process in the somatic cells, while HDR mainly occurs during S and G2 phases of the cell cycle (Puchta, 2005). When DNA is introduced into plant cells, the frequency of illegitimate recombination events are typically 105–107 times higher than that of homologous recombination (Paszkowski et al., 1988; Lee et al., 1990; Offringa et al., 1990; Halfter et al., 1992; Hrouda and Paszkowski, 1994; Miao and Lam, 1995; Risseeuw et al., 1995). As a result, the frequency of replacement or targeted integration via HDR is much lower in comparison to random integration (Symington and Gautier, 2011), and reports describing successful gene replacement or site-specific trait gene integration through SSNs-mediated HDR in plants are very limited. Several reviews have described the rapid development and applications of the SSNs system in plants (Voytas and Gao, 2014; Belhaj et al., 2015; Osakabe and Osakabe, 2015; Kumar et al., 2016; Ma et al., 2016; Schiml and Puchta, 2016; Weeks et al., 2016). Here, we present an overview of the recent research advances mainly focusing on development and applications of precise GT in plants using the three recently developed SSN systems, ZFNs, TALENs, and CRISPR/Cas9 reagents, and the potential mechanisms underlying HDR events in plant cells as well as the challenges and future perspectives in implementing precise genome modification through SSNs-mediated GT for crop improvement.
Ssns-Mediated Gene Targeting in Plants
Ever since HDR was demonstrated to be feasible in plant cells in 1988 (Paszkowski et al., 1988), various classical GT strategies have been attempted to achieve HDR in plants (Paszkowski et al., 1988; Offringa et al., 1990; Zhu et al., 2000; Terada et al., 2002; Okuzaki and Toriyama, 2004; Shaked et al., 2005; Nishizawa-Yokoi et al., 2015). The induction of a DSB at a specific locus can significantly increase the frequency of homologous recombination up to more than 100-fold (Puchta et al., 1996). By using of a site-specific synthetic nuclease, meganuclease I-SceI, to induce DSB at the target locus, HDR events were successfully generated in several plant species, such as Arabidopsis, tobacco, rice, and tomato (Puchta et al., 1993, 1996; Beetham et al., 1999; Reiss et al., 2000; Siebert and Puchta, 2002; Fauser et al., 2012; Kwon et al., 2012). The emergence of three programmable SSNs, such as ZFNs, TALENs, and CRISPR/Cas9 has revolutionized the precise genome modification in a more controlled manner in plants. Over the last several years, GT has been achieved in higher plants with a varied degree of success (Table 1). Below, we summarized the recent research findings in precise GT in higher plants using the three different SSN systems described above.
Table 1
| Method | Plant species | Target gene | Donor | Delivery method | Homology-directed repair (HDR) event | Gene targeting (GT) phenotype | Reference |
|---|---|---|---|---|---|---|---|
| ZFNs | Nicotiana tabacum | Defective β-Glucuronidase gene (GUS):NPTII (transgene) | Restoring GUS function | Bombardment | Yes | GUS expression | Wright et al., 2005 |
| Arabidopsis thaliana | PRps5a-gfp/gus cassette (transgene) | Hpt coding region replaced by gfp coding region | Agrobacterium | Yes | GFP expression | de Pater et al., 2009 | |
| Nicotiana tabacum | Endo-chitinase gene CHN50 | PAT expression cassette | Agrobacterium | Yes | Herbicide resistances | Cai et al., 2009 | |
| Nicotiana tabacum | ALS | ALS with mutant site | Electroporation | Yes | Herbicide resistances | Townsend et al., 2009 | |
| Zea mays | IPK1 | PAT expression cassette/PAT gene no promoter | Silicon carbide whiskers | Yes | Herbicide resistances | Shukla et al., 2009 | |
| Arabidopsis thaliana | Mutated GUS expression cassette (transgene) | No donor | Agrobacterium | Yes | GUS expression | Even-Faitelson et al., 2011 | |
| Arabidopsis thaliana | Protoporphyrinogen oxidase (PPO) | T-DNA harboring an incomplete PPO gene | Agrobacterium | Yes | Insensitive to the herbicide butafenacil | de Pater et al., 2013 | |
| Arabidopsis thaliana | ADH1 | ADH1 fragment with a insertion of 68 bp and deletion of 12 bp | Agrobacterium | Yes | N.A | Qi et al., 2013 | |
| Arabidopsis thaliana | Quasipalindromic QQR ZFN recognition sites | Promoter-less hpt gene | Agrobacterium | Yes | Hygromycin resistance | Weinthal et al., 2013 | |
| Nicotiana tabacum | Quasipalindromic QQR ZFN recognition sites | Promoter-less hpt gene | Agrobacterium | Yes | Hygromycin resistance | Weinthal et al., 2013 | |
| Zea mays | TLPs | AAD1 expression cassette | Bombardment | Yes | Herbicide resistances | Ainley et al., 2013 | |
| Nicotiana tabacum | Defective GUS (transgene) | Restoring gus:nptII gene (Geminivirus-based replicons) | Agrobacterium | Yes | GUS expression and kanamycin resistance | Baltes et al., 2014 | |
| TALENs | Nicotiana tabacum | ALS | ALS with mutant site | PEG | No | N.A | Zhang et al., 2013 |
| Hordeum vulgare | GFP (transgene) | Truncated yfp fragment | Bombardment | No | YFP expression | Budhagatapalli et al., 2015 | |
| Solanum lycopersicum | Anthocyanin mutant 1 (ANT1) | 35S promoter upstream of the endogenous ANT1 coding sequence | Agrobacterium | Yes | Purple plant tissue | Cermak et al., 2015 | |
| Solanum tuberosum | ALS1 | ALS with mutant site (Geminivirus-based replicons) | Agrobacterium | Yes | Herbicide resistances | Butler et al., 2016 | |
| Oryza sativa | ALS | ALS with mutant site | Bombardment | Yes | Herbicide resistances | Li et al., 2016 | |
| CRISPR/Cas9 | Arabidopsis thaliana | DGU.US, IU.GUS (transgene) | No donor | Agrobacterium | Yes | GUS expression | Fauser et al., 2014 |
| Arabidopsis thaliana | ADH1 | NptII expression cassette | Agrobacterium | Yes | Kanamycin resistance | Schiml et al., 2014 | |
| Solanum lycopersicum | Anthocyanin mutant 1 (ANT1) | 35S promoter upstream of the endogenous ANT1 coding sequence (Geminivirus-based replicons) | Agrobacterium | Yes | Purple plant tissue | Cermak et al., 2015 | |
| Glycine max | ALS1 | ALS with mutant site | Bombardment | No | N.A | Li et al., 2015 | |
| Glycine max | Genomic sites DD43 on chromosome 4 | Hpt expression cassette | Bombardment | Yes | Hygromycin resistances | Li et al., 2015 | |
| Zea mays | ALS2 | ALS with mutant site | Bombardment | Yes | Herbicide resistances | Svitashev et al., 2015 | |
| Zea mays | Liguleless-1 (LIG1) | PAT expression cassette | Bombardment | Yes | Herbicide resistances | Svitashev et al., 2015 | |
| Linum usitatissimum | EPSPS | EPSPS with mutant site | PEG | Yes | Herbicide resistances | Sauer et al., 2016 | |
| Solanum tuberosum | ALS1 | ALS with mutant site (Geminivirus-based replicons) | Agrobacterium | Yes | Herbicide resistances | Butler et al., 2016 | |
| Zea mays | ARGOS8 | GOS2 promoter | Bombardment | Yes | Improved grain yield under drought stress conditions | Shi et al., 2016 | |
| Oryza sativa | ALS | ALS with mutant site | Agrobacterium | Yes | Herbicide resistances | Endo et al., 2016 | |
| Oryza sativa | ALS | ALS with mutant site | Bombardment | Yes | Herbicide resistances | Sun et al., 2016 |
Gene targeting in diverse plant species by employing different sequence-specific nucleases (SSNs).
HDR event, obtained plants through HDR.
