- 1Department of Aquatic Bioscience, Faculty of Bio-Industry, Tokyo University of Agriculture, Abashiri, Japan
- 2Laboratory of Aquaculture, Genetics and Genomics, Division of Marine Life Science, Faculty of Fisheries Sciences, Hokkaido University, Hakodate, Japan
The marine red seaweed Pyropia yezoensis has a haploid-diploid life cycle wherein two heteromorphic generations, a haploid gametophyte and a diploid sporophyte, are reciprocally generated from conchospores and carpospores, respectively. When we treated gametophytic blades of P. yezoensis with H2O2, discharge of asexual monospores was accelerated, resulting in increased numbers of gametophytic clones. Production of sporophytes without fertilization of male and female gametes was also observed. These findings indicate that oxidative stress can induce vegetative cells to develop into monospores that produce gametophytes asexually and can sometimes prompt carpospores to develop into sporophytes. The discovery of oxidative stress-triggered asexual reproduction and -apogamy will stimulate progress in studies of life-cycle regulation in P. yezoensis.
Introduction
Plants are multicellular organisms that exhibit alternation of ontogenies, such as haploid gametophyte and diploid sporophyte generations, during their life cycles (Coelho et al., 2011a; Bowman et al., 2016; Horst and Reski, 2016), such that a single nuclear genome operates two different developmental programs (Friedman, 2013). Developmental programs for haploid and diploid generations are initiated by meiosis to produce haploid spores and fertilization of male and female gametes to produce diploid spores, respectively. However, homeotic mutations that induce apomixis, i.e., a switch between generation without fertilization or meiosis, have been reported in terrestrial plants (Bell, 1992; Schmidt et al., 2015). Apomixis encompasses two developmental processes, namely apospory (the occurrence of a gametophyte from a sporophyte without meiosis) and apogamy (the occurrence of a sporophyte from a gametophyte without fertilization). Thus, apomixis is a highly useful tool with which to elucidate the regulatory mechanisms of reprograming required for generation switching.
The life-cycle of the red seaweed Pyropia yezoensis, previously referred as Porphyra yezoensis and recently renamed according to the novel classification of Bangiales (Sutherland et al., 2011), has been extensively studied and comprises the reciprocal appearance of free-living haploid gametophytes and diploid sporophytes as leafy blades and filamentous conchocelis as shown in Figure 1 (Sahoo et al., 2002; Shimizu et al., 2008; Blouin et al., 2011; Mikami et al., 2012; Herron et al., 2013). Like other organisms, P. yezoensis requires fertilization and meiosis for the transitions from gametophyte to sporophyte and from sporophyte to gametophyte, respectively (Figure 1). Despite the accumulation of knowledge about its life cycle, mechanisms regulating the generation-to-generation transitions in P. yezoensis have not been well studied to date. The present work sought to provide information about the effect of reactive oxygen species on P. yezoensis reproduction, which is not known. We here found that oxidative stress can promote the generation switch through apogamy in P. yezoensis.