GT in Plants Using ZFNs
Zinc finger nucleases, as the first generation of SSNs, were used to edit plant genomes (Smith et al., 2000; Bibikova et al., 2002; Lloyd et al., 2005; Zhang et al., 2010; Zhang and Voytas, 2011; Kumar et al., 2015; Petolino, 2015). By fusing the DNA cleavage domain from the restriction enzyme FokI to the highly variable DNA binding domain (DBD) of different zinc finger transcription factors to form different ZFNs, different target sites in the DNA can be recognized and cleaved (Figure 1A) (Kim et al., 1996). GT was demonstrated to be feasible in Arabidopsis and tobacco by inducing a DSB with a ZFN (Wright et al., 2005; Cai et al., 2009; de Pater et al., 2009, 2013; Townsend et al., 2009; Even-Faitelson et al., 2011; Qi et al., 2013; Weinthal et al., 2013; Baltes et al., 2014). So far, only two cases reported the successful GT for integration or stacking herbicide resistances gene(s) in a crop plant (maize). For example, simultaneous expression of ZFNs and delivery of a heterologous donor molecule led to precise targeted insertion of an herbicide tolerance gene expression cassette at the inositol 1,3,4,5,6-pentakisphosphate 2-kinase (IPK1) locus in maize (Shukla et al., 2009). Combination of the engineered ZFNs with modular “trait landing pads” (TLPs) could enable the site-specific transgene integration and traits stacking in crop plants (Ainley et al., 2013). For example, an herbicide resistance gene, phosphinothricin acetyltransferase (pat), along with TLPs was integrated into maize genome in the first round of transformation. Then, a second herbicide resistance gene, aryloxyalkanoate dioxygenase (aad1), flanked by sequences homologous to the integrated TLPs, along with a corresponding ZFN expression construct, was precisely targeted to the genomic locus of previous integrated pat following a second round of transformation, resulting in a sequential stack. Up to 5% of the embryo-derived transgenic events contained the aad1 transgene integrated precisely at the TLP which was directly adjacent to the pat transgene (Ainley et al., 2013). The ability to stack multiple trait genes at a single locus by ZFNs-mediated GT to enable simple inheritance addresses a significant agricultural challenge.
FIGURE 1
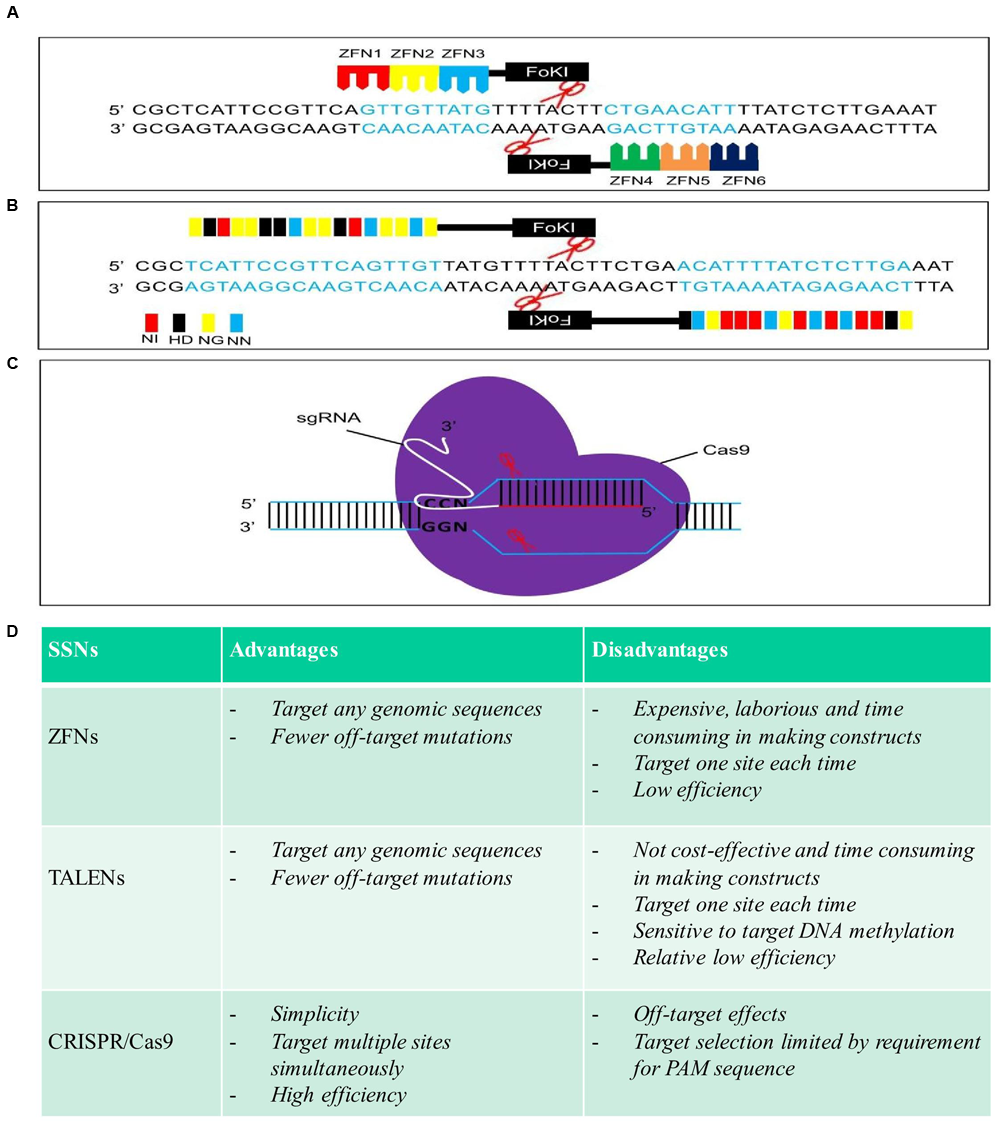
Schematic structures, advantages, and disadvantages of zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and clustered regularly interspaced short palindromic repeat (CRISPR)/Cas9. (A), (B), and (C) are schematic structures of ZFNs, TALENs, and CRISPR/Cas9 in the process of DNA cleavage, respectively. (D) Advantages and disadvantages of each sequence-specific nuclease (SSN).
GT in Plants Using TALENs
Over the past few years, TALENs have emerged as the reagent of choice in genome engineering in plants (Bogdanove and Voytas, 2011). Like ZFNs, TALENs are chimeric proteins produced by fusing an engineered DNA-binding domain with the catalytic domain of FokI endonuclease, which cleaves as a dimer (Figure 1B) (Christian et al., 2010; Li et al., 2011). TALENs and ZFNs, therefore, work in a same way in that two monomers bind opposing strands of DNA separated by a spacer of an appropriate length, allowing FokI to dimerize and cleave DNA. One of the advantages of TALENs over ZFNs is that the DBD can be easily engineered to recognize virtually any DNA sequence (Miller et al., 2011). So far, TALENs have been applied successfully for genome editing of a variety of different plant species, such as Arabidopsis, brachypodium, tobacco, tomato, rice, barley, wheat, and maize (Mahfouz et al., 2011; Shan et al., 2013; Zhang et al., 2013; Wang et al., 2014; Budhagatapalli et al., 2015; Cermak et al., 2015; Butler et al., 2016; Li et al., 2016), among which, only a few cases reported the recovery of precisely edited plants in GT experiments (Table 1). For example, TALENs introduced targeted mutations in acetolactate synthase (ALS) in 30% of transformed cells in tobacco protoplast, and the frequencies of targeted gene insertion reached at 14% (Zhang et al., 2013). The feasibility of precise modification of a target DNA sequence which resulted in a predicted alteration of gene function was also demonstrated in barley, when green fluorescent protein gene (gfp) specific TALENs along with a repair template were introduced into barley calli, conversion of gfp into yellow fluorescent protein gene (yfp) via HDR was achieved in three of 100 calli bombarded (Budhagatapalli et al., 2015). A strong promoter was inserted upstream of a gene controlling anthocyanin biosynthesis, precise modification of the tomato genome was achieved through either TALENS or CRISPR/Cas9 using geminivirus replicons, resulting in overexpression and ectopic accumulation of pigments in tomato tissues (Cermak et al., 2015). The same strategy was also successful applied in generation of herbicide resistant potato plants by introducing one point mutation in potato ALS1 gene through gene replacement (Butler et al., 2016). Recently, through Agrobacterium-mediated transformation, herbicide resistant rice plants were recovered by introducing double point mutations in rice ALS through TALENs-mediated gene replacement with an efficiency of 1.4–6.3% (Li et al., 2016).
However, it is worth to mention that both ZFNs and TALENs need tandem repeats in their DNA-binding domains that engineered to recognize specific DNA sequences in the genome to generate DSBs. In this case, a new chimeric protein must be engineered for each new target sequence of interest (Figures 1A,B). This has been a major hurdle to the wider application of these two SSNs because engineering new protein is a very complicate process, non-cost effective, time-consuming and is not feasible in most laboratories (Figure 1D).