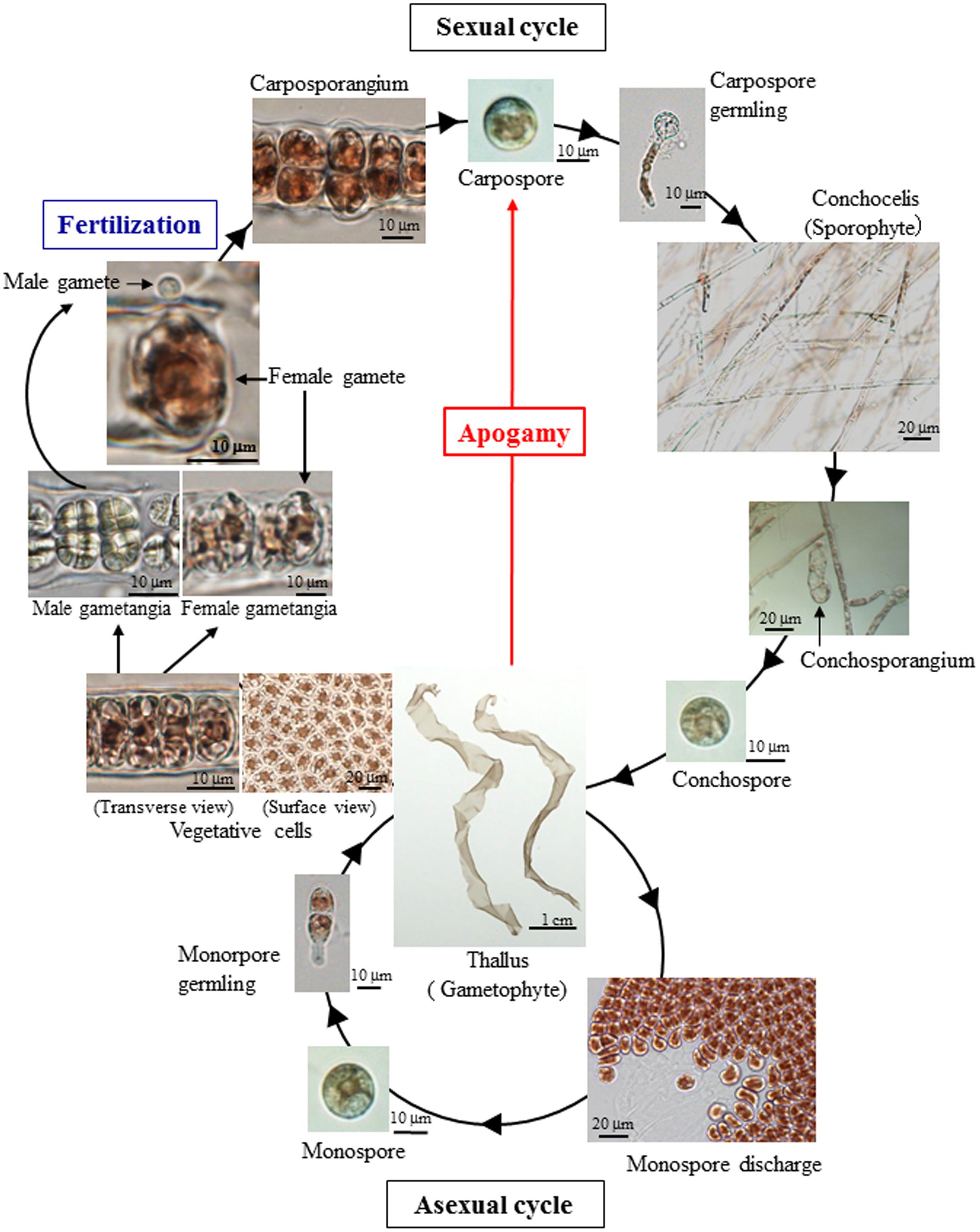
FIGURE 1. Life cycle of the marine red seaweed Pyropia yezoensis. Monospores and carpospores are released from an asexual gametophyte and fertilization-dependent carposporangium, respectively. However, apogamy produces carpospores directly from gametophyte without fertilization.
Materials and Methods
Gametophytic blades of P. yezoensis strain U-51 were cultured in PES medium, which was made using filtered natural seawater with PES [Provasoli’s enriched seawater; Provasoli (1968] solution, under 60 μmol/m2/s irradiance with a photocycle of 10 h light and 14 h dark at 15°C. The PES medium was continuously bubbled with filter-sterilized air and renewed weekly. Gametophytes of ca. 10 mm length (whole blades) were used for experiments. H2O2 was dissolved in distilled water (DW) to create a 0.1 M stock solution. We employed total six blades per experiment by dividing into three sets (two individuals per set) to perform standing-culture using three upper wells of a 6-well culture dish (Iwaki Sci Tech Div., Asahi Techno Glass, Japan) containing 5 mL PES medium for 2 weeks at 15°C with addition of the H2O2 solution at working concentrations indicated in the text or DW corresponding to the maximum volume of the H2O2 stock solution. The concentration of the solutions did not exceed 1% after addition to the medium. Culture medium was renewed weekly by replacing gametophytes to a new well containing new medium. After H2O2 treatment, the numbers of monospore germlings, carpospore germlings and non-germinating spores in each well were counted under an inverted light microscope (CKX-41, Olympus, Tokyo, Japan) equipped with a camera (DP26, Olympus).
Results and Discussion
When each set of two P. yezoensis gametophytes was treated with 0, 0.5, or 1.0 mM H2O2, production and release of asexual monospores was accelerated (Figure 2A), although effects of H2O2 varied among experiments (Table 1). Thus, oxidative stress is one factor promoting monospore discharge for asexual propagation. In plants, it is well known that oxidative stress enhances photosynthesis (Foyer and Shigeoka, 2011) and stimulates Ca2+ influx (Mori and Schroeder, 2004; Rentel and Knight, 2004). We previously reported that a Ca2+ influx that requires photosynthetic activity is responsible for monospore discharge in P. yezoensis (Takahashi et al., 2010). Thus, it is possible that H2O2 treatment of gametophytic thallus activates photosynthesis-dependent Ca2+ influx to promote release of monospores. This possibility would suggest that P. yezoensis may harbor H2O2-dependent Ca2+ transporters.
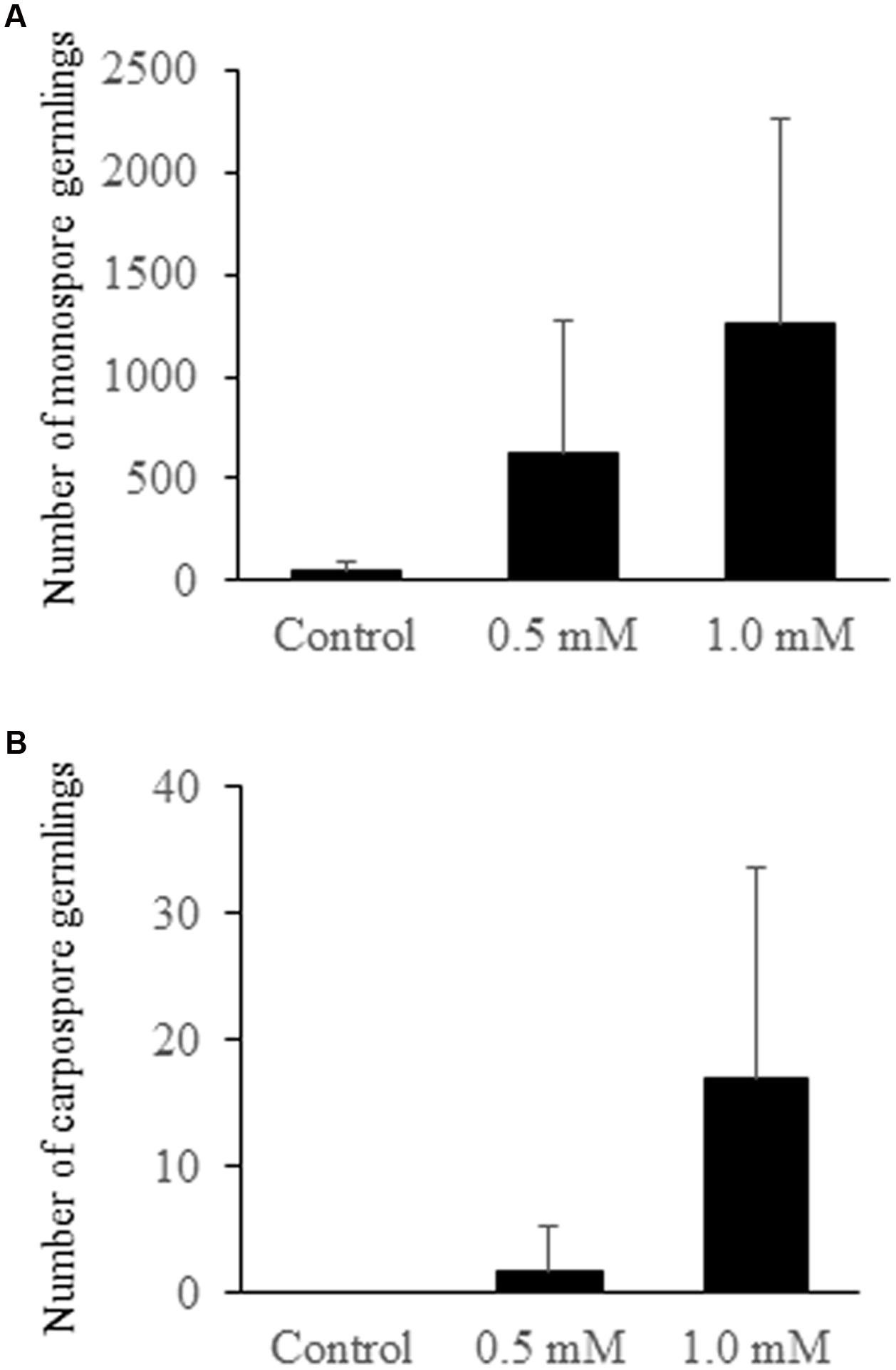
FIGURE 2. Number of germlings from monospores and carpospores released from H2O2-treated gametophytic thallus. (A) Monospore germlings. (B) Carpospore germlings. Error bars indicated ±SD of four independent experiments (see Table 1).