GT in Plants Using CRISPR/Cas9
As a third generation of designed SSNs, the emergence of CRISPR/Cas9 has revolutionized genome editing because of its specificity, simplicity, and versatility (Cong et al., 2013; Feng et al., 2014; Gao and Zhao, 2014; Zhou et al., 2014; Lawrenson et al., 2015; Ma et al., 2015, 2016; Xie et al., 2015; Sun et al., 2016). The CRISPR/Cas9 system uses a single guide RNA (sgRNA) to direct the Cas9 endonuclease to the complementary target DNA, and only a new sgRNA is needed for a new target site of interest whilst the nuclease itself remains unmodified (Figure 1C) (Jinek et al., 2012; Gaj et al., 2013). Thus, the CRISPR/Cas9 system surpasses ZFNs and TALENs, for its simplicity, versatility and high efficiency, and has been successfully applied in precise genome modification in many organisms including plants (Figure 1D) (Doudna and Charpentier, 2014; Ma et al., 2016; Weeks et al., 2016). However, the majority of these studies in plants reported genome editing via NHEJ to generate random loss-of-function mutations or gene knock-outs (Ma et al., 2016). Although gene replacement or GT could be potentially achieved through HDR after CRSIPR/Cas9 generates a DSB at specific gene loci, it remains very challenging to make use of HDR in plants through CRISPR/Cas9-mediated genome editing (Svitashev et al., 2015; Sun et al., 2016). The successful gene replacement or GT has been documented so far in a limited number of studies in Arabidopsis, rice, maize and flax (Linum usitatissimum) (Table 1). The CRISPR/Cas9 system can be used as nuclease for in planta GT in Arabidopsis (Schiml et al., 2014). ALS is a key enzyme for the biosynthesis of branched chain amino acids and which is a major target for agriculturally important herbicides including chlorsulfuron and bispyribac-sodium (BS). Substitution of proline 165 with serine in the ALS2 gene using either single-stranded oligonucleotides or double-stranded DNA vectors as repair templates yielded chlorsulfuron resistant maize plants via CRISPR/Cas9-mediated gene editing. However, the efficiency of generation of herbicide resistant plants was very low. Among 1000 calli bombarded, only nine calli recovered were able to regenerate herbicide resistant plants (Svitashev et al., 2015). In another parallel experiment with HDR-mediated gene insertion at an endogenous liguleless-1 gene (L1G) target site in maize, co-delivery of both Cas9-sgRNA and donor DNA either separately or as a single vector through bombardment resulted in a frequency of 2.5–4% of target insertion, respectively (Svitashev et al., 2015). A repair construct and the CRISPR/Cas9 expression vector targeting the rice ALS gene were transferred into rice calli either separately or sequentially through Agrobacterium-mediated transformation, resulted in herbicide resistant rice plants with the ratio of GT frequency of 0.323% (Endo et al., 2016). In our previous study, by using dual sgRNAs in combination with two sources of DNA repair templates, one released from T-DNA in planta and the other co-bombarded as free double-stranded DNA, to promote HDR-mediated ALS gene replacement in rice, multiple homozygous herbicide resistant rice plants in T0 generation were successfully recovered via CRISPR/Cas9-mediated HDR for simultaneous in planta point substitutions of two amino acid residues, tryptophan 548 and serine 627 in the rice ALS with leucine and isoleucine (Sun et al., 2016). Maize ARGOS8 is a negative regulator of ethylene responses. The maize GOS2 promoter was inserted into the 5’ untranslated region of the native ARGOS8 gene to replace the native promoter of ARGOS8 through CRISPR/Cas9-mediated HDR. Precise promotor replacement at the ARGOS8 locus resulted in increased grain yield by five bushels per acre under stress conditions (Shi et al., 2016). An herbicide tolerance trait was also developed in flax (L. usitatissimum) by precise modification of the 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) gene through a co-delivery of CRISPR/Cas9 system and the single-stranded oligonucleotides (ssODN) template into protoplast with the frequency of precise EPSPS edited events ranged between 0.09 and 0.23% (Sauer et al., 2016). Most recently, through bombardment of a vector harboring the CRISPR/Cas9 system and donor template, glyphosate-resistant rice plants was generated by CRISPR/Cas9-mediated gene replacement of the intron region of EPSPS gene at an efficiency of 2.0% (Li et al., 2016).
The Potential Mechanisms Underlying HDR Inside Plant Cells
In principle, there are three main mechanisms of DSB repair involving the use of a homologous template: single-strand annealing (SSA) (Figure 2A), synthesis-dependent strand annealing (SDSA) (Figure 2B), and the so-called double-strand break repair (DSBR) model (Puchta and Fauser, 2014). Following DSB induction in all pathways, single-stranded overhangs are produced via exonuclease-catalyzed resection. In the case of the SSA mechanism, both ends of the break carry complementary sequences. These molecules can then anneal to one another to form a chimeric DNA molecule with the 3’-overhangs be trimmed. As a consequence, the sequences flanking the complementary sequences will be lost (Puchta and Fauser, 2014). SSA can, in principle, also occur between two DNA molecules that are not linked. These molecules could be transfected plasmid DNAs or T-DNAs as well as broken chromosomes (Puchta and Hohn, 1991a; Tinland et al., 1994; Pacher et al., 2007). This mechanism is not conservative since all sequence information between the respective repeats is lost, SSA can proceed in somatic plant cells as efficient as NHEJ (Knoll et al., 2012). In the case of DSBR and SDSA, the homologous repair template can be supplied in cis or trans. Following the DSB induction, 3’-end invasion of a single strand into a homologous double strand occurs, resulting in a D-loop (Figure 2B). Reparative synthesis is initiated using the newly paired strand as a template. Whereas in SDSA, the genetic information of a homologous sequence is only copied to one strand, leading to no loss of sequence information although the reaction sometimes results in gene conversion (Puchta and Fauser, 2013), in DSBR, DNA synthesis occurs at both broken ends, respectively, so that genetic information is copied from both strands of the homologous sequences, respectively, thus may lead to a crossover event. DSBR is a prominent mechanism for meiotic recombination (Osman et al., 2011). In our previous study with ALS gene replacement in rice, evaluation of the HDR events demonstrated that while most of the HDR events faithfully copied the genetic information of the donor template, some only carried the substitutions at 5′-end and some harbored the conversion at the 3′-end, and no crossover detected in our case (Sun et al., 2016). Our result is in consistence with other GT experiments in which SDSA may represent the major class of GT events and is probably a predominant mechanism underlying HDR event as well as to a combination of HDR and NHEJ events (Puchta et al., 1996; Reiss et al., 2000; Wright et al., 2005). As the SDSA pathway is beneficial for genome stability, it seems to be a predominant pathway responsible for conservative HDR in somatic plant cells (Puchta, 1998; Osman et al., 2011). So far, a majority of successful GT experiments employed the DNA repair template flanked with homologous arms at each end. A positive correlation was found between the HDR rates and the lengths of overlapping homology (up to 1200 bp) of the transfected supercoiled circular or linearized plasmids, with a significantly decreased HDR rate was observed when the overlap of both substrates was reduced to 456 bp or less (Puchta and Hohn, 1991b).
FIGURE 2
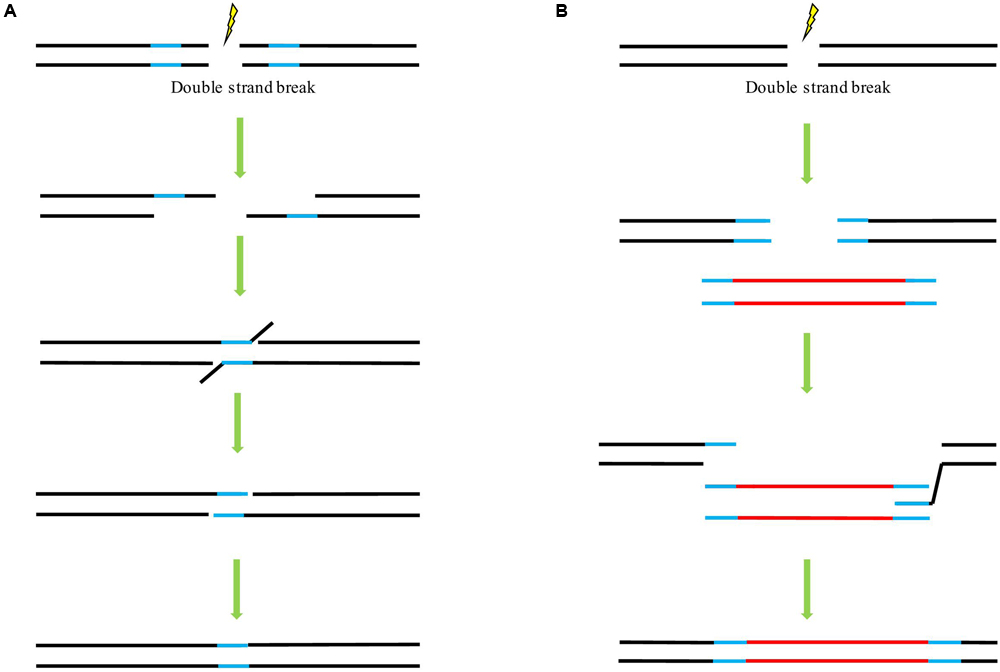
The potential mechanisms underlying double strand break (DSB) repair upon the availability of a homologous template. (A) Single-strand annealing (SSA) pathway of homology-directed repair (HDR). (B) Synthesis-dependent strand annealing (SDSA) pathway of HDR. Lines in blue indicate the homologous sequences. Lines in red indicate the foreign sequences.