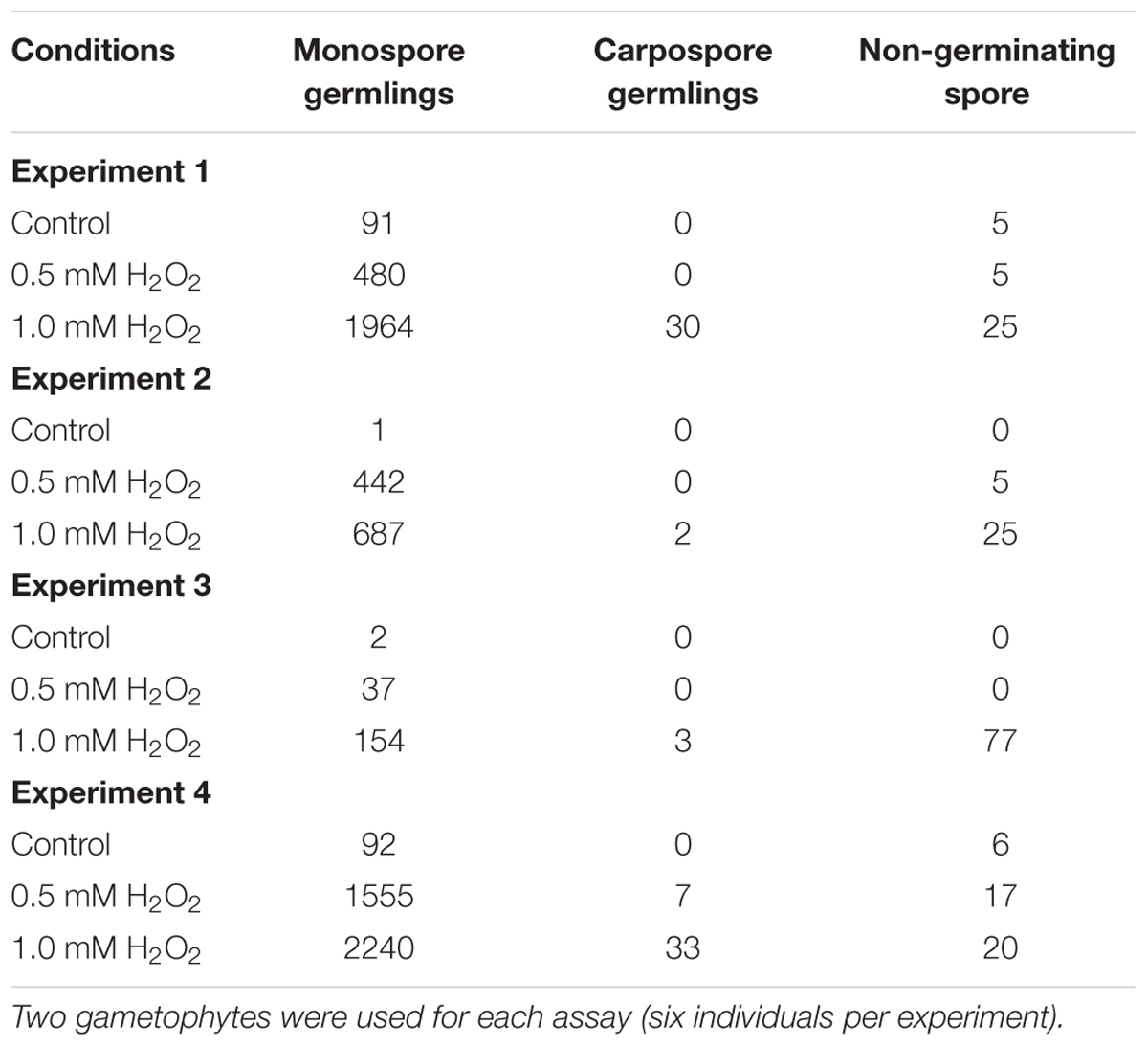
TABLE 1. Number of germlings from monospores and carpospores released from H2O2-treated gametophytic thallus in four independent experiments.
We also observed an induction of apogamy resulting in the production of sporophytes from released spores without development and fertilization of male and female gametes (Figure 3A), although at low-frequency (Figure 2B; Table 1). Since the apogamous sporophytes generated conchosporangia from which conchospores were produced and developed into normal gametophytes (Figures 3B–E), carpospores generated under oxidative stress conditions were indistinguishable from those produced in conchosporangia via the normal life cycle (Figure 1), suggesting diploidy of apogamous sporophytes, although it should be confirmed. Thus, oxidative stress has the potential to reprogram the developmental fate of a gametophytic vegetative cell to produce carpospores from which sporophytes develop. This finding represents the first evidence of abiotic stress-induced apogamy in seaweeds.