Challenges and Future Perspectives
One major obstacle in performing GT for crop improvement is its low efficiency because repair through NHEJ predominates in plant somatic cells and competes with the HDR pathway (Figure 3) (Puchta et al., 1996; Qi et al., 2013). Other challenges including plant species recalcitrance to tissue culture and transformation, and thus low frequency of stably transformed events limit efforts to use genome editing for genetic improvement in some crop species and genotypes (Figure 3) (Shrawat and Lorz, 2006; Hiei et al., 2014; for review, please see Altpeter et al., 2016). Moreover, unintended random integration of donor template should be identified, suppressed or segregated because of biosafety concerns in application of these edited crop plants in a breeding practice (Figure 3) (Altpeter et al., 2016; Kumar et al., 2016). Consequently, substantial efforts have been devoted in the last few years to meet the above challenges.
FIGURE 3
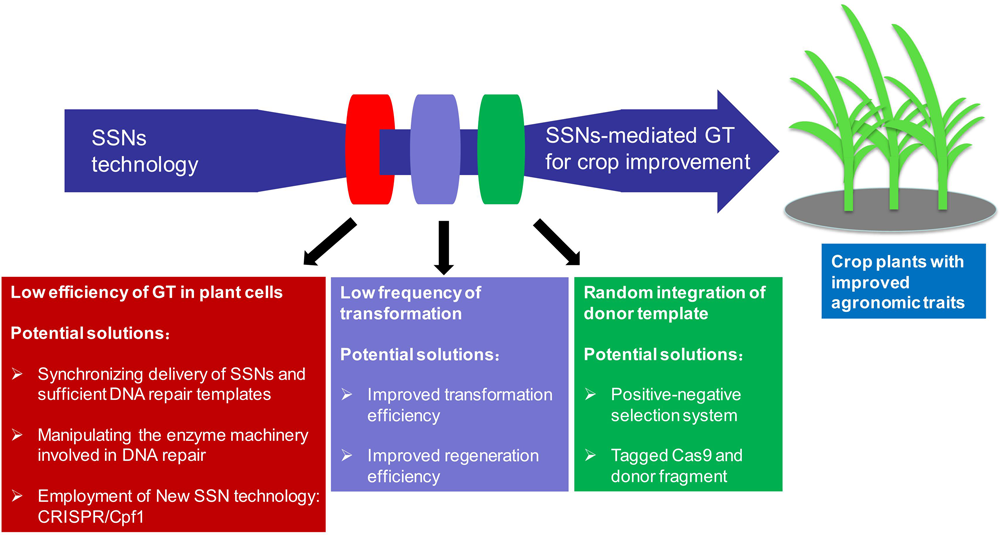
Current challenges and future perspectives in applying SSNs-mediated gene targeting (GT) for crop improvement.
Synchronizing Delivery of SSNs and Sufficient DNA Repair Templates
In theory, synchronizing the DSB induction by SSNs and delivery of the HDR template is essential for the occurrence of HDR in plant cells (Baltes et al., 2014; Schiml et al., 2014; Endo et al., 2016; Sun et al., 2016). To increase the frequency of HDR-mediated GT, timing the induction DSBs on the target gene to coincide with the delivery of sufficient HDR template is crucial for the successful GT events. Therefore, how to deliver the SSNs and DNA repair templates for HDR represent hurdles to the efficient achievement of GT (Figure 3). Protoplasts can be transformed with both SSNs and DNA repair template at high efficiency (Wright et al., 2005; Townsend et al., 2009; Zhang et al., 2013), however, for most plant species, especially major cereal crops, regeneration of plants from cultured protoplasts is still not feasible. An efficient way to supply the pant cell with a matrix for HDR-mediated DSB repair is to use an incoming T-DNA from Agrobacterium tumefaciens or transfected plasmid DNA (Puchta and Fauser, 2015). A sophisticated system, in planta GT, to enhance gene replacement was first established in 2012 using the meganuclease I-SceI (Fauser et al., 2012), then successfully applied in Arabidopsis using the CRISPR/Cas9 reagent by the same group in 2014 (Schiml et al., 2014). It is based on a transgene carrying both sequences homologous to the flanking sequences of the target locus and two recognition sites for a SSN, which also cuts the locus of interest in plant genome. In planta GT system allows the simultaneous release of a linear GT vector and the induction of a DSB at the target locus. Under this strategy, the GT vector can be designed for the site-specific integration of transgenes or to modify the target gene/locus in a predefined manner (Puchta and Fauser, 2015). Considering the low DNA titers delivered by Agrobacterium-mediated gene transfer, biolistic gene transfer may be superior for HDR-mediated GT by simultaneously delivery of both SSNs and DNA repair template and providing larger quantities of repair template. By using the same strategy, dual sgRNAs in combination of two sources of DNA repair templates, one released from T-DNA in planta and the other co-bombarded as free double-stranded DNA, to promote HDR-mediated ALS gene replacement, multiple homozygous GT rice plants were successfully recovered in T0 generation with a highest frequency up to 10%, whereas only hemizygous lines were recovered after two rounds of BS selection in our Agrobacterium experiments (Sun et al., 2016). Thus, it would be interesting to investigate the effects of different delivery methods and parameters on GT in crop plants. Furthermore, to overcome the challenges of inefficient transformation and plant regeneration systems in a majority of crop species or genotypes, intra-genomic homologous recombination through intra-genomic mobilization by crossing the SSNs transgenic lines with the transgenic plants harboring stable integrated donor DNA template, is an alternative effective strategy not only for GT, but also for gene stacking (Kumar et al., 2016). In addition, taking advantage of geminivirus replicon, a GT enhancement of greater than two orders of magnitude has been achieved (Baltes et al., 2014). A T-DNA construct harboring the minimal parts necessary for geminivirus replication, a ZFN and a donor template was used to transform tobacco. After transformation, the rolling circle replication of the replicon was initiated at the large intergenic regions (LIRs) that flank the T-DNA construct, leading to the circularization of the construct. Thereafter, the ZFN was expressed and induced a DSB in a defective target gene. GT events occurred using the supplied correct donor template sequence, copying it via GT in the target gene and leading to gene restoration, and thus the donor template was replicated multiple times (Baltes et al., 2014). Heritable gene replacement was achieved by using this strategy in tomato at frequencies 10-fold higher than traditional methods of DNA delivery (i.e., Agrobacterium). Both TALENs and CRISPR/Cas9 achieved GT with more than two-thirds of the insertions were precise, and had no unanticipated sequence modifications (Cermak et al., 2015). This method overcomes the efficiency barrier that has made GT in plants challenging.