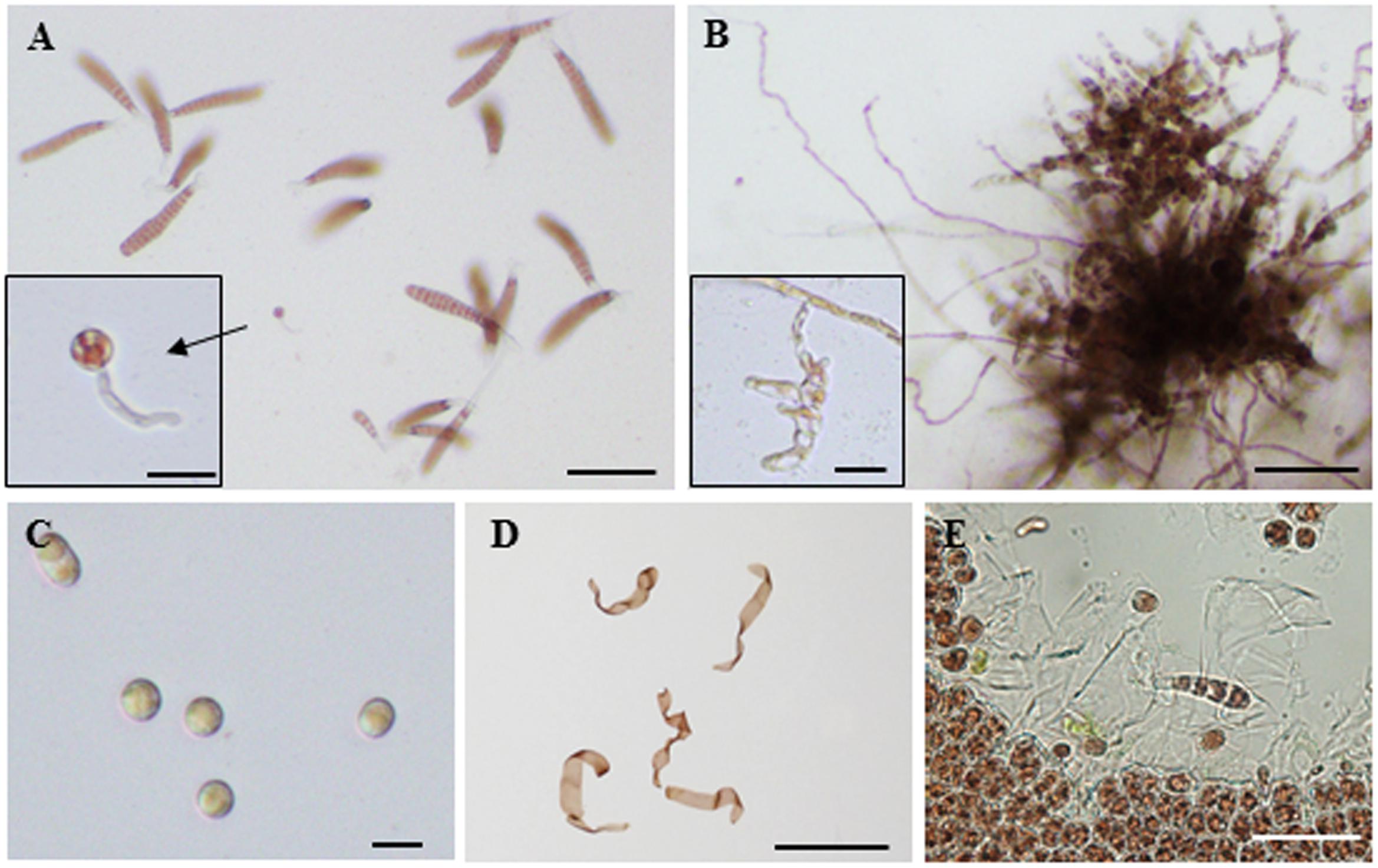
FIGURE 3. Oxidative stress-triggered apogamy in Pyropia yezoensis. (A) Sporophyte and gametophytes released from spores from H2O2-treated gametophytic thallus. Most spores were monospores that developed into gametophytes. Enlarged view shows a sporophyte. (B) Filamentous sporophyte and conchosporangia (enlarged view) developed from a carpospore produced by apogamy. (C) Conchospores produced from conchosporangia. (D) Gametophytes developed from conchospores. (E) Discharge of monospores from gametophytes developed from apogamy-derived conchospores and normally growing monospore germlings. Scale bars = 100 μm (A,B,E), 20 μm (C and enlarged views in A and B), and 1 mm (D).
Previously, it was reported that sporophyte development from gametophytic cells could be artificially induced in the red seaweed Pyropia pseudolinearis when free cells were prepared by treatment of thallus with allantoin followed by homogenization (Saito et al., 2008). Allantoin is a purine metabolite (Bai et al., 2006) that activates jasmonic acid (JA) signaling in an abscisic acid (ABA)-dependent manner in Arabidopsis thaliana (Watanabe et al., 2014; Takagi et al., 2016). Although P. yezoensis lacks endogenous JA, it contains ABA (Mikami et al., 2016). It is possible that allantoin stimulates ABA biosynthesis in P. yezoensis cells, which might accelerate monospore production. In light of our results, wounding-dependent production of H2O2 might influence apogamy, because homogenization of allantoin-treated thallus was required for preparation of free cells (Saito et al., 2008). Indeed, sporophyte development by apogamy has been observed when protoplasts were prepared from gametophytes by artificial digestion of the cell wall (Waaland et al., 1990; Ar Gall et al., 1993). Our observation of H2O2-induced apogamy is consistent with these findings. Alternatively, the function of allantoin as a nitrogen source in algae (Anita et al., 1980; Prasad, 1983) suggests the involvement of nutritional conditions in apogamy in P. yezoensis. Therefore, it is necessary to examine whether ABA- and nitrogen-rich conditions induce apogamy in P. yezoensis and P. pseudolinearis to identify factors related to the initiation of apogamy in red seaweeds.
Elucidation of the relationship between the commitment to a developmental fate and the regulation of life-cycle progression is central to understanding the transitions between life-cycle generations. The H2O2-triggered apogamy identified in the present study suggests the presence of master regulators positively and negatively controlling ontogenies of life cycle generations in P. yezoensis as in terrestrial plants and the brown alga (Peters et al., 2008; Mosquna et al., 2009; Okano et al., 2009; Coelho et al., 2011b; Sakakibara et al., 2013; Horst et al., 2016). Since H2O2 treatment of released monospores did not produce any sporophytes (Takahashi and Mikami, unpublished), the fate of asexual spores appears to be fixed when they are released. Thus, we propose that precursors of thallus-derived unicellular spores have a competency to develop into both gametophyte and sporophyte, and that oxidative stress sometimes stimulates the selection of the conchospore developmental program before spore release. It is possible that distinct factors determining the early developmental process of monospores or carpospores before spore release might exist in P. yezoensis. In fact, we have already demonstrated that development of gametophytes starts with an asymmetrical cell division of the monospore to produce functionally distinct vegetative and rhizoid cells (Li et al., 2008) and also observed that filamentous conchocelis is produced by budding of the initial filament from a carpospore and subsequent elongation via symmetrical cell division and branching (unpublished). Therefore, reprogramming of developmental patterns might be closely related to switching of genetic programs regulating asymmetrical and symmetrical cell division. In addition, as in moss and brown seaweed (Peters et al., 2008; Mosquna et al., 2009; Okano et al., 2009; Coelho et al., 2011a,b; Sakakibara et al., 2013; Horst and Reski, 2016; Horst et al., 2016), it is possible that P. yezoensis has master regulators governing the expression of genes required for determination of gametophyte and sporophyte identities through asymmetrical and symmetrical cell division, respectively. However, these factors remain to be identified.
Identification of master regulators and their target genes, both of which would be involved in determination of the developmental fate of unicellular spores from thallus, in P. yezoensis would help in understanding the relationships between expression of genetic programs regulating ontogenies of each generation and activation of the master regulators. In this respect, the artificial induction of apogamy reported in the present study has the potential to provide a break-through model system. In fact, apogamy in red seaweeds has been reported in Bangia fuscopurpurea and Pyropia haitanensis as spontaneously occurring (Notoya and Iijima, 2003; Yan et al., 2007), suggesting that apogamy is a natural strategy for generation switching in certain red seaweeds. By contrast, P. yezoensis apparently lacks this strategy, which is an advantage for investigating the molecular mechanisms regulating transitions of life-cycle generations in non-apomictic plants, like A. thaliana and Physcomitrella patens (Mosquna et al., 2009; Okano et al., 2009; Sakakibara et al., 2013; Horst et al., 2016).
The oxidative stress-dependent apogamy we have discovered in P. yezoensis provides novel insight into the developmental plasticity of the transitions between gametophytes and sporophytes in the seaweed life cycle and could be a good model for the study of life-cycle regulation.
Author Contributions
MT performed the experiments and collected the data. MT and KM designed the research, analyzed the data, and wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgment
We are grateful to the Marine Resources Research Center of Aichi Fisheries Research Institute for kindly providing P. yezoensis strain U51.