Manipulating the Enzyme Machinery Involved in DNA Repair to Enhance GT in Plants
Once DSB induced, DNA repair through NHEJ predominates in somatic cells and competes with the HDR pathway. Suppression of core components of the NHEJ pathway or enhancement of key elements of HDR machinery can be used to increase frequencies of HDR (Figure 3) (Puchta et al., 1996; Qi et al., 2013). Expression of a bacterial RecA gene in plants stimulated HDR in tobacco (Reiss et al., 2000). During HDR-mediated DSB repair in eukaryotes, creation of a single-stranded DNA (ssDNA) overhang via resection of a 5′ end is an initial step. RAD51 can polymerize on this ssDNA to search for a homologous sequence so that the gapped sequence is then repaired using another undamaged homologous DNA strand as template (Kwon et al., 2012). Therefore, the presence of RAD51 is extremely important for SDSA in Arabidopsis, but not for SSA. The same is true for the SWI2/SNF2 chromatin remodeler AtRAD54 (Roth et al., 2012). Expression of a yeast RAD54 gene led to an increase in GT efficiency in Arabidopsis (Shaked et al., 2005; Even-Faitelson et al., 2011). The fact that expression of a rice OsRecQl4 (a gene encoding bloom helicase counterpart) and/or rice exonuclease 1 could enhance intra-chromosomal HDR was taken as an indication that these proteins might, in fact, be involved in end resection in plants (Kwon et al., 2012). Indeed, Arabidopsis plants with a deficit of RECQ4A showed some deficiency in both the SSA and SDSA pathways (Knoll and Puchta, 2011). Instead of heterologous expression of HDR-related proteins, GT efficiency could also be increased by suppression of proteins involved in NHEJ, which led to a hyper-recombination phenotype in Arabidopsis (Endo et al., 2006; Hartung et al., 2007; Knoll and Puchta, 2011; Kwon et al., 2012; Recker et al., 2014). DNA repair proteins, such as KU70/80 and Ligase 4 (Lig4) are involved in classic NHEJ, suppression of KU70 or Lig4 function increased the frequency of ZFNs-mediated GT 5- to 16-fold and threefold to fourfold in Arabidopsis ku70 and lig4 mutants, respectively (Qi et al., 2013). HDR activity in calli with a Lig4 deficiency background was twofold to threefold higher than that in control calli in rice. Combination of the induced DSBs via SSNs at a target gene and suppression of NHEJ-related genes or treatment with Lig4 inhibitors can be expected to enhance synergistically the frequency of GT in rice (Endo et al., 2016). However, one obstacle with the manipulation of the HDR repair machinery is that this may lead to a destabilization of the genome, as higher HDR efficiencies can also lead to undesirable recombination events between repetitive sequence motives that are abundantly present in plant species with larger and complex genomes (Steinert et al., 2016).
Enriching GT Events Using Positive-Negative Selection System
Positive-negative selection (PNS) is an alternative approach to enrich HDR-mediated GT events; it can eliminate NHEJ effectively by expression of a negative-selection marker gene (Figure 3) (Shimatani et al., 2015). The single copy Waxy locus was targeted for classical GT (knock-in) using a PNS vector carrying the hygromycin phosphotransferase gene (hpt) for positive selection followed by the effective transcriptional stop signal of the maize transposon En/Spm, which was positioned between the Waxy homologous sequences, and one negative selection gene DT-A (diphtheria toxin A-fragment from Corynebacterium diphtheriae) flanked with the homologous sequence at both ends (Terada et al., 2002). Since then, the endogenous rice genes at more than 10 loci have been targeted (Terada et al., 2007; Yamauchi et al., 2009; Moritoh et al., 2012; Ono et al., 2012; Ozawa et al., 2012; Dang et al., 2013; Osakabe et al., 2014). Using a combination of neomycin phosphotransferase II (nptII) and an antisense nptII construct, a universally applicable PNS system for GT in plants was established, although negative selection with this system is relatively less efficient compared with DT-A (Nishizawa-Yokoi et al., 2015). It is worth to note that although this strategy has been only documented in classical GT experiment, it is expected that combination of SSNs-mediated GT with PNS strategy may facilitate the enrichment and recovery of GT events in plants.
Toward Successful Implementation of Precise Genome Modification through SSNs-Mediated GT for Crop Improvement
Over the last several years, genome manipulation has been revolutionized by the development of three types of SSNs for the control induction of DSBs and thus offers a great promise for harnessing plant genes in crop improvement. However, only a handful of studies reported the precise modification of an endogenous gene for knock-in or target gene replacement in crop plants due to the fact that GT has not been established as a feasible technique in a majority of laboratories. To establish a routine GT system in crop plants, synchronization of DSB induction and delivery of sufficient DNA repair template is crucial for successful GT (Schiml et al., 2014; Endo et al., 2016; Sun et al., 2016). Virus replicon-based GT could also be a good choice to provide sufficient donor template (Baltes et al., 2014; Cermak et al., 2015). For crop species recalcitrant to transformation and regeneration, intra-chromosome HDR will be an effective alternative strategy (Kumar et al., 2016). Furthermore, suppression of core components of the NHEJ pathway or enhancement of key elements of HDR machinery can be used to increase frequencies of GT (Puchta et al., 1996; Qi et al., 2013; Puchta and Fauser, 2015). In addition, to date, most of the GT events reported in plants were either herbicide resistant genes or selectable marker genes in which positive selection for GT is much easier such that precise insertion or gene replacement of the donor in the target locus conferred resistance to selection and avoidance of random integration of the donor (Table 1). The PNS selection system might be an attractive strategy to increase the GT efficiency of non-selectable target genes through enrichment of the DSB-induced GT events. Moreover, most recently, a new RNA-guided genome engineering tool, the CRISPR/Cpf1 system, was reported to have properties different from those of the CRISPR/Cas9 systems in that the CRISPR/Cpf1 system has a single RNA-guided endonuclease lacking tracrRNA, 5′ T-rich protospacer adjacent motif (PAM), and a staggered DSB with 4 or 5-nt overhang in contrast to the blunt ends generated by Cas9 (Zetsche et al., 2015). This structure of the cleavage product could be particularly advantageous for facilitating NHEJ-based gene insertion into the plant genome because the DNA insert could be designed to integrate into the genome in a proper orientation (Zetsche et al., 2015). Specifically, Cpf1 could provide an effective way to precisely introduce DNA into the genome via NHEJ mechanism in somatic cells in which genome editing via HDR mechanisms is especially challenging (Chan et al., 2011). At last, regeneration and transformation efficiency, and issues of regulation must also be taken into consideration when selecting a transformation strategy for a given crop species (Altpeter et al., 2016). Both the CRISPR/Cas9 array and the donor templates could be tagged by fluorescence proteins and tracked and eliminated following segregation in the progeny to avoid the random integration of donor fragments and obtain Cas9-free lines (Gao et al., 2016). However, this tagging strategy will only feasible for the integrations of intact constructs. For the integration of fragmented SSN expression cassettes or GT donor molecules, genome-wide sequencing would solve this problem and should not be a major cost issue for sequenced organisms; especially if these developed new varieties will be commercialized in the future. Nonetheless, combination of the different strategies discussed above will be expected to make GT more efficient in crop plants (Figure 3). And it is tempting to propose that in the long run, precise genome modification through SSNs-mediated GT will greatly facilitate crop improvement by fully exploiting the agronomic traits important alleles within a gene pool or even beyond species boundaries.
Statements
Author contributions
YWS and JYL wrote the manuscript; LQX revised the manuscript.
Acknowledgments
Some work mentioned in this review is partly funded by the Ministry of Science and Technology of China (grant no. 2016YFD0100500 and 2016YFD0102003) and the Ministry of Agriculture of China (grant no. 2016ZX08010-003). YWS is supported by a GSCAAS-ULg Joint Ph.D. Program.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1
Ainley W. M. Sastry-Dent L. Welter M. E. Murray M. G. Zeitler B. Amora R. et al (2013). Trait stacking via targeted genome editing.Plant Biotechnol. J.111126–1134. 10.1111/pbi.12107
2
Altpeter F. Springer N. M. Bartley L. E. Blechl A. E. Brutnell T. P. Citovsky V. et al (2016). Advancing crop transformation in the era of genome editing.Plant Cell281510–1520. 10.1105/tpc.16.00196
3
Baltes N. J. Gil-Humanes J. Cermak T. Atkins P. A. Voytas D. F. (2014). DNA replicons for plant genome engineering.Plant Cell26151–163. 10.1105/tpc.113.119792
4
Baltes N. J. Voytas D. F. (2015). Enabling plant synthetic biology through genome engineering.Trends Biotechnol.33120–131. 10.1016/j.tibtech.2014.11.008
5
Beetham P. R. Kipp P. B. Sawycky X. L. Arntzen C. J. May G. D. (1999). A tool for functional plant genomics: chimeric RNA/DNA oligonucleotides cause in vivo gene-specific mutations.Proc. Natl. Acad. Sci. U.S.A.968774–8778. 10.1073/pnas.96.15.8774
6
Belhaj K. Chaparro-Garcia A. Kamoun S. Patron N. J. Nekrasov V. (2015). Editing plant genomes with CRISPR/Cas9.Curr. Opin. Biotechnol.3276–84. 10.1016/j.copbio.2014.11.007
7
Bibikova M. Golic M. Golic K. G. Carroll D. (2002). Targeted chromosomal cleavage and mutagenesis in drosophila using zinc-finger nucleases.Genetics1611169–1175.
8
Bogdanove A. J. Voytas D. F. (2011). TAL effectors: customizable proteins for DNA targeting.Science3331843–1846. 10.1126/science.1204094
9
Budhagatapalli N. Rutten T. Gurushidze M. Kumlehn J. Hensel G. (2015). Targeted modification of gene function exploiting homology-directed repair of TALEN-mediated double-strand breaks in barley.G3 51857–1863. 10.1534/g3.115.018762
10
Butler N. M. Baltes N. J. Voytas D. F. Douches D. S. (2016). Geminivirus-mediated genome editing in potato (Solanum tuberosum L.) using sequence-specific nucleases.Front. Plant Sci.7:1045. 10.3389/fpls.2016.01045
11
Cai C. Q. Doyon Y. Ainley W. M. Miller J. C. Dekelver R. C. Moehle E. A. et al (2009). Targeted transgene integration in plant cells using designed zinc finger nucleases.Plant Mol. Biol.69699–709. 10.1007/s11103-008-9449-7
12
Cermak T. Baltes N. J. Cegan R. Zhang Y. Voytas D. F. (2015). High-frequency, precise modification of the tomato genome.Genome Biol.16: 232. 10.1186/s13059-015-0796-9
13
Chan F. Hauswirth W. W. Wensel T. G. Wilson J. H. (2011). Efficient mutagenesis of the rhodopsin gene in rod photoreceptor neurons in mice.Nucleic Acids Res.395955–5966. 10.1093/nar/gkr196
14
Christian M. Cermak T. Doyle E. L. Schmidt C. Zhang F. Hummel A. et al (2010). Targeting DNA double-strand breaks with TAL effector nucleases.Genetics186757–761. 10.1534/genetics.110.120717
15
Cong L. Ran F. A. Cox D. Lin S. Barretto R. Habib N. et al (2013). Multiplex genome engineering using CRISPR/Cas systems.Science339819–823. 10.1126/science.1231143
16
Dang T. T. Shimatani Z. Kawano Y. Terada R. Shimamoto K. (2013). Gene editing a constitutively active OsRac1 by homologous recombinationbased gene targeting induces immune responses in rice.Plant Cell Physiol.542058–2070. 10.1093/pcp/pct147
17
de Pater S. Neuteboom L. W. Pinas J. E. Hooykaas P. J. Van Der Zaal B. J. (2009). ZFN-induced mutagenesis and gene-targeting in Arabidopsis through Agrobacterium-mediated floral dip transformation.Plant Biotechnol. J.7821–835. 10.1111/j.1467-7652.2009.00446.x
18
de Pater S. Pinas J. E. Hooykaas P. J. Van Der Zaal B. J. (2013). ZFN-mediated gene targeting of the Arabidopsis protoporphyrinogen oxidase gene through Agrobacterium-mediated floral dip transformation.Plant Biotechnol. J.11510–515. 10.1111/pbi.12040
19
Doudna J. A. Charpentier E. (2014). The new frontier of genome engineering with CRISPR-Cas9.Science346: 1258096. 10.1126/science.1258096
20
Endo M. Ishikawa Y. Osakabe K. Nakayama S. Kaya H. Araki T. et al (2006). Increased frequency of homologous recombination and T-DNA integration in Arabidopsis CAF-1 mutants.EMBO J.255579–5590. 10.1038/sj.emboj.7601434
21
Endo M. Mikami M. Toki S. (2016). Biallelic gene targeting in rice.Plant Physiol.170667–677. 10.1104/pp.15.01663
22
Even-Faitelson L. Samach A. Melamed-Bessudo C. Avivi-Ragolsky N. Levy A. A. (2011). Localized egg-cell expression of effector proteins for targeted modification of the Arabidopsis genome.Plant J.68929–937. 10.1111/j.1365-313X.2011.04741.x
23
Fauser F. Roth N. Pacher M. Ilg G. Sanchez-Fernandez R. Biesgen C. (2012). In planta gene targeting.Proc. Natl. Acad. Sci. U.S.A.1097535–7540. 10.1073/pnas.1202191109
24
Fauser F. Schiml S. Puchta H. (2014). Both CRISPR/Cas-based nucleases and nickases can be used efficiently for genome engineering in Arabidopsis thaliana.Plant J.79348–359. 10.1111/tpj.12554
25
Feng Z. Mao Y. Xu N. Zhang B. Wei P. Yang D. L. et al (2014). Multigeneration analysis reveals the inheritance, specificity, and patterns of CRISPR/Cas-induced gene modifications in Arabidopsis.Proc. Natl. Acad. Sci. U.S.A.1114632–4637. 10.1073/pnas.1400822111
26
Gaj T. Gersbach C. A. Barbas C. F. III (2013). ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering.Trends Biotechnol.31397–405. 10.1016/j.tibtech.2013.04.004
27
Gao X. Chen J. Dai X. Zhang D. Zhao Y. (2016). An effective strategy for reliably isolating heritable and Cas9-free Arabidopsis mutants generated by CRISPR/Cas9-mediated genome editing.Plant Physiol.1711794–1800. 10.1104/pp.16.00663
28
Gao Y. Zhao Y. (2014). Self-processing of ribozyme-flanked RNAs into guide RNAs in vitro and in vivo for CRISPR-mediated genome editing.J. Integr. Plant Biol.56343–349. 10.1111/jipb.12152
29
Halfter U. Morris P.-C. Willmitzer L. (1992). Gene targeting in Arabidopsis thaliana.Mol. Gen. Genet.231186–193.
30
Hartung F. Suer S. Puchta H. (2007). Two closely related RecQ helicases have antagonistic roles in homologous recombination and DNA repair in Arabidopsis thaliana.Proc. Natl. Acad. Sci. U.S.A.10418836–18841. 10.1073/pnas.0705998104
31
Hiei Y. Ishida Y. Komari T. (2014). Progress of cereal transformation technology mediated by Agrobacterium tumefaciens.Front. Plant Sci.5:628. 10.3389/fpls.2014.00628
32
Hrouda M. Paszkowski J. (1994). High fidelity extrachromosomal recombination and gene targeting in plants.Mol. Gen. Genet.243106–111. 10.1007/BF00283882
33
Jinek M. Chylinski K. Fonfara I. Hauer M. Doudna J. A. Charpentier E. (2012). A programmable dual-RNA–guided DNA endonuclease in adaptive bacterial immunity.Science337816–821. 10.1126/science.1225829
34
Kim Y. G. Cha J. Chandrasegaran S. (1996). Hybrid restriction enzymes: zinc finger fusions to Fok I cleavage domain.Proc. Natl. Acad. Sci. U.S.A.931156–1160. 10.1073/pnas.93.3.1156
35
Knoll A. Higgins J. D. Seeliger K. Reha S. J. Dangel N. J. Bauknecht M. et al (2012). The Fanconi anemia ortholog FANCM ensures ordered homologous recombination in both somatic and meiotic cells in Arabidopsis.Plant Cell241448–1464. 10.1105/tpc.112.096644
36
Knoll A. Puchta H. (2011). The role of DNA helicases and their interaction partners in genome stability and meiotic recombination in plants.J. Exp. Bot.621565–1579. 10.1093/jxb/erq357
37
Kumar S. Alabed D. Worden A. Novak S. Wu H. Ausmus C. et al (2015). A modular gene targeting system for sequential transgene stacking in plants.J. Biotechnol.20712–20. 10.1016/j.jbiotec.2015.04.006
38
Kumar S. Barone P. Smith M. (2016). Gene targeting and transgene stacking using intra genomic homologous recombination in plants.Plant Methods12:11. 10.1186/s13007-016-0111-0
39
Kwon Y. I. Abe K. Osakabe K. Endo M. Nishizawa-Yokoi A. Saika H. et al (2012). Overexpression of OsRecQl4 and/or OsExo1 enhances DSB-induced homologous recombination in rice.Plant Cell Physiol.532142–2152. 10.1093/pcp/pcs155
40
Lawrenson T. Shorinola O. Stacey N. Li C. Østergaard L. Patron N. et al (2015). Induction of targeted, heritable mutations in barley and Brassica oleracea using RNA-guided Cas9 nuclease.Genome Biol.16: 258. 10.1186/s13059-015-0826-7
41
Lee K. Y. Lund P. Lowe K. Dunsmuir P. (1990). Homologous recombination in plant cells after Agrobacterium-mediated transformation.Plant Cell2415–425. 10.1105/tpc.2.5.415
42
Li T. Huang S. Jiang W. Z. Wright D. Spalding M. H. Weeks D. P. et al (2011). TAL nucleases (TALNs): hybrid proteins composed of TAL effectors and FokI DNA-cleavage domain.Nucleic Acids Res.39359–372. 10.1093/nar/gkq704
43
Li T. Liu B. Chen C. Y. Yang B. (2016). TALEN-mediated homologous recombination produces site-directed DNA base change and herbicide-resistant rice.J. Genet. Genomics43297–305. 10.1016/j.jgg.2016.03.005
44
Li Z. Liu Z. B. Xing A. Moon B. P. Koellhoffer J. P. Huang L. et al (2015). Cas9-guide RNA directed genome editing in soybean.Plant Physiol.169960–970. 10.1104/pp.15.00783
45
Lloyd A. Plaisier C. L. Carroll D. Drews G. N. (2005). Targeted mutagenesis using zinc-finger nucleases in Arabidopsis.Proc. Natl. Acad. Sci. U.S.A.1022232–2237. 10.1073/pnas.0409339102
46
Ma X. Zhang Q. Zhu Q. Liu W. Chen Y. Qiu R. et al (2015). A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot Plants.Mol. Plant81274–1284. 10.1016/j.molp.2015.04.007
47
Ma X. Zhu Q. Chen Y. Liu Y. G. (2016). CRISPR/Cas9 platforms for genome editing in plants: developments and applications.Mol. Plant9961–974. 10.1016/j.molp.2016.04.009
48
Mahfouz M. M. Li L. Shamimuzzaman M. Wibowo A. Fang X. Zhu J. K. (2011). De novo-engineered transcription activator-like effector (TALE) hybrid nuclease with novel DNA binding specificity creates double-strand breaks.Proc. Natl. Acad. Sci. U.S.A.1082623–2628. 10.1073/pnas.1019533108
49
Miao Z. H. Lam E. (1995). Targeted disruption of the TGA3 locus in Arabidopsis thaliana.Plant J.7359–365. 10.1046/j.1365-313X.1995.7020359.x
50
Miller J. C. Tan S. Qiao G. Barlow K. A. Wang J. Xia D. F. et al (2011). A TALE nuclease architecture for efficient genome editing.Nat. Biotechnol.29143–148. 10.1038/nbt.1755
51
Moritoh S. Eun C. H. Ono A. Asao H. Okano Y. Yamaguchi K. et al (2012). Targeted disruption of an orthologue of domains rearranged methylase 2, OsDRM2, impairs the growth of rice plants by abnormal DNA methylation.Plant J.7185–98. 10.1111/j.1365-313X.2012.04974.x
52
Nishizawa-Yokoi A. Nonaka S. Osakabe K. Saika H. Toki S. (2015). A universal positive-negative selection system for gene targeting in plants combining an antibiotic resistance gene and its antisense RNA.Plant Physiol.169362–370. 10.1104/pp.15.00638
53
Offringa R. De Groot M. J. Haagsman H. J. Does M. P. Van Den Elzen P. J. Hooykaas P. J. (1990). Extrachromosomal homologous recombination and gene targeting in plant cells after Agrobacterium mediated transformation.EMBO J.93077–3084.