References
Anita, N. J., Berland, B. R., Bonin, D. J., and Maestrini, S. Y. (1980). Allantoin as nitrogen source for growth of marine benthic microalgae. Phycologia 19, 103–109. doi: 10.2216/i0031-8884-19-2-103.1
Ar Gall, E. (Le Gall, Y.), Chiang, Y.-M., and Kloareg, B. (1993). Isolation and regeneration of protoplasts from Porphyra dentata and Porphyra crispate. Eur. J. Phycol. 28, 277–283. doi: 10.1080/09670269300650391
Bai, C., Reilly, C. C., and Wood, B. W. (2006). Nickel deficiency disrupts metabolism of ureides, amino acids, and organic acid of young pecan foliage. Plant Physiol. 140, 433–443. doi: 10.1104/pp.105.072983
Bell, P. R. (1992). Apospory and apogamy: implication for understanding the plant life cycle. Int. J. Plant Sci. 153, 123–136. doi: 10.1086/297070
Blouin, N. A., Brodie, J. A., Grossman, A. C., Xu, P., and Brawley, S. H. (2011). Porphyra: a marine crop shaped by stress. Trends Plant Sci. 11, 29–37. doi: 10.1016/j.tplants.2010.10.004
Bowman, J. L., Sakakibara, K., Furumizu, C., and Dierschke, T. (2016). Evolution in the cycles of life. Annu. Rev. Genet. 50, 133–154. doi: 10.1146/annurev-genet-120215-035227
Coelho, S. M., Godfroy, O., Arun, A., Le Corguillé, G., Peters, A. F., and Cock, J. M. (2011a). Genetic regulation of life cycle transitions in the brown alga Ectocarpus. Plant Signal. Behav. 6, 1858–1860. doi: 10.4161/psb.6.11.17737
Coelho, S. M., Godfroy, O., Arun, A., Le Corguillé, G., Peters, A. F., and Cock, J. M. (2011b). OUROBOROS is a master regulator of the gametophyte to sporophyte life cycle transition in the brown alga Ectocarpus. Proc. Natl. Acad. Sci. U.S.A. 108, 11518–11523. doi: 10.1073/pnas.1102274108
Foyer, C. H., and Shigeoka, S. (2011). Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 155, 93–100. doi: 10.1104/pp.110.166181
Friedman, W. E. (2013). One genome, two ontogenies. Science 339, 1045–1046. doi: 10.1126/science.1234992
Herron, M. D., Rashidi, A., Shelton, D. E., and Driscoll, W. W. (2013). Cellular differentiation and individuality in the ‘minor’ multicellular taxa. Biol. Rev. Camb. Philos. Soc. 88, 844–861. doi: 10.1111/brv.12031
Horst, N. A., Katz, A., Pereman, I., Decker, E. L., Ohad, N., and Reski, R. (2016). A single homeobox gene triggers phase transition, embryogenesis and asexual reproduction. Nat. Plants 2:15209. doi: 10.1038/nplants.2015.209
Horst, N. A., and Reski, R. (2016). Alternation of generations - unravelling the underlying molecular mechanism of a 165-year-old botanical observation. Plant Biol. 18, 549–551. doi: 10.1111/plb.12468
Li, L., Saga, N., and Mikami, K. (2008). Phosphatidylinositol 3-kinase activity and asymmetrical accumulation of F-actin are necessary for establishment of cell polarity in the early development of monospores from the marine red alga Porphyra yezoensis. J. Exp. Bot. 59, 3575–3586. doi: 10.1093/jxb/ern207
Mikami, K., Li, L., and Takahashi, M. (2012). “Monospore-based asexual life cycle in Porphyra yezoensis,” in Porphyra yezoensis: Frontiers in Physiological and Molecular Biological Research, ed. K. Mikami (New York: Nova Science Publishers), 15–37.
Mikami, K., Mori, I. C., Matsuura, T., Ikeda, Y., Kojima, M., Sakakibara, H., et al. (2016). Comprehensive quantification and genome survey reveal the presence of novel phytohormone action modes in red seaweeds. J. Appl. Phycol. 28, 2539–2548. doi: 10.1007/s10811-015-0759-2
Mori, I. C., and Schroeder, J. I. (2004). Reactive oxygen species activation of plant Ca2+ channels. A signaling mechanism in polar growth, hormone transduction, stress signaling, and hypothetically mechanotransduction. Plant Physiol. 135, 702–708. doi: 10.1104/pp.104.042069
Mosquna, A., Katz, A., Decker, E. L., Rensing, S. A., Reski, R., and Ohad, N. (2009). Regulation of stem cell maintenance by the Polycomb protein FIE has been conserved during land plant evolution. Development 136, 2433–2444. doi: 10.1242/dev.035048
Notoya, M., and Iijima, N. (2003). Life history and sexuality of archeospore and apogamy of Bangia atropurpurea (Roth) Lyngbye (Bangiales, Rhodophyta) from Fukaura and Enoshima, Japan. Fish. Sci. 69, 799–805.
Okano, Y., Aono, N., Hiwatashi, Y., Murata, T., Nishiyama, T., Ishikawa, T., et al. (2009). A polycomb repressive complex 2 gene regulates apogamy and gives evolutionary insights into early land plant evolution. Proc. Natl. Acad. Sci. U.S.A. 106, 16321–16326. doi: 10.1073/pnas.0906997106
Peters, A. F., Scornet, D., Ratin, M., Charrier, B., Monnier, A., Merrien, Y., et al. (2008). Life-cycle- generation-specific developmental processes are modified in the immediate upright mutant of the brown alga Ectocarpus siliculosus. Development 135, 1503–1512. doi: 10.1242/dev.016303
Prasad, P. V. D. (1983). Hypoxanthine and allantoin as nitrogen sources for the growth of some freshwater green algae. New Phytol. 93, 575–580. doi: 10.1111/j.1469-8137.1983.tb02708.x
Provasoli, L. (1968). “Media and prospects for the cultivation of marine algae,” in Proceedings of the U.S.-Japan Conference: Cultures and Collections of Algae, eds A. Watanabe and A. Hattori (Hakone: Japanese Society of Plant Physiology), 63–75.
Rentel, M. C., and Knight, M. R. (2004). Oxidative stress-induced calcium signaling in Arabidopsis. Plant Physiol. 135, 1471–1479. doi: 10.1104/pp.104.042663
Sahoo, D., Tang, X., and Yarish, C. (2002). Porphyra} – the economic seaweed as a new experimental system. Curr. Sci. 83, 1313–1316.
Saito, A., Mizuta, H., Yasui, H., and Saga, N. (2008). Artificial production of regenerable free cells in the gametophyte of Porphyra pseudolinearis (Bangiales, Rhodophyceae). Aquaculture 281, 138–144. doi: 10.1016/j.aquaculture.2008.06.012
Sakakibara, K., Ando, S., Yip, H. K., Tamada, Y., Hiwatashi, Y., Murata, T., et al. (2013). KNOX2 genes regulate the haploid-to-diploid morphological transition in land plants. Science 339, 1067–1070. doi: 10.1126/science.1230082
Schmidt, A., Schmid, M. W., and Grossniklaus, U. (2015). Plant germline formation: common concepts and developmental flexibility in sexual and asexual reproduction. Development 142, 229–241. doi: 10.1242/dev.102103
Shimizu, A., Morishima, K., Kobayashi, M., Kunimoto, M., and Nakayama, I. (2008). Identification of Porphyra yezoensis (Rhodophyta) meiosis by DNA quantification using confocal laser scanning microscopy. J. Appl. Phycol. 20, 83–88. doi: 10.1007/s10811-007-9184-5
Sutherland, J. E., Lindstrom, S. C., Nelson, W. A., Brodie, J., Lynch, M. D. J., Hwang, M. S., et al. (2011). A new look at an ancient order: generic revision of the Bangiales (Rhodophyta). J. Phycol. 47, 1131–1151. doi: 10.1111/j.1529-8817.2011.01052.x
Takagi, H., Ishiga, Y., Watanabe, S., Konishi, T., Egusa, M., Akiyoshi, N., et al. (2016). Allantoin, a stress-related purine metabolite, can activate jasmonate signaling in a MYC2-regulated and abscisic acid-dependent manner. J. Exp. Bot. 67, 2519–2532. doi: 10.1093/jxb/erw071
Takahashi, M., Saga, N., and Mikami, K. (2010). Photosynthesis-dependent extracellular Ca2+ influx triggers an asexual reproductive cycle in the marine red macroalga Porphyra yezoensis. Am. J. Plant Sci. 1, 1–11. doi: 10.4236/ajps.2010.11001
Waaland, J. R., Dickson, L. G., and Watson, B. A. (1990). Protoplast isolation and regeneration in the marine red alga Porphyra nereocystis. Planta 181, 522–528. doi: 10.1007/BF00193005
Watanabe, S., Matsumoto, M., Hakomori, Y., Takagi, H., Shimada, H., and Sakamoto, A. (2014). The purine metabolite allantoin enhances abiotic stress tolerance through synergistic activation of abscisic acid metabolism. Plant Cell Environ. 37, 1022–1036. doi: 10.1111/pce.12218
Keywords: apogamy, oxidative stress, life cycle, generation switch, Pyropia yezoensis
Citation: Takahashi M and Mikami K (2017) Oxidative Stress Promotes Asexual Reproduction and Apogamy in the Red Seaweed Pyropia yezoensis. Front. Plant Sci. 8:62. doi: 10.3389/fpls.2017.00062
Received: 21 October 2016; Accepted: 11 January 2017;
Published: 27 January 2017.
Edited by:
Stefan A. Rensing, University of Marburg, GermanyReviewed by:
Nayelli Marsch-Martinez, Centro de Investigacion y de Estudios Avanzados del Instituto Politecnico Nacional, MexicoJonas Collén, Université Pierre et Marie Curie, France
Copyright © 2017 Takahashi and Mikami. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Koji Mikami, a29taWthbWlAZmlzaC5ob2t1ZGFpLmFjLmpw
 Megumu Takahashi
Megumu Takahashi Koji Mikami
Koji Mikami