54
Okuzaki A. Toriyama K. (2004). Chimeric RNA/DNA oligonucleotide-directed gene targeting in rice.Plant Cell Rep.22509–512. 10.1007/s00299-003-0698-2
55
Ono A. Yamaguchi K. Fukada-Tanaka S. Terada R. Mitsui T. Iida S. (2012). A null mutation of ROS1a for DNA demethylation in rice is not transmittable to progeny.Plant J.71564–574. 10.1111/j.1365-313X.2012.05009.x
56
Osakabe K. Nishizawa-Yokoi A. Ohtsuki N. Osakabe Y. Toki S. (2014). A mutated cytosine deaminase gene, codA (D314A), as an efficient negative selection marker for gene targeting in rice.Plant Cell Physiol.55658–665. 10.1093/pcp/pct183
57
Osakabe Y. Osakabe K. (2015). Genome editing with engineered nucleases in plants.Plant Cell Physiol.56389–400. 10.1093/pcp/pcu170
58
Osman K. Higgins J. D. Sanchez-Moran E. Armstrong S. J. Franklin F. C. (2011). Pathways to meiotic recombination in Arabidopsis thaliana.New Phytol.190523–544. 10.1111/j.1469-8137.2011.03665.x
59
Ozawa K. Wakasa Y. Ogo Y. Matsuo K. Kawahigashi H. Takaiwa F. (2012). Development of an efficient agrobacterium-mediated gene targeting system for rice and analysis of rice knockouts lacking granule-bound starch synthase (Waxy) and b1,2-xylosyltransferase.Plant Cell Physiol.53755–761. 10.1093/pcp/pcs016
60
Pacher M. Schmidt-Puchta W. Puchta H. (2007). Two unlinked double-strand breaks can induce reciprocal exchanges in plant genomes via homologous recombination and nonhomologous end joining.Genetics17521–29. 10.1534/genetics.106.065185
61
Paszkowski J. Baur M. Bogucki A. Potrykus I. (1988). Gene targeting in plants.EMBO J.74021–4026.
62
Petolino J. F. (2015). Genome editing in plants via designed zinc finger nucleases.In Vitro Cell. Dev. Biol. Plant511–8. 10.1007/s11627-015-9663-3
63
Puchta H. (1998). Repair of genomic double-strand breaks in somatic plant cells by one-sided invasion of homologous sequences.Plant J.13331–339. 10.1046/j.1365-313X.1998.00035.x
64
Puchta H. (2005). The repair of double-strand breaks in plants: mechanisms and consequences for genome evolution.J. Exp. Bot.561–14.
65
Puchta H. Dujon B. Hohn B. (1993). Homologous recombination in plant cells is enhanced by in vivo induction of double strand breaks into DNA by a site-specific endonuclease.Nucleic Acids Res.215034–5040. 10.1093/nar/21.22.5034
66
Puchta H. Dujon B. Hohn B. (1996). Two different but related mechanisms are used in plants for the repair of genomic double-strand breaks by homologous recombination.Proc. Natl. Acad. Sci. U.S.A.935055–5060. 10.1073/pnas.93.10.5055
67
Puchta H. Fauser F. (2013). Gene targeting in plants: 25 years later.Int. J. Dev. Biol.57629–637. 10.1387/ijdb.130194hp
68
Puchta H. Fauser F. (2014). Synthetic nucleases for genome engineering in plants: prospects for a bright future.Plant J.78727–741. 10.1111/tpj.12338
69
Puchta H. Fauser F. (2015). “Double-strand break repair and its application to genome engineering in plants,” inAdvances in New Technology for Targeted Modification of Plant GenomesedsZhangF.PuchtaH.ThomsonJ. G. (New York, NY: Springer) 1–20.
70
Puchta H. Hohn B. (1991a). The mechanism of extrachromosomal homologous DNA recombination in plant cells.Mol. Gen. Genet.2301–7. 10.1007/BF00290641
71
Puchta H. Hohn B. (1991b). A transient assay in plant cells reveals a positive correlation between extrachromosomal recombination rates and length of homologous overlap.Nucleic Acids Res.192693–2700. 10.1093/nar/19.10.2693
72
Qi Y. Zhang Y. Zhang F. Baller J. A. Cleland S. C. Ryu Y. et al (2013). Increasing frequencies of site-specific mutagenesis and gene targeting in Arabidopsis by manipulating DNA repair pathways.Genome Res.23547–554. 10.1101/gr.145557.112
73
Recker J. Knoll A. Puchta H. (2014). The Arabidopsis thaliana homolog of the helicase RTEL1 plays multiple roles in preserving genome stability.Plant Cell264889–4902. 10.1105/tpc.114.132472
74
Reiss B. Schubert I. Köpchen K. Wendeler E. Schell J. Puchta H. (2000). RecA stimulates sister chromatid exchange and the fidelity of double-strand break repair, but not gene targeting, in plants transformed by Agrobacterium.Proc. Natl. Acad. Sci. U.S.A.973358–3363. 10.1073/pnas.97.7.3358
75
Risseeuw E. Offringa R. Franke-Van Dijk M. E. Hooykaas P. J. (1995). Targeted recombination in plants using Agrobacterium coincides with additional rearrangements at the target locus.Plant J.7109–119. 10.1046/j.1365-313X.1995.07010109.x
76
Roth N. Klimesch J. Dukowic-Schulze S. Pacher M. Mannuss A. Puchta H. (2012). The requirement for recombination factors differs considerably between different pathways of homologous double-strand break repair in somatic plant cells.Plant J.72781–790. 10.1111/j.1365-313X.2012.05119.x
77
Sauer N. J. Narvaez-Vasquez J. Mozoruk J. Miller R. B. Warburg Z. J. Woodward M. J. et al (2016). Oligonucleotide-mediated genome editing provides precision and function to engineered nucleases and antibiotics in plants.Plant Physiol.1701917–1928. 10.1104/pp.15.01696
78
Schiml S. Fauser F. Puchta H. (2014). The CRISPR/Cas system can be used as nuclease for in planta gene targeting and as paired nickases for directed mutagenesis in Arabidopsis resulting in heritable progeny.Plant J.801139–1150. 10.1111/tpj.12704
79
Schiml S. Puchta H. (2016). Revolutionizing plant biology: multiple ways of genome engineering by CRISPR/Cas.Plant methods12:8. 10.1186/s13007-016-0103-0
80
Shaked H. Melamed-Bessudo C. Levy A. A. (2005). High-frequency gene targeting in Arabidopsis plants expressing the yeast RAD54 gene.Proc. Natl. Acad. Sci. U.S.A.10212265–12269. 10.1073/pnas.0502601102
81
Shan Q. Wang Y. Chen K. Liang Z. Li J. Zhang Y. et al (2013). Rapid and efficient gene modification in rice and Brachypodium using TALENs.Mol. Plant61365–1368. 10.1093/mp/sss162
82
Shi J. Gao H. Wang H. Lafitte H. R. Archibald R. L. Yang M. et al (2016). ARGOS8 variants generated by CRISPR-Cas9 improve maize grain yield under field drought stress conditions.Plant Biotechnol. J.10.1111/pbi.12603[Epub ahead of print]
83
Shimatani Z. Nishizawa-Yokoi A. Endo M. Toki S. Terada R. (2015). Positive–negative-selection-mediated gene targeting in rice.Front. Plant Sci.5:748. 10.3389/fpls.2014.00748
84
Shrawat A. K. Lorz H. (2006). Agrobacterium-mediated transformation of cereals: a promising approach crossing barriers.Plant Biotechnol. J.4575–603. 10.1111/j.1467-7652.2006.00209.x
85
Shukla V. K. Doyon Y. Miller J. C. Dekelver R. C. Moehle E. A. Worden S. E. et al (2009). Precise genome modification in the crop species Zea mays using zinc-finger nucleases.Nature459437–441. 10.1038/nature07992
86
Siebert R. Puchta H. (2002). Efficient repair of genomic double-strand breaks by homologous recombination between directly repeated sequences in the plant genome.Plant Cell141121–1131. 10.1105/tpc.001727
87
Smith J. Bibikova M. Whitby F. G. Reddy A. R. Chandrasegaran S. Carroll D. (2000). Requirements for double-strand cleavage by chimeric restriction enzymes with zinc finger DNA-recognition domains.Nucleic Acids Res.283361–3369. 10.1093/nar/28.17.3361
88
Steinert J. Schiml S. Puchta H. (2016). Homology-based double-strand break-induced genome engineering in plants.Plant Cell. Rep.351429–1438. 10.1007/s00299-016-1981-3
89
Sun Y. Zhang X. Wu C. He Y. Ma Y. Hou H. et al (2016). Engineering herbicide-resistant rice plants through CRISPR/Cas9-mediated homologous recombination of acetolactate synthase.Mol. Plant9628–631. 10.1016/j.molp.2016.01.001
90
Svitashev S. Young J. K. Schwartz C. Gao H. Falco S. C. Cigan A. M. (2015). Targeted mutagenesis, precise gene editing, and site-specific gene insertion in maize using Cas9 and guide RNA.Plant Physiol.169931–945. 10.1104/pp.15.00793
91
Symington L. S. Gautier J. (2011). Double-strand break end resection and repair pathway choice.Annu. Rev. Genet.45247–271. 10.1146/annurev-genet-110410-132435
92
Terada R. Johzuka-Hisatomi Y. Saitoh M. Asao H. Iida S. (2007). Gene targeting by homologous recombination as a biotechnological tool for rice functional genomics.Plant Physiol.144846–856. 10.1104/pp.107.095992
93
Terada R. Urawa H. Inagaki Y. Tsugane K. Iida S. (2002). Efficient gene targeting by homologous recombination in rice.Nat. Biotechnol.201030–1034. 10.1038/nbt737
94
Tinland B. Hohn B. Puchta H. (1994). Agrobacterium tumefaciens transfers single stranded T-DNA into the plant cell nucleus.Proc. Natl. Acad. Sci. U.S.A.918000–8004. 10.1073/pnas.91.17.8000
95
Townsend J. A. Wright D. A. Winfrey R. J. Fu F. Maeder M. L. Joung J. K. et al (2009). High-frequency modification of plant genes using engineered zinc-finger nucleases.Nature459442–445. 10.1038/nature07845
96
Voytas D. F. Gao C. (2014). Precision genome engineering and agriculture: opportunities and regulatory challenges.PLoS Biol.12:e1001877. 10.1371/journal.pbio.1001877
97
Wang Y. Cheng X. Shan Q. Zhang Y. Liu J. Gao C. et al (2014). Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew.Nat. Biotechnol.32947–951. 10.1038/nbt.2969
98
Weeks D. P. Spalding M. H. Yang B. (2016). Use of designer nucleases for targeted gene and genome editing in plants.Plant Biotechnol. J.14483–495. 10.1111/pbi.12448
99
Weinthal D. M. Taylor R. A. Tzfira T. (2013). Nonhomologous end joining-mediated gene replacement in plant cells.Plant Physiol.162390–400. 10.1104/pp.112.212910
100
Wright D. A. Townsend J. A. Winfrey R. J. Irwin P. A. Rajagopal J. Lonosky P. M. et al (2005). High-frequency homologous recombination in plants mediated by zinc-finger nucleases.Plant J.44693–705. 10.1111/j.1365-313X.2005.02551.x
101
Xie K. Minkenberg B. Yang Y. (2015). Boosting CRISPR/Cas9 multiplex editing capability with the endogenous tRNA-processing system.Proc. Natl. Acad. Sci. U.S.A.1123570–3575. 10.1073/pnas.1420294112
102
Yamauchi T. Johzuka-Hisatomi Y. Fukada-Tanaka S. Terada R. Nakamura I. Iida S. (2009). Homologous recombination-mediated knock-in targeting of the MET1a gene for a maintenance DNA methyltransferase reproducibly reveals dosage-dependent spatiotemporal gene expression in rice.Plant J.60386–396. 10.1111/j.1365-313X.2009.03947.x
103
Zetsche B. Gootenberg J. S. Abudayyeh O. O. Slaymaker I. M. Makarova K. S. Essletzbichler P. et al (2015). Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system.Cell163759–771. 10.1016/j.cell.2015.09.038
104
Zhang F. Maeder M. L. Unger-Wallace E. Hoshaw J. P. Reyon D. Christian M. et al (2010). High frequency targeted mutagenesis in Arabidopsis thaliana using zinc finger nucleases.Proc. Natl. Acad. Sci. U.S.A.10712028–12033. 10.1073/pnas.0914991107
105
Zhang F. Voytas D. F. (2011). Targeted mutagenesis in Arabidopsis using zinc-finger nucleases.Methods Mol. Biol.701167–177. 10.1007/978-1-61737-957-4_9
106
Zhang Y. Zhang F. Li X. Baller J. A. Qi Y. Starker C. G. et al (2013). Transcription activator-like effector nucleases enable efficient plant genome engineering.Plant Physiol.16120–27. 10.1104/pp.112.205179
107
Zhou H. Liu B. Weeks D. P. Spalding M. H. Yang B. (2014). Large chromosomal deletions and heritable small genetic changes induced by CRISPR/Cas9 in rice.Nucleic Acids Res.4210903–10914. 10.1093/nar/gku806
108
Zhu T. Mettenburg K. Peterson D. J. Tagliani L. Baszczynski C. L. (2000). Engineering herbicide-resistant maize using chimeric RNA/DNA oligonucleotides.Nat. Biotechnol.18555–558. 10.1038/75435
Summary
Keywords
clustered regularly interspersed short palindromic repeats (CRISPR)/Cas9, crops, double strand breaks (DSBs), gene targeting (GT), homology-directed repair (HDR), sequence-specific nucleases (SSNs), transcription activator-like effector nucleases (TALENs), zinc finger nucleases (ZFNs)
Citation
Sun Y, Li J and Xia L (2016) Precise Genome Modification via Sequence-Specific Nucleases-Mediated Gene Targeting for Crop Improvement. Front. Plant Sci. 7:1928. doi: 10.3389/fpls.2016.01928
Received
30 September 2016
Accepted
05 December 2016
Published
20 December 2016
Volume
7 - 2016
Edited by
Frank Hartung, Julius Kühn-Institut, Germany
Reviewed by
Andrzej Miroslaw Pacak, Adam Mickiewicz University in Poznañ, Poland; Friedrich Fauser, Carnegie Institution for Science, USA
Updates
Copyright
© 2016 Sun, Li and Xia.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lanqin Xia, xialanqin@caas.cn
This article was submitted to Plant Biotechnology, a section of the journal Frontiers in Plant Science
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